Pediatric intrathecal baclofen management during the COVID-19 pandemic in the US and Canada
Abstract
The COVID-19 pandemic has been a challenge to healthcare systems around the world. Within pediatric rehabilitation medicine, management of intrathecal baclofen has been particularly challenging. This editorial reviews how programs in the US and Canada coped with the quickly changing healthcare environment and how we can learn from this pandemic to be prepared for future crises.
1.Introduction
Intrathecal baclofen (ITB) therapy is used in the management of global hypertonicity, including both spasticity and dystonia, via an implantable infusion system. The pump delivers a precise amount of baclofen directly into the intrathecal space via a catheter. ITB has been utilized in children with cerebral palsy (CP), brain injury, and spinal cord injury since the early 1990s for the management of spasticity and has been increasingly used in children with global dystonia as well [1]. It was initially used in the early 1990s in an investigational manner and was later FDA approved for use in children with spasticity of cerebral origin in June 1996.
Withdrawal is a life-threatening complication associated with sudden discontinuation of intrathecal baclofen secondary to failure to refill the drug reservoir or the interruption of medication flow due to mechanical failure of the system. Timely refills, watchful monitoring, and clear communication between patients, families, and providers are essential in avoiding withdrawal. System malfunction is rare; however, because it can be life threatening, early recognition is crucial to minimize morbidity [2, 3].
When the COVID-19 pandemic reached the United States (US) and Canada in March 2020, with initial cases in January 2020, many providers were required to change their practice in order to limit exposure of patients and their families, as well as that of healthcare providers. Healthcare systems in many jurisdictions were mandated by public health orders to restrict service to urgent and emergent care only. Large numbers of healthcare providers were redeployed to respond to the needs of their local institutions in managing their local COVID-19 outbreak. Given the morbidity and mortality associated with withdrawal from ITB, it was evident that baclofen pump management would need to continue in a way that would be safe for both providers and patients. Providers caring for these patients needed to quickly adapt in order to ensure services were available for routine maintenance of refilling ITB pumps, management of pump complications, and surgical interventions as they became necessary. Given the changing healthcare and social milieu, many patients and families also needed reassurance that their medical team was still available, especially given the level of complexity of this population at baseline.
2.Methods
A survey of Pediatric ITB providers was performed in late April of 2020 to review the challenges and adjustments that allowed for continued care of ITB patients during the COVID-19 pandemic. The survey was distributed as a Google form to 250 providers at approximately 84 programs through the Pediatric ITB Network and the Pediatric Rehabilitation Facebook group Representatives from 29 programs responded, yielding a 35% response rate. Only one response was allowed per site. Results were compiled into an Excel spreadsheet and basic descriptive statistics were performed.
3.Results
Responding programs varied in geographic region, number of providers, and number of patients; the programs represented a variety of practice sites including pediatric and general academic centers, rehabilitation hospitals, community hospitals, and private practice settings (Table 1). The findings revealed that many challenges were addressed, including limiting patient and provider exposure, ensuring that routine pump maintenance was performed in time, adjusting typical clinic schedules for pump management, and addressing ITB complications in a timely and effective manner.
Table 1
Characteristics of surveyed pediatric intrathecal baclofen pump programs (
| Geographic Region | ||
| Canada | 2 | 7% |
| Midwest | 6 | 21% |
| Northeast | 7 | 24% |
| South | 8 | 28% |
| West | 6 | 21% |
| Facility Type | ||
| Community Hospital | 2 | 7% |
| Freestanding Rehabilitation Hospital | 3 | 10% |
| General Academic Center | 5 | 17% |
| Pediatric Academic Center | 17 | 59% |
| Private Practice | 2 | 7% |
| Number of Refill Providers | ||
| 1 | 2 | 7% |
| 2 to 4 | 17 | 59% |
| 5 to 9 | 6 | 21% |
| 10 or more | 4 | 14% |
| Number of Patients with ITB Pumps | ||
| Less than 20 | 6 | 21% |
| 20 to 49 | 7 | 24% |
| 50 to 99 | 8 | 28% |
| 100 or more | 8 | 28% |
Figure 1.
ITB program changes during the COVID-19 pandemic.
