U.S. Tax Credits to Promote Practical Proactive Preventative Care for Parkinson’s Disease
Abstract
Persons with Parkinson’s disease (PD) and society at large can profit from a strategic investment into a forward leaning, practical, preventative, and proactive multidisciplinary care policy. The American healthcare system is not easily bent to accommodate this type of care, and thus a tax benefit is an attractive option. An individual federal income tax benefit of $6200 each year for every person residing in the US with a diagnosis of PD, could among other offerings provide monthly access to a licensed clinical social worker and access to mental health services. The implementation of more coordinated care has the potential reduce the burden of depression, anxiety, and demoralization. Personal training would also be covered and directed by physical and occupational therapists. The combination of home-based and telemedicine services would have the added benefit of improving access. The tax benefit would also provide access to a dietician. This type of care strategy could be designed to proactively identify early signs of aspiration and urinary tract infections to ‘head off’ significant morbidity. A $6200/year individual tax benefit for those diagnosed with PD will thus translate into more fall prevention, more care in the home setting, less hospitalizations, less depression, less anxiety, less demoralization, better diets, and less persons placed in nursing facilities. Additionally, this tax benefit will provide the potential for billions of dollars in savings to the healthcare system. A tax benefit for PD is a practical preventative and proactive strategy which can serve to advantage both this generation and the next.
There is amassing evidence strongly supporting a multidisciplinary approach for the implementation of proactive preventative care for persons with Parkinson’s disease [1–5]. There is, however, no mechanism in the health care system to facilitate this approach. Failure to implement such a tactic will lead to countless unrealized benefits including reduction in falls, hip fractures, and nursing home placements.
Implementation of multidisciplinary care will provide billions of dollars in economic and societal savings. A tax benefit is an attractive solution because the American healthcare system is not easily bent to accommodate practical proactive preventative care for Parkinson’s disease. We must think ‘out of the box’ and implement a more immediate and feasible solution. I propose providing a federal income tax benefit each year for every person residing in the United States with a diagnosis of Parkinson’s disease (Fig. 1).
Fig. 1
Cost of Parkinson’s disease and benefits of a tax credit.
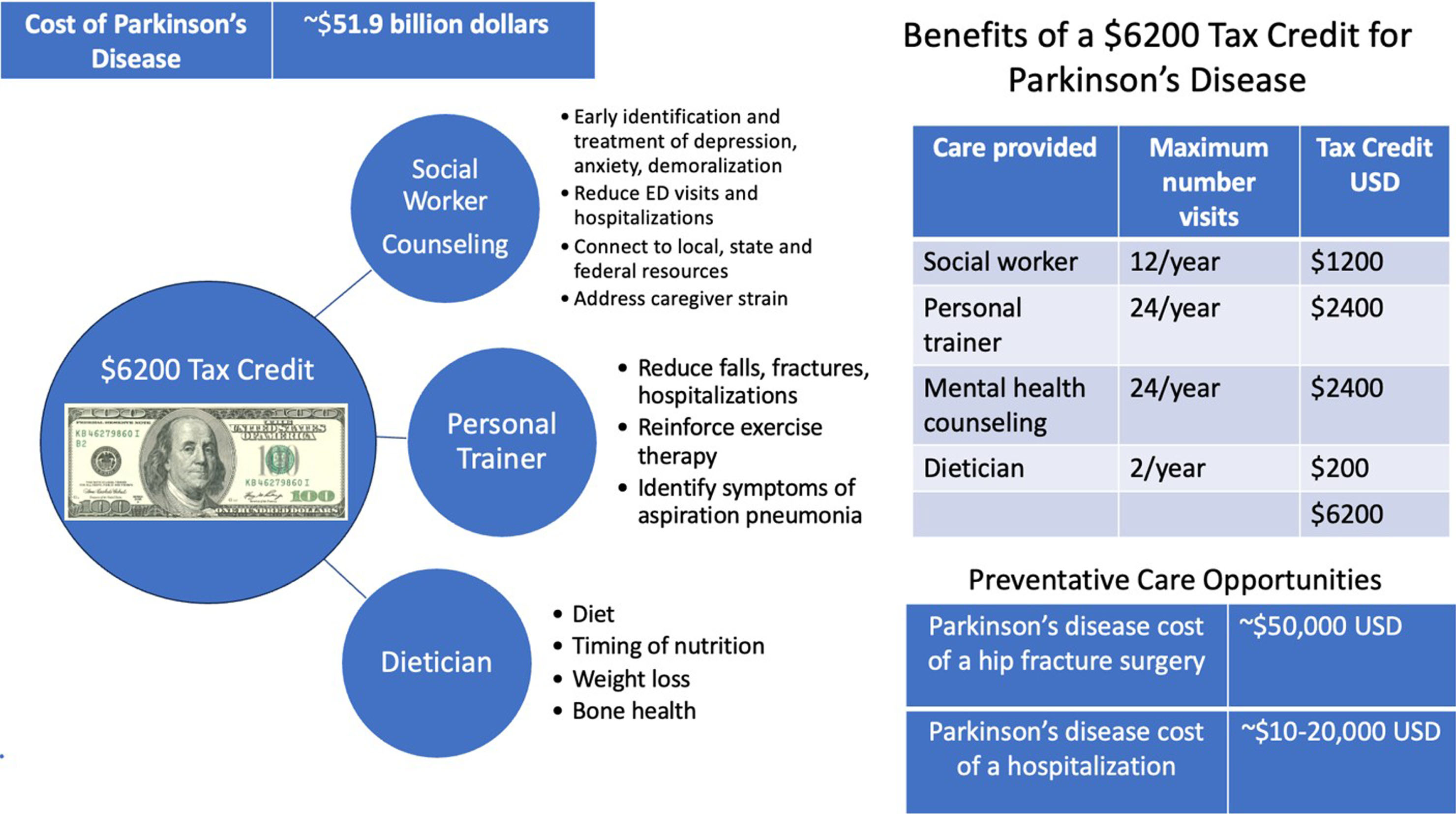
What are the economic consequences of Parkinson’s disease? The total accrued expenses have the potential to cripple Medicare and to devastate the private payor system. Parkinson’s is the fastest growing neurological disease and one of the most expensive [6–11]. A recent economic burden study of Parkinson’s disease was conducted in 2017. The results revealed an estimated one million individuals in the United States are currently diagnosed and represent a total combined economic burden of $51.9 billion dollars. This calculation represented a combination of direct medical expenses ($25.4 billion) and indirect non-medical costs ($26.5 billion) [12]. The impact of indirect costs is meaningful and worthy of consideration. These