Advice to People with Parkinson’s in My Clinic: Orthostatic Hypotension
Abstract
Orthostatic hypotension (OH) is the most common manifestation of cardiovascular autonomic dysfunction in Parkinson’s disease. In this viewpoint, we discuss five practical questions regarding OH in Parkinson’s disease: 1) How common is the problem? 2) Why should people with Parkinson’s disease and providers care about OH? 3) What are the symptoms of OH? 4) How to confirm a diagnosis of OH? And 5) How to treat OH? OH is an important non-motor symptom of Parkinson’s disease for which we have available treatments to significantly mitigate morbidity and possibly positively impact the disease course.
HOW COMMON IS ORTHOSTATIC HYPOTENSION IN PARKINSON’S DISEASE?
Orthostatic hypotension (OH) is a large decrease in blood pressure on standing in response to decreased venous return to the heart.1 It is considered to be neurogenic when the person has persistent OH secondary to insufficient norepinephrine release from the sympathetic postganglionic neurons upon standing up.1 Three main types of OH have been described: initial OH, defined by a transient drop in systolic blood pressure (SBP) by 40 mmHg and/or diastolic blood pressure (DBP) by 20 mmHg within 15 seconds of standing from a supine position; classic OH, defined by a drop in SBP≥20 mmHg and/or DBP≥10 mmHg within 3 minutes of standing, and delayed OH when the drop in blood pressure becomes apparent after a prolonged standing posture (>3 minutes).1 The estimated prevalence of classic OH in individuals with an established clinical diagnosis of Parkinson’s disease (PD) ranges from 30% to 65%.2,3 Classic OH becomes more frequent as the disease progresses.4 Importantly, OH can be the initial manifestation of the disease5 and there is evidence of subclinical abnormalities of blood pressure regulation within the first year after initial diagnosis in approximately 10% of people with PD.6 The prevalence of initial OH and delayed OH in PD is unknown. Delayed OH may progress to classic OH in PD.7 The clinician should consider the diagnosis of multiple system atrophy (MSA) in a patient with OH and parkinsonism, especially if there is urinary incontinence and/or retention and erectile dysfunction. At the early stages of the disease, the distinction between PD and MSA can be challenging and laboratory and imaging testing may be helpful.8 This Viewpoint will focus on PD.
WHY DOES IT MATTER?
A higher prevalence of autonomic dysfunction has been associated with a more malignant phenotype of PD.9 The presence of OH in PD is associated with more rapid disease progression, shorter survival time, and falls.10,11 The consequences of falls are devastating and include restriction of activities of daily living, fear of falling, fractures, and high levels of caregiver stress.12 Orthostatic hypotension also increases the risk of dementia in PD,13,14 possibly related to long-term recurring cerebral hypoperfusion. However, OH is likely not the only culprit. Supine hypertension (defined as a supine SBP≥140 mmHg and/or diastolic BP of≥90 mmHg) is found in up to 50% of people with autonomic failure, and it has been associated with a higher burden of white matter disease, renal failure, and higher prevalence of left ventricular hypertrophy.15 Therefore, the treatment of OH needs an individualized approach to avoid or reduce hypotensive episodes without inducing hypertension which increases cardiovascular morbidity and mortality in the long term. Furthermore, blood pressure variability according to the position of the body (i.e., hypotension while standing and hypertension when lying down) may be the cause of increased cardiovascular risk and dementia in people with PD. Falls are another significant complication of OH, especially in PD where gait difficulties and postural instability are common.11 At this time, aerobic exercise is the only intervention that has been shown to possibly slow disease progression in PD.16 Treatment of OH and supine hypertension has the potential to prevent or delay complications of PD including falls, cognitive impairment, and possibly progression of motor symptoms.
WHAT ARE THE SYMPTOMS OF ORTHOSTATIC HYPOTENSION IN PARKINSON’S DISEASE?
