Update on bone fragility in spina bifida
Abstract
BACKGROUND CONTEXT:
Patients with spina bifida (SB) are at risk for pathological fractures and low bone mineral density (BMD).
PURPOSE AND METHODS:
This article reviews the literature and provides a comprehensive overview of how the characteristics of SB and its associated comorbidities intersect with bone fragility to identify possible pathophysiological mechanisms of fractures and low BMD.
RESULTS:
Bone fragility occurs early in the life of patients with SB as a result of a disturbance that determines changes in bone shape, quantity, and quality, as poor mineralization reduces bone stiffness. Bone fragility in SB occurs due to local and systemic factors and may be considered a state of impaired bone quality of multifactorial aetiology, with complex interacting influences of neurological, metabolic, and endocrinological origins and the presence of smaller bones. Bone fragility should be evaluated globally according to skeletal age and Tanner staging. The phases of the evolution of Charcot joints seem to intercept the evolution of epiphyseal fractures. Charcot arthropathy in SB may be initiated by the occurrence of repetitive trauma and fractures in epiphyseal and subepiphyseal regions, where there is a deficit of bone mineralization and greater bone mass deficits.
CONCLUSION:
Bone fragility in MMC potentially has a multifactorial neuro-endocrinological-metabolic-renal dimension, with smaller bones, lower bone mass, and mineralization deficits affecting bone strength.
1.Introduction
Acquiring adequate bone mass during childhood is a prerequisite for bone health in adulthood. If a threshold of low bone mass is reached, the presence of osteoporosis is considered. Implicit in the concept of peak bone mass is that osteoporosis, an adult disease, has antecedents in childhood and adolescence; thus, understanding the determinants of bone mass acquisition is an important aspect of its prevention [1, 2, 3]. Furthermore, skeletal adaptations that occur during childhood and adolescence are less reversible than adaptations that occur in adulthood [4]. Prior studies have demonstrated an 11–30% frequency of fractures in paediatric patients with spina bifida (SB) [5, 6, 7, 8, 9, 10, 11, 12], highlighting the importance of evaluating this topic. This narrative review will outline a comprehensive overview of how the characteristics of SB and its associated comorbidities intersect with bone fragility to identify possible pathophysiological mechanisms of fractures and low bone mineral density (BMD). As a primary endpoint, we evaluate factors/disease-specific risk factors associated with the occurrence of fractures, BMD, and bone fragility: paraplegia and alterations of sensitivity, immobility/inactivity, dietary intake of calcium and vitamin D and serum levels of vitamin D, urinary calcium excretion, secondary hyperparathyroidism, chronic renal failure (CRF), metabolic acidosis and urinary diversion surgery/augmentation cystoplasty, endocrinological diseases, metabolic syndrome, as well as anthropometric measures and mechanostat theory. The association between the occurrence of fractures and low levels of BMD is analysed. As a secondary endpoint, markers of bone metabolism were assessed, as well as other issues such as using Charcot arthropathy to determine whether physiopathology intercepts bone fragility. In addition, we attempt to establish an integrated explanation of the causes and mechanisms involved in bone fragility according to the above-cited factors to address the evolution of the incidence of fractures until adulthood in patients with SB and to provide recommendations for the management of bone fragility.
2.Methods
The search strategy of this review can be divided into two phases.
In the first phase, we followed a structured method to establish a PUBMED search in which studies were identified by focusing on domains concerning the incidence and risk factors of fractures and BMD. Bibliographic research was carried out up to 07/04/2018. We considered the review previously published by Marreiros et al. [8]. In the domain titled “incidence and risk of fracture”, we searched using the keywords myelomeningocele (MMC) or SB and fracture. We included only observational studies with more than two patients and studies recruiting children and/or adults and excluded studies with heterogeneous disease samples. To identify an association among BMD and the occurrence of fractures and the risk factors of low BMD, we searched using the keywords meningomyelocele or SB cystica and bone density. Due to the scarcity of publications on this topic, we did not exclude studies with heterogeneous samples. We excluded studies that recruited only adults. We included articles that evaluated BMD and/or bone mineral content (BMC). For heterogeneous samples, studies were included only if they included at least six patients with SB. In both domains, we included additional articles that were not obtained using this search strategy but were known by the author to meet the inclusion criteria. The search was restricted to English articles. Articles were excluded if they were not available in PUBMED or Portuguese libraries.
In the second phase, we also selected other articles that evaluated risk factors with a potential impact on bone health (fractures and BMD) in SB but did not analyse this correlation. Little research has been conducted on this disease, which has various concurrent pathologies that may influence one another with potential health impacts on bone, and there is a lack of research on these comorbidities and their impacts on bone health in SB. Therefore, in this paper we discuss articles focused on the adult and pediatric age group in order to evaluate the relationships between SB (and its comorbidities) and factors impacting bone health.
3.Results
In the literature review, the search focusing on fracture: Spina bifida [MeSH] OR Myelomeningocele [MeSH] AND Fracture [MeSH] yielded 128 articles. Of these, 21 met the inclusion criteria [6, 7, 9, 10, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28]. Although not yielded by the search criteria, two additional articles on this topic were identified and included [5, 11]. Thus, 23 articles about fractures and SB were analysed in detail.
The search focusing on BMD: Meningomyelocele [Mesh] OR Spina Bifida Cystica [Mesh] AND Bone Density [Mesh] yielded 17 articles. Of these, 11 articles met the inclusion criteria [13, 14, 15, 16, 17, 29, 30, 31, 32, 33, 34]. Although not yielded by the search criteria, five additional articles on this topic were identified and included [35, 36, 37, 38, 39]. Thus, 16 articles about BMD and SB were analysed in detail.
The remaining articles were selected in the second phase.
4.Discussion
4.1Risk factors for fractures in SB
Risk factors for the occurrence of fractures in SB include high levels of neurological involvement [6, 7, 9, 10, 18], patients in wheelchairs [6, 7, 13, 28], the presence of hypercalciuria [15], the presence of contractures [18], and high body fat content and body mass index (BMI) values [15]. An association between secondary inactivity due to cast immobilization and/or operative orthopaedic procedures and the occurrence of fractures was detected [6, 7, 8, 9, 10, 11, 12, 19, 20, 40]. The occurrence of a spontaneous first fracture increases the risk of a second fracture [7].
4.2BMD assessment
Bone densitometry has been the technique of choice for the evaluation of BMD in studies involving patients with MMC. However, the available studies have used either single-energy beam absorptiometry [13, 36, 37] or dual-energy beam absorptiometry (DXA) [14, 29, 31, 33, 35] as well as different types of apparatuses, making it difficult to compare the results.
4.2.1Evaluation of BMD in SB – general concepts
A decrease in BMD is correlated with an increase in the neurological level of the lesion [17, 35, 36, 41] and with lower ambulatory ability [14, 33, 36, 41].
Reported mean BMD values for all sites examined in the lower extremities, lumbar region and forearms were approximately 1 to 3 standard deviation (SD) scores below the mean of age- and gender-matched individuals with typical development (TD) [13, 14, 15, 16, 33, 34, 35, 39, 41]. The selected studies had at least one mean Z-score with an SD between
4.3Low BMD (local vs. systemic factors and pattern)
4.3.1Local pattern (below the neurological level of injury)
Several studies have used DXA to measure BMD in the lower extremities of children with SB [42]. Research using DXA scans has documented that children with SB are predisposed to a lower BMD in the distal femoral region than their typically developing peers [16, 41] as well as a lower BMD in the femoral neck [14, 17, 29, 32]; this predisposition is especially pronounced in children who are non-ambulatory [16, 41]. There are local factors involved due to the deficit of muscular strength caused by neurological injury that compromise BMD and increase the risk of occurrence of complications such as extremity fractures below the neurological level of injury [15]. Since Frost’s introduction of the concept of the “mechanostat”, it has been accepted that bone mass and architecture are regulated in response to the local strains engendered by functional loading [43, 44].
4.3.2Systemic patterns
Several authors have reported BMD in the distal radius, a site with a lower frequency of SB fractures [13, 36]. Quan et al. [13]assessed BMD in the distal radius in patients with MMC with an average age of 11.1
4.4Factors that interfere with bone mass
The regulatory influences that determine bone mass can be divided into several categories: 1) genetic; 2) local, referring to stress and strain as a result of mechanical loads; 3) neuroendocrine/humoural; and 4) neural [46, 47]. Recognizing the presence of other comorbidities/conditions with a possible impact on bone health in patients with MMC, we considered the following additional factors: diet, environment, chronic kidney disease (CKD), acidosis due to urinary diversion/augmentation cystoplasty, and other factors.
4.4.1Endocrine factors
4.4.1.1. Hormonal factors
Hydrocephalus and associated central nervous system anomalies are present in 80 to 90% of patients and are assessed as risk factors for endocrine disorders in MMC as a result of hypothalamic-pituitary dysfunction. In particular, growth hormone (GH) deficiency has been documented in SB [48, 49]. Certain studies have found that adults with childhood-onset GH deficiency (GHD) also have a lower BMD than healthy controls [50]. In other studies, premature adrenarche and central precocious puberty were identified in SB [51, 52, 53, 54, 55, 56]. Thus, there are factors that occur in this pathology that may contribute to an apparently high BMD for chronological age (CA), such as the occurrence of precocious puberty [16], but show no difference if adjusted for bone age (BA) [28].
