Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2024 Update
Abstract
Background:
For the past five years, our annual reports have been tracking the clinical development of new drug-based therapies for the neurodegenerative condition of Parkinson’s disease (PD). These reviews have followed the progress both of “symptomatic treatments” (ST – improves/reduces symptoms of the condition) and “disease-modifying treatments” (DMT – attempts to delay/slow progression by addressing the underlying biology of PD). Efforts have also been made to further categorize these experimental treatments based on their mechanisms of action and class of drug.
Methods:
A dataset of clinical trials for drug therapies in PD using trial data downloaded from the ClinicalTrials.gov online registry was generated. A breakdown analysis of all the studies that were active as of January 31st, 2024, was conducted. This analysis involved categorizing the trials based on both the mechanism of action (MOA) and the drug target.
Results:
There were 136 active Phase 1–3 trials evaluating drug therapies for PD registered on ClinicalTrials.gov, as of January 31, 2024. Of these trials, 76 (56%) were classified as ST trials and 60 (44%) were designated DMT. More than half (58%) of the trials were in Phase 2 testing stage, followed by Phase 1 (30%) and Phase 3 (12%). 35 of the trials were registered since our last report, with the remaining 101 trials appearing in at least one earlier report.
Conclusions:
The drug development pipeline for PD remains in a robust state with a wide variety of approaches being developed and evaluated in Phase 1 and 2. Yet again, however, only a limited number of DMTs are transitioning to Phase 3.
Plain Language Summary
The development of new medical therapies, particularly for neurodegenerative conditions, is a long process that involves multiple phases of testing before a treatment is approved for use in a doctor’s clinic. The first phase assesses the short-term safety of a drug – most often in healthy volunteers but sometimes in people affected by the disease. The second phase explores the short-term safety and preliminary efficacy of the agent in people affected by the disease of interest, and the third phase investigates long-term safety and efficacy in a large group of people affected by the disease. For a disease like Parkinson’s disease, where the causes of the condition are not well understood, drugs targeting different biological pathways need to be tested to determine which ones may be useful in treating the symptoms, and which could be administered to slow down or stop the progression of the condition. Here, we provide an annual report on the current landscape of both these clinical testing efforts. In total, we reviewed 136 active studies evaluating therapies for Parkinson’s disease registered on a clinical trial database called ‘ClinicalTrials.gov’. Of these trials, approximately 55% were testing experimental symptomatic treatments, while the rest were focused on slowing down disease progression. More than half (58%) of the studies were in the second phase of clinical testing (short-term safety and preliminary efficacy), but only three studies were found to be testing treatments to stop the progression of Parkinson’s in the Phase 3 testing. We concluded that the drug development pipeline for Parkinson’s is robust, but more progress needs to be made with late-stage testing of treatments to slow the disease.
INTRODUCTION
Recent progress to improve and accelerate the Parkinson’s disease (PD) drug development pipeline has been significant. In 2023, we saw considerable advances in the development of biomarkers for PD. These include publications describing biomarker approaches important for assessing PD-relevant biology, such as validation of the alpha-synuclein seeding assay as a sensitive and specific measure of PD-associated synucleinopathy1 and development of a blood-based mitochondrial damage assay which may prove particularly valuable for testing biological impact of drugs targeting LRRK2.2 Additional advances may increase ability to assess dopamine system dysfunction, such as detection of elevated DOPA decarboxylase levels in cerebrospinal fluid3 and data showing potential of vesicular monoamine transporter 2 brain imaging as a measure of early PD progression.4 These new measurement tools offer potential for identifying specific biological features, forms and progression of PD and indeed it is encouraging to note that some of these approaches are already being employed in ongoing clinical trials (for example, the biotech company Vaxxinity employed the alpha-synuclein seeding assay in the Phase 1 testing of their alpha-synuclein targeting vaccine UB-312).5
As a result of these advances in biomarker research, in 2023 there was also a renewed drive towards the establishment of biologic staging systems.6 Although there have been clinical staging criteria for PD since the late 1960s,7 new frameworks based on molecular changes could represent a fundamental shift in how we define neurodegenerative conditions in general. Two biological definition systems have been proposed in early 2024, both centering on the presence or absence of pathological alpha-synuclein.8,9 Further development of these frameworks and additional advances and validation of novel biomarkers will hopefully improve clinical trial designs. As an example, The Michael J. Fox Foundation for Parkinson’s Research will pilot the concept of neuronal synuclein disease (NSD) rather than historically defined ‘prodromal PD’ in its ‘Path to Prevention’ (P2P) Platform Trial.10
In 2023, we also saw great strides in expanding diversity in PD research with the discovery of a genetic risk factor in the GBA1 gene specific to people of African ancestry.11 Given current understanding of PD is dominated by datasets derived largely from individuals of European ancestry, a shift in attention towards non-European populations provides novel insights into both ethnic and sex-specific differences in genetic risk factors.12–15 It has also drawn attention to the profound need for more robust data collection among under-represented communities.16,17 Such projects encourage improvement in equitable access and diverse patient participation in clinical trials.18
The ultimate measure of impact for all this progress is to observe advances in new therapy development for PD. Similar to annual reviews of experimental agents in clinical trials for Alzheimer’s,19 Lewy body dementia,20 and Huntington’s disease21 research, for the last five years we have produced an annual report seeking to provide a better understanding of the PD drug development pipeline. In this current account, we hope to add to the growing collection of data, highlight progress, point out areas of concern, and stimulate greater awareness and engagement in the clinical trial process.
METHODS
The methods used in this analysis are those of our previous reports22 with some minor adjustments. A diagram of the workflow is presented in Fig. 1. Clinical trial data were downloaded from ClinicalTrials.gov on January 31, 2024, based on the following search criteria:
• Condition: Parkinson disease
• Study type: Interventional
• Phase: Early Phase 1, Phase 1, Phase 2, Phase 3
• Status parameter: “Recruiting”, “Not yet recruiting”, “Active, not recruiting”, or “Enrolling by invitation”.
Fig. 1
A schematic outlining the data collection and analysis.
