Why individuals with cerebral palsy are at higher risk for respiratory complications from COVID-19
Abstract
Respiratory dysfunction is a leading cause of morbidity and mortality in individuals with cerebral palsy (CP). In children and adults with CP, movement and physical function is always affected. Yet, many clinicians overlook potential for impaired movement and function of the diaphragm muscle (DIAm) in individuals with CP. Since individuals with pre-existing respiratory disorders are at greater risk for respiratory complications if they contract COVID-19, understanding potential risks to individuals with CP is important. In this review we present research on respiratory function and DIAm force generation in children with CP. We compare this clinical work to basic science research investigating phrenic motor neuron and DIAm motor unit dysfunction in an animal model with CP symptoms, the spa mouse. Finally, we integrate the clinical and basic science work in respiratory function in CP, discussing potential for individuals with CP to have severe respiratory symptoms from COVID-19.
1.What is the relationship between respiratory complications and the diaphragm muscle (DIAm) in individuals with cerebral palsy (CP)?
For clinicians who see and diagnose infants and children with CP, a question that frequently arises from families is “will my child be able to walk?”. Yet, for individuals with CP, respiratory dysfunction, not their inability to walk, is the greatest contributor to their morbidity and mortality [1, 2, 3, 4]. CP is a clinically recognized syndrome defined as a permanent disorder of movement or posture that occurs during the development of the fetal or infant brain [5]. The movement difficulties in individuals with CP can be classified based on the limbs that are primarily affected such as hemiplegic, diplegic, and quadriplegic CP and on the type of movement difficulty including spastic (80–85% of individuals), dyskinetic (less than 10% of individuals), and ataxic (less than 5% of individuals), though other subtypes and classifications exist [6, 7, 8, 9, 10]. However, one muscle that is often neglected when discussing impairments of movement and posture is the diaphragm muscle (DIAm). In addition to being the major inspiratory muscle for breathing, the DIAm is also critical for coughing and sneezing which are high force expulsive behaviors needed for airway protection and clearance [11, 12, 13, 14, 15]. In one registry review, 58% of children with CP had a daily cough or wheeze, 10% had obstructive sleep apnea, 40% had a cough with drinking, and 20% had abnormalities on clinical pulmonary exam [16]. Furthermore, adults with CP have a greater risk of respiratory disease than adults in the general population [17, 18]. The DIAm plays a critical role in reducing susceptibility to respiratory infections in two ways: 1) during the pharyngeal phase of swallowing, the DIAm generates a large negative intrathoracic pressure necessary to propel a food bolus through the pharynx and into the esophagus, with failure resulting in aspiration; and 2) coughing and sneezing, where the DIAm contributes to increased intra-abdominal pressure necessary to expel aspirates and phlegm from the airway [11, 19, 20, 21]. Therefore, we propose that we should educate families of children with CP that their child may also have an impaired cough, which can increase their child’s susceptibility to respiratory complications.
2.What complications are seen with COVID-19?
Figure 1.
Respiratory parameters in pulmonary function testing. Total lung capacity is the amount of air in the lungs after maximal inspiration and is about 4 L to 6 L in adults with volumes varying depending on age, sex, and body composition [93, 94, 95]. Tidal volume is the amount of air inhaled and exhaled during quiet breathing. In healthy adults, tidal volume is between 400–500 ml, approximately 10% of the total lung volume [96]. Vital capacity is the maximal amount of air that can be exhaled after maximal inhalation [97]. Vital capacity is composed of the tidal volume, inspiratory reserve volume, and expiratory reserve volume. Two common measures for vital capacity are the forced vital capacity which is the maximal amount of air that can be exhaled at maximum speed and effort following maximal inspiration, and slow vital capacity which is the maximal amount of air that can be slowly exhaled following maximal inspiration. Forced expiratory volume at 1 second is the maximal amount of air that can be exhaled in 1 second following maximal inspiration [98]. Residual volume is the amount of air remaining in the lungs after maximal expiration.
![Respiratory parameters in pulmonary function testing. Total lung capacity is the amount of air in the lungs after maximal inspiration and is about 4 L to 6 L in adults with volumes varying depending on age, sex, and body composition [93, 94, 95]. Tidal volume is the amount of air inhaled and exhaled during quiet breathing. In healthy adults, tidal volume is between 400–500 ml, approximately 10% of the total lung volume [96]. Vital capacity is the maximal amount of air that can be exhaled after maximal inhalation [97]. Vital capacity is composed of the tidal volume, inspiratory reserve volume, and expiratory reserve volume. Two common measures for vital capacity are the forced vital capacity which is the maximal amount of air that can be exhaled at maximum speed and effort following maximal inspiration, and slow vital capacity which is the maximal amount of air that can be slowly exhaled following maximal inspiration. Forced expiratory volume at 1 second is the maximal amount of air that can be exhaled in 1 second following maximal inspiration [98]. Residual volume is the amount of air remaining in the lungs after maximal expiration.](https://content.iospress.com:443/media/prm/2020/13-3/prm-13-3-prm200746/prm-13-prm200746-g001.jpg)
Effective and coordinated coughing and sneezing are particularly important when an individual contracts an acute respiratory illness. More recently, the general population has become aware of the devastating effects of an acute respiratory illness with the outbreak of the coronavirus SARS-CoV-2, now known as COVID-19 which was declared a pandemic by the World Health Organization on March 11, 2020 [22]. The working case definition of COVID-19 is a severe acute respiratory illness with fever and respiratory symptoms such as cough and shortness of breath. While respiratory symptoms are the most devastating, other symptoms have also been reported including gastrointestinal, cardiac, vascular, and neurologic [23, 24]. In a report modeling epidemiologic susceptibility to symptomatic infection and death, the peak age of susceptibility was found to be 60 years and older. Death from symptomatic infections was also found to rise dramatically at this age [25]. Unfortunately, the presence of pre-existing conditions such as respiratory, cardiac, or neuromuscular disorders was not included in this modeling. However, in another report, higher fatality rates were noted in those with pre-existing conditions including chronic respiratory disease, cardiovascular disease, diabetes, and hypertension [26].
Interestingly, children appear to have milder symptoms than adults, and many present asymptomatically [27, 28]. There is limited information regarding the impact of pre-existing conditions in children on symptoms or severity of COVID-19. One case series of 34 children who tested positive for COVID-19 reported that none had pre-existing conditions [29]. However, in another study describing outcomes of 48 children with COVID-19 who were admitted to pediatric intensive care units (PICU), 40 (83%) had pre-existing conditions [30]. Of those with pre-existing conditions, 21 (44%) were considered “medically complex” defined as having long-term dependence on technology and/or tracheostomy and/or developmental delay and/or genetic differences and/or having chronic lung disease [30]. Overall, 38% required intubation or tracheostomy ventilation, 13% required extracorporeal therapy (ECMO) with 4% of the children dying with 32% still hospitalized at the time this report was published [30]. While CP was not a diagnosis this study addressed, it seems likely that some in the “medically complex” group would have had CP.
