Management and treatment of musculoskeletal problems in adults with cerebral palsy: Experience gained from two lifespan clinics
1Introduction
Cerebral palsy (CP) is a non-progressive disorder of the central nervous system. Worldwide, approximately 17 million people are living with some manifestation of CP [1]. Due to improvements in early care for premature infants, there are now more adults than pediatric patients with CP. Its musculoskeletal manifestations are progressive due to the long-term effects of spasticity on the musculoskeletal system. As children, interventions are performed to maintain the current level of mobility according to the Gross Motor Function Classification System (GMFCS). As adults, treatment revolves around symptomatic management of deformities secondary to the long-term effects of spasticity, dystonia, and asymmetric biomechanical forces. In addition, many other biopsychosocial problems arise from access to knowledgeable healthcare and preventive health screening to workforce integration, independence, and social representation.
The GMFCS classification scheme has been revolutionary for demonstrating natural history, outcomes, and treatment guidelines. A patient’s GMFCS classification can be established as early as two years of age. McCormick et al. reviewed 103 records of adults with CP and cross-matched their adult GMFCS classification to school age from the record review. The 12 to 18-year-old GMFCS scheme was most accurate for adulthood. There was a 0.96 positive predictive value for wheelchair ambulators and 0.88 for ambulatory adults with CP [2]. Continued usage of the GMFCS schema helps to guide treatment and decision-making in adults with CP.
The senior authors (HC and DR) have managed lifespan clinics for adults with CP at Rady Children’s Hospital and Columbia University Medical Center for over 30 years. During this time, they have cared for over 5000 patients. This paper relates their experience over this time.
1.1General health
With healthcare quality improving and the initiation of gastrostomy tube feeding over the last three decades, mortality in CP patients has decreased, and life expectancy has improved [3–6].
The secondary health conditions and associated conditions with CP are frequently ignored or overlooked by practitioners due to their primary non-progressive neurologic disorder. The lack of centers dedicated to providing care for adults with CP results in a “care cliff.” Adolescents or young adults with CP can no longer be seen by their long-term pediatric providers, and a significant barrier to care exists due to the paucity of adult providers who treat CP and its manifestations. Patients are abruptly left with no experienced providers due to aging out of the pediatric system and falling off the “care cliff” to an underserved medical community. Secondarily, inadequate primary health screening has led to a higher incidence of chronic medical conditions. Adults with CP have higher incidences of chronic or recurrent pain, gastroesophageal reflux disease (GERD), respiratory disease, dental problems, Alzheimer’s disease, Parkinson’s disease, depression, bipolar disorder, and other comorbid conditions [7–9]. Stroke risk is increased, and cardiovascular death risk is four times higher than in the general population [4, 10]. Although early studies suggested an increased incidence of cancer in adults with CP, more recent literature does not find an increased incidence [4]. In contrast, epilepsy, which affects 20–30% of children with CP, tends to regress in adulthood. Therefore, the need for anti-epileptic medication may need to be addressed as the patient ages.
Women’s health issues are often not addressed. The lack of women’s health screening has led to decreased long-term monitoring in the form of mammograms, pap smears, and general women’s health [9]. Breast cancer is reported three times higher than in the typically developing population, and this may be secondary to a lack of screening mammography [4]. Access to hormonal contraceptives for dysmenorrhea, hygiene, and sexual health should be discussed and driven by the family and primary treatment team [11].
Bone density is decreased in patients with CP [9, 12, 13]. The smaller cross-sectional size of long bones in patients with CP weakens the bone, as strength is related to the radius of the fourth power [13]. The risk of fragility fractures in young to middle-aged adults with CP is similar to worse than those of elderly, typically developing females [12]. The risk of nonunion increases as the bone density decreases. Although a mandatory consideration, the incidence of nonunion for femoral derotations has been low with modern load-sharing devices [14]. Patients should be screened, educated, and considered for bone-strengthening medical therapies. Fragility fractures can decrease ambulatory function and risk general medical decompensation and mortality.
As patients with CP age, ambulatory capabilities decrease at two described time points. First, progressive crouch sets in at about 20–25 years of age due to weakness and changes in spastic muscles, and inhibits the patient from pacing with their peers. A second nadir is at 40–45 years old when secondary musculoskeletal joint degeneration leads to chronic pain, fatigue, and deconditioning [8, 9]. Chronic pain and fatigue plague the adult CP population and should be screened appropriately.
The GMFCS level of an adult patient with CP will gradually decrease over time [9, 10]. GMFCS I and II patients are expected to have higher preservation of assist-free or limited-assist mobilization as they age. In one longitudinal study following adults with CP, 39% were independent ambulators at 20, whereas only 25% retained ambulation in the 60-year-old cohort [15]. In contrast, a recent study of 106 adults with CP and a 13-year follow-up demonstrated slower gait speeds but no other decompensation in other gait or patient-reported metrics [16]. The literature should be interpreted cautiously due to the heterogeneous presentation of adults with CP. A safe assumption is that at least 25% will decline in mobility, with a poorer prognosis in those with worse initial gait ability, bilateral motor involvement, older age, and higher overall pain and fatigue levels [17].
2Tone management
In addition to expected functional decline over time, the adult with CP gradually trends toward more dystonic features, especially in the higher level GMFCS patients [9]. Botulinum toxin-A continues to be used into adulthood. Protocols are similar to that in the pediatric population. Adults with worsening or painful dystonia benefit from repeat injections in the most affected motor groups every three to four months. A myriad of other medications are used for tone management. Baclofen, levodopa, tizanidine, and dantrolene all have a role in managing spasticity and dystonia [9]. Phenol injections into the motor branches of nerves can also be used for movement disorders in locations such as the hip or shoulder [9].
Intrathecal baclofen (ITB) therapy administered via a subcutaneous pump is increasingly used for spasticity and dystonia management. The literature is limited in the setting of specific use in adults with CP. ITB may help decrease the incidence of bony surgery as adults if implanted before an adolescent growth spurt, potentially preventing further torsional deformity through spasticity reduction [18]. Caregiver ease of care, pain, comfort, and positioning are all reported to improve in adults with ITB pumps [7]. A comparative study of GMFCS III and IV patients in their 30 s between ITB and deep brain stimulation (DBS) found significant improvements in patient-reported outcome measures for the 36-Item Short Form Survey (SF-36), pain, satisfaction, and quality of life [19]. ITB has been associated with improved walking speed and mobility outcome scores in ambulatory adult CP or stroke patients [7, 20]. While it can be effective, ITB therapy has a high adverse event and complication rate requiring revision or removal of 20–30% [10, 21]. The long-term role of ITB in adult CP patients is still in question, and future studies are needed to delineate further the indication for initiation, weaning, and continued benefits versus risks of use.
2.1Single event multi-level surgery (SEMLS) in adults with CP
SEMLS is a therapeutic approach to limit surgical exposure and maximize efficacy in CP patients. It has a well-documented use and safety in the pediatric CP population. Residual deformity in adulthood is common, in one series around 60%, with 10% needing secondary intervention [16]. Multiple deformities require an identical approach in adults with CP as that of pediatric CP patients. Soft tissue lengthening has reduced efficacy in adults with CP. Muscle changes over time and with age is replaced by fibrotic connective tissue. Prolonged rehabilitation and recovery are expected in adult CP patients compared to children. Delays in ambulation recovery can take up to 20 months postoperatively [14]. Planning for the long rehabilitation course may require coordinating the assistance of long-term acute care or specialized nursing facilities.
3Spine
Scoliosis is present in over 60% of adults with CP. The progression of deformity after skeletal maturity can approximate one degree per year if below, or two degrees per year if above, 40–50 degrees of coronal deformity [9, 22, 23]. In a cohort of 292 young adults with CP, scoliosis was strongly correlated with GMFCS level and degree of dystonia. Curves over 40 degrees were almost exclusively seen in GMFCS IV and V patients and were 18.2 times more likely in dystonic versus spastic CP patients [23]. Scoliosis is present in 70–80% of GMFCS IV or V adults [23]. As curves decompensate over time, pain in the convexity, gastrointestinal or respiratory status difficulties, and loss of function can occur. Non-operative modalities include seating adjuncts, supportive bracing, anti-inflammatory medications, and physical therapies. Pediatric neuromuscular scoliosis is relatively painless. Adults may develop increasing rigidity and pain as the facet joints degenerate and the posterior spinal extensors become fibrotic. The pain, positioning difficulties, ulcer formation, and secondary medical comorbidities from the curve all highlight the importance of addressing spinal deformity. Fifty degrees of scoliosis remains a suitable threshold for operative intervention.
Post-operative complications in scoliosis for adults with CP remain high and are consistent with pediatric CP patients. A review of adults with CP in the 90-day postoperative period recently demonstrated a 32% complication rate, with a 13% readmission rate in over 1000 adults with CP [24]. Complications remain high even during primary admission. Urinary tract infections and respiratory complications reportedly represent 22% and 7% respectively of the total [24]. Overall, the adult data is consistent with pediatric CP scoliosis complication profiles. Yaszay et al. recently reported pediatric CP patients having a 36% major complication and 14% reoperation rate at a two-year follow-up [25].
Spondylolisthesis occurs much more frequently in ambulatory patients with CP than in typically developing counterparts. Estimates in ambulatory adults with CP are 20–30% irrespective of dystonia, which is four times higher than the general population [9, 26]. The incidence in non-ambulatory adults with CP is near zero, with one series of 143 patients reporting no occurrence in their cohort [27]. However, the authors are aware of several cases in their practices. Chronic low back pain is often dismissed as merely a secondary effect of spasticity in the adult CP patient. A workup starting with plain radiographs can help identify any underlying spinal pathology. These may be difficult to interpret in the setting of severe lordosis, and computed tomography (CT) may be necessary to assess this condition. Hyperextension and axial rotation with anterior pelvic tilt are thought to be contributory to the stress fractures leading to spondylolisthesis. Muscle spasticity exacerbates and accelerates the decompensation of the facets. Prevention of this by aggressive soft tissue management through injections or lengthenings can help prevent fatigue fractures of the pars interarticularis [9].
Cervical stenosis occurs at a higher incidence in adults with CP than in the typically developing population. Harada et al. reported an eight-fold increase in cervical disc degeneration and mid-cervical instability, predisposing to stenosis and neurologic deterioration [28]. Cervical myelopathy occurs in the 40 s, earlier than the general population [29, 30]. Poor head control, increased muscle tone, and abnormal gait predispose to cervical degeneration [29]. Athetoid CP, in particular, is a predisposing risk factor to cervical instability, myelopathy, and functional deterioration [9, 29, 31]. One study reported a rate of cervical myelopathy or degeneration of 36% in athetotic patients versus 7.5% in spastic CP patients [32]. Myelopathy diagnosis is delayed on average 15 months and presents with increased spasticity, functional deterioration, upper extremity weakness, and vesicosphinteric reflex dysfunction [32, 33]. Vigilance and careful assessment are crucial to eliciting critical changes in the patient’s function. The caregiver often remains the best litmus test of function. Any loss of function should immediately cause one to assess for cervical pathology.
