Causes of death among people with myelomeningocele: A multi-institutional 47-year retrospective study
Abstract
PURPOSE:
This study aimed to analyze organ system-based causes and non-organ system-based mechanisms of death (COD, MOD) in people with myelomeningocele (MMC), comparing urological to other COD.
METHODS:
A retrospective review was performed of 16 institutions in Canada/United States of non-random convenience sample of people with MMC (born > = 1972) using non-parametric statistics.
RESULTS:
Of 293 deaths (89% shunted hydrocephalus), 12% occurred in infancy, 35% in childhood, and 53% in adulthood (documented COD: 74%). For 261 shunted individuals, leading COD were neurological (21%) and pulmonary (17%), and leading MOD were infections (34%, including shunt infections: 4%) and non-infectious shunt malfunctions (14%). For 32 unshunted individuals, leading COD were pulmonary (34%) and cardiovascular (13%), and leading MOD were infections (38%) and non-infectious pulmonary (16%). COD and MOD varied by shunt status and age (p < = 0.04), not ambulation or birthyear (p > = 0.16). Urology-related deaths (urosepsis, renal failure, hematuria, bladder perforation/cancer: 10%) were more likely in females (p = 0.01), independent of age, shunt, or ambulatory status (p > = 0.40). COD/MOD were independent of bladder augmentation (p = >0.11). Unexplained deaths while asleep (4%) were independent of age, shunt status, and epilepsy (p >= 0.47).
CONCLUSION:
COD varied by shunt status. Leading MOD were infectious. Urology-related deaths (10%) were independent of shunt status; 26% of COD were unknown. Life-long multidisciplinary care and accurate mortality documentation are needed.
1Introduction
Despite significant improvements in acute myelomeningocele (MMC) care and greater dedication to lifetime models of care, premature mortality remains significant. The annual mortality rate is 0.3-0.4% after infancy [1-5]. Sudden and unexpected death is a rare but sinister reality that understandably concerns patients and families alike. Little is known about the characteristics of people with MMC who died and their organ-based causes of death (COD) and more specific mechanisms of death (MOD). Patient-centered outcomes mandate that both COD and MOD be more carefully captured and studied.
While MMC impacts multiple body systems, historically, the principal COD centered on urologic and neurologic complications. Each of these domains has specific MOD that partially depend on age. Urologic COD include renal failure, urosepsis, bladder cancer, or perforation (among patients who have undergone bladder augmentation). Among historical COD, renal failure has been the most common.[6, 7] The introduction of clean intermittent catheterization (CIC) in 1972 [17] markedly reduced the risks of renal failure and urosepsis. Neurologic causes include brainstem dysfunction, which is also described as Chiari II crisis, and hydrocephalus/shunt failure. Mortality is highest during infancy and brainstem failure and sepsis predominate as MOD. Traditionally, about 80% of patients with MMC require treatment for hydrocephalus, which has usually involved implantation of a ventricular shunt. Obstruction failure is an unfortunately common shunt complication and may result in elevated intracranial pressure and stress to an already compromised nervous system. As such, shunt failures are important MOD that underline neurological COD.
Studies of modern cohorts of people with MMC report a spectrum of neurological, pulmonary, urological, and other COD [2, 8-16]. Some people with MMC have been reported to die from an “unexplained death,” which occurs when people die suddenly while asleep, without an attributable known cause [15]. Small series suggest this may be more frequent among adolescents and adults with shunted hydrocephalus due to shunt-related respiratory arrest from brain stem dysfunction. Importantly, given the multi-system nature of MMC, many studies reporting COD have relied on death certificate diagnoses. These are often nonspecific (e.g., “spina bifida”) or just one of many contributors to a person’s death (e.g., respiratory failure).
The aim of this study was to describe mortality and COD/MOD in deceased people with MMC born after 1971 (CIC was introduced in 1972) [17]. The hypotheses included the following:
(1) COD varied with shunt status and age,
(2) deaths from renal failure were rare (< = 5%) and from urological causes infrequent (< = 10%), and
(3) unexplained deaths were rare (< = 5%) and associated with shunted hydrocephalus.
2Methods
This was a retrospective study of a non-random convenience sample of people with MMC born after December 31, 1971, who died before January 1, 2020, at 16 institutions in Canada and the United States. Data were obtained from geographically distributed centers known to the investigators to have a pediatric and/or adult spina bifida clinic. Four additional centers were approached (two in Canada, two in the United States) but declined to participate. All institutions followed the same data collection process using a survey developed by the authors. Patients with unknown shunt status (n = 8) and syndromal conditions that included spinal dysraphism (e.g., cloaca, cloacal exstrophy, imperforate anus, VATER syndrome, caudal regression, lipo/meningocele), genetic anomalies (e.g., trisomy 13/18, mitochondrial disorder) or cardiac anomalies (e.g., tetralogy of Fallot, severe pulmonary hypertension) were excluded [18].
2.1Collaboration structure
A multidisciplinary team of MMC care experts (including pediatric urology, adult urology, neurosurgery, developmental pediatrics, and nursing) from participating institutions iteratively developed the research plan. Each institution contributed > = 5 records with local Institutional Review Board (IRB) approval (median 16 records/center).
2.2Data collection
Data were obtained from available medical records (most recent baseline characteristics prior to death), family phone calls, obituaries, death certificates, and, when permitted by IRB, a publicly accessible genealogical resource (ancestry.com). Deidentified data was submitted via RedCap to the lead author’s institution. After each center submitted an initial five records, a data quality review was performed; the data collection sheet was revised to address deficiencies identified and the deficient records resubmitted. Duplicate records (n = 2) were consolidated based on gender, surgical history, birth/death years for patients who were followed at different participating centers at some point in their lives.
2.3Cause of death classification
Each site submitted free-text detailed descriptions of the clinical course leading to death with proposed organ system-based COD (e.g., neurological, urological). In consultation with the local team, lead and senior authors amended COD by consensus if classification appeared unclear (21% of patients). Every attempt was made to follow published World Health Organization COD assignment guidelines [19]. Unknown COD were classified in two subgroups: “unexplained deaths” (unexpected deaths while asleep, without attributable known cause) and “other unknown deaths” (including “spina bifida” and “multiple congenital anomalies” after the neonatal period). MOD described the underlying etiology sometimes spanning organ systems (e.g., infections, non-infectious pulmonary disease).
2.4Statistics
All primary analyses and hypotheses were drafted and approved a priori by the multidisciplinary study team. Additional exploratory analyses were included to address other clinically relevant questions.
Anticipating potentially low counts of some outcomes, a Fisher’s exact test was used to analyze categorical variables (e.g., death in infancy/childhood/adulthood, shunted/unshunted, community ambulators/non-ambulators). When analyzing risk factors for COD/MOD, a binary age at death variable (child vs. adult) and five categories of COD/MOD were used (the three most common, other, and unknown). This was done to prevent age-shunt subgroups from becoming too small for meaningful clinical and statistical analysis. Wilcoxon rank-sum test was used to analyze continuous variables without assuming a normal distribution. For all tests described below, the listed groups were compared to each other (p = 0.05, Stata, StataCorp, College Station, TX, USA).
2.5Sensitivity analyses
To control for patients dying before baseline characteristics were apparent (e.g., ascribing non-ambulation to infants), the authors performed several additional comparisons. To achieve this for shunt and epilepsy status, those dying in the first year of life were excluded, as this is when hydrocephalus management decisions are typically made and seizures noted. To achieve this for ambulatory status, those dying in the first four years of life were excluded, as children under four may not be clearly assessable for ambulatory status. No statistically or clinically significant differences were noted (data not shown).
3Results
3.1Population characteristics
A total of 293 patients born after 1971 who died before 2020 were identified. Half were born after 1991. Overall, 50% were male and 11% were community ambulators (14% among > = 4-year-olds) (Table 1, Online Appendix). Overall, 32% had documented developmental delay and 31% epilepsy.
Table 1
Population characteristics
| Patient characteristic | Shunted | Unshunted | Overall |
| (n = 261) | (n = 32) | (n = 293) | |
| Male gender (%) | 129 (49%) | 18 (56%) | 147 (50%) |
| Community ambulators (%)* | 27 (10%) | 5 (16%) | 32 (11%) |
| Mortality data | |||
| Median age at death (years, IQR) | 20 (9-27) | <1 (<1-10) | 19 (5-27) |
| Infants (<1 year old) | 18 (7%) | 17 (53%) | 35 (12%) |
| Children (1-17.9 years old) | 94 (36%) | 8 (25%) | 102 (35%) |
| Adults (18 years old or older) | 149 (57%) | 7 (22%) | 156 (53%) |
| Location where death occurred (%) | |||
| Hospital with multidisciplinary MMC care | 101 (39%) | 21 (66%) | 122 (42%) |
| Home/group home | 39 (15%) | 7 (22%) | 46 (16%) |
| Hospital without multidisciplinary MMC care | 34 (13%) | 1 (3%) | 35 (12%) |
| Hospice | 11 (4%) | 0 (0%) | 11 (4%) |
| Long-term care/nursing home | 2 (1%) | 0 (0%) | 2 (1%) |
| Other (work, roadway) | 2 (1%) | 0 (0%) | 2 (1%) |
| Unknown | 72 (28%) | 3 (9%) | 75 (26%) |
| Autopsy performed (%) | 23 (9%) | 7 (22%) | 30 (10%) |
| Hydrocephalus management | |||
| Documented hydrocephalus (%) | 261 (100%) | 20 (63%) | 281 (96%) |
| Endoscopic third ventriculostomy (%) | 17 (7%) | 3 (15%) | 20 (8%) |
| Shunt revision (%) | 180 (71%) | n/a | 180 (61%) |
| Bladder management* | |||
| History of incontinent vesicostomy (%) | 46 (18%) | 1 (3%) | 47 (16%) |
| Augmented bladder | 71 (27%) | 6 (19%) | 77 (26%) |
| Skin and bone care* | |||
| Skin ulcers in the final 12 months of life (%) | 57 (23%) | 1 (3%) | 58 (20%) |
| Bone fractures in the final 12 months of life (%) | 8 (2%) | 1 (3%) | 9 (3%) |
| Baseline pulmonary function (among those who died after age one year)* | n = 243 | n = 15 | n = 258 |
| BiPap/CPap (%) | 33 (14%) | 4 (27%) | 37 (14%) |
| Supplemental oxygen (%) | 44 (18%) | 3 (20%) | 47 (18%) |
| Tracheostomy (%) | 29 (12%) | 2 (13%) | 31 (12%) |
| None (%) | 111 (46%) | 7 (47%) | 118 (46%) |
| Adult health and social characteristics* | n = 149 | n = 7 | n = 156 |
| Obesity (%) | 43 (29%) | 2 (29%) | 45 (29%) |
| Hypertension (%) | 38 (26%) | 2 (29%) | 40 (26%) |
| Chronic kidney disease (%) | 32 (21%) | 2 (29%) | 34 (22%) |
| History of depression (%) | 31 (21%) | 2 (29%) | 33 (21%) |
| History of anxiety (%) | 15 (10%) | 1 (14%) | 16 (10%) |
| History of opioid addiction (%) | 5 (3%) | 1 (14%) | 6 (4%) |
| History of alcohol addiction (%) | 2 (1%) | 1 (14%) | 3 (2%) |
| History of suicidal ideation or attempts (%) | 4 (3%) | 1 (14%) | 5 (3%) |
| Living arrangement (%) | |||
| Alone | 4 (3%) | 0 (0%) | 4 (3%) |
| With own family (i.e., with spouse/partner) | 9 (6%) | 3 (43%) | 12 (8%) |
| Parents/caregivers | 65 (44%) | 1 (14%) | 66 (42%) |
| Group home | 11 (7%) | 2 (29%) | 13 (8%) |
| Long-term care facility | 9 (6%) | 0 (0%) | 9 (6%) |
*Some data was missing (unknown). The full characteristics are available in the Online Appendix. Totals may not add up to 100% due to rounding. IQR: interquartile range; MMC: myelomeningocele.
Median age at death was 19 years old (interquartile range [IQR] 5-27). Half of deaths occurred after 2012. Deaths occurred in infancy (<1 year old: 12%), childhood (1-17.9 : 35%) and adulthood (18+: 53%). Withdrawal of care preceded all four neonatal deaths and 20 of 31 (65%) deaths between one and 12 months old.
Deaths were most common in hospitals with multidisciplinary MMC care (42%), followed by homes/group homes (16%) and hospitals without multidisciplinary MMC care (12%). Overall, 24% of patients underwent surgery within 30 days before death. Details of death were primarily obtained from medical records (92%), institutional databases (11%), parents/caregivers (9%), and death certificates (10%). Autopsy was documented in 10%.
COD were undocumented in 26%. Comparing the proportion of unknown COD across three types of locations where deaths occurred, unknown causes were less common for deaths occurring in a hospital (8%) compared to other (43%) or unknown locations (51%, p < 0.001).
3.1.1Hydrocephalus management
Overall, 89% of patients had shunted hydrocephalus. Unshunted patients were dichotomous: infants too ill to be shunted and healthier, older individuals who did not require shunting. Shunt revision rate was 71% (median three revisions, IQR 1-5). Last revision predated death by four years (median, IQR 1-12).
3.1.2Bladder and bowel management
At some point in life, 16% had an incontinent vesicostomy and 6% had it at time of death. Overall, 60% were on CIC, 26% had an augmented bladder, and 6% had an incontinent diversion. Overall, 8% had a colostomy; 20% had a Malone antegrade continence enema (MACE).
3.1.3Skin and bone care
Overall, 30% had a history of skin ulcer debridement (67% of them within 12 months of death), and 3% had a bone fracture in the year before death.
3.1.4Baseline pulmonary function
Baseline characteristics for 258 patients who died after age one included the following: 14% on bilevel/continuous positive airway pressure (BiPap/CPap), 18% on supplementary oxygen, and 12% had a tracheostomy. Among those who died after the age of four years old, 24% had ever had a sleep study performed (24% of this group had it done in the final year of life).
3.1.5Adult health and social characteristics
Obesity was documented in 29% of adults, hypertension in 26%, and chronic kidney disease in 22%. Records also indicated a history of depression (21%), anxiety (10%), opioid addiction (4%), alcohol addiction (2%), and suicidality (3%). At the time of death, adults lived with parents/caregivers (42%), in a group home (8%), or with a spouse/partner (8%) (unknown: 33%). Primary care providers were documented for 67% (none: 2%, unknown: 31%).
3.2COD/MOD
3.2.1Shunted patients
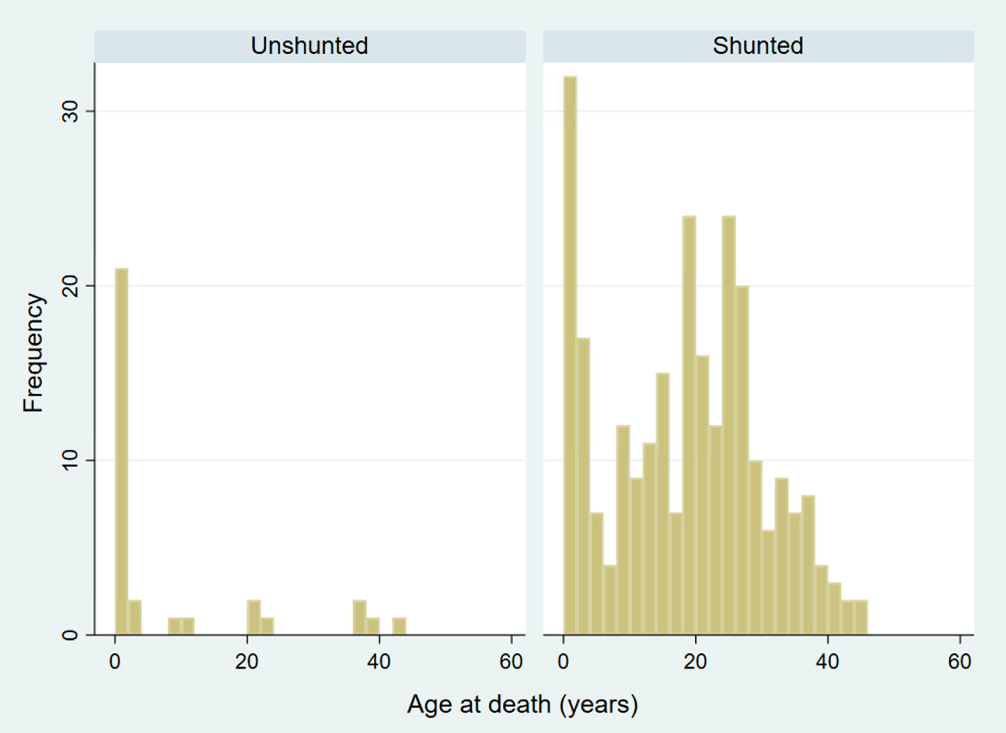
The median age at death for 261 people with shunted MMC was 20 years old (IQR 9-27). Age at death appeared to have a bimodal distribution, with an initial peak in the first years of life, followed by another peak in the third decade (Fig. 1).
Fig. 1
Distribution of ages at time of death among shunted and unshunted people with myelomeningocele (p < 0.0001).
Leading organ-based COD for 261 people with shunted MMC were neurological, followed by pulmonary, urological, and gastrointestinal (Fig. 2). The most common MOD were infections (34%), non-infectious shunt malfunction (14%), and non-infectious pulmonary disease (6%) (Table 2). The most common infections were pneumonias (32% of all infections, 45% were aspiration), urosepsis (17% of infections), ulcers/wounds, peritonitis without bladder perforation, and shunt infections. It is unknown to what degree these infections represented potential final common MOD pathways versus an initial and responsible illness.
Fig. 2
Organ-based causes of death among people with myelomeningocele. Neurological (shunt infection, encephalitis, Chiari II malformation, seizure-related: status epilepticus, SUDEP: sudden unexpected death in epilepsy), Pulmonary (pneumonia, non-infectious pulmonary disease), Urological (urosepsis, bladder perforation, renal failure, exsanguination form hematuria, bladder cancer), Gastrointestinal (peritonitis not due to bladder perforation, non-infectious: ischemic bowel, bowel obstruction, high ostomy output, gastrointestinal bleeding, colon cancer), Cardiovascular (infectious: endocarditis, venous access device infection, congenital abnormalities, non-infectious non-congenital: pulmonary embolus, cerebrovascular accident, intracranial hemorrhage, congenital heart failure, etc.), Other (trauma, cancer, complication of pregnancy, multi-drug toxicity)
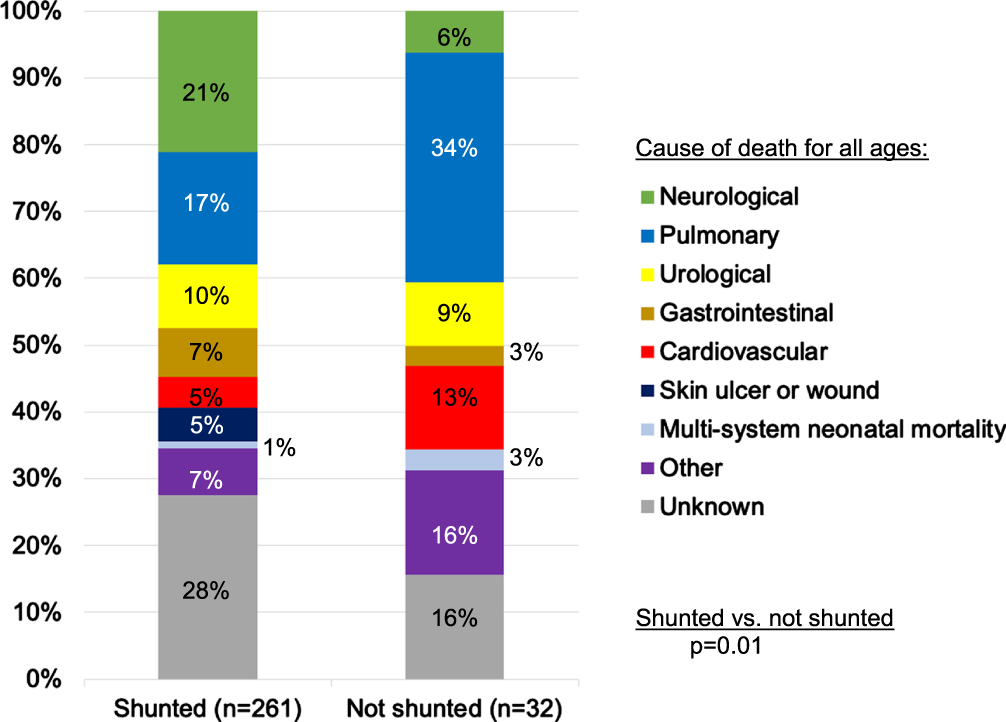
Table 2
Mechanisms of death among people with myelomeningocele with or without shunted hydrocephalus (p= 0.003)
| Mechanism of death | Shunted | Unshunted | Overall |
| (n = 261) | (n = 32) | (n = 293) | |
| Infections | 90 (34%) | 12 (38%) | 102 (35%) |
| Pneumonia (aspiration) | 29 (13) | 6 (3) | 35 (16) |
| Urosepsis | 15 | 1 | 16 |
| Skin ulcer or wound | 13 | 0 | 13 |
| Peritonitis, no bladder perforation | 12 | 1 | 13 |
| Peritonitis, bladder perforation | 1 | 0 | 1 |
| Shunt infection | 11 | 0 | 11 |
| Encephalitis | 0 | 1 | 1 |
| Endocarditis | 1 | 0 | 1 |
| Venous access device | 1 | 0 | 1 |
| Unknown source | 7 | 3 | 10 |
| Non-infectious shunt malfunction | 37 (14%) | 0 (0%) | 37 (13%) |
| Chiari II malformation | 0 (0%) | 1 (3%) | 1 (0.3%) |
| Non-infectious pulmonary disease | 15 (6%) | 5 (16%) | 20 (7%) |
| Cardiovascular disease (congenital) | 0 (0.0%) | 1 (3%) | 1 (0.3%) |
| Cardiovascular disease (non-congenital) | 10 (4%) | 3 (9%) | 13 (4%) |
| Pulmonary embolus | 4 | 0 | 4 |
| Cerebrovascular accident | 3 | 0 | 3 |
| Intracranial hemorrhage | 1 | 1 | 2 |
| Other | 2* | 2** | 4 |
| Renal failure | 7 (3%) | 2 (6%) | 9 (3%) |
| Exsanguination from gross hematuria | 1 (0.3%) | 0 (0%) | 1 (0.3%) |
| Seizure-related | 7 (3%) | 0 (0%) | 7 (2%) |
| Status epilepticus | 4 | 0 | 4 |
| Other seizure-related cause | 2 | 0 | 2 |
| Sudden unexpected death in epilepsy (SUDEP) | 1 | 0 | 1 |
| Trauma | 7 (3%) | 0 (0%) | 7 (2%) |
| Accidental | 6 | 0 | 6 |
| Non-accidental (suicide) | 1 | 0 | 1 |
| Non-infectious gastrointestinal | 6 (2%) | 0 (0%) | 6 (2%) |
| Ischemic bowel | 4 | 0 | 4 |
| Other (small bowel obstruction, gastrointestinal bleeding) | 2 | 0 | 2 |
| Cancer | 4 (2%) | 1 (3%) | 5 (2%) |
| Hematogenous cancer | 2*** | 1**** | 3 |
| Transitional cell carcinoma of the bladder | 1 | 0 | 1 |
| Colon cancer | 1 | 0 | 1 |
| Complication of pregnancy | 1 (0.3%) | 0 (0%) | 1 (0.3%) |
| Multi-drug toxicity | 1 (0.3%) | 0 (0%) | 1 (0.3%) |
| Drug overdose (opioid) | 0 (0%) | 1 (3%) | 1 (0.3%) |
| Neonatal mortality (multi-system) | 3 (1%) | 1 (3%) | 4 (1%) |
| Unknown cause of death | 72 (28%) | 5 (16%) | 77 (26%) |
| Unexplained deaths (unexpected deaths while asleep, without attributable known cause) | 11 | 2 | 13 |
| Other unknown (including ‘spina bifida’ and ‘multiple congenital anomalies’) | 61 | 3 | 64 |
*Congestive heart failure, myocardial infarction. **Cardiac arrest, portal vein thrombosis. ***Acute lymphoblastic leukemia, lymphoblastic t-cell lymphoma. ****Myelodysplastic syndrome. Totals may not add up to 100% due to rounding.
