Transforming the Perioperative Treatment Paradigm in Non-Metastatic RCC—A Possible Path Forward
Abstract
In 2017, there is no adjuvant systemic therapy proven to increase overall survival in non-metastatic renal cell carcinoma (RCC). The anti-PD-1 antibody nivolumab improves overall survival in metastatic treatment refractory RCC and is generally tolerable. Mouse solid tumor models have revealed a benefit with a short course of neoadjuvant PD-1 blockade compared to adjuvant therapy. Two ongoing phase 2 studies of perioperative nivolumab in RCC patients have shown preliminary feasibility and safety with no surgical delays or complications. The recently opened PROSPER RCC trial (A Phase 3 RandOmized Study Comparing PERioperative Nivolumab vs. Observation in Patients with Localized Renal Cell Carcinoma Undergoing Nephrectomy; EA8143) will examine if the addition of perioperative nivolumab to radical or partial nephrectomy can improve clinical outcomes in patients with high risk localized and locally advanced RCC. With the goal of increasing cure and recurrence-free survival (RFS) rates in non-metastatic RCC, we are executing a three-pronged, multidisciplinary approach of presurgical priming with nivolumab followed by resection and adjuvant PD-1 blockade. We plan to enroll 766 patients with clinical stage ≥T2 or node positive M0 RCC of any histology in this global, randomized, unblinded, phase 3 National Clinical Trials Network study. The investigational arm will receive two doses of nivolumab 240 mg IV prior to surgery followed by adjuvant nivolumab for 9 months. The control arm will undergo the current standard of care: surgical resection followed by observation. Patients are stratified by clinical T stage, node positivity, and histology. The trial is powered to detect a 14.4% absolute benefit in the primary endpoint of RFS from the ASSURE historical control of 55.8% to 70.2% at 5 years (HR = 0.70). The study is also powered to detect a significant overall survival benefit (HR 0.67). Key safety, feasibility, and quality of life endpoints are incorporated. PROSPER RCC exemplifies team science with a host of planned correlative work to investigate the impact of the baseline immune milieu and changes after neoadjuvant priming on clinical outcomes.
HISTORY OF PERIOPERATIVE SYSTEMIC THERAPY FOR NON-METASTATIC OR UNRESECTABLE RCC
The rationale for adjuvantly administering an effective systemic therapy is clear—despite surgery, an unacceptably high percentage of patients recur and die from this disease [1]. In 2017, there is no perioperative therapy that has been proven to increase overall survival over surgery alone in non-metastatic disease. In the last 30 years, 13 randomized controlled studies have investigated adjuvant systemic therapies but only one trial has met its primary endpoint [2, 3]. These studies have encompassed nearly 6500 patients and have tested a wide variety of systemic therapies from cytokines to chemotherapy to vaccines to more modern targeted therapies against carbonic anhydrase IX (CA-IX), vascular endothelial growth factor (VEGF) and its receptor (VEGFR), and mammalian target of rapamycin (mTOR) [2]. The majority of studies before 2004 employed observation as a control whereas the more contemporary trials have used placebo.
Moving the targeted therapies against the hypoxia-inducible factor (HIF) axis forward to the adjuvant setting made sense given their wide reaching clinical benefits in upwards of 80% of patients with RCC in the metastatic setting [4]. As of June 2017, six phase 3 adjuvant studies have been executed with three reported [5]. The frontrunner was ECOG 2805, the ASSURE study, a National Clinical Trial Network (NCTN) led trial, which randomized close to 2000 patients who had undergone radical nephrectomy to sunitinib/placebo, sorafenib/placebo, or placebo/placebo. Patients with disease of all histologies were permitted and enrollment targeted UISS intermediate high and very high patients (pT1 grade 3-4, T2-4, or TanyN+) [1, 6]. Unfortunately, no DFS or overall survival benefit was observed. Five year DFS ranged from 54–56% and 5 year OS from 77–81%. Notably, after 1332 of the 1943 patients were enrolled, the study was amended to reduce the starting doses of both sunitinib and sorafenib given intolerability.
The S-TRAC study was the second study to report and also tested one year of adjuvant VEGFR blockade with sunitinib [3]. In this industry sponsored study led by Dr. Ravaud and colleagues, 615 patients with M0 clear cell RCC of higher risk by UISS stage (pT2N0 grade 3-4 or pT3-4N0, or pTxN1) were enrolled. No pT1 or non-clear cell disease was permitted. Patients were randomized to sunitinib 50 mg daily for 4 weeks on and two weeks off or placebo for one year after nephrectomy. No starting dose reductions were allowed and dose reductions on study were limited to only one dose level (37.5 mg/day) as opposed to the ASSURE trial which had permitted reductions to 25 mg daily. The S-TRAC study revealed a greater than 1 year median benefit in DFS at 6.8 years with sunitinib versus 5.6 years with placebo (p = 0.03, HR 0.76, 95% CI 0.59–0.98) on blinded central review. The 3 and 5 year DFS rates were also statistically improved at 64.9% vs. 59.5% and 59.3% vs. 51.3% respectively. Notably, the investigator-assessed DFS difference was not statistically significant though a similar greater than 1 year benefit in median DFS was observed. Overall survival information was reportedly immature but was estimated as having no difference at a median follow-up of 5.4 years with a HR 1.01 (95% CI 0.7–1.4, p = 0.938).