Orthostatic hypotension is not defined by symptoms; it can be symptomatic or asymptomatic. Typical symptoms associated with OH may be postural lightheadedness, dizziness, vision changes (tunnel or blurred), syncope, or near-syncope.17 Some people report atypical postural symptoms such as fatigue or cognitive dysfunction,18 pain in the shoulders and neck (also known as “coat hanger” pain), or even dyspnea or akathisia.19 These symptoms can vary from day to day, without a clear explanation. The description of symptoms is subjective and some people may not recognize specific symptoms, but when hypotensive will report a strong urge to sit down or lie down. People also may not report symptoms of OH unless asked specifically. Individuals with neurogenic OH often relate that their symptoms are worst in the morning, upon heat exposure, after eating a large meal (post-prandial lightheadedness and hypotension), or during or after exercise (exercise-induced and exaggerated post-exercise hypotension, respectively). Information gathered from spouses, other family members, or caregivers may be the most informative with evidence of witnessed episodes of pallor or unresponsiveness. Clinical observation can suggest a tendency for hypotension. Someone sitting in a chair with his or her legs twisted around each other may have learned from experience that doing this mitigates orthostasis on standing (“pretzel leg sign”).20 Shifting the body weight back and forth while standing or fidgeting the legs while seated can also activate the muscle pump on the lower extremity to increase venous return to the heart.19,20 Coming to the appointment with a water bottle and sipping on it regularly may be a clue on the benefit of increased fluid intake to minimize orthostatic intolerance secondary to OH (“water bottle sign”).20 Intolerance of starting or increasing dopaminergic therapy can be a sign that OH is a significant problem for the patient and should be considered a red flag for underlying autonomic failure.21
HOW TO SCREEN FOR ORTHOSTATIC HYPOTENSION?
The clinician must be familiar with the diagnosis of various forms of OH. Orthostatic hypotension can easily be identified, and people with PD should have supine and standing blood pressure and heart rate measurements during clinic visits. A decreased heart rate response to OH is a surrogate marker of a neurogenic etiology;22,23 however, in some cases, postural tachycardia is preserved in neurogenic OH.24 For routine screening, we recommend measuring blood pressure and heart rate in the supine position, after a period of rest of at least 3 to 5 minutes, and then while standing at 1 minute and 3 minutes25 (Fig. 1). Measuring blood pressure and heart rate before a full minute has passed can be valuable if there is a history of syncope or falling immediately upon standing. Repeating measurements beyond 3 minutes may be appropriate in some cases. The blood pressure cuff should be kept at the level of the heart, especially during changes in posture. Cuff size should be based on arm diameter. The patient should be comfortable, relaxed, and not speaking or tensing limb muscles. We encourage people with PD to ask all their physicians to take orthostatic vital signs if only their seated blood pressure is measured. Sitting-to-standing (rather than lying-to-standing) measurements may be used for the initial evaluation of OH in a busy clinic without the necessary space to measure supine blood pressure, but it is at the expense of lower sensitivity.26 Measuring orthostatic blood pressure changes during a supine-to-stand maneuver should be preferred to a sit-to-stand maneuver because of important methodological considerations. First, it is established that the sit-to-stand maneuver elicits a smaller blood pressure drop in comparison to the supine-to-stand maneuver.27 Therefore, OH is likely underdiagnosed using a sit-to-stand protocol. The optimal blood pressure cutoffs for a diagnosis of OH are also different when using a sit-to-stand maneuver (≥15/7 mmHg threshold) and have not been validated in PD.27 Second, a supine-to-stand or supine-to-tilt protocol was used to demonstrate that a failure to increase the heart rate by at least 1 beat per minute for every 2 mmHg drop in systolic blood pressure suggests a neurogenic etiology (ΔHR/ΔSBP < 0.5 bpm·mmHg-1).22,23 Thus, you cannot conclude that someone has neurogenic OH from this calculation unless blood pressure changes are measured supine to standing. Finally, supine hypertension cannot be detected with a sit-to-stand OH protocol. This is clinically important because supine hypertension is common in people with autonomic failure and should be managed appropriately. Screening for OH without screening for supine hypertension looks at only one side of the coin. Systematic measurement of orthostatic blood pressure and heart rate using a supine-to-stand maneuver is feasible in people with PD without adding unnecessary time and provides invaluable information about cardiovascular autonomic function.
Fig. 1
Proposed flowchart for first-line diagnostic investigation of orthostatic hypotension in Parkinson’s disease. A1c, glycated hemoglobin; BMP, basic metabolic panel; BP, blood pressure; CBC, complete blood count; HR, heart rate; OH, orthostatic hypotension; PD, Parkinson’s disease; SPEP, serum protein electrophoresis; TSH, thyroid stimulating hormone; UPEP, urine protein electrophoresis. ΔHR/ΔSBP = difference in HR divided by the difference in SBP from supine to standing; if < 0.5 bpm.mmHg-1 it suggests neurogenic orthostatic hypotension.
