A confounding pediatric spinal cord injury: Anterior, central, or both?
Abstract
Pediatric spinal cord injury (SCI) most commonly affects the cervical region. Central cord syndrome most often occurs in the lower cervical injury due to hyperextension injury, while anterior cord syndrome is primarily due to vascular infarction after hyperextension injury. An unusual case of a pediatric patient who physically presented with central cord syndrome but radiologically had evidence of anterior spinal artery syndrome is described.
A two-year-old male presented after a fall from three feet with flaccid upper extremities and dysesthesias but maintained functional strength in bilateral lower extremities. Although his clinical presentation was that of central cord syndrome, he was found to have an anterior spinal artery infarct spanning from C2-T3 with a ligamentous injury at C3 and an incidental finding of Chiari I malformation on MRI. Given the negative evaluation for a cardiac or hematologic source of embolus and normal angiography, it was theorized that compression of vertebral arteries by previously undiagnosed Chiari I malformation in the setting of trauma could have made the patient more vulnerable to this complication. During inpatient rehabilitation, he regained scapular movement and shoulder flexion. However, he regained distal movement in supination, wrist extension, and finger flexion instead of the more usual proximal-to-distal motor recovery observed in SCI. While he had a relative sparing of strength in his legs, he had impaired proprioception and balance, leading to gait impairment.
This case highlights the complexity of pediatric cervical SCI diagnosis and prognostication. While classic SCI subtypes are well described, many pediatric and adult patients will present and recover in unexpected ways. All with SCI should be evaluated thoroughly for common etiologies and transitioned to rehabilitation therapies to assist in recovery.
1Acute care presentation
A two-year-old male with normal development and no prior medical history presented to acute care after an unwitnessed fall, jumping from approximately three feet high. After the fall, his mother immediately noted that the patient could not move his neck or bilateral upper extremities. However, there was preservation of spontaneous movement of the lower extremities. Upon arrival at the hospital, the patient was admitted to the pediatric intensive care unit, and his physical exam was remarkable for bilateral upper extremity flaccid paraplegia with areflexia and dysesthesia to light touch. The patient had intact cranial nerve function, including swallowing capabilities; however, given that he had not been toilet trained at this time, it was difficult to gauge whether there was bowel or bladder dysfunction.
