Impact of Physical Exercise on Levodopa Therapy Across Parkinson’s Disease Stages
Abstract
Background:
Levodopa is the gold standard of treatment in Parkinson’s disease (PD). Its clinical effect changes as the disease progresses. Wearing off is a frequent first manifestation of motor fluctuations. Some patients with advanced PD report faster wearing off after physical exercise.
Objective:
The aim was to assess if pharmacokinetics of levodopa is influenced by physical exercise in patients with different disease advancement.
Methods:
22 patients with PD (12 untreated with levodopa and 10 with motor fluctuations) and 7 healthy controls (HC) were included. Plasma samples were collected at 9 fixed timepoints following administration of levodopa/benserazide 200/50 mg for two days: rest day and standardized physical exercise day. Clinical assessment with Unified Parkinson Disease Rating Scale part III (UPDRS III) was performed in fixed timepoints. Liquid chromatography-tandem mass spectrometry was used to measure levodopa concentrations.
Results:
No differences between the HC, levodopa naïve and advanced PD groups were observed regarding selected pharmacokinetic parameters. In advanced PD and HC no differences in pharmacokinetic parameters of levodopa with and without effort were observed. In levodopa naïve PD group higher mean residence time after rest than after exercise (168.9±48.3 min vs. 145.5±50.8 min; p = 0.026) was observed. In advanced PD group higher UPDRS III score (14.45±5.5 versus 20.9±6.1 points, p = 0.04) was observed after exercise.
Conclusions:
The deterioration of motor status of advanced PD patients after physical effort is not reflected by changes in pharmacokinetics but rather mediated by central mechanisms.
Plain Language Summary
Background:
Levodopa is an important treatment for Parkinson’s disease (PD). As the disease gets worse, levodopa’s effects change. A common problem is “wearing off,” where the medicine stops working sooner than expected. Some advanced PD patients say this happens faster after they exercise.
Study goals:
The study aimed to find out if exercise changes how the body processes levodopa in patients at different stages of PD.
Methods:
Participants: 22 PD patients (12 not yet on levodopa and 10 advanced patients- treated with levodopa with “wearing off”) and 7 healthy people.
Procedure: Participants took levodopa/benserazide (200/50 mg) on two days: a rest day and an exercise day. Each day blood samples were collected at 9 set times to measure levodopa levels. PD symptoms were assessed using a scale called UPDRS III at specific times.
Testing: Levodopa levels in blood were measured using a technique called liquid chromatography-tandem mass spectrometry.
Results:
No differences: There were no differences in how the body processed levodopa among healthy people, untreated PD patients, and advanced PD patients.
Exercise impact: For both healthy people and advanced PD patients, exercise did not change how the body processed levodopa.
Untreated patients: In PD patients not yet on levodopa, the medicine stayed in the body longer after rest compared to after exercise.
Advanced PD patients: These patients had worse PD symptoms after exercise, but this was not due to changes in how levodopa was processed.
Conclusion:
The worsening of symptoms in advanced PD patients after exercise is not because of changes in levodopa levels. It likely involves other factors in the brain.
INTRODUCTION
Levodopa, since its introduction in Parkinson’s disease (PD) therapy, for over 40 years remains the gold standard of PD treatment [1]. Unfortunately, after the initial period of mostly consistent positive motor response to treatment, patients develop motor fluctuations [2, 3]. These include “off” periods when parkinsonian symptoms (mainly rigidity, tremor, bradykinesia) reappear during the day. While the mechanisms leading to fluctuations are complex, loss of ability of dopaminergic neurons to store dopamine is among critical ones. Results of STRIDE-PD study indicated, that such features as lower age at onset, nominal levodopa dose, and higher Unified Parkinson’s Disease Rating Scale (UPDRS) part II score significantly predispose to earlier appearance of wearing off symptoms [3]. As the disease progresses, the short plasma half-life of levodopa and diminished striatal dopamine synthesis and storage mean that alterations in absorption, metabolism, and distribution of the drug will immediately translate into clinical effects [2].
Current strategies aim at improving pharmacokinetics of the drug include introduction of peripheral inhibitors of L-amino acid decarboxylase (carbidopa or benserazide), catechol-o-methyltranferase (COMT) inhibitors (entacapone, tolcapone, opicapone), monoamine oxidase B inhibitors (rasagiline, selegiline, safinamide), as well as forms of constant dopaminergic stimulation for advanced PD patients [4, 5]. Constant dopaminergic stimulation with levodopa is currently most achieved by intestinal gel infusion of levodopa-carbidopa formulation for intestinal administration or, MORE recently, levodopa-entacapone-carbidopa intestinal gel [6, 7].
It is reported that physical exercise improve PD patients’ outcomes in various aspects of PD symptoms in the long term [8]. These include aerobic exercise, tai chi, dancing, and water exercises among many others [8–11]. Exercise and physical therapy are considered an important factor contributing to reduction of PD symptoms as well as possible decrease of dyskinesia [12–14]. Cardio exercises such as spinning bike are often practiced by PD patients due to its feasibility, low cost, and safety. It was also proved to be beneficial in terms of neuroprotection in PD [15]. Animal studies indicate that this might be achieved by restoration of striatal cdk5 activity, decrease of Thr75/Thr34 phosphorylation ratio and increased c-Fos expression [16]. In daily clinical practice PD patients should always be encouraged to maintain regular physical activity [8, 17, 18]. Nevertheless, some patients with PD report increase of PD symptoms during/after physical activity or impression that wearing off appears faster (clinical observation). This observation as well as possible antidyskinetic effect of exercises may suggest, that exercise influence pharmacokinetics of levodopa. In the past a few clinical trials aimed at assessing the effect of exercise on levodopa concentration in the blood after physical activity was conducted, but they did not differentiate patients with regards to disease severity and treatment duration [19–23]. This lack of differentiation may be a reason why the results of these studies are inconclusive. It can be hypothesized that short-term response to physical effort and its influence of levodopa bioavailability may differ between levodopa naïve PD patients and those already treated with levodopa and manifesting treatment-related motor fluctuations. Such distinction was not performed before. A paper by Sciacca et al. indicates that levodopa naïve patients may particularly benefit from achieving a sustained long duration response to levodopa associated with motor learning. This combination is proposed to have a positive effect on neuroplasticity [24].
