Positive Surgical Margins After Partial Nephrectomy: A Systematic Review and Meta-Analysis of Comparative Studies
Abstract
Objective:
We performed an update of previous reviews of the literature to provide an overview on incidence, predictive factors, management and prognosis of positive surgical margins (PSMs) after partial nephrectomy (PN) including recent surgical series and studies comparing different approaches and techniques.
Material and methods:
A literature search was performed from January 2013 to January 2018 using the Medline database. The search strategy included a free-text protocol using the term “nephron-sparing surgery” OR “partial nephrectomy” AND “positive surgical margins” across the title and abstract fields of the records. From each selected study, we extracted the following data: number of analyzed patients, study design, approach and surgical technique used, PSMs rate, pathological features, type of PSMs treatment, mean (median) follow-up duration and final patient status. Meta-analysis was conducted using Review Manager software v. 5.2 (Cochrane Collaboration, Oxford, UK).
Results:
We selected a total of 36 (48%) studies. All studies were retrospective and the best statistical method used for comparison was the matched-pair analysis (level 4). Overall, 45,786 patients treated with PN were included in the selected studies. PSMs were reported in a total of 3,093 (6.7%) patients. The mean estimated PSMs rate was 7%, 5% and 4.3% in patients who underwent robot-assisted PN (RAPN), laparoscopic PN (LPN) and open PN (OPN), respectively. Comparative studies showed a significant advantage in favor of OPN compared with minimally invasive approach, while RAPN showed more favourable PSMs risk compared with LPN (odds ratio 3.02, 95% confidence intervals 2.05–4.45). No differences were detected stratifying data according to other surgical or tumor-related factors. Tumor size, nuclear grading and pT3a stage represent the most important predictors of PSMs. In 6,809 patients, follow-up data were available. Only 101 (1.4%) local recurrences and 88 (1.3%) distant recurrences were observed both in PSMs and negative surgical margins subgroups. PSMs were associated with a significant increased risk of local recurrence with a significant impact on local recurrence-free survival and metastasis-free survival. However, a significant impact on cancer-specific and overall survival could not be demonstrated.
Conclusions:
Studies published in the last 5 years confirmed that PSMs after PN are a rare condition. Although PSMs increase the risk of local and distant recurrence, their influence on cancer-specific and overall survival seems to be limited. Close surveillance should be strongly recommended as initial treatment of patients with PSMs after PN.
INTRODUCTION
The aim of nephron-sparing surgery (NSS) is the complete removal of the renal tumor preserving the largest possible part of healthy renal parenchyma. The absence of cancer cells at the level of inked parenchymal excision surface is an important early oncologic outcome. Therefore, the evaluation of positive surgical margins (PSMs) is considered as a quality metric for patients treated with NSS. Indeed, the presence of PSMs could be the expression of persistent disease predisposing local recurrence and/or distant progression. When physicians is faced with PSMs, management can be challenging and controversial, ranging from surveillance to radical nephrectomy.
Previous non-systematic reviews of the literature evaluating studies published until 2013 showed that PSMs after partial nephrectomy (PN) is a rare condition, reported in 0–7% of patients after open PN (OPN), in 1–4% after laparoscopic PN (LPN) and in 4–6% after robot-assisted PN (RAPN) [1, 2]. Few data were available about other potential risk factors, e.g. tumor size, stage or grade. Considering that the majority of these patients did not experience local or distant tumor recurrence at an intermediate term follow-up, previous studies concluded that secondary partial or radical nephrectomy might not be necessary [1]. However, surveillance may not be appropriate in patients showing both PSMs and high-grade disease [2, 3].
In agreement with the international guidelines, in the last 5 years an increasing number of large (cT1b-2) and complex renal tumors have been treated with NSS using mainly laparoscopic approaches. Notably, in our experience, we also witnessed a progressive reduction of the thickness of the peritumoral healthy parenchyma removed according to some authors popularizing the simple enucleation (SE) [4].
Herein, we performed an update of previous reviews of the literature to provide an overview on incidence, predictive factors, management and prognosis of PSMs after PN including recent surgical series and studies comparing different approaches and techniques.
METHODS
A literature search was performed including studies published from January 2013 to January 2018 using the Medline database. The search strategy included a free-text protocol using the term “nephron-sparing surgery” OR “partial nephrectomy” AND “positive surgical margins” across the title and abstract fields of the records.
The following limits were used: humans and publications written in English. Three authors (GG, AC and VF) separately reviewed the records to select non-comparative surgical series and randomized or non-randomized controlled studies reporting the percentage of PSMs in patients who underwent either OPN, LPN or RAPN for renal cell carcinoma (RCC), with any discrepancy resolved by open discussion. Only studies reporting on more than 50 cases were selected. Other significant studies cited in the reference lists of the selected papers were evaluated, as well as relevant studies published after the systematic search. Studies published only as abstracts and reports from meetings were not included in the review.
The selected studies were grouped in two categories: 1) studies only reporting on the rate of PSMs after PN and 2) studies reporting on predictors, management and follow-up of PSMs.
All the papers were distinguished according to the 2011 level of evidence for therapy studies: systematic review of randomized trials or n-of-1 trials (level 1); randomized trials or observational studies with dramatic effects (level 2); non-randomized controlled cohort/follow-up studies (level 3); case series, case-control studies, or historically controlled studies (level 4); and mechanism-based reasoning (level 5) [5].
From each selected study, we extracted the following data: number of analyzed patients, study design, approach and surgical technique used, PSMs rate, pathological features, type of PSMs treatment, mean (median) follow-up duration and final patient status. All data retrieved from the selected studies were recorded in an electronic database. Quality control of the electronic data recording was performed on a random sample of papers (accounting for about 15% of the articles)
Meta-analysis was conducted using Review Manager software v. 5.2 (Cochrane Collaboration, Oxford, UK). Specifically, statistical heterogeneity was tested using the χ2 test. A value of p < 0.10 was used to indicate heterogeneity. In the case of a lack of heterogeneity, fixed-effects models were used for meta-analyses. The results were expressed as odds ratio (OR) with 95% confidence intervals (CIs).
RESULTS
From 75 records initially identified, we selected 36 (48%) studies meeting the criteria of this search. No randomized controlled trials were available. Eleven (30.5%) studies were comparative studies evaluating different surgical approaches. Specifically, 5 studies compared LPN versus RAPN, 3 studies OPN versus RAPN, and 3 studies OPN versus minimally invasive procedures (LPN and RAPN). Two (5.5%) studies compared no ischemia versus different ischemia techniques. All comparative studies were retrospective and the best statistical method used for comparison was the matched-pair analysis (level 4). Figure 1 shows the flowchart of the systematic review and the factors evaluated into the studies.
Fig.1
Flow-chart of the systematic review. LPN = laparoscopic partial nephrectomy; MIPN = minimally invasive partial nephrectomy; OPN = open partial nephrectomy; PSMs = positive surgical margins; RAPN = robot-assisted partial nephrectomy; SE = simple enucleation; SPN = standard partial nephrectomy.
Overall 45,786 PNs were included in the 36 selected studies. PSMs were reported in a total of 3,093 (6.7%) patients. The mean PSMs rate reported in the selected studies ranged from 0.7% to 10.1% (Table 1). Data concerning RAPN were evaluable in 22 studies including a total of 26,606 cases. In this subgroup of patients, 1,884 (7%) PSMs were reported. Mean PSMs rate reported in RAPN series ranged between 0–8.5% (Table 2). Ten studies including 2,013 cases reported PSM rates after LPN. A total of 104 (5%) cases with PSMs were reported. Mean PSMs rate reported in LPN series ranged between 1–12% (Table 3a). OPN data were available in 7 studies including a total of 7.126 patients. In this subgroup of patients, PSMs were observed in 313 (4.3%) cases. Mean PSMs rate reported in OPN series ranged between 1.8–18% of cases (Table 3b).
