Ethical Considerations for Identifying Individuals in the Prodromal/Early Phase of Parkinson’s Disease: A Narrative Review
Abstract
The ability to identify individuals in the prodromal phase of Parkinson’s disease has improved in recent years, raising the question of whether and how those affected should be informed about the risk of future disease. Several studies investigated prognostic counselling for individuals with isolated REM sleep behavior disorder and have shown that most patients want to receive information about prognosis, but autonomy and individual preferences must be respected. However, there are still many unanswered questions about risk disclosure or early diagnosis of PD, including the impact on personal circumstances, cultural preferences and specific challenges associated with different profiles of prodromal symptoms, genetic testing or biomarker assessments. This narrative review aims to summarize the current literature on prognostic counselling and risk disclosure in PD, as well as highlight future perspectives that may emerge with the development of new biomarkers and their anticipated impact on the definition of PD.
Plain Language Summary
An important goal of Parkinson’s disease research is to diagnose the disease at an earlier stage, even before the typical motor symptoms appear, in the so-called ‘prodromal phase’. Currently, there are no treatments available that can slow down or prevent disease progression in this early phase, even though many of the early symptoms are treatable. This raises ethical questions about whether people want to know their future risk of Parkinson’s and, if so, how this information should be given. This article summarizes the current state of knowledge, but also open questions about risk disclosure in the prodromal phase of Parkinson’s. Previous studies have shown that many people with early symptoms of Parkinson’s would like to know their risk, but that the individual’s wish to know (or not to know) must first be ascertained and respected. Future studies need to find out whether very early diagnosis of Parkinson’s might have an impact on people affected, for example in terms of psychological stress or anxiety, and whether cultural background might influence attitudes to risk disclosure. Furthermore, it is expected that in the future it will be possible to make an early diagnosis of Parkinson’s using specific new techniques, e.g., by testing spinal fluid. It is of utmost importance to find out if and how test results of these new techniques should be communicated to patients, even if they do not lead to direct medical treatment.
INTRODUCTION
In recent years, converging findings from neuropathological and clinical studies indicate that the clinical diagnosis of Parkinson’s disease (PD) is preceded by years of neurodegeneration. These early years and in particular the prodromal phase of the disease have increasingly been the subject of focus, as they present an opportunity for early treatment with anticipated disease-modifying therapies. Previously defined risk factors (that predispose for the development of PD) and prodromal markers (as indicators of already ongoing neurodegeneration) have been used to build prodromal PD cohorts around the world.1–5 However, ethical implications of the identification and recruitment of individuals in the prodromal phase have only gained attention during recent years. Currently, there are two fundamental limitations to the detection of prodromal PD that raise ethical concerns6–12 (Fig. 1A):
Fig. 1
Pillars of risk disclosure and early diagnosis in Parkinson’s disease. The points mentioned in this figure were developed based on the review of the literature of Parkinson’s disease and other neurodegenerative diseases (in particular Alzheimer’s disease) and derived from empirically relevant factors.
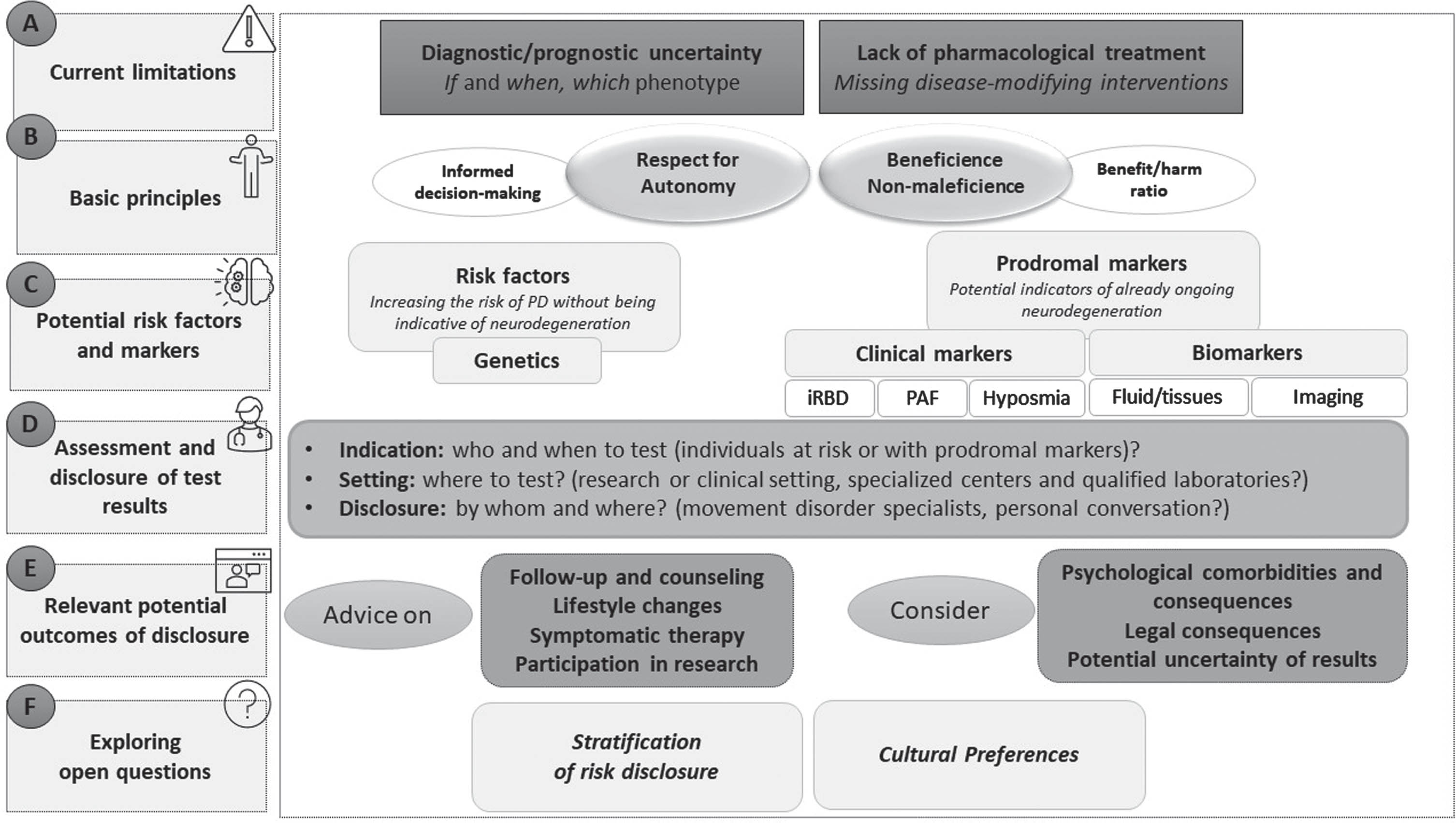
(1) Uncertainty: although knowledge on the diagnostic value of clinical markers and biomarkers is constantly increasing, there is still prognostic uncertainty regarding if and when fully manifest clinical symptoms of PD will occur during life. While the diagnosis of isolated REM sleep behavior disorder (iRBD) in combination with the assessment of an α-synuclein biomarker (see below) seems to be a promising tool to diagnose prodromal PD,13–16 prediction of risk in the setting of other less-specific prodromal symptoms is more challenging. Similarly, uncertainty can come with the assessment of genetic risk factors, which include genetic variants associated with modest risk and mutations associated with incomplete penetrance.