3.2.2Unshunted patients
There were 32 people who were unshunted at the time of death. Of these, 17 (53%) were younger than one year, eight (25%) were between 1-17, and seven (22%) were 18 years or older. Most unshunted individuals died in the first year of life, which was a younger age compared to shunted individuals (p < 0.001). Distribution of age at death was skewed to infancy, with remaining deaths distributed at older ages (Fig. 1).
Leading organ-based COD were pulmonary, followed by cardiovascular, urological, and neurological (Fig. 2). Looking at all categories of organ-based COD, they differed between shunted and unshunted patients (p = 0.01): unshunted patients were more likely to die of pulmonary (34% vs. 17%) and cardiovascular causes (13% vs. 5%), but less likely of neurological causes (6% vs. 21%), with similar urological deaths (9% vs. 10%).
The most common MOD were infections (38%), non-infectious pulmonary (16%), and non-congenital cardiovascular disease (9%) (Table 2). Looking at all categories of MOD, they differed between shunted and unshunted patients (p = 0.003). Compared to shunted patients, unshunted patients were more likely to die of non-infectious pulmonary causes, but a similar proportion died of infections.
Five patients with unshunted MMC were community ambulators and died at median 37 years. Deaths were due to infection (unknown source), cancer (myelodysplastic syndrome), opioid overdose, and two unknown causes (including one unexplained death).
3.3Associations with shunt status, age, birthdate and ambulatory status
To prevent subgroups from becoming too small for meaningful clinical and statistical analysis, five COD/MOD categories were compared: the three most common ones, other, and unknown. The most common COD were neurological, pulmonary, and urological. The most common MOD were infection, non-infectious shunt malfunction, and non-infectious pulmonary disease.
3.3.1COD
Comparing neurological, pulmonary, urological, other, and unknown COD, COD differed with shunt status (p = 0.03) and with age (p = 0.046), but not with community ambulatory status (p = 0.16) or birthyear (< = 1992 [median] vs. >1992, p = 0.68).
Associations between COD and shunt status were examined after adjusting for age, and age after adjusting for shunt status (Table 3). Shunted adults were less likely than shunted children to die of neurological causes (13% vs. 31%, p = 0.02).
Table 3
Causes and mechanisms of death for people with myelomeningocele and known shunt status, stratified by age
| Shunted | Unshunted | |||
| Children (n = 112) | Adults (n = 149) | Children (n = 25) | Adults (n = 7) | |
| Cause of death | ||||
| Neurological | 35 (31%) | 20 (13%) | 2 (8%) | 0 (0%) |
| Pulmonary | 16 (14%) | 28 (19%) | 9 (36%) | 2 (29%) |
| Urological | 10 (9%) | 15 (10%) | 2 (8%) | 1 (14%) |
| Other | 23 (21%) | 42 (28%) | 8 (32%) | 3 (43%) |
| Unknown | 28 (25%) | 44 (30%) | 4 (16%) | 1 (14%) |
| p = 0.02 | p = 0.99 | |||
| ↑– | p = 0.02 | –↑ | ||
| ↑– | p = 0.68 | –↑ | ||
| Mechanism of death | ||||
| Infection | 29 (26%) | 61 (41%) | 9 (36%) | 3 (43%) |
| Non-infectious shunt malfunction | 23 (21%) | 14 (9%) | 0 (0%) | 0 (0%) |
| Non-infectious pulmonary disease | 7 (6%) | 8 (5%) | 5 (20%) | 0 (0%) |
| Other | 25 (28%) | 22 (15%) | 7 (28%) | 3 (43%) |
| Unknown | 28 (25%) | 40 (30%) | 4 (16%) | 1 (14%) |
| p = 0.01 | p = 0.78 | |||
| ↑_ | p = 0.01 | _↑ | ||
| ↑_ | p = 0.40 | _↑ | ||
Totals may not add up to 100% due to rounding.
Shunted children were more likely than unshunted children to die of neurological causes (31% vs. 8%), and less likely to die of pulmonary causes (14% vs. 36%) and other causes (21% vs. 32%, p = 0.02). Comparing shunted to unshunted adults was limited by low statistical power (p = 0.99).
On exploratory analysis, neurological, pulmonary, urological, other, and unknown COD differed with sex: females appeared less likely than males to die of pulmonary causes (14% vs. 23%) and more likely from urological causes (14% vs. 5%, p = 0.03). Sex was not associated with different distributions of the five COD categories when it was analyzed after adjusting for either shunt status or age (p=>0.05).
3.3.2Mechanism of death
Comparing infection, non-infectious shunt malfunction, non-infectious pulmonary disease, other, and unknown MOD, MOD differed with shunt status (p = 0.01) and age (p = 0.03), but not with ambulatory status (p = 0.06) or birthyear (p = 0.61). On exploratory analysis, MOD did not differ based on sex, including similar risk of death from infections (males: 37% vs. females: 33%, p = 0.77). Infections were the most common MOD for people who died in hospitals (hospital: 48%, other: 30%, unknown: 12%, p < 0.001).
Associations between MOD and shunt status were examined after adjusting for age, and age after adjusting for shunt status (Table 3). Shunted adults were more likely than shunted children to die of infection (41% vs. 26%), and less of non-infectious shunt malfunction (9% vs. 21%) and other causes (15% vs. 28%, p = 0.01). Although limited by low statistical power, unshunted adults and children had a similar distribution of MOD (p = 0.78).
Shunted children were less likely than unshunted children to die of infections (26% vs. 36%) and non-infectious pulmonary disease (6% vs. 20%). Lethal non-infectious shunt malfunctions, from which the non-shunted group is obviously spared (21% vs. 0%, p = 0.01), were an important MOD in this group. Comparing shunted to unshunted adults was limited by low power (p = 0.40).
