Specialized Allied Health Care for Parkinson’s Disease: State of the Art and Future Directions
Abstract
People with Parkinson’s disease (PD) experience a range of progressive motor and non-motor symptoms, that negatively affect their daily functioning, social participation and quality of life. Allied health therapies have emerged as an effective treatment approach—complementary to pharmacological and neurosurgical treatments—which reduces the impact of PD in daily life. In this article, we propose criteria for what constitutes specialized allied health care for PD, and we review allied health research in PD in terms of meeting these criteria and its outcomes for monodisciplinary approaches as well as multi- or interdisciplinary allied health interventions. We focus on the three most studied allied health disciplines in PD: physical therapy, occupational therapy and speech-language therapy. Overall, the available evidence underscores the importance and potential benefits of specialized allied health care for people with PD. Our proposed criteria and recommendations for future research might help in further delineating specialized allied health care.
INTRODUCTION
People with Parkinson’s disease (PD) experience progressive motor and non-motor symptoms, that negatively impact on their daily activities, social participation and quality of life.1,2 Within multidisciplinary care, pharmacological and neurosurgical treatments are well established and effective in alleviating symptoms such as bradykinesia, rigidity and tremor.3 However, these interventions have limited effect on other symptoms, such as impaired balance, cognition and oral motor functions. These symptoms negatively impact daily life functioning and can put patients at increased risk for potentially serious complications, such as hip fractures or aspiration pneumonia.4 Over the past decades, allied health interventions have emerged as an effective complementary treatment approach in the multidisciplinary care of people with PD to reduce the impact of PD on daily functioning and to prevent medical complications. While there is no universally accepted definition of allied health care, three allied health disciplines are commonly recognized and most frequently involved in the context of PD care. These are physical therapy, occupational therapy, and speech-language therapy. More recently, the role of other allied health professionals such as dieticians psychologists, social workers and sexologists is emerging.5,6
Because of the complexity of PD, there is a growing awareness that the care for people with PD should be tailored to the specific needs of affected individuals. In this article, we describe what constitutes “specialized allied health care” for PD. We subsequently review studies of specialized physical therapy, occupational therapy and speech-language therapy, and evaluate the extent to which these studies meet the proposed criteria for specialized care. Our work includes an unstructured review of the literature on monodisciplinary allied health approaches as well as a structured review of the literature on multi- or interdisciplinary allied health interventions. Finally, we outline remaining knowledge gaps in further defining and supporting specialized allied health care for PD.
WHAT CONSTITUTES SPECIALIZED ALLIED HEALTH CARE IN PARKINSON’S DISEASE
Specialized care generally refers to high-quality care that is designed and tailored for a specific disease or patient population and delivered by professionals with a special interest and competence in that area.7 As far as we are aware, there is no consensus statement on specific criteria to distinguish “specialized” versus “generic” allied health care in PD. Here, we propose a set of criteria to operationalize “specialized allied health care” in PD (Table 1).
Table 1
Proposed criteria of specialized allied health care in Parkinson’s disease
| Criterium | Examples of operationalization |
| 1. Provided by allied health professionals (AHPs) with specific expertise in PD | – AHPs have a substantial caseload of patients with PD.8 – AHPs completed a PD-specific training course and use evidence-based resources in PD (guidelines).9 |
| 2. Personalized | – The interventions are tailored towards the personal needs of and specific to the context of the person with PD.14,15 |
| 3. PD-specific: Considers the specific motor and non-motor characteristic features and complexity of PD in choice of assessment and intervention options | – Targets bradykinesia, hypokinesia, freezing with exercise and strategies that increase attention and trigger initiation, amplitude and rhythm.8,16 – Addresses the impact of fluctuating symptom severity during the day related to wearing off &on/off fluctuations.19 – Compensates for reduced internal feedback on motor functioning (e.g. posture, gait, speech loudness).83,84 – Targets and considers reduced ability in multitasking, changes in functional cognition and learning ability.16–18 – Targets and considers of the impact of other non-motor symptoms such as fatigue, apathy, anxiety, depression, vision problems and incontinence.8,24 |
| 4. Delivered in appropriate treatment context to enhance learning | – Interventions to improve functional mobility (skills) and activities are task-oriented and (also) trained in a setting where the skill is usually needed.20,21 |
| 5. Delivered in appropriate dose to enhance physiological adaptation or learning new skills and strategies | – Exercise for physiological adaptations according to the generally accepted training principles.22 – Skills practice (e.g. motor learning): multiple times a week.12,20,21,23 – Strategy training: multiple times per week.22 – Providing regular follow up.85 |
| 6. Delivered within appropriate multidisciplinary care | – Combination or sequential involvement of AHPs to meet the specific needs and goals of the individual.8,24,25 – The care of a AHP aligns with the medical, nursing and psychosocial care of the person.8,25 |
First, the allied health professionals (AHPs) providing specialized care must have expertise in PD to understand the specific clinical manifestations of PD, the (potential) working mechanism of interventions, and how to tailor and deliver these interventions. They need to integrate PD guidelines into their clinical practice, and be aware of what other disciplