Nutritional Predictors of Perioperative Complications and Mortality Following Nephrectomy for Renal Malignancies: A Population-Based Analysis
Abstract
Introduction and Objective:
Conflicting data exists regarding the impact of body mass index (BMI) on postoperative outcomes following surgery for renal malignancies (RM). Herein, we investigated associations between obesity, hypoalbuminemia, and/or significant weight loss in the preoperative period, and risk complications and mortality within 30 days of radical (RN) or partial nephrectomy (PN).
Materials and Methods:
Review of the American College of Surgeons National Surgical Quality Improvement Program database identified 8,618 patients treated with PN or RN for RM between 2005 and 2012. Univariate and multivariable logistic regression models were developed to assess associations between hypoalbuminemia (<3.5 g/dl), >10% weight loss within 6 months of surgery, obesity (BMI >30 kg/m2), and 30-day major complications and mortality.
Results:
Median BMI was 29.2 kg/m2 with 24.9%, 11.9%, and 8.2% having class I, II, and III obesity, respectively. Weight loss of >10% was observed in 2.6% and 15.4% had preoperative albumin<3.5 g/dl. There were 1,802 complications (20.9%) and 88 deaths within 30 days (1.0%). On multivariable analysis, BMI ≥40 kg/m2 (OR 1.3, p = 0.04), >10% weight loss (OR 1.9, p < 0.001) and hypoalbuminemia (OR 1.5, p < 0.001) were independently associated with 30-day complications, while only >10% weight loss was independently associated with 30-day mortality (OR 2.4, p = 0.03).
Conclusions:
Extreme obesity, hypoalbuminemia, and significant weight loss were independently associated with risk of significant complications following PN or RN while only significant preoperative weight loss was associated with early mortality, underscoring the need to further understand the utility of moderating these risk factors in the perioperative period.
INTRODUCTION
In 2017, over 40,000 men and 23,000 women in the United States will receive a diagnosis of cancer involving the kidney and renal pelvis [1]. Surgical resection is the cornerstone of treatment for localized renal malignancy (RM) [2]. Nephron-sparing surgery or partial nephrectomy (PN) is the standard of care in small, localized RM, while larger, central, or endophytic tumors often warrant radical nephrectomy (RN). Extirpation of RM via either PN or RN can be approached using either an open or minimally invasive approach [2].
Preoperative nutritional status is increasingly acknowledged to be of critical importance with respect to postoperative outcomes and perioperative risk stratification. Indeed, malnutrition has been associated with an increased risk of postoperative complications and mortality in the pediatric, cardiac, orthopedic, and general surgery literature [3–8]. Malnutrition may be characterized using variable definitions, including unintentional weight loss, low body mass index (BMI) according to the World Health Organization criteria (BMI;<18.5 kg/m2) as well as hypoalbuminemia (<3.5 g/dl) [9]. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) define malnutrition on the basis of 6 characteristics: insufficient energy intake, weight loss, loss of muscle mass, loss of subcutaneous fat, localized or generalized fluid accumulation, and diminished functional status, of which 2 must be present to diagnose malnutrition [10]. Regarding serum albumin, A.S.P.E.N. demonstrated that this acute phase protein does not consistently change with weight loss or caloric restriction and may not be a reliable indicator of malnutrition [10–13]. Therefore, we present albumin as a possible limitation in our discussion.
In the urologic literature, poor preoperative nutritional status appears to be strongly associated with oncologic outcomes following surgery, including cancer-specific and overall survival [9, 14–18]. Additionally, among patients with metastatic RM undergoing cytoreductive nephrectomy, malnutrition as defined by hypoalbuminemia has been associated with perioperative mortality [15]. However, beyond this report, the association between preoperative nutritional status and early postoperative morbidity and mortality following PN and RN across stages of RM remains inadequately characterized. Furthermore, there is conflicting data regarding associations between obesity, as defined by BMI >30 kg/m2, and perioperative outcomes after PN and RN, as some authors have reported increased perioperative morbidity among patients with obesity [16, 17], while other reports have failed to find any such association [16, 19, 20].
Understanding potentially modifiable risk factors for perioperative complications is of the utmost importance. Therefore, the objective of this study was to test the hypothesis that malnutrition, as defined by BMI-based criteria for underweight (BMI<18.5 kg/m2), hypoalbuminemia, and/or significant weight loss in the preoperative period is associated with increased risk of complications and mortality within 30 days following surgery, using a population-based cohort of patients with RM treated with either PN or RN. Additionally, a secondary objective was to assess how these perioperative outcomes were associated with increasing severity of obesity, according to BMI.
MATERIALS AND METHODS
We performed a retrospective review of the American College of Surgeons National Quality Improvement Program (ACS-NSQIP) database from 2005–2012. This is a nationally validated, prospectively maintained dataset that includes 135 patient-level clinical and surgical variables and tracks 30-day complications and mortality for major surgical procedures.
PN and RN cases performed for RM were extracted from the database using ICD-9 codes for RM and Current Procedure Terminology codes for RN (50545, 50230, 50220, 50225, 50234, 50236, 50543, 50546, 50548, 50549) and PN (50240). To minimize the risk of confounding related to coincident procedures that may have increased the complexity of the surgery, and therefore the potential risk for complications, cases were excluded for concurrent procedures that were likely to be unrelated to a primary diagnosis of RM.
Preoperative nutritional factors extracted included preoperative albumin (<3.5 g/dl vs. >3.5 g/dl), documented weight loss >10% within 6 months prior to surgery and preoperative BMI (categorized as underweight, normal weight, overweight, class I, II, and III obesity as ≤18.5, 18.5–24.9, 25–29.9, 30–34.9, 35–39.9, and ≥40 kg/m2 respectively).
29.1Statistical analysis
Clinical, tumor, demographic, and treatment-related factors were compared between patients across the three variables related to nutritional status, including age, sex, race/ethnicity, medical comorbidities (including pulmonary comorbidity [dyspnea or chronic obstructive pulmonary disease], heart disease [congestive heart failure, history of myocardial infarction (MI), prior percutaneous catheterization, previous cardiac surgery, history of angina], baseline paralysis, steroid use, requirement for dialysis, history of stroke, peripheral vascular disease requiring revascularization or amputation, presence of ascites, American Society of Anesthesia (ASA) classification, history of substance abuse, functional status, exposure to preoperative chemotherapy or radiotherapy, and whether or not the patient had disseminated cancer at the time of surgery. Surgical factors included PN vs. RN, laparoscopic vs. open approach, and whether or not the patient underwent a concurrent lymphadenectomy.
Continuous features were described with means (standard deviation [SD]) and categorical data was described with numbers (percentages). A subset of the medical comorbidities included in the analysis (history of MI, prior percutaneous catheterization, previous cardiac surgery, history of angina, paralysis, history of stroke, peripheral vascular disease, alcohol use, and chemotherapy or radiotherapy) were phased out from collection by NSQIP in 2011 and as therefore only available for a subset of our cohort. Missing values were handled as follows: for descriptive continuous statistics, only the patients for whom the variable was available were included in that variable’s distribution. In the univariate and multivariable modeling, for features with a significant number of patients with unknown values, missing data was included as a separate “unknown” category, or if only missing for a small number, was collapsed with the normal category and or excluded for continuous data, as specified in the multivariable tables. In total, 29 patients were excluded from the final multivariable modeling due to missing data.
The outcomes of interest included major and minor complications and mortality within 30 days of surgery. Complications included cardiac arrest, MI, pneumonia, ventilator >48 hours, reintubation, deep surgical site infection (SSI), organ space SSI, superficial SSI, sepsis or septic shock, deep vein thrombosis, pulmonary embolism, renal insufficiency or failure, return to the operating room, intraoperative or postoperative transfusion with 72 hours, wound disruption, cerebrovascular accident/stroke, coma >24 hours, peripheral nerve injury, 30-day mortality, and urinary tract infection. The proportion of patients who required transfusion were assessed but not included in the final models. Univariate and multivariable logistic regression were performed to evaluate associations between clinical, demographic, and surgical factors and the outcomes of interest. The final multivariable models were developed using forward selection of variables independently associated with the outcomes of interest, at the 5% level of significance. Due to the low prevalence of 30-day mortality, variables for the model of 30-day mortality were further selected based on clinical significance to avoid over-fitting. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary NC).
RESULTS
A total of 8,618 patients who underwent PN or RN for RM between 2005–2012 were identified, with a median age of 62 (interquartile range [IQR]: 53–71). The study cohort included 5,254 (61.1%) males and 6,384 (74.1%) non-Hispanic white patients. An open surgical approach was utilized in 3,689 patients (42.8%), and 208 patients (2.4%) underwent concurrent lymphadenectomy. Cytoreductive surgery was performed in 374 patients (4.3%, coded as “disseminated cancer” in the NSQIP dataset). PN was undertaken in 1,435 (16.7%) patients. In total, 5,354 patients (62.2%) met criteria for ASA Class III-V, and 174 (2.0%) of patients were either partially or totally dependent with respect to functional status. Preoperative demographic, clinical, and comorbidity data is summarized in Table 1.
Table 1
Demographic, clinical, and treatment-related factors
| Feature (N available) | Number (%) or Median (Interquartile Range, IQR) |
| Age (N = 8618) | 62 (53,71) |
| Sex (N = 8604) | |
| Male | 5254 (61.1) |
| Female | 3350 (38.9) |
| Race/Ethnicity (N = 8618) | |
| Non-Hispanic White | 6384 (74.1) |
| Hispanic White | 411 (4.8) |
| Black or African American | 714 (8.3) |
| Asian, Native Hawaiian, or Pacific Islander | 172 (2.0) |
| American Indian or Alaska Native | 28 (0.3) |
| Other or Unknown | 909 (10.5) |
| Year of Operation (N = 8618) | |
| 2005 | 8 (0.1) |
| 2006 | 48 (0.6) |
| 2007 | 173 (2.0) |
| 2008 | 474 (5.5) |
| 2009 | 792 (9.2) |
| 2010 | 1100 (12.8) |
| 2011 | 2661 (30.9) |
| 2012 | 3362 (39.0) |
| Surgical Approach (N = 8618) | |
| Laparoscopic | 4929 (57.2) |
| Open | 3689 (42.8) |
| Concurrent Lymphadenectomy (N = 8618) | |
| No | 8410 (97.6) |
| Yes | 208 (2.4) |
| Partial Nephrectomy (N = 8618) | 1435 (16.7) |
| ASA Class (N = 8610) | |
| I/II | 3256 (37.8) |
| III/IV/V | 5354 (62.2) |
| Concurrent procedures (N = 8618) | |
| Thoracic procedure | 13 (0.2) |
| Chest tube | 7 (0.1) |
| Vascular repair/reconstruction | 125 (1.5) |
| Removal of thrombus | 42 (0.5) |
| Splenectomy | 44 (0.5) |
| Resection/repair of diaphragm | 17 (0.2) |
| Bowel resection | 107 (1.2) |
| Lysis of adhesions | 92 (1.1) |
| Bowel repair | 4 (0.0) |
| Liver biopsy/resection | 62 (0.7) |
| Cholecystectomy | 134 (1.6) |
| Pancreatic resection | 25 (0.3) |
| Hernia repair | 93 (1.1) |
| Adrenalectomy | 104 (1.2) |
| Current smoker within 1 year (N = 8618) | 1732 (20.1) |
| Diabetes mellitus (N = 8618) | |
| Insulin | 526 (6.1) |
| Non-insulin (oral) | 1141 (13.2) |
| Hypertension (N = 8618) | 5617 (65.2) |
| Preoperative hematocrit (N = 8312) | 40.2 (36.7, 43.3) |
| Preoperative creatinine (N = 8329) | 1.0 (0.8, 1.21) |
| Preoperative GFR (N = 8227) | 71.9 (54.8, 86.9) |
| Preoperative GFR Category (N = 8227) | |
| Normal (≥90 ml/min/1.732) | 1735 (21.1) |
| CKD 2 (eGFR 60–89 ml/min/1.732) | 3883 (47.2) |
| CKD 3 (eGFR 30–59 ml/min/1.732) | 2081 (25.3) |
| CKD 4 (eGFR 15–29 ml/min/1.732) | 200 (2.4) |
| CKD 5 (eGFR<15 ml/min/1.732) | 328 (4.0) |
| Preoperative chemotherapy/systemic therapy (N = 4706) | 69 (1.5) |
| Preoperative radiotherapy (N = 4668) | 23 (0.5) |
| Paralysis (hemiplegia, paraplegia, quadruplegia (N = 4706) | 49 (1.0) |
| Disseminated Cancer/Metastatic disease (N = 8618) | 374 (4.3) |
| Steroid use for chronic condition (N = 8618) | 379 (4.4) |
| Stroke (CVA, stroke with/without neurological deficits (N = 4705) | 181 (3.8) |
| History of revascularization/amputation for peripheral vascular disease (N = 4706) | 63 (1.3) |
| Alcohol consumption >2 drinks/day in the 2 weeks prior to admission (N = 4725) | 162 (3.4) |
| Dyspnea (N = 8618) | |
| At rest | 43 (0.5) |
| With moderate exertion | 818 (9.5) |
| No | 7757 (90.0) |
| Functional status (N = 8590) | |
| Independent | 8416 (98.0) |
| Partially Dependent | 154 (1.8) |
| Totally Dependent | 20 (0.2) |
| Pulmonary comorbidity (dyspnea or chronic obstructive pulmonary disease) (N = 8618) | 1132 (13.1) |
| Heart disease (congestive heart failure, myocardial infarction, previous percutaneous coronary intervention, cardiac surgery, angina) (N = 4732) | 588 (12.4) |
| Angina in the 1 month prior to surgery (N = 4706) | 36 (0.8) |
The prevalence of the nutritional features of interest (BMI, preoperative albumin level and preoperative weight loss) are summarized in Table 2. The median BMI of the study cohort was 29.2 kg/m2, with 2,126 (24.9%), 1,018 (11.9%), and 704 (8.2%) with class I, II, and III obesity respectively. Thus, the overall prevalence of obesity in the study cohort was 45.0% (n = 3,848) while 2,936 (34.0%) of the population were overweight, and 95 (1.1%) were underweight. Significant (>10%) weight loss in the 6 months preceding surgery was observed in 223 (2.6%) patients. Serum albumin was available in 5,163 patients (59.9%). Median preoperative albumin was 4 g/dL (IQR: 3.7, 4.3; range 1–7.1). Of patients with available serum albumin, 796 (15.4%) had albumin<3.5 g/dL. Figure 1 demonstrates the distribution of the two nutritional features of interest according to BMI category. Patients who were underweight according to BMI category (BMI<18.5 kg/m2) had the highest proportion of hypoalbuminemia and significant weight loss as a percentage of their total body weight in the 6 months preoperatively (p < 0.001 for both).