Table 1
Incidence of PSMs reported in the 36 studies selected in the present systematic review
| Authors | Study | Cases | Approach | PSMs rate | PSMs cases |
|---|---|---|---|---|---|
| (n) | (%) | (n) | |||
| Kaczmarek, 2013 [6] | Case series | 886 | RAPN | 3% | 26 |
| Porpiglia, 2013 [7] | Case series | 206 | LPN | 2.9% | 6 |
| Roushias, 2013 [8] | Case series | 128 | OPN/LPN | 10.1% | 13 |
| Williams, 2013 [9] | Comparative | 86 | LPN Vs RAPN | 9.3% | 8 |
| Khalifeh, 2013 [10] | Case series | 943 | RAPN | 2.2% | 21 |
| Zargar, 2014 [11] | Comparative | 125 | OPN Vs RAPN | 4% | 5 |
| Jang, 2014 [12] | Comparative | 127 | LPN Vs RAPN | 0.7% | 1 |
| Akca, 2014 [13] | Case series | 110 | RAPN | 1.8% | 2 |
| Longo, 2014 [14] | Case series | 280 | OPN/LPN | 4.2% | 12 |
| Carneiro, 2015 [15] | Comparative | 347 | LPN Vs RAPN | 2.9% | 10 |
| Curtiss, 2015 [16] | Case series | 228 | RAPN | 2.1% | 5 |
| Kim, 2015 [17] | Comparative | 390 | LPN Vs RAPN | 1.5% | 6 |
| Komninos, 2015 [18] | Case series | 162 | RAPN | 4.9% | 8 |
| Lista, 2015 [19] | Case series | 339 | RAPN | 6.5% | 22 |
| Serni, 2015 [20] | Case series | 84 | RAPN | 3.6% | 3 |
| Zargar, 2015 [21] | Comparative | 1831 | LPN Vs RAPN | 6% | 109 |
| Tabayoyong, 2015 [22] | Comparative | 11587 | OPN Vs MIPN | 7% | 811 |
| Dong, 2016 [23] | Case series | 270 | LPN | 2.2% | 6 |
| Ito, 2016 [24] | Case series | 156 | LPN | 3.7% | 6 |
| Malkoc, 2016 [25] | Comparative | 110 | OPN Vs RAPN | 4.5% | 5 |
| Novara, 2016 [26] | Case series | 465 | RAPN | 4.5% | 21 |
| Paulucci, 2016 [27] | Case series | 665 | RAPN | 3.6% | 24 |
| Roberts, 2016 [28] | Case series | 50 | LPN | 4% | 2 |
| Shah, 2016 [29] | Case series | 1240 | PN | 7.8% | 97 |
| Wang, 2016 [30] | Case series | 117 | PN | 8.5% | 10 |
| Bigot, 2017 [31] | National database | 234 | PN | 6% | 14 |
| Hennessey, 2017 [32] | Case series | 249 | RAPN | 3.2% | 8 |
| Khene, 2017 [33] | Case series | 216 | RAPN | 2.8% | 6 |
| Matos, 2017 [34] | Case series | 60 | OPN/LPN | 6.6% | 4 |
| Maurice, 2017 [35] | Comparative | 605 | OPN Vs RAPN | 4.3% | 26 |
| Veeratterapillay, 2017 [36] | Case series | 250 | RAPN | 7.3% | 18 |
| Xia, 2017 [37] | National database | 18724 | RAPN | 8.5% | 1597 |
| Bansal, 2017 [38] | National database | 1103 | PN | 6.4% | 70 |
| Marchinena, 2017 [39] | Comparative | 314 | OPN Vs MIPN | 7% | 22 |
| Delto, 2018 [40] | Case series | 1236 | RAPN | 4.6% | 56 |
| Petros, 2018 [41] | Comparative | 1863 | OPN Vs MIPN | 1.8% | 33 |
| Analysis | 36 studies | 45786 | 0.7–10,1% | 3093 (6.7%) |
LPN = laparoscopic partial nephrectomy; MIPN = minimally invasive partial nephrectomy; OPN = open partial nephrectomy; PSMs = positive surgical margins; RAPN = robot-assisted partial nephrectomy.
Table 2
Incidence of PSMs in 22 studies reporting data after RAPN
| Authors | Cases | Approach | PSMs rate | PSMs cases |
|---|---|---|---|---|
| (n) | (%) | (n) | ||
| Kaczmarek, 2013 [6] | 886 | RAPN | 3% | 26 |
| Williams, 2013 [9] | 27 | RAPN | 4% | 1 |
| Khalifeh, 2013 [10] | 943 | RAPN | 2.2% | 21 |
| Zargar, 2014 [11] | 40 | RAPN | 7% | 3 |
| Jang, 2014 [12] | 89 | RAPN | 0% | 0 |
| Akca, 2014 [13] | 110 | RAPN | 1.8% | 2 |
| Carneiro, 2015 [15] | 44 | RAPN | 2.2% | 1 |
| Curtiss, 2015 [16] | 228 | RAPN | 2.1% | 5 |
| Kim, 2015 [17] | 195 | RAPN | 1.5% | 3 |
| Komninos, 2015 [18] | 162 | RAPN | 4.9% | 8 |
| Lista, 2015 [19] | 339 | RAPN | 6.5% | 22 |
| Serni, 2015 [20] | 84 | RAPN | 3.6% | 3 |
| Zargar, 2015 [21] | 1185 | RAPN | 3.2% | 38 |
| Malkoc, 2016 [25] | 54 | RAPN | 5.6% | 3 |
| Novara, 2016 [26] | 465 | RAPN | 4.5% | 21 |
| Paulucci, 2016 [27] | 665 | RAPN | 3.6% | 24 |
| Hennessey, 2017 [32] | 249 | RAPN | 3.2% | 8 |
| Khene, 2017 [33] | 216 | RAPN | 2.8% | 6 |
| Maurice, 2017 [34] | 415 | RAPN | 4.5% | 18 |
| Veeratterapillay, 2017 [36] | 250 | RAPN | 7.3% | 18 |
| Xia, 2017 [37] | 18724 | RAPN | 8.5% | 1597 |
| Delto, 2018 [40] | 1236 | RAPN | 4.6% | 56 |
| Analysis | 26606 | 22 studies | 0 – 8.5% | 1884 (7%) |
PSMs = positive surgical margins; RAPN = robot-assisted partial nephrectomy.
Table 3
Incidence of PSMs in studies reporting data after LPN
| Authors | Cases | Approach | PSMs rate | PSMs cases |
|---|---|---|---|---|
| (n) | (%) | (n) | ||
| Porpiglia, 2013 [7] | 206 | LPN | 2.9% | 6 |
| Roushias, 2013 [8] | 90 | LPN | 6% | 5 |
| Williams, 2013 [9] | 59 | LPN | 12% | 7 |
| Jang, 2014 [12] | 38 | LPN | 2.6% | 1 |
| Carneiro, 2015 [15] | 303 | LPN | 2.3% | 7 |
| Kim, 2015 [17] | 195 | LPN | 1% | 2 |
| Zargar, 2015 [21] | 646 | LPN | 9.7% | 62 |
| Dong, 2016 [23] | 270 | LPN | 2.2% | 6 |
| Ito, 2016 [24] | 156 | LPN | 3.7% | 6 |
| Roberts, 2016 [28] | 50 | LPN | 4% | 2 |
| Analysis | 2013 | 10 studies | 1–12% | 104 (5%) |
LPN = laparoscopic partial nephrectomy; PSMs = positive surgical margins.