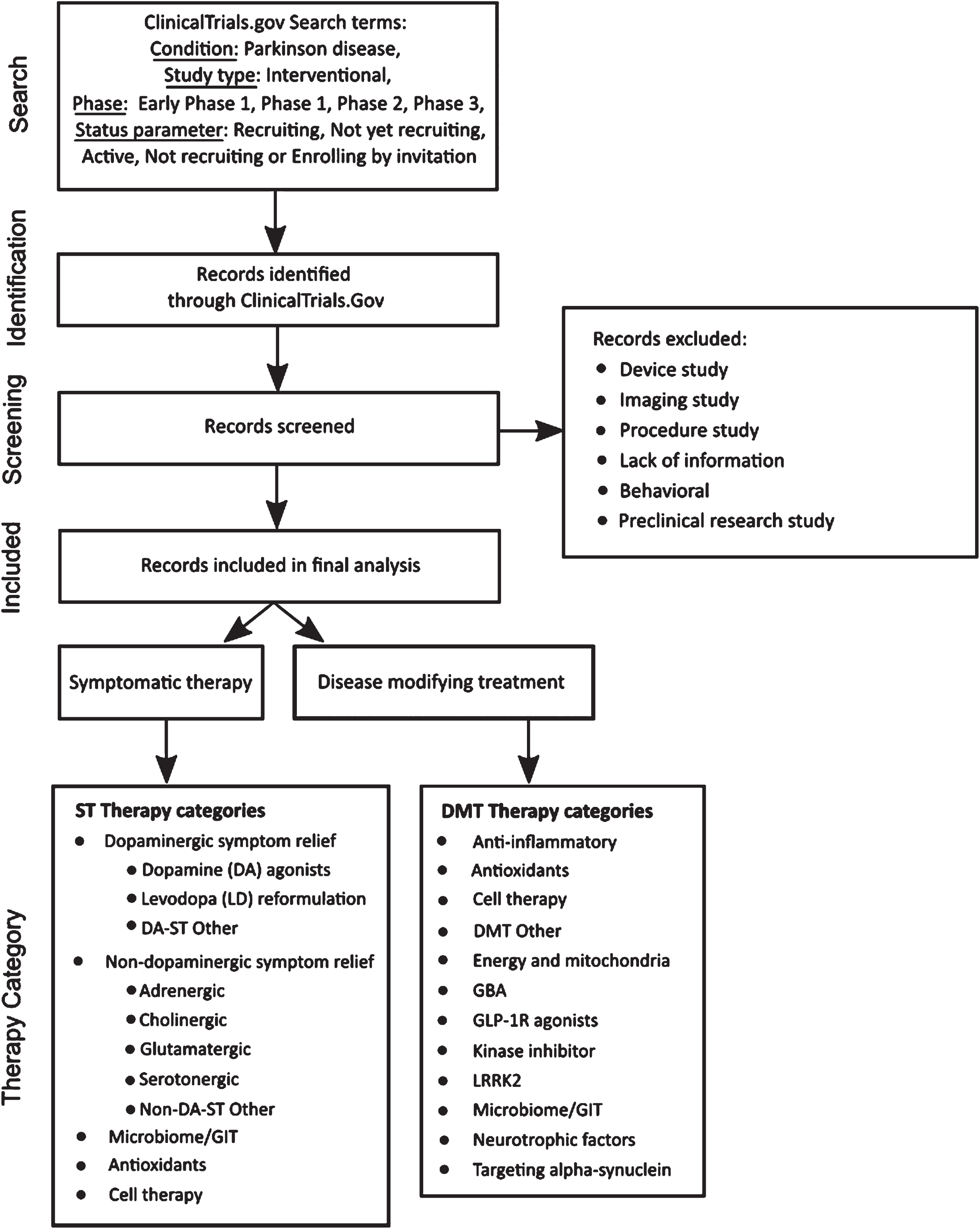
In our analysis, trials designated as “Early Phase 1” were grouped with Phase 1 trials. “Phase 1/Phase 2” trials were also combined with Phase 1 trials, and those described as “Phase 2/Phase 3” merged with Phase 2 trials. As explained in our last report, the focus for data analysis was ClinicalTrials.gov, so additional WHO registry data were not included.
Trial and therapeutic categorization
As of January 31, 2024, we downloaded records of 179 active interventional clinical trials meeting our search criteria from the ClinicalTrials.gov website. Trials evaluating medical devices, biomarkers, or behavioral and other lifestyle interventions were excluded, as were those that did not have reliable supporting public information available online. This filtering removed 43 trials from the dataset. The remaining trials were classed as either “symptomatic treatments” (ST – improves or reduces symptoms of the condition) or “disease-modifying treatments” (DMT – attempts to delay or slow progression by targeting the underlying biology of PD). Trials were then assigned to categories using an iterative process that considered both the mechanism of action (MOA) and the drug target. Our approach to therapeutic categorization continues to evolve as we aim to improve the analysis. This categorization has only changed slightly this year, with NMDA-based symptomatic relief grouped being within the Glutamatergic category.
The therapeutic agent in each trial was classified using the coding framework detailed in Fig. 1. First, agents were classified as either a novel (i.e. not yet approved for any indication) or existing compound. Existing compounds were further classified as either a “repurposed” agent, a “reformulation”, or a “new claim”. Repurposed compounds are those already approved by regulatory authorities for use in a different disease or condition that are now being evaluated for use in PD. Reformulated compounds involve drugs already in use for PD that have the potential to be delivered in alternate ways, such as subcutaneously or inhaled. Compounds seeking a new claim involve testing of drugs already approved for treating specific PD symptoms for use in the treatment of additional PD symptoms.
The therapeutic categories applied to this year’s analysis are:
- ‘Dopaminergic symptom relief therapies’ (MOA) apply to ST agents that either restore, replace or mimic the neurotransmitter dopamine, and include the following subcategories:
- DA agonist
- Levodopa (LD) reformulation
- DA-ST Other
- ‘Non-dopaminergic symptom relief therapies’ (MOA) applies to ST agents that target neurotransmitter systems other than dopamine and include:
- Adrenergic
- Cholinergic
- Glutamatergic
- Serotonergic
- Non-DA-ST Other
- ‘Anti-inflammatory’ (MOA) – agents seeking to reduce inflammatory processes.
- ‘Antioxidants’ (MOA) – agents focused on reducing oxidative stress.
- ‘Cell therapy’ (MOA) – trials including either intracerebral cell transplantation or peripheral delivery of cells.
- ‘DMT Other’ (MOA) – therapies with a MOA that did not match another category.
- ‘Energy and mitochondria’ (MOA) – agents seeking to stimulate improvements in mitochondrial function.
- ‘GBA’ (MOA) – agents focused on enhancing the activity of glucocerebrosidase (GCase).
- ‘GLP-1R agonists’ (MOA) – a specific class of drugs activating the glucagon-like peptide-1 receptor.
- ‘Kinase inhibitor’ (MOA) – agents blocking specific kinase activity.
- ‘LRRK2’ (target) – agents seeking to reduce or inhibit the activity of LRRK2.
- ‘Microbiome/GIT’ (target) – agents specifically targeting the activity of the gastrointestinal tract (GIT).
- ‘Neurotrophic factors’ (MOA) – therapies involving the delivery of growth factors such as GDNF or CDNF.
- ‘Targeting alpha-synuclein’ (target) – molecules specifically focused on preventing alpha-synuclein aggregation, or disaggregation of existing complexes.
ST trials were further segmented by target symptoms (e.g., motor symptoms, sleep, and psychosis) based on sponsor-provided information available in the trial registry. If the allocation of the symptom descriptor was not available in the trial registration, sponsor-provided information was used and, if ambiguity remained, the consensus of the authors of this report was used. For the ‘Cell therapy’ category, unless otherwise advised by ClinicalTrials.gov records, we allocated intracerebral cell transplantation trials to the ST group (as a form of DA replacement) and trials involving the peripheral delivery of cells to the DMT group.
The primary source for our analyses is ClinicalTrials.gov from which we have extracted data on active trials. Drug development programs follow a sequential path and include periods where one trial has been completed, and work is underway to start the next one. Some projects have been announced publicly but are not yet registered. These so-called “Inbetweeners” have therefore not been included in the analyses, but it is important to acknowledge their presence in the pipeline. In addition, four important and active trials are not registered on ClinicalTrials.gov but on other registries. These, together with 47 Inbetweener programs, are included in the supplementary information to this paper. The primary source for this information was the Parkinson’s Hope List (bit.ly/ParkinsonsHopeList).
