What Survival Benefits are Needed to Make Adjuvant Sorafenib Worthwhile After Resection of Intermediate- or High-Risk Renal Cell Carcinoma? Clinical Investigators’ Preferences in the SORCE Trial
Abstract
Background:
Decisions about adjuvant therapy involve trade-offs between possible benefits and harms.
Objective:
We sought to determine the survival benefits that clinical investigators would judge as sufficient to warrant treatment with adjuvant sorafenib in the SORCE trial after nephrectomy for apparently localised renal cell carcinoma (RCC).
Methods:
A subset of clinical investigators in the SORCE trial completed a validated questionnaire that elicited the minimum survival benefits they judged sufficient to warrant one year of adjuvant sorafenib in scenarios with hypothetical baseline survival times of 5 years and 15 years, and baseline survival rates at 5 years of 65% and 85%.
Results:
The 100 participating SORCE investigators had a median age of 42 years, and 74 were male. For one year of sorafenib versus no therapy, the median benefits in survival times the investigators judged sufficient to warrant treatment were an extra nine months beyond five years and an extra 12 months beyond 15 years; the median benefits in survival rates were an extra 5% beyond baseline survival rates of both 65% and 85% at five years. The patients recruited in the SORCE trial by these investigators judged smaller benefits sufficient to warrant adjuvant sorafenib for both survival rate scenarios (p≤0.0001). The survival benefits the investigators judged sufficient to warrant one year of adjuvant therapy with sorafenib for RCC were similar to those of other clinicians considering three months of adjuvant chemotherapy for lung cancer, but smaller than those of clinicians considering six months of adjuvant chemotherapy for breast cancer.
Conclusion:
SORCE investigators judged larger benefits necessary to warrant adjuvant sorafenib than their patients. The benefits required by the investigators were similar or smaller than those other clinicians considered sufficient to warrant adjuvant chemotherapy for other cancers. Clinicians should recognise that their patients and colleagues may have preferences that differ from their own when considering the potential benefits and harms of adjuvant treatment.
INTRODUCTION
The management of renal cell carcinoma (RCC) has been revolutionised by the availability of multiple drugs that improve survival for patients with metastatic disease. Sorafenib and sunitinib, both multikinase inhibitors, were the first US FDA approved agents for metastatic RCC since the cytokines [1, 2]. By 2017, twelve drugs were approved for this indication by the US FDA. The activity of these agents in the metastatic setting provided a strong rationale for testing in the adjuvant setting. In 2017 sunitinib was the first agent to gain FDA approval for adjuvant treatment of RCC at high risk of recurrence after nephrectomy, and was based on an improvement in disease free survival (DFS) rather than overall survival (OS) [3].
Adjuvant therapy for RCC has the potential to prevent or delay recurrence (and thereby improve OS times and survival rates), but also to cause side effects and be inconvenient for patients. Multikinase inhibitors have different patterns of toxicities than conventional cytotoxic drugs. These “targeted therapies” often have toxicities of low severity, but which can cause significant distress when treatment is prolonged over many months or years [4, 5]. These toxicities become even more important in the adjuvant setting, where patients have no symptoms of cancer and may already have been cured by surgery.
Decisions about adjuvant therapy involve trade-offs between possible benefits and harms, including the inconvenience of treatment. Most cancer patients favour joint decision-making with their doctor, rather than having either party make a decision on their own [6, 7]. Shared decision-making requires that clinicians understand the preferences of their patients, as well as their own [8]. Studies of patients’ preferences have shown they judge small survival benefits sufficient to make adjuvant chemotherapy worthwhile in a range of common cancers [9–13] Studies of clinicians have shown they judge larger benefits than patients necessary to make adjuvant chemotherapy worthwhile [13]. Studies of clinicians’ preferences for adjuvant therapy in RCC have not been reported.
In this study, we sought to determine the minimum survival benefits that investigators in the SORCE trial (NCT00492258) judged sufficient to make adjuvant sorafenib worthwhile after nephrectomy for intermediate- or high-risk localised RCC, and the factors associated with their judgements. We were also interested in comparing their judgements with those of their patients with RCC, and with those of clinicians considering adjuvant therapies for other cancers reported in our previous studies [14].
METHODS
We conducted a cross-sectional study nested within the SORCE trial, an international, double-blind, placebo-controlled, phase 3 trial of 1711 patients comparing adjuvant sorafenib for one year, three years, or observation only (placebo), after resection of localised RCC at intermediate- or high-risk of recurrence according to the Leibovich score [15]. The SORCE trial is expected to report on its primary outcome of DFS in 2018. We surveyed clinical investigators (medical oncologists and urologists) recruiting patients to the SORCE trial at all participating sites in Australia and at selected sites in the United Kingdom (UK). Human Research Ethics Committee / Institutional Review Board approval was obtained from each participating site, and all participants provided written informed consent. This preferences sub-study was led and conducted by the Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP) and the National Health and Medical Research Council of Australia Clinical Trials Centre (NHMRC CTC) at the University of Sydney, in collaboration with the Medical Research Council Clinical Trials Unit at University College London (MRC CTU at UCL), UK.
