Abstracts from the Seventeenth International Kidney Cancer Symposium, 2nd-3rd November 2018, Miami, Florida
CONTENTS
01 A decision analysis of screening for renal cancer using focused renal ultrasound S1
02 A phase II trial of intermittent nivolumab in patients (pts) with metastatic renal cell carcinoma (mRCC) who have received prior anti-angiogenic therapy (NCT03126331) S1
03 A Phase III study of atezolizumab vs placebo as adjuvant therapy in patients with renal cell carcinoma at high risk of recurrence following resection (IMmotion010) S2
04 A Prospective Study of Pediatric Renal Cell Carcinoma: A Report From the Children’s Oncology Group (COG) Study AREN0321 S3
05 An evaluation of the role of cytoreductive nephrectomy in patients with sarcomatoid RCC S4
06 An evolutionary comprehensive psychiatric approach to prolong survival: Revival after brain seizure & craniotomy of mRCC (Stage IV mRCC with rare VHL mutation) current patient use case & journey S5
07 Analysis of First-line Sunitinib Treatment Duration on Clinical Outcomes in Patients with Metastatic Renal Cell Carcinoma (mRCC) Receiving Subsequent Immuno-oncology (IO) Checkpoint Inhibitors S6
08 Association of VHL mutation status and clinical response to pazopanib in Mexican patients with clear renal cell carcinoma S7
09 Blood based biomarkers and their association with overall survival (OS) and progression-free survival (PFS) in patients with metastatic renal cell carcinoma (mRCC) treated with immunotherapy (IO) S7
10 Body mass index (BMI) as a predictor of treatment outcome for patients receiving systemic therapy for metastatic renal cell carcinoma (mRCC) S10
11 Body mass index (BMI) as a prognostic indicator of survival in metastatic renal cell carcinoma (mRCC) patients treated with immune checkpoint inhibitors (ICI) S10
12 Characterization of Response to Nivolumab Plus Ipilimumab or Sunitinib in Patients With Previously Untreated Advanced Renal Cell Carcinoma: CheckMate 214 S12
13 CheckMate 214 Retrospective Analyses of Nivolumab Plus Ipilimumab or Sunitinib in IMDC Intermediate/Poor-risk Patients With Previously Untreated Advanced Renal Cell Carcinoma With Sarcomatoid Features S13
14 Clonality estimates of oncogenic events and identification of ccRCC subtypes S14
15 Coordinated pembrolizumab and high dose IL-2 (5-in-a-row schedule) schedule for therapy of metastatic clear cell renal cancer, a single center, single arm trial. NCT02964078 S15
16 Cytoreductive nephrectomy for non-clear cell RCC: NLR predicts survival outcomes S16
17 Disparities in the Management of Clinical T1a and T1b Renal Masses Amoung Pateints in the National Cancer Database (NCDB) S17
18 Identification of novel epidermal growth factor receptor (EGFR) splice variants in clear cell renal cell carcinoma S18
19 Immune response in patients treated with autologous dendritic cells transduced with AdGMCA9 (DC-AdGMCAIX) in patients with metastatic renal cell carcinoma from the phase I, open label, dose escalation and cohort expansion study S18
20 Ipilimumab plus Nivolumab (Ipi/Nivo) as Salvage Therapy in Patients with Immunotherapy (IO)-Refractory Metastatic Renal Cell Carcinoma (mRCC) S19
21 Mucinous tubular and spindle-cell carcinoma (MSTCC) of the kidney: Patient characteristics, genomic profile, and treatment outcome S20
22 Neutrophil-to-lymphocyte ratio predicts recurrence-free survival in unclassified renal cell carcinoma S21
23 Nivolumab for the treatment of Metastatic Renal cancer-Retrospective Audit study S21
24 Overall and progression-free survival in metastatic renal cell carcinoma (mRCC) patients treated with first-line tyrosine kinase inhibitors (TKI) followed by second-line immunotherapy S22
25 Patient-reported Outcomes Among Those Taking Pazopanib for Metastatic Renal Cell Carcinoma (mRCC) in a Community Oncology Setting S23
26 Pazopanib-induced liver toxicity in metastatic renal cell carcinoma (mRCC) patients: impact of UGT1A1 polymorphism on pazopanib dose reduction, safety and patient outcome S24
27 Phase 1b study (COSMIC-021) of cabozantinib in combination with atezolizumab: Results of the dose escalation stage in patients with treatment-naïve advanced renal cell carcinoma (RCC) S24
28 Phase 2 Study of Sequential First-line Pazopanib (PAZ) Followed by Everolimus (EVE) in Patients (pts) with Advanced or Metastatic Renal Cell Carcinoma (RCC) (CATChEz Study) S25
29 Phase II Trial of Intermittent Therapy in Patients (pts) with Metastatic Renal Cell Carcinoma (mRCC) Treated with Front-line Ipilimumab and Nivolumab (Ipi/Nivo) S26
30 Predictors of Receiving a Lymph Node Dissection at the Time of Surgery for Non-Metastatic Renal Cell Carcinoma S26
31 Preliminary investigation of Radiogenomics in sarcomatoid dedifferentiation of renal cell carcinoma S27
32 Prognostic Implications in Renal Cell Carcinoma Survival: Large Cohort Evidence (Surveillance Epidemiology and End Results) Dataset S28
33 PROSPER: A phase III randomized study comparing perioperative nivolumab (nivo) vs. observation in patients with localized renal cell carcinoma (RCC) undergoing nephrectomy (ECOG-ACRIN 8143) S28
34 Racial differences in clinical outcomes of metastatic renal cell carcinoma patients treated with immune checkpoint inhibitors S29
35 Real-World Effectiveness and Tolerability of Pazopanib as First Targeted Therapy in Metastatic Renal Cell Carcinoma: A Retrospective Chart Review in Latin America S30
36 Safety and activity of immune checkpoint inhibitors in patients with advanced renal cell carcinoma (RCC) and pre-existing autoimmune disorders (AD) S31
37 Sarcomatoid Features in Renal Cell Carcinoma: Rethinking the Stage-Survival Paradigm S31
38 Second-Line VEGF Receptor TKI Outcomes after First-Line Immune Checkpoint Blockade in Metastatic Renal Cell Carcinoma S33
39 Sites of distant metastatic disease and association with clinical outcomes in metastatic renal cell carcinoma (mRCC) patients treated with immune checkpoint inhibitors (ICI) S33
40 The EVERPRO Study: Final Results of a Non-Interventional Study Evaluating the Quality of Life (QoL) in Second-Line Treatment of Metastatic Renal Cell Carcinoma (mRCC) with Everolimus S35
41 The Oncologic Outcome of Partial and Radical Nephrectomy in Localized Sarcomatoid Renal Cell Carcinoma S35
42 Treatment of metastatic Non clear cell Renal Cell Carcinoma (nccRCC) with ipilimumab and nivolumab S36
43 Treatment-Free Survival Following Discontinuation of First-Line Nivolumab Plus Ipilimumab or Sunitinib in Patients With Advanced Renal Cell Carcinoma: CheckMate 214 Analysis S37
44 Trial in Progress – Children’s Oncology Group (COG) Study AREN 1721: A Randomized Phase 2 Trial of Axitinib/Nivolumab combination therapy vs single agent Axitinib or Nivolumab for Translocation Renal Cell Carcinoma (tRCC) across all age groups S38
Abstracts
01A decision analysis of screening for renal cancer using focused renal ultrasound
Rossi, Sabrina
Author Company: University of Cambridge
Background and aims: Screening for renal cell carcinoma (RCC) has been identified as a key research priority, however several uncertainties remain. This thesis aims to perform a decision analysis to determine the optimal screening population based on the current available evidence and the value of performing further research into this topic.
Methods: Screening was defined as a “one-off” focused renal ultrasound scan, delivered by technicians in primary care. A cohort simulation model was developed to compare screening asymptomatic individuals versus the standard of care, adopting a National Health Service perspective. The expected lifetime costs, quality adjusted life years (QALYs) and incremental cost-effectiveness ratio (ICER) were determined, using a 3.5% discount rate. Different screening populations were simulated, based on age and gender, to determine the optimal screening strategy. Probabilistic sensitivity analysis was performed to assess uncertainty. The expected value of perfect information (EVPI) and perfect parameter information (EVPPI) were determined.
Results: Given a prevalence of RCC of 0.17% (0.09-0.27%), screening individuals aged 55 years (both genders) resulted in an ICER of £26,125/QALY; with a 64% probability of being below £30,000. The key determinants of cost-effectiveness were age, gender, the prevalence of RCC and the cost of ultrasound. Reducing the cost of the screening ultrasound from £37 to £20, would lower the ICER to £18,088/QALY for 55-year olds and £27,947/QALY for 65-year olds. Given a willingness to pay threshold of £30,000, the population EVPI was £273 million. The prevalence of RCC and the stage distribution of RCC detected by screening were the parameters with the highest population EVPPI (£24.7 and £24.5 million respectively).
Conclusion: This work represents the first decision analysis of population screening for RCC. Uncertainty remains regarding the true prevalence of RCC by age and gender. The EVPI suggests further research into this topic may be a good use of NHS resources.
02A phase II trial of intermittent nivolumab in patients (pts) with metastatic renal cell carcinoma (mRCC) who have received prior anti-angiogenic therapy (NCT03126331)
Ornstein, M.D., MA, Moshe C.
Author Company: Cleveland Clinic
Co Authors: Wood, Laura S.; Allman, Kimberly D.; Martin, Allison; Gilligan, Timothy D.; Garcia, Jorge A.; Rini, Brian I.
Background: Nivolumab is approved for mRCC pts who have received prior anti-angiogenic therapy but the duration of therapy required for sustained clinical benefit is unknown. A phase II clinical trial to investigate the feasibility of intermittent nivolumab dosing was conducted (NCT03126331).
Methods: Pts with mRCC of any histology who received at least one prior anti-angiogenic therapy were treated with nivolumab (240mg every 2 weeks or 480mg every 4 weeks). Pts with = 10% reduction in tumor burden (TB) following 12 weeks had nivolumab held, with re-staging CT scans approximately every 12 weeks. Nivolumab was re-initiated in those patients with an increase in TB = 10% and again held with = 10% TB reduction. This intermittent nivolumab dosing continued until RECIST PD while on nivolumab. Pts not initially achieving at least a 10% reduction in TB continued nivolumab per standard of care. The primary objective was feasibility of intermittent nivolumab, defined as the proportion of pts eligible for intermittent therapy who elect to receive intermittent nivolumab. The alternative hypothesis is a feasibility rate of > 80% vs. the null hypothesis of < 50%. Forty pts provides 80% power based on a two-sided exact test with a .05 type I error. With the approval of the combination of ipilimumab & nivolumab (Ipi/Nivo) in front-line mRCC and a host of other frontline immunotherapy combination trials, this cohort was closed prior to completed pre-planned approval. A separate cohort is opening to include pts who receive Ipi/Nivo.
Results: Fourteen pts with mRCC were included; 93% male, median age 65, all had prior nephrectomy, 93% clear-cell histology, 93% KPS = 80%, and 86% were intermediate-risk by IMDC criteria. Metastatic sites were typical for mRCC. Twelve pts (86%) received only one prior anti-angiogenic therapy (8 sunitinib; 2 pazopanib; 2 axitinib). Two pts received 3 prior therapies. In total, four (26%) of the fourteen pts met criteria for intermittent therapy and all entered the intermittent phase. Median TB decrease for pts entering intermittent phase was -48% (range, -22 to -91%). One patient restarted therapy after a 12 week break given some non-PD growth in non-target lesions and remains on nivolumab. Three patients remain off therapy with sustained treatment response for a median of 18 weeks (range, 12-36) off therapy. No pt had RECIST-defined PD while on treatment break.
Conclusions: This prospective experience of intermittent nivolumab dosing in mRCC supports further investigation of intermittent immunotherapy dosing strategies in RCC. Updated efficacy and outcome data will be presented.
03A Phase III study of atezolizumab vs placebo as adjuvant therapy in patients with renal cell carcinoma at high risk of recurrence following resection (IMmotion010)
Pal, M.D., Sumanta Kumar
Author Company: City of Hope Comprehensive Cancer Center
Co Authors: Uzzo, Robert G. (Fox Chase Cancer Center); Rini, Brian (Cleveland Clinic); Albiges, Laurence (Institut Gustave- Roussy); Suárez, Cristina; Shen, Xiaodong; Qiu, Jiaheng (Genentech); Hashimoto, Kenji (Roche); Bex, Axel (The Netherlands)
Introduction: In early-stage renal cell carcinoma (RCC), nephrectomy is the standard of care. However, for patients with stage II or III disease, the 5-year relapse rate after surgery is 30%-40%, with survival and recurrence correlating with tumor stage and grade. Currently, the role for adjuvant therapy after nephrectomy in patients who have had complete tumor resection is limited; observation is standard. In a randomized Phase II study of first-line metastatic RCC (IMmotion150, NCT01984242), treatment with single-agent atezolizumab (anti–PD-L1) resulted in an objective response rate of 25% for intent-to-treat patients and 28% for patients with PD-L1 expression on = 1% of tumor-infiltrating immune cells (IC; McDermott, et al. Nat Med. 2018). In addition, atezolizumab was well tolerated, supporting its potential use in the adjuvant setting. IMmotion010, a Phase III, multicenter, randomized, placebo-controlled, double-blinded trial, will evaluate the efficacy and safety of atezolizumab as adjuvant therapy in patients with RCC who are at high risk of recurrence following resection (NCT03024996).
Materials and methods: IMmotion010 is enrolling patients with RCC (clear cell or sarcomatoid histologies) who have undergone nephrectomy (radical or partial) and are at high risk of recurrence (T2 Grade 4, T3a Grade 3-4, T3b/c any Grade, T4 any Grade or TxN+ any Grade) or who have had complete resection of limited metachronous/synchronous metastasis. Eligible patients must show no residual disease or evidence of metastases by CT scan at enrollment. ECOG PS = 1 and PD-L1–evaluable tumor specimens will also be required. Patients will be randomized 1:1 to receive atezolizumab 1200 mg IV q3w or placebo IV q3w for 16 cycles or 1 year; disease stage (T2/T3a vs T3b/c/T4/N+ vs metastasectomy), region (North America [excluding Mexico] vs other countries) and PD-L1 IC expression status (< 1% vs = 1%, per SP142 IHC assay) will be used as stratification factors. The primary endpoint is independent review facility (IRF)-assessed disease-free survival (DFS), defined as the time from randomization to the first documented recurrence event (local recurrence, new primary RCC, distant metastasis) or death. Secondary endpoints include overall survival, investigator (INV)-assessed DFS, IRF-assessed and INV-assessed DFS in patients with = 1% PD-L1 IC, disease-specific survival, distant metastasis-free survival and IRF-assessed DFS and INV-assessed DFS at 3 years. Ethical committee approval has been obtained. Safety and biomarkers will also be evaluated. Target enrollment is 664 patients across ˜200 sites worldwide. The planned analysis of the primary endpoint will occur when 50% of patients have had a DFS event. Planned protocol amendments will be presented.
04A Prospective Study of Pediatric Renal Cell Carcinoma: A Report From the Children’s Oncology Group (COG) Study AREN0321
Cost, M.D., Nicholas G.
Author Company: University of Colorado School of Medicine
Co Authors: James I. Geller, Mariana Cajaiba, Yueh-Yun Chi, Elizabeth J. Perlman, Yeonil Kim, Elizabeth A. Mullen, Richard D. Glick, Geetika Khanna, Najat C. Daw, Peter F. Ehrlich, Conrad V. Fernandez, Jeffrey S. Dome
Background: Although renal cell carcinoma (RCC) is the second most common pediatric kidney cancer, no prospective clinical trials have been conducted. AREN0321 sought to establish biological, epidemiological, and outcome data for pediatric RCC in a prospective manner, and to confirm that completely resected pediatric RCC, including those with N1 disease, has a favorable prognosis without adjuvant medical therapy.
Methods: From 2006 to 2012, patients <30yr old with centrally reviewed pathology confirmation of RCC were prospectively enrolled. Patients with completely resected disease, including those with N1 disease, were not offered adjuvant therapy. Patients with incompletely resected disease were treated per local investigator choice. Data on demographics, histology, stage, surgery, and oncologic outcome were evaluated.
Results: Sixty-eight patients enrolled (39 male; median age 13.0 yr (range 0.17 - 22.1)). Stage distribution (minus 1 patient without available staging data) was: I (26, 38.8%), II (7, 10.4%), III (26, 38.8%) and IV (8, 11.9%). Sixty-five (95.6%) had definitive surgery of the kidney tumor and 60 (88.2%) attained a complete response with a combination of systemic therapy and surgical resection. Fifty-eight (85.3%) had resection of the renal primary and all other known sites of disease at diagnosis and 2 (2.9%) had initial nephrectomy followed by eventual resection of all residual disease. For the 8 patients that never had all disease completely resected, 6 were stage IV, 1 stage III, and 1 stage indeterminate. Definitive renal tumor surgery included radical nephrectomy (53 (81.5%)) and partial nephrectomy (12 (18.5%)), via an open approach in 50 (76.9%) and laparoscopic in 15 (23.1%). Histology was: TFE-associated RCC (tRCC; 35, 51.4%), RCC NOS/other (22, 32.4%), Renal Medullary Carcinoma (RMC; 6, 8.8%), and papillary RCC (5, 7.4%). Lymph node (LN) status was: N0 (21 (30.9%)), N1 (21 (30.9%)), and Nx (26 (38.2%)). Histology for patients with N1 disease was: TRCC (13/35, 37.1%), RCC NOS/other (5/22, 22.7%) and RMC (3/6, 50%).
Four-year EFS and OS were 80.2% (95% CI 69.6-90.9) and 86.3% (76.9-95.7), respectively. Four-year EFS and OS for those with Stage I-III disease that had all disease completely resected at diagnosis were: 87.2% (95% CI 77.0 – 97.4) and 94.6% (87.6-100), respectively. Specifically, in those with nodal-spread only, 16 patients had N1M0 disease and 15 (93.8%) had this completely resected (12 tRCC, 3 RCC NOS/other), for which the 4yr EFS and OS was 87.5% (68.3-100) and 93.8% (79.2-100), respectively. Data regarding medical treatments for those with unresectable or relapsed disease were limited.
Conclusion: Favorable outcomes can be achieved without adjuvant therapy in children and adolescents with completely resected, locally-advanced disease, independent of LN status. Future study of patients with M1 RCC is needed to optimize treatment for this group, as is planned for tRCC (COG AREN1721).
Disclaimer: A version of this abstract was presented at an American Society of Clinical Oncology (ASCO) meeting, and thus ASCO holds the copyright to the abstract. A credit line must be published with the re-publication of the abstract as follows: “© 2018 American Society of Clinical Oncology, Inc. Reused with permission. This abstract was accepted and previously presented at the 2018 ASCO Annual Meeting. All rights reserved.
Table
Survival outcomes
| n | 4yr EFS (95% CI) | p-value | 4yr OS (95% CI) | p-value | |
| Histology | 68 | <0.001 | <0.001 | ||
| tRCC | 82.7% (68.6-96.7) | 88.3% (75.9-100) | |||
| RCC NOS/other | 84.2% (66.0-100) | 95.2% (84.7-100) | |||
| RMC | 33.3% (0-71.1) | 33.3% (0-71.1) | |||
| Papillary RCC | 100% | 100% | |||
| Age | 68 | 0.36 | 0.46 | ||
| ≤13yo | 75.7% (59.7-91.7), | 84.8% (71.0-98.6) | |||
| >13yo | 82.4% (67.9-96.8) | 90.3% (78.8-100) | |||
| Stage1 | 58 | 0.29 | 0.72 | ||
| I | 92.2% (80.8-100) | 96.0% (87.8-100) | |||
| II | 100% | 100% | |||
| III | 78.6% (60.2-96.9) | 91.6% (78.7-100) |
1 ߝ 58 Stage I-III pts with complete resection
05An evaluation of the role of cytoreductive nephrectomy in patients with sarcomatoid RCC
Silagy AW, Blum KA, Mano R, Gupta S, Marcon J, Dinatale RG, Tickoo SK, Coleman JA, Russo P, Hakimi AA.
Urology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY.
Introduction: Renal cell carcinoma with sarcomatoid dedifferentiation (sRCC) is often associated with metastatic disease at presentation and a uniformly poor prognosis; thus, the role of cytoreductive nephrectomy in this tumor subtype is contentious. We sought to evaluate the outcomes of patients with sRCC who have undergone cytoreductive nephrectomy to determine which pre-operative factors predict survival outcomes.
Methods: After obtaining IRB approval, the medical records of 514 patients with sRCC were systematically reviewed for treatment, metastatic patterns and survival outcomes. Patients who had distant metastases at nephrectomy or developed metastatic disease within 30 days after the procedure were considered to have undergone a cytoreductive nephrectomy. Univariate and multivariate cox regression analyses were used to identify significant predictors of overall and cancer specific survival.
Results: 225 patients underwent a cytoreductive nephrectomy, with a median age of 59 years (IQR 52-66). 51 patients had a biopsy prior to nephrectomy, with a sensitivity for sRCC of 64.7%. Lung/pleura and abdominal cavity were the most common locations for metastases.
