Therapeutic Sequencing in Metastatic Renal Cell Carcinoma
Abstract
The influx of multiple novel therapeutic options in the mRCC field has brought a challenge for treatment sequencing in this disease. In the past few years, cabozantinib, nivolumab and the combination of lenvatinib and everolimus have been approved in the second-line setting. As there is no direct comparison between these agents and the studies have failed to show improved benefit among a biomarker-selected patient population, appropriate patient selection based on clinical factors for individualized therapy is critical. Herein we provide a comprehensive overview of current data from each agent through the discussion of disease biology, clinical trials, potential biomarkers and distilling future perspectives in the field.
INTRODUCTION
Renal cell carcinoma (RCC) is the most frequent renal malignant neoplasm with an incidence of 15.6 cases per 100.000 men and women per year and an estimation of 62.700 new cases in 2016 in the US, [1] accounting for 90% of kidney cancers [2]. While 81% of new cases are diagnosed as locorregional disease, the Surveillance, Epidemiology, and End Results (SEER) database shows that roughly 16% present with de novo metastatic disease [3] and around 20% who undergo nephrectomy for localized disease will eventually relapse [4].
The treatment of metastatic disease has evolved greatly over the past decade with a deeper understanding that the biology of both sporadic and hereditary RCCs are driven by von Hippel-Lindau (VHL) tumor suppressor gene alterations. [5]. These alterations lead to hypoxia-inducible factor (HIF) and vascular endothelial growth factor (VEGF) overexpression, resulting in increased angiogenesis, cell growth and cell survival [5]. Targeting this pathway has led to multiple approvals of new agents such as sunitinib, pazopanib, bevacizumab and axitinib [6–10]. Also, downstream of VEGF is the mammalian target of rapamycin (mTOR) pathway which is now known to play a critical role in RCC development [11]. Agents targeting the mTOR pathway, such as everolimus and temsirolimus, have also been shown to improve outcomes and integrated into the treatment arsenal of mRCC [12–14]. Over the past year, we have had another influx of newly approved agents to the mRCC treatment panorama, such as lenvatinib, [15] nivolumab, [16] and cabozantinib [17, 18]. Collectively, the improvements provided by these innovative drugs have granted an overall survival (OS) approaching 30months [19].
While the principal decision in the past several years had been whether to continue VEGF-directed therapy in the second-line setting or to switch to mTOR inhibitors, now, however, the decision now is much more complex with a wide array of new therapies available.
The current review will focus on distilling the data for the three newer FDA-approved therapies in mRCC (nivolumab, cabozantinib and lenvatinib) through a discussion of disease biology, clinical data and available biomarkers, and also offer what we feel represents an optimal sequencing approach.
LENVATINIB
Disease biology
The ability to generate new blood vessels is an essential part of cancer progression and metastasis formation [20]. Angiogenesis is driven by stimulatory factors, such as VEGF, platelet derived growth factor (PDGF), basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-b). VEGF binds to tyrosine kinase domain of vascular endothelial growth factor receptors (VEGFR) 1, 2 or 3, with VEGFR-2 being the most important angiogenesis promoter. VEGFR-3 is important to activatelymphangiogenesis [21, 22].
Since tumors depend on angiogenesis to sustain growth, agents targeting pro-angiogenic factors have been widely studied in oncology. In this context, lenvatinib (E7080), an oral multitargeted tyrosine kinase inhibitor of VEGFR 1–3, fibroblast growth factor receptor (FGFR) 1–4, platelet derived growth factor receptor alpha (PDGFR-α), RET and c-KIT has been developed. The mechanism of action of lenvatinib involves modulation of angiogenesis by inhibiting VEGFR-2 and lymphangiogenesis by targeting VEGFR-3. Its distinct capability to control angiogenesis also relies on its strength of inhibiting FGFR-1, which may play a role in resistance to VEGFR inhibitors [23]. Importantly, inhibition of lymphangiogenesis through VEGFR-3 has also been shown to suppress lymph node metastasis as well as lung metastasis in mammary tumor models [24].
Other authors were able to demonstrate the ability of lenvatinib to inhibit tumor cell migration and invasion [25]. Lenvatinib has a direct oncogenic effect on tumor cell proliferation by inhibiting RET, c-KIT and PDGFR-α and is active in the tumor microenvironment by blocking PDGFR-α and FGFR.
Phase I data
Phase I data assessing the safety and tolerability of lenvatinib was analyzed in a trial that enrolled 82 patients with advanced, refractory solid malignancies, including 8 patients with RCC. The drug was administered in escalating doses from 0.2 mg to 32 mg once daily in a 28-day cycles. The maximum tolerated dose (MTD) was defined as 25 mg, and the most prevalent toxicities were diarrhea (45% ), hypertension (40% ), nausea (37% ), stomatitis (32% ) and proteinuria (26% ). Dose alterations were required in 54% of patients receiving the MTD. Partial response (PR) was reached in 9% of patients and stable disease (SD) in 46% [26].
Additionally, a phase 1b trial assessed safety, MTD and preliminary antitumor activity of lenvatinib combined with everolimus in advanced or metastatic RCC patients. Twenty patients were enrolled in a 3+3 dose escalating design, starting with 12 mg of lenvatinib and further progressing to 18 mg and 24 mg thereafter combined with 5 mg of everolimus once a day in 28-day cycles. The MTD established for lenvatinib in combination with everolimus was 18 mg once daily. The most important toxicities of any grade reported in all cohorts were fatigue (60% ), mucositis (50% ), proteinuria, diarrhea, vomiting, hypertension and nausea (40% each). Rates of PR and SD were 33% and 50%, respectively [27]. Toxicities were consistent with those previously reported for each single agent alone and considered manageable, permitting the initiation of a phase 2 trial.
Phase 2 data
A randomized phase 2 trial was conducted in 5 countries comparing the combination of lenvatinib and everolimus with each drug alone. The study enrolled a total of 153 patients (51 in the combination arm [L+E], and 50 and 52 in single agent arms everolimus [E] and lenvatinib [L], respectively). The primary objective was progression-free survival (PFS) and secondary measures included OS, objective response rate (ORR), safety and tolerability of thecombination.
Median PFS for the combination was 14.6 months, 5.5 months for everolimus alone and 7.4 months for lenvatinib alone. Although the combination significantly prolonged PFS compared to single-agent everolimus (HR 0.40; p = 0.0005), the difference was not significant compared to lenvatinib alone (HR 0.66; p = 0.12). The ORR was 43% (L+E), 27% (L) and 6% (E) and SD was 41% (L+E), 27% (L) and 52% (E). Median OS was 25.5 months, 19.1 months and 15.4 months, respectively, with the difference between the combination and single agent everolimus being statistically significant (HR 0.51; p = 0.024), while no difference was detected between the two single agents.
The most frequent adverse events (AE) of all grades for the combination were similar to the previous phase 1 study, with diarrhea (65% ), decreased appetite (45% ), fatigue (45% ), vomiting (37% ) and nausea (35% ) being higher than those observed with either agent alone. Rates of grade 3 and 4 AEs were seen in 71% of patients on the combination and described as follows: constipation (37% ), diarrhea (20% ), fatigue (14% ) and hypertension (14% ). Importantly, two grade 5 AEs were deemed related to lenvatinib (one seen with the combination and one seen with lenvatinib alone). Unfortunately, rates of discontinuation were not reported on this trial, although authors did report on the rate of dosereductions required for the combination (71% ).
The impressive and unanticipated benefit in OS is notable and rarely seen in the second-line setting for this disease, especially considering the proportion of poor Memorial Sloan Kettering Cancer Center (MSKCC) risk patients enrolled in the trial (38–44% ). However, it must be approached with caution considering the nature of a phase 2 trial, with a small sample size and lack of power to detect a difference in OS.
NIVOLUMAB
Targeting the immune system to induce an endogenous anti-tumor immune response is the goal of immunotherapy. The recognition of inhibitory signaling pathways that restrain T cell function and favors cancer progression and evasion has been the basis of development of immune checkpoint inhibitors such as nivolumab in recent years.
Nivolumab is a fully human monoclonal antibody that targets programmed death-1 (PD-1, CD279), an inhibitory co-receptor expressed on antigen-activated and exhausted T and B cells, [28] which interacts with two known ligands, B7-H1 and B7-DC (also known as programmed death ligand 1 [PD-L1] and programmed death ligand 2 [PD-L2], respectively). The expression of B7-H1/PD-L1 has been implicated with immune resistance [29, 30] and is associated with poorer outcomes in epithelial malignancies [31–33].
Nivolumab blocks the downregulation of the cellular immune response caused by PD-L1, enhancing T-cell activation and anti-tumor activity [33].