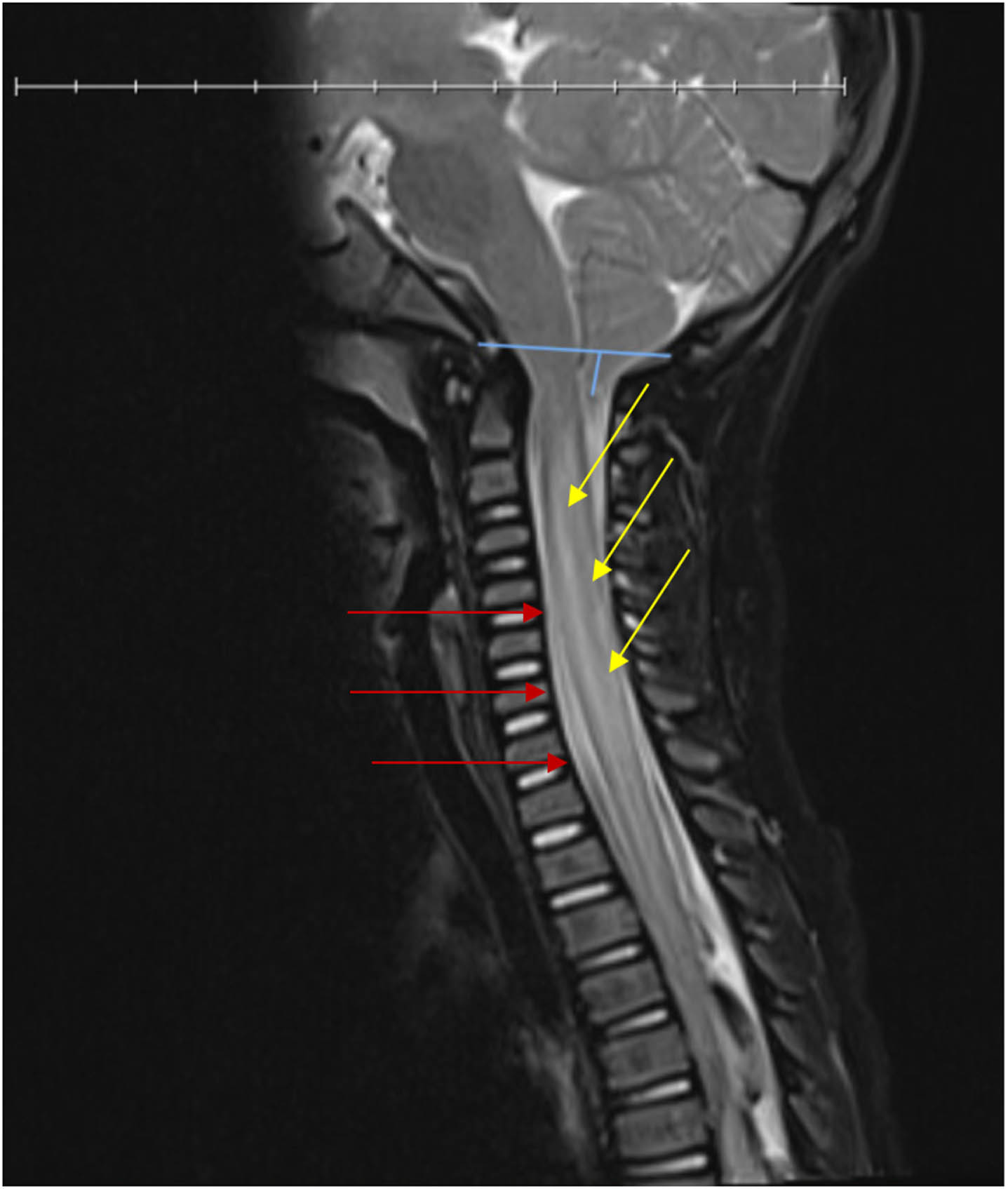
Initial work included magnetic resonance imaging (MRI) of the cervical spine, which showed cervical spinal cord swelling with cord signal change and diffusion restriction from levels C2-T3 with a ligamentous injury at C3. There was also an incidental finding of Chiari I malformation (Fig. 1). Radiographic interpretation by neurosurgery and radiology reported an anterior spinal artery (ASA) infarction in the setting of the anatomic predisposition of Chiari I. Although not standard care and still considered controversial management, pediatric neurosurgery recommended dexamethasone therapy for six days for spinal cord swelling and Miami J cervical collar for six weeks without surgical intervention [1–3]. Pediatric vascular consultants suggested that although rare, sensitivity to ASA strokes has been theorized with Chiari malformations as the vertebral arteries pass through the foramen magnum and could theoretically be vulnerable to compression at that site. Computerized tomography angiography (CTA) of the head and neck provides an unreliable visualization of the ASA; however, the patient’s CTA was obtained and showed no branch occlusion, vascular injury, arteritis, or aneurysm to the anterior and posterior circulation. In addition, his cardiac workup was negative for secondary causes of stroke. The patient was admitted to the acute care hospital for seven days and due to his functional deficits secondary to the new SCI, he was transferred to inpatient rehabilitation (IPR), where he remained for six weeks for a comprehensive therapy program.
Fig. 1
T2 weighted MRI of cervical spinal cord showing swelling with cord signal changes (right, yellow arrows) and diffusion restriction from levels C2-T3 with a ligamentous injury (left, red arrows) and Chiari I malformation (top, blue lines).
1.1IPR course
During his time at IPR, the patient worked extensively with occupational therapy (OT) due to the primary involvement of the upper extremities. Upon admission to IPR, his physical exam revealed bilateral upper extremity hypersensitivity with flaccid paralysis, areflexia, and absent Hoffman sign. He showed bilateral lower extremity spontaneous movements with good muscle strength, could support his weight, and ambulated with assistance. He also had brisk reflexes in the bilateral lower extremities, left greater than right, with few beats of ankle clonus present. Further neurologic assessment was primarily observational, as standardized assessment was limited due to the patient’s age and developmental level.