It is well recognized that bone loss accelerates in hypogonadal states [57]. The presence of hypogonadism has been detected in patients with MMC [28]. However, this condition may not have a considerable association with SB; in fact, Decter et al. [58] demonstrated that men with SB have normally distributed serum testosterone levels despite the possibility of a hypothalamic-pituitary-gonadal axis abnormality secondary to congenital hydrocephalus.
4.4.1.2. Obesity and metabolic syndrome
In an observational cross-sectional study that included children with MMC and healthy children, DXA measurements of the percentage of fat in the trunk, arms, legs, and whole body were performed. Children with MMC had higher than normal total body fat and leg fat in comparison to healthy children, but only children with higher-level lesions exhibited increased trunk fat [59]. In a cross-sectional study, Shepherd et al. [60] evaluated body composition and measures of obesity in 59 subjects with MMC aged 0.3–29 years via anthropometry, measures of body cell mass (BCM), and intra- and extracellular water (ICW and ECW). After 3–4 years of age, children with MMC demonstrated low levels of total body potassium (TBK), BCM, and total body water; anomalous water distribution (increased ECW and decreased ICW); and elevated percentages of body fat for those of their age and weight. These findings were more pronounced in females and in those with high-level lesions. It appears that during infancy and early childhood, children with MMC have a body composition similar to that of TD children, but after the age of four years BCM and lean mass fail to grow normally and the relative deficit in lean tissue becomes compensated for by increased adipose tissue [60].
Mita et al. [61] showed that the percentage of body fat in patients with SB younger than five years of age was similar to that in age-matched normal children; however, 58% of patients older than six years had an increased percentage of body fat.
In a retrospective cross-sectional study including 30 patients with MMC aged 6–17 years, the incidence of fractures was correlated significantly with BMI and body fat content. Since fractures had no association with activity, locomotion, clinical picture, or level/anatomical location of the lesion, the authors hypothesized that increased fat deposition and excessive body mass may contribute to the pathomechanism of bone fragility in children with MMC [15]. Since children with higher-level lesions have increased trunk fat, these findings may be suggestive of a greater risk of developing obesity-related diseases [59]. A prospective cross-sectional study of 28 children with MMC and 58 controls without physical disability, aged six to 13 years, revealed a higher percentage of total body and trunk fat in the children with MMC (particularly those with higher-level lesions) than in the controls even though the two groups had similar BMI values. In addition to obesity, the evaluation of metabolic syndrome components showed that children with MMC tended to have higher triglycerides and lower high-density lipoproteins (HDL) than controls; 15% of the MMC group was classified as having metabolic syndrome. Considering that lumbar involvement was associated with abnormalities in the lipid profile, bone metabolism markers, and metabolic syndrome markers, whereas youths with sacral levels had values similar to those of healthy controls, the degree of these abnormalities may be associated with neurosegmental level [62]. In a cross-sectional investigation, Pollock et al. [3] compared bone mass among overweight adolescents aged 14–18 years with and without cardiometabolic risk factors (CMRs). BMC was reduced by 5.4% in adolescents that had at least one component of CMR compared with that in those without any risk factors and by 6.3% when two or more CMRs were present. When examining groups of overweight adolescents together, Pollock et al. [3] found that the visceral fat area was inversely associated with whole-body BMC, but there was no association between the total body fat mass or subcutaneous fat area and whole-body BMC. Thus, the adiposity phenotype, which is evaluated based on visceral versus subcutaneous fat, appears to affect the relationship between adiposity and bone mass [3, 63].
Recognizing the relevance of the higher percentage of total body and trunk fat in MMC in children with higher-level lesions [59, 62], it is expected that some observations identified in the general population with obesity but without neural tube defects could occur in MMC with implications for bone mass. Overall, the aforementioned findings suggested the presence of inherent factors in SB that emerge and change with age, such as an adiposity phenotype, which could affect bone health.
4.4.2Nervous system
Individuals with MMC often exhibit motor and sensory neurological deficits below the level of the lesion [64]. A well-recognized effect of neurologic disease is the loss of bone in the regions affected by paresis or paralysis [46]. In addition to the consequences of motoneuron injury in SB with regard to bone health, other potential causes of low bone mass in SB include the loss of sympathetic and sensory innervation of bone [28]. Furthermore, the link between leptin signalling in the hypothalamus and the sympathetic regulation of bone turnover is recognized [65]. However, the impact of hypothalamic dysregulation due to complex CNS anomalies in MMC [66] can interfere with the pattern of leptin secretion in MMC.
4.4.3Chronic kidney disease
Congenital and acquired CKD may lead to a disordered regulation of mineral metabolism with subsequent alterations of bone remodelling and growth [67]. The term renal osteodystrophy (ROD) refers to a large spectrum of abnormalities of skeletal homeostasis related to CRF [67], including osteitis fibrosa, osteomalacia, mixed lesions, adynamic bone disease, and osteoporosis [68].
In a five-year retrospective observational study of medical data from 54 children with MMC, renal parenchyma damage progression in renal scintigraphy was noted in 12 children (22.2%), and the deterioration of estimated glomerular filtration rate (eGFR) values was noted in 11 (30.4%) patients, of whom six (11.1%) children qualified for higher CKD stages after follow-up [69]. Additionally, the data obtained by Norman et al. [70] from children aged two to 18 years with renal disease showed the occurrence of ROD early in the course of CRF with glomerular filtration rate (GFR) values of 45 to 50 mL/minute/1.73 m
4.4.4Intestinal urinary diversion/augmentation cystoplasty – acidosis and impact on BMD
Children with MMC are at risk for acidosis from various sources, including nutritional aspects contributing to metabolic changes that may be related to dysphagia (associated with Arnold-Chiari malformation), chronic wound healing and/or chronic infection, pulmonary insufficiency due to restrictive lung disease (associated with severe scoliosis or kyphosis), and/or GFR of less than 30% [71, 72].
Previous studies have supported the premise that intestinal urinary diversion/enterocystoplasty may adversely affect bone mineralization [29, 73, 74]. Koch et al. [29] evaluated 93 patients with MMC, 30 of whom underwent urinary diversion (ileal or colonic), while 63 were treated with intermittent catheterization. In this study, bone demineralization was detected in DXA in patients treated with urinary diversion, but there was no significant difference from controls treated with intermittent catheterization. There was a higher incidence of intermittent metabolic acidosis among patients with urinary diversion than among those who used urinary catheterization [29].
Haas et al. [41] used a cross-sectional observational study to assess the BMD of the lateral distal femur of children with congenital spinal dysfunction. Forty-four children aged six to 18 years with congenital spinal dysfunction (35 with MMC) were enrolled in the study. The authors did not identify a correlation between augmentation cystoplasty and BMD of the distal femur [41].
Adams et al. [71] retrospectively evaluated a cohort of paediatric patients with MMC and bladder augmentation enteroplasty. The analysis included 71 children with a history of ileal or colonic enteroplasty. No statistically significant differences were found between the preoperative and postoperative laboratory values, including pH, HCO
In an observational study, Mingin et al. [30] evaluated 22 patients with MMC and 11 with bladder exstrophy to examine changes in calcium metabolism, height, bone chemistry, and BMD. Children with MMC who were and were not augmented were compared to individuals of the same sex, age, ambulatory status, and similar level of the lesion as well as to those without MMC. In the augmented MMC group, there were 13 patients: seven patients with a mean age of 13 years underwent ileal augmentation and six patients with a mean age of 13 years had gastric augmentation. A significant difference was detected in serum bicarbonate and chloride levels between ileal and non-augmented MMC patients. The bicarbonate levels were significantly lower in six out of seven patients in the ileal augmented SB group. This finding combined with a lower pH and elevated chloride is significant for metabolic acidosis. There were no other significant differences in percentiles for height, laboratory studies, or BMD measurements among the groups (SB control, SB gastric, SB ileal, and normal) [30].
Boylu et al. [32] evaluated BMD after ileal augmentation cystoplasty in eight patients with neurogenic bladder with MMC and seven patients with non-neurogenic bladder with a mean age of 10.2
The study reported by Taskinen et al. [38] investigated 54 consecutive patients with bladder augmentation, of whom 42 belonged to the neurogenic group (including 33 patients with MMC), and 12 belonged to the non-neurogenic group and presented with bladder exstrophy. The median age of the patients was 12.6 (5.8–32.5) years at the time of augmentation. Forty-five patients (83%) had ileocystoplasty, six had ileocoecal cystoplasty, and three had sigmacystoplasty. Six (11%) patients had acidosis, but it is not clear whether they had a diagnosis of MMC. Of the 54 patients, 34 (63%) had a reduced areal BMD, 20 (37%) had mild osteopaenia, and 14 (26%) had severe osteopaenia. Low areal BMDs were more common in the neurogenic than in the non-neurogenic group [38].
The results of these studies are not directly comparable because the studies used different designs and methodologies and had non-overlapping objectives. Thus, the results of the presented studies do not allow the establishment of a definitive causal link between metabolic acidosis after urinary diversion or bladder augmentation and a decrease in BMD in SB.
4.4.5Calcium and vitamin D deficiencies - environmental and dietary factors
Children with SB may have unique predisposing factors to vitamin D deficiency, such as immobility and consequent decreased sun exposure, dietary limitations, and CKD [35, 40, 62, 69, 75, 76, 77, 78]. Dietary and metabolic factors include the intake of vitamin-D-rich foods and the association between obesity and 25-OHD levels, respectively [62, 79, 80]. To our knowledge, only three studies have applied a food frequency questionnaire to determine calcium intake [14, 17, 37] Theoretically, the absorption of fat-soluble vitamins, including vitamin D, may be impaired after ileocystoplasty. However, in the study by Taskinen et al. [38], approximately 42% of patients had moderate to severe vitamin D deficiency. The prevalence of vitamin D deficiency did not differ from that in the general population [38, 81, 82].