The aim of our study was to assess the effect of standardized physical exercise on pharmacokinetics of levodopa/benserazide in three groups: 1) early PD patients with no previous levodopa treatment (levodopa-naïve PD, LNPD); 2) PD patients treated with levodopa and experiencing wearing off fluctuations (Advanced PD, APD); 3. healthy control group (HC). The differences between pharmacokinetic parameters in the groups were considered the primary outcome, while change in the UPDRS part III after physical effort, reflecting patients’ motor status was also assessed as a secondary outcome.
METHODS
Patients
The study was performed in accordance with the declaration of Helsinki. The protocol of the study was approved by the Ethics committee of the Medical University of Warsaw (KB/8/2017) and written informed consent was obtained from all participants.
Twenty-two patients with diagnosis of PD and 7 HC were included in the study. PD was diagnosed based on MDS diagnostic criteria for idiopathic PD by Postuma et al. [25]. PD patients were divided into 2 groups. LNPD consisted of 12 patients with early PD, which did not receive levodopa treatment previously. APD group consisted of 10 patients treated with levodopa, with motor fluctuations. Seven age and sex-matched HC were recruited among patients’ families as well as hospital personnel who volunteered to participate in the study. None of the HC members displayed symptoms of neurodegenerative disorder. Clinical characteristic of all three groups (LNPD, APD and HC) is provided in Table 1. All PD patients were admitted to the hospital for the assessments. HC group was assessed for two days but was not hospitalized for the entire duration of the study.
Table 1
Demographic characteristics of Parkinson’s disease patients and healthy controls
| LNPD group | APD group | Healthy control | |
| N | 12 | 10 | 7 |
| Age±SD (range) [y] | 59.2±11.0 (43–75) | 55.5±11.2 (41–73) | 55.2±16.7 (52–74) |
| Sex (male) | 5/12 | 6/10 | 3/7 |
| PD duration±SD [y] | 1.5±0.9 | 8.7±5.9 | n/a |
| LEDD±SD [mg] | 0 | 1180±460 | n/a |
| UPDRS III off | 19.7±13.9 | 26.9±8.4 | n/a |
| Dopamine agonist treatment | 0/12 | 5/10 | n/a |
LNPD, levodopa-naïve Parkinson’s disease; APD, advanced Parkinson’s disease; UPDRS III off, Unified Parkinson’s Disease Rating scale part III assessed in “off” state; LEDD, Levodopa Equivalent daily dose; PD, Parkinson’s disease; SD, standard deviation; y, years; SD, standard deviation; mg, milligrams; n/a, non applicable.
Exclusion criteria included cardiac arrhythmias, inability, or medical contraindications to perform a spinning session on a stationary bike or to receive oral levodopa, and treatment with COMT inhibitors. For HC group exclusion criteria were diagnosis of PD or any other neurodegenerative disease, inability, or medical contraindications to perform a spinning session on a stationary bike or to receive orallevodopa.
Procedures
Patients were administered orally standard release levodopa/benserazide in the form of commercially available Madopar (Roche) capsule (200 mg levodopa and 50 mg benserazide). Patients were asked to refrain from their daily levodopa and other PD treatments for 12 h prior to the task on both days, to achieve clinical “off” state. On the day of assessments patients were also asked to refrain from smoking and eating protein products which could influence absorption of levodopa and its clinical efficacy [26, 27]. All patients received standard hospital breakfast in the morning of the assessment (a sandwich and a cup of tea), 1 h prior to levodopa administration.
Two blood collection sessions were performed. At the beginning of each session patients had intravenous cannula (size 16–18 G) inserted into the vein in the forearm to minimize number of venous punctures during sample collections. Blood samples were collected at 0, 20, 40, 60, 90, 120, 150, 180 and 240 min after administration of 250 mg levodopa/benserazide. Blood samples of 10 ml were collected in EDTA-test tubes containing 100μl of 0.5% sodium disulfate solution and centrifuged. Obtained plasma was immediately frozen in –20°C for further analysis.
UPDRS scale part III (motor symptoms) was a clinical examination of parkinsonian symptoms and was performed before, and 60, 180, and 240 min after oral admission of levodopa [28]. The UPDRS III raters (A.M., M.F.) were not blinded regarding patients’ exercise status.
Exercise schedule
During the rest day (RD) patients were asked to refrain from any physical activity and rest in bed or chair for a total time of sample collection. During physical exercise day (PE) patients were asked to perform spinning session on a cycle ergometer with control of the heart rate by a hand pulse sensor. Their maximal heart rate was calculated as 220 minus patient’s age [29]. Patients were asked to exercise within 55 to 85 percent of their maximum heart rate for total of 60 min. Exercises were divided into 40 min (0–40 min since levodopa/benserazide administration) and then after a 20-min break additional 20 min (minutes 60–80 since levodopa/benserazide administration) spinning sessions.
Analytical method
Plasma sample (200μl) was mixed with 50μl of internal standard (levodopa-D3, 500 ng/ml, TRC Chemicals, Canada) for 5 min on the vortex. Next, 240μl of 0.4M perchloric acid was added, mixed for 1 min centrifuged (10 min, at 10,000 g). The supernatant was diluted with water (4 : 1, v/v) and 10μL aliquot was injected into the LC-MS/MS.
Instrumental analysis was performed using an Agilent 1260 Infinity System (Agilent Technologies, Santa Clara, CA, USA), equipped with a degasser, an autosampler and a binary pump, coupled to a QTRAP 4000 hybrid triple quadrupole/linear ion trap mass spectrometer (AB Sciex, Framingham, MA, USA). The turbo ion spray source was operated in positive mode. The curtain gas, ion source gas 1 and ion source gas 2, were set at 241 kPa, 275 kPa, 345 kPa and “high” instrument units (4.6×10–5 Torr), respectively. The ion spray voltage, source temperature and declustering potential were 5,500 V, 600°C, and 40 V respectively. The target compounds were analyzed in multiple reaction monitoring (MRM) mode. The quantitative MRM transitions, and collision energy (CE) are m/z 198/2152 (CE = 19 V) and m/z 201/155 (CE = 19 V) for levodopa and the internal standard, respectively. Chromatographic separation was achieved with a Kinetex C18 Column (100 mm × 4.6 mm, 2.6μm) from Phenomenex (Torrance, USA). The column was maintained at 40°C at a flow rate of 0.5 mL min–1. The mobile phases consisted of water with 0.2% formic acid as eluent A, and acetonitrile with 0.2% formic acid as eluent B. The gradient (% B) was as follows: 0 min. 5%, 3 min. 5%, 4.5 min. 95% and 6.5 min. 95%. The injection volume was 10μL.