Fig.1
Preoperative Serum Albumin and Weight loss >10% within the 6 months prior to surgery according to body mass index group.
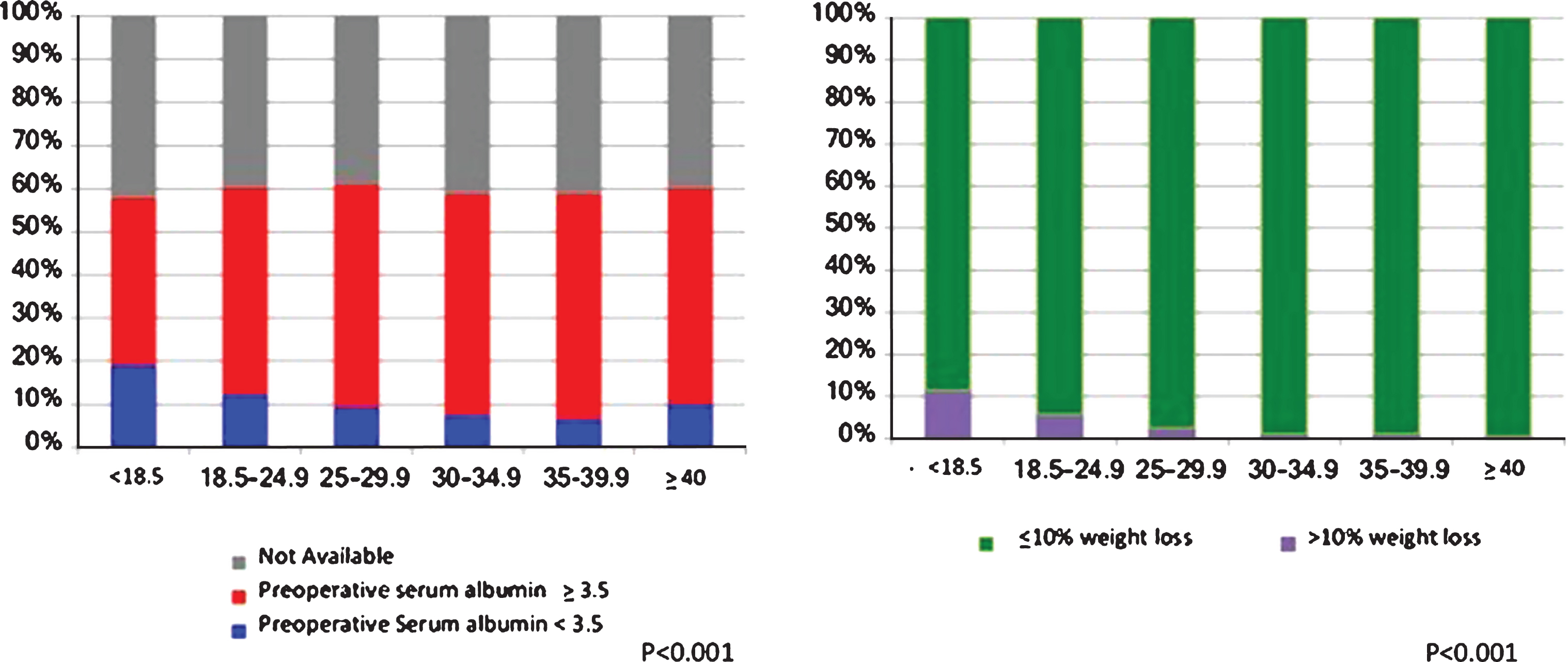
Table 2
Distribution of Nutritional Factors and Obesity across the cohort
| Nutritional Characteristic | Median (IQR; range) or N (%) |
| Body Mass Index (kg/m2; N = 8552) | 29.2 (25.7, 33.7; 10.9 – 86.9) |
| BMI Category (WHO Classification) (N = 8552) | |
| <18.5 | 95 (1.1) |
| 18.5–24.9 | 1673 (19.6) |
| 25.0–29.9 | 2936 (34.3) |
| 30.0–34.9 | 2126 (24.9) |
| 35.0–39.9 | 1018 (11.9) |
| ≥40 | 704 (8.2) |
| >10% loss of body weight in the last 6 months (N = 8617) | 223 (2.6) |
| Preoperative serum albumin (g/dL; N = 5163) | 4.0 (3.7, 4.3; 1.0–7.1) |
| Preoperative albumin category | |
| <3.5 g/dL | 796 (15.4) |
| ≥3.5 g/dL | 4367 (84.6) |
Complications occurred in 1,802 of 8,618 patients (20.9%) within 30 days postoperatively and there were 88 deaths (1.0%). Mean post-operative length of stay was 4.4 days (SD 4.0, range 0–82). Complications necessitating reoperation were observed in 253 patients (2.9%) within an average of 9.9 days (SD 8.3, range 0–30). Readmission within 30 days was available for 5,758 patients (2011–2012 only) and was observed in 370 patients treated in that time period (6.4%). Respiratory complications (pneumonia, use of ventilator >48 hours, and reintubation) occurred in 237 patients (2.8%). The prevalence of specific complications by type are detailed in Table 3. Blood transfusions were administered in 1,170 (13.6%). In total, excluding bleeding or transfusion-related complications, 626 (7.3%) patients experienced a single complication, 153 (1.8%) of patients experienced two complications, and 163 (1.9%) experienced three or more complications after PN or RN.
Table 3
Operative details and 30-day Complications and Mortality (N = 8618)
| Operative Detail | Mean (SD) or N(%) |
| Total Operative Time (minutes) | 184.0 (85.1) |
| Transfusion | 1170 (13.6) |
| Return to the Operative Room (N = 8617) | 253 (2.9) |
| Days from Principal Operative Procedure to Unplanned Reoperation | 9.9 (8.3; range 0–30) |
| Post-operative Length of Stay | 4.4 (4.01; range 0–82) |
| Readmissions (Available 2011 – 2012 only; N = 5758) | 370 (6.4) |
| Complications | N(%) |
| Cardiac Arrest requiring CPR | 46 (0.5) |
| Myocardial Infarction | 58 (0.7) |
| Pneumonia | 121 (1.4) |
| Requirement for ventilation >48 hours | 91 (1.1) |
| Unplanned intubation | 124 (1.4) |
| Deep Incision Surgical Site Infection | 25 (0.3) |
| Organ Space Surgical Site Infection | 46 (0.5) |
| Sepsis or Septic Shock | 142 (1.6) |
| Deep Vein Thrombosis/Thrombophlebitis/DVT Requiring Therapy | 67 (0.8) |
| Pulmonary Embolism | 42 (0.5) |
| DVT or PE | 100 (1.2) |
| Progressive Renal Insufficiency | 86 (1.0) |
| Superficial Surgical Site Infection | 105 (1.2) |
| Wound disruption | 35 (0.4) |
| Urinary Tract Infection | 146 (1.7) |
| Stroke/CVA with neurological deficit | 28 (0.3) |
| Coma >24 hours | 1 (0.0) |
| Peripheral nerve injury | 3 (0.0) |
| Death within 30 days | 88 (1.0) |
| Multiple complications (excluding bleeding/transfusion) | |
| 0 | 7676 (89.1) |
| 1 | 626 (7.3) |
| 2 | 153 (1.8) |
| 3 | 76 (0.9) |
| 4 | 37 (0.4) |
| 5 | 23 (0.3) |
| 6 | 19 (0.2) |
| 7 | 7 (0.1) |
| 8 | 1 (0.0) |
Univariate and multivariable logistic regression models detailing associations between the nutritional and clinical factors of interest with the outcomes of 30-day complications (excluding transfusions) and 30-day mortality are presented in Table 4a and 4b, respectively. On multivariable analysis, class III obesity compared to normal weight (Odds Ratio [OR] 1.28, 95% Confidence Interval [CI] 1.01–1.62; p = 0.04), >10% weight loss within 6 months (OR 1.87, 95% CI 1.27–2.55; p < 0.0001), and serum albumin<3.5 g/dL (OR 1.47, 95% CI 1.22–1.78; p < 0.0001) were independently associated with increased risk of moderate-to-severe complications within 30 days of surgery (Table 4A). The final multivariable model was adjusted for laparoscopic vs. open approach, PN vs. RN, age, concurrent lymphadenectomy, ASA class, year of operation, preoperative hematocrit and eGFR, and preoperative comorbidities (DM, metastatic disease, functional status, pulmonary comorbidity, dialysis treatment, and presence of ascites). The c-index for the model was 0.75.
Regarding mortality within 30 days of surgery, only >10% weight loss in the 6 months prior to surgery (OR 2.39, 95% CI 1.11–5.14; p = 0.03) was statistically significantly associated with the outcome of interest, while preoperative serum albumin<3.5 g/dL (OR 1.72, 95% CI 0.98–3.00; p = 0.06) did not demonstrate a complete association with 30-day mortality. Conversely, there were no significant associations between obesity or underweight and 30-day mortality, after adjusting for surgical approach, age, ASA class, eGFR, metastatic disease burden, and smoking (Table 4B). The c-index for this model was 0.83.
Table 4A
Univariate and Multivariable Logistic Regression Models for Postoperative Complications within 30 days of surgery
| Univariate Models | Multivariable Model | |||
| Feature | Odds Ratio (95% Confidence Interval) | p-value | Odds Ratio (95% Confidence Interval) | p-value |
| BMI Category (ref = normal) | ||||
| Underweight (<18.5) | 0.94 (0.57–1.55) | 0.82 | 0.64 (0.37–1.12) | 0.12 |
| Overweight (25.0 – 29.9) | 0.83 (0.72–0.96) | 0.01 | 0.92 (0.78–1.08) | 0.29 |
| Obese Class I (30.0 – 34.9) | 0.81 (0.70–0.95) | 0.009 | 0.92 (0.77–1.10) | 0.35 |
| Obese Class II (35.0 – 39.9) | 0.79 (0.65–0.96) | 0.02 | 0.88 (0.71–1.09) | 0.25 |
| Obese Class III (>40.0) | 1.09 (0.89–1.34) | 0.41 | 1.28 (1.01–1.62) | 0.04 |
| Unknown | 1.25 (0.72–2.17) | 0.44 | 0.85 (0.44–1.64) | 0.62 |
| >10% loss of body weight within 6 months (ref = no) | 3.32 (2.54–4.34) | <0.001 | 1.87 (1.37–2.55) | <0.001 |
| Preoperative Serum Albumin (ref>3.5) | ||||
| <3.5 | 3.21 (2.73–3.76) | <0.001 | 1.47 (1.22–1.78) | <0.001 |
| Unknown | 1.00 (0.89–1.12) | 0.98 | 0.99 (0.87–1.13) | 0.90 |
| Preoperative Serum Albumin (continuous) | 0.38 (0.35–0.44) | <0.001 | ||
| Partial Nephrectomy (ref = Radical Nephrectomy) | 1.09 (0.95–1.25) | 0.23 | 0.72 (0.61–0.84) | <0.001 |
| Laparoscopic Nephrectomy (ref = Lap) | 3.14 (2.82–3.50) | <0.001 | 3.38 (2.97–3.85) | <0.001 |
| Lymphadenectomy (ref = no) | 2.53 (1.91–3.36) | <0.001 | 1.78 (1.30–2.44) | <0.001 |
| Age (per 5-year increase) | 1.12 (1.09–1.14) | <0.001 | ||
| Sex (ref = female) | ||||
| Male | 1.07 (0.96–1.19) | 0.20 | ||
| Unknown | 2.20 (0.74–6.59) | 0.16 | ||
| Race (ref = Non-Hispanic White) | ||||
| American Indian/Alaska Native | 1.50 (0.66–3.40) | 0.34 | ||
| Asian/Native Hawaiian/Pacific Islander | 0.95 (0.66–1.39) | 0.81 | ||
| Black or African American | 1.09 (0.90–1.31) | 0.37 | ||
| Hispanic White | 0.79 (0.61–1.03) | 0.08 | ||
| Other/Unknown | 0.91 (0.76–1.08) | 0.29 | ||
| Year of Operation | 0.91 (0.87–0.94) | <0.001 | 0.96 (0.93–1.00) | 0.08 |
| ASA Class 3–5 (ref = 1-2 or unknown) | 2.27 (2.02–2.56) | <0.001 | 1.50 (1.31–1.72) | <0.001 |
| Current smoker within 1 year of surgery (ref = no) | 0.85 (0.74–0.97) | 0.01 | ||
| Diabetes Mellitus (ref = no) | ||||
| Insulin-dependent | 2.16 (1.78–2.61) | <0.001 | 1.54 (1.24–1.92) | <0.001 |
| Treated with oral agents | 1.29 (1.11–1.50) | <0.001 | 1.07 (0.91–1.26) | 0.43 |
| Hypertension requiring medication (ref = no) | 1.48 (1.32–1.66) | <0.001 | ||
| Preoperative Hematocrit (ref = normal or unknown: male 42+, female 38+) | ||||
| Very low (male<32, female<28) | 9.48 (7.67–11.72) | <0.001 | 4.65 (3.64–5.95) | <0.001 |
| Low (male 32–41.9, female 28–37.9) | 2.09 (1.87–2.34) | <0.001 | 1.51 (1.33–1.71) | <0.001 |
| Preoperative eGFR (ref = eGFR>90 ml/min/1.73 m2) | ||||
| CKD 2 (60–89) | 0.97 (0.83–1.13) | 0.66 | 0.93 (0.79–1.10) | 0.42 |
| CKD 3 (30–59) | 2.09 (1.79–2.45) | <0.001 | 1.46 (1.22–1.75) | <0.001 |
| CKD 4 (15–29) | 4.88 (3.60–6.62) | <0.001 | 2.70 (1.88–3.88) | <0.001 |
| CKD 5 (<15) | 2.12 (1.62–2.78) | <0.001 | 1.82 (1.10–3.01) | 0.02 |
| Unknown | 1.07 (0.80–1.43) | 0.66 | 1.14 (0.83–1.57) | 0.42 |
| Disseminated Cancer (ref = no) | 2.49 (2.01–3.08) | <0.001 | 1.54 (1.20–1.97) | <0.001 |
| Functional Status (ref = independent) | ||||
| Partially dependent | 2.63 (1.90–3.64) | <0.001 | 1.53 (1.06–2.21) | 0.02 |
| Totally dependent | 9.10 (3.49–23.72) | <0.001 | 3.74 (1.28–10.90) | 0.02 |
| Dyspnea (ref = no) | ||||
| At rest | 4.62 (2.53–8.43) | <0.001 | ||
| With moderate exertion | 1.50 (1.36–1.88) | <0.001 | ||
| History of severe COPD (ref = no) | 1.62 (1.32–1.99) | <0.001 | ||
| Pulmonary comorbidity dyspnea or COPD) (ref = no) | 1.66 (1.44–1.91) | <0.001 | 1.18 (1.00–1.38) | 0.048 |
| Heart disease (ref = no) | ||||
| Yes | 1.55 (1.28–1.88) | <0.001 | ||
| Unknown | 0.84 (0.75–0.94) | 0.002 | ||
| Paralysis (ref = no) | ||||
| Hemiplegia/paraplegia/quadriplegia | 2.19 (1.23–3.91) | 0.008 | ||
| Unknown | 0.81 (0.73–0.90) | <0.001 | ||
| Steroid use for a chronic condition (ref = no) | 1.44 (1.14–1.81) | 0.002 | ||
| Stroke (CVA/stroke with or without neurological deficit) (ref = no) | ||||
| Yes | 1.65 (1.20–2.28) | 0.002 | ||
| Unknown | 0.82 (0.74–0.91) | <0.001 | ||
| Currently on dialysis (ref = no) | 1.62 (1.28–2.03) | <0.001 | 0.65 (0.41–1.04) | 0.07 |
| History of revascularization/amputation for peripheral vascular disease (ref = no) | ||||
| Yes | 2.61 (1.58–4.32) | <0.001 | ||
| Unknown | 0.81 (0.73–0.90) | <0.001 | ||
| Ascites (ref = no) | 8.17 (3.32–20.06) | <0.001 | 2.83 (0.98–8.14) | 0.05 |
| History of angina within 1 month before surgery (ref = no) | ||||
| Yes | 1.32 (0.64–2.75) | 0.46 | ||
| Unknown | 0.80 (0.72–0.89) | <0.001 | ||
The multivariable model was developed with forward selection. The C-index for the multivariable model is 0.75.