3.4Urology-related deaths
Overall, 10% of COD were urological. They occurred at a median of 20 years old (IQR 9-27), similar to the age for all other COD (median 19, IQR 4-27, z = -0.73, p = 47). Urosepsis (5%) and renal failure (3%) were the most common, followed by exsanguination from hematuria in an anticoagulated patient on dialysis with no identifiable tumor on cystoscopy of gastrocystoplasty (1), bladder perforation (1) and bladder cancer (1). A 46-year-old shunted male not on CIC and with no bladder augmentation died of metastatic transitional cell carcinoma. Two deaths could be directly attributed to bladder augmentation (hematuria, perforation), representing 3% of all deaths among only the augmented patients.
Urological COD were not associated with age (infancy: 3% of deaths, childhood: 11%, adulthood: 10%, p = 0.40), birthyear (< = 1992 : 11% vs. >1992 : 8%, p = 0.43), shunt status (no: 9% vs. yes: 10%, p = 0.99) or ambulatory status (yes: 13%, no: 9%, unknown: 11%, p = 0.71).
On exploratory analysis, compared to all other COD, death from urological causes was more likely in females than males (14% vs. 5%, p = 0.01). Urosepsis was the most common urological cause of death among 146 females (9%), followed by renal failure (5%). Death from urosepsis occurred in 3% of 147 males, followed by renal failure (1%). Urological COD were not associated with death location (hospital: 10%, other: 11%, unknown: 7%, p = 0.60).
3.5Unexplained deaths (unexpected cause while asleep)
Overall, 4% of deaths were unexplained (autopsy: 0%). These were not associated with age (infancy: 0%, childhood: 6%, adulthood: 4%, p = 0.47), shunt status (shunted: 4%, unshunted: 6%, p = 0.64), history of epilepsy (yes: 3%, no: 5%, unknown: 6%, p = 0.69), or gender (female: 3% vs. male: 6%, p = 0.26, exploratory analysis).
3.6Exploratory analyses
3.6.1Death among unshunted patients undergoing endoscopic third ventriculostomy only, n = 3 (exploratory)
Three boys died from unknown COD at the age of one, one and three years old, having undergone a third ventriculostomy without subsequent shunting. In terms of MOD, one of the one-year-old boys experienced an unexplained death while asleep and the three year old died from an infection from an unknown source. When the preceding analysis was repeated with these three children reclassified as shunted, no clinically or statistically significant changes were noted in the results (data not shown).
3.6.2Disease severity over time (children who died before 2002, n = 51) (exploratory)
Since patients born earlier could potentially be older than those born later, this could bias the analysis. Therefore, an exploratory analysis was performed of only those dying in childhood who were born between 1972 and 2001. This gave all individuals in this exploratory analysis a theoretical opportunity to reach the age of 18 years old. The 24 children born from 1972 to 1992 were not significantly different from 27 children born from 1993 to 2001 in terms of age (medians: 12 vs. 12 years old, z = -0.76, p = 0.46), shunt status (100% vs. 100%) and community ambulation (6% to 27%, p = 0.10).
3.6.3Deaths in childhood (children who died after their first birthday, n = 258) (exploratory)
Excluding 35 deaths in infancy, 40% of patients died in childhood. Compared to those who died in adulthood, death in childhood was not associated with shunt status (shunted: 39%, unshunted: 53%, p = 0.29) or ambulatory status (community ambulators: 31%, non-ambulators: 32%, unknown: 29%, p = 0.99).
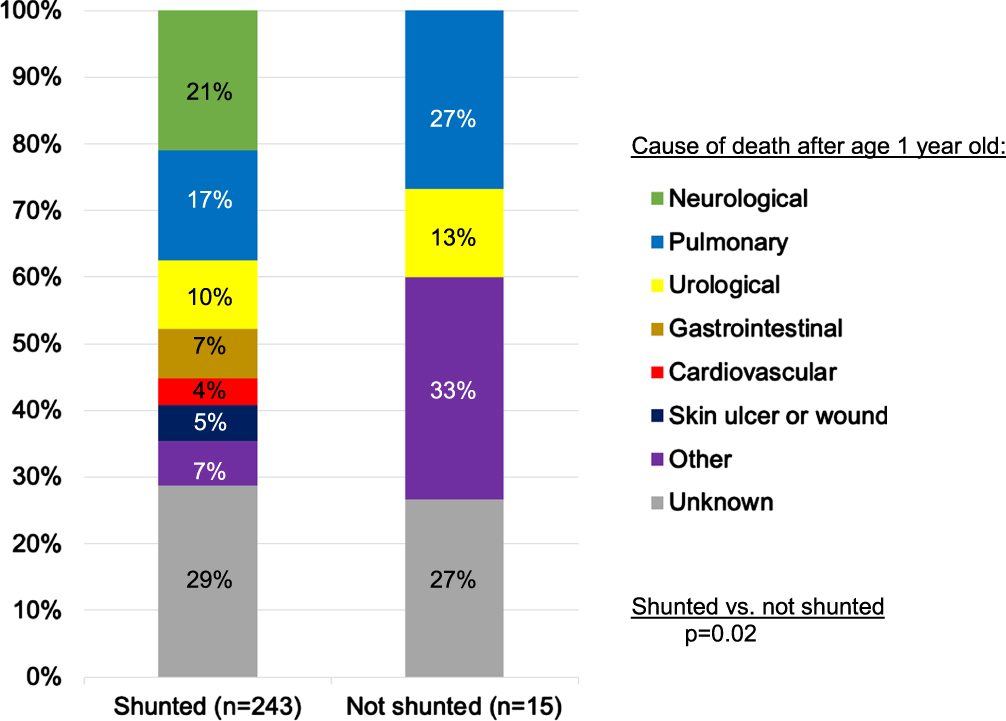
Results of COD and MOD for those who died after infancy were similar to the main analysis. COD still varied with shunt status (p = 0.02) (Fig. 3). While the distribution of COD was similar for shunted patients in this subgroup, compared to the main analysis of deaths at all ages, unshunted patients who died after infancy primarily died of pulmonary and urological causes (40%), rather than pulmonary and cardiovascular causes (47%). Infectious MOD were most common, regardless of shunt status (shunted: 35%, unshunted: 53%, p = 0.32).
Fig. 3
Organ-based causes of death among people with shunted hydrocephalus and myelomeningocele who died after one year of age.
3.6.4Deaths among patients with augmented bladders (n = 77) (exploratory)
Of the entire population, 77 (26%) had an augmented bladder. In this group, 47% were male and the median age at death was 23 years old (IQR 18.5-27). Most individuals (92%) were shunted, 17% were community ambulators, and 80% used a catheterizable channel. Further surgical data was not collected.