(2) Consequence of disclosure: So far early risk disclosure is not clearly advantageous from the perspective of treatment. Although non-motor symptoms occurring in the prodromal phase of PD often require symptomatic treatment, which may justify early diagnosis in some way17,18, an evidence-based intervention that might prevent or slow down the disease in the prodromal phase does not yet exist.
However, as research on the prodromal phase of PD is crucial for further developments to counter the disease, it is of utmost importance to navigate these ethical issues and define clear guidelines for research and clinical practice. Within this review we first summarize current discussion points and results of completed studies on ethical issues around prognostic counseling in prodromal PD, with the objective to provide a framework for risk disclosure in early/prodromal PD that can be applied in both research and clinical settings. Following the rapid growth in knowledge of the last few years, this review additionally aims to address unanswered questions and future perspectives of early diagnosis of PD, that must be addressed in future studies as a basis for advanced risk disclosure guidelines.
STATE OF THE ART
Basic principles of early risk disclosure in PD
Autonomy
Previous publications on risk disclosure in prodromal PD, including experts’ and patients’ opinions, clearly emphasized that respect for patient autonomy must be a central component of prognostic counseling7,8,19–23 (Fig. 1B). That implies that the patient’s wish to know has to be respected to the same extent than the wish not to know. To allow an informed decision-making, the counselor should clearly indicate potential advantages and disadvantages of receiving a prognosis. The decision-making process may require a period of reflection and follow-up meetings, moreover, patients who initially refuse to be informed about their risk should be offered the possibility of a future risk disclosure.
The respect for autonomy is challenged by unresolved issues on how to proceed with incidental information of prodromal symptoms or risk factors, including for example the diagnosis of iRBD being discovered during evaluation for a primary presentation of obstructive sleep apnea9,24,25 or the finding of a genetic risk variant for PD, e.g., within direct-to-consumer genetic testing (DTC-GT). If patients seek medical advice due to burdensome symptoms or a positive family history for PD, they generally have time beforehand to inform themselves on potential outcomes of assessments and actively sought clarification, while patients who receive information in above scenarios do so without preparation. So far, there is no consensus for (prodromal) PD if incidental diagnosis should be conveyed in the same way as for primary diagnosis. The most appropriate approach in clinical practice at present is to inform patients in advance of the possibility that the planned examinations (e.g., polysomnography) may generate incidental findings.7,12,26,27
Finally, the above-mentioned ethical guidelines to respect patient autonomy have been mainly discussed for a clinical setting, while they are not directly comparable with research settings. A particular challenge is the recruitment of individuals from the general population, where the screening process for studies on prodromal PD sometimes actively point out specific prodromal signs or risk factors, which were not previously considered relevant by the participant. In this scenario, ethicists should be involved early in the study design.7 In general, disclosure of results and transparency regarding reasons for enrollment are recommended, if preceded by a shared decision making and patient education.7
Beneficence and nonmaleficence
The second and third key ethical principles for prognostic counseling are the consideration of beneficence and nonmaleficence to promote patient benefit and avoid harm7–10,12,23,28,29 (Fig. 1B).
Nonmaleficence: psychological consequences. Especially when considering the above-mentioned major limitations of early diagnosis in PD arguments against disclosure must be considered. In this regard, the most critical aspect that could jeopardize a positive “benefit/harm ratio” is the potential psychological impact that risk disclosure could have on an individual (Fig. 1E). This potential consequence of risk disclosure presents a particular challenge as the individual’s response depends not only on potentially predictable, but also on many unpredictable factors, which have been highlighted in areas of cancer, Huntington’s disease, or Alzheimer’s disease (AD)30–38 (Table 1). Among others the health literacy of the individual and the capacity of the healthcare system to provide long-term support are important factors that play a role in the response and can be anticipated. However, a number of unpredictable factors, such as the individual’s personality, cultural values or previous experiences of serious illness, can lead to an undesirable reaction following disclosure in the short or long term, including hopelessness, depression or even suicidal ideation.34,39,40 Thus, the strategy of disclosure should be based on optimizing the predictable factors and being prepared for the potential challenges posed by unpredictable factors. To avoid unwanted consequences, parallels can be drawn with bad news conversations in medical care.41 In this context, it is recommended to divide disclosure of results in three stages, including steps to take before, during and following disclosure (Table 2). Key points that should be considered include beyond others i) before: the selection of an appropriate setting and experienced health-care provider for the talk, ii) during: the use of an appropriate tone and language of the presenter, while allowing questions and checking for misunderstandings, iii) after: the investigation of potential effects of risk disclosure, ideally within follow-up meetings. In some cases, disclosure can be accomplished in several visits and the healthcare provider should even consider watchful waiting and postpone the disclosure if the timing is not considered right. A quick and direct confrontation without empathy and without letting the individual comprehend the meaning and indication of future risk may trigger negative reactions and is strongly discouraged.
Table 1
Factors that potentially influence an individual‘s reaction to disclosure
| Predictable factors | Unpredictable factors |
| Age of the individual | Personality of the individual |
| Education and health literacy of the individual | Expectations of the individual from life |
| Medical and family history of the individual | Cultural values of the individual |
| Current mental health and mood of the individual | Overall mental health and vulnerability of the individual |
| Competence of the healthcare provider | Timing of the disclosure for the individual |
| Level of trust with the healthcare provider | Independence of the individual in life |
| Socioeconomic status of the individual | Family issues of the individual |
| The capacity of the healthcare system for long-term physical and mental support | Past experiences of the individual with serious diseases |
Factors mentioned in this Table are based on the literature in the fields of cancer, Huntington’s disease, and Alzheimer’s disease.
