Pain Fluctuations in Parkinson’s Disease and Their Association with Motor and Non-Motor Fluctuations
Abstract
Background:
Pain fluctuations are a characteristic phenomenon in advanced Parkinson’s disease (PD), but their temporal association with motor and non-motor symptom (NMS) fluctuations remains largely enigmatic. Moreover, data on their importance for disease severity perception and health-related quality-of-life (hr-QoL) is limited.
Objective:
To dissect pain fluctuations with respect to pain type and frequency patterns, and their association with motor and non-motor fluctuations.
Methods:
Prospective observational cohort study in advanced PD assessing symptom fluctuations by simultaneous hourly ratings using the PD Home diary (Off, On, Dyskinetic state), a pain diary (assessing 9 pain types) and a non-motor diary (10 key NMS) based on validated instruments.
Results:
Forty-seven out of 55 eligible participants with fluctuating PD (51% men, median age 65, median disease duration 10 years) had sufficient datasets (>95% of hours) from 2 consecutive days. Pain was reported in 35% of waking hours with clear circadian rhythm peaking in early morning Off periods and clustering during motor Off state (49% of Off state hours with pain). Main NMS co-fluctuating with pain were “Fatigue” and “Inner Restlessness”. Simultaneous assessment of global disease severity by participants revealed that pain was associated with worse disease severity only in motor On and Dyskinetic state but not in Off state, which translated into significant correlations of daily pain times with hr-QoL only during motor On and Dyskinetic state.
Conclusions:
Aside from treating motor Off periods, specific recognition of pain particularly during motor On and Dyskinetic state comprises an important aspect for disease management in advanced PD.
Plain Language Summary
Oscillations of the frequency and severity of pain over the day (also called pain fluctuations) are common in advanced Parkinson’s disease (PD). However, their relationship with oscillations of motor and other non-motor symptoms remains unclear. Moreover, only very little data exists on how pain impacts disease severity perception and quality of life for the patients. The present study thus aimed to better understand pain fluctuations and their association with motor and non-motor symptoms in advanced PD. We conducted a prospective observational cohort study in advanced PD patients. Participants rated their symptoms hourly on two consecutive days using three diaries: the PD Home diary (for motor fluctuations), a pain diary (assessing several pain types), and a non-motor diary covering 10 key non-motor symptoms. Pain occurred during 35% of waking hours with a clear circadian rhythm peaking in the early morning and clustering during motor “Off” states as characterized by pronounced motor symptoms. The main non-motor symptoms associated with pain were “Fatigue” and “Inner Restlessness.” Interestingly, pain severity correlated with health-related quality of life only during motor “On” state (defined as a state with good mobility and motor function) and “Dyskinetic” state characterized by the occurrence of involuntary movements, but not during motor “Off” periods. In conclusion, in managing advanced PD, recognizing pain during motor “On” and Dyskinetic states is crucial beyond just addressing motor “Off” periods. This understanding can significantly impact disease management and improve patients’ quality of life.
INTRODUCTION
Pain in Parkinson’s disease (PD) has received increasing attention during recent years, but is still not completely characterized and its treatment is frequently carried out in an undifferentiated manner.1–6 Pain in PD is frequent with 40– 85% of patients complaining about pain1,6–8 and comprises various pain types, such as dystonic, musculoskeletal, central and radicular pain, which can accumulate and change over the disease course in a single patient, further complicating its management.2–4,9,10 Indeed, pain is ranked top 4 and 6 of the most bothersome symptoms in early and late PD, respectively, and significantly impairs health-related quality-of-life (hr-QoL).10–14 Consistent with complex pain patterns in PD, the underlying pathophysiological mechanisms are heterogeneous and include neuropathic, nociceptive and nociplastic pain.2–4,9,15,16
In addition to the already complex construct of pain in PD, pain is known to fluctuate in conjunction with motor fluctuations in advanced PD— similar to other non-motor symptoms (NMS). The first reports on pain fluctuations date back to 1986 describing pain during motor Off state in a series of cases as well as in a cohort of 95 PD patients.8,16 In this cohort with moderate PD, 30% of all patients and 65% of patients with pain experienced pain in correlation with motor disability with the most severe pain reported in motor Off state.8 In subsequent studies, 7– 30% of patients in general PD cohorts and in 34– 65% of patients with motor fluctuations complained about fluctuating pain with more severe pain in motor Off state.8,17–25 Although only very few studies investigated fluctuations of various types of pain, most pain types such as musculoskeletal pain, dystonic pain and radicular pain seem to fluctuate in conjunction with motor fluctuations.8 In addition, cross-sectional studies show that pain is more frequent in patients experiencing levodopa-induced dyskinesia (LID).19,26 However, previous studies did not assess pain intra-individually across the various motor states, but only on the cohort level using general pain scales such as the MDS-NMS/NMF or the Wearing-Off questionnaire.18,21 Thus, only limited systematic data on circadian occurrence, timing and kinetics of fluctuations of the various types of pain as well as on their co-fluctuation with other NMS are available. Moreover, the importance of specific pain fluctuations for disease severity perception by patients and for the impact on their hr-QoL remain to be determined.
We here report data on pain fluctuations from the VALIDATE-PD study,27,28 which was designed to apply patient-centered diary data and wrist-wearable accelerometry for objective motor diary assessment in fluctuating PD in a routine clinical environment (see Fig. 1 for study synopsis). Our main aim in this report was to dissect timing and pain type patterns of pain fluctuations in direct relation to motor and other non-motor fluctuations. We thus assessed pain fluctuations using a diary comprising nine questions assessing the various types of pain combined with simultaneous hourly performed motor ratings by the participants using the PD Home diary27 and the non-motor diary introduced by Ossig and colleagues.29 Importantly, the pain diary applied the questions originally used by the validated King’s PD pain questionnaire (KPPQ).12,30 We then estimated fluctuations of pain and its various types with respect to four different diary outcomes (Fig. 1): (1) Temporal agreement of pain and its types with motor and non-motor states and their relation to global disease impression. (2) Agreement of pain with motor Off episodes (Off state episode following motor On state).28,31 (3) Percentage daily times (PDTs) with pain on the participant level independent of motor fluctuations as well as with respect to PDTs for motor and non-motor states. (4) Proportion of hours with pain as percentage of total hours in the respective motor state (percentage pain times, PPTs) as a measure of pain distribution patterns within the three motor states independent of the specific motor state frequencies.
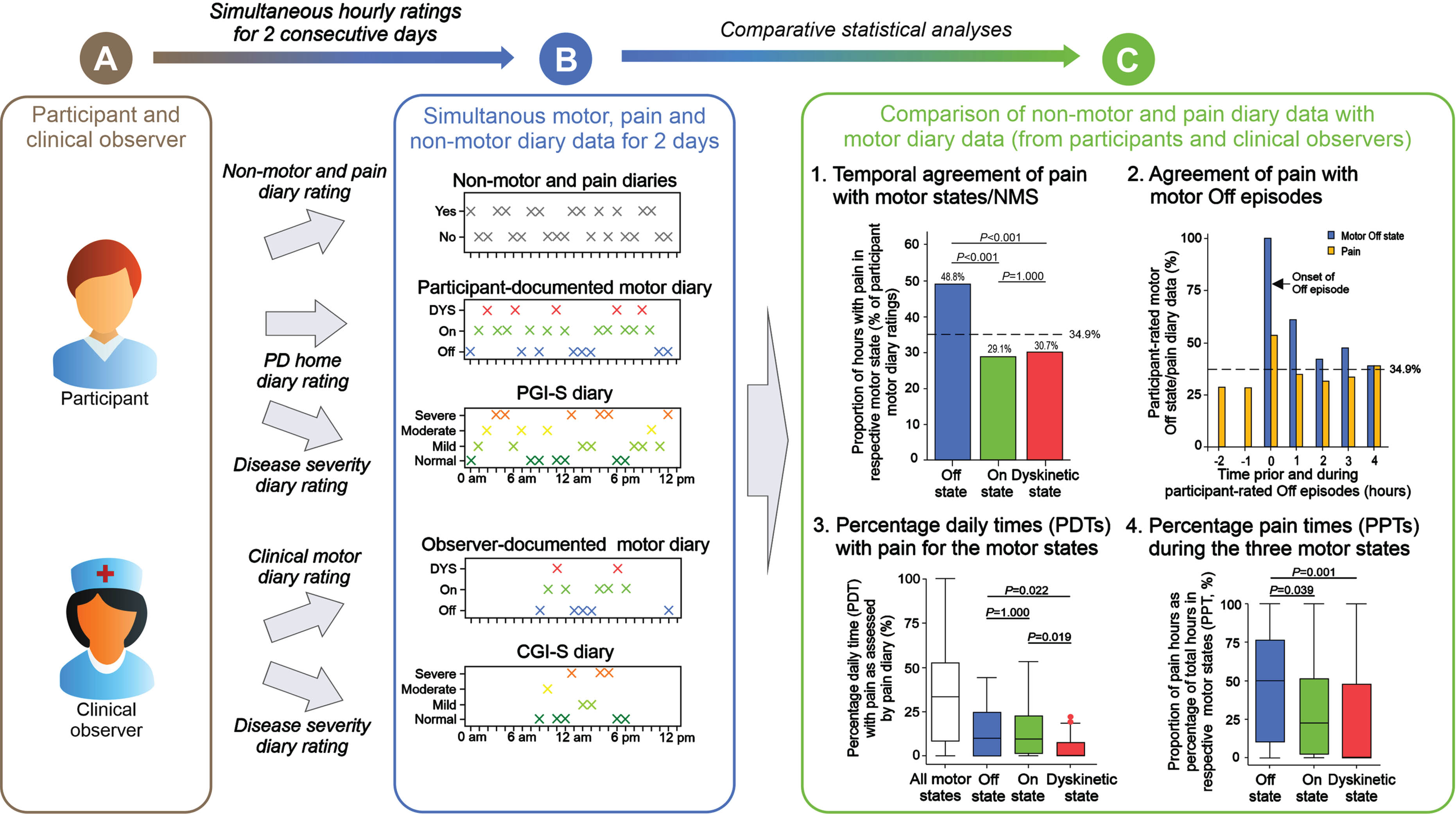
Fig. 1
Graphical synopsis of the diary study for assessment of pain fluctuations in Parkinson’s disease. The present study is a substudy of the VALIDATE-PD study and designed to assess pain fluctuations in conjunction with motor and non-motor fluctuations in fluctuating Parkinson’s Disease (PD) using diary ratings. (A) Symptom fluctuations were simultaneously assessed by study participants and an experienced clinical observer. (B) Pain and the other non-motor symptoms were assessed by participants using pain and NMS diaries based on the questions of the King’s PD Pain Questionnaire and motor function was assessed by participants and clinical observer using the PD Home diary (Off state, On state, Dyskinetic state). In addition, disease severity was estimated using the Patient Global Impression of Severity (PGI-S) and the Clinical Global Impression of Severity (CGI-S) scales as diary ratings. All diary ratings were assessed for awake hours for participant ratings and simultaneously for all hour time periods between 8 am and 6 pm for clinical observer ratings on 2 consecutive days. (C) Comparative statistical analyses using the pain and non-motor diaries as well as the PD home motor diary ratings from the participant were applied to analyze pain fluctuations for (1) temporal agreement of pain and its various types with motor and non-motor symptoms on the hour level, (2) detection of pain during Off episodes following a motor On phase, (3) assessment of percentage daily times (PDTs) with pain for all motor states together as well as for the three motor states separately on the participant level, and (4) the proportion of hours with pain as percentage of total hours in the respective motor state (percentage pain times, PPTMotorstate) on the participant level. Ancillary analyses used the PD home diary data as documented by the clinical observer. The design of this synopsis was adapted from Löhle and colleagues.28
METHODS
Study protocol approval and patient consents
We here report data on pain fluctuations from the German cohort of the prospective, observational cohort VALIDATE-PD study, which was conducted at two hospital centers in Germany (University Medicine Rostock, Movement Disorder Clinic Beelitz-Heilstätten).27 The study was approved by the institutional review boards of both participating centers (ethic committee registry numbers A 2017-0115 for Rostock and AS 84(bB)/2018 for Beelitz-Heilstätten). All participants provided written informed consent prior to study participation.