He had minimal functional improvement in both upper extremities in his first four weeks of IPR. During multiple OT sessions, neuromuscular electrical stimulation was trialed to attempt flaccid musculature recruitment without success. Despite several attempts during each session, therapists observed no recruitment in the upper extremities. This pattern is typical of a lower motor neuron (LMN) SCI; however, he otherwise clinically did not appear to have areflexic bowel and bladder, which are also associated with a LMN cord injury. Although he was not toilet trained before the injury, post-void residuals were measured during the first week of admission and remained at zero. He also had regular, soft bowel movements and only required polyethylene glycol intermittently throughout his admission. The International Standards for Neurological Classification of SCI (ISNCSCI) was deferred due to age, inability to participate, and not being toilet trained[4, 5].
After three weeks in IPR, he began to show motor activation in bilateral finger flexors, with the right side stronger than the left. For recruitment patterns, he gained trapezius first, with the right side returning greater than the left during the fourth week. Also, within this time, the anterior deltoids showed a similar pattern of laterality. During the course of the fifth week, he started exhibiting wrist extension and supination despite not displaying any return of his biceps or triceps. He utilized supination to compensate for weak finger flexors to assist with wrist extension for a tenodesis grasp, observed more on the right side, allowing him a functional yet partial grasp while in IPR. In the fourth week, he utilized shoulder hike and trunk rotation to swing his arms to compensate for no elbow flexion or extension, but this did not become functional until the sixth week of his IPR stay, during which he demonstrated increased muscle activation distally. Activation of the deltoid improved to antigravity during the sixth week of his IPR stay, but he continued to have bilateral anterior shoulder subluxation limiting his range of motion; therefore, custom shoulder support was fabricated. His transient upper extremities dysesthesias were treated with gabapentin and other desensitization techniques, and the patient was eventually tapered off medication without residual symptoms.
The patient was also evaluated and treated by physical therapy (PT) due to decreased upper extremity activation impacting gross motor function, such as absent protective upper extremity responses in case of loss of balance. In addition, he was found to have impaired lower extremity and truncal coordination for his age with standing and ambulation, thereby requiring maximal assistance for transfers. He progressed in balance and gait training with bilateral ankle foot orthoses to increase safety and lower his risk of subsequent falls. Parents were also provided with a gait harness to assist with safe ambulation.
1.2Six-month clinic follow-up
Upon evaluation in the clinic and through outpatient therapy notes review, the patient had progressed to achieve muscle recruitment in elbow flexion, which had not been observed in IPR. Regaining biceps muscles to partial antigravity up to 90 degrees in elbow flexion increased his functionality as he could now carry objects using bilateral upper extremities in a supinated position. However, at this time, he was not able to bring hand to mouth. As demonstrated in Table 1, with PT, he progressed to going from supine to sit, ascending stairs with a handheld assist, and descending stairs scooting for safety. He had developed increasing bilateral upper extremity weight bearing by assuming quadruped from a prone or sitting position to promote protective response capabilities. After six months of continued therapy, the patient also demonstrated falling safely while tucking his head with self-paced walking.
Table 1
Patient’s muscle recovery and therapy progress
| IPR Discharge | Six-month progress | Eleven-month progress | |
| Shoulder flexion | - RUE: 10° with compensatory strategies | - RUE: 0–45° AG (can obtain 70° with lordosis and arm swing) | - BUE: >90° gravity eliminated |
| Elbow flexion | - None bilaterally | - RUE: 60–70° AG | - BUE: 90° AG |
| - LUE: 50–60° AG | |||
| Grasping | - RUE: Using tenodesis and supination | - RUE: Pincer using lightweight objects | - RUE: Independent |
| - LUE: Unable to maintain with tenodesis | - LUE: Min A | ||
| Upper extremity functionality | - Unable to carry objects | - Picks object with BUE to carry | - Min A for increased participation with feeding, goal continued for consistency |
| - Requires max A for hand to mouth with RUE | - Consistently grasps to retrieve items with RUE | ||
| Supine to sit | - Mod A | - Supervision with setup of BUE | - No change * |
| Gait | - 150 ft CGA and bilateral AFOs | - Supervision | - No change * |
| Stairs | - Max A | - Supervision, scooting for safety | - No change * |
IPR: Inpatient rehabilitation, RUE: Right upper extremity, LUE: Left upper extremity, BUE: Bilateral upper extremities, AG: Against gravity, Min A: Minimal assistance, Max A: Maximal assistance, Mod A: Moderate assistance, CGA: Contact guard assist, AFO: Ankle-foot orthoses. *Patient discharged from physical therapy after nine months of ongoing therapies.