Statistics
The pharmacokinetic parameters were calculated using non-compartmental analysis tool of PKSolver, a freely available menu-driven add-in program for Microsoft Excel written in Visual Basic for Applications (VBA) [30]. The area under the plasma concentration versus time curve (AUC) was calculated by the linear trapezoidal method. The apparent terminal elimination rate constant, λz, was obtained by linear regression of the log-linear terminal phase of the concentration-time profile by using at least three non-zero declining concentrations in terminal phase with a correlation coefficient of > 0.8. The terminal half-life value (t1/2) was calculated using the equation (ln2)×λz.
The selected pharmacokinetic parameters of levodopa: half time (t1/2), time-to-peak (Tmax), area under the curve from dosing time to infinity (AUC 0-inf), mean residence time (MRT), maximum serum concentration (Cmax), volume of distribution (Vz) and clearance (Cl) were compared between three groups (HC, LNPD, APD) and two days: PE, when physical exercise were performed during blood collection, and RD, when patients were resting for the whole study duration. Additionally, the influence of the exercise within the groups was analyzed.
Statistical tests were performed using Statistica 13.5 (StatSoft). The data are presented as the mean±standard deviation (SD) or median±interquartile range (IQR). Normality of data distribution was tested by using the Shapiro-Wilk test, and homogeneity of variance by using Levene’s test. Logarithmic transformation was performed for data without normal distribution, and non-parametric tests were used if normal distribution was not achieved. All parameters were assessed statistically using χ2, Student’s t-test, Mann Whitney U-test, ANOVA (with post-hoc least significant difference (LSD) analysis), or Kruskal-Wallis test (with post hoc Dunn test). Missing data were imputed with nearest neighbor method. The p-value<0.05 was considered as statistically significant.
The principal component analysis and heatmaps were performed using Metaboanalyst 5.0. Before the analysis, log-transformation of the data to remove heteroscedasticity and correct for skewed data distribution was performed. The heatmap with standardized features was used to visualize differences between pharmacokinetic parameters across samples. Cluster analysis of metabolites was based on squared Euclidean distance and Ward’s method.
RESULTS
No differences between the groups were detected between HC, LNPD and APD regarding selected pharmacokinetic parameters. The data summarizing the pharmacokinetic findings is presented in Table 2. The effect of physical effort on levodopa pharmacokinetic parameters was also calculated for the whole cohort (LNPD, APD, and HC consolidated). The mean score achieved in UPDRS part III after 180 min since levodopa administration was significantly higher after PE (M = 16.5, SD = 9.7 points) than in the RD (M = 12.5, SD = 6.8 points, p = 0.005). There were no other significant differences in Tmax, t1/2, Cmax, AUC 0-inf, Vz, Cl or MRT 0-inf between RD and PE for the whole group.
Table 2
Comparison between clinical and pharmacokinetic parameters median
| LNPD | APD | HC | p | |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Tmax RD [min] | 105 (50–120) | 50 (40–60) | 90 (40–90) | 0.1 |
| Tmax PE [min] | 75 (40–120) | 65 (40–120) | 60 (40–120) | 0.93 |
| t1/2 RD [min] | 77.2 (63.4–85.4) | 75.1 (60.7–88.5) | 61.6 (45.9–75.4) | 0.15 |
| t1/2 PE [min] | 67.0 (55.7–81.4) | 65.3 (56.7–80.1) | 53.6 (51.0–93.3) | 0.43 |
| Cmax RD [μg/ml] | 2.3 (1.4–3.2) | 3.0 (2.4–4.0) | 2.0 (1.4–3.5) | 0.38 |
| Cmax PE [μg/ml] | 2.9 (1.5–3.1) | 2.5 (2.2–4.0) | 2.7 (2.0–3.4) | 0.66 |
| AUC 0-inf RD [mg/ml*min] | 0.33 (0.2–0.5) | 0.36 (0.28–0.4) | 0.27 (0.2–0.4) | 0.54 |
| AUC 0-inf PE [mg/ml*min] | 0.33 (0.2–0.4) | 0.36 (0.32–0.4) | 0.29 (0.3–0.4) | 0.45 |
| MRT 0-inf RD [min] | 158 (134–189) | 130.4 (107.6–154.9) | 133.3 (114–155) | 0.19 |
| MRT 0-inf PE [min] | 133 (114–172) | 137.8 (122.5–176.4) | 126 (103–165) | 0.78 |
| Vz RD [l] | 72 (50–92) | 54 (39–85) | 60 (44–99) | 0.64 |
| Vz PE [l] | 55 (49–85) | 53 (48–66) | 57 (45–88) | 0.41 |
| Cl RD [l/min] | 36.6 (26.4–54) | 33.6 (30–42.6) | 44.4 (31.8–62.4) | 0.54 |
| Cl PE [l/min] | 36.6 (28.8–59.4) | 33 (30–37.8) | 42 (34.2–44.4) | 0.45 |
Statistical analysis was performed using ANOVA after the transformation of variables. IQR, interquartile range; LNPD, levodopa-naive Parkinson’s disease; APD, advanced Parkinson’s disease; HC, healthy control; RD, Rest Day; PE, Physical exercise day; Tmax, Time to peak drug concentration; t1/2, half-life time; Cmax, maximum serum concentration; AUC 0-inf, the area under the concentration-time curve from dosing (time 0) to infinity; MRT 0-inf, mean residence time from dosing (time 0) to infinity; Vz, volume of distribution; Cl, clearance; min, minutes; mg, milligrams; μg, micrograms; ml, milliliters; l, liters.
In the next step separate analysis of effect of physical effort on pharmacokinetic parameters and clinical symptoms in each group (LNPD, APD, HC) was performed. The results are presented in Table 3 and Supplementary Table 1. In LNPD group a significantly higher MRT in the RD than in the PE day (M = 168.9, SD = 48.3 min and M = 145.5, SD = 50.8 min respectively; p = 0.026) was observed. This reflects longer residence of levodopa in patients’ body in LNPD group during rest. Figure 1A summarizes effect of physical effort on MRT within all three groups. In APD, no significant differences in pharmacokinetic parameters of levodopa with and without effort were detected. Significantly higher score in UPDRS part III in 180 min after exercise was observed in PE day than in RD (M = 20.9 points, SD = 6.1 and M = 14.45 points, SD = 5.5, p = 0.04) for APD group. The results are presented in Table 3 and summarized in Fig. 1B. In HC group no significant impact of physical exercises on pharmacokinetics of the drug was reported. The summary of the results in HC are presented in supplementary Table 1.
Fig. 1
Selected pharmacokinetic parameters comparison between study groups. A) Statistically significant differences in the MRT parameter with and without effort were observed for the levodopa nïve Parkinson’s disease (LNPD) group. No significant difference was observed in advanced Parkinson disease (APD) group and healthy control (HC). B) Influence of exercise on the UPDRS III score after 180 min. In APD group UPDRS III score was significantly higher at 180 min during physical exercise day than during rest day. HC, healthy control; LNPD, levodopa naïve Parkinson’s Disease; APD, advanced Parkinson’s disease; MRT, mean residence time; UPDRS, Unified Parkinson Disease Rating Scale-part III; min, minutes. Significant differences are marked with *. Significant p-value is considered < 0.05.
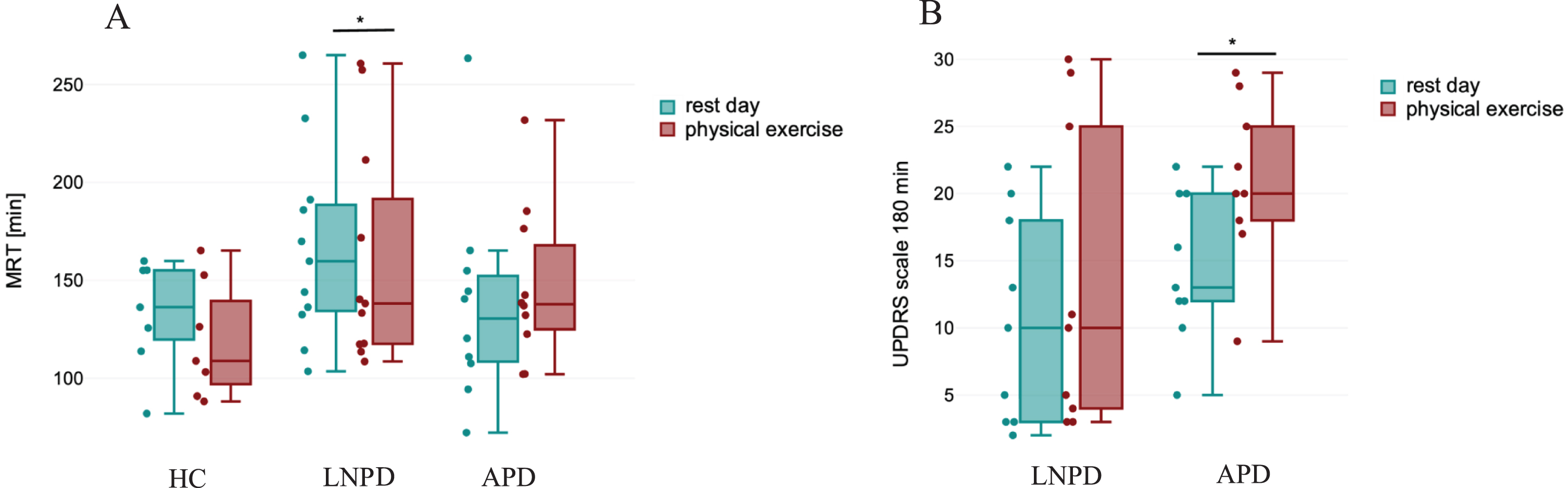
Table 3
Impact of the physical effort on clinical and pharmacokinetic parameters in levodopa-naive Parkinson’s patients’ and advanced Parkinson’s disease patients’ subgroups
| LNPD | ||||
| Wilcoxon test | ||||
| T | Z | p | ||
| UPDRS III 180 min RD vs. PE [score] | 8 | 1.7 | 0.09 | |
| Tmax RD vs. PE [min] | 12 | 0.8 | 0.4 | |
| t1/2 RD vs. T1/2 PE [min] | 21 | 1.1 | 0.29 | |
| Vz RD vs. PE [l] | 24 | 0.8 | 0.42 | |
| Cl RD vs. PE [l/min] | 19 | 1.2 | 0.21 | |
| T-Student test | T | Mean RD (SD) | Mean PE (SD) | p |
| UPDRS III 0 min RD vs. PE | 1.69 | 19.7 (13.9) | 17.7 (10.6) | 0.12 |
| Cmax RD vs. PE [μg/ml] | 0.44 | 2.4 (0.1) | 2.6 (0.1) | 0.67 |
| AUC 0-inf RD vs. AUC 0-inf PE [mg/ml*min] | 1.18 | 0.3 (0.1) | 0.3 (0.2) | 0.27 |
| MRT 0-inf RD vs. MRT 0-inf PE [min] | 2.62 | 168.9 (48.3) | 145.5 (50.8) | 0.026* |
| APD | ||||
| T-Student test | T | Mean RD (SD) | Mean PE(SD) | p |
| UPDRS III 0 min RD vs. PE [score] | 0.68 | 26.9 (8.4) | 24.8 (7.8) | 0.52 |
| UPDRS III 180 min RD vs. PE [score] | –2.43 | 14.5 (5.5) | 20.9 (6.1) | 0.04* |
| TmaxRD vs. PE [min] | –1.12 | 57 (29.1) | 74 (37.5) | 0.29 |
| t1/2 RD vs. PE [min] | 0.54 | 78.0 (28.4) | 71.1 (23.1) | 0.6 |
| Cmax RD vs. PE [μg/ml] | 0.52 | 3.1 (1.1) | 2.9 (1) | 0.62 |
| MRT 0-inf RD vs. PE [min] | –0.44 | 137.4 (52.7) | 147.1 (40.2) | 0.67 |
| AUC 0-inf RD vs. PE [mg/ml*min] | 0.02 | 0.4 (0.1) | 0.4 (0.6) | 0.9 |
| Vz RD vs. PE [l] | 0.74 | 65.3 (30.4) | 57 (20.4) | 0.48 |
| Cl RD vs. PE [l/min] | ||||
| 0.81 | 35.0 (9.4) | 33.5 (5) | 0.44 | |
LNPD, Levodopa-naïve Parkinson’s disease; APD, Advanced Parkinson’s disease; RD, Rest Day; PE, Physical effort day; UPDRS, Unified Parkinson’s Disease Rating Scale score; Tmax, Time to peak drug concentration; t1/2, half life time; Cmax, maximum serum concentration; AUC 0-inf, the area under the concentration-time curve from dosing (time 0) to infinity; MRT 0-t, mean residence time from dosing (time 0) to time t; Vz, volume of distribution; Cl, clearance; min, minutes; mg, milligrams; μg, micrograms; ml, milliliters; l, liters; SD, standard deviation; vs., versus. Significant differences are marked with*.
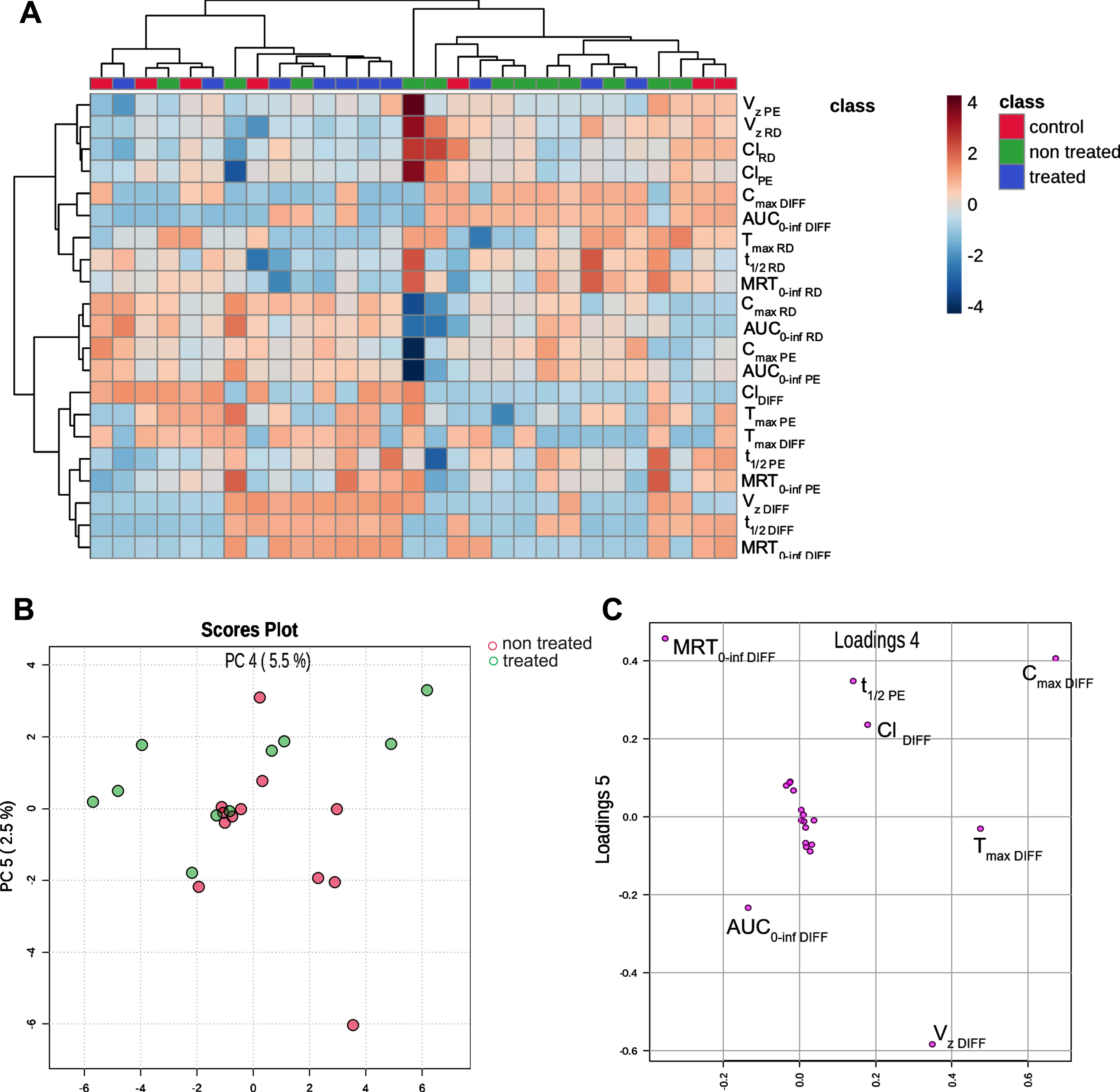
Chemometric analysis, specifically hierarchical clustering, revealed distinctions in parameter profiles between the treated and non-treated groups (Fig. 2). The examined parameters exhibited significant variability within the group and were evenly distributed into two distinct clusters. The observation was further substantiated through principal component analysis, where Principal Components 4 and 5 demonstrated the most effective differentiation between the treated and non-treated groups. It is evident that the primary contributor to the separation of the treated and non-treated groups is the parameter MRT diff, which represents the relative difference between MRT with and without physical effort (Fig. 2).
Fig. 2
A) Heat map visualization of differences in pharmacokinetic parameters in three different groups. The deeper the blue color, the lower value of the parameter compared to other patients analyzed. The deeper the red color, the higher the level. The parameters with similar behavior across the samples were clustered. Scores Plot B and Loading Plot C illustrate Principal Component Analysis of pharmacokinetic parameters after levodopa administration to treated and non-treated patients. Tmax, Time to peak drug concentration; t1/2, half-life time; Cmax, maximum serum concentration; AUC 0-inf- the area under the concentration-time curve from dosing (time 0) to infinity; MRT 0-inf, mean residence time from dosing (time 0) to infinity t; DIFF, relative difference between a parameter with and without physical effort; Vz, volume of distribution; Cl, clearance; PE, Physical exercise day, RD, rest day.
Additional investigation was performed regarding additional clinical factors which could influence levodopa pharmacokinetics. In dopamine agonist (DA)-treated group some pharmacokinetic parameters (Tmax, Cmax and MRT) differed significantly between PE and RD. These include higher Cmax (M = 4μg/ml, SD = 0.6 vs. M = 2.8μg/ml, SD = 1.1, p = 0.02) in DA treated group in RD than PE. In the group untreated with DA, significantly higher MRT with PE than on RD (M = 181.6 min, SD = 48.9 vs. M = 140.3, SD = 33.8, p = 0.02) was observed. The differences are presented in Supplementary Table 2 and Supplementary Figures 1–3.
DISCUSSION
To our knowledge, this study marks the pioneering investigation into levodopa pharmacokinetics across varying stages of PD advancement: encompassing early-stage, drug-naïve patients alongside those with advanced PD [31]. Similar methods of assessment of levodopa pharmacokinetic were already applied in smaller groups without such divisions, reaching generally similar conclusions.