3.How does DIAm neuromotor control in individuals with CP relate to increased susceptibility to respiratory complications with COVID-19?
3.1Respiratory and pulmonary function
For children and adults with CP, the potential for respiratory impairment is typically considered in those with severe physical symptoms, such as those in the Gross Motor Functional Classification System (GMFCS) levels IV and V (i.e., those who function primarily from a wheelchair base) [31, 32]. Individuals with severe CP symptoms are more likely to have difficulties with sialorrhea, swallowing, recurrent respiratory infections, hypoventilation in sleep, and need for tracheostomy [33, 34]. Similarly, these same risks are prevalent in the more intensively studied population of individuals with spinal cord injury [35, 36, 37]. For these children, and many others with CP, pulmonary function testing (PFTs) cannot be reliably performed due to severity of motor dysfunction, cognitive impairment, or simply the inability to perform the testing. However, there have been some recent studies addressing pulmonary function in children with mild CP [38, 39, 40, 41]. For review, Figure 1 shows an example of respiratory parameters.
In a study involving children approximately 5 to 12 years of age, 35% with CP could not perform the task of breath holding [42]. Those with CP who could hold their breath were only able to do so for less than half the amount of time as typically developing children, likely related to a significantly smaller inspiratory volume [42]. Furthermore, the ability to breath hold and the volume of inspired air was further impaired in children with choreoathetoid CP [42]. In a study of children with hemiplegic and diplegic CP (GMFCS I-III, mean age
3.2DIAm pressure generation
Activation of the DIAm serves to create a negative intra-thoracic pressure and a positive intra-abdominal pressure, with the trans-diaphragmatic pressure (
Figure 2.
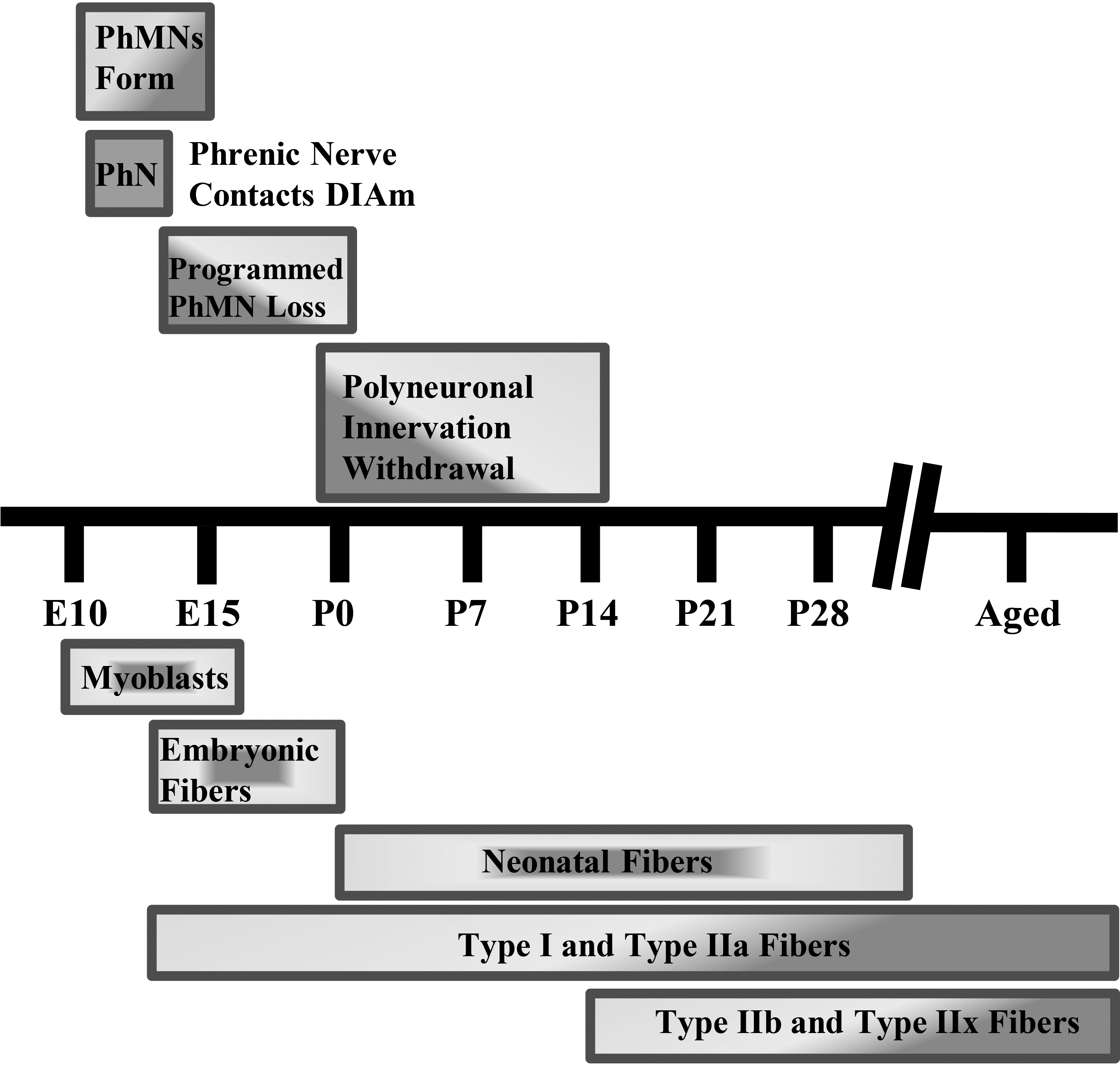
Diaphragm motor unit development timeline in rodents. Phrenic motor neuron (PhMN) and diaphragm (DIAm) developmental timing in rodents. Rodent gestation is approximately 18–20 days. PhMN development begins around embryonic day 10 (E10), with the phrenic nerve contacting the DIAm around the same time. For the DIAm, myoblasts form around E12, rapidly transitioning to immature embryonic and neonatal muscle fibers with emergence of Type I and IIa muscle fibers. Type IIb and/or IIx DIAm fibers are the last to emerge and do so around postnatal day 14 (P14). Type I, IIa, IIb, and/or IIx are the complement of fiber types present by maturity.