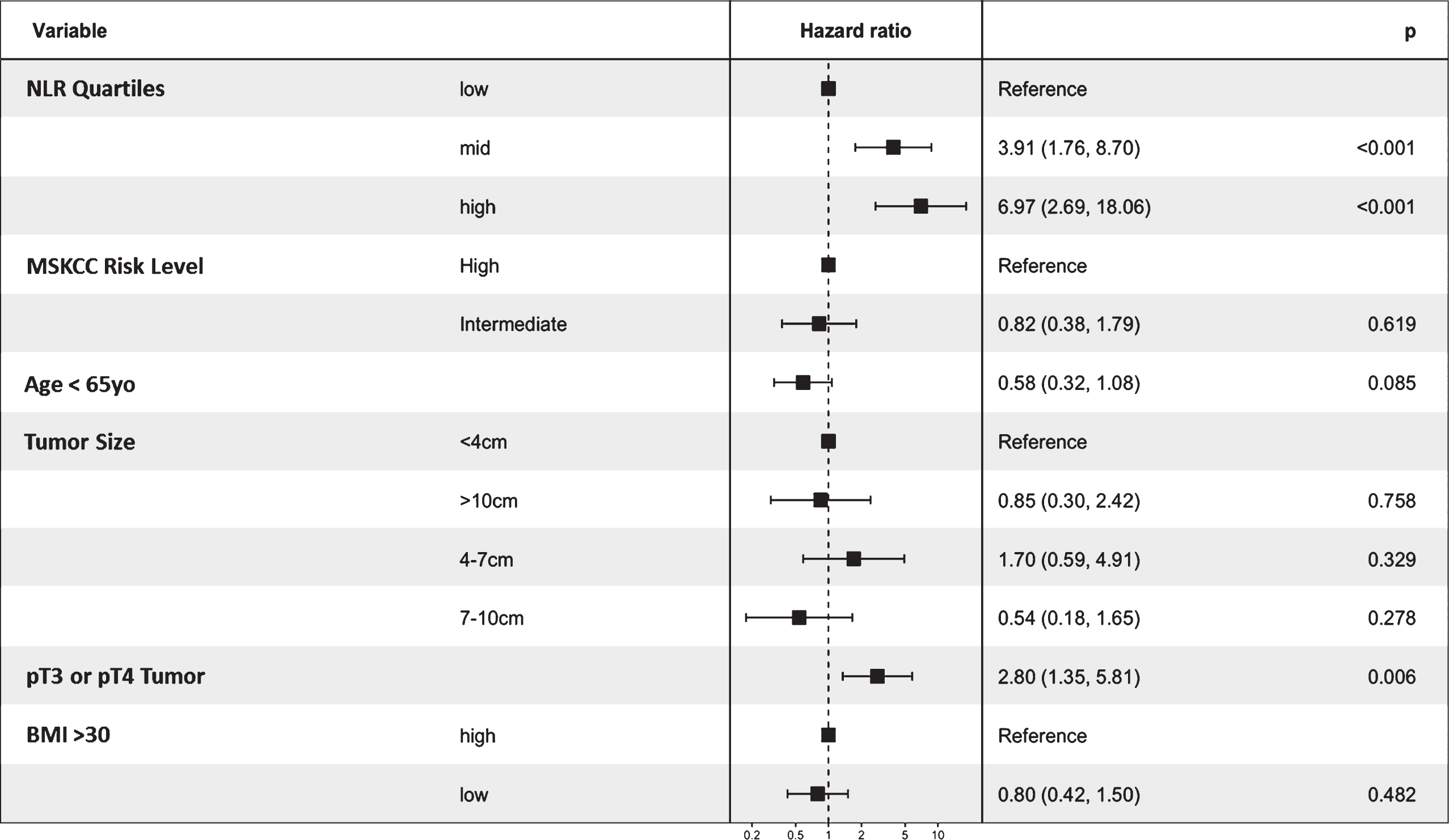
Median follow up time for survivors was 13 months. Estimated 2- and 5-year overall survival were 33.2% (95% CI: 27.1%-40.8%) and 16.3% (95% CI: 11.2%-23.7%), respectively. On multivariate cox regression analysis metastases to multiple organs (HR=4.39; 95% CI 1.22-15.85; p=0.024), appendicular bone involvement (HR=2.33; 95% CI 1.13-4.80; p=0.022), lung/pleural metastases (HR=1.83; 95% CI 1.11-3.01; p=0.018) and tumor size >10cm (HR=1.59; 95% CI 1.07-2.36; p=0.021) were significant predictors for worse overall survival, with non-clear cell histology trending towards significance (p=0.084). N0/NX status (HR=0.38; 95% CI 0.23-0.60; p<0.001) was a protective factor for OS and CSS (Figure 1).
Figure 1.
Multivariate Cox regression model of predictors of cancer specific survival in a cohort of patients with metastatic sRCC who underwent cytoreductive nephrectomy (n=225)
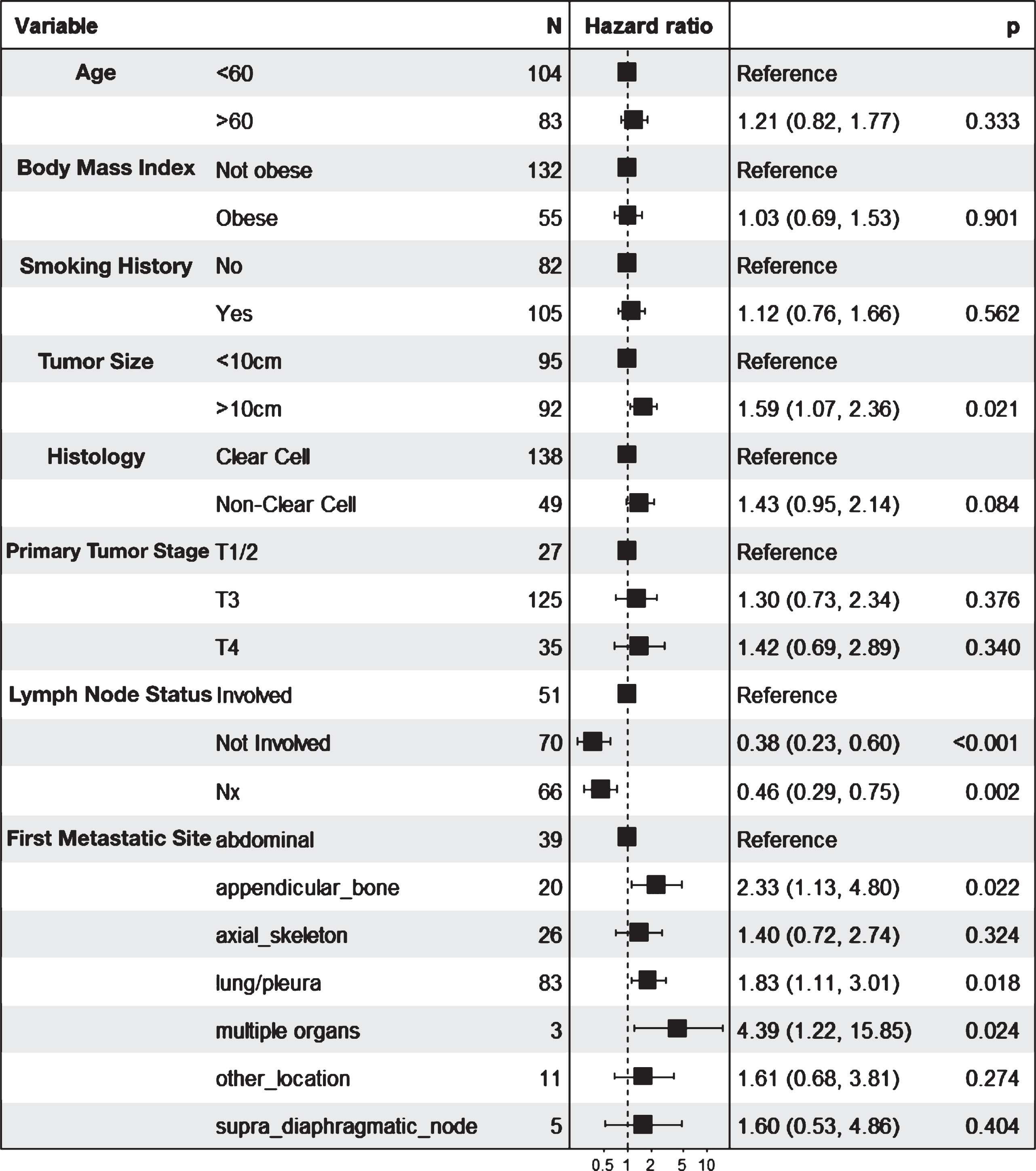
Conclusion: Patients undergoing cytoreductive nephrectomy for sRCC have an overall poor outcome. Patients with a single metastatic site that does not involve the appendicular bones and lung/pleura, a tumor size <10cm and no evidence of nodal disease have a better outcome following cytoreductive nephrectomy and may benefit from the procedure.
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
06An evolutionary comprehensive psychiatric approach to prolong survival: Revival after brain seizure & craniotomy of mRCC (Stage IV mRCC with rare VHL mutation) current patient use case & journey
Govindasamy, Ph.D., Murugesan
Author Company: Arizona State University
Co Authors: Hegde, Shura (University of Cincinnati)
As a patient and health care innovative researcher, challenged with Stage IV mRCC cancer journey (from 2015). The journey started with Mayo Clinic (Arizona) with radical nephrectomy, continued with trials at (first line & second line immuno therapy trials) Memorial Sloan Kettering (MSKCC -New York) and actively pursuing with MD Anderson Houston with advanced trial options. Poor Prognosis after the brain tumor, brain seizure, craniotomy with radiation exposed to depression, anxiety and aloofness provided an evidence of the psychiatrist issues and support and consultation is absolutely necessary to elevate the levels of the patient knowledge, understanding, importance of mental hygiene and anti-depressive for the continuing journey. All ended up with the 12 different Kidney Oncologists (at all of the above three cancer institutions) and consultation from multi-disciplinary doctors. Co-author leading psychiatrist (who tackled and suffered his 4-year-old son’s Wills tumor 10 years ago) consulted to advise and advance the evolutionary radical approach(es) to improve the patient’s quality of life. This use case or current patient journey with psychiatry approach(es) will be analyzed and shared with evidence to improve kidney cancer patient’s revival after poor prognosis. The sharing of the journey provides a new outlook and proofs to similar advanced patients with life threatening surgeries during mRCC cancer prolonged survival.
07Analysis of First-line Sunitinib Treatment Duration on Clinical Outcomes in Patients with Metastatic Renal Cell Carcinoma (mRCC) Receiving Subsequent Immuno-oncology (IO) Checkpoint Inhibitors
Wells, John C.
Author Company: University of Calgary
Co Authors: Jeffrey Graham, MD1; Benoit Beuselinck, MD, PhD2; Georg A Bjarnason, MD, FRCPC3; Frede Donskov, MD, DMSci4; Aaron Hansen, MBBS5; Rana McKay, MD6; Ulka Vaishampayan, MD7; Guillermo De Velasco, MD, PhD8; Mei S. Duh, MPH, ScD9; Lynn Huynh, MPH, MBA, DrPH9; Catherine Nguyen, MPH9; Giovanni Zanotti, MSc, PharmD10; Krishnan Ramaswamy, PhD10; Toni Choueiri, MD11; Daniel Y C Heng, MD, MPH, FRCPC1 1University of Calgary, Calgary, Canada 2University Hospital Leuven, Leuven, Belgium 3Sunnybrook Health Sciences Centre, Toronto, Canada 4Aarhus University Hospital, Aarhus, Denmark 5Princess Margaret Cancer Centre, Toronto, Canada 6University of California San Diego, San Diego, CA, 7Karmanos Cancer Institute, Detroit, MI, 8University Hospital 12 de Octubre, Madrid, Spain 9Analysis Group, Inc., Boston, MA, 10Pfizer, Inc., New York, NY, USA 11Dana-Farber Cancer Institute, Boston, MA
Background: Sunitinib (SUN) is commonly used for first-line (1L) treatment of mRCC. This study aims to assess the effect of 1L SUN duration on clinical outcomes in second-line (2L) IO.
Methods: Using a subset of the International mRCC Database Consortium (IMDC) dataset from 7 clinical centers, outcomes assessed between patients (pts) treated with 1L SUN for = 6 months (mos) vs < 6 mos were: overall survival (OS: time from IO initiation to death), time to treatment failure (TTF: time from IO initiation to next line of therapy or death), and physician-assessed objective tumor response. OS and TTF were analyzed by Kaplan Meier analysis and time-varying Cox proportional hazards model adjusting for age, sex, and IMDC risk score. ?2 trend test was used to compare proportion of tumor responses in 1L and 2L.
Results: Among 161 study pts, median 1L SUN duration was 11.0 mos. The = 6 mos group (n=116) tended to be older with better IMDC risk than the < 6 mos group (n=45) (mean age: 63 vs 58 years, p=0.004; IMDC favorable: 10% vs 3%, p=0.18; IMDC intermediate: 83% vs 67%, p=0.04). Median OS was numerically higher in = 6 mos vs < 6 mos groups (OS: 24.9 vs 17.5 mos, p=0.15). In adjusted analysis, a 57% significant reduction in hazard of death in = 6 mos vs < 6 mos groups (adjusted hazard ratio [aHR]: 0.43, p=0.03) was observed. However, no significant association was observed between 1L SUN and 2L IO treatment failure (aHR: 0.85, p=0.57) or tumor response (p=0.48) (Table 1).
Table 1.
Summary of OS and TTF among Patients with 1L SUN ≥ 6 mos vs < 6 mos Before 2L IO Therapy and Tumor Response to 2L IO Therapy Grouped by Best Response to 1L SUN
| Unadjusted | Adjusted | |||
| ≥ 6 mos 1L SUN median (mos) | < 6 mos 1L SUN median (mos) | ≥ 6 mos vs < 6 mos 1L SUN HR (95% CI) | ||
| OS | 24.9 | 17.5 | 0.43 (0.20, 0.92), p=0.03 | |
| TTF | 9.2 | 8.0 | 0.85 (0.50, 1.47), p=0.57 | |
| Tumor Response | Number of Patients Grouped by Best Response in 1L SUN | |||
| ORR (N=26) | SD (N=30) | PD (N=41) | ||
| % Response in 2L IO based on 1L SUN Response | ORR | 23% | 20% | 12% |
| SD | 42% | 57% | 15% | |
| PD | 35% | 23% | 73% | |
Adjusted analyses models included age, sex, and IMDC risk scores. Overall survival (OS), hazard ratio (HR), confidence interval (CI), time to treatment failure (TTF), objective response rate (ORR) which includes complete and partial response, stable disease (SD), progressive disease (PD)
Conclusions: Pts receiving 1L SUN = 6 mos had better adjusted OS compared to those who received < 6 mos. Although this was adjusted by prognostic factors, there may be other covariates that may impact results. There appears to be no significant association between 1L SUN duration and clinical outcomes of TTF and tumor response in 2L IO.
08Association of VHL mutation status and clinical response to pazopanib in Mexican patients with clear renal cell carcinoma
Gonzalez-Cavazos, Adriana Carolina
Author Company:
Co Authors: Ibarra-Sanchez, Hector Eduardo; Gallegos-Gonzalez, Elena Yareli; Luna-Aguirre, Claudia Maribel; Juarez-Zuñiga, Eliseo; Soto-Medina, Francisco; Barrera-Saldaña, Hugo Alberto
Clear renal cell carcinoma (ccRCC) is the most common renal cancer in Mexico, with around 4,000 new cases each year, representing 75% of renal cancer cases. In ccRCC, the VHL (Von Hippel Lindau) tumor suppressor gene is the most frequently mutated gene. The functional absence of VHL protein (pVHL) allows accumulation of the hypoxia inducible factor (HIF) and activation of genes associated in different cellular pathways, such as the vascular endothelial growth factor (VEGF) gene, which regulates angiogenesis, a fundamental event in the process of tumor growth and metastasis. VEGF binds to its tyrosine kinase receptor (VEGFR) in vascular endothelial cells, inducing their permeability, proliferation and migration. Over the past few years, different anti-angiogenic treatments that inhibit the tyrosine kinase activity of VEGFR have improved the clinical response in metastatic ccRCC. One of these treatments is pazopanib (Votrient®, Novartis), an orally medicine approved by the US FDA as a first-line treatment for patients with metastatic ccRCC. This inhibitor competes with ATP for binding to the intracellular side of VEGFR1-3, PDGFRa-®, FGFR1-3 and c-KIT. Although several clinical trials have evaluated the association between VHL alterations and clinical outcomes in patients with ccRCC treated with anti-VEFG therapies, the relation hasn’t been established with accuracy. The major limitation of these studies is the heterogenic agents included in the same analysis. The aims of this study are to determine mutation frequency of the VHL gene and to find an association to the clinical response in Mexican patients with ccRCC treated with pazopanib as a single agent. To compare the progression-free survival (PFS) with the VHL mutation status, we categorized two different groups of patients: one with PFS = 6 months and another with PFS < 6 months. Mutation status of the FFPE collected samples (n==30) will be analyzed by Sanger sequencing. To detect significant difference between the two PFS groups we will perform a Student’s t-test. The results of this study will allow to determine whether VHL mutation status function as a biomarker to predict the clinical outcome of ccRRC patients treated with pazopanib.
09Blood based biomarkers and their association with overall survival (OS) and progression-free survival (PFS) in patients with metastatic renal cell carcinoma (mRCC) treated with immunotherapy (IO)
Shabto, Julie M
Author Company: Emory University
Co Authors: Dylan J. Martini1,2, Yuan Liu3, Bradley C. Carthon1,2, Emilie Elise Hitron1,2, Greta Anne Russler1,2, Meagan Barbee1,4, Haydn Kissick2,5, Wayne B. Harris1,2, Omer Kucuk1,2, Viraj A. Master5, Mehmet Asim Bilen1,2
1Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA
2Winship Cancer Institute of Emory University, Atlanta, GA, USA
3Departments of Biostatistics and Bioinformatics, Emory University, Atlanta, GA, USA
4Department of Pharmaceutical Services, Emory University School of Medicine, Atlanta, GA, USA
5Department of Urology, Emory University School of Medicine, Atlanta, GA, USA
Background: Blood based biomarkers, such as neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte (MLR), and platelet-to-lymphocyte ratio (PLR), have been explored as prognostic indicators for response to IO. We investigated the association between these biomarkers and clinical outcomes in patients with mRCC receiving IO-based therapy.
Methods: We completed a retrospective analysis of 100 patients with mRCC who were treated with IO-based therapy at Winship Cancer Institute of Emory University from 2015 to 2018. Overall survival (OS) and progress-free survival (PFS) were measured from first dose of IO to date of death or hospice referral and clinical or radiographic progression, respectively. MLR, NLR, and PLR were collected at baseline and 6 (+/-2) weeks (6W) after first dose of IO. Multivariate analysis (MVA) was carried out using Cox proportional hazard model. Covariates included age, gender, clear cell RCC (ccRCC), number of metastatic sites, and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group. MLR, NLR, and PLR were log transformed and treated as continuous variables in Cox model and were dichotomized at median in Kaplan-Meier method.
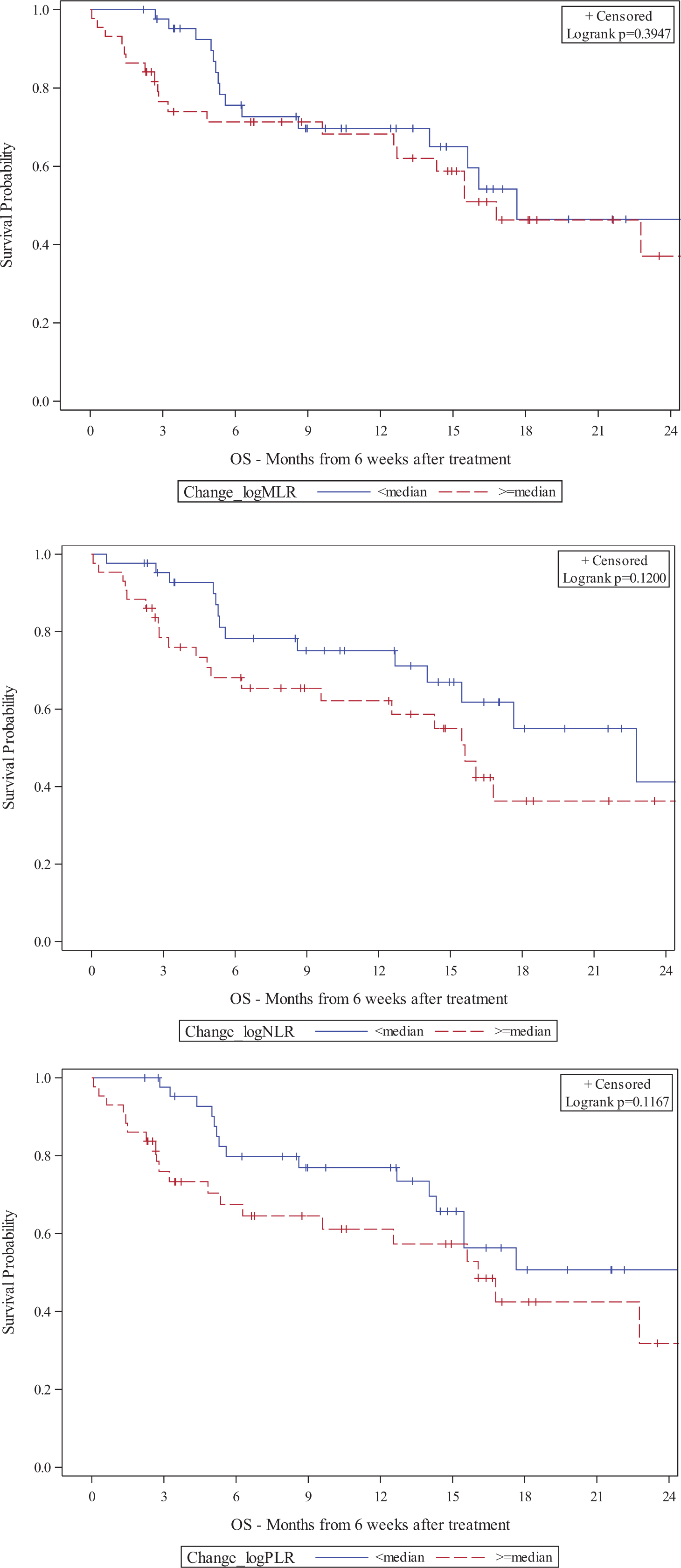
Results: The median patient age was 65 and most (71%) received anti-PD-1 monotherapy. High baseline MLR was associated with shorter OS (HR: 2.23) while early increase in MLR was associated with shorter OS and PFS (HR: 2.86, 2.49) (Table 1). Early increase in NLR and PLR also portended shorter OS (HR: 2.67, 2.71). Patients with higher baseline MLR had a shorter median OS (16.9 vs. 29.7 months) and patients with higher baseline PLR had a shorter median OS (15.7 vs. 29.7 months) (Figure 1). Figures 2 and 3 show Kaplan-Meier plots of the association between MLR, NLR, and PLR at 6W or 6W change and OS, respectively. Early increases in these biomarkers were significantly associated with shorter OS and PFS.Conclusions: Baseline and early change in MLR, NLR, and PLR may predict clinical outcomes in patients receiving IO-based therapy. These results may warrant a larger study to investigate the prognostic value of biomarkers, particularly MLR, for mRCC patients on IO-based therapy.