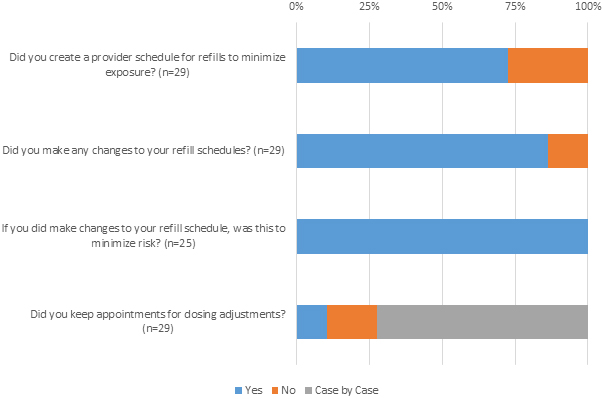
3.1Limiting patient risk of exposure
In order to create an environment of least possible risk for these medically fragile patients, a number of different strategies were employed by the ITB teams, as shown in Fig. 1. Some brought the patients to a less trafficked clinic site, especially if a hospital-based clinic meant increased risk because of on-site COVID-19 care. Other teams increased the time between daily scheduled visits to avoid patient overlap, roomed their patients immediately upon arrival, and avoided waiting areas. Some preemptively coordinated and clustered care based on alarm date, successfully consolidating refill visits to a condensed schedule. For example, some centers created single clinic sessions just for pump refills to avoid potential “sick” visits, but separation by time and space was still employed to prevent potential transmission. Based on the size of the ITB program, some consolidated to as little as one to two days per month with larger programs requiring more days. All patients/families were pre-screened for symptoms of illness prior to appointments and proper PPE and isolation were used. The number of people, including caregivers, allowed in the procedure room was minimized to allow for social distancing. In some cases, an increased number of exam rooms were utilized to ensure that patients had minimal contact with each other. In locations where clinic space was shared between disciplines, specific dates were set aside for ITB refills to minimize patient’ exposure to other populations. Cleaning of spaces was standard among survey participants. In certain areas, appointments were adjusted when possible to avoid bringing patients out of their homes and into a medical setting during the peak of the pandemic in their geographic region. In some cases, this meant bringing them in earlier than originally scheduled for regular pump maintenance.
Another strategy was utilization of home refill companies, which dramatically limited exposure because the patient did not have to leave their home. In our survey, a total of 76% of respondents reported that their ITB program is located within an academic center (59% of programs were located specifically within pediatric academic centers). Catchment areas for these centers can be quite large and in states with a preponderance of rural areas, there may be only one or two centers that have a provider who manages pediatric intrathecal baclofen pumps. This situation prompted some providers to increase efforts to utilize home refill programs. Prior to the COVID-19 pandemic, availability of home refills was often limited by geography and insurance coverage. Finally, the medication itself could be manipulated by changing the concentration or supplementing a lower dose with enteral therapy to increase the time between refills. Patients with appointments for dose adjustments were typically addressed on a case by case basis and deferred if possible after discussion with them and their families (Fig. 1).
FDA regulations state that for any intrathecal drug delivery pump, the reservoir should be emptied and refilled every 6 months, regardless of the volume of medication in the reservoir at that time. Additionally, the manufacturers of non-compounded intrathecal baclofen (i.e. Gablofen
In some rare cases, patients were not able to make it to their home institutions for refills and required urgent refills from other local providers or home refill agencies. This was reported to be particularly prevalent in Florida, where many families live for the winter while having permanent residences in other locations.
3.2Limiting provider risk of exposure
Centers caring for ITB patients made many adjustments to limit the provider exposure to illnesses. Of those surveyed, 72% reported schedules for pump refills were adjusted to limit risk of exposure (Fig. 1). The centers assessed the number of providers who were proficient in pump refills and many reported limiting the number of providers in the clinic on a given day. Separating providers or creating isolated teams helped decrease the risk of many providers being exposed and later quarantined. For some, this meant working for one week, then isolating for one to two weeks. Losing some or all of the ITB team at any institution would place significant stress on the remaining providers while potentially increasing the risk of morbidity to patients. Ensuring backup providers were available in case primary providers needed to self-isolate was also a common response in the survey. In some cases, residents and fellows remained involved in the refill procedure after being educated about the utility of clustering care as well as the maximum importance of maintaining safety for all. However, many institutions removed all trainees to minimize exposure.
Many practices also reported changes to clinic templates in order to limit exposure for providers, sometimes including consolidating pump refill clinics to a single day per week or month. This also allowed providers to minimize use of PPE, an important goal of many healthcare systems during this time. These schedule changes involved not only changing a patient’s appointment but also confirming that the new appointment was scheduled prior to the reservoir becoming empty. For the centers that managed over 100 patients, this was a task that involved many man-hours. On some teams, certain providers absorbed the bulk of the caseload in order to protect others deemed “high risk.”