Table 2
Recommendations for risk disclosure for future Parkinson’s disease
| Before disclosure |
| Consider potential differences between research and clinical setting. |
| Make certain that your assessments are standardized and (if applicable) comprise adequate quality control measures. |
| Make sure that the individual has been educated on potential consequences of risk disclosure, has chosen to receive information and given informed consent prior to the test. |
| Evaluate and communicate the level of certainty of the test. If there is a doubt, consider a re-test. |
| Confirm that the individual does not have manifest cardinal motor or dementia symptoms, which allow a clinical diagnosis. |
| Review the individual’s medical history and comorbidities that might affect the reaction to risk disclosure. |
| Evaluate the status of knowledge of the individual on disease and determine the level of detail in disclosure. |
| Evaluate the psychological status and preparedness of the individual. Delay disclosure if needed. |
| Be aware that disclosure is not a brief encounter but a process. It may be broken into bits of information given in several visits if needed. |
| Prepare a quiet and private setting, prevent interruption. |
| Make sure that the person disclosing the news is experienced and has extensive knowledge of the parameters investigated. |
| Remind the individual the option that family members may, but du not necessarily have to attend. |
| Consider potential aspects of judicial protection, including insurance policies or information of employers. |
| During disclosure |
| Balance your tone between being straightforward (conveying the information that is meant to be given) and sensitive (being aware of the individual’s psychological situation). Be honest but emphasize positive aspects. |
| Deliver the information starting from basics to more complicated ones. Check for understanding in each step. Avoid medical jargon and provide enough time to allow the individual to process the received information. |
| Make sure that the strengths and limitations of the test are understood. |
| Assure that questions are welcomed. |
| Be ready for potential questions and comments resulting from prior internet search. |
| Offer a plan for self-care and self-efficacy including lifestyle changes. |
| Remind that psychological assistance can be offered if needed. |
| Discuss potential symptomatic therapies. |
| Discuss recruitment for clinical studies, including potential disease-modifying drug trials. |
| Summarize at the end the most important information and check again for potential misunderstandings or questions. |
| Following disclosure |
| Document the disclosure discussion. |
| Schedule a follow-up plan. |
| Consider potential effects of risk disclosure upon the patient (or research participant) |
| Consider additional tests if required. |
| Inform that some symptoms may be wrongly attributed to a synucleinopathy. |
| Update the individual if a new diagnostic test is available (such as biomarker testing in PD). |
| Provide educational material and contact information. |
| Establish a healthcare support network plan. |
Recommendations mentioned in this Table are based on review of the literature on neurodegenerative diseases and bad-news conversations and derived from empirically relevant factors for building a disclosure strategy.
But even if all measures are taken to consider predictable and unpredictable factors, a negative reaction is still possible. In this context, it is positive that studies in individuals with prodromal (iRBD) and clinical PD have shown that those affected are generally in favor of risk disclosure.11,21,23,42 Moreover, follow-up studies in AD reported no significant increase in depression or anxiety following risk disclosure.114,115 However, the available data for PD so far are cross-sectional and limited in terms of cultural and diagnostic diversity. Future studies should investigate the attitudes of underrepresented populations and the long-term psychological consequences of risk disclosure in PD in more depth.
Beneficence: opportunities of risk disclosure. Information about the risk of future PD also includes potential opportunities and can be less burdensome for patients, if specific already established recommendations are followed6,11,21,27 (Fig. 1E). These recommendations include as basic aspects that (i) patients/probands should be offered regular follow-ups, (ii) prognostic counseling should be accompanied by advice on potential effects of lifestyle changes, (iii) symptomatic therapy for prodromal symptoms should be considered, (iv) possibilities to participate in research should be explained, and (v) potential uncertainties of results should be clearly communicated.
Most importantly, prognostic counseling should be adapted to current knowledge about the prognostic significance of specific prodromal and risk markers. The following sections therefore discuss specific markers that are currently of particular importance for prodromal research, as they are most commonly used to recruit prodromal cohorts worldwide (Fig. 1C).4,5,44
Specific risk factors, prodromal markers, and biomarkers
Risk factors
Genetics. There has been tremendous progress in uncovering genetic contributions to PD and more than 90 risk loci have been identified.45–47 There are ∼10 genes in which mutations are sufficient to cause monogenic forms of PD,49,50 but many of these are rare or very rare, and as such opportunities for risk disclosure are few. However, variants in some genes are relatively common and convey non-trivial risk of future PD. Of these, variants in LRRK2 and GBA1 are most frequently used for recruitment of at-risk cohorts. The commonest variant of LRRK2, G2019 S, is associated with an odds ratio up to 2051 and of carriers living to 80 years old, approximately 25–30% will develop PD.51 For GBA1 the situation is more complicated, while heterozygous variants such as T369M are relatively mild (ORs 1.2–2.2), others such as L444P are more deleterious.52,53 Again, in GBA1 variant carriers the prevalence of PD at age 80 years is approximately 20%.54 Given these many (so far known) different risk estimates and the many potential other factors that may influence individual PD risk, including environmental influences or lifestyle, genetic susceptibility testing for PD is subject to many uncertainties. It is therefore still an important question whether and how genetic susceptibility should be disclosed, taking into account the possible psychological consequences, but also the possible stigmatization or burden on the family.55 A study on genetic testing of asymptomatic carriers of the GBA1/LRRK2 variants showed that those who tested positive were more likely to have negative psychological reactions and were more worried about the impact on potential family members.56 To meet this ethical challenge, respect for autonomy should be prioritized. However, with predictive genetic testing in particular, the individual’s decision whether or not to know must be an informed one, and the uncertainties mentioned above must be clearly communicated in advance. It is very important that trained experts who have comprehensive knowledge of the variants and their predictive value carry out the risk assessment.57 These principles are specifically challenged by DTC-GT, including for example initiatives like 23andMe, a DTC-GT company, that has been giving customers information about their LRRK2 G2019 S status and GBA1 N370 S status for > 10 years. DCT can lead to misunderstandings of results, especially as clients often do not discuss the results with health care providers.58,59 In this context, it was found that even among clinical PD patients, knowledge about genetic testing is very limited and an overestimation of risk is often observed.60 On the other hand, several GBA1 trials are launching or already underway,61 and it has been noted that predictive tests for PD are considered to be significantly more acceptable when clinical trials are available.62
Additionally, it possible to calculate polygenic risk scores (PRS) from the number of risk alleles for each GWAS-significant variant associated with PD. Although PRS estimates individual genetic liability towards PD due to common variation, it still lacks acceptable diagnostic or predictive capability. PRS alone is therefore not particularly useful for early detection and are not clinically meaningful to warrant disclosure to individuals currently.45,63,64
In conclusion, there remains much work to be done to determine which genetic results are sufficiently important to feed back to individuals and the circumstances under which this should be done. Most importantly, affected individuals should be included in further studies and longitudinal data are needed to assess long-term effects of risk disclosure.
Clinical prodromal markers
REM Sleep behavior disorder (RBD). RBD is a parasomnia characterized by the presence of “acting-out” complex vocal and/or motor behaviors in sleep and vivid dreams.42,65 When it occurs in isolation in older adults (iRBD), individuals are at a high risk of developing PD, DLB, or MSA. Large studies with longitudinal follow-up indicate a new diagnosis rate of PD/DLB/MSA of approximately 6.3% annually, meaning that ∼73% have developed PD/DLB/MSA at 12 years of follow-up.66 It is thus generally accepted that in older adults, iRBD represents an early stage of α-synuclein-related disorder.
There is a lack of consensus about what information should be imparted to patients with iRBD, both at the point of diagnosis and during follow-up, given the above limitations and basic principles of autonomy, beneficence and nonmaleficence. In recent studies that sought the views of patients with iRBD, ∼90% wanted to receive information about prognosis.21,23 Of those, most wanted to receive this information at the point of diagnosis and from their clinician, citing the importance of maintaining trust between doctor and patient. Given significant research interest in iRBD, there is a large volume of material online that patients can access.23,44 Failure to explore whether a patient wishes to receive this information risks erosion of trust between clinician and patient. Patients value honesty and several recommended delivering information in a stepwise manner, with the authors endorsing careful communication to impart facts without use of unnecessarily ‘triggering’ phrases (e.g., ‘neurodegenerative’ or volunteering exact phenoconversion rates). While these were the first two studies to assess the views of patients with iRBD, similar surveys have been conducted with patients with PD and with clinicians who see patients with iRBD.6,11,22,29,67 These broadly found agreement with one another, that disclosure is situation-specific and heavily nuanced. All found that disclosure was predicated on establishing whether the patient wants to know or not (autonomy) and that risk disclosure should not be foisted on a patient (nonmaleficence). However, although the respect for autonomy is paramount, previous studies have shown that in clinical settings, less than 50% of patients diagnosed with iRBD were asked about their preferences.10,23 Probing the patient’s wishes by asking them whether they wish to know about prognostic risk, when in terms of timing, and in how much detail, should be the starting point when discussing the diagnosis of iRBD.