RESULTS
There were 136 active Phase 1–3 trials evaluating drug therapies for PD registered on ClinicalTrials.gov as of January 31, 2024, that met our selection criteria. This dataset is not a complete list of active trials during this period as other clinical studies are not registered on ClinicalTrials.gov. Of these 136 trials, (Supplemental file), 101 (74%) were represented in one or more of our prior annual reviews. The remaining 35 trials were registered on ClinicalTrials.gov after the 2023 review and were added to this year’s pipeline analysis. When the nature and phase status of the 136 studies were reviewed, 76 (56%) were classified as ST trials and 60 (44%) were designated DMT. More than half (58%) of the trials were in Phase 2 testing, followed by Phase 1 (30%) and Phase 3 (12%). (Table 1).
Table 1
Number of active PD drug trials by phase and ST/DMT categorization (as of January 31st, 2024, ClinicalTrials.gov)
| Phase 1 | Phase 2 | Phase 3 | Total | % | |
| ST | 19 | 44 | 13 | 76 | 56% |
| DMT | 22 | 35 | 3 | 60 | 44% |
| Total | 41 | 79 | 16 | 136 | |
| % | 30% | 58% | 12% |
A breakout of the 136 trials by therapeutic category (Table 2) showed about half (66 trials) were testing either dopaminergic STs (23 trials) or non-dopaminergic neurotransmitter STs (43 trials). There were 11 cell therapy trials (8%), all in Phase 1, of which five were DMT and six were ST. Further classification breakdown of DMT and ST trials is found in Tables 4 and 5, respectively. The agents being tested in each phase are presented in Fig. 2, showing the variety of therapies and categories under evaluation. Novel and repurposed compounds were being tested in a similar number of trials (each at 38% of the 136 total trials), with reformulated compounds appearing in about one-fifth of the studies and new claims in 5% (Table 3).
Table 2
Number of active PD drug trials by therapeutic category and phase (as of January 31, 2024, ClinicalTrials.gov)
| Category | Phase 1 | Phase 2 | Phase 3 | Total | % |
| Anti-inflammatory | 5 | 2 | 0 | 7 | 5% |
| Antioxidant | 0 | 2 | 0 | 2 | 1% |
| Cell therapy | 11 | 0 | 0 | 11 | 8% |
| Energy and mitochondria | 0 | 4 | 0 | 4 | 3% |
| GBA | 2 | 4 | 1 | 7 | 5% |
| GLP-1R agonist | 1 | 3 | 1 | 5 | 4% |
| Kinase inhibitor | 1 | 4 | 0 | 5 | 4% |
| LRRK2 | 1 | 1 | 0 | 2 | 1% |
| Microbiome/GIT | 2 | 3 | 1 | 6 | 4% |
| Neurotrophic factors | 2 | 2 | 0 | 4 | 3% |
| Other DMT | 2 | 6 | 0 | 8 | 6% |
| Targeting aSN | 1 | 8 | 0 | 9 | 7% |
| Dopamine agonist | 3 | 4 | 6 | 13 | 10% |
| Dopaminergic – LD reformulation | 1 | 1 | 3 | 5 | 4% |
| Dopaminergic other | 4 | 0 | 1 | 5 | 4% |
| Non-DA adrenergic | 0 | 3 | 1 | 4 | 3% |
| Non-DA cholinergic | 1 | 3 | 1 | 5 | 4% |
| Non-DA glutamatergic | 1 | 8 | 0 | 9 | 7% |
| Non-DA serotonergic | 1 | 7 | 1 | 9 | 7% |
| Non-DA other | 2 | 14 | 0 | 16 | 12% |
| Total | 41 | 79 | 16 | 136 | 100% |
Table 3
Active PD drug trials by phase and therapeutic novelty (as of January 31, 2024. ClinicalTrials.gov)
| Novel | Repurposed | Reformulation | New claim | Total | |
| Phase 1 | 18 | 8 | 14 | 1 | 41 |
| Phase 2 | 29 | 40 | 6 | 4 | 79 |
| Phase 3 | 4 | 4 | 6 | 2 | 16 |
| Total | 51 | 52 | 26 | 7 | 136 |
| % | 38% | 38% | 19% | 5% |
Table 4
Disease-modifying (DMT) trials listed based on their therapy categories by phase and by drug novelty (active DMT trials, as of January 31, 2024, ClinicalTrials.gov)
| DMT categories | # DMT trials | % DMT trials | Category/Phase breakout | Category/Novelty breakout | |||||
| Phase 1 | Phase 2 | Phase 3 | Novel | Repurp | Reform | New claim | |||
| Anti-inflammatory | 6 | 10% | 5 | 1 | 2 | 3 | 1 | ||
| Antioxidant | 2 | 3% | 2 | 1 | 1 | ||||
| Cell therapy | 5 | 8% | 5 | 2 | 3 | ||||
| Energy and mitochondria | 4 | 7% | 4 | 3 | 1 | ||||
| GBA | 7 | 12% | 2 | 4 | 1 | 2 | 5 | ||
| GLP-1R agonist | 5 | 8% | 1 | 3 | 1 | 2 | 3 | ||
| Kinase inhibitor | 5 | 8% | 1 | 4 | 3 | 2 | |||
| LRRK2 | 2 | 3% | 1 | 1 | 2 | ||||
| Microbiome/GIT | 3 | 5% | 2 | 1 | 1 | 2 | |||
| Neurotrophic factors | 4 | 7% | 2 | 2 | 3 | 1 | |||
| Other DMT | 8 | 13% | 2 | 6 | 1 | 5 | 2 | ||
| Targeting aSN | 9 | 15% | 1 | 8 | 8 | 1 | |||
| Total DMT trials | 60 | 22 | 35 | 3 | 25 | 25 | 8 | 2 | |
| % DMT trials | 100% | 37% | 58% | 5% | 42% | 42% | 13% | 3% | |
Table 5
Symptomatic (ST) trials listed based on their therapy categories by phase and by drug novelty (active ST trials, as of January 31, 2024, ClinicalTrials.gov)
| Category/Phase breakout | Category/Novelty breakout | ||||||||
| ST categories | # ST trials | % ST trials | Phase 1 | Phase 2 | Phase 3 | Novel | Repurp | Reform | New claim |
| Anti-inflammatory | 1 | 1% | 1 | 1 | |||||
| Cell therapy | 6 | 8% | 6 | 6 | |||||
| Microbiome/GIT | 3 | 4% | 3 | 2 | 1 | ||||
| Dopamine agonist | 13 | 17% | 3 | 4 | 6 | 7 | 1 | 5 | |
| Dopaminergic – LD reformulation | 5 | 7% | 1 | 1 | 3 | 5 | |||
| Other DA ST | 5 | 7% | 4 | 1 | 3 | 1 | 1 | ||
| Adrenergic | 4 | 5% | 3 | 1 | 3 | 1 | |||
| Cholinergic | 5 | 7% | 1 | 3 | 1 | 4 | 1 | ||
| Glutamatergic | 9 | 12% | 1 | 8 | 6 | 3 | |||
| Serotonergic | 9 | 12% | 1 | 7 | 1 | 1 | 5 | 3 | |
| Other non-DA ST | 16 | 21% | 2 | 14 | 6 | 10 | |||
| Total ST trials | 76 | 19 | 44 | 13 | 26 | 27 | 18 | 5 | |
| % of ST trials | 100% | 25% | 58% | 17% | 34% | 36% | 24% | 7% | |
Fig. 2
A schematic of all of the agents in active clinical trials for PD, registered on ClinicalTrials.gov as of the January 31st, 2024 – 107 agents being tested in 136 trials. [#] after agent name indicates trial count, e.g., Apomorphine.2 The pie graph is divided up according to the % of total trials, with ST Phase 1 : 14%, ST Phase 2 : 32%, ST Phase 3 : 10%, DMT Phase 1 : 16%, DMT Phase 2 : 26%, and DMT Phase 3 : 2%.