Given the disparate results of these two large randomized trials that studied the same drug, possible etiologies behind the discrepancy include differences in dose exposure, risk status and stage as well as the allowance of non-clear cell histologies and the lack of central review in ASSURE. Given S-TRAC’s later execution, greater experience with the drug and management of its toxicities could have led to higher sunitinib exposure. To investigate these possibilities, the ASSURE investigators undertook post hoc subset analyses based on dose, histology and stage. No differences were observed in DFS or overall survival even in the patients who were exposed to higher doses or when the analysis was restricted to high risk, clear cell disease [7].
The latest study to report is the industry sponsored PROTECT trial where Motzer and colleagues [8] evaluated 52 weeks of pazopanib compared to placebo. Eligibility based on stage was similar to S-TRAC and required pT2N0 grade 3-4 or pT3-4N0, or pTxN1 disease. Similar to the ASSURE study, there were issues with toxicity with the full dosing of pazopanib. After enrollment of 403 patients, the study was amended to decrease the starting dose to 600 mg due to tolerability issues and high treatment discontinuation rate due to adverse events. Subsequently, 1135 patients were enrolled at the 600 mg/placebo dose. The primary endpoint of the study was also amended from DFS at 800 mg to 600 mg and ultimately did not reach statistical or clinical significance (HR 0.86, 95% CI: 0.7–1.06, p = 0.16) in the ITT600 mg subset of patients. However, secondary analyses of the ITT800 mg subset and in all patients at any dose were positive with HR 0.69 and 0.80 respectively. Overall survival in the ITT600 mg group was not statistically significant (HR 0.76, 95% 0.57–1.09, p = 0.16). Thus, it appears pazopanib is not effective at a tolerable dose. The investigators did not recommend adjuvant pazopanib nor does the study sponsor (Novartis) intend to apply for an adjuvant indication [9].
The bottom line is that despite S-TRAC being the first phase 3 study [3] to demonstrate a positive DFS improvement with adjuvant therapy in RCC, many physicians may be reluctant to incorporate it into practice without proof of a definite overall survival benefit due to concerns over the associated toxicity and potential detriments on quality of life during the year of adjuvant treatment. Treatment discontinuation due to toxicity occurred in 44% of patients in the ASSURE sunitinib arm, 28% in S-TRAC, and 35–39% in PROTECT on pazopanib 600 mg and 800 mg respectively. The degree of benefit and impact on quality of life appear to matter significantly to many patients, advocates, and their physicians. The European Association of Urology (EAU) has published consensus guidelines based on evidence review and the opinions of leaders in kidney cancer and patient advocates that have recommended against adjuvant sunitinib given the low benefit-to-harm ratio and the lack of evidence for an overall survival benefit [10]. Pfizer has submitted an application for this indication to the FDA, which is under review. Given the controversial results among the three studies, careful discussion and shared decision making between patients and their doctors will be key to the rational application of sunitinib in the adjuvant setting.
The high frequency of treatment discontinuations for toxicity in all reported adjuvant VEGFR TKI studies and evidence of decreased quality of life during that year sparks the question of whether delayed treatment given only to those who need it at time of recurrence may produce similar outcomes. The outcomes of the other adjuvant TKI and mTOR inhibitor studies such as ATLAS, EVEREST, and SORCE and eventual meta-analyses of these data could be informative. Perhaps even more critical is interrogation of tissue and serum to identify biomarkers better than stage that will help select patients who definitely need the added systemic therapy.
ENGAGING THE IMMUNE SYSTEM TO SYNERGIZE WITH SURGERY
As we await those results, the field has moved forward with testing the latest immunooncologic agents, namely the checkpoint inhibitors against the PD-1 pathway and CTLA-4. This shift is supported by the positive results of the phase 3 study showing the survival benefit of the anti-PD-1 antibody nivolumab compared to everolimus in patients whose disease was refractory to VEGF targeted therapies [11]. Nivolumab remains the only checkpoint inhibitor approved in RCC and is in the metastatic VEGF targeted therapy treatment-refractory setting. Given its ability to increase survival in more advanced disease and its general tolerability, incorporating nivolumab earlier in the micrometastatic setting where disease burden is smaller and the immune system may be more intact makes sense.
As of June 2017, there are multiple immunotherapy perioperative trials planned (www.clinicaltrials.gov). These include at least 3 pure adjuvant studies capitalizing on the standard paradigm of adjuvant therapy after resection. The furthest along is IMmotion 010, which is testing the ability of the PD-L1 antibody atezolizumab to increase DFS over placebo in patients with high-risk RCC who undergo nephrectomy or complete metastectomy (NCT03024996; Study Chairs; S. Pal, R. Uzzo). Two additional studies on the horizon include pembrolizumab vs. placebo (NCT03142334; PI: Choueiri) and the first combination phase 3 study investigating nivolumab/ipilimumab vs. placebo (NCT03138512; PI: Motzer).