SORCE investigators’ preferences were elicited using a self-completed paper questionnaire completed soon after their respective hospital was activated for participation in the SORCE trial. The questionnaire was based on our previous work [10] and used hypothetical scenarios to elicit the minimum survival benefit that clinicians judged sufficient to warrant (make worthwhile): one year of adjuvant sorafenib versus no adjuvant sorafenib; and, three years of adjuvant sorafenib versus one year of adjuvant sorafenib. Two types of scenarios were used to evaluate one year of sorafenib versus no sorafenib; one type featured differing survival times, and the other type featured differing survival rates. Survival time scenarios asked participants to choose between a baseline survival time without the side effects and inconvenience of sorafenib (e.g. 5 years) versus a series of longer survival times with the side effects and inconvenience of sorafenib for one year, ranging from an extra one month to an extra 15 years. Survival rate scenarios asked participants to choose between a baseline survival rate at 5 years without the side effects and inconvenience of sorafenib (e.g. 65%) or a series of survival rates at 5 years with the side effects and inconvenience of sorafenib for one year. The survival rates with sorafenib ranged from an extra 1% to a maximum survival rate of 100%. The endpoint for each scenario was the minimum benefit for which the investigator chose sorafenib rather than placebo. Additional survival time scenarios were used to evaluate the benefits needed to warrant extending the duration of sorafenib from one year to three years. The baseline survival times (5 years and 15 years) and survival rates (65% and 85% at 5 years) were based on data from previous trials and chosen to reflect the range of typical prognoses for patients with resected intermediate and high risk RCC [16, 17].
To compare the absolute benefits in OS judged sufficient in scenarios using survival times versus survival rates, we expressed both as hazard ratios (HRs), a measure of relative benefit. To assess the plausibility of these benefits expressed as HRs, we compared them with the hypothesised HR of 0.75 for DFS specified in the SORCE trial protocol. We assumed that a plausible benefit for OS would be smaller (HR closer to 1) than that hypothesised for DFS.
Analysis methods were chosen to accommodate the skewed distributions expected with preference data. The Wilcoxon rank sum test was used to perform comparisons between groups, the Wilcoxon signed rank test was used to perform comparisons within groups, and normal score transformed preference data was modelled using linear regression to explore predictors of preference disposition. Preference disposition was characterised as the average response to the two survival time questions used to evaluate one year of adjuvant therapy with sorafenib.
We assumed a sample size of 100 clinicians, which yields 95% confidence intervals no wider than±10% for percentages based on all clinicians.
RESULTS
Preferences were elicited from 100 SORCE investigators: 75 medical oncologists and 25 urologists. The majority were from Australia (n = 76). Table 1 summarises their baseline characteristics. Seventy-four were male, and their median age was 42 years (range 27 to 62).
Table 1
Baseline characteristics of SORCE investigators
| Characteristic | Doctors n = 100 |
|---|---|
| Age (years) | |
| <30 | 2 |
| 30–39 | 38 |
| 40–49 | 48 |
| 50–59 | 8 |
| ≥60 | 1 |
| Missing | 3 |
| Gender | |
| Male | 74 |
| Female | 26 |
| Specialist | |
| Medical Oncologist | 75 |
| Urologist | 25 |
| Marital status | |
| Married/De Facto | 83 |
| Separated | 2 |
| Widowed | 1 |
| Single | 10 |
| Missing | 4 |
| Children | |
| Yes | 73 |
| No | 23 |
| Missing | 4 |
| Dependents | |
| Dependent children | 71 |
| Dependent people | 58 |
| Close friend/relative died from cancer | 54 |
The absolute survival benefits that SORCE investigators judged sufficient to warrant one year of adjuvant sorafenib are shown in Fig. 1. The required survival benefits varied widely, spanning almost the entire available range from 0.1% to 20% beyond a baseline survival rate at five years of 65%, from 0.1% to 15% beyond a baseline survival rate at five years of 85%, from one day to seven years beyond a baseline survival time of five years, and from one day to 15 years beyond a baseline survival time of 15 years. The median (IQR) survival benefit judged sufficient to warrant one year of adjuvant sorafenib for each scenario was: nine months (six to 18) beyond five years; 12 months (nine to 24) beyond 15 years; 5% (five to 10) beyond a five year survival rate of 65%; and, 5% (four to 10) beyond a five year survival rate of 85%. The HRs (relative benefits) corresponding to these absolute benefits are shown in Table 2.