4.4.6Other factors from an anthropometric perspective: Can smaller bones explain bone fragility?
Wald et al. [83] evaluated 189 singleton infants with SB cystica and showed that the mean birth weight was statistically significantly lower than the mean birth weight of 3,816 singleton infants without neural tube defects. Most, if not all, of this difference in birth weight was due to the low weight for gestational age of the SB infants [83]. In an observational, cross-sectional, controlled study with 114 patients with shunted hydrocephalus (17 patients with SB) and 73 healthy subjects, aged five to 20 years, Lopponen et al. [84] described the growth pattern of hydrocephalic children. Patients with SB were already shorter than control subjects at birth. However, in a retrospective study with 109 patients with MMC aged 3.2–21.0 years, Trollmann et al. [53] showed that length at birth generally ranged within expected values for the age group, independent of the level of the lesion [53]. An inspection of the means of leg lengths and shank circumferences suggested that infants born with MMC and age-matched infants with TD showed very similar values at one month. In contrast, at 12 months, infants born with MMC exhibited shorter leg lengths and smaller shank circumferences than those with TD [85]. Studies have documented that children with MMC have a short stature [17, 30, 33, 37, 53, 54, 86, 87, 88], particularly those with high lesion neurological levels [87, 89], with significantly lower values for height in immobilized than in mobilized patients [17, 33] compared with the values for controls [17, 62, 90], and they tended to weigh less than other children of the same age [54, 59, 86]. However, the comparison of weight with height suggested that children with MMC were relatively obese [54, 86].
Recognizing that patients with MMC are shorter, a hypothesis can be posed regarding whether smaller bones can contribute to bone fragility.
4.5Implications for bone age
The study of Lopponen et al. [84] cited above found a retarded linear growth in prepuberty in hydrocephalic children and an earlier adolescent growth spurt, leading to a decreased final height and increasing obesity during adolescence. All these characteristics were even more pronounced in hydrocephalic children with SB compared to the other patients with hydrocephalus [84]. The reduced GH secretion during prepuberty is likely to contribute to slow growth at that stage, while reduced GH secretion in puberty may result in a suboptimal adolescent growth spurt concomitantly with the acceleration of pubertal maturation, leading to the early fusion of the epiphyses [49, 91]. The relative BA was retarded in prepubertal shunted hydrocephalic patients, and it was accelerated in pubertal patients compared with that in the control subjects [84]. In a retrospective study of 109 patients with MMC aged 3.2 to 21 years, in addition to the advanced development of secondary sex characteristics (central precocious puberty in five girls), a marked acceleration of BA developed upon reaching puberty [53]. Greene et al. [54] documented a retarded BA in MMC patients prior to the age of nine years and an acceleration between the ages of nine and 10 years [53, 54]. In the study reported by Elias et al. [52], four of five among 32 girls younger than 10 years of age with MMC and precocious puberty had an advanced BA by 1.8 to 2.5 years. In the case series by Rotenstein et al. [88], all seven prepubertal patients with neural tube defects, aged 5.4 to 13.1 years, had delays in BA.
4.6Metabolic bone markers
In the study by Van Speybroeck et al. [62], 93% of MMC participants were 25-hydroxy vitamin D [25(OH)D] insufficient or deficient and showed a trend towards higher levels of phosphate, while the control group without MMC had a rate of 78%. Okurowska-Zawada et al. [35] and Baum et al. [75] detected values of 25(OH)D insufficiency and deficiency at 97% and 75% in SB, respectively [35, 75]. The higher fat mass in the MMC group than in the controls in the Van Speybroeck et al. [62] study and the inverse relationship of 25(OH)D levels in Caucasian children to total adiposity, metabolic syndrome, and hypertension [80] may help explain this difference. Martinelli et al. [39] documented significantly lower serum 25(OH)D in patients with SB than in the healthy population, and the patients also presented with hypophosphatemia. Okurowska-Zawada et al. [35] detected the presence of a negative correlation between the serum concentration of 25(OH)D, BMI and osteoporosis and a significant positive correlation between 25(OH)D and phosphorus. Although Martinelli et al. [39] emphasized the need for vitamin D supplementation in all patients with SB, Kafadar et al. [17] showed no differences between MMC patients and age- and gender-matched healthy children in terms of calcium intake, vitamin D prophylaxis, sun exposure, or in serum 25(OH)D levels [17].
Quan et al. [13] found higher levels of urinary calcium excretion in the non-ambulatory MMC patients than that of ambulatory MMC patients. Okurowska-Zawada et al. [15] reported a significant correlation between the incidence of fractures in children with MMC and an increase in 24-hour urinary calcium excretion levels in the presence of normal renal function [15]. However, Kafadar et al. [17] did not detect differences between patients with MMC and controls or between the different levels of injury with respect to urinary Ca
Taskinen et al. [38] reported a significant negative correlation between the serum 25(OH)D level and parathormone (PTH) plasma concentrations, which was consistent with the known effects of vitamin D deficiency on bone loss. However, Okurowska-Zawada et al. [35] did not find a correlation between the serum levels of 25(OH)D and PTH. Additionally, Van Speybroeck et al. [62] showed that children with MMC had lower PTH levels than controls, despite lower calcium, 25(OH)D, and alkaline phosphatase (AP) levels. According to Van Speybroeck et al. [62], the obtained data suggests an alteration in the sensing mechanism or response of the parathyroid gland to normal physiological stimuli in patients with MMC. As AP levels are generally elevated in vitamin D deficiency, the meaning of the lower levels of AP in the MMC group than compared to the controls is not known. Van Speybroeck et al. [62] hypothesized that because they did not measure 1,25 OH vitamin D (1,25(OH)
The cytokine-like hormone leptin, which is secreted by fat cells, is an important candidate molecule for linking changes in body composition with bone formation and bone resorption [65]. Van Speybroeck et al. [62] showed that abnormalities in leptin in patients with MMC compared with controls were greatest in the highest neurosegmental group; thus, the midlumbar and above group had more trunk fat and significantly higher values for leptin than did controls [62]. In a controlled cross-sectional study to examine spontaneous leptin secretion in patients with MMC and GHD, serum leptin levels were studied in 10 prepubertal MMC patients with GHD (CA 6.21
In an observational cross-sectional study of 33 patients aged four to 17 years, nine (27.3%) were obese, while seven had a thoracolumbar level and seven had a midlumbar level of neurological involvement. Okurowska-Zawada et al. [35] showed that almost all patients had osteocalcin above-normal limits. As we will describe below, some markers such as osteocalcin are cleared by the kidneys; thus, as the presence of renal injury in patients with SB in the Okurowska-Zawada et al. [35] study is not described, the observed values may be overestimated [35, 93].
In the study by Kafadar et al. [17], significant changes were detected in the urinary excretion of deoxypyridinoline in patients with sacral and thoracic-level MMC compared with that in healthy controls. In contrast, no changes were identified in the parameters of bone formation (osteocalcin, AP and vitamin D) [17]. Quan et al. [13] detected higher urinary pyridinoline levels in patients with MMC with gait impairment. These data suggest that BMD reduction in MMC patients is associated with accelerated bone resorption and consequently with factors related to osteoporosis rather than factors related to bone formation [17].
The difficulty associated with evaluating bone metabolism markers in different studies in paediatric MMC patients arises from the complexity of the different study designs and samples with distinct age ranges. Furthermore, bone turnover markers are largely a reflection of linear growth and not bone turnover per se [94]. In BMD research in SB, studies have evaluated 25(OH)D [35, 39, 62, 75], 1,25(OH)
Considering the frequency of renal injury in SB, that bone turnover markers and reference ranges in patients with CKD do not exist and that some markers are cleared by the kidney (osteocalcin, procollagen type I amino propeptide monomer, and collagen type 1 cross-linked C-telopeptide), their application in ROD diagnostics is challenging [93]. In addition, data in adult patients with spinal cord injury demonstrate that serum creatinine is often significantly decreased as a result of disuse muscle atrophy, which commonly accompanies muscle denervation [95, 96]. Studies of MMC use serum creatinine to measure renal function, which may underestimate the presence of renal failure due to the deficit in lean tissue [60, 69, 78], and the consequent effects on the clearance of the parameters of bone metabolism may not be evaluated in light of lower renal function.