1.3Eleven-month therapy progress
The patient continued OT sessions three times a week for 26 weeks. In his most recent monthly summary of progress, when tested in positions that allow for impacts of gravity to be minimized, such as side lying or supine, he demonstrated a 2/5 strength on manual muscle testing (MMT), compared to his IPR stay when he was not attaining a 1/5 in gravity-eliminated plane. During this 11-month evaluation, he improved elbow flexion to bring items from hand to mouth in standing with decreased compensatory strategies. As described in Table 1, the patient achieved hand-to-mouth functionality; however, the goal is still ongoing to achieve consistency. He had previously demonstrated a functional partial grasp during his IPR stay, but at this time, his upper extremity function had also been enhanced by his ability to grasp objects with his right hand consistently.
2Discussion
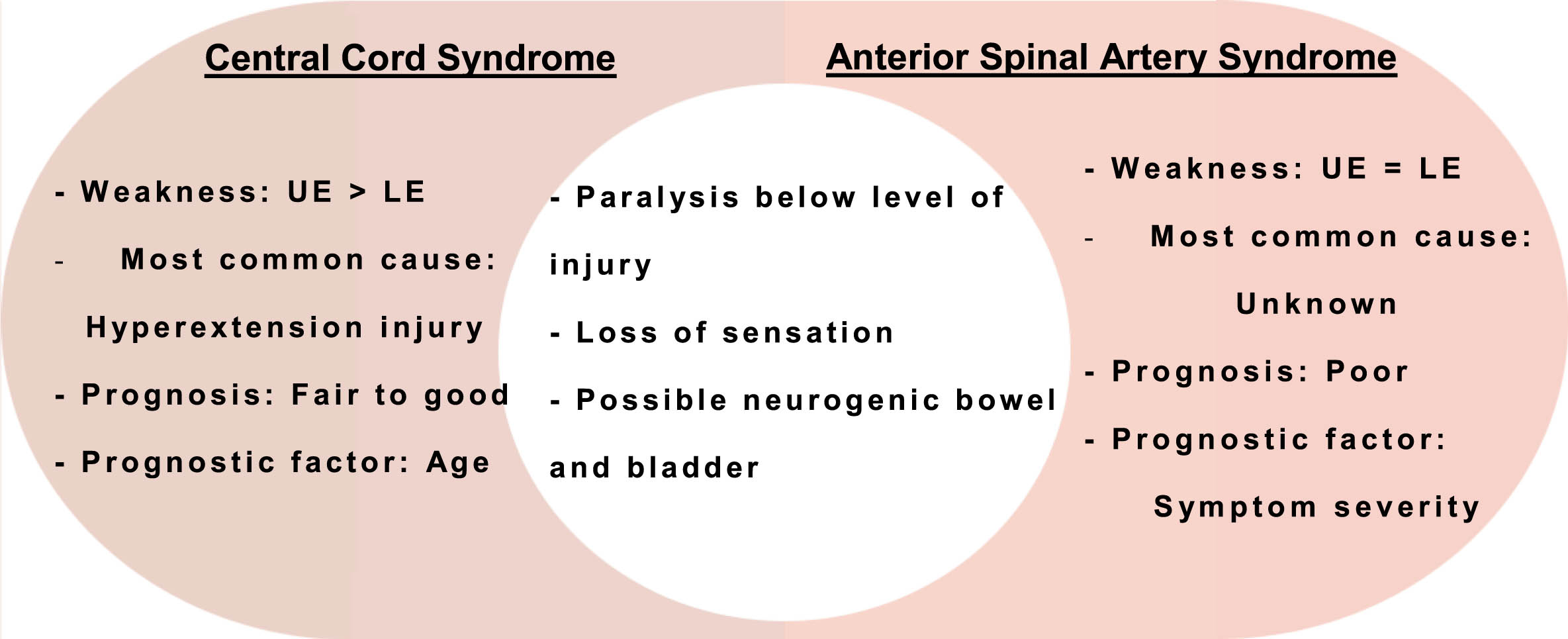