The results of performed analysis suggest that physical exercise does not significantly influence pharmacokinetics of levodopa. The significant pharmacokinetic difference observed was higher MRT observed in RD than during PE, only in LNPD group. The observed clinical effect of physical exercise- higher UPDRS III score in APD group- was not reflected by significant changes in pharmacokinetic parameters.
Only one paper by Carter et al. reported that exercises change the rate of levodopa absorption [23]. Authors reported delay in levodopa absorption in 5/10 patients and increase in 3/10. No change was only observed in 2 patients. This was not observed in our study. A paper by Reuter et al. assessed effect of physical exercise on absorption on 100 mg levodopa/25 mg benserazide combination admitted orally [19]. Twelve patients with motor fluctuations resulting from treatment were included. Authors reported no difference in the mean basal levodopa plasma concentration between day with and without exercises, which is consistent with our findings. Mouradian et al. performed a study on 4 patients, receiving intravenous constant infusion of levodopa [22]. They assessed the effect of acute physical exercise (walking on a treadmill with increasing velocity) on levodopa plasma levels. The results were consistent with other findings and showed no change in levodopa pharmacokinetics. In a paper by Muhlack et al. authors reported, also consistently with our results and other studies, similar levodopa bioavailability (AUC, Tmax, Cmax) on PE day and RD [20]. They included 12 male patients. Interestingly, they found a significantly better motor status (lower UPDRS III score) of patients 120 and 150 min after administration of levodopa with physical exercise. In our study, UPDRS part III assessments were conducted 180 min since levodopa administration, revealing a notable decline in the APD group (M = 20.9 points, SD = 6.1 on the PE day, and M = 14.45 points, SD = 5.5 on the RD; p = 0.04). Muhlack et al. hypothesized that exercise improves the motor response to treatment by increasing levodopa passage to the brain over blood-brain barrier or increase in endogenous dopamine synthesis and release into nigrostriatal region [20]. The group characteristic indicated that all patients received levodopa treatment prior to participation in the study. It can be however suspected by the mean disease duration of 4.38±3.04 years that the group was less advanced than our APD subgroup (M = 8.7, SD = 5.9 years of disease duration), which could potentially account for the difference observed in the outcomes.
In our study, no positive immediate clinical effect of exercise on UPDRS part III was reported. Our finding is consistent with findings by Goetz et al. [21]. Authors correlated levodopa pharmacokinetics and UPDRS part III score in 10 patients. They did not observe improvement in UPDRS after exercise and reported similar bioavailability of levodopa with and without physical exercise.
Similarly to other authors, measures were taken to limit external factors influencing levodopa bioavailability. The meals patients obtained to limit influence of food were standardized. There are however numerous factors involved in levodopa bioavailability, such as age, diet, occurrence of gastroparesis and constipations or gastrointestinal microbiota composition [32–34]. Senek et al. mention COMT gene polymorphism rs4680 and DCC gene polymorphisms rs921451 and rs3837091 as important for levodopa pharmacokinetics, in individuals with higher DDC and COMT enzyme activity having higher levodopa Cl/F [35]. Another suggested mechanism leading to malabsorption of levodopa from the intestine is decreased enteral blood flow during exercise. However, it should be emphasized that this causes only minimal disruption in drug absorbance and was not observed in our study or otherpapers [36].
The most important aspect of our study is a comparison of effect of physical exercise on patients in different stages of PD as well as in healthy controls. Such distinction was not performed in previous studies assessing exercise and levodopa bioavailability. Inclusion of healthy subjects aimed at assessing effect of impaired gastric mobility on levodopa bioavailability [37]. No significant correlation between changes in drug bioavailability and patients’ parkinsonian symptoms was detected. We hypothesize that deterioration in UPDRS III in APD group with PE, not reflected by pharmacokinetic differences may have numerous causes. Firstly, it can be mediated by central nervous system (CNS) rather than peripheral mechanisms [38]. CNS mechanisms leading to wearing off include dopaminergic presynaptic, dopaminergic postsynaptic and glutamatergic receptor alterations [38, 39]. Presynaptic dopaminergic neurodegeneration leads to non-physiological storage and release of dopamine. In LNPD with shorter disease duration, central synthesis of dopamine as well as the ability to store the drug in dopaminergic neurons remains preserved to some extent, which may prevent from wearing off symptoms [38, 40]. Postsynaptic mechanisms may also lead to wearing off and sudden off phenomenon [41]. These include upregulation of D2 receptor-mediated “indirect” striatopallidal function and downregulation of Dl receptor- mediated “direct” striatonigral function. This also explains why “off” periods are still present in patients treated with continuous dopaminergic therapies such as rotigotine transdermal patch, apomorphine subcutaneous infusions or levodopa-carbidopa and levodopa-entacapone-carbidopa intestinal gel infusions [42]. Lack of correlation between levodopa clearance and appearance of parkinsonian symptoms was also observed in a study by Fabbrini et al. comparing patients never treated with levodopa with patients with stable motor response, as well as those with wearing off and on/off phenomenon [38].
The effect of treatment with DA (ropinirole) on patients with PD with and without motor fluctuations was assessed by Adamiak-Giera et al. [43]. The authors reported no difference in levodopa pharmacokinetics regarding status of ropinirole treatment. However, the design of the study did not include any physical exercise. We observed that additional treatment with DA may influence levodopa pharmacokinetics. Specifically, we observed significantly shorter residence of levodopa in the system (reflected by lower MRT of 105.9±26.4 min vs. 181.6±48.9 min p < 0.05) and shorter time to reach peak drug concentration (Tmax of 48±10.9 min vs. 100±33.5 min, p < 0.05) in DA-treated group when resting, comparing to patients untreated with DA. This difference was not observed during the day when exercises were performed. To our best knowledge this was not reported before. The addition of DA to levodopa is a frequent practice in patients with PD and may lead to reduction of total levodopa equivalent dose [44, 45]. DA may also be beneficial in treatment of non-motor symptoms of PD [46, 47].
The limitation of our study includes limited sample size and lack of blinding of clinicians regarding patients’ status. Blinding could influence UPDRS III results in the PD groups. Other non-motor symptoms of PD such as fatigue, pain, gastroenteric symptoms and sleep quality could also be included for a more detailed assessment.