TRANSFORMING THE PARADIGM
However, if we consider that the mechanism of action of PD-1 blockade relies on antigen (tumor) and the presence of PD-1/-L1 driven immune cells, there is strong rationale to change the current practice of strictly adjuvant administration and prime the immune system prior to surgery. Specifically, we know that in some kidney cancers, there is an ongoing but unsuccessful anti-tumor T cell response in the primary tumor, microenvironment surrounding the tumor, and the lymph nodes supporting the tumor and affected kidney (Fig. 1) [12]. Work by Woo et al. has shown that in mice models, anti-tumor effector cells may proliferate in these areas after PD-1 blockade [13]. Theoretically, they can then traffic to distant sites where they can eliminate metastatic disease. Effector T cells also have the potential to transform to memory cells and create a surveillance “squad” capable of continual suppression or elimination of metastatic disease or new primaries. Upfront nephrectomy will eliminate the bulk of antigen (tumor) as well as nearby effector cells (“the army”), which produce the necessary immunostimulatory cytokines and could result in a less potent response to adjuvant PD-1 pathway blockade alone. This theory is supported by MacFarlane et al.’s observation of increased presence of circulating PD-1+ myelomonocytic, effector T and natural killer (NK) subsets before nephrectomy for localized RCC, and a subsequent significant reduction after primary tumor resection back to baseline levels in all immune cell types in the majority of patients who had elevated levels pre-surgery [14].
Fig.1
Rationale to prime the immune system with PD-1 blockade prior to nephrectomy. At baseline, it is thought that there is an ongoing but ineffective inflammatory T cell response against the primary tumor. Preclinical work suggests PD-1 blockade may induce proliferation of anti-tumor T cells in the tumor, tumor microenvironment, and lymph nodes supporting the tumor. These antigen specific T cells can then traffic to distant sites through the lymphatic and circulatory system where they can eliminate metastatic disease. They can transform to memory cells capable of continual suppression or elimination of metastatic disease or new primaries. Removal of the kidney tumor by nephrectomy before priming would eliminate the majority of tumor antigen as well as nearby effector cells, which produce the necessary immunomodulatory cytokines, and could result in a less potent response to adjuvant PD-1 pathway blockade alone.
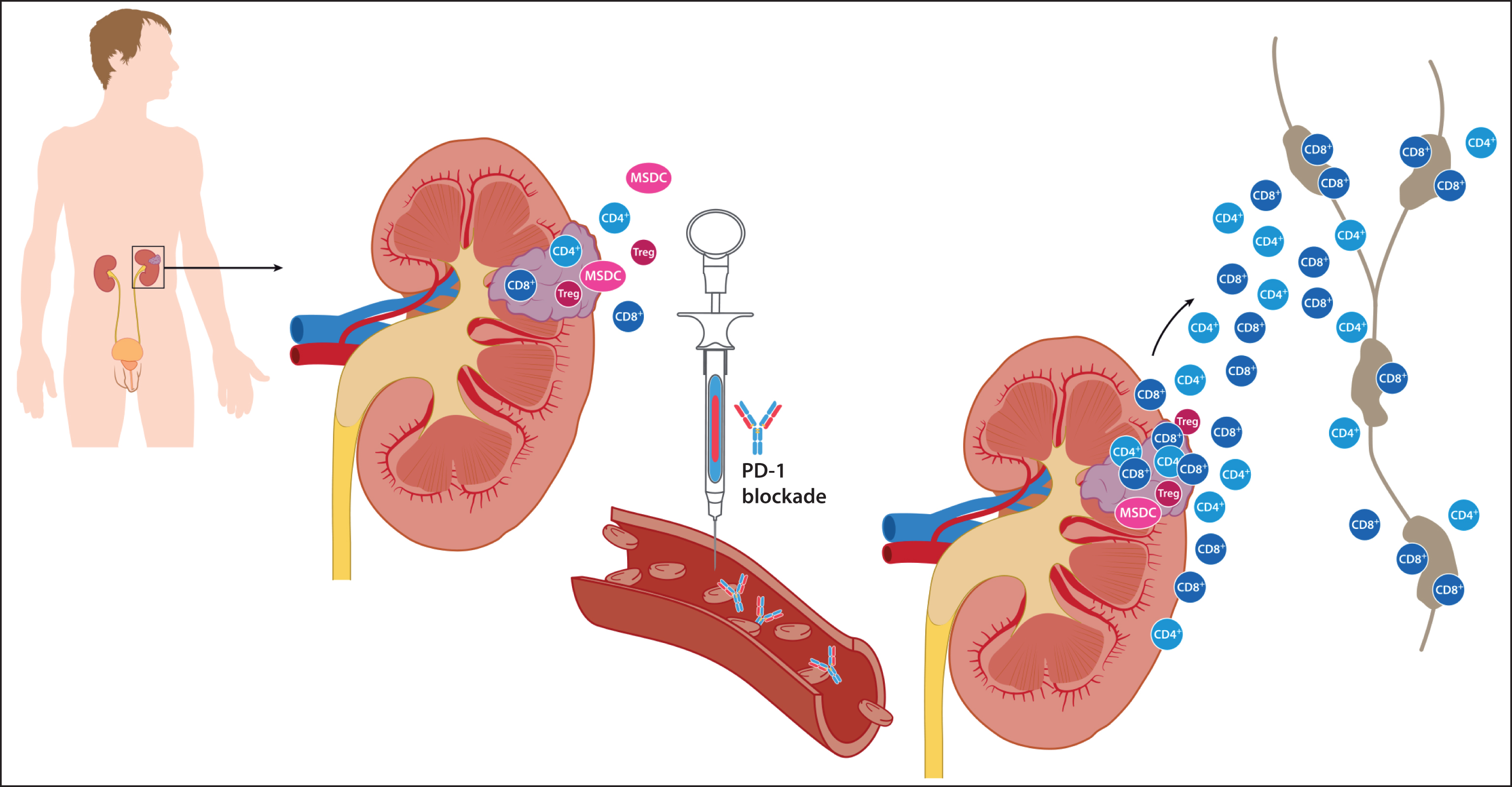
Preclinical evidence is emerging about the potential therapeutic efficacy of a neoadjuvant approach over pure adjuvant admininistration. Liu et al. compared neoadjuvant and adjuvant dosing of various immunotherapeutics using two aggressive solid tumor mouse models 4T1.2 and E0771 [15]. In one of the 4T1.2 models, they found that a short course of neoadjuvant immunotherapy in the form of two doses of PD-1 blockade +/– anti-CD-137 significantly improved survival compared to two