Dietary Interventions in Parkinson’s Disease
Abstract
Several dietary patterns and nutritional supplements have been linked to the development, progression, and symptomatic treatment of Parkinson’s disease (PD). Most of the evidence, at this point, is preliminary and based largely on observational studies. Interventional studies are scarce, so the evidence on effectiveness remains inconclusive. Dietary interventions could, analogous to exercise, potentially have a beneficial effect on disease symptoms as well as on the progression of the disease and should therefore be researched in high quality studies. Further work is also needed to study whether dietary interventions, when applied to an at-risk population, have any potential to postpone the onset of manifest PD. In this paper, we summarize all ongoing clinical trials on dietary interventions in PD. We found 10 ongoing studies, all aimed at a different intervention. These studies are mostly exploratory in nature or represent phase I or phase II trials focusing on safety, biological responses, and symptomatic effects. Taken together, we conclude that research on dietary interventions in persons with PD is still in its early days. The results of the various ongoing trials are expected to generate new hypotheses and will help to shape the agenda for future research on this important topic.
INTRODUCTION
Parkinson’s disease (PD) is a complex neurodegenerative disorder characterized neuropathologically by degeneration of dopaminergic neurons, as well as lesions outside the nigrostriatal circuitry [1]. These neurodegenerative changes lead to a wide range of motor- and non-motor symptoms, some of which are responsive to dopaminergic treatment. While the underlying disease mechanisms causing this neurodegeneration have not been fully clarified, treatment of the disease remains merely symptomatic by offering dopamine replacement therapy (medication for almost all individuals, and deep brain surgery for selected cases) [1]. Several potentially disease modifying drugs have been studied, but none of these have thus far been shown to alter the progressive course of the disease [2-4]. In the absence of disease modifying pharmacological approaches, increasing attention is being paid to non-pharmacological management of the disease [5]. Most work has so far focused on exercise, which can have positive effects on a wide range of symptoms such as gait, balance, and mood [6, 7]. Exercise can also alleviate a range of non-motor symptoms, including, e.g., sleep [8] or depression [9]. At this moment, high-intensity aerobic exercise is hypothesized to be one of the very few intervention that could potentially impact the progression of PD [10-12]. The evidence for this assumption is based on a combination of animal studies [13, 14], exploratory randomized controlled trials in humans [15], neuroimaging studies showing evidence for exercise-induced cerebral plasticity in persons with PD [15] and longitudinal cohort studies [16, 17].
Longitudinal cohort studies have also identified other lifestyle factors that are related to the development, progression, or symptomatic treatment of PD, including diet and strategies to manage stress [18]. In this present paper, we focus on dietary patterns and nutritional supplements (which we will refer to as dietary interventions). One of the reasons why dietary interventions are generating such enormous interest is that, in recent years, the relationship between the gut microbiome and PD development has become clearer [18-21]. Another reason is the possible link between exposure to pesticides on the one hand, and the risk of developing PD on the other hand [22, 23]. Humans can be exposed to pesticides in various ways, and exposure via the food chain is one of them. The latter notion actually directly links pesticides again to changes in gut microbiome, as is highlighted in an elegant recent review in this journal [24]. Indeed, in clinical practice, in patient forums and on social media, we see many people with PD (PwPD) who are deeply interested in the subject of nutrition, and they have many questions on what diet they should follow, or what supplements they should take.
Taken together, nutrition is potentially important in PD for at least three reasons. First, dietary factors can play a role in the preclinical phase by determining the risk of developing PD, either in a negative way or in a positive way (because of protective elements in food). Indeed, several dietary products have been associated with a decreased risk of future development of PD, such as adherence to the Mediterranean diet and the regular consumption of coffee or flavonoids; conversely, other dietary products such as dairy products have been associated with an increased risk [25, 26]. Metabolic syndrome is another risk factor associated with a higher risk of developing PD [27], and this is of course also linked to diet.
Second, diet can be important in people who have already developed symptomatic PD. One important factor to consider in the management of PD is the relationship between protein intake and absorption of levodopa in the gut (gastrointestinal absorption) and the brain (blood-brain barrier). Because dietary proteins split into amino acids after digestion, they compete with the absorption of levodopa when taken together. This may lead to reduced effectiveness of the dopaminergic medication and response fluctuations [28]. People with PD are therefore advised not to take proteins (e.g., dairy products) around their medication intake. This can, however, lead to insufficient protein intake which then impacts negatively on muscle quality and function [29]. Also, diet can affect the rate of gastric emptying, which is an increasingly recognized cause of unpredictable response fluctuations, such as dose failures or delayed “on” periods [30]. Another common and vexing problem for people with PD is constipation [31, 32], which is often one of the earliest symptoms, and which often even antedates the manifestations of motor parkinsonism. Constipation also impacts the absorption and efficacy of levodopa, in part because of secondary small intestinal bacterial overgrowth (SIBO) that may lead to production of enzymes that convert levodopa into dopamine within the lumen of the gut, thereby hampering its entry into the brain [33], Conversely, appropriate nutrition (i.e., sufficient fiber and liquid consumption) plays an important role in the management of constipation and the prevention of SIBO [26, 34, 35]. But many people with PD do not eat well enough, e.g., because the food does not taste well due to hyposmia, or because constipated patients avoid meals as these may cause gastrointestinal complaints such as bloating, abdominal pain, or winds. Inadequate diet then also impacts on the nutritional status, potentially leading to underweight and malnutrition [34, 36].
Finally, nutrition may potentially have a disease modifying effect because of its impact on mitochondrial function, inflammation, and immune responses [37]. Moreover, recent research suggests that dysregulation of the gut microbiome may play a role in the pathogenesis of the disease, for example by enhancing inflammation systemically and possibly also in the central nervous system [19, 20]. This raises the exciting possibility that adequate nutrition could potentially impact on the development and progression of PD, but this proactive idea has thus far never been tested in controlled studies.
Besides some small pilot studies on different kinds of dietary patterns, i.e., the ketogenic diet [38] or Mediterranean diet [39], and studies of supplements, i.e., vitamins and antioxidants [37], the current evidence on nutrition in PD is mostly observational in nature [26, 34]. So, overall, the evidence on the merits of any PD-specific diet(s) remains inconclusive [40]. Following the example of exercise, the time is now ripe to take nutrition to the test, just as one would do when evaluating a new drug, and to do this in high quality randomized clinical trials. In this review, we will summarize all ongoing clinical trials on a nutritional intervention (dietary pattern or nutritional supplement) in PD. In doing so, we hope to offer more insights into where this field is headed and which gaps need to be filled with solid scientific research.
OVERVIEW OF RANDOMIZED CONTROLLED TRIALS ON NUTRITION
We searched Clinicaltrials.gov for interventional studies in PD with the terms “NUTRITION’’ or “DIET” and “Parkinson’s disease”. Studies were only included if their status was “not yet recruiting”, “recruiting”, or “active, not recruiting”. Studies were excluded if it was not an interventional study, a combined intervention with exercise, or including another population than people with PD (Fig. 1). This resulted in the inclusion of 11 studies. After the start of the review process, 1 study was terminated because of withdrawal of funding. This open label pilot study on the biological responses of supplementing short-chain fatty acid (BUTTER study) was therefore excluded from this paper. The general characteristics of the included 10 studies are described in Table 1.