Fig.1
Cumulative proportions of SORCE investigators judging specified survival benefits sufficient to (A) warrant 1 year of adjuvant sorafenib given baseline survival rates at 5-years without adjuvant sorafenib of either 65% or 85% (B) warrant 1 year of adjuvant sorafenib given baseline survival times without adjuvant sorafenib of either 5 years or 15 years and (C) warrant 3 years of adjuvant sorafenib given baseline survival times with 1 year of adjuvant sorafenib of either 5 years or 15 years.
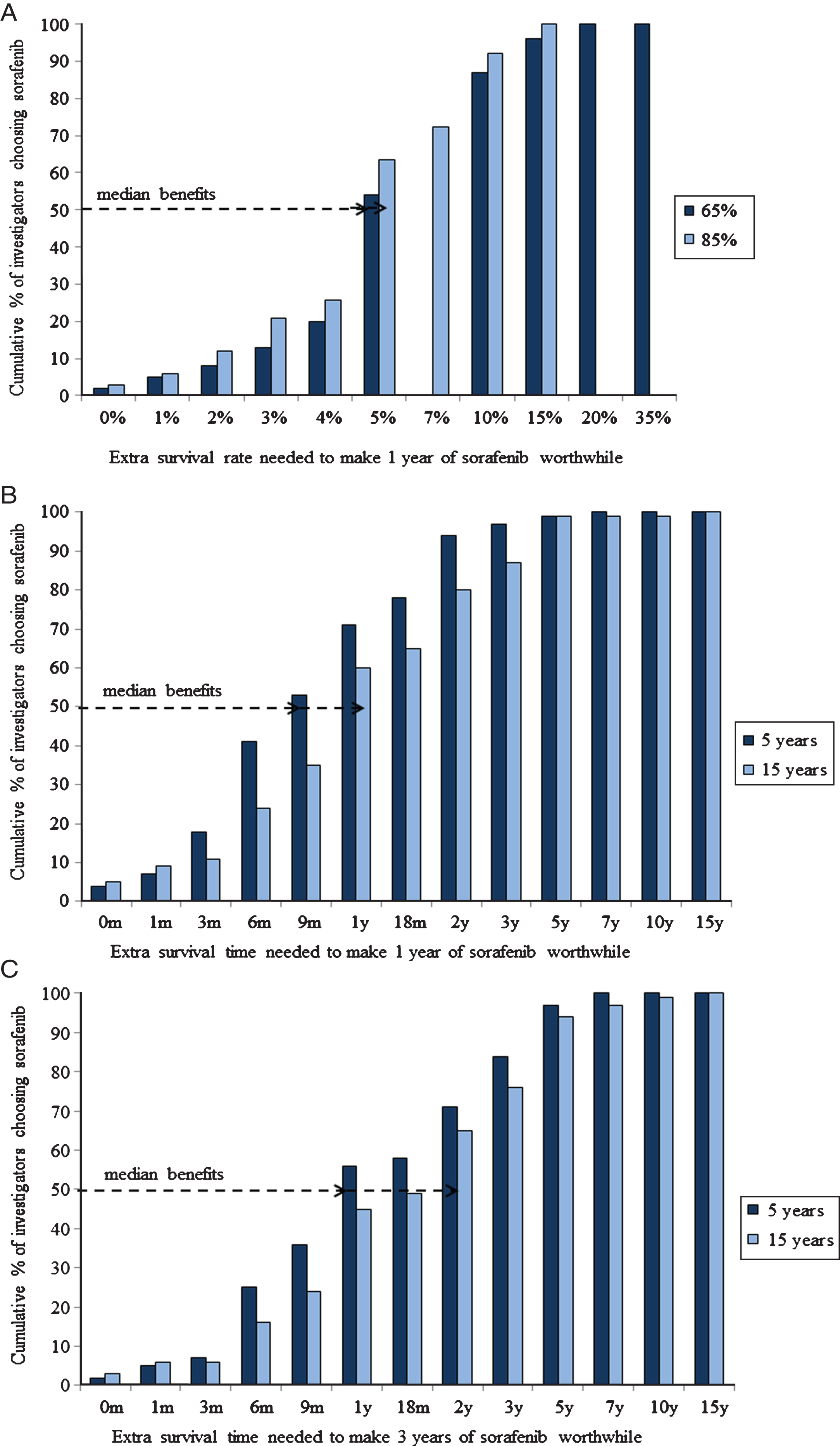
Table 2
Relative survival benefits judged sufficient to warrant 1 year of adjuvant sorafenib
| Prognosis | Baseline prognosis without | Corresponding median HR (IQR) | Percentage of clinicians who judged sufficient |
|---|---|---|---|
| expressed as | adjuvant sorafenib | for benefits judged sufficient to | a benefit in overall survival that was smaller |
| warrant 1 year of adjuvant | than that hypothesised for disease | ||
| sorafenib | free survival (i.e. HR > 0.75) | ||
| Survival time | 5 years | 0.87 (0.77 to 0.91) | 78 |
| 15 years | 0.94 (0.88 to 0.95) | 87 | |
| Survival rate | 65% | 0.83 (0.67 to 0.83) | 54 |
| 85% | 0.65 (0.32 to 0.72) | 21 |
The survival benefits that SORCE investigators judged sufficient to warrant three years versus one year of adjuvant sorafenib are shown in Fig. 1. The survival benefits judged sufficient to make the extra two years of adjuvant sorafenib worthwhile were greater than those required to make the first year of adjuvant sorafenib worthwhile (p < 0.0001 for both scenarios).