costs include future earnings losses due to premature death, reduced employment, disease related absenteeism, disease related lack of productivity at work (presenteeism) and losses in volunteer productivity. The author’s calculation was inclusive of paid daily non-medical care, home modifications, motor vehicle modifications and other expenses and it also included $4.8 billion dollars paid in disability income. In sum, the growing Parkinson’s related economic expenses will be massive and future costs will likely continue to mushroom when weighing the increasing number of cases of early onset Parkinson’s disease and their years of required care.
There are many critical services not covered under Medicare, Medicaid, or commercial insurance. Not surprisingly, these critical services are inadequately accessed. Additionally, many services are not feasible for persons with Parkinson’s disease living with mobility challenges and those without adequate transportation. These services include licensed clinical social workers, mental health professionals (including counselors), personal trainers and dieticians. A tax benefit will make these critical services and their integrated benefits affordable and accessible both for Parkinson’s disease and for early onset Parkinson’s disease.
Licensed clinical social workers are crucial for both persons with neurodegenerative diseases and for their families. Regular consultations with social workers will connect persons with Parkinson’s disease and their families to current and emerging local, state, and federal programs. Social workers facilitate wellness and success in navigating both the healthcare system and the disease. Additionally, many licensed clinical social workers provide counseling services, similar to what would be provided by counseling psychologists. Why then use a social worker? First, psychiatrists are not counselors. Psychiatrists provide medications and guidance on mental health strategies. Second, accessing a psychiatrist or a psychologist in most U.S. geographies is difficult, and many payors will not cover services; citing mental health ‘carve outs.’ Finally, when you are living with a neurodegenerative disease, its progressive nature translates to a rapidly changing landscape and a social worker possesses the ideal skillset to navigate all of the emerging challenges. A tax credit opens access to social workers and to those certified to provide individual and group counseling services. Providing a benefit to cover the expense of a licensed clinical social worker once a month, has the potential to improve non-motor Parkinson’s symptoms, which have been shown in studies to be more disabling than many of the visible motor symptoms [13, 14]. Regular access to a social worker will facilitate early and more timely referrals to neurologists and psychiatrists. Earlier referrals will translate into proactive identification and treatment of severe depression, anxiety and demoralization, all of which commonly contribute to Parkinson’s hospitalizations, morbidity and in some cases, even death. Additionally, research strongly supports that caregiver strain occurs in the majority of Parkinson’s cases and largely goes unaddressed [15]. More frequent hospitalizations and emergency room visits are highly dependent on the skillset and disposition of the caregiver, so it is appropriate that we focus attention on their empowerment and well-being. This tax benefit will close the neglected gap in addressing caregiver strain.
Proactive prevention of the hospitalization of persons with Parkinson’s disease represents one of the greatest opportunities for reducing morbidity and mortality and for facilitating care within the home and outpatient clinic settings. Prevention of falling, early intervention for infections and prevention of aspiration pneumonia are all achievable goals. We must, however, offer a proactive preventative strategy which places appropriate emphasis on continuous monitoring, which when coupled to available physician and rehabilitation services will lead to care remaining in an outpatient and home setting, and thus improve outcomes and reduce costs. Collective research strongly supports the idea that consistent exercise with appropriate supplementation by physical, occupational and speech/swallow therapy may lead to better outcomes [16–19]. Providing a tax benefit will incentivize persons with Parkinson’s to integrate an ‘alternating week personal trainer strategy’ into a proactive Parkinson’s disease plan. Many personal trainers will meet persons with Parkinson’s disease in their homes, thus this strategy has an added benefit of improving access. Additionally, regular visits with certified personal trainers will reinforce the crucial importance and benefits of continuous exercise for Parkinson’s disease. Finally, personal trainers can provide a link to physicians and rehabilitation services and can be utilized to facilitate more timely referrals to address emerging issues, which if unaddressed, may lead to falls, fractures, hospitalizations, morbidity and in some cases mortality.
The current health care system does not provide access and reimbursement for dieticians. Emerging evidence has revealed that diet impacts medication absorption, the microbiome and various symptoms in the Parkinson’s disease complex [20–23]. Reduction or timing of protein intake has a potential to improve absorption of Parkinson’s medications. Timing and coordination of nutrition with medication is important and underappreciated. There is emerging evidence that Mediterranean and other diets may impact both health and wellness as well as Parkinson’s symptoms [24–30]. Additionally, there is a slow continuous weight loss in Parkinson’s disease, and this leads to frailty and to downstream consequences such as a higher risk for bone fractures [31–36]. Dieticians can address this issue proactively, neutralize weight loss and facilitate appropriate referrals for bone health [37, 38]. Finally, constipation is one of the most common, disabling, and undertreated symptoms of Parkinson’s disease [39, 40]. Access to a dietician just twice a year has the potential to improve quality of life and to promote healthier living.
How impactful could a $6200 a year tax benefit be for Parkinson’s disease? Let’s walk through both the cost and the impact. Access to a licensed clinical social worker once a month at an approximate cost of ∼$100 per visit would cost ∼$1200 a year. The person with Parkinson’s would benefit with access to local, state, and federal services and benefit from counseling, including combined visits with the caregiver. The social worker will proactively monitor and facilitate referrals to assure more timely access to physicians and rehabilitation services. Additionally, a tax credit of ∼$100 per visit applied every other week to access psychological counseling services through telemedicine would cost ∼$2400 a year and reduce the burden of depression, anxiety, and demoralization. Frequent visits to counseling therapists will serve as a monitoring tool for triggering earlier referrals to psychiatrists and neurologists. Earlier treatment translates into an opportunity to reduce hospitalizations, attempted suicides, and deaths. The use of a tax credit for personal trainers twice a month at an approximate cost of ∼$2400 a year will have a strong potential to reduce falls, fractures, hospitalizations, morbidity, and mortality. Regular meetings with personal trainers will provide the reinforcement and monitoring of exercise; a powerful and underutilized evidence-based treatment. Finally, adding a benefit for twice a year dietician services at an approximate cost of ∼$200 ($100 per visit) will address the delicate balance of nutrition with medication absorption, as well as address constipation, tackle the perils of frailty and facilitate faster referrals and coordination in cases of rapid weight loss. Social workers, counselors, personal trainers, and dieticians can all become proficient in inquiring about coughing when eating and in identifying early signs of aspiration and urinary tract infections.
If 75% of the 1 million persons in the United States diagnosed with Parkinson’s disease cashed in the full tax credit of $6,200 a year this would add up to ∼4–5 billion dollars. The current cost of Parkinson’s disease each year in the United States is 51.9 billion dollars [41], and thus this tax benefit would likely pay for itself by applying a conservative minimum 10% savings in direct and indirect healthcare expenditures. One powerful example of potential savings will be in prevention of hip fractures, which cost the American Healthcare system ∼$50,000 per person. About 1/3 of persons with Parkinson’s will experience at least one hip fracture within 10 years of diagnosis [42]. The opportunity for improvement each year will be billions of dollars for the system.
Hospitalization is another enormous expense for the healthcare system at over 7 billion dollars a year [12], yet we have failed to provide adequate preventative care. One-third of persons with Parkinson’s disease will experience a hospital encounter each year, and one-half of those will experience a repeat encounter [43–45]. Preventing hospitalizations in Parkinson’s disease will translate into billions of dollars in healthcare savings. The cost will climb, especially in individuals who cannot return to a home setting and will inevitably ‘run up’ staggering and in many cases unaffordable bills. We know in Parkinson’s disease, that among those hospitalized, 44% will never return to their pre-hospitalization functional status [44].
In summary, a $6200 tax benefit for those diagnosed with Parkinson’s disease will translate into more fall prevention, more care provided in the home setting, less hospitalizations, less depression, less anxiety, less demoralization, better diets, and less persons placed in nursing facilities. If those benefits are not enough to convince you of the merits of a tax credit, then consider the billions of dollars in savings to the healthcare system. An additional consideration is the untapped opportunity for policymakers and politicians to press pesticide and chemical companies for remunerative support of the tax credit. For generations, taxpayers have paid for the likely sequelae of the toxicants that chemical companies have produced. It is time to end those subsidies. Instead, companies should pay for the tax credits needed to cover the health care consequences that are increasingly tied to their products. Such action will at a minimum reduce the financial burden on patients and their families. In addition, these payments could change the calculus of manufacturers and incent them to produce products that improve rather than harm health. These companies should ideally contribute, given their generation of billions of dollars in benefit through the manufacturing and distribution of products associated with the later occurrence of Parkinson’s disease. The time has come for a carefully crafted tax credit for Parkinson’s disease. We need to promote a practical preventative and proactive strategy that will advantage both this generation and the next.
ACKNOWLEDGMENTS
The author works at the University of Florida Parkinson’s Foundation Center of Excellence.
FUNDING
The author has no funding to report.
CONFLICT OF INTEREST
Dr. Okun serves as Medical Advisor the Parkinson’s Foundation, and has received research grants from NIH, Parkinson’s Foundation, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun’s research is supported by: R01 NS131342 NIH R01 NR014852, R01NS096008, UH3NS119844, U01NS119562. Dr. Okun is PI of the NIH R25NS108939 Training Grant. Dr. Okun has received royalties for publications with Hachette Book Group, Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology and JAMA Neurology. Dr. Okun has participated in CME and educational activities (past 12–24 months) on movement disorders sponsored by WebMD/Medscape, RMEI Medical Education, American Academy of Neurology, Movement Disorders Society, Mediflix and by Vanderbilt University. The institution and not Dr. Okun receives grants from industry. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. Research projects at the University of Florida receive device and drug donations.