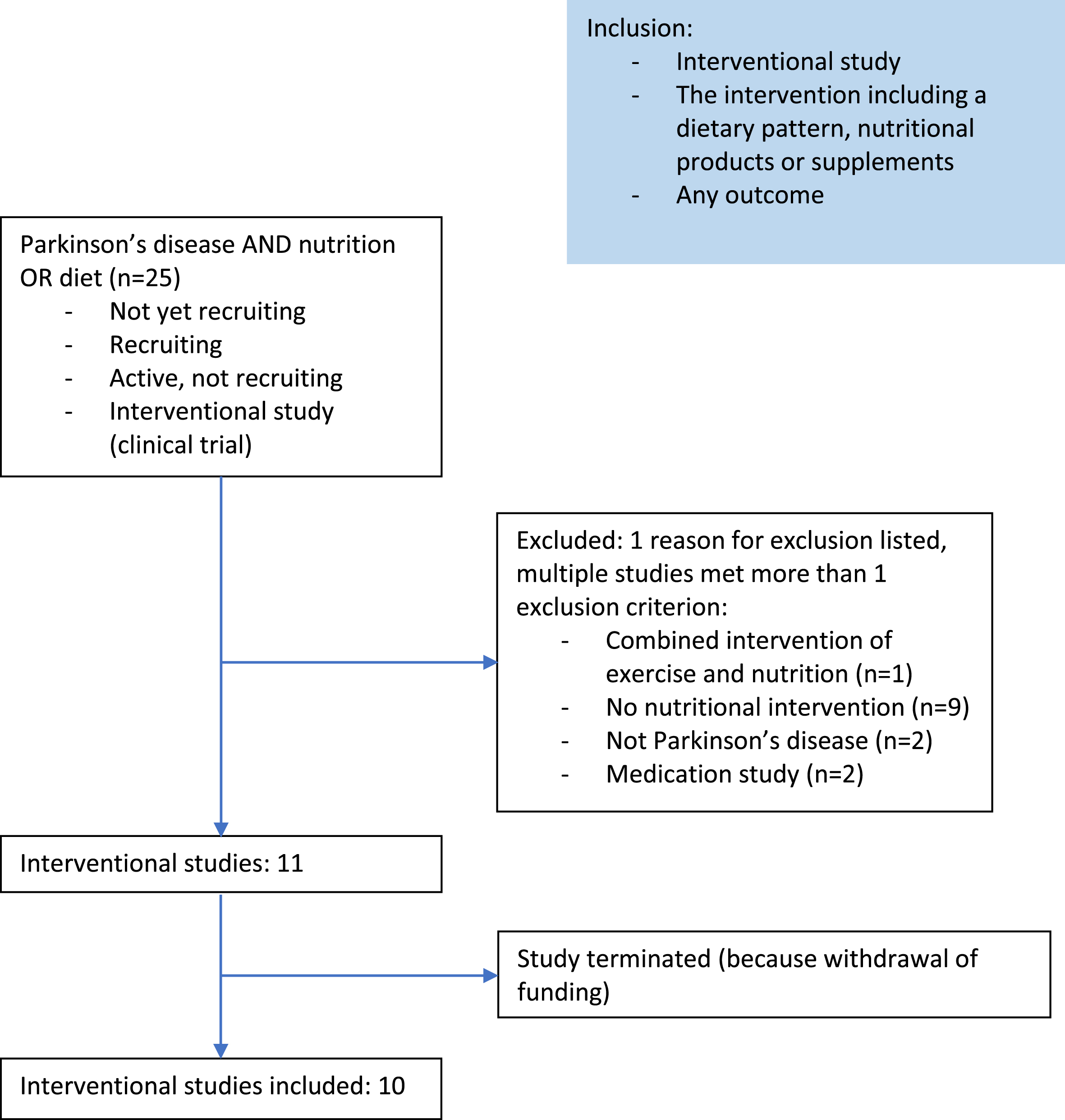
Table 1
Baseline demographics of the first 108 PwPs enrolled on the HBC pathway
| Study | Sponsor | ID | Main aim - dietary pattern, supplement, or other | Intervention | Phase | Estimated enrolment | Estimated completion date | |
| 1. | KIM | University of British Columbia | NCT05469997 | Safety – dietary pattern | Two 8-week dietary interventions (the MeDi-MCT and MeDi-KD diets) separated by an 8-week washout period. | N/A | 50 | July 2024 |
| 2. | Optimizing Protein Patterns for Skeletal Muscle Preservation and Sleep in the Medical Management of Parkinson Disease | University of Alabama at Birmingham | NCT05437640 | Clinical effects – dietary pattern | Two 2-week dietary interventions (the PCD and PRD diets) separated by a 1-week washout period (receiving one-on-one education and supportive materials). | N/A | 20 | June 30, 2024 |
| 3. | NRO | University of Florida | NCT04829760 | Safety and clinical effects – supplement (fiber) | 10 grams daily of the dietary supplement 1) psyllium or 2) coarse wheat bran or 3) maltodextrin (placebo) for 8 weeks | N/A | 79 | April 28, 2024 |
| 4. | N-DOSE | Haukeland University Hospital | NCT05589766 | Biological response - supplement (vitamin B3) | The dietary supplement Nicotinamide Riboside (NR) up to 3000 mg daily in total or a placebo tablet (no active ingredients), administered twice daily for 12 weeks. | N/A | 80 | December 31, 2024 |
| 5. | GFREEPARK | General University Hospital, Prague | NCT05238545 | Clinical effects - dietary pattern | A gluten-free diet or a regular gluten-containing diet for 12 months | N/A | 90 | December 31, 2025 |
| 6. | Brain Small Chain Fatty Acid Metabolism in Parkinson Disease: Ketones | University of Michigan | NCT05778695 | Biological response and clinical effects – supplement (ketones) | Dietary supplement: Ketone Ester (R)-3-Hydroxybutyl (R)-3-Hydroxybutyrate (KetoneAid) for 30 days. Days 1-7: 12.5 g (25 mL) and on day 8: 25 g (50 mL) three times daily (TID) as tolerated. | 1 | 30 | May 2025 |
| 7. | Tocotrienols in Parkinson’s Disease (PD) | National Neuroscience Institute | NCT04491383 | Clinical effects – supplement (vitamin E) | Tocovid Suprabio (HOV-12020) or a placebo of 200 mg, twice a day for 12 months. | 2 | 100 | December 2024 |
| 8. | Resistant Maltodextrin for Gut Microbiome in Parkinson’s Disease: Safety and Tolerability Study | Northwestern University | NCT03667404 | Safety and tolerability – supplement (carbohydrate) | Dietary supplements resistant maltodextrin (RM) powder or maltodextrin powder 25 g during days 1-7 and 50 g during days 8-28 | 2 | 30 | June 30, 2023 |
| 9. | W-Domus | Milko Zanini | NCT04983290 | Clinical effects – other | A set of foods with modified textures or their regular foods for 4 months. | N/A | 140 | May 30, 2022 |
| 10. | ExpoBiome | Andreas Michalsen | NCT04847011 | Biological response – other | A 5-10 day fasting period with a dietary energy supply 350-400 kcal per day with fruit and vegetable juices or an established fasting-mimicking diet of 600-800 kcal according to Longo et al. | N/A | 180 | May 2023 |
Population
Samples sizes vary between 20 and 180. Most studies include people with PD older than 40 years and in Hoehn and Yahr stage 1-3. Two studies are not only including people with PD, but also other neurological conditions, such as Multiple System Atrophy and Lewy Body Dementia. One study even includes people with a condition that is not neurological: Rheumatoid Arthritis.
Interventions
Three of the ongoing trials are studying a dietary pattern: the Mediterranean diet versus an adapted Mediterranean – ketogenic diet (study 1), consistent protein intake versus redistributed protein intake and a gluten free diet versus a gluten containing diet. Five different supplements are being studied (fiber, vitamin B3, ketones, vitamin E, and carbohydrate), all in early stages focusing on safety, biological responses, and preliminary clinical effects. Finally, one study evaluates the clinical effects of modified food textures for people with dysphagia and one study evaluates the biological response of fasting.
Primary and secondary outcome measures
A great variety of outcomes is included in the different studies. We see a mix of outcomes related to metabolism and the biological response to a supplement and clinical outcomes (i.e., disease symptoms and quality of life). Also, the microbiome is included as an outcome in some studies. None of the studies explicitly aims to study disease progression.