The relative OS benefits SORCE investigators judged sufficient to warrant one year of adjuvant sorafenib were compared with the hypothesised benefit in DFS specified in the trial sample size justification (HR 0.75), and are shown in Table 2. For the survival time scenarios, more than 75% of investigators judged that the survival benefit sufficient to warrant one year of adjuvant sorafenib was smaller (HR closer to 1) than the hypothesised benefit in DFS. However, for the survival rate scenarios, less than 55% of investigators judged that the survival benefit sufficient to warrant one year of adjuvant sorafenib was smaller (HR closer to 1) than the hypothesized benefit in DFS.
The baseline characteristics of SORCE investigators’ shown in Table 1 were not associated with the survival benefits they judged sufficient to make 1 year of adjuvant sorafenib worthwhile.
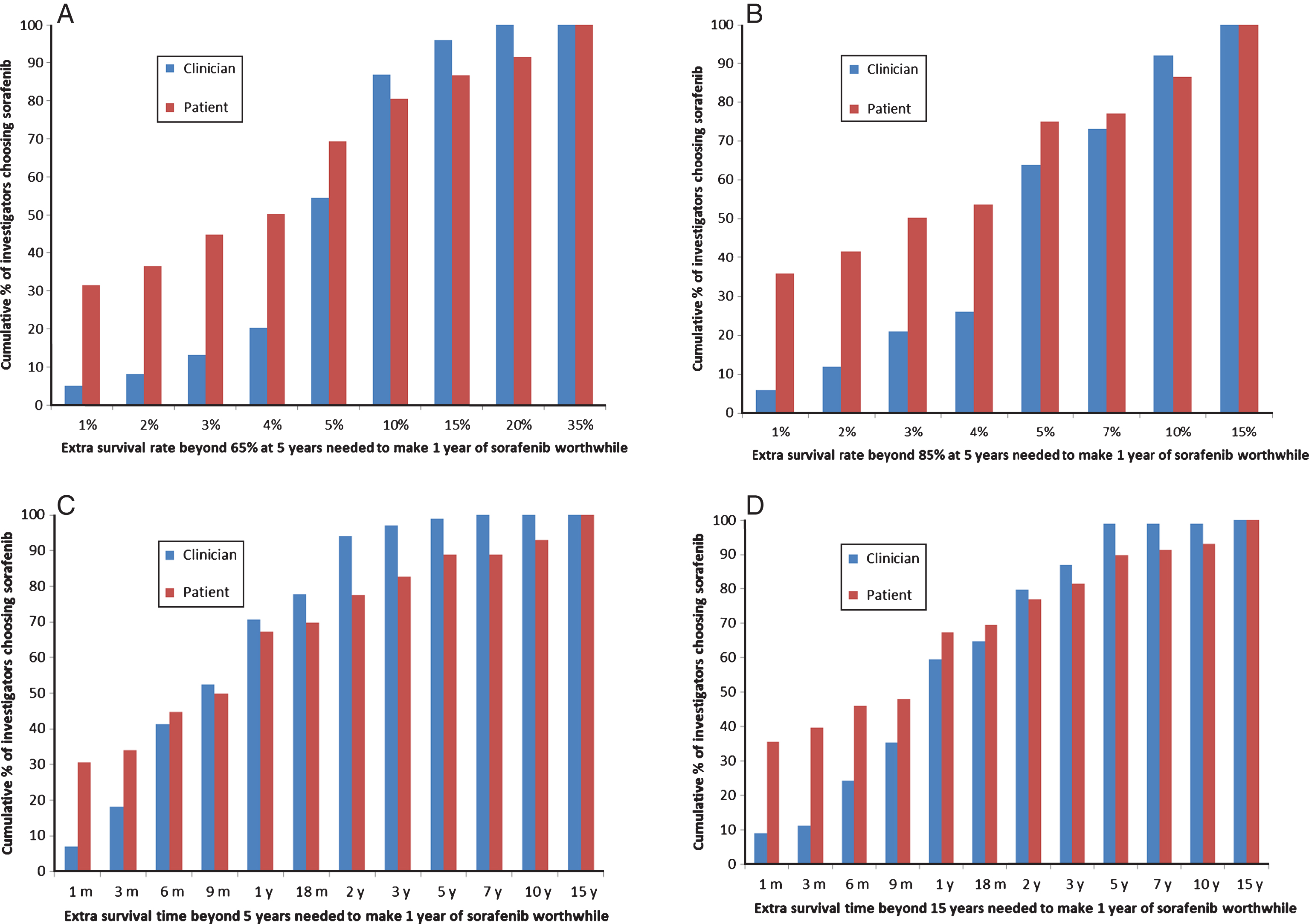
SORCE investigators judged larger survival benefits necessary to warrant adjuvant sorafenib than their patients participating in the trial (prior to starting treatment) [18], particularly in the survival rate scenarios (Fig. 2). The differences in survival benefits judged sufficient to make one year of sorafenib worthwhile by investigators versus patients were statistically significant for the baseline five year survival rates of 65% (p = 0.0001) and 85% (p < 0.0001), and for the baseline survival time of 15 years (p = 0.010), but not for the baseline survival time of five years (p = 0.64). These differences were largely due to investigators being less likely than patients to judge very small benefits sufficient in all scenarios; 2% vs 21% (p < 0.0001) respectively judged sufficient an extra 1% beyond a five year survival rate of 65%; 3% vs 23% (p < 0.0001) judged sufficient an extra 1% beyond an 85% five year survival rate; 7% vs 31% (p < 0.0001) judged sufficient an extra one month beyond five years; and 5% vs 26% (p < 0.0001) judged sufficient an extra one month beyond15 years.
Fig.2
A, B, C, D. Comparisons of the proportions of SORCE investigators versus patients judging specified survival benefits sufficient to make 1 year of adjuvant sorafenib worthwhile given baseline survival rates at 5 years of either 65% (A) or 85% (B). Comparisons of the proportion of SORCE investigators versus patients judging specified survival benefits sufficient to make 1 year of adjuvant sorafenib worthwhile given baseline survival times of 5 years (C) and 15 years (D).
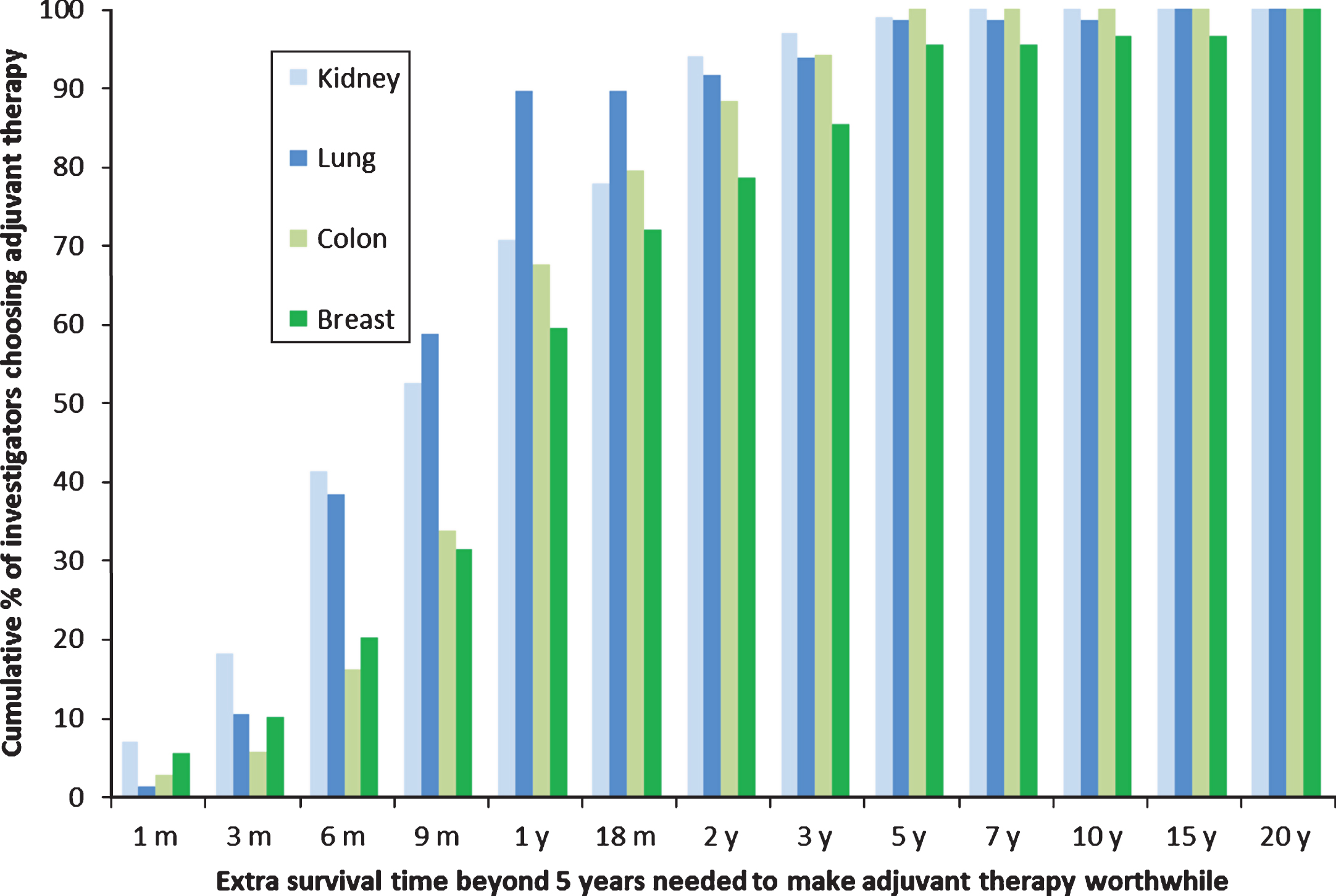
The survival benefits SORCE investigators judged sufficient to warrant one year of adjuvant sorafenib for RCC in this study were compared with the survival benefits judged sufficient to make three to six months of adjuvant chemotherapy worthwhile in similar studies of clinicians who treat lung, breast, and colon cancer (Fig. 3) [14]. The survival benefits judged sufficient to warrant one year of adjuvant sorafenib in RCC were similar to those judged sufficient to warrant three months of adjuvant chemotherapy in lung cancer (median benefits of nine months beyond five years, p = 0.95), but smaller than those judged sufficient to warrant six months of adjuvant chemotherapy in breast cancer and perhaps colon cancer (median benefits of nine months for RCC versus 12 months for breast and colon cancer beyond five years, p = 0.02 and 0.10 respectively).
Fig.3
Cumulative proportions of different clinicians who treat kidney, lung, colon or breast cancer judging specified benefits in overall survival sufficient to warrant adjuvant therapy given a baseline survival time of 5 years without adjuvant therapy. The types of cancer and adjuvant therapy were: kidney, 1 year of sorafenib; colon, 6 months of FOLFOX; breast, 6 months of AC and CMF; and lung, 3 months of cisplatin and vinorelbine.
DISCUSSION
Most SORCE investigators who participated judged moderate survival benefits sufficient to warrant one year of adjuvant sorafenib after resection of a RCC at intermediate- to high-risk of recurrence. The interquartile ranges demonstrate that 25% of investigators judged an extra six to nine months beyond survival times of five or 15 years respectively as sufficient, while 75% of investigators judged an extra 18 to 24 months beyond survival times of five or 15 years respectively as sufficient. In the survival rate scenarios 25% of investigators judged an extra 5% and 4% beyond a five year survival rate of 65% and 85% respectively as sufficient, while 75% of investigators judged an extra 10% beyond a five year survival rate of both 65% and 85% as sufficient. Larger benefits were required to warrant a second and third year of adjuvant sorafenib, than one year.
The characteristics of SORCE investigators were not associated with their preferences for adjuvant sorafenib in RCC, consistent with our previous studies of clinicians’ preferences for adjuvant chemotherapy in lung and endometrial cancer [13, 14].