Table 1:
MVA† of MLR, NLR, and PLR at baseline, 6W, and change with OS and PFS
| OS | PFS | ||||
| HR (CI) | p-value | HR (CI) | p-value | ||
| Log(MLR) | Baseline | 2.23 (1.08-4.60) | 0.03* | 0.99 (0.62-1.59) | 0.976 |
| 6W | 2.92 (1.68-5.06) | <0.001* | 1.41 (0.82-2.44) | 0.216 | |
| Change | 2.86 (1.44-5.68) | 0.003* | 2.49 (1.14-5.43) | 0.022* | |
| Log(NLR) | Baseline | 1.08 (0.77-1.52) | 0.637 | 1.01 (0.71-1.44) | 0.962 |
| 6W | 2.08 (1.25-3.46) | 0.005* | 1.01 (0.66-1.56) | 0.952 | |
| Change | 2.67 (1.14-0.024) | 0.024* | 1.85 (0.85-4.05) | 0.124 | |
| Log(PLR) | Baseline | 1.62 (0.82-3.23) | 0.168 | 0.90 (0.60-1.37) | 0.624 |
| 6W | 2.17 (1.25-3.78) | 0.006* | 1.16 (0.67-1.99) | 0.599 | |
| Change | 2.71 (1.02-7.22) | 0.046* | 2.36 (0.99-5.59) | 0.052 | |
†The multivariable model controlled for gender, IMDC risk group, number of different metastatic sites, age, and ccRCC
*statistical significance at alpha < 0.05
Figure 1.
Kaplan-Meier plots of baseline MLR, NLR, and PLR cut by median and OS
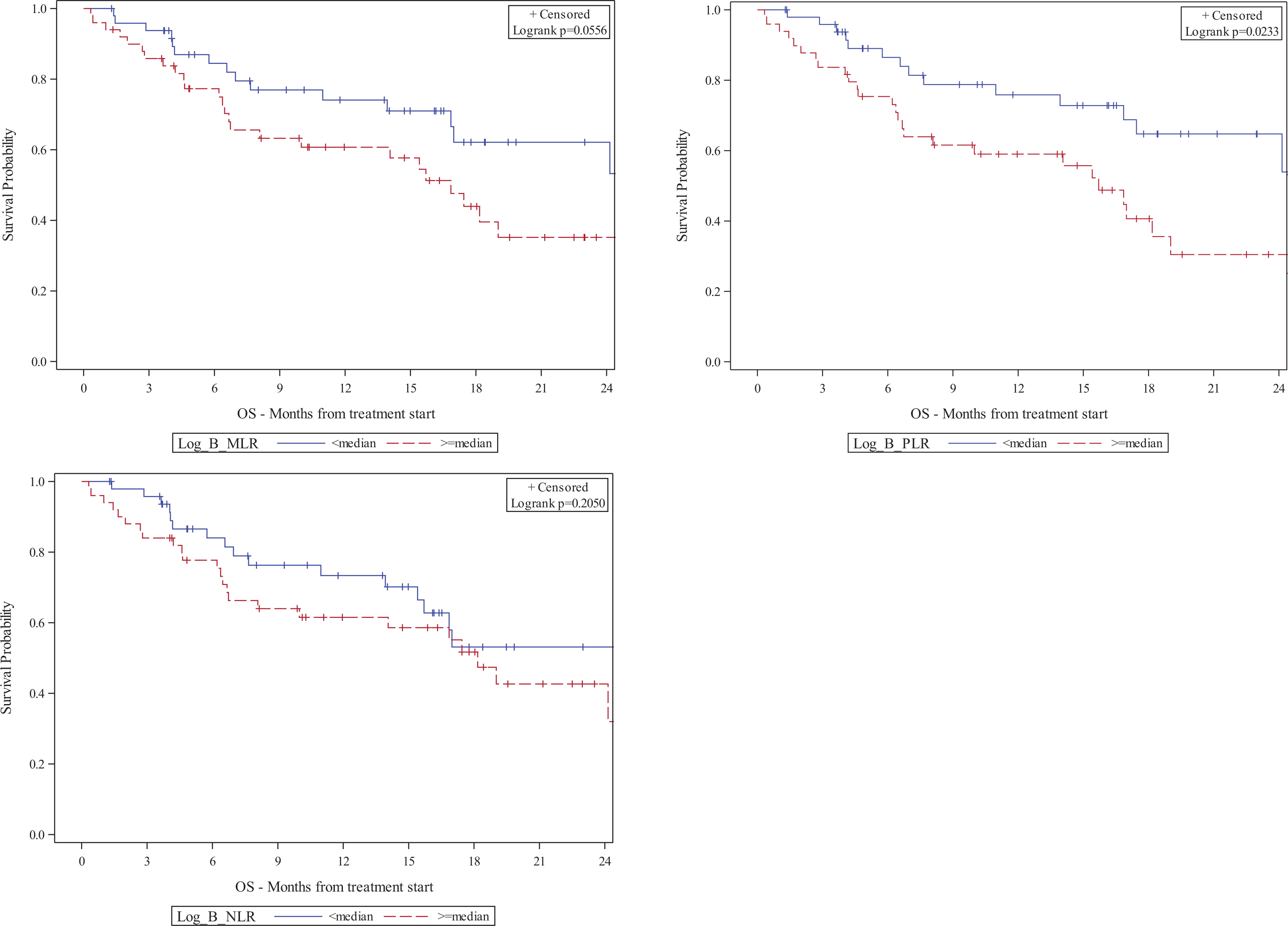
Figure 2.
Kaplan-Meier plots of MLR, NLR, and PLR at 6W cut by median and OS
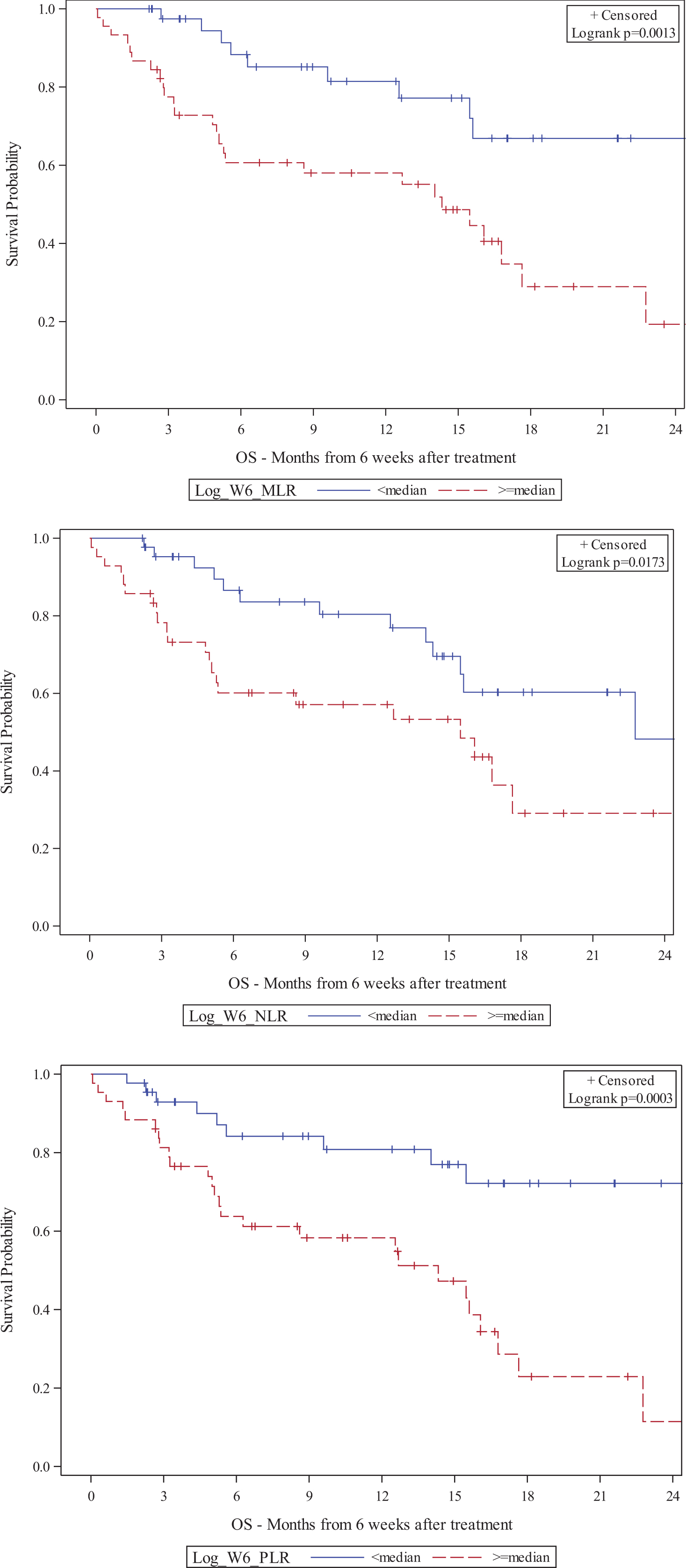
Figure 3.
Kaplan-Meier plots of change in MLR, NLR, and PLR cut by median and OS
10Body mass index (BMI) as a predictor of treatment outcome for patients receiving systemic therapy for metastatic renal cell carcinoma (mRCC)
Bergerot, Paulo
Author Company: City of Hope Comprehensive Cancer Center
Co Authors: Bergerot, Cristiane; Philip, Errol; Meza, Luis; Dizman, Nazli; Hsu, JoAnn; Pal, Sumanta
Background: High body mass index (BMI) has been identified as a predictor of treatment outcomes for mRCC patients receiving targeted therapy with vascular endothelial growth factor-tyrosine kinase inhibitors (VEGF-TKIs). In this study we sought to determine whether a similar trend can be observed with mammalian target of rapamycin (mTOR) inhibitors and with immunotherapy (IO).
Methods: Demographic and clinical data of patients with RCC were collected from medical charts at a single institution over a period of 8 years in this retrospective study. BMI was characterized as high (=25 kg/m2) versus low (<25 kg/m2). The Kaplan-Meier method was used to estimate the difference in OS, with comparisons based on BMI and by treatment type (VEGF-TKI, mTOR and IO).
Results: A total of 379 cases who received systemic treatment for mRCC were identified from institutional database. The majority of patients (65%) had high BMI, 73% were male, and the median age was 65 (range, 33-90). In total, 86% of patients had undergone nephrectomy. VEGF-TKI was the most frequent type of systemic therapy rendered (61%), followed by mTOR inhibitors (22%) and IO (17%). Among patients who were treated with VEGF-TKIs, the median OS was 23.0 months (95% CI: 19.7-26.2) and 36.0 months (95% CI: 19.3-53.3) for those with low BMI and high BMI (P=0.01) respectively. Patients treated with mTOR inhibitors demonstrated a similar result, with a median OS of 18.0 months (95% CI: 10.0-25.9) for patients with low BMI versus 24.0 months (95% CI: 14.8-33.1) for patients with high BMI (P=0.02). In contrast, patients treated with IO and with low BMI had a median OS of 55.0 months (95% CI: 33.7-76.7) versus 22.9 months (95% CI: 17.7-28.1) among patients with high BMI (P=0.19).
Conclusions: Patients with mRCC with high BMI who were treated with VEGF-TKIs and mTOR inhibitors had improved OS, confirming previous findings. The inverse trend, however, was observed among patients receiving IO, although this result was not statistically significant. These findings highlight the need to reassess this phenomenon in the context of immune checkpoint inhibitors.
11Body mass index (BMI) as a prognostic indicator of survival in metastatic renal cell carcinoma (mRCC) patients treated with immune checkpoint inhibitors (ICI)
Martini, Dylan J
Author Company: Emory University
Co Authors: Shabto, Ph.D., Julie M; Liu, Yuan; Carthon, Bradley C; Hitron, Emilie Elise; Russler, Greta Anne; Barbee, Meagan; Kissick, Haydn; Harris, Wayne B; Kucuk, Omer; Master, Viraj A; Bilen, Mehmet Asim
Background: The association between BMI and clinical outcomes has been studied in cancer patients, but the effect of BMI on mRCC patients treated with ICI is not known. We explored the prognostic value of BMI in mRCC patients treated with ICI.
Methods: We performed a retrospective analysis of 100 mRCC patients treated with ICI at Winship Cancer Institute from 2015-2018. Overall survival (OS) and progression-free survival (PFS) were measured from ICI-initiation to date of death and clinical or radiographic progression, respectively. BMI was obtained at baseline and 6 (+/-2) weeks (6W) after ICI-initiation. Cox proportional hazard model and Kaplan-Meier method were used for association with OS and PFS. BMI was analyzed as a categorical variable (BMI < 25 or BMI >/= 25).
Results: Approximately two-thirds of the patients (66%) male and the median age was 65 years. The majority of patients (78%) had ccRCC histology. The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group distribution was: 16% favorable, 60% intermediate, and 24% poor. Treatment consisted predominantly (71%) of anti-PD-1 monotherapy. The median baseline BMI was 26.7 and most patients (61%) were overweight or obese at baseline (BMI >/= 25). The median OS and PFS was significantly longer (both p < 0.05) for patients with baseline BMI >/= 25 per Kaplan-Meier estimation (Figures 1-2). Increased 6W BMI was significantly associated with longer median OS (p=0.01) and trended towards longer PFS (p=0.19) (Figures 3-4). Patients with a baseline BMI < 25 trended towards shorter OS and PFS compared to patients with baseline BMI >/= 25 (Table 1). Increased 6W BMI showed a trend towards longer OS.
Figure 1.
Kaplan-Meier plot of association between BMI and OS
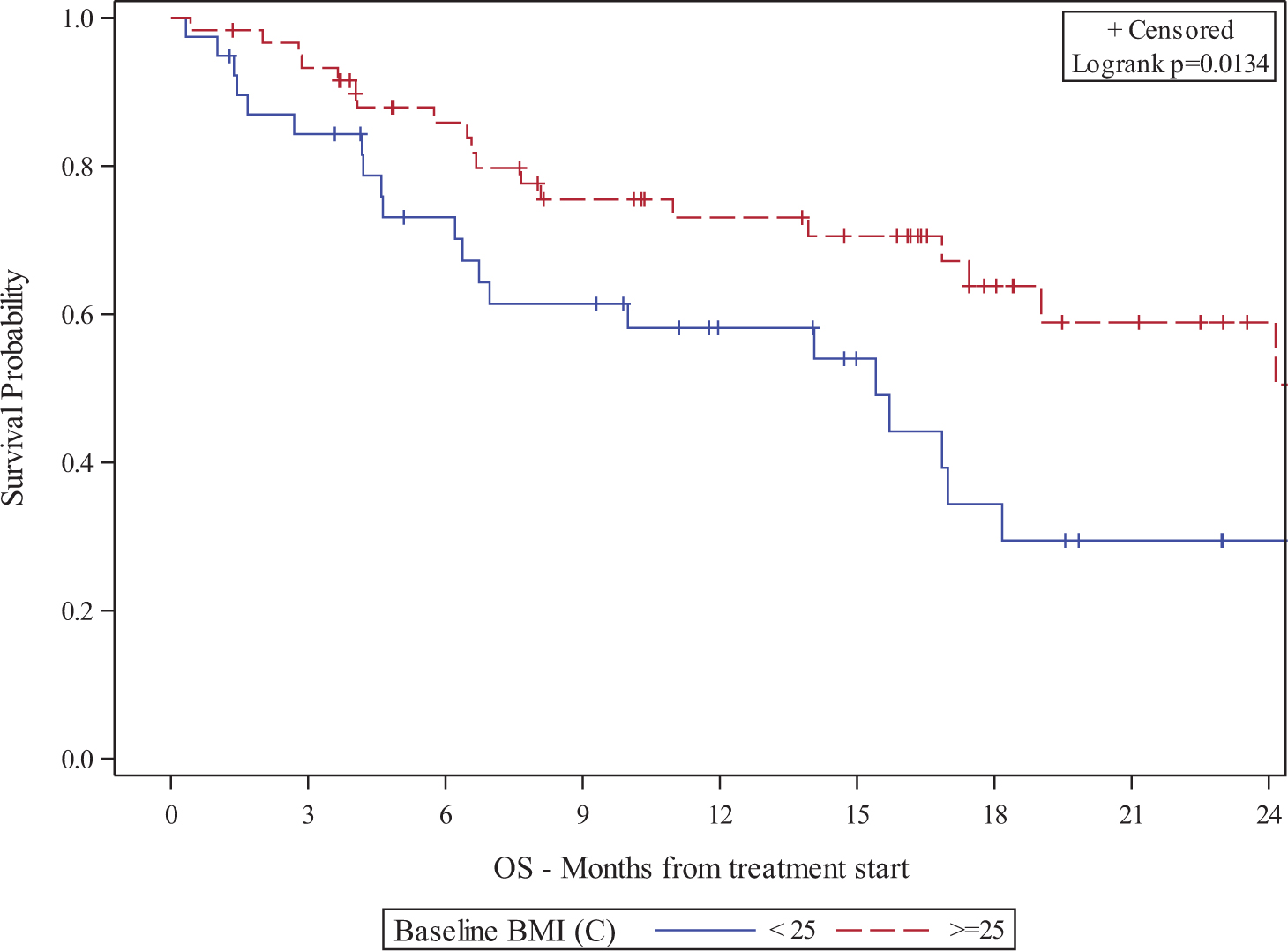
Figure 2.
Kaplan-Meier plot of association between BMI and PFS
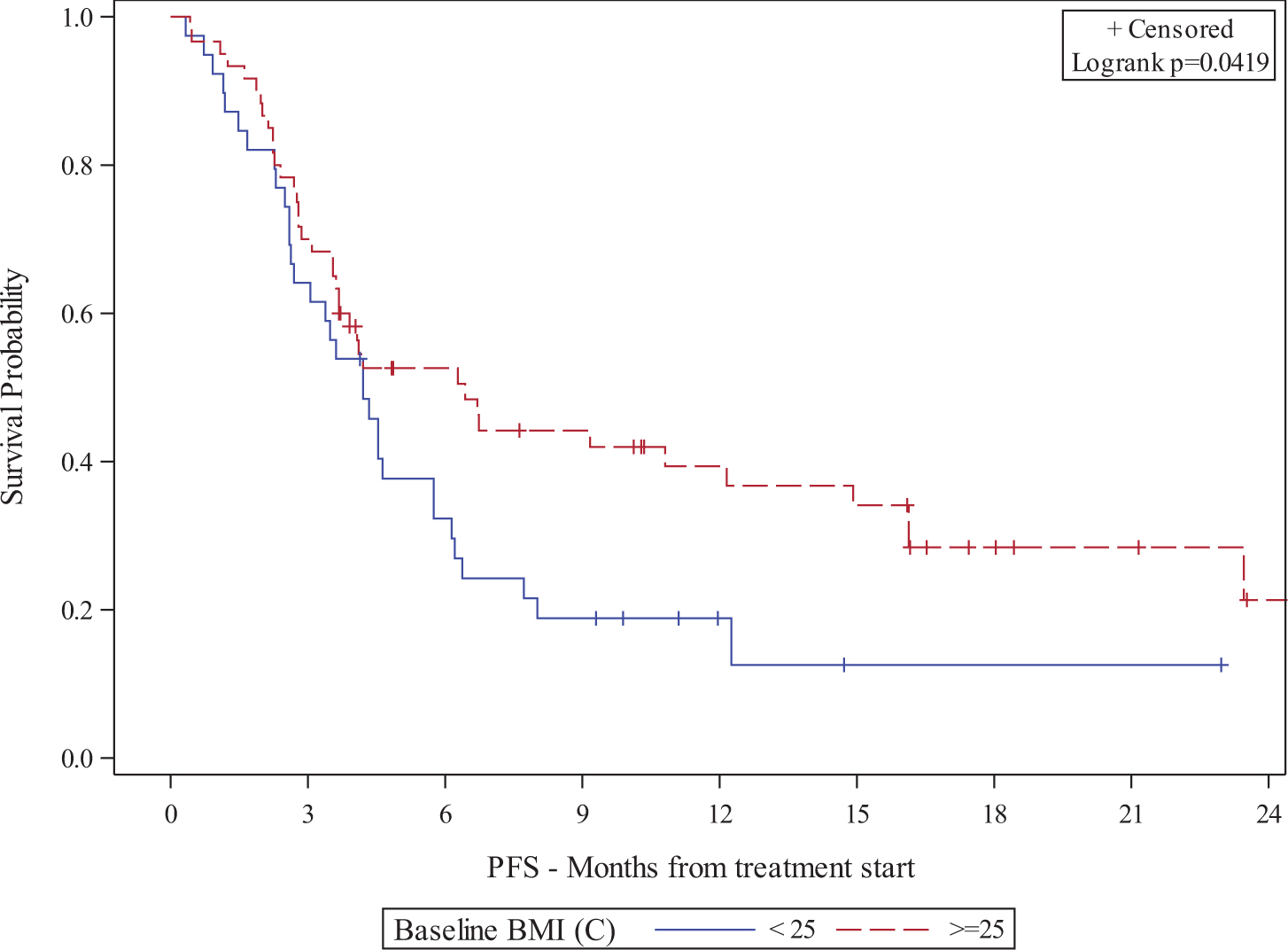
Figure 3.