Table 4B
Univariable and Multivariable Logistic Regressions for Death within 30 days
| Univariate Models | Multivariable Model | |||
| Feature | Odds Ratio (95% Confidence Interval) | p-value | Odds Ratio (95% Confidence Interval) | p– value |
| BMI Category (ref = normal) | ||||
| Underweight (<18.5) | 1.31 (0.31–5.60) | 0.71 | 1.11 (0.25–4.92) | 0.89 |
| Overweight (25.0 – 29.9) | 0.46 (0.26–0.81) | 0.007 | 0.56 (0.31–1.01) | 0.05 |
| Obese Class I (30.0 – 34.9) | 0.43 (0.23–0.82) | 0.01 | 0.64 (0.33–1.24) | 0.19 |
| Obese Class II (35.0 – 39.9) | 0.91 (0.48–1.72) | 0.78 | 1.55 (0.78–3.07) | 0.21 |
| Obese Class III (>40.0) | 0.52 (0.22–1.28) | 0.15 | 0.98 (0.38–2.50) | 0.96 |
| Unknown | 0.94 (0.13–7.01) | 0.95 | 0.44 (0.05–3.94) | 0.46 |
| >10% loss of body weight within 6 months (ref = no) | 4.43 (2.19–8.94) | <0.001 | 2.39 (1.11–5.14) | 0.03 |
| Preoperative Serum Albumin (ref>3.5) | ||||
| <3.5 | 3.39 (2.01–5.72) | <0.001 | 1.72 (0.98–3.00) | 0.06 |
| Unknown | 0.90 (0.55–1.47) | 0.67 | 0.94 (0.56–1.57) | 0.80 |
| Preoperative Serum Albumin (continuous) | 0.35 (0.25–0.49) | <0.001 | ||
| Partial Nephrectomy (ref = Radical Nephrectomy) | 0.71 (0.38–1.34) | 0.30 | ||
| Laparoscopic Nephrectomy (ref = Open) | 2.36 (1.53–3.65) | <0.001 | 2.30 (1.47–3.60) | <0.001 |
| Lymphadenectomy (ref = no) | 2.47 (0.99–6.16) | 0.05 | ||
| Age (per 5-year increase) | 1.34 (1.22–1.47) | <0.001 | 1.38 (1.23–1.55) | <0.001 |
| Male Sex (ref = female) | 1.53 (0.96–2.42) | 0.07 | ||
| Race (ref = Non-Hispanic White) | ||||
| Asian/Native Hawaiian/Pacific Islander | 1.11 (0.27–4.57) | 0.89 | ||
| Black or African American | 0.80 (0.35–1.85) | 0.60 | ||
| Hispanic White | 0.93 (0.34–2.55) | 0.88 | ||
| Other/Unknown | 0.94 (0.47–1.90) | 0.87 | ||
| Year of Operation | 0.88 (0.76–1.01 | 0.07 | ||
| ASA Class 3–5 (ref = 1-2 or unknown) | 7.15 (3.30–15.48) | <0.001 | 3.64 (1.64–8.07) | 0.002 |
| Current smoker within 1 year of surgery (ref = no) | 1.17 (0.71–1.93) | <0.001 | 1.76 (1.03–3.02) | 0.04 |
| Diabetes Mellitus (ref = no) | ||||
| Insulin-dependent | 1.93 (0.96–3.91) | 0.07 | ||
| Treated with oral agents | 1.68 (0.98–2.89) | 0.06 | ||
| Hypertension requiring medication (ref = no) | 1.71 (1.05–2.80) | 0.03 | ||
| Preoperative Hematocrit (continuous) | 0.90 (0.87–0.93) | <0.001 | ||
| Preoperative eGFR (ref = eGFR>90 ml/min/1.73 m2) | ||||
| CKD 2 (60–89) | 0.82 (0.42–1.62) | 0.57 | 0.69 (0.34–1.38) | 0.29 |
| CKD 3 (30–59) | 2.13 (1.12–4.07) | 0.02 | 1.12 (0.56–2.24) | 0.74 |
| CKD 4 (15–29) | 5.52 (2.26–13.48) | 0.002 | 2.31 (0.90–5.99) | 0.08 |
| CKD 5 (<15) | 3.31 (1.36–8.05) | 0.008 | 3.06 (1.22–7.70) | 0.02 |
| Unknown | 0.68 (0.15–3.03) | 0.61 | 0.67 (0.14–3.15) | 0.62 |
| Disseminated Cancer (ref = no) | 5.90 (3.48–10.02) | <0.001 | 5.33 (3.02–9.41) | <0.001 |
| Functional Status (ref = independent) | ||||
| Partially dependent | 6.81 (3.35–13.86) | <0.001 | ||
| Totally dependent | 12.20 (2.78–53.47) | <0.001 | ||
| Dyspnea (ref = no) | ||||
| At rest | 5.07 (1.20–21.33) | 0.03 | ||
| Moderate exertion | 1.55 (0.84–2.86) | 0.16 | ||
| Heart disease (ref = no) | ||||
| Yes | 4.48 (2.58–7.77) | <0.001 | ||
| Unknown | 1.04 (0.64–1.68) | 0.89 | ||
| Paralysis (ref = no) | ||||
| Hemiplegia/paraplegia/quadriplegia | 1.78 (0.24–13.1) | 0.57 | ||
| Unknown | 0.73 (0.47–1.12) | 0.15 | ||
| Steroid use for a chronic condition (ref = no) | 2.84 (1.46–5.52) | 0.002 | ||
| Stroke (CVA/stroke with or without neurological deficit) (ref = no) | ||||
| Yes | 2.02 (0.72–5.66) | 0.18 | ||
| Unknown | 0.78 (0.51–1.22) | 0.28 | ||
| Univariate Models | Multivariable Model | |||
| Currently on dialysis (ref = no) | 1.92 (0.88–4.19) | 0.10 | ||
| History of revascularization/amputation for peripheral vascular disease (ref = no) | ||||
| Yes | 7.92 (3.05–20.58) | <0.001 | ||
| Unknown | 0.78 (0.50–1.22) | 0.27 | ||
| Ascites (ref = no) | 9.90 (2.28–42.99) | 0.002 | ||
| History of angina within 1 month before surgery (ref = no) | ||||
| Yes | 5.12 (1.20–21.88) | 0.03 | ||
| Unknown | 0.74 (0.48–1.15) | 0.18 | ||
To better characterize patients with a BMI >40 kg/m2, >10% preoperative weight loss, and a preoperative albumin <3.5 g/dl, contingency tables were included to compare groups based on age, sex, race, smoking status, preoperative comorbidities, laparoscopic vs. open approach, ASA class, metastatic vs. non-metastatic disease, and PN vs. RN. Patients with a BMI >40 kg/m2 were more likely to be aged 50–59 (n = 226; p < 0.001), received a radical nephrectomy (n = 570; p < 0.001), and had a history of hypertension requiring medication (n = 549; p < 0.001; Table 5). Significant preoperative weight loss was associated with receipt of radical nephrectomy (n = 212; p < 0.001), an open surgical approach (n = 133; p < 0.001), and ASA class 3–5 (n = 157; p = 0.010; Table 6). Preoperative hypoalbuminemia was associated with age 60–69 (n = 247; p < 0.001), ASA class 3–5 (n = 656; p < 0.001), and history of hypertension requiring medication (n = 555; p = 0.002; Table 7).
Table 5
Relationship of body mass index by category and variables of interest
| BMI Category (WHO classification) (with unknown) | |||||||||
| Characteristic/ feature | All (n = 8618) | <18.5 (n = 95) | 18.5–4.9 (n = 1673) | 25.0–29.9 (n = 2936) | 30.0–34.9 (n = 2126) | 35.0–39.9 (n = 1018) | 40+ (n = 704) | Unknown (n = 66) | P-value |
| Age | <0.001 | ||||||||
| No. (%) used | 8618 (100.0%) | 95 (100.0%) | 1673 (100.0%) | 2936 (100.0%) | 2126 (100.0%) | 1018 (100.0%) | 704 (100.0%) | 66 (100.0%) | |
| Mean (SD) | 61.53 (12.88) | 60.71 (15.58) | 63.19 (14.65) | 62.78 (12.84) | 61.36 (11.67) | 58.78 (11.58) | 56.74 (11.43) | 63.76 (13.49) | |
| Median (IQR) | 62 (53,71) | 62 (51,73) | 64 (54,74) | 63 (55,72) | 62 (54,70) | 59.5 (52,67) | 57 (49,65) | 62.5 (52,75) | |
| Range | 17 to 90 | 19 to 90 | 17 to 90 | 18 to 90 | 22 to 90 | 19 to 88 | 22 to 89 | 36 to 90 | |
| Age Category, no. (row %) | <0.001 | ||||||||
| <40 | 457 | 7 (1.5) | 114 (24.9) | 131 (28.7) | 82 (17.9) | 63 (13.8) | 57 (12.5) | 3 (0.7) | |
| 40–49 | 1039 | 14 (1.3) | 172 (16.6) | 324 (31.2) | 255 (24.5) | 142 (13.7) | 126 (12.1) | 6 (0.6) | |
| 50–59 | 2136 | 20 (0.9) | 349 (16.3) | 673 (31.5) | 545 (25.5) | 304 (14.2) | 226 (10.6) | 19 (0.9) | |
| 60–69 | 2564 | 24 (0.9) | 418 (16.3) | 866 (33.8) | 708 (27.6) | 331 (12.9) | 205 (8.0) | 12 (0.5) | |
| 70–79 | 1764 | 21 (1.2) | 397 (22.5) | 661 (37.5) | 432 (24.5) | 157 (8.9) | 78 (4.4) | 18 (1.0) | |
| 80+ | 658 | 9 (1.4) | 223 (33.9) | 281 (42.7) | 104 (15.8) | 21 (3.2) | 12 (1.8) | 8 (1.2) | |
| Gender, no. (row %) | <0.001 | ||||||||
| Not available | 14 | 1 | 4 | 5 | 3 | 1 | |||
| Male | 5254 | 36 (0.7) | 904 (17.2) | 1994 (38.0) | 1390 (26.5) | 588 (11.2) | 303 (5.8) | 39 (0.7) | |
| Female | 3350 | 58 (1.7) | 765 (22.8) | 937 (28.0) | 733 (21.9) | 430 (12.8) | 400 (11.9) | 27 (0.8) | |
| Race/ethnicity (all years), no. (row %) | <0.001 | ||||||||
| Non-Hispanic White | 6384 | 66 (1.0) | 1225 (19.2) | 2134 (33.4) | 1615 (25.3) | 773 (12.1) | 528 (8.3) | 43 (0.7) | |
| Hispanic White | 411 | 3 (0.7) | 67 (16.3) | 165 (40.1) | 97 (23.6) | 39 (9.5) | 36 (8.8) | 4 (1.0) | |
| Black or African American | 714 | 13 (1.8) | 121 (16.9) | 225 (31.5) | 168 (23.5) | 114 (16.0) | 67 (9.4) | 6 (0.8) | |
| Asian, Native Hawaiian or Pacific Islander | 172 | 3 (1.7) | 65 (37.8) | 70 (40.7) | 23 (13.4) | 5 (2.9) | 5 (2.9) | 1 (0.6) | |
| American Indian or Alaska Native | 28 | 0 (0.0) | 6 (21.4) | 8 (28.6) | 10 (35.7) | 3 (10.7) | 1 (3.6) | 0 (0.0) | |
| Other or Unknown | 909 | 10 (1.1) | 189 (20.8) | 334 (36.7) | 213 (23.4) | 84 (9.2) | 67 (7.4) | 12 (1.3) | |
| Year of Operation, no. (row %) | 0.017 | ||||||||
| 2005 | 8 | 0 (0.0) | 1 (12.5) | 4 (50.0) | 2 (25.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | |
| 2006 | 48 | 1 (2.1) | 18 (37.5) | 15 (31.3) | 3 (6.3) | 7 (14.6) | 3 (6.3) | 1 (2.1) | |
| 2007 | 173 | 2 (1.2) | 40 (23.1) | 56 (32.4) | 34 (19.7) | 25 (14.5) | 15 (8.7) | 1 (0.6) | |
| 2008 | 474 | 8 (1.7) | 110 (23.2) | 156 (32.9) | 124 (26.2) | 39 (8.2) | 32 (6.8) | 5 (1.1) | |
| 2009 | 792 | 13 (1.6) | 147 (18.6) | 278 (35.1) | 183 (23.1) | 94 (11.9) | 66 (8.3) | 11 (1.4) | |
| 2010 | 1100 | 10 (0.9) | 216 (19.6) | 347 (31.5) | 298 (27.1) | 131 (11.9) | 93 (8.5) | 5 (0.5) | |
| 2011 | 2661 | 27 (1.0) | 530 (19.9) | 921 (34.6) | 652 (24.5) | 319 (12.0) | 188 (7.1) | 24 (0.9) | |
| 2012 | 3362 | 34 (1.0) | 611 (18.2) | 1159 (34.5) | 830 (24.7) | 403 (12.0) | 306 (9.1) | 19 (0.6) | |
| Lap vs Open (nephrectomy cohort only), no. (row %) | 0.49 | ||||||||
| Lap | 4929 | 46 (0.9) | 973 (19.7) | 1682 (34.1) | 1213 (24.6) | 565 (11.5) | 410 (8.3) | 40 (0.8) | |
| Open | 3689 | 49 (1.3) | 700 (19.0) | 1254 (34.0) | 913 (24.7) | 453 (12.3) | 294 (8.0) | 26 (0.7) | |
| Lymphadenectomy, no. (row %) | 0.035 | ||||||||
| No | 8410 | 92 (1.1) | 1639 (19.5) | 2859 (34.0) | 2059 (24.5) | 1002 (11.9) | 693 (8.2) | 66 (0.8) | |
| Yes | 208 | 3 (1.4) | 34 (16.3) | 77 (37.0) | 67 (32.2) | 16 (7.7) | 11 (5.3) | 0 (0.0) | |
| Partial nephrectomy, no. (row %) | <0.001 | ||||||||
| No | 7183 | 87 (1.2) | 1447 (20.1) | 2449 (34.1) | 1747 (24.3) | 820 (11.4) | 570 (7.9) | 63 (0.9) | |