4.7Aetiopathogenesis of fractures in MMC
4.7.1Site of fracture
Ralis et al. [97] studied 17 dissected tibiae from infants with SB who died with paralysis and foot deformities (at ages between 32 foetal weeks and 10 postnatal weeks) and in 14 tibiae from non-SB controls (12th foetal week to 13th postnatal week) to evaluate changes in the cross-sectional shape, size, bone and amount of unmineralized osteoid tissue. In addition, 12 tibiae from young experimental rats with myotomy of the foot dorsiflexors and foot plantiflexors were studied to determine the role of experimental muscle imbalance in the dynamic remodelling of the developing long bones. The study detected an atrophic cortex, with a decrease in both the number of the Haversian system and remodelling cavities as well as an increase in the quantity of bone osteoid in the SB patients. Delayed mineralization of the newly laid bone matrix would lead to the softening of the new bone matrix and osteoid-rich subepiphyseal and metaphyseal regions. Since fractures in SB may occur in these regions, the discovery of paralytic rickets may help explain their cause due to diminished mechanical bone properties, and its occurrence early in life may point to an underdiagnosed type of hereditary rickets. In an observational case series, Edvardsen [98] demonstrated that the growth plates and epiphyses of the lower extremities of children with MMC could be severely traumatized during daily walking activities as well as by passive joint movements. Roberts et al. [99] suggested that repetitive microtrauma in non-sensitive limbs could produce micromovements in the zone of transformation of the cartilage, compromise the calcification process, and lead to the widening of the proximal non-calcified portion of the physis [99]. Failure to use orthoses for walking in patients requiring these aids constitutes a risk factor with regard to epiphysiolysis [100, 101]. Kumar et al. [21] reported that epiphyseal fractures occur most often in patients in a low lumbar ambulatory group, but they can occur at any level of motor paraplegia [21, 101]. James showed that due to the fragility of the bone, the insertions of the gastrocnemius and of the hamstrings around the knee may cause the displacement of the epiphyses when these muscles are spastic and the knee joint is repeatedly forced straight, as occurs during the application of splints [5].
In support of the hypothesis of bone fragility in the epiphysis, Horenstein et al. [42] evaluated bone mass throughout the length of the tibia via computed tomography in a sample of 257 children aged 6–17 years (
4.7.2Prenatal and postnatal factors associated with bone fragility
In a retrospective study that included 80 newborns with SB where six sustained fractures during the neonatal period, all of the six patients with fractures had both contractures and a neurological level equal to or higher than L3. Boytim et al. [18] found that neonates with these two characteristics had a 17% chance of sustaining a neonatal fracture. The fractures occurred predominantly in the lower limbs and particularly in the femur. The cause of fractures in the six patients was related to birth injury in one patient, believed to be related to vigorous physical therapy in two patients, and undetermined in three patients [18]. These results suggest that at higher levels of neurologic involvement, some important factors influencing bone fragility are already present in early life, thus increasing the risk of fragility fractures in the lower limbs. Lee et al. [34] studied the development of BMC in infants with MMC who did or did not receive upright supported stepping practice (USSP) and in infants with TD. The authors tested 36 infants: 14 with TD, 13 with MMC, and nine with MMC who received USSP, all aged one to 18 months. At birth, the whole-body BMC was similar, but differences emerged with age; values for infants with MMC were slightly lower than those for infants with TD. The BMC for legs and arms was lower for infants with MMC than for infants with TD. Between the MMC groups, the group that received USSP showed increases in the BMC with age. Infants with MMC who did not receive USSP showed no change in the leg or arm BMC. These results indicate the presence of early compromise of bone accrual in patients with MMC; thus, the lower BMC mean values start in infancy [34] and extend with advancing age until they peak at 19 years of age [13], establishing a potential for cascading effects including osteopaenia, osteoporosis, and fractures. The uterine environment creates buoyancy and environmentally induced motions that may trigger motility [34], which may explain the findings of Ralis et al. [97], who found that the mean values of changes in total square area, cortical thickness, number of Haversian systems, and bone remodelling cavities in the controls (TD infants) continually increased with age, while those from infants with SB decreased after birth. Postnatally, gravity increases the demands of energy and neural control in order to create movement [34]. As such, it is possible that there are factors that compete with and contribute to the aetiopathogenesis of fractures in the MMC and bone fragility in the neonatal period, which express themselves as the presence of increased osteoid and mineralization deficits (referred to as paralytic rickets by Ralis et al. [97]) and a decrease in cortical thickness and circumference [97], with the commitment of bone mass accrual post-birth with low BMC [34].
4.7.3Role of endocrinological factors in radial bone growth and bone mineral accrual
In dictating radial bone growth through a periosteal formation modelling deficit, hormonal factors may compete for this outcome in SB. Oestrogen acts to inhibit periosteal modelling such that at puberty, the extent of formation modelling is decreased in girls relative to boys, but it also prevents remodelling from removing bone from the endocortical surface and widening the marrow cavity. In contrast, the absence of oestrogen promotes bone resorption on the endocortical surface. Boys exhibit a spike in GH and insulin-like growth factor-1 (IGF-1) during puberty, which, along with increasing levels of testosterone, stimulates periosteal growth. These gender-specific hormonal differences result in men having larger bone diameters when peak bone mass is attained [4]. Beyond hypogonadism, which is more common in males [28], individuals with SB may exhibit decreased anabolic GH/IGF-1 activity [49]. The loss of anabolic GH/IGF-1 action in SB may disturb the capacity to increase the pool of calcium in order to mineralize bone through calcium absorption from the intestines, which is particularly crucial during the most rapid BMC increase in the two years before and after the time of peak height velocity [4].
4.7.4Suggestion for an integrated model of bone fragility factors
The broad spectrum of neuromotor deficits and distribution of comorbidities may in part justify the diversity of fracture aetiopathogenesis in this population. The aetiology of bone fragility in MMC can be identified based on postnatal and later factors associated with bone fragility, local and systemic factors of mixed aetiology due to the formation deficit, defective bone mineralization, and increased bone loss. Therefore, three different pathophysiological conditions may be lumped together in MMC and act synergistically to decrease radiological (areal) BMD: decreased formation deficit/bone size, osteomalacia/rickets, and osteopaenia [102].
A short stature and diminished bone circumference suggest longitudinal and radial growth commitment, respectively, during growth and development in childhood and adolescence in SB [17, 30, 37, 53, 54, 60, 62, 86, 87, 88, 90, 97, 103]. Horenstein et al. [42] demonstrated that the mean normalized bone volume in the Non-AmbSB group was significantly lower than that in the AmbSB and TD groups in the proximal epiphysis, distal epiphysis, and diaphysis. The occurrence of smaller and thinner bones suggests that a peak bone mass is not achieved.
Figure 1.
Aetiopathogenesis and evolution of fractures across age in SB.
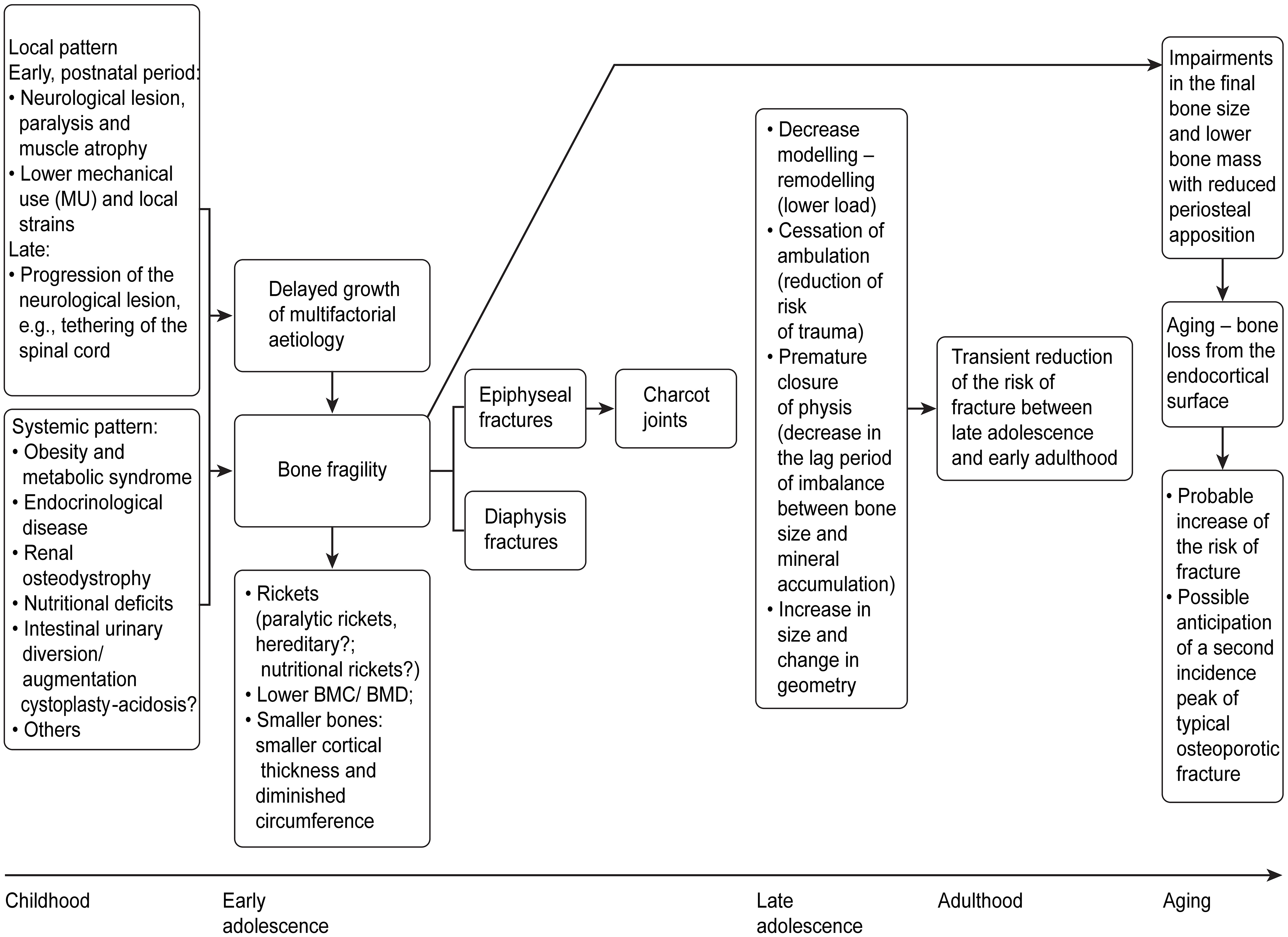
In addition to the mineralization deficit within the context of paralytic rickets and reduced bone mass [34, 97] with a deficit in radial growth confirming that the modelling problems occur early in life in SB, as suggested by Ralis et al. [97], the cross-sectional shape of the tibia midshaft cortex may also change in response to altered mechanical forces. Over time, the growth process remains compromised, conditioning the short bones, and systemic factors will concomitantly arise (infancy, childhood and adolescence); some are already present but may increase in severity with age and potentially interfere with bone health. Some studies such as those by Quan et al. [13] and Kafadar et al. [17] underscore a state of accelerated bone resorption according to bone metabolic markers in samples with a mean age of 11.1
It should be emphasized that classification in local and systemic factors is not indisputable. In fact, the bone response to a mechanical stimulus is threshold driven and is called a mechanostat, with set points termed by Frost as the minimum effective strains [4, 44]. Set points are likely determined by many factors in the hormonal and metabolic environment, and they should not be viewed as fixed at a particular strain level or strain rate [4, 44]. We acknowledge the limitations of these exposures and emphasize that a causal link between bone fragility and all of the factors described above has not yet been conclusively established in MMC and may arise from inference caused by studies in individuals without SB (Fig. 1).