Kaplan-Meier plot of association between BMI change at 6W and OS
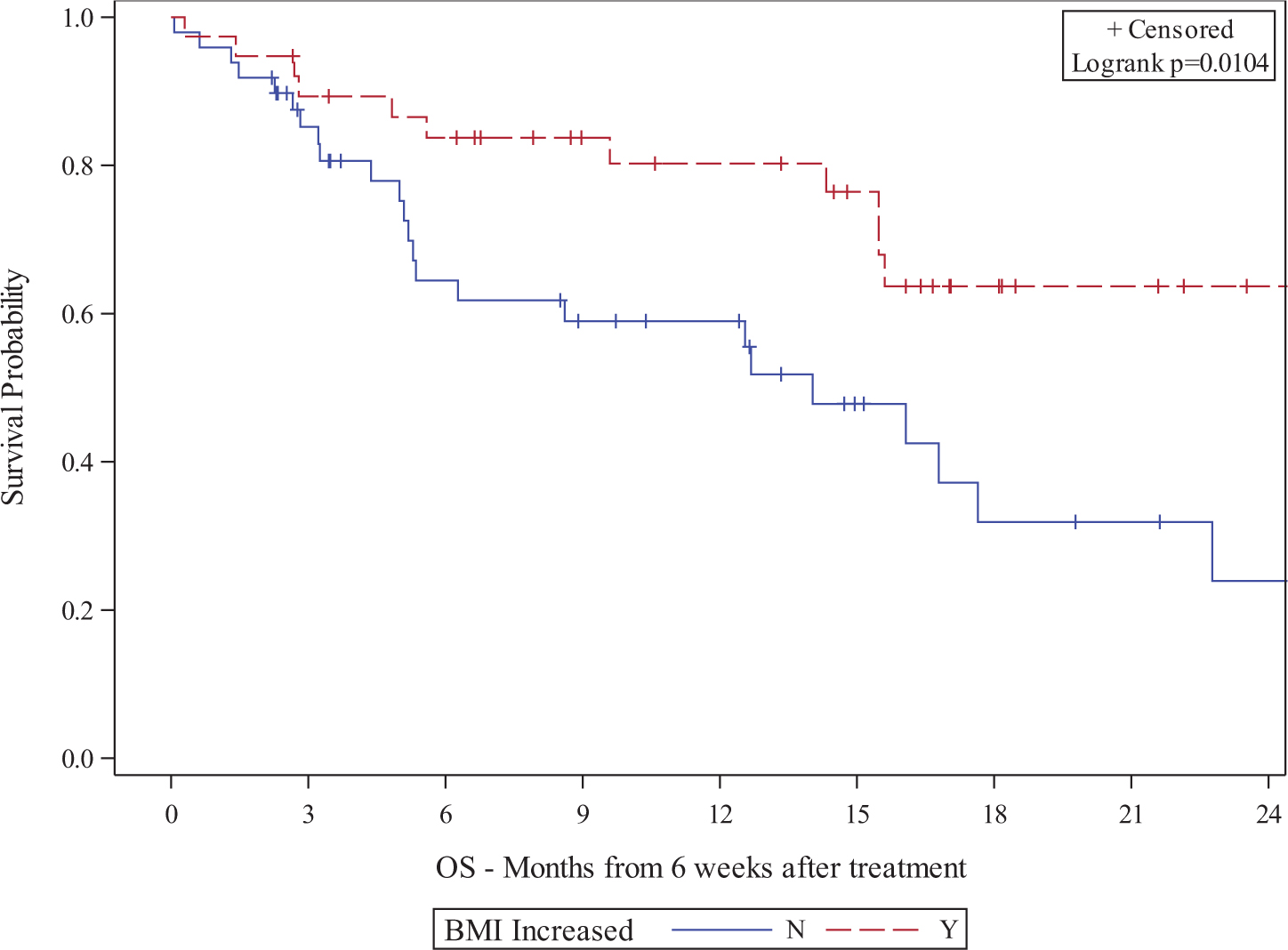
Figure 4.
Kaplan-Meier plot of association between 6W BMI change and PFS
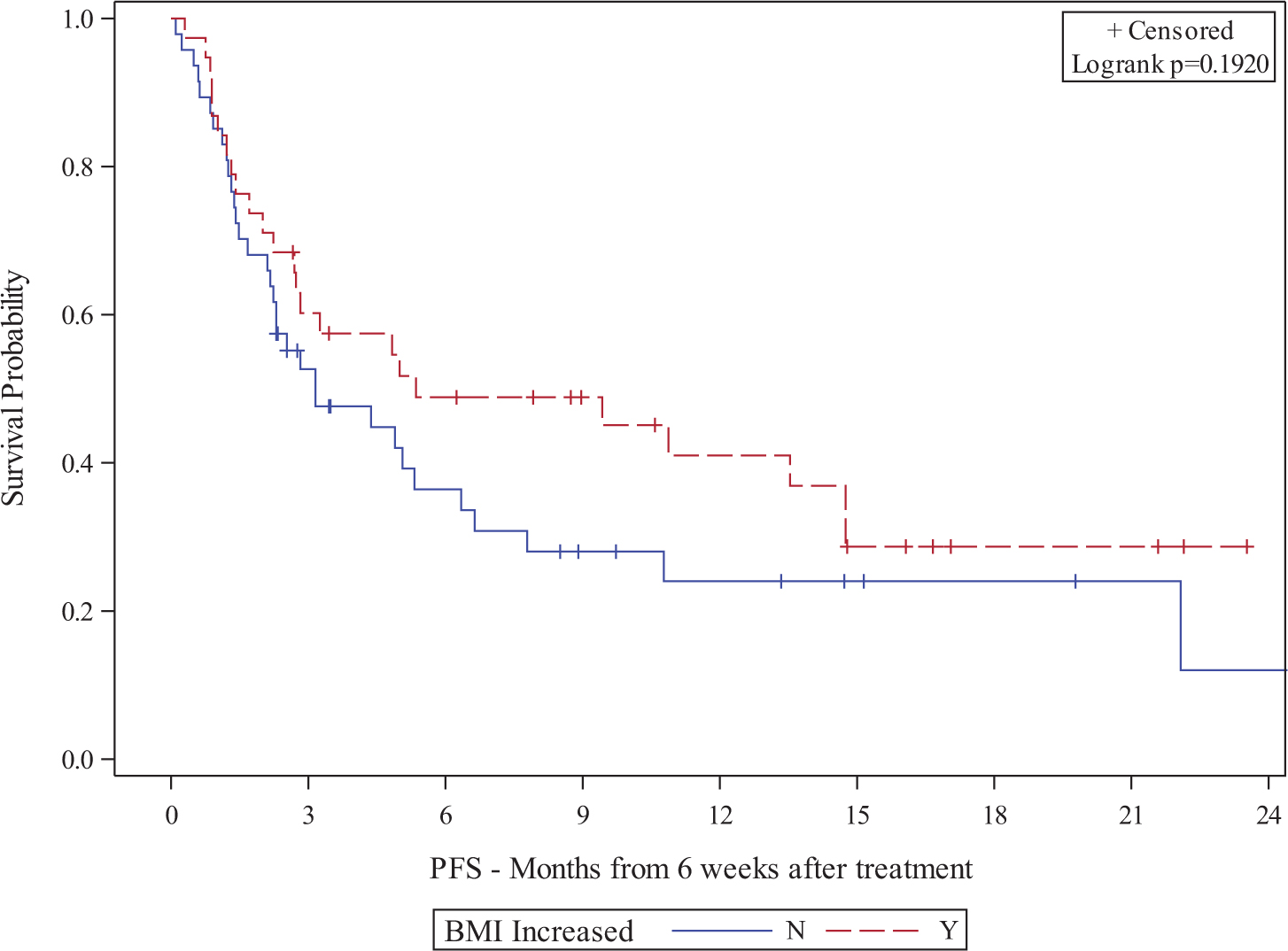
Table 1:
MVA† of association between BMI and survival
| OS | PFS | |||
| HR (CI) | p-value | HR (CI) | p-value | |
| Baseline BMI | ||||
| Normal or underweight (BMI < 25, n=39) | 1.98 (0.98-3.98) | 0.056 | 1.63 (0.96-2.77) | 0.073 |
| Median Survival: 15.4 months | Median Survival: 4.2 months | |||
| Overweight or Obese (BMI 3 25, n=60) | 1 | 1 | 1 | 1 |
| Median Survival: Not reached | Median Survival: 6.4 months | |||
| BMI Change at 6W | ||||
| Not Increased (n=50) | 1.85 (0.88-3.89) | 0.104 | 0.87 (0.48-1.59) | 0.657 |
| Median Survival: 14 months | Median Survival: 3.2 months | |||
| Increased (n=40) | 1 | 1 | ||
| Median Survival: Not reached | Median Survival: 5.4 months | |||
†Multivariable model controlled for gender, race, IMDC risk group, number of distant metastases, age, and ccRCC histology
*statistical significance at alpha < 0.05.
Conclusions: Increased BMI may be associated with improved survival in mRCC patients treated with ICI. Further studies are needed to validate these results and elucidate the biological relationship between adiposity, the tumor microenvironment, and the immune system in patients treated with immunotherapy.
12Characterization of Response to Nivolumab Plus Ipilimumab or Sunitinib in Patients With Previously Untreated Advanced Renal Cell Carcinoma: CheckMate 214
Rini, M.D., Brian I.
Author Company: Cleveland Clinic - Taussig Cancer Institute
Co Authors: Tannir, Nizar M.1; Escudier, Bernard3; McDermott, David F.1; Grimm, Marc-Oliver17; Porta, Camillo18; Powles, Thomas10; Kollmannsberger, Christian11; Gurney, Howard14; Tykodi, Scott S15; Harrison, Michael5; Heng, Daniel Y.C12.; Grünwald, Viktor13; Choueiri, Toni K.6; Mekan, Sabeen16; McHenry, Brent M.; Hammers, Hans J.7; Motzer, Robert J.9; George, Saby8
Affiliations: 1Beth Israel Deaconess Medical Center, 2MD Anderson Cancer Center, 3Institute Gustav Roussy, 5Duke University Cancer Center, 6Dana-Farber Cancer Institute,7UT Southwestern, 8Roswell Park Cancer Center, 9Memorial Sloan Kettering Cancer Center, 10Bart’s Cancer Institute, 11BBCA Vancouver Cancer Centre; 12University of Calgary, 13Medical School Hannover, 14Macquarie University, 15University of Washington Medical Center, 16Long Island Jewish Medical Center, 17Universitatsklinikum Jena, 18IRCCS San Matteo University Hospital Foundation
Background: Nivolumab plus ipilimumab (N+I) demonstrated superior objective response rate (ORR) and overall survival (OS) vs sunitinib (S) in patients with IMDC intermediate/poor-risk advanced renal cell carcinoma (aRCC) in the phase 3 CheckMate 214 trial. Further characterization of response may inform clinical practice.
Methods: In CheckMate 214, patients with previously untreated aRCC were randomly assigned 1:1 to N 3 mg/kg + I 1 mg/kg every 3 weeks for 4 doses then N 3 mg/kg every 2 weeks or S 50 mg once daily for 4 weeks on, 2 weeks off. Efficacy, safety, and quality of life (QoL) were explored in intermediate/poor-risk patients with complete response or partial response to N+I or S.
Results: At 25.2 months median follow-up, confirmed ORR per independent radiology review committee in intermediate/poor-risk patients was 42% for N+I vs 27% for S (P<0.001; Table), with 36% vs 18% of patients achieving best tumor reduction =50% with N+I vs S. Of N+I vs S responders, 72% vs 63% have ongoing response, 47% and 34% remain on treatment, and 53% and 66% discontinued, most often for disease progression (N+I, 22%; S, 40%) or toxicity (N+I, 23%; S, 13%). N+I responders received a median of 21.0 months of treatment (vs 3.8 months for N+I nonresponders). Response lasting =18 months was seen in 13% of N+I and 4% of S patients. Grade 3-4 treatment-related adverse events (TRAEs) occurred in 52% of N+I and 68% of S responders. Mean change from baseline at 24 weeks in Functional Assessment of Cancer Therapy–Kidney Symptom Index 19 score was 3.0 in N+I responders (better) vs -0.7 in S responders (worse). Updated 3-year data on responders, including use of subsequent therapies, will be presented.
| Outcome | N+I intermediate/poor-risk patients | S intermediate/poor-risk patients | ||||
| Total n=425 | CR n=40 | PR n=137 | Total n=422 | CR n=5 | PR n=107 | |
| BOR (95% CI), % | 42 (37-47) | 9 | 32 | 27 (22-31) | 1 | 25 |
| Median (range) time to response, months | 2.8 (0.9-11.3) | 4.4 (2.4-22.1) | 2.8 (1.4-11.3) | 3.0 (0.6-15.0) | 5.6 (3.0-6.9) | 3.1 (0.6-15.0) |
| Median (95% CI) duration of response, months | NR (21.8-NE) | NR | NR (18.8-NE) | 18.2 (14.8- NE) | NR | 18.2 (13.9-NE) |
| Patients with ongoing response in responders, n/N (%) | 128/177 (72) | 34/40 (85) | 94/137 (69) | 71/112 (63) | 5/5 (100) | 66/107 (62) |
| 12-month PFS rate (95% CI), % | 50 (44-55) | 97 (83-100) | 81 (73-86) | 43 (37-48) | 100 (100-100) | 79 (69-86) |
| 18-month OS rate (95% CI), % | 78 (74-81) | 100 (100-100) | 94 (89-97) | 68 (63-72) | 100 (100-100) | 92 (85-96) |
BOR, best overall response; CI, confi dence interval; CR, complete response; NE, not estimable; NR, not reached; PFS, progression-free survival; PR, partial response
Conclusions: ORR and OS were significantly improved with N+I compared with S in patients with intermediate/poor-risk aRCC in CheckMate 214. Responses to N+I were more likely to be complete responses and were more durable than responses to S. High-grade TRAEs were less frequent and QoL was better in N+I responders compared with S responders.
Originally presented at ESMO Congress 2018; Munich.
13CheckMate 214 Retrospective Analyses of Nivolumab Plus Ipilimumab or Sunitinib in IMDC Intermediate/Poor-risk Patients With Previously Untreated Advanced Renal Cell Carcinoma With Sarcomatoid Features
McDermott, M.D., David F.
Author Company: Beth Israel Deaconess Medical Center/Harvard Medical School
Co Authors: Motzer, Robert J.1; Arén Frontera, Osvaldo9 (United States); George, Saby2; Powles, Thomas3; Donskov, Frede7; Harrison, Michael5; Rodriguez-Cid, Jeronimo8; Ishii, Yuko; McHenry, M. Brent; Mekan, Sabeen6; Tannir, Nizar M.4
1Memorial Sloan Kettering Cancer Center, 2Roswell Park Cancer Center, 3Bart’s Cancer Center, 4MD Anderson Cancer Center, 5Duke University Cancer Center, 6Long Island Jewish Medical Center, 7Aarhus University Hospital, 8INSTITUTO NACIONAL DE ENFERMEDADES RESPIRATORIAS, 9Centro Investigación Clínica Bradford Hill
Background: Patients with advanced renal cell carcinoma (aRCC) with sarcomatoid features (+sRCC) have poor prognoses. Previous studies of anti-VEGF systemic therapy in +sRCC patients demonstrated suboptimal outcomes, underscoring the need for more effective treatment options. Nivolumab plus ipilimumab (N+I) demonstrated superior objective response rate (ORR) and overall survival (OS) versus sunitinib (S) in previously untreated patients with International Metastatic RCC Database Consortium (IMDC) intermediate/poor-risk advanced RCC in the phase 3 CheckMate 214 trial. A retrospective exploratory analysis of efficacy and safety of N+I versus S in CheckMate 214 +sRCC patients was performed.
Methods: The presence of sarcomatoid features was retrospectively assessed by manual keyword search for “sarcomatoid” in CheckMate 214 patients who had available local pathology reports accompanying tumor samples. Key efficacy outcomes are presented for +sRCC patients by treatment arm (N+I vs S). Safety data will be presented.
Results: A total of 825 of 847 intention-to-treat patients had local pathology reports available; 111 IMDC intermediate/poor-risk +sRCC patients were identified. A total of 59 and 52 intermediate/poor-risk +sRCC patients were treated with N+I and S, respectively. Baseline characteristics were balanced between arms. However, 44% and 50% of N+I- and S-treated +sRCC patients had =1% PD-L1 expression at baseline, notably higher than observed in all intermediate/poor-risk patients (N+I, 26%; S, 29%). At a median follow-up of 25.2 months, confirmed ORR per independent review (RECIST v1.1) in +sRCC patients was 57% (N+I) versus 23% (S). Additionally, complete responses (24% vs 0%), OS, progression-free survival (PFS), and duration of response (DOR) were all higher with N+I than with S in +sRCC patients (Table).
| +sRCC Intermediate/poor risk | Total Intermediate/poor risk | |||
| N+I N=59a | S N=52 | N+I N=425 | S N=422 | |
| ORR, n (%) | 33 (57) | 12 (23) | 177 (42) | 112 (27) |
| Complete response | 14 (24) | 0 (0) | 40 (9) | 5 (1) |
| Partial response | 19 (33) | 12 (23) | 137 (32) | 107 (25) |
| Stable disease | 6 (10) | 20 (38) | 133 (31) | 188 (45) |
| Progressive disease | 14 (24) | 12 (23) | 83 (20) | 72 (17) |
| Unable to determine/ not reported | 5 (9) | 8 (15) | 32 (8) | 50 (12) |
| DOR,b median (95% CI), months | NR (20.7-NE) | 12.9 (7.2-NE) | NR (21.8-NE) | 18.2 (14.8-NE) |
| PFS, median (95% CI), months | 12.0 (6.3-NE) | 5.1 (4.1-7.0) | 11.6 (8.7-15.5) | 8.4 (7.0-10.8) |
| HR (95% CI), 0.60 (0.36-1.01) | HR (99.1% CI), 0.82 (0.64-1.05) | |||
| OS, median (95% CI), months | 24.8 (23.0-NE) | 13.6 (8.9-20.9) | NR (28.2-NE) | 26.0 (22.1-NE) |
| HR (95% CI), 0.51 (0.30-0.86) | HR (99.8% CI), 0.63 (0.44-0.89) | |||
aN=58 for ORR and best overall response
bReported in patients with a response; N=33 (+sRCC N+I); N=12 (+sRCC S); N=177 (total N+I); N=112 (total S) CI, confi dence interval; HR, hazard ratio; NE, not estimable; NR, not reached
Conclusions: In this retrospective exploratory analysis of CheckMate 214, N+I demonstrated promising efficacy (including a 24% complete response rate) and prolonged survival compared with S in previously untreated advanced +sRCC patients with available pathology reports. Prospective phase 3b/4 studies are ongoing to confirm the efficacy and safety of N or N+I for the treatment of +sRCC patients
14Clonality estimates of oncogenic events and identification of ccRCC subtypes
DiNatale, M.D., Renzo
Author Company: Memorial Sloan Kettering Cancer Center
Co Authors: Reznik E2,4, Yoo A3, Marcon J1, Silagy AW1, Roy M1, Blum KA1, Coleman JA1, Russo P1, Hakimi AA1
1Urology Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York City, NY 2Computational Biology Center, Center for Molecular Oncology, Memorial Sloan-Kettering Cancer Center, New York City, NY 3Department of Urology, SUNY Downstate Medical Center, New York City, NY 4Authors contributed equally.
Introduction: The genomic events underlying ccRCC have been extensively studied in next-generation sequencing studies. However, ccRCC tumors are very heterogeneous which complicates the identification of potential biomarkers.Recent multiregional sampling studies have proposed that the evolution of ccRCC follows constrained trajectories that determine the overall disease course. It has been suggested that identification of clonal driver events may help categorize tumors into specific evolutionary subtypes. However, many of these studies either lack robust statistical methods or don’t consider clonality estimates. We aimed to use clonality estimates of driver events to analyze a cohort of 176 single-site biopsies with proven ccRCC.
Methods: We identified 267 patients with proven ccRCC who had undergone targeted-panel next-generation sequencing at our center. Patients with incomplete clinical data were excluded, the final cohort consisted of 176 single-biopsy samples. Mutation calling was performed using our previously-validated institutional pipeline. Annotation of oncogenic variants was done using OncoKB. Allele-specific copy-number (CN) and purity estimates were computed using the FACETS package. Clonality of a specific event was calculated based on the allele frequency, purity and CN estimates. Consensus clustering analysis was performed using a binary matrix of clonal driver events and the raw segmentation data. Clinical outcomes were compared between clusters. Overall and recurrence-free survival estimates were computed using the Kaplan-Meier method. Cox models were used to calculate inter-cluster survival differences. All analyses were performed in R v3.5.0
Results: After selecting the best combination of clusters and penalty parameters. Fiveclusters were identified. When evaluating the fraction of copy-number altered genome in each cluster, we evidenced a significant difference between them (ANOVA,p<0.001). We then proceeded to evaluate overall survival differences between the clusters. Particularly, there was a significant difference in OS between clusters 1 and 2 (Cox,p=0.02).
Conclusions: Clonality estimates from single-site biopsy samples allows characterization of ccRCC subgroups that correlate with survival outcomes. Inclusion of additional parameters and other data types may improve outcome prediction.
15Coordinated pembrolizumab and high dose IL-2 (5-in-a-row schedule) schedule for therapy of metastatic clear cell renal cancer, a single center, single arm trial. NCT02964078.
Chatzel, Jonathan
Author Company: Moffitt Cancer Center
Co Authors: Swank, Jennifer; Ludlow, Steven; Lombardi, Kristina; Croft, Cortlin; Artigas, Yesenia; Hart, Sarah; Johnson, Elaine; Zhang, Jingsong; Jain, Rohit; Fishman, M.D., Mayer
1. DF McDermott, et al., Clin Cancer Res. 2014, p 1520.
2. G Fyfe et al. J Clin Onc. 1995 13(3):695.
3. DF McDermott et al. (meeting presentation) J Clin Onc , 2018. 36(15_suppl): 4500.
4. TK Choueiri et al. Clin Cancer Res. 2016: 2839.
5. J Brayer and M Fishman. J. Immunotherapy 2014. 37(3): 187.
6. DA Vaena et al. (meeting presentation ) 2018 J Clin Onc 2018 36, 2018 (suppl; abstr TPS3115)
7. KA Margolin. Seminars Oncology 2000; 27(2):194.
Background: Ligation or blockade of IL-2 receptor and of PD-1 receptor may change lymphocyte behavior to cause meaningful disease regression in cancers with diverse histology and sites of origin. Single agent objective response rates of 14-25% have been reported for IL-2 therapy of metastatic clear cell RCC (ccRCC) [1, 2]. A major response rate of 33.6% was observed in pembrolizumab treated ccRCC patients [3].
Nivolumab treated ccRCC patients were observed to have intratumoral migration of lymphocytes early after therapy [4]. A case report of IL-2 induced major regression that had been immediately preceded by no change on nivolumab therapy suggested that combining the two means of lymphocyte stimulation could be effective [5]. Other trials combining IL-2 receptor agonists (NKTR-214) and PD-1 blockade have also reported regression of ccRCC [6]. Two distinctive attributes of high dose IL-2 as a therapy are the required inpatient stay related to hypotension and cytokine release syndrome risks and, critically, the durability of the complete responses [1] [7].