Phase 1 data
In a phase 1 trial, 39 patients with advanced, treatment-refractory solid malignancies were included in a dose-escalation six-patient cohort design, starting with 0.3, 1.0, 3.0 or 10mg/kg, followed by an expansion cohort of 15 patients treated with a dose of 10 mg/kg. Primary objectives were safety, tolerability, MTD and pharmacokinetics. Secondary objectives included anti-tumor activity, pharmacodynamics and immunological endpoints.
As no dose-limiting toxicities were seen, MTD could not be defined. The treatment was well tolerated and most frequent AEs were decreased CD4+ lymphocyte count (35.9% ), lymphopenia (25.6% ), fatigue and musculoskeletal events (15.4% each). Only one grade 3 AE was observed in a patient who developed colitis, which responded to steroids and infliximab [34].
Phase 1b biomarker data
Several studies have attempted to define biomarkers that predict response to nivolumab in order to better select patients who are more likely to benefit from treatment. A prospective biomarker study included 91 patients with mRCC on nivolumab and assessed serum chemokines, tumor infiltrate lymphocytes (TIL), gene expression, T cell repertoire (TCR), and other biomarkers potentially related to clinical outcomes both at baseline and following treatment with nivolumab [35]. Patients were treated with different doses of nivolumab (0.3, 2 or 10 mg/kg Q3W). All patients had a baseline biopsy and repeated biopsies at cycles 2 and 8. PD-L1 expression was assessed by immunohistochemistry and regarded as positive if ≥5%.
Of 56 evaluable baseline biopsies, 32% were PD-L1+. Overall, median OS was not reached for PD-L1+ and was 23.4 months for PD-L1- patients. One-year OS for PD-L1+ and PD-L1- patients were both 71%, although 2-year OS was 64% and 48%, respectively. Importantly, patients with significant tumor response (≥20% reduction in tumor burden) had baseline 1.3-fold differential expression of 311 genes, suggesting that infiltrating immune cells may mediate nivolumab response. Also of note, PD-L1 expression emerged as a potential biomarker for anti-PD-1 treatment[36, 37].
Phase 2 data
A total of 168 patients with clear cell mRCC who had previously failed agents targeting the VEGF pathway were randomly assigned to nivolumab at three different doses of 0.3, 2 or 10 mg/kg once every 3 weeks, with randomization stratified by risk categories as well as number of prior regimens. The primary endpoint was comparison of dose-response relationship of PFS across arms. Secondary endpoints were ORR, time to response (TTR), duration of response (DOR), OS and safety. Other exploratory endpoints were included, such as immune-related PFS (based on immune-related RECIST criteria) and tumor PD-L1 expression [38].
Median PFS was 2.7 months, 4 months and 4.2 months for 0.3, 2 and 10 mg/kg arms, respectively. No dose-response relationship was detected (p = 0.9). Median immune-related PFS (irPFS) was 4.3 months, 5.4 months and 6.9 months in each group (p = 0.6). Median OS was 18.2 months, 25.5 months and 24.7 months in the 0.3, 2 and 10 mg/kg groups, with favorable risk patients achieving longer OS as compared to intermediate and poor risk patients. For PD-L1+ patients, median PFS, ORR and OS were 4.9 months, 31% and not reached, respectively, while the same outcomes were 2.9 months, 18% and 18.2 months for PD-L1- patients.
It is important to mention that the degree of benefit observed in the study was higher than anticipated based on PFS numbers, which may reflect the actual mechanism of action of immunotherapy in that appropriate time is required for the host immune system to mount an anti-tumor immune response. This is also reflected in the longer irPFS observed compared to traditional RECIST-based PFS. Although increased benefit was observed in PD-L1+ patients, those who were PD-L1- still benefited from the treatment.
The safety profile was consistent with that previously seen in the phase 1 study, with most AEs being of low grade in severity. Together with results of safety and efficacy from the previous phase 1 study, the data from this phase 2 trial supported the establishment of a dose of 3 mg/kg every 2 weeks for nivolumab.
Phase 3 data
Based on the impressive findings from phase 1 and 2 trials, a large multi-institutional phase 3 study was undertaken comparing nivolumab with everolimus. A total of 821 patients with advanced clear-cell RCC who had failed one or two prior regimens were enrolled and randomized to receive nivolumab (3 mg/kg every 2 weeks) or everolimus (10 mg once daily). Primary endpoint was OS and secondary endpoints were OR and safety.
Patients on nivolumab achieved an OS of 25 months, compared to 19.6 months on everolimus (HR 0.73; p = 0.002). ORR was higher in the nivolumab group compared to everolimus group (25% and 5%, respectively; p < 0.001), with 31% of responders presenting with ongoing responses at 12 months. Similar PFS was observed between treatment arms (4.6 and 4.4 months with nivolumab and everolimus, respectively; p = 0.11). Benefit from the treatment was seen irrespective of PD-L1 expression, with PD-L1 ≥1% patients presenting a mOS of 21.8 months as compared to 27.4 months of those with PD-L1<1% on nivolumab. As such, PD-L1 was not able to predict treatment outcomes.
Safety profile also favored nivolumab, with fewer patients reporting grade 3/4 AEs (19% vs 37% for nivolumab and everolimus, respectively) and patients less frequently discontinuing the drug due to toxicities. Importantly, side effects reported in this publication reflect drug related adverse events, and not all-cause adverse events. This data established nivolumab as an effective second or third-line option for mRCC patients, leading to FDA approval of the drug in 2015.
CABOZANTINIB
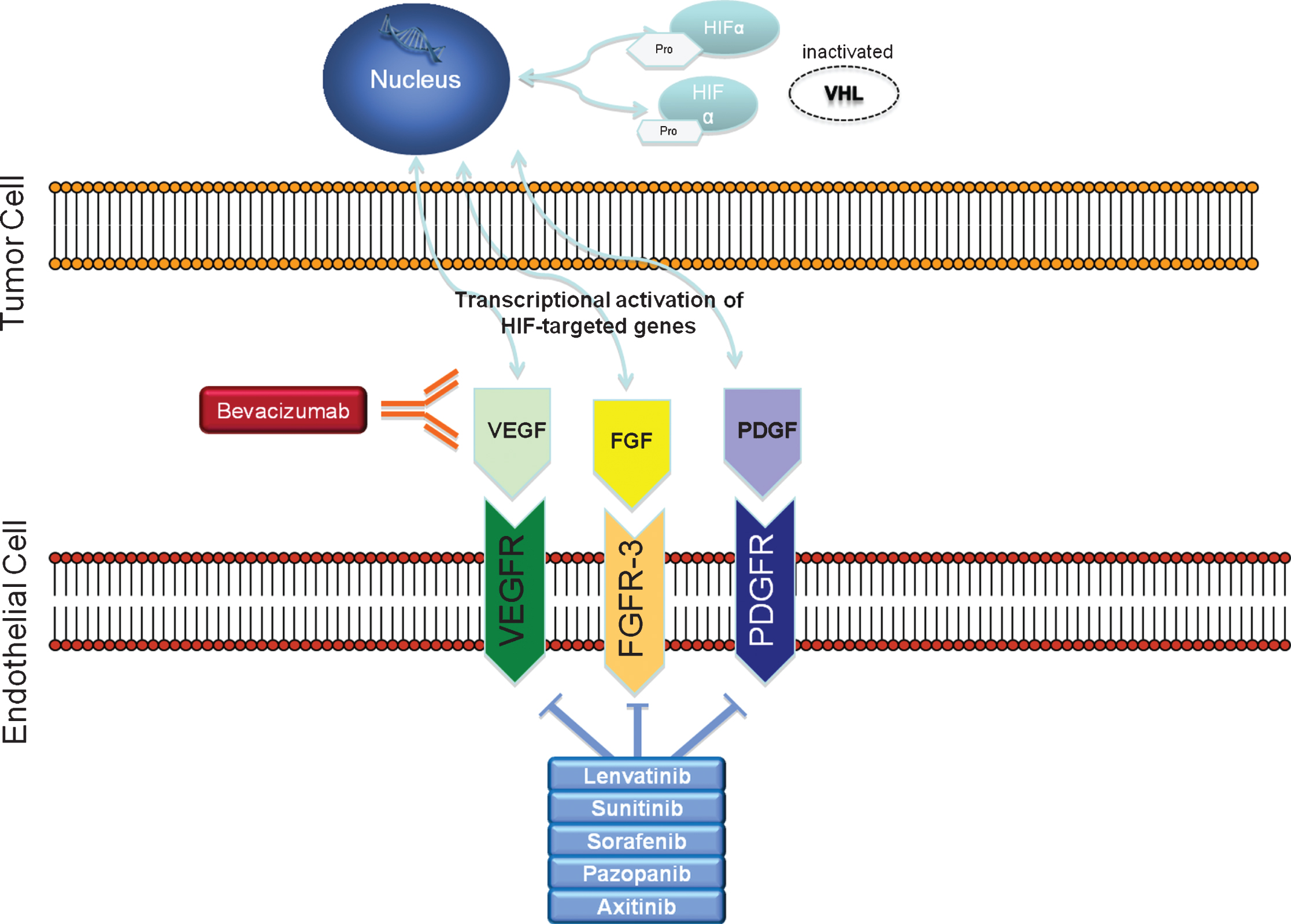
Cabozantinib is an oral inhibitor of multiple tyrosine kinases, including MET, AXL and VEGFR, which, as a consequence of the VHL gene dysfunction, becomes upregulated in clear-cell RCC. Overexpression of MET and AXL products has been associated with poor prognosis in RCC and found to be present upon progression on first-line VEGFR inhibitors, suggesting this is an important alternative proangiogenic and invasive signaling pathway developed by these tumors [39–43]. As such, targeting both VEGFR and MET pathways simultaneously provides advantages over targeting a single pathway alone (Fig. 1) [44, 45].