Pure autonomic failure (PAF). Pure autonomic failure (PAF) is an α-synucleinopathy that is usually associated with neurogenic orthostatic hypotension (OH), generally presenting in middle to older aged adults with syncopal attacks or severe presyncopal symptoms.68 It has been shown that PAF is often a prodromal stage of other α-synucleinopathies, with involvement initially limited to the peripheral autonomic nervous system. In one large series, overall 24% phenoconverted to PD, DLB, or MSA.69 There is substantial overlap between PAF and iRBD, which occurs in 72% of patients with PAF.70 However, while the ethical implications of prognostic counseling in iRBD have received increasing attention, no study to date has addressed the views of patients with PAF. It can be assumed that the conclusions drawn from the iRBD studies mentioned above can be transferred to individuals presenting with a combination of PAF and iRBD. However, given the potentially lower phenoconversion rates reported to date for PAF itself, this higher prognostic uncertainty complicates the ethical issue of risk disclosure. Apart from applying the basic principles of autonomy and beneficence/non-maleficence, it seems important to consider individual patient characteristics and communicate potential uncertainties when a patient desires information about a link to α-synucleinopathies. Further specific studies on patient preferences in PAF populations need to be conducted.
Hyposmia. Hyposmia is well recognized as an early feature of PD.71,72 Observational studies suggest a significantly increased incidence of PD in individuals who scored the lowest in the smell tests compared to individuals with preserved/best smell scores (∼8 vs. 38–54 per 10,000 person-years) corresponding to an OR of 4–6.1,73–75 Several longitudinal cohort studies have used smell tests to identify people at future risk of PD, but, as a screening measure, objective smell tests under-perform in isolation in population-based screening programs.76,77 However, as novel biomarkers for PD emerge in the form of α-synuclein seed amplification assays (SAA, see below), it is clear that hyposmia appears closely related to SAA-positive status in patients with established PD.13 Moreover, while iRBD is relatively rare in the general population and diagnosis is expensive because it necessitates overnight sleep studies, wide-scale testing of hyposmia is feasible and cost-effective. It can therefore be expected that hyposmia screening, coupled with biomarkers such as SAA, will be used even more frequently as screening method for the detection of prodromal PD. However, despite the frequent use of hyposmia-testing in cohort studies, there has been little research to understand the implications of risk disclosure for hyposmia. This is particularly critical given the possible other causes of hyposmia (including beyond others the association with AD) and the significantly lower predictive value compared to iRBD. It is therefore important to inform patients and participants about uncertainties and potential implications prior to testing. Future studies should address potential consequences of hyposmia testing to investigate the benefit-harm-ratio.
Biomarkers
In recent years, there have been rapid technical advances in prognostic and diagnostic biomarkers for PD, including fluid/tissue-based biomarkers and imaging. One of the most promising techniques is the amplification of pathological α-synuclein using SAAs, with CSF-SAA in particular reaching a very high sensitivity and specificity to distinguish clinical PD from healthy controls.14,16,78–95 Several studies have investigated α-synuclein-SAAs in prodromal cohorts, i.e., individuals with iRBD or hyposmia, and showed highly promising results to identify individuals already in the prodromal phase of PD.14–16,78,86,96–99 Interestingly, these studies did not report their policies regarding disclosure of test results and there was virtually no debate about the ethical implications of biomarker assessment in PD.
Advances in the assessment of fluid biomarkers is comparable to developments in AD, where CSF and blood have been intensively investigated to diagnose prodromal and even preclinical stages of AD. In contrast to the lack of debate in PD, the technical advances in biomarkers100 and the steps toward a biological definition of AD101 have led to a vivid discussion how biomarker results should be disclosed.102,103 So far, several studies in AD investigated the potential impact of disclosing (liquid) biomarkers and found no severe harm following disclosure, while some positive effects, including lifestyle changes, have been observed.104 However, it should be noted that individual views and perceptions may vary considerably, and although consequences such as anxiety or depression have rarely been reported, they are not non-existent.105 Moreover, risks of stigmatization and discrimination have been discussed.103,106 To date, ethical recommendations for AD support the disclosure of biomarker results in research,107,109 provided, of course, that the wishes of the recipient have been explored beforehand. However, use of preclinical/prodromal biomarkers in clinical practice110 will bring new ethical challenges, including potential legal and social implications, e.g., insurance policy issues.32 Therefore, standardized methods for the assessment of biomarkers and disclosure of results in a clinical setting need to be prepared in advance. The following requirements and key issues for biomarker disclosure have been proposed for AD and can be derived for PD43,104,111 (Fig. 1D): i) A clear distinction should be made between the use of biomarkers in research and in clinical setting. Diagnostic validity must be clearly established before transition to clinical practice, which is still pending for many α-synuclein biomarkers used in research. ii) The indication for the use of biomarkers must be defined, and especially outside of research it must be clarified in which populations and at what time-point biomarker tests should be offered. iii) Risk assessment might affect personal rights and interests, such as health insurance. Judicial protection should be considered when implementing guidelines for the clinical use of biomarkers. iv) For future clinical use there must be clear standards for the environment in which the tests are performed, i.e., specialized centers with standardized techniques and quality control measures. Currently, global access to standardized α-synuclein SAAs has not yet been established. v) In both research and clinical practice, patients/probands must be adequately educated prior to testing and patient education should include clear statements about the limitations of the biomarker.
In addition to fluid and tissue-based biomarkers, dopaminergic imaging (FDG-PET and DAT-SPECT) is currently the biomarker with the highest predictive value for PD.1. Although dopaminergic imaging has been used for years in studies of prodromal cohorts, again in contrast to AD and Amyloid-PETs,106,112,113 no guidelines for disclosure of imaging results have yet been established. To date, it can only be inferred from AD studies that disclosure of imaging results in the context of clinical trials is most likely safe and therefore feasible if desired by participants.113–115 However, in clinical practice, if a person shows prodromal symptoms such as iRBD or hyposmia, there is no consensus on the use of dopaminergic imaging, which is handled very differently. It is therefore of utmost importance to establish clear guidelines for clinical practice as to when dopaminergic imaging should be used when a prodromal phase is suspected.
OPEN QUESTIONS AND FUTURE PERSPECTIVES
While the above-mentioned previous studies and ethical debates already form a usable basis to address ethical implications of risk disclosure in PD, the following important questions remain unanswered so far and need to be addressed in future studies (Fig. 1F).