In contrast to pathology involving the lower spine and hip, cervical disease occurs most frequently in GMFCS I-II, 70% of the time in one cross-sectional study [32]. The mid-cervical spine from C4 to C7 is the most common stenosis level [32]. CT and cervical spine MRI are necessary to confirm the diagnosis. Serial MRI surveillance for cervical myelopathy has been recommended for high-risk athetotic patients every two years as they enter early adulthood [9]. Botulinum toxin injection can also be used as an adjunct to assist in cervical dystonia to delay cervical spondylosis. Surgical intervention is recommended for progressive symptoms, failure of non-operative management, or sudden loss of function.
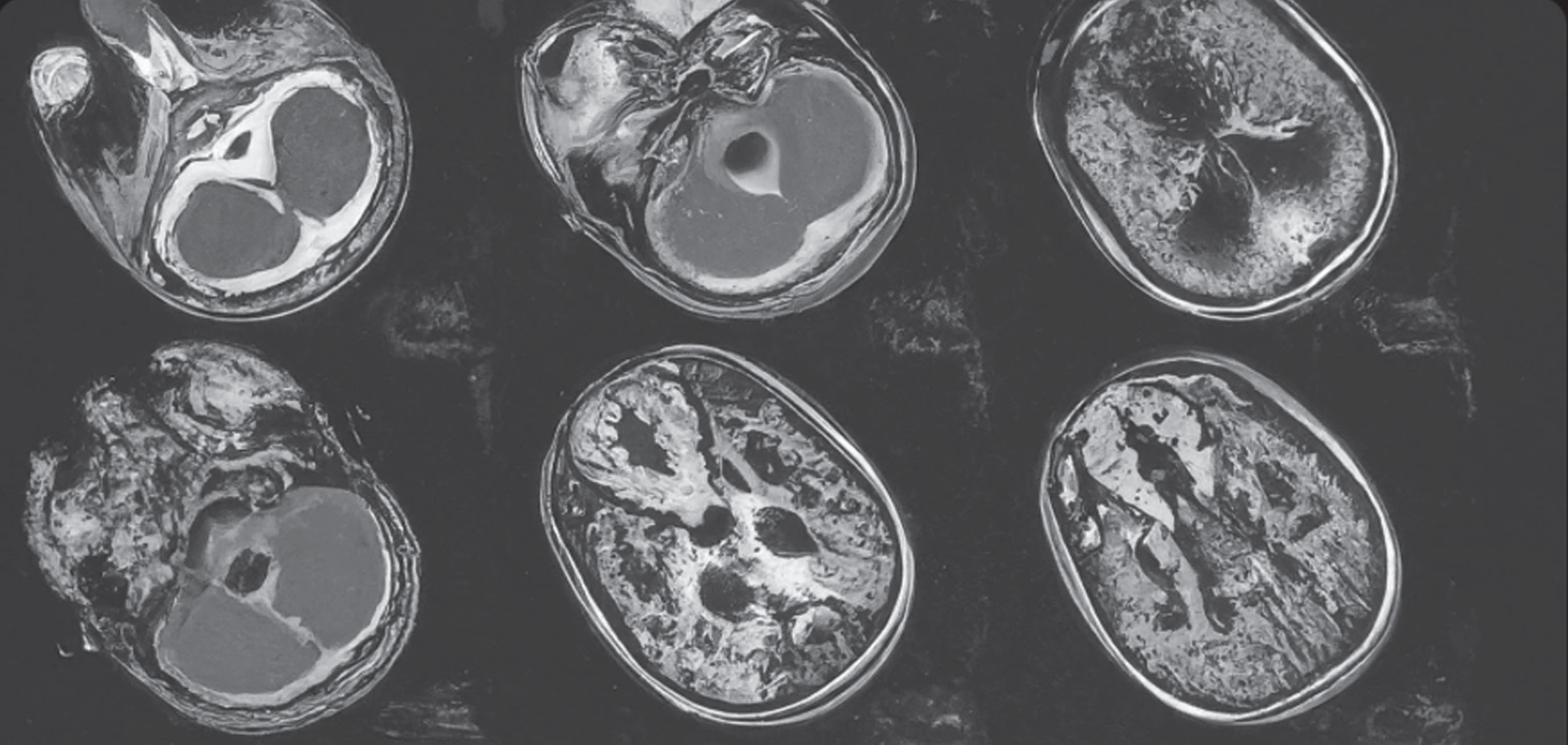
Hydrocephalus can also present with an acute loss of function. Periventricular damage in some patients with CP often leaves ventriculomegaly in the cerebral cortex. This can cause occult “normal pressure” hydrocephalus that may be unmasked as the patient ages [34, 35]. Most commonly, this is unveiled with a late-onset foot deformity or increased frequency of cerebrospinal fluid (CSF) leaks in patients with ITB pumps [35]. When the thecal sac is newly instrumented with a catheter, the occult hydrocephalus will now have an opening to cause CSF leaks as it tries to normalize the intrathecal pressure gradient. Any adult CP patient with an unexpected loss of function, increased spasticity, or weakness should be screened for cervical myelopathy and hydrocephalus (Case 1).
3.1Case 1
A 55-year-old retired postal worker (GMFCS II) presented with increased weakness in his upper and lower extremities as well as occasional urinary incontinence. His sister stated that he was having difficulty with activities of daily living, including feeding himself. He noted that his gait had deteriorated, and he had to use a walker. He also thought he was having memory lapses. His physical examination demonstrated 2+ reflexes but generalized Grade 4 muscle testing. He had not had a prior neurological examination, so it was unknown if this was a new finding. However, he and his sister believed that it was a progression from when he retired several years prior to this. As part of his diagnostic workup, an MRI of the brain and the cervical spine was obtained. He was found to have hydrocephalus which responded well to catheter placement (ventriculoperitoneal shunt). His strength improved, as did his urinary incontinence. However, he was still using a walker at his five-year follow-up.
Fig. 1
MRI of 45-year-old man with progressive loss of function of upper and lower extremities as well as urinary incontinence.
4Hip
Hip subluxation followed by dislocation and secondary acetabular dysplasia occurs linearly from lower to higher GMFCS level patients. Hemiplegic GMFCS I patients have less than 10% risk, while quadriplegic GMFCS V patients have over a 90% risk [9, 36]. Mild hip displacement forms a pseudo acetabulum, often asymptomatic in adolescence and adulthood. As the hip degenerates and becomes osteoarthritic, the hip can become severely symptomatic. A cohort study of 98 young adults with CP demonstrated a 72% incidence of pain, directly correlating to the degree of residual hip deformity and displacement [37]. Other studies have reported varying rates of pain in chronically dislocated hips, up to 52% [38]. With improved methods of measuring pain in nonverbal CP patients, the accuracy of diagnosis and treatment is improving with a renewed interest in hip preservation.
4.1Case 2: 26-year-old man with quadriplegic spastic CP GMFCS IV
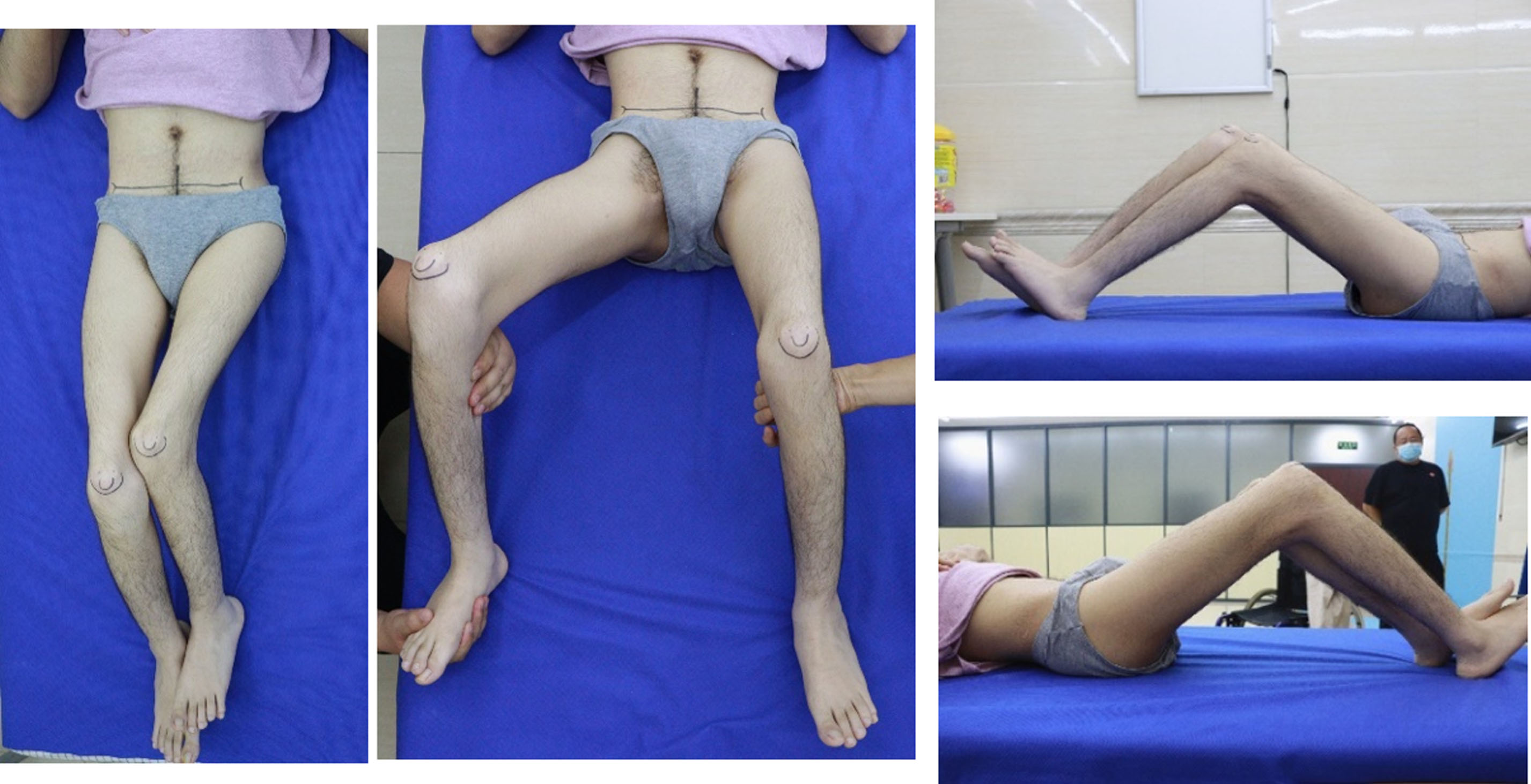
This 26-year-old graduate student presented with increasing left hip and bilateral knee pain and decreasing function. He could walk short distances with a walker until hip pain intervened at age 10. He is now a wheelchair ambulator (Fig. 2) and is dependent on a caregiver for transfers. Treatment consisted of intermittent physical therapy. There was no surgical intervention, no botulinum toxin, and no casting. He had lost the ability to perform standing transfers secondary to pain in the left hip, pain in both knees, and generalized weakness.
Fig. 2
Patient with a caregiver at university.

The patient was admitted for evaluation by rehabilitation medicine. A period of physical therapy and medication therapies was initiated. He responded to oral baclofen and gabapentin with less pain and lower tone. His startle response was partially suppressed. The flexion contractures were severe, and the left hip and bilateral knee pain prevented standing transfers. He remained entirely dependent on transfers requiring lifting from bed to chair, chair to toilet, and chair to bath (Fig. 3).
