Modulation of Cerebellar Oscillations with Subthalamic Stimulation in Patients with Parkinson’s Disease
Abstract
Background:
Deep brain stimulation (DBS) targeting the subthalamic nucleus (STN) has emerged as a potent treatment for alleviating motor symptoms in Parkinson’s disease (PD). Despite its effectiveness, the impact of high frequency STN-DBS on cerebellar oscillations remains unclear, posing an intriguing challenge for neural modulation. Given the direct and indirect connections between the STN and cerebellum, we investigated whether STN-DBS affects cerebellar oscillations.
Objective:
To observe the effects of STN-DBS on cerebellar oscillations in patients with PD.
Methods:
We recruited 15 PD patients receiving STN-DBS. Electroencephalographic (EEG) signals were recorded from cerebellar regions during resting-state conditions in both the OFF-DBS and STN-DBS conditions. Our analyses centered on spectral features, particularly theta and beta oscillations, guided by prior research and correlation tests to investigate the relationship between oscillatory changes and motor symptom severity.
Results:
In the mid-cerebellar (Cbz) region, we observed a significant increase in the relative power in all frequency bands, including theta and beta oscillations during STN-DBS, showing the global effect of DBS. Importantly, the correlation results indicated significant associations between mid-cerebellar (Cbz) beta power during the OFF condition and motor severity, which were not evident during STN-DBS. Interestingly, correlations between beta power and motor severity were not observed at the mid-occipital (Oz) and mid-frontal (Cz) regions. Notably, signal similarity analyses demonstrated no evidence of volume conduction effects between the mid-cerebellar (Cbz) and nearby mid-occipital (Oz) regions.
Conclusions:
While these findings provide valuable insights into the complex interplay between STN-DBS and neural oscillations, further research is essential to decipher their precise functional significance and clinical implications. Understanding these intricacies may contribute to the optimization of DBS therapies for PD.
Plain Language Summary
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is an effective treatment for reducing motor symptoms in Parkinson’s disease (PD). However, its effects on brain activity, specifically in the cerebellum, are not well understood. This study aimed to investigate how STN-DBS affects cerebellar brain waves in PD patients. We recruited 15 PD patients undergoing STN-DBS and recorded their brain activity including cerebellar region using EEG. We compared the brain wave patterns during periods when the DBS was turned OFF and when it was turned ON, focusing on specific brain wave frequencies (theta and beta). The results showed a significant increase in brain wave power across all frequencies in the mid-cerebellar region during STN-DBS. Additionally, there was a strong link between beta power in the cerebellum and motor symptom severity when DBS was OFF, which was not present when DBS was ON. This relationship was specific to the cerebellum and not found in other brain regions. The findings suggest that STN-DBS significantly alters cerebellar brain activity and that these changes are related to improvements in motor symptoms. However, more research is needed to fully understand the functional significance and potential clinical applications of these findings for optimizing DBS treatment in PD patients.
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by motor and non-motor symptoms.1 While these symptoms primarily result from the degeneration of dopaminergic neurons in the substantia nigra, research has shown that other brain regions, including the cerebellum2,3 and the subthalamic nucleus (STN),4,5 also play significant roles in the pathophysiology of the symptomology of PD. The cerebellum is usually associated with voluntary movement, motor coordination, balance, and cognitive functions.6 Emerging evidence also suggests the involvement of cerebellar activity in motor and non-motor symptoms of PD.7,8 Specifically, abnormal oscillatory activities in the cerebellum, particularly in the theta (4–7 Hz) and beta (13–30 Hz) frequency ranges, have been observed in patients with PD and these abnormal oscillations are thought to contribute to motor and non-motor dysfunctions.9–11
Deep brain stimulation (DBS) is a surgical treatment option to alleviate motor and non-motor symptoms of PD that involves the implantation of electrodes into the subcortical regions, such as the STN.12 While the exact mechanisms underlying the therapeutic effects of DBS are not fully understood, one proposed hypothesis is that it modulates abnormal cerebellar oscillations.13,14 Studies in both animals and humans have demonstrated the presence of direct and indirect anatomical connections between the STN and cerebellar regions.15,16 Some studies suggest the existence of direct connections between the STN and specific regions of the cerebellum, such as the cerebellar cortex and the deep cerebellar nuclei.17 In addition, the cerebellum has indirect connections to the STN through the pedunculopontine nucleus (PPN) in the brainstem. These connections are part of the complex neural circuitry within the brain that plays a role in motor control and coordination. The cerebellum is involved in fine-tuning motor movements, and the STN is implicated in processes related to movement, particularly in the context of conditions like PD, and the PPN serves as an intermediary in this network of connections.18 The outputs of the basal ganglia and the cerebellum influence many of the same cortical areas by projecting to distinct thalamic nuclei and communicate with the cortex through different thalamic pathways.16 Interestingly, rodent studies have shown that high-frequency stimulation of the STN can activate deep cerebellar nuclei19 and increase spike activity in those deep cerebellar nuclei, which is likely from reduced afferent activity of Purkinje cells.20 An MRI study demonstrated the functional recruitment of subcortical-cerebellar pathways during the movement task.21 Additionally, DBS was found to have modulatory effects on several basal ganglia pathways, along with the ability to modulate local cortical and cerebellar circuits.22 Altogether, it seems plausible that STN-DBS may modulate cerebellar oscillatory activity due to the direct connections between STN and cerebellar areas. This modulation is hypothesized to be associated with the improvement of PD symptoms observed in patients receiving STN-DBS. However, the effects of therapeutic STN-DBS on cerebellar oscillations in patients with PD have not been demonstrated. Here, we collected cerebellar EEG signals during a resting-state condition in patients with PD during OFF-DBS and high-frequency STN-DBS.