Investigators required larger survival benefits to warrant adjuvant sorafenib than their patients in the SORCE trial. Although the preferences of both investigators and patients varied over the entire available range, patients were more likely to judge very small benefits sufficient to warrant adjuvant sorafenib. This corresponds with our group’s previous studies showing that clinicians generally require larger benefits than patients to make adjuvant chemotherapy worthwhile except in lung cancer [8, 13].
The benefits the SORCE investigators judged sufficient to warrant adjuvant sorafenib in RCC were similar to those of other clinicians judging three months of adjuvant chemotherapy after resection of localised lung cancer (p = 0.95), but smaller than those required by other clinicians judging six months of adjuvant chemotherapy for breast and perhaps colon cancer (p = 0.02 and 0.10 respectively). We expected the benefits required to warrant adjuvant sorafenib would be smaller than those required to warrant adjuvant chemotherapy for breast and colorectal cancer, but were surprised that they were similar to those required to warrant three months of adjuvant chemotherapy for lung cancer.
The main strengths of this study are its elicitation of preferences using validated methods within a randomised controlled trial allowing direct comparisons of investigators and the patients they recruited using standardised information presented to all participants. The main limitation is the inherent selection bias of surveying investigators in a clinical trial whose views might differ from those of other clinicians. The scenarios provided were based on hypothetical baseline survival times and rates, and may not reflect an individual patient’s prognosis. The number of urologists was small so we had limited power to detect differences in their preferences compared with those of medical oncologists.
The SORCE trial was powered to detect a HR for DFS of 0.75. Effects on OS are likely to be less extreme (HR closer to 1). SORCE investigators judged smaller benefits (HR closer to 1) sufficient to warrant adjuvant sorafenib in scenarios described with survival times rather than survival rates. These smaller benefits in scenarios described with survival times were more consistent with the hypothesised benefits in DFS specified in the SORCE trial statistical considerations. This was surprising because clinicians discussing survival after curative surgery for cancer typically express the outcomes in terms of survival rates. Survival times are more commonly used to discuss outcomes in metastatic cancer when the life expectancy is shorter. This raises the counterintuitive hypothesis that clinicians might provide more realistic estimates by expressing the potential benefits of adjuvant therapy in terms of survival times rather than survival rates.