| Yes | 1435 | 8 (0.6) | 226 (15.7) | 487 (33.9) | 379 (26.4) | 198 (13.8) | 134 (9.3) | 3 (0.2) | |
| ASA class, no. (row %) | <0.001 | ||||||||
| Not available | 8 | 1 | 5 | 1 | 1 | ||||
| 1-No Disturb | 156 | 2 (1.3) | 51 (32.7) | 77 (49.4) | 19 (12.2) | 5 (3.2) | 1 (0.6) | 1 (0.6) | |
| 2-Mild Disturb | 3100 | 22 (0.7) | 656 (21.2) | 1189 (38.4) | 803 (25.9) | 286 (9.2) | 125 (4.0) | 19 (0.6) | |
| 3-Severe Disturb | 4836 | 64 (1.3) | 855 (17.7) | 1503 (31.1) | 1195 (24.7) | 664 (13.7) | 519 (10.7) | 36 (0.7) | |
| 4-Life Threat | 517 | 7 (1.4) | 109 (21.1) | 162 (31.3) | 108 (20.9) | 62 (12.0) | 59 (11.4) | 10 (1.9) | |
| 5-Moribund | 1 | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| ASA class (1-2 vs 3-4), no. (row %) | <0.001 | ||||||||
| Not available | 8 | 1 | 5 | 1 | 1 | ||||
| 1 or 2-No or Mild Disturb | 3256 | 24 (0.7) | 707 (21.7) | 1266 (38.9) | 822 (25.2) | 291 (8.9) | 126 (3.9) | 20 (0.6) | |
| 3, 4, or 5-Severe Disturb, Life Threat, or Moribund | 5354 | 71 (1.3) | 965 (18.0) | 1665 (31.1) | 1303 (24.3) | 726 (13.6) | 578 (10.8) | 46 (0.9) | |
| BMI | <0.001 | ||||||||
| No. (%) used | 8552 (99.2%) | 95 (100.0%) | 1673 (100.0%) | 2936 (100.0%) | 2126 (100.0%) | 1018 (100.0%) | 704 (100.0%) | 0 (0.0%) | |
| Mean (SD) | 30.26 (6.87) | 16.98 (1.55) | 22.75 (1.61) | 27.47 (1.41) | 32.21 (1.43) | 37.12 (1.42) | 45.68 (6.42) | () | |
| Median (IQR) | 29.18 (25.68,33.71) | 17.33 (16.72,18.11) | 23.01 (21.67,24.14) | 27.49 (26.23,28.68) | 32.02 (30.98,33.37) | 36.89 (35.96,38.19) | 43.59 (41.36,47.67) | (,) | |
| Range | 10.93 to 86.85 | 10.93 to 18.50 | 18.51 to 24.99 | 25.01 to 30.00 | 30.01 to 35.00 | 35.01 to 39.97 | 40.02 to 86.85 | ||
| BMI Category (WHO classification), no. (row %) | <0.001 | ||||||||
| Not available | 66 | 66 | |||||||
| <18.5 | 95 | 95 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 18.5–24.9 | 1673 | 0 (0.0) | 1673 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 25.0–29.9 | 2936 | 0 (0.0) | 0 (0.0) | 2936 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 30.0–34.9 | 2126 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2126 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 35.0–39.9 | 1018 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1018 (100.0) | 0 (0.0) | 0 (0.0) | |
| 40+ | 704 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 704 (100.0) | 0 (0.0) | |
| >10% loss body weight in last 6 months, no. (row %) | <0.001 | ||||||||
| Not available | 1 | 1 | |||||||
| No | 8394 | 84 (1.0) | 1576 (18.8) | 2861 (34.1) | 2102 (25.0) | 1007 (12.0) | 700 (8.3) | 64 (0.8) | |
| Yes | 223 | 11 (4.9) | 96 (43.0) | 75 (33.6) | 24 (10.8) | 11 (4.9) | 4 (1.8) | 2 (0.9) | |
| Current smoker within one year, no. (row %) | <0.001 | ||||||||
| No | 6886 | 60 (0.9) | 1264 (18.4) | 2336 (33.9) | 1749 (25.4) | 831 (12.1) | 592 (8.6) | 54 (0.8) | |
| Yes | 1732 | 35 (2.0) | 409 (23.6) | 600 (34.6) | 377 (21.8) | 187 (10.8) | 112 (6.5) | 12 (0.7) | |
| Diabetes mellitus with oral agents or insulin, no. (row %) | <0.001 | ||||||||
| Insulin | 526 | 1 (0.2) | 40 (7.6) | 130 (24.7) | 148 (28.1) | 109 (20.7) | 93 (17.7) | 5 (1.0) | |
| Non-Insulin/Oral | 1141 | 7 (0.6) | 122 (10.7) | 319 (28.0) | 331 (29.0) | 186 (16.3) | 169 (14.8) | 7 (0.6) | |
| None | 6951 | 87 (1.3) | 1511 (21.7) | 2487 (35.8) | 1647 (23.7) | 723 (10.4) | 442 (6.4) | 54 (0.8) | |
| Hypertension requiring medication, no. (row %) | <0.001 | ||||||||
| No | 3001 | 51 (1.7) | 829 (27.6) | 1073 (35.8) | 609 (20.3) | 264 (8.8) | 155 (5.2) | 20 (0.7) | |
| Yes | 5617 | 44 (0.8) | 844 (15.0) | 1863 (33.2) | 1517 (27.0) | 754 (13.4) | 549 (9.8) | 46 (0.8) | |
| Surgical Specialty, no. (row %) | <0.001 | ||||||||
| General Surgery | 535 | 11 (2.1) | 148 (27.7) | 164 (30.7) | 104 (19.4) | 64 (12.0) | 37 (6.9) | 7 (1.3) | |
| Cardiac Surgery | 1 | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Gynecology | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Neurosurgery | 4 | 0 (0.0) | 0 (0.0) | 2 (50.0) | 1 (25.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | |
| Orthopedics | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| ENT | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Plastics | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Thoracic | 6 | 0 (0.0) | 0 (0.0) | 3 (50.0) | 1 (16.7) | 2 (33.3) | 0 (0.0) | 0 (0.0) | |
| Urology | 8010 | 83 (1.0) | 1506 (18.8) | 2749 (34.3) | 2004 (25.0) | 943 (11.8) | 666 (8.3) | 59 (0.7) | |
| Vascular | 59 | 0 (0.0) | 18 (30.5) | 17 (28.8) | 15 (25.4) | 8 (13.6) | 1 (1.7) | 0 (0.0) | |
| Ophthalmology | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Oral Surgery | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Podiatry | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other | 1 | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Pre-operative serum albumin | <0.001 | ||||||||
| No. (%) used | 5163 (59.9%) | 55 (57.9%) | 1007 (60.2%) | 1790 (61.0%) | 1248 (58.7%) | 598 (58.7%) | 422 (59.9%) | 43 (65.2%) | |
| Mean (SD) | 3.95 (0.56) | 3.69 (0.75) | 3.89 (0.63) | 3.98 (0.57) | 4.00 (0.54) | 3.99 (0.48) | 3.85 (0.50) | 3.76 (0.67) | |
| Median (IQR) | 4 (3.7,4.3) | 3.9 (3.2,4.2) | 4 (3.6,4.3) | 4.1 (3.7,4.4) | 4.1 (3.7,4.4) | 4 (3.7,4.3) | 3.9 (3.6,4.2) | 3.9 (3.5,4.2) | |
| Range | 1 to 7.1 | 1.6 to 5 | 1 to 6.3 | 1.2 to 5.9 | 1.3 to 7.1 | 1.8 to 6.4 | 1.6 to 4.9 | 2.3 to 4.9 | |
| Pre-operative serum albumin category, no. (row %) | <0.001 | ||||||||
| Not available | 3455 | 40 | 666 | 1146 | 878 | 420 | 282 | 23 | |
| <3.5 | 796 | 18 (2.3) | 205 (25.8) | 269 (33.8) | 159 (20.0) | 66 (8.3) | 70 (8.8) | 9 (1.1) | |
| 3.5+ | 4367 | 37 (0.8) | 802 (18.4) | 1521 (34.8) | 1089 (24.9) | 532 (12.2) | 352 (8.1) | 34 (0.8) | |
| Pre-operative hematocrit | <0.001 | ||||||||
| No. (%) used | 8312 (96.4%) | 89 (93.7%) | 1619 (96.8%) | 2827 (96.3%) | 2055 (96.7%) | 982 (96.5%) | 676 (96.0%) | 64 (97.0%) | |
| Mean (SD) | 39.73 (5.26) | 36.66 (5.76) | 38.70 (5.35) | 39.98 (5.23) | 40.22 (5.25) | 40.32 (4.94) | 39.46 (4.85) | 36.68 (6.63) | |
| Median (IQR) | 40.2 (36.7,43.3) | 36.7 (32.6,39.6) | 39.1 (35.6,42.2) | 40.5 (37,43.5) | 40.8 (37.4,43.7) | 40.65 (37.4,43.6) | 39.7 (36.4,43) | 37.55 (32.6,41.85) | |
| Range | 11.8 to 59 | 21.8 to 52 | 13.8 to 57.3 | 12.1 to 58.2 | 11.8 to 59 | 14.8 to 55.5 | 23 to 52.8 | 12.8 to 46.5 | |
| Pre -operative hematocrit category, no. (row %) | <0.001 | ||||||||
| Not available | 318 | 7 | 58 | 113 | 73 | 36 | 29 | 2 | |
| Very low (Male<32; Female<28) | 425 | 10 (2.4) | 119 (28.0) | 140 (32.9) | 88 (20.7) | 35 (8.2) | 22 (5.2) | 11 (2.6) | |
| Low (Male 32–41.9; Female 28–37.9) | 3701 | 47 (1.3) | 771 (20.8) | 1259 (34.0) | 876 (23.7) | 418 (11.3) | 296 (8.0) | 34 (0.9) | |
| Normal (Male 42+; Female 38+) | 4174 | 31 (0.7) | 725 (17.4) | 1424 (34.1) | 1089 (26.1) | 529 (12.7) | 357 (8.6) | 19 (0.5) | |
| Pre-operative serum creatinine | <0.001 | ||||||||
| No. (%) used | 8239 (95.6%) | 90 (94.7%) | 1601 (95.7%) | 2796 (95.2%) | 2034 (95.7%) | 982 (96.5%) | 672 (95.5%) | 64 (97.0%) | |
| Mean (SD) | 1.30 (1.42) | 1.24 (1.34) | 1.37 (1.66) | 1.32 (1.43) | 1.32 (1.38) | 1.26 (1.30) | 1.10 (0.82) | 1.67 (2.04) | |
| Median (IQR) | 1 (0.8,1.21) | 0.805 (0.7,1.1) | 0.94 (0.8,1.2) | 1 (0.81,1.24) | 1 (0.82,1.22) | 0.95 (0.8,1.2) | 0.94 (0.79,1.195) | 1.01 (0.8129,1.32) | |
| Range | 0.2 to 15 | 0.23 to 8.39 | 0.3 to 15 | 0.3 to 14.69 | 0.2 to 14.13 | 0.3 to 15 | 0.47 to 13.31 | 0.37 to 9.7 | |
| Pre-operative GFR | 0.003 | ||||||||
| No. (%) used | 8227 (95.5%) | 89 (93.7%) | 1597 (95.5%) | 2792 (95.1%) | 2032 (95.6%) | 982 (96.5%) | 671 (95.3%) | 64 (97.0%) | |
| Mean (SD) | 71.31 (28.70) | 83.85 (49.37) | 71.38 (29.77) | 70.33 (26.02) | 70.80 (30.85) | 73.04 (27.73) | 72.79 (26.02) | 69.20 (38.50) | |
| Median (IQR) | 71.90 (54.80,86.88) | 78.16 (60.04,100.46) | 72.65 (54.25,88.27) | 71.28 (55.23,85.51) | 71.04 (54.97,85.65) | 74.41 (55.47,90.06) | 72.03 (54.73,89.69) | 70.65 (44.56,83.02) | |
| Range | 3.13 to 595.90 | 6.27 to 359.94 | 3.72 to 298.15 | 3.30 to 316.08 | 3.13 to 595.90 | 3.37 to 226.92 | 4.20 to 186.09 | 5.00 to 192.20 | |
| Pre-operative GFR category, no. (row %) | <0.001 | ||||||||
| Not available | 391 | 6 | 76 | 144 | 94 | 36 | 33 | 2 | |
| Normal: eGFR> = 90 mL/min/1.73 m2 | 1735 | 25 (1.4) | 369 (21.3) | 527 (30.4) | 391 (22.5) | 246 (14.2) | 165 (9.5) | 12 (0.7) | |
| CKD 2: eGFR 60–89 mL/min/1.7 3 m2 | 3883 | 42 (1.1) | 715 (18.4) | 1369 (35.3) | 998 (25.7) | 437 (11.3) | 294 (7.6) | 28 (0.7) | |
| CKD 3: eGFR 30–59 mL/min/1.73 m2 | 2081 | 13 (0.6) | 377 (18.1) | 734 (35.3) | 519 (24.9) | 239 (11.5) | 183 (8.8) | 16 (0.8) | |
| CKD 4: eGFR 15–29 mL/min/1.73 m2 | 200 | 4 (2.0) | 50 (25.0) | 55 (27.5) | 42 (21.0) | 28 (14.0) | 19 (9.5) | 2 (1.0) | |
| CKD 5: eGFR<15 mL/min/1.73 m2 | 328 | 5 (1.5) | 86 (26.2) | 107 (32.6) | 82 (25.0) | 32 (9.8) | 10 (3.0) | 6 (1.8) | |
| Chemotherapy for malignancy in< = 30 days pre-op, no. (row %) | 0.12 | ||||||||
| Not available | 3912 | 46 | 722 | 1399 | 965 | 442 | 319 | 19 | |
| No | 4637 | 48 (1.0) | 934 (20.1) | 1508 (32.5) | 1150 (24.8) | 573 (12.4) | 377 (8.1) | 47 (1.0) | |
| Yes | 69 | 1 (1.4) | 17 (24.6) | 29 (42.0) | 11 (15.9) | 3 (4.3) | 8 (11.6) | 0 (0.0) | |
| Radiotherapy for malignancy in last 90 days, no. (row %) | 0.70 | ||||||||
| Not available | 3950 | 46 | 733 | 1413 | 973 | 444 | 322 | 19 | |
| No | 4645 | 49 (1.1) | 935 (20.1) | 1512 (32.6) | 1150 (24.8) | 571 (12.3) | 381 (8.2) | 47 (1.0) | |
| Yes | 23 | 0 (0.0) | 5 (21.7) | 11 (47.8) | 3 (13.0) | 3 (13.0) | 1 (4.3) | 0 (0.0) | |
| Paralysis (hemiplegia, paraplegia, quadriplegia), no. (row %) | 0.081 | ||||||||
| Not available | 3912 | 46 | 722 | 1399 | 965 | 442 | 319 | 19 | |
| No | 4657 | 48 (1.0) | 934 (20.1) | 1522 (32.7) | 1153 (24.8) | 574 (12.3) | 379 (8.1) | 47 (1.0) | |
| Yes | 49 | 1 (2.0) | 17 (34.7) | 15 (30.6) | 8 (16.3) | 2 (4.1) | 6 (12.2) | 0 (0.0) | |
| Disseminated cancer, no. (row %) | <0.001 | ||||||||
| No | 8244 | 89 (1.1) | 1591 (19.3) | 2798 (33.9) | 2046 (24.8) | 980 (11.9) | 683 (8.3) | 57 (0.7) | |
| Yes | 374 | 6 (1.6) | 82 (21.9) | 138 (36.9) | 80 (21.4) | 38 (10.2) | 21 (5.6) | 9 (2.4) | |
| Steroid use for chronic condition, no. (row %) | <0.001 | ||||||||
| No | 8239 | 90 (1.1) | 1563 (19.0) | 2821 (34.2) | 2055 (24.9) | 969 (11.8) | 678 (8.2) | 63 (0.8) | |
| Yes | 379 | 5 (1.3) | 110 (29.0) | 115 (30.3) | 71 (18.7) | 49 (12.9) | 26 (6.9) | 3 (0.8) | |
| Stroke (CVA/stroke with or without neurological deficit), no. (row %) | 0.18 | ||||||||
| Not available | 3913 | 46 | 722 | 1400 | 965 | 442 | 319 | 19 | |
| No | 4524 | 47 (1.0) | 899 (19.9) | 1484 (32.8) | 1122 (24.8) | 555 (12.3) | 371 (8.2) | 46 (1.0) | |
| Yes | 181 | 2 (1.1) | 52 (28.7) | 52 (28.7) | 39 (21.5) | 21 (11.6) | 14 (7.7) | 1 (0.6) | |
| Currently on dialysis (pre-op), no. (row %) | <0.001 | ||||||||
| No | 8244 | 88 (1.1) | 1578 (19.1) | 2808 (34.1) | 2043 (24.8) | 978 (11.9) | 690 (8.4) | 59 (0.7) | |
| Yes | 374 | 7 (1.9) | 95 (25.4) | 128 (34.2) | 83 (22.2) | 40 (10.7) | 14 (3.7) | 7 (1.9) | |
| History of revascularization/amputation for periph. vascular disease, no. (row %) | 0.85 | ||||||||
| Not available | 3912 | 46 | 722 | 1399 | 965 | 442 | 319 | 19 | |
| No | 4643 | 49 (1.1) | 936 (20.2) | 1514 (32.6) | 1149 (24.7) | 568 (12.2) | 381 (8.2) | 46 (1.0) | |
| Yes | 63 | 0 (0.0) | 15 (23.8) | 23 (36.5) | 12 (19.0) | 8 (12.7) | 4 (6.3) | 1 (1.6) | |
| EtOH>2 drinks/day in 2 wks before admission, no. (row %) | 0.19 | ||||||||
| Not available | 3893 | 46 | 721 | 1389 | 962 | 440 | 316 | 19 | |
| No | 4563 | 48 (1.1) | 912 (20.0) | 1485 (32.5) | 1127 (24.7) | 566 (12.4) | 379 (8.3) | 46 (1.0) | |
| Yes | 162 | 1 (0.6) | 40 (24.7) | 62 (38.3) | 37 (22.8) | 12 (7.4) | 9 (5.6) | 1 (0.6) | |
| Dyspnea, no. (row %) | <0.001 | ||||||||
| At rest | 43 | 1 (2.3) | 10 (23.3) | 12 (27.9) | 8 (18.6) | 6 (14.0) | 6 (14.0) | 0 (0.0) | |
| Moderate exertion | 818 | 11 (1.3) | 126 (15.4) | 221 (27.0) | 205 (25.1) | 121 (14.8) | 129 (15.8) | 5 (0.6) | |
| No | 7757 | 83 (1.1) | 1537 (19.8) | 2703 (34.8) | 1913 (24.7) | 891 (11.5) | 569 (7.3) | 61 (0.8) | |
| Functional Status, no. (row %) | <0.001 | ||||||||
| Not available | 28 | 1 | 8 | 5 | 9 | 2 | 3 | ||
| Independent | 8416 | 87 (1.0) | 1621 (19.3) | 2876 (34.2) | 2087 (24.8) | 1002 (11.9) | 682 (8.1) | 61 (0.7) | |
| Partially Dependent | 154 | 5 (3.2) | 38 (24.7) | 50 (32.5) | 27 (17.5) | 12 (7.8) | 19 (12.3) | 3 (1.9) | |
| Totally Dependent | 20 | 2 (10.0) | 6 (30.0) | 5 (25.0) | 3 (15.0) | 2 (10.0) | 0 (0.0) | 2 (10.0) | |
| History of severe COPD, no. (row %) | 0.012 | ||||||||
| No | 8141 | 82 (1.0) | 1568 (19.3) | 2776 (34.1) | 2023 (24.8) | 966 (11.9) | 663 (8.1) | 63 (0.8) | |
| Yes | 477 | 13 (2.7) | 105 (22.0) | 160 (33.5) | 103 (21.6) | 52 (10.9) | 41 (8.6) | 3 (0.6) | |
| Pulmonary comorbidity (dyspnea or COPD), no. (row %) | <0.001 | ||||||||
| No | 7486 | 75 (1.0) | 1478 (19.7) | 2604 (34.8) | 1855 (24.8) | 862 (11.5) | 552 (7.4) | 60 (0.8) | |
| Yes | 1132 | 20 (1.8) | 195 (17.2) | 332 (29.3) | 271 (23.9) | 156 (13.8) | 152 (13.4) | 6 (0.5) | |
| Ascites, no. (row %) | 0.28 | ||||||||
| No | 8596 | 94 (1.1) | 1665 (19.4) | 2929 (34.1) | 2123 (24.7) | 1016 (11.8) | 703 (8.2) | 66 (0.8) | |
| Yes | 22 | 1 (4.5) | 8 (36.4) | 7 (31.8) | 3 (13.6) | 2 (9.1) | 1 (4.5) | 0 (0.0) | |
| Heart disease (CHF, MI, previous PCI, previous cardiac surgery, angina), no. (row %) | 0.11 | ||||||||
| Not available | 3886 | 46 | 716 | 1392 | 959 | 439 | 316 | 18 | |
| No | 4144 | 47 (1.1) | 846 (20.4) | 1346 (32.5) | 1017 (24.5) | 494 (11.9) | 352 (8.5) | 42 (1.0) | |
| Yes | 588 | 2 (0.3) | 111 (18.9) | 198 (33.7) | 150 (25.5) | 85 (14.5) | 36 (6.1) | 6 (1.0) | |
| History of angina in 1 month before surgery, no. (row %) | 0.83 | ||||||||
| Not available | 3912 | 46 | 722 | 1399 | 965 | 442 | 319 | 19 | |
| No | 4670 | 49 (1.0) | 943 (20.2) | 1528 (32.7) | 1150 (24.6) | 570 (12.2) | 383 (8.2) | 47 (1.0) | |
| Yes | 36 | 0 (0.0) | 8 (22.2) | 9 (25.0) | 11 (30.6) | 6 (16.7) | 2 (5.6) | 0 (0.0) | |
Table 6
Relationship of weight loss >10% within the 6 months prior to surgery and variables of interest
| >10% loss body weight in last 6 months | ||||
| Characteristic/feature | All (n = 8618) | No (n = 8394) | Yes (n = 223) | P-value |
| Age | 0.080 | |||
| No. (%) used | 8618 (100.0%) | 8394 (100.0%) | 223 (100.0%) | |
| Mean (SD) | 61.53 (12.88) | 61.49 (12.88) | 63.02 (12.90) | |
| Median (IQR) | 62 (53,71) | 62 (53,71) | 63 (55,73) | |
| Range | 17 to 90 | 17 to 90 | 26 to 88 | |
| Age Category, no. (row %) | 0.012 | |||
| <40 | 457 | 446 (97.6) | 11 (2.4) | |
| 40–49 | 1039 | 1019 (98.1) | 20 (1.9) | |
| 50–59 | 2136 | 2075 (97.1) | 61 (2.9) | |
| 60–69 | 2564 | 2509 (97.9) | 55 (2.1) | |
| 70–79 | 1764 | 1717 (97.3) | 46 (2.6) | |
| 80+ | 658 | 628 (95.4) | 30 (4.6) | |
| Gender, no. (row %) | 0.83 | |||
| Not available | 14 | 13 | 1 | |
| Male | 5254 | 5120 (97.4) | 134 (2.6) | |
| Female | 3350 | 3261 (97.3) | 88 (2.6) | |
| Race/ethnicity (all years), no. (row %) | 0.38 | |||
| Non-Hispanic White | 6384 | 6219 (97.4) | 164 (2.6) | |
| Hispanic White | 411 | 404 (98.3) | 7 (1.7) | |
| Black or African American | 714 | 698 (97.8) | 16 (2.2) | |
| Asian, Native Hawaiian or Pacific Islander | 172 | 167 (97.1) | 5 (2.9) | |
| American Indian or Alaska Native | 28 | 26 (92.9) | 2 (7.1) | |
| Other or Unknown | 909 | 880 (96.8) | 29 (3.2) | |
| Year of Operation, no. (row %) | 0.55 | |||
| 2005 | 8 | 8 (100.0) | 0 (0.0) | |
| 2006 | 48 | 47 (97.9) | 1 (2.1) | |
| 2007 | 173 | 165 (95.4) | 8 (4.6) | |
| 2008 | 474 | 459 (96.8) | 15 (3.2) | |
| 2009 | 792 | 773 (97.6) | 19 (2.4) | |
| 2010 | 1100 | 1065 (96.8) | 34 (3.1) | |
| 2011 | 2661 | 2593 (97.4) | 68 (2.6) | |
| 2012 | 3362 | 3284 (97.7) | 78 (2.3) | |
| Lap vs Open (nephrectomy cohort only), no. (row %) | <0.001 | |||
| Lap | 4929 | 4838 (98.2) | 90 (1.8) | |
| Open | 3689 | 3556 (96.4) | 133 (3.6) | |
| Lymphadenectomy, no. (row %) | <0.001 | |||
| No | 8410 | 8200 (97.5) | 209 (2.5) | |
| Yes | 208 | 194 (93.3) | 14 (6.7) | |
| Partial nephrectomy, no. (row %) | <0.001 | |||
| No | 7183 | 6970 (97.0) | 212 (3.0) | |
| Yes | 1435 | 1424 (99.2) | 11 (0.8) | |
| ASA class, no. (row %) | 0.090 | |||
| Not available | 8 | 8 | ||
| 1-No Disturb | 156 | 152 (97.4) | 4 (2.6) | |
| 2-Mild Disturb | 3100 | 3038 (98.0) | 62 (2.0) | |
| 3-Severe Disturb | 4836 | 4693 (97.0) | 142 (2.9) | |
| 4-Life Threat | 517 | 502 (97.1) | 15 (2.9) | |
| 5-Moribund | 1 | 1 (100.0) | 0 (0.0) | |
| ASA class (1-2 vs 3-4), no. (row %) | 0.010 | |||
| Not available | 8 | 8 | ||
| 1 or 2-No or Mild Disturb | 3256 | 3190 (98.0) | 66 (2.0) | |
| 3, 4, or 5-Severe Disturb, Life Threat, or Moribund | 5354 | 5196 (97.0) | 157 (2.9) | |
| BMI | <0.001 | |||
| No. (%) used | 8552 (99.2%) | 8330 (99.2%) | 221 (99.1%) | |
| Mean (SD) | 30.26 (6.87) | 30.38 (6.86) | 25.77 (5.37) | |
| Median (IQR) | 29.18 (25.68,33.71) | 29.33 (25.78,33.83) | 25.27 (21.99,28.41) | |
| Range | 10.93 to 86.85 | 10.93 to 86.85 | 11.94 to 49.48 | |
| BMI Category (WHO classification), no. (row %) | <0.001 | |||
| Not available | 66 | 64 | 2 | |
| <18.5 | 95 | 84 (88.4) | 11 (11.6) | |
| 18.5–24.9 | 1673 | 1576 (94.2) | 96 (5.7) | |
| 25.0–29.9 | 2936 | 2861 (97.4) | 75 (2.6) | |
| 30.0–34.9 | 2126 | 2102 (98.9) | 24 (1.1) | |
| 35.0–39.9 | 1018 | 1007 (98.9) | 11 (1.1) | |
| 40+ | 704 | 700 (99.4) | 4 (0.6) | |
| >10% loss body weight in last 6 months, no. (row %) | <0.001 | |||
| Not available | 1 | |||
| No | 8394 | 8394 (100.0) | 0 (0.0) | |
| Yes | 223 | 0 (0.0) | 223 (100.0) | |
| Current smoker within one year, no. (row %) | 0.010 | |||
| No | 6886 | 6723 (97.6) | 163 (2.4) | |
| Yes | 1732 | 1671 (96.5) | 60 (3.5) | |
| Diabetes mellitus with oral agents or insulin, no. (row %) | 0.99 | |||
| Insulin | 526 | 512 (97.3) | 14 (2.7) | |
| Non-Insulin/Oral | 1141 | 1112 (97.5) | 29 (2.5) | |
| None | 6951 | 6770 (97.4) | 180 (2.6) | |
| Hypertension requiring medication, no. (row %) | 0.029 | |||
| No | 3001 | 2908 (96.9) | 93 (3.1) | |
| Yes | 5617 | 5486 (97.7) | 130 (2.3) | |
| Surgical Specialty, no. (row %) | 0.030 | |||
| General Surgery | 535 | 515 (96.3) | 20 (3.7) | |
| Cardiac Surgery | 1 | 1 (100.0) | 0 (0.0) | |
| Gynecology | 1 | 1 (100.0) | 0 (0.0) | |