Contingency plans were made for treating patients who either tested positive for COVID-19 or became a patient under investigation (PUI). Most survey respondents delayed refills for patients who were COVID-19 positive or PUIs when possible. Those who were able to delay refills on COVID-19 positive patients tried to get 1–2 negative repeat tests before bringing in the patients for refills. In addition to delaying refills, other risk reduction strategies included performing the refill in the emergency department where more robust PPE, such as N95 masks and controlled air purifying respirators (CAPRs), was likely to be found, utilizing negative pressure ventilation rooms, masking the patient as well as the provider, and following hospital and CDC guidelines. Challenges were noted related to the constantly changing recommendations around PPE use.
3.3Ensuring pump maintenance performed in time
Routine surgeries were postponed by most institutions, including pump replacement for end of battery life. The elective replacement indicator (ERI) identifies when the pump has 90 days of service remaining. An audible alarm begins and must be adjusted by a clinician using a programmer. Within the next 90 days, the pump must be replaced or the therapy changed to oral medication to avoid withdrawal. Replacement surgeries for ERI, especially for children, are routinely scheduled several months prior to the ERI date. This preemptive cushion along with the 90-day window following the ERI allowed for some postponement of these semi-elective surgeries in the time of COVID-19. As surgical centers begin to reopen for elective surgeries, these patients are being rescheduled to avoid withdrawal issues. The risk of exposure in a hospital or surgical center is also decreased by presenting outside of the peak of that region’s COVID-19 curve. Placement of new pumps has largely been avoided with the rare exception of end-of-life cancer treatment, hepatic artery infusion, dystonic crisis, or paroxysmal sympathetic hyperactivity that has not responded to enteral or intravenous medications. Of note, during the COVID-19 pandemic, there has also been an unrelated hold on Medtronic SynchroMed II pump production in the US since the latest version is lacking FDA approval for a new component, leading to limitations on new elective pump placements.
3.4Addressing ITB complications
In the case of baclofen pump and catheter complications, some providers have partnered with local pediatricians and hospitals for initial work up to prevent a child from unnecessary travel or hospitalization. Some ITB teams attempted to prolong the time until formal baclofen pump troubleshooting by using enteral medications for symptom management. In many cases, significant effort was made to prevent unnecessary visits to an urgent care or emergency department by attempting to troubleshoot via telemedicine and by seeing patients urgently in clinic. Patients presenting with possible withdrawal were evaluated and treated appropriately. Vigilance in these evaluations mitigated the possibility of a potential life-threatening situation.
It has been previously documented that patients during the COVID-19 pandemic were less likely to seek medical help for acute illnesses [5]. An American College of Emergency Physicians’ survey found that 74% of patients were afraid of contracting COVID-19 when they sought treatment for a non-COVID condition and 30% avoided seeking treatment [6]. Most of our centers reported that they spent extra time educating patients and families about ITB withdrawal symptoms and reiterating contact information to prevent complications or morbidity due to delayed care.
In many regions, providers are managing children with baclofen pumps not only locally, but also in more rural areas or even surrounding states and provinces. These circumstances lend themselves to challenges surrounding baclofen pump refills, complications, and even insurance coverage for telemedicine visits or home refill companies. In selective cases, patients were provided letters of medical necessity to cross state or provincial borders to attend refill appointments.
4.Discussion and recommendations
4.1Pump and ITB management
Recommendations from this group include: maximizing the efficiency of visits by scheduling them carefully, consolidating care to minimize both patient and provider risk, utilizing community providers and home infusion companies for local pump refills, avoiding emergency or urgent care visits and hospitalizations, and being prepared to manage a patient who cannot get a refill or undergo troubleshooting. Additionally, the FDA requires intrathecal pump reservoirs to be emptied and refilled at least every 6 months and both Gablofen
4.2Communication
The COVID-19 pandemic highlights the importance of having robust communication in place for families of patients with ITB pumps, as well as standard procedures. It is crucial to always review overdose/withdrawal symptoms and emergency procedures at every encounter with families in order to ensure they know when to seek additional care. One thing to consider is an “ITB Action Plan;” similar action plans are common in other areas of pediatrics, such as asthma management, to prepare families in case of worsening symptoms. Such a plan would act as a guide for families to decide on the correct intervention for their child. It is also important to communicate with those caring for the patient, including primary care physicians, on-call ITB pump providers, and of course with patients and families.