Follow-up duration
The duration of the interventions varies from 1 week to 12 months.
Blinding
Of the 10 studies included, three are performed double blind, and four single blind (1 participant blinded, 3 assessor blinded). The remaining three studies are open label studies. Two of them do not include a control group.
Compliance
Very little attention is being paid to compliance. Only two studies (on dietary patterns) mention that participants will be guided by a professional in adhering to the study regimen. Only one study mentions monitoring compliance.
IPD sharing statement
None of the studies indicated to be sharing Individual participant data (IPD).
1. Ketogenic Diet Interventions in Parkinson’s Disease: Safeguarding the Gut Microbiome (KIM)
Title: Ketogenic Diet Interventions in Parkinson’s Disease: Safeguarding the Gut Microbiome
Phase: N/A
Objective: To investigate the safety of a modified Mediterranean-ketogenic intervention that is thought to be safer than the traditional Ketogenic Diet.
Status: Not yet recruiting
Clinicaltrials.gov ID: NCT05469997
Sponsor: University of British Columbia
Collaborators: Weston Family Foundation
Estimated enrolment: 50
Estimated completion date: July 2024
Study design: A crossover study with two 8-week interventions. Participants (n=50) will be randomized into either the Mediterranean Diet- Medium-Chain Triglyceride oil (MeDi-MCT) or the Mediterranean Diet-Ketogenic Diet (MeDi-KD) group. Eight weeks of intervention will be followed by an 8-week washout period where no interventions will be applied. Subsequently, the participants will receive the other intervention for 8 weeks.
Intervention: The MeDi-MCT intervention consists of a Mediterranean diet. In addition, participants will be asked to take two daily doses of medium-chain triglyceride oil (MCT oil - Nutiva Inc. approved by Health Canada, NPN: 80086912). Participants will start the intervention by taking 5 mL of MCT oil twice daily for the first day and then gradually increase the dose to 14 g by the end of week 1. When tolerated, doses may increase to 20 mL of MCT twice daily.
Participants in the MeDi-KD group will receive a modified Mediterranean-ketogenic diet. The ketogenic component of the diet includes limiting the intake of carbohydrates to about 10% of all calories consumed per day. The ketogenic ratio (the ratio of fat to carbohydrates) will be gradually increased during the first week from 1:1 to 3:1. The Mediterranean component of this dietary pattern includes consuming green leafy vegetables, nuts, and olive oil, while limiting the consumption of processed or fried food, red meat, full-fat dairy, and sweets.
Outcome measures: Primary: Between group differences after each 8-week intervention in: 1) measures of fecal and serum calprotectin, and 2) biomarkers of gut health, i.e., short-chain fatty acid production, gut-barrier integrity, and microbial composition.
Secondary: Retention and adherence. Retention will be measured by the number of participants who complete the study. Adherence is quantified by the percentage of time spent in clinically relevant levels of ketosis (>0.5 mmol/L) by each participant throughout each intervention, as measured using daily breath ketone analyses.
Other outcomes: Changes from baseline after each intervention in: motor and non-motor PD symptoms (MDS-UPDRS; Movement Disorders Society-Unified Parkinson’s Disease Rating Scale), fatigue (Fatigue Severity Scale), apathy (Starkstein Apathy Scale), depressive symptoms (Beck Depression Inventory II), anxiety (Parkinson’s Anxiety Scale (PAS)), quality of life (Parkinson’s disease questionnaire-39), capacity for performing physical activities (Physical Activity Scale for Individuals with Physical Disabilities), stool consistency (Bristol Stool Chart), constipation and irritable bowel symptoms (Rome III module).
Dietary habits before the study are measured using the Canadian version of the Diet History questionnaire. Cognitive function is measured using the National Health Institute Toolbox-Cognitive battery (NIHTB-CB)).
Comments: This open label, crossover study will give important insights in the safety of and adherence to the Mediterranean diet and an adjusted ketogenic diet. Even though both interventions are well described and a measure of adherence is included, it remains unclear how the interventions are delivered and how participants are motivated to adhere to the diet under study. While many exploratory outcomes on potential effects will be measured, this study is not designed to draw conclusions on effectiveness.
Results: The estimated study completion date is July 2024
References: -
2. Optimizing Protein Patterns for Skeletal Muscle Preservation and Sleep in the Medical Management of Parkinson Disease
Title: Optimizing Protein Patterns for Skeletal Muscle Preservation and Sleep in the Medical Management of Parkinson Disease
Phase: N/A
Objective: To study the preliminary impact of dietary protein patterns on markers of skeletal muscle health and drug efficacy in Parkinson disease.
Status: Recruiting
Clinicaltrials.gov ID: NCT05437640
Sponsor: University of Alabama at Birmingham
Collaborators: No information provided
Estimated enrolment: 20
Estimated completion date: June 30, 2024
Study design: This study is single-blinded (outcomes assessor) crossover intervention study. Eligible people with PD (n=20) are randomly assigned to either the Protein Consistent Diet (PCD) or the Protein Redistribution Diet (PRD) for 2 weeks. This period is followed by a 1-week washout period and then the other diet will be followed for another 2 weeks. Outcome measures will be assessed at days 0, 14, 21, and 35.
Intervention: The PRD-diet consists of consuming 10 grams or less of protein until the evening meal. The evening meal will be high in proteins to meet the protein needs (as determined by a dietician). The PCD-diet incudes instructions to consume 20-30 grams of protein per meal. Participants will receive one-on-one education by a registered dietician and will receive diet prescriptions and meal plans.
Outcome measures: Primary: Change from baseline to 5-week follow-up in markers of skeletal muscle metabolism: Serum Growth Differentiation Factor 15 and Serum Fibroblast Growth Factor 21 (GDF15 and FGF21), handgrip strength (digital dynamometer), sleep efficiency (actigraphy), and motor symptoms (MDS-UPDRS part II).
Secondary: Change from baseline to 5-week follow-up in physical activity (actigraphy) and total PD symptoms (total MDS-UPDRS score).
Comments: This is a small open label crossover study which defined five primary outcomes. It seems like a comparison is being made between baseline and follow-up and no specific between group comparisons are being made. It is unclear how the two interventions will be compared. Moreover, the study includes very short interventions of 2 weeks and only a 1 week wash out period.
Results: The estimated study completion date is June 30, 2024
References: -
3. The Effect of Psyllium and Wheat Bran on Body Weight in People with Parkinson’s Disease and Constipation Symptoms (NRO)
Title: The Effect of Psyllium and Wheat Bran on Body Weight in People with Parkinson’s Disease and Constipation Symptoms (NRO)
Phase: N/A
Objective: Comparing the safety and efficacy of two common fiber supplements, psyllium and wheat bran on changes in body weight, nutrition status, and bowel function in people with PD with constipation.
Status: Recruiting
Clinicaltrials.gov ID: NCT04829760
Sponsor: University of Florida
Collaborators: No information provided
Estimated enrolment: 79
Estimated completion date: April 28, 2024
Study design: A single-blinded (participant) randomized controlled trial with 3 parallel groups. After a 2-week run-in period, participants (n=79) will be randomized to receive 10 grams daily of 1) psyllium, 2) coarse wheat bran, or 3) maltodextrin (placebo) for 8 weeks. Assessments will be performed at the beginning and end of the 8-week intervention period.
Intervention: Participants will consume two doses of their allocated supplement per day for 8 weeks. The supplement can be added to food participants normally eat or beverages they drink (psyllium and maltodextrin).
Outcome measures: Primary: Body weight at 8 weeks.
Secondary: Digestive health (Gastrointestinal Symptom Rating Scale), laxative use, stool frequency, stool consistency (Bristol Stool Form Scale), body composition (bioelectrical impedance spectroscopy), appetite (Council on Nutrition Appetite Questionnaire (CNAQ), nutrition risk (Patient Generated- Subjective Global Assessment (PG-SGA)), constipation-related Quality of Life (Patient Assessment of Constipation- Quality of Life questionnaire), non-motor symptoms (Non-Motor Symptom Scale for Parkinson’s Disease (NMSS)), Parkinson’s disease related Quality of Life (Parkinson’s disease Questionnaire 39 (PDQ-39)), handgrip strength (dynamometer).