![A schematic of all of the agents in active clinical trials for PD, registered on ClinicalTrials.gov as of the January 31st, 2024 – 107 agents being tested in 136 trials. [#] after agent name indicates trial count, e.g., Apomorphine.2 The pie graph is divided up according to the % of total trials, with ST Phase 1 : 14%, ST Phase 2 : 32%, ST Phase 3 : 10%, DMT Phase 1 : 16%, DMT Phase 2 : 26%, and DMT Phase 3 : 2%.](https://content.iospress.com:443/media/jpd/2024/14-5/jpd-14-5-jpd240272/jpd-14-jpd240272-g002.jpg)
Among the 60 DMT trials, nine were focused on alpha-synuclein, eight on other targets (Other DMT), seven on GBA, and six on Inflammation, accounting for 50% of the DMT trials (Table 4). Trials of anti-inflammatory agents increased from three in 2023 to seven in 2024, due to new trials targeting NLRP3, a key component of the inflammasome.23 Trials targeting alpha-synuclein numbered nine, compared to 14 in 2023. The reduction was due to five trials completing and one reaching unknown status; with one study added to the category: MThera’s Phase 2 for MT101-5. Of the completed trials, three are included in the “Inbetweeners” list. The number of trials targeting GBA has increased from three in 2023 to seven in 2024.
Two categories, GBA and GLP-1R agonists, had active trials in Phase 1, 2, and 3. One other category, Microbiome/GIT, also had a trial in Phase 3. There was an equal number of Novel and Repurposed trials (25 each) among the DMT studies, with Reformulations accounting for eight trials and New Claims appearing in two trials within the Other DMT category. The Anti-inflammatory, Cell therapy and Microbiome/GIT categories were also strategies being tested in ST trials (Table 5).
Among the 76 ST trials, 23 (31%) were evaluating dopaminergic symptom relief therapies (Dopamine agonists, LD reformulations, and Other DA), while 43 (57%) were targeting non-dopaminergic neurotransmitter systems (Adrenergic, Cholinergic, Glutamatergic, Serotonergic, Other non-DA ST; Table 5). The remaining 10 trials were in the Anti-inflammatory (one trial), Cell therapy (six trials) and Microbiome/GIT (three trials) categories which, as noted above, also contains agents being evaluated in DMT trials. There was a similar number of Novel and Repurposed studies (26 and 27, respectively) among the ST trials, which coincidentally was close to the number of Novel (25) and Repurposed (25) DMT trials shown in Table 4. About a quarter of ST trials were Reformulations, with the remaining 7% (five trials) testing New Claims.
Trials by target symptoms
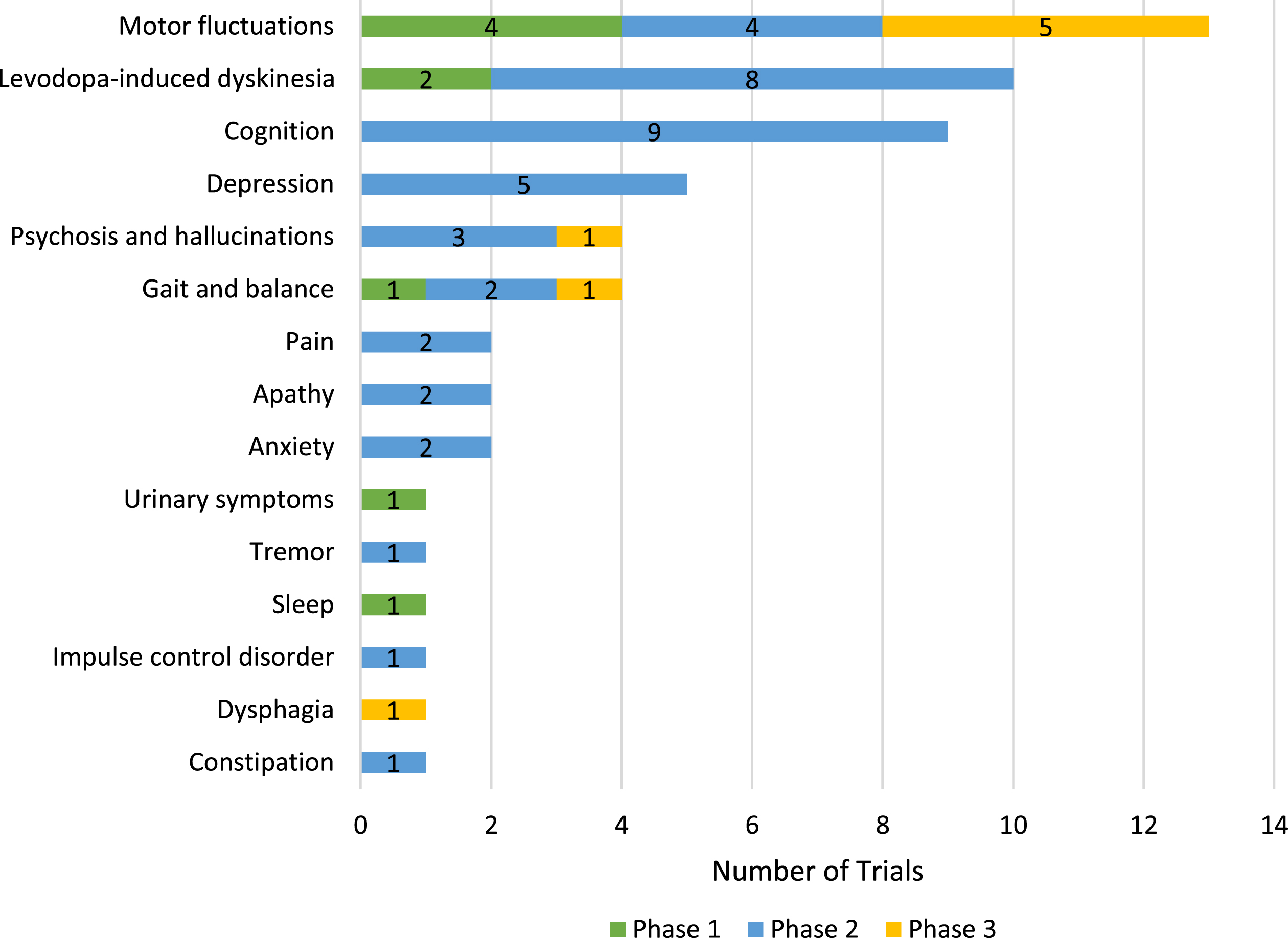
Of the 76 symptomatic trials, 23 are described (based on title, brief summary or endpoints in the ClinicalTrials.gov listing) as evaluating general motor and/or non-motor symptoms. The balance of symptomatic trials (53) are evaluating one or more specific targeted symptoms (Fig. 3) with motor fluctuations (defined here as off episodes with or without dyskinesia) being the most common targeted symptom (13 trials) followed by levodopa-induced dyskinesia (10), cognitive function (9), depression (5), gait and balance (4) and psychosis/hallucinations (4). Most of the targeted symptom trials are in Phase 2 with only eight Phase 3 trials (five for motor fluctuations and one each in gait and balance, psychosis, and dysphagia).