| Neurosurgery | 4 | 4 (100.0) | 0 (0.0) | |
| Orthopedics | 0 | 0 (0.0) | 0 (0.0) | |
| ENT | 1 | 1 (100.0) | 0 (0.0) | |
| Plastics | 0 | 0 (0.0) | 0 (0.0) | |
| Thoracic | 6 | 6 (100.0) | 0 (0.0) | |
| Urology | 8010 | 7812 (97.5) | 197 (2.5) | |
| Vascular | 59 | 53 (89.8) | 6 (10.2) | |
| Ophthalmology | 0 | 0 (0.0) | 0 (0.0) | |
| Oral Surgery | 0 | 0 (0.0) | 0 (0.0) | |
| Podiatry | 0 | 0 (0.0) | 0 (0.0) | |
| Other | 1 | 1 (100.0) | 0 (0.0) | |
| Pre-operative serum albumin | <0.001 | |||
| No. (%) used | 5163 (59.9%) | 5003 (59.6%) | 159 (71.3%) | |
| Mean (SD) | 3.95 (0.56) | 3.97 (0.55) | 3.42 (0.76) | |
| Median (IQR) | 4 (3.7,4.3) | 4.03 (3.7,4.3) | 3.5 (2.9,4) | |
| Range | 1 to 7.1 | 1 to 7.1 | 1.5 to 5.9 | |
| Pre-operative serum albumin category, no. (row %) | <0.001 | |||
| Not available | 3455 | 3391 | 64 | |
| <3.5 | 796 | 718 (90.2) | 78 (9.8) | |
| 3.5+ | 4367 | 4285 (98.1) | 81 (1.9) | |
| Pre-operative hematocrit | <0.001 | |||
| No. (%) used | 8312 (96.4%) | 8095 (96.4%) | 216 (96.9%) | |
| Mean (SD) | 39.73 (5.26) | 39.84 (5.21) | 35.64 (5.35) | |
| Median (IQR) | 40.2 (36.7,43.3) | 40.3 (36.9,43.3) | 35.95 (32,39.6) | |
| Range | 11.8 to 59 | 11.8 to 59 | 18.8 to 49.2 | |
| Pre-operative hematocrit category, no. (row %) | <0.001 | |||
| Not available | 318 | 310 | 8 | |
| Very low (Male<32; Female<28) | 425 | 391 (92.0) | 34 (8.0) | |
| Low (Male 32–41.9; Female 28–37.9) | 3701 | 3566 (96.4) | 135 (3.6) | |
| Normal (Male 42+; Female 38+) | 4174 | 4127 (98.9) | 46 (1.1) | |
| Pre-operative serum creatinine | 0.61 | |||
| No. (%) used | 8239 (95.6%) | 8027 (95.6%) | 211 (94.6%) | |
| Mean (SD) | 1.30 (1.42) | 1.31 (1.42) | 1.26 (1.39) | |
| Median (IQR) | 1 (0.8,1.21) | 1 (0.8,1.21) | 0.96 (0.77,1.21) | |
| Range | 0.2 to 15 | 0.2 to 15 | 0.3 to 13.25 | |
| Pre-operative GFR | 0.061 | |||
| No. (%) used | 8227 (95.5%) | 8016 (95.5%) | 210 (94.2%) | |
| Mean (SD) | 71.31 (28.70) | 71.22 (28.57) | 74.97 (32.95) | |
| Median (IQR) | 71.90 (54.80,86.88) | 71.90 (54.85,86.72) | 72.13 (54.31,94.16) | |
| Range | 3.13 to 595.90 | 3.13 to 595.90 | 4.65 to 298.15 | |
| Pre-operative GFR category, no. (row %) | 0.13 | |||
| Not available | 391 | 378 | 13 | |
| Normal: eGFR> = 90 mL/min/1.73 m2 | 1735 | 1677 (96.7) | 58 (3.3) | |
| CKD 2: eGFR 60–89 mL/min/1.73 m2 | 3883 | 3798 (97.8) | 84 (2.2) | |
| CKD 3: eGFR 30–59 mL/min/1.73 m2 | 2081 | 2025 (97.3) | 56 (2.7) | |
| CKD 4: eGFR 15–29 mL/min/1.73 m2 | 200 | 195 (97.5) | 5 (2.5) | |
| CKD 5: eGFR<15 mL/min/1.73 m2 | 328 | 321 (97.9) | 7 (2.1) | |
| Chemotherapy for malignancy in< = 30 days pre-op, no. (row %) | 0.002 | |||
| Not available | 3912 | 3809 | 103 | |
| No | 4637 | 4523 (97.5) | 113 (2.4) | |
| Yes | 69 | 62 (89.9) | 7 (10.1) | |
| Radiotherapy for malignancy in last 90 days, no. (row %) | 0.12 | |||
| Not available | 3950 | 3847 | 103 | |
| No | 4645 | 4526 (97.4) | 118 (2.5) | |
| Yes | 23 | 21 (91.3) | 2 (8.7) | |
| Paralysis (hemiplegia, paraplegia, quadriplegia), no. (row %) | 1.00 | |||
| Not available | 3912 | 3809 | 103 | |
| No | 4657 | 4537 (97.4) | 119 (2.6) | |
| Yes | 49 | 48 (98.0) | 1 (2.0) | |
| Disseminated cancer, no. (row %) | <0.001 | |||
| No | 8244 | 8059 (97.8) | 184 (2.2) | |
| Yes | 374 | 335 (89.6) | 39 (10.4) | |
| Steroid use for chronic condition, no. (row %) | 0.17 | |||
| No | 8239 | 8029 (97.5) | 209 (2.5) | |
| Yes | 379 | 365 (96.3) | 14 (3.7) | |
| Stroke (CVA/stroke with or without neurological deficit), no. (row %) | 0.47 | |||
| Not available | 3913 | 3810 | 103 | |
| No | 4524 | 4410 (97.5) | 114 (2.5) | |
| Yes | 181 | 174 (96.1) | 6 (3.3) | |
| Currently on dialysis (pre-op), no. (row %) | 0.82 | |||
| No | 8244 | 8029 (97.4) | 214 (2.6) | |
| Yes | 374 | 365 (97.6) | 9 (2.4) | |
| History of revascularization/amputation for periph. vascular disease, no. (row %) | 0.68 | |||
| Not available | 3912 | 3809 | 103 | |
| No | 4643 | 4524 (97.4) | 118 (2.5) | |
| Yes | 63 | 61 (96.8) | 2 (3.2) | |
| EtOH>2 drinks/day in 2 wks before admission, no. (row %) | 0.61 | |||
| Not available | 3893 | 3790 | 103 | |
| No | 4563 | 4447 (97.5) | 115 (2.5) | |
| Yes | 162 | 157 (96.9) | 5 (3.1) | |
| Dyspnea, no. (row %) | <0.001 | |||
| At rest | 43 | 40 (93.0) | 3 (7.0) | |
| Moderate exertion | 818 | 774 (94.6) | 44 (5.4) | |
| No | 7757 | 7580 (97.7) | 176 (2.3) | |
| Functional Status, no. (row %) | <0.001 | |||
| Not available | 28 | 28 | ||
| Independent | 8416 | 8206 (97.5) | 209 (2.5) | |
| Partially Dependent | 154 | 140 (90.9) | 14 (9.1) | |
| Totally Dependent | 20 | 20 (100.0) | 0 (0.0) | |
| History of severe COPD, no. (row %) | 0.17 | |||
| No | 8141 | 7934 (97.5) | 206 (2.5) | |
| Yes | 477 | 460 (96.4) | 17 (3.6) | |
| Pulmonary comorbidity (dyspnea or COPD), no. (row %) | <0.001 | |||
| No | 7486 | 7317 (97.7) | 168 (2.2) | |
| Yes | 1132 | 1077 (95.1) | 55 (4.9) | |
| Ascites, no. (row %) | 0.11 | |||
| No | 8596 | 8374 (97.4) | 221 (2.6) | |
| Yes | 22 | 20 (90.9) | 2 (9.1) | |
| Heart disease (CHF, MI, previous PCI, previous cardiac surgery, angina), no. (row %) | 0.25 | |||
| Not available | 3886 | 3783 | 103 | |
| No | 4144 | 4042 (97.5) | 101 (2.4) | |
| Yes | 588 | 569 (96.8) | 19 (3.2) | |
| History of angina in 1 month before surgery, no. (row %) | 0.23 | |||
| Not available | 3912 | 3809 | 103 | |
| No | 4670 | 4551 (97.5) | 118 (2.5) | |
| Yes | 36 | 34 (94.4) | 2 (5.6) | |
Table 7
Relationship of preoperative serum albumin and variables of interest
| Preoperative serum albumin category (with unknown) | |||||
| Characteristic/feature | All (n = 8618) | <3.5 (n = 796) | 3.5+ (n = 4367) | Unknown (n = 3455) | P-value |
| Age | <0.001 | ||||
| No. (%) used | 8618 (100.0%) | 796 (100.0%) | 4367 (100.0%) | 3455 (100.0%) | |
| Mean (SD) | 61.53 (12.88) | 63.36 (12.93) | 61.37 (12.73) | 61.30 (13.01) | |
| Median (IQR) | 62 (53,71) | 64 (55,72.5) | 62 (53,70) | 62 (53,71) | |
| Range | 17 to 90 | 22 to 90 | 17 to 90 | 18 to 90 | |
| Age Category, no. (row %) | <0.001 | ||||
| <40 | 457 | 31 (6.8) | 233 (51.0) | 193 (42.2) | |
| 40–49 | 1039 | 77 (7.4) | 535 (51.5) | 427 (41.1) | |
| 50–59 | 2136 | 192 (9.0) | 1080 (50.6) | 864 (40.4) | |
| 60–69 | 2564 | 247 (9.6) | 1314 (51.2) | 1003 (39.1) | |
| 70–79 | 1764 | 156 (8.8) | 908 (51.5) | 700 (39.7) | |
| 80+ | 658 | 93 (14.1) | 297 (45.1) | 268 (40.7) | |
| Gender, no. (row %) | 0.11 | ||||
| Not available | 14 | 7 | 7 | ||
| Male | 5254 | 462 (8.8) | 2654 (50.5) | 2138 (40.7) | |
| Female | 3350 | 334 (10.0) | 1706 (50.9) | 1310 (39.1) | |
| Race/ethnicity (all years), no. (row %) | <0.001 | ||||
| Non-Hispanic White | 6384 | 588 (9.2) | 3315 (51.9) | 2481 (38.9) | |
| Hispanic White | 411 | 33 (8.0) | 209 (50.9) | 169 (41.1) | |
| Black or African American | 714 | 97 (13.6) | 352 (49.3) | 265 (37.1) | |
| Asian, Native Hawaiian or Pacific Islander | 172 | 14 (8.1) | 106 (61.6) | 52 (30.2) | |
| American Indian or Alaska Native | 28 | 4 (14.3) | 16 (57.1) | 8 (28.6) | |
| Other or Unknown | 909 | 60 (6.6) | 369 (40.6) | 480 (52.8) | |
| Year of Operation, no. (row %) | <0.001 | ||||
| 2005 | 8 | 3 (37.5) | 4 (50.0) | 1 (12.5) | |
| 2006 | 48 | 3 (6.3) | 26 (54.2) | 19 (39.6) | |
| 2007 | 173 | 28 (16.2) | 87 (50.3) | 58 (33.5) | |
| 2008 | 474 | 40 (8.4) | 254 (53.6) | 180 (38.0) | |
| 2009 | 792 | 61 (7.7) | 374 (47.2) | 357 (45.1) | |
| 2010 | 1100 | 90 (8.2) | 529 (48.1) | 481 (43.7) | |
| 2011 | 2661 | 270 (10.1) | 1429 (53.7) | 962 (36.2) | |
| 2012 | 3362 | 301 (9.0) | 1664 (49.5) | 1397 (41.6) | |
| Lap vs Open (nephrectomy cohort only), no. (row %) | <0.001 | ||||
| Lap | 4929 | 363 (7.4) | 2612 (53.0) | 1954 (39.6) | |
| Open | 3689 | 433 (11.7) | 1755 (47.6) | 1501 (40.7) | |
| Lymphadenectomy, no. (row %) | <0.001 | ||||
| No | 8410 | 779 (9.3) | 4288 (51.0) | 3343 (39.8) | |
| Yes | 208 | 17 (8.2) | 79 (38.0) | 112 (53.8) | |
| Partial nephrectomy, no. (row %) | <0.001 | ||||
| No | 7183 | 728 (10.1) | 3601 (50.1) | 2854 (39.7) | |
| Yes | 1435 | 68 (4.7) | 766 (53.4) | 601 (41.9) | |
| ASA class, no. (row %) | <0.001 | ||||
| Not available | 8 | 4 | 4 | ||
| 1-No Disturb | 156 | 5 (3.2) | 85 (54.5) | 66 (42.3) | |
| 2-Mild Disturb | 3100 | 135 (4.4) | 1545 (49.8) | 1420 (45.8) | |
| 3-Severe Disturb | 4836 | 546 (11.3) | 2511 (51.9) | 1779 (36.8) | |
| 4-Life Threat | 517 | 109 (21.1) | 222 (42.9) | 186 (36.0) | |
| 5-Moribund | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) | |
| ASA class (1-2 vs 3-4), no. (row %) | <0.001 | ||||
| Not available | 8 | 4 | 4 | ||
| 1 or 2-No or Mild Disturb | 3256 | 140 (4.3) | 1630 (50.1) | 1486 (45.6) | |
| 3, 4, or 5-Severe Disturb, Life Threat, or Moribund | 5354 | 656 (12.3) | 2733 (51.0) | 1965 (36.7) | |
| BMI | <0.001 | ||||
| No. (%) used | 8552 (99.2%) | 787 (98.9%) | 4333 (99.2%) | 3432 (99.3%) | |
| Mean (SD) | 30.26 (6.87) | 29.23 (7.24) | 30.36 (6.75) | 30.36 (6.90) | |
| Median (IQR) | 29.18 (25.68,33.71) | 27.82 (24.43,32.80) | 29.35 (25.88,33.71) | 29.26 (25.74,33.89) | |
| Range | 10.93 to 86.85 | 16.09 to 61.14 | 10.93 to 86.85 | 13.71 to 79.88 | |
| BMI Category (WHO classification), no. (row %) | <0.001 | ||||