Fig.1
Renal cell carcinoma‘s disease biology: inactivated VHL gene leads to overexpression of HIF. Genes activated by HIF transcript growth factors such as VEGF, PDGF and FGF.
Phase 1 data
Twenty five heavily pretreated mRCC patients were enrolled in an open-arm phase 1 study assessing safety and tolerability of cabozantinib starting with 140 mg once daily in a 28-day cycle. The vast majority of patients had received at least one VEGF-inhibitor (88% ) and risk categories were represented as follows: 80% of intermediate risk, 8% of poor risk and 12% of good risk [46].
Most frequently reported grade 3/4 AEs were hypophosphatemia (40% ), fatigue (20% ), hyponatremia (20% ), diarrhea (12% ) and lipase increased (12% ) and 24% of patients discontinued the drug due to adverse events. Seven patients (28% ) developed a partial response and 52% had stable disease as their best response. Median PFS was 12.9 months and median OS was 15.0 months. Based on frequent dose reductions to manage tolerability and the continued clinical benefit with the lower dose observed in this trial as well as in others [47, 48], the dose of 60 mg was adopted for further studies in mRCC patients.
Phase 3 data
Based on encouraging results from the aforementioned phase 1 study, a large multi-institutional phase 3 trial was conducted comparing cabozantinib 60 mg once daily with everolimus 10 mg once daily in patients who had previously failed one or more anti-VEGF therapies (METEOR trial) [49]. A total of 658 patients were enrolled, with 45% of them defined as favorable, 42% as intermediate and 13% as poor risk. No cross-over was allowed.
At first interim analysis, the reported median PFS was longer with cabozantinib with 7.4 months as compared to 3.8 months with everolimus (HR 0.58; P < 0.001), showing an impressive 42% reduction in the rate of progression or death. While objective responses were seen in 21% of patients on cabozantinib and 5% in everolimus (P < 0.001), SD was achieved in 62% in each group and PD in 14% of cabozantinib group and 27% in everolimus group. The safety profile did not differ from previous studies, with hypertension (15% ), diarrhea 11% ) and fatigue (9% ) as the most common grade 3/4 AEs. 71% of patients developed grade 3 and 4 AEs. Although toxicities were reported as manageable, 60% of patients receiving cabozantinib required dose reductions due to toxicities, leading to 9% of them discontinuing the drug as compared to 10% of discontinuation rate among everolimus-treated patients. In this scenario, dose reduction was a successful way of maintaining most of the patients on cabozantinib.
In a subsequent publication of the study’s final results, cabozantinib was shown to prolong OS in comparison to everolimus (21.4 months vs 16.5 months, respectively; HR 0.66; p = 0.00026). Results of PFS and OR were similar to those previously reported [18]. An attempt was made to establish a potential predictive biomarker for cabozantinib by analyzing MET expression by immunohistochemistry, but results suggest that MET expression do not correlate with clinical benefit in this population.
OPTIMAL THERAPEUTIC SEQUENCING
As all of these new therapeutic agents have become available as second-line options for mRCC patients with no direct comparison between them, it is now much more difficult to establish how best to sequence these agents. Here we present cross-trial comparisons of reported clinical outcomes of the discussed drugs (Table 1). We acknowledge that cross-trial comparisons are subject to biases and pitfalls and that only clinical trials are able to address these issues when comparing agents against each other. However, in order to help the decision-making process when choosing a second-line agent for mRCC, it is necessary to break down the trials and highlight strengths and weaknesses of each agent. With so many options, it is critical that we individualize therapy in order to maximize efficacy and tolerability while simultaneously minimizing costs. Here we present a discussion of potential advantages of each of the newly approved therapies.
Table 1
Cross-trial comparisons of second-line agents in mRCC
| RECORD-112 | CheckMate 02516 | METEOR18 | Randomized | |
| Phase 215 | ||||
| Regimen | Everolimus vs placebo | Nivolumab | Cabozantinib vs everolimus | Lenvatinib + everolimus |
| vs everolimus | vs lenvatinib vs everolimus | |||
| Patients, N | 416 | 821 | 658 | 153 |
| Risk group, % | ||||
| Favorable | 29% | 36% | 43% | 23% |
| Intermediate | 56% | 49% | 41% | 37% |
| Poor | 14% | 15% | 15% | 40% |
| Median OS, mo | 14.8 m vs 14.4 m | 25 m vs 19.6 m | 21.4 m vs 16.5 m | 25.5 m vs 19.1 m vs 15.4 m |
| HR 0.87 (p = 0.16) | HR 0.73 (p = 0.002) | HR 0.66 (p < 0.001) | HR 0.51 (p = 0.024) | |
| Median PFS, mo | 4.9 m vs 1.87 m | 4.6 m vs 4.4 m | 7.4 m vs 3.8 m | 14.6 m vs 7.4 m vs 5.5 m |
| HR 0.33 (p < 0.001) | HR 0.58 (p < 0.001) | HR 0.40 (P < 0.001) | ||
| ORR (% ) | 1.8% vs 0% | 25% vs 5% | 17% vs 3% | 43% vs 27% vs 6% |
| PD as Best Response | 31.4% vs 67.6% | 35% vs 28% | 14% vs 27% | 4% vs 6% vs 24% |
Nivolumab has some potential advantages over cabozantinib and the combination of lenvatinib plus everolimus. These advantages are related to DOR, low toxicity rates and quality of life (QoL) issues. Regarding the DOR, for instance, previous studies with nivolumab have shown the capability to elicit durable responses both in melanoma and renal cell carcinoma patients. Clinical trials in melanoma reported median DOR as long as 2 years [50, 51]. This has also been shown in mRCC patients, with approximately one third of patients enrolled in the phase 1 study surviving for 5 years and one third surviving for 3 years in the phase 2 trial [52]. Of note, as has also been shown previously in other diseases, a significant proportion of patients with longer survival were those who had stable or even PD as their best responses at first assessment, a common phenomenon observed with immunotherapies [53]. This is highlighted by a subgroup analysis from the phase 2 trial of nivolumab where patients were treated beyond first progression based on RECIST. Among 154 patients who were classified as non-responders at first assessment, 36 were treated beyond progression with 25 (69% ) of them experiencing disease stabilization or tumor regression on subsequent assessments [54]. In the phase 3 CheckMate 025 study, continuation of nivolumab was also permitted after RECIST progression as long as clinical benefit was observed, with treatment discontinuation in the case of a subsequent progression. Of those patients treated beyond first progression, 14% showed ≥30 percent tumor burden reduction thereafter [16, 55]. Of course, these numbers are small and many are skeptical as to whether pseudoprogression is a real phenomenon in mRCC.
However, in the CheckMate 025 trial, the median DOR was 12 months for nivolumab and the same for everolimus. At data cut-off, 48% of those receiving nivolumab had ongoing responses, compared to 45% of those receiving everolimus. Among responders, 31% and 27% had ongoing responses for 12 months or longer in the nivolumab and everolimus groups, respectively. A recent analysis of QoL measures of the phase 3 trial of nivolumab in mRCC reported that more patients in the nivolumab group had meaningful improvements in QoL, while patients on everolimus had a significant deterioration [56]. Limitations with nivolumab include a low response rate (RR) and high rates of primary PD, translating into a short PFS (not statistically different from everolimus). For instance, the RR with nivolumab of 25% is considerably low compared to the combination of lenvatinib and everolimus (RR 43% ), but quite similar to the RR of cabozantinib (17% ). The rate of primary PD in the phase 3 study of nivolumab was high, reaching 35%, as compared to cabozantinib (14% ) and the combination of lenvatinib with everolimus (4% ). PFS with nivolumab was 4.6 months as compared to 4.4 months with everolimus. These observations turn the combination of lenvatinib with everolimus into a very attractive option for those patients requiring high responses in order to control symptoms, while making nivolumab much less interesting in thisscenario.