Participants
Participants were eligible for the study if they were over 30 years old, had been diagnosed with PD according to the United Kingdom PD Society Brain Bank criteria, suffered from motor fluctuations observed by the treating physician and/or documented on part 4 of the Movement Disorder Society-revised Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) and were able to provide written informed consent. Even though there is no consensus on the definition of the advanced stage of PD, we herein define advanced PD based on the occurrence of motor fluctuations and/or dyskinesia.32 Exclusion criteria comprised the existence of clinical signs for secondary or atypical parkinsonian syndromes, inability to complete questionnaires and/or patient diaries, lack of cooperation during the study procedures, presence of dementia (defined as scores on the Montreal Cognitive assessment [MoCA] < 21)33 and/or relevant psychotic symptoms, ongoing treatment with advanced/invasive therapies (deep brain stimulation, subcutaneous apomorphine and levodopa-carbidopa intestinal gel) as well as the presence of miscellaneous diseases impairing the patient’s ability for consenting, participation and judgment.
Baseline assessments
The baseline assessments included cognitive screening with the MoCA,33 clinical evaluation using the MDS-UPDRS and assessment of non-motor symptoms using the Non-Motor Symptom Scale (NMSS) and the King’s PD Pain Scale (KPPS). Furthermore, all participants were asked to fill-out the 19-item Wearing-off Questionnaire (WOQ-19), Non-Motor Symptoms Questionnaire (NMSQuest), King’s PD Pain Questionnaire, Beck Depression Inventory version 2 (BDI-II) and 39-item Parkinson’s disease Questionnaire (PDQ-39).
Diary assessments
Motor diary assessments. After inclusion into the study, all participants received detailed instructions on the PD home diary and watched a training video explaining all functional states with particular focus on the difference between tremor and dyskinesia.34 Participants were then asked to indicate their predominant status during hour time periods for two consecutive days using the categories Asleep, “Off” (Off state), “On without dyskinesia” (On state), “On with non-troublesome dyskinesia”, and “On with troublesome dyskinesia”. While diary data for both participants and the observer was initially collected using the categories originally defined by Hauser and co-workers,35 we eventually combined the categories “On with non-troublesome dyskinesia” and “On with troublesome dyskinesia” into the category “On with dyskinesia” (Dyskinetic state) for analysis, since the distinction between non-troublesome and troublesome dyskinesia could only be made by patients.
On both consecutive days, participants were observed by an experienced physiotherapist (A.B.), who had been trained to identify motor complications in advanced PD patients in the participating hospitals and acquired MDS certification as qualified UPDRS rater prior to the start of the study. The observer acted as single rater and independently evaluated motor states half-hourly throughout daytime (8.00 am through 6.00 pm) based on his clinical observations during a 7-meter version of the Timed Up and Go test (7m-TUGT),36 taking into account global bradykinesia, tremor, dyskinesia and gait function. The observer was also instructed to dismiss any attempts from patients to get assistance with their own ratings. If not stated otherwise, presented data reflect results on PD Home motor diary data assessed by the participants.
Non-motor symptom diary assessment. On study day 1, participants were asked to rate eleven key NMS (psychiatric NMS: anxiety, depressive mood, inner restlessness, difficulties with concentration, hallucinations; fatigue; autonomic NMS: excessive sweating, sialorrhea, bladder urgency and dizziness; sensory NMS: pain) as present or absent during awake time using the same questions as the NMSQuest as already introduced by Ossig and colleagues.29 During the training session, patients were instructed as to how the different functional states are defined and how the diary should be completed (placing a tick mark on a daily diary card every 60 minutes reflecting their predominant status over the prior hour period; for time asleep, the diary was completed upon awakening). Of note, if NMS were rated as “present” the NMS state in that hour was interpreted herein as NMS Off state and if NMS were rated as “absent” the state was interpreted as NMS On state to allow for an easier comparison with motor states.
Pain diary assessment. On day 2, participants were asked to rate pain in general and seven pain subdomains (pain around joints, pain related to internal organ or deep within the body (visceral pain), dyskinetic pain, painful cramps in a region during “Off” periods, generalized “Off” period pain, pain when chewing, pain due to grinding teeth, burning sensation in the mouth, burning pain in the limbs, shooting pain/pins & needles) as present or absent during awake time using similar questions as the German version of the KPPQ.30 Pearson correlation tests comparing pain diary frequencies (expressed as percentage daily times with pain, PDTPain) with the corresponding frequencies as reported in King’s PD Pain Scale (KPPS)37 were used as a measure of convergent validity of pain diary data (Table 2). The overall pain frequency and all pain subtype domain frequencies except visceral pain showed acceptable Pearson correlation coefficient (r) values higher than 0.30.38 The item “Dyskinetic pain” was a major exception with a Pearson correlation coefficient of only – 0.06.
Global disease severity impression diaries. In addition to the motor and non-motor diaries, patients and observer rated disease severity in individual motor states hourly using 7-point versions of Patient Global Impression of Severity scale (PGI-S) and Clinical Global Impression of Severity scale (CGI-S), respectively, ranging from “normal” to “extremely ill”. For simplicity, we condensed the original 7 grades of disease severity on both scales to four categories: “normal” (containing the original grade ‘normal’), “mild” (comprising the original grades ‘borderline ill’ and ‘mildly ill’), “moderate” (containing the original grade ‘moderately ill’) and “severe” (comprising the original grades ‘profoundly ill, ‘severely ill’ and extremely ill’).
Aggregated data calculated from diary ratings. On the participant level, percentage daily times (PDT) were calculated from the diary ratings by normalizing the number of hours per day in individual motor states (PDTOff, PDTOn and PDTDyskinetic) or the number of hours with pain/NMS ratings (PDTPain or PDTNMS) to the total waking day hour ratings and expressed in percentage of waking day hours. The analyses of PDTPain for the three motor states separately resulted in PDTPain/Off, PDTPain/On and PDTPain/Dyskinetic. To analyse the pain distribution patterns within the three motor states independent of the specific motor state frequencies, the proportion of hours with pain as percentage of total hours in the respective motor state per day were calculated (percentage pain times, PPTOff, PPTOn and PPTDyskinetic).
Adherence to diary assessments and asleep hours
All VALIDATE-PD study participants who had 2 days of diary datasets with at least 46 hour time periods including sleeping hours (>95% of all hour periods) and at least 21 hour time periods for observer dairy ratings between 8 : 00 and 18 : 00 (>95% of all hour periods) with simultaneous patient/observer motor diary data and NMS/pain diary data were included in the final study analyses leading to analyzable datasets from a total of 47 participants.
In total, we analyzed 2,216 hour periods with patient diary entries (98.2% of all hour periods; day 1 : 1,089 [96.5%], day 2 : 1,126 [100%]). 711 time periods (32.1% of all time periods) rated as Asleep by the participants were excluded from further analyses. 448 (29.7% of waking day periods) hour time periods were rated by the participants as as motor Off state, 749 (49.7%) as On state and 309 (20.5%) as Dyskinetic state. From observer diaries, we analyzed 1,026 hour periods during the waking day (99.2% of all hour periods between 8 am and 6 pm; day 1 : 515 [99.6%], day 2 : 511 [98.8%]) with 20 (1.9%) hour periods being excluded due to the rating “Asleep”. 279 (27.7% of all periods) hour time periods rated by observers were classified as motor Off state, 358 (35.6%) as On state and 369 (36.7%) as Dyskinetic state.
These numbers translated into 688 hour periods (92.7% of all waking day time periods) with complete simultaneous ratings of motor states in patient diaries and NMS diary data from day 1, and 728 (95.3%) hour periods with simultaneous participant-ratings and pain diary data (day 2). For observer diaries, we analyzed 498 hour periods (99.0%) with complete simultaneous ratings of motor states and NMS diary data (day 1), and 501 (99.6%) hour periods with simultaneous motor ratings and pain diary data (day 2).