Other outcomes: changes in quality of life related to digestion (Digestion-associated quality of life questionnaire), stress (10-point severity Likert scale), physical activity (International Physical Activity Questionnaire).
Comments: This single-blind placebo-controlled study will give valuable information on supplements to treat constipation in people with PD. Unfortunately, body weight was selected as primary outcome, instead of constipation. In addition, PD motor symptoms are not taken into account. Because constipation may impact levodopa efficacy, an effect on motor symptoms may be expected. Finally, it is not clear how adherence is assessed.
Results: The estimated study completion date is April 28, 2024
References: -
4. N-DOSE: A Dose Optimization Trial of Nicotinamide Riboside in Parkinson’s Disease
Title: N-DOSE: A Dose Optimization Trial of Nicotinamide Riboside in Parkinson’s Disease
Phase: N/A
Objective: Determining the optimal biological dose of nicotinamide riboside (NR) to achieve maximal cerebral nicotinamide adenine dinucleotide (NAD) levels, or maximal expression increase in Nicotinamide Riboside Related Pattern (NRRP), or maximal proportion of magnetic resonance spectroscopy MRS-responders, in the absence of unacceptable toxicity.
Status: Recruiting
Clinicaltrials.gov ID: NCT05589766
Sponsor: Haukeland University Hospital
Collaborators: No information provided
Estimated enrolment: 80
Estimated completion date: December 31, 2024
Study design: This is a 12- week randomized double-blinded (participant and investigator) placebo-controlled study. Individuals with PD (n = 80) will be randomized in a 1:1:2 manner to either placebo group (n = 20), 1000 mg of NR daily (n = 20) or to a dose-escalation group where NR 1000 mg daily will be administered during week 1-4, NR 2000 mg daily during week 5-8 and NR 3000 mg daily during week 9-12 (n = 40). Outcomes will be assessed at baseline, 4 weeks, 8 weeks, and 12 weeks. Cerebrospinal fluid will be collected at baseline and 12 weeks.
Intervention: All participants receive a dietary supplement: NR up to 3000 mg daily in total or a placebo tablet with no active ingredients. NR and placebo are identical in taste, form, and appearance. All supplements are administered twice daily for 12 weeks.
Outcome measures: Primary: The between-visit differences in cerebral NAD levels (31P-Magnetic resonance spectroscopy), CSF NAD and related metabolite levels (HPLC-MS metabolomics, or the NADmed method), expression of the Nicotinamide Riboside Related Pattern (NRRP) (FDG-PET) and the proportion of MRS responders (Defined as participants displaying significant increase in the Nicotinamide Riboside Related Pattern (NRRP) on FDG-PET.).
Secondary: Frequency and severity of adverse events, changes in health-related quality of life (EuroQol 5L), NAD-metabolites in whole blood and CSF (HPLC-MS and the NADmed method), Non-motor symptoms (MDS-NMS), cognition (MoCA), gastrointestinal dysfunction (modified GIDS-PD), PD symptoms (MDS-UPDRS).
Other outcomes: Change in gene and protein expression levels related to lysosomal and proteasomal function (RNA sequencing), proteomics (LC-MS)), levels of one carbon metabolism metabolites (HPLC-MS metabolomics in whole blood and CSF), levels of monoamine neurotransmitters in CSF, genomic distribution of DNA methylation and levels of DNA methylation (Illumina Infinium MethylationEPIC Kit), levels of histone acetylation and genomic distribution of histone acetylation (immunoblotting and chromatin immunoprecipitation sequencing (ChIPseq)), gut microbiome composition (assessed by metagenomics in fecal samples), fecal metabolomics and levels of inflammatory cytokines in serum and CSF (ELISA method).
Comments: This is a well-designed placebo-controlled and double-blinded study to investigate the optimal dosing of NR supplements, which has already been studied on tolerability and safety. Adherence and how this is monitored is not described. Multiple primary endpoints are selected. While this study will give insights in the biological response to NR treatment, which is hypothesized to have a disease modifying potential, clinical effects need to be further studied.
Results: The estimated study completion date is December 31, 2024
References: -
5. The Effect of Gluten-free Diet on Parkinsonism (GFREEPARK)
Title: The Effect of Gluten-free Diet on Clinical Symptoms, Immune Parameters and Metabolome in Neurodegenerative Diseases with Alfa-synucleinopathy (PD And multiple system atrophy- MSA)
Phase: N/A
Objective: Testing the effect of gluten-free diet in people with PD and MSA. Another research line studies the effects in an animal model.
Status: Recruiting
Clinicaltrials.gov ID: NCT05238545
Sponsor: General University Hospital, Prague
Collaborators: Czech Academy of Sciences
Estimated enrolment: 90
Estimated completion date: December 31, 2025
Study design: A randomized controlled rater-blinded study comparing a gluten-free diet group with a regular gluten-containing diet group. Assessments are performed at baseline, and at 1, 5, 3, 6, 9, 12, and 13 months follow-up.
Intervention: Participants in the gluten-free diet groups avoid foods containing gluten, participants in the other group are not restricted.
Outcome measures: Primary: Change in severity of the clinical symptoms (MDS-UPDRS for PD, and UMSARS for MSA).
Secondary: Change in cognition (Montreal Cognitive Assessment), severity of autonomic symptoms (Autonomic Scale for Outcomes in Parkinson’s Disease), spatio-temporal parameters of the gait (investigated by GaitRite system), quality of sleep (Pittsburgh Sleep Quality Index), mood (Beck depression inventory), change in quality of life (Quality of life questionnaire).
Comments: This study includes both people with PD and MSA. It is unclear how many people of both populations will be included. In addition, no description is given on how participants will be guided in adhering to a gluten-free diet, nor how adherence will be monitored. This also applies to the control group for which it is unclear what their diet exactly contains; having no restrictions and no blinding leads to a risk of contamination. Follow-up measurements are performed until 13 months after the start of the intervention, which is a substantial period of time, and adequate for the primary endpoint; clinical disease symptoms. It has not been clearly described whether the intervention is also performed for 12 months.
Results: The estimated study completion date is December 31, 2025
References: -
6. Brain Small Chain Fatty Acid Metabolism in Parkinson Disease: Ketones
Title: Brain Small Chain Fatty Acid Metabolism in Parkinson Disease: Ketones
Phase: 1
Objective: To investigate the effects of a ketone ester (KetoneAid) on glucose metabolism, cognitive functioning, clinical functioning, and imaging in people with PD and Parkinson’s Disease Dementia (PDD), Lewy Body Dementia (LBD) and healthy controls.
Status: Recruiting
Clinicaltrials.gov ID: NCT05778695
Sponsor: University of Michigan
Collaborators: Farmer Family Foundation
Estimated enrolment: 30
Estimated completion date: May 2025
Study design: This is a small exploratory non-randomized open-label pilot study. Participants (n=30) will take the ketone ester supplement for 30 days +/- 7 days. For days 1 through 7, participants will take 12.5 g (25 mL) of the ketone ester supplement (KetoneAid) TID, and on day 8, the dose will be increased to 25 g (50 mL). The supplement will be taken three times daily as tolerated. Ketone Ester dose will be titrated down to a tolerated level if necessary.
Intervention: Participants take a dietary supplement: Ketone Ester (R)-3-Hydroxybutyl (R)-3-Hydroxybutyrate (KetoneAid) for 30 days.
Outcome measures: Primary: Average glucose metabolism (measured via continuous glucose monitor), Fluorodeoxyglucose (FDG) positron emission tomography (PET) brain and Clinical Dementia Rating scale score.