REFERENCES
[1] | Radder DLM , Nonnekes J , van Nimwegen M , Eggers C , Abbruzzese G , Alves G , Browner N , Chaudhuri KR , Ebersbach G , Ferreira JJ , Fleisher JE , Fletcher P , Frazzitta G , Giladi N , Guttman M , Iansek R , Khandhar S , Klucken J , Lafontaine AL , Marras C , Nutt J , Okun MS , Parashos SA , Munneke M , Bloem BR ((2020) ) Recommendations for the organization of multidisciplinary clinical care teams in Parkinson’s disease. J Parkinsons Dis 10: , 1087–1098. |
[2] | van der Eijk M , Bloem BR , Nijhuis FA , Koetsenruijter J , Vrijhoef HJ , Munneke M , Wensing M , Faber MJ ((2015) ) Multidisciplinary collaboration in professional networks for PD a mixed-method analysis. J Parkinsons Dis 5: , 937–945. |
[3] | van der Marck MA , Munneke M , Mulleners W , Hoogerwaard EM , Borm GF , Overeem S , Bloem BR , IMPACT study group ((2013) ) Integrated multidisciplinary care in Parkinson’s disease: A non-randomised, controlled trial (IMPACT). Lancet Neurol 12: , 947–956. |
[4] | van der Marck MA , Bloem BR , Borm GF , Overeem S , Munneke M , Guttman M ((2013) ) Effectiveness of multidisciplinary care for Parkinson’s disease: A randomized, controlled trial. Mov Disord 28: , 605–611. |
[5] | Post B , van der Eijk M , Munneke M , Bloem BR ((2011) ) Multidisciplinary care for Parkinson’s disease: Not if, but how!. Postgrad Med J 87: , 575–578. |
[6] | Schiess N , Cataldi R , Okun MS , Fothergill-Misbah N , Dorsey ER , Bloem BR , Barretto M , Bhidayasiri R , Brown R , Chishimba L , Chowdhary N , Coslov M , Cubo E , Di Rocco A , Dolhun R , Dowrick C , Fung VSC , Gershanik OS , Gifford L , Gordon J , Khalil H , Kuhn AA , Lew S , Lim SY , Marano MM , Micallef J , Mokaya J , Moukheiber E , Nwabuobi L , Okubadejo N , Pal PK , Shah H , Shalash A , Sherer T , Siddiqui B , Thompson T , Ullrich A , Walker R , Dua T ((2022) ) Six action steps to address global disparities in Parkinson Disease: A World Health Organization priority. JAMA Neurol 79: , 929–936. |
[7] | Bloem BR , Henderson EJ , Dorsey ER , Okun MS , Okubadejo N , Chan P , Andrejack J , Darweesh SKL , Munneke M ((2020) ) Integrated and patient-centred management of Parkinson’s disease: A network model for reshaping chronic neurological care. Lancet Neurol 19: , 623–634. |
[8] | Tarolli CG , Zimmerman GA , Auinger P , McIntosh S , Horowitz RK , Kluger BM , Dorsey ER , Holloway RG ((2020) ) Symptom burden among individuals with Parkinson disease: A national survey. Neurol Clin Pract 10: , 65–72. |
[9] | Dorsey ER , Sherer T , Okun MS , Bloem BR ((2018) ) The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 8: , S3–S8. |
[10] | Dorsey ER , Bloem BR ((2018) ) The Parkinson pandemic-a call to action. JAMA Neurol 75: , 9–10. |
[11] | Dorsey R ST , Okun MS , Bloem BR ((2020) ) Ending Parkinson’s Disease: A Prescription for Action, Public Affairs, New York. |
[12] | Yang JX , Chen L ((2017) ) Economic burden analysis of Parkinson’s disease patients in China. Parkinsons Dis 2017: , 8762939. |
[13] | Bock MA , Brown EG , Zhang L , Tanner C ((2022) ) Association of motor and nonmotor symptoms with health-related quality of life in a large online cohort of people with Parkinson disease. Neurology 98: , e2194–e2203. |
[14] | Berganzo K , Tijero B , Gonzalez-Eizaguirre A , Somme J , Lezcano E , Gabilondo I , Fernandez M , Zarranz JJ , Gomez-Esteban JC ((2016) ) Motor and non-motor symptoms of Parkinson’s disease and their impact on quality of lifeand on different clinical subgroups. Neurologia 31: , 585–591. |
[15] | Oguh O , Kwasny M , Carter J , Stell B , Simuni T ((2013) ) Caregiver strain in Parkinson’s disease: National Parkinson Foundation Quality Initiative study. Parkinsonism Relat Disord 19: , 975–979. |