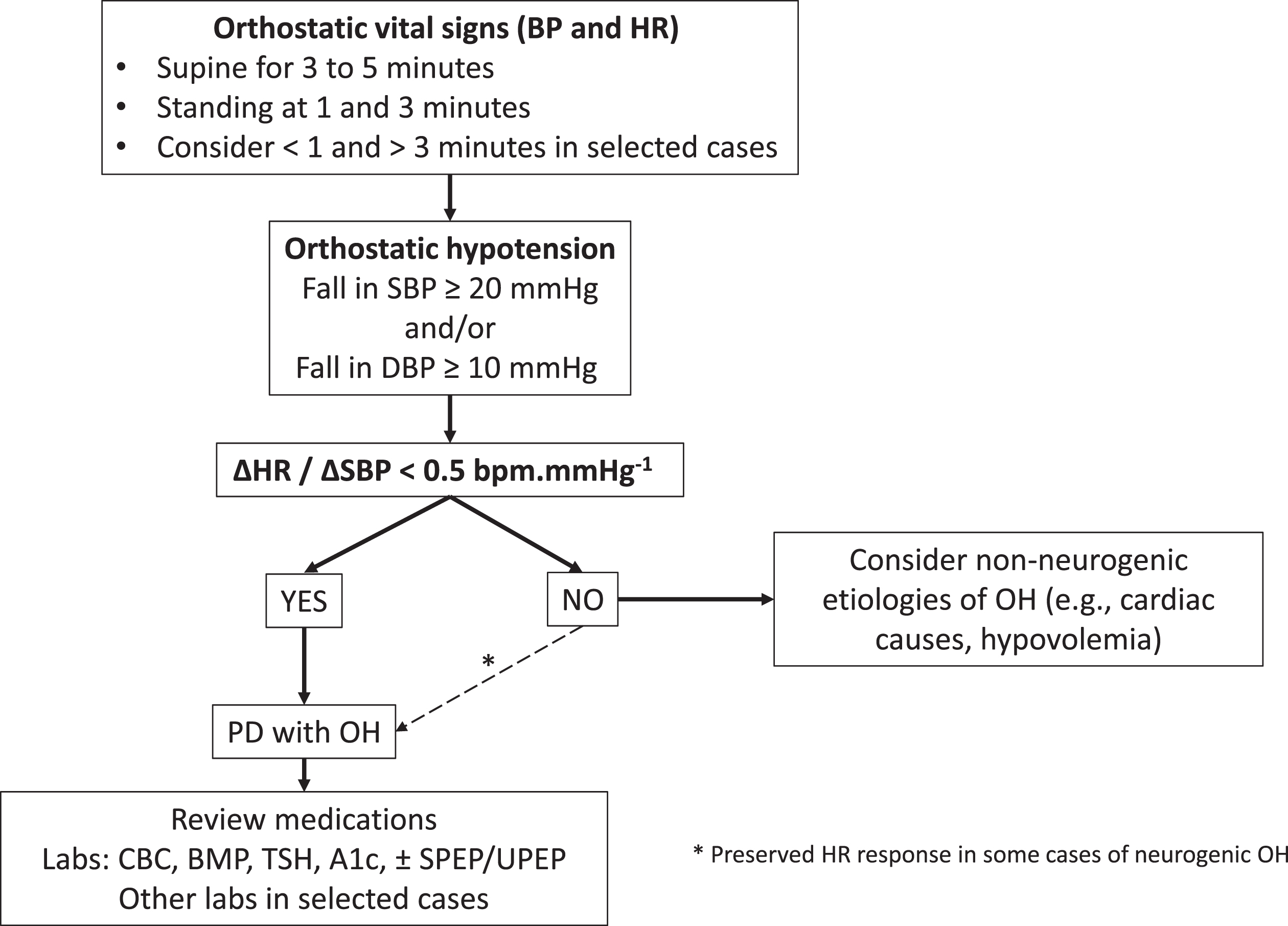
A few additional considerations are worth discussing. Detection of OH in someone with advanced PD who has difficulty standing up can be challenging and neurogenic OH may be suggested by some degree of drop in blood pressure from supine to seated. The absence of OH at a given point in time does not exclude the presence of noradrenergic failure with the potential for intermittent neurogenic OH.25 Factors such as the time of day, hydration status, medication effect, ambient temperature, and meals influence the hemodynamic response to orthostatic stress.1 For these reasons, morning assessments may be more sensitive, and when the results are uncertain, repeated measurements at home and in the office on separate days may be necessary. Home blood pressure monitoring has emerged as an effective and convenient means of measuring blood pressure. In general, wrist devices are less accurate than upper-arm devices.28 Although not as accurate as office blood pressure measurement, home blood pressure monitoring devices are usually sufficient for assessing changes in blood pressure and should prompt a more detailed assessment in the office.29 Ambulatory blood pressure monitoring is a valuable tool to assess OH and supine hypertension and may predict the development of falls, dementia, and hospitalization in PD.30 Detailed autonomic function testing can help investigate the severity of autonomic failure, but given the ability to diagnose OH using the algorithm listed in Fig. 1, autonomic testing is not necessary in most cases. Furthermore, beat-to-beat measurement of blood pressure using a finger cuff may be unreliable due to tremor. Other tests should be guided by the history and physical examination. We recommend screening for anemia because it may contribute to OH and it is also a common complication of autonomic failure. Screening for treatable causes of autonomic neuropathy such as diabetes, monoclonal gammopathy, and thyroid disorders is also recommended (Fig. 1).