Table 3b
Incidence of PSMs in studies reporting data after OPN
| Authors | Cases (n) | Approach | PSMs rate (%) | PSMs cases (n) |
|---|---|---|---|---|
| Roushias, 2013 [8] | 38 | OPN | 18% | 13 |
| Zargar, 2014 [11] | 85 | OPN | 9% | 5 |
| Tabayoyong, 2015 [22] | 5094 | OPN | 4.9% | 249 |
| Malkoc, 2016 [25] | 56 | OPN | 3.6% | 2 |
| Maurice, 2017 [35] | 190 | OPN | 4.2% | 8 |
| Marchinena, 2017 [39] | 142 | OPN | 6.3% | 9 |
| Petros, 2018 [41] | 1521 | OPN | 1.8% | 27 |
| Analysis | 7126 | 7 studies | 1.8–18% | 313 (4.3%) |
OPN = open partial nephrectomy; PSMs = positive surgical margins.
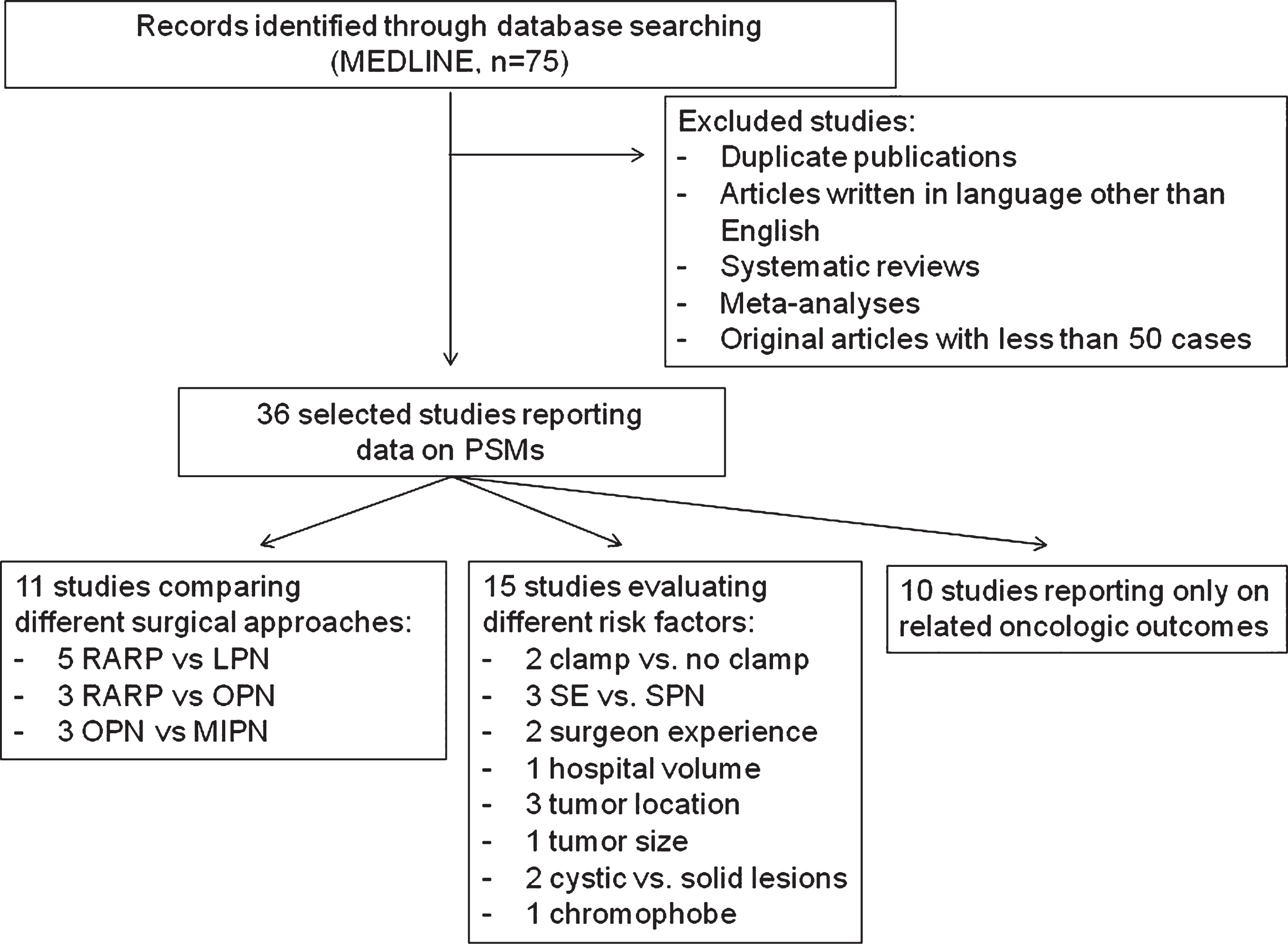
Only two comparative studies showed significant differences in terms of PSM rate between different surgical approaches. Specifically, Tabayoyong et al. reported lower PSMs rate in patients who underwent OPN compared with those receiving a minimally invasive approach [22]. Similarly, Zargar et al. reported a lower PSMs rate in OPN compared with RAPN [11]. Table 4 summarizes data reported in the remaining 9 comparative studies. Cumulative analysis of studies comparing OPN versus RAPN showed overlapping results between the two techniques in terms of positive surgical margins (Fig. 2). Conversely, cumulative analysis comparing LPN versus RAPN showed significant advantages in favor of RAPN (OR 3.02, 95% CIs 2.05–4.45) (Fig. 3).
Fig.2
Cumulative analysis of studies comparing open partial nephrectomy vs robot-assisted partial nephrectomy in terms of positive surgical margins rates. OPN = open partial nephrectomy; RAPN = robot-assisted partial nephrectomy.
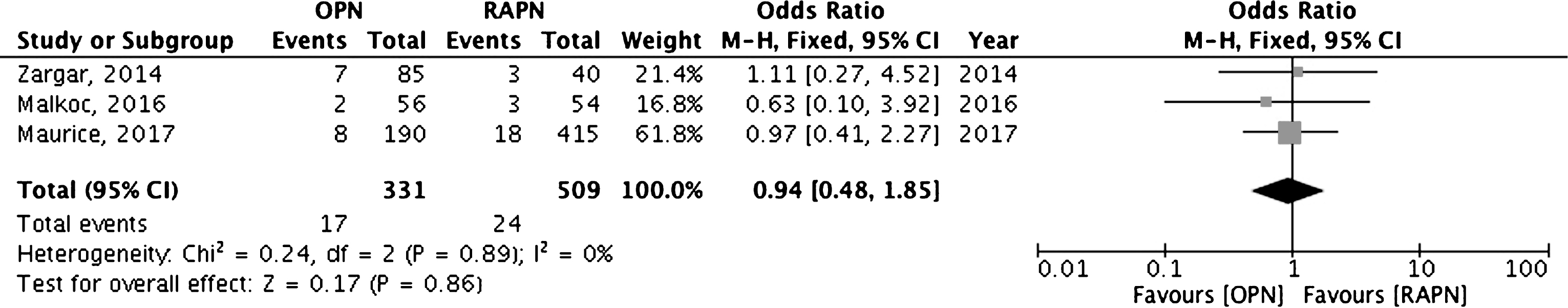
Fig.3
Cumulative analysis of studies comparing laparoscopic partial nephrectomy vs robot-assisted partial nephrectomy in terms of positive surgical margins rates. LPN = laparoscopic partial nephrectomy; RAPN = robot-assisted partial nephrectomy.