Secondary: Root mean square of center of pressure (COP) during the instrumented Sway test (iSWAY) eyes open condition, cognition (Montreal Cognitive Assessment), mobility (Timed Up and Go Test), balance (Mini Balance Evaluation Systems test), gait speed (Protokinetics Zeno™ Walkway), cognition (Wechsler Adult Intelligence Scale III and IV (WAIS-III, WAIS IV) Digit Symbol Coding test, Parkinson’s Disease (PD)-Cognitive Rating Scale, Boston Naming Test, California Verbal Learning Test-II, Stroop Color Word Interference Test, Delis-Kaplan Executive Function System (DKEFS) Trail Making Test, DKEFS Sorting, Digit Span test, Matrix Reasoning test, Benton Judgement of Line Orientation scores, the Controlled Oral Word Association or “FAS” test, reaction time test.
Comments: This is also a small phase 1 study without a control group. The study includes different targe populations, i.e., people with PD, PDD, LBD, and healthy controls. The study includes many secondary cognitive outcomes. Positive findings may support future target engagement studies of ketone supplementation in PD.
Results: The estimated study completion date is May 2025
References:
7. Tocotrienols in Parkinson’s Disease (PD)
Title: Tocotrienols in Parkinson’s Disease (PD): A Pilot, Randomized, Placebo-controlled Trial
Phase: 2
Objective: Investigating the effects of tocotrienols (HOV-12020) on motor and non-motor symptoms in people with PD.
Status: Recruiting
Clinicaltrials.gov ID: NCT04491383
Sponsor: National Neuroscience Institute
Collaborators: Hovid Berhad
Estimated enrolment: 100
Estimated completion date: December 2024
Study design: This randomized placebo-controlled double-blind trial comparing oral tocotrienols (400 mg/day) or placebo for 52 weeks. Assessments (questionnaires) will be performed at baseline, at week 52 and week 104. Additional PD staging using MDSUPDRS (Part III) and Hoehn & Yahr (H&Y) will be conducted at Week 26 and week 78. Blood samples will be collected to evaluate PD biomarkers and for safety monitoring (liver function, renal function, and hematology).
Intervention: Participants will either be given the supplement Tocovid Suprabio (HOV-12020) or a placebo of 200 mg, twice a day for 12 months.
Outcome measures: Primary: Mean change from Baseline to Week 104 in MDS-UPDRS.
Secondary: Mean change from baseline to week 104 in disease severity, individual cognitive domain z scores on comprehensive neuropsychological testing, MDS-UPDRS total score, quality of life (Parkinson’s Disease Questionnaire-39), MDS-UPDRS part II and III, mean change in levels of blood-based biomarkers (including total antioxidant status TAS, oxidative stress biomarkers and αsynuclein), difference of type and incidence of Adverse Events (AEs) and Serious AEs, Schwab and England Activities of Daily Living (SE-ADL) scale.
Comments: This is an interesting phase II study, evaluating the clinical effects of a dietary supplement over a long period of time in a relatively large sample. In addition to the clinical outcomes, also blood biomarkers are taken in order to gain insight in biological working mechanisms. It is unclear how adherence to the supplement and general nutritional intake is monitored.
Results: The estimated study completion date is December 2024
References: -
8. Resistant Maltodextrin for Gut Microbiome in Parkinson’s Disease: Safety and Tolerability Study
Title: Gut Microbial Remodeling with Resistant Maltodextrin for Motor and Non-motor Symptoms in Parkinson’s Disease: Safety and Tolerability Study
Phase: 2
Objective: To examine the safety and tolerability of resistant maltodextrin (RM), a prebiotic non-digestible fiber, and its effect on the microbiome and motor in non-motor symptoms and PD.
Status: Active, not recruiting
Clinicaltrials.gov ID: NCT03667404
Sponsor: Northwestern University
Collaborators: University of Illinois at Chicago
Estimated enrolment: 30
Estimated completion date: June 30, 2023
Study design: A randomized, parallel-group double-blinded (participants and investigators) controlled trial comparing resistant maltodextrin (RM) to maltodextrin (an easily digestible glucose polysaccharide) over 4 weeks.
Intervention: Participants will either receive 25 g of resistant maltodextrin (RM) powder or maltodextrin powder during days 1-7 and 50 g during days 8-28. Every morning, the supplement is dissolved in 8 oz of water.
Outcome measures: Primary: Adverse events (based on diary reports, phone calls and in-person assessments).
Secondary: Gut microbial remodeling (change in fecal butyrate-producing bacteria based on high-throughput amplicon sequencing of the V4 variable region of the microbial 16s ribosomal ribonucleic acid (RNA).
Comments: This phase 2 study will inform us on the safety and tolerability of resistant maltodextrin. It will also give some preliminary insights in its impact on gut microbial remodeling. It is a pity that no clinical outcomes are investigated.
Results: The estimated study completion date was June 30, 2023
References:
9. Outcomes to the Nutritional Need of Patients with Parkinson’s Disease (W-Domus)
Title: Clinical Trial to Assess the Effectiveness of the Food Plan Consisting of Products with Modified Consistency Called (Weancare-Domus) in Changing the Quality of Life in Patients With Parkinson’s Disease
Phase: N/A
Objective: To improve the nursing-care management and to prevent nutritional deficits in people with PD with dysphagia.
Status: Not yet recruiting
Clinicaltrials.gov ID: NCT04983290
Sponsor: Milko Zanini
Collaborators: Azienda Sanitaria Locale 3 Genovese
Estimated enrolment: 140
Estimated completion date: May 30, 2022
Study design: This is a randomized controlled cross-over trial comparing a food plan with modified consistency (Weancare-Domus) with diet as usual during 4 months.
Intervention: Participants in the intervention group will receive a set of foods with modified textures for the complete 4-month period. They will be followed by personnel form the dietary service to verify compliance. The control group will continue with its own feeding for the entire observation period of the experimental group.
Outcome measures: Primary: change in non-motor symptoms (Novel Non-Motor Symptoms Scale for Parkinson’s Disease - NNMS).
Secondary: Suction and ab-ingestis events related to textured food change (Novel Non-Motor Symptoms Scale for Parkinson’s Disease - Domain 6: Gastrointestinal tract).
Other: Functional parameters related to malnutrition, gait speed, grip strength, muscle mass, Body Mass Index (BMI), arm circumference, biomarkers (plasma cholinesterase concentration (U/ml), plasma transferrin concentration (mg/dL), plasma albumin concentration (g/dL), lymphocyte count in 1 microliter (µL) of blood), nutritional status evaluation (measured by phase angle and derived body composition data and Mini Nutritional Assessment Score (MNA) score) and bolus transit time evaluation.
Comments: This is a large study including 140 people with PD and dysphagia. The study is single blinded, and it is unclear whether this is a parallel group comparison study or a crossover trial. Compliance in the intervention group is verified but is not operationalized. In addition, the primary outcome may not be the most appropriate outcome since the study aims to prevent nutritional deficits.
Results: The estimated study completion date was May 30, 2022
References:
10. Deciphering the Impact of Exposures from the Gut Microbiome-derived Molecular Complex in Human Health and Disease (ExpoBiome)
Title: Deciphering the Impact of Exposures from the Gut Microbiome-derived Molecular Complex in Human Health and Disease
Phase: N/A
Objective: To study the impact of fasting on clinical functioning, the immune system and gut microbiota in people with PD or rheumatoid arthritis (RA).
Status: Recruiting
Clinicaltrials.gov ID: NCT04847011
Sponsor: Andreas Michalsen
Collaborators: Luxembourg Centre for Systems Biomedicine and Paracelsus-Elena-Klinik Kassel
Estimated enrolment: 180
Estimated completion date: May 2023
Study design: This is a non-randomized open-label clinical trial. First, a quantitative, integrated multi-omics analysis will be performed. Then, people with PD and RA will be assigned to the intervention or control group. The intervention is performed for 5-11 days. Healthy controls will not be given an intervention. Outcome assessments are performed at 12-month follow-up.