HOW TO TREAT ORTHOSTATIC HYPOTENSION?
General recommendations
The four steps recommended by the American Autonomic Society and the National Parkinson Foundation in the treatment of neurogenic OH are the following: 1) review and adjust existing medications; 2) implement nonpharmacological measures, and, if needed, 3) use single-drug treatments before 4) combining pharmacologic approaches.31 The main goals are to improve standing time, relieve orthostatic symptoms, and improve activities of daily living without causing excessive supine hypertension. A careful review of the medication list and dietary supplements is mandatory to detect medications that can affect autonomic function. Common culprits are diuretics, α-antagonists, antihypertensive drugs, and calcium channel blockers, among others. Nonpharmacological management is a first-line treatment in the management of OH in PD (Fig. 2). Convenience or safety should be considered (i.e., comorbid severe heart failure may limit fluid and salt intake).1 Nonpharmacological interventions work by expanding blood volume (increasing fluid intake to at least 2 L per day and increasing salt intake to 8– 10 g daily), decreasing nocturnal pressure natriuresis (raising the head of the bed), decreasing venous pooling (using an abdominal binder, strengthening of the core musculature or leg muscles with recumbent exercise), or inducing a pressor response (drinking 16 ounces of cold water) (Fig. 2).1,32 Because symptoms of OH are typically worse in the early morning due to nocturnal pressure natriuresis, drinking a large glass of cold water before getting out of bed may be beneficial. Nonpharmacologic measures should be maximized before starting— and must be continued after— initiating pharmacotherapy. The pharmacologic management of OH and supine hypertension should be individualized (Table 1).33,34 There have been no head-to-head comparison studies to guide the initial choice of OH treatments. Droxidopa and midodrine are the only drugs approved by the Food and Drug Administration for the treatment of OH and are often used first-line in people with PD. Droxidopa is not available in Europe. Supine position should be avoided within 4 hours of taking these medications to avoid the risk of supine hypertension.34 Other medications are used off-label for the treatment of OH. Pyridostigmine may improve standing blood pressure without worsening supine hypertension. Furthermore, pyridostigmine may also help constipation, another common autonomic manifestation of PD. Fludrocortisone should be used with caution in people with congestive heart failure and onset of action occurs over 3– 7 days.34 Fludrocortisone may cause severe hypokalemia, which requires regular blood monitoring. If combination therapy is warranted, titrate the second agent from its lowest starting dose. In people with supine hypertension, small studies have shown the potential benefit of nonpharmacological measures such as the application of an abdominal heating pad or continuous positive airway pressure.35 People with supine hypertension may be encouraged to eat a carbohydrate-rich snack before bedtime and sleep with the head of the bed raised up to 10 degrees (13-inch elevation, the highest degree of bed tilt that can usually be tolerated).35 People should be cautious when getting out of a tilted bed during the night and in the morning to avoid falls. For persistent supine hypertension, medications may be necessary (Table 1). The ideal treatment would control nocturnal hypertension, reduce natriuresis, and improve morning orthostatic tolerance. Of the medications listed in Table 1, only losartan and clonidine reduce nocturnal natriuresis and none improve morning orthostatic tolerance. The use of clonidine to treat supine hypertension should be done with caution, as rebound hypertension has been described with this drug. The treatment of supine hypertension should be individualized. In our experience, losartan can be an effective first-line treatment for supine hypertension, but other short-acting antihypertensives might be considered (Table 1), while diuretics and alpha-adrenoceptor inhibitors should be avoided. Because most people with PD suffer from nocturia, treatment with antihypertensive medications during the night may increase the risk of falling when they go to the bathroom. We recommend people with PD with OH use a bedside commode.