4.7.5Weight of local and systemic factors in fracture aetiopathogenesis from a clinical perspective
For clinicians, the central quandary remains that despite the existence of systemic factors associated with a low BMD, involving both the appendicular and axial skeleton, fractures occur predominantly topographically in the paretic zone [7, 13, 16, 36, 41]. Contrary to the possible infrequency of vertebral involvement [7, 8, 23], studies have shown a low BMD in the lumbar spine [15, 17, 32, 35, 39]. It is possible that most studies did not evaluate spine X-rays for the detection of asymptomatic vertebral fractures, which may be an under-recognized problem in this population.
4.8Other fracture features in SB
Children with SB cystica are particularly liable to sustain fractures, which usually heal with substantial callus formation [5, 12, 27]. Extensive subperiosteal haemorrhage is seen in many cases of fracture associated with spinal cord lesions and may be facilitated by the flaccidity of the denervated muscles [22]. Additionally, a lack of pain sensation may delay the immobilization of the affected limb. Continued motion may lead to periosteal stripping by haemorrhage with extension of the callus far up the shaft. A lack of weight-bearing activity leads to the failure of remodelling and a resultant flared or widened metaphysis. Healed fractures may show cystic expansion with honeycombing, coarse or irregular trabeculation, and a thinning of the cortex [22, 24, 104].
4.9Factors that may contribute to Charcot joints
Charcot arthropathy was described in association with SB, and all cases described in a case series of 16 patients with SB involved the lower limbs [105], which suggests the importance of neurological lesions in its aetiology. The phases of the evolution of Charcot joints seem to overlap with the evolution of epiphyseal fractures: acute fragmentation, widening of the physeal line, and finally excessive callus formation and destruction [22, 25, 106, 107]. Stress on the lower limbs due to gait (given a deficit of bone mineralization and bone mass in the epiphyseal regions [42, 97]), decreased muscle mass [60], increased intramuscular fat [59], strength deficit, and progressive ligament lassitude may contribute to the increased likelihood of these fractures. Additionally, the growing cartilage of the physis is liable to undergo blood supply loss under conditions of trauma to the joint surroundings. Furthermore, the loss of sensation in patients with MMC is a physiological handicap that permits damage to the joint surroundings in the absence of pain protection [98, 100]. Thus, the reason for the susceptibility to damage of the epiphyseal and subepiphyseal regions is a combination of anatomical and physiological factors: mineralization deficits, greater deficits in the bone mass of the epiphyses, lower blood supply to the growth plate, and a loss of pain sensation and the subsequent susceptibility to trauma [16, 42, 97, 98, 100]. Consequently, MMC may represent a model combining elements of the neurotraumatic theory [100, 105, 108], the neurovascular theory [105, 108], the mineralization deficit and low bone mass during the origin of epiphyseal and subepiphyseal fractures, and the ensuing origin of Charcot arthropathy.
4.10Explanatory hypotheses for why the prevalence of fractures in MMC seems to decrease in late adolescence and in adulthood
Trinh et al. [28] detected a progressive decrease in the incidence of fractures in SB from infancy to adulthood in a retrospective cohort study of 146 individuals with SB aged two years or older, and they found fracture rates in children (ages 2–10), adolescents (ages 11–18), and adults (age
4.10.1The cerebral palsy model may suggest potential mechanisms for the decreased incidence of fractures observed in MMC
Extrapolating observations from a population that can serve as a model, while recognizing its specificity and the need for caution, Binkley et al. [109] used peripheral quantitative computed tomography to determine bone measurement in patients with cerebral palsy (CP) and two age-adjusted and sex-adjusted controls randomly selected from a database of healthy children aged 2.6 to 20.8 years. The authors observed that children with CP showed a decrease in periosteal circumference and appeared to have a higher cortical volumetric BMD (vBMD) than did control individuals with higher cortical thicknesses [109]. Cortical porosity is increased during periods of high modelling and remodelling, which can be expected with bone-loading activities during growth [109, 110]. Since patients with CP may not be able to participate in bone-loading activity, modelling-remodelling is likely to be decreased, leading to a reduction in periosteal circumference and increased vBMD (decreased cortical porosity) [109]. Whether these phenomena occur in MMC remains uncertain. Williams et al. [111] reported the cessation of ambulation in children with MMC and high-level paralysis between six and nine years of age, which may simultaneously lead to decreased bone-loading activity and decreased participation in situations with a higher risk of fracture injuries. Longitudinal growth occurs faster than mineral accrual during puberty, making the skeleton virtually osteopenic [4], and premature closure of the physis may occur as a result of epiphyseal injuries in patients with MMC [10, 25]. Thus, we can postulate that the abrupt termination of linear growth may favour the improvement of cortical BMD and decrease the lag period due to the imbalance between bone size and mineral accumulation in conjunction with or independent from the hypothetical modelling-remodelling commitment, and both processes may decrease the fracture rate in late adolescence and early adulthood. Furthermore, Parfitt described that a temporary skeletal debt is incurred by removing calcium from cortical bone to supply some of the calcium needed for the rapidly growing metaphyses of the long bones prior to epiphyseal fusion [110]. Another hypothesis may be that the increase in the size and change in geometry of bone with ageing together with the completion of bone modelling [28] contributed to an improvement of bone strength and improved adaptation of muscles and bones in adulthood due to the lower habitual loading forces, thereby reducing the risk of fracture [112].
During ageing, bone is preferentially lost from the endocortical surface, with limited periosteal apposition of no more than a few millimetres. Thus, if periosteal apposition is affected during the critical growing years, as may occur in MMC, bone strength will be reduced in the ageing skeleton, considering the occurrence of accelerated loss on the endocortical surface [4, 113]. Impairment in the final bone size may occur upon completion of growth. The average lifespan of patients with MMC may increase with current and future improvements in health care. These findings may be indicative of future fracture risk at an advanced age in MMC and allow the prediction of the anticipated second incidence peak of typical osteoporotic fractures [28] (Fig. 1).
4.11Management of bone fragility in MMC – pitfalls and misconceptions
Quan et al. [13] found that the BMD Z-score of the patients with bone fractures was significantly lower than that of the remaining patients. However, not all studies have identified a relationship between low BMD and a history of fractures in MMC [14, 15, 36, 41]. In addition to the small sample sizes and convenience sampling, the retrospective nature of the information concerning fractures [13, 14, 15, 16, 29, 35, 36, 41, 114], and the retrospective [16, 29, 38, 41] and cross-sectional design of most BMD studies in MMC [13, 14, 15, 29, 30, 32, 33, 35, 36, 37, 38, 39, 41], according to Apkon et al. [14], may be due to the average age of the children (nine years and eight months). If an older population had been studied, a relationship might have existed, since results suggest that older children with a history of fractures have lower Z-scores for the femoral neck than do older children with no history of fractures [14]. In fact, Rosenstein et al. [36] demonstrated an effect of age on BMD in the lower extremities according to the neurological level of injury, identifying a greater tibia and metatarsal BMD in those with more distal neurological levels, with proportionally greater differences as patients aged. In the present study, the mean age was similar to that in the study by Apkon et al. [14], which may have prevented the detection of a relationship between BMD and the fracture event [36]. Furthermore, the use of fracture as the end point in bone density studies requires very large numbers of subjects [16, 114].
4.12Prevention of fractures
4.12.1Non-pharmacological measures- nutritional and mobility programmes
Due to the complex aetiology of obesity and its increased prevalence in individuals with SB, it is critical to initiate prevention efforts early with a multifactorial approach for this at-risk population [60, 115].
Even a limited amount of walking appears to be beneficial for building bone mass and volume in this population, particularly in the epiphyses (trabecular bone sites) [42, 116]. Lee et al. [34] demonstrated that daily USSP can improve the BMC in as early as during the first year of life. Mazur et al. [26] compared two groups of patients with high-level SB: 36 patients who had participated in a walking programme and 36 patients that had been prescribed a wheelchair early in life. The patients who walked early had fewer fractures [26]. Still, gait training should be accompanied by orthotic support due to the intrinsic fragility at the epiphyses and subepiphyseal regions [42, 97]. These measures may also prevent the early closure of the physis and enhance bone growth. However, the use of splints may increase the risk of injuries in the clinical setting of knee tonus imbalances and contractures [5, 10]. In this context, the role of orthoses should be individualized and not applied to all patients.