3.3Swallowing and aspiration
The DIAm is not only important for sustaining ventilation and intermittent coughing and sneezing, it also plays an important role in swallowing, preventing aspiration, and reducing the risk of aspiration pneumonia. Swallowing is a complicated process that includes precise coordination of muscles from the mouth to the DIAm. During swallowing, the DIAm is important in generating negative intrathoracic pressure to facilitate propulsion of a food or liquid bolus. Peak DIAm activation during swallowing appears to occur just prior to the start of the pharyngeal phase [55]. In anesthetized, spontaneously breathing cats, negative esophageal pressure (due to DIAm activation) increases by over 40% during the pharyngeal phase of swallowing [19]. Prior to phrenic motor neuron and DIAm activation during swallowing, there is increased activation of inspiratory pre-motor neurons in the dorsal medulla of the brain in anesthetized, spontaneously breathing cats [20]. Thus, the negative intrathoracic pressure generated by the DIAm contributes to the rapid movement of the bolus of food or water from the mouth to the esophagus. If a bolus, portion of a bolus, or saliva is inadvertently aspirated, a reflex is triggered that causes a sudden deep inspiration followed by a forceful cough to clear the irritant (i.e., the aspiration reflex) [56]. The DIAm is the key muscle in the aspiration reflex involved with both the deep inspiration and forceful cough. In individuals with CP, if DIAm neuromotor control and force generation is impaired, then the efficacy of the aspiration reflex is also impaired, with dire consequences. An effective cough is not only critical for airway clearance in aspiration, but also at times of respiratory infection. Thus, without an effective cough, one is at greater risk of respiratory infection and when a respiratory infection occurs, impaired airway clearance potentially results in a prolonged course of recovery.
4.What are the mechanisms for DIAm impairment in CP?
4.1DIAm and phrenic motor neurons during development
For the vast majority of individuals with CP, the developmental injury or abnormality that results in physical impairments occurs in utero or in the early postnatal period. The timing of this developmental injury or abnormality is not only when the brain and spinal cord are vulnerable to disruption, but also motor neurons and motor units, including phrenic motor neurons and DIAm motor units [57, 58, 59, 60, 61, 62, 63, 64]. In fact, in the original description of CP, it was termed a “cerebro-spinal disorder” in acknowledgement of the importance of motor units to the etiology and pathophysiology of CP [65, 66].
In mammals, the absolute number of motor neurons in the spinal cord reaches a maximum at commencement of the third trimester, after which a process of developmental loss occurs into the early perinatal period until the optimal number of motor neurons is achieved (Fig. 2) [59, 60, 64]. However, changes in inputs to motor neurons during this developmental period can dramatically alter the final number of motor neurons [67, 68, 69, 70, 71]. During the perinatal period and into maturity, motor neurons, including phrenic motor neurons, undergo differential growth which appears to correspond with the emergence of motor unit diversity [62]. The differential growth of motor neurons is necessary as a range of motor neuron sizes are required to carry out different motor behaviors and provide for an orderly, graded recruitment of different motor unit types (Fig. 3).
Figure 3.
Diaphragm motor unit properties. A full complement of motor units is required for the diaphragm (DIAm) to effectively perform activities ranging from eupneic breathing to cough/Valsalva (A).Smaller motor neurons innervate the fatigue resistant Type I and IIa muscle fibers creating slow (S) and fatigue resistant (FR) motor units needed for sustaining eupnea (A,B). Larger motor neurons innervating Type IIx and IIb muscle fibers form the fatigue intermediate (FInt) and fast fatigable (FF) motor units, respectively (B). FInt and FF motor units are recruited for high force behaviors, such as those needed for an effective cough (A). In animal models of CP, there are fewer larger phrenic motor neurons with reduced specific force generation, which coincides with reduced DIAm pressure generation in humans with CP, suggesting impairment of high force behaviors (A).
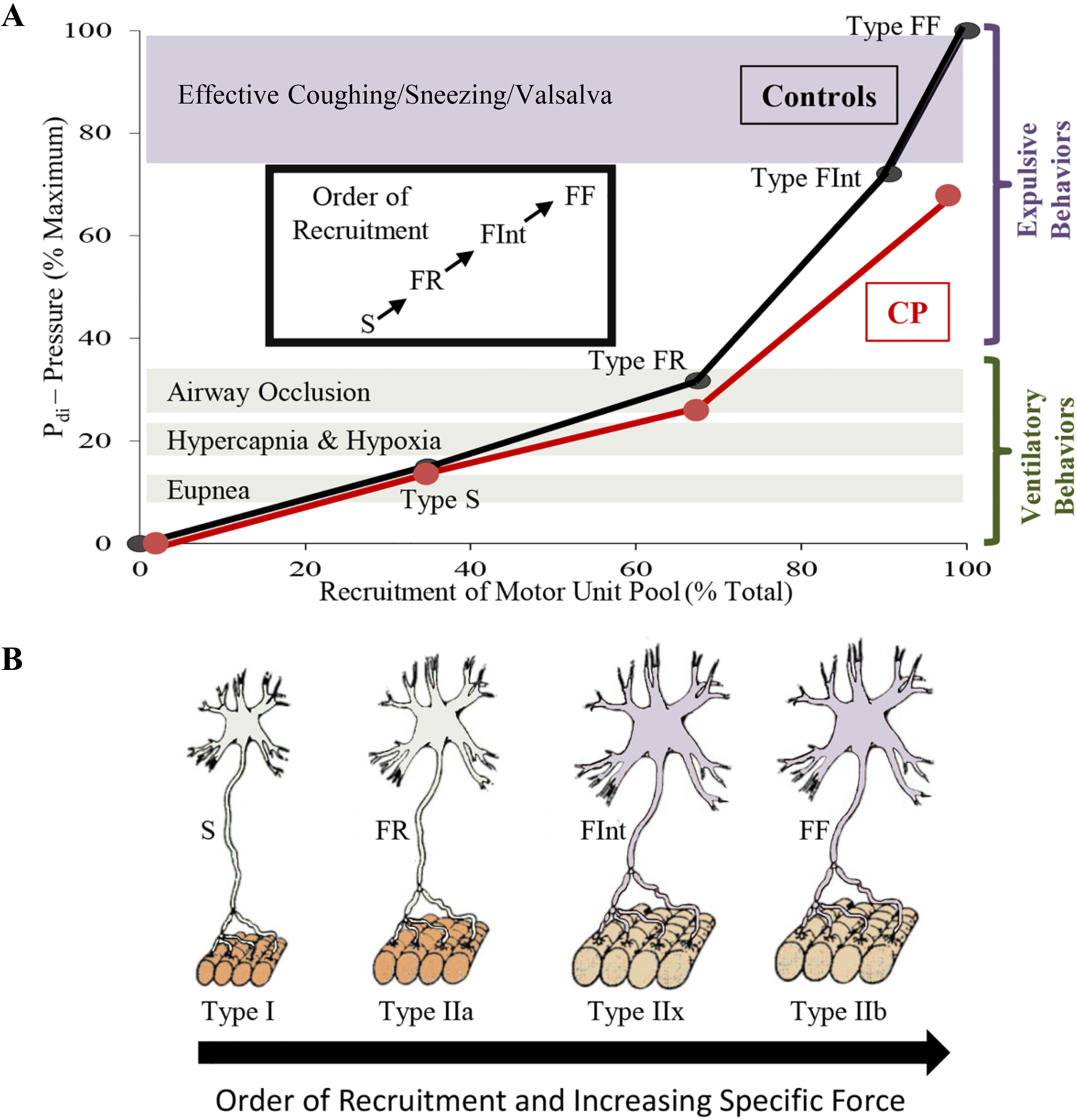