| Not available | 66 | 9 | 34 | 23 | |
| <18.5 | 95 | 18 (18.9) | 37 (38.9) | 40 (42.1) | |
| 18.5–24.9 | 1673 | 205 (12.3) | 802 (47.9) | 666 (39.8) | |
| 25.0–29.9 | 2936 | 269 (9.2) | 1521 (51.8) | 1146 (39.0) | |
| 30.0–34.9 | 2126 | 159 (7.5) | 1089 (51.2) | 878 (41.3) | |
| 35.0–39.9 | 1018 | 66 (6.5) | 532 (52.3) | 420 (41.3) | |
| 40+ | 704 | 70 (9.9) | 352 (50.0) | 282 (40.1) | |
| >10% loss body weight in last 6 months, no. (row %) | <0.001 | ||||
| Not available | 1 | 1 | |||
| No | 8394 | 718 (8.6) | 4285 (51.0) | 3391 (40.4) | |
| Yes | 223 | 78 (35.0) | 81 (36.3) | 64 (28.7) | |
| Current smoker within one year, no. (row %) | 0.11 | ||||
| No | 6886 | 629 (9.1) | 3458 (50.2) | 2799 (40.6) | |
| Yes | 1732 | 167 (9.6) | 909 (52.5) | 656 (37.9) | |
| Diabetes mellitus with oral agents or insulin, no. (row %) | <0.001 | ||||
| Insulin | 526 | 88 (16.7) | 254 (48.3) | 184 (35.0) | |
| Non-Insulin/Oral | 1141 | 106 (9.3) | 610 (53.5) | 425 (37.2) | |
| None | 6951 | 602 (8.7) | 3503 (50.4) | 2846 (40.9) | |
| Hypertension requiring medication, no. (row %) | 0.002 | ||||
| No | 3001 | 241 (8.0) | 1496 (49.9) | 1264 (42.1) | |
| Yes | 5617 | 555 (9.9) | 2871 (51.1) | 2191 (39.0) | |
| Surgical Specialty, no. (row %) | |||||
| General Surgery | 535 | 94 (17.6) | 266 (49.7) | 175 (32.7) | |
| Cardiac Surgery | 1 | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Gynecology | 1 | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Neurosurgery | 4 | 0 (0.0) | 2 (50.0) | 2 (50.0) | |
| Orthopedics | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| ENT | 1 | 0 (0.0) | 1 (100.0) | 0 (0.0) | |
| Plastics | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Thoracic | 6 | 0 (0.0) | 6 (100.0) | 0 (0.0) | |
| Urology | 8010 | 691 (8.6) | 4058 (50.7) | 3261 (40.7) | |
| Vascular | 59 | 10 (16.9) | 34 (57.6) | 15 (25.4) | |
| Ophthalmology | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Oral Surgery | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Podiatry | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) | |
| Pre-operative serum albumin | <0.001 | ||||
| No. (%) used | 5163 (59.9%) | 796 (100.0%) | 4367 (100.0%) | 0 (0.0%) | |
| Mean (SD) | 3.95 (0.56) | 2.95 (0.44) | 4.14 (0.36) | () | |
| Median (IQR) | 4 (3.7,4.3) | 3.1 (2.7,3.3) | 4.1 (3.9,4.4) | (,) | |
| Range | 1 to 7.1 | 1 to 3.4 | 3.5 to 7.1 | ||
| Pre-operative serum albumin category, no. (row %) | <0.001 | ||||
| Not available | 3455 | 3455 | |||
| <3.5 | 796 | 796 (100.0) | 0 (0.0) | 0 (0.0) | |
| 3.5+ | 4367 | 0 (0.0) | 4367 (100.0) | 0 (0.0) | |
| Pre-operative hematocrit | <0.001 | ||||
| No. (%) used | 8312 (96.4%) | 792 (99.5%) | 4340 (99.4%) | 3180 (92.0%) | |
| Mean (SD) | 39.73 (5.26) | 34.35 (5.81) | 40.40 (4.79) | 40.14 (4.97) | |
| Median (IQR) | 40.2 (36.7,43.3) | 34 (30.3,38.3) | 40.7 (37.5,43.5) | 40.6 (37.4,43.5) | |
| Range | 11.8 to 59 | 13.8 to 57.3 | 11.8 to 59 | 12.1 to 56.3 | |
| Pre-operative hematocrit category, no. (row %) | <0.001 | ||||
| Not available | 318 | 4 | 34 | 280 | |
| Very low (Male<32; Female<28) | 425 | 194 (45.6) | 115 (27.1) | 116 (27.3) | |
| Low (Male 32–41.9; Female 28–37.9) | 3701 | 471 (12.7) | 1864 (50.4) | 1366 (36.9) | |
| Normal (Male 42+; Female 38+) | 4174 | 127 (3.0) | 2354 (56.4) | 1693 (40.6) | |
| Pre-operative serum creatinine | <0.001 | ||||
| No. (%) used | 8239 (95.6%) | 788 (99.0%) | 4355 (99.7%) | 3096 (89.6%) | |
| Mean (SD) | 1.30 (1.42) | 1.70 (1.93) | 1.24 (1.23) | 1.30 (1.50) | |
| Median (IQR) | 1 (0.8,1.21) | 1.045 (0.8,1.5) | 0.98 (0.8,1.2) | 0.99275 (0.8,1.2) | |
| Range | 0.2 to 15 | 0.3 to 13.9 | 0.2 to 15 | 0.23 to 15 | |
| Pre-operative GFR | <0.001 | ||||
| No. (%) used | 8227 (95.5%) | 788 (99.0%) | 4348 (99.6%) | 3091 (89.5%) | |
| Mean (SD) | 71.31 (28.70) | 65.07 (34.70) | 72.19 (28.57) | 71.67 (26.94) | |
| Median (IQR) | 71.90 (54.80,86.88) | 64.09 (43.75,84.25) | 72.85 (55.64,87.20) | 71.96 (56.16,86.88) | |
| Range | 3.13 to 595.90 | 3.74 to 298.15 | 3.30 to 595.90 | 3.13 to 359.94 | |
| Pre-operative GFR category, no. (row %) | <0.001 | ||||
| Not available | 391 | 8 | 19 | 364 | |
| Normal: eGFR> = 90 mL/min/1.73 m2 | 1735 | 161 (9.3) | 930 (53.6) | 644 (37.1) | |
| CKD 2: eGFR 60–89 mL/min/1.73 m2 | 3883 | 275 (7.1) | 2092 (53.9) | 1516 (39.0) | |
| CKD 3: eGFR 30–59 mL/min/1.73 m2 | 2081 | 230 (11.1) | 1093 (52.5) | 758 (36.4) | |
| CKD 4: eGFR 15–29 mL/min/1.73 m2 | 200 | 50 (25.0) | 96 (48.0) | 54 (27.0) | |
| CKD 5: eGFR<15 mL/min/1.73 m2 | 328 | 72 (22.0) | 137 (41.8) | 119 (36.3) | |
| Chemotherapy for malignancy in< = 30 days pre-op, no. (row %) | 0.003 | ||||
| Not available | 3912 | 392 | 2172 | 1348 | |
| No | 4637 | 393 (8.5) | 2155 (46.5) | 2089 (45.1) | |
| Yes | 69 | 11 (15.9) | 40 (58.0) | 18 (26.1) | |
| Radiotherapy for malignancy in last 90 days, no. (row %) | 0.96 | ||||
| Not available | 3950 | 397 | 2198 | 1355 | |
| No | 4645 | 397 (8.5) | 2159 (46.5) | 2089 (45.0) | |
| Yes | 23 | 2 (8.7) | 10 (43.5) | 11 (47.8) | |
| Paralysis (hemiplegia, paraplegia, quadriplegia), no. (row %) | 0.55 | ||||
| Not available | 3912 | 392 | 2172 | 1348 | |
| No | 4657 | 398 (8.5) | 2175 (46.7) | 2084 (44.7) | |
| Yes | 49 | 6 (12.2) | 20 (40.8) | 23 (46.9) | |
| Disseminated cancer, no. (row %) | <0.001 | ||||
| No | 8244 | 712 (8.6) | 4200 (50.9) | 3332 (40.4) | |
| Yes | 374 | 84 (22.5) | 167 (44.7) | 123 (32.9) | |
| Steroid use for chronic condition, no. (row %) | <0.001 | ||||
| No | 8239 | 735 (8.9) | 4178 (50.7) | 3326 (40.4) | |
| Yes | 379 | 61 (16.1) | 189 (49.9) | 129 (34.0) | |
| Stroke (CVA/stroke with or without neurological deficit), no. (row %) | 0.30 | ||||
| Not available | 3913 | 392 | 2172 | 1349 | |
| No | 4524 | 386 (8.5) | 2103 (46.5) | 2035 (45.0) | |
| Yes | 181 | 18 (9.9) | 92 (50.8) | 71 (39.2) | |
| Currently on dialysis (pre-op), no. (row %) | <0.001 | ||||
| No | 8244 | 706 (8.6) | 4218 (51.2) | 3320 (40.3) | |
| Yes | 374 | 90 (24.1) | 149 (39.8) | 135 (36.1) | |
| History of revascularization/amputation for periph. vascular disease, no. (row %) | 0.007 | ||||
| Not available | 3912 | 392 | 2172 | 1348 | |
| No | 4643 | 392 (8.4) | 2173 (46.8) | 2078 (44.8) | |
| Yes | 63 | 12 (19.0) | 22 (34.9) | 29 (46.0) | |
| EtOH>2 drinks/day in 2 wks before admission, no. (row %) | 0.65 | ||||
| Not available | 3893 | 392 | 2153 | 1348 | |
| No | 4563 | 391 (8.6) | 2143 (47.0) | 2029 (44.5) | |
| Yes | 162 | 13 (8.0) | 71 (43.8) | 78 (48.1) | |
| Dyspnea, no. (row %) | <0.001 | ||||
| At rest | 43 | 10 (23.3) | 20 (46.5) | 13 (30.2) | |
| Moderate exertion | 818 | 120 (14.7) | 409 (50.0) | 289 (35.3) | |
| No | 7757 | 666 (8.6) | 3938 (50.8) | 3153 (40.6) | |
| Functional Status, no. (row %) | <0.001 | ||||
| Not available | 28 | 3 | 13 | 12 | |
| Independent | 8416 | 744 (8.8) | 4291 (51.0) | 3381 (40.2) | |
| Partially Dependent | 154 | 37 (24.0) | 60 (39.0) | 57 (37.0) | |
| Totally Dependent | 20 | 12 (60.0) | 3 (15.0) | 5 (25.0) | |
| History of severe COPD, no. (row %) | 0.014 | ||||
| No | 8141 | 735 (9.0) | 4125 (50.7) | 3281 (40.3) | |
| Yes | 477 | 61 (12.8) | 242 (50.7) | 174 (36.5) | |
| Pulmonary comorbidity (dyspnea or COPD), no. (row %) | <0.001 | ||||
| No | 7486 | 632 (8.4) | 3792 (50.7) | 3062 (40.9) | |
| Yes | 1132 | 164 (14.5) | 575 (50.8) | 393 (34.7) | |
| Ascites, no. (row %) | <0.001 | ||||
| No | 8596 | 782 (9.1) | 4363 (50.8) | 3451 (40.1) | |
| Yes | 22 | 14 (63.6) | 4 (18.2) | 4 (18.2) | |
| Heart disease (CHF, MI, previous PCI, previous cardiac surgery, angina), no. (row %) | <0.001 | ||||
| Not available | 3886 | 384 | 2161 | 1341 | |
| No | 4144 | 336 (8.1) | 1958 (47.2) | 1850 (44.6) | |
| Yes | 588 | 76 (12.9) | 248 (42.2) | 264 (44.9) | |
| History of angina in 1 month before surgery, no. (row %) | 0.11 | ||||
| Not available | 3912 | 392 | 2172 | 1348 | |
| No | 4670 | 398 (8.5) | 2183 (46.7) | 2089 (44.7) | |
| Yes | 36 | 6 (16.7) | 12 (33.3) | 18 (50.0) | |
DISCUSSION
Postoperative complications have been reported to affect up to one quarter of patients following surgery for RM and are associated with both in-hospital mortality and total hospitalization costs [21]. In this study, we assessed the association of BMI and malnutrition with major complications and mortality within 30 days of surgery in 8,618 patients undergoing PN or RN for RM. In this study cohort, the median BMI was 29.2 kg/m2, with an overall prevalence of class I– III obesity of 45% while only 1.1% were underweight. Significant weight loss (>10%) was observed in 2.6% of patients and 15.4% of patients for whom albumin was available had a preoperative albumin of <3.5 g/dl. As for our outcomes of interest, a total of 1,802 complications (20.9%) and 88 deaths (1.0%) were observed within 30 days after surgery. Additionally, we observed an increased risk of complications with RN compared to PN on multivariable modeling. This may be due to an increased risk of chronic kidney disease with RN compared to PN [22] or due to lower complexity related to smaller tumors. Unfortunately, we were unable to account for tumor size, stage, or surgical complexity due to limitations of tumor-specific covariates in the dataset.