Intervention: Participants (n=180) with PD or RA will either have no intervention or they will undergo a 5-10 day fasting period with a dietary energy supply 350-400 kcal per day with fruit and vegetable juices or, if not feasible, an established fasting-mimicking diet of 600-800 kcal. There is also a healthy control group receiving no intervention.
Outcome measures: Primary: Gut microbiota Characterization (via molecular typing of the gut microbiota using sequencing and high-throughput analysis from stool samples (metagenomics, meta transcriptomics, metaproteomic, metabolomics)).
Secondary: Resting blood pressure, heart rate, abdominal circumference, Waist to Hip Ratio, Body Mass Index (kg/m2), Disease Activity Score 28, Health Assessment Questionnaire (HAQ), Simplified Disease Activity Index Score (SDAI), Hannover Functional Ability Questionnaire (Funktionsfragebogen Hannover, FFbH-R), MDS-UPDRS, Parkinson’s Disease Sleep Scale-2 (PDSS-2), Parkinson’s Disease Questionnaire-39 (PDQ-39), Non-motor symptoms questionnaire (NMSQ), Non Motor Symptoms Scale (NMSS), Stress questionnaire (Cohen Perceived Stress Scale, CPSS), Quality of Life questionnaire (WHO-5), Hospital Anxiety and Depression Scale (HADS), Mood questionnaire (Profile of Mood States, POMS). [Change over baseline to 12 months] Sociodemographic Measurements. [Baseline] Behavioral Factors, such as: Physical inactivity, coffee, health promoting activities, alcohol consumption and smoking status. Dietary behavior, expectation questions, differential blood count, Hepatic transaminases (GPT, GOT) and Gamma glutamyl transpeptidase (y-GT), Bilirubine (total, direct, indirect in mg/dL), Total protein in grams per liter (g/L), Albumine in grams per liter (g/L), Creatinine in µmol per liter (µmol/L), estimated glomerular filtration rate (eGFR) in milliliter per minute (mL/min), Alkaline Phosphatase in units per liter (U/L), Urea in milligrams per deciliter (mg/dL), blood lipids and fasting glucose (triglycerides (mmol/L), total cholesterol (mmol/L), LDL (mmol/L), HDL (mmol/L), fasting glucose (mmol/L)), HbA1C (mmol/mol Hb, %), TSH (mU/L), IGF-1 (ng/mL), Insulin (mU/L), High sensitive CrP (mg/L), Rheumatoid factor (RF, IgM) (U/mL), Anti-cyclic citrullinated peptide (ACPA) (U/mL), Zonulin (ng/mL), Fatty acid binding protein 2 (FABP2) (pg/mL), Plasma Calprotectin (µg/g), Fecal Calprotectin (µg/g), Phenotyping of immune cells, Urine metabolomics (10 ml midstream urine) and oral microbiota analysis in saliva.
Comments: This is an open label and non-randomized study. The sample is quite large but includes three different populations: people with PD, people with RA, and healthy controls. How treatment allocation takes place and whether the first phase of immunophenotyping plays a role in allocation is unclear. Outcomes are assessed at 12-month follow-up, while the intervention only lasts for 5-10 days. While the study will give insights in the microbiome and longitudinal changes, drawing conclusions on the effects of fasting will probably be difficult.
Results: The estimated study completion date was May 2023
References: -
DISCUSSION
This review shows that research on dietary interventions in PD is still in its early days. We found a limited number of 10 ongoing phase I, phase II, or even more exploratory trials (i.e., open label, no control group). These studies will not be able to definitively answer critical questions about effectiveness but are expected to further shape our ideas about which dietary patterns or supplements may be worth investigating in a more rigorous way. In addition, most of these studies will look into the working mechanisms and biological responses which will offer essential information on the potential of disease modification induced by nutritional supplements. Unfortunately, very little attention is being paid to compliance, which can be quite a challenge for nutritional interventions.
The ongoing trials all investigate a different dietary pattern or nutritional supplement. Considering the dietary patterns, the Mediterranean diet and ketogenic diet are popular and have been subject of previous studies, but without conclusive evidence [26, 41]. Observational studies previously suggested that the Mediterranean diet is associated with a delay in disease onset [42] and a reduction in disease progression [37]. A previous randomized controlled trial showed that the Mediterranean diet positively affected executive functioning, attention, language, and memory [39]. The Mediterranean diet is further thought have an anti-inflammatory effect, to reduce oxidative stress, C reactive protein, and fasting insulin, and to improve gut microbiota [26]. The exact mechanisms have, however, not been elucidated. Current work will not help to unravel these potential neurobiological effects since only one study on the Mediterranean diet is currently being performed (study 1). This study primarily aims to assess the safety and adherence of this intervention. Moreover, this study combines the Mediterranean diet with a ketogenic diet. The ketogenic diet is based on a high percentage of lipids and a low percentage of carbohydrates and proteins. A concern of the ketogenic diet is that it reduces appetite and carries a risk of malnutrition. It is also difficult diet to adhere to in the long term because of the effort it requires to prepare meals and the consequences related to the social aspects of eating together. A pilot randomized controlled trial showed that it is safe for people with PD to follow a ketogenic diet for 8 weeks. The diet also improved non-motor symptoms [43]. A combination with the Mediterranean diet, as is currently being studied, may be a more attractive alternative on the long term. This new study (study 1, Table 1) will give insights into the safety, adherence, and retention of this combined dietary pattern. Other ways to reach a ketogenic state is by supplementation of ketones (ongoing study 11) or by fasting (ongoing study 6). As such, this ongoing work will give more insights into the biological mechanisms of these two options. None of these studies is primarily aiming to study the effectiveness of ketogenesis. The other dietary pattern currently being studied is a gluten free diet (study 5, Table 1), which has not been studied before in PD.
Among the supplements, we found studies supplementing vitamin B3, vitamin E, carbohydrate, and fiber. While the additional value of vitamin intake in PD remains controversial, some vitamins have been proposed to have a beneficial effect. Vitamin B3 is hypothesized to have a neuroprotective effect through multiple mechanisms, e.g., an anti-inflammatory effect, oxidative stress reduction, and perhaps dopamine suppletion [44]. In an open label effectiveness trial, 12 months of daily 250 mg vitamin B3 supplementation resulted in reduced PD motor symptoms (primary outcome), increased handwriting size, mood, and postural control and decreased fatigue [45]. The ongoing study included here (study 4, Table 1) is a double-blind placebo-controlled study, which uses a much higher dosage (of up to 3000 mg per day) than in the previous study aiming to investigate optimal dosage and safety. Vitamin E has also been shown to have some positive effects on disease symptoms [46] and on the inflammatory profile in small sample of people with PD [47]. The large DATATOP study in the 90’s, however, in which deprenyl and vitamin E were provided, did not show a potential for disease modification [48-50]. The study included here (study 7, Table 1) is a phase II study including a larger cohort and a longer follow-up period. Importantly, this study will evaluate clinical as well as mechanistic outcomes. There is little evidence on the potential role of macronutrients (carbohydrates and fats) in the development and progression of PD [26]. Study 8 will give some preliminary evidence on supplementing carbohydrates. Interestingly, we did not find any ongoing clinical trial on for example caffeine (despite its fairly consistent negative association with the risk of developing PD), minerals, proteins or flavonoids, for all of which associations with PD have been found previously [18, 26, 51].
At this point, no specific recommendations on diet or supplements can be given based on rigorous scientific research. However, healthy nutrition in general is important for everyone, including people with PD who improve their functioning by reducing obstipation (thereby increasing levodopa efficiency) and by preventing malnutrition, deficiencies, and weight loss. Two ongoing studies specifically aim at reducing obstipation (study 3) and preventing malnutrition by offering food with different textures to people with dysphagia (study 10). A study that would be of great interest is to evaluate the effect of offering personalized guidance by an expert dietician to people with PD, giving them individualized advice tailored to their usual food intake and diet-related symptoms or problems (e.g., obstipation, timing of protein intake). Although this type of intervention is not intended to alter the course of the disease, it could potentially have an enormous clinical effect because of the various symptomatic improvements [41].