Stratification of risk disclosure
Given the differences in life expectancy, disease severity, caregiver burden and treatment options,116–118 it seems to be of great importance to affected persons which type of α-synucleinopathy they will develop. The question therefore arises whether (i) risk disclosure should not only contain information about the possible development of PD but should also mention the possibility of DLB or MSA, and whether (ii) current studies allow a prognosis as to which of the diseases is to be expected and whether this prognosis should be communicated.
Several studies suggest that certain clinical symptoms may indicate the development of specific α-synucleinopathies. For example, individuals with iRBD or PAF have a higher risk of developing MSA if they present with normal olfactory performance,70,119 while the most important clinical variable associated with DLB so far is still prodromal cognitive impairment.66,120 However, there is still a considerable prognostic uncertainty so that prognostic statements regarding the manifest type of α-synucleinopathy should be made very cautiously.12 In addition, the question of whether the possibility of future MSA or DLB should be included in prognostic counseling has been controversially discussed and cannot be answered on the basis of the literature available to date.121 In view of the lack of literature, one possible approach remains to first explore the desired depth of information of the person concerned and clearly communicate the existing uncertainties.
The second uncertainty faced by people with expected prodromal PD and physicians who disclose risk is when clinical conversion will occur. Recent evidence has suggested that certain clinical and imaging characteristics likely indicate an higher risk for near term conversion to PD, DLB, or MSA,66,122–124 including beyond others abnormal motor markers or dopamine transporter (DaT) uptake scans.66,124 However, so far it has not been reported, in which way these potential prognostic factors should be included in prognostic counseling. Again, so far it can only be assumed that this aspect of risk stratification should also be adapted to the individual desire for detailed information and should be communicated with caution until more evidence is available. Future studies on patient perceptions should urgently investigate whether risk stratification is desired by patients and explore the potential benefits or harms of this information.
Cultural preferences
One acknowledged aspect of risk disclosure for future PD is that it should be tailored according to personal preferences and values of the individual. This requires consideration of the cultural, economic, social, and educational background of the individual, which varies immensely. Similar concerns were raised in other fields, where delivering “bad news” is frequent. For instance, while in Western populations a complete and straightforward disclosure of cancer diagnosis or prognosis is preferred, this may not be the case in certain in African, Asian, or Hispanic communities.125 So far, the available data on disclosure of future PD indicates that the attitudes of German and Turkish PD patients towards risk disclosure are similar.11,67 Likewise, results of studies investigating the psychological impact of disclosing imaging or CSF AD biomarker status seem to be parallel in Western and Japanese societies.114,126,127 While these reports are encouraging when it comes to mitigating the effect of culture on disclosure, they are by no means sufficient. The current literature on risk disclosure is derived mainly from European and North American populations, which constitute only 17% of the global population, and they are biased by culture/geography.
An all-encompassing, worldwide guideline for risk disclosure in PD is impossible, as it is not feasible to take account of all the values of different cultures. Thus, data from underrepresented populations are critically needed to understand whether and how cultural values and attitudes play a role in disclosure and counseling, a caveat that has also been highlighted in the field of AD.32,102 This would allow local institutions to develop culture-specific guidelines for risk disclosure and prognostic counseling that would embrace both universal ethical values and cultural sensitivities.
CONCLUSION
Disclosing the risk of future PD signifies the beginning of a new period for the individual, which has several negative and positive facets. The period starting from the moment of disclosure until the clinically manifest disease will be long and subject to many unanswered questions and unsolved issues that have yet to be discovered and discussed. Recent advances in biomarker studies will change our understanding of PD and their currently defined phases, ushering in the era of a biologically defined disease rather than a clinical diagnosis.78,128–130 Although the regular use of biomarkers for early diagnosis of PD is to be expected, the discussion on the ethical implications of these scientific advances is still lacking and lags far behind the progress made in AD research. In addition, long-term psychological consequences of risk disclosure, cultural and personal preferences and potential challenges coming with risk stratification have not been addressed sufficiently so far. However, the worldwide recruitment of prodromal cohorts offers a great opportunity to involve those affected in the process at an early stage. It should therefore be an important goal to determine the perspectives and wishes of patients to address these challenges and open questions in the future.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
Alastair Noyce is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
REFERENCES
1. | Heinzel S , Berg D , Gasser T , et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord (2019) ; 34: : 1464–1470. |
2. | Berg D , Postuma RB , Adler CH , et al. MDS research criteria for prodromal Parkinson’s disease: MDS Criteria for Prodromal PD. Mov Disord (2015) ; 30: : 1600–1611. |
3. | Bestwick JP , Auger SD , Simonet C , et al. Improving estimation of Parkinson’s disease risk-the enhanced PREDICT-PD algorithm. NPJ Parkinsons Dis (2021) ; 7: : 33. |
4. | Noyce AJ , Bestwick JP , Silveira-Moriyama L , et al. PREDICT-PD: Identifying risk of Parkinson’s disease in the community: methods and baseline results. J Neurol Neurosurg Psychiatry (2014) ; 85: : 31–37. |
5. | Jennings D , Siderowf A , Stern M , et al. Conversion to Parkinson disease in the PARS hyposmic and dopamine transporter–deficit prodromal cohort. JAMA Neurol (2017) ; 74: : 933–940. |
6. | Marcinkowska A , Bogucki A , Kroemeke A , et al. To know or not to know? Opinions of patients with Parkinson’s disease on disclosing risk of phenoconversion in RBD. Neurol Neurochir Pol (2023) ; 57: : 438–443. |
7. | Dommershuijsen LJ , Darweesh SKL , Luik AI , et al. Ethical considerations in screening for rapid eye movement sleep behavior disorder in the general population. Mov Disord (2020) ; 35: : 1939–1944. |