The role of adjuvant targeted therapy for RCC remains controversial and of substantial interest. The SORCE trial is expected to report on its primary endpoint of DFS in 2018. The ATLAS trial of adjuvant axitinib versus placebo has recently been stopped at a planned interim analysis due to futility with detailed efficacy and safety data awaited (unpublished data, Pfizer Press Release, April 10, 2018). Three other randomised trials addressing this question have reported results. The S-TRAC trial (one year of sunitinib versus placebo, n = 615) showed an improvement in its primary endpoint of DFS (medians 5.6 years versus 6.8 years, HR 0.76; p = 0.03), but there was no difference in OS after a median follow-up of 5.4 years, with additional follow-up required for the final analysis of OS [3]. The ASSURE trial (one year of sunitinib versus one year of sorafenib versus placebo, n = 1943) showed no differences in either its primary endpoint of DFS (for sunitinib versus placebo the HR was 1.02; p = 0.80, for sorafenib versus placebo the HR was 0.97; p = 0.72), or in OS [19]. The PROTECT trial (one year of pazopanib versus placebo, n = 1538) did not show a statistically significant improvement in its primary endpoint of DFS (HR 0.86; p = 0.17) [20].
Our study used scenarios based on OS rather than DFS to assess the minimum benefits the SORCE investigators judged sufficient to warrant adjuvant sorafenib after resection of a RCC. The FDA recently approved sunitinib for adjuvant treatment of RCC at high risk of recurrence after nephrectomy, based on DFS in the S-TRAC trial [3]. We chose to use OS rather than DFS in order to assess the investigators judgement of the ultimate goal of adjuvant treatment, rather than a surrogate. An improvement in DFS without an improvement in OS means that all patients who receive adjuvant treatment will experience immediate toxicity where patients by definition will have no symptoms of cancer and will also experience shorter post-progression survival. It is important to consider the overall disease and treatment course as well as the toxicities associated with any treatment when weighing the benefits and harms of treatment.
Significant treatment toxicity was reported in all of the three trials with published data, with more than 60% of participants on active treatment in S-TRAC and ASSURE experiencing a grade three or four toxicity, and more than a quarter discontinuing study drug because of adverse events [3, 19]. Toxicity also occurred in PROTECT, leading to a reduction in the starting dose of pazopanib from 800 mg to 600 mg daily [20]. The toxicity reported in these three trials highlights the importance of considering the potential benefits, harms, and each individual patient’s preferences when making decisions about adjuvant targeted therapy.
In summary, investigators in the SORCE trial judged moderate survival benefits sufficient to warrant one year of adjuvant sorafenib after resection of apparently localised RCC at intermediate- to high-risk of recurrence, and required larger benefits to warrant a second and third year of treatment. Smaller, more plausible, benefits were judged sufficient when scenarios were expressed as survival times rather than survival rates. SORCE investigators required larger benefits than the patients they recruited in the trial. Clinicians thinking and talking about adjuvanttherapy should consider the differences between their own preferences and those of their patients andcolleagues.
FUNDING
This work was supported by funding from Cancer Australia for ANZUP and the NHMRC Clinical Trials Centre. The SORCE trial was funded globally by Cancer Research UK, Bayer Pharmaceuticals and the Medical Research Council UK, and partly supported in Australia and New Zealand by an untied grant from Bayer Australia. Bayer had no input into the design, conduct, analysis, or reporting of this study. ID Davis is supported by an NHMRC Practitioner Fellowship (APP1102604). NJ Lawrence received support from an NHMRC Postgraduate Scholarship (Australia), Goldman Sachs New Zealand Fellowship (New Zealand) and a Top-Up PhD Scholarship from Sydney Catalyst (University of Sydney and Cancer Institute NSW, Australia).
The NHMRC Clinical Trials Centre is supported by an Australian NHMRC Program Grant (APP1037786).
DISCLOSURES
ID Davis is a member/ chair of following industry advisory boards: Pfizer Renal Cell Carcinoma, Novartis, BMS ipilimumab, BMS anti-PD1, Bayer Nexavar, Roche Genitourinary Cancers, AstraZeneca Immunotherapy, and Eisai. No personal remuneration is received for any of this work. All payments or honoraria are paid directly to ANZUP Cancer Trials Group. ID Davis is a director and chair of the Board of ANZUP Cancer Trials Group. No personal remuneration is received for any of this work. ANZUP led the SORCE trial in Australia with funding support from Bayer Australia. ANZUP has received funds from Novartis to perform the EVERSUN trial. ANZUP has funding agreements to support clinical trials in renal cell carcinoma from MSD, Amgen and BMS.