Statistics
Statistical analysis was performed using IBM SPSS Statistics software version 27 (IBM Corporation, New York, USA). Values are provided as numbers (percentages) or median (interquartile range, IQR), as appropriate. Boxplots are shown with a central mark at the median, bottom, and top edges of the boxes at 25th and 75th percentiles, respectively, whiskers out to the most extreme points within 1.5 times the interquartile range, and outliers scoring more than 1.5×IQR but at most 3×IQR outside the quartiles. Pairwise exclusion was used for missing values. For statistical comparisons, we used Kruskal-Wallis tests or Friedman tests with post-hoc Wilcoxon signed-rank tests with adjustment for multiple comparisons as indicated. p-values<0.05 were considered to be statistically significant. All diary sets were included for analysis. Individual time periods were excluded from analysis if there was no response or more than one response on either diary, or if the patient indicated they were asleep on one diary but not the other. For all analyses except for the 24-hours distribution analyses, hours marked as asleep or switches from or towards asleep were omitted from the calculations.
Association of pain with demographic/clinical data and motor and non-motor diary data. Assessments on the hour time level were analyzed using χ2 tests comparing pain and no-pain hours or all three motor states with post-hoc pair-wise comparisons χ2 tests test with Bonferroni adjustment.
To cluster pain and the other NMS we used hierarchical clustering of simultaneously occurring NMS using average linkage between groups as the agglomeration rule and size difference as the (dis)similarity measure using IBM SPSS Statistics software version 27.39–41 The size difference quantifies the difference in the number of observations present on one item but absent on the other. In the context of binary data as in the present cluster analyses, size difference is an index of asymmetry that ranges from 0 to 1: A value of 0 indicates perfect symmetry (both variables are equally distributed), while a value closer to 1 indicates greater asymmetry (one variable dominates over the other). The formula to calculate size difference is as follows: size difference=(|b-c|)/n, with n representing the total number of observations and b and c represent the diagonal cells corresponding to observations present on one item but absent on the other and (|b-c|) the absolute difference between the two diagonal cells.
On the participant level, Friedman tests with post-hoc Wilcoxon signed-rank tests with Bonferroni correction for multiple comparisons were applied to compare pain times between the three motor stwtes. Pearson’s correlation tests were used for correlations of PDTs and PPTs and demographic and clinical data in all motor states or in individual motor states. Pearson’s correlation coefficient |r| < 0.3 was considered a weak, |r| = 0.3– 0.59 a moderate and |r|≥0.6 a strong correlation.
Temporal connection between pain and motor Off episodes. For analyzing the temporal connection between pain and Off episodes as rated by PD Home diaries or clinical observers, Off episodes (defined herein as an motor Off state of at least 1 hour duration following a motor On period of at least 2 hours) from all participants were synchronized by summation of all Off state periods using the first hour of the motor Off period as the trigger event (start of Off episode). The Off state ratings were then cross-classified with pain diary ratings by putting them into 2×2 contingency tables for each hour motor Off state interval. All diary sets were included for analysis. Individual time periods were excluded from analysis if there was no response or more than one response on either diary.
Association of pain diary data with hr-QoL. Pearson’s correlation tests and multiple linear regression modelling was used to test the association of pain times from pain diary ratings and PDQ-39 as a measure of hr-QoL. Since hr-QoL had been reported to potentially correlate with various demographic and clinical parameters,42–45 we adjusted the correlations of pain diary data and PDQ-39 for the variables age, sex, symptom duration, MDS-UPDRS part III motor score as a measure of disease severity, and included BDI-2 and MoCA as independent covariates into the models. Multicollinearity of candidate variables were excluded by Pearson correlation tests (|r|<0.5).
RESULTS
Demographic and clinical data
We screened 55 participants for eligibility of whom 47 (85%) were successfully included into the pain study according to inclusion and exclusion criteria. Seven participants were excluded since they reached Montreal Cognitive Assessment (MoCA) scores below 21 points at screening (n = 2) or were not able to sufficiently adhere to motor (n = 2) or pain/NMS assessments (n = 3). One participant declined further participation due to undisclosed reasons after the screening visit. Demographic and clinical characteristics of the cohort are displayed in Table 1, further details in Supplementary Table 1). In brief, complete datasets were available from 24 male (51%) and 23 female (49%) PD patients with a median age of 65 years. Participants had been diagnosed with PD about 10 years prior to the study and were suffering from motor fluctuations since 61 months. During structured interviews, patients reported a wide range of motor fluctuations, in particular wearing-off (94%), nocturnal off (85%) and peak-dose dyskinesia (77%). Clinical scores and antiparkinsonian medication were representative for a patient population suffering from advanced PD (Table 1). 14 (30%) patients had joint or spinal osteoarticular degeneration (see Supplementary Table 1 for concomitant diseases). For data on diary adherence and data quantity, please refer to the Participants and Methods section.
Table 1
Demographic and clinical characteristics of study cohort
| Overall cohort (n = 47) | |
| Male/Female, n (%) | 24 (51%)/23 (49%) |
| Age, Median (IQR), y | 65 (58–73) |
| Disease duration, Median (IQR), y | 10 (8–15) |
| Symptom duration, Median (IQR), y | 12 (9–17) |
| Duration of fluctuations, Median (IQR), mo | 61 (34–106) |
| Hypokinetic fluctuations | 75 (40–114) |
| Hyperkinetic fluctuations | 38 (25–53) |
| Medication | |
| Levodopa daily dose (mg per day), Median (IQR) | 500 (425–700) |
| Total LED (mg per day), Median (IQR) | 1,325 (1,025–1,667) |
| Clinical scales | |
| MDS-UPDRS Total score On state, Median (IQR) | 64 (52–83) |
| Part I | 13 (7–16) |
| Part II | 16 (10–20) |
| Part III | 28 (20–40) |
| Part IV | 9 (7–11) |
| Hoehn &Yahr stage, Median (IQR) | 2.5 (2–3) |
Values are provided as number (percentages), median (interquartile range, IQR), or mean (standard deviation, SD). LED, Levodopa equivalent dose. MDS-UPDRS: Movement Disorder Society-revised version of the Unified Parkinson’s Disease Rating Scale. Levodopa equivalent doses were calculated according to Jost et al.60
Temporal association of pain types with motor symptoms
To assess pain in every hour of the waking day, we used the NMS diary as reported earlier plus pain question (to compare the occurrence of pain with that of other NMS)29 on study day 1 and the pain diary using questions from the German version of the King’s PD Pain Questionnaire (KPPQ)12,30 on study day 2 to determine the various pain types. Analyzing these pain diary data on the time level, participants experienced pain on the two study days in 34.9% of waking hours independent of the motor state (day 1 using the NMS diary pain item: 27.5%; day 2 using the pain diary: 42.0%; Fig. 2a). Using the pain diary assessment on study day 2, the median number of pain types (max. 9 pain items) in each hour was 1 (IQR: 1– 2; range: 1– 6) with “Musculoskeletal pain” being by far the most frequent type of pain (29.6% of waking hours), followed by “Shooting pain/pins & needles” (10.5%), “Generalized Off pain” (10.3%) and “Off period dystonia in a specific region” (7.8%; Table 2).
Fig. 2
Temporal association of pain and its various types with simultaneous PD home motor diary states as rated by the participants. (a) Proportions of hourly diary ratings of pain by participants with respect to simultaneous PD home motor diary ratings (motor Off state, motor On state, Dyskinetic state). Note that participants reported the presence of pain in 34.9% of all ratings (dotted line as reference). Values are percentages. p-values above the diagram are from post-hoc χ2 tests with Bonferroni adjustment (p-value from χ2 test comparing all three motor states:<0.001). (b) Proportion of hourly participant diary ratings of the various pain types with respect to simultaneous PD home motor diary ratings (motor Off state, motor On state, Dyskinetic state). Values are percentages. *p < 0.05 and ***p < 0.001 are from χ2 tests comparing all three motor states, while $/#/§ represents p < 0.05, $$/##/§§p < 0.01 and $$$/###/§§§p < 0.001 for pair-wise comparisons as displayed in upper left corner applying χ2 tests test with Bonferroni adjustment. Data are based on 1,506 simultaneous hourly ratings (67.9% of all time periods and 98.2% of waking hours on 2 consecutive days) in (a) and 764 simultaneous hourly ratings (67.7% of all time periods and 100% of waking hours on 1 day) in (b) by 47 participants.