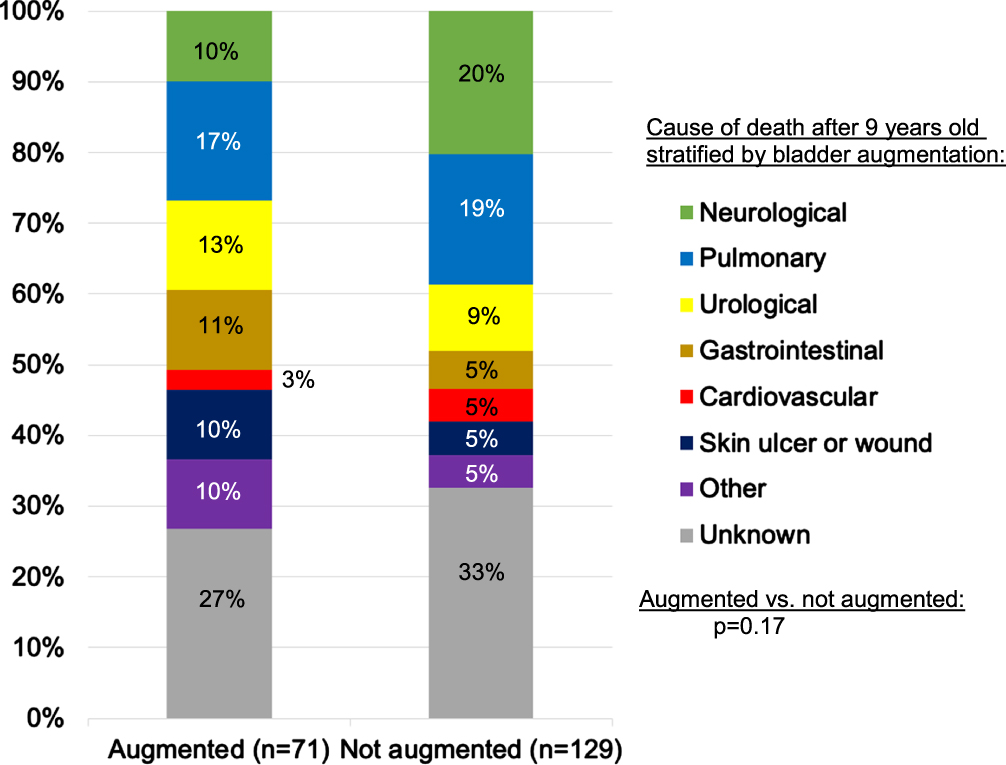
For 71 shunted augmented patients, the most common organ-based COD were pulmonary (17%), urological (13%), and gastrointestinal (11%) (Fig. 4). Urological deaths included urosepsis (6), renal failure, exsanguination form gross hematuria, and bladder perforation (one each). Comparing pulmonary, urological, gastrointestinal, other, and unknown COD, COD were similar between males and females (p = 0.19), although 6% of 33 males and 16% of 38 females died of urological causes.
Fig. 4
Organ-based causes of death among people with shunted hydrocephalus and myelomeningocele who died at or after nine years old, the age of the youngest augmented patient to die.
Looking at MOD, 48% of shunted augmented patients died of infections (including peritonitis without bladder perforation: 10%, pneumonia: 10%, ulcer/wound: 10%, urosepsis: 9%, shunt infections: 4%, bladder perforation: 1%, endocarditis: 1%, and unknown: 3%). Another 7% died of non-infectious pulmonary disease and 4% of non-infectious shunt malfunction (unknown: 27%).
Since augmentation is not performed in early childhood, 71 shunted augmented patients were compared to 129 shunted non-augmented patients who died at or after nine years old (the age of the youngest augmented patient to die). Ages of the two groups were comparable (medians: 24 vs. 23 years old, p = 0.36). While augmented patients were less likely to die of neurological causes (10% vs. 20%), this did not reach statistical significance (p = 0.17) (Fig. 4) and neither did any differences in MOD (p = 0.11). Interestingly, 3% of the 70 shunted non-augmented males and 17% of 59 females died of urological causes, similar to the proportions observed among augmented patients.
Six unshunted augmented patients died of urologic (2), pulmonary (1), other (2), and unknown (1) causes. The most common mechanisms were infectious (3: pneumonia, urosepsis, unknown source), renal failure (1), drug overdose (1), and unknown (1).
4Discussion
COD and MOD in MMC varied with shunt status and age. Neurological causes were leading COD among shunted people, followed by pulmonary causes. Pulmonary causes were leading COD for unshunted people, followed by cardiovascular causes. Half of unshunted individuals died from these two causes. Since impairment in one body system may contribute to decline in others, these findings may reflect interactions of acute and chronic conditions. Mechanistically, a third of deaths were infection-related, regardless of shunt status. Shunted children were more likely to die of neurological causes than either shunted adults or unshunted children, and less likely to die of infections. Shunt presence likely marks more severe neurological impairment, even when not a direct contributor of COD. Overall, urological COD were infrequent, but they appeared to be more common among females, particularly deaths secondary to urosepsis and renal failure. These data underline the need for effective strategies to prevent urinary tract infections and renal deterioration, particularly among females with MMC. Deaths directly from bladder augmentation were rare. Augmentation did not predispose patients to urological COD or COD/MOD different than non-augmented age-matched individuals.
Unexplained deaths occurring while asleep without a known cause were rare (4%, 13/293). This is consistent with 2% (2/71) of deaths in an older cohort reported by Oakeshott et al., which met the definition used here [20]. In contrast, Jernigan et al. reported that 55% (6/11) of deaths among adults with MMC were unexplained, emphasizing the need to corroborate findings of smaller studies [15]; all six of these patients were all shunted females. Unexplained deaths were also reported in people with hydrocephalus from multiple causes [21]. Contrary to the hypothesis, similar proportions of unexplained deaths were noted regardless of shunt status, age, epilepsy, and gender. While MMC predisposes people to abnormal breathing during sleep [22], sleep apnea or brain MRI results were not available. It must be noted that patients with MMC are generally at higher risk for potential causes of sudden and sleep-related death, including cardiac arrhythmias, pulmonary emboli, anaphylaxis, and epilepsy. Without autopsy studies on this subset of patients, the role that sleep-disordered breathing contributes to risk can only be speculated upon.
Multiple studies report on historically and clinically heterogenous spina bifida populations (MMC and non-MMC) born before 1972. COD classifications vary, making direct comparisons challenging. However, these findings concur with others when applying the same COD/MOD classification to ten papers reporting deaths beyond the neonatal period for patients born after 1971 (Table 4) [2, 8-16]. Reporting a total of 328 deaths, most were single center series and seven reported deaths specifically for MMC [2, 8, 9, 11, 13, 14]. Neurological COD were the most common in 5/9 studies (second in two), accounting for 6-57% of deaths across all studies. Urological causes were the most common in two studies (second in two), accounting for 0-44% of deaths across all studies. Pulmonary causes were the most common in one study (second in three), accounting for 0-29% of deaths across all studies. A study of 182 deaths by Kancherla et al. had insufficient data to determine individual COD/MOD, but the most common COD in that study were neurological [5]. McDowell et al. reported that 75-100% of patients with shunted MMC and symptomatic Chiari II malformations had neurological COD [23]. Cai et al. reported multiple COD for 48 deaths, citing pulmonary to be the most common, followed by neurological [24]. Infections were the most common MOD in 8/10 studies, accounting for 17-57% of deaths per study [2, 8-12, 14, 16]. These studies must be interpreted with caution, as many relied on death certificate documentation. Many patients with MMC who die experience a chain of events that sometimes includes an admission for infection (e.g., wound), complicated by respiratory compromise (e.g., requiring intubation), then possibly secondary organ failure (e.g., acute kidney injury) and continues until the patient succumbs. Whether the provider who signed the death certificate elected to name the inciting, the final, or all the contributors to mortality on the death certificate is unknown. In this study, an intensive chart review was conducted by providers skilled in MMC care to try to adjudicate this issue. Ultimately, however, there remain many patients who have multifactorial COD due to the complexity of their disease.