doses of adjuvant blockade with the same drugs or control IgG. Specifically, they reported that 50% of the 4T1.2 mice that received the combination neoadjuvantly displayed long-term survival compared to no survivors in the adjuvant arm. Further interrogation of these murine models revealed that the life-prolonging effects were plausibly due to increased and proliferating tumor specific CD8+ T cells that had an effector/memory phenotype, and produced IFN-γ and TNF in the peripheral blood and distant organs [15]. Germane to our proposed neoadjuvant strategy, the researchers noted that the primary tumor was required for expansion of tumor specific T cells. They did not observe the same degree of expansion of tumor-specific T cells following adjuvant blockade. Elevated tumor specific CD8+ T cells in blood early after neoadjuvant dosing was predictive of greater survival and thus, may be a readily accessible biomarker for the clinic especially with therapies reliant upon effector T cell activity.
There are at least two ongoing proof of concept phase 2 studies of perioperative PD-1 blockade in non-metastatic RCC patients that are evaluating 3-4 doses of neoadjuvant nivolumab (NCT02575222 PI M. Allaf; NCT02595918 PI M. Voss). The Hopkins study has revealed preliminary feasibility and safety with no surgical delays or complications (Allaf 2016 International Kidney Cancer Association Symposium). Additional presurgical studies are underway in a variety of metastatic and non-metastatic settings that are testing checkpoint combinations with anti–PD-1 and –CTLA-4 therapies such as durvalumab with or without tremelimumab (NCT02762006, PI B. Rini) and nivolumab with or without ipilimumab or bevacizumab (NCT02210117, PI P. Sharma).
Proof of principle can also be seen in other cancers. Impressive pathologic complete response rates (pCR) have been recently reported in breast cancer and lung cancers with neoadjuvant PD-1 blockade. In a small feasibility study of early stage non-small cell lung cancer (NSCLC), 2 doses of neoadjuvant nivolumab induced pCRs in 43% of patients (n = 21) with no surgical delays (NCT02259621) [16]. In responding tumors, increased tumor infiltration of PD1+CD8+ T cells compared to the pre-nivolumab biopsy was observed. Baseline exome sequencing of the tumor specimens highlighted that both neoantigen and tumor mutation burden corresponded with pathologic response. The ongoing CheckMate 816 phase 3 study is testing this this strategy in randomized fashion (NCT02998528) Similarly in non-metastatic triple negative breast cancer (TNB), the phase 2 I-SPY 2 study demonstrated a tripling in pCR rates from 20% to 60% with the addition of neoadjuvant pembrolizumab compared to standard chemotherapy alone (NCT01042379) [17] prompting the development of KEYNOTE522, a phase 3 study to definitively address the question. The study will randomize patients with early stage TNB to pembrolizumab plus chemotherapy vs. placebo plus chemotherapy followed by 27 weeks of pembrolizumab or placebo adjuvantly after surgery (NCT03036488).