Many have hypothesized that nivolumab is best placed in the setting of patients with more indolent disease, where a trial of immunotherapy could be safely conducted. However, exploring the HRs for benefit, it actually appears that nivolumab is better in those patients with poor-risk disease (HR 0.89, 0.76 and 0.47 for favorable, intermediate and poor-risk patients, respectively). Paradoxically, cabozantinib performs better in the good- and intermediate-risk disease cohorts for PFS (HR 0.51, 0.47 and 0.70 for favorable, intermediate and poor-risk, respectively).
Specific subgroups may derive increased benefit with one drug over another. This is the case for mRCC patients with bone metastasis, for which there is compelling evidence to support the use of cabozantinib. The presence of bone metastasis is a poor prognostic feature [57, 58] and, although it is a frequent site of metastasis in RCC, [59] it is often resistant to antiangiogenic agents [58]. Preclinical models have provided clues of the participation of both MET and VEGF pathways in promoting bone remodeling [60]. In these models, cabozantinib has shown activity in the modulation of the bone microenvironment, promoting bone remodeling by inhibiting osteoclast differentiation as well as indirectly stimulating osteoblasts [61]. Subsequently, clinical studies of cabozantinib demonstrated the drug’s efficacy in prostate cancer patients with bone metastasis. In a phase II randomized study in men with castration-resistant prostate cancer, cabozantinib showed an impressive response of 68% on a bone scan and was also associated with pain relief in 64% of the patients, [47] a result also seen in another study that reported a similar high bone scan resolution rate [48].
Based on these compelling data, authors of METEOR explored the specific benefit of cabozantinib in bone metastasis of RCC patients in a subset analysis. Exploratory endpoints included bone scan response, incidence of skeletal-related events and changes in bone turnover markers. Of the 658 patients included in the METEOR trial, 142 had bone metastasis, including 112 with concomitant bone and visceral metastasis. Median PFS was 7.4 months in those with bone disease and 5.6 months for those with bone and visceral disease treated with cabozantinib as compared to 2.7 months and 1.9 months with everolimus, respectively, with HR of 0.33 (bone; 95% CI 0.21–0.51) and 0.26 (bone+visceral; 95% CI 0.16–0.43). Scan response was 18% withcabozantinib vs 10% with everolimus. A reduction in the rate of post-randomization skeletal-related events was observed (16% vs 34% with cabozantinib and everolimus, respectively), and a reduction in bone turnover markers was also observed in favor of cabozantinib [62].
Another subset of patients that requires special attention is those with brain metastasis. Brain metastasis is another key adverse prognostic factor, with a low median OS similar to the OS of poor risk patients [63, 64]. Although no specific subgroup analysis has been performed, these patients were included in the METEOR trial and excluded from CheckMate 025. In this scenario, the only available evidence for treating these patients pertains to cabozantinib. In the absence of data for the other drugs on this specific subgroup, cabozantinib may be preferred as it is backed byliterature.
Regarding age subgroups, two different subset analysis of CheckMate 025 trial have been reported. While the original publication reported on results according to three different age strata (<65, 65–75 and ≥75 years of age), the subsequent publication used a different stratification on which age groups were divided in <65 and ≥65 years. Importantly, although the former showed a lower benefit among the elderly population (HR for OS of 0.78, 0.64 and 1.23, for <65, 65–75 and ≥75 years of age, respectively), the latter manuscript results did not support this association with outcomes, rather showing similar benefit among the two groups (HR for OS of 0.78 and 0.74 for <65 and ≥65 years). It is therefore unclear whether age represents a negative predictive factor for nivolumab benefit in mRCC.
The high response rate seen with the combination of lenvatinib with everolimus (43% ) is compelling, making it very attractive for patients who are symptomatic and have high disease burden. However, one must consider that the approval of the combination was based on a phase 2 trial and that the lack of phase 3 data should be taken into account. Many would argue that a higher level of evidence would pend in favor of cabozantinib or nivolumab as preferred choices. Additionally, the high rate of toxicity seen with this combination precludes its widespread use, especially for those patients with borderline performance status, fragile and/or older.
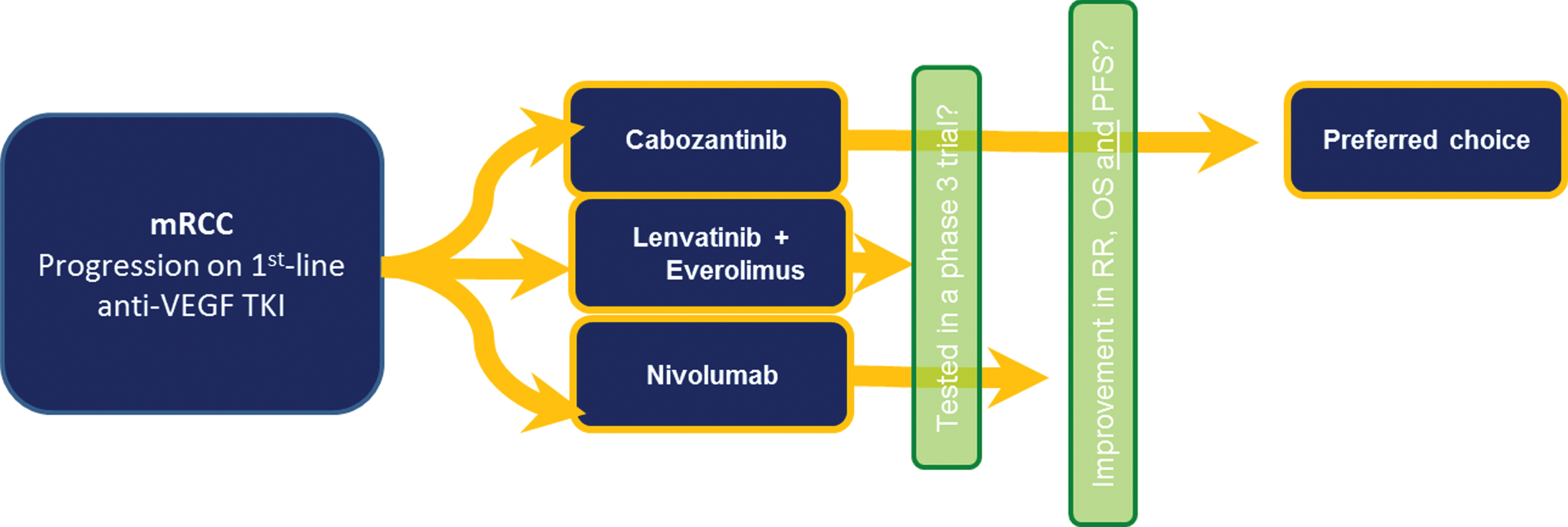
Although there is no clear winner between all three treatment strategies commented herein, cabozantinib may be the preferred second-line agent until we have biomarkers, especially for those aforementioned subgroups that derived increased benefit, that is, elderly, bone metastasis, brain metastasis, good and intermediate risk patients (Fig. 2).
Fig.2
Suggested considerations when choosing the second-line agent in mRCC.
POTENTIAL BIOMARKERS
The development of predictive biomarkers is essential in the era of personalized medicine, allowing physicians to choose the right option for the appropriate patient with mRCC. In this disease, genomic profiling has added valuable information about potential biomarkers in clear-cell RCC patients. Data from the RECORD-3 clinical trial (which evaluated first-line sunitinib followed by everolimus versus the reverse sequence) revealed that a mutation in PBRM1 was associated with a comparable PFS benefit between everolimus and sunitinib (11.5 months versus 11.0 months, respectively), while patients with wild-type PBRM1 derived increased benefit with sunitinib as compared to everolimus (8.3 months versus 5.3 months, respectively). Also, in the same study, alterations KDM5C could predict for activity of VEGF inhibition, with KDM5C-mutated patients presenting a median PFS of 20.6 months with sunitinib as compared to 9.8 months with everolimus in the first-line setting [65].
Another study explored the association of genomic alterations with response to anti-VEGF agents. Exceptional responders to anti-VEGF agents more commonly bore alterations in KDM5C, PBRM1, and VHL, as reported in the study. Although these findings require prospective validation, they provide support for the use of comprehensive genomic profiling when selecting appropriate treatments in clinicalpractice [66].