MATERIALS AND METHODS
We recruited 15 PD subjects with bilateral STN DBS electrodes for this study. All participants provided their written informed consent and procedures were approved by the University of Iowa Institutional Review Board in accordance with the declaration of Helsinki. To ensure that participants fit the requirements for the United Kingdom PD Society Brain Bank criteria, all diagnoses were confirmed by an independent neurologist. All participants were assessed during their regular administration of prescribed levodopa medication. PD individuals experiencing levodopa-induced involuntary movements or tremor at the resting-state were excluded from the study. The severity of the disease in PD participants was evaluated using the motor section of the Unified Parkinson’s Disease Rating Scale (mUPDRS). A summary of participant characteristics can be found in Table 1.
Table 1
Summary of Participant Characteristics
| OFF-DBS | HF-DBS | t-value (p) | |
| Gender (M/F) | 13/2 | – | – |
| Age, y | 63.4±4.77 | – | – |
| DD | 9.53±1.01 | – | – |
| LEDD (mg) | 955±181 | – | – |
| MOCA | 23.1±1 | – | – |
| mUPDRS | 39.8±5.32 | 23.6±3.53 | 3.82 (0.002)** |
Values shown as mean±SEM. DD, disease duration; LEDD, Levodopa equivalent daily dose; MOCA, Montreal Cognitive Assessment; mUPDRS, motor portion of the Unified Parkinson’s Disease Rating Scale. MOCA was performed when DBS was ON with therapeutic settings. **p < 0.01 for paired samples t-test.
EEG data during resting-state were collected using a 64-channel cap at a 500 Hz sampling rate using a 0.1 Hz high-pass filter with Pz used as a reference. This cap was customized to include left cerebellar (Cb1), right cerebellar (Cb2), and mid-cerebellar (Cbz) electrodes positioned over the posterior fossa corresponding to the medial aspects of cerebellar lobules VII, VIII, and IX23,24 (Supplementary Figure 1). During the resting state task, participants were seated comfortably with their eyes open for 180 s while we collected EEG signals. In addition, participants either received STN-DBS at a high frequency (120–175 Hz, see Table 2) or received 0 Hz stimulation (OFF). The patients were fully aware of the STN-DBS being in the OFF condition throughout the study. They were not blinded, as it was important for ethical reasons to ensure they felt comfortable during the periods when the stimulation was turned off, which was often physically noticeable. We observed that the stimulation frequency varied across patients, ranging from 110 to 175 Hz. This variability is common in clinical settings and is typically tailored to each patient’s specific therapeutic needs. Despite this range, all applied stimulation frequencies effectively induced therapeutic effects and modulated both cortical and subcortical oscillations.25,26 These findings underscore the robustness of the therapeutic effects of DBS across different stimulation parameters. This information highlights the adaptability of DBS in achieving desired clinical outcomes despite heterogeneous stimulation settings.27 The order of stimulation was counterbalanced across participants and there was a 20-min washout period between stimulation types.
Table 2
Summary of DBS Settings
| Right STN | Left STN | |||||
| Subject | Frequency | Pulse Width | Amplitude | Frequency | Pulse Width | Amplitude |
| 1 | 130 Hz | 60 μs | 1.0 V | 130 Hz | 60 μs | 1.0 V |
| 2 | 130 Hz | 60 μs | 1.6 V | 130 Hz | 60 μs | 2.15 V |
| 3 | 150 Hz | 90 μs | 3.8 V | 150 Hz | 80 μs | 3.5 V |
| 4 | 130 Hz | 90 μs | 1.5 V | 130 Hz | 90 μs | 5.6 V |
| 5 | 130 Hz | 60 μs | 1.7 V | 130 Hz | 60 μs | 1.4 V |
| 6 | 110 Hz | 100 μs | 4.35 V | 110 Hz | 150 μs | 4.25 V |
| 7 | 120 Hz | 60 μs | 4 V | 120 Hz | 70 μs | 3.4 V |
| 8 | 130 Hz | 90 μs | 3.3 V | 130 Hz | 90 μs | 3.2 V |
| 9 | 130 Hz | 60 μs | 1.1 V | 130 Hz | 60 μs | 1.45 V |
| 10 | 140 Hz | 110 μs | 3.9 V | 140 Hz | 110 μs | 3.9 V |
| 11 | 175 Hz | 60 μs | 2.5 V | 175 Hz | 60 μs | 2.3 V |
| 12 | 125 Hz | 60 μs | 1.95 V | 125 Hz | 60 μs | 1.4 V |
| 13 | 160 Hz | 90 μs | 2.2 V | 160 Hz | 90 μs | 2.4 V |
| 14 | 140 Hz | 70 μs | 4.05 V | 140 Hz | 60 μs | 1.95 V |
| 15 | 130 Hz | 90 μs | 3.4 V | 130 Hz | 70 μs | 3.2 V |
STN-DBS can induce transient sensory effects that might potentially influence study outcomes. To mitigate this influence, we adopted a precautionary measure. Following any alteration in stimulation frequency, we implemented a waiting period of at least 20 min before initiating the data collection to achieve the best ON-DBS response. This waiting period allowed for the gradual dissipation of any lingering effects from the previous stimulation settings. Notably, aside from changes in stimulation frequency, all other stimulation parameters remained consistent. This included the maintenance of the same electrode contacts, stimulation amplitude, and pulse width as determined based on each participant’s individualized, chronic DBS settings that produced the optimal clinical response. This ensured that any effects observed during the resting period were mainly due to changes in stimulation frequency (OFF vs. ON) and were not influenced by simultaneous adjustments in other stimulation parameters.
After data collection, EEG signals were processed in EEGLAB. We removed electrodes Fp1, Fp2, FT9, FT10, TP9, and TP10 due to their susceptibility to eye-blink and muscle artifact. Data were band-pass filtered (0.1–50 Hz), re-referenced to the average, and epoched into 4-s consecutive epochs. Initially, we applied MATLAB-based functions DBSFILT (https://sourceforge.net/projects/dbsfilt/files/)28 toremove DBS-induced artifacts. This function comprises three key steps: temporal filtering, spike detection using the Hampel identifier, and spike removal through interpolation.29,30 This approach effectively enhances data quality by addressing unwanted DBS-related artifacts (Supplementary Figure 2). Later, bad epochs and artifacts were removed from the dataset using the FASTER,31 as well as the pop_rejchan function and ICA for eye-blinks in EEGLAB.32 In addition to removing channels Fp1, Fp2, FT9, FT10, TP9, and TP10, ICA was employed to further remove eye-blink artifacts and ensure that nearby channels were not affected by residual artifacts.