Our study reveals some interesting directions for future research. Future studies should be focused on closer understanding of central mechanisms leading to wearing off and sudden off symptoms. Some peripheral mechanisms reducing bioavailability of levodopa can also be addressed. These include Helicobacter pylori eradication or gut microbiota modifications such as reduction of gastrointestinal Enterococcus faecalis (i.e., the major tyrosine decarboxylase producer) or Eggerthella lenta (i.e., a strain responsible for dehydroxylating dopamine to m-tyramine) [48].
Conclusions
We conclude that physical exercise did not influence levodopa pharmacokinetics at large. They may however lead to faster immediate motor deterioration in APD patients. We also observed some significant change in MRT in LNPD. Obtained results suggest that exercise may have different effect on clinical symptoms and pharmacokinetics of levodopa depending on the stage of the disease. While patients should be encouraged to include moderate physical exercises in their daily routine due to their numerous positive effects in PD, it should probably be paired with appropriate increase of levodopa dose during physical activity period.
ACKNOWLEDGMENTS
We would like to acknowledge nurses Anna Gutowska and Ewa Nawrocka for their involvement in blood sample collection.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230384.
REFERENCES
[1] | Cotzias GC , Van Woert MH , Schiffer LM ((1967) ) Aromatic amino acids and modification of parkinsonism. N Engl J Med 276: , 374–379. |
[2] | Nutt JG ((2008) ) Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord 23: , S580–S584. |
[3] | Warren Olanow C , Kieburtz K , Rascol O , Poewe W , Schapira AH , Emre M , Nissinen H , Leinonen M , Stocchi F , Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease (STRIDE-PD) Investigators ((2013) ) Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord 28: , 1064–1071. |
[4] | Fox SH , Katzenschlager R , Lim SY , Barton B , De Bie RM , Seppi K , Coelho M , Sampaio C , Movement Disorder Society Evidence-Based Medicine Committee ((2018) ) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33: , 1248–1266. |
[5] | Armstrong MJ , Okun MS ((2020) ) Diagnosis and treatment of Parkinson disease: A review. JAMA 323: , 548–560. |
[6] | Othman AA , Rosebraugh M , Chatamra K , Locke C , Dutta S ((2017) ) Levodopa-carbidopa intestinal gel pharmacokinetics: Lower variability than oral levodopa-carbidopa. J Parkinsons Dis 7: , 275–278. |
[7] | Nyholm D , Jost WH ((2022) ) Levodopa-entacapone-carbidopa intestinal gel infusion in advanced Parkinson’s disease: Real-world experience and practical guidance. Ther Adv Neurol Disord 15: , 17562864221108018. |
[8] | van der Kolk NM , de Vries NM , Kessels RP , Joosten H , Zwinderman AH , Post B , Bloem BR ((2019) ) Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol 18: , 998–1008. |
[9] | Rawson KS , McNeely ME , Duncan RP , Pickett KA , Perlmutter JS , Earhart GM ((2019) ) Exercise and Parkinson disease: Comparing tango, treadmill, and stretching. J Neurol Phys Ther 43: , 26–32. |
[10] | Zhu M , Zhang Y , Pan J , Fu C , Wang Y ((2020) ) Effect of simplified Tai Chi exercise on relieving symptoms of patients with mild to moderate Parkinson’s disease. J Sports Med Phys Fitness 60: , 282–288. |
[11] | Pérez de la Cruz S ((2017) ) Effectiveness of aquatic therapy for the control of pain and increased functionality in people with Parkinson’s disease: A randomized clinical trial. Eur J Phys Rehabil Med 53: , 825–832. |
[12] | Reuter I , Engelhardt M , Stecker K , Baas H ((1999) ) Therapeutic value of exercise training in Parkinson’s disease. Med Sci Sports Exerc 31: , 1544–1549. |
[13] | Frazzitta G , Bertotti G , Morelli M , Riboldazzi G , Pelosin E , Balbi P , Boveri N , Comi C , Turla M , Leva S , Felicetti G , Maestri R ((2012) ) Rehabilitation improves dyskinesias in Parkinsonian patients: A pilot study comparing two different rehabilitative treatments. Neurorehabilitation 30: , 295–301. |
[14] | Farashi S , Kiani L , Bashirian S ((2021) ) Effect of exercise on Parkinson’s disease tremor: A meta-analysis study. Tremor Other Hyperkinet Mov (N Y) 11: , 15. |
[15] | Alberts JL , Linder SM , Penko AL , Lowe MJ , Phillips M ((2011) ) It is not about the bike, it is about the pedaling: Forced exercise and Parkinson’s disease. Exerc Sport Sci Rev 39: , 177–186. |
[16] | Aguiar AS , Moreira ELG , Hoeller AA , Oliveira PA , Córdova FM , Glaser V , Walz R , Cunha RA , Leal RB , Latini A , Prediger RDS ((2013) ) Exercise attenuates levodopa-induced dyskinesia in 6-hydroxydopamine-lesioned mice. Neuroscience 243: , 46–53. |
[17] | Mahlknecht P , Georgiev D , Akram H , Brugger F , Vinke S , Zrinzo L , Hariz M , Bhatia KP , Hariz G-M , Willeit P ((2018) ) Parkinsonian signs in patients with cervical dystonia treated with pallidal deep brain stimulation. Brain 141: , 3023–3034. |
[18] | Speelman AD , van de Warrenburg BP , van Nimwegen M , Petzinger GM , Munneke M , Bloem BR ((2011) ) How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol 7: , 528–534. |
[19] | Reuter I , Harder S , Engelhardt M , Baas H ((2000) ) The effect of exercise on pharmacokinetics and pharmacodynamics of levodopa. Mov Disord 15: , 862–868. |
[20] | Muhlack S , Welnic J , Woitalla D , Müller T ((2007) ) Exercise improves efficacy of levodopa in patients with Parkinson’s disease. Mov Disord 22: , 427–430. |
[21] | Goetz CG , Thelen JA , MacLeod C , Carvey PM , Bartley EA , Stebbins GT ((1993) ) Blood levodopa levels and Unified Parkinson’s Disease Rating Scale function: With and without exercise. Neurology 43: , 1040–1040. |