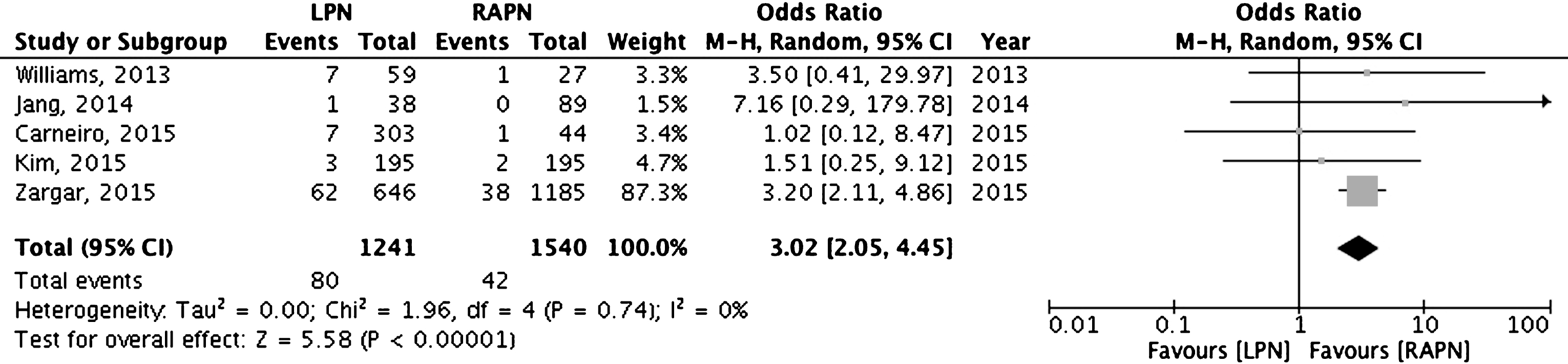
Table 4
Incidence of PSMs reported in studies comparing the different surgical approaches
| Authors | Approaches | PSMs cases | PSMs rate | p value |
|---|---|---|---|---|
| Zargar, 2014 [11] | OPN (n = 85) | 7 | 9% | Not significant |
| RAPN (n = 40) | 3 | 6.7% | ||
| Malkoc, 2016 [25] | OPN (n = 56) | 2 | 3.6% | Not significant |
| RAPN (n = 54) | 3 | 5.6% | ||
| Maurice, 2017 [35] | OPN (n = 190) | 8 | 4.2% | Not significant |
| RAPN (n = 415) | 18 | 4.5% | ||
| Tabayoyong, 2015 [22] | OPN (n = 5094) | 249 | 4.9% | <0.001 |
| MIPN (n = 6493) | 551 | 8.5% | ||
| Marchinena, 2017 [39] | OPN (n = 142) | 9 | 6.3% | Not significant |
| MIPN (n = 172) | 13 | 7.5% | ||
| Petros, 2018 [41] | OPN (n = 1521) | 27 | 1.8% | Not significant |
| MIPN (n = 342) | 6 | 1.8% | ||
| Williams, 2013 [9] | LPN (n = 59) | 7 | 12% | Not significant |
| RAPN (n = 27) | 1 | 4% | ||
| Jang, 2014 [12] | LPN (n = 38) | 1 | 2.6% | Not significant |
| RAPN (n = 89) | 0 | 0% | ||
| Carneiro, 2015 [15] | LPN (n = 303) | 7 | 2.3% | Not significant |
| RAPN (n = 44) | 1 | 1.7% | ||
| Kim, 2015 [17] | LPN (n = 195) | 3 | 1.5% | Not significant |
| RAPN (n = 195) | 2 | 1% | ||
| Zargar, 2015 [21] | LPN (n = 646) | 62 | 9.7% | <0.001 |
| RAPN (n = 1185) | 38 | 3.2% |
LPN = laparoscopic partial nephrectomy; MIPN = minimally invasive partial nephrectomy; OPN = open partial nephrectomy; PSMs = positive surgical margins; RAPN = robot-assisted partial nephrectomy.
Table 5 summarizes PSMs rates stratified according to the investigated surgical-related factors (Table 5). Surgical experience did not influence PSMs rate in a single-surgeon LPN series [7] as well as no differences were observed among expert surgeons and fellows in a single-center RAPN series [33]. Conversely, hospital volume impacts significantly on PSM rate as reported by National Cancer Database [37]. Two studies stratified PSM rates according to clamp or off-clamp techniques [18, 27]. Cumulative analysis of previous studies did not demonstrate significant differences between off-clamp, selective clamping and main artery clamping techniques (Fig. 4).
Fig.4
Cumulative analysis of studies comparing clamp versus off-clamp techniques.
Table 5
Incidence of PSMs in studies stratifying data according to surgical-related factors
| Authors | Approaches | Comparison | Subgroups | PSMs rate (%) | p value |
|---|---|---|---|---|---|
| Porpiglia, 2013 [7] | LPN | Surgeon experience | Group 1 (n = 50) | 3.9% | Not significant |
| Group 2 (n = 50) | 1.9% | ||||
| Group 3 (n = 50) | 1.9% | ||||
| Group 4 (n = 50) | 3.8% | ||||
| Khene, 2017 [33] | RAPN | Surgeon experience | Expert surgeons (n = 127) | 3.1% | Not significant |
| Fellows (n = 89) | 2.2% | ||||
| Xia, 2017 [37] | RAPN | hospital volume | Very low volume (n = 3693) | 11.8% | <0.001 |
| Low volume (n = 3719) | 9.5% | ||||
| Medium volume (n = 3833) | 9.4% | ||||
| High volume (n = 3649) | 7.5% | ||||
| Very high volume (n = 3830) | 4.6% | ||||
| Komninos, 2015 [18] | RAPN | Ischemia methods | Off-clamp (n = 23) | 0% | Not significant |
| Selective Clamp (n = 25) | 8.6% | ||||
| Main artery clamp (n = 114) | 6.5% | ||||
| Paulucci, 2016 [27] | RAPN | Ischemia methods | Selective Clamp (n = 66) | 3% | Not significant |
| Main artery clamp (n = 132) | 5.7% | ||||
| Longo, 2014 [14] | OPN/LPN | Simple enucleation Vs standard PN | SE (n = 145) | 1.4% | 0.02 |
| SPN (n = 145) | 6.9% | ||||
| Dong, 2016 [23] | LPN | Simple enucleation Vs standard PN | SE (n = 98) | 1% | Not significant |
| SPN (n = 98) | 3.1% | ||||
| Wang, 2016 [30] | PN | Simple enucleation Vs standard PN | SE (n = 59) | 17.2% | <0.001 |
| SPN (n = 58) | 0% |
LPN = laparoscopic partial nephrectomy; OPN = open partial nephrectomy; PN = partial nephrectomy; PSMs = positive surgical margins; RAPN = robot-assisted partial nephrectomy.
Conflicting results were reported in studies comparing SE with SPN. While Longo et al. reported a significant lower PSMs rate in the SE group compared with the SPN one, Wang et al. observed a higher percentage of PSMs in patients who underwent SE compared with SPN [14, 30]. Finally, Dong et al. did not report differences between the two surgical techniques [23]. Cumulative analysis showed overlapping results between the two techniques (Fig. 5).
Fig.5
Cumulative analysis of studies comparing simple enucleation versus standard partial nephrectomy. SE = simple enucleation; SPN = standard partial nephrectomy.