8. | Arnaldi D , Antelmi E , St Louis EK , et al. Idiopathic REM sleep behavior disorder and neurodegenerative risk: To tell or not to tell to the patient? How to minimize the risk? Sleep Med Rev (2017) ; 36: : 82–95. |
9. | Feinstein MA , Sharp RR , Sandness DJ , et al. Physician and patient determinants of prognostic counseling in idiopathic REM sleep-behavior disorder. Sleep Med (2019) ; 62: : 80–85. |
10. | Malkani RG and Wenger NS . REM sleep behavior disorder as a pathway to dementia: if, when, how, what, and why should physicians disclose the diagnosis and risk for dementia. Curr Sleep Med Rep (2021) ; 7: : 57–64. |
11. | Schaeffer E , Rogge A , Nieding K , et al. Patients’ views on the ethical challenges of early Parkinson disease detection. Neurology (2020) ; 94: : e2037–e2044. |
12. | Stefani A , Mozersky J , Kotagal V , et al. Ethical aspects of prodromal synucleinopathy prognostic counseling. Semin Neurol (2023) ; 43: : 166–177. |
13. | Siderowf A , Concha-Marambio L , Lafontant D-E , et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol (2023) ; 22: : 407–417. |
14. | Rossi M , Candelise N , Baiardi S , et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol (2020) ; 140: : 49–62. |
15. | Iranzo A , Fairfoul G , Ayudhaya ACN , et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol (2021) ; 20: : 203–212. |
16. | Poggiolini I , Gupta V , Lawton M , et al. Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain (2021) ; 145: : 584–595. |
17. | Schrag A , Horsfall L , Walters K , et al. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol (2015) ; 14: : 57–64. |
18. | Simonet C , Bestwick J , Jitlal M , et al. Assessment of risk factors and early presentations of Parkinson disease in primary care in a diverse UK population. JAMA Neurol (2022) ; 79: : 359–369. |
19. | St Louis EK Prognostic counseling for patients with idiopathic/isolated REM sleep behavior disorder: should we tell them what’s coming? Yes. Mov Disord Clin Pract (2019) ; 6: : 667–668. |
20. | Vertrees S and Greenough GP . Ethical considerations in REM sleep behavior disorder. Continuum (Minneap Minn) (2013) ; 19: (1 Sleep Disorders), 199–203. |
21. | Gossard TR , Teigen LN , Yoo S , et al. Patient values and preferences regarding prognostic counseling in isolated REM sleep behavior disorder. Sleep (2023) ; 46: : zsac244. |
22. | Kayis G , Yilmaz R , Arda B , et al. Risk disclosure in prodromal Parkinson’s disease – A survey of neurologists. Parkinsonism Relat Disord (2023) ; 106: : 105240. |
23. | Pérez-Carbonell L , Simonet C , Chohan H , et al. The views of patients with isolated rapid eye movement sleep behavior disorder on risk disclosure. Mov Disord (2023) ; 38: : 1089–1093. |
24. | Fernández-Arcos A , Iranzo A , Serradell M , et al. The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: a study in 203 consecutive patients. Sleep (2016) ; 39: : 121–132. |
25. | Frauscher B , Gschliesser V , Brandauer E , et al. REM sleep behavior disorder in 703 sleep-disorder patients: the importance of eliciting a comprehensive sleep history. Sleep Med (2010) ; 11: : 167–171. |
26. | Green RC , Berg JS , Grody WW , et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med (2013) ; 15: : 565–574. |
27. | Smedinga M , Darweesh SKL , Bloem BR , et al. Towards early disease modification of Parkinson’s disease: a review of lessons learned in the Alzheimer field. J Neurol (2021) ; 268: : 724–733. |
28. | Rees RN , Acharya AP , Schrag A , et al. An early diagnosis is not the same as a timely diagnosis of Parkinson’s disease. F1000Res (2018) ; 7: : F1000 Faculty Rev-1106. |
29. | Teigen LN , Sharp RR , Hirsch JR , et al. Specialist approaches to prognostic counseling in isolated REM sleep behavior disorder. Sleep Med (2021) ; 79: : 107–112. |
30. | Erickson CM and Largent EA . Diagnosing preclinical and prodromal neurodegenerative diseases-the clinical is political. JAMA Neurol (2024) ; 81: : 439–440. |
31. | Erickson CM , Chin NA , Johnson SC , et al. Disclosure of preclinical Alzheimer’s disease biomarker results in research and clinical settings: Why, how, and what we still need to know. Alzheimers Dement (Amst) (2021) ; 13: : e12150. |
32. | Erickson CM , Clark LR , Ketchum FB , et al. Implications of preclinical Alzheimer’s disease biomarker disclosure for US policy and society. Alzheimers Dement (Amst) (2022) ; 14: : e12339. |
33. | Vanderschaeghe G , Schaeverbeke J , Bruffaerts R , et al. From information to follow-up: Ethical recommendations to facilitate the disclosure of amyloid PET scan results in a research setting. Alzheimers Dement (N Y) (2018) ; 4: : 243–251. |
34. | McCusker EA and Loy CT . Huntington disease: the complexities of making and disclosing a clinical diagnosis after premanifest genetic testing. Tremor Other Hyperkinet Mov (N Y) (2017) ; 7: : 467. |
35. | Klitzman R , Thorne D , Williamson J , et al. Disclosures of Huntington disease risk within families: patterns of decision-making and implications. Am J Med Genet A (2007) ; 143A: : 1835–1849. |
36. | Zheng Y , Lei F and Lei B . Cancer diagnosis disclosure and quality of life in elderly cancer patients. Healthcare (Basel) (2019) ; 7: : 163. |
37. | Mori M , Lin C-P , Cheng S-Y , et al. Communication in cancer care in Asia: a narrative review. JCO Glob Oncol (2023) ; 9: : e2200266. |
38. | Raicher I and Caramelli P . Diagnostic disclosure in Alzheimer’s disease: a review. Dement Neuropsychol (2008) ; 2: : 267–271. |
39. | Mai AS , Chao Y , Xiao B , et al. Risk of suicidal ideation and behavior in individuals with Parkinson disease: a systematic review and meta-analysis. JAMA Neurol (2024) ; 81: : 10–18. |
40. | Ou R , Wei Q , Hou Y , et al. Suicidal ideation in early-onset Parkinson’s disease. J Neurol (2021) ; 268: : 1876–1884. |
41. | Jalali R , Jalali A and Jalilian M . Breaking bad news in medical services: a comprehensive systematic review. Heliyon (2023) ; 9: : e14734. |
42. | Kang S-H , Yoon I-Y , Lee SD , et al. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep (2013) ; 36: : 1147–1152. |
43. | McCusker EA and Loy CT . Scientific, ethical, and practical considerations for the testing and disclosure of Alzheimer disease biomarkers. Neurology (2023) ; 100: : 993–994. |
44. | Sixel-Döring F . Prognostic counseling for patients with idiopathic/isolated rapid eye movement sleep behavior disorder: should we tell them what’s coming? No. Mov Disord Clin Pract (2019) ; 6: : 669–671. |
45. | Nalls MA , Blauwendraat C , Vallerga CL , et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol (2019) ; 18: : 1091–1102. |
46. | Loesch DP , Horimoto ARVR , Heilbron K , et al. Characterizing the genetic architecture of Parkinson’s disease in Latinos. Ann Neurol (2021) ; 90: : 353–365. |
47. | Foo JN , Chew EGY , Chung SJ , et al. Identification of risk loci for Parkinson disease in Asians and comparison of risk between Asians and Europeans: a genome-wide association study. JAMA Neurol (2020) ; 77: : 746–754. |