Experts often compare two types of SCI: ASA syndrome (ASAS) and central cord syndrome (CCS). Injury to the center of the spinal cord usually causes CCS, while damage to the front part of the spinal cord typically results in ASAS (Fig. 2). Both types of injuries can cause paralysis below the level of injury, loss of sensation, and possible bowel or bladder dysfunction (Fig. 3). However, CCS can produce more significant weakness in the arms than in the legs [6]. While the patient presented with physical manifestations of the incomplete SCI CCS, such as upper extremity flaccid paralysis and dysesthesias, he had radiographic evidence of ASAS [7].
Fig. 2
Axial drawings of spinal cord for visual demonstration of (A) simultaneous anterior and posterior spinal cord compression affects the medial aspect of the lateral corticospinal tracts in central cord syndrome and (B) loss of blood flow to the anterior spinal artery leads to involvement of the anterior 2/3 of the spinal cord, as observed in anterior spinal artery syndrome.
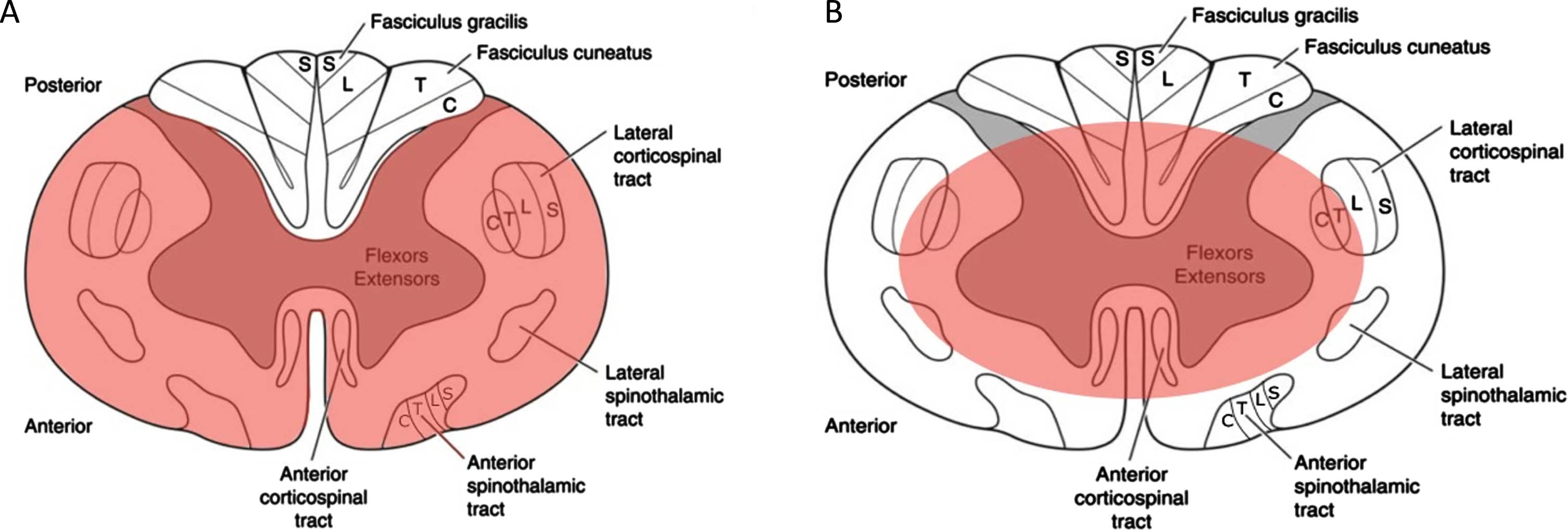
Fig. 3
Comparison and contrast of CCS and ASAS. UE: upper extremity; LE: lower extremity; CCS: central cord syndrome; ASAS: anterior spinal artery syndrome.