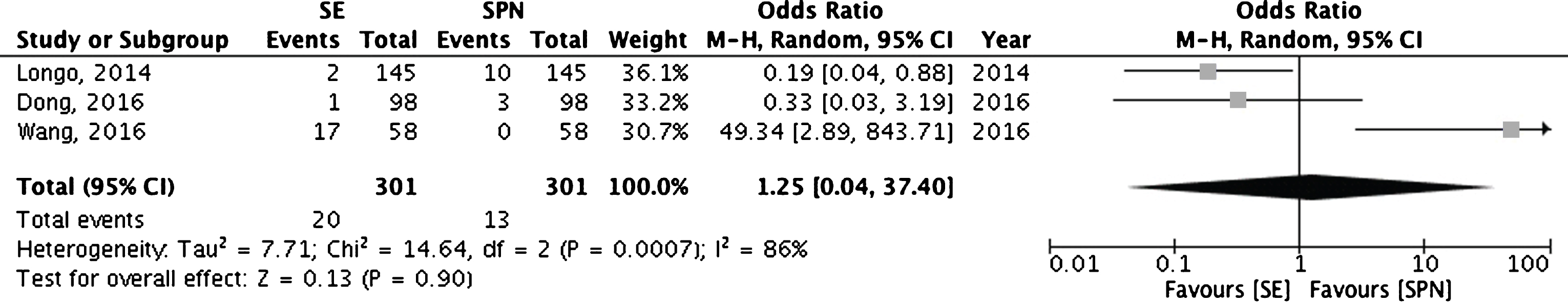
Table 6 summarizes PSMs rates stratified according to the evaluated tumor-related factors. Risk categories according nephrometry systems, tumor size, and cystic or solid tumor feature did not influence PSMs rates [8, 13, 16, 26, 34, 40].
Table 6
Incidence of PSMs in studies stratifying data according to tumor-related factors
| Authors | Approaches | Comparison | Subgroups | PSMs rate (%) | p value |
|---|---|---|---|---|---|
| Roushias, 2013 [8] | OPN/LPN | Tumor location | Low risk (n = 55) | 1.9% | Not significant |
| Moderate risk (n = 68) | 7.8% | ||||
| High risk (n = 5) | 40% | ||||
| Curtiss, 2015 [16] | RAPN | Tumor location | Endophytic (n = 30) | 0% | Not significant |
| Esophytic (n = 267) | 2.4% | ||||
| Matos, 2017 [34] | OPN/LPN | Tumor location | Low risk (n = 7) | 14.3% | Not significant |
| Moderate risk (n = 42) | 4.8% | ||||
| High risk (n = 22) | 4.5% | ||||
| Delto, 2018 [40] | RAPN | Tumor size | cT1a (n = 940) | 4.5% | Not significant |
| cT1b (n = 294) | 3.3% | ||||
| cT2a (n = 22) | 4.5% | ||||
| Akca, 2014 [13] | RAPN | Cystic Vs Solid lesions | Cystic lesions (n = 55) | 0% | Not significant |
| Solid lesions (n = 55) | 4.2% | ||||
| Novara, 2016 [26] | RAPN | Cystic Vs Solid lesions | Cystic lesions (n = 54) | 7.4% | Not significant |
| Solid lesions (n = 411) | 4.2% | ||||
| Bigot, 2017 [31] | PN | Chromophobe | 6% |
LPN = laparoscopic partial nephrectomy; OPN = open partial nephrectomy; PN = partial nephrectomy; PSMs = positive surgical margins; RAPN = robot-assisted partial nephrectomy.
Considering the potential predictors of PSMs, Tabayoyong et al. clearly demonstrated that tumor 2–3 cm in size (OR 1.21, p = 0.03), LPN approach (OR 1.8, p < 0.001) and RAPN approach (OR 1.79, p < 0.001) were the most important predictors for PSMs after PN also when data were adjusted for hospital volume [22]. More recently, Bansal et al. reported that pT3a stage and G3-4 nuclear grade were independent predictors of PSMs after PN [38].
Only 14 of the 36 selected studies reported data on the oncologic follow-up (range 12–62 months) (Table 7). These studies included 6,809 patients with 314 (4,6%) PSMs. Only 101 (1,4%) local recurrences and 88 (1,3%) distant recurrences were observed during the follow-up both in PSMs and negative surgical margins (NSMs) subgroups.
Table 7
Studies reporting on PSMs status and oncologic outcomes
| Authors | Cases | PSMs rate | Comparison | Follow-up | Local recurrence | Distant recurrence | Notes |
|---|---|---|---|---|---|---|---|
| (%) | (months) | n (%) | n (%) | ||||
| Khalifeh, 2013 [10] | 943 | 2.2% | PSMs (n = 21) | 17 | 2 (9.5%) | 2 (9.5%) | Statistically significant difference (p < 0.001) |
| NSMs (n = 922) | 7 (0.7%) | 2 (0.2%) | |||||
| Shah, 2016 [29] | 1240 | 7.8% | PSMs (n = 97) | 33 | 8 (8.2%) | 9 (9.2%) | PSMs, tumor size, G3-4 and pT3a were independent predictors of LR |
| NSMs (n = 1143) | 34 (2.9%) | 24 (2%) | |||||
| Marchinena, 2017 [39] | 314 | 7% | PSMs (n = 22) | 24 | 2 (9%) | 2 (9%) | PSMs and G3-4 were independent predictors of LR |
| NSMs (n = 292) | 4 (1.3%) | 6 (2%) | |||||
| Bansal, 2017 [38] | 1103 | 6.4% | PSMs (n = 68) | 15 | 2 (2.9%) | 5 (7.3%) | PSMs have not significant role on disease progression |
| NSMs (n = 935) | 19 | 28 (2.9%) | 21 (2.2%) | ||||
| Petros, 2018 [41] | 1863 | 1.8% | PSMs (n = 34) | 62 | 4 (11.7%) | 5 (14.7%) | PSMs impact on local- and metastatic-RFS. No impact on OS |
| NSMs (n = 100) | |||||||
| Serni, 2015 [20] | 84 | 3.6% | None | 54 | 1 (1.2%) | 3 (3,5%) | 5-yr RFS 90,8%; CSS 96,1%; OS 88% |
| Dong, 2016 [23] | 270 | 2.2% | SE (n = 98) | 39,5 | 1 (1%) | 1 (1%) | Non-statistically significant difference |
| SPN (n = 98) | 36,9 | 1 (1%) | 2 (2%) | ||||
| Ito, 2016 [24] | 132 | 3.7% | None | 45 | 1 (0.7%) | ||
| Malkoc, 2016 [25] | 110 | 4.5% | OPN (n = 56) | 24,2 | 1 (2.1%) | 2 (4.3%) | Non-statistically significant difference |
| RAPN (n = 54) | 19,4 | 2 (4.9%) | 3 (7.3%) | ||||
| Wang, 2016 [30] | 117 | 8.5% | SE vs SPN | 20 | 0 | 0 | |
| Bigot, 2017 [31] | 234 | 6% | Chromophobe | 35 | 2 (0.8%) | 0 | |
| Hennessey, 2017 [32] | 249 | 3.2% | None | 12 | 0 | 0 | |
| Veeratterapillay, 2017 [36] | 250 | 7.3% | None | 12 | 1 (0.4%) | 1 (0.4%) | |
| Delto, 2018 [40] | 1236 | 4.6% | Tumor size | 12 | RFS: 99.6% (T1a); 100% (T1b); 91.7% (T2a) |
CSS = cancer-specific survival; LR = local recurrence; NSMs = negative surgical margins; OPN = open partial nephrectomy; OS = overall survival; PSMs = positive surgical margins; RAPN = robot-assisted partial nephrectomy; RFS = recurrence-free survival; SE = simple enucleation; SPN = standard partial nephrectomy.