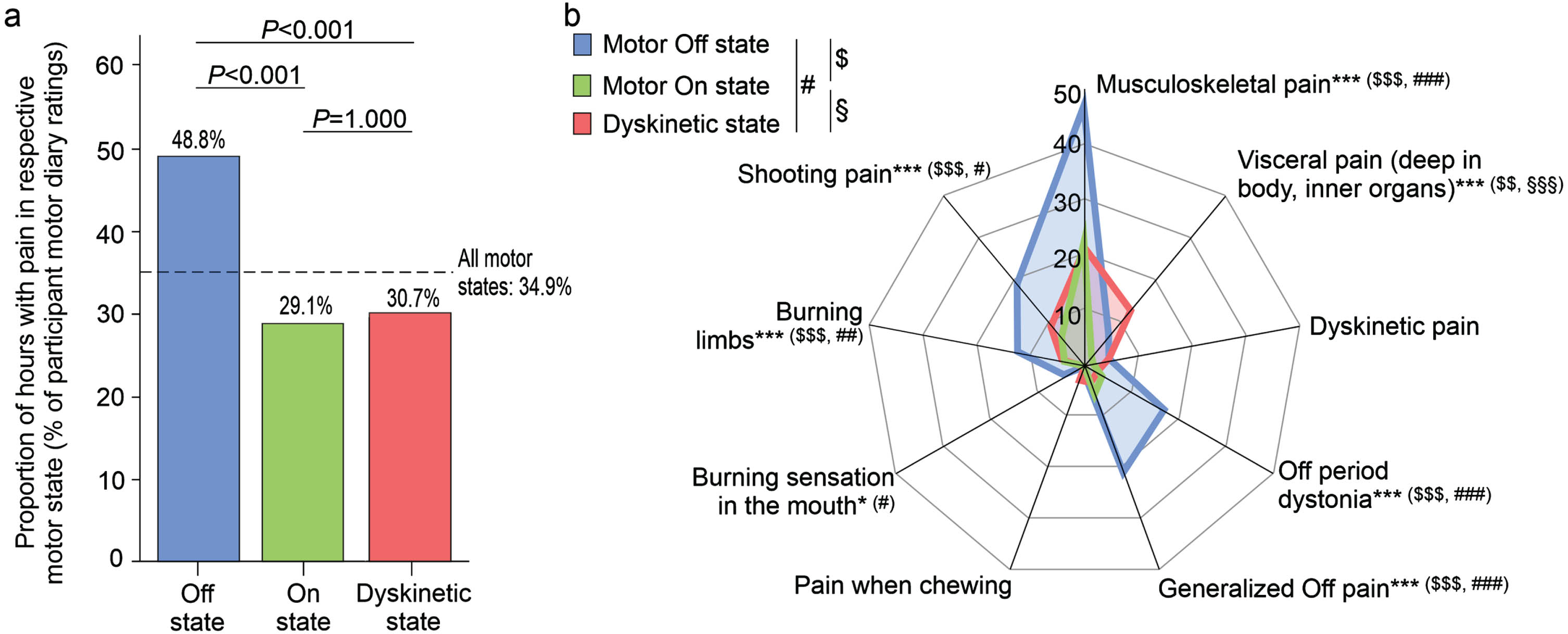
Table 2
Pain type characteristics of study cohort as assessed by pain diary ratings
| Item/Domain | Pain diary$ | KPPS# | Pain diary vs. KPPS§ | |||
| Time | Participant level (%), | Frequency score (n, %), | Frequency×Severity score | Frequency | Frequency× | |
| level (%) | Median [IQR] | Median [IQR] | (n, %), Median [IQR] | score, ρ | Severity score, ρ | |
| 1. Pain around joints | 29.6% | 6.7% [0.0% – 50.0%] | 2 (50%) [0 (0%) – 3 (75%)] | 2 (17%) [0 (0%) – 6 (50%)] | 0.449** | 0.454** |
| Domain 1. Musculoskeletal pain | 29.6% | 6.7% [0.0% – 50.0%] | 2 (50%) [0 (0%) – 3 (75%)] | 2 (17%) [0 (0%) – 6 (50%)] | 0.449** | 0.454** |
| 2. Pain deep within the body | – | – | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | – | – |
| 3. Pain related to internal organ | – | – | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | – | – |
| Domain 2. Visceral pain | 5.8% | 0.0% [0.0% – 6.3%] | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | 0.187 | 0.225 |
| 4. Dyskinetic pain | 3.2% | 0.0% [0.0% – 0.0%] | 0 (0%) [0 (0%) – 0 (50%)] | 0 (0%) [0 (0%) – 3 (25%)] | – 0.062 | – 0.101 |
| 5. Off period dystonia in a specific region | 7.8% | 0.0% [0.0% – 8.3%] | 1 (25%) [0 (0%) – 3 (75%)] | 1 (8%) [0 (0%) – 6 (50%)] | 0.273* | 0.299* |
| 6. Generalized “off” period pain | 10.3% | 0.0% [0.0% – 0.0%] | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | 0.222 | 0.210 |
| Domain 3. Fluctuation– related pain | 16.6% | 0.0% [0.0% – 30.8%] | 2 (17%) [0 (0%) – 3 (25%)] | 6 (17%) [0 (0%) – 9 (25%)] | 0.544** | 0.486** |
| 7. PLM or RLS– associated pain | – | – | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | – | – |
| 8. Pain while turning in bed | – | – | 0 (0%) [0 (0%) – 3 (75%)] | 0 (0%) [0 (0%) – 8 (67%)] | – | – |
| Domain 4. Nocturnal pain | – | – | 1 (13%) [0 (0%) – 4 (50%)] | 1 (5%) [0 (0%) – 7 (29%)] | – | – |
| 9. Pain when chewing | 1.5% | 0.0% [0.0% – 19.0%] | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | 0.385** | 0.385** |
| 10. Pain due to grinding teeth | – | – | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | – | – |
| 11. Burning sensation in the mouth | 2.1% | 0.0% [0.0% – 21.6%] | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | 0.492** | 0.492** |
| Domain 5. Oro-facial pain | – | – | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | – | – |
| 12. Burning pain in the limbs | 6.1% | 0.0% [0.0% – 43.0%] | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | 0.206 | 0.206 |
| 13. Generalized lower abdominal pain | – | – | 0 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | – | |
| Domain 6. Discoloration, oedema/swelling | – | – | 2 (0%) [0 (0%) – 0 (0%)] | 0 (0%) [0 (0%) – 0 (0%)] | – | – |
| 14. Shooting pain/pins &needles | 10.5% | 0.0% [0.0% – 11.1%] | 0 (0%) [0 (0%) – 2 (50%)] | 0 (0%) [0 (0%) – 2 (17%)] | 0.342* | 0.328* |
| Domain 7. Shooting pain/pins &needles | 10.5% | 0.0% [0.0% – 11.1%] | 0 (0%) [0 (0%) – 2 (50%)] | 0 (0%) [0 (0%) – 2 (17%)] | 0.342* | 0.328* |
| Total Score | – | – | 8 (14%) [4 (7%) – 11 (20%)] | 12 (7%) [7 (4%) – 24 (14%)] | – | – |
| Total Score with pain diary items only | 42.0% | 35.3% [6.7% – 69.2%] | 6 (17%) [3 (8%) – 9 (25%)] | 9 (7%) [6 (5%) – 18 (14%)] | 0.531** | 0.468** |
KPPS, King’s Parkinson’s Disease Pain Scale. $We included domains into the pain diary data and ordered the questions according to the ordering of the KPPS for easy comparison of the two scales. Pain diary was assessed on study day 2 only. #Numbers in parentheses are percentages of total item/domain score. Data on KPPS are from Löhle and colleagues 30 and used here for correlation with pain diary results. §Compared are the pain diary data at the participant level with the Frequency score and the combined Frequency×Severity score from KPPS data using the Spearman rank correlation test (*p < 0.05 and **p < 0.001).
We then analyzed pain frequencies with respect to motor states from participant PD Home diary data (Fig. 2a; for motor state frequencies, refer to Participants and Methods section). Pain was more frequent on motor Off state as compared to motor ON and Dyskinetic state, while we did not detect any differences between On and Dyskinetic state. When analyzing the various pain types, most types showed significant differences in their frequency between the three different motor states except “Dyskinetic pain” and “Pain when chewing” (Fig. 2b). Pain frequency patterns were similar as compared to overall pain with more frequent pain reported in motor Off state as compared to On and Dyskinetic state with “Visceral pain (deep in body, inner organs)” as the only exception (Fig. 2b): This pain type was least frequent in motor On state and more common in Dyskinetic than in motor Off state. Comparing pain and its types with motor states as rated by clinical observers revealed similar pain type patterns (Supplementary Figure 1).
Pain occurrence showed a clear circadian rhythm peaking in the early morning period with up to 62% of hours with pain. This pattern closely mirrored periods with motor Off but not Dyskinetic periods (see Supplementary Text for details). In addition to the comparison of pain and motor diary data from hour periods, we also assessed the timing of pain occurrence during Off episodes 31: We did not detect any association of pain occurrence with the beginning and the further course of Off episodes (Supplementary Text).
Temporal association of pain with other non-motor symptoms
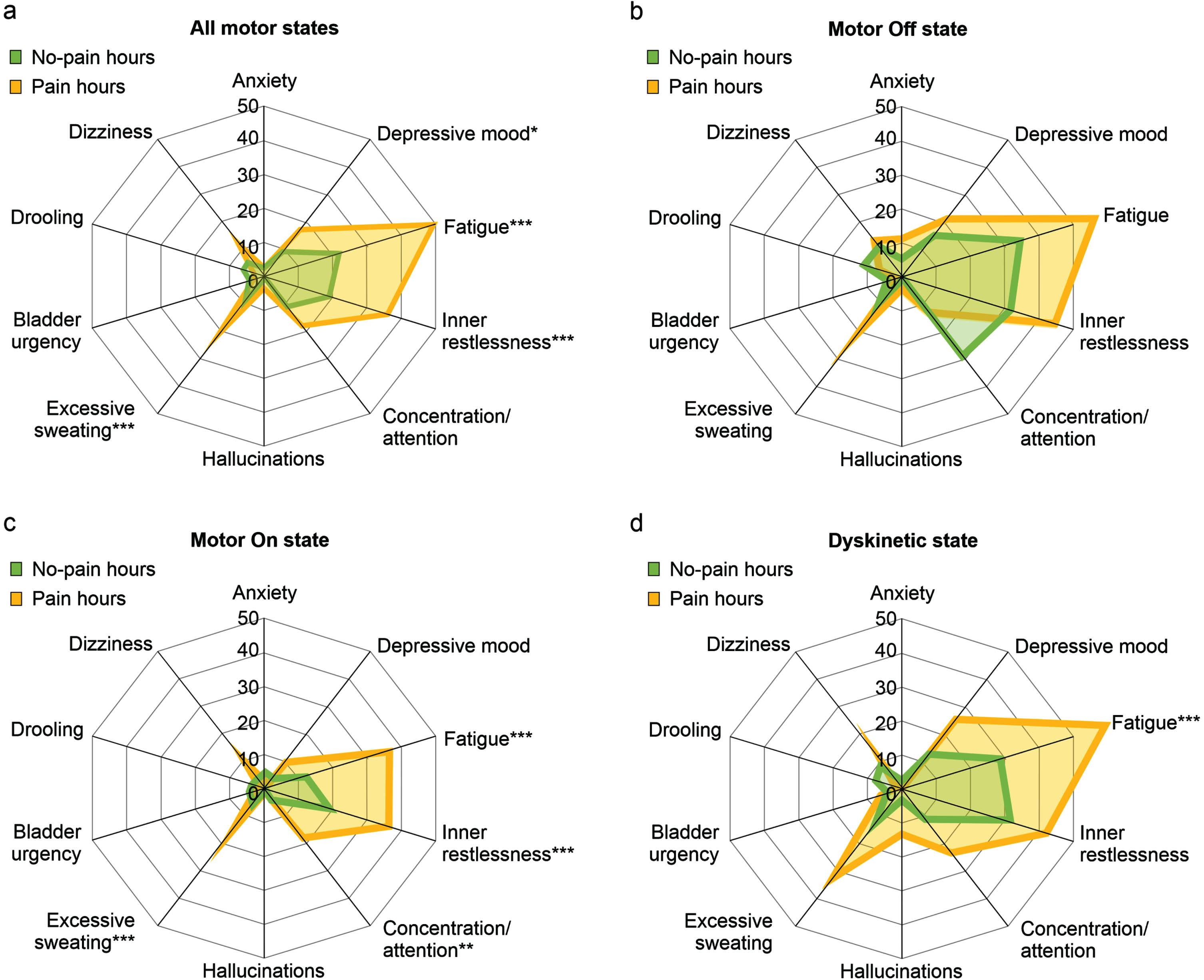
We then compared no-pain and pain hours of study day 1 with respect to the co-occurrence of other NMS (Fig. 3). When analyzing all motor states together, we detected significantly increased frequencies of “Depressive mood”, “Fatigue”, “Inner restlessness” and “Excessive sweating” but not of the remaining NMS in pain as compared to no-pain hours (Fig. 3a). When analyzing the association of pain and other NMS for the three motor states separately, we did not detect any differences between pain and no-pain hours in motor Off state, but only increased frequencies of “Fatigue”, “Inner restlessness”, “Concentration/attention” and “Excessive sweating” in motor On state and only increased frequency of “Fatigue” in Dyskinetic state (Fig. 3b– d). Cluster analyses largely confirmed the association of pain with “Fatigue” and “Inner restlessness” when analyzing all motor states together or the three motor states separately (Supplementary Text). Together, pain was particularly associated with “Fatigue” and “Inner restlessness” than other fluctuating NMS on the hour level.