The current overview shows that various different nutritional interventions are being studied, mainly with respect to their biological response, safety and preliminary clinical effects. These are important steps that must be taken before moving towards larger and more rigorously designed clinical trials, which come with great challenges because of numerous methodological considerations. A first challenge relates to how a dietary intervention is delivered. Especially when a dietary pattern is being studied, the options of ‘feeding’ (i.e., providing all foods or ingredients during the study period) or ‘dietary counseling’ both have advantages and disadvantages. Especially when counseling is performed, the exact dosing becomes more difficult to monitor and tracking compliance will be challenging. Feeding, on the other hand, is more expensive, certainly for the undoubtedly large-scale studies that will be necessary, and also not realistic when considering future implementation strategies in real life [52]. For studies evaluating a supplement, adherence is less complicated, but still challenging. It is also easier to design a placebo intervention when testing supplements, thus ascertaining blinding, while this is practically impossible when studying a dietary pattern. Only one of the studies included in this overview clearly indicated how adherence will be monitored (study 1). Surprisingly, none of the studies explicitly stated how they will register dietary intake which may obviously be a serious confounder in this type of study [52].
While there is increasing evidence that diet could play an important role in the development, progression and management of clinical manifestations of person with PD. Diet may also empower PwPD in self-management. The field clearly needs more high-quality research. This is not an easy task, given the great variety in potentially important diets and nutritional supplements, the great number of possible working mechanisms and the many methodological challenges related to studying nutrition. We expect that the ongoing studies reviewed here will result in valuable information that can help to design future clinical trials. Research on diet and nutrition in PD is still in its’ early days but holds great promise and opportunities for the future.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The Center of Expertise for Parkinson & Movement Disorders was supported by a center of excellence grant of the Parkinson’s Foundation.
NmdV reports grants from The Netherlands Organisation for Health Research and Development (ZonMw), The Michael J Fox Foundation, and Verily Lifes Sciences.
CONFLICT OF INTEREST
BRB has received honoraria from serving on the scientific advisory board for AbbVie, Biogen, and UCB; has received fees for speaking at conferences from AbbVie, Zambon, Roche, GE Healthcare, and Bial; and has received research support from the Netherlands Organization for Scientific Research, The Michael J. Fox Foundation, UCB, AbbVie, the Stichting Parkinson Fonds, the Hersenstichting Nederland, the Parkinson’s Foundation, Verily Life Sciences, Horizon 2020, the Topsector Life Sciences and Health, the Gatsby Foundation, and the Parkinson Vereniging. BRB currently serves as co-Editor-in-Chief of this journal but was not involved in the peer-review process nor had access to any information regarding its peer review. He also serves on the editorial board of Practical Neurology and Digital Biomarkers.
NMdV is an Editorial Board member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer review.
All other authors have no conflict of interest to report.
REFERENCES
[1] | Bloem BR , Okun MS , Klein C ((2021) ) Parkinson’s disease. Lancet 397: , 2284–2303. |
[2] | McFarthing K , Buff S , Rafaloff G , Dominey T , Wyse RK , Stott SRW ((2020) ) Parkinson’s disease drug therapies in the clinical trial pipeline: 2020. J Parkinsons Dis 10: , 757–774. |
[3] | McFarthing K , Rafaloff G , Baptista M , Mursaleen L , Fuest R , Wyse RK , Stott SRW ((2022) ) Parkinson’s disease drug therapies in the clinical trial pipeline: 2022 update. J Parkinsons Dis 12: , 1073–1082. |
[4] | McFarthing K , Rafaloff G , Baptista MAS , Wyse RK , Stott SRW ((2021) ) Parkinson’s disease drug therapies in the clinical trial pipeline: 2021 update. J Parkinsons Dis 11: , 891–903. |
[5] | Bloem BR , de Vries NM , Ebersbach G ((2015) ) Nonpharmacological treatments for patients with Parkinson’s disease. Mov Disord 30: , 1504–1520. |
[6] | Radder DLM , Ligia Silva de Lima A , Domingos J , Keus SHJ , van Nimwegen M , Bloem BR , de Vries NM ((2020) ) Physiotherapy in Parkinson’s disease: a meta-analysis of present treatment modalities. Neurorehabil Neural Repair 34: , 871–880. |
[7] | Ernst M , Folkerts AK , Gollan R , Lieker E , Caro-Valenzuela J , Adams A , Cryns N , Monsef I , Dresen A , Roheger M , Eggers C , Skoetz N , Kalbe E ((2023) ) Physical exercise for people with Parkinson’s disease: a systematic review and network meta-analysis. Cochrane Database Syst Rev 1: , CD013856. |
[8] | Amara AW , Wood KH , Joop A , Memon RA , Pilkington J , Tuggle SC , Reams J , Barrett MJ , Edwards DA , Weltman AL , Hurt CP , Cutter G , Bamman MM ((2020) ) Randomized, controlled trial of exercise on objective and subjective sleep in Parkinson’s disease. Mov Disord 35: , 947–958. |
[9] | Cusso ME , Donald KJ , Khoo TK ((2016) ) The impact of physical activity on non-motor symptoms in Parkinson’s disease: a systematic review. Front Med (Lausanne) 3: , 35. |
[10] | Schootemeijer S , van der Kolk NM , Bloem BR , de Vries NM ((2020) ) Current perspectives on aerobic exercise in people with Parkinson’s disease. Neurotherapeutics 17: , 1418–1433. |
[11] | Gamborg M , Hvid LG , Dalgas U , Langeskov-Christensen M ((2022) ) Parkinson’s disease and intensive exercise therapy - An updated systematic review and meta-analysis. Acta Neurol Scand 145: , 504–528. |
[12] | Li JA , Loevaas MB , Guan C , Goh L , Allen NE , Mak MKY , Lv J , Paul SS ((2023) ) Does exercise attenuate disease progression in people with Parkinson’s disease? A systematic review with meta-analyses. Neurorehabil Neural Repair 37: , 328–352. |
[13] | Petzinger GM , Fisher BE , McEwen S , Beeler JA , Walsh JP , Jakowec MW ((2013) ) Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol 12: , 716–726. |
[14] | Jang Y , Koo JH , Kwon I , Kang EB , Um HS , Soya H , Lee Y , Cho JY ((2017) ) Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson’s disease mice. Brain Res 1655: , 186–193. |
[15] | Johansson ME , Cameron IGM , Van der Kolk NM , de Vries NM , Klimars E , Toni I , Bloem BR , Helmich RC ((2022) ) Aerobic exercise alters brain function and structure in Parkinson’s disease: a randomized controlled trial. Ann Neurol 91: , 203–216. |
[16] | Yoon SY , Suh JH , Yang SN , Han K , Kim YW ((2021) ) Association of physical activity with all-cause mortality in Parkinson’s disease: Importance of total amount and maintenance. JAMA Neurol 78: , 1446–1453. |