Fig. 3
A physical exam revealed a fixed adduction shortening of the left thigh segment, bilateral knee flexion contractures, bilateral hip flexion contractures, and patella alta.
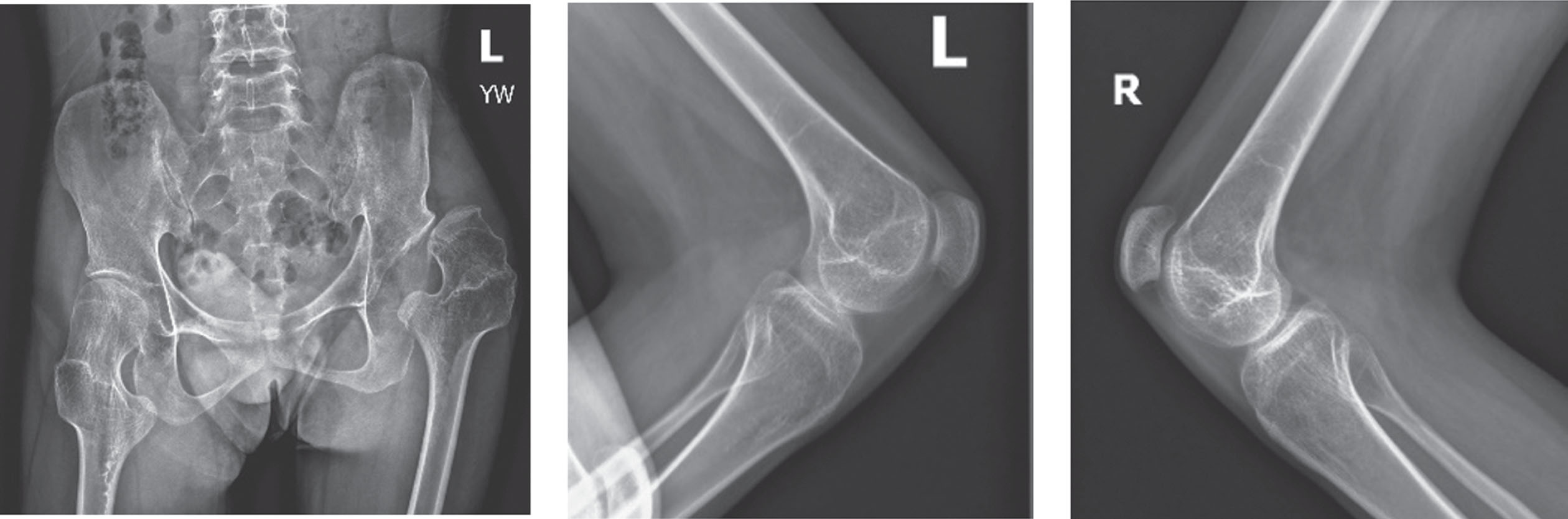
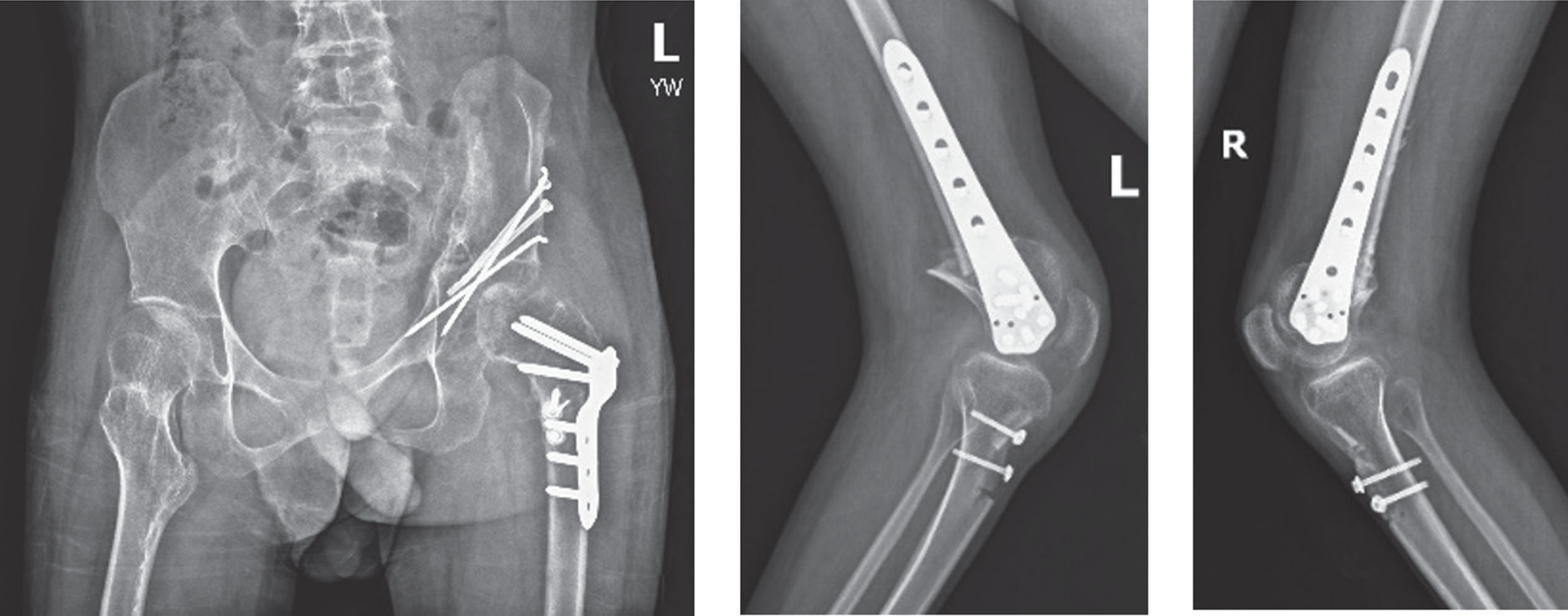
His preoperative x-rays revealed a dislocation of the left hip and patella alta, along with documentation of his knee flexion contracture (Fig. 4).
Fig. 4
Preoperative x-rays reveal dislocation of the left hip, bilateral knee flexion contractures, and patella alta.
After a period of inpatient rehabilitation, medication, and workup, a decision was made with the patient and his family to perform corrective surgery. A proximal femoral varus, shortening, extension, and rotation osteotomy was performed on the left, along with bilateral extension osteotomies of the distal femurs. Patellar advancement was also performed. The surgery substantially improved the alignment (Figs. 5 and 6).
Fig. 5
Postoperative alignment with improvement of hip extension, knee extension, and patella alta.
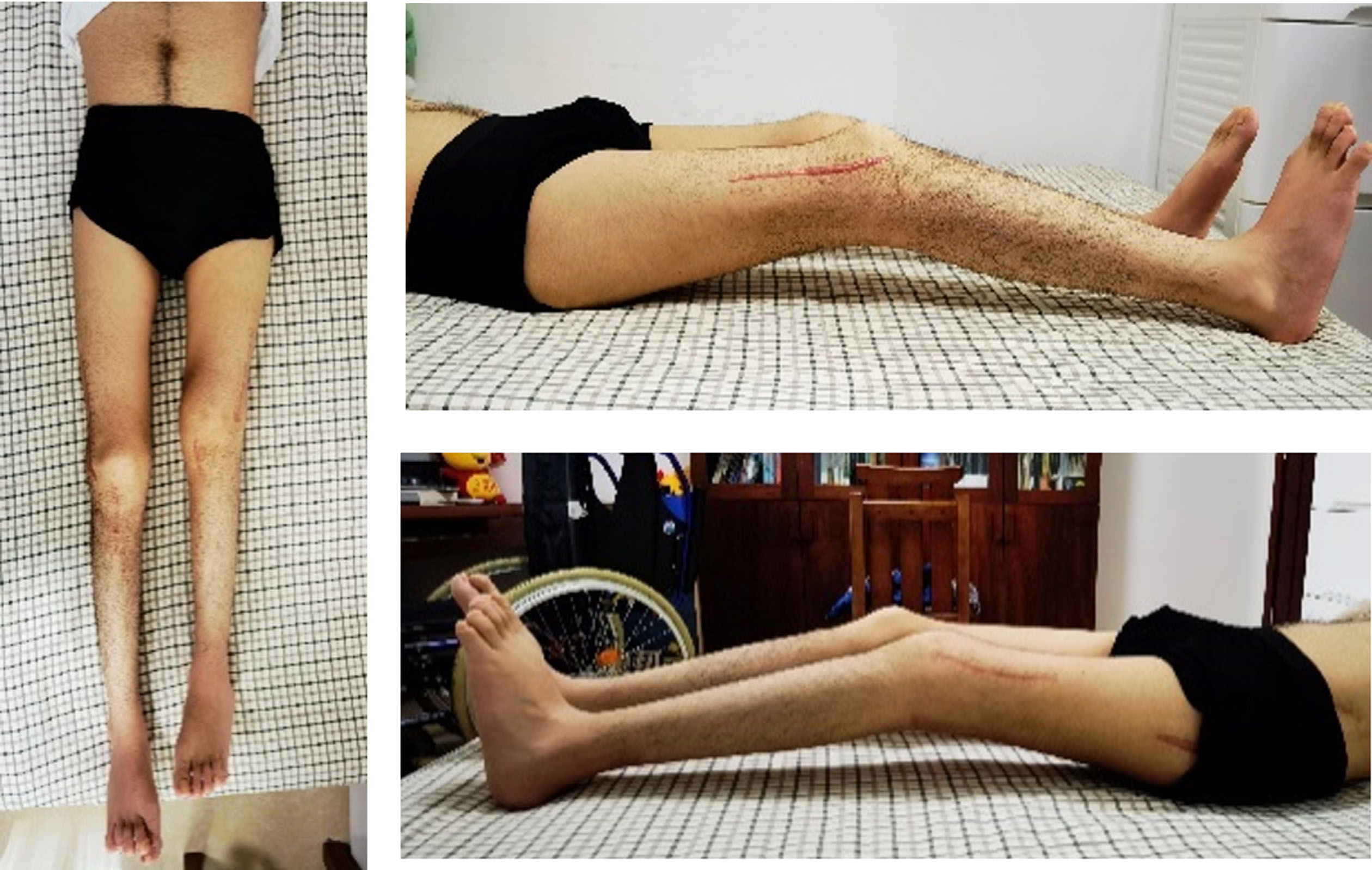
Fig. 6
Postoperative x-rays after proximal femoral extension, varus, and rotation osteotomy, distal femur extension osteotomy, and patellar advancement.
Post-operative rehabilitation continued inpatient. His hip pain and knee pain were relieved. He continued oral baclofen. Two years later, he could perform standing transfers and take steps when supported. He was pleased with his improvement in function and comfort. His endurance in sitting was also improved substantially, secondary to better tone control and the absence of hip and knee pain.
Hip reconstruction in GMFCS IV and V is controversial. In this case of a highly intelligent, motivated patient, his desire to perform standing transfers would be more likely achieved by providing him with a stable, mobile hip. The Chiari pelvic osteotomy has provided more acetabular coverage of the femoral head. Treating the flexion contractures was, of course, a necessity to meet the goal of standing transfers. The knee surgery was successful in eliminating his pain.