Fig. 3
Temporal association of pain with other non-motor symptoms on the hour level from pain and non-motor diary ratings by the participants. Proportions of hourly diary ratings of various non-motor symptoms by participants with respect to simultaneous pain diary ratings by the participants. Upper left diagram (a) represents data for all motor states, while the other diagrams display data for motor Off state (b), motor On state (c) and Dyskinetic state (d) as assessed by PD home diary. Values are percentages. *p < 0.05, **p < 0.01, and ***p < 0.001 from χ2 tests comparing pain and no-pain hours. Data are based on 1,089 simultaneous hourly ratings (68.1% of all time periods and 96.5% of waking hours on 1 day) by 47 participants.
Impact of pain on global disease severity impression
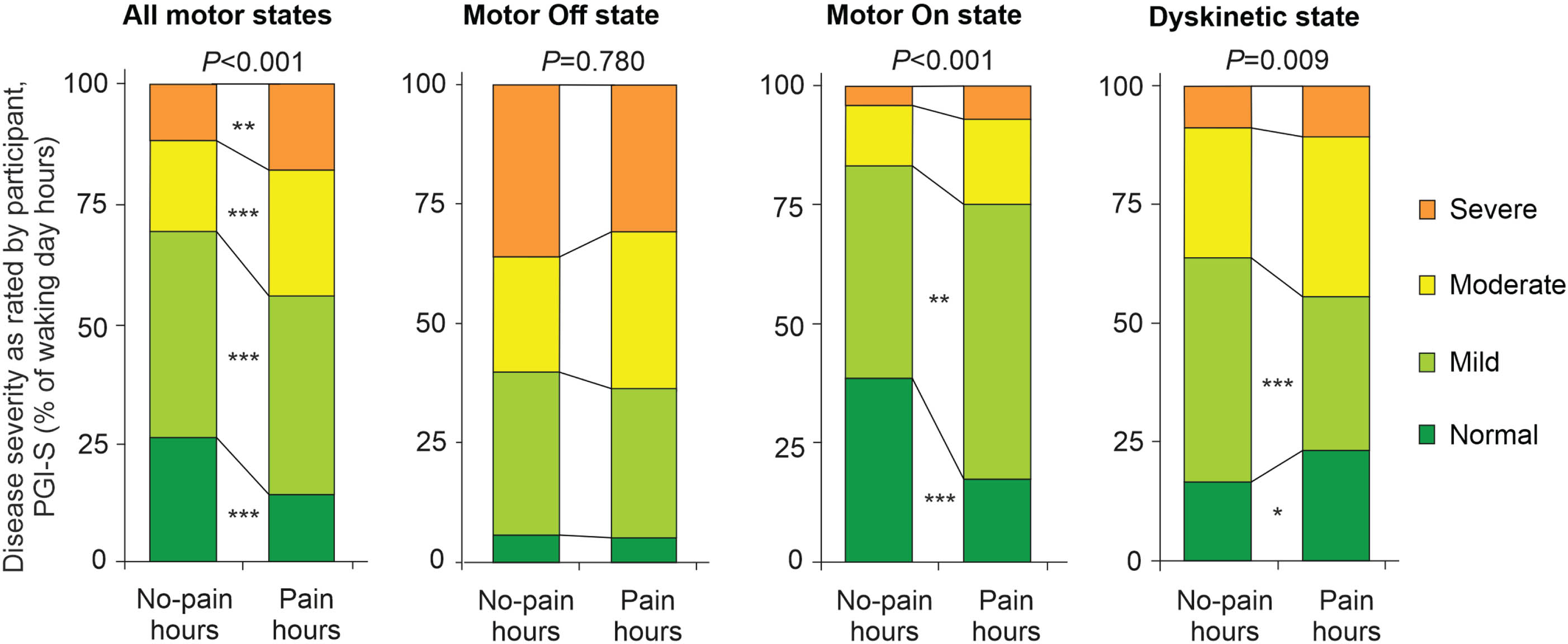
Global disease severity as perceived by participants was assessed by simultaneous hourly ratings of patient global impression of severity (PGI-S; Fig. 4). PGI-S ratings displayed higher disease burden in pain hours as compared to no-pain hours when analyzing all motor states (Fig. 4a). These differences are essentially driven by higher disease burden in pain as compared to no-pain hours exclusively in motor On and Dyskinetic state hours (Fig. 4b– d). In contrast, hourly ratings of clinical global impression of severity by the clinical observer (CGI-S) did not show relevant differences between pain and no-pain hours (Supplementary Figure 2).
Fig. 4
Perception of disease severity by participants on the hour level with respect to pain diary data and motor states. Perception of disease severity as rated by participants on the Patient Global Impression of Severity (PGI-S) on the hour level. Illustration of the distribution of PGI-S ratings with respect to simultaneous pain ratings using the levels “Normal” (deep green color), “Mild” (bright green color), “Moderate” (yellow color) and “Severe” (orange color). The analyses were performed for all motor states (left diagram) and for motor Off state, motor On state and Dyskinetic state (from left to right diagram) as assessed by simultaneous PD home motor diary ratings. All PGI-S values were originally assessed with 7 severity grades, but then condensed to four levels to enhance clarity of the figure (please refer to Methods for more details). Values are percentages. p-values above the diagrams are from χ2 tests comparing all PGI-S scores in pain versus no-pain hours. *p < 0.05, **p < 0.01, and ***p < 0.001 are from pair-wise comparisons of PGI-S score applying χ2 tests with Bonferroni adjustment. Data are based on 1,506 simultaneous hourly ratings (67.9% of all time periods and 99.2% of waking hours on 2 consecutive days) by 47 participants.
Pain fluctuations on the participant level
To analyze pain fluctuations on the participant level, we first analyzed the percentage daily time with pain for all motor states (PDTPain; Fig. 5a): The median PDTPain was 35.3% (IQR: 6.7– 69.2%; for PDTs of the various types of pain, refer to Table 2). We then compared PDTPain separately for each motor state, which revealed significant differences between motor Off or On state when compared to Dyskinetic state, but not between motor Off and On state (Fig. 5a). Of note, the analyses of PDTsPain during the different motor states did not only depend on the pain times per day but also on the PDTs spent in the different motor states and therefore do not allow the direct comparison of the pain patterns during the different motor states.
Fig. 5
Percentage daily times with pain (PDTPain) with respect to motor states and percentage pain time during specific motor states (PPT). (a) Percentage daily times with pain (PDTPain) from pain diary ratings for all motor states (white box) and with respect to simultaneous PD home motor diary ratings (motor Off state [blue box], motor On state [green box], Dyskinetic state [red box]). (b) Percentage motor state time with pain for each motor state normalized to motor state hours (percentage pain time during the specific motor states; PPTMotorstate) for the three motor states from simultaneous PD home motor diary ratings (motor Off state, On state, Dyskinetic state). Boxplots are shown with a central mark at the median, bottom, and top edges of the boxes at 25th and 75th percentiles, respectively, whiskers out to the most extreme points within 1.5 times the interquartile range, and outliers scoring more than 1.5×IQR but at most 3×IQR outside the quartiles. Displayed p-values are from Friedman tests with post-hoc Wilcoxon signed-rank tests with Bonferroni correction for multiple comparisons. (c) 3D correlation analysis of PPTMotorstate for the three motor states. Solid yellow line represents the regression line and dotted yellow lines display the crossing of the regression line at PPTOff = 0% and at PPTOff = 100% for easy interpretation of the 3D course of the regression line. Data are based on 1,506 simultaneous hourly ratings (67.9% of all time periods and 98.2% of waking hours on 2 consecutive days) by 47 participants.
![Percentage daily times with pain (PDTPain) with respect to motor states and percentage pain time during specific motor states (PPT). (a) Percentage daily times with pain (PDTPain) from pain diary ratings for all motor states (white box) and with respect to simultaneous PD home motor diary ratings (motor Off state [blue box], motor On state [green box], Dyskinetic state [red box]). (b) Percentage motor state time with pain for each motor state normalized to motor state hours (percentage pain time during the specific motor states; PPTMotorstate) for the three motor states from simultaneous PD home motor diary ratings (motor Off state, On state, Dyskinetic state). Boxplots are shown with a central mark at the median, bottom, and top edges of the boxes at 25th and 75th percentiles, respectively, whiskers out to the most extreme points within 1.5 times the interquartile range, and outliers scoring more than 1.5×IQR but at most 3×IQR outside the quartiles. Displayed p-values are from Friedman tests with post-hoc Wilcoxon signed-rank tests with Bonferroni correction for multiple comparisons. (c) 3D correlation analysis of PPTMotorstate for the three motor states. Solid yellow line represents the regression line and dotted yellow lines display the crossing of the regression line at PPTOff = 0% and at PPTOff = 100% for easy interpretation of the 3D course of the regression line. Data are based on 1,506 simultaneous hourly ratings (67.9% of all time periods and 98.2% of waking hours on 2 consecutive days) by 47 participants.](https://content.iospress.com:443/media/jpd/2024/14-7/jpd-14-7-jpd240026/jpd-14-jpd240026-g005.jpg)
To get better insights into pain distribution patterns within the three motor states independent of the specific motor state frequencies, we thus calculated the proportions of hours with pain as percentage of total hours in the respective motor state (percentage pain times, PPTsMotorstate; Fig. 5b). PPTOff was significantly higher as compared to PPTOn and PPTDyskinetic, while the latter two states did not show any differences. We found significant moderate to strong correlations of PPTs of the three motor states among each other with r ranging from 0.383 (PPTOff versus PPTDyskinetic) through 0.579 (PPTOff versus PPTOn) to 0.696 (PPTOn versus PPTDyskinetic; p < 0.05; Fig. 5c) showing interdependences of pain times across the three motor states.