We performed spectral analyses on each epoch using the pwelch method, employing a 1-second window with 50% overlap in window length. The average spectral power across all epochs was then computed for each recording. Later, relative power (frequency-specific power/total spectral power) was computed across the theta (4–7 Hz) and beta (13–30 Hz) frequency bands since we were primarily interested in examining the effect of HF-DBS on cerebellar theta and beta oscillations. However, we also examined the delta (1–4 Hz), alpha (7–13 Hz), and gamma (30–50 Hz) frequency bands (see Supplementary Material). In addition to examining cerebellar electrodes, we also compared a nearby occipital electrode with the cerebellar leads in separate analyses10,33 to examine the conduction effects (see Supplementary Material). We exported the mean relative power values of frequency bands for each participant in both the HF-DBS and OFF-DBS conditions. These mean power values were subsequently used for all analyses.
We performed separate 2 (condition: OFF-DBS, HF-DBS) x 3 (electrode: Cb1, Cbz, Cb2) repeated measure ANOVAs in the theta (4–7 Hz) and beta (13–30 Hz) frequency bands to determine the effects of HF-DBS on cerebellar theta and beta oscillations. Moreover, we investigated potential associations between mid-cerebellar (Cbz) theta/beta oscillations and mUPDRS scores (UPDRSIII) in both HF-DBS and OFF-DBS conditions through Pearson’s correlations. To emphasize the specificity of the mid-cerebellar (Cbz) region, we extended our analysis to include similar correlation assessments for the mid-occipital (Oz) and mid-frontal (Cz) regions (see Supplementary Material). Additionally, we performed signal similarity analyses, comparing mid-cerebellar (Cbz) signals with neighboring mid-occipital (Oz) signals across delta, alpha, and gamma frequencies. Finally, we examined the similarity between mid-cerebellar (Cbz) and mid-occipital (Oz) signals using a 2 (condition: OFF-DBS, HF-DBS) x 2 (electrode: Cbz, Oz) rmANOVA as well as various similarity analyses, including coherence estimates, phase coherence, and cross-spectrum phase analysis. Coherence estimates measure linear correlation in the frequency domain, identifying synchronized frequency bands. Phase coherence analyzes the consistency of phase differences over time, revealing phase-locking and functional relationships. Cross-spectrum phase analysis examines phase differences across frequencies, exposing lead-lag relationships and synchronized frequency bands. By applying these techniques, we uncovered synchronization, coupling, and coordination between Cbz and nearby Oz signals.
RESULTS
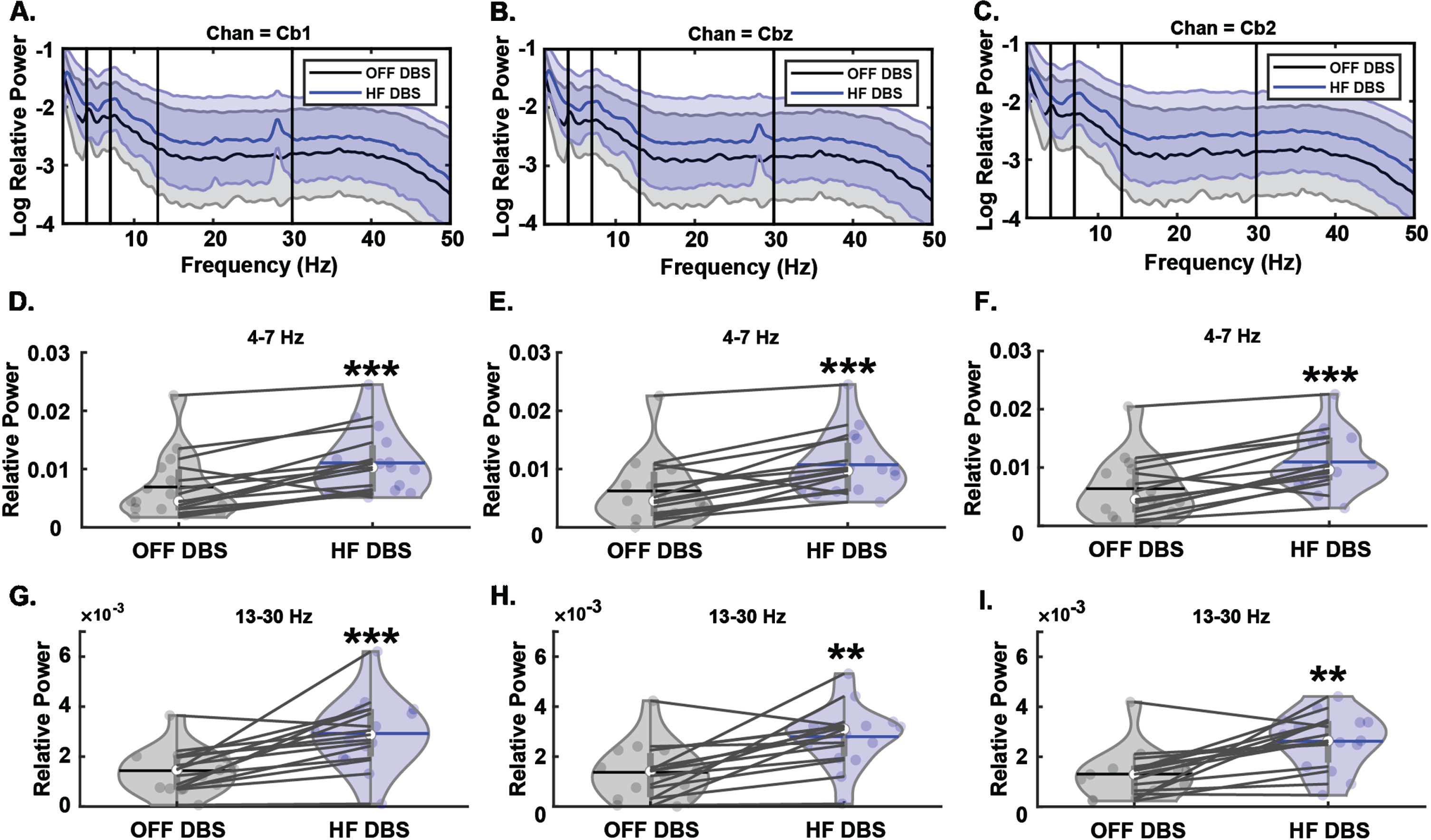
In the theta band, we observed a main effect of condition (F1,14 = 28.14, p < 0.001) with the HF-DBS condition producing greater relative power compared to the OFF-DBS condition (Fig. 1). There was no main effect of electrode (F2,28 = 0.29, p = 0.75) nor was there an interaction between condition and electrode (F2,28 = 0.95, p = 0.4). In the beta band, there was also a main effect of condition (F1,14 = 19.01, p < 0.001) with HF-DBS producing greater relative power compared to OFF-DBS (Fig. 1). Similar to theta, there was no main effect of electrode (F2,28 = 1.50, p = 0.24) nor an interaction between these factors (F2,28 = 0.24, p = 0.79). These results demonstrate that HF-DBS produces increased theta and beta power over cerebellar electrode sites, but these increases do not localize to a single electrode in the cerebellar cluster.
Fig. 1
Effects of HF STN-DBS on cerebellar theta and beta oscillations. A–C) Spectral power differences between OFF-DBS and HF-DBS at Cb1, Cbz, and Cb2. D–F) HF STN-DBS increases relative theta-band power across Cb1, Cbz, and Cb2 cerebellar electrode sites. G–I) HF STN-DBS increases relative beta-band power across Cb1, Cbz, and Cb2 cerebellar electrode sites. **p < 0.01, ***p < 0.001 vs. OFF-DBS. In violin plots, horizontal lines and white circles represent the mean and median values, respectively.
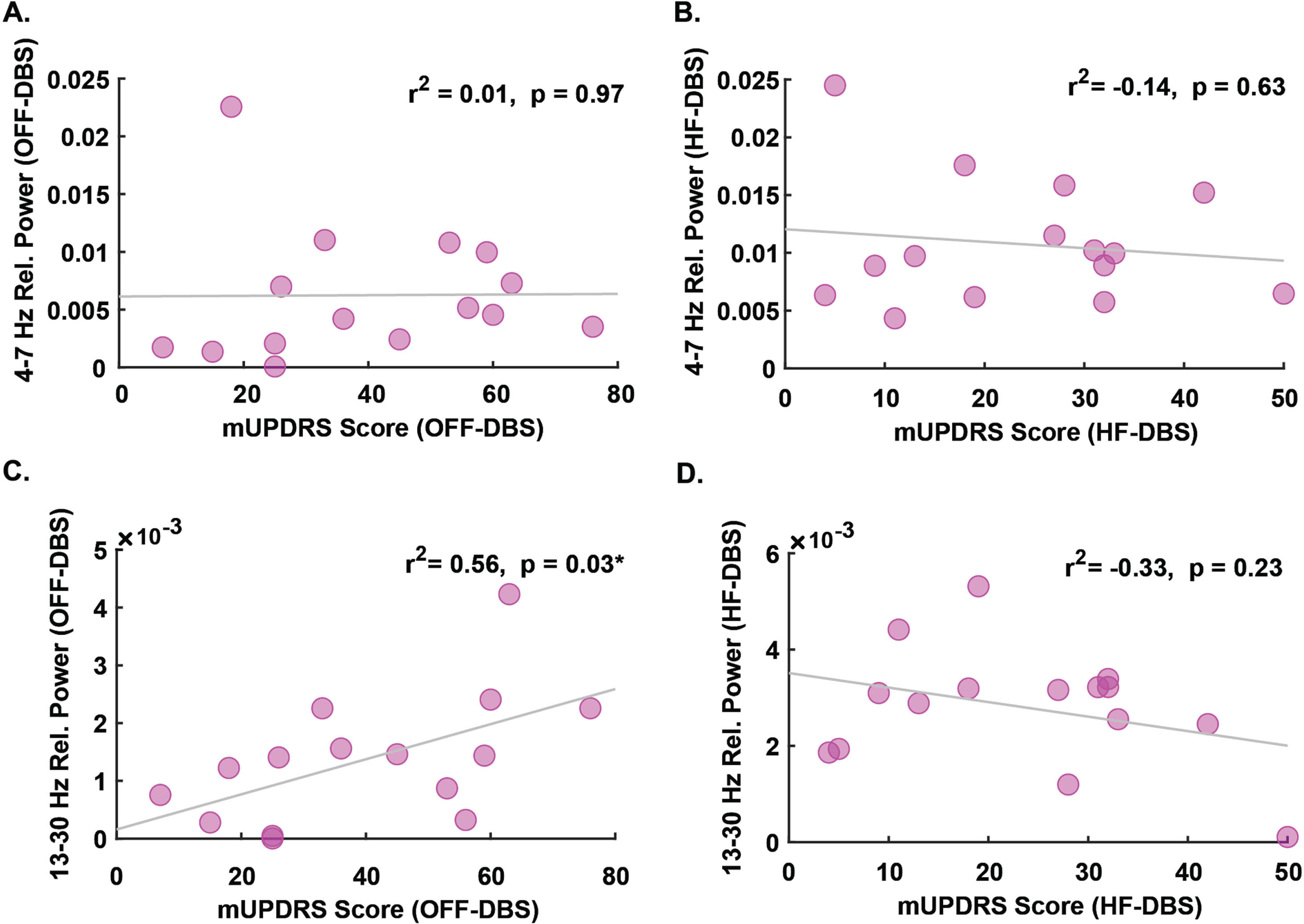
In addition to examining changes in relative power, we also examined associations between mid-cerebellar (Cbz) power and mUPDRS scores. We found no association between mid-cerebellar (Cbz) theta power and mUPDRS scores in either the OFF-DBS condition (r2 = 0.01, p = 0.97, Fig. 2A) or the HF-DBS condition (r2 = –0.14, p = 0.63, Fig. 2B). In the beta band, there was an association between mid-cerebellar (Cbz) beta power during the OFF-DBS condition and mUPDRS scores (r2 = 0.56, p = 0.03, Fig. 2 C), though no association was observed during the HF-DBS condition (r2 = –0.33, p = 0.23, Fig. 2D). Correlation analyses between mUPDRS scores and each cerebellar electrode across all frequency bands can be viewed in Table S1 (see Supplementary Material). These striking findings illustrate that the baseline associations between cerebellar beta oscillations and PD severity are diminished during HF-DBS and can be recorded from cerebellar locations.