[17] | Tsukita K , Sakamaki-Tsukita H , Takahashi R ((2022) ) Long-term effect of regular physical activity and exercise habits in patients with early Parkinson disease. Neurology 98: , e859–e871. |
[18] | Ascherio A , Schwarzschild MA ((2016) ) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15: , 1257–1272. |
[19] | Kleine Bardenhorst S , Cereda E , Severgnini M , Barichella M , Pezzoli G , Keshavarzian A , Desideri A , Pietrucci D , Aho VTE , Scheperjans F , Hildebrand F , Weis S , Egert M , Karch A , Vital M , Rübsamen N ((2023) ) Gut microbiota dysbiosis in Parkinson disease: A systematic review and pooled analysis. Eur J Neurol 30: , 3581–3594. |
[20] | Nielsen SD , Pearson NM , Seidler K ((2021) ) The link between the gut microbiota and Parkinson’s Disease: A systematic mechanism review with focus on α-synuclein transport. Brain Res 1769: , 147609. |
[21] | Ascherio A , Schwarzschild MA ((2019) ) Lifestyle and Parkinson’s disease progression. Mov Disord 34: , 7–8. |
[22] | Brouwer M , Huss A , van der Mark M , Nijssen PCG , Mulleners WM , Sas AMG , van Laar T , de Snoo GR , Kromhout H , Vermeulen RCH ((2017) ) Environmental exposure to pesticides and the risk of Parkinson’s disease in the Netherlands. Environ Int 107: , 100–110. |
[23] | Kab S , Spinosi J , Chaperon L , Dugravot A , Singh-Manoux A , Moisan F , Elbaz A ((2017) ) Agricultural activities and the incidence of Parkinson’s disease in the general French population. Eur J Epidemiol 32: , 203–216. |
[24] | Kulcsarova K , Bang C , Berg D , Schaeffer E ((2023) ) Pesticides and the microbiome-gut-brain axis: convergent pathways in the pathogenesis of Parkinson’s disease. J Parkinsons Dis 13: , 1079–1106. |
[25] | Gao X , Chen H , Fung TT , Logroscino G , Schwarzschild MA , Hu FB , Ascherio A ((2007) ) Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr 86: , 1486–1494. |
[26] | Bianchi VE , Rizzi L , Somaa F ((2023) ) The role of nutrition on Parkinson’s disease: a systematic review. Nutr Neurosci 26: , 605–628. |
[27] | Li LY , Liu SF , Zhuang JL , Li MM , Huang ZP , Chen YH , Chen XR , Chen CN , Lin S , Ye LC ((2023) ) Recent research progress on metabolic syndrome and risk of Parkinson’s disease. Rev Neurosci 34: , 719–735. |
[28] | Rusch C , Flanagan R , Suh H , Subramanian I ((2023) ) To restrict or not to restrict? Practical considerations for optimizing dietary protein interactions on levodopa absorption in Parkinson’s disease. NPJ Parkinsons Dis 9: , 98. |
[29] | Wang L , Xiong N , Huang J , Guo S , Liu L , Han C , Zhang G , Jiang H , Ma K , Xia Y , Xu X , Li J , Liu JY , Wang T ((2017) ) Protein-restricted diets for ameliorating motor fluctuations in Parkinson’s disease. Front Aging Neurosci 9: , 206. |
[30] | Marrinan S , Emmanuel AV , Burn DJ ((2014) ) Delayed gastric emptying in Parkinson’s disease. Mov Disord 29: , 23–32. |
[31] | Makaroff L , Gunn A , Gervasoni C , Richy F ((2011) ) Gastrointestinal disorders in Parkinson’s disease: prevalence and health outcomes in a US claims database. J Parkinsons Dis 1: , 65–74. |
[32] | Rossi M , Merello M , Perez-Lloret S ((2015) ) Management of constipation in Parkinson’s disease. Expert Opin Pharmacother 16: , 547–557. |
[33] | Beckers M , Bloem BR , Verbeek MM ((2022) ) Mechanisms of peripheral levodopa resistance in Parkinson’s disease. NPJ Parkinsons Dis 8: , 56. |
[34] | M ÓB , Smith MD , Tenison E , Henderson EJ , Lithander FE ((2022) ) Parkinson’s disease: the nutrition perspective. Proc Nutr Soc 81: , 12–26. |
[35] | Leta V , Klingelhoefer L , Longardner K , Campagnolo M , Levent H , Aureli F , Metta V , Bhidayasiri R , Chung-Faye G , Falup-Pecurariu C , Stocchi F , Jenner P , Warnecke T , Ray Chaudhuri K ((2023) ) Gastrointestinal barriers to levodopa transport and absorption in Parkinson’s disease. Eur J Neurol 30: , 1465–1480. |
[36] | Kacprzyk KW , Milewska M , Zarnowska A , Panczyk M , Rokicka G , Szostak-Wegierek D ((2022) ) Prevalence of malnutrition in patients with Parkinson’s disease: a systematic review. Nutrients 14: , 5194. |
[37] | Mischley LK , Lau RC , Bennett RD ((2017) ) Role of diet and nutritional supplements in Parkinson’s disease progression. Oxid Med Cell Longev 2017: , 6405278. |
[38] | Grammatikopoulou MG , Tousinas G , Balodimou C , Anastasilakis DA , Gkiouras K , Dardiotis E , Evangeliou AE , Bogdanos DP , Goulis DG ((2022) ) Ketogenic therapy for Parkinson’s disease: A systematic review and synthesis without meta-analysis of animal and human trials. Maturitas 163: , 46–61. |
[39] | Paknahad Z , Sheklabadi E , Derakhshan Y , Bagherniya M , Chitsaz A ((2020) ) The effect of the Mediterranean diet on cognitive function in patients with Parkinson’s disease: A randomized clinical controlled trial. Complement Ther Med 50: , 102366. |
[40] | Knight E , Geetha T , Burnett D , Babu JR ((2022) ) The role of diet and dietary patterns in Parkinson’s disease. Nutrients 14: , 4472. |
[41] | Rees J , Ryan J , Laws M , Devine A ((2023) ) A comprehensive examination of the evidence for whole of diet patterns in Parkinson’s disease: a scoping review. Nutr Neurosci, doi: 10.1080/1028415X.2023.2233727. |
[42] | Molsberry S , Bjornevik K , Hughes KC , Healy B , Schwarzschild M , Ascherio A ((2020) ) Diet pattern and prodromal features of Parkinson disease. Neurology 95: , e2095–e2108. |
[43] | Phillips MCL , Murtagh DKJ , Gilbertson LJ , Asztely FJS , Lynch CDP ((2018) ) Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov Disord 33: , 1306–1314. |
[44] | Wuerch E , Urgoiti GR , Yong VW ((2023) ) The promise of niacin in neurology. Neurotherapeutics 20: , 1037–1054. |
[45] | Chong R , Wakade C , Seamon M , Giri B , Morgan J , Purohit S ((2021) ) Niacin enhancement for Parkinson’s disease: an effectiveness trial. Front Aging Neurosci 13: , 667032. |
[46] | Taghizadeh M , Tamtaji OR , Dadgostar E , Daneshvar Kakhaki R , Bahmani F , Abolhassani J , Aarabi MH , Kouchaki E , Memarzadeh MR , Asemi Z ((2017) ) The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem Int 108: , 183–189. |
[47] | Tamtaji OR , Taghizadeh M , Aghadavod E , Mafi A , Dadgostar E , Daneshvar Kakhaki R , Abolhassani J , Asemi Z ((2019) ) The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin Neurol Neurosurg 176: , 116–121. |
[48] | ((1996) ) Impact of deprenyl and tocopherol treatment on Parkinson’s disease in DATATOP subjects not requiring levodopa. Parkinson Study Group. Ann Neurol 39: , 29–36. |
[49] | ((1996) ) Impact of deprenyl and tocopherol treatment on Parkinson’s disease in DATATOP patients requiring levodopa. Parkinson Study Group. Ann Neurol 39: , 37–45. |
[50] | Shoulson I ((1998) ) DATATOP: a decade of neuroprotective inquiry. Parkinson Study Group. Deprenyl And Tocopherol Antioxidative Therapy Of Parkinsonism. Ann Neurol 44: , S160–166. |
[51] | Ascherio A , Schwarzschild MA ((2017) ) Dietary antioxidants and Parkinson’s disease. Mov Disord 32: , 1501–1503. |
[52] | Staudacher HM , Yao CK , Chey WD , Whelan K ((2022) ) Optimal design of clinical trials of dietary interventions in disorders of gut-brain interaction. Am J Gastroenterol 117: , 973–984. |