Coinciding with motor neuron development, myoblasts transition to myotubes and finally to immature (embryonic) muscle fibers (Fig. 2). Innervation of DIAm by the phrenic nerve appears very early during myoblast migration and myotube formation. Initially several phrenic motor neurons contact a single developing DIAm fiber (polyneuronal innervation); thus, developing DIAm fibers are influenced by several motor neurons (neurotrophic influence) (Fig. 2). The developmental loss of motor neurons coincides with the loss of polyneuronal innervation of muscle fibers such that by the second postnatal week in rodents, each muscle fiber is innervated by only a single motor neuron (Fig. 2) [64, 72]. During the perinatal period and into maturity, muscle fiber type transitions from embryonic to neonatal to adult fiber types with the emergence and growth of more fatigable fast twitch (type IIx and/or IIb) muscle fibers being the final event to occur [64, 72, 73, 74]. In the adult DIAm, type I, IIa, IIx and/or IIb fibers are all present in varying proportions depending on species. Type I and IIa DIAm fibers comprise fatigue resistant slow (type S) and fast (type FR) motor units, respectively, which are innervated by smaller, more excitable phrenic motor neurons (Fig. 3). These fatigue resistant type S and FR DIAm motor units are recruited for inspiratory behaviors including eupnea and breathing stimulated by hypoxia and hypercapnia (Fig. 3). Type IIx and/or IIb DIAm fibers comprise fast fatigue-intermediate (type FInt) and fast fatigable (type FF) motor units, which are innervated by larger, less excitable phrenic motor neurons. These more fatigable type FInt and FF DIAm motor units generate greater specific force (force normalized for muscle fiber cross-sectional area) and are only recruited to generate the higher pressures required for effective coughing, sneezing or Valsalva-type maneuvers that are typically of short duration (Fig. 3) [64, 75].
4.2DIAm and phrenic motor neurons in maturity
Properties of motor neurons and their motor units have not been well studied in CP, in part due to the lack of animal models with physical symptoms similar to individuals with CP [66]. A second major barrier has been the enormous animal husbandry hurdles that have to be overcome in some of the perinatal injury/hypoxia animal models, which unlike the majority of individuals with CP have very poor survival with death before adolescence [66]. While animal models of CP do not completely recapitulate human disorders, they can provide insight regarding mechanisms underlying the symptoms of CP.
Although CP is a neuro-developmental disorder, there is only one study that has estimated the impact of CP on the final number of motor neurons in adults. In this study, an electrophysiological technique, the Motor Unit Number Index (MUNIX), was used to estimate the number of motor units in the hypothenar muscle of adults with and without CP [76]. Interestingly, this study estimated that there are 21% fewer hypothenar muscle motor units in adults with CP compared to adults without CP (168
Recently, we investigated motor neuron loss and motor unit properties in a mouse model of CP symptoms, the spa mouse. The spa mouse has a homozygous insertion of LINE-1 in the beta subunit of the glycine receptor gene resulting in a splicing error of this subunit [79]. The spa mouse is smaller in size than wild type and heterozygote littermates, and most importantly, it develops symptoms of CP such as hypertonicity and exaggerated startle response by 2–4 weeks of age [80]. This developmental period coincides with the postnatal emergence of symptoms in humans with CP. In a recent study evaluating the number of motor neurons innervating the tibialis anterior muscle in adult spa mice, we found 61% fewer motor neurons compared to wild type mice [81]. In addition, the surviving motor neurons in spa mice were smaller, reflecting a disproportionate loss of larger motor neurons [81]. In another recent study, we found that there are
In both the tibialis anterior muscle and DIAm in spa mice, we found that there was increased susceptibility to neuromuscular transmission failure compared to wild type mice [83, 84, 85]. With neuromuscular transmission failure, the neural signal from the motor neuron fails to evoke muscle fiber contraction, a form of fatigue [86]. In these studies, we found that in spa mice neuromuscular transmission failure in the tibialis anterior and DIAm was apparent even during initial nerve stimulation, and that it worsened during repeated nerve stimulation, especially at higher frequencies of stimulation [83, 84, 85]. Also, these studies also showed that there were no differences in the total number of muscle fibers in the tibialis anterior muscle [83] or in the DIAm (preliminary observation) in spa mice, and that each muscle fiber is innervated (i.e. neuromuscular junctions are present). Thus, with fewer motor neurons and the same number of innervated muscle fibers, the innervation ratio (i.e., number of muscle fibers innervated by a single motor neuron) was greater for both tibialis anterior and DIAm in spa mice. An expanded innervation ratio suggests that the increased susceptibility to neuromuscular transmission failure in spa mice reflects a failure of action potential propagation at the increased number of axonal branch points [86, 87, 88, 89].