Fig. 2
Association between mid-cerebellar theta and beta and PD severity. No association between relative theta-band power and mUPDRS scores during A) OFF-DBS or B) HF-DBS. C) Relative beta-band power is positively associated with mUPDRS scores during OFF-DBS. D) No association between relative beta-band power and mUPDRS during HF-DBS. mUPDRS, motor portion of the Unified Parkinson’s Disease Rating Scale. *p < 0.05.
In addition to the theta and beta frequencies, we also examined the delta (1–4 Hz), alpha (7–13 Hz), and gamma (30–50 Hz) frequencies. In all three frequency bands, we observed main effects of condition, with HF-DBS facilitating increased relative power across cerebellar electrode sites (delta: F1,14 = 11.23, p = 0.005; alpha: F1,14 = 46.63, p < 0.001; gamma: F1,14 = 10.06, p = 0.007; Fig. S3, see Supplementary Material). Similar to the theta and beta frequency bands, no main effects of electrode (delta: F2,28 = 0.036, p = 0.96; alpha: F2,28 = 0.51, p = 0.61; gamma: F2,28 = 0.53, p = 0.59) nor interactions between electrode and condition were observed (delta: F2,28 = 1.88, p = 0.17; alpha: F2,28 = 2.36, p = 0.11; gamma: F2,28 = 0.82, p = 0.45).
Though our primary interest was to determine whether HF-DBS produces changes in resting-state theta and beta oscillations in PD subjects at cerebellar electrode sites, we also examined mid-occipital (Oz) electrode site in relation to the mid-cerebellar (Cbz) site. When examining the occipital site, we observed main effects of condition in multiple frequency bands (delta: F1,14 = 14.86, p = 0.002; theta: F1,14 = 40.85, p < 0.001; alpha: F1,14 = 24.95, p < 0.001; beta: F1,14 = 13.64, p = 0.002; gamma: F1,14 = 10.32, p = 0.006; Fig. S4, see Supplementary Material). There were also main effects of electrode for theta (F1,14 = 4.47, p = 0.05), alpha (F1,14 = 5.24, p = 0.038), beta (F1,14 = 5.01, p = 0.042), and gamma (F1,14 = 9.05, p = 0.009), but no effect of electrode for delta (F1,14 = 0.56, p = 0.47). Relative power for theta, alpha, beta, and gamma frequency bands for the mid-cerebellar (Cbz) electrode was higher compared to the mid-occipital (Oz) electrode. No interaction effects were observed across all frequency bands (delta: F1,14 = 0.052, p = 0.82; theta: F1,14 = 2.32, p = 0.15; alpha: F1,14 = 0.16, p = 0.23; beta: F1,14 = 0.003, p = 0.96; gamma: F1,14 = 1.28, p = 0.28). These additional analyses demonstrate that HF-DBS produces increased relative power across multiple electrode sites and frequency bands. In addition, the main effects of electrode between cerebellar and occipital sites indicate separate and distinct signals at each site despite their proximity. Notably, similar to our previous work, this can be further visualized via similarity analyses between Cbz and Oz using cross-correlation, coherence estimates, phase coherence, and cross-spectrum phase analysis (Supplementary Figure 5).10
Considering the remarkable finding that mid-cerebellar (Cbz) beta power not only associates with PD motor severity but also diminishes after HF STN-DBS, we also sought to examine whether this relationship existed at mid-occipital (Oz) or mid-central (Cz) sites, which would indicate a non-specific, global effect. We found no associations between mid-occipital (Oz) beta power and mUPDRS in either the OFF-DBS or HF-DBS conditions. Similarly, we found no associations between mid-central (Cz) beta power and mUPDRS in either condition (see Table 3). Importantly, these findings demonstrate that although HF-DBS produces a global increase in power across multiple frequency bands and electrode locations, the association and subsequent change in association with mUPDRS scores is localized to the mid-cerebellar (Cbz) electrode site. Furthermore, this association diminishes after applying HF-DBS, a finding which may have widespread implications for the impact of HF-DBS on cerebellar function in PD.
Table 3
Associations between relative EEG power and mUPDRS scores
| Cbz | Oz | Cz | |
| Theta (4– 7 Hz) | |||
| OFF-DBS | 0.01 (0.97) | –0.01 (0.97) | –0.1 (0.72) |
| HF-DBS | –0.14 (0.63) | –0.22 (0.43) | –0.34 (0.22) |
| Beta (13– 30 Hz) | |||
| OFF-DBS | 0.56 (0.03)* | 0.37 (0.17) | 0.03 (0.93) |
| HF-DBS | – 0.33 (0.23) | –0.2 (0.47) | –0.06 (0.84) |
Values shown as r2 (p-value). mUPDRS, motor portion of the Unified Parkinson’s Disease Rating Scale. * p < 0.05 for Pearson’s correlation coefficient of determination.
Additionally, we performed frequency and correlation analyses (as described above) on the right and left sensorimotor channels. Our results indicated that DBS-ON did not significantly modulate beta band power in the left sensorimotor region (t14 = –2.0, p = 0.065; Supplementary Table 2). Moreover, no significant correlations were found between beta power and mUPDRS scores in either the right or left sensorimotor channels (p > 0.05; SupplementaryTable 3).