4.12.2Calcium and vitamin D and pharmacological treatment
Recognizing the importance of calcium and vitamin D in bone health, the fact that the American Academy of Pediatrics does not recommend a reliance on sun exposure for vitamin D synthesis in the skin [39, 79, 117], as well as the prevalence of the insufficiency or deficiency of vitamin D in observational studies of SB [35, 39, 62, 75, 79] and the fact that adequate nutrition may have the greatest impact during periods of rapid bone turnover, such as during bone growth, the need for prophylactic measures to address nutrient inadequacies (vitamin D or calcium deficiency) early in life may potentiate the achievement of peak height and bone mass [4] and prevent these fragility fractures.
Given the risk of increased calcium intake in MMC and considering the possibility of exacerbating chronic constipation in children with neurogenic gut, as well as the risks of hypercalciuria and renal calculi in children with recurrent urinary tract infections and mobility impairment [8, 13, 15, 79, 118], advice on the intake of foods rich in calcium and calcium supplementation should be based on individualized assessment.
However, supplementing deficient nutrients may be an overly simplistic approach to addressing bone fragility, as the influence of hormonal changes occurring during a particular life stage may overwhelm any effects of nutrition on bone [4]. Physical examinations of growth and pubertal and evaluations of hormone levels that influence bone growth and mineral accrual should be performed [48, 51, 53, 55, 119, 120, 121].
Trinh et al. [28] recommend that SB patients who have undergone urological intervention with intestinal segments undergo urinary calcium excretion studies. The presence of hypercalciuria should alert the clinician to the risk of osteomalacia and metabolic acidosis. A trial of bicarbonate therapy may be useful in these situations [28].
Recognizing the role of kidney disease in this pathology, it is critical to identify paediatric patients at risk for kidney disease and to optimize follow-up for prevention [78]. In established disease, it seems possible that in the current era, the decision to treat and to monitor the treatment response in a CKD patient managed with vitamin D analogues or anti-resorptive or osteoanabolic agents may not be completely possible without a bone biopsy [93]. However, PTH should not be considered since it has a black box warning against its use in children and adolescents because it has caused osteosarcoma in a significant proportion of young rats [122, 123].
5.Conclusion
Bone fragility in MMC potentially has a multifactorial neuro-endocrinological-metabolic-renal dimension, with smaller bones, lower bone mass, and mineralization deficits affecting bone strength. These data emphasize the multiple and complex uncertainties regarding our understanding of the pathophysiology of bone fragility in paediatric patients with MMC. It is possible that the pathophysiology of bone fragility is different according to age and varies among patients, since the presence and severity of each factor with implications for bone fragility may vary. This patient-to-patient variation poses complex questions concerning the clinical management of bone fragility in SB, thereby suggesting the need for individualized assessment.
Future research investigating MMC and bone health should focus on identifying the factors that exacerbate the risk of bone fragility and how those factors interact with the specific skeletal envelopes/bone surfaces, the types and mechanisms of fracture involved during a long life, and the evaluation of the aetiology of low BMD (decreased bone size, low bone mineralization, and osteopaenia) and its impact on bone fragility. It is important to clearly separate these three entities, as the strategies for their prevention and treatment are different [102], and it is important to consider these entities in establishing guidelines for the prevention and treatment of bone fragility in MMC.
Conflict of interest
The authors declare that they have no conflict of interest.
References
[1] | Carey DE, Golden NH. Bone health in adolescence. Adolesc Med State Art Rev (2015) ; 26: (2): 291-325. |
[2] | Hui SL, Slemenda CW, Johnston CC, Jr. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest (1988) ; 81: (6): 1804-9. |
[3] | Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong Y. Adolescent obesity, bone mass, and cardiometabolic risk factors. J Pediatr (2011) ; 158: (5): 727-34. |
[4] | Burr DB, Allen MR. Basic and Applied Bone Biology. London: Elsevier Science; (2014) . |
[5] | James CCM. Fractures of the lower limbs in spina bifida cystica: A survey of 44 fractures in 122 children. Dev Med Child Neurol (1970) ; 12: : 88-93. |
[6] | Akbar M, Bresch B, Raiss P, Fürstenberg CH, Bruckner T, Seyler T, et al. Fractures in myelomeningocele. J Orthop Traumatol (2010) ; 11: (3): 175-82. |
[7] | Marreiros H, Monteiro L, Loff C, Calado E. Fractures in children and adolescents with spina bifida: The experience of a Portuguese tertiary-care hospital. Dev Med Child Neurol (2010) ; 52: (8): 754-9. |
[8] | Marreiros H, Loff C, Calado E. Osteoporosis in paediatric patients with spina bifida. J Spinal Cord Med (2012) ; 35: (1): 9-21. |
[9] | Lock TR, Aronson DD. Fractures in patients who have myelomeningocele. J Bone Joint Surg Am (1989) ; 71: (8): 1153-7. |
[10] | Parsch K. Origin and treatment of fractures in spina bifida. Eur J Pediatr Surg (1991) ; 1: (5): 298-305. |
[11] | Drennan JC, Freehafer AA. Fractures of the lower extremities in paraplegic children. Clin Orthop Relat Res (1971) ; 77: : 211-7. |
[12] | Quilis AN. Fractures in children with myelomeningocele. Acta Orthop Scand (1974) ; 45: (6): 883-97. |
[13] | Quan A, Adams R, Ekmark E, Baum M. Bone mineral density in children with myelomeningocele. Pediatrics (1998) ; 102: (3): E34. |
[14] | Apkon SD, Fenton L, Coll JR. Bone mineral density in children with myelomeningocele. Dev Med Child Neurol (2009) ; 51: (1): 63-7. |
[15] | Okurowska-Zawada B, Konstantynowicz J, Kulak W, Kaczmarski M, Piotrowska-Jastrzebska J, Sienkiewicz D, et al. Assessment of risk factors for osteoporosis and fractures in children with meningomyelocele. Adv Med Sci (2009) ; 54: (2): 247-52. |
[16] | Szalay EA, Cheema A. Children with spina bifida are at risk for low bone density. Clin Orthop Relat Res (2011) ; 469: (5): 1253-7. |
[17] | Kafadar I, Kilic BA, Yilmaz FK, Kilic M. Bone mineral density in pediatric patients with meningomyelocele. Childs Nerv Syst (2016) ; 32: (1): 111-9. |
[18] | Boytim MJ, Davidson RS, Charney E, Melchionni JB. Neonatal fractures in myelomeningocele patients. J Pediatr Orthop (1991) ; 11: (1): 28-30. |
[19] | Drabu KJ, Walker G. Stiffness after fractures around the knee in spina bifida. J Bone Joint Surg Br (1985) ; 67: (2): 266-7. |
[20] | Drummond DS, Moreau M, Cruess RL. Post-operative neuropathic fractures in patients with myelomeningocele. Dev Med Child Neurol (1981) ; 23: (2): 147-50. |
[21] | Kumar SJ, Cowell HR, Townsend P. Physeal, metaphyseal, and diaphyseal injuries of the lower extremities in children with myelomeningocele. J Pediatr Orthop (1984) ; 4: (1): 25-7. |
[22] | Hyre HM, Stelling CB. Radiographic appearance of healed extremity fractures in children with spinal cord lesions. Skeletal Radiol (1989) ; 18: (3): 189-92. |
[23] | Dosa NP, Eckrich M, Katz DA, Turk M, Liptak GS. Incidence, prevalence, and characteristics of fractures in children, adolescents, and adults with spina bifida. J Spinal Cord Med (2007) ; 30: (1): S5-9. |
[24] | Khoury JG, Morcuende JA. Dramatic subperiosteal bone formation following physeal injury in patients with myelomeningocele. Iowa Orthop J (2002) ; 22: : 94-8. |
[25] | Rodgers WB, Schwend RM, Jaramillo D, Kasser JR, Emans JB. Chronic physeal fractures in myelodysplasia: Magnetic resonance analysis, histologic description, treatment, and outcome. J Pediatr Orthop (1997) ; 17: (5): 615-21. |
[26] | Mazur JM, Shurtleff D, Menelaus M, Colliver J. Orthopaedic management of high-level spina bifida. Early walking compared with early use of a wheelchair. J Bone Joint Surg Am (1989) ; 71: (1): 56-61. |