Fig. 3
Symptom-specific targets of active PD drug trials as of January 31, 2024, ClinicalTrials.gov. Some trials are targeting multiple specific symptoms and are counted several times in this figure.
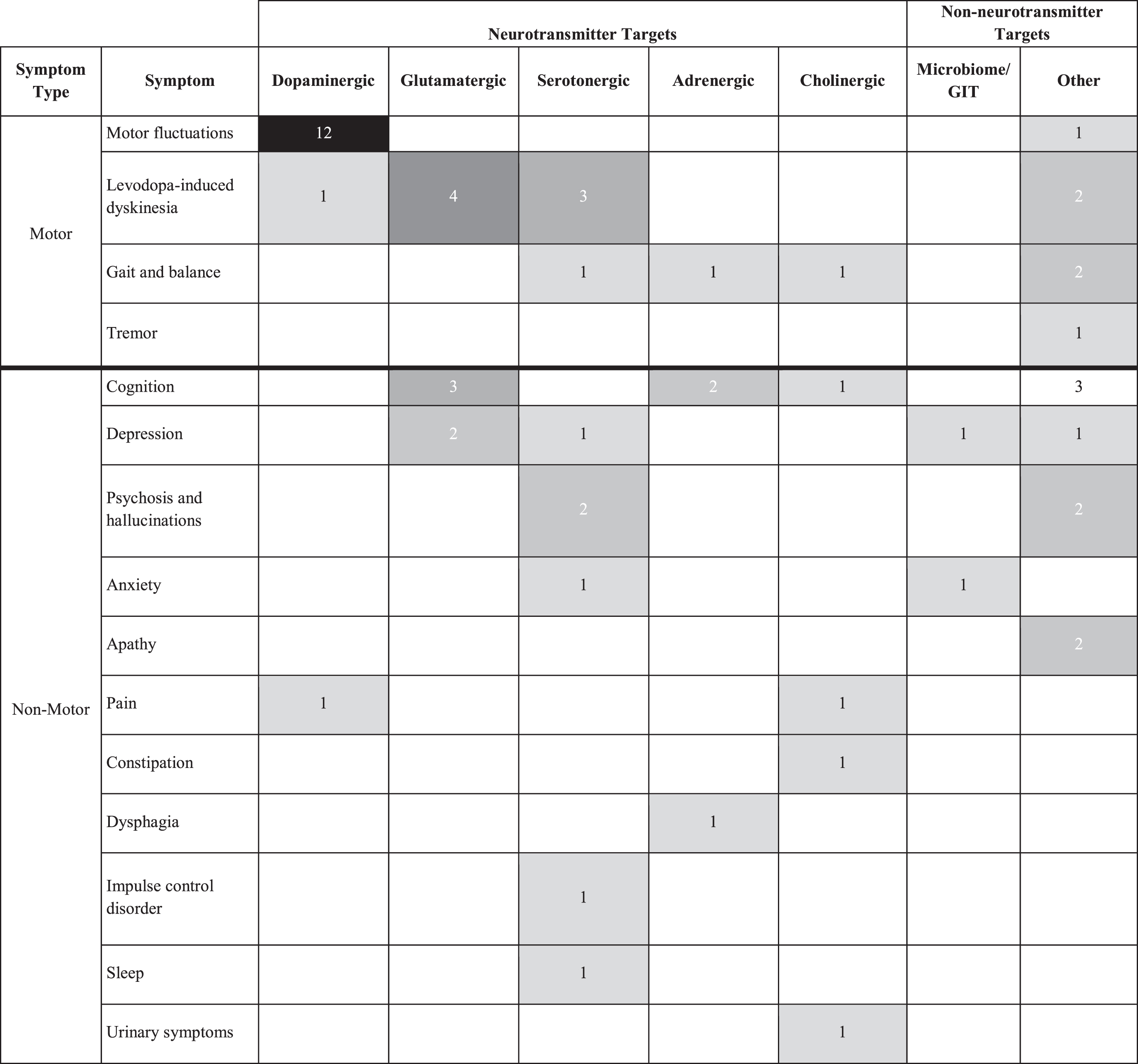
Agents from several therapeutic categories are being evaluated in the symptomatic trials (Table 6). Dopaminergic agents are the subject of the greatest number of trials with most of these for motor fluctuations. Levodopa-induced dyskinesias are the target for trials with agents from four therapeutic categories including multiple trials with glutamatergic and serotonergic agents. Cognition, depression, gait and balance, and psychosis and hallucinations are all symptoms for which agents from at least three therapeutic categories are being studied in active trials.
Table 6
Symptomatic Trials by Target Symptom and Therapeutic Category (active PD drug trials, as of January 31, 2024, ClinicalTrials.gov). Note that some trials are targeting more than one specific symptom and are counted several times in this table
 |
Trials newly registered
Over the last few years, there has been a significant increase in the number of newly registered trials evaluating anti-inflammatory therapeutics. In this report there are seven anti-inflammatory agents in clinical trials, of which five were newly registered (Table 7). There were also four newly registered clinical trials assessing cell therapies, demonstrating that this is an area of increased research activity. Five Phase 1 studies were both registered and completed in 2023 and, as they were not in the active trial dataset at the cutoff point, they were excluded from the overall analysis. These trials were the Cyclops levodopa delivery system from PureIMS (NCT06037590); Herantis’ HER-096, a CDNF mimic (NCT05915427); MTR101-5 from MThera, an inhibitor of alpha-synuclein aggregation (NCT05844787); adipose-derived stromal vascular fraction cells from Wake Forest University in Nicaragua (NCT05699161); and Lundbeck’s Lu Af28996, a D1/D2 agonist (NCT06004180).