Considering the preclinical observations, principles behind PD-1 pathway blockade, the early clinical feasibility and safety across multiple solid tumors, and the known need for an effective perioperative therapy, the ongoing PROSPER RCC study (EA8143) is designed to optimize for success for our patients (NCT 03055013, Fig. 2, Table 1). We believe that the trifecta of priming, surgery, and adjuvant administration is necessary to achieve improved clinical outcomes. While presurgical priming with PD-1 blockade is required for efficacy, two neoadjuvant doses may not be sufficient for micrometastatic tumor elimination, and thus additional adjuvant therapy is warranted. We will employ nivolumab monotherapy as there is no proven PD-1 blocking combination therapy at the current time and while the results from the combination therapy trials that have been reported show promising efficacy, it can come at the expense of a range of toxicity. While this toxicity may be warranted in the more advanced metastatic setting when we know definitively that disease is present, a finer balance may be needed in the adjuvant setting where some patients are cured with surgery alone. At the time of this report, nivolumab remains the PD-1 inhibitor that is the farthest ahead in metastatic RCC and is an established safe and effective treatment for VEGF targeted therapy refractory disease [11]. PD-L1 tumor testing did not pan out as a predictive marker in the phase 3 study, [11] and currently there in no biomarker which can add to stage to predict patients at higher risk of recurrence. As such, PROSPER RCC targets a higher risk population using AJCC stage as there is no validated predictive marker at present. Finally, tumor biopsy is mandatory prior to randomization to ensure the correct diagnosis of RCC and is considered a standard of care. The resultant tissue will also enable a wealth of unparalleled correlative science.
Fig.2
Schema of The PROSPER RCC Study. A phase III randomized study comparing perioperative nivolumab vs. observation in patients with localized renal cell carcinoma undergoing nephrectomy (PROSPER RCC) NCT 03055013.
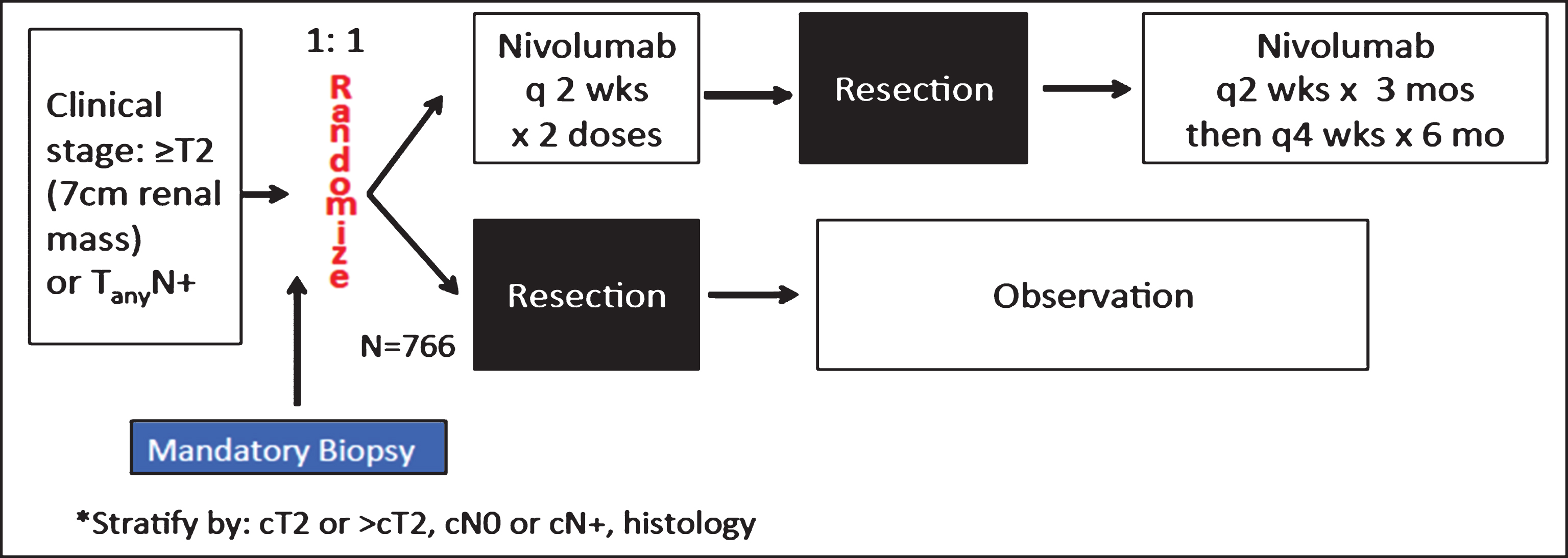
Table 1
Choices made to optimize for success for our patients in PROSPER RCC
| •The trifecta is necessary—presurgical priming with PD-1 blockade is required for efficacy |
| •2 neoadjuvant doses of PD-1 inhibition may not be sufficient to eliminate micrometastatic disease and additional adjuvant therapy is warranted |
| •Nivolumab monotherapy is established as safe and effective in second line metastatic RCC and will be employed as there is currently no proven PD-1 blocking combination therapy |
| •A higher risk population by stage will be targeted but will be unselected by PD-L1 or other metric as there is no validated predictive marker at present |
| •Patients will not be subjected to placebo |
| •A mandatory upfront biopsy will ensure correct RCC diagnosis but also permits unparalleled correlative science |
The overarching objective of the PROSPER RCC trial is to implement a three-pronged approach in high risk RCC to increase cures and recurrence-free survival rates. To accomplish this goal, we aim to harness the immune system first by presurgical priming with the tolerable immunostimulant nivolumab followed by standard of care partial or radical nephrectomy and adjuvant PD-1 blockade with nivolumab. Ultimately, if proven successful, a confirmatory study to tease out the impact of the added neoadjuvant strategy over a strictly adjuvant approach may need to be considered especially if the ongoing pure adjuvant monotherapy studies with other PD-1 pathway inhibitors are positive.