Four studies reported oncologic data stratified according to surgical margin status after PN [10, 29, 39, 38]. In three studies, the presence of PSMs was associated with a significant increased risk of local recurrence [10, 29, 39]. Specifically, multivariable analyses demonstrated that PSMs are a strong predictor of local recurrence especially in combination with high nuclear grade of the tumor [29, 39]. Recently, Petros et al. reported a significant impact of PSMs on local recurrence-free survival and metastasis-free survival, failing to demonstrate a significant impact on overall survival [41]. Conversely, Bansal et al. failed to demonstrate a significant role of PSMs on disease progression at multivariable analysis [38].
DISCUSSION
PSMs after PN for RCC remain a rare condition regardless of the approach and surgical technique used. Minimally invasive procedures as well as tumor size, pT3a stage and high nuclear grade seem to be the most important factors associated with an increased risk of PSMs after PN. Although some studies confirmed the predictive role of PSM on risk of local and distant recurrences, only few patients showed these unfavorable prognostic conditions. Moreover, PSMs were shown not to impact on cancer-specific and overall survival. For this reason, a close surveillance strategy is justified in the majority of patients with PSMs after PN. Salvage surgical treatments should be considered in patients with locally advanced or high-grade tumors associated with PSMs.
Similarly to the previous non-systematic review of the literature, our research confirmed that the majority of the available studies were single-center or multicenter surgical series or low-quality comparative studies. Therefore, the level of evidence supporting data of the present systematic review is of low quality (level 4).
Previous non-systematic reviews including studies published between 2001 and 2012 showed an overall PSMs rate ranging from 0 to 7% after elective NSS. Our data showed a PSMs rate of 7% with studies reporting values between 0.7 to 10.1%. Interestingly, in the last 5 years the majority of patients included in the studies underwent a RAPN (n = 26,606), followed by OPN (n = 7,126) and LPN (n = 2,013), respectively.
Several surgical-related factors were investigated as potential predictors of PSMs rate. According to the different approach, previous reviews showed PSMs rates ranging between 0–7% after OPN, 0.7–4% after LPN, and 3.9–5.7% after RAPN [1, 2]. Our analysis showed that PSMs rate was 7%, 5% and 4.3% after RAPN, LPN and OPN, respectively, with ranges between 0–8.5%, 1–12% and 1.8–18%, respectively.
Moreover, non-randomized studies comparing OPN versus LPN or LPN versus RAPN reported overlapping PSM rates [1, 2]. The majority of available comparative studies showed overlapping results among the different approaches with exception of two large studies comparing OPN versus MIPN and LPN versus RAPN, respectively. Zargar et al. reported a significant lower risk of PSMs in patients who underwent RAPN compared with LPN [21] and Tabayoyogo et al. a significant lower risk of PSMs in patients who underwent OPN compared with MIRP. In the same study, LPN and RAPN turned out to be independent predictors of PSM at multivariable analysis also when data were adjusted for hospital volume [22]. Considering the wide diffusion of laparoscopic techniques in the last years, findings of the latter study should be confirmed in large, well-conducted, comparative studies.
Ischemia allows better visualization of the tumor limits facilitating complete tumor resection and minimizing the potential risk of PSMs. Conversely, prolonged warm ischemia time can impact negatively on the postoperative renal function. In 2006 Duvdevani et al. reported a PSMs rate of 0.6% in patients performing PN with warm ischemia and 4.2% in those receiving a clampless technique. Therefore, they concluded that no vascular occlusion could increase significantly the risk of PSMs during PN [42]. However, other studies failed to demonstrate significant differences in terms of PSMs rates in patients who underwent no ischemia or warm ischemia during PN [43, 44]. We selected 2 surgical series stratifying data according to the different methods used to manage the vascular pedicle during PN. Both studies failed to demonstrate significant difference between clamp or clampless approaches [18, 27].
Studies included in the previous non-systematic reviews clearly showed that a minimal tumor-free margin is sufficient to avoid PSMs after PN. Moreover, in 2011 Minervini et al. reported lower PSMs rate after SE compared with SPN technique in a retrospective, multicenter study comparing 537 SE cases with 982 SPN cases [45]. These data supported the diffusion of SE instead of SPN. Data collected in the present systematic review are conflicting. Longo et al. in a multicenter study enrolling both OPN and LPN showed a significantly lower PSMs rate in patients who underwent SE compared with those receiving SPN [14]. Conversely, Dong et al. observed similar PSMs rates after SE and SPN in a matched-pair analysis comparing 98 SE and 98 SPN cases [23]. Interestingly, a recent pathological study comparing the two techniques showed a significant higher PSMs rate after SE in comparison with SPN. We believe that further well-done studies should be performed before drawing the definitive conclusion that SE equals minimal PN in terms of oncologic safety.
Several tumor-related factors were investigated as potential predictors of PSMs after PN. In details, tumor size and tumor location as well as histologic subtype and nuclear grading were extensively evaluated.
Studies evaluating the impact of tumor size on PSMs rate showed conflicting and inconclusive results [1, 2]. While some studies demonstrated similar PSMs rates in case of tumors≤ or >4 cm [46, 47], Yossepowitch et al. observed that increasing tumor size was associated with lower incidence of PSMs at uni- and multivariable analyses [48]. More recently, Delto et al. analyzed the impact of tumor size in a large series of RAPN reporting similar PSMs rates regardless of tumor diameter [40]. We agree that an inaccurate estimation of tumor extension as well as absence or incomplete development of pseudocapsule and the accidental disintegration of the resection margins are more likely to occur in smaller tumors.
Until 2012, few studies evaluated the impact of tumor location on the risk of PSMs after PN. All studies were strongly limited by the lack of use of standardized and reproducible nephrometry systems introduced in 2009 [49, 50]. The arbitrary definition of tumor location strongly limited definitive conclusions in previous non-systematic reviews [1]. Moreover, available studies reached contrasting conclusions. Some studies showed comparable PSM rates for central and peripheral tumors [51], others a higher risk for central located tumors [52, 53]. In the last years, some studies stratified PSM rates according to the risk categories defined by nephrometry scores [8, 16, 34]. No study demonstrated a significant impact of nephrometry score on risk of PSMs.
Controversial data were reported concerning the potential correlation between histologic subtype and risk of PSMs. In a study published in 2007, Kwon et al. concluded that tumor histology was not predictive of surgical margin status. However, in this series the Authors observed a higher risk of local recurrence in patients with PSMs and aggressive histologic subtypes [54]. Other Authors observed a higher frequency of high-grade tumors in patients with PSMs compared with those with NSMs [52]. Recent studies confirmed the important role of high nuclear grading to predict the risk of PSMs and local recurrence [29, 39]. Two studies compared cystic versus solid lesions showing overlapping results in terms of PSM rates [13, 26].
Frozen-section analysis was adopted for several years with the intent to reduce the risk of PSMs during PN. However, the accuracy of frozen sections was always questionable considering the consistent percentage of false-negative or inconclusive results that did not correlate with the final pathological examination [55]. Conversely, Timsit et al. in 2006 demonstrated a robust concordance between macroscopic surgical margins evaluation by the surgeons and final histologic examination [56]. No studies reconsidered the role of frozen-section analysis in the search period of this systematic review.