In preliminary work, we found that maximum DIAm specific force is reduced by
The reduction in the number of phrenic motor neurons in spa mice is similar to the age-related loss of phrenic motor neurons observed in rats. Old rats (24 months old) have 22% fewer phrenic motor neurons with the surviving phrenic motor neurons being 19% smaller compared to young adult rats (6 months old) [90]. In congruence with the tibialis anterior muscle and preliminary DIAm studies in spa mice, [83, 84, 85] old rats display increased failure of neuromuscular transmission upon initial stimulation and greater failure of neuromuscular transmission following repetitive phrenic nerve stimulation [91]. Old rats also showed a 22% reduction in the apposition of pre-synaptic terminals at the post-synaptic endplate of neuromuscular junctions innervating type IIx and/or IIb DIAm fibers compared to type I and IIa fibers [91]. This suggests that in old rats the loss of larger phrenic motor neurons results in denervation of type IIx and/or IIb fibers. In contrast, in spa mice, the extent of apposition of pre- and post-synaptic elements of neuromuscular junctions was not different in type IIx and/or IIb DIAm fibers when compared to wild type. Thus, the loss of phrenic motor neurons may occur much earlier during the perinatal period before the emergence of type IIx and/or IIb fibers (Fig. 2). In the DIAm of older rats, the increased susceptibility to neuromuscular transmission failure may also reflect increased axonal branching resulting in failure at branch points, and also a disturbance at the neuromuscular junction. Despite the loss of phrenic motor neurons and impairment in neuromuscular transmission, the
5.Conclusions
Children with CP, regardless of the severity of their locomotor symptoms, have impairments in respiratory function due to impaired DIAm pressure generation that continue into adulthood. In an animal model of CP symptoms, there are fewer large phrenic motor neurons which comprise more fatigable DIAm motor units, and are required for airway clearance behaviors such as coughing and sneezing. In addition, neuromuscular transmission at these more fatigable motor units is impaired further limiting the performance of these more forceful behaviors of the DIAm. Thus, this animal work provides insight to the pathophysiology of impaired respiratory function in individuals with CP. Interestingly, the pattern of a loss of larger motor neurons in concert with reduced effectiveness of neuromuscular transmission in the spa mouse model of CP symptoms has a remarkable resemblance to observations with aging. Advanced age and pre-existing respiratory comorbidities are significant risk factors for respiratory complications from COVID-19. Thus, if an individual with CP contracts COVID-19 and develops respiratory symptoms, they should be counseled that they could be at higher risk of progression to severe respiratory symptoms. In order to reduce the risk of contracting COVID-19, we need to be vigilant in encouraging and supporting families of children with CP and individuals who have CP to practice physical distancing, good hand hygiene, minimizing trips to public places such as stores and restaurants, and thorough cleaning of any equipment that is used outside the home (i.e. walkers and wheelchairs).
Acknowledgments
This work was supported by National Institutes of Health grants R01-AG044615 (GCS), R01-HL146114 (GCS), an Australian National Health & Medical Research Council CJ Martin Early Career Fellowship (MJF), and Mr. and Mrs. Richard and Rosemary Crandall (JEB).
Conflict of interest
The authors have no conflicts of interest to report.
Abbreviations
| CP | Cerebral Palsy |
|---|---|
| DIAm | diaphragm muscle |
| type FF | fast fatigable |
| type FInt | fast fatigue-intermediate |
| type FR | fatigue resistant fast |
| type S | fatigue resistant slow |
| FEV | forced expiratory volume at 1 second |
| FVC | forced vital capacity |
| GMFCS | Gross Motor Function Classification System |
| MEP | maximum expiratory pressure |
|---|---|
| MIP | maximum inspiratory pressure |
| MUNIX | motor unit number index |
| PEF | peak expiratory flow |
| PICU | pediatric intensive care unit |
| PFT | pulmonary function testing |
| SVC | slow volume capacity |
| TV | tidal volume |
| P | trans-diaphragmatic pressure |
References
[1] | Duruflé-Tapin A, Colin A, Nicolas B, Lebreton C, Dauvergne F, Gallien P. Analysis of the medical causes of death in cerebral palsy. Ann Phys Rehabil Med. (2014) Feb; 57: (1): 24-37. doi: 10.1016/j.rehab.2013.11.002. |
[2] | Hutton JL. Cerebral palsy life expectancy. Clin Perinatol. (2006) Jun; 33: (2): 545-55. doi: 10.1016/j.clp.2006.03.016. |
[3] | Maudsley G, Hutton JL, Pharoah PO. Cause of death in cerebral palsy: a descriptive study. Arch Dis Child. (1999) Nov; 81: (5): 390-4. doi: 10.1136/adc.81.5.390. |
[4] | Pilla M, Langlois NEI, Byard RW. Causes of death in a series of decedents with cerebral palsy in a medicolegal context. Aust J Forensic Sci. (2018) ; 50: (4): 428-34. |
[5] | Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. (2007) Feb; 109: : 8-14. |
[6] | Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. (2005) Mar 94: (3): 287-94. doi: 10.1111/j.1651-2227.2005.tb03071.x. |
[7] | Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. (2017) Sep 1; 171: (9): 897-907. doi: 10.1001/jamapediatrics.2017.1689. |
[8] | Schiariti V, Fowler E, Brandenburg JE, Levey E, Mcintyre S, Sukal-Moulton T, et al. A common data language for clinical research studies: the National Institute of Neurological Disorders and Stroke and American Academy for Cerebral Palsy and Developmental Medicine Cerebral Palsy Common Data Elements Version 10. recommendations. Dev Med Child Neurol. (2018) Oct; 60: (10): 976-986. doi: 10.1111/dmcn.13723. |
[9] | Christensen D, Van Naarden Braun K, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning – Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol. (2014) Jan; 56: (1): 59-65. doi: 10.1111/dmcn.12268. |