Table 4
Causes and mechanisms of death among people with myelomeningocele in modern published series
| Paper | Data source | Country | Included patients born before 1972 | Number of deaths reported | MMC among dead patients | Shunted among dead patients | Median age at death (range, years) | Cause of death | Most common mechanism of death | ||||
| Neurological | Pulmonary | Urological (renal failure) | Other | Unknown | |||||||||
| McDonnell et al., 2000 | Single center | United Kingdom | Unknown | 18 | Unknown | Unknown | 28 (n/a) (adults only) | 22-39% * | 0-17% * | 33-44% * (22%) | 39-55% * | 0% | Infection, non-congenital cardiovascular, renal failure (22% each) |
| Bowman et al., 2001 | Single center | United States | No | 28 | 100% | Unknown (86% in 118 MMC patients) | 1 to 5 (range < 1->15) | > = 50% * | n/a | n/a | n/a | n/a | Hindbrain dysfunction (> = 46% *) |
| Jernigan et al., 2012 | Single center | United States | No | 11 | 100% | Unknown (92% in 106 MMC patients) | 24 (mean, SD 3) (adults only) | 18% | 18% | 0% (0%) | 64% | 0% | Unexplained death: unknown cause while asleep (55%) |
| Malakounides et al., 2013 | Single center | United Kingdom | Yes (> = 1970) | 7 | Unknown | Unknown | 14 (0-25) | 29-57% * | 0-29% * | 0-14% * (0%) | 43-71% * | 29% | Infection (57%) |
| Szymanski et al., 2015 | Single center | United States | No | 28 | 100% ** | 96% ** | 22 (9-38) | 25% | 18% | 14% (7%) | 39% | 4% | Infection (57%) |
| Borgstedt-Bakke et al., 2017 | National registry | Denmark | Yes (> = 1970) | 27 | 100% | Unknown | 1 (0-39) | 11% | 19% | 7% (0%) | 26% | 37% | Infection (48%) |
| Dicianno et al., 2018 | Single center | United States | Yes (∼half) | 36 | 100% | Unknown (73% hydrocephalus in 48 MMC/non-MMC patients) | 42 (mean, range 24-83) | 6-17% * | 8-25% * | 0-17% * (0%) | 3-19% * | 67% | Infection (17%) |
| North et al., 2018 | Single center | Canada | Yes (> = 1971) | 18 | 100% | Unknown (84% in all 101 MMC patients) | n/a (13 for entire cohort of 83 patients) | 22-28% * | 0-6% * | 6-11% * (6%) | 6-11% * | 61% | Infection (17-22%)* |
| Protzenko et al., 2019 | Single center | Brazil | No | 17 | 100% | Unknown (84% in all 231 MMC patients) | n/a (7 for entire cohort of 384 patients) | 35% | 0% | 29% (6-29%)* | 0% | 35% | Infection (29-53%)* |
| Peyronnet et al., 2020 | National registry of in-hospital deaths | France | Yes (∼half) | 138 | 95% | Unknown (39% in all 138 MMC/non-MMC patients) | 41 (IQR 25-52) | 14% | 17% | 17% (5%) | 38% | 14% | Infection (37%) |
| Current study | Multi-center | United States &Canada | No | 261 | 100% | 100% (shunted group) | <1 (IQR < 1-10) | 21% | 17% | 10% (3%) | 24% | 28% | Infection (34%) |
| 32 | 100% | 0% (unshunted group) | 20 (IQR 9-27) | 6% | 34% | 9% (6%) | 35% | 16% | Infection (38%) | ||||
*Exact number not calculable due to lack of data granularity. **Based on review of original data (not in published manuscript). Totals may not add up to 100% due to rounding. IQR: interquartile range; MMC: myelomeningocele; SD: standard deviation.
While in this study COD/MOD differed based on shunt status and age, few previous papers have stratified their results using these variables. Contrary to previous reports, shunted patients in this study were not at a decreased risk of urological COD [10] or earlier death [9]. While Oakeshott et al. noted that urological COD were associated with higher lesions, the same relationship with ambulatory status was not observed in this study [25]. Similar to Kalucy et al., no significant changes were noted in COD/MOD in the last five decades [3].
Only 4% of countries have published population- or hospital-based MMC mortality data [26]. While this study presented large data from the United States and Canada, one in four COD was unknown, underscoring the need for long-term multidisciplinary care and a national MMC mortality database. Individuals with MMC, and their families, should be encouraged to request clarification of COD/MOD and autopsies when they are unclear or unexpected.
Given the high prevalence of congenital abnormalities, as well as poor ambulatory and pulmonary function, this study sample had more severe disease than the general MMC population [27]. Importantly, all patients in this study died prematurely within the first five decades of life. Healthier individuals with MMC born at comparable dates were not included in the study precisely because of lower mortality.
This study had several limitations. As it was based on a convenience sample, the study likely overrepresented patients with multiple comorbidities followed by multidisciplinary spina bifida teams. The unshunted group was heterogenous, consisting of ill infants who died with unshunted hydrocephaly and, typically older, children without hydrocephalus. This aligns with clinical observations in which a small cohort of severely affected newborns succumb to a variety of different mechanisms, including Chiari crisis, pneumonia, and sepsis. Attempting to ascertain this difference, the subgroup of those who died after one year of age was analyzed (Section 3.6.3). Rather than considering the unshunted group as a “control,” the comparison of shunted and unshunted patients was one of two groups who underwent different neurosurgical management. Most of the medical record data was extracted from specialty clinic notes rather than primary care visits: a third of adults did not even have a primary care provider documented. For that reason, estimates of overall health and psychological characteristics of adults (Section 3.1.5) were most likely underestimates. The results were limited by the study sample, including that some MOD, such as bladder cancer, typically occur beyond the age of this sample. Nonetheless, these findings are at least partially generalizable, since much MMC care in the United States and Canada, especially recently, occurs at tertiary academic centers like those participating in this study. Being a muti-institutional study, it captured some geographical and other differences between participating sites and is therefore more generalizable than single center series [2, 8, 9, 11, 13, 14].
Given limited sample size, only three main hypothesis-driven analyses were performed. Exploratory analyses were included to set the stage for further studies. Whether due to insufficient documentation or varying levels of assessment between institutions, several variables, including level of lesion and adherence with therapy, were not captured. Undoubtedly, assigning a single cause or mechanism of death oversimplified the clinical reality that multiple organ systems often contributed to mortality.
This retrospective study focused on the numerator of mortality: how deaths occurred and risk factors for dying of a particular cause when death occurred. Since living patients were not included, mortality risks and risk factors could not be evaluated. Consequently, eliciting potential preventative measures of mortality was beyond the scope of this study. Large cohort studies that include the National Spina Bifida Patient Registry are needed to determine these.
In line with recent MMC mortality studies [2, 8-12, 14, 16], a fourth of COD/MOD were unknown, suggesting a potentially significant margin of error. It is unclear whether the proportions of COD/MOD would change if these were all known. This would only occur if certain COD/MOD were more likely to be unknown than others, which may not be true. In addition, some COD/MOD were based only on family reports, and few patients underwent an autopsy. No data were available to guide speculations about deaths with unknown causes or mechanisms. For those with documented COD/MOD, potential misclassification could not be assessed since autopsies were rarely performed. Therefore, the results represented the best current level of evidence.
5Conclusions
For those patients with known COD, neurologic COD were most common in shunted patients while pulmonary COD were most common in unshunted patients. Importantly, 26% had unknown COD. About 10% of deaths were urology-related. Infections were the most common MOD. Since much MMC care in the United States and Canada occurs at tertiary academic centers and the study involved multiple such centers, the results are likely generalizable.
Conflict of interest
None.
Ethical considerations
Internal review board approval:
Boston Children’s Hospital: P00018022
Children’s Hospital of Philadelphia: 12-009259
Cleveland Clinic: 19-1044
Duke University: 34501 and 103887
Mayo Clinic: 17-006368
Nationwide Children’s Hospital: STUDY00000534
Riley Hospital for Children at Indiana University Health: 1907150500
University of British Columbia: H15-00885
University of Colorado: 19-1937
University of Texas Southwestern: exempt
University of Oklahoma: 11210
University of Alabama at Birmingham: 300003966
Texas Children’s Hospital/Baylor College of Medicine: H-46590
Vanderbilt University: 191700
Lurie Children’s Hospital of Chicago: 2020-3371
University of Alberta: 00093941
Acknowledgments
Stuart Bauer, MD, Department of Urology, Boston Children’s Hospital, Boston, Massachusetts, for his assistance and guidance. No funding to report.