Design: This is a single-institution, single arm design addresses safety and feasibility of the combination of IL-2 and pembrolizumab in the treatment of metastatic ccRCC. Subjects are treated on four nine-week block, as follows: Pembrolizumab is given on weeks 1, 4, and 7 of each block. Patients are admitted for 5 doses of high dose Il-2 (given over 3 days) on weeks 2, 3, 5, and 6 of blocks 2 and 3. Safety is monitored by a Pocock boundary of .05 likelihood of 0.15 dose limiting toxicity rate. Scans for efficacy are checked at baseline and at the end of each 9 weeks block, and at 2-3 months intervals after the completion of treatment. The hypothesis for the sample size is at least a 45% major response rate (null hypothesis <20%). Accrual was 26 patients from 4/2017 through 8/2018.
Major eligibility criteria include irRECIST measurable metastatic ccRCC; stress test without ischemia, acceptable pulmonary function testing, creatinine under 1.5 x upper limit (or clearance over 60 mg/ml/min) no uncontrolled CNS disease; zero or one prior therapies in last 12 months, and no prior PD-1 pathway treatment.
Correlative studies will include: PD-L1 assays of responders vs nonresponders, leukocyte subsets at treatment points (pretreatment; after pembrolizumab monotherapy; after 10 doses of IL-2; after all IL-2), and a nanostring evaluation of cytokine profiles of flow sorted CD8+ lymphocytes
Sponsorship: Prometheus IIT 15PLK02(funding); Merck MISP 52587 (provision of pembrolizumab); Moffitt Cancer Center (regulatory sponsor, IND #132688.).
16Cytoreductive nephrectomy for non-clear cell RCC: NLR predicts survival outcomes
Silagy, M.D., Ph.D., Andrew
Author Company: Memorial Sloan Kettering Cancer Center
Co Authors: Silagy AW, Chen YB, Mano R, Dinatale RG, Blum KB, Marcon J, Sanchez A, Coleman JA, Russo P, Hakimi AA.Urology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY.
Introduction: Cytoreductive nephrectomy (CN) is selectively utilized for the management of metastatic RCC (mRCC). Recently, the CARMENA trial failed to show benefit in the use of upfront CN for intermediate- and poor-risk clear cell RCC emphasizing the importance of patient selection. Few reports evaluated the clinical benefit for CN in non-clear cell RCC (nccRCC). We analyzed CN in nccRCC to report treatment outcome and identify pre-operative characteristics of patients that respond best to CN.
Methods: We queried our prospectively collated nephrectomy database for mRCC patients with a nccRCC histology who underwent CN at MSKCC from 1990-2018 (total n=122). All available pathology specimens were re-reviewed by genitourinary pathologists. Sixteen patients reclassified as clear cell histology and 5 patients with inadequate follow-up were excluded from the study cohort.
Pre-operative clinicopathological factors and subsequent treatment and survival outcomes were recorded. The pre-operative Neutrophil to Lymphocyte Ratio (NLR) was calculated and analyzed as a continuous variable, grouped into quartiles (Q1, Q2-3 and Q4) and grouped according to previously published cutoffs (<3 and <4.5).
The Kaplan-Meier method was used to estimate survival. A multivariate cox regression analysis was performed to identify statistically significant pre-operative predictors of survival.
Results: The study cohort included 101 nccRCC patients treated with CN; 65.7% of the cohort were male, the median age was 61 (IQR: 48-69).
Median follow-up was 13.5 months (IQR: 3-30.5). 80 patients died at a median time of 11.5 months. Estimated 2- and 5-year overall survival were 31.7% and 7.9%, respectively.
Patients with lower NLR had longer overall survival on Kaplan-Meier; p<0.001 (Figure 1). On multivariate cox-regression analysis, an elevated NLR was a significant predictor of cancer-specific survival when evaluated as a continuous variable, categorized in quartiles (Q1: Reference, NLR Q2-Q3: (HR 4.23 95%CI [1.43,12.47]; p=0.009), NLR Q4: (HR 6.21 95%CI [1.94,19.86.30]; p=0.002) and based on the cutoff values >3 and >4.5 (Figure 2). Tumor T-stage (T3, T4 vs. T1, T2) was also found to be a significant predictor of cancer specific survival.
Figure 1.
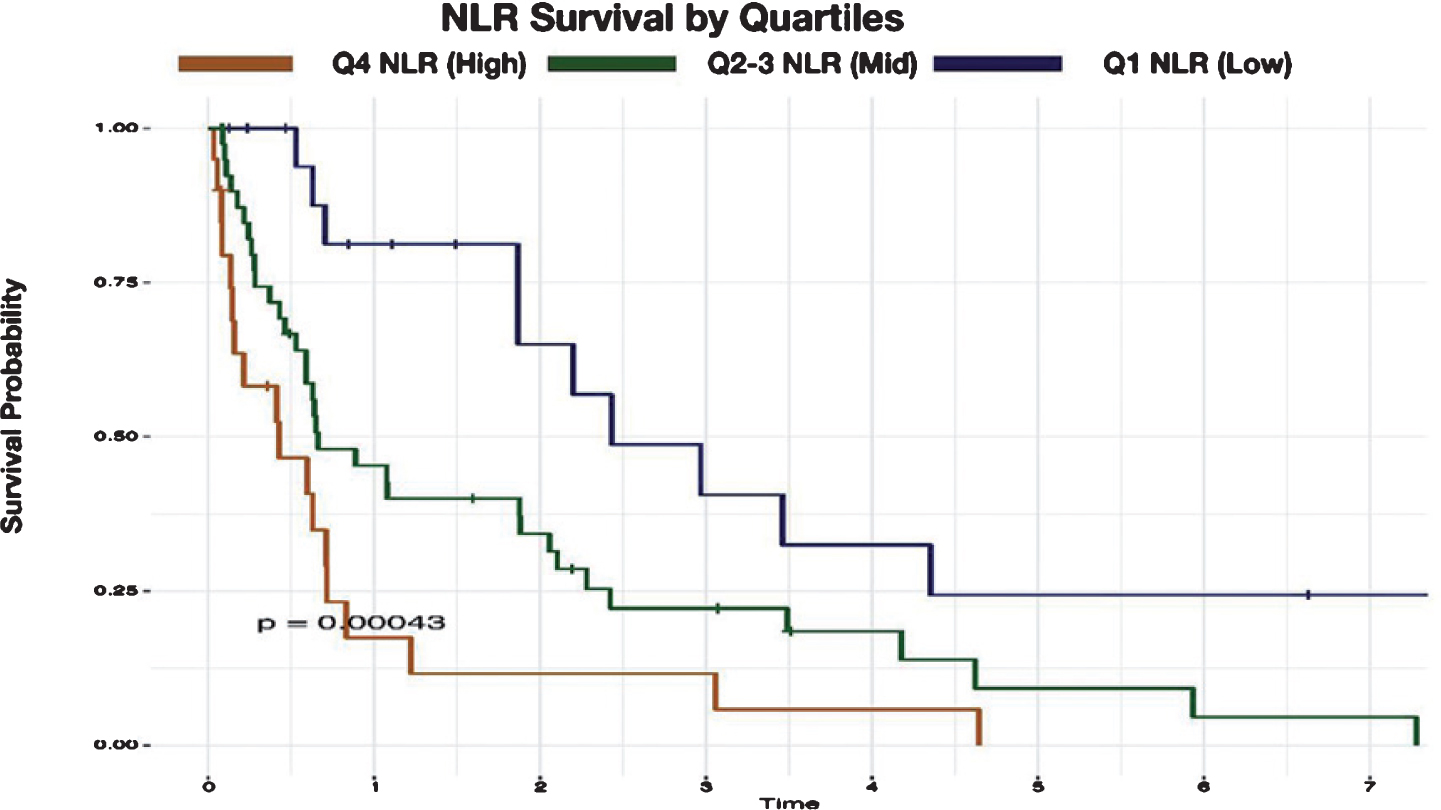
Figure 2.
Conclusion: The outcome of CN for nccRCC is poor. Patients with the highest quartile of pre-operative NLR may have worse survival when adjusting for established clinicopathologic prognostic features.
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
17Disparities in the Management of Clinical T1a and T1b Renal Masses Amoung Pateints in the National Cancer Database (NCDB)
Sterling, M.D., Joshua
Author Company: Robert Wood Johnson University Hospital
Co Authors: Rivera-Núñez, Zorimar; Farber, Nicholas; Kim, Sinae; Radadia, Kushan; Modi, Parth; Goyal, Sharad; Parikh, Rahul; Weiss, Robert; Kim, Isaac; Elsamra, Sammy; Jang, Thomas; Singer, Eric
Introduction: The 2017 AUA guideline for management of renal cell carcinoma (RCC) recommends prioritizing partial nephrectomy (PN) for the treatment of most clinical T1a (cT1a) tumors, using PN for clinical T1b (cT1b) tumors when feasible, and performing a minimally invasive surgery (MIS) when possible. Since cT1 RCC is a heterogeneous disease, we evaluated patterns of care in this population to examine factors associated with receipt of PN.
Methods: We queried the NCDB from 2010-2014 to identify patients treated surgically for cT1N0M0 RCC. Patient socio-demographics, clinical characteristics, and treatment parameters were examined for the cT1a and cT1b cohorts. Logistic regression models examined factors associated with receipt of PN.
Results: Our study population included 69,694 patients, 44,043 cT1a and 25,651 cT1b. In the cT1a cohort, 70% of tumors were treated with a PN and 30% with a RN; 35% of patients underwent an open procedure and 65% had MIS. In the cT1b cohort, 32% of tumors were treated with a PN and 68% with a RN; 38% of patients received an open operation and 62% had MIS. Patients with the following characteristics, in both ct1a and ct1b populations, were less likely to have a PN: income <$62,000, Medicare insurance, and those treated outside an academic hospital. African American patients in the cT1a cohort were less likely to receive a PN (Odd ratio (OR): 0.77, 95% CI: 0.72-0.83), while those in the cT1b group were more likely to receive a PN (OR: 1.20 95% CI: 1.09-1.32). For both cohorts, the farther a patient traveled to a treatment center the higher the likelihood a PN was performed.
Conclusions: While most patients with cT1 tumors had MIS, 30% of patients with cT1a tumors did not receive PN. This identifies an opportunity for improvement in the management of cT1a patients. We found treatment disparities for patients of lower household income, those without private insurance, and those treated outside academic centers, as they were less likely to receive PN. We also found patients who traveled farther for treatment were more likely to receive PN. Additional research into the impact of regionalization of RCC surgery on PN access, utilization, and outcomes is warranted.
18Identification of novel epidermal growth factor receptor (EGFR) splice variants in clear cell renal cell carcinoma
Zaman, Saif
Author Company: USF/Moffitt Cancer Center
Co Authors: Teer, Jamie; Zhang, Jingsong; Knepper, Todd; Spiess, Philippe; Sexton, Wade; Smith, Matthew; Fishman, Mayer; Magliocco, Tony; Pow-Sang, Julio; Poch, Michael; Gilbert, Scott; Boyle, Theresa; Manley, Brandon
Introduction: It is well established that alterations of epidermal growth factor receptor (EGFR) are associated with the development and progression of epithelial tumors across several cancer types. Alternative splicing and alterations of EGFR splice sites can cause translational changes and EGFR alterations have demonstrated associations with clinical and therapeutic outcomes in several malignancies.
Methods: Our institutional CLIA approved next generation targeted sequencing assay Moffitt STAR™ includes both DNA and RNA analyses. This assay has two targeted components. DNA sequencing is employed for identification of substitutions; small insertion/deletions and copy number variants and RNA sequencing is employed for identification of gene fusions and splice site variants. DNA and RNA extracted by DNA and RNA FFPE Allprep (Qiagen, Inc.) were prepared into sheared DNA and cDNA. The regions of interest were hybridized using the Trusight Tumor 170 (Illumina, Inc) library prep kit. Sequencing was performed using the Illumina NextSeq 500 instrument. Data was analyzed using the Illumina BaseSpace Enterprise TST170 app v1.0 and a customized analysis pipeline within the Clinical Genomics Workspace software platform from PierianDx.
Results: We identified a previously unreported EGFR gene splice variant, c.2470-188_c.2470-2 between exons 20 and 21 in four of eight renal cell carcinomas subjected to this sequencing assay. All four tumors were clear cell renal cell carcinoma (ccRCC) and the tissue examined was from three primary renal tumors (average tumor size 10.1cm) and from one L2 epidural tumor metastasectomy. The average age of the patients at time of surgery was 60 years. To date, one patient had localized disease without recurrence at 6 months follow up, one presented with metastatic disease and two patients developed metastatic disease on surveillance. All variants were identified at the RNA level without obvious corresponding DNA alterations. There was an average of 106 unique reads (average of 7.7% of all reads) for these cases supporting this variant. A representative case with corresponding Shashimi plot is demonstrated in Figure 1. This splice variant has not been detected in the approximately 150 other solid tumors cases that have been analyzed with this assay thus far.
Conclusion: We present four cases of ccRCC with a novel EGFR splice variant. Evaluation of the possible downstream effects of this alteration and possible clinical implications is currently underway.
19Immune response in patients treated with autologous dendritic cells transduced with AdGMCA9 (DC-AdGMCAIX) in patients with metastatic renal cell carcinoma from the phase I, open label, dose escalation and cohort expansion study
Faiena, M.D., Izak
Author Company: UCLA
Co Authors: Zomorodian, Nazy; Berent - Maoz, Beata; Sachadeva, Ankush; Bot, Adrian; Kabinnavar, Fairooz; Said, Jonathan; Cheung - Lau, Gardenia; Pang, Jia; Macabali, Mignonette; Chodon, Tinle; Wang, Xiaoyan; Cabrera, Paula; Kaplan - Lezco, Paula; Liu, Sandy; Comin - Anduix, Begonia; Pantuck, Allan; Belldegrun, Arie; Chamie, Karim; Drakaki, Alexandra
Background: Patients with metastatic RCC were treated in a phase I trial with autologous dendritic cells transduced by a replication deficient adenovirus comprised of GM-CSF+CAIX. Nine patients in three dose escalation cohorts (5, 15, and 50 X 106 cells/administration) were injected based on a 3+3 design.
Methods: An enzyme-linked immunospot (ELISpot) assay was used to determine the frequency of CAIX-specific IFN-? producing T cells in blood. 15-mer overlapping peptides from CAIX protein, AdV5-pepton, and controls (+/-) were plated in Elispot plates pre-coated with anti-IFN-? antibody. Subsequent to assay development, the number of T-cells responding to CAIX was calculated as above the lower limit of detection (LLD) (7 spots). After subtracting the backgrounds, fold change was calculated with respect baseline. The criterion for positive immunological response was defined as the mean fold change plus two. Further assessment included immunohistochemistry (IHC) staining of tissue from patients #4 (with PD) and #8 (with SD) for CAIX, CD4/8, Ki67, GrZ8, PD1/L1. The samples were scored based on percent positivity and staining intensity. Tissue was obtained from the primary tumor prior to vaccination, and the target tumor at the end of the study period (18 months).
Results: ELISpot showed consistently positive responses against CAIX upon vaccination with DC vaccine, more prominently in patients in cohort 3 (high dose) as well as in those with longer time to progression (figure 1). None of the treated patients showed an objective response. However, patient #8 who achieved stable disease (SD) lasting 18 months had more than 2-fold change in immune response over baseline on day 35 and 60 after the first vaccination cycle. All nine patients showed different degrees of immunological reaction to AdV5 at baseline and elevation at the end of the study. IHC showed that both patients had high CAIX expression in primary tumor and on the target lesion post vaccination. Immune infiltrates were seen at baseline in both subjects, with predominant CD4/8 T-cells in patient #8 with a high PD-1 expression in infiltrating lymphocytes without PD-L1 expression in the tumor environment.
Conclusion: DC-AdGMCAIX vaccination may elicit robust immunologic response against CAIX in patients with ccRCC. The findings of high PD-1 expression in the patient with SD in both the primary tumor and target lesion warrants future efforts to explore how combination therapies with biological response modifiers may further enhance clinical responses.
20Ipilimumab plus Nivolumab (Ipi/Nivo) as Salvage Therapy in Patients with Immunotherapy (IO)-Refractory Metastatic Renal Cell Carcinoma (mRCC)
Allman MSN CNP, Kimberly
Author Company: Cleveland Clinic
Co Authors: Authors: Kimberly Allman MSN CNP1, Anita Gul MD 1, Moshe C. Ornstein MD1, Ruby Gupta MD1, Jessica Ball RN1, Laura Wood RN MSN OCN1, Jorge A. Garcia MD1, Dendra von Merveldt RN2, Hans Hammers, MD, PhD2, Brian I. Rini MD1
1Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, USA
2UT Southwestern, Dallas, TX, USA
Background: Ipi/Nivo is a standard of care in previously untreated mRCC and has shown activity in patients (pts) with RCC previously-treated with VEGF inhibitors (Hammers et al. JCO). The activity of Ipi/nivo in patients failing prior IO is unknown.
Methods: Pts with mRCC treated at Cleveland Clinic or UT Southwestern with salvage Ipi/Nivo after having progressed on prior IO were retrospectively reviewed. Ipi/Nivo was administered as per CHECKMATE 214. Computed tomography imaging was obtained at baseline and every12 weeks to assess disease response per RECIST 1.1 criteria. Baseline patient characteristics, outcome to therapy and adverse effects as per CTCAE v5.0 were collected from the electronic medical record.
Results: A total of 14 patients were identified. The median age was 60 (range, 49-78), all of which were male with clear cell histology and ECOG PS 0-2. IMDC risk group at time of salvage Ipi/Nivo initiation was favorable risk (4 pts), intermediate risk (9) and poor risk (1). The median number of prior systemic therapies was 3 (range, 1-6). Pts received a variety of prior IO therapies including nivolumab monotherapy (6 pts), avelumab/axitinib (2 pts), previous Ipi/Nivo (2 pts), nivolumab/HIF inhibitor (1 pt), atezolizumab/IFN (1 pt), pembrolizumab/bevacizumab (1 pt) and an oral adenosine inhibitor (1 pt). The median time on prior IO was 14 months (range, 1-21) with a best response to prior IO of CR in 1 pt, PR in 4 pts and SD in 5 pts (3 of whom had minor disease regression) and progressive disease (3 pts). Seven pts have reached restaging on salvage Ipi/Nivo demonstrating 3 PRs, 1 SD and 3 PD. No major unexpected toxicities were observed.
Conclusions: Ipi/Nivo is feasible and safe in IO-refractory mRCC population with preliminary evidence of anti-tumor activity. Updated response data will be presented.
21Mucinous tubular and spindle-cell carcinoma (MSTCC) of the kidney: Patient characteristics, genomic profile, and treatment outcome.
Ged, M.D., Ph.D., Yasser
Author Company: Memorial Sloan Kettering Cancer Center
Co Authors: Ying-bei Chen, Andrea Knezevic, Maria I. Carlo, Chung-Han Lee, Darren R. Feldman, A. Ari Hakimi, Sujata Patil, Paul Russo, Martin H.Voss, Robert J. Motzer
Background: MTSCC is a rare subtype of kidney cancer, first recognized as a distinct entity in the 2004 WHO classification of renal tumors. While typically indolent, rare cases with high grade transformation or sarcomatoid differentiation have been reported. There is no defined standard treatment for metastatic cases. We report on the clinical presentations, genomic profile and outcomes of patients (pts) with MTSCC managed at our institution.
Methods: The Memorial Sloan Kettering Cancer Center database was queried and clinical data extracted for all pts with MTSCC between 01/01/2004 to 06/01/2018. All identified cases were reviewed by a pathologist (Y.C.). Next generation sequencing (NGS) with MSK-IMPACT was performed in a subset of pts who had tumor samples available for study.
Results: A total of 25 pts were identified; clinical features are summarized below (table). All pts underwent primary tumor-directed management including 23 pts with nephrectomy (Radical, n=7, Partial, n=16) and 2 pts with cryoablation. Metastases were diagnosed in 6 pts (24%), three of which had de novo metastatic disease and time from presentation to development of metastatic disease for the other 3 pts was 6.7, 9.9 and 15.5 months (mos). 5 of 6 pts with metastatic disease had sarcomatoid or high grade histological features compared to 0 of 19 non-metastatic pts (83% vs 0%, p < 0.001, Fisher’s Exact Test). Commonest sites of metastatic disease included bone (n=4, 67%) and lung (n=3,50%). 3-year overall survival from diagnosis with MTSCC was 84.8% (95% CI: 59.6, 94.9) for all pts with a median follow-up time for survivors of 3.9 years (range:1 mos, 10.3 years). Three deaths occurred, all from metastatic disease. 4 pts received first line VEGF TKI with a time to treatment failure (TTF) of 1.6, 3.0, 3.8 and 30.6 mos with 1 pt achieving long term response on sunitinib. 3 pts received nivolumab in the third line setting with a TTF of 1.0, 2.4 and 4 mos. NGS was performed in 5 pts: most frequent altered gene was NF2 (n=2, 40%). Germline alterations were detected in 2 pts (40%) including CHEK2 and BRCA2. Mismatch repair (MMR) deficiency was detected in 1 pt.