[10] | Shevell M, Dagenais L, Oskoui M. The epidemiology of cerebral palsy: new perspectives from a Canadian registry. Semin Pediatr Neurol. (2013) Jun; 20: (2): 60-4. doi: 10.1016/j.spen.2013.06.008. |
[11] | Fogarty MJ, Sieck GC. Evolution and Functional Differentiation of the Diaphragm Muscle of Mammals. Compr Physiol. (2019) Mar 14; 9: (2): 715-766. doi: 10.1002/cphy.c180012. |
[12] | Fogarty MJ, Mantilla CB, Sieck GC. Breathing: Motor Control of Diaphragm Muscle. Physiology (Bethesda). (2018) Mar 1; 33: (2): 113-126. doi: 10.1152/physiol.00002.2018. |
[13] | Greising SM, Mantilla CB, Sieck GC. Functional Measurement of Respiratory Muscle Motor Behaviors Using Transdiaphragmatic Pressure. Methods Mol Biol. (2016) ; 1460: : 309-19. doi: 10.1007/978-1-4939-3810-0_21. |
[14] | Greising SM, Mantilla CB, Medina-Martínez JS, Stowe JM, Sieck GC. Functional impact of diaphragm muscle sarcopenia in both male and female mice. Am J Physiol Lung Cell Mol Physiol. (2015) Jul 1; 309: (1): L46-52. doi: 10.1152/ajplung.00064.2015. |
[15] | Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol. (2011) Oct 15; 179: (1): 57-63. doi: 10.1016/j.resp.2011.06.028. |
[16] | Reddihough DS, Baikie G, Walstab JE. Cerebral palsy in Victoria, Australia: mortality and causes of death. J Paediatr Child Health. (2001) Apr; 37: (2): 183-6. doi: 10.1046/j.1440-1754.2001.00644.x. |
[17] | Ryan JM, Peterson MD, Matthews A, Ryan N, Smith KJ, O’Connell NE, et al. Noncommunicable disease among adults with cerebral palsy: A matched cohort study. Neurology. (2019) Oct 1; 93: (14): e1385-e1396. doi: 10.1212/WNL.0000000000008199. |
[18] | Ryan JM, Peterson MD, Ryan N, Smith KJ, O’connell NE, Liverani S, et al. Mortality due to cardiovascular disease, respiratory disease, and cancer in adults with cerebral palsy. Dev Med Child Neurol. (2019) Aug; 61: (8): 924-928. doi: 10.1111/dmcn.14176. |
[19] | Pitts T, Gestreau C, Rose MJ, Davenport PW, Morris KF, Bolser DC. The mechanical advantage of negative intra-thoracic pressure during swallow. The FASEB Journal. (2013) ; 27: (1_supplement): 93014. |
[20] | Pitts T, Poliacek I, Rose MJ, Reed MD, Condrey JA, Tsai H-W, et al. Neurons in the dorsomedial medulla contribute to swallow pattern generation: Evidence of inspiratory activity during swallow. PLoS One. (2018) Jul 19; 13: (7): e0199903. doi: 10.1371/journal.pone.0199903. |
[21] | Reed MD, English M, English C, Huff A, Poliacek I, Musselwhite MN, et al. The Role of the Cerebellum in Control of Swallow: Evidence of Inspiratory Activity During Swallow. Lung. (2019) Apr; 197: (2): 235-240. doi: 10.1007/s00408-018-00192-2. |
[22] | World Health Organization. WHO director-general’s opening remarks at the media briefing on COVID 19-11 March 2020 2020. Available from: https//www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19–11-march-2020. |
[23] | Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. (2020) Jun; 10: (6): 537-540. doi: 10.1542/hpeds.2020-0123. |
[24] | Vetter P, Vu DL, L’Huillier AG, Schibler M, Kaiser L, Jacquerioz F. Clinical features of COVID-19. BMJ. (2020) Apr 17; 369: : m1470. doi: 10.1136/bmj.m1470. |
[25] | Wu JT, Leung K, Bushman M, Kishore N, Niehus R, de Salazar PM, et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. (2020) Apr; 26: (4): 506-510. doi: 10.1038/s41591-020-0822-7. |
[26] | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. (2020) Apr 7; 323: (13): 1239-1242. doi: 10.1001/jama.2020.2648. |
[27] | Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. (2020) Jun; 145: (6): e20200702. doi: 10.1542/peds.2020-0702. |
[28] | Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. (2020) May; 39: (5): 355-368. doi: 10.1097/INF.0000000000002660. |
[29] | Wang XF, Yuan J, Zheng YJ, Chen J, Bao YM, Wang YR, et al. [Retracted: Clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen]. Zhonghua Er Ke Za Zhi. (2020) Feb 17; 58: : E008. doi: 10.3760/cma.j.issn.0578-1310.2020.0008. |
[30] | Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. (2020) May 11. doi: 10.1001/jamapediatrics.2020.1948. |
[31] | Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Arch Dis Child. (2003) Jan; 88: (1): 75-8. doi: 10.1136/adc.88.1.75. |
[32] | Proesmans M. Respiratory illness in children with disability: a serious problem? Breathe (Sheff). (2016) Dec; 12: (4): e97-e103. doi: 10.1183/20734735.017416. |
[33] | Boel L, Pernet K, Toussaint M, Ides K, Leemans G, Haan J, et al. Respiratory morbidity in children with cerebral palsy: an overview. Dev Med Child Neurol. (2019) Jun; 61: (6): 646-653. doi: 10.1111/dmcn.14060. |
[34] | Pruitt DW, Tsai T. Common medical comorbidities associated with cerebral palsy. Phys Med Rehabil Clin N Am. (2009) Aug; 20: (3): 453-67. doi: 10.1016/j.pmr.2009.06.002. |
[35] | Arora S, Flower O, Murray NPS, Lee BB. Respiratory care of patients with cervical spinal cord injury: a review. Crit Care Resusc. (2012) Mar; 14: (1): 64-73. |
[36] | Tollefsen E, Fondenes O. Respiratory complications associated with spinal cord injury. Tidsskr Nor Laegeforen. (2012) May 15; 132: (9): 1111-4. doi: 10.4045/tidsskr.10.0922. |
[37] | Fogarty MJ, Sieck GC. Spinal cord injury and diaphragm neuromotor control. Expert Rev Respir Med. (2020) May; 14: (5): 453-464. doi: 10.1080/17476348.2020.1732822. |
[38] | Kwon H-Y. Comparison of differences in respiratory function and pressure as a predominant abnormal movement of children with cerebral palsy. J Phys Ther Sci. (2017) Feb; 29: (2): 261-265. doi: 10.1589/jpts.29.261. |
[39] | Kwon YH, Lee HY. Differences of respiratory function in children with spastic diplegic and hemiplegic cerebral palsy, compared with normally developed children. J Pediatr Rehabil Med. (2013) ; 6: (2): 113-7. doi: 10.3233/PRM-130246. |
[40] | Kwon YH, Lee HY. Differences of the Truncal Expansion and Respiratory Function between Children with Spastic Diplegic and Hemiplegic Cerebral Palsy. J Phys Ther Sci. (2013) Dec; 25: (12): 1633-5. doi: 10.1589/jpts.25.1633. |