Table 7
Category breakout of trials registered since last report (active as of January 31, 2024 and posted after January 31, 2023, ClinicalTrials.gov)
| DMT category | # trials | ST category | #s trials | |
| Phase 1 | Anti-inflammatory | 3 | Cell therapy | 3 |
| Cell therapy | 1 | Cholinergic | 1 | |
| GBA | 1 | Glutamatergic | 1 | |
| GLP1-R agonist | 1 | Other non-DA ST | 1 | |
| Kinase inhibitor | 1 | Serotonergic | 1 | |
| Other DMT | 2 | |||
| Phase 2 | Anti-inflammatory | 1 | Anti-inflammatory | 1 |
| GBA | 2 | Cholinergic | 1 | |
| Kinase inhibitor | 1 | DA agonist | 2 | |
| Neurotrophic factors | 1 | Glutamatergic | 1 | |
| Other DMT | 1 | Other non-DA ST | 4 | |
| Phase 3 | GBA | 1 | Serotonergic | 1 |
| Total | 18 | 17 |
Target enrollment
The target participant enrollment across the 136 active PD drug trials was 16,649, with 60% of participants sought for Phase 2 trials and one-third for Phase 3 trials (Table 8). Of note, eight of the 136 trials each have an enrollment target of at least 500 and together account for 32% (5286 participants) of the total 16,640 target enrollment. These eight trials include four Phase 2 DMT trials and four Phase 3 ST trials. Specifically, the four Phase 2 DMT trials are (i) BIIB122 sponsored by Biogen/Denali (target of 640), (ii) Prasinezumab sponsored by Hoffman La Roche (target of 586), (iii) K0706 sponsored by Sun Pharma (target of 506) and (iv) Rasagaline (Phase 2/3) sponsored by Zhejiang University (target of 732). The four Phase 3 ST trials are (i) Rivastigmine sponsored by University of Bristol (target of 600) and (ii) three Tavapadon trials from Cerevel with targets of 1200, 500, and 522. Cerevel also has a fourth Tavapadon Phase 3 trial (target of 296), so collectively the four Tavapadon trials comprise 2822 (60%) of the ST Phase 3 trial enrollments (4698).
Table 8
Target enrollment for drug trials by phase and ST/DMT; n = 136 active PD drug trials, with the number of trials per phase in parentheses (as of January 31, 2024, ClinicalTrials.gov)
| DMT enrollment | ST enrollment | Total | % enrollment | |
| Phase 1 | 966 (22) | 435 (19) | 1401 (41) | 8% |
| Phase 2 | 6097 (35) | 3863 (44) | 9960 (79) | 60% |
| Phase 3 | 590 (3) | 4698 (13) | 5288 (16) | 32% |
| Total | 7653 (60) | 8996 (76) | 16649 (136) | |
| % enrollment | 46% | 54% |
Completed trials, duration and delays
Of the 139 active trials on January 31, 2023, only 24 (17%) were completed by January 31, 2024. Sixteen of the 24 completed trials were Phase 1 with four each in Phase 2 and Phase 3. Most (70%, 97 /139) of the active trials from January 31, 2023, were still active a year later, while 1% (2/139) were terminated and 12% (16/139) had an unknown status. [i] One of the terminated trials was in Phase 3 (NCT05418673 for BIIB122 in LRRK2 variant carriers which was folded into NCT05348785, a Phase 2 trial of BIIB122 originally targeting only idiopathic PD) as were two of the trials with unknown status. These studies were testing memantine (NCT03858270) and Lingzhi (Ganoderma – NCT03594656), both non-industry trials of repurposed agents. Completion rates were similar for DMT trials (19%) and ST trials (16%) as were the rate still active (65% vs. 74%) and those with unknown status (13% vs. 11%).
There were 12 completed DMT trials (Table 9) but most (9 trials) were in Phase 1 with two in Phase 2 and only one in Phase 3. As of the time of writing, at least the initial results have been released for seven of these trials. The rasagiline Phase 2 study was negative, but results from the other six trials (mesenchymal stem cells, Anle138b, NE3107, IkT-148009, lithium, and UB-312) were reported to be supportive of further development for these agents.
Table 9
Disease-modifying clinical trials that have completed since the McFarthing et al. (2023) report22
| Phase 1 | Phase 2 | Phase 3 |
| Anle138b NCT05532358 NCT04685265 | Mesenchymal stem cells NCT04506073 | Buntanetap NCT05357989 |
| Hypoestoxide NCT04858074 | Rasagiline NCT02789020 | |
| NE3107 NCT05083260 | ||
| ikT-148009 NCT04350177 | ||
| Human Amniotic Epithelial Stem Cells NCT04414813 | ||
| UCB7853 NCT04651153 | ||
| Lithium NCT04273932 | ||
| UB-312 NCT04075318 |
For the 24 completed trials as of January 31, 2024, the average time from study start to completion was 29 months. Phase 1 trials averaged 23 months with Phase 2 at 36 months and Phase 3 at 42 months. Note that ranges were broad (e.g., 12 to 66 months for Phase 2 and 16 to 66 months for Phase 3) with industry studies generally faster than others, which is also the case in studies for Alzheimer’s disease.19 Average study duration was similar for DMT vs ST trials (28 and 30 months, respectively) with analysis of duration by phase limited by the small numbers of trials. Delays in completion date were posted during the year for 41% (57/139) of the trials active on January 31, 2023. The average delay was 10 months. There was little difference in these metrics by phase, but delays were somewhat more common for ST trials (45%) versus DMT trials (37%).
Trials due to complete in 2024
There are 75 trials (55%) in the dataset with estimated completion dates before the end of 2024 (22 Phase 1 trials, 46 Phase 2 trials and seven Phase 3 trials). Note, however, that estimated completion dates are often not kept up to date by many sponsors. For example, 30 of the 75 trials have estimated completion dates prior to January 31, 2024 when our data set of active trials was extracted from ClinicalTrials.gov. Of the 75 trials in this group, 27 (20%) are classified as disease modifying (12 Phase 1, 14 Phase 2 and 1 Phase 3). Of note, the one DMT Phase 3 trial is a large study of exenatide ER (NCT04232969) and the Phase 2 DMT group includes four trials with sample sizes≥120 patients offering prospects for important results to become available in the near future.
DISCUSSION
This is our fifth consecutive annual report describing the changing clinical development pipeline for PD. When compared with our previous commentaries,22,24–26 we report relative stability in the number of trials that were active on January 31st, 2024. For example, the number of PD trials captured in our analyses (136 in the current 2024 dataset) has not varied greatly throughout the years we have been producing these reports, with 139 included in 2023, 147 in 2022, 142 in 2021 and 145 in 2020. The change in the number of active trials from year to year depends on the number of (i) new trials registered in the year, (ii) the number of trials completed or terminated, and (iii) the number of trials that dropped off because their status was unknown (i.e., registry information was not updated in at least 2 years). The net effect of these explains the fairly consistent number of active trials in the datasets across our five reports. The number of separate Phase 1 trials (41 on the current study cutoff date, 30%) have consistently hovered between 30% and 35% of the total number of PD trials across our analyses. Phase 2 trials (79 in the current study year, 58%) have never been lower than 46% of the total over this time period, and Phase 3 trials (16 in the current study year, 12%) have also maintained a relatively constant proportion within their five-year range of 12%–19%.