STUDY DESIGN SPECIFICS
PROSPER RCC (EA8143) is a global, randomized, unblinded, phase 3 National Clinical Trials Network study supported by Bristol-Myers Squibb. All histologies of RCC are permitted and 766 patients will be enrolled. Mandatory biopsy of the primary tumor is required unless already performed prior to registration. Patients will undergo 1 : 1 randomization to either the study arm or the control arm stratified by clinical T and N stage as well as histology. Should the biopsy be non-diagnostic, patients may still be randomized as a good faith effort will have been made, and they will be stratified as “unknown” histology. Non-clear cell enrollment will be limited to 15% such that the study has adequate power to evaluate the clear cell subset of patients in terms of DFS and overall survival but their inclusion will allow us to gain our first exploratory experience of perioperative PD-1 blockade in patients with non-clear cell disease.
Eligibility requires patients with clinical T2 or any node positive RCC of any histology with no evidence of metastatic disease who are planned for nephrectomy (partial or radical) (Table 2). They must have good ECOG performance status 0 or 1 and adequate end organ function including hematologic, liver, renal, neurologic and endocrine parameters. Patients cannot have active or suspected autoimmune disorders with the exception of vitiligo, diabetes mellitus type 1, hypothyroidism on stable hormone replacement, psoriasis, or past endocrine conditions that are not expected to recur. They cannot have any conditions requiring steroids >10 mg prednisone or its equivalent daily. Adequately treated Stage I or II cancers for which the patient is in complete remission for 3 years and not on any adjuvant therapy are generally permitted with some limitations. Full eligibility requirements are publically available on www.clinicaltrials.gov.
Table 2
Key eligibility criteria of PROSPER RCC (EA8143)
| Key Inclusion Criteria | Key Exclusion Criteria |
| •Clinical T2 or any node positive RCC | •No active or suspected autoimmune disorders |
| •No evidence of metastatic disease (M0) | - Exceptions: vitiligo, diabetes type 1, hypothyroid on stable hormone |
| •Clear cell or non-clear cell histology | replacement, psoriasis, conditions not expected to recur |
| - Non-clear cell will be limited to 15% | •No disorders requiring steroids >10 mg prednisone or its equivalent daily |
| •Planned for nephrectomy (partial or radical) | |
| •ECOG performance status 0 or 1 | •Limitations on prior cancers |
| •Adequate end organ function—hematologic, hepatic, renal, neurologic |
*See www.clincialtrials.gov for full listing.
Eligible patients will be randomized 1 : 1 to the investigational arm or control (Fig. 2). The study arm will receive nivolumab 240 mg IV for 2 doses prior to surgery followed by nivolumab adjuvantly for 9 months (every 2 weeks for 3 months followed by every 4 weeks for 6 months). The control arm will undergo the current standard of care: surgical resection followed by observation. The decision to increase the dosing interval to monthly after 6 adjuvant doses was to enhance quality of life for the patients. It is rational given the proven tolerability of 10 mg/kg dosing every 2 weeks [18] and based on the company’s PK modeling and simulations that it would be safe and effective once a steady state was achieved with 240 mg every 2 weeks (BMS communication).
The control arm is based on the current standard of care in 2017, which is nephrectomy followed by surveillance/observation. While the S-TRAC study reported a DFS benefit, it was in a higher risk clear cell population and is contrasted with a larger negative study, ASSURE. That data combined with the lack of a proven overall survival benefit, noted decreased quality of life during the year of therapy, and no definite knowledge that early administration is better than delayed therapy provided our rationale for observation after nephrectomy as the control arm. The lack of a placebo control was the result of much interactive debate with patient advocates and key opinion leaders in the field. As PROSPER RCC is a registration study, the FDA has officially weighed in on its design and agreed with these choices. To counteract the potential for evaluation time bias [19] with lack of blinding, interim analyses monitoring timeliness of scans and uniformity of scan intervals among the arms as well as planned sensitivity analyses have been integrated and will assure the robustness of the outcome. Scans will also be banked for eventual blinded central review if warranted.
STATISTICAL CONSIDERATIONS
The primary endpoint is RFS as defined as the time from randomization to disease recurrence or death from any cause. Patients who do not undergo surgery or whose surgery does not render them disease-free or who have other evidence of disease after surgery will be included in the RFS analysis as events occurring on Day 1. Patients without recurrence will be censored at the date of last disease evaluation. We estimate accrual at 264 patients/year for 2.9 years with an 2.1 additional years of follow-up.