Conclusions: MTSCC is characterized by localized tumors treated successfully with tumor directed therapy. However, pts with high grade histological features were more likely to develop metastatic disease, with one long term responder to sunitinib and 0 of 3 pts responding to nivolumab.
| Characteristic | N | Median (range) or Frequency (%) |
| Age at diagnosis, years | 25 | 58 (21, 74) |
| Female gender | 25 | 16 (64%) |
| Primary tumor size, cm | 22 | 5.7 (1.3, 16.2) |
| Primary stage | 22 | |
| pT1 | 11 (50%) | |
| pT2 | 8 (36%) | |
| pT3a | 2 (9%) | |
| pT3b | 1 (5%) | |
| Symptoms at presentation | 21 | |
| None | 13 (62%) | |
| Localized | 7 (33%) | |
| Systemic | 1 (5%) |
22Neutrophil-to-lymphocyte ratio predicts recurrence-free survival in unclassified renal cell carcinoma
Marcon, M.D., Julian
Author Company: Memorial Sloan Kettering Cancer Center
Co Authors: DiNatale RG1, Ghanaat M1, Silagy AW1, Mano R1, Blum KA1, Reznik E2, Coleman JA1, Russo P1, Hakimi AA1, Chen YB3
1 Urology Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York City, NY 2 Computational Biology Center, Center for Molecular Oncology, Memorial Sloan-Kettering Cancer Center, New York City, NY 3 Department of Pathology, Memorial Sloan-Kettering Cancer Center, New York City, NYFundingThis research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Introduction/Background: Unclassified renal cell carcinoma (uRCC) is a rare non-clear cell RCC variant, which comprises clinically and genomically different tumor entities. The neutrophil-to-lymphocyte ratio (NLR) as a marker of inflammatory host response has recently shown a significant association with patient prognosis in multiple neoplasms. We aimed to investigate the association between NLR and time to disease recurrence in patients with uRCC and no evidence of distant metastatic disease at the time of diagnosis.
Material and methods: After obtaining IRB approval we queried our institutional nephrectomy database and identified 113 patients with loco-regional uRCC with complete demographic data, a preoperative complete blood count obtained within one month prior to nephrectomy and comprehensive follow-up data. 47 patients with an oncocytic variant of uRCC and 10 patients with concomitant secondary neoplasms and/or active or chronic infectious diseases were excluded, leaving 56 patients for analysis. NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count (both in K/µl). Time to recurrence was analyzed using the log-rank test and Cox proportional-hazards model.
Results: The study cohort included a total of 33 men and 23 women with a median age of 58.4 years (IQR: 44.1-66.9). Median follow-up for the whole cohort was 27.7 months. 14 patients developed disease recurrence at a median time of 9.93 months (IQR: 5.2-19.4). Median NLR was 2.56 (IQR: 1.75-3.38). The third quartile of the NLR was used to stratify patients into two subgroups with high and low NLR values. The log-rank test showed a significantly shorter interval to disease recurrence for the high NLR subgroup (p = 0.0057, Fig. 1). Cox regression analysis yielded a statistically significant association between NLR and time to recurrence (HR=1.58, 95%CI: 1.14-2.18, p = 0.0056).
Conclusion: Our study demonstrated a significant association between NLR and time to disease recurrence in patients with uRCC. Validation of these findings in other uRCC cohorts should be performed to confirm the predictive value of NLR in this setting.
23Nivolumab for the treatment of Metastatic Renal cancer-Retrospective Audit study
Jain, M.D., Ankit
Author Company: New Cross Hospital
Co Authors: 1Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, USA
Background: Nivolumab, PD-1 inhibitor has shown promising overall survival and good tolerance in clinical trials for patients with advanced metastatic renal cell cancer. This study tried to analyse survival statistics and tolerance to treatment in clinical practice setting.
Methods: A retrospective analysis of eight patients with metastatic renal cell cancer was carried out in a teaching hospital in West Midlands,UK. A descriptive analysis of data was carried out with respect to demographics, adverse events and survival statistics.
Results: Eight patients (7 male, 1 female) with metastatic renal cancer who received Nivolumab between July 2016 and September 2017 were identified for this study. Median age was 61.5 years (range 43 – 70). Six patients had clear cell histology, one had poorly differentiated carcinoma and one did not have histology available. 6 patients received Nivolumab as 2nd line therapy, 1 received as 3rd line and 1 as 4th line. Seven out of the eight patients received prior Tyrosine Kinase Inhibitor therapy. Median duration of treatment was 14.5 cycles (range 8 – 32). Five adverse events were noted in four patients. All were of grades 1-2. One patient had colitis and pruritis, two had arthralgia and one had pruritis alone. Two patients (colitis and arthralgia) required oral steroids for the treatment of adverse events. Median time to develop any adverse event was 15.5 weeks (range 4 -36). Overall response rate was 50% (n=4) with three patients showing partial response and one patient showing stable disease. Median overall survival was not reached in this group.
Conclusion: With the main limitation being the small number of patients included, this study demonstrates that Nivolumab is well tolerated and has good overall response rates which is in line with the findings in clinical trials. These findings need to be validated in bigger patient cohorts in clinical practice.
24Overall and progression-free survival in metastatic renal cell carcinoma (mRCC) patients treated with first-line tyrosine kinase inhibitors (TKI) followed by second-line immunotherapy
Ratto, M.D., Barbara*
Author Company: Novartis Pharmaceutical Corporation
Co Authors: Derek Tang1, Luca Dezzani1, Nicholas Robert2, Bismark Baidoo2, Jennifer Frytak2, Nicholas J. Vogelzang3
1Novartis Pharmaceutical Corporation, East Hanover, NJ
2McKesson Specialty Health, The US Oncology Network, The Woodlands, TX
3Comprehensive Cancer Centers of Nevada, Las Vegas, NV
*corresponding author
Background: There are several treatment options available for mRCC, including TKIs and immunotherapy. The purpose of this study was to assess overall and progression-free survival in mRCC patients treated with second-line (2L) nivolumab, after first-line (1L) TKI, in a real-world community oncology setting.
Methods: This retrospective cohort study examined stage IV adult RCC patients who initiated 2L nivolumab between 12/01/2015-09/30/2016 and were previously treated with a 1L TKI in US Oncology Network (USON) clinics. USON’s iKnowMedTM (iKM) EHR database was used to identify eligible patients and obtain relevant data. Chart review was conducted to abstract treatment information from medical charts. Study follow-up extended through 06/30/2017 for a minimum of 9-months of follow up. Progression-free survival (PFS) and overall survival (OS) were estimated from initiation of 1L TKIs and from initiation of 2L nivolumab, using standard Kaplan-Meier methods. For PFS, death or disease progression were considered an event. Patients who were lost to follow up were censored on last contact or study end date. An intention-to-treat approach was employed.
Results: Table 1 presents characteristics of the 105 eligible patients treated with 1L TKIs followed by 2L nivolumab. From the initiation of 1L therapy, unadjusted median OS for TKIs was 26.7 months (95% CI: 23.6-30.3), and PFS for 1L treatment was 9.23 months (95% CI: 7.0-10.6). From 2L nivolumab initiation, median OS was 13.6 months (95% CI: 11.9-16.6) and PFS for 2L was 6.3 months (95% CI: 4.67-8.41).
Table 1.
Characteristics of mRCC patient population
| Characteristic | Overall N=105 n (%) |
| Age at index | |
| <45 | 5 (4.8%) |
| 45-65 | 54 (51.4%) |
| >65 | 46 (43.8%) |
| Median (Range) | 63.4 (34.1-87.4) |
| Race | |
| Caucasian | 90 (85.7%) |
| Black | 5(4.8%) |
| Other/Not documented | 10 (9.5%) |
| Sex | |
| Female | 32(30.5%) |
| Male | 73(69.5%) |
| Stage at initial diagnosis | |
| I | 9 (8.6%) |
| II | 10 (9.5%) |
| III | 17 (16.2%) |
| IV | 61 (58.1%) |
| Not Documented | 8 (7.6%) |
| ECOG performance status at baseline | |
| 0 | 15 (14.3%) |
| 1 | 47 (44.8%) |
| 2 | 10 (9.5%) |
| 3 | 1 (1.0%) |
| Not Documented | 32 (30.5%) |
| Heng Risk Category at baseline | |
| Favorable | 10 (9.5%) |
| Intermediate | 30 (28.6%) |
| Poor | 12 (11.4%) |
| Not Documented | 53 (50.5%) |
| Follow-Up Time (months) | |
| Median from 1L initiation (Range) | 16.2 (2.3,114.8) |
| Median from 2L initiation (Range) | 5.6 (0.0,48.1) |
Note. Index=initiation date of 1L therapy; baseline=closest measure within ±30 days of index date
Conclusions: While OS from 1L TKI initiation is numerically similar to published estimates from clinical trials, OS from 2L nivolumab initiation appeared numerically shorter in our study. The phase 3 trial comparing nivolumab to everolimus reported a median OS of 25 months (30% of patients were alive at data cut-off) in the nivolumab group, whereas in our study, median OS from nivolumab initiation was only ~14 months (with ~40% of patients censored). However, our study population was slightly older and generally had lower performance scores at treatment initiation. Median PFS from nivolumab initiation was similar to both trial data and real-world evidence from the Italian Nivolumab Renal Cell Cancer Early Access Program. The results of this study may help improve our understanding of the effectiveness of TKIs followed by nivolumab in real-world practice.
25Patient-reported Outcomes Among Those Taking Pazopanib for Metastatic Renal Cell Carcinoma (mRCC) in a Community Oncology Setting
Fisher, Maxine1
Author Company: Vector Oncology
Co Authors: 1 Sameer R. Ghate,2 Paul J. Miller,1 Mark S. Walker,1 Ilia L. Ferrusi,2 Neeraj Agarwal3
1Vector Oncology, Memphis, TN, USA; 2Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 3Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA
Introduction/objective: Targeted therapy is widely used in mRCC to improve survival and health-related quality of life (HRQOL). Existing HRQOL data in patients with mRCC comes from clinical trials. This study investigated the impact of pazopanib on mRCC patients’ HRQOL in the United States’ community oncology setting.
Methods: Medical records data from adult patients with mRCC starting pazopanib between 1/1/2009–9/30/2016 were retrospectively collected. HRQOL was assessed for first-line pazopanib patients using the Patient Care Monitor (PCM), an 86-item patient-reported outcomes survey, administered as part of routine care in participating practices, which resulted in HRQOL data for 20%–30% of patients. Study endpoints included composite scores (General Physical Symptoms, Treatment Side Effects, Acute Distress, Impaired Ambulation, and Impaired Performance). Linear mixed models (LMM) were used to assess change in PCM scores during first-line therapy. Covariates included baseline clinical and demographic variables.
Results: Among 109 first-line pazopanib patients identified, 43 had PCM data (>200 observations). Median age of respondents was 67 (range 47–86) years; 70% were male, 81% were white, and 19% were African American. Initial RCC diagnosis was most commonly stage IV (37.2%). Overall, at baseline, 7.0% of patients had impaired performance status (PS) and 11.6% had poor Heng Score. Most metastases occurred in lung (65.1%) and bone (30.2%). LMM for pazopanib as first-line therapy showed no clinically meaningful changes over time for HRQOL scores. Among patients with baseline impaired PS, General Physical Symptoms (P = 0.072), Impaired Ambulation (P = 0.036), and Impaired Performance (P = 0.007) scores were worse during therapy. Other covariates were unchanged.
Conclusions: This study, using real-world retrospective data, showed no change over time in HRQOL for patients with mRCC after starting on pazopanib, suggesting that pazopanib treatment did not worsen HRQOL. Despite the small sample size, results appear clinically meaningful and the self-reported HRQOL was consistent with physician-rated performance status at baseline.
26Pazopanib-induced liver toxicity in metastatic renal cell carcinoma (mRCC) patients: impact of UGT1A1 polymorphism on pazopanib dose reduction, safety and patient outcome
Henriksen, M.D., Jakob N.
Author Company: Aarhus University Hospital
CO-Authors: Pernille Bøttger3, Carina K Hermansen2, Søren A Ladefoged3, Peter H
Nissen3, Stephen H Dutoit4, Thomas L Fink2, Frede Donskov2
Departments of Clinical Pharmacology1, Clinical Oncology2, Clinical Biochemisty3 and
Histopathology4, Aarhus University Hospital, Denmark
Background: Pazopanib may induce liver toxicity in patients with mRCC leading to treatmentinterruption and discontinuation. We assessed impact of a TA repeat polymorphism in the UGT1A1gene encoding Uridine Diphosphate Glucuronosyltransferase 1A1, (TA6/TA7 and TA7/TA7 vsnormal TA6/TA6) for liver toxicity, dose reduction and patient outcome.
Patients and methods: Patients with mRCC treated with first line pazopanib developing livertoxicity was genotyped for the UGT1A1 polymorphism. Genomic DNA was isolated from EDTAstabilised blood samples. Following PCR the UGT1A1 polymorphisms were assessed by fragmentanalyses using an Applied Biosystems 3500 Genetic Analyzer. Liver toxicity was assessedaccording to Common Terminology Criteria for Adverse Events (CTCAE) v. 4.0. Progression freesurvival (PFS) and overall survival (OS) were assessed by Kapplan-Meier method.
Results: A total of 261 patients received first line pazopanib. Of these, 34 (13%) patientsdeveloped liver toxicity after median 29 days (range 5-155 days); grade 4, 3 and 2 alanineaminotransferase (ALT) increased in 1, 15 and 7 patients, respectively, and grade 4 and 3 bilirubinincreased in 1 and 1 patient, respectively. UGT1A1 assessment demonstrated 18 (53%) patientswere TA6/TA7, 7 (21%) patients were TA7/TA7, and 9 (26%) patients with normal TA6/TA6.UGT1A1 polymorphism was associated with PFS, median PFS TA6/TA6 5.5 mo, TA6/TA7 34.2mo, TA7/TA7 22.3 mo, and unknown UGT1A1 status 9.2 mo; UGT1A1 polymorphisms combinedvs TA6/TA6, p=0.033. UGT1A1 was also associated with OS, median OS TA6/TA6 8.1 mo,TA6/TA7 or TA7/TA7 not reached, and unknown UGT1A1 status 16.6 mo; UGT1A1polymorphisms combined vs normal or unknown status, p=0.03. No association with best objectiveresponse was noted. Liver toxicity resulted in pazopanib interruption in median 23 days (range 7-215 days). Patients with UGT1A1 polymorphism had pazopanib safely resumed in ultra-low dosesbased on the degree of liver toxicity and UGT1A1 polymorphism, with highest dose reductions inpatients with TA7/TA7 to pazopanib 200 mg every third day or 200 mg every second day.
Conclusion: Managing liver toxicity in mRCC patients treated with pazopanib may be improved byUGT1A1 assessment. UGT1A1 polymorphisms are associated with improved outcomes despitesubstantial dose reduction.
27Phase 1b study (COSMIC-021) of cabozantinib in combination with atezolizumab: Results of the dose escalation stage in patients with treatment-naïve advanced renal cell carcinoma (RCC)
Agarwal, Neeraj
Author Company: Huntsman Cancer Institute
Co Authors: Ulka Vaishampayan,2 Marjorie Green,3 Flavia di Nucci,3 Pei-Yun Chang,4 Christian Scheffold,4 Sumanta Pal,5 for the XL184-021 study group
1Huntsman Cancer Institute, University of Utah, Salt Lake City, UT; 2Karmanos Cancer Center, Detroit, MI; 3Genentech, South San Francisco, CA; 4Exelixis Inc., Alameda, CA; 5City of Hope, Duarte, CA
Background: Cabozantinib (C) is an inhibitor of multiple receptor tyrosine kinases involved in tumor cell proliferation, neovascularization, and immune cell regulation, including MET, VEGFRs, and TAM family of kinases (TYRO3, MER, and AXL). Preclinical/clinical studies suggest that C promotes an immune-permissive environment that may facilitate synergistic effects with checkpoint inhibitors. This Phase 1b study evaluates C in combination with the programmed death ligand (PD-L1) targeting antibody atezolizumab (A) in patients with solid tumors (NCT03170960).
Methods: Safety and clinical activity of C (2 dose levels: 40 mg, 60 mg QD) + A (1200 mg Q3W) administered in 3-week cycles were evaluated in a 3+3 dose escalation design. Safety data of all patients and criteria for dose limiting toxicity (DLT) determined the recommended dose (RD) for a subsequent expansion stage. Tumor response was assessed by CT/MRI and bone scan (RECIST v 1.1).
Results: 12 patients with treatment-naïve advanced RCC (mostly clear-cell subtype) were treated in the dose escalation stage (6 at each dose level). At data cutoff, all patients were actively receiving study treatment (range, 3–12 cycles). There were no DLTs or serious adverse events (AEs) in either C+A dose cohort. Most AEs were Grade 1-2 in severity including immune-related AEs. Grade 3 AEs were experienced by 7 patients and included 3 events of hypertension, 2 events each of diarrhea and hypophosphatemia, and 1 pulmonary embolism. There were no Grade 4/5 AEs. Among 10 patients investigator-assessed confirmed objective response rate was 50% (1 complete response, 4 partial responses [PRs]); 2 additional patients had unconfirmed PRs with only 1 tumor assessment at data cut-off.
Conclusions: C+A is well tolerated and shows encouraging anti-tumor activity in advanced RCC. C 40 mg QD + A 1200 mg Q3W was selected as the RD for expansion in multiple solid tumor cohorts including RCC.
Keywords: cabozantinib, atezolizumab, renal cell carcinoma, solid tumors
Other information to provide at submission: The study was supported by Exelixis, Inc.
28Phase 2 Study of Sequential First-line Pazopanib (PAZ) Followed by Everolimus (EVE) in Patients (pts) with Advanced or Metastatic Renal Cell Carcinoma (RCC) (CATChEz Study)
de Souza, Paul
Author Company: University of Western Sydney School of Medicine
Co Authors: Shirley Wong,2 Sanjeev Sewak,3 Dusan Kotasek,4 Bhumsuk Keam,5 Jinsoo Chung,6 Jackie Han,7 Luca Dezzani,7 Qasim Ahmad,7 Paul Mainwaring8
2Western Health, Footscray, Victoria, Australia; 3Peninsula and Southeast Oncology, Frankston, Victoria, Australia; 4Adelaide Cancer Centre, Kurralta Park and University of Adelaide, South Australia, Australia; 5Seoul National University Cancer Research Institute and Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea; 6Department of Urology, National Cancer Center, Goyang, Korea; 7Novartis Pharmaceuticals Corporation, East Hanover, New Jersey; 8 Centre for Personalized Nanomedicine, Australian Institute for Bioengineering and Nanotechnology (AIBN), The University of Queensland, Australia
Introduction/objective: EVE following failure of sorafenib or sunitinib for RCC was first approved by the FDA in 2009. CATChEz (NCT01545817) was designed to test the activity of EVE following first-line PAZ in pts with advanced or metastatic RCC who had not received prior systemic therapy.
Methods: From 2012 to 2016, pts received first-line PAZ followed by EVE until progressive disease (PD), death, unacceptable toxicity, consent withdrawal, or study termination. Pts with PD during or within 6 months of stopping PAZ were eligible for EVE. Pts off study treatment were evaluated for PD, survival, and updates on anticancer treatment every 8 weeks until death or end of study. The primary efficacy endpoint was median progression-free survival (mPFS) for the second-line EVE treatment period; secondary endpoints included other survival measures, and safety evaluations were for second-line EVE and grade 3/4 toxicities attributable to PAZ and EVE.
Results: Of 74 pts who started first-line PAZ, 38 received =1 dose of second-line EVE. The primary endpoint of mPFS from the start of second-line EVE and the secondary endpoint of mPFS with first-line PAZ (Table) were consistent with previous reports; no unexpected adverse events (AEs) were reported. All pts had =1 treatment-emergent AE, 83.8% had grade =3 AEs, and 71.6% had serious AEs. Of 34 total deaths, 29 were due to PD and 5 were due to AEs (2 related to EVE [lower respiratory tract infection; pulmonary sepsis]; 3 unrelated to study treatment).
Conclusions: Efficacy and safety outcomes were consistent with published phase 3 data. The CATChEz study supports sequential first-line use of PAZ followed by EVE for the treatment of pts with advanced or metastatic RCC.
29Phase II Trial of Intermittent Therapy in Patients (pts) with Metastatic Renal Cell Carcinoma (mRCC) Treated with Front-line Ipilimumab and Nivolumab (Ipi/Nivo)
Ornstein, M.D., MA, Moshe C.
Author Company: Cleveland Clinic
Co Authors: Wood, Laura S.; Wei, Wei (Auston); Allman, Kimberly D.; Martin, Allison; Garcia, Jorge A.; Gilligan, Timothy D.; Rini, Brian I.
Background: The combination of Ipi/Nivo is approved for previously untreated intermediate and poor risk mRCC. Two primary questions for pts treated with Ipi/Nivo are duration of therapy required for sustained clinical benefit and the safety of re-induction with ipilimumab for pts who progress while on maintenance nivolumab (Nivo). A phase II trial is thus planned (NCT03126331).
Methods: Pts with treatment-naïve mRCC who receive up to four doses of induction Ipi/Nivo followed by 24 weeks (wks) of maintenance Nivo and achieve stable disease (SD), complete response (CR), or partial response (PR) will be eligible for inclusion. Patients who achieve SD will continue with Nivo maintenance per standard of care while those who achieve a PR or CR will enter an observation period off therapy. All pts will be restaged every 12 wks. Upon disease progression, patients will be re-challenged with 2 doses of Ipi/Nivo every 3 wks followed by CT imaging. Patients may receive 1 or 2 more doses of re-induction ipilimumab and nivolumab depending on response to first 2 re-induction doses. Following re-induction, pts with PD will be removed from the trial, SD pts will receive maintenance Nivo, and pts who achieve a CR/PR will enter an observation phase. Correlative blood and tissue samples will be obtained at pre-planned time-points to assess for biomarkers of response and resistance.The study objectives are (a) to estimate success rate of observation in pts who achieve a CR/PR, in which success is defined by 50% of CR/PR pts who withhold therapy for at least 9 months, and (b) to estimate toxicity rate (i.e. rate of grade = 3 treatment-related AEs) in patients undergoing re-induction. Forty pts will be enrolled (estimated, 20 CR/PR and 20 SD) such that the half-width of 95% confidence interval (CI) around success rate will not exceed 0.23. Early termination rules for toxicity are integrated as well. Summary statistics of success status and toxicity will be provided in frequencies and percentages. Toxicity and success rates will be estimated along with 95% CIs. Logistic regression model will be used to explore effects of patient and tumor characteristics on success status and toxicity.
30Predictors of Receiving a Lymph Node Dissection at the Time of Surgery for Non-Metastatic Renal Cell Carcinoma
Radadia, Kushan
Author Company:
Co Authors: Rivera-Nunez, Zorimar; Kim, Sinae; Farber, Nicholas; Sterling, Joshua; Modi, Parth; Goyal, Sharad; Parikh, Rahul; Weiss, Robert; Kim, Isaac; Elsamra, Sammy; Jang, Thomas; Singer, Eric
Introduction: The benefit of a lymph node dissection (LND) in renal cell carcinoma (RCC) remains poorly defined. Despite this uncertainty, the American Urological Association (AUA) guideline on localized renal cancer recommends that LND be performed for staging purposes when there is suspicion of regional lymphadenopathy on imaging. Using the National Cancer Database (NCDB), we examined factors associated with the receipt of LND at the time of kidney surgery.