We observed that morbid obesity, significant weight loss within 6 months, and serum albumin <3.5 g/dl were independently associated with an increased risk of complications 30 days of surgery. However, significant weight loss was the only nutritional factor independently significantly associated with an increased rate of mortality within 30 days of surgery while there appeared to be a trend with respect to preoperative hypoalbuminemia and early postoperative death. These results highlight the importance of assessing preoperative factors of malnutrition in patients undergoing PN or RN for RM.
The relationship between nutritional status and surgical outcomes has been previously described in patients treated for cancers of the kidney. Morgan and colleagues reported that nutritional deficiency was independently associated with overall mortality (Hazard Ratio [HR] 2.41, 95% CI 1.40–4.18) and disease-specific mortality (HR 2.76, 95% CI 1.17–6.50) in 369 patients undergoing surgical treatment for kidney cancer [14]. Nutritional deficiency was defined as a BMI of <18.5 kg/m2, serum albumin <3.5 g/dl, or preoperative weight loss >5%. Overall survival was 58.5% in the nutritionally deficient cohort vs. 85.4% in controls (p < 0.001). Disease-specific survival was 80.4% and 94.7%, respectively (p < 0.001).
Similarly, Abel and colleagues described that a preoperative serum albumin <3.4 g/dl was a predictor of 90-day mortality on univariate analysis in 162 patients undergoing surgical treatment for kidney cancer with concomitant IVC thrombus above the hepatic veins (OR 8.61, 95% CI 1.81–40.94; p = 0.01) [15]. On multivariable analysis serum albumin <3.4 g/dl was an independent predictor of 90-day mortality (OR 10.13, 95% CI 1.56–65.64; p = 0.02). Within this cohort, 17 patients (10.5%) underwent surgical treatment with a preoperative serum albumin <3.4 g/dl. The authors recommended delaying surgical treatment until serum albumin levels were within normal limits.
The association between preoperative nutritional status and surgical complications for other genitourinary malignancies have also been described in the literature. Johnson and colleagues reported that poor preoperative nutritional status, defined by serum albumin <3.5 g/dl, was an independent predictor of having a postoperative complication in 1,213 patients who underwent radical cystectomy for bladder cancer on multivariable analysis (p = 0.03) [9]. Respiratory complications were most significantly associated with a serum albumin <3.5 g/dl (19% vs. 6%; p < 0.01). BMI was not found to be an independent predictor of increased overall complication rate on multivariable analysis; however, an increased BMI was significantly associated with developing a superficial wound infection (p < 0.001), would dehiscence (p = 0.01), renal insufficiency (p = 0.01), and returning to the OR (p = 0.05) on univariate analysis.
Our study has certain limitations related to its retrospective study design that must be addressed. The use of registry data may be limited due to abstractor accuracy and limited variables or granularity of the data [23]. Importantly, the NSQIP database does not provide tumor stage, grade, and size data, which is a limitation when using this database. The NSQIP database also provides only 30-day outcomes, and not 90-day outcomes. Furthermore, we observed that serum albumin data was only available in 60% of the cohort, which may introduce bias into the study results. As noted, those patients for whom albumin was missing were included in the overall models as a separate category, however, this likely represents a heterogeneous group. Furthermore, albumin is an acute phase reactant that can fluctuate with inflammation and as a single measurement may be an unreliable indicator of nutritional status [24]. Additionally, when assessing true body composition, BMI is nonspecific and does not differentiate excessive adipose tissue from lean muscle mass, which may have implications for both oncologic outcomes as well as perioperative morbidity [25–27]. For example, BMI has been demonstrated to mask sarcopenia, which has been associated with both increased mortality and postoperative complications in kidney cancer [28], as well as in breast, rectal, esophageal, and head and neck malignancies [29–32].
Despite these limitations, to our knowledge this is the first study that demonstrated the association of morbid obesity, hypoalbuminemia and significant weight loss with 30-day complications and/or mortality in a large population-based cohort undergoing PN or RN for RM. This data demonstrates implications for preoperative risk stratification based upon a patient’s nutritional status. To better characterize patients at risk for postoperative complications, we recommend assessing preoperative serum albumin as a marker of malnutrition. Furthermore, patients at risk for adverse outcomes may potentially be identified preoperatively permitting prehabilitative and perioperative nutritional interventions to be implemented accordingly. Surgical rehabilitation includes the use of aerobic and resistant exercises and nutritional optimization to a preoperative albumin of >4 g/dl to improve postoperative outcomes [33, 34]. In a recent randomized control trial, preoperative nutritional interventions were compared in patients undergoing colorectal resection [35]. Patients that received immunonutrient-enriched supplementation 7 days preoperatively and 5 days postoperatively had reduced postoperative complications compared to patients that received standard high-caloric supplementation. Herein, we suggest including nutritional optimization preoperatively into the medical management of all oncologic patients undergoing surgical treatment for RM.
Preoperative nutritional status appears to be an important aspect when assessing and treating oncologic patients, particularly when surgery is the primary treatment. Morbid obesity and poor nutritional status, measured by serum albumin level and significant preoperative weight loss, were all significantly associated with increased moderate-to-severe postoperative complications and/or mortality. Finally, these findings suggest the importance of preoperative risk stratification and counseling prior to surgical treatment of RM. Future work is necessary to better evaluate the correlation of nutritional status and surgical complications in patients undergoing surgical treatment for kidney cancer in a prospective study design.
AUTHORS’ CONTRIBUTION
Karan Arora: Manuscript writing/editing
Kristine T. Hanson: Protocol/project development, Data collection, Data analysis, Manuscript writing/editing
Elizabeth B. Habermann: Protocol/project development, Data collection, Data analysis, Manuscript writing/editing
Matthew K. Tollefson: Protocol/project development, Data collection, Data analysis
Sarah P. Psutka: Protocol/project development, Data collection, Data analysis, Manuscript writing/editing
FUNDING SOURCES
None.
CONFLICT OF INTEREST DISCLOSURES
The authors declare that they have no conflict of interest.
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2017. CA Cancer J Clin. (2017) ;677–30. |
[2] | Motzer RJ , Jonasch E , Agarwal N et al., NCCN Clinical Practice Guidelines in Oncology: Kidney Cancer Version 2.2017. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, (2016) . |
[3] | Buzby G , Mullen J , Matthews D et al., Prognostic nutritional index in gastrointestinal surgery. Am J Surg (1980) ;139160–7. |
[4] | Gibbs J , Cull W , Henderson W et al., Preoperative serum albumin level as a preditor of operative mortality and morbidity: Results from the National VA Surgical Risk Study. Arch Surg. (1999) ;13436–42. |
[5] | Burden S , Todd C , Hill J et al., Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev. (2012) ;11CD008879. |
[6] | Sabzi F , Faraji R . Effect of body mass index on postoperative complications in beating coronary artery surgery. Ethiop J Health Sci. (2016) ;26: :509–16. |
[7] | Ihle C , Freude T , Bahrs C et al., Malnutrition – An underestimated factor in the inpatient treatment of traumatology and orthopedic patients: A prospective evaluation of patients. Injury. (2017) ;48: :628–36. |
[8] | Ross F , Latham G , Joffe D et al., Preoperative malnutrition is associated with increased mortality and adverse outcomes after pediatric cardiac surgery. Cardiology in the Young. (2017) ;1–10. |
[9] | Johnson DC , Riggs SB , Nielsen ME et al., Nutritional predictors of complications following radical cystectomy. World J Urol. (2015) ;33: :1129–37. |
[10] | White JV , Guenter P , Jensen G et al., Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. (2012) ;112: :730–8. |
[11] | American Dietetic Association Evidence Analysis Library: Does serum albumin correlate with weight loss in four models of prolonged protein-energy restriction: Anorexia nervosa, non-malabsorptive gastric partitioning bariatric surgery, calorie-restricted diets or starvation, http://www.adaevidencelibrary.com/conclusion.cfm?conclusionstatementid=251263&highlight=albumin&home=1. Accessed January 10, 2018. |
[12] | American Dietetic Association Evidence Analysis Library: Does serum prealbumin correlate with weight loss in four models of prolonged protein-energy restriction: Anorexia nervosa, non-malabsorptive gastric partitioning bariatric surgery, calorie-restricted diets or starvation, http://www.adaevidencelibrary.com/conclusion.cfm?conclusion_statement_id=251313&highlight=prealbumin&home=1. Accessed January 10, 2018. |
[13] | Skipper A , Ferguson M , Thompson K et al., Nutrition screening tools: An analysis of the evidence. Journal of Parenteral and Enteral Nutrition. (2012) ;36: :292–8. |
[14] | Morgan TM , Tang D , Stratton KL et al., Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol. (2011) ;59: :923–8. |
[15] | Abel EJ , Thompson RH , Margulis V et al., Perioperative outcomes following surgical resection of renal cell carcinoma with inferior vena cava thrombus extending above the hepatic veings: A contemporary multicenter experience. Eur Urol. (2014) ;66: :584–92. |
[16] | Isac WE , Autorino R , Hillyer SP et al., The impact of body mass index on surgical outcomes of robotic partial nephrectomy. BJU Int. (2012) ;110: :E997–1002. |
[17] | Hua X , Ying-Ying C , Zu-Jun F et al., Obesity, hypertension and diabetes mellitus affect complication rate of different nephrectomy techniques. Actas Urol Es. (2014) ;38: :640–6. |
[18] | Charles PC , Matthew HG , James RT et al., Does sarcopenia impact complications and overall survival in patients undergoing radical nephrectomy for stage III and IV kidney cancer? Journal of Endourology (2016) ;30: :229–36. |
[19] | Naeem N , Petros F , Sukumar S et al., Robot-assisted partial nephrectomy in obese patients. J Endourol. (2011) ;25: :101–105. |
[20] | Komninos C , Tuliao P , Koo KC et al., Obesity is not associated with increased operative complications in single-site robotic partial nephrectomy. Yonsei Med J. (2015) ;56: :382–387. |
[21] | Kim SP , Leibovich BC , Shah ND et al., The relationship of postoperative complications with in-hospital outcomes and costs after renal surgery for kidney cancer. BJU Int. (2012) . |
[22] | Kim SP , Thompson RH , Boorjian SA et al., Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: A systematic review and meta-analysis. J Urol. (2012) ;188: :51–7. |
[23] | Johnson EK , Nelson CP . Utility and pitfalls in the use of administrative databases for outcomes assessment. J Urol. (2013) ;190: :17–8. |
[24] | Psutka SP , Carrasco A , Schmidt GD et al., Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer-specific and all-cause mortality. Cancer. (2014) ;120: :2910. |
[25] | Psutka SP , Moynagh MM , Cheville JC et al., The impact of excess fat mass on mortality after radical nephrectomy for renal cell carcinoma: Beyond body mass index. J Urol. (2015) ;193: :e427. |
[26] | De Schutter A , Lavie CJ , Milani RV . The impact of obesity on risk factors and prevalence and prognosis of coronary heart disease-the obesity paradox. Prog Cardiovasc Dis. (2014) ;56: :401–8. |
[27] | Martin L , Birdsell L , Macdonald N et al., Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. (2013) ;311539–47. |
[28] | Psutka SP , Boorjian SA , Moynagh MR et al., Decreased skeletal muscle mass is associated with an increased risk of mortality after radical nephrectomy for localized renal cell cancer. J Urol. (2016) ;195: :270–6. |
[29] | Shachar SS , Deal AM , Weinberg M et al., Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res. (2017) ;23: :658–65. |
[30] | Choi SY , Yoo S , You D et al., Prognostic factors for survival of patients with synchronous or metachronous brain metastasis of renal cell carcinoma. Clin Genitourin Cancer. (2017) . |
[31] | Nakashima Y , Saeki H , Nakanishi R et al., Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg. (2017) . |
[32] | Wendrich AW , Swartz JE , Bril SI et al., Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. (2017) ;71: :26–33. |
[33] | Carli F , Ferreira V . Prehabilitation: A new area of integration between geriatricians, anesthesiologists, and exercise therapists. Aging Clinical and Experimental Research. (2018) ;1–4. |
[34] | Kaya SO , Akcam TI , Ceylan KC et al., Is preoperative protein-rich nutrition effective on postoperative outcome in non-small cell lung cancer surgery? A prospective randomized study. J Cardiothorac Surg. (2016) ;11: :14. |
[35] | Moya P , Soriano-Irigaray L , Ramirez JM et al., Perioperative standard oral nutrition supplements versus immunonutrition in patients undergoing colorectal resection in an enhanced recovery (ERAS) protocol. Medicine. (2016) ;95: :e3704. |