We are pleased to report that the most recent figures to January 31st 2024 show there is a continuation of the previously wide diversity of therapeutic approaches that tackle a range of biological mechanisms of action. As with our 2023 report, this year, amongst the symptomatic therapies being pursued in the clinic, we describe a diverse selection of ‘Dopaminergic (DA, LD and Other)’ as well as ‘Non-dopaminergic symptom relief therapies’ (i.e. those that target neurotransmitter systems other than dopamine, and which include Adrenergic, Cholinergic, Glutaminergic, Serotonergic and Other approaches). A similar diverse pattern of widely different therapeutic approaches exists in this year’s results across the 60 disease-modifying therapies we report. Of note is that the number of anti-inflammatory trials between 2023 and 2024 has doubled to six, a trend which may continue in subsequent years as inflammation is now considered to be important in the pathogenesis of PD and in the trajectory of disease progression.27 The number of DMT GBA trials has also increased sharply, from three in 2023 to seven in 2024, and with more reaching Phase 2 and Phase 3 stages. Clinical trials exploring disease-modifying therapies targeting alpha-synuclein in PD patients changed from 14 in 2023 to nine in 2024, Eight of these trials are in Phase 2, whereas in 2023, six were in Phase 2. There was a slight fall this year in the number of microbiome/gastrointestinal trials exploring the therapeutic management of the gut/brain axis in PD, from eight ongoing disease-modifying therapeutic trials in 2023, to six. Twelve cell therapies trials were reported as ongoing in our 2023 report (10 in Phase 1, and two in Phase 2), whereas by 2024 this had decreased slightly to 11; these remain mainly focused on early Phase 1 studies (with none in Phase 2 or 3). The number of GLP-1 receptor agonist trials remained at five in both 2023 (four Phase 2 and one Phase 3) and in 2024 (one in Phase 1, three in Phase 2 and one in Phase 3). The recent publication of the encouraging results of the lixisenatide disease modification trial,28 will likely draw attention to upcoming results of additional trials of Bydureon (exenatide) in PD patients (a Phase 2: NCT04305002 and a Phase 3: NCT04232969) expected in late 2024/early 2025.
In the 2023 report, there were six Phase 3 DMT trials, but in this latest analysis, there are now three, one of which has only 66 participants (NCT05576818). Of the other three that dropped off the list, two became “unknown” in status (these studies were testing memantine (NCT03858270) and Lingzhi (Ganoderma – NCT03594656)). The other Phase 3 study was the Biogen trial of the LRRK2 inhibitor BIIB122 (Lighthouse study – NCT05418673). Due to the complexity of the study, Biogen decided to fold this trial into their Phase 2 study of BIIB122 in an idiopathic cohort (Luma Study – NCT05348785).29
There were a number of trials this year of potential importance to the field that we placed in the Inbetweener category (in-between the conclusion of a PD trial and the commencement of a follow-up trial). It is interesting to note that three inflammasome (NLRP3) inhibitors are being oriented towards future clinical testing in PD cohorts (with a Phase 2 study of dapansutrile set to start in 2025).30 There are also new clinical trials being prepared for LRRK2 inhibition, including the first PROTAC approach to be tested in PD with ARV-102 which is being developed by Arvinas.31 In addition to anticipated trials, there are also Inbetweeners for which results are expected, such as the first Australian Parkinsons Mission trial in PD patients (APM1) testing nilvadipine, alogliptin and albuterol, which should report in 2025.32
The 2024 analysis also highlighted the large number of trials targeting the relief of specific PD symptoms. This is of great importance to people living with PD when, quite often, it may be a small number of the potential constellation of symptoms that actually have the greatest impact on quality of life.33 Many of these are non-motor symptoms or complications resulting from medicines. The most prominent specific symptom in the pipeline is dyskinesia, closely followed by the psychiatric symptoms of cognition (including PD dementia), depression, and psychosis and hallucinations. It was encouraging to observe in our analysis that cognition, depression, gait and balance, and psychosis/hallucinations are all symptoms for which agents from at least three therapeutic categories are being evaluated in clinical trials. It was also interesting to note that there are at least four clinical programs developing continuous levodopa delivery therapies in the Inbetweeners group, in addition to introduction of the AbbVie Foslevodopa-foscarbidopa (Produodopa) in certain geographies.34
In summary, the results of the 2024 PD drug therapies in the clinical trial pipeline analysis indicate that there is a wide variety of experimental therapeutics being assessed. While there are encouraging trends across the report (such as an increasing focus on non-motor symptoms and broader number of DMT interventions coming to clinical testing), there is still limited transition of DMTs from Phase 2 to the crucial Phase 3 stage. This may be due to the limited understanding of PD biology, but we hope the number of Phase 3 studies will increase in coming years with the setting up and initiation of Multi-Arm, Multi-Stage clinical trial platforms aimed at accelerating the development of new therapies for PD.35 By assessing multiple agents against a single placebo and having continuous evaluation of the results to allow seamless transition between phases of testing, it is hoped that such initiatives will not only allow more rapid testing of novel therapies, but also provide patients with more opportunity to engage with the research. The contribution of the PD community is essential, and we acknowledge the value of their commitment and involvement.
ACKNOWLEDGMENTS
The authors would like to thank Professor Tanya Simuni of Northwestern University and Helen Matthews of Cure Parkinson’s for reading the manuscript and providing constructive feedback. The authors would also like to thank all of the trial participants and their families, and the researchers involved in the ongoing clinical research for PD.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Abbreviations
AAV | Adeno-associated virus |
ADMS | Adipose-Derived Mesenchymal Stem Cells |
AINSCDA | Autologous induced neural stem cell-derived DA precursor cells |
Botox | Botulinum toxin type A |
CDNF | Cerebral dopamine neurotrophic factor |
CHM | Chinese herbal medicine treatment |
DA-ST | Dopaminergic symptomatic therapy |
DMT | Disease Modifying Therapies |
EJS-ACT PD | The Edmond J. Safra Accelerating Clinical Treatments for Parkinson’s Disease |
FMT | Fecal Microbiota Transfer |
GCase | Glucocerebrosidase |
GDNF | Glial cell-derived neurotrophic factor |
GIT | Gastrointestinal Tract |
GLP-1 R | Glucagon-like peptide 1 receptor |
hAESCs | Human Amniotic Epithelial Stem Cells |
HDAP | Human Dopaminergic Progenitor Cells |
hESC | Human embryonic stem cells |
LD/CD | Levodopa and carbidopa |
LID | Levodopa-induced dyskinesia |
LRRK2 | Leucine-rich repeat kinase 2 |
MOA | Mechanisms of Action |
NMDA | N-methyl-D-aspartate |
NMS | Non-motor symptoms |
PASCs | Pluripotent Adipose Stem Cells |
PD | Parkinson’s Disease |
PROTAC | Proteolysis targeting chimera |
ST | Symptomatic Therapy |
WHO | World Health Organization |
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-240272.