Materials and methods: The NCDB was queried for non-metastatic patients who underwent partial nephrectomy or nephrectomy for RCC from 2010 to 2014. Patient socio-demographics, clinical characteristics, and treatment factors were extracted. Logistic regression models were used to examine factors associated with the receipt of LND.
Results: We identified 110,963 patients who underwent surgery for RCC, of whom 11,867 (11%) had LND performed at the time of surgery. Clinical lymph node (cLN) and pathologic lymph node (pLN) information were available in 11,300 patients, of which 1,725 were preoperatively staged as having positive cLN.In the entire study population, patients who were cLN positive were approximately 19 times more likely to receive a LND at the time of surgery (OR: 18.68, 95%CI: 16.62-21.00). Among patients who were cLN negative (n=106,370), patients who received care at an academic/research institution (OR: 1.58, 95%CI: 1.43-1.74), traveled farther (>31 miles) to a treatment center (OR: 1.22, 95%CI: 1.14-1.30), and had a higher clinical tumor (cT) stage (cT2-4, OR range: 4.87-11.1) were more likely to undergo a LND despite being cLN negative. Patients who underwent robotic or laparoscopic surgery were less likely to receive a LND compared to open surgery (OR: 0.73, 95%CI: 0.69-0.78 and OR: 0.60, 95%CI: 0.59-0.66 respectively) (Table 1).
Conclusion: The greatest single predictor of LND receipt is being cLN positive. Among patients who are cLN negative, predictors of undergoing LND include treatment center type, distance to the treatment center, and cT stage. The impact of treatment center type and location on access to and outcomes from RCC surgery need further investigation. Additional studies to determine the accuracy of clinical staging and assess novel preoperative imaging modalities that evaluate nodal involvement are indicated
31Preliminary investigation of Radiogenomics in sarcomatoid dedifferentiation of renal cell carcinoma
Marcon, M.D., Julian
Author Company: Memorial Sloan-Kettering Cancer Center
Co Authors: DiNatale RG1, Silagy AW1, Mano R1, Blum KA1, Reznik E3, Coleman JA1, Russo P1, Duzgol C2, Akin O2, Hakimi AA1
1 Urology Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York City, NY
2 Department of Radiology, Memorial Sloan-Kettering Cancer Center, New York City, NY
3 Computational Biology Center, Center for Molecular Oncology, Memorial Sloan-Kettering Cancer Center, New York City, NY
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Introduction/Background: Sarcomatoid dedifferentiation of renal cell carcinoma (sRCC) can occur in any RCC subtype and is associated with aggressive tumor biology and poor prognosis. The association between imaging features and genomic mutations has been examined in clear cell RCC and has revealed specific radiogenomic subgroups with prognostic value. This study aims to correlate imaging features with genomic findings in the sRCC setting and explore their association with survival.
Material and Methods: After obtaining IRB approval we analyzed data for 25 patients who underwent nephrectomy with a histological diagnosis of sRCC between April 2012 and October 2017. All patients had a preoperative CT scan available for review and underwent comprehensive molecular testing using next-generation targeted gene sequencing (MSK-IMPACT). The top mutated genes in our cohort were evaluated (VHL, TERT, PTEN, BAP1, PBRM1, SETD2, ARID2, TP53). Segmentation analysis tracking was performed using TeraRecon iNtuition® v4.4.13 software to determine tumor volume, RECIST 1.1 tumor diameter and mean attenuation values. The presence of multiple morphological features was also evaluated (e.g. locoregional lymphadenopathy, renal vein invasion). Statistical analysis included Fisher’s exact test and Wilcoxon test for the comparison of genomic findings and imaging features, Cox proportional-hazards model and log-rank test were used for survival analysis.
Results: The cohort included 19 men and 6 women at a median age of 52 years (IQR: 46-58). Median patient follow-up of the whole cohort was 25.9 months (IQR 12-37.6). 10 patients died of kidney cancer within the observation period. There was a significant association between tumor volume and mutation in the TP53 gene (p=0.029, Fig. 1A). No significant associations were found between genomic alterations and survival. However, higher tumor volume and radiographic presence of locoregional lymphadenopathy were significantly associated with worse survival (HR=7.85, 95% CI: 1.5-40, p=0.013, q=0.035 and HR: 7.26, 95% CI: 1.9-27.5, p=0.004, q=0.025, respectively, Fig. 1B).
Conclusion: The association between TP53 mutation and tumor size in the current cohort may suggest that TP53 is an important driver event in large sRCC tumors. Importantly, size was the most significant clinical predictor for overall survival. Further studies in larger patient cohorts are required to validate these findings.
32Prognostic Implications in Renal Cell Carcinoma Survival: Large Cohort Evidence (Surveillance Epidemiology and End Results) Dataset.
Masire, Phatsimo1
Author Company: Nemours Children’s Health Care System
Co Authors: Authors:, Eaton A, Dabney K, Holmes L Jr
Affiliation: 1 Nemours Children’s Health Care System, 2 Alma College, 3 Thomas Jefferson University, 4 University of Delaware
Purpose: Renal cell carcinoma (RCC) is a rare but severe and aggressive pediatric malignant neoplasm. While incidence is uncommon, survival is relatively low compared to acute lymphocytic leukemia and Wilms Tumor. The incidence of RCC and mortality vary by prognostic factors including though not limited to sex, race, area of residence, tumor size, grade, age and primary therapies. We aimed to assess area of residence as well as other prognostic factors in pediatric RCC (pRCC) survival.
Methods: A retrospective cohort design was utilized to examine the event-free survival of children (0-19 years) with RCC using the Surveillance Epidemiology and End Result Data, 1973-2015. The survival function was based on the time dependent variable, namely survival months. To assess the predictors of survival, we used Cox proportional hazard model after testing the assumption that the hazard rate remained constant over time.
Results: Between 1973-2015, there were 174 cases of pRCC, of whom 49 died (28.2%). With respect to area of residence, mortality was higher in urban (46.7%) relative to metropolitan (26.4%). Relative to whites (17.2%), mortality was higher amongst blacks (47.0%). A sizable survival difference was observed with blacks relative to whites. Compared to whites, blacks were almost three times as likely to die, Hazard Ratio (HR) = 2.90, 95% Confidence Interval (CI) = 1.56-5.31. Survival was associated with sex, with males 21% more likely to die (HR = 1.21, 95% CI 0.69-2.11). Similarly, there was a nexus with age at tumor diagnosis and survival. Although imprecise, children ages 1-4, 5-9, 10-14 were 72%, 50%, and 21% less likely to die compared to children ages 15-19. Tumor grade, education, and income were prognostic in survival, although imprecise. The conjoint effect of area of residence and race illustrated excess risk of dying in urban relative to metropolitan areas. In the metropolitan area, the risk of dying was almost 3 times as likely for blacks compared to whites (HR = 2.78, 95% CI 1.45-5.43); in urban areas there was more black survival disadvantage, HR = 4.18, 95% CI 0.84-20.80. After controlling for age, sex, education, and insurance, the risk of dying increased amongst blacks with RCC in metropolitan areas, a-HR = 3.37, 99% CI 1.35-8.44. Similarly, in the urban areas, after adjustment for age, sex, and insurance, there was an increased risk of dying for blacks compared to whites, a-HR = 8.87, 99% CI 2.77-281.0.
Conclusion: Pediatric RCC indicated a survival disadvantage of black male children, implication of race as a prognostic factor in pRCC survival. Additionally, area of residence significantly influenced racial differences in mortality.
33PROSPER: A phase III randomized study comparing perioperative nivolumab (nivo) vs. observation in patients with localized renal cell carcinoma (RCC) undergoing nephrectomy (ECOG-ACRIN 8143).
Harshman, M.D., Lauren C.
Author Company: Dana-Farber Cancer Institute, Harvard Medical School
Co Authors: Puligandla, Maneka; Haas, Naomi10; Allaf, Mohamad11; Drake, Charles2; McDermott, David13; Signoretti, Sabina1; Cella, David5; Gupta, Rajan; Bhatt, Rupal13; Van Allen, Eliezer1; Shuch, Brian6; Choueiri, Toni1; Lara, Primo1; Kapoor, Anil11; Heng, Daniel7; Jewett, Michael4; George, Daniel8; Michaelson, Dror9; Leibovich, Bradley3; Wagner, Lynne5; Carducci, Michael11
Affiliations: 1Dana-Farber Cancer Institute, 2Columbia University Medical Center, 3Mayo Clinic, 4Princess Margaret Cancer Centre, 5Northwestern University, 6Yale University, 7UC Davis Comprehensive Cancer Center, 7University of Calgary, 8Duke University Medical Center, 9Massachusetts General Hospital, 10University of Pennsylvania, 11Johns Hopkins Hospital, 12McMaster University, 13Beth Israel Deaconess Medical Center
Background: As of 2018, there is no standard adjuvant systemic therapy that increases overall survival (OS) over surgery alone for patients with non-metastatic RCC. Nivolumab (nivo), an antibody against PD-1, improves overall survival in metastatic RCC and is well tolerated. Mouse solid tumor models have revealed an OS benefit to a short course of PD-1 blockade when given neoadjuvantly compared to adjuvantly. Two ongoing phase 2 studies of perioperative nivo in M0 RCC patients are showing preliminary feasibility and safety with no surgical delays (NCT02575222; NCT02595918). The PROSPER RCC trial will examine if administering perioperative nivo with radical or partial nephrectomy can increase cures, time to recurrence and survival in patients with high risk localized and locally advanced RCC.
Methods: This global, unblinded, phase 3 National Clinical Trials Network study is accruing patients with clinical stage =T2 or node positive M0 RCC of any histology. Tumor biopsy prior to randomization is mandatory to ensure RCC and will also permit unparalleled, in depth correlative science. The investigational arm will receive two doses of nivo prior to surgery followed by adjuvant nivo for 9 months (q2 wks x 3 mo followed by q4 wks x 6 mo). The control arm will receive standard of care surgical resection followed by observation. Randomization will be stratified by clinical T stage, node positivity, and histology. With 766 patients, there is 84.2% power to detect a 14% absolute benefit in recurrence-free survival (RFS) at 5 years assuming the ASSURE historical control of ~56% to 70% (HR = 0.70). The study is also powered to evaluate a significant increase in overall survival (HR 0.67). Safety, feasibility, and quality of life endpoints critical to adjuvant therapy considerations will be evaluated. PROSPER RCC exemplifies team science with a wealth of embedded correlative work aimed at investigating the impact of the baseline immune milieu, the changes induced by neoadjuvant anti-PD-1 priming, and how both correlate with clinical outcomes. Clinical trial information: NCT03055013
34Racial differences in clinical outcomes of metastatic renal cell carcinoma patients treated with immune checkpoint inhibitors
Author Name: Martini, Dylan J
Author Company: Emory University
Co Authors: Shabto, Julie M; Liu, Yuan; Carthon, Bradley C; Hitron, Emilie Elise; Russler, Greta Anne; Barbee, Meagan; Kissick, Haydn; Harris, Wayne B; Kucuk, Omer; Master, Viraj A; Bilen, Mehmet Asim
Background: Racial differences in cancer outcomes have been documented in several malignancies. There is data suggesting that there may be underlying differences in tumor biology between races, but there is limited data in this area in patients treated with immunotherapy. In this study, we explored the association between race and clinical outcomes in metastatic renal cell carcinoma (mRCC) patients treated with immune checkpoint inhibitors (ICI).
Methods: We performed a retrospective analysis of 100 mRCC patients treated with ICI at Winship Cancer Institute of Emory University from 2015-2018. Overall survival (OS) and progression-free survival (PFS) were measured from the first dose of ICI to date of death and clinical or radiographic progression, respectively. An objective response (OR) was defined as a best response of partial response (PR) or complete response (CR). Cox proportional hazard model and Kaplan-Meier method were used for association with OS/PFS, and logistic regression model for OR. Covariates included age, gender, clear cell RCC (ccRCC), number of sites of distant metastatic disease, and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group.Results: Approximately two-thirds (66%) of patients were males and the median age was 65 years. Most patients (78%) had ccRCC and the majority of patients had either zero (31%) or one (45%) line of prior systemic therapy in the metastatic setting. Most patients (83%) had at least two distant sites of metastatic disease. The IMDC risk group distribution was: favorable (16%), intermediate (60%), and poor (24%). The majority of patients (71%) received anti-PD-1 monotherapy. Black patients made up 17% of this sample of patients. Non-black patients had a significantly longer PFS and showed a trend towards longer OS and a higher chance of OR (Table 1). Black patients also had a shorter median OS (17.0 vs 24.1 months, p=0.225) and PFS (3.5 vs. 5.7 months, p=0.003) when compared to non-black patients per Kaplan-Meier estimation (Figures 1-2).
Table 1:
MVA† of racial differences in clinical outcomes
| OS | PFS | OR** | ||||
| Race | HR (CI) | p-value | HR (CI) | p-value | OR (CI) | p-value |
| Non-Black (n=83) | 0.80 (0.36-1.79) | 0.594 | 0.39 (0.21-0.72) | 0.002* | 1.31 (0.34-5.12) | 0.695 |
| Median Survival: 24.1 months | Median Survival: 5.7 months | Response rate: 22.9% | ||||
| Black (n=17) | - | - | - | - | - | - |
| Median Survival: 17.0 months | Median Survival: 3.5 months | Response rate: 17.6% | ||||
†The multivariable model was built by controlling for gender, IMDC risk group, number of sites of distant metastases, age, and ccRCC
*statistical significance at alpha < 0.05.
** Objective response: the probability of PR+CR were modeled.
Conclusions: There may be racial differences in tumor biology of mRCC patients that affect clinical responses to ICI. Future studies are needed to validate these results and investigate the relationship between underlying biological differences between races, the tumor microenvironment, and the immune system.
35Real-World Effectiveness and Tolerability of Pazopanib as First Targeted Therapy in Metastatic Renal Cell Carcinoma: A Retrospective Chart Review in Latin America
Author Name: Muniz Queiroz, David
Author Company: ICESP
Co Authors: Ratto, Barbara; Yang, Hongbo; Zhao, Jing; Signorovitch, James; Dezzani, Luca; Salman, Pamela; Lema, Mauricio; Lopera, Diego; Lerzo, Guillermo; del Castillo, Cesar; Chacon, Matias; Martins, Ana; Campos, Saul
Background: Pazopanib is one of the most commonly used first-line (1L) treatment for metastatic renal cell carcinoma (mRCC) and was first approved for mRCC in Latin America in 2010. Limited real-world data is available on mRCC patients who received pazopanib as 1L treatment in Latin America.
Methods: A retrospective chart review was conducted among oncology care centers in Argentina, Brazil, Chile, Colombia, and Mexico. Medical charts were reviewed for adult mRCC patients who initiated pazopanib as 1L therapy between 01/2011 and 03/2016. Patient characteristics, treatment pattern, overall survival (OS), progression-free survival (PFS), and adverse events were evaluated.
Results: 156 charts of mRCC patients receiving pazopanib as 1L treatment were reviewed (29, 54, 27, 28 and 18 patients from Argentina, Brazil, Chile, Colombia, and Mexico, respectively). Mean age at initial mRCC diagnosis was 61.6 years. Median duration from initial mRCC diagnosis to pazopanib initiation was 2.2 months. 64.1% of the patients were covered by public payers. Among 153 patients with ECOG score, 34.0%, 43.1%, and 22.9% of the patients had an ECOG score of 0, 1, and =2, respectively. Among 132 patients with Memorial Sloan Kettering Cancer Center score, proportion with favorable, intermediate, and poor risk was 19.7%, 61.4%, and 18.9%, respectively. Median initial daily dose of pazopanib was 800 mg. Median follow-up since pazopanib initiation was 16.6 months. 16.7% of the patients remained on pazopanib treatment at the time of data extraction and clinical progression was the main discontinuation reason; median time on treatment was 10.0 months. 28.8% of the patients had subsequent therapy; the most commonly used second therapies were everolimus (9.6%) and axitinib (5.8%). Median PFS was 10.8 months. Median OS in the overall population was 16.9 months and varied across countries (Argentina: 26.8; Brazil: 10.0; Chile: not reached; Colombia: 14.4; Mexico: 26.3 months). The most commonly reported adverse events were diarrhea (44.9%), asthenia/fatigue (43.6%), and nausea (28.8%).
Conclusions: In this retrospective analysis of oncology practice data in five Latin American countries, pazopanib was used for 1L mRCC treatment in a clinically diverse patient population. Real-world patterns of PFS and tolerability were on par with previous clinical studies of pazopanib in mRCC.
36Safety and activity of immune checkpoint inhibitors in patients with advanced renal cell carcinoma (RCC) and pre-existing autoimmune disorders (AD).
Martinez Chanza, M.D., Nieves
Author Company: Dana-Farber Cancer Institute
Co Authors: Flippot, Ronan; Alaiwi, Sarah Abou; Nassar, Amin; Vitale, Pier N; Kaymakcalan, Marina D; Hamid, Anis; Wei, M.D., MAS, Xiao; McGregor, Brad; Choueiri, Toni K; Harshman, Lauren C
Background: Checkpoint inhibitors (CPI) exhibit robust clinical activity in advanced RCC. However, the safety of their administration in patients with pre-existing AD is less clear as these patients have been underrepresented in clinical trials. We retrospectively characterized the safety and clinical activity of CPI in RCC patients with AD.
Methods: Medical records from advanced RCC patients treated with CPI at Dana-Farber Cancer Institute were reviewed. We captured baseline characteristics and clinical outcomes. Objective response rate (ORR) was assessed by RECIST principles. Overall survival (OS) was estimated using the Kaplan-Meier method.
Results: We identified 176 patients with advanced RCC who had received CPI at our institution. At immunotherapy initiation, 25 patients (14%) with clear-cell (64%) and non-clear cell (36%) histology had documented pre-existing AD. These included: dermatologic (36%), rheumatologic (32%), endocrine (24%), and renal (8%) disorders. Median follow-up was 15 months (range: 1-42). Of these patients, 12% had clinically active but stable AD; 4% required concurrent immune-modulatory agents. The majority were IMDC intermediate/poor risk RCC (84%). Most had CPI as part of combination therapy (56%) while 44% received monotherapy. CPI was most frequently employed as a 1st (48%) or 2nd line treatment (40%). After CPI initiation, a third of patients experienced AD exacerbations (n=8, 32%). Psoriatic (38%) and polymyalgia rheumatic (25%) flares were the most frequent. Most events were grade 1-2 (87.5%); only 1 patient had a grade 3 event. Systemic corticosteroids were required in 25% of cases; no patient required other immunosuppressive agents (e.g. DMARDs). New immune-related adverse events (irAEs) occurred in 12 patients (48%) including two grade 3 toxicities. Hypothyroidism was the most common new irAE (33%). 33% required treatment with systemic corticosteroids. Immunotherapy was permanently discontinued for AD exacerbations in 13% and for new irAEs in 17%. An additional 13% of patients with AD exacerbations and 17% with new irAEs had CPI interruptions but were able to resume therapy. At the time of analysis, 50% of AD exacerbations and 58% of new irAEs had resolved. Median time from CPI initiation to AD flare was 90 days (range: 38-270) and time to new onset irAE was 64 days (range: 10-180). Median immunotherapy exposure was 4.5 months (range: 1-27). Progression was the main cause of treatment discontinuation (52%), followed by toxicity (20%). ORR was 40%. Median OS was not reached. The 12-month OS rate was 79% (95%CI 56.7-90.8).
Conclusion: Checkpoint inhibitors can be safe and active among patients with pre-existing AD and advanced RCC. AD exacerbations were manageable with drug interruptions and systemic steroids. Most patients with AD were able to safely receive treatment without significant toxicity and experienced clinical benefit. Larger scale retrospective analyses are ongoing and prospective investigation is warranted that includes patients with AD.
37Sarcomatoid Features in Renal Cell Carcinoma: Rethinking the Stage-Survival Paradigm
Blum, M.D., Kyle A.
Author Company: Memorial Sloan Kettering Cancer Ctr
Co Authors: Renzo G. DiNatale1, Alejandro Sanchez1, Andrew Silagy1, Julian Marcon1, Roy Mano1, Sounak Gupta2, Satish K. Tickoo2, Paul Russo1 and A. Ari Hakimi1
1 Urology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY
2Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY
Funding Source: Supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
Introduction: Sarcomatoid features (sRCC) are present in approximately 4% of renal cell carcinoma cases. This rare sub-variant is associated with extremely poor clinical outcomes. Due to its rarity, limited studies have evaluated the impact of sarcomatoid differentiation among patients, especially with lower stage pT1-2 disease. We herein report survival trends stage-for-stage between sRCC and non-sRCC patients who underwent nephrectomy.
Methods: After IRB approval, we queried our prospectively maintained database for patients who underwent partial or radical nephrectomy between 2000-2017. Patients were divided into four groups for analysis: non-sRCC pT1-2; non-sRCC pT3-4; sRCC pT1-2 and sRCC pT3-4. All patients included were NxMx. Clinicopathological outcomes including sex, race, age, primary histology, lymph node involvement and margin status were compared between groups using chi-squared and two-tailed t-tests. Overall survival rates were analyzed by constructing Kaplan-Meier curves, p-values were calculated using log-rank tests and fitting Cox proportional hazards models for adjusted analyses.
Results: In a total of 3,850 cases available for analysis, sRCC was identified in 168 (4.4%) patients. Mean overall follow-up for the entire cohort was 59.9 months (range 1-192). Lower stage (pT1-2) was present in 33 (19.6%) sRCC patients and in 2,644 (71.8%) non-sRCC patients. Cancer-Specific Survival (CCS) between groups was worse in patients with sarcomatoid features regardless of pT stage (p<0.0001), figure 1. Of note, CSS was worse in lower stage sRCC pT1-2 patients than in higher stage non-sRCC pT3-4 patients. Overall survival (OS) results were similar, with sarcomatoid tumors yielding poorer estimates on survival analysis (p<0.0001).