Association of pain fluctuations with non-motor fluctuations on the participant level
When comparing the PDTPain and PDTs with other NMS from diary assessments at study day 1, we solely detected a significant correlation between PDTPain and PDTFatigue with r = 0.427 (p = 0.003, Pearson correlation test) when analysing all motor states (Supplementary Table 2). This association was also exclusively observed when correlating PDTsPain with PDTsFatigue or the corresponding PPTs for the three motor states separately (r values ranging from 0.345 to 0.530, P < 0.05; Supplementary Table 2).
Association of pain with health-related quality of life (hr-QoL)
We detected significant mild-to-moderate correlations of PDTPain/On and PDTPain/Dyskinetic but not PDTPain or PDTPain/Off with PDQ-39 sum scores as a measure of hr-QoL with Pearson correlation coefficients between 0.281 and 0.381 (Supplementary Table 4). Similar patterns of correlations were observed for pain time in the three motor states when expressed as PPTs (Supplementary Table 4). These significant correlations persisted after the deletion of subscore 8 (“Bodily pain/discomfort”) from PDQ-39 sum scores (r values: 0.261– 0.320; Supplementary Table 4). These correlations survived adjustment for potential demographic and clinical confounders by multivariate linear regression modelling (see Supplementary Text for details).
Analyses of pain frequency with other demographic and clinical characteristics did not reveal any associations of pain frequency parameters, neither with sex nor with other demographic or clinical parameters (regardless whether times of pain were expressed as PDTs or PPTs; Supplementary Table 3). Of note, osteoarthritis as a concomitant condition had also no influences on pain diary measures (p≥0.05; Mann-Whitney U-test).
Pain fluctuations on the cohort level
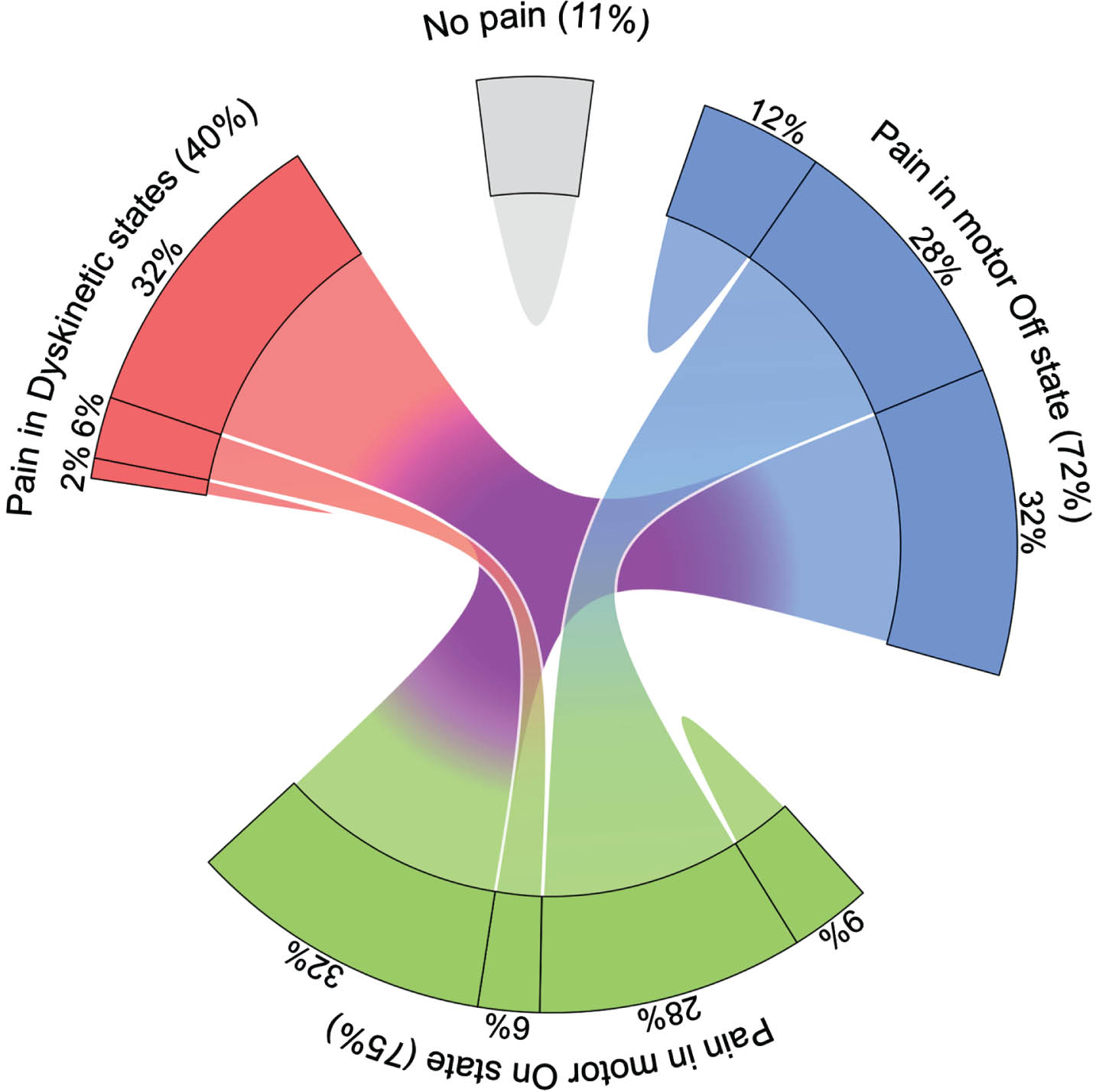
Using the pain diaries as the pain assessment tool, 89% of all participants experienced at least one hour of pain during the two days of pain assessment (Table 2). The chord diagram in Fig. 6 displays the many-to-many relationship between participants with pain in motor Off, On and/or Dyskinetic state: 32% of participants complained about pain in all motor states, while 28% complained about pain in Off and On state, but only 6% in On and Dyskinetic state. Complaints of pain in only one of the three motor states was rather infrequent and only 2% of participants complained about pain only in Dyskinetic state.
Fig. 6
Proportion of participants with Parkinson’s disease reporting pain with respect to motor states. The chord diagram shows the many-to-many relationship between participants with pain as reported in pain diary with respect to motor Off, motor On and/or Dyskinetic state as rated by participants using the PD home diary. Participants were classified as having pain in the various motor states if they reported at least one hour of pain in the pain diary in the respective motor state as simultaneously rated in the PD Home motor diary. Note that only 11% of participants reported no pain at all. Data are based on 1,506 simultaneous hourly ratings (67.9% of all time periods and 99.2% of waking hours on 2 consecutive days) by 47 participants.
DISCUSSION
The primary finding of this prospective observational multi-center cohort study in 47 participants with advanced PD (defined herein based on the presence of motor fluctuations and/or dyskinesia)32 is that pain is very frequent and present in one third of the waking day with a clear circadian rhythm showing a peak in the early morning Off period and a clustering during motor Off state hours. Most pain types were fluctuating in conjunction with motor fluctuations including “Musculoskeletal pain”, “Visceral pain”, “Off period dystonia”, “Generalized Off pain”, “Burning sensation in the mouth”, “Burning limbs” and “Shooting pain”. The main NMSs co-fluctuating with pain were “Fatigue” and “Inner Restlessness”. Synchronizing pain diary data with motor Off episodes did not show any close temporal association of pain with motor Off episodes. Unexpectedly, simultaneous co-assessment of global disease severity impression by participants (PGI-S) revealed that pain is associated with more severe perception of illness only in motor On and Dyskinetic state but not in motor Off state, which translated into significant correlations of daily pain times with hr-QoL only for pain during motor On state and Dyskinetic state.
Although pain fluctuations in advanced PD have received increasing research attention during recent years, circadian occurrence, timing and kinetics of pain subtype fluctuations as well as their co-fluctuation with other NMS remain largely enigmatic. Moreover, the importance of fluctuations of specific types of pain for disease severity perception and hr-QoL were mainly unclear. Since previous studies used the motor state as the basis for investigating frequency and severity of pain fluctuations,8,17–25 these studies drew conclusions neither on circadian occurrence patterns of pain (as shown for the motor symptoms using the PD home diary since more than 10 years)27,34,35,46,47 nor on temporal dependency of pain and motor/NMS fluctuations. Here we used a pain diary assessing nine pain types in simultaneous conjunction with standard motor and non-motor diaries27,29,31 in order to close this data gap. This is the first use of a pain diary following our former studies on NMS diaries29,31 using questions of the KPPQ as a validated assessment tool for pain in PD.12,30 Although we did not have the problem of diary fatigue in the present study due to restriction to two study days and close surveillance of the patient during the study27,28 leading to high diary adherence, it is relatively evident from previous studies that participants have more difficulties in rating their NMS than their motor state on an hourly basis.29 There is however, to our knowledge, no other tool available to assess pain and its subtypes with high frequency (several times per day) and the validation of such data will be challenging due to the lack of suitable external validation criteria. However, convergent validities of diary data on fluctuating NMS with independent measures of related constructs were in general moderate.29 Although no data on the pain diary from healthy subjects or non-fluctuating PD patients are available, the correlations of pain diary data (PDTs with pain and its types) with the corresponding quantitative pain frequency assessments using the King’s PD Pain Scale (KPPS) further support the suitability of the pain diary assessment (with the item “Dyskinetic pain” as a major exception, see Participants and Methods section).