4.3Systems planning
This pandemic highlights that all ITB practices need to have a plan in place for safely managing a patient who has any transmissible illness, including, but not limited to COVID-19. They must include a plan for the potential decrease in available providers. Developing one that allows for adjustment of clinic schedules to benefit both the patients and the providers is of utmost importance. Any plan should be flexible enough to deal with the rapidly changing environment of these complicated situations. Based on the results of the survey, we recommend prioritizing safety of patients and providers by identifying proper locations to perform refills in potentially ill patients, limiting exposure as much as possible, and creating space, both in time and distance, for clinic visits. Providers should be separated as well, so that an entire team does not simultaneously contract a transmissible illness. It may be beneficial in some cases for groups of providers to consult with their institutio’s infection prevention and control teams to have a plan in place for future pandemics.
In addition to practical considerations for safety from accidental virus transmission, it would be highly beneficial for programs to have a way to access alarm dates and most recent refill dates for all patients managed in their program. This would allow program directors and coordinators to triage patients efficiently to confirm that none have been missed, resulting in alarms, or worse, empty pump reservoirs and baclofen withdrawal. Such an organization system would be beneficial in pandemics as well as natural disasters or any other unplanned disruptions in medical care.
4.4ITB training
While we did not directly survey programs to see how trainees were affected by the COVID-19 pandemic with regards to ITB training, we felt that it is important to address this here. A successful academic ITB program must meet the needs of trainees in a safe and efficient manner, teaching them spasticity and dystonia management, including ITB pump management, and how to treat a patient safely with confirmed or suspected COVID-19. This may involve more training in a simulated environment to maintain the breadth of skills and education.
The ever-changing protocols for our training programs necessitate regular communication with our local, regional and national educational leaders to advocate for our trainees.
4.5The utility of the Pediatric ITB Network
The Pediatric ITB Network was established in 2015 and meets annually with the goal of improving the care of children utilizing ITB pumps. This network has facilitated significant collaboration between pediatric ITB programs leading to shared ideas, policies, care approaches, and troubleshooting. Perhaps most importantly, the network brings together experts to provide clinical advice and support in the management of pediatric ITB. During the COVID-19 pandemic, the Pediatric ITB network was able to rely on its members to share unique solutions and develop best practices for managing unprecedented challenges in an efficient and rapid manner.
5.Conclusion
Baclofen pumps are often placed in patients who are already medically complex and therefore at higher risk of illness at baseline. These devices require close supervision and time sensitive management by a specially trained medical professional. Baclofen pump management can occur safely in the midst of a pandemic by creating new systems-level practices for provider rotation, patient scheduling, and minimizing exposure to both providers and patients. ITB programs must have robust protocols in place to ensure the safe delivery of ITB therapy. Such protocols will facilitate necessary modifications when novel public health threats arise. These also give confidence to providers, patients, families and health system administrators that patient safety is a high priority. Urgent or emergent situations in the care of children and young adults with baclofen pumps require ITB teams to think critically about management and safety. Clear communication with the ITB team, patients and families and local providers about necessary changes enhances care and safety during the COVID-19 pandemic and beyond.
Acknowledgments
There was no funding used to produce this article.
Conflict of interest
The authors have no conflicts of interest.
References
[1] | Albright AL, Ferson SS. Intrathecal baclofen therapy in children. Neurosurg Focus. (2006) ; 21: (2): 1–6. doi: 10.3171/foc.2006.21.2.4. |
[2] | Fernandes P, Dolan L, Weinstein SL. Intrathecal baclofen withdrawal syndrome following posterior spinal fusion for neuromuscular scoliosis: a case report. The Iowa Orthop J. (2008) ; 28: : 77–80. |
[3] | Zuckerbraun NS, Ferson SS, Albright AL, Vogeley E. Intrathecal baclofen withdrawal; emergent recognition and management. Pediatr Emerg Care. (2004) ; 2: (11): 759–64. doi: 10.1097/01.pec.0000144919.08619.95. |
[4] | Moberg-Wolff E. Potential clinical impact of compounded versus noncompounded intrathecal baclofen. Arch Phys Med Rehabil. (2009) ; 90: : 1815–20. doi: 10.1016/j.apmr.2009.05.018. |
[5] | Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer F, et al. Reduction in st-segment elevation cardiac catheterization laboratory activations in the united states during COVID-19 pandemic. J Am Coll Cardiol. 75: (22): 2871–2. doi: 10.1016/j.jacc.2020.04.011. |
[6] | American College of Emergency Physicians COVID 19 Survey. Accessed on May 28, (2020) . Available from: https://www.emergencyphysicians.org/globalassets/emphysicians/all-pdfs/acep-mc-covid19-april-poll-analysis.pdf. |