REFERENCES
1. | Siderowf A , Concha-Marambio L , Lafontant D-E , et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: A cross-sectional study. Lancet Neurol (2023) ; 22: : 407–417. |
2. | Qi R , Sammler E , Gonzalez-Hunt CP , et al. A blood-based marker of mitochondrial DNA damage in Parkinson’s disease. Sci Transl Med (2023) ; 15: : eabo1557. |
3. | Pereira JB , Kumar A , Hall S , et al. DOPA decarboxylase is an emerging biomarker for Parkinsonian disorders including preclinical Lewy body disease. Nat Aging (2023) ; 3: : 1201–1209. |
4. | Beauchamp LC , Dore V , Villemagne VL , et al. Using (18)F-AV-133 VMAT2 PET Imaging to Monitor Progressive Nigrostriatal Degeneration in Parkinson Disease. Neurology (2023) ; 101: : e2314–e2324. |
5. | Vaxxinity Demonstrates Target Engagement of Toxic Alpha-Synuclein in Parkinson’s Patients, https://ir.vaxxinity.com/news-releases/news-release-details/vaxxinity-demonstrates-target-engagement-toxic-alpha-synuclein (2023, accessed 13 June 2024). |
6. | Chahine LM , Merchant K , Siderowf A , et al. Proposal for a Biologic Staging System of Parkinson’s Disease. J Parkinsons Dis (2023) ; 13: : 297–309. |
7. | Hoehn MM , Yahr MD . Parkinsonism: Onset, progression and mortality. Neurology (1967) ; 17: : 427–442. |
8. | Höglinger GU , Adler CH , Berg D , et al. A biological classification of Parkinson’s disease: The SynNeurGe research diagnostic criteria. Lancet Neurol (2024) ; 23: : 191–204. |
9. | Simuni T , Chahine LM , Poston K , et al. A biological definition of neuronal α-synuclein disease: Towards an integrated staging system for research. Lancet Neurol (2024) ; 23: : 178–190. |
10. | Michael J. Fox Foundation. Path to Prevention (P2P) Platform Trial: A Phase 2A, Randomized, Double Blind, Placebo Controlled Study to Evaluate Investigational Interventions in Early Stage Neuronal Alpha-Synuclein Disease (NSD), https://www.ppmi-info.org/sites/default/files/docs/P2P-013%20stand%20alone%20synopsis%20and%20SOA%201Apr24.pdf (2024). |
11. | Rizig M , Bandres-Ciga S , Makarious MB , et al. Identification of genetic risk loci and causal insights associated with Parkinson’s disease in African and African admixed populations: A genome-wide association study. Lancet Neurol (2023) ; 22: : 1015–1025. |
12. | Kim JJ , Vitale D , Otani DV , et al. Multi-ancestry genome-wide association meta-analysis of Parkinson’s disease. Nat Genet (2024) ; 56: : 27–36. |
13. | Okunoye O , Ojo OO , Abiodun O , et al. MAPT allele and haplotype frequencies in Nigerian Africans: Population distribution and association with Parkinson’s disease risk and age at onset. Parkinsonism Relat Disord (2023) ; 113: : 105517. |
14. | Pan H , Liu Z , Ma J , et al. Genome-wide association study using whole-genome sequencing identifies risk loci for Parkinson’s disease in Chinese population. NPJ Parkinsons Dis (2023) ; 9: : 22. |
15. | Leal TP , Rao SC , French-Kwawu JN , et al. X-Chromosome Association Study in Latin American Cohorts Identifies New Loci in Parkinson’s Disease. Mov Disord (2023) ; 38: : 1625–1635. |
16. | Walker R , Fothergill-Misbah N , Kariuki S , et al. Transforming Parkinson’s Care in Africa (TraPCAf): Protocol for a multimethodology National Institute for Health and Care Research Global Health Research Group project. BMC Neurol (2023) ; 23: : 373. |
17. | Towns C , Richer M , Jasaityte S , et al. Defining the causes of sporadic Parkinson’s disease in the global Parkinson’s genetics program (GP2). NPJ Parkinsons Dis (2023) ; 9: : 131. |
18. | Corneli A , Hanlen-Rosado E , McKenna K , et al. Enhancing Diversity and Inclusion in Clinical Trials. Clin Pharmacol Ther (2023) ; 113: : 489–499. |
19. | Cummings J , Zhou Y , Lee G , et al. Alzheimer’s disease drug developemt pipeline: 2024 Alzheimers Dement (N Y) (2024) ; 10: : e12465. |
20. | MacDonald S , Shah AS , Tousi B . Current Therapies and Drug Development Pipeline in Lewy Body Dementia: An Update. Drugs Aging (2022) ; 39: : 505–522. |
21. | Van de Roovaart HJ , Nguyen N , Veenstra TD . Huntington’s Disease Drug Development: A Phase 3 Pipeline Analysis. Pharmaceuticals (Basel); 16: . Epub ahead of print 24 October 2023. DOI: 10.3390/ph16111513. |
22. | McFarthing K , Buff S , Rafaloff G , et al. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2023 Update. J Parkinsons Dis (2023) ; 13: : 427–439. |
23. | Singh J , Habean ML , Panicker N . Inflammasome assembly in neurodegenerative diseases. Trends Neurosci (2023) ; 46: : 814–831. |
24. | McFarthing K , Buff S , Rafaloff G , et al. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020. J Parkinsons Dis (2020) ; 10: : 757–774. |
25. | McFarthing K , Rafaloff G , Baptista MAS , et al. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2021 Update. J Parkinsons Dis (2021) ; 11: : 891–903. |
26. | McFarthing K , Rafaloff G , Baptista M , et al. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2022 Update. J Parkinsons Dis (2022) ; 12: : 1073–1082. |
27. | Tansey MG , Wallings RL , Houser MC , et al. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol (2022) ; 22: : 657–673. |
28. | Meissner WG , Remy P , Giordana C , et al. Trial of Lixisenatide in Early Parkinson’s Disease. N Engl J Med (2024) ; 390: : 1176–1185. |
29. | Biogen Provides Update on Parkinson’s Disease Clinical Development Program, https://investors.biogen.com/newsreleases/news-release-details/statement-biogen-provides-update-parkinsons-disease-clinical (2023, accessed 13 June 2024). |
30. | Cure Parkinson’s. Phase 2 clinical trial of dapansutrile for Parkinson’s confirmed, https://cureparkinsons.org.uk/2024/02/phase-2-clinical-trial-of-dapansutrile-for-parkinsons-confirmed/ (2024, accessed 13 June 2024). |
31. | Arvinas Announces First-in-Human Dosing of ARV-102, an Investigational PROTAC® Protein Degrader for Neurodegenerative Disease, https://ir.arvinas.com/news-releases/news-release-details/arvinas-announces-first-human-dosing-arv-102-investigational (2024, accessed 13 June 2024). |
32. | Australian Parkinson’s Mission, https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375958&isReview=true (accessed 13 June 2024). |
33. | Port RJ , Rumsby M , Brown G , et al. People with Parkinson’s Disease: What Symptoms Do They Most Want to Improve and How Does This Change with Disease Duration? J Parkinsons Dis (2021) ; 11: : 715–724. |
34. | AbbVie Launches PRODUODOPA® (foslevodopa/foscarbidopa) for People Living with Advanced Parkinson’s Disease in the European Union Open a printable version of this pageEmail the URL of this page to a friend, https://news.abbvie.com/-01-09-AbbVie-Launches-PRODUODOPA-R-foslevodopa-foscarbidopa-for-People-Living-with-Advanced-Parkinsons-Disease-in-the-European-Union (2024, accessed 13 June 2024). |
35. | Foltynie T , Gandhi S , Gonzalez-Robles C , et al. Towards a multi-arm multi-stage platform trial of disease modifying approaches in Parkinson’s disease. Brain (2023) ; 146: : 2717–2722. |