48. | Kim JJ , Vitale D , Otani DV , et al. Multi-ancestry genome-wide association meta-analysis of Parkinson’s disease. Nat Genet (2024) ; 56: : 27–36. |
49. | Klein C and Westenberger A . Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med (2012) ; 2: : a008888. |
50. | Day JO and Mullin S . The genetics of Parkinson’s disease and implications for clinical practice. Genes (Basel) (2021) ; 12: : 1006. |
51. | Healy DG , Falchi M , O’Sullivan SS , et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol (2008) ; 7: : 583–590. |
52. | Zhang Y , Shu L , Sun Q , et al. Integrated genetic analysis of racial differences of common GBA variants in Parkinson’s disease: a meta-analysis. Front Mol Neurosci (2018) ; 11: : 43. |
53. | Gan-Or Z , Amshalom I , Kilarski LL , et al. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology (2015) ; 84: : 880–887. |
54. | Balestrino R , Tunesi S , Tesei S , et al. Penetrance of glucocerebrosidase (GBA) mutations in Parkinson’s disease: a kin cohort study. Mov Disord (2020) ; 35: : 2111–2114. |
55. | Tan E-K and Jankovic J . Genetic testing in Parkinson disease: promises and pitfalls. Arch Neurol (2006) ; 63: : 1232–1237. |
56. | Verbrugge J , Cook L , Miller M , et al. Outcomes of genetic test disclosure and genetic counseling in a large Parkinson’s disease research study. J Genet Counsel (2021) ; 30: : 755–765. |
57. | Manrique de Lara A , Soto-Gómez L , Núñez-Acosta E , et al. Ethical issues in susceptibility genetic testing for late-onset neurodegenerative diseases. Am J Med Genet B Neuropsychiatr Genet (2019) ; 180: : 609–621. |
58. | Pal G , Cook L , Schulze J , et al. Genetic testing in Parkinson’s disease. Mov Disord (2023) ; 38: : 1384–1396. |
59. | Roberts JS , Patterson AK and Uhlmann WR . Genetic testing for neurodegenerative diseases: Ethical and health communication challenges. Neurobiol Dis (2020) ; 141: : 104871. |
60. | Falcone DC , Wood EM , Xie SX , et al. Genetic testing and Parkinson disease: assessment of patient knowledge, attitudes, and interest. J Genet Couns (2011) ; 20: : 384–395. |
61. | Senkevich K , Rudakou U and Gan-Or Z . New therapeutic approaches to Parkinson’s disease targeting GBA, LRRK2 and Parkin. Neuropharmacology (2022) ; 202: : 108822. |
62. | Dahodwala N , Connolly J , Farmer J , et al. Interest in predictive testing for Parkinson’s disease: Impact of neuroprotective therapy. Parkinsonism Relat Disord (2007) ; 13: : 495–499. |
63. | Jacobs BM , Belete D , Bestwick J , et al. Parkinson’s disease determinants, prediction and gene-environment interactions in the UK Biobank. J Neurol Neurosurg Psychiatry (2020) ; 91: : 1046–1054. |
64. | Nalls MA , McLean CY , Rick J , et al. Diagnosis of Parkinson’s disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol (2015) ; 14: : 1002–1009. |
65. | Haba-Rubio J , Frauscher B , Marques-Vidal P , et al. Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep (2018) ; 41: : zsx197. |
66. | Postuma RB , Iranzo A , Hu M , et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain (2019) ; 142: : 744–759. |
67. | Yilmaz R , Dilek GR , Kayis G , et al. Disclosing the news of future risk of Parkinson’s disease: What do patients think? Parkinsonism Relat Disord (2023) ; 116: : 105895. |
68. | Coon EA , Singer W and Low PA . Pure autonomic failure. Mayo Clin Proc (2019) ; 94: : 2087–2098. |
69. | Coon EA , Mandrekar JN , Berini SE , et al. Predicting phenoconversion in pure autonomic failure. Neurology (2020) ; 95: : e889–e897. |
70. | Kaufmann H , Norcliffe-Kaufmann L , Palma J-A , et al. Natural history of pure autonomic failure: A United States prospective cohort. Ann Neurol (2017) ; 81: : 287–297. |
71. | Ponsen MM , Stoffers D , Booij J , et al. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol (2004) ; 56: : 173–181. |
72. | Haehner A , Hummel T , Hummel C , et al. Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov Disord (2007) ; 22: : 839–842. |
73. | Ross GW , Petrovitch H , Abbott RD , et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol (2008) ; 63: : 167–173. |
74. | Chen H , Shrestha S , Huang X , et al. Olfaction and incident Parkinson disease in US white and black older adults. Neurology (2017) ; 89: : 1441–1447. |
75. | Bestwick JP , Auger SD , Schrag AE , et al. Optimising classification of Parkinson’s disease based on motor, olfactory, neuropsychiatric and sleep features. NPJ Parkinsons Dis (2021) ; 7: : 87. |
76. | Goodwin GR , Bestwick JP and Noyce AJ . The potential utility of smell testing to screen for neurodegenerative disorders. Expert Rev Mol Diagn (2022) ; 22: : 139–148. |
77. | Wilson JMG and Jungner G . Principles and practice of screening for disease. Geneva: World Health Organization, (1968) . |
78. | Siderowf A , Concha-Marambio L , Lafontant D-E , et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol (2023) ; 22: : 407–417. |
79. | Groveman BR , Orrù CD , Hughson AG , et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun (2018) ; 6: : 7. |
80. | Fairfoul G , McGuire LI , Pal S , et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol (2016) ; 3: : 812–818. |
81. | Brockmann K , Quadalti C , Lerche S , et al. Association between CSF alpha-synuclein seeding activity and genetic status in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol Commun (2021) ; 9: : 175. |
82. | Bargar C , Wang W , Gunzler SA , et al. Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol Commun (2021) ; 9: : 62. |
83. | Hall S , Orrù CD , Serrano GE , et al. Performance of αSynuclein RT-QuIC in relation to neuropathological staging of Lewy body disease. Acta Neuropathol Commun (2022) ; 10: : 90. |
84. | Russo MJ , Orru CD , Concha-Marambio L , et al. High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol Commun (2021) ; 9: : 179. |
85. | Wang Z , Becker K , Donadio V , et al. Skin α-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol (2021) ; 78: : 30–40. |
86. | Kuzkina A , Panzer C , Seger A , et al. Dermal real-time quaking-induced conversion is a sensitive marker to confirm isolated rapid eye movement sleep behavior disorder as an early α-synucleinopathy. Mov Disord (2023) ; 38: : 1077–1082. |
87. | Kang UJ , Boehme AK , Fairfoul G , et al. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov Disord (2019) ; 34: : 536–544. |
88. | Orrù CD , Ma TC , Hughson AG , et al. A rapid α-synuclein seed assay of Parkinson’s disease CSF panel shows high diagnostic accuracy. Ann Clin Transl Neurol (2021) ; 8: : 374–384. |
89. | Singer W , Schmeichel AM , Shahnawaz M , et al. Alpha-synuclein oligomers and neurofilament light chain predict phenoconversion of pure autonomic failure. Ann Neurol (2021) ; 89: : 1212–1220. |
90. | Quadalti C , Calandra-Buonaura G , Baiardi S , et al. Neurofilament light chain and α-synuclein RT-QuIC as differential diagnostic biomarkers in parkinsonisms and related syndromes. NPJ Parkinsons Dis (2021) ; 7: : 93. |