Fig. 2
Non-pharmacological measures for orthostatic hypotension and supine hypertension.
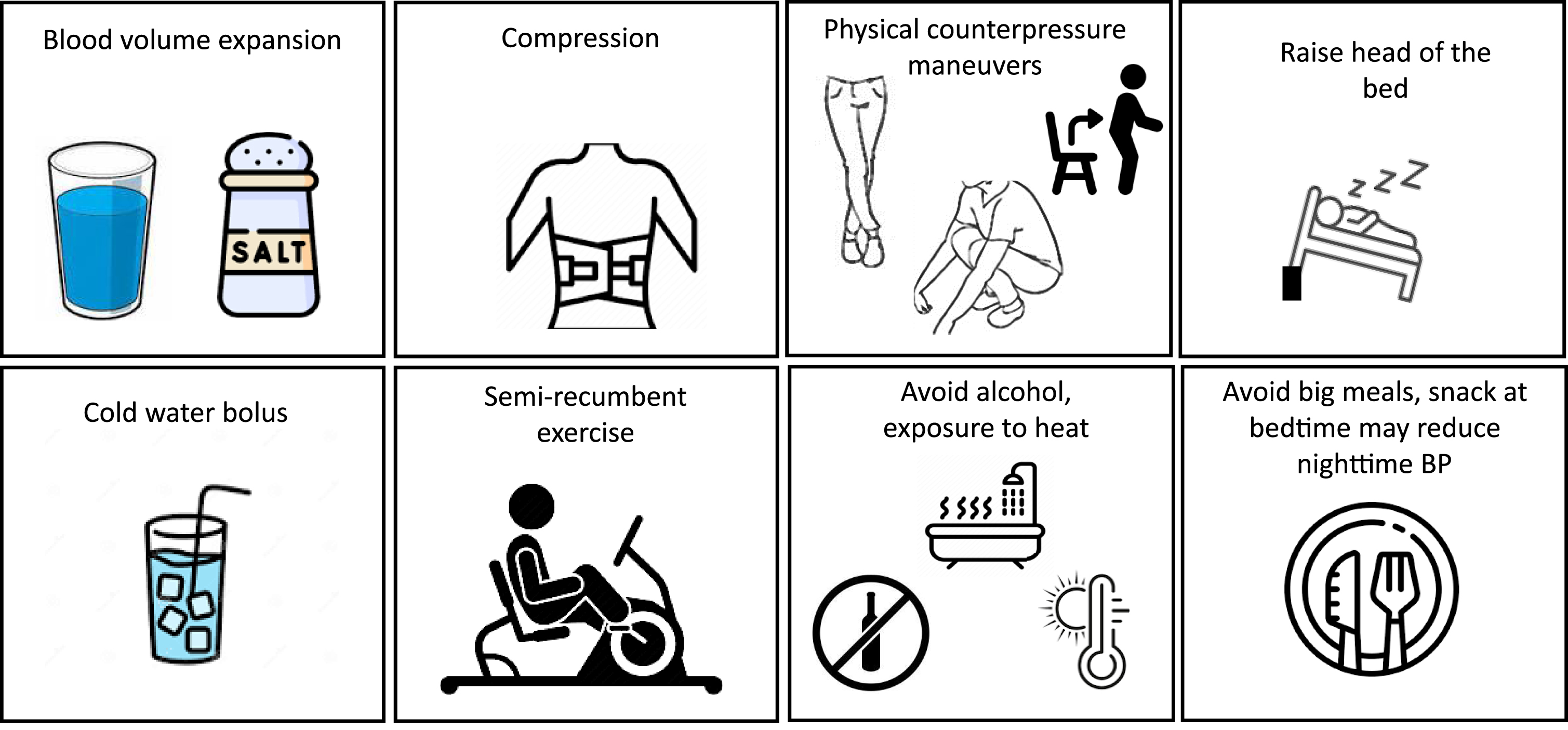
Table 1
Pharmacological agents to treat orthostatic hypotension and supine hypertension in Parkinson’s disease
| Orthostatic hypotension | |||
| Drug | Mechanism of action | Typical dosing for OH | Side effects |
| Midodrine | Alpha-1 agonist | Tablet: 2.5 mg, 5 mg, 10 mg Dosage: 2.5 to 10 mg, orally, 3-4 times per day | Scalp pruritus, piloerection, dysuria, paresthesia, supine hypertension |
| Droxidopa | Precursor of norepinephrine | Tablet: 100 mg, 200 mg, 300 mg Dosage: 100 to 600 mg, orally, 3 times per day | Headaches, hypertension, dizziness, nausea |
| Fludrocortisone | Mineralocorticoid. Promotes increased reabsorption of sodium and loss of potassium from renal distal tubules. | Tablet: 0.1 mg Dosage: 0.1 to 0.2 mg, orally, daily in the morning | Cardiac failure, cardiomegaly, edema, hypertension, hypokalemia |
| Pyridostigmine | Acetylcholinesterase inhibitor | Tablet: 30 mg, 60 mg ER: 180 mg Dosage: 30 to 60 mg 3 times daily, orally, or 180 mg ER daily, orally, in the morning | Diarrhea, abdominal pain, muscle contraction/twitching, increase secretion |
| Atomoxetine | Norepinephrine transporter inhibitor | Tablet: 18 mg daily, orally, in the morning | Hyperhidrosis, abdominal pain, erectile dysfunction, drowsiness, hypertension |
| Erythropoietin | Erythropoiesis-stimulating agents | 50 U per kilogram of body weight three times a week | Hypertension |
| Supine hypertension | |||
| Consider if supine BP consistently > 160 to 170 mmHg despite implementation of conservative measures. Use at bedtime (supine position should be avoided during the daytime) | |||
| Drug | Mechanism of action | Typical dosing for SH | Side effects |
| Losartan | Angiotensin receptor blockers, decrease nocturnal diuresis | Tablet: 25 to 50 mg | Hypotension, cough, edema |
| Nitroglycerin patch | Nitrate vasodilator | Patch 0.1 mg/h to 0.4 mg/h | Headache, hypotension, flushing, skin irritation |
| Nebivolol | Beta-blocker | Tablet: 5 mg, 10 mg, 20 mg | Headache, fatigue, hypotension, diarrhea, bronchospasm |
| Sildenafil | Selective inhibitor of cGMP-specific phosphodiesterase | Tablet: 25 mg to 50 mg | Flushing, diarrhea, dyspepsia, headache, back pain, hypotension |
| Clonidine | Stimulation of the pre-synaptic alpha 2 adrenoceptors, decrease nocturnal diuresis | Tablet 0.1 to 0.3 mg | Hypotension, contact dermatitis, abdominal pain |
| Nifedipine | Blocks voltage-dependent L-type calcium channels | Tablet: 30, 60, 90 mg | Flushing, peripheral edema, heartburn, hypotension |