E Hovey is a member of the Janssen Advisory Board for prostate cancer.
T Eisen declared current employment with Astra Zeneca and Vice President of Astra Zeneca. Research grants from Astra Zeneca, Pfizer, Bayer to Dr Eisen, and from Astra Zeneca to institution.
MR Stockler declared research grants from Astellas, Bayer, Astra Zeneca, Pfizer, Celgene, BMS, Merck, and Amgen all to institution.
All other authors declared no conflict of interest.
REFERENCES
[1] | Motzer RJ , Hutson TE , Tomczak P , . Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. (2007) ;356: (2):115–24. |
[2] | Escudier B , Eisen T , Stadler WM , . Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. (2007) ;356: (2):125–34. |
[3] | Ravaud A , Motzer RJ , Pandha HS , . Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. (2016) ;375: (23):2246–54. |
[4] | Larkin JM , Pyle LM , Gore ME Fatigue in renal cell carcinoma: The hidden burden of current targeted therapies. Oncologist. (2010) ;15: (11):1135–46. |
[5] | Kollmannsberger C , Mitchell T Selected toxicities of targeted therapies: Presentation and management. Seminars in Oncology. (2013) ;40: (4):499–510. |
[6] | Hubbard G , Kidd L , Donaghy E Preferences for involvement in treatment decision making of patients with cancer: A review of the literature. European Journal of Oncology Nursing: The Official Journal of European Oncology Nursing Society. (2008) ;12: (4):299–318. |
[7] | Moth E , McLachlan SA , Veillard AS , . Patients’ preferred and perceived roles in making decisions about adjuvant chemotherapy for non-small-cell lung cancer. Lung Cancer (Amsterdam, Netherlands). (2016) ;95: , 8–14. |
[8] | Blinman P , Hughes B , Crombie C , . Patients’ and doctors’ preferences for adjuvant chemotherapy in resected non-small-cell lung cancer: What makes it worthwhile? European Journal of Cancer (Oxford, England: 1990). (2015) ;51: (12):1529–37. |
[9] | Simes RJ , Coates AS Patient preferences for adjuvant chemotherapy of early breast cancer: How much benefit is needed? J Natl Cancer Inst Monogr. (2001) );30 146–52. |
[10] | Blinman P , Duric V , Nowak AK , . Adjuvant chemotherapy for early colon cancer: What survival benefits make it worthwhile? European Journal of Cancer (Oxford, England: 1990). (2010) ;46: (10):1800–7. |
[11] | Blinman P , Alam M , Duric V , McLachlan SA , Stockler MR Patients’ preferences for chemotherapy in non-small-cell lung cancer: A systematic review. Lung Cancer (Amsterdam, Netherlands). (2010) ;69: (2):141–7. |
[12] | Duric VM , Stockler MR , Heritier S , . Patients’ preferencesfor adjuvant chemotherapy in early breastcancer: What makes AC and CMF worthwhilenow? Annals of Oncology: Official Journal of the European Societyfor Medical Oncology / ESMO. (2005) ;16: (11):1786–94. |
[13] | Blinman P , Mileshkin L , Khaw P , . Patients’ and clinicians’ preferences for adjuvant chemotherapy in endometrial cancer: An ANZGOG substudy of the PORTEC-3 intergroup randomised trial. Br J Cancer. (2016) ;115: (10):1179–85. |
[14] | Blinman P , McLachlan SA , Nowak AK , . Lung cancer clinicians’ preferences for adjuvant chemotherapy in non-small-cell lung cancer: What makes it worthwhile? Lung Cancer (Amsterdam, Netherlands). (2011) ;72: (2):213–8. |
[15] | Leibovich BC , Blute ML , Cheville JC , . Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: A stratification tool for prospective clinical trials. Cancer. (2003) ;97: (7):1663–71. |
[16] | Clark JI , Atkins MB , Urba WJ , . Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: A cytokine working group randomized trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2003) ;21: (16):3133–40. |
[17] | Messing EM , Manola J , Wilding G , . Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: An Eastern Cooperative Oncology Group/Intergroup trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2003) ;21: (7):1214–22. |
[18] | Blinman P , Davis ID , Martin A , . Patients’ preferences for adjuvant sorafenib after resection of renal cell carcinoma in the SORCE trial: What makes it worthwhile?. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO. (2017) ); mdx715-mdx. |
[19] | Haas NB , Manola J , Uzzo RG , . Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN EA double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. (2016) ;387: (10032):2008–16. |
[20] | Motzer RJ , Haas NB , Donskov F , . Randomized phase III trialof adjuvant pazopanib versus placebo after nephrectomy in patientswith locally advanced renal cell carcinoma (RCC) (PROTECT). Journal of Clinical Oncology: Official Journal of the AmericanSociety of Clinical Oncology. (2017) ;35: (15_suppl):4507. |