With 766 patients, there is 84.2% power to detect a 14.4% absolute benefit in RFS. We employed the ASSURE historical control of 55.8% and are aiming to increase that to 70.2% at 5 years (HR = 0.70) with perioperative PD-1 blockade [6]. Planned key secondary analyses include overall survival and RFS specifically in clear cell patients. There is an 80% power to detect an increase in 5 year overall survival from 78.7% to 85.2% (HR 0.67) and 82% power to detect a 13% increase in RFS in the clear cell subset (HR 0.70). Key safety, feasibility, and quality of life endpoints are incorporated and are critical factors in the eventual overall analysis in this generally otherwise healthy patient population who could be cured with surgery alone.
INTEGRATED CORRELATIVE STUDIES
PROSPER RCC exemplifies team science with a host of planned correlative work to investigate the clinical significance of the baseline immune milieu and changes after neoadjuvant priming with the hopes of identifying predictive biomarkers and gene expression patterns. The study currently brings together a team of basic scientists, immunologists, geneticists, pathologists, radiologists, cardiologists, and endocrinologists. Additional collaborations are welcomed.
IHC and cytokine analysis will evaluate the impact of baseline inflammation and the predictive role of pre-existing intratumoral CD8+ T cells. We will catalogue whether priming increases trafficking of effector T cells to the tumor and induces proliferation and whether tumor PD-L1 expression adaptively increases after nivolumab indicative of local tumor infiltrating lymphocytes on the attack. Whole exome sequencing will be employed to identify neoepitope signatures and characterize mutational patterns and frequency. T cell proliferation assays will be performed to assess response to neoantigens. Nanostring will be used to identify predictive gene expression patterns. Cardio-oncology and endo-oncology substudies are planned in an effort to understand how PD-1 blockade may impact cardiac and bone health and tease out mechanisms behind these effects.
CLOSING THOUGHTS
Surgical monotherapy does not cure a significant number of high risk, non-metastatic RCC patients given the presence of micrometastatic disease at the time of resection. Few advances have been seen with the addition of adjuvant VEGF inhibition and the field is moving forward with testing of the latest immuno-oncologic agents especially those targeting the PD-1 pathway. There is strong preclinical evidence that the mechanism behind PD-1 blockade relies on antigen. Thus, priming the immune system with a stimulant such as nivolumab, when there is a greater burden of tumor antigen present makes sense. Further, the possible science and potential discovery with the addition of neoadjuvant priming is priceless and PROSPER RCC has integrated thoughtful correlative work to identify biomarkers that predict need and efficacy with this trimodal approach. Most importantly, this novel paradigm requires an evolution from our current pure adjuvant approach, which is bolstered by the recognition that this strategy has not advanced the field in the last 30 years in terms of increasing overall survival over surgery alone. We assert that a little hard work upfront to modify our workflow and integrate the PROSPER RCC multidisciplinary approach may mean a significant difference in overall survival for our patients. Key to PROSPER’s success will be cohesive teamwork between the patients, urologists, medical oncologists, pathologists, and scientists.
CONFLICTS OF INTEREST
Potential COI: LC Harshman is the Principal Investigator of the PROSPER RCC trial. All co-authors are co-investigators on the study or were involved in its design.
Harshman LC: Advisory: Bayer, Genentech, Dendreon, Pfizer, Medivation/Astellas, Kew Group, Theragene; Corvus; Research to the institution: Bayer, Sotio, Bristol-Myers Squib, Merck, Takeda, Dendreon/Valient, Jannsen, Medivation/Astellas, Genentech, Pfizer; CME: PER; Travel accommodations: Sanofi.
Drake CG: Advisory/consultant: BMS, Compugen, Roche/Genentech, Regeneron, AZ Medimmune and Merck. Co-inventor on patents licensed from Johns Hopkins to AZ/Medimmune and to BMS.
Haas NB: Advisory: Exelixis, Novartis.
Manola J: None.
Puligandla M: None.
Signoretti S: Research Support: Exelixis, AstraZeneca; Consultant: Merck, AstraZeneca, Verastem.
Cella D: Consultant/advisory: Bayer, Pfizer, Merck, Astellas, BMS, GSK, Novartis, Puma Research to Institution: Bayer, Pfizer, Novartis, Alexion, BMS.
Gupta RT: Bayer Pharma AG (Consultant and Speakers Bureau), Invivo Corp. (Consultant), Halyard Health (Consultant).
Bhatt R: None.
Van Allen E: Advisory: Genome Medical, Tango Therapeutics, Novartis, Genentech/Roche; Research support: Novartis, BMS. Lara P: Consultant for BMS.
Choueiri TK: Research Funding: AstraZeneca, BMS, Exelixis, Genentech, GSK, Merck, Novartis, Peloton, Pfizer, Roche, Tracon, Eisai; Consulting/Advisory Role: AstraZeneca, Bayer, BMS, Cerulean, Eisai, Foundation Medicine Inc., ExelixisGenentech/Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, Prometheus Labs.
Kapoor A: Research funding: Pfizer Oncology, Novartis Oncology, BMS and Roche.
Heng DYC: Advisory: BMS, Pfizer, Novartis, Exelixis.
Shuch B: None.