Our study design allowed estimation of pain frequencies on the time/hour level, the participant level and the cohort level. On the time level, we observed a high frequency of 28% of waking day hour periods with pain when using general NMS diary assessment and 42% when using a specific pain diary. It well known that pain frequencies largely depend on the assessment tool, which is one presumed factor for the high variance of pain frequencies in PD. Pain displayed a clear circadian rhythm peaking in the early morning Off period and a clustering during motor Off state hours. On the participant level, diary data showed similar results with a median PDTPain of 35% of the waking day with the highest proportion of pain during motor Off state. These figures are in line with previous studies investigating pain in motor Off and particularly in early morning Off periods.8,18,22,23,48 The 100% frequency of pain during the night hours (Supplementary Figure 3) suggests that pain during Off state is the major reason for early awaking and does not represent a specific circadian association of pain and motor Off state. Indeed, the stable distribution of the proportion of pain time within the three motor states over the waking day further supports the notion that there is no circadian rhythm of pain perception in the different motor states. Together, the recognition of early morning motor/pain Off represents an important aspect for disease management in PD since clinical trial data are available showing efficacy of long-acting dopaminergic strategies for this indication.49–51
On the cohort level, 89% of the participants complained of at least one hour of pain within the two days of diary assessment. This figure is in the upper range of pain frequency (40– 85%),1,6–8 which is easily explained by both the novel and presumably sensitive method of pain assessment by diaries and the cohort characteristics of the present study consisting of advanced fluctuating PD patients.52 Pain fluctuation measures on the cohort level indicated that pain is as frequent in motor Off state as in motor On state (72– 75% of cohort), but less frequent in Dyskinetic state (40% of cohort). One-third of the cohort had pain in all three motor states, but only very few patients complained of pain only in one specific motor state (2% in Dyskinetic state and up to 12% in Off state). This pain distribution pattern across an advanced PD cohort is similar to previous reports using other pain assessment tools such as the visual analogue scale or the NMSS pain item.8,22,23
The most frequent pain type by far was “Musculoskeletal pain” (30% of waking day time) followed by “Fluctuation-related pain” (17%) and “Shooting pain/pins & needles” (11%). This pattern is similar to previous studies on pain types in advanced PD12,30,37 when measured with the KPPS, again supporting the validity of our pain diary approach.
Most pain types including the items “Musculoskeletal pain”, “Off period dystonia”, “Generalized Off pain”, “Burning sensation in the mouth”, “Burning limbs” and “Shooting pain” were fluctuating in conjunction with motor fluctuations with the highest frequencies reported in motor Off state. In contrast, the item “Visceral pain” was the single item with higher frequencies in both pathological motor states (Off and Dyskinetic state) with even the highest frequency in Dyskinetic state. The mechanisms behind this phenomenon are unclear, particularly because visceral pain is considered a nociplastic pain type, which is usually associated with hypodopaminergic states such dopamine agonist withdrawal syndrome or motor/non-motor Off periods.23,53,54 Cross-sectional studies also show the association of pain and dyskinesia with pain being more common in patients experiencing levodopa-induced dyskinesia,19,26 but, to the best of our knowledge, there is no report available from the literature on the association of specific pain subtypes and dyskinesia. Although we are not able to draw any conclusions on mechanisms behind various pain types during their fluctuations, studies using pain sensitivity assessment suggest common mechanisms of pain and dyskinesia.55
The item “Dyskinetic pain” did not fluctuate at all and did not show any association with the Dyskinetic state. The reason for this discrepancy is unclear, but might be related to the very low frequency of this pain subtype and/or the limited validity of this item in the diary setting. The results on this particular pain subtype should therefore be interpreted with caution.
The simultaneous assessment of pain and other NMS showed that the items “Fatigue” and “Inner Restlessness” “Depression” and “Excessive sweating” co-fluctuate with pain when analyzing all motor states together, but separate analysis of the three motor states separately and clustering techniques revealed “Fatigue” and “Inner Restlessness” as main NMS co-fluctuating with pain. On the participant level, PDTs with pain were exclusively correlated with PDTs with fatigue emphasizing the specific association of pain and fatigue. The cross-sectional study performed by Hagell and Brundin also detected an association of pain with fatigue.56 The association of pain fluctuations with neuropsychiatric symptoms and inner restlessness fits closely to the concept of nociplastic pain as a third mechanism of pain in PD.53,57
The present study found that pain was associated with more severe global disease perception only in motor On and Dyskinetic state but not in motor Off state. This pattern of pain and disease severity was not detected with observer ratings, most likely because the clinical observer was not able to pick up the symptom pain. This association translated into significant correlations of daily pain times with hr-QoL again only for pain during motor On state and Dyskinetic state. This particular pattern of disease perception and hr-QoL results with respect to pain might be mediated by the phenomenon that pain is overruled by motor symptoms in Off state but gains importance for disease severity perception and hr-QoL during phases with good motor performance. Similar associations of NMS and hr-QoL with a particular importance of NMS in motor On state were already reported for depression, anxiety and fatigue.23 The many available studies on the importance of NMS for hr-QoL need to be interpreted in light of these results and motor performance and therapy need to be included into interpretation of the importance of NMS for hr-QoL.11,58
Our study has several limitations: Firstly, our study cohort consisted of a rather heterogeneous and small sized inpatient cohort from two movement disorder centers, which is however similar to other larger cohorts investigating NMS in advanced PD.23,59 Moreover, we only recruited PD patients already experiencing motor fluctuations and intentionally excluded patients who screened positive for dementia to ensure proper understanding of motor and NMS states and adherence to the hourly ratings. These aspects could limit generalizability of our results to a greater population including patients with cognitive dysfunction. Secondly, we used diary assessments to evaluate pain fluctuations facing all issues known to limit data from diary ratings (for special aspects of the pain diary, refer to the detailed discussion above). Moreover, the pain diary does not meet all requirements, especially the complexity of pain in PD, and in many areas still follows the traditional terminology used in pain therapy and not a mechanistic classification.57 This might further limit the transferability of our clinical results to mechanistic conclusions. However, we took several precautions to ensure a high validity of our study results: We trained all patients how to depict motor and NMS fluctuations prior to the diary recordings. We also used an instructional video to enhance understanding of different motor states and utilized an adopted version of the PD home diary with pictograms, which has shown to be preferred by patients in comparison to the original version.27 Additionally, we also performed simultaneous clinical observer motor diary ratings with a single rater approach to control for timely and complete diary ratings by the participants.
In conclusion, pain is a very frequent NMS in advanced fluctuating PD with a clear circadian rhythm showing a peak in the early morning Off period and a strong association with motor Off state over the waking day. However, there is no close temporal association of pain with motor Off episodes. Pain is co-fluctuating in particular with “Fatigue” and “Inner Restlessness”. In fluctuating PD patients, pain during motor On and Dyskinetic state but not motor Off state is an important factor for perceived global disease severity and reduced hr-QoL. This finding is of particular interest, because fluctuating NMS including pain are usually treated in the first step by optimizing dopaminergic (motor) therapy, which in particular addresses pain in motor Off state but not in motor On state. The recognition and subsequent specific therapy of pain associated with motor On/Dyskinetic state therefore seems an important aspect for disease management in advanced PD. Future clinical trials on pain in advanced PD should take pain fluctuations and particularly On state pain into consideration.
ACKNOWLEDGMENTS
The authors would like to thank all patients for their willingness to participate in our study.
FUNDING
The trial was not supported by financial sponsors. The corresponding author had the final responsibility for the decision to submit for publication.
CONFLICT OF INTEREST
The authors declare no competing financial and non-financial interest concerning the research related to the manuscript. A.S. has received funding from the Deutsche Forschungsgemeinschaft (German Research Association) and the Helmholtz-Association outside the present study. He has received honoraria for presentations/advisory boards/consultations from Global Kinetics Corporation (manufacturer of the PKG), Esteve, Desitin, Lobsor Pharmaceuticals, STADA, Bial, RG Gesellschaft, Zambon, NovoNordisk and AbbVie outside the present study. He has received royalties from Kohlhammer Verlag and Elsevier Press. He serves as an editorial board member of Stem Cells International. A.B. has nothing to disclose. F.G. has received honoraria from AbbVie, BIAL, Merz, and STADA outside the submitted work. P.O. has received funding from AbbVie, Lund University Medical Faculty, Multipark, the Swedish Parkinson Foundation, Health Care Region Skåne, and Åhlens Foundation outside of the present work. He has received honoraria for lectures and expert advice from AbbVie, Bial, Britannia, Ever Pharma, Global Kinetics, Lobsor, Nordic Infucare, Stada, and Zambon outside of the present study. He has received royalties from UNI-MED Verlag. G.E. has received honoraria for advisory Boards, consultancy and presentations from AbbVie Pharma, BIAL, Biogen GmbH, Desitin Pharma, STADA Pharma, Neuroderm Inc., Licher GmbH, UCB Pharma, and Zambon Pharma outside of the present work. He has received royalties from Kohlhammer Verlag and Thieme Verlag. M.L. has received honoraria for presentations from Novartis Pharma and STADA Pharma outside of the present study.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-240026.