91. | Compta Y , Painous C , Soto M , et al. Combined CSF α-SYN RT-QuIC, CSF NFL and midbrain-pons planimetry in degenerative parkinsonisms: From bedside to bench, and back again. Parkinsonism Relat Disord (2022) ; 99: : 33–41. |
92. | Martinez-Valbuena I , Visanji NP , Olszewska DA , et al. Combining skin α-synuclein real-time quaking-induced conversion and circulating neurofilament light chain to distinguish multiple system atrophy and Parkinson’s disease. Mov Disord (2022) ; 37: : 648–650. |
93. | Shahnawaz M , Tokuda T , Waragai M , et al. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurology (2017) ; 74: : 163–172. |
94. | Kuzkina A , Bargar C , Schmitt D , et al. Diagnostic value of skin RT-QuIC in Parkinson’s disease: a two-laboratory study. NPJ Parkinsons Dis (2021) ; 7: : 99. |
95. | Kluge A , Bunk J , Schaeffer E , et al. Detection of neuron-derived pathological α-synuclein in blood. Brain (2022) ; 145: : 3058–3071. |
96. | Iranzo A , Mammana A , Muñoz-Lopetegi A , et al. Misfolded α-synuclein assessment in the skin and CSF by RT-QuIC in isolated REM sleep behavior disorder. Neurology (2023) ; 100: : e1944–e1954. |
97. | Concha-Marambio L , Weber S , Farris CM , et al. Accurate detection of α-synuclein seeds in cerebrospinal fluid from isolated rapid eye movement sleep behavior disorder and patients with Parkinson’s disease in the DeNovo Parkinson (DeNoPa) Cohort. Mov Disord (2023) ; 38: : 567–578. |
98. | Stefani A , Iranzo A , Holzknecht E , et al. Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain (2021) ; 144: : 1118–1126. |
99. | Okuzumi A , Hatano T , Matsumoto G , et al. Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nat Med (2023) ; 29: : 1448–1455. |
100. | Mattsson-Carlgren N , Salvadó G , Ashton NJ , et al. Prediction of longitudinal cognitive decline in preclinical Alzheimer disease using plasma biomarkers. JAMA Neurol (2023) ; 80: : 360–369. |
101. | Jack CR , Bennett DA , Blennow K , et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement (2018) ; 14: : 535–562. |
102. | van der Schaar J , Visser LNC , Bouwman FH , et al. Considerations regarding a diagnosis of Alzheimer’s disease before dementia: a systematic review. Alzheimers Res Ther (2022) ; 14: : 31. |
103. | Schicktanz S , Schweda M , Ballenger JF , et al. Before it is too late: professional responsibilities in late-onset Alzheimer’s research and pre-symptomatic prediction. Front Hum Neurosci (2014) ; 8: : 921. |
104. | van der Schaar J , Visser LNC , Ket JCF , et al. Impact of sharing Alzheimer’s disease biomarkers with individuals without dementia: A systematic review and meta-analysis of empirical data. Alzheimers Dement (2023) ; 19: : 5773–5794. |
105. | Draper B , Peisah C , Snowdon J , et al. Early dementia diagnosis and the risk of suicide and euthanasia. Alzheimers Dement (2010) ; 6: : 75–82. |
106. | Stites SD , Milne R and Karlawish J . Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimers Dement (Amst) (2018) ; 10: : 285–300. |
107. | Kim SYH , Karlawish J and Berkman BE . Ethics of genetic and biomarker test disclosures in neurodegenerative disease prevention trials. Neurology (2015) ; 84: : 1488–1494. |
108. | Mozersky J , Sankar P , Harkins K , et al. Comprehension of an elevated amyloid positron emission tomography biomarker result by cognitively normal older adults. JAMA Neurol (2018) ; 75: : 44–50. |
109. | Harkins K , Sankar P , Sperling R , et al. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther (2015) ; 7: : 26. |
110. | Teunissen CE , Verberk IMW , Thijssen EH , et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol (2022) ; 21: : 66–77. |
111. | Largent EA , Grill JD , O’Brien K , et al. Testing for Alzheimer disease biomarkers and disclosing results across the disease continuum. Neurology (2023) ; 100: : 1010–1019. |
112. | Roberts JS , Christensen KD and Green RC . Using Alzheimer’s disease as a model for genetic risk disclosure: implications for personal genomics. Clin Genet (2011) ; 80: : 407–414. |
113. | Kim H and Lingler JH . Disclosure of amyloid PET scan results: A systematic review. Prog Mol Biol Transl Sci (2019) ; 165: : 401–414. |
114. | Grill JD , Raman R , Ernstrom K , et al. Short-term psychological outcomes of disclosing amyloid imaging results to research participants who do not have cognitive impairment. JAMA Neurol (2020) ; 77: : 1504–1513. |
115. | de Wilde A , van Buchem MM , Otten RHJ , et al. Disclosure of amyloid positron emission tomography results to individuals without dementia: a systematic review. Alzheimers Res Ther (2018) ; 10: : 72. |
116. | Goldstein DS , Holmes C , Sharabi Y , et al. Survival in synucleinopathies. Neurology (2015) ; 85: : 1554–1561. |
117. | Savica R , Grossardt BR , Bower JH , et al. Survival and causes of death among people with clinically diagnosed synucleinopathies with parkinsonism: a population-based study. JAMA Neurol (2017) ; 74: : 839–846. |
118. | Rigby T , Johnson DK , Taylor A , et al. Comparison of the caregiving experience of grief, burden, and quality of life in dementia with Lewy bodies, Alzheimer’s disease, and Parkinson’s disease dementia. J Alzheimers Dis (2021) ; 80: : 421–432. |
119. | Wenning GK , Stankovic I , Vignatelli L , et al. The Movement Disorder Society Criteria for the diagnosis of multiple system atrophy. Mov Disord (2022) ; 37: : 1131–1148. |
120. | McKeith IG , Ferman TJ , Thomas AJ , et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology (2020) ; 94: : 743–755. |
121. | Schaeffer E , Toedt I , Köhler S , et al. Risk disclosure in prodromal Parkinson’s disease. Mov Disord (2021) ; 36: : 2833–2839. |
122. | Postuma RB , Iranzo A , Hogl B , et al. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol (2015) ; 77: : 830–839. |
123. | Postuma RB , Gagnon J-F , Bertrand J-A , et al. Parkinson risk in idiopathic REM sleep behavior disorder. Neurology (2015) ; 84: : 1104–1113. |
124. | Joza S , Hu MT , Jung K-Y , et al. Progression of clinical markers in prodromal Parkinson’s disease and dementia with Lewy bodies: a multicentre study. Brain (2023) ; 146: : 3258–3272. |
125. | Mitchell JL . Cross-cultural issues in the disclosure of cancer. Cancer Pract (1998) ; 6: : 153–160. |
126. | Wake T , Tabuchi H , Funaki K , et al. Disclosure of amyloid status for risk of Alzheimer disease to cognitively normal research participants with subjective cognitive decline: a longitudinal study. Am J Alzheimers Dis Other Demen (2020) ; 35: : 1533317520904551. |
127. | Taswell C , Donohue C , Mastwyk MT , et al. Safety of disclosing amyloid imaging results to MCI and AD patients. |
128. | Simuni T , Chahine LM , Poston K , et al. A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research. Lancet Neurol (2024) ; 23: : 178–190. |
129. | Höglinger GU , Adler CH , Berg D , et al. A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria. Lancet Neurol (2024) ; 23: : 191–204. |
130. | Cardoso F , Goetz CG , Mestre TA , et al. A statement of the MDS on biological definition, staging, and classification of Parkinson’s disease. Mov Disord (2024) ; 39: : 259–266. |