Despite the arduous rehabilitation process lasting one year, the patient was very pleased with the outcome and returned to university.
Once the hip has degenerated, there are multiple salvage options available. Proximal femoral resection (PFR) for chronically dislocated hips was first described by Castle in 1978, consisting of resection to the lesser trochanter and interposition arthroplasty of the hip capsule and abductor tendon [39]. McCarthy modified this procedure to emphasize extraperiosteal resection to limit heterotopic bone formation and more distal femoral resection [40]. These techniques lead to complete pain relief in 50–90% of cases and near 100% improvement in sitting tolerance [38]. Difficulties with proximal femoral migration and heterotopic ossification led McHale to adopt a subtrochanteric valgus osteotomy (SVO) technique for CP [41]. This osteotomy has been adapted with or without femoral head resection. It offloads the painful, arthritic part of the hip joint, leading to similar pain-relieving and sitting balance results as the PFR with improved femoral migration [38, 42]. The prominence of both the implant and greater trochanter in a patient population with at-risk soft tissues has been the main drawbacks of SVO. In addition, there is a high risk for secondary pressure necrosis of the skin and subsequent infection at prominent sites around the hips.
Furthermore, the PFR and SVO techniques have been associated with 20–30% revision rates [38, 42]. Most recently, a new iteration of PFR spares the muscular attachments to the trochanter with surgical hip dislocation. It integrates secondary compartmentalization of the proximal femur resection from the acetabulum. This new technique has demonstrated lower revision rates, good sitting tolerance, preserved motion, and proximal femoral migration consistent with modified versions of the McHale procedure [43]. Assessing the patient’s household ambulatory capabilities is critical before choosing a surgical technique. For example, suppose one is a crawler around the house, depending on weight bearing through the knees for mobility. In this case, proximal femoral resection is recommended in order to maintain this functional mobility [9]. A variety of salvage options with mixed results in the literature leaves it to the provider’s clinical judgment to determine the optimal surgical technique for relieving painful hip dislocation in a degenerated or deformed joint.
Hip arthrodesis and interposition prosthetic arthroplasty are less frequently used salvage options in the adult CP population. Arthrodesis has been associated with good pain relief, around 80% on a systematic review. However, a complication profile over 100% in the literature has led to this procedure being not recommended [38]. Arthroplasty has been utilized for salvage options using multiple techniques, including total hip, shoulder hemiarthroplasty, dual mobility technology, and femoral resurfacing. Survivorship has been reported to be up to 81% at 15 years [44, 45]. Dislocation rates due to spasticity have been reported from 25% to 28%. Early series with new dual mobility technology which increases the arc of motion free of impingement have reduced this rate to zero [38]. In a recent review, over 2,000 total hip arthroplasties were performed in the CP population nationwide over ten years [46]. Due to preserved ambulation, GMFCS II to III patients with hip degeneration are ideal for an arthroplasty solution. As new implant technology improves the high complication profile, the use of arthroplasty will continue to be revisited.
5Lower extremity abnormalities
Multiple deformities of the lower extremity can persist into adulthood from ineffective preventive care. The inability to maintain flexibility and provide adequate soft tissue lengthening or correction of lever arm dysfunction leaves persistent torsional or angular deformity in the femur, knee, ankle, patella, or foot.
Patella alta is a common condition that persists. This is often present with knee flexion contractures. Persistent rectus femoris spasticity can worsen the patella alta over time and contribute to patellar maltracking, dislocation, or patellar avulsion fracture. Quadriceps stretching, use of a patellar stabilizing brace, or alignment procedures can be beneficial. Distal and proximal patellar realignment with patellar tendon transfer, shortening, or distal femoral extension osteotomies can be performed. If not addressed over time, these issues will lead to patellofemoral arthritis.
5.1Case 3: 24-year-old woman with CP and crouch gait
A 24-year-old woman with mixed-tone diplegia, GMFCS III, presented with a progressive crouch gait and increasing anterior knee pain that had become disabling. Her complaints were primarily about the pain and the physical difficulty of walking, which limited her ambulation to about 50 meters, but she was also concerned about her posture and appearance.
Gestation was 28 weeks, and there was perinatal distress. At age six, she underwent bilateral Achilles tendon and hamstring lengthening at another institution. She walked independently at the age of seven years. She began having anterior knee pain at age eight.
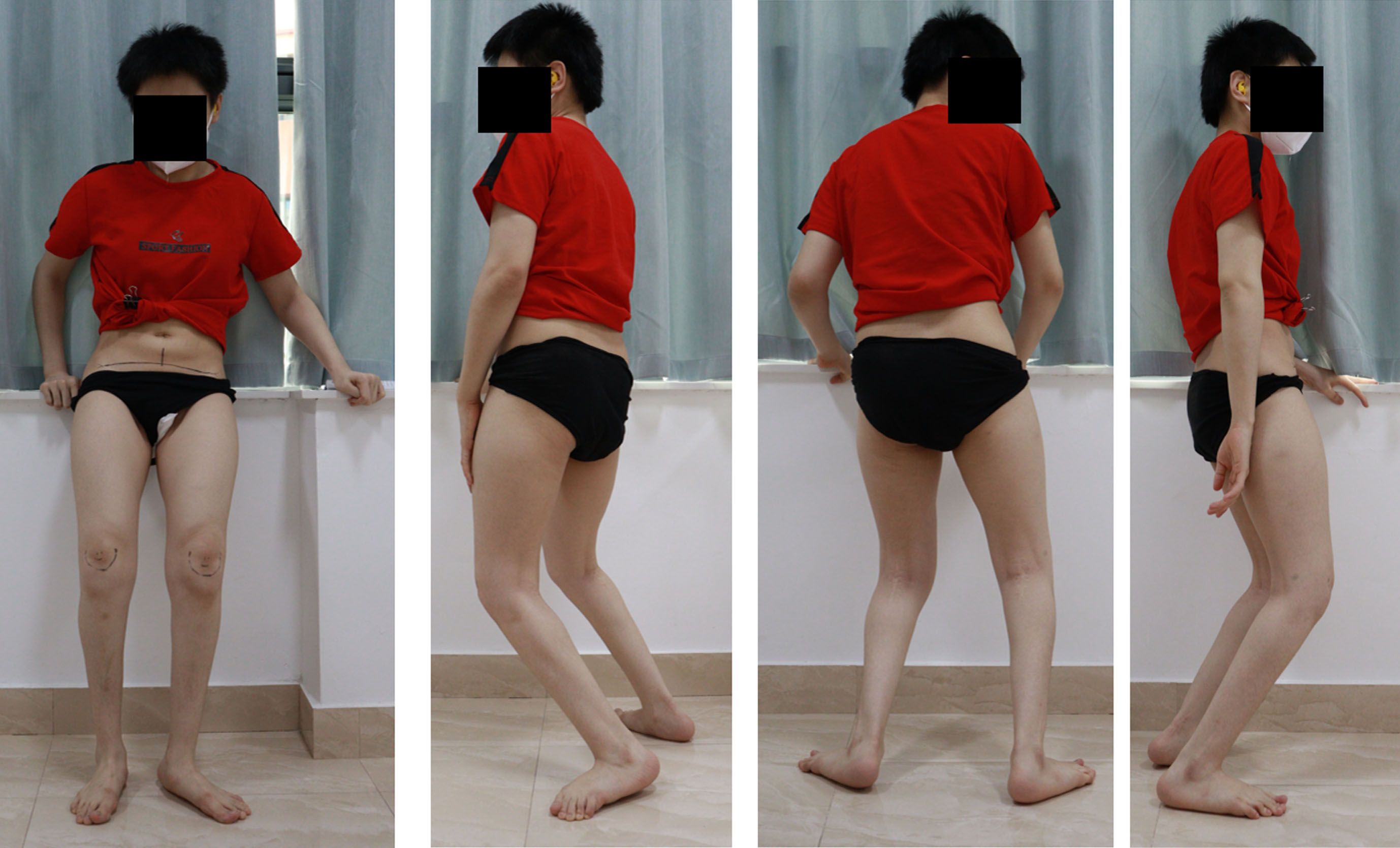
She was evaluated in Guangzhou in 2022. At the time of that evaluation, she complained of constant anterior knee pain when standing or walking. She felt the crouch was getting worse. Her standing posture revealed flexed hip, flexed knee, and ankle dorsiflexion (Fig. 7).
Fig. 7
Standing posture reveals flexed hip, flexed knee, and ankle dorsiflexion with anterior pelvic rotation.
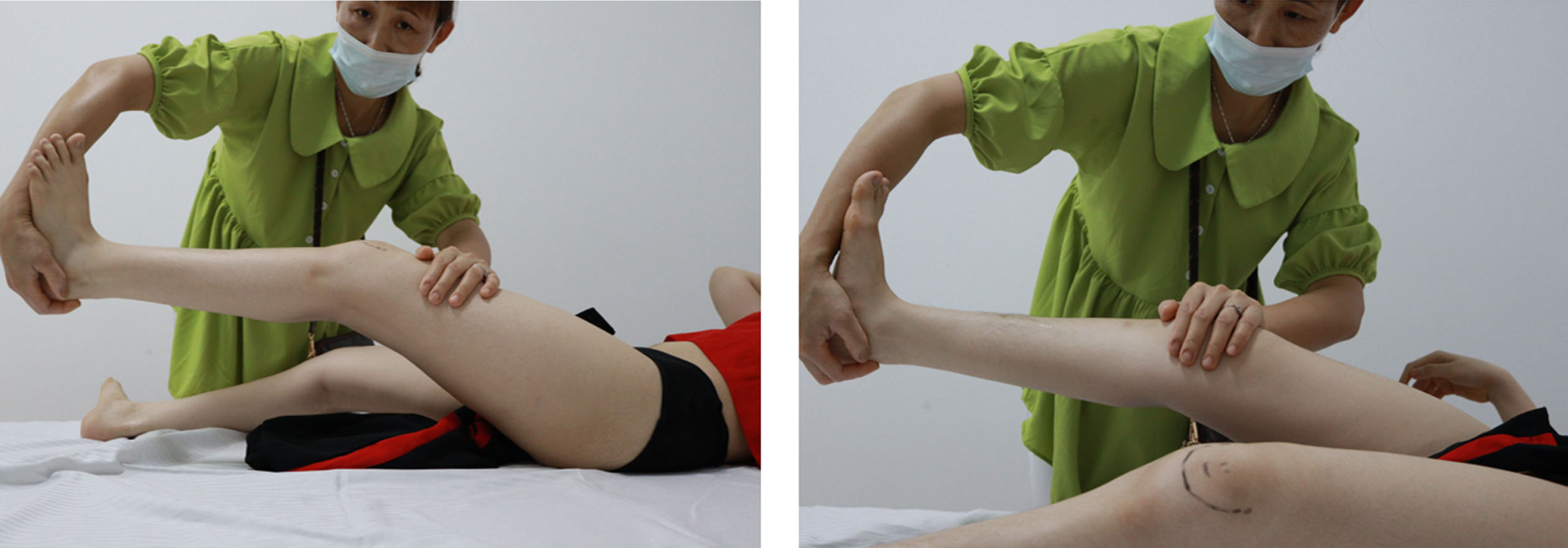
She had bilateral positive Ely (Fig. 8) and positive Thomas test with 20-degree hip flexion contractures (Fig. 9). Knee flexion contractures measured 25 degrees (Fig. 10).
Fig. 8
Bilateral positive Ely Test.
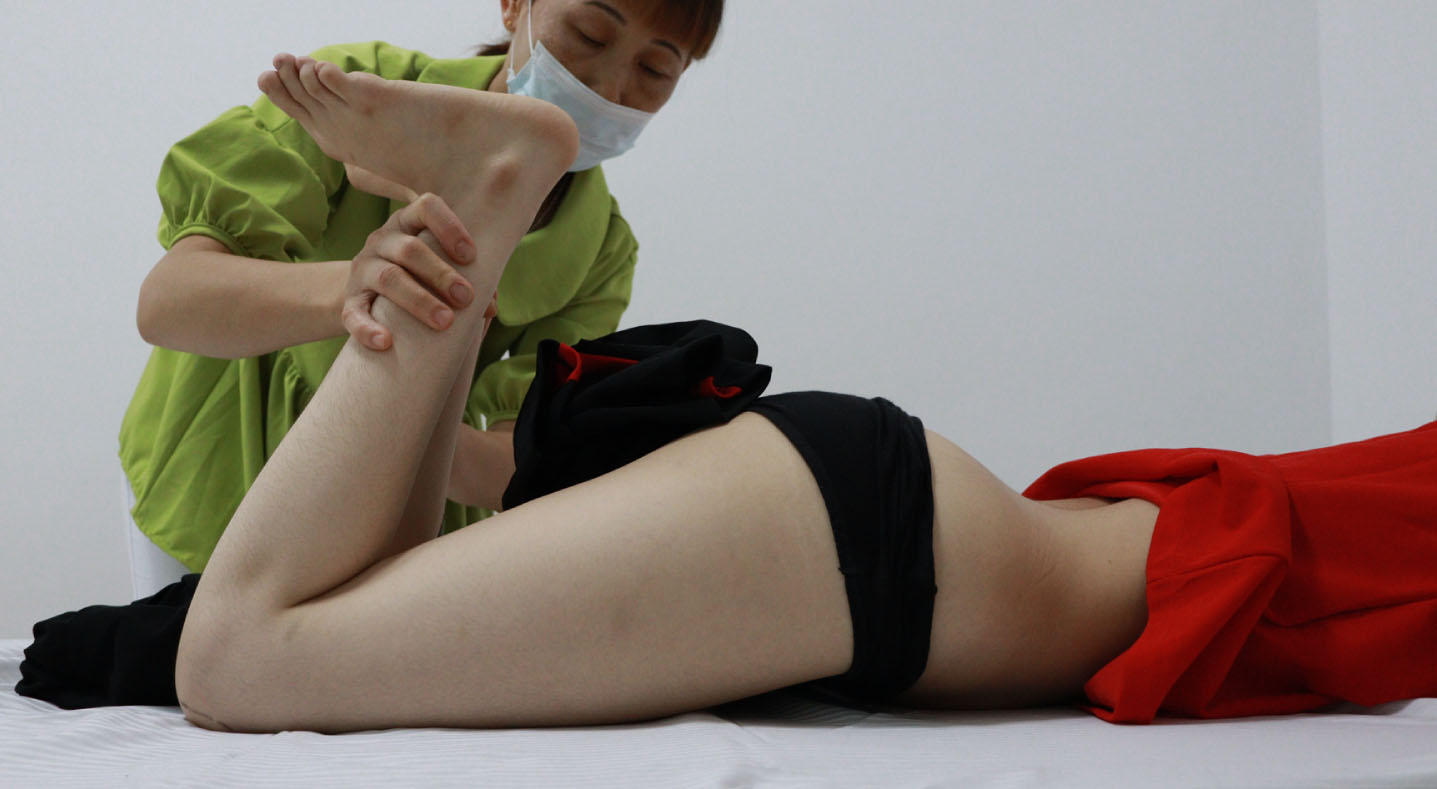
Fig. 9
Bilateral positive Thomas test.
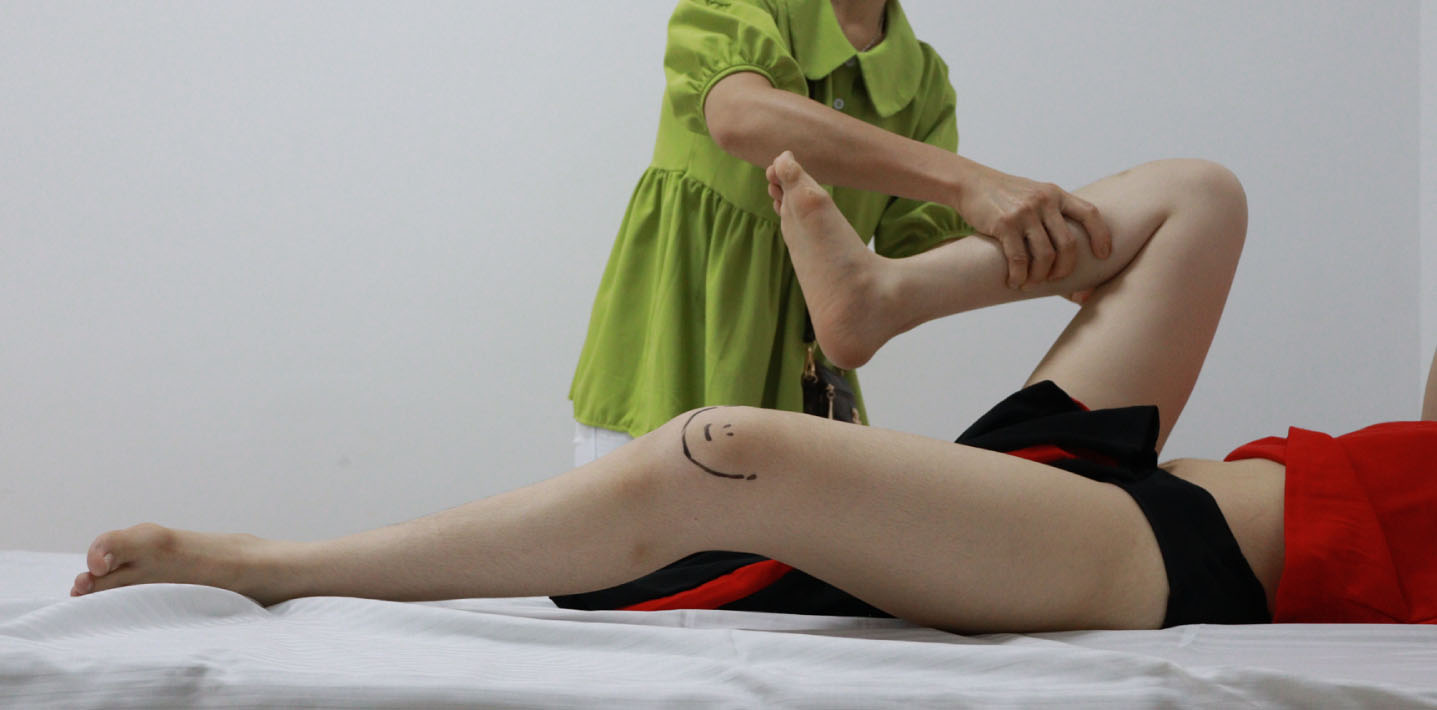
Fig. 10
Bilateral knee flexion contractures 25 degrees.
The inpatient hospital course included rehabilitation medicine evaluation, administration of baclofen and gabapentin, brace trials, and evaluation with ambulatory aids. A decision was reached with the patient and her family to perform surgical procedures. Proximal femoral varus, extension, and rotation osteotomies were performed along with distal femur extension osteotomy and patellar advancement. The right side was performed first (Fig. 7), and the left was performed three months later (Fig. 8).
Fig. 11
Preoperative imaging revealed good coverage of the hips, knee flexion, and patella alta. On the CT anteversion, the left was 37 degrees and the right 28 degrees. The neck shaft angle was 131 degrees bilaterally.
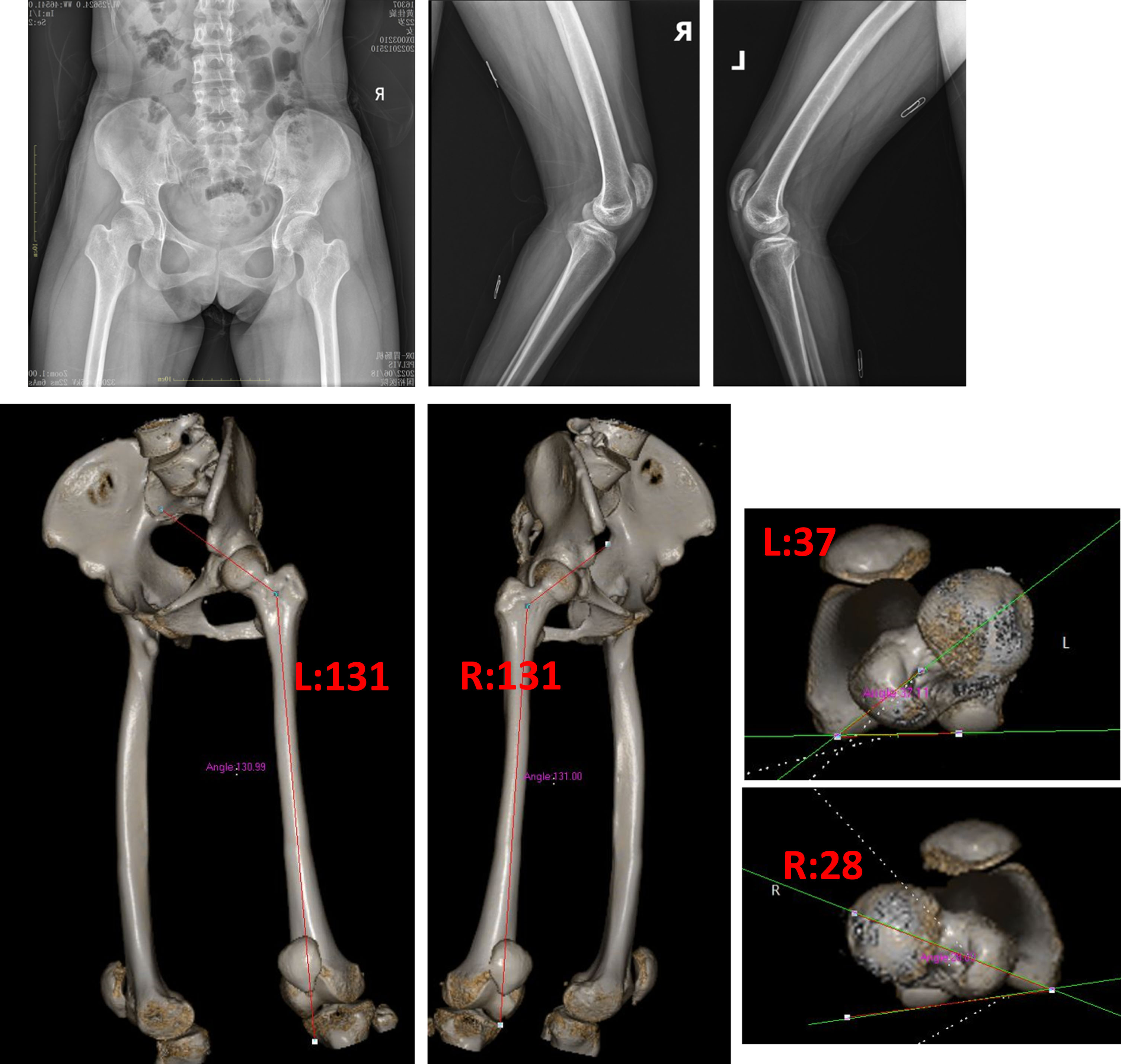
Fig. 12
Right side reconstruction with proximal femur extension rotation osteotomy, distal femur extension osteotomy, and patellar advancement.