Other datasets imply that alterations in mTOR, TSC1, TSC2 may predict exceptional responses to everolimus as they were more common among responders (28% ) than non-responders (11% ) in one study that retrospectively analyzed tumor DNA from patients who experienced distinct clinical benefit with mTOR inhibitors. We could speculate that these patients could potentially be better candidates for lenvatinib/everolimus [67]. However, it is important to note that a great proportion of responders did not show any mTOR alterations (56% ).
The great outcomes seen with immunotherapies in many solid tumors have led to the exploration of multiple immune biomarkers in this field. Several noteworthy studies have shed light on the association between mutational load and response to immunotherapies, especially in lung cancer and melanoma. Somatic mutations lead to a generation of neoantigens, which will ultimately be recognized by immune cells as non-self proteins, generating an anti-tumor immune response [68]. This response can be harnessed by checkpoint inhibitors, such as anti-CTLA4 or anti-PD-1 agents. A study with non-small cell lung cancer patients treated with pembrolizumab showed that elevated mutational burden was associated with increased overall response rate, PFS and durable clinical benefit (partial or stable response lasting more than 6 months). This study also highlighted that high mutational burden and quantity of neoantigens per tumor correlated with an increased benefit from pembrolizumab in the cohort analyzed [69]. Similarly, in melanoma, mutational burden and the consequent generation of neoepitopes strongly correlated with response to anti-CTLA4 agents [68]. Other reports called attention to increased benefit with pembrolizumab in mismatch deficient tumors, as they usually bear as much as 20 times greater the number of mutations [70].
While melanoma and lung cancer are solid malignancies recognized to bear a high number of mutations, RCC is a disease with a comparatively low mutational burden. Nevertheless, data for the association between mutational landscape and response to immunotherapies in RCC patients is growing, although still not definitive, as some studies showed conflicting results. In one study, the neoantigen load and mutational burden correlated with ORR and durable clinical benefit rate (compared to non-responders) in mRCC patients treated with nivolumab monotherapy, but not for patients receiving nivolumab in combination with ipilumumab [71]. In a different study, a higher number of somatic mutations and a higher number of mutation-associated neoantigens were found in exceptional responders to PD-1 blockade in RCC patients [72]. In contrast to the previous cited studies, however, deVelasco et al reported that nivolumab benefit was not associated with a higher number of mutations, neoantigens or signatures of immune infiltration among mRCC patients, differently from other tumortypes [73].
Still in the field of immune biomarkers, the predictive value of PD-L1 expression has been extensively explored among solid malignancies with good responses to immunotherapeutic compounds. In some solid tumors, [51, 74] but especially in lung and bladder cancers, [75] PD-L1 expression has emerged as another potential biomarker for anti-PD-1 response, although it is yet to be proven in ccRCC, as results have been variable.
In a phase I trial of atezolizumab that included a cohort of RCC patients, a higher PD-L1 expression (defined as ≥1% ) correlated with increased ORR compared to lesser expressors (ORR of 20% and 10%, respectively, but reaching 38% in those with PD-L1 ≥10% ) [76]. Based on the data presented, we must conclude that the utilization of PD-L1 as a biomarker for immunotherapy so far is still an unresolved issue, with many different factors competing for this: the appropriate cutoff is not well established, best location within tumor microenvironment to assess is not defined (whether it should be in tumor tissue or in TIL) and the timing, as there is clearly a diversity when assessing it in the primary tumor or later when metastatic sites develop [77–79]. Additionally, various modifications within tumor microenvironment occur over time as patients are sequentially exposed to different agents and disease advances [80, 81]. At the same time, it is now clear that other factors influence on response to immunotherapies since some PD-L1 –patients happen to benefit from the treatment and some PD-L1 + patients do not [36].
FUTURE DIRECTIONS
Future perspectives include a multitude of combinatorial regimens intended to maximize clinical benefit. A wide range of approaches are currently being tested, involving either immunotherapies alone, immunotherapies with anti-VEGF agents (TKIs or monoclonal antibodies) or other combinations with innovational approaches, such as vaccines (Table 2). The rationale for combined immunotherapy and VEGF agents is based not only on efficacy of each therapy alone, but also on the fact that VEGF has been implicated with immune suppression, so VEGF blockage may further facilitate the activity of immunotherapies [82, 83].
Table 2
Selected Ongoing Clinical Trials in mRCC
| Immunotherapy + Anti-VEGF | ||||||
| Clinical Trial | Phase | Population | Experimental arm | Control | Endpoints | Accrual Goal |
| NCT02853331 | III | Front-line | Pembrolizumb 200 mg q3 w IV + Axitinib 5 mg BID | Sunitinib 50 mg qd (4 weeks on, 2 weeks off) | 1st = PFS, OS; 2nd = ORR, DCR, AEs. | 840 |
| NCT 02811861 | III | Front-line | Everolimus 5 mg qd + Lenvatinib 18 mg qd or Pembrolizumab 200 mg q3 w IV + Lenvatinib 20 mg qd | Sunitinib 50 mg qd (4 weeks on, 2 weeks off) | 1st = PFS; 2nd = OS, ORR, TTF, AEs, | 735 |
| NCT02420821 | III | Front-line | Atezolizumab 1200 mg IV Days 1 and 22 every 42 days +/–Bevacizumab 15 mg/kg | Sunitinib 50 mg qd (4 weeks on, 2 weeks off) | 1st: PFS, OS; 2nd: ORR, DOR, QoL measures, AEs, PK measures | 830 |
| NCT02684006 | III | Front-line | Avelumab 10 mg/kg q2 w + Axitinib 5 mg BID | Sunitinib 50 mg qd (4 weeks on, 2 weeks off) | 1st = PFS; 2nd = ORR, time to tumor response, DOR, QoL measures, PK/PD measures | 583 |
| Combination of immunotherapies | ||||||
| NCT02231749 | III | Front-line | Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg q3 w followed by nivolumab 3 mg/kg q2w | Sunitinib 50 mg qd (4 weeks on, 2 weeks off) | 1st = PFS, OS; 2nd = ORR, AEs | 1070 |
| NCT02089685 | I/II | Front-line | Pembrolizumab 200mg IV q3W + peguilated IFN SC qw | Pembrolizumab | 1st = PFS, safety; 2nd = OS, ORR, DOR | 343 |
Dual immune checkpoint inhibition with the combination of nivolumab with ipilimumab have already called much attention in patients with metastatic untreated melanoma, on which this combination demonstrated impressive RR and gains in PFS [84, 85]. Its role in mRCC patients has been recently suggested in a phase I study (NCT01472081). Patients were exposed to a series of three different doses of the combination in two dosing cohorts (nivolumab 3 mg/kg with ipilimumab 1 mg/kg; nivolumab 1 mg/kg with ipilimumab 3 mg/kg; nivolumab 3 mg/kg with ipilimumab 3 mg/kg; all given in a 3-weekly basis), followed by a maintenance phase of nivo alone (nivo 3 mg/kg every 2 weeks until progression). The ORR, median PFS and median DOR were 43%, 36 weeks and 54 weeks, respectively, for the nivolumab 3 mg/kg with ipilimumab 1 mg/kg arm [86]. This combination is also being evaluated in a phase III trial against sunitinib in the first-line setting, which has completed its accrual phase and results are anticipated (NCT02231749). In the same phase 1 study, two other arms were included with nivolumab combined with either pazopanib or sunitinib. Although a high ORR was seen with both pazopanib (45% ) and sunitinib (53% ), a high rate of grade ≥3 toxicities were also reported (73% in sunitinib arm and 60% in pazopanib arm) and precluded the subsequent continuation of the former arm pazopanib, mainly due to liver toxicity (40% ) [87].
Pembrolizumab, another anti-PD-1 agent that has gained approval for metastatic melanoma, is also being evaluated in mRCC. Two randomized phase II studies are ongoing. One of them is testing pembrolizumabalone or in combination with pegylated interferon-α (NCT02089685). In the other study, it is being tested alone or in combination with pazopanib (NCT02014636). A randomized phase III study evaluating efficacy and safety of pembrolizumab in combination with axitinib compared to sunitinib monotherapy is also underway (NCT02853331). Additionally, another phase 3 trial is exploring the association lenvatinib + everolimus versus pembrolizumab + lenvatinib versus sunitinib alone in the first-line setting (NCT02811861).