[16] | Cui W , Li D , Yue L , Xie J ((2023) ) The effects of exercise dose on patients with Parkinson’s disease: A systematic review and meta-analysis of randomized controlled trials. J Neurol 270: , 5327–5343. |
[17] | Omar Ahmad S , Longhurst J , Stiles D , Downard L , Martin S ((2023) ) A meta-analysis of exercise intervention and the effect on Parkinson’s Disease symptoms. Neurosci Lett 801: , 137162. |
[18] | Ernst M , Folkerts AK , Gollan R , Lieker E , Caro-Valenzuela J , Adams A , Cryns N , Monsef I , Dresen A , Roheger M , Eggers C , Skoetz N , Kalbe E ((2023) ) Physical exercise for people with Parkinson’s disease: A systematic review and network meta-analysis. Cochrane Database Syst Rev 1: , CD013856. |
[19] | Choi HY , Cho KH , Jin C , Lee J , Kim TH , Jung WS , Moon SK , Ko CN , Cho SY , Jeon CY , Choi TY , Lee MS , Lee SH , Chung EK , Kwon S ((2020) ) Exercise therapies for Parkinson’s disease: A systematic review and meta-analysis. Parkinsons Dis 2020: , 2565320. |
[20] | Bianchi VE , Rizzi L , Somaa F ((2023) ) The role of nutrition on Parkinson’s disease: A systematic review. Nutr Neurosci 26: , 605–628. |
[21] | Lister T ((2020) ) Nutrition and lifestyle interventions for managing Parkinson’s disease: A narrative review. J Mov Disord 13: , 97–104. |
[22] | Gaenslen A , Gasser T , Berg D ((2008) ) Nutrition and the risk for Parkinson’s disease: Review of the literature. J Neural Transm (Vienna) 115: , 703–713. |
[23] | Rusch C , Flanagan R , Suh H , Subramanian I ((2023) ) To restrict or not to restrict? Practical considerations for optimizing dietary protein interactions on levodopa absorption in Parkinson’s disease. NPJ Parkinsons Dis 9: , 98. |
[24] | Maraki MI , Yannakoulia M , Xiromerisiou G , Stefanis L , Charisis S , Giagkou N , Kosmidis MH , Dardiotis E , Hadjigeorgiou GM , Sakka P , Scarmeas N , Stamelou M ((2023) ) Mediterranean diet is associated with a lower probability of prodromal Parkinson’s disease and risk for Parkinson’s disease/dementia with Lewy bodies: A longitudinal study. Eur J Neurol 30: , 934–942. |
[25] | Bisaglia M ((2022) ) Mediterranean diet and Parkinson’s disease. Int J Mol Sci 24: , 42. |
[26] | Fox DJ , Park SJ , Mischley LK ((2022) ) Comparison of associations between MIND and Mediterranean diet scores with patient-reported outcomes in Parkinson’s disease. Nutrients 14: , 5185. |
[27] | Solch RJ , Aigbogun JO , Voyiadjis AG , Talkington GM , Darensbourg RM , O’Connell S , Pickett KM , Perez SR , Maraganore DM ((2022) ) Mediterranean diet adherence, gut microbiota, and Alzheimer’s or Parkinson’s disease risk: A systematic review. J Neurol Sci 434: , 120166. |
[28] | Rusch C , Beke M , Tucciarone L , Nieves C Jr. , Ukhanova M , Tagliamonte MS , Mai V , Suh JH , Wang Y , Chiu S , Patel B , Ramirez-Zamora A , Langkamp-Henken B ((2021) ) Mediterranean diet adherence in people with Parkinson’s disease reduces constipation symptoms and changes fecal microbiota after a 5-week single-arm pilot study. Front Neurol 12: , 794640. |
[29] | Rusch C , Beke M , Tucciarone L , Dixon K , Nieves C Jr. , Mai V , Stiep T , Tholanikunnel T , Ramirez-Zamora A , Hess CW , Langkamp-Henken B ((2021) ) Effect of a Mediterranean diet intervention on gastrointestinal function in Parkinson’s disease (the MEDI-PD study): Study protocol for a randomised controlled trial. BMJ Open 11: , e053336. |