| Eplerenone | Mineralocorticoid receptor antagonist | Tablet: 50 mg | Hyperkalemia, hyponatremia, hypertriglyceridemia |
Specific considerations
Approximately one to two-thirds of individuals with OH report no symptoms or minimal symptoms relative to the magnitude of their fall in blood pressure, a phenomenon called “hypotension unawareness”.36 Differences in cerebral blood flow regulation between people exhibiting symptoms of OH and those who do not may explain this phenomenon. The role of pressor medications in these cases is controversial, but the discovery of asymptomatic OH should prompt clinicians to review the medication list carefully and consider the possible role of OH as a risk factor for cognitive impairment, fatigue, and gait dysfunction.37 Levodopa, the gold standard treatment for the motor symptoms of PD, decreases blood pressure in PD with and without OH.38 Lower standing blood pressure with levodopa may increase the risks of falls and syncope. Hypotension has also been documented with other treatments for PD including dopamine agonists,39 amantadine, and selegiline. In people with PD with significant OH, some antiparkinsonian medications should be reduced to the minimum therapeutic dose or discontinued in a sequential manner starting with the highest risk-to-benefit ratio. Generally, dose reduction or discontinuation of dopamine agonists is attempted first, followed by amantadine, and monoamine oxidase B inhibitors. Levodopa should ideally be continued with optimal treatment of OH. A reasonable practical goal for the treatment of supine hypertension is a regimen that maintains supine blood pressure under 160 mmHg systolic and 90 mmHg diastolic. Postprandial hypotension is defined as a fall of at least 20 mmHg in SBP (usually in the sitting position) within two hours of eating.40 The optimal regimen to manage symptomatic postprandial hypotension has not been defined. However, modification of meals may be helpful in selected cases (avoiding large meals, ingesting meals low in carbohydrates, avoiding alcohol, and drinking water before and with meals).40 In selected cases, medications such as acarbose or somatostatin can be discussed to treat postprandial hypotension, but the effect on symptoms has not been adequately evaluated. Similarly, the optimal management of hypotension during and following exercise is unknown.41 For people with PD with OH, we recommend starting with seated or semi-recumbent exercise and drinking water before, during, and after exercise. The timing of pressor medications in relationship to exercise may be individualized.