CCS is most commonly caused by hyperextension injury leading to simultaneous anterior and posterior spinal cord compression, affecting the medial aspect of the lateral corticospinal tracts. CCS generally has a better prognosis, with 90% of motor recovery in three years, regardless of management [6]. It is worth highlighting that pediatric SCI patients generally experience better outcomes [4]. Usually, improvement follows an ascending, proximal to distal pattern [6]. In contrast, this patient had distal muscle recovery with minimal proximal recovery before his discharge from IPR.
ASAS most commonly occurs as a complication of aortic surgery leading to loss of blood flow to the ASA, which supplies the anterior two-thirds of the spinal cord [7]. Similar to adults, the cause of ASA infarction in pediatric patients is related to a reduction in blood flow resulting from hypotension or vascular blockage, which can be caused by traumatic or iatrogenic factors, as well as thrombotic or embolic diseases. However, nontraumatic spinal cord thromboembolism is uncommon in children, and its causes are poorly understood. Commonly theorized sources include fibrocartilaginous embolism (often after minor trauma), systemic hypotension, parainfectious vasculopathy, and cardioembolic disease, among many other causes [8]. Short interval follow-up MRI of the cervical spine could have helped with diagnostic certainty by ensuring the expected evolution of the cord injury, including the scan contrast uptake would be expected with a spinal infarct. Due to the need for anesthesia for MRI, the team did not pursue a short interval scan.
A potential increased risk of ischemia has also been proposed in the setting of Chiari I. Two case reports were found in the literature suggesting Chiari I as a possible risk factor of ASA ischemia in adults, one asymptomatic and the other with classic symptoms of tetraparesis [9, 10]. Further investigation is necessary as no literature has been found on the pediatric population.
Additional rare diagnoses that could explain the presentation of the patient presented in this case are Man-in-barrel syndrome (MIBS) and Bell’s cruciate paralysis, as these cases are also significant for significant weakness in the bilateral upper extremities and preserved strength in the bilateral neck and lower extremities. MIBS most commonly occurs in the setting of bilateral watershed infarcts due to hypoperfusion affecting the most distal branches of the anterior cerebral artery and middle cerebral artery that supply upper extremity motor fibers in the brain [11]. Bilateral symmetric damage isolated to the upper extremity motor fibers anywhere else in the brain, brainstem, or cervical spinal could also contribute to MIBS; in this case, there was observed sensory involvement of the upper extremities, as well as lower extremity lack of coordination and impaired proprioception that was not consistent with the patient’s age and had been present since his injury, suggesting involvement of some lower extremity fibers. Similarly, Bell’s cruciate paralysis was unlikely as it is also associated with various degrees of lower cranial nerve palsies and comatose periods due to the patient’s cervicomedullary involvement [12].
An ISNCSCI exam cannot be accurately completed in children under five years old due to their inability to understand complex instructions such as MMT, understand the difference between light touch and pinprick, and participate in the rectal portion of the examination [4, 5]. Prior toilet training and continence play an essential role in the algorithm to determine impairment [5]. In this two-year-old patient, additionally, compliance with the exam was further challenged by age-appropriate stranger anxiety in the setting of hospitalization trauma and difficulty following complex and specific directions. Clinical practice in this age group suggests that careful observation of patients’ motor and sensory functions without the use of complex directions is an alternative way to complete a physical examination for SCI patients [13]. However, age-appropriate stranger anxiety is a commonly encountered obstacle for pediatric physicians. Consequently, more research is needed to develop a standardized measurement tool to observe and isolate voluntary function from reflexive findings [3, 13].