DISCUSSION
Our findings present intriguing insights into the impact of therapeutic HF STN-DBS on cerebellar oscillatory activity in individuals with PD. We observed a comprehensive effect of DBS on cerebellar oscillations, including well studied theta and beta frequencies, demonstrating an increase in power compared to the OFF-DBS condition. Notably, the increased beta band power correlated with PD motor symptoms in the OFF-DBS condition, and this correlation diminished in the ON-DBS condition, specifically within the mid-cerebellar (Cbz) region as opposed to mid-occipital (Oz) or mid-frontal (Cz) regions. These results suggest that STN-DBS may indeed exert a modulatory influence on cerebellar oscillations.
Theta and beta oscillations have been implicated in various motor and cognitive processes in PD.34,35 Theta oscillations are associated with memory, attention, and sensorimotor integration.36 Beta oscillations are closely linked to motor control and have been shown to play a role in the generation and suppression of movement.37,38 The observed increase in both theta and beta power over cerebellar electrode sites during HF-DBS raises questions about their specific functional significance in PD. The increase in theta and beta power during HF-DBS may suggest enhanced synchronization or altered information processing within cerebellar circuits. Interestingly, a magnetoencephalography study observed increased cortical theta power during the resting state after HF-STN-DBS.39 This study also demonstrated increased average power in the 1 to 48 Hz range in whole cortex after stimulation. Prior investigations have shown that STN-DBS is capable of dampening cortical and subcortical beta oscillations, which have been correlated with improvements in upper-limb motor function.37,40 Nevertheless, the impact of HF STN-DBS on lower-limb movements and the associated beta oscillations has yielded less consistent results.41,42 Some studies have reported an increase in cortical beta oscillations following STN DBS, particularly during the immediate post-stimulation period. This phenomenon is sometimes referred to as “beta rebound.” However, it has been suggested that this increase is usually temporary and is followed by a subsequent decrease.43,44 Overall, one potential interpretation of our results is that STN-DBS affects the cerebello-thalamo-cortical network,16 potentially leading to improved motor control and symptom relief in PD. It appears that network-level modulation via STN-DBS may indirectly influence cerebellar oscillations, similar to its impact on cortical oscillations. However, the effects on cerebellar oscillations may vary due to the intricate network connections and interactions with other nuclei. Therefore, further research is needed to unravel the precise functional consequences of these oscillatory changes and their relationship to clinical outcomes.
Aberrant oscillatory activity, especially in the theta frequency range, has been observed in both the STN and the cerebellum in PD.33,38 Dysregulated synchronization between these structures may play a pivotal role in the manifestation of motor symptoms in PD. Given the anatomical connections between the STN and cerebellum, it is plausible that the STN-cerebellar connection serves as a regulatory mechanism, coordinating oscillatory activity between these regions. The modulation of cerebellar oscillations by STN-DBS, as observed in our study, may represent an avenue through which abnormal synchronization is corrected or mitigated. An intriguing possibility is that the STN-cerebellar connection serves as a compensatory mechanism in response to motor deficits in PD. The cerebellum, with its well-established role in motor coordination, may provide alternative pathways for motor information processing when the basal ganglia circuitry is impaired.16 In this context, the observed increase in theta and beta power during HF-DBS might reflect a functional adaptation to enhance motor coordination and compensate for basal ganglia dysfunction.
Notably, our study examined the relationship between cerebellar oscillations and the motor symptoms of PD as assessed by the mUPDRS. Interestingly, we found no significant association between theta power and mUPDRS scores in either the OFF-DBS or HF-DBS conditions. This lack of association suggests that changes in cerebellar theta oscillations may not directly correlate with the motor symptom severity of PD in the context of STN-DBS. However, in the beta band, we observed a significant association between beta power during the OFF-DBS condition and mUPDRS scores. This association, which reflects increased beta power being associated with more severe motor symptoms, underscores the potential relevance of cerebellar beta oscillations as a biomarker of motor impairment in PD, similar to motor cortical and sub-cortical beta oscillations.37 Intriguingly, this association was no longer significant during the HF-DBS condition. Here, we focused our correlation analysis on the mid-cerebellar (’Cbz’) electrode that was based on its anatomical proximity to relevant neural circuits involved in motor control. Even prior studies have shown the involvement of mid-cerebellar region in motor control and its potential relationship with motor severity in PD.10,33,45 This observation raises the possibility that HF-DBS may disrupt the established relationship between beta network and motor symptoms in PD, highlighting a potential mechanism through which STN-DBS exerts its therapeutic effects.
Moreover, the correlation between beta band power and motor severity was specifically observed in cerebellar regions. Intriguingly, no comparable association was found between mid-occipital (Oz) beta power or mid-frontal (Cz) beta power and motor severity in individuals with PD. This particular localization of the association within cerebellar regions suggests a unique relationship between beta oscillations and motor symptoms in PD patients. The absence of a similar correlation in other brain regions, such as mid-occipital (Oz) and mid-frontal (Cz) areas, emphasizes the specificity of this finding to the cerebellar region. These observations underscore the regional specificity in the modulation of beta band power and its link to motor severity, providing valuable insights into the intricate neural dynamics underlying PD.
Notably, the comprehensive examination of cerebellar oscillatory activity across other frequency bands such as delta, alpha, and gamma, reveals the wider impact of therapeutic HF-DBS on neural dynamics within the cerebellum. Our findings reveal that HF-DBS exerts a pervasive influence on cerebellar oscillations, extending beyond the previously discussed theta and beta frequency bands. In all three frequency bands (delta, alpha, and gamma), we observed significant main effects of DBS condition, with HF-DBS inducing a consistent increase in relative power across cerebellar regions. This widespread impact suggests that HF-DBS has the capacity to modulate oscillatory activity in a multifaceted manner within the cerebellum similar to the cortical region.39 However, our analysis did not reveal any main effects of cerebellar region or interactions between cerebellar region and DBS for any of the frequency bands examined. The lack of electrode-specific effects suggests that the modulation of cerebellar oscillations during HF-DBS is a distributed phenomenon, likely involving interconnected cerebellar regions. Each of the other frequency bands we explored— delta, alpha, and gamma— has distinct functional implications.46,47 The increase in power across these diverse frequency bands during HF-DBS suggests a global alteration in cerebellar function, potentially impacting attention, sensory processing, and cognitive functions.