Residual tumor at the surgical margins due to inadequate tumor resection is a potential factor increasing the risk of local recurrence. Studies included in previous non-systematic reviews showed conflicting results about the role of PSMs as risk factor of local and/or distant disease recurrence. Briefly, in 2008 Yossepowitch et al. reported overlapping results in terms of local recurrence in patients with PSMs or NSMs after a median follow-up of 40 months [48]. Similar results were reported in a series of patients with a longer follow-up [57]. Conversely, other studies showed that local recurrence was significantly higher in patients with PSMs as well as the time of disease recurrence seems to be shorter than in patients with NSMs [52, 58]. Studies published after 2013 showed that patients with PSMs had a significantly increased risk of local recurrence. Specifically, two studies showed at multivariable analyses that PSMs status is a strong predictor of local recurrence especially together with high nuclear grading of the tumor [29, 39]. Conversely, Bansal et al. did not confirm the role of PSMs on disease progression at multivariable analysis [38].
No study in the previous reviews reported a negative impact of PSMs on cancer-specific or overall survival [1, 2], Recently, Petros et al. reported a significant impact of PSMs status on local recurrence-free survival and metastasis-free survival. Conversely, no effects on overall survival were reported [41].
Management of patients with PSMs is challenging. Radical nephrectomy, repeat PN, ablative therapies or observation must be considered and discussed with patients. Very few data are available about the management of PSMs. Therefore, no definitive conclusions or strong recommendations can be proposed. In agreement with previous non-systematic reviews of the literature, we believe that observation is the most appropriate option for the management of PSMs after PN. However, it is important to highlight that a standardized and shared follow-up scheme for these patients is still lacking.
CONCLUSIONS
Studies published in the last 5 years confirmed that PSMs after PN for renal tumors are a rare condition. Tumor size, pT3a stage, nuclear grade 3–4 and minimally invasive procedures have been considered as potential risk factors for PSMs. Data concerning the risk of PSMs after SE are still conflicting. Therefore, whenever possible the excision of a minimal rim of healthy parenchyma around the tumor should be preferred.
Although PSMs increase the risk of local and distant recurrence, their influence on cancer-specific and overall survival seems to be limited. Close surveillance should be strongly recommended as initial treatment of patients with PSMs after PN. Conversely, radical nephrectomy or repeat PN should be considered in patients with PSMs and high-grade and/or aggressive histologic subtypes.
REFERENCES
[1] | Marszalek M , Carini M , Chlosta P , et al., Positive surgical margins after nephron-sparing surgery. Eur Urol. (2012) ;61: :757–63. |
[2] | Borghesi M , Brunocilla E , Schiavina R , Martorana G .Positive surgical margins after nephron-sparing surgery for renal cell carcinoma: Incidence, clinical impact, and management. Clin Genitourin Cancer. (2013) ;11: :5–9. |
[3] | Steinestel J , Steffens S , Steinestel K and Schrader AJ Positive surgical margins in nephron-sparing surgery: Risk factors and therapeutic consequences. World J Surg Oncol. (2014) ;12: :252. |
[4] | Minervini A , Tuccio A , Masieri L , et al., Endoscopic robot-assisted simple enucleation (ERASE) for clinical T1 renal masses: Description of the technique and early postoperative results. Surg Endosc. (2015) ;29: :1241–9. |
[5] | Howick J , Mebius A .In search of justification for the unpredictability paradox. Trials. (2014) ;15: :480. |
[6] | Kaczmarek BF , Tanagho YS , Hillyer SP , et al., Off-clamp robot-assisted partial nephrectomy preserves renal function: A multi-institutional propensity score analysis. Eur Urol. (2013) ;64: :988–93. |
[7] | Porpiglia F , Bertolo R , Amparore D , Fiori C .Margins, ischaemia and complications rate after laparoscopic partial nephrectomy: Impact of learning curve and tumour anatomical characteristics. BJU Int. (2013) ;112: :1125–32. |
[8] | Roushias S , Vasdev N , Ganai B , et al., Can the R.e.N.a.L nephrometry score preoperatively predict postoperative clinical outcomes in patients undergoing open and laparoscopic partial nephrectomy? Curr Urol. (2013) ;7: :90–7. |
[9] | Williams SB , Kacker R , Alemozaffar M , et al., Robotic partial nephrectomy versus laparoscopic partial nephrectomy: A single laparoscopic trained surgeon’s experience in the development of a robotic partial nephrectomy program. World J Urol. (2013) ;31: :793–8. |
[10] | Khalifeh A , Kaouk JH , Bhayani S , et al., Positive surgical margins in robot-assisted partial nephrectomy: A multi-institutional analysis of oncologic outcomes (leave no tumor behind). J Urol. (2013) ;190: :1674–9. |
[11] | Zargar H , Bhayani S , Allaf ME , et al., Comparison of perioperative outcomes of robot-assisted partial nephrectomy and open partial nephrectomy in patients with a solitary kidney. J Endourol. (2014) ;28: :1224–30. |
[12] | Jang HJ , Song W , Suh YS , et al., Comparison of perioperative outcomes of robotic versus laparoscopic partial nephrectomy for complex renal tumors (RENAL nephrometry score of 7 or higher). Korean J Urol. (2014) ;55: :808–13. |
[13] | Akca O , Zargar H , Autorino R , et al., Robotic partial nephrectomy for cystic renal masses: A comparative analysis of a matched-paired cohort. Urology. (2014) ;84: :93–8. |
[14] | Longo N , Minervini A , Antonelli A , et al., Simple enucleation versus standard partial nephrectomy for clinical T1 renal masses: Perioperative outcomes based on a matched-pair comparison of 396 patients (RECORd project). Eur J Surg Oncol. (2014) ;40: :762–8. |
[15] | Carneiro A , Sivaraman A , Sanchez-Salas R , et al., Evolution from laparoscopic to robotic nephron sparing surgery: A high-volume laparoscopic center experience on achieving ‘trifecta’ outcomes. World J Urol. (2015) ;33: :2039–44. |
[16] | Curtiss KM , Ball MW , Gorin MA , et al., Perioperative outcomes of robotic partial nephrectomy for intrarenal tumors. J Endourol. (2015) ;29: :293–6. |
[17] | Kim JH , Park YH , Kim YJ , et al., Perioperative and long-term renal functional outcomes of robotic versus laparoscopic partial nephrectomy: A multicenter matched-pair comparison. World J Urol. (2015) ;33: :1579–84. |
[18] | Komninos C , Shin TY , Tuliao P , et al., Renal function is the same 6 months after robot-assisted partial nephrectomy regardless of clamp technique: Analysis of outcomes for off-clamp, selective arterial clamp and main artery clamp techniques, with a minimum follow-up of 1 year. BJU Int. (2015) ;115: :921–8. |
[19] | Lista G , Buffi NM , Lughezzani G , et al., Margin, ischemia, and complications system to report perioperative outcomes of robotic partial nephrectomy: A European Multicenter Observational Study (EMOS project). Urology. (2015) ;85: :589–95. |
[20] | Serni S , Vittori G , Frizzi J , et al., Simple enucleation for the treatment of highly complex renal tumors: Perioperative, functional and oncological results. Eur J Surg Oncol. (2015) ;41: :934–40. |