Jewett M: Advisory: Pfizer, Ipsen, Consultant: Theralase Therapeutics Inc Research Support: Olympus Inc.
George D: Research Support: Astrazeneca; Consultant, Steering Committee: Myovant Sciences, Inc
Michaelson D: Advisory: Pfizer, Novartis, Exelixis.
Carducci MA: Advisory: Astellas, Pfizer, Roche/Genentech, Merck, AbbVIe Research to Institution: Astra Zeneca, EMD Serrano, Pfizer, Bristol-Meyers Squibb, Gilead
McDermott D: Consultant: Kidney Cancer Research: BMS, Pfizer, Merck, Novartis, Eisai, Exelixis, Array BioPharm, Genentech BioOncology; Research Support: Kidney Cancer Research: Prometheus.
Allaf M: Research support: Progenics.
ACKNOWLEDGMENTS
We would like to acknowledge the many hundreds of patients, investigators, and study staff that will make up the PROSPER RCC team. We recognize and appreciate Bristol-Myers Squibb for supporting this study and their medical team chiefly Ian Waxman MD and Corina Taitt MD. We especially thank the ECOG-ACRIN and NCTN operations teams for aiding our execution of this registration study.
REFERENCES
[1] | Zisman A , Pantuck AJ , Dorey F , et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol (2001) ;19: :1649–57. |
[2] | Harshman LXW , Moreira RB , Bosse D , Ares GR , Sweeney C , Choueiri TK . Evaluation of disease-free survival as an intermediate metric for overall survival in localized renal cell carcinoma: A trial-level meta-analysis. J Clin Oncol (2017) ;35: (suppl; abstr 4585). |
[3] | Ravaud A , Motzer RJ , Pandha HS , et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med (2016) . |
[4] | Choueiri TK , Motzer RJ . Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med (2017) ;376: :354–66. |
[5] | Pal SK , Haas NB . Adjuvant therapy for renal cell carcinoma: Past, present, and future. Oncologist (2014) ;19: :851–9. |
[6] | Haas NB , Manola J , Uzzo RG , et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet (2016) ;387: :2008–16. |
[7] | Haas NB , Manola J , Dutcher JP , et al. Adjuvant treatment for high-risk clear cell renal cancer: Updated results of a high-risk subset of the ASSURE randomized trial. JAMA Oncol (2017) . |
[8] | Motzer RJ HN , Donskov F , Gross-Goupil M , Varlamov S , Kopyltsov E , Lee JL , Melichar B , Rini BI , Choueiri TK , Zemanova M , Wood LA , Fahlenkamp D , Reaume MN , Stenzl A , Bao W , Aimone P , Doehn C , Russo P , Sternberg CN . Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with locally advanced renal cell carcinoma (RCC) (PROTECT). J Clin Oncol (2017) ;35: (suppl; abstr 4507). |
[9] | PROTECT RCC Study. https://www.novartis.com/sites/www.novartis.com/files/2017-01-interim-financial-report-en.pdf |
[10] | Bex A , Albiges L , Ljungberg B , et al. Updated European association of urology guidelines regarding adjuvant therapy for renal cell carcinoma. Eur Urol (2017) ;71: :719–22. |
[11] | Motzer RJ , Escudier B , McDermott DF , et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med (2015) ;373: :1803–13. |
[12] | Harshman LC , Choueiri TK , Drake C , et al. Subverting the B7-H1/PD-1 pathway in advanced melanoma and kidney cancer. Cancer J (2014) ;20: :272–80. |
[13] | Woo SR , Turnis ME , Goldberg MV , et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res (2012) ;72: :917–27. |
[14] | Macfarlane AWt , Jillab M , Plimack ER , et al. PD-1 expression on peripheral blood cells increases with stage in renal cell carcinoma patients and is rapidly reduced after surgical tumor resection. Cancer Immunol Res (2014) ;2: :320–31. |
[15] | Liu J , Blake SJ , Yong MC , et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov (2016) ;6: :1382–99. |
[16] | Chaft JE FP, Smith KN, Anagnostou V, Cottrell T, Taube JM, Rekhtman N, Merghoub T, Jones DR, Hellmann MD, Yang SC, Broderick S, Rusch VW, Velculescu VE, Topalian SL, Pardoll DM, Brahmer JR. Neoadjuvant nivolumab in early-stage, resectable non-small cell lung cancers. J Clin Oncol (2017) ;35: (suppl; abstr 8508). |
[17] | Nanda RLM , Yau C , Asare S , Hylton N , Van’t Veer L , Perlmutter J , Wallace AM , Chien AJ , Forero-Torres A , Ellis E , Han H , Clark AS , Albain KS , Boughey JC , Elias AD , Berry DA , Yee D , DeMichele A , Esserman L . Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J Clin Oncol (2017) ;35: (suppl; abstr 506). |
[18] | Topalian SL , Hodi FS , Brahmer JR , et al. Safety, Activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine (2012) . |
[19] | Schluchter MD . Methods for the analysis of informatively censored longitudinal data. Stat Med (1992) ;11: :1861–70. |