Conclusion: Patients with lower stage pT1-2 sRCC demonstrated worse CSS when compared to even higher stage pT3-T4 non-sRCC, regardless of primary histology. Our data suggests that the presence of sarcomatoid features, even at low stage, is a significant marker for poor oncologic outcomes. Based on this observed aggressiveness, sarcomatoid features in lower stage RCC may require more vigilant surveillance and possible inclusion in adjuvant therapy trials.
38Second-Line VEGF Receptor TKI Outcomes after First-Line Immune Checkpoint Blockade in Metastatic Renal Cell Carcinoma
Author Name: Kotecha, M.D., Ritesh
Author Company: Memorial Sloan Kettering Cancer Center
Co Authors: Shah, Amishi2; Lemke, Emily2; Chandramohan, Anuradha2; Chaim, Joshua1; Xiao, Lianchun2; Gao, Jianjun2; Campbell, Matthew2; Zurita, Amado2; Wang, Jennifer2; Corn, Paul2; Jonasch, Eric2; Sharma, Padmanee2; Voss, Martin1; Tannir, Nizar2
1Memorial Sloan Kettering Cancer Center; 2MD Anderson Cancer Center
Background: As immune checkpoint blockade (IO) therapy moves to the first-line setting (1L), VEGFR TKIs are increasingly used in second-line settings (2L) in metastatic renal cell carcinoma (RCC) patients. Whether this practice affects 2L VEGFR TKI response or cancer specific outcomes is unclear.
Patients & Methods: We conducted a retrospective review of metastatic RCC patients treated with 2L VEGFR TKI after disease progression on 1L IO therapy. Patients were treated at Memorial Sloan Kettering Cancer Center and MD Anderson Cancer Center between December 2015 and February 2018. Patient characteristics, treatment history and toxicity were compiled from the medical record. Response was assessed by blind radiologist review using RECIST v1.1. Analysis was then conducted using log-rank and Fisher’s exact test, with progression-free (PFS) and overall survival (OS) outcomes generated using Kaplan-Meier method.
Results: 70 patients were identified with a mean age of 59 at diagnosis of metastatic RCC. By IMDC risk scoring, 8 patients (11%) had favorable, 48 patients (69%) had intermediate, and 14 (20%) patients had poor-risk disease. All patients received 1L IO therapy with a median duration of 25.3 weeks, including nivolumab (17%), nivolumab plus ipilumumab (47%), and combination anti-PD-(L)1 plus bevacizumab (36%). All patients had evidence of progression and resolution of grade 3/4 adverse effects (AEs), and were started on 2L VEGFR TKI therapy with pazopanib (27%), sunitinib (9%), axitinib (36%), or cabozantinib (28%). On 2L VEGFR TKI, 1 patient achieved a complete response (1.5%), 27 patients achieved partial responses (39.7%), and 36 patients had stable disease (52.9%), for a total disease control rate of 94%. 2L VEGFR TKI median PFS was 14.1 months (95% CI: 10.8, NA), and estimated 1-year OS was 79.6% (95% CI: 70.2 – 90.3). Median duration of 2L VEGFR TKI was 10.8 months, and individual IL IO therapy choice did not significantly affect 2L VEGFR TKI response or survival outcomes. Observed AEs were consistent with the safety profile of all VEGFR TKIs, with no treatment related deaths.
Conclusions: As front-line therapies continue to emerge in metastatic RCC, this retrospective analysis reveals encouraging efficacy and safety for the use of 2L VEGFR TKI after 1L IO, with findings comparable to historical controls for 1L VEGFR TKI therapy.
39Sites of distant metastatic disease and association with clinical outcomes in metastatic renal cell carcinoma (mRCC) patients treated with immune checkpoint inhibitors (ICI)
Martini, Dylan J
Author Company: Emory School of Medicine, Winship Cancer Institute – not In
Co Authors: Julie M. Shabto1,2, Yuan Liu3, Bradley C. Carthon1,2, Emilie Elise Hitron1,2, Greta Anne Russler1,2, Meagan Barbee1,2, Haydn Kissick2,4, Wayne B. Harris1,2, Omer Kucuk1,2, Viraj A. Master4, Mehmet Asim Bilen1,2
1Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA
2Winship Cancer Institute of Emory University, Atlanta, GA, USA
3Departments of Biostatistics and Bioinformatics, Emory University, Atlanta, GA, USA
4Department of Urology, Emory University School of Medicine, Atlanta, GA, USA
Background: Liver metastases have been associated with poor outcomes in oncology patients. We investigated the association between metastatic sites and clinical outcomes in mRCC patients treated with ICI.
Methods: We completed a retrospective review of 100 mRCC patients who received ICI at Winship Cancer Institute from 2015-2018. Overall survival (OS) and progression-free survival (PFS) were calculated from date of ICI-initiation to date of death and clinical or radiographic progression, respectively. Objective response (OR) was defined as best response of partial response (PR) or complete response (CR). Metastatic sites were collected from radiology reports and clinical notes and included brain, bone, liver, lung, and lymph node. Cox proportional hazard model and Kaplan-Meier method were used for association with OS or PFS, and logistic regression model was applied to OR.Results: The patients were predominantly (66%) male and the median age was 65 years. Most patients (78%) had ccRCC histology. The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk groups were: favorable (16%), intermediate (60%), and poor (24%). Most patients (71%) received anti-PD-1 monotherapy. The majority (83%) had two or more sites of distant metastatic disease. The distribution of metastatic sites were: brain (17%), bone (37%), liver (25%), lung (71%), and lymph node (56%). Patients with 0-1 metastatic sites had significantly longer OS and trended towards longer PFS and higher chance of OR (Table 1). Patients who had liver metastases had significantly shorter OS and trended towards shorter PFS and lower chance of OR. Kaplan-Meier plots of association between metastatic sites and survival are shown in Figures 1-4.
Table 1:
MVA† of metastatic sites and clinical outcomes
| OS | PFS | OR** | |||||
| HR(CI) | p-value | HR(CI) | p-value | OR(CI) | p-value | ||
| Number of Metastatic Sites | 0-1 (n=17) | 0.24 (0.05-1.09) | 0.064 | 0.41 (0.19-0.89) | 0.024* | 2.31 (0.70-7.63) | 0.168 |
| Median Survival: Not reached | Median Survival: 16.1 months | Response Rate: 35% | |||||
| 2+ (n=83) | - | - | - | - | - | - | |
| Median Survival: 16.9 months | Median Survival: 4.1 months | Response Rate: 19% | |||||
| No Liver Metastases (n=75) | 0.29 (0.13-0.63) | 0.002* | 0.59 (0.33-1.05) | 0.072 | 1.33 (0.38-4.64) | 0.650 | |
| Median Survival: 29.7 months | Median Survival: 4.6 months | Response Rate: 24% | |||||
| Liver Metastases (n=25) | - | - | - | - | - | - | |
| Median Survival: 6.7 months | Median Survival: 3.6 months | Response Rate: 16% | |||||
†Multivariable model controlled for gender, IMDC risk group, number of distant metastases, age, and ccRCC
*statistical significance at alpha < 0.05.
** Objective response: probability of PR+CR were modeled.
Conclusions: These results suggest that liver metastases are a poor prognostic factor for mRCC patients treated with ICI, but should be validated in a larger study. Future studies should explore the combination of immunotherapy and liver-directed therapy for patients with hepatic metastases.
40The EVERPRO Study: Final Results of a Non-Interventional Study Evaluating the Quality of Life (QoL) in Second-Line Treatment of Metastatic Renal Cell Carcinoma (mRCC) with Everolimus
Goebell, MD, Peter J.
Author Company: Universitaebklinik Grlangen
Co Authors: 1 Edwin Herrmann,2 Christian Doehn,3 Norbert Walter Marschner,4 Marc-Oliver Grimm,5 Michael Thomas Scheffler,6 Anja Rogler,7 Lothar Bergmann8
1University Hospital Erlangen, Erlangen, Germany; 2University Hospital Muenster, Muenster, Germany; 3Urologikum Lübeck, Lübeck, Germany; 4Medical Practice for Oncology and Hematology, Freiburg, Germany; 5University Hospital Jena, Jena, Germany; 6Praxis Dr. Scheffler, Zwickau, Germany; 7Novartis Pharma GmbH, Nürnberg, Germany; 8University Hospital Frankfurt AM, Frankfurt, Germany
Introduction/objective: Although the prognosis for patients with mRCC remains poor, median overall survival has improved since 2006, probably due to the introduction of tyrosine kinase inhibitors (TKIs). Everolimus treatment following first-line vascular endothelial growth factor (VEGFR)-TKI was demonstrated to be efficacious and safe and is recommended by current guidelines. Reassuringly, adequate QoL has become a major goal of therapy, especially in the second-line setting. Hence, QoL and the time associated with everolimus treatment were the major factors assessed in this study.
Methods: Adult patients scheduled for on-label everolimus treatment after first-line VEGFR-TKI or bevacizumab were eligible. QoL was assessed by means of the NCCN-FACT FKSI-19 questionnaire. Additionally, time to administer treatment and associated limitations to QoL were evaluated using a specifically designed questionnaire that assessed total treatment duration and treatment type, among other things.
Results: 202 patients were evaluable for analyses. Median treatment duration was 19.1 weeks [95% CI, 16.4–23.0] and median time to progression was 26.0 weeks [95% CI, 23.3–32.6]. The FKSI-19 median total score remained stable during treatment. The median time effort spent on total therapy was 20 hours per patient. Most patients reported ‘no’, ‘a little,’ or ‘moderate’ limitations in their daily, social, and professional life. Two months after treatment start, 65 patients reported ‘no’ or ‘a little’ time burden due to therapy. 167 patients experienced 804 adverse events (AEs) in total. The most commonly reported AEs related to everolimus were dyspnea, fatigue, and anemia (all-grade).
Conclusions: Everolimus therapy was associated with maintained QoL and acceptable therapy duration for most patients. The observed safety profile was consistent with previous data and does not impact the benefit-risk balance of everolimus. This study supports previously published data demonstrating that everolimus is an effective and safe treatment option following a VEGFR-targeted drug. Clinical trial information: 2284.
41The Oncologic Outcome of Partial and Radical Nephrectomy in Localized Sarcomatoid Renal Cell Carcinoma
Blum, M.D., Kyle A.
Author Company: Memorial Sloan Kettering Cancer Ctr
Co Authors: Andrew W. Silagy MD1, Renzo G. DiNatale MD1, Roy Mano MD1, Julian Marcon MD1, Sounak Gupta2, Satish Tickoo2, Paul Russo MD1, A. Ari Hakimi MD1
1 Urology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY
2Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY
Funding Source: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
Introduction: Sarcomatoid dedifferentiation is present in approximately 4% of patients with renal cell carcinoma (sRCC) and portends a poor prognosis. In conventional RCC, partial nephrectomy yields equivalent oncologic outcomes compared to radical nephrectomy. It is unclear if this paradigm remains true within aggressive sRCC tumors. We sought to assess the oncological efficacy of partial nephrectomy compared to radical nephrectomy in a cohort of localized, non-metastatic sRCC patients.
Methods: This is a retrospective study evaluating ¬¬¬¬patients with sRCC who underwent partial or radical nephrectomy between 1986-2018. Patients were excluded if they were metastatic at the time of nephrectomy or within 30 days after their nephrectomy, did not have sarcomatoid features on final pathology, or had missing information related to their M or N status. The final cohort was comprised of 159 patients with localized, non-metastatic (N0M0) sRCC. Cox proportional hazards models were used to compare recurrence-free survival (RFS) and cancer-specific survival (CSS) in patients undergoing radical and partial nephrectomies while adjusting for known preoperative variables.
Results: The study cohort included 106 males and 53 females with a median age of 59.68 (IQR 50.8-67.2). One-hundred thirty-one patients underwent radical nephrectomy and 28 patients underwent partial nephrectomy. Median follow up for the entire cohort was 27.2 months (IQR: 11.9 – 48.1 months. Estimated 5-year recurrence-free survival were 33.8% and 20.9% for partial and radical nephrectomy, respectively and estimated 5-year cancer-specific survival were 69.5% and 40.6%, respectively. On multivariable analysis for RFS, no significant difference in outcome was observed between partial or radical nephrectomy (Figure 1a). On multivariable analysis for CSS radical nephrectomy was associated with a worse outcome compared to partial nephrectomy (HR=2.67, 95% CI 1.02-6.96, p=0.045) after correcting for age, sex, BMI, smoking history, histology, stage and margin status. Independent predictors of improved survival included clear cell histology, and male sex while positive smoking history predicted a worse CSS (Figure 1b).
Conclusion: The current findings suggest the oncological outcomes of partial nephrectomy are not worse than those of radical nephrectomy in a cohort of patients with localized sarcomatoid RCC. Under currently utilized selection criteria, partial nephrectomy may be safely used for patients with sarcomatoid RCC.
42Treatment of metastatic Non clear cell Renal Cell Carcinoma (nccRCC) with ipilimumab and nivolumab
Gul, Anita
Author Company: Cleveland Clinic, Taussig Cancer Institute
Co Authors: Authors: Anita Gul MD 1, Kimberly Allman MSN CNP1, Moshe C. Ornstein MD1, Jessica Ball RN1, Laura Wood RN MSN OCN1, Jorge A. Garcia MD1, Dendra von Merveldt RN2, Hans Hammers MD PhD2, Brian I. Rini MD1
Affiliation: 1Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, USA, 2UT Southwestern, Dallas, TX.
Background: Currently, the standard treatment of metastatic ncc RCC is a VEGF TKI, although clinical outcomes remain poor. The activity of Ipi/Nivo in patients (pts) with metastatic ncc RCC is not well characterized.
Methods: Pts with metastatic nccRCC who were treated with Ipi/Nivo at Cleveland Clinic or UT Southwestern were retrospectively reviewed. Ipi/Nivo was administered as per CHECKMATE 214. Computed tomography imaging was obtained at baseline and every12 weeks to assess disease response per RECIST 1.1 criteria. Baseline patient characteristics, outcome to therapy and adverse effects as per CTCAE v5.0 were collected from the electronic medical record.
Results: A total of 9 patients with metastatic ncc RCC histology were identified. The median age was 49 years (range, 32-68). Pts had metastatic adenocarcinoma of renal origin not otherwise specified (2), unclassified (2), papillary (2) chromophobe (2) and medullary histology (1). Five pts had ECOG PS 0 and 4 pts had ECOG PS 1. Eight pts were male and one female. IMDC risk group at time of initiation of Ipi/Nivo was intermediate (8 pts) and poor (1 pt). Six patients received Ipi/ Nivo as first line treatment, two pts received Ipi/Nivo after prior TKI and one pt received Ipi/ Nivo as third line treatment after prior chemotherapy and nivolumab monotherapy. Five patients have reached restaging scans with two pts demonstrating partial response (PR) and three pts demonstrating progression (PD). One patient experienced grade 2 diarrhea after 4 cycles of Ipi/ Nivo and required prednisone. One patient experienced grade 1 hepatotoxicity after 3 cycles of Ipi/ Nivo and required prednisone. One patient experienced grade 2 pancreatitis requiring steroids after one dose of Ipi/Nivo.
Conclusions: Ipi/Nivo is feasible and safe in patients with metastatic nccRCC with preliminary evidence of anti-tumor activity. Updated response data will be presented.
43Treatment-Free Survival Following Discontinuation of First-Line Nivolumab Plus Ipilimumab or Sunitinib in Patients With Advanced Renal Cell Carcinoma: CheckMate 214 Analysis
McDermott, M.D., David F.
Author Company: Beth Israel Deaconess Medical Center/Harvard Medical School
Co Authors: Rini, Brian1; Motzer, Robert J2.; Tannir, Nizar M.3; Escudier, Bernard4; Kollmannsberger, Christian K.14; Hammers, Hans J8; Porta, Camillo9; George, Saby5; Donskov, Frede11; Gurney, Howard P.12; Grimm, Marc-Oliver13; Harrison, Michael6; Hutson, Thomas E.8; Doan, Justin10; Yang, Shuo10; Rao, Sumati10; Mekan, Sabeen; Ambavane, Apoorva; Powles, Thomas7
Affilliations: 1Cleveland Clinic, 2Memorial Sloan Kettering Cancer Center, 3MD Anderson Cancer Center, 4Institut Gustave Roussy, 5Roswell Park Cancer Center, 6Duke University Cancer Center, 7Bart’s Cancer Center, 8UT Southwestern, 9IRCCS San Matteo University Hospital Foundation, 10Bristol-Myers Squibb, 11Aarhus University Hospital, 12Westmead Hospital and Macquarie University, 13University of Jena, 14BCCA Vancouver Cancer Centre
Background: Patients with metastatic melanoma who discontinue nivolumab plus ipilimumab (N+I) may experience sustained clinical benefit and a delayed need for subsequent therapy. In this analysis, treatment-free survival (TFS) was retrospectively analyzed using data from the phase 3 CheckMate 214 trial, in which N+I demonstrated superior efficacy vs sunitinib (S) in patients with International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate/poor-risk advanced renal cell carcinoma (aRCC).
Methods: In CheckMate 214, patients with previously untreated clear-cell aRCC were randomized 1:1 to receive N 3 mg/kg + I 1 mg/kg every 3 weeks for 4 doses followed by N 3 mg/kg every 2 weeks, or S 50 mg daily orally for 4 weeks (6-week cycles). TFS was defined as the time from last dose of N+I or S to the start of subsequent systemic therapy or death. All randomized patients with IMDC intermediate/poor-risk aRCC (N+I, 425; S, 422) were analyzed. Kaplan–Meier curves and log-rank tests were used to compare TFS between N+I and S.
Results: With median overall survival follow-up of 25.2 months, patients in the N+I arm had significantly longer time from randomization to subsequent systemic therapy or death than patients in the S arm (median, 15.4 vs 8.5 months; P<0.0001); 2 years after randomization, 42% vs 19% of patients were alive and not requiring subsequent therapy. Overall, 320 (75%) N+I patients and 359 (85%) S patients discontinued treatment, most commonly due to disease progression (N+I, 42%; S, 58%) or study drug–related adverse events (N+I, 23%; S, 11%). In patients who discontinued, TFS was significantly longer with N+I than with S (P<0.0001); 18 months after discontinuation, 19% of N+I patients vs 4% of S patients remained treatment-free. TFS was also significantly longer with N+I than with S, irrespective of best overall response on study (P<0.0001). At 18 months after discontinuation, 48% of N+I patients vs 6% of S patients with complete/partial response were still free of subsequent treatment; at the same time point, 13% of N+I patients vs 4% of S patients with stable disease remained treatment-free.
Conclusions: The use of N+I was associated with a significantly longer TFS beyond treatment discontinuation in patients with IMDC intermediate/poor-risk aRCC and irrespective of whether patients achieved response or disease control. TFS should be considered along with traditional efficacy measures when evaluating treatment options for aRCC.
Originally presented at ESMO Congress 2018; Munich. 874P.
Funding: Bristol-Myers Squibb
44Trial in Progress – Children’s Oncology Group (COG) Study AREN 1721: A Randomized Phase 2 Trial of Axitinib/Nivolumab combination therapy vs single agent Axitinib or Nivolumab for Translocation Renal Cell Carcinoma (tRCC) across all age groups.
Cost, M.D., Nicholas G.
Author Company: University of Colorado School of Medicine
Co Authors: Geller JI, Cost NG, Chi YY, Perlman E, Cajaiba M, Khanna G, Mullen E, Kalapurakal J, Tracy E, Ehrlich PF, Dome J and Fernandez CV.
Background: Translocation Renal Cell Carcinoma (tRCC) was formally recognized by the WHO in 2004 as a distinct, typically translocation-associated, RCC with characteristic morphology and immunohistochemical (IHC) expression of TFE3 or TFEb. tRCC occurs in all races, and accounts for 1 5% of RCC, typically in adolescents and young adults. The dominant presentation pattern for tRCC is one of advanced stage and rapid fatality, pointing to an aggressive cancer. A minority of patients have a more indolent course. Anti-vascular endothelial growth factor (anti-VEGF) receptor tyrosine kinase inhibitors (RTKi) and programmed death-1 (PD-1)/PD-1 ligand (PD-L1) inhibitors (anti-PD1) are established first-line treatments for patients with metastatic or unresectable renal cell carcinoma (RCC). However, neither have been systematically investigated in tRCC.
Methods: This is the first prospective therapeutic study focused on tRCC, combining the resources of the COG and adult cooperative groups (Alliance, ECOG, NRG, SWOG) through the NCI’s National Clinical Trials Support Network. Efficacy data for VEGF RTKi and PD1/PD-L1 targeted therapy, the two key RCC therapeutic targets, will be assessed prospectively specifically in tRCC, and the behavior of tRCC comprehensively described across all ages.
Study patients must have histologically-confirmed unresectable or metastatic tRCC diagnosed using WHO-defined criteria. Additional pertinent inclusion criteria are: Age = 1 year old at enrollment, the ability to swallow oral pills whole, measurable disease as defined by RECIST v1.1, and inability to undergo complete surgical resection of the disease. Patients receiving prior therapy with axitinib or nivolumab, or other VEGF or PD1/PDL1 targeted therapies are excluded.
There are three study arms: A: Axitinib, B: Nivolumab, and C: Combination. The study is powered to detect a difference of 4-7 months in progression free survival (PFS) between either single agent vs the combination, with 25 patients in each arm. To accommodate this design, randomization to treatment arms will be 1:1:1. Randomization will incorporate stratification for age (<18 vs =18) and prior systemic therapy.
Retrospective central pathology review with TFE IHC will be performed to confirm each case is evaluable as tRCC. Axitinib will be started as 5mg PO BID for patients =18yr, and 2.4mg/m2 PO BID for patients <18yr. Axitinib dose escalation will be permitted. Nivolumab dose will be 240mg IV q 2wk in adults, and 3mg/kg IV q2wk in children. Combination therapy will be given with agents given at full single agent doses as above.
The primary endpoint will be an assessment of PFS. The Objective Response Rate, defined as complete plus partial responses, will also be evaluated and compared. The accrual rate on this study is anticipated to approximate 25 tRCC patients annually based on estimates of tRCC prevalence, access to treatment centers, and enrollment patterns. This will require an accrual duration of approximately 3 years. Interim analyses will include both toxicity and efficacy metrics. The total study duration will be 5 years, which includes a potential 2 years of therapy in responding patients.