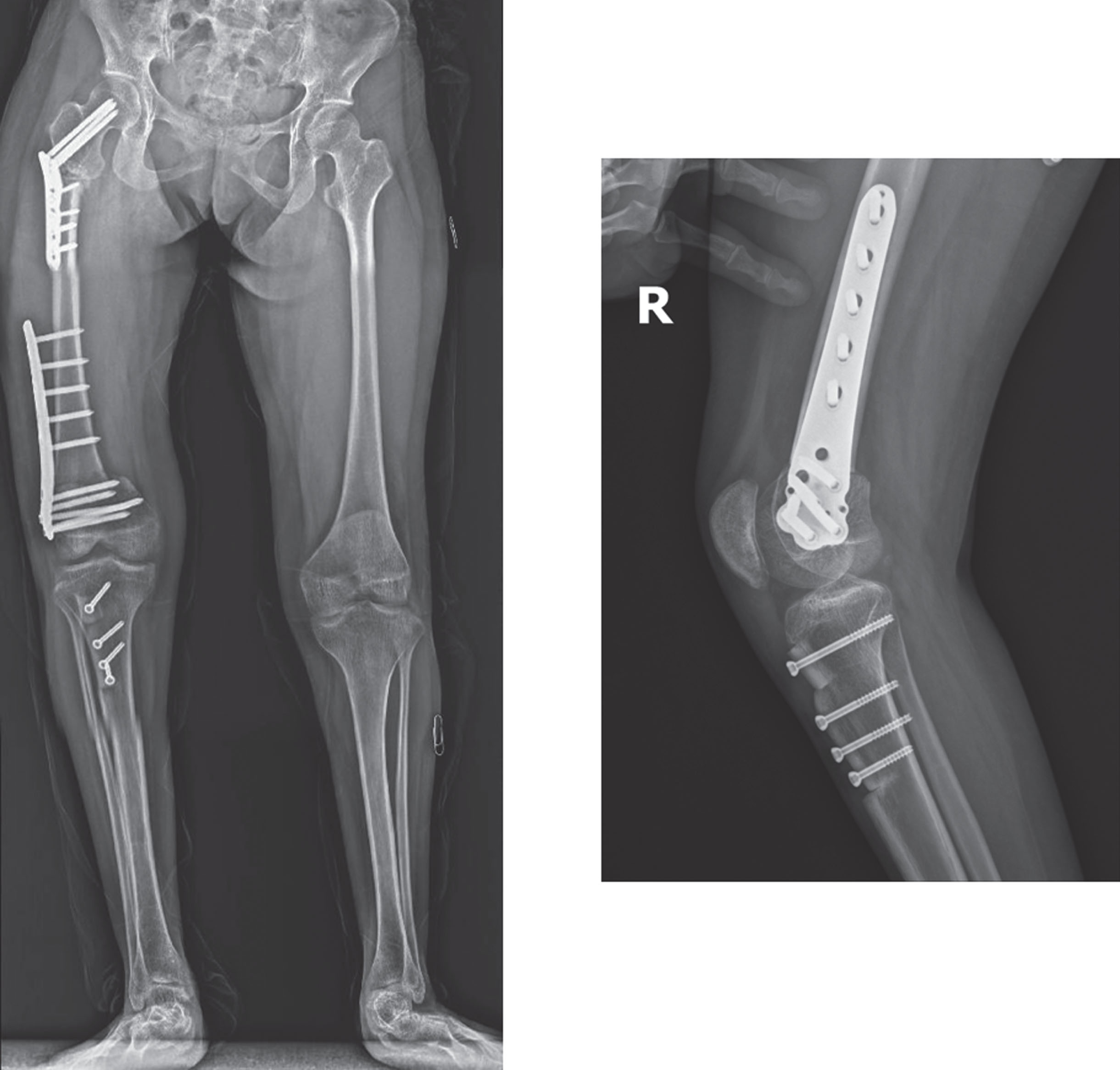
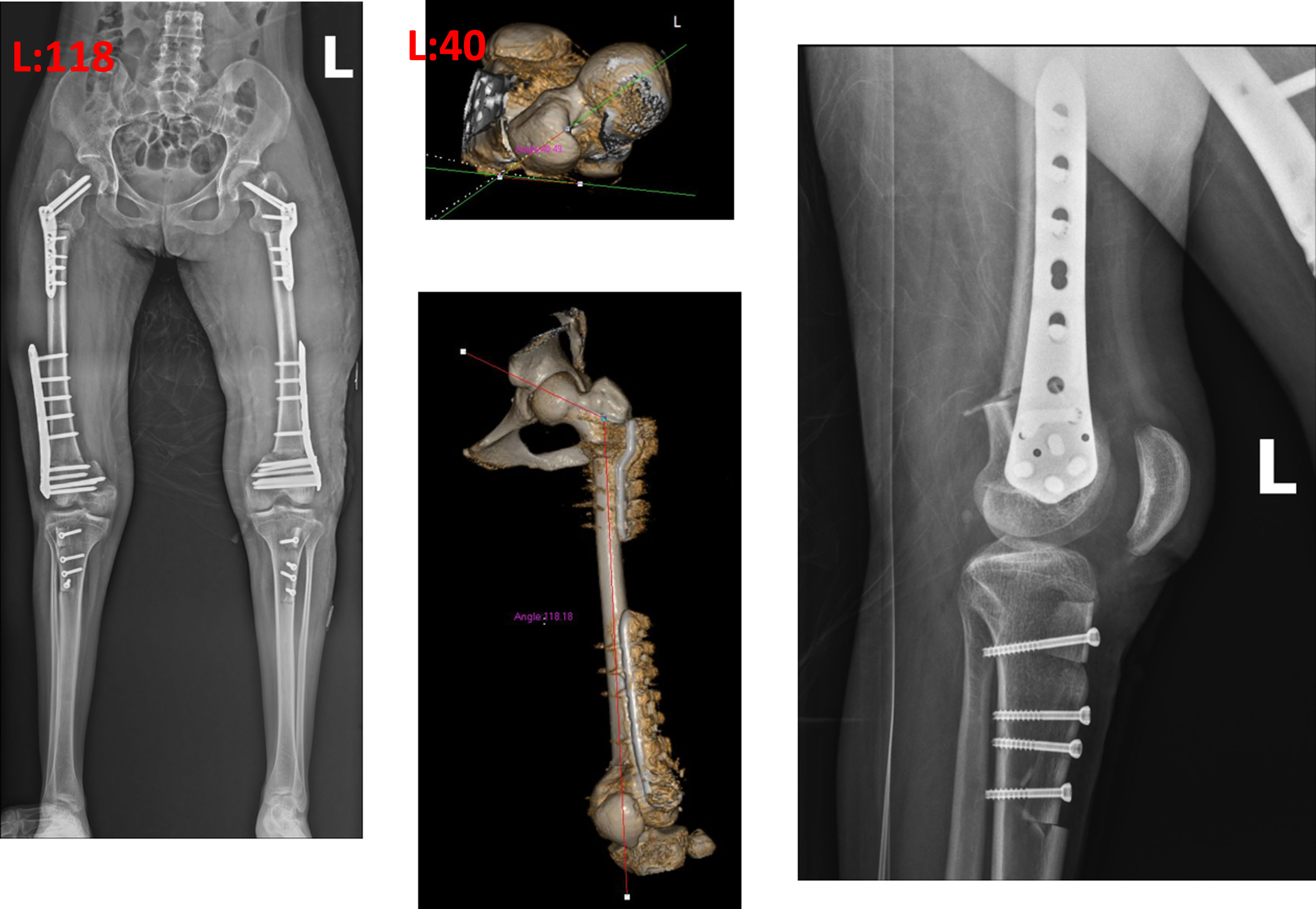
Fig. 13
The left side was performed three months after the right with varus, extension, rotation osteotomy of the proximal femur, extension osteotomy of the distal femur, and advancement of the patella. The neck shaft angles of 131 were reduced to about 120 degrees. The anteversion was slightly increased to account for the external rotation of the limb.
Five months after the first surgery, the osteotomies were healed. The knee pain was completely relieved, and she and her family were pleased with the improved posture (Fig. 14). She was continuing with rehabilitation. Her velocity has not been restored, and her endurance remains limited. Concerns about foot position remain, but further surgical treatment is not contemplated until she completely recovers from her recent surgeries.
Fig. 14
Postoperative position.
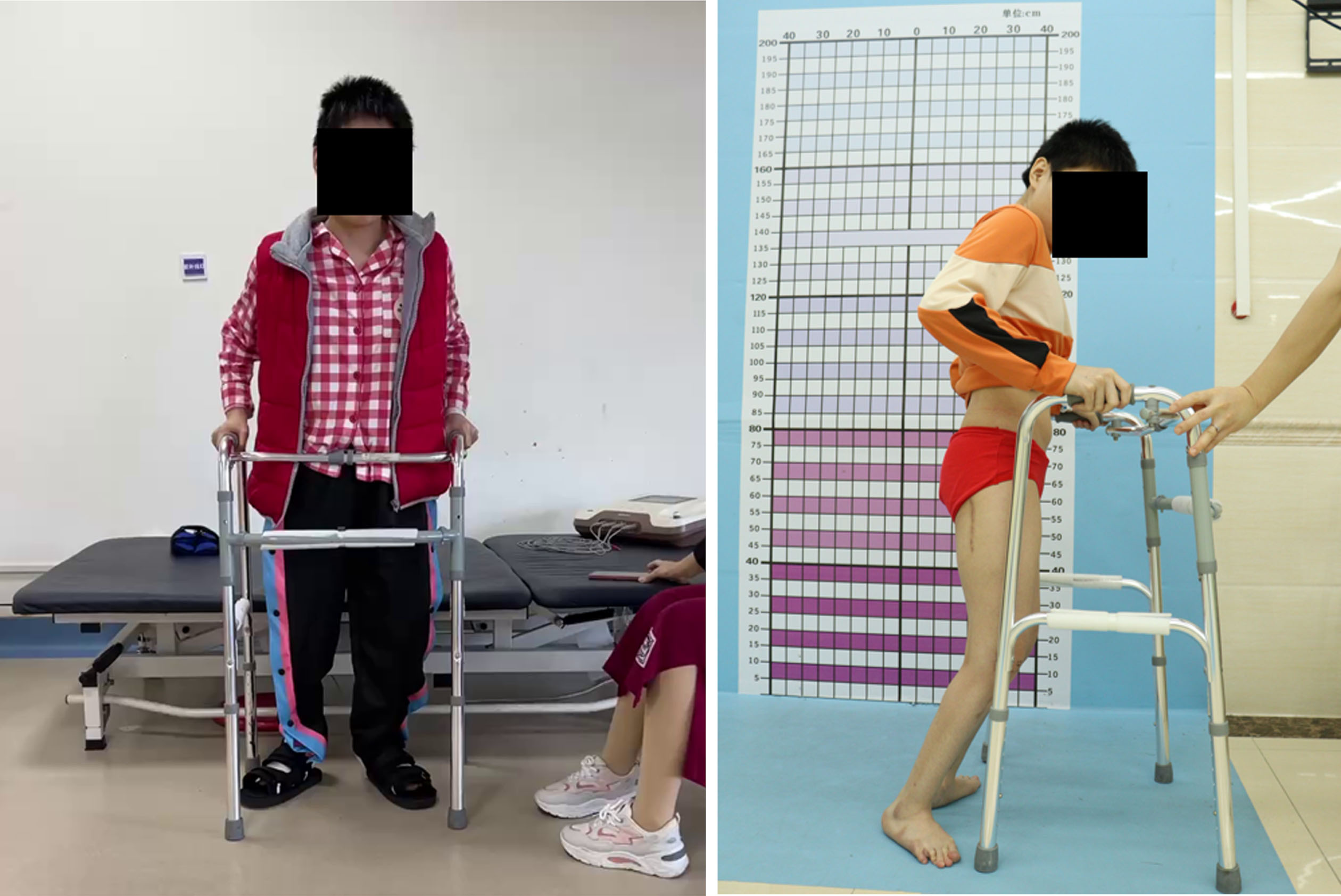
Crouch gait is a result of profoundly disturbed lever arms and results in progressive loss of function with increasing pain and decreasing endurance. Treating early in the younger child can result in good outcomes with less dramatic surgical intervention, but performing these procedures in the older patient is worthwhile. The upper age limits for this type of intervention have yet to be discovered.