[27] | Reikeras O, Hellum C. Fractures in children with myelomeningocele. Arch Orthop Trauma Surg (1981) ; 98: (1): 25-8. |
[28] | Trinh A, Wong P, Brown J, Hennel S, Ebeling PR, Fuller PJ, et al. Fractures in spina bifida from childhood to young adulthood. Osteoporos Int (2017) ; 28: (1): 399-406. |
[29] | Koch MO, McDougal WS, Hall MC, Hill DE, Braren HV, Donofrio MN. Long-term metabolic effects of urinary diversion: A comparison of myelomeningocele patients managed by clean intermittent catheterization and urinary diversion. J Urol (1992) ; 147: (5): 1343-7. |
[30] | Mingin GC, Nguyen HT, Mathias RS, Shepherd JA, Glidden D, Baskin LS. Growth and metabolic consequences of bladder augmentation in children with myelomeningocele and bladder exstrophy. Pediatrics (2002) ; 110: (6): 1193-8. |
[31] | Quan A, Adams R, Ekmark E, Baum M. Bone mineral density in children with myelomeningocele: Effect of hydrochlorothiazide. Pediatr Nephrol (2003) ; 18: (9): 929-33. |
[32] | Boylu U, Horasanli K, Tanriverdi O, Kendirci M, Gumus E, Miroglu C. Evaluation of bone mineral density after ileocystoplasty in children with and without myelomeningocele. Pediatr Surg Int (2006) ; 22: (4): 375-9. |
[33] | Ausili E, Focarelli B, Tabacco F, Fortunelli G, Caradonna P, Massimi L, et al. Bone mineral density and body composition in a myelomeningocele children population: Effects of walking ability and sport activity. Eur Rev Med Pharmacol Sci (2008) ; 12: (6): 349-54. |
[34] | Lee DK, Muraszko K, Ulrich BD. Bone mineral content in infants with myelomeningocele, with and without treadmill stepping practice. Pediatr Phys Ther (2016) ; 28: (1): 24-32. |
[35] | Okurowska-Zawada B, Kozerska A, Zelazowska B, Kulak W, Wasilewska A, Wysocka J. Serum 25-hydroxyvitamin D, osteocalcin, and parathormone status in children with meningomyelocele. Neuropediatrics (2012) ; 43: (6): 314-9. |
[36] | Rosenstein BD, Greene WB, Herrington RT, Blum AS. Bone density in myelomeningocele: The effects of ambulatory status and other factors. Dev Med Child Neurol (1987) ; 29: (4): 486-94. |
[37] | Polito C, del Gaizo D, di Manso G, Stabile D, del Gado R. Children with myelomeningocele have shorter stature, greater body weight, and lower bone mineral content than healthy children. Nutr Res (1995) ; 15: (11): 1605-11. |
[38] | Taskinen S, Fagerholm R, Makitie O. Skeletal health after intestinal bladder augmentation: findings in 54 patients. BJU Int (2007) ; 100: (4): 906-10. |
[39] | Martinelli V, Dell’Atti C, Ausili E, Federici E, Magarelli N, Leone A, et al. Risk of fracture prevention in spina bifida patients: Correlation between bone mineral density, vitamin D, and electrolyte values. Childs Nerv Syst (2015) ; 31: (8): 1361-5. |
[40] | Marreiros H, Loff C, Calado E. Who needs surgery for pediatric myelomeningocele? A retrospective study and literature review. J Spinal Cord Med (2015) ; 38: (5): 626-40. |
[41] | Haas RE, Kecskemethy HH, Lopiccolo MA, Hossain J, Dy RT, Bachrach SJ. Lower extremity bone mineral density in children with congenital spinal dysfunction. Dev Med Child Neurol (2012) ; 54: (12): 1133-7. |
[42] | Horenstein RE, Shefelbine SJ, Mueske NM, Fisher CL, Wren TA. An approach for determining quantitative measures for bone volume and bone mass in the pediatric spina bifida population. Clin Biomech (2015) ; 30: (7): 748-54. |
[43] | Sugiyama T, Price JS, Lanyon LE. Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone (2010) ; 46: (2): 314-21. |
[44] | Frost HM. Bone “mass” and the “mechanostat”: A proposal. Anat Rec (1987) ; 219: (1): 1-9. |
[45] | Mazur JM, Stillwell A, Menelaus M. The significance of spasticity in the upper and lower limbs in myelomeningocele. J Bone Joint Surg Br (1986) ; 68: (2): 213-7. |
[46] | Qin W, Bauman WA, Cardozo CP. Evolving concepts in neurogenic osteoporosis. Curr Osteoporos Rep (2010) ; 8: (4): 212-8. |
[47] | Zaidi M. Skeletal remodeling in health and disease. Nat Med (2007) ; 13: (7): 791-801. |
[48] | Rotenstein D, Reigel DH. Growth hormone treatment of children with neural tube defects: results from 6 months to 6 years. J Pediatr (1996) ; 128: (2): 184-9. |
[49] | Lopponen T, Saukkonen AL, Serlo W, Tapanainen P, Ruokonen A, Knip M. Reduced levels of growth hormone, insulin-like growth factor-I and binding protein-3 in patients with shunted hydrocephalus. Arch Dis Child (1997) ; 77: (1): 32-7. |
[50] | Tritos NA. Focus on growth hormone deficiency and bone in adults. Best Pract Res Clin Endocrinol Metab (2017) ; 31: (1): 49-57. |
[51] | Meyer S, Landau H. Precocious puberty in myelomeningocele patients. J Pediatr Orthop (1984) ; 4: (1): 28-31. |
[52] | Elias ER, Sadeghi-Nejad A. Precocious puberty in girls with myelodysplasia. Pediatrics (1994) ; 93: (3): 521-2. |
[53] | Trollmann R, Dorr HG, Strehl E, Katalinic A, Beyer R, Wenzel D. Growth and pubertal development in patients with meningomyelocele: A retrospective analysis. Acta Paediatr (1996) ; 85: (1): 76-80. |
[54] | Greene SA, Frank M, Zachmann M, Prader A. Growth and sexual development in children with meningomyelocele. Eur J Pediatr (1985) ; 144: (2): 146-8. |
[55] | Trollmann R, Strehl E, Dorr HG. Precocious puberty in children with myelomeningocele: Treatment with gonadotropin-releasing hormone analogues. Dev Med Child Neurol (1998) ; 40: (1): 38-43. |
[56] | Perrone L, del Gaizo D, D’Angelo E, Rea L, Di Manso G, del Gado R. Endocrine studies in children with myelomeningocele. J Pediatr Endocrinol (1994) ; 7: (3): 219-23. |
[57] | Golds G, Houdek D, Arnason T. Male hypogonadism and osteoporosis: The effects, clinical consequences, and treatment of testosterone deficiency in bone health. Int J Endocrinol (2017) ; 2017: : 4602129. |
[58] | Decter RM, Furness PD, 3rd, Nguyen TA, McGowan M, Laudermilch C, Telenko A. Reproductive understanding, sexual functioning and testosterone levels in men with spina bifida. J Urol (1997) ; 157: (4): 1466-8. |
[59] | Mueske NM, Ryan DD, van Speybroeck AL, Chan LS, Wren TAL. Fat distribution in children and adolescents with myelomeningocele. Dev Med Child Neurol (2015) ; 57: (3): 273-8. |
[60] | Shepherd K, Roberts D, Golding S, Thomas BJ, Shepherd RW. Body composition in myelomeningocele. Am J Clin Nutr (1991) ; 53: (1): 1-6. |
[61] | Mita K, Akataki K, Itoh K, Ono Y, Ishida N, Oki T. Assessment of obesity of children with spina bifida. Dev Med Child Neurol (1993) ; 35: (4): 305-11. |
[62] | van Speybroeck A, Mueske NM, Mittelman SD, Kremer RK, Ryan DD, Wren TA. Fasting serum blood measures of bone and lipid metabolism in children with myelomeningocele for early detection of cardiovascular and bone fragility risk factors. J Spinal Cord Med (2017) ; 40: (2): 193-200. |
[63] | Kalkwarf HJ. Adiposity and bone: The influence of subcutaneous versus visceral fat and insulin resistance. J Pediatr (2011) ; 158: (5): 698-700. |
[64] | Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM. Spina bifida. Nat Rev Dis Primers (2015) ; 1: : 15007. |
[65] | Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int (2008) ; 19: (7): 905-12. |
[66] | Trollmann R, Dörr HG, Gröschl M, Blum WF, Rascher W, Dötsch J. Spontaneous nocturnal leptin secretion in children with myelomeningocele and growth hormone deficiency. Horm Res Paediatr (2002) ; 58: (3): 115-9. |
[67] | Gigante C, Borgo A, Corradin M. Correction of lower limb deformities in children with renal osteodystrophy by guided growth technique. J Child Orthop (2017) ; 11: (1): 79-84. |
[68] | Massy Z, Drueke T. Adynamic bone disease is a predominant bone pattern in early stages of chronic kidney disease. J Nephrol (2017) ; 30: (5): 629-34. |
[69] | Miklaszewska M, Korohoda P, Zachwieja K, Wolnicki M, Mizerska-Wasiak M, Drozdz D, et al. Can we further improve the quality of nephro-urological care in children with myelomeningocele? Int J Environ Res Public Health (2016) ; 13: (9): E876. |
[70] | Norman ME, Mazur AT, Borden S, Gruskin A, Anast C, Baron R, et al. Early diagnosis of juvenile renal osteodystrophy. J Pediatr (1980) ; 97: (2): 226-32. |
[71] | Adams RC, Vachha B, Samuelson ML, Keefover-Hicks A, Snodgrass WT. Incidence of new onset metabolic acidosis following enteroplasty for myelomeningocele. J Urol (2010) ; 183: (1): 302-5. |