REFERENCES
1. | Broen MP , Braaksma MM , Patijn J , et al. Prevalence of pain in Parkinson’s disease: a systematic review using the modified QUADAS tool. Mov Disord (2012) ; 27: : 480–484. |
2. | Viseux FJF , Delval A , Simoneau M , et al. Pain and Parkinson’s disease: Current mechanism and management updates. Eur J Pain (2023) ; 27: : 553–567. |
3. | Wasner G and Deuschl G. Pains in Parkinson disease--many syndromes under one umbrella. Nat Rev Neurol (2012) ; 8: : 284–294. |
4. | Rukavina K , Cummins TM , Chaudhuri KR , et al. Pain in Parkinson’s disease: Mechanism-based treatment strategies. Curr Opin Support Palliat Care (2021) ; 15: : 108–115. |
5. | Karnik V , Farcy N , Zamorano C , et al. Current status of pain management in Parkinson’s disease. Can J Neurol Sci (2020) ; 47: : 336–343. |
6. | Defazio G , Gigante A , Mancino P , et al. The epidemiology of pain in Parkinson’s disease. J Neural Transm (Vienna) (2013) ; 120: : 583–586. |
7. | Tai YC and Lin CH. An overview of pain in Parkinson’s disease. Clin Park Relat Disord (2020) ; 2: : 1–8. |
8. | Goetz CG , Tanner CM , Levy M , et al. Pain in Parkinson’s disease. Mov Disord (1986) ; 1: : 45–49. |
9. | Naisby J , Lawson RA , Galna B , et al. Trajectories of pain over 6 years in early Parkinson’s disease: ICICLE-PD. J Neurol (2021) ; 268: : 4759–4767. |
10. | Politis M , Wu K , Molloy S , et al. Parkinson’s disease symptoms: the patient’s perspective. Mov Disord (2010) ; 25: : 1646–1651. |
11. | Gallagher DA , Lees AJ and Schrag A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord (2010) ; 25: : 2493–2500. |
12. | Martinez-Martin P , Rizos AM , Wetmore J , et al. First comprehensive tool for screening pain in Parkinson’s disease: the King’s Parkinson’s Disease Pain Questionnaire. Eur J Neurol (2018) ; 25: : 1255–1261. |
13. | Winter Y , von Campenhausen S , Arend M , et al. Health-related quality of life and its determinants in Parkinson’s disease: results of an Italian cohort study. Parkinsonism Relat Disord (2011) ; 17: : 265–269. |
14. | Rahman S , Griffin HJ , Quinn NP , et al. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord (2008) ; 23: : 1428–1434. |
15. | Antonini A , Tinazzi M , Abbruzzese G , et al. Pain in Parkinson’s disease: facts and uncertainties. Eur J Neurol (2018) ; 25: : 917–e969. |
16. | Quinn NP , Koller WC , Lang AE , et al. Painful Parkinson’s disease. Lancet (1986) ; 1: : 1366–1369. |
17. | Kurihara K , Fujioka S , Kawazoe M , et al. Fluctuating pain in Parkinson’s disease: Its prevalence and impact on quality of life. eNeurologicalSci (2021) ; 25: : 100371. |
18. | Rodriguez-Blazquez C , Schrag A , Rizos A , et al. Prevalence of non-motor symptoms and non-motor fluctuations in Parkinson’s disease using the MDS-NMS. Mov Disord Clin Pract (2021) ; 8: : 231–239. |
19. | Tinazzi M , Del Vesco C , Fincati E , et al. Pain and motor complications in Parkinson’s disease. J Neurol Neurosurg Psychiatry (2006) ; 77: : 822–825. |
20. | Gunal DI , Nurichalichi K , Tuncer N , et al. The clinical profile of nonmotor fluctuations in Parkinson’s disease patients. Can J Neurol Sci (2002) ; 29: : 61–64. |
21. | Seki M , Takahashi K , Uematsu D , et al. Clinical features and varieties of non-motor fluctuations in Parkinson’s disease: a Japanese multicenter study. Parkinsonism Relat Disord (2013) ; 19: : 104–108. |
22. | Storch A , Schneider CB , Klingelhofer L , et al. Quantitative assessment of non-motor fluctuations in Parkinson’s disease using the Non-Motor Symptoms Scale (NMSS). J Neural Transm (Vienna) (2015) ; 122: : 1673–1684. |
23. | Storch A , Schneider CB , Wolz M , et al. Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology (2013) ; 80: : 800–809. |
24. | Martinez-Fernandez R , Schmitt E , Martinez-Martin P , et al. The hidden sister of motor fluctuations in Parkinson’s disease: A review on nonmotor fluctuations. Mov Disord (2016) ; 31: : 1080–1094. |
25. | Nebe A and Ebersbach G. Pain intensity on and off levodopa in patients with Parkinson’s disease. Mov Disord (2009) ; 24: : 1233–1237. |
26. | Vela L , Facca A , Lyons KE , et al. Pain and Parkinson’s disease. Mov Disord (2002) ; 17: : S154–S155. |
27. | Löhle M , Bremer A , Gandor F , et al. Validation of the PD home diary for assessment of motor fluctuations in advanced Parkinson’s disease. NPJ Parkinsons Dis (2022) ; 8: : 69. |
28. | Lohle M , Timpka J , Bremer A , et al. Application of single wrist-wearable accelerometry for objective motor diary assessment in fluctuating Parkinson’s disease. NPJ Digit Med (2023) ; 6: : 194. |
29. | Ossig C , Sippel D , Fauser M , et al. Assessment of nonmotor fluctuations using a diary in advanced Parkinson’s disease. J Parkinsons Dis (2016) ; 6: : 597–607. |
30. | Löhle M , Jost WH , Bremer A , et al. Intercultural translation and application of the German version of King’s Parkinson’s Disease Pain Questionnaire in fluctuating Parkinson’s disease. Acta Neurol Scand (2024) ; 2024: : 052552. |
31. | Ossig C , Sippel D , Fauser M , et al. Timing and kinetics of nonmotor fluctuations in advanced Parkinson’s disease. J Parkinsons Dis (2017) ; 7: : 325–330. |
32. | Aslam S , Manfredsson F , Stokes A , et al. “Advanced” Parkinson’s disease: A review. Parkinsonism Relat Disord (2024) ; 123: : 106065. |
33. | Dalrymple-Alford JC , MacAskill MR , Nakas CT , et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology (2010) ; 75: : 1717–1725. |
34. | Hauser RA , Russ H , Haeger DA , et al. Patient evaluation of a home diary to assess duration and severity of dyskinesia in Parkinson disease. Clin Neuropharmacol (2006) ; 29: : 322–330. |
35. | Hauser RA , Friedlander J , Zesiewicz TA , et al. A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol (2000) ; 23: : 75–81. |
36. | Podsiadlo D and Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc (1991) ; 39: : 142–148. |
37. | Chaudhuri KR , Rizos A , Trenkwalder C , et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation, Mov Disord (2015) ; 30: : 1623–1631. |
38. | Fayers PM and Machin D. Quality of life. Assessment, analysis and interpretation. Chichester: Wiley, (2000) . |
39. | George D and Mallery P. IBM SPSS Statistics 27 Step by Step -- A Simple Guide and Reference. Boca Raton, FL, USA: Routledge Taylor & Francis Group, (2022) . |
40. | Tamasauskas D , Sakalauskas V and Kriksciuniene D. Evaluation framework of hierarchical clustering methods for binary data. 12th International Conference on Hybrid Intelligent Systems. Pune, India: IEEE, (2012) , p. 421–426. |
41. | Pratsinakis ED , Karapetsi L , Ntoanidou S , et al. Comparison of hierarchical clustering methods for binary data from SSR and ISSR molecular markers. In: Chadjipadelis T, Lausen B, Markos A, et al. (eds) Data Analysis and Rationality in a Complex World. Springer, (2019) , pp. 233–241. |
42. | Lacy B , Piotrowski HJ , Dewey RBJr., et al. Severity of depressive and motor symptoms impacts quality of life in Parkinson’s disease patients at an academic movement clinic: A cross-sectional study. Clin Park Relat Disord (2023) ; 8: : 100180. |
43. | Cassidy I , Doody O and Meskell P. Exploring factors that influence HRQoL for people living with Parkinson’s in one region of Ireland: A cross-sectional study. BMC Geriatr (2022) ; 22: : 994. |
44. | He L , Lee EY , Sterling NW , et al. The key determinants to quality of life in Parkinson’s disease patients: results from the Parkinson’s Disease Biomarker Program (PDBP). J Parkinsons Dis (2016) ; 6: : 523–532. |
45. | Moore O , Kreitler S , Ehrenfeld M , et al. Quality of life and gender identity in Parkinson’s disease. J Neural Transm (Vienna) (2005) ; 112: : 1511–1522. |
46. | Hauser RA , Deckers F and Lehert P. Parkinson’s disease home diary: further validation and implications for clinical trials. Mov Disord (2004) ; 19: : 1409–1413. |
47. | Timpka J , Lohle M , Bremer A , et al. Objective observer vs. patient motor state assessments using the PD home diary in advanced Parkinson’s disease. Front Neurol (2022) ; 13: : 935664. |
48. | Rizos A , Martinez-Martin P , Odin P , et al. Characterizing motor and non-motor aspects of early-morning off periods in Parkinson’s disease: an international multicenter study. Parkinsonism Relat Disord (2014) ; 20: : 1231–1235. |
49. | Aldred J , Freire-Alvarez E , Amelin AV , et al. Continuous subcutaneous foslevodopa/foscarbidopa in Parkinson’s disease: safety and efficacy results from a 12-month, single-arm, open-label, phase 3 study. Neurol Ther (2023) ; 12: : 1937–1958. |
50. | Kassubek J , Chaudhuri KR , Zesiewicz T , et al. Rotigotine transdermal system and evaluation of pain in patients with Parkinson’s disease: a post hoc analysis of the RECOVER study. BMC Neurol (2014) ; 14: : 42. |
51. | Trenkwalder C , Kies B , Rudzinska M , et al. Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord (2011) ; 26: : 90–99. |
52. | Barone P , Antonini A , Colosimo C , et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord (2009) ; 24: : 1641–1649. |
53. | Marques A , Attal N , Bouhassira D , et al. How to diagnose parkinsonian central pain? Parkinsonism Relat Disord (2019) ; 64: : 50–53. |
54. | Warren N , O’Gorman C , Lehn A , et al. Dopamine dysregulation syndrome in Parkinson’s disease: a systematic review of published cases. J Neurol Neurosurg Psychiatry (2017) ; 88: : 1060–1064. |
55. | Lim SY , Farrell MJ , Gibson SJ , et al. Do dyskinesia and pain share common pathophysiological mechanisms in Parkinson’s disease? Mov Disord (2008) ; 23: : 1689–1695. |
56. | Hagell P and Brundin L. Towards an understanding of fatigue in Parkinson disease. J Neurol Neurosurg Psychiatry (2009) ; 80: : 489–492. |
57. | Mylius V , Perez Lloret S , Cury RG , et al. The Parkinson disease pain classification system: results from an international mechanism-based classification approach. Pain (2021) ; 162: : 1201–1210. |
58. | Kakimoto A , Kawazoe M , Kurihara K , et al. Impact of non-motor fluctuations on QOL in patients with Parkinson’s disease. Front Neurol (2023) ; 14: : 1149615. |
59. | Stocchi F , Antonini A , Barone P , et al. Early DEtection of wEaring off in Parkinson disease: The DEEP study. Parkinsonism Relat Disord (2014) ; 20: : 204–211. |
60. | Jost ST , Kaldenbach MA , Antonini A , et al. Levodopa dose equivalency in Parkinson’s disease: updated systematic review and proposals. Mov Disord (2023) ; 38: : 1236–1252. |