[22] | Mouradian M , Juncos J , Serrati C , Fabbrini G , Palmeri S , Chase T ((1987) ) Exercise and the antiparkinsonian response to levodopa. Clin Neuropharmacol 10: , 351–355. |
[23] | Carter JH , Nutt JG , Woodward WR ((1992) ) The effect of exercise on levodopa absorption. Neurology 42: , 2042–2042. |
[24] | Sciacca G , Mostile G , Disilvestro I , Donzuso G , Nicoletti A , Zappia M ((2023) ) Long-duration response to levodopa, motor learning, and neuroplasticity in early Parkinson’s disease. Mov Disord 38: , 626–635. |
[25] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[26] | Camargo SM , Vuille-dit-Bille RN , Mariotta L , Ramadan T , Huggel K , Singer D , Götze O , Verrey F ((2014) ) The molecular mechanism of intestinal levodopa absorption and its possible implications for the treatment of Parkinson’s disease. J Pharmacol Exp Ther 351: , 114–123. |
[27] | Frankel JP , Kempster PA , Bovingdon M , Webster R , Lees AJ , Stern GM ((1989) ) The effects of oral protein on the absorption of intraduodenal levodopa and motor performance. J Neurol Neurosurg Psychiatry 52: , 1063–1067. |
[28] | Fahn S , Elton R , Members of the UPDRS Development Committee ((1987) ) Unified Parkinson’s disease rating scale. In Recent Developments in Parkinson’s Disease Vol. 2: , Fahn S, Marsden CD, Calne DB, Goldstein M, eds. McMellam Health Care Information, Florham Park, pp. 153–163. |
[29] | Riebe DE , Jonathan K; , Liguori Gary; , Magal Meir ((2018) ) Chapter 6: General principles of exercise prescription. In ACSM’s Guidelines for Exercise Testing and Prescription. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA, pp. 143–179. |
[30] | Zhang Y , Huo M , Zhou J , Xie S ((2010) ) PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99: , 306–314. |
[31] | Malaty IA , Martinez-Martin P , Chaudhuri KR , Odin P , Skorvanek M , Jimenez-Shahed J , Soileau MJ , Lindvall S , Domingos J , Jones S ((2022) ) Does the 5– 2-1 criteria identify patients with advanced Parkinson’s disease? Real-world screening accuracy and burden of 5– 2-1-positive patients in 7 countries. BMC Neurol 22: , 35. |
[32] | Robertson D , Wood N , Everest H , Monks K , Waller D , Renwick A , George C ((1989) ) The effect of age on the pharmacokinetics of levodopa administered alone and in the presence of carbidopa. Br J Clin Pharmacol 28: , 61–69. |
[33] | Maini Rekdal V , Bess EN , Bisanz JE , Turnbaugh PJ , Balskus EP ((2019) ) Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 364: , eaau6323. |
[34] | Leta V , Klingelhoefer L , Longardner K , Campagnolo M , Levent HÇ , Aureli F , Metta V , Bhidayasiri R , Chung-Faye G , Falup-Pecurariu C ((2023) ) Gastrointestinal barriers to levodopa transport and absorption in Parkinson’s disease. Eur Neurol 30: , 1465–1480. |
[35] | Senek M , Nyholm D , Nielsen EI ((2020) ) Population pharmacokinetics of levodopa gel infusion in Parkinson’s disease: Effects of entacapone infusion and genetic polymorphism. Sci Rep 10: , 18057. |
[36] | Khazaeinia T , Ramsey AA , Tam YK ((2000) ) The effects of exercise on the pharmacokinetics of drugs. J Pharm Pharm Sci 3: , 292–302. |
[37] | Kurlan R , Rothfield KP , Woodward WR , Nutt JG , Miller C , Lichter D , Shoulson I ((1988) ) Erratic gastric emptying of levodopa may cause “random” fluctuations of parkinsonian mobility. Neurology 38: , 419–421. |
[38] | Fabbrini G , Juncos J , Mouradian MM , Serrati C , Chase TN ((1987) ) Levodopa pharmacokinetic mechanisms and motor fluctuations in Parkinson’s disease. Ann Neurol 21: , 370–376. |
[39] | Calon F , Rajput AH , Hornykiewicz O , Bédard PJ , Di Paolo T ((2003) ) Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson’s disease. Neurobiol Dis 14: , 404–416. |
[40] | de la Fuente-Fernández R , Sossi V , Huang Z , Furtado S , Lu J-Q , Calne DB , Ruth TJ , Stoessl AJ ((2004) ) Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: Implications for dyskinesias. Brain 127: , 2747–2754. |
[41] | Bravi D , Mouradian MM , Roberts JW , Davis TL , Sohn YH , Chase TN ((1994) ) Wearing-off fluctuations in Parkinson’s disease: Contribution of postsynaptic mechanisms. Ann Neurol 36: , 27–31. |
[42] | Rota S , Urso D , van Wamelen DJ , Leta V , Boura I , Odin P , Espay AJ , Jenner P , Chaudhuri KR ((2022) ) Why do ‘OFF’ periods still occur during continuous drug delivery in Parkinson’s disease? Transl Neurodegener 11: , 43. |
[43] | Adamiak-Giera U , Jawień W , Pierzchlińska A , Białecka M , Kobierski JD , Janus T , Gawrońska-Szklarz B ((2021) ) Pharmacokinetics of levodopa and 3-O-methyldopa in parkinsonian patients treated with levodopa and ropinirole and in patients with motor complications. Pharmaceutics 13: , 1395. |
[44] | Whone AL , Watts RL , Stoessl AJ , Davis M , Reske S , Nahmias C , Lang AE , Rascol O , Ribeiro MJ , Remy P , Poewe WH , Hauser RA , Brooks DJ ((2003) ) Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol 54: , 93–101. |
[45] | Pahwa R , Lyons KE , Hauser RA ((2004) ) Ropinirole therapy for Parkinson’s disease. Expert Rev Neurother 4: , 581–588. |
[46] | Pagonabarraga J , Piñol G , Cardozo A , Sanz P , Puente V , Otermín P , Legarda I , Delgado T , Serrano C , Balaguer E , Aguirregomozcorta M , Álvarez R , Kulisevsky JJ ((2015) ) Transdermal rotigotine improves sleep fragmentation in Parkinson’s disease: Results of the multicenter, prospective SLEEP-FRAM Study. Parkinsons Dis 2015: , 131508. |
[47] | Wang Y , Jiang DQ , Lu CS , Li MX , Jiang LL ((2021) ) Efficacy and safety of combination therapy with pramipexole and levodopa vs levodopa monotherapy in patients with Parkinson disease: A systematic review and meta-analysis. Medicine (Baltimore) 100: , e27511. |
[48] | Mulroy E , Bhatia KP ((2019) ) The gut microbiome: A therapeutically targetable site of peripheral levodopa metabolism. Mov Disord Clin Pract 6: , 547–548. |