The prognosis for untreated crouch gait is not good, as subsequent pain and ineffective lever arms hasten the decline of ambulatory function. Treatment to restore normal sagittal alignment can improve posture and function. Decreasing anterior pelvic tilt may help prevent the onset of intractable lumbar spine pain seen in CP patients with severe anterior pelvic tilt.
Total knee arthroplasty (TKA) is a salvage solution in the ambulatory CP patient. Unfortunately, only one series of 15 adult CP patients undergoing TKA demonstrated reliable outcomes with improved gait and functioning scores [47]. A recent nationwide inpatient database identified 2,200 subjects with CP who had total knee arthroplasty over ten years [46]. With careful assessment of perioperative comorbidities and complications and modern arthroplasty techniques, this is becoming a more prevalent treatment option for GMFCS I-III adults who are ambulators.
Lever arm dysfunction can persist or recur into adulthood, secondary to persistent torsional spasticity on the muscle-tendon units. Femoral anteversion and foot deformities are the most common in the GMCS II and III populations [16]. Residual femoral anteversion or external tibial torsion can contribute to lever arm dysfunction, pain, and difficulty with ambulation. CT scan or MRI with torsional assessment should be performed with a careful physical examination to identify rotational abnormalities. In a SEMLS setting, 66% of the time, the derotation was the intended amount with a 34% rate of slight under or over-correction with excellent overall outcomes in foot progression angle, anteversion, and clinical hip internal and external rotation. [14]. With skeletal maturity, femoral or tibial derotation over intramedullary nail implants is recommended to allow early weight bearing and mobilization and prevent delayed healing or nonunion. Although the femur can be derotated proximally or distally, proximal derotation is more accurate in the adult CP population [14, 48].
6Foot
Residual deformities of the foot are commonly encountered. The transient, flexible deformities of childhood progress into fixed skeletal deformations. Tibial torsion, pes planovalgus, and hallux valgus predominate the deformities seen in adults with CP. It is crucial to systematically evaluate the lower extremities, consistent with all CP patients [49]. Weight-bearing foot and ankle radiographs should always be obtained to assess for midfoot collapse, the severity of forefoot pronation or supination, talocalcaneal alignment, and any secondary ankle valgus contributing to the deformity.
Recurrent equinus is a common problem, leading to decreased gait mechanics or contributing to a late onset or recurrent foot deformity. In adult patients, the secondary capsular stiffness often prevents full anatomic correction to a neutral foot. However, in adults, gastrocnemius and soleus recessions are viable treatment options that restore kinetic and kinematic gait parameters, and even secondary articular deformity [50]. The posterior chain should be addressed in foot reconstructions in equinus settings.
Hallux valgus with metatarsophalangeal joint subluxation or dislocation is commonly symptomatic and the most common foot pathology addressed surgically in adults with CP. Although no specific literature exists on adults with CP, the adolescent CP population can be extrapolated. In ambulatory CP, the deformity is symptomatic hallux valgus. In non-ambulatory CP, the deformity is a dorsal bunion from the metatarsophalangeal subluxation and calcaneus [51]. The child with CP diverges between corrective metatarsal osteotomy for ambulatory patients, and arthrodesis for non-ambulatory patients [52]. In the adult CP patient, the secondary joint stiffness at presentation precludes any joint preserving options in the adult patient. Therefore, a first metatarsophalangeal arthrodesis is a durable option for all GMFCS levels.
In the ambulatory GMFCS I through III patient, the degree of deformity should be balanced with the patient’s activity level for deciding between joint-preserving bony reconstruction and triple arthrodesis. In non-ambulatory CP patients, double or triple arthrodesis is a durable surgical option for obtaining a plantigrade braceable foot for simulated weight-bearing and wheelchair use.
7Summary
The adult patient with CP faces a difficult situation with a lack of preventative or primary medical or surgical care. The impetus is on the providers, encouraging specialization and access to care through continuity clinics and consistent providers. Spine and hip pathology are a primary consideration. New loss of function or clinical deterioration should prompt screening for myelopathy or hydrocephalus as a cause. In the authors’ experience, a SEMLS approach is safe with an expected prolonged rehabilitation course. Lever arm dysfunction is primarily addressed with femoral derotation or foot reconstructions. Foot deformities are the most common surgically addressed sites for adults with CP. It is critical that more research into the long-term outcomes of these surgeries, as well as a more systematic approach to the general and musculoskeletal assessment and treatment of adults with CP, should be the focus of future research.
Key Evidence
• Murphy KP. The Adult with Cerebral Palsy. Orthopedic Clinics of North America. 2010;41(4):595-605.
• Kim HC, Oh SH, Oh JK, Ha Y. Surgical Strategies and Perioperative Considerations for Cervical Deformity with Cerebral Palsy: A Comprehensive Review of the Literature. Neurospine. 2022;19(4):868-875.
• Jameson R, Rech C, Garreau de Loubresse C. Cervical myelopathy in athetoid and dystonic cerebral palsy: retrospective study and literature review. Eur Spine J. 2010;19(5):706-712.
• Shaw KA, Hire JM, Cearley DM. Salvage Treatment Options for Painful Hip Dislocations in Nonambulatory Cerebral Palsy Patients. JAAOS - Journal of the American Academy of Orthopaedic Surgeons. 2020;28(9):363.
• Krach LE. Intrathecal baclofen use in adults with cerebral palsy. Developmental Medicine & Child Neurology. 2009;51(s4):106-112
Conclusions
• Adults outnumber pediatric patients with CP, with starkly decreased access to adult healthcare due to a lack of treatment centers or providers specializing in CP care.
• Adults with CP have higher rates and presentations of general medical problems, including malignancies and cardiovascular and renal disorders.
• Baclofen in intrathecal or oral form or botulinum toxin injections are the primary adjuvants for tone management.
• Progressive loss of function, weakness of the upper extremities, changes in spasticity, and bladder dysfunction should prompt a work-up for cervical myelopathy or occult hydrocephalus.
• Athetotic CP patients are at the highest risk for cervical spine pathology.
• Scoliosis can continue to progress into adulthood and become symptomatic; the earlier the treatment, the better to prevent medical decompensation.
• Spastic dislocated hips have a high rate of becoming symptomatic; multiple salvage options are available as treatment.
• Intramedullary devices should be utilized for derotational osteotomies to decrease the incidence of nonunion due to poor bone health.
Conflict of interest
Henry Chambers, MD, is a consultant for Abbvie Corporation. The other authors have no conflicts of interest.
Ethical considerations
This paper is exempt from Institutional Review Board.