[41] | Kwon YH, Lee HY. Differences of Respiratory Function According to Level of the Gross Motor Function Classification System in Children with Cerebral Palsy. J Phys Ther Sci. (2014) Mar; 26: (3): 389-91. doi: 10.1589/jpts.26.389. |
[42] | McPherson KA, Kenny DJ, Koheil R, Bablich K, Sochaniwskyj A, Milner M. Ventilation and swallowing interactions of normal children and children with cerebral palsy. Dev Med Child Neurol. (1992) Jul; 34: (7): 577-88. doi: 10.1111/j.1469-8749.1992.tb11488.x. |
[43] | Greising SM, Sieck DC, Sieck GC, Mantilla CB. Novel method for transdiaphragmatic pressure measurements in mice. Respir Physiol Neurobiol. (2013) Aug 1; 188: (1): 56-9. doi: 10.1016/j.resp.2013.04.018. |
[44] | Medina-Martínez JS, Greising SM, Sieck GC, Mantilla CB. Semi-automated assessment of transdiaphragmatic pressure variability across motor behaviors. Respir Physiol Neurobiol. (2015) Aug 15; 215: : 73-81. doi: 10.1016/j.resp.2015.05.009. |
[45] | Khurram OU, Fogarty MJ, Rana S, Vang P, Sieck GC, Mantilla CB. Diaphragm muscle function following midcervical contusion injury in rats. J Appl Physiol (1985). (2019) Jan 1; 126: (1): 221-230. doi: 10.1152/japplphysiol.00481.2018. |
[46] | Khurram OU, Fogarty MJ, Sarrafian TL, Bhatt A, Mantilla CB, Sieck GC. Impact of aging on diaphragm muscle function in male and female Fischer 344 rats. Physiol Rep. (2018) Jul; 6: (13): e13786. doi: 10.14814/phy2.13786. |
[47] | Evans JA, Whitelaw WA. The Assessment of Maximal Respiratory Mouth Pressures In Adults. Respir Care. (2009) Oct; 54: (10): 1348-59. |
[48] | Gayraud J, Ramonatxo M, Rivier F, Humberclaude V, Petrof B, Matecki S. Ventilatory parameters and maximal respiratory pressure changes with age in Duchenne muscular dystrophy patients. Pediatr Pulmonol. (2010) Jun; 45: (6): 552-9. doi: 10.1002/ppul.21204. |
[49] | Fogarty MJ, Sieck GC. Diaphragm muscle adaptations in health and disease. Drug Discov Today Dis Models. Summer (2019) ; 29-30: : 43-52. doi: 10.1016/j.ddmod.2019.10.002. |
[50] | Wang H-Y, Chen C-C, Hsiao S-F. Relationships between respiratory muscle strength and daily living function in children with cerebral palsy. Res Dev Disabil. Jul-Aug (2012) ; 33: (4): 1176-82. doi: 10.1016/j.ridd.2012.02.004. |
[51] | Keles MN, Elbasan B, Apaydin U, Aribas Z, Bakirtas A, Kokturk N. Effects of inspiratory muscle training in children with cerebral palsy: a randomized controlled trial. Braz J Phys Ther. Nov-Dec (2018) ; 22: (6): 493-501. doi: 10.1016/j.bjpt.2018.03.010. |
[52] | Gay PC, Westbrook PR, Daube JR, Litchy WJ, Windebank AJ, Iverson R. Effects of Alterations in Pulmonary Function and Sleep Variables on Survival in Patients With Amyotrophic Lateral Sclerosis. Mayo Clin Proc. (1991) Jul; 66: (7): 686-94. doi: 10.1016/s0025-6196(12)62080-1. |
[53] | Schmidt EP, Drachman DB, Wiener CM, Clawson L, Kimball R, Lechtzin N. Pulmonary predictors of survival in amyotrophic lateral sclerosis: use in clinical trial design. Muscle Nerve. (2006) Jan; 33: (1): 127-32. doi: 10.1002/mus.20450. |
[54] | Bennett S, Siritaratiwat W, Tanrangka N, Bennett MJ, Kanpittaya J. Diaphragmatic mobility in children with spastic cerebral palsy and differing motor performance levels. Respir Physiol Neurobiol. (2019) Aug; 266: : 163-170. doi: 10.1016/j.resp.2019.05.010. |
[55] | Hårdemark Cedborg AI, Sundman E, Bodén K, Hedström HW, Kuylenstierna R, Ekberg O, et al. Co-ordination of spontaneous swallowing with respiratory airflow and diaphragmatic and abdominal muscle activity in healthy adult humans. (2009) Apr; 94: (4): 459-68. doi: 10.1113/expphysiol.2008.045724. |
[56] | Tomori Z, Widdicombe JG. Muscular, bronchomotor and cardiovascular reflexes elicited by mechanical stimulation of the respiratory tract. J Physiol. (1969) Jan; 200: (1): 25-49. doi: 10.1113/jphysiol.1969.sp008680. |
[57] | Dennis MJ, Ziskind-Conhaim L, Harris AJ. Development of neuromuscular junctions in rat embryos. Dev Biol. (1981) Jan 30; 81: (2): 266-79. doi: 10.1016/0012-1606(81)90290-6. |
[58] | Harris AJ. Embryonic growth and innervation of rat skeletal muscles. I. Neural regulation of muscle fibre numbers. Philos Trans R Soc Lond B Biol Sci. (1981) Jul 16; 293: (1065): 257-77. doi: 10.1098/rstb.1981.0076. |
[59] | Harris AJ, McCaig CD. Motoneuron death and motor unit size during embryonic development of the rat. J Neurosci. (1984) Jan; 4: (1): 13-24. doi: 10.1523/JNEUROSCI.04-01-00013.1984. |
[60] | Sheard P, McCaig CD, Harris AJ. Critical periods in rat motoneuron development. Dev Biol. (1984) Mar; 102: (1): 21-31. doi: 10.1016/0012-1606(84)90171-4. |
[61] | Rodier PM. Chronology of Neuron Development: Animal Studies and their Clinical Implications. Dev Med Child Neurol. (1980) Aug; 22: (4): 525-45. doi: 10.1111/j.1469-8749.1980.tb04363.x. |
[62] | Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol (1985). (2000) Aug; 89: (2): 563-72. doi: 10.1152/jappl.2000.89.2.563. |
[63] | Prakash YS, Smithson KG, Sieck GC. Growth-related alterations in motor endplates of type-identified diaphragm muscle fibres. J Neurocytol. (1995) Mar; 24: (3): 225-35. doi: 10.1007/BF01181536. |
[64] | Mantilla CB, Fahim MA, Brandenburg JE, Sieck GC. Functional Development of Respiratory Muscles. In: Polin RA, Abman SH, Rowitch DH, Beneithz WE, Fox WW, editors. Fetal and Neonatal Physiology. 5th ed. Philadelphia, PA: Elsevier, (2017) . |
[65] | Little WJ. On the influence of abnormal parturition, difficult labor, premature birth and asphyxia neonatorum on the mental and physical condition of the child, especially in relation to deformities. Transactions of the Obstetrical Society of London. 1862: (3): 293. |
[66] | Brandenburg JE, Fogarty MJ, Sieck GC. A Critical Evaluation of Current Concepts in Cerebral Palsy. Physiology (Bethesda). (2019) May 1; 34: (3): 216-229. doi: 10.1152/physiol.00054.2018. |
[67] | Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci. (2010) ; 33: : 409-40. doi: 10.1146/annurev.neuro.051508.135722. |