[21] | Zargar H , Allaf ME , Bhayani S , et al., Trifecta and optimal perioperative outcomes of robotic and laparoscopic partial nephrectomy in surgical treatment of small renal masses: A multi-institutional study. BJU Int. (2015) ;116: :407–14. |
[22] | Tabayoyong W , Abouassaly R , Kiechle JE , et al., Variation in Surgical Margin Status by Surgical Approach among Patients Undergoing Partial Nephrectomy for Small Renal Masses. J Urol. (2015) ;194: :1548–53. |
[23] | Dong W , Lin T , Li F , et al., Laparoscopic Partial Nephrectomy for T1 Renal Cell Carcinoma: Comparison of Two Resection Techniques in a Multi-institutional Propensity Score-Matching Analysis. Ann Surg Oncol. (2016) ;23: :1395–402. |
[24] | Ito H , Makiyama K , Kawahara T , et al., Impact of Accidental Tumor Incision During Laparoscopic Partial Nephrectomy on the Oncologic and Clinical Outcomes. Clin Genitourin Cancer. (2016) ;14: :e291–e297. |
[25] | Malkoc E , Ramirez D , Kara O , et al., Robotic and open partial nephrectomy for localized renal tumors larger than 7 cm: A single-center experience. World J Urol. (2017) ;35: :781–7. |
[26] | Novara G , La Falce S , Abaza R , et al., Robot-assisted partial nephrectomy in cystic tumours: Analysis of the Vattikuti Global Quality Initiative in Robotic Urologic Surgery (GQI-RUS) database. BJU Int. (2016) ;117: :642–7. |
[27] | Paulucci DJ , Rosen DC , Sfakianos JP , et al., Selective arterial clamping does not improve outcomes in robot-assisted partial nephrectomy: A propensity-score analysis of patients without impaired renal function. BJU Int. (2017) ;119: :430–5. |
[28] | Roberts J , Wong J , Haxhimolla H , Kua B .Laparoscopic nephron sparing surgery: An Australian experience. ANZ J Surg. (2016) ;86: :926–9. |
[29] | Shah PH , Moreira DM , Okhunov Z , et al., Positive Surgical Margins Increase Risk of Recurrence after Partial Nephrectomy for High Risk Renal Tumors. J Urol. (2016) ;196: :327–34. |
[30] | Wang L , Hughes I , Snarskis C , et al., Tumor enucleation specimens of small renal tumors more frequently have a positive surgical margin than partial nephrectomy specimens, but this is not associated with local tumor recurrence. Virchows Arch. (2017) ;470: :55–61. |
[31] | Bigot P , Bernhard JC , Flamand V , et al., Localized chromophobe carcinomas treated by nephron-sparing surgery have excellent oncologic outcomes. Urol Oncol. (2017) ;35: :35.e15–35.e19. |
[32] | Hennessey DB , Wei G , Moon D , et al., Strategies for success: A multi-institutional study on robot-assisted partial nephrectomy for complex renal lesions. BJU Int. (2018) ;121: (Suppl 3):40–7. |
[33] | Khene ZE , Peyronnet B , Bosquet E , et al., Does training of fellows affect peri-operative outcomes of robot-assisted partial nephrectomy? BJU Int. (2017) ;120: :591–9. |
[34] | Matos AC , Dall’Oglio MF , Colombo JR Jr , et al., Predicting outcomes in partial nephrectomy: Is the renal score useful? Int Braz J Urol. (2017) ;43: :422–31. |
[35] | Maurice MJ , Ramirez D , Kara Ö , et al., Optimum outcome achievement in partial nephrectomy for T1 renal masses: A contemporary analysis of open and robot-assisted cases. BJU Int. (2017) ;120: :537–43. |
[36] | Veeratterapillay R , Addla SK , Jelley C , et al., Early surgical outcomes and oncological results of robot-assisted partial nephrectomy: A multicentre study. BJU Int. (2017) ;120: :550–5. |
[37] | Xia L , Pulido JE , Chelluri RR , et al., Hospital volume and outcomes of robot-assisted partial nephrectomy. BJU Int. (2018) ;121: :900–7. |
[38] | Bansal RK , Tanguay S , Finelli A , et al., Positive surgical margins during partial nephrectomy for renal cell carcinoma: Results from Canadian Kidney Cancer information system (CKCis) collaborative. Can Urol Assoc J. (2017) ;11: :182–7. |
[39] | Marchiñena PG , Tirapegui S , Gonzalez IT , et al., Positive surgical margins are predictors of local recurrence in conservative kidney surgery for pT1 tumors. Int Braz J Urol. (2018) ;44: :475–82. |
[40] | Delto JC , Paulucci D , Helbig MW , et al., Robot-assisted partial nephrectomy for large renal masses: A multi-institutional series. BJU Int. (2018) ;121: :908–15. |
[41] | Petros FG , Metcalfe MJ , Yu KJ , et al., Oncologic outcomes of patients with positive surgical margin after partial nephrectomy: A 25-year single institution experience. World J Urol. (2018) ;36: :1093–101. |
[42] | Duvdevani M , Mor Y , Kastin A , et al., Renal artery occlusion during nephron-sparing surgery: Retrospective review of 301 cases. Urology. (2006) ;68: :960–3. |
[43] | Thompson RH , Lane BR , Lohse CM , et al., Comparison of warm ischemia versus no ischemia during partial nephrectomy on a solitary kidney. Eur Urol. (2010) ;58: :331–6. |
[44] | Smith GL , Kenney PA , Lee Y , Libertino JA .Non-clamped partial nephrectomy: Techniques and surgical outcomes. BJU Int. (2011) ;107: :1054–8. |
[45] | Minervini A , Ficarra V , Rocco F , et al., Simple enucleation is equivalent to traditional partial nephrectomy for renal cell carcinoma: Results of a nonrandomized, retrospective, comparative study. J Urol. (2011) ;185: :1604–10. |
[46] | Patard JJ , Shvarts O , Lam JS , et al., Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. (2004) ;171: :2181–5. |
[47] | Porpiglia F , Fiori C , Bertolo R , Scarpa RM .Does tumour size really affect the safety of laparoscopic partial nephrectomy? BJU Int. (2011) ;108: :268–73. |
[48] | Yossepowitch O , Thompson RH , Leibovich BC , et al., Positive surgical margins at partial nephrectomy: Predictors and oncological outcomes. J Urol. (2008) ;179: :2158–63. |
[49] | Kutikov A , Uzzo RG .The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. (2009) ;182: :844–53. |
[50] | Ficarra V , Novara G , Secco S , et al., Preoperative aspects and dimensions used for an anatomical (PADUA) classification} of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. (2009) ;56: :786–93. |
[51] | Frank I , Colombo JR Jr , Rubinstein M , et al., Laparoscopic partial nephrectomy for centrally located renal tumors. J Urol. (2006) ;175: :849–52. |
[52] | Bensalah K , Pantuck AJ , Rioux-Leclercq N , et al., Positive surgical margin appears to have negligible impact on survival of renalcell carcinomas treated by nephron-sparing surgery. Eur Urol. (2010) ;57: :466–71. |
[53] | Venkatesh R , Weld K , Ames CD , et al., Laparoscopic partial nephrectomy for renal masses: Effect of tumor location. Urology. (2006) ;67: :1169–74. |
[54] | Kwon EO , Carver BS , Snyder ME , Russo P .Impact of positive surgical margins in patients undergoing partial nephrectomy for renal cortical tumours. BJU Int. (2007) ;99: :286–9. |
[55] | Breda A , Stepanian SV , Liao J , et al., Positive margins in laparoscopic partial nephrectomy in 855 cases: A multi-institutional survey from the United States and Europe. J Urol. (2007) ;178: :47–50. |
[56] | Timsit MO , Bazin JP , Thiounn N , et al., Prospective study of safety margins in partial nephrectomy: Intraoperative assessment and contribution of frozen section analysis. Urology. (2006) ;67: :923–6. |
[57] | López-Costea MÁ , Bonet X , Pérez-Reggeti J , et al., Oncological outcomes and prognostic factors after nephron-sparing surgery in renal cell carcinoma. Int Urol Nephrol. (2016) ;48: :681–6. |
[58] | Bernhard JC , Pantuck AJ , Wallerand H , et al., Predictive factors for ipsilateral recurrence after nephron-sparing surgery in renal cell carcinoma. Eur Urol. (2010) ;57: :1080–6. |