[72] | Greenbaum L. Pathophysiology of body fluids and fluid therapy. in: Nelson Textbook of Pediatrics. Kliegman RM, Stanton BMD, Geme JS, Schor NF, Behrman RE. editors. Philadelphia: WB Saunders Co.; (2007) . p. 299-300. |
[73] | Boyd JD. Chronic acidosis secondary to ureteral transplantation. Am J Dis Child (1931) ; 42: (2): 366-371. |
[74] | Hossain M. The osteomalacia syndrome after colocystoplasty; a cure with sodium bicarbonate alone. Br J Urol (1970) ; 42: (2): 243-5. |
[75] | Baum M, Stein D, Feldman H, Hobbs N, Gordon C. Vitamin D deficiency in children with spina bifida. in: Second World Congress on Spina Bifida Research and Care. Las Vegas; (2012) . |
[76] | Chagas CEA, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients (2012) ; 4: (1): 52-67. |
[77] | Grant WB. Epidemiology of disease risks in relation to vitamin D insufficiency. Prog Biophys Mol Biol (2006) ; 92: (1): 65-79. |
[78] | Filler G, Gharib M, Casier S, Lödige P, Ehrich JH, Dave S. Prevention of chronic kidney disease in spina bifida. Int Urol Nephrol (2012) ; 44: (3): 817-27. |
[79] | Mazur LJ, Wilsford LD, Rosas L, Sullivan E. Low 25-hydroxyvitamin D Levels in children with spina bifida. South Med J (2016) ; 109: (1): 31-5. |
[80] | Pacifico L, Anania C, Osborn JF, Ferraro F, Bonci E, Olivero E, et al. Low 25(OH)D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur J Endocrinol (2011) ; 165: (4): 603-11. |
[81] | Valimaki VV, Alfthan H, Lehmuskallio E, Loyttyniemi E, Sahi T, Stenman UH, et al. Vitamin D status as a determinant of peak bone mass in young Finnish men. J Clin Endocrinol Metab (2004) ; 89: (1): 76-80. |
[82] | Lehtonen-Veromaa MK, Mottonen TT, Nuotio IO, Irjala KM, Leino AE, Viikari JS. Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: A 3-y prospective study. Am J Clin Nutr (2002) ; 76: (6): 1446-53. |
[83] | Wald NJ, Cuckle HS, Boreham J, Althouse R. Birth weight of infants with spina bifida cystica. Br J Obstet Gynaecol (1980) ; 87: (7): 578-81. |
[84] | Lopponen T, Saukkonen AL, Serlo W, Lanning P, Knip M. Slow prepubertal linear growth but early pubertal growth spurt in patients with shunted hydrocephalus. Pediatrics (1995) ; 95: (6): 917-23. |
[85] | Teulier C, Smith BA, Kubo M, Chang CL, Moerchen V, Murazko K, et al. Stepping responses of infants with myelomeningocele when supported on a motorized treadmill. Phys Ther (2009) ; 89: (1): 60-72. |
[86] | Hayes-Allen MC. Obesity and short stature in children with myelomeningocele. Dev Med Child Neurol Suppl (1972) ; 27: : 59-64. |
[87] | Rosenblum MF, Finegold DN, Charney EB. Assessment of stature of children with myelomeningocele, and usefulness of arm-span measurement. Dev Med Child Neurol (1983) ; 25: (3): 338-42. |
[88] | Rotenstein D, Reigel DH, Flom LL. Growth hormone treatment accelerates growth of short children with neural tube defects. J Pediatr (1989) ; 115: (3): 417-20. |
[89] | Rotenstein D, Adams M, Reigel DH. Adult stature and anthropomorphic measurements of patients with myelomeningocele. Eur J Pediatr (1995) ; 154: (5): 398-402. |
[90] | Belt-Niedbala BJ, Ekvall S, Cook CM, Oppenheimer S, Wessel J. Linear growth measurement: A comparison of single arm-lengths and arm-span. Dev Med Child Neurol (1986) ; 28: (3): 319-24. |
[91] | Lopponen T, Saukkonen AL, Serlo W, Tapanainen P, Ruokonen A, Knip M. Accelerated pubertal development in patients with shunted hydrocephalus. Arch Dis Child (1996) ; 74: (6): 490-6. |
[92] | Mornet E. Hypophosphatasia. Metabolism (2018) ; 82: : 142-55. |
[93] | McNerny EMB, Nickolas TL. Bone quality in chronic kidney disease: Definitions and diagnostics. Curr Osteoporos Rep (2017) ; 15: (3): 207-13. |
[94] | Ward LM, Konji VN, Ma J. The management of osteoporosis in children. Osteoporos Int (2016) ; 27: (7): 2147-79. |
[95] | Thomassen SA, Johannesen IL, Erlandsen EJ, Abrahamsen J, Randers E. Serum cystatin C as a marker of the renal function in patients with spinal cord injury. Spinal Cord (2002) ; 40: (10): 524-8. |
[96] | Filler G, Gharib M, Casier S, Lodige P, Ehrich JH, Dave S. Prevention of chronic kidney disease in spina bifida. Int Urol Nephrol (2012) ; 44: (3): 817-27. |
[97] | Ralis ZA, Ralis HM, Randall M, Watkins G, Blake PD. Changes in shape, ossification and quality of bones in children with spina bifida. Dev Med Child Neurol Suppl (1976) ; 37: : 29-41. |
[98] | Edvardsen P. Physeo-epiphyseal injuries of lower extremities in myelomeningocele. Acta Orthop Scand (1972) ; 43: (6): 550-7. |
[99] | Roberts JA, Bennet GC, MacKenzie JR. Physeal widening in children with myelomeningocele. J Bone Joint Surg Br (1989) ; 71: (1): 30-2. |
[100] | Gyepes MT, Newbern DH, Neuhauser EB. Metaphyseal and physeal injuries in children with spina bifida and meningomyeloceles. Am J Roentgenol Radium Ther Nucl Med (1965) ; 95: : 168-77. |
[101] | Cuxart A, Iborra J, Melendez M, Pages E. Physeal injuries in myelomeningocele patients. Paraplegia (1992) ; 30: (11): 791-4. |
[102] | Schoenau E, Saggese G, Peter F, Baroncelli GI, Shaw NJ, Crabtree NJ, et al. From bone biology to bone analysis. Horm Res (2004) ; 61: (6): 257-69. |
[103] | Sharrard WJ. Paediatric Orthopaedics and Fractures. Oxford: Blackwell; (1971) . |
[104] | Katz JF. Spontaneous fractures in paraplegic children. J Bone Joint Surg Am (1953) ; 35: (1): 220-6. |
[105] | Nagarkatti DG, Banta JV, Thomson JD. Charcot arthropathy in spina bifida. J Pediatr Orthop (2000) ; 20: (1): 82-7. |
[106] | Gillies CL, Hartung W. Fracture of the tibia in spina bifida vera: report of two cases. Radiology (1938) ; 31: : 621-3. |
[107] | Bruckner FE, Howell A. Neuropathic joints. Semin Arthritis Rheum (1972) ; 2: (1): 47-9. |
[108] | Alpert SW, Koval KJ, Zuckerman JD. Neuropathic arthropathy: Review of current knowledge. J Am Acad Orthop Surg (1996) ; 4: (2): 100-8. |
[109] | Binkley T, Johnson J, Vogel L, Kecskemethy H, Henderson R, Specker B. Bone measurements by peripheral quantitative computed tomography (pQCT) in children with cerebral palsy. J Pediatr (2005) ; 147: (6): 791-6. |
[110] | Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos Int (1994) ; 4: (6): 382-98. |
[111] | Williams EN, Broughton NS, Menelaus MB. Age-related walking in children with spina bifida. Dev Med Child Neurol (1999) ; 41: (7): 446-9. |
[112] | Hogler W, Shaw N. Childhood growth hormone deficiency, bone density, structures and fractures: Scrutinizing the evidence. Clin Endocrinol (Oxf) (2010) ; 72: (3): 281-9. |
[113] | Seeman E. Periosteal bone formation – a neglected determinant of bone strength. N Engl J Med (2003) ; 349: (4): 320-3. |
[114] | Li Z, Chines AA, Meredith MP. Statistical validation of surrogate endpoints: Is bone density a valid surrogate for fracture? J Musculoskelet Neuronal Interact (2004) ; 4: (1): 64-74. |
[115] | Polfuss M, Bandini LG, Sawin KJ. Obesity prevention for individuals with spina bifida. Curr Obes Rep (2017) ; 6: (2): 116-26. |
[116] | Abramson AS. Bone disturbances in injuries to the spinal cord and cauda equina (paraplegia) their prevention by ambulation. J Bone Joint Surg Am (1948) ; 30: (4): 982-7. |
[117] | Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab (2011) ; 96: (1): 53-8. |
[118] | Matlaga BR, Kim SC, Watkins SL, Kuo RL, Munch LC, Lingeman JE. Changing composition of renal calculi in patients with neurogenic bladder. J Urol (2006) ; 175: (5): 1716-9. |
[119] | Trollmann R, Strehl E, Wenzel D, Dorr HG. Does growth hormone (GH) enhance growth in GH-deficient children with myelomeningocele? J Clin Endocrinol Metab (2000) ; 85: (8): 2740-3. |
[120] | Binkovitz LA, Henwood MJ, Sparke P. Pediatric dual-energy X-ray absorptiometry: Technique, interpretation, and clinical applications. Semin Nucl Med (2007) ; 37: (4): 303-13. |
[121] | Trinh A, Wong P, Sakthivel A, Fahey MC, Hennel S, Brown J, et al. Fat-bone interactions in adults with spina bifida. J Endocr Soc (2017) ; 1: (10): 1301-11. |
[122] | Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol (2002) ; 30: (3): 312-21. |
[123] | Bachrach LK, Ward LM. Clinical review 1: Bisphosphonate use in childhood osteoporosis. J Clin Endocrinol Metab (2009) ; 94: (2): 400-9. |