Supplementary material
[1] The Appendix part is available in the electronic version of this article: https://dx.doi.org/10.3233/PRM-220086.
References
[1] | Tennant PW , Pearce MS , Bythell M , Rankin J . 20-year survival of children born with congenital anomalies: a population-based study. Lancet. (2010) :375: (9715) 649–56. https://doi.org/10.1016/S0140-6736(09)61922-X. |
[2] | Borgstedt-Bakke JH , Fenger-Gron M , Rasmussen MM . Correlation of mortality with lesion level in patients with myelomeningocele: a population-based study. J Neurosurg Pediatr. (2017) :19: (2):227–31. doi: 10.3171/2016.8.PEDS1654. |
[3] | Kalucy M , Bower C , Stanley F , Burton P . Survival of infants with neural tube defects in Western Australia 1966-1990. Paediatr Perinat Epidemiol. (1994) :8: (3):334–51. doi: 10.1111/j.1365-3016.1994.tb00467.x. |
[4] | Wong LY , Paulozzi LJ . Survival of infants with spina bifida: a population study. Paediatr Perinat Epidemiol. (2001) :15: (4):374–8. doi: 10.1046/j.1365-3016.2001.00371.x. |
[5] | Kancherla V , Druschel CM , Oakley GP Population-based study to determine mortality in spina bifida: New York State Congenital Malformations Registry, 1983 to 2006. Birth Defects Res A Clin Mol Teratol. (2014) :100: (8):563–75. doi: 10.1002/bdra.23259. |
[6] | Hunt GM . A study of deaths and handicap in a consecutive series of spina bifida treated unselectively from birth. Z Kinderchir. (1983) :38: (Suppl 2):100–2. doi: 10.1055/s-2008-1063090. |
[7] | Singhal B , Mathew KM . Factors affecting mortality and morbidity in adult spina bifida. Eur J Pediatr Surg. (1999) :9: (Suppl 1):31–2. doi: 10.1055/s-2008-1072310. |
[8] | Protzenko T , Bellas A , Pousa MS , et al. Reviewing the prognostic factors in myelomeningocele. Neurosurg Focus.. (2019) :47: (4):E2. doi: 10.3171/2019.7.FOCUS19462. |
[9] | Dicianno BE , Sherman A , Roehmer C , Zigler CK . Co-morbidities Associated With Early Mortality in Adults With Spina Bifida. Am J Phys Med Rehabil. (2018) :97: (12):861–5. doi: 10.1097/PHM.0000000000000964. |
[10] | Peyronnet B , Gao F , Brochard C , et al. Urologic Disorders are Still the Leading Cause of In-hospital Death in Patients With Spina Bifida. Urology. (2020) :137: :200–4. doi: 10.1016/j.urology.2019.11.006. |
[11] | Szymanski KM , Misseri R , Whittam B , et al. Mortality after bladder augmentation in children with spina bifida. J Urol. (2015) :193: (2):643–9. doi: 10.1016/j.juro.2014.07.101. |
[12] | McDonnell GV , McCann JP . Why do adults with spina bifida and hydrocephalus die? A clinic-based study. Eur J Pediatr Surg. (2000) :10: (Suppl 1):31–2. doi: 10.1055/s-2008-1072411. |
[13] | Bowman RM , McLone DG , Grant JA , Tomita T , Ito JA . Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg. (2001) :34: (3):114–20. doi: 10.1159/000056005. |
[14] | North T , Cheong A , Steinbok P , Radic JA . Trends in incidence and long-term outcomes of myelomeningocele in British Columbia. Childs Nerv Syst. (2018) :34: (4):717–24. doi: 10.1007/s00381-017-3685-6. |
[15] | Jernigan SC , Berry JG , Graham DA , et al. Risk factors of sudden death in young adult patients with myelomeningocele. J Neurosurg Pediatr. (2012) :9: (2):149–55. doi: 10.3171/2011.11.PEDS11269. |
[16] | Malakounides G , Lee F , Murphy F , Boddy SA . Single centre experience: long term outcomes in spina bifida patients. J Pediatr Urol. (2013) :9: (5):585–9. doi: 10.1016/j.jpurol.2013.02.015. |
[17] | Lapides J , Diokno AC , Silber SJ , Lowe BS . Clean, intermittent self-catheterization in the treatment of urinary tract disease. J Urol. (1972) :107: (3):458–61. doi: 10.1016/s0022-5347(17)61055-3. |
[18] | Wilkes JK , Whitehead WE , Wang Y , Morris SA . Congenital Heart Disease and Myelomeningocele in the Newborn: Prevalence and Mortality. Pediatr Cardiol. (2021) :42: (5):1026–32. doi: 10.1007/s00246-021-02576-3. |
[19] | ICD-10 Mortality Manual 2a. Albany, NY: WHO Publications; 2014. Available from: https://www.cdc.gov/nchs/data/dvs/2a 2014.pdf |
[20] | Oakeshott P , Hunt GM , Poulton A , Reid F . Expectation of life and unexpected death in open spina bifida: a 40-year complete, non-selective, longitudinal cohort study. Dev Med Child Neurol. (2010) :52: (8):749–53. doi: 10.1111/j.1469-8749.2009.03543.x. |
[21] | Rickert CH , Grabellus F , Varchmin-Schultheiss K , Stoss H , Paulus W . Sudden unexpected death in young adults with chronic hydrocephalus. Int J Legal Med. (2001) :114: (6):331–7. doi: 10.1007/s004140000196. |
[22] | Shellhaas RA , Kenia PV , Hassan F , Barks JDE , Kaciroti N , Chervin RD . Sleep-Disordered Breathing among Newborns with Myelomeningocele. J Pediatr. (2018) :194: :244–247e1. doi: 10.1016/j.jpeds.2017.10.070. |
[23] | McDowell MM , Blatt JE , Deibert CP , Zwagerman NT , Tempel ZJ , Greene S . Predictors of mortality in children with myelomeningocele and symptomatic Chiari type II malformation. J Neurosurg Pediatr. (2018) :21: (6):587–96. doi: 10.3171/2018.1.PEDS17496. |
[24] | Cai B , McDermott S , Wang Y , et al. Skin Ulcers and Mortality Among Adolescents and Young Adults With Spina Bifida in South Carolina During 2000-2010. J Child Neurol. (2016) :31: (3):370–7. doi: 10.1177/0883073815596611. |
[25] | Oakeshott P , Reid F , Poulton A , Markus H , Whitaker RH , Hunt GM . Neurological level at birth predicts survival to the mid-40s and urological deaths in open spina bifida: a complete prospective cohort study. Dev Med Child Neurol. (2015) :57: (7):634–8. doi: 10.1111/dmcn.12698. |
[26] | Kancherla V , Weakland AP , Xu SY , Walani SR . Scorecard for spina bifida research, prevention, and policy: Score analysis by Human Development Index and WHO region. Prev Med. (2019) :123: :1–7. doi: 10.1016/j.ypmed.2019.02.020. |
[27] | Sawin KJ , Liu T , Ward E , et al. The National Spina Bifida Patient Registry: profile of a large cohort of participants from the first 10 clinics. J Pediatr. (2015) :166: (2):444–50.e1. doi: 10.1016/j.jpeds.2014.09.039. |