WHAT DO WE TELL PEOPLE WITH PARKINSON’S IN MY CLINIC?
Orthostatic hypotension is common in PD and associated with more rapid disease progression, shorter survival time, falls, and increased risk of dementia. Clinicians and people with PD should be familiar with the “typical” and “atypical” symptoms of OH. Measurement of orthostatic vital signs should be done systematically at every office visit. The treatment of OH and supine hypertension should be individualized and is based on nonpharmacological measures and pharmacotherapy if needed. Future studies should investigate the impact of personalized treatment of OH and supine hypertension on disease progression.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Numbers U01NS113851 and K23NS123506. Research is also supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. It is also supported by a generous philanthropic gift in honor of Howard Gilbert and a gift from the JCS Family Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
Dr. McKee is a consultant for Ceraxis Inc.
REFERENCES
1. | Wieling W , Kaufmann H , Claydon VE , et al.. Diagnosis and treatment of orthostatic hypotension. Lancet Neurol (2022) ; 21: : 735–746. |
2. | Velseboer DC , de Haan RJ , Wieling W , et al.. Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord (2011) ; 17: : 724–729. |
3. | Hiorth YH , Pedersen KF , Dalen I , et al.. Orthostatic hypotension in Parkinson disease: A 7-year prospective population-based study. Neurology (2019) ; 93: : e1526–e1534. |
4. | Kim JB , Kim BJ , Koh SB , et al.. Autonomic dysfunction according to disease progression in Parkinson’s disease. Parkinsonism Relat Disord (2014) ; 20: : 303–307. |
5. | Kaufmann H , Norcliffe-Kaufmann L , Palma JA , et al.. Natural history of pure autonomic failure: A United States prospective cohort. Ann Neurol (2017) ; 81: : 287–297. |
6. | Baschieri F , Sambati L , Guaraldi P , et al.. Neurogenic orthostatic hypotension in early stage Parkinson’s disease: New insights from the first 105 patients of the BoProPark study. Parkinsonism Relat Disord (2021) ; 93: : 12–18. |
7. | Gibbons CH and Freeman R . Clinical implications of delayed orthostatic hypotension: A 10-year follow-up study. Neurology (2015) ; 85: : 1362–1367. |
8. | Kauppila LA , Ten Holter SEM , van de Warrenburg B , et al. A guide for the differential diagnosis of multiple system atrophy in clinical practice. J Parkinsons Dis (2022) ; 12: : 2015–2027. |
9. | Merola A , Romagnolo A , Dwivedi AK , et al.. Benign versus malignant Parkinson disease: the unexpected silver lining of motor complications. J Neurol (2020) ; 267: : 2949–2960. |
10. | De Pablo-Fernandez E , Tur C , Revesz T , et al.. Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol (2017) ; 74: : 970–976. |
11. | Fanciulli A , Campese N , Goebel G , et al.. Association of transient orthostatic hypotension with falls and syncope in patients with Parkinson disease. Neurology (2020) ; 95: : e2854–e2865. |
12. | Fasano A , Canning CG , Hausdorff JM , et al.. Falls in Parkinson’s disease: A complex and evolving picture. Mov Disord (2017) ; 32: : 1524–1536. |
13. | Longardner K , Bayram E and Litvan I . Orthostatic hypotension is associated with cognitive decline in Parkinson disease. Front Neurol (2020) ; 11: : 897. |
14. | Ruiz Barrio I , Miki Y , Jaunmuktane ZT , et al.. Association between orthostatic hypotension and dementia in patients with Parkinson disease and multiple system atrophy. Neurology (2023) ; 100: : e998–e1008. |
15. | Palma JA , Redel-Traub G , Porciuncula A , et al.. The impact of supine hypertension on target organ damage and survival in patients with synucleinopathies and neurogenic orthostatic hypotension. Parkinsonism Relat Disord (2020) ; 75: : 97–104. |
16. | Corcos DM , Lamotte G , Luthra NS , et al.. Advice to people with Parkinson’s in my clinic: exercise. J Parkinsons Dis (2024) ; 14: : 609–617. |
17. | Lamotte G and Lenka A . Orthostatic hypotension in Parkinson disease: what is new? Neurol Clin Pract (2022) ; 12: : e112–e115. |