Upon reviewing the literature on SCIs, it has become evident that most information available is based on adult cases [4]. It is crucial to note that age plays a significant role in achieving better outcomes for CCS, with individuals under 50 tending to fare better [6]. However, for ASAS, the severity of symptoms determines the recovery rate, and even with a milder presentation, neurological recovery to the prior baseline is minimal [7]. In general, it is challenging to predict pediatric SCI recovery. In this patient, it is particularly challenging to predict what long-term functionality will look like based on his clinical features of CCS with imaging findings of ASA infarct and Chiari I, his unusual muscle recovery pattern, and the lack of documentation in the literature of these syndromes in children. The reporting of this case intends to raise awareness of new traumatic SCIs in pediatric patients and the difficulties faced in understanding recovery and prognosis in the rehabilitation of this patient population.
Acknowledgments
The authors have no acknowledgments.
Conflicts of interest
The authors have no conflicts of interest to report.
Ethical considerations
This study, as a case report, is exempt from Institutional Review Board approval. Caregiver consent was not sought as identifying markers were removed from case report.
References
[1] | Bracken MB . Steroids for acute spinal cord injury. Cochrane Database Syst Rev. (2012) ;1: (1):CD001046. doi: 10.1002/14651858.CD001046.pub2 |
[2] | Canseco JA , Karamian BA , Bowles DR , et al. Updated review: The steroid controversy for management of spinal cord injury. World Neurosurg. (2021) ;150: :1–8. doi: 10.1016/j.wneu.2021.02.116 |
[3] | Wang JZ , Yang M , Meng M , Li ZH . Clinical characteristics and treatment of spinal cord injury in children and adolescents. Chin J Traumatol. (2023) ;26: (1):8–13. doi: 10.1016/j.cjtee.2022.04.007 |
[4] | Powell A , Davidson L . Pediatric spinal cord injury: A review by organ system. Phys Med Rehabil Clin N Am. (2015) ;26: (1):109–32. doi: 10.1016/j.pmr.2014.09.002 |
[5] | Roberts TT , Leonard GR , Cepela DJ . Classifications in brief: American spinal injury association (ASIA) impairment scale. Clin Orthop Relat Res. (2017) ;475: (5):1499–504. doi: 10.1007/s11999-016-5133-4 |
[6] | Ameer MA , Tessler J , Munakomi S , Gillis C . Central cord syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; (2024) [updated 13 August 2023; cited 6 September 2023]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441932 |
[7] | Pearl NA , Weisbrod LJ , Dubensky L . Anterior cord syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; (2024) https://www.ncbi.nlm.nih.gov/books/NBK559117/ |
[8] | Bar C , Cheuret E , Bessou P , Pedespan JM . Childhood idiopathic spinal cord infarction: Description of 7 cases and review of the literature. Brain Dev. (2017) ;39: (10):818–27. doi: 10/1016/j.braindev.2017.05.009 |
[9] | Kelly MP , Guillaume TJ , Lenke LG . Spinal deformity associated with chiari malformation. Neurosurg Clin N Am. (2015) ;26: (4):579–85. doi: 10.1016/j.nec.2015.06.005 |
[10] | Mashriqi F , Loukas M , Oskouian RJ , D’Antoni AV , Tubbs RS . Chiari I malformation and spinal cord ischemia in a cadaver. Cureus. (2017) ;9: (8):e1567. doi: 10.7759%2Fcureus.1567 |
[11] | Boodle J , Emmady P . Man in barrel syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; (2024) [updated 12 June 2023; cited 7 May 2024]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559186/ |
[12] | Hopkins B , Khanna R , Dahdaleh NS . Revisiting cruciate paralysis: A case report and systematic review. J Craniovertebr Junction Spine. (2016) ;7: (4):265–72. doi: 10.4103/0974-8237.193262 |
[13] | Calhoun CL , Gaughan JP , Ross S , Mulcahey MJ . A pilot study of observational motor assessment in infants and toddlers with spinal cord injury. Pediatr Phys Ther. (2009) ;21: (1):62–7. doi: 10.1097/PEP.0b013e31818f5bbd |