[30] | Cassani E , Barichella M , Ferri V , Pinelli G , Iorio L , Bolliri C , Caronni S , Faierman SA , Mottolese A , Pusani C , Monajemi F , Pasqua M , Lubisco A , Cereda E , Frazzitta G , Petroni ML , Pezzoli G ((2017) ) Dietary habits in Parkinson’s disease: Adherence to Mediterranean diet. Parkinsonism Relat Disord 42: , 40–46. |
[31] | Yoon SY , Heo SJ , Lee HJ , Shin J , Kim YW , Yang SN , Park YG ((2022) ) Initial BMI and weight loss over time predict mortality in Parkinson disease. J Am Med Dir Assoc 23: , 1719.e1–1719.e7. |
[32] | Ghourchian S , Gruber-Baldini AL , Shakya S , Herndon J , Reich SG , von Coelln R , Savitt JM , Shulman LM ((2021) ) Weight loss and weight gain in Parkinson disease. Parkinsonism Relat Disord 83: , 31–36. |
[33] | Yong VW , Tan YJ , Ng YD , Choo XY , Sugumaran K , Chinna K , Md Shah MN , Raja Aman RRA , Moy FM , Mohd Ramli N , Grossmann M , Lim SY , Tan AH ((2020) ) Progressive and accelerated weight and body fat loss in Parkinson’s disease: A three-year prospective longitudinal study. Parkinsonism Relat Disord 77: , 28–35. |
[34] | Cersosimo MG , Raina GB , Pellene LA , Micheli FE , Calandra CR , Maiola R ((2018) ) Weight loss in Parkinson’s disease: The relationship with motor symptoms and disease progression. Biomed Res Int 2018: , 9642524. |
[35] | Ma K , Xiong N , Shen Y , Han C , Liu L , Zhang G , Wang L , Guo S , Guo X , Xia Y , Wan F , Huang J , Lin Z , Wang T ((2018) ) Weight loss and malnutrition in patients with Parkinson’s disease: Current knowledge and future prospects. Front Aging Neurosci 10: , 1. |
[36] | Wills AM , Li R , Perez A , Ren X , Boyd J , NINDS NET-PD Investigators ((2017) ) Predictors of weight loss in early treated Parkinson’s disease from the NET-PD LS-1 cohort. J Neurol 264: , 1746–1753. |
[37] | Torsney KM , Noyce AJ , Doherty KM , Bestwick JP , Dobson R , Lees AJ ((2014) ) Bone health in Parkinson’s disease: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 85: , 1159–1166. |
[38] | Jones PD , Stone D , Jones S , Lewis B ((2010) ) Parkinson’s disease and bone health. Age Ageing 39: , 278. |
[39] | Yao L , Liang W , Chen J , Wang Q , Huang X ((2023) ) Constipation in Parkinson’s disease: A systematic review and meta-analysis. Eur Neurol 86: , 34–44. |
[40] | Adams-Carr KL , Bestwick JP , Shribman S , Lees A , Schrag A , Noyce AJ ((2016) ) Constipation preceding Parkinson’s disease: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 87: , 710–716. |
[41] | Yang W , Hamilton JL , Kopil C , Beck JC , Tanner CM , Albin RL , Ray Dorsey E , Dahodwala N , Cintina I , Hogan P , Thompson T ((2020) ) Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Parkinsons Dis 6: , 15. |
[42] | Huyke-Hernandez FA , Parashos SA , Schroder LK , Switzer JA ((2022) ) Hip fracture care in Parkinson disease: A retrospective analysis of 1,239 patients. Geriatr Orthop Surg Rehabil 13: , 21514593221118225. |
[43] | Zeldenrust F , Lidstone S , Wu S , Okun MS , Cubillos F , Beck J , Davis T , Lyons K , Nelson E , Rafferty M , Schmidt P , Dai Y , Marras C ((2020) ) Variations in hospitalization rates across Parkinson’s Foundation Centers of Excellence. Parkinsonism Relat Disord 81: , 123–128. |
[44] | Shahgholi L , De Jesus S , Wu SS , Pei Q , Hassan A , Armstrong MJ , Martinez-Ramirez D , Schmidt P , Okun MS ((2017) ) Hospitalization and rehospitalization in Parkinson disease patients: Data from the National Parkinson Foundation Centers of Excellence. PLoS One 12: , e0180425. |
[45] | Hassan A , Wu SS , Schmidt P , Dai Y , Simuni T , Giladi N , Bloem BR , Malaty IA , Okun MS , NPF-QII Investigators ((2013) ) High rates and the risk factors for emergency room visits and hospitalization in Parkinson’s disease. Parkinsonism Relat Disord 19: , 949–954. |