18. | Centi J , Freeman R , Gibbons CH , et al.. Effects of orthostatic hypotension on cognition in Parkinson disease. Neurology (2017) ; 88: : 17–24. |
19. | Cheshire WP Jr. . Hypotensive akathisia: autonomic failure associated with leg fidgeting while sitting. Neurology (2000) ; 55: : 1923–1926. |
20. | Goldstein DS and Cheshire WP Jr. . The autonomic medical history. Clin Auton Res (2017) ; 27: : 223–233. |
21. | Cani I , Guaraldi P , Giannini G , et al.. Levodopa-induced orthostatic hypotension in parkinsonism: A red flag of autonomic failure. Eur J Neurol (2024) ; 31: : e16061. |
22. | Norcliffe-Kaufmann L , Kaufmann H , Palma JA , et al.. Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol (2018) ; 83: : 522–531. |
23. | Balagny P , Wanono R , d’Ortho MP , et al.. Reply to validation of the new diagnostic tests for neurogenic orthostatic hypotension. Ann Neurol (2018) ; 84: : 957–958. |
24. | Low P and Singer W . The arterial baroreflex in neurogenic orthostatic hypotension. Clin Auton Res (2023) ; 33: : 81–82. |
25. | Fanciulli A , Jordan J , Biaggioni I , et al.. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res (2018) ; 28: : 355–362. |
26. | Lim KB , Lim SY , Hor JW , et al.. Orthostatic hypotension in Parkinson’s disease: Sit-to-stand vs. supine-to-stand protocol and clinical correlates. Parkinsonism Relat Disord (2024) ; 123: : 106980. |
27. | Cooke J , Carew S , O’Connor M , et al.. Sitting and standing blood pressure measurements are not accurate for the diagnosis of orthostatic hypotension. QJM (2009) ; 102: : 335–339. |
28. | Casiglia E , Tikhonoff V , Albertini F , et al.. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension (2016) ; 68: : 896–903. |
29. | Juraschek SP , Vyavahare M , Cluett JL , et al.. Comparison of home and office blood pressure devices in the clinical setting. Am J Hypertens (2024) ; 37: : 342–348. |
30. | Vallelonga F , Valente M , Tangari MM , et al.. Hypotensive episodes at 24-h ambulatory blood pressure monitoring predict adverse outcomes in Parkinson’s disease. Clin Auton Res (2024) ; 34: : 281–291. |
31. | Gibbons CH , Schmidt P , Biaggioni I , et al.. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol (2017) ; 264: : 1567–1582. |
32. | Jordan J , Shannon JR , Black BK , et al.. The pressor response to water drinking in humans: a sympathetic reflex? Circulation (2000) ; 101: : 504–509. |
33. | Juraschek SP , Cortez MM , Flack JM , et al.. Orthostatic hypotension in adults with hypertension: a scientific statement from the American Heart Association. Hypertension (2024) ; 81: : e16–e30. |
34. | Olshansky B and Muldowney J . Cardiovascular safety considerations in the treatment of neurogenic orthostatic hypotension. Am J Cardiol (2020) ; 125: : 1582–1593. |
35. | Park JW , Okamoto LE and Biaggioni I . Advances in the pathophysiology and management of supine hypertension in patients with neurogenic orthostatic hypotension. Curr Hypertens Rep (2022) ; 24: : 45–54. |
36. | Palma JA , Gomez-Esteban JC , Norcliffe-Kaufmann L , et al.. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord (2015) ; 30: : 639–645. |
37. | Lenka A , Lamotte G and Beach P . Asymptomatic orthostatic hypotension in synucleinopathies: to treat or not to treat? Clin Auton Res (2024) ; 34: : 25–29. |
38. | Earl T , Jridi A , Thulin PC , et al.. Effect of levodopa on postural blood pressure changes in Parkinson disease: a randomized crossover study. Clin Auton Res (2024) ; 34: : 117–124. |
39. | Kujawa K , Leurgans S , Raman R , et al.. Acute orthostatic hypotension when starting dopamine agonists in Parkinson’s disease. Arch Neurol (2000) ; 57: : 1461–1463. |
40. | Jansen RW and Lipsitz LA . Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Ann Intern Med (1995) ; 122: : 286–295. |
41. | Low DA , Vichayanrat E , Iodice V , et al.. Exercise hemodynamics in Parkinson’s disease and autonomic dysfunction. Parkinsonism Relat Disord (2014) ; 20: : 549–553. |