Atezolizumab, a monoclonal antibody that targets PD-L1, has shown activity in mRCC and is being evaluated in phase II as well as in phase III trials. First data came from a phase I study, on which 70 patients with mRCC were treated with atezolizumab, showing a RR of 15% and a median DOR of 17 months [88]. This agent was also studied in combination with a VEGF agent (bevacizumab). A phase Ib study showed promising results with this association with an ORR of 40% and a good safety profile, leading to a subsequent phase II trial that has just recently been presented. A total of 305 patients were accrued to one of three arms in the first-line setting: atezolizumab with (n = 101) or without bevacizumab (n = 103) or sunitinib (n = 101). The HR for PFS (the primary endpoint of the study) among the intention-to-treat population was 1.0 (P = 0.98) for combination group versus sunitinib and 1.19 (P = 0.35) for atezolizumab alone versus sunitinib. Promising results were shown, however, for PD-L1+ patients (HR 0.64 for combination versus sunitinib, P = 0.095 and 1.03 for atezolizumab versus sunitinib, P = 0.91) [89]. The corresponding phase III trial exploring this combination in the front-line setting is under way (NCT02420821).
Avelumab, a monoclonal antibody that targets PD-L1, is another immunotherapeutic compound that is being tested in conjunction with a targeted agent. A phase III trial (JAVELIN Renal 101) is currently open and assigning patients to avelumab plus axitinib or sunitinib alone in the first-line setting (NCT02684006).
Novel approaches targeting the immune system include vaccines. A phase II study with the autologous dendritic cell-based vaccine AGS-003 was studied in combination with sunitinib in intermediate and poor-risk advanced untreated clear cell RCC patients. A total of 21 patients were enrolled and the study showed a median PFS of 11 months and median OS of 30 months [90]. Based on these results, a phase III trial was conducted in which mRCC patients undergoing debulking nephrectomy are subsequently randomized to sunitinib plus AGS-003 (given in 8 intradermal injections in 12 months followed by boosters every 3 months) or sunitinib alone until disease progression (NCT01582672). A recent press release suggests that this study did not meet its primary endpoint of achieving improved overall survival [91]. Another cancer vaccine, IMA901, which is based on multiple tumor-associated peptides and given after a single-dose cyclophosphamide, has been tested in conjunction with both GM-CSF and sunitinib in first-line setting for mRCC. A phase II trial demonstrated an increased OS in patients with immune responses pre-treated with cyclophosphamide (hazard ratio = 0.38, P = 0.040) [92]. However, a phase III trial recently published failed to corroborate an OS benefit with the addition of IMA901 to first-line in HLA-A*02 positive patients of favorable or intermediate-risk. In fact, the HR favored the control arm [93, 94].
CONCLUSIONS
Much like the era in which sequencing of second-line axitinib and everolimus was frequently discussed, we are now approaching a point when the same debate centers on use of cabozantinib, nivolumab or lenvatinib with everolimus. However, as the previous section underscores, combination therapies are likely to emerge in the first-line space. If regimens such as nivolumab with ipilimumab or anti-VEGF therapies with PD-1 inhibitors supplant sunitinib, the second-line debate skews to what treatment options would be most relevant following immunotherapeutic agents. Biomarkers (cited herein and beyond) would likely become key decision points in discerning appropriate therapy.
CONFLICT OF INTEREST
The authors have no conflict of interest to report
ACKNOWLEDGMENTS
None
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2016. CA Cancer J Clin (2016) ;66: (1):7–30. PubMed PMID: 26742998. |
[2] | Ljungberg B , Campbell SC , Choi HY , Jacqmin D , Lee JE , Weikert S , et al. The epidemiology of renal cell carcinoma. Eur Urol (2011) ;60: (4):615–21. PubMed PMID: 21741761. |
[3] | SEER Cancer Statistics Review, 1975-2013, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Internet]. |
[4] | Stukalin I , Alimohamed N , Heng DY . Contemporary treatment of metastatic renal cell carcinoma. Oncol Rev (1582) ;10: (1):295. PubMed PMID: 27471582. Pubmed Central PMCID: PMC4943094. |
[5] | Kim WY , Kaelin WG . Role of VHL gene mutation in human cancer. J Clin Oncol (2004) ;22: (24):4991–5004. PubMed PMID: 15611513. |
[6] | Motzer RJ , Hutson TE , Cella D , Reeves J , Hawkins R , Guo J , et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. The New England Journal of Medicine (2013) ;369: (8):722–31. |
[7] | Sternberg CN , Davis ID , Mardiak J , Szczylik C , Lee E , Wagstaff J , et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol (2010) ;28: (6):1061–8. PubMed PMID: 20100962. |
[8] | Motzer RJ , Escudier B , Tomczak P , Hutson TE , Michaelson MD , Negrier S , et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. The Lancet Oncology (2016) ;14: (6):552–62. |
[9] | Motzer RJ , Hutson TE , Tomczak P , Michaelson MD , Bukowski RM , Oudard Sp , et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology (2009) ;27: (22):3584–90. |
[10] | Rini BI , Bellmunt J , Clancy J , Wang K , Niethammer AG , Hariharan S , et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J Clin Oncol (2014) ;32: (8):752–9. PubMed PMID: 24297945. |
[11] | Pal SK , Figlin RA , Reckamp KL . The role of targeting mammalian target of rapamycin in lung cancer. Clin Lung Cancer (2008) ;9: (6):340–5. PubMed PMID: 19073516. |
[12] | Motzer RJ , Escudier B , Oudard S , Hutson TE , Porta C , Bracarda S , et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors. Cancer (2010) ;116: (18):4256–65. |
[13] | Motzer RJ , Escudier B , Oudard Sp , Hutson TE , Porta C , Bracarda S , et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. The Lancet (2008) ;372: (9637):449–56. |
[14] | Hudes G , Carducci M , Tomczak P , Dutcher J , Figlin R , Kapoor A , et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med (2007) ;356: (22):2271–81. PubMed PMID: 17538086. |
[15] | Motzer RJ , Hutson TE , Glen H , Michaelson MD , Molina A , Eisen T , et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol (2015) ;16: (15):1473–82. PubMed PMID: 26482279. Epub 2015/10/21. eng. |
[16] | Motzer RJ , Escudier B , McDermott DF , George S , Hammers HJ , Srinivas S , et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. The New England Journal of Medicine (2015) ;373: (19):1803–13. |
[17] | Choueiri TK , Escudier B , Powles T , Mainwaring PN , Rini BI , Donskov F , et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. New England Journal of Medicine (2015) ;373: (19):1814–23. |
[18] | Choueiri TK , Escudier B , Powles T , Tannir NM , Mainwaring PN , Rini BI , et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol (2016) ;17: (7):917–27. PubMed PMID: 27279544. Epub 2016/06/10. Eng. |
[19] | Gong J , Gerendash B , Dizman N , Khan A , Pal SK . Advances in treatment of metastatic renal cell carcinoma. Curr Opin Urol (2016) ;26: (5):439–46. PubMed PMID: 27467136. |
[20] | Carmeliet P , Jain RK . Angiogenesis in cancer and other diseases. Nature (2000) ;407: (6801):249–57. PubMed PMID: 11001068. |
[21] | Dvorak HF . Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol (2002) ;20: (21):4368–80. PubMed PMID: 12409337. |
[22] | Karkkainen MJ , Makinen T , Alitalo K . Lymphatic endothelium: A new frontier of metastasis research. Nat Cell Biol (2002) ;4: (1):E2–5. PubMed PMID: 11780131. |
[23] | Stjepanovic N , Capdevila J . Multikinase inhibitors in the treatment of thyroid cancer: Specific role of lenvatinib. Biologics (2014) ;8: :129–39. PubMed PMID: 24748771. Pubmed Central PMCID: PMC3990290. |
[24] | Matsui J , Funahashi Y , Uenaka T , Watanabe T , Tsuruoka A , Asada M . Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res (2008) ;14: (17):5459–65. PubMed PMID: 18765537. |