One of the key questions emerging from our study revolves around the functional significance of the STN-cerebellar connection. The basal ganglia-thalamocortical circuit is central to motor control, and the cerebellum is another vital contributor to this process. Speculation arises regarding the role of the STN-cerebellar connection in modulating and integrating information within this broader motor circuit, potentially aiding in fine-tuning motor output. This integration might facilitate coordination and motor planning, contributing to the overall motor function of individuals with PD.
Our investigation also included a critical examination of signal similarities between the mid-cerebellar (Cbz) EEG signals and mid-occipital (Oz) EEG signals to assess the possibility of volume conduction effects. The absence of any significant similarities between these signals serves as compelling evidence against the presence of volume conduction. This observation further reinforces the notion that the alterations in oscillatory activity observed in the mid-cerebellum (Cbz) and mid-occipital (Oz) regions during high-frequency STN DBS are likely not due to the propagation of electrical activity from one region to the other. Instead, these changes in oscillatory dynamics appear to be intrinsic to the respective brain regions and could reflect the complex network-level effects of STN DBS. This finding is important as it underscores the specificity and localized nature of the observed oscillatory alterations, supporting the idea that STN DBS exerts distinct influences on different neural regions, each with its unique functional consequences.
Moreover, it is important to acknowledge the limitations of our study. Our sample size was relatively small, and our analysis focused on resting-state EEG data. Future studies with larger cohorts and more comprehensive assessments of cerebellar function, such as during motor or cognitive tasks, may provide a more comprehensive understanding of the effects of STN-DBS on cerebellar oscillations. Another notable limitation of our study is the variability in DBS settings used during the experiments. The stimulation frequencies (110 Hz–175 Hz) were not systematically controlled or constrained. This lack of uniformity in DBS parameters could potentially lead to different physiological responses. Future research should aim to systematically explore the impact of various DBS settings on physiological outcomes to better understand their specific effects. This would help to reduce variability and improve the interpretability and reproducibility of the outcomes. Additionally, exploring the temporal dynamics and causal relationships within the cerebello-thalamo-cortical network through techniques like dynamic causal modeling or combined EEG with neuroimaging approaches could further elucidate the mechanisms at play.
Conclusion
Our findings provide compelling evidence that modulating cerebellar oscillations through STN DBS in PD patients holds promise for improving both motor and non-motor symptoms via modulating cortical and subcortical pathways. By addressing abnormal oscillations within the cerebellum, STN-DBS may contribute to the amelioration of a wide range of impairments associated with PD, from motor dysfunction to cognitive deficits.9–11 However, to employ the full potential of this approach, ongoing research is imperative. Further investigations into the underlying mechanisms of cerebellar oscillations, their interaction with basal ganglia circuits, and their precise relationship to clinical outcomes are necessary to optimize the therapeutic application of DBS in PD.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was supported by the Division of Basic Biomedical Sciences at the University of South Dakota, Vermillion, SD, USA and by F32AG069445 to RCC from NIA.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY
All raw data and codes are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-240065.
REFERENCES
1. | Jankovic J . Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry (2008) ; 79: : 368–376. |
2. | O’Callaghan C , Hornberger M , Balsters JH , et al. Cerebellar atrophy in Parkinson’s disease and its implication for network connectivity. Brain (2016) ; 139: : 845–855. |
3. | Wu T and Hallett M . The cerebellum in Parkinson’s disease. Brain (2013) ; 136: : 696–709. |
4. | Eisenstein SA , Koller JM , Black KD , et al. Functional anatomy of subthalamic nucleus stimulation in Parkinson disease. Ann Neurol (2014) ; 76: : 279–295. |
5. | Knight EJ , Testini P , Min HK , et al. Motor and nonmotor circuitry activation induced by subthalamic nucleus deep brain stimulation in patients with Parkinson disease: intraoperative functional magnetic resonance imaging for deep brain stimulation. Mayo Clin Proc (2015) ; 90: : 773–785. |
6. | Strick PL . The cerebellum: the cerebellum and neural control. Science (1985) ; 229: : 547. |
7. | Lewis MM , Galley S , Johnson S , et al. The role of the cerebellum in the pathophysiology of Parkinson’s disease. Can J Neurol Sci (2013) ; 40: : 299–306. |
8. | Wu T , Liu J , Hallett M , et al. Cerebellum and integration of neural networks in dual-task processing. Neuroimage (2013) ; 65: : 466–475. |
9. | Bosch TJ , Espinoza AI and Singh A . Cerebellar oscillatory dysfunction during lower-limb movement in Parkinson’s disease with freezing of gait. Brain Res (2023) ; 1808: : 148334. |
10. | Bosch TJ , Groth C , Eldridge TA , et al. Altered cerebellar oscillations in Parkinson’s disease patients during cognitive and motor tasks. Neuroscience (2021) ; 475: : 185–196. |
11. | Bosch TJ , Kammermeier S , Groth C , et al. Cortical and cerebellar oscillatory responses to postural instability in Parkinson’s disease. Front Neurol (2021) ; 12: : 752271. |
12. | Benabid AL . Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol (2003) ; 13: : 696–706. |