[68] | Lin S, Landmann L, Ruegg MA, Brenner HR. The role of nerve- versus muscle-derived factors in mammalian neuromuscular junction formation. J Neurosci. (2008) Mar 26; 28: (13): 3333-40. doi: 10.1523/JNEUROSCI.5590-07.2008. |
[69] | Mantilla CB, Sieck GC. Trophic factor expression in phrenic motor neurons. Respir Physiol Neurobiol. (2008) Dec 10; 164: (1-2): 252-62. doi: 10.1016/j.resp.2008.07.018. |
[70] | Fogarty MJ, Smallcombe KL, Yanagawa Y, Obata K, Bellingham MC, Noakes PG. Genetic deficiency of GABA differentially regulates respiratory and non-respiratory motor neuron development. PLoS One. (2013) ; 8: (2): e56257. doi: 10.1371/journal.pone.0056257. |
[71] | Fogarty MJ, Yanagawa Y, Obata K, Bellingham MC, Noakes PG. Genetic absence of the vesicular inhibitory amino acid transporter differentially regulates respiratory and locomotor motor neuron development. Brain Struct Funct. (2015) Jan; 220: (1): 525-40. doi: 10.1007/s00429-013-0673-9. |
[72] | Mantilla CB, Sieck GC. Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J Appl Physiol (1985). (2008) Jun; 104: (6): 1818-27. doi: 10.1152/japplphysiol.01192.2007. |
[73] | Geiger PC, Bailey JP, Mantilla CB, Zhan W-Z, Sieck GC. Mechanisms underlying myosin heavy chain expression during development of the rat diaphragm muscle. J Appl Physiol (1985). 2006: Dec; 101: (6): 1546-55. doi: 10.1152/japplphysiol.00221.2006. |
[74] | Johnson BD, Wilson LE, Zhan WZ, Watchko JF, Daood MJ, Sieck GC. Contractile properties of the developing diaphragm correlate with myosin heavy chain phenotype. J Appl Physiol (1985). (1994) Jul; 77: (1): 481-7. doi: 10.1152/jappl.1994.77.1.481. |
[75] | Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol (1985). (2000) Aug; 89: (2): 695-703. doi: 10.1152/jappl.2000.89.2.695. |
[76] | Marciniak C, Li X, Zhou P. An examination of motor unit number index in adults with cerebral palsy. J Electromyogr Kinesiol. (2015) Jun; 25: (3): 444-50. doi: 10.1016/j.jelekin.2015.02.007. |
[77] | Fatehi F, Grapperon A-M, Fathi D, Delmont E, Attarian S. The utility of motor unit number index: A systematic review. Neurophysiol Clin. (2018) Oct; 48: (5): 251-259. doi: 10.1016/j.neucli.2018.09.001. |
[78] | Ngo ST, Baumann F, Ridall PG, Pettitt AN, Henderson RD, Bellingham MC, et al. The relationship between Bayesian motor unit number estimation and histological measurements of motor neurons in wild-type and SOD1(G93A) mice. Clin Neurophysiol. (2012) Oct; 123: (10): 2080-91. doi: 10.1016/j.clinph.2012.01.028. |
[79] | Kingsmore SF, Giros B, Suh D, Bieniarz M, Caron MG, Seldin MF. Glycine receptor beta-subunit gene mutation in spastic mouse associated with LINE-1 element insertion. Nat Genet. (1994) Jun; 7: (2): 136-41. doi: 10.1038/ng0694-136. |
[80] | Chai CK. Hereditary Spasticity in Mice. Journal of Heredity. (1961) ; 52: (5): 241-3. |
[81] | Brandenburg JE, Gransee HM, Fogarty MJ, Sieck GC. Differences in Lumbar Motor Neuron Pruning in an Animal Model of Early Onset Spasticity. J Neurophysiol. (2018) Aug 1; 120: (2): 601-609. doi: 10.1152/jn.00186.2018. |
[82] | Brandenburg JE, Fogarty MJ, Brown AD, Sieck GC. Phrenic Motor Neuron Loss in an Animal Model of Early Onset Hypertonia. J Neurophysiol. (2020) May 1; 123: (5): 1682-1690. doi: 10.1152/jn.00026.2020. |
[83] | Fogarty MJ, Sieck GC, Brandenburg JE. Impaired neuromuscular transmission of the tibialis anterior in a rodent model of hypertonia. J Neurophysiol. (2020) May 1; 123: (5): 1864-1869. doi: 10.1152/jn.00095.2020. |
[84] | Brandenburg J, Fogarty M, Sieck G. Evaluation of diaphragm neuromuscular transmission and fatigue in a mouse model of cerebral palsy: TB-SP09. Dev Med Child Neurol. (2019) ; 61: . |
[85] | Brandenburg JE, Fogarty MJ, Sieck GC. Neuromuscular transmission failure in an animal model of early onset spasticity. Society for Neuroscience Annual Meeting. (2018) ; Program #675.11. |
[86] | Sieck GC, Prakash YS. Fatigue at the neuromuscular junction: Branch point vs. presynaptic vs. postsynaptic mechanisms. New York, NY: Plenum Press, (1995) . |
[87] | Fournier M, Alula M, Sieck GC. Neuromuscular transmission failure during postnatal development. Neurosci Lett. (1991) Apr 15; 125: (1): 34-6. doi: 10.1016/0304-3940(91)90124-c. |
[88] | Johnson BD, Sieck GC. Differential susceptibility of diaphragm muscle fibers to neuromuscular transmission failure. J Appl Physiol (1985). 1993: Jul; 75: (1): 341-8. doi: 10.1152/jappl.1993.75.1.341. |
[89] | Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. (1998) Jul; 21: (7): 887-95. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. |
[90] | Fogarty MJ, Omar TS, Zhan W-Z, Mantilla CB, Sieck GC. Phrenic Motor Neuron Loss in Aged Rats. J Neurophysiol. (2018) May 1; 119: (5): 1852-1862. doi: 10.1152/jn.00868.2017. |
[91] | Fogarty MJ, Gonzalez Porras MA, Mantilla CB, Sieck GC. Diaphragm Neuromuscular Transmission Failure in Aged Rats. J Neurophysiol. (2019) Jul 1; 122: (1): 93-104. doi: 10.1152/jn.00061.2019. |
[92] | Fogarty MJ, Mantilla CB, Sieck GC. Impact of sarcopenia on diaphragm muscle fatigue. Exp Physiol. (2019) Jul; 104: (7): 1090-1099. doi: 10.1113/EP087558. |
[93] | Maiolo C, Mohamed EI, Carbonelli MG. Body composition and respiratory function. Acta Diabetol. (2003) Oct; 40: Suppl 1: S32-8. doi: 10.1007/s00592-003-0023-0. |
[94] | DeMuth GR, Howatt WF, Hill BM. I. Lung Volumes. Pediatr. (1965) ; 35: (1): 162-76. |
[95] | Lazarus R, Gore CJ, Booth M, Owen N. Effects of body composition and fat distribution on ventilatory function in adults. Am J Clin Nutr. (1998) Jul; 68: (1): 35-41. doi: 10.1093/ajcn/68.1.35. |
[96] | Hallett S, Ashhurst JV. Tidal Volume. (2020) . In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. Available from: https//www.ncbi.nlm.nih.gov/books/NBK482502/. |
[97] | David S, Sharma S. Vital Capacity. 2019 5/5/2020. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. Available from: https//www.ncbi.nlm.nih.gov/books/NBK541099/. |
[98] | David S, Edwards CW. Forced Expiratory Volume. (2019) . In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. Available from: https//www.ncbi.nlm.nih.gov/books/NBK540970/. |