[25] | Glen H , Mason S , Patel H , Macleod K , Brunton VG . E7080, a multi-targeted tyrosine kinase inhibitor suppresses tumor cell migration and invasion. BMC Cancer (2011) ;11: :309. PubMed PMID: 21781317. Pubmed Central PMCID: PMC3154179. |
[26] | Boss DS , Glen H , Beijnen JH , Keesen M , Morrison R , Tait B , et al. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer (2012) ;106: (10):1598–604. PubMed PMID: 22516948. Pubmed Central PMCID: PMC3349182. |
[27] | Molina AM , Hutson TE , Larkin J , Gold AM , Wood K , Carter D , et al. A phase 1b clinical trial of the multi-targeted tyrosine kinase inhibitor lenvatinib (E7080) in combination with everolimus for treatment of metastatic renal cell carcinoma (RCC). Cancer Chemother Pharmacol (2013) ;73: (1):181–9. PubMed PMID: 24190702. Pubmed Central PMCID: Pmc3889692. Epub 2013/11/06. eng. |
[28] | Keir ME , Butte MJ , Freeman GJ , Sharpe AH . PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol (2008) ;26: :677–704. PubMed PMID: 18173375. |
[29] | Iwai Y , Ishida M , Tanaka Y , Okazaki T , Honjo T , Minato N . Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A (2002) ;99: (19):12293–7. PubMed PMID: 12218188. Pubmed Central PMCID: PMC129438. |
[30] | Dong H , Strome SE , Salomao DR , Tamura H , Hirano F , Flies DB , et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med (2002) ;8: (8):793–800. PubMed PMID: 12091876. |
[31] | Thompson RH , Kuntz SM , Leibovich BC , Dong H , Lohse CM , Webster WS , et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res (2006) ;66: (7):3381–5. PubMed PMID: 16585157. |
[32] | Thompson RH , Gillett MD , Cheville JC , Lohse CM , Dong H , Webster WS , et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A (2004) ;101: (49):17174–9. PubMed PMID: 15569934. Pubmed Central PMCID: PMC534606. |
[33] | Hamanishi J , Mandai M , Iwasaki M , Okazaki T , Tanaka Y , Yamaguchi K , et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A (2007) ;104: (9):3360–5. PubMed PMID: 17360651. Pubmed Central PMCID: PMC1805580. |
[34] | Brahmer JR , Drake CG , Wollner I , Powderly JD , Picus J , Sharfman WH , et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol (2010) ;28: (19):3167–75. PubMed PMID: 20516446. Pubmed Central PMCID: PMC4834717. |
[35] | Choueiri TK , Fishman MN , Escudier B , McDermott DF , Drake CG , Kluger H , et al. Immunomodulatory Activity of Nivolumab in Metastatic Renal Cell Carcinoma. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research (2016) ;22: (22):5461–71. PubMed PMID: 27169994. Pubmed Central PMCID: PMC5106340. Epub 2016/11/01. eng. |
[36] | Choueiri TK , Fishman MN , Escudier BJ , Kim JJ , Kluger HM , Stadler WM , et al. Immunomodulatory activity of nivolumab in previously treated and untreated metastatic renal cell carcinoma (mRCC): Biomarker-based results from a randomized clinical trial. J Clin Oncol (2014) ;32: :15_suppl, 5012-5012. |
[37] | Toni K , Choueiri MNF , Escudier B , McDermott DF , Kluger HM , Stadler WM , Perez-Gracia JL , McNeel DG , Curti BD , Harrison MR , Plimack ER , Appleman LJ , Fong L , Drake CG , Young TC , Chasalow SD , Ross-Macdonald P , Simon JS , Walker D , Sznol M . Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma (mRCC): Association of biomarkers with clinical outcomes. J Clin Oncol (2015) ;33: :15_suppl, 4500-4500. |
[38] | Motzer RJ , Rini BI , McDermott DF , Redman BG , Kuzel TM , Harrison MR , et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol (2015) ;33: (13):1430–7. PubMed PMID: 25452452. Epub 2014/12/03. eng. |
[39] | Gibney GT , Aziz SA , Camp RL , Conrad P , Schwartz BE , Chen CR , et al. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol (2012) ;24: (2):343–9. PubMed PMID: 23022995. Pubmed Central PMCID: 3551486. Epub 2012/10/02. eng. |
[40] | Gustafsson A , Martuszewska D , Johansson M , Ekman C , Hafizi S , Ljungberg B , et al. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res (2009) ;15: (14):4742–9. PubMed PMID: 19567592. |
[41] | Rankin EB , Fuh KC , Castellini L , Viswanathan K , Finger EC , Diep AN , et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci U S A (2014) ;111: (37):13373–8. PubMed PMID: 25187556. Pubmed Central PMCID: PMC4169907. |
[42] | Zhou L , Liu XD , Sun M , Zhang X , German P , Bai S , et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene (2016) ;35: (21):2687–97. PubMed PMID: 26364599. Pubmed Central PMCID: PMC4791213. |
[43] | Shojaei F , Lee JH , Simmons BH , Wong A , Esparza CO , Plumlee PA , et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res (2010) ;70: (24):10090–100. PubMed PMID: 20952508. |
[44] | Harshman LC , Choueiri TK . Targeting the hepatocyte growth factor/c-Met signaling pathway in renal cell carcinoma. Cancer J (2013) ;19: (4):316–23. PubMed PMID: 23867513. |
[45] | Pinato DJ , Chowdhury S , Stebbing J . TAMing resistance to multi-targeted kinase inhibitors through Axl and Met inhibition. Oncogene (2016) ;35: (21):2684–6. PubMed PMID: 26434595. |
[46] | Choueiri TK , Pal SK , McDermott DF , Morrissey S , Ferguson KC , Holland J , et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol (2014) ;25: (8):1603–8. PubMed PMID: 24827131. Epub 2014/05/16. eng. |
[47] | Smith DC , Smith MR , Sweeney C , Elfiky AA , Logothetis C , Corn PG , et al. Cabozantinib in patients with advanced prostate cancer: Results of a phase II randomized discontinuation trial. J Clin Oncol (2013) ;31: (4):412–9. PubMed PMID: 23169517. Pubmed Central PMCID: PMC4110249. Epub 2012/11/22. eng. |
[48] | Lee RJ , Saylor PJ , Michaelson MD , Rothenberg SM , Smas ME , Miyamoto DT , et al. A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin Cancer Res (2013) ;19: (11):3088–94. PubMed PMID: 23553848. Pubmed Central PMCID: PMC3684567. |
[49] | Choueiri TK , Escudier B , Powles T , Mainwaring PN , Rini BI , Donskov F , et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. The New England Journal of Medicine (2015) ;373: (19):1814–23. |
[50] | Topalian SL , Sznol M , McDermott DF , Kluger HM , Carvajal RD , Sharfman WH , et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol (2014) ;32: (10):1020–30. PubMed PMID: 24590637. Pubmed Central PMCID: PMC4811023. |
[51] | Topalian SL , Hodi FS , Brahmer JR , Gettinger SN , Smith DC , McDermott DF , et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med (2012) ;366: (26):2443–54. PubMed PMID: 22658127. Pubmed Central PMCID: PMC3544539. |
[52] | McDermott DF , Motzer RJ , Atkins MB , et al. Long-term overall survival (OS) with nivolumab in previously treated patients with advanced renal cell carcinoma (aRCC) from phase I and II studies. Abstract 4507, American Society of Clinical Oncology 2016 Annual Meeting. 34: (15) suppl pp. 4507-4507. |
[53] | Chiou VL , Burotto M . Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol (2015) ;33: (31):3541–3. PubMed PMID: 26261262. Pubmed Central PMCID: PMC4622096. |
[54] | George S , Motzer RJ , Hammers HJ , Redman BG , Kuzel TM , Tykodi SS , et al. Safety and Efficacy of Nivolumab in Patients With Metastatic Renal Cell Carcinoma Treated Beyond Progression: A Subgroup Analysis of a Randomized Clinical Trial. JAMA Oncol (2016) ;2: (9):1179–86. PubMed PMID: 27243803. |
[55] | Escudier B , Motzer RJ , Sharma P , Wagstaff J , Plimack ER , Hammers HJ , et al. Treatment Beyond Progression in Patients with Advanced Renal Cell Carcinoma Treated with Nivolumab in CheckMate 025. Eur Urol (2017) . pii: S0302-2838(17)30265-8 [Epub ahead of print] PubMed PMID: 28410865. |
[56] | Cella D , Grunwald V , Nathan P , Doan J , Dastani H , Taylor F , et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate A randomised, open-label, phase 3 trial. Lancet Oncol (2016) ;17: (7):994–1003. PubMed PMID: 27283863. |
[57] | Escudier BJ , Powles T , Motzer RJ , et al. Efficacy of cabozantinib (C) vs everolimus (E) in patients (pts) with advanced renal cell carcinoma (RCC) and bone metastases (mets) from the phase III METEOR study. J Clin Oncol (Meeting Abstracts) (2016) ;34: (15):suppl4558. |
[58] | Beuselinck B , Oudard S , Rixe O , Wolter P , Blesius A , Ayllon J , et al. Negative impact of bone metastasis on outcome in clear-cell renal cell carcinoma treated with sunitinib. Ann Oncol (2011) ;22: (4):794–800. PubMed PMID: 20937648. |