References
[1] | Rutkowski S , Riehle E . Access to Employment and Economic Independence in Cerebral Palsy. IPhysical Medicine and Rehabilitation Clinics of North America. (2009) ;20: (3):535–47. doi: 10.1016/j.pmr.2009.06.003. |
[2] | McCormick A , Brien M , Plourde J , Wood E , Rosenbaum P , McLean J . Stability of the Gross Motor Function Classification System in adults with cerebral palsy. IDevelopmental Medicine & Child Neurology. (2007) ;49: (4):265–9. doi: 10.1111/j.1469-8749.2007.00265.x. |
[3] | Strauss D , Shavelle R . Life expectancy of adults with cerebral palsy. IDevelopmental Medicine & Child Neurology. (1998) ;40: (6):369–75. doi: 10.1111/j.1469-8749.1998.tb08211.x. |
[4] | Ryan J , Peterson M , Ryan N , et al Mortality due to cardiovascular disease, respiratory disease, and cancer in adults with cerebral palsy. IDev Med Child Neurol.924-8. (2019) ;61: (8):924–8. doi: 10.1111/dmcn.14176. |
[5] | Strauss D , Shavelle R , Reynolds R , Rosenbloom L , Day S . Survival in cerebral palsy in the last 20 years: Signs of improvement? IDev Med Child Neuro. (2007) ;49: (2):l86–92. doi: 10.1111/j.1469-8749.2007.00086.x. |
[6] | Strauss D , Brooks J , Rosenbloom L , Shavelle R . Life expectancy in cerebral palsy: An update. IDevelopmental Medicine & Child Neurology. (2008) ;50: (7):487–93. doi: 10.1111/j.1469-8749.2008.03000.x. |
[7] | Krach LE . Intrathecal baclofen use in adults with cerebral palsy. IDevelopmental Medicine & Child Neurology. (2009) ;51: (s4):106–12. doi: 10.1111/j.1469-8749.2009.03422.x. |
[8] | Murphy KP , Molnar GE , Lankasky K . Medical and Functional Status of Adults Wth Cerebral Palsy. IDevelopmental Medicine & Child Neurology. (1995) ;37: (12):1075–84. doi: 10.1111/j.1469-8749.1995.tb11968.x. |
[9] | Murphy KP . The Adult with Cerebral Palsy. IOrthopedic Clinics of North America. (2010) ;41: (4):595–605. doi: 10.1016/j.ocl.2010.06.007. |
[10] | Smith SE , Gannotti M , Hurvitz EA , et al Adults with Cerebral Palsy Require Ongoing Neurologic Care: A Systematic Review. IAnnals of Neurology. (2021) ;89: (5):860–71. doi: 10.1002/ana.26040. |
[11] | Flavin M , Shore BJ , Miller P , Gray S . Hormonal Contraceptive Prescription in Young Women With Cerebral Palsy. IJ Adolesc Health. (2019) ;65: (3):405–9. doi: 10.1016/j.jadohealth.2019.03.010. |
[12] | Whitney DG , Clines GA , Leis AM , Caird MS , Hurvitz EA . Five-year risk of fracture and subsequent fractures among adults with cerebral palsy. IBone Re. (2022) ;17: 101613.doi: 10.1016/j.bonr.2022.101613. |
[13] | Whitney DG , Caird MS , Clines GA , Hurvitz EA , Jepsen KJ . Clinical bone health among adults with cerebral palsy: Moving beyond assessing bone mineral density alone. IDevelopmental Medicine & Child Neurology. (2022) ;64: (4):469–75. doi: 10.1111/dmcn.15093. |
[14] | Putz C , Wolf SI , Geisbüsch A , Niklasch M , Döderlein L , Dreher T . Femoral derotation osteotomy in adults with cerebral palsy. IGait & Posture. (2016) ;49: 290–6. doi: 10.1016/j.gaitpost.2016.06.034. |
[15] | Strauss D , Ojdana K , Shavelle R , Rosenbloom L . Decline in function and life expectancy of older persons with cerebral palsy. INeuroRehabilitation. (2004) ;19: (1):69–78. doi: 10.3233/NRE-2004-19108. |
[16] | Saisongcroh T , Shrader MW , Lennon N , Church C , Sees JP , Miller F . Residual Deformity and Outcome of Ambulatory Adults With Cerebral Palsy: A Long-term Longitudinal Assessment. IJournal of Pediatric Orthopaedics. (2022) ;42: (4):215.doi: 10.1097/BPO.0000000000002057. |
[17] | Morgan P , McGinley J . Gait function and decline in adults with cerebral palsy: A systematic review. IDisabil Rehabil. (2014) ;36: (1):1–9. doi: 10.3109/09638288.2013.775359. |
[18] | Gerszten PC , Albright AL , Johnstone GF . Intrathecal baclofen infusion and subsequent orthopedic surgery in patients with spastic cerebral palsy. IJ Neurosurg.. (1009) ;88: (6):1009–13. doi: 10.3171/jns.1998.88.6.1009. |
[19] | Kim JH , Jung NY , Chang WS , Jung HH , Cho SR , Chang JW . Intrathecal Baclofen Pump Versus Globus Pallidus Interna Deep Brain Stimulation in Adult Patients with Severe Cerebral Palsy. IWorld Neurosurgery. (2019) ;126: e550–e556. doi: 10.1016/j.wneu.2019.02.092. |
[20] | Francisco GE , Boake C . Improvement in walking speed in poststroke spastic hemiplegia after intrathecal baclofen therapy: A preliminary study11A commercial party with a direct financial interest in the results of the research supporting this article has conferred or will confer a financial benefit on the author or 1 or more of the authors. IArchives of Physical Medicine and Rehabilitation. (2003) ;84: (8):1194–9. doi: 10.1016/S0003-9993(03)00134-5. |
[21] | Tassëel Ponche S , Ferrapie AL , Chenet A , et al Intrathecal baclofen in cerebral palsy. IA retrospective study of 25 wheelchair-assisted adults. Annals of Physical and Rehabilitation Medicine.483-98. (2010) ;53: (8):483–98. doi: 10.1016/j.rehab.2010.07.007. |
[22] | Thometz JG , Simon SR ,Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. JBJS.(1988) ;70: (9), 1290. |
[23] | Willoughby KL , Ang SG , Thomason P , et al Epidemiology of scoliosis in cerebral palsy: A population-based study at skeletal maturity. IJournal of Paediatrics and Child Health. (2022) ;58: (2):295–301. doi: 10.1111/jpc.15707. |
[24] | Fields M , Lee NJ , McCormick K , et al A national analysis on complications and readmissions for adult cerebral palsy patients undergoing primary spinal fusion surgery. IEur Spine J.718-25. (2022) ;31: (3):718–2. doi: 10.1007/s00586-021-07089-4. |
[25] | Yaszay B , Bartley CE , Sponseller PD , et al Major complications following surgical correction of spine deformity in 257 patients with cerebral palsy. ISpine Deform. (2020) ;8: (6):1305–- 12. doi: 10.1007/s43390-020-00165-7. |
[26] | Harada T , Ebara S , Anwar MM ,et al The lumbar spine in spastic diplegia. IA radiographic study. J Bone Joint Surg Br. (1993) ;75: (4):534–7. doi: 10.1302/0301-620X.75B4.8331105. |
[27] | Rosenberg NJ , Bargar WL , Friedman B . The incidence of spondylolysis and spondylolisthesis in nonambulatory patients. ISpine (Phila Pa1976). (1981) ;6: (1):35–8. doi: 10.1097/00007632-198101000-00005. |
[28] | Harada T , Ebara S , Anwar MM , et al The cervical spine in athetoid cerebral palsy. IA radiological study of 180 patients. J Bone Joint Surg Br. (1996) ;78: (4), 613–9. |
[29] | Kim HC , Oh SH , Oh JK , Ha Y . Surgical Strategies and Perioperative Considerations for Cervical Deformity With Cerebral Palsy: A Comprehensive Review of the Literature. INeurospine. (2022) ;19: (4):868–75. doi: 10.14245/ns.2244956.478. |
[30] | Kim GU , Ahn MW , Lee GW . Combined Anterior-Posterior Fusion Versus Posterior Alone Fusion for Cervical Myelopathy in Athetoid-Cerebral Palsy. IGlobal Spine J.-22. (2022) ;12: (8):1715–22. doi: 10.1177/2192568220987535. |
[31] | Ando N , Ueda S . Functional deterioration in adults with cerebral palsy. IClin Rehabil. (2000) ;14: (3):300–6. doi: 10.1191/026921500672826716. |
[32] | Hung CW , Matsumoto H , Ball JR ,et al Symptomatic cervical spinal stenosis in spastic cerebral palsy. IDevelopmental Medicine & Child Neurology. (2020) ;62: (10):1147–53. doi: 10.1111/dmcn.14607. |
[33] | Jameson R , Rech C , Garreau de Loubresse C . Cervical myelopathy in athetoid and dystonic cerebral palsy: Retrospective study and literature review. IEur Spine J. (2010) ;19: (5):706–12. doi: 10.1007/s00586-009-1271-7. |
[34] | Bret P , Chazal J . Chronic (“normal pressure”) hydrocephalus in childhood and adolescence. IA review of 16 cases and reappraisal of the syndrome. Childs Nerv Syst. (1995) ;11: (12):687–91. doi: 10.1007/BF00262232. |
[35] | Albright AL , Ferson S , Carlos S . Occult hydrocephalus in children with cerebral palsy. INeurosurgery. (2005) ;56: (1):93–6discussion 96-97. doi: 10.1227/01.neu.0000144779.32401.a2. |
[36] | Soo B , Howard JJ , Boyd RN , et al Hip displacement in cerebral palsy. IJ Bone Joint Surg Am. (2006) ;88: (1):121–9. doi: 10.2106/JBJS.E.00071. |
[37] | Wawrzuta J , Willoughby KL , Molesworth C , et al Hip health at skeletal maturity: A population-based study of young adults with cerebral palsy. IDev Med Child Neurol. (1273) ;58: (12):1273–80. doi: 10.1111/dmcn.13171. |
[38] | Shaw KA , Hire JM , Cearley DM . Salvage Treatment Options for Painful Hip Dislocations in Nonambulatory Cerebral Palsy Patients. IJAAOS - Journal of the American Academy of Orthopaedic Surgeons. (2020) ;28: (9):363.doi: 10.5435/JAAOS-D-19-00349. |
[39] | Castle ME , Schneider C . Proximal femoral resection-interposition arthroplasty. IJ Bone Joint Surg Am. (1978) ;60: (8), 1051–4. |
[40] | McCarthy RE , Simon S , Douglas B , Zawacki R , Reese N . Proximal femoral resection to allow adults who have severe cerebral palsy to sit. IJBJS. (1988) ;70: (7), 1011. |
[41] | McHale KA , Bagg M , Nason SS . Treatment of the chronically dislocated hip in adolescents with cerebral palsy with femoral head resection and subtrochanteric valgus osteotomy. IJ Pediatr Ortho. (1990) ;10: (4), 504–9. |
[42] | Godfrey J , McGraw J , Kallur A , Silva S , Szalay E . A Modification to the McHale Procedure Reduces Operative Time and Blood Loss. IJournal of Pediatric Orthopaedics. (2016) ;36: (8):e89 10.1097/BPO.0000000000000634. |
[43] | Bauer JM , Butler MP , Schoenecker PL , Schoenecker JG . Trochanteric-sparing Proximal Femoral Resection for Arthritic Spastic Hips. ITechniques in Orthopaedics.. (2020) ;35: (1):51–62 10.1097/BTO.0000000000000324. |
[44] | Houdek MT , Watts CD , Wyles CC , Trousdale RT , Milbrandt TA , Taunton MJ . Total Hip Arthroplasty in Patients with Cerebral Palsy: A Cohort Study Matched to Patients with Osteoarthritis. IJBJS. (2017) ;99: (6):488.doi: 10.2106/JBJS.16.00528. |
[45] | Raphael BS , Dines JS , Akerman M , Root L . Long-term followup of total hip arthroplasty in patients with cerebral palsy. IClin Orthop Relat Res.-54. (2010) ;468: (7):1845–54. doi: 10.1007/s11999-009-1167-1. |
[46] | Moon AS , Pinto MC , Cichos KH , McGwin GJ , Ponce BA , Ghanem ES . Total Joint Arthroplasty in Patients With Cerebral Palsy. IJAAOS - Journal of the American Academy of Orthopaedic Surgeons. (2020) ;28: (4):171.doi:10.5435/JAAOS-D-18-00828. |
[47] | Houdek MT , Watts CD , Wyles CC , Trousdale RT , Milbrandt TJ , Taunton MJ . Total Knee Arthroplasty in Patients With Cerebral Palsy: A Matched Cohort Study to Patients With Osteoarthritis. IJAAOS - Journal of the American Academy of Orthopaedic Surgeons. (2017) ;25: (5):381.doi:10.5435/JAAOS-D-16-00437. |
[48] | Niklasch M , Boyer ER , Novacheck T , Dreher T , Schwartz M . Proximal versus distal femoral derotation osteotomy in bilateral cerebral palsy. IDev Med Child Neurol. (2018) ;60: (10):1033–7. doi: 10.1111/dmcn.13910. |
[49] | Davids JR . The Foot and Ankle in Cerebral Palsy. IOrthopedic Clinics of North America. (2010) ;41: (4):579–93. doi: 10.1016/j.ocl.2010.06.002. |
[50] | Putz C , Mertens EM , Wolf SI ,et al Equinus Correction During Multilevel Surgery in Adults With Cerebral Palsy. IFoot Ankle Int. (2018) ;39: (7):812–0. doi: 10.1177/1071100718765161. |
[51] | van de Velde SK , Cashin M , Johari R , Blackshaw R , Khot A , Graham HK . Symptomatic hallux valgus and dorsal bunion in adolescents with cerebral palsy: Clinical and biomechanical factors. IDev Med Child Neurol. (2018) ;60: (6):624–8. doi: 10.1111/dmcn.13724. |
[52] | Lewis TL , Patel K , Shepherd KL , MacInnes P , Ray R , Kokkinakis M . Hallux valgus surgery in children with cerebral palsy: A systematic review. IFoot Ankle Surg. (2022) ;28: (4):476–82. doi: 10.1016/j.fas.2021.12.009. |