13. | Bhuvanasundaram R , Krzyspiak J and Khodakhah K . Subthalamic nucleus modulation of the pontine nuclei and its targeting of the cerebellar cortex. J Neurosci (2022) ; 42: : 5538–5551. |
14. | Hanssen H , Steinhardt J , Munchau A , et al. Cerebello-striatal interaction mediates effects of subthalamic nucleus deep brain stimulation in Parkinson’s disease. Parkinsonism Relat Disord (2019) ; 67: : 99–104. |
15. | Bostan AC , Dum RP and Strick PL . The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A (2010) ; 107: : 8452–8456. |
16. | Bostan AC and Strick PL . The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci (2018) ; 19: : 338–350. |
17. | Pelzer EA , Hintzen A , Goldau M , et al. Cerebellar networks with basal ganglia: feasibility for tracking cerebello-pallidal and subthalamo-cerebellar projections in the human brain. Eur J Neurosci (2013) ; 38: : 3106–3114. |
18. | Aravamuthan BR , Muthusamy KA , Stein JF , et al. Topography of cortical and subcortical connections of the human pedunculopontine and subthalamic nuclei. Neuroimage (2007) ; 37: : 694–705. |
19. | Moers-Hornikx VM , Vles JS , Tan SK , et al. Cerebellar nuclei are activated by high-frequency stimulation of the subthalamic nucleus. Neurosci Lett (2011) ; 496: : 111–115. |
20. | Sutton AC , O’Connor KA , Pilitsis JG , et al. Stimulation of the subthalamic nucleus engages the cerebellum for motor function in parkinsonian rats. Brain Struct Funct (2015) ; 220: : 3595–3609. |
21. | Kasparek T , Rehulova J , Kerkovsky M , et al. Cortico-cerebellar functional connectivity and sequencing of movements in schizophrenia. BMC Psychiatry (2012) ; 12: : 17. |
22. | Kahan J , Mancini L , Flandin G , et al. Deep brain stimulation has state-dependent effects on motor connectivity in Parkinson’s disease. Brain (2019) ; 142: : 2417–2431. |
23. | Andersen LM , Jerbi K , Dalal SS . Can EEG and MEG detect signals from the human cerebellum? Neuroimage (2020) ; 215: : 116817. |
24. | Todd NPM , Govender S , Colebatch JG . The human electrocerebellogram (ECeG) recorded non-invasively using scalp electrodes. Neurosci Lett (2018) ; 682: : 124–131. |
25. | Fasano A , Daniele A and Albanese A . Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol (2012) ; 11: : 429–442. |
26. | Kuhn AA , Kupsch A , Schneider GH , et al. Reduction in subthalamic 8–35Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci (2006) ; 23: : 1956–1960. |
27. | Volkmann J , Moro E , Pahwa R . Basic algorithms for the programming of deep brain stimulation in Parkinson’s disease. Mov Disord (2006) ; 21: Suppl 14: S284–S289. |
28. | Lio G , Thobois S , Ballanger B , et al. Removing deep brain stimulation artifacts from the electroencephalogram: Issues, recommendations and an open-source toolbox. Clin Neurophysiol (2018) ; 129: : 2170–2185. |
29. | Allen DP . A frequency domain Hampel filter for blind rejection of sinusoidal interference from electromyograms. J Neurosci Methods (2009) ; 177: : 303–310. |
30. | Allen DP , Stegemoller EL , Zadikoff C , et al. Suppression of deep brain stimulation artifacts from the electroencephalogram by frequency-domain Hampel filtering. Clin Neurophysiol (2010) ; 121: : 1227–1232. |
31. | Nolan H , Whelan R , Reilly RB . FASTER: fully automated statistical thresholding for EEG artifact rejection. J Neurosci Methods (2010) ; 192: : 152–162. |
32. | Delorme A and Makeig S . EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods (2004) ; 134: : 9–21. |
33. | Bosch TJ , Groth C and Singh A . Resting-state low-frequency cerebellar oscillations can be abnormal in Parkinson’s disease. Cerebellum (2022) ; 21: : 1139–1143. |
34. | Singh A , Cole RC , Espinoza AI , et al. Frontal theta and beta oscillations during lower-limb movement in Parkinson’s disease. Clin Neurophysiol (2020) ; 131: : 694–702. |
35. | Singh A , Richardson SP , Narayanan N , et al. Mid-frontal theta activity is diminished during cognitive control in Parkinson’s disease. Neuropsychologia (2018) ; 117: : 113–122. |
36. | Cavanagh JF and Frank MJ . Frontal theta as a mechanism for cognitive control. Trends Cogn Sci (2014) ; 18: : 414–421. |
37. | Singh A . Oscillatory activity in the cortico-basal ganglia-thalamic neural circuits in Parkinson’s disease. Eur J Neurosci (2018) ; 48: : 2869–2878. |
38. | Brittain JS , Sharott A and Brown P . The highs and lows of beta activity in cortico-basal ganglia loops. Eur J Neurosci (2014) ; 39: : 1951–1959. |
39. | Cao C , Li D , Jiang T , et al. Resting state cortical oscillations of patients with Parkinson disease and with and without subthalamic deep brain stimulation: a magnetoencephalography study. J Clin Neurophysiol (2015) ; 32: : 109–118. |
40. | Little S and Brown P . The functional role of beta oscillations in Parkinson’s disease. Parkinsonism Relat Disord (2014) ; 20: Suppl 1: S44–S48. |
41. | Conway ZJ , Silburn PA , Perera T , et al. Low-frequency STN-DBS provides acute gait improvements in Parkinson’s disease: a double-blinded randomised cross-over feasibility trial. J Neuroeng Rehabil (2021) ; 18: : 125. |
42. | Bosch TJ , Cole RC , Bezchlibnyk Y , et al. Effects of very low- and high-frequency subthalamic stimulation on motor cortical oscillations during rhythmic lower-limb movements in Parkinson’s disease patients. J Parkinsons Dis (2023) ; 13: : 549–561. |
43. | Oswal A , Beudel M , Zrinzo L , et al. Deep brain stimulation modulates synchrony within spatially and spectrally distinct resting state networks in Parkinson’s disease. Brain (2016) ; 139: : 1482–1496. |
44. | Whitmer D , de Solages C , Hill B , et al. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Hum Neurosci (2012) ; 6: : 155. |
45. | Kumar A , Lin CC , Kuo SH , et al. Physiological recordings of the cerebellum in movement disorders. Cerebellum (2023) ; 22: : 985–1001. |
46. | Klimesch W . alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci (2012) ; 16: : 606–617. |
47. | Fries P . Rhythms for cognition: communication through coherence. Neuron (2015) ; 88: : 220–235. |