[59] | Coleman RE . Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Ress (2006) ;12: (20 Pt 2):6243s–9s. PubMed PMID: 17062708. |
[60] | Aftab DT , McDonald DM . MET and VEGF: Synergistic targets in castration-resistant prostate cancer. Clin Transl Oncol (2011) ;13: (10):703–9. PubMed PMID: 21975330. Pubmed Central PMCID: PMC4422050. |
[61] | Pantano F , Fioramonti M , Iuliani M , et al. Biological effects of cabozantinib on bone microenvironment. J Clin Oncol (2016) ;34: :e23004-e23004. |
[62] | Escudier BJTP , Motzer RJ , Olencki T , Aren OR , Oudard S , Bracarda S , Tomczak P , Castellano DE , Appleman LJ , Drabkin HA , Vaena DA , Milwee S , Youkstetter J , Choueiri TK , editor Efficacy of cabozantinib (C) vs everolimus (E) in patients (pts) with advanced renal cell carcinoma (RCC) and bone metastases (mets) from the phase III METEOR study. American Society of Clinial Oncology Anual Meeting 2016 (2016) ;Chicago. |
[63] | Motzer RJ , Bacik J , Mazumdar M . Prognostic factors for survival of patients with stage IV renal cell carcinoma: Memorial sloan-kettering cancer center experience. Clin Cancer Res (2004) ;10: (18 Pt 2):6302S–3S. PubMed PMID: 15448021. |
[64] | Culine S , Bekradda M , Kramar A , Rey A , Escudier B , Droz JP . Prognostic factors for survival in patients with brain metastases from renal cell carcinoma. Cancer (1998) ;83: (12):2548–53. PubMed PMID: 9874462. |
[65] | Hsieh J , Chen D , Wang P , et al. Identification of efficacy biomarkers in a large metastatic renal cell carcinoma (mRCC) cohort through next generation sequencing (NGS): Results from RECORD-3. J Clin Oncol (2015) ;33: :4509. |
[66] | Ho TH , et al. Correlation between findings from comprehensive genomic profiling and targeted therapy response in metastatic renal clear cell carcinoma. Journal of Clinical Oncology 34: , no. 2_suppl (January 2016) 570-570. (DOI: 10.1200/jco.2016.34.2-suppl.570). |
[67] | Kwiatkowski DJ , Choueiri TK , Fay AP , Rini BI , Thorner AR , de Velasco G , et al. Mutations in TSC1, TSC2, and MTOR are associated with response to rapalogs in patients with metastatic renal cell carcinoma. Clin Cancer Res (2016) ;22: (10):2445–52. PubMed PMID: 26831717. Pubmed Central PMCID: PMC4976069. |
[68] | Snyder A , Makarov V , Merghoub T , Yuan J , Zaretsky JM , Desrichard A , et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med (2014) ;371: (23):2189–99. PubMed PMID: 25409260. Pubmed Central PMCID: PMC4315319. |
[69] | Rizvi NA , Hellmann MD , Snyder A , Kvistborg P , Makarov V , Havel JJ , et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (2015) ;348: (6230):124–8. PubMed PMID: 25765070. Pubmed Central PMCID: PMC4993154. |
[70] | Machiels J-PH , Haddad RI , Fayette J , Licitra LF , Tahara M , Vermorken JB , et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. The Lancet Oncology (2015) ;16: (5):583–94. |
[71] | Voss M. H. , et al. Mutation burden and tumor neoantigens in RCC patients (pts) treated with nivolumab. J Clin Oncol (2016) ;34: :514. |
[72] | Ball MW , et al. Clinical, pathologic, and genomic profiles of exceptional responders to anti–PD1 therapy in renal cell carcinoma. J Clin Oncol (2016) ;34: :625. |
[73] | de Velasco G , et al. Integrated genomic correlates of response to PD-1 inhibitor nivolumab in metastatic renal cell carcinoma (mRCC). ASCO Meeting Abstracts (2016) ;34: :545. |
[74] | Taube JM , Klein A , Brahmer JR , Xu H , Pan X , Kim JH , et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res (2014) ;20: (19):5064–74. PubMed PMID: 24714771. Pubmed Central PMCID: PMC4185001. |
[75] | Rosenberg JE , Hoffman-Censits J , Powles T , van der Heijden MS , Balar AV , Necchi A , et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet (2016) ;387: (10031):1909–20. PubMed PMID: 26952546. Epub 2016/03/10. eng. |
[76] | McDermott DF , Sznol M , Sosman JA , Soria JC , Gordon MS , Hamid O , et al. Immune correlates and long term follow up of a Phase Ia study of MPDL3280A, an engineered PD-L1 antibody, in patients with metastatic renal cell carcinoma (mRCC); Proceedings of the European Society of Medical Oncology Meeting; Madrid, Spain. (2014) . |
[77] | Callea M , Albiges L , Gupta M , Cheng SC , Genega EM , Fay AP , et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res (2015) ;3: (10):1158–64. PubMed PMID: 26014095. Pubmed Central PMCID: PMC4596765. Epub 2015/05/28. Eng. |
[78] | Jilaveanu LB , Shuch B , Zito CR , Parisi F , Barr M , Kluger Y , et al. PD-L1 Expression in Clear Cell Renal Cell Carcinoma: An Analysis of Nephrectomy and Sites of Metastases. J Cancer (2014) ;5: (3):166–72. PubMed PMID: 24563671. Pubmed Central PMCID: PMC3931264. |
[79] | Gerlinger M , Rowan AJ , Horswell S , Larkin J , Endesfelder D , Gronroos E , et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England Journal of Medicine (2012) ;366: (10):883–92. PubMed PMID: 22397650. Pubmed Central PMCID: PMC4878653. Epub 2012/03/09. eng. |
[80] | Di Napoli A , Signoretti S . Tissue biomarkers in renal cell carcinoma: Issues and solutions. Cancer (2009) ;115: (10 Suppl)::2290–7. PubMed PMID: 19402057. Pubmed Central PMCID: PMC3809842. |
[81] | Taube JM , Anders RA , Young GD , Xu H , Sharma R , McMiller TL , et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med (2012) ;4: (127):127ra37. PubMed PMID: 22461641. Pubmed Central PMCID: PMC3568523. |
[82] | Kusmartsev S , Su Z , Heiser A , Dannull J , Eruslanov E , Kubler H , et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res (2008) ;14: (24):8270–8. PubMed PMID: 19088044. |
[83] | Shrimali RK , Yu Z , Theoret MR , Chinnasamy D , Restifo NP , Rosenberg SA . Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res (2010) ;70: (15):6171–80. PubMed PMID: 20631075. Pubmed Central PMCID: PMC2912959. |
[84] | Larkin J , Chiarion-Sileni V , Gonzalez R , Grob JJ , Cowey CL , Lao CD , et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New England Journal of Medicine (2015) ;373: (1):23–34. |
[85] | Ma P , Chesney J , Pavlick AC , Robert C , Grossmann K , McDermott D , et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. New England Journal of Medicine (2015) ;372: (21):2006–17. |
[86] | Hammers HJ , Plimack ER , Infante JR , et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma. J Clin Oncol (2014) ;32: (suppl; abstr 4504):5s. |
[87] | Amin A , Plimack ER , Infante JR , et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). ASCO Meeting Abstracts (2014) ;32: (15 suppl):5010. |
[88] | McDermott DF , Sosman JA , Sznol M , Massard C , Gordon MS , Hamid O , et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: Long-term safety, clinical activity, and immune correlates from a phase ia study. J Clin Oncol (2016) ;34: (8):833–42. PubMed PMID: 26755520. |
[89] | McDermott DFMR , Rini BI , et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). J Clin Oncol (2017) ;35: :6_suppl, 431-431. |
[90] | Amin A , Dudek AZ , Logan TF , Lance RS , Holzbeierlein JM , Knox JJ , et al. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results. J Immunother Cancer (2015) ;3: :14. PubMed PMID: 25901286. Pubmed Central PMCID: PMC4404644. |
[91] | Zhou Z , Zhang H , Xu Z , Li W , Dang C , Song Y . Nomogram predicted survival of patients with adenocarcinoma of esophagogastric junction. World J Surg Oncol (2015) ;13: :197. PubMed PMID: 26055624. Pubmed Central PMCID: PMC4465317. |
[92] | Walter S , Weinschenk T , Stenzl A , Zdrojowy R , Pluzanska A , Szczylik C , et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med (2012) ;18: (8):1254–61. PubMed PMID: 22842478. |
[93] | Rini B , Stenzl A , Zdrojowy R , et al. Results from an open-label, randomized, controlled phase 3 study investigating IMA901 multipeptide cancer vaccine in patients receiving sunitinib as first-line therapy for advanced/metastastic RCC. Abstract 17LBA European Society for Medical Oncology (2015) . Vol 51: , Supp 3, Page S718. |
[94] | Rini BI , et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. Published Online, October 03, (2016) ;17: (11):1599–1611. |




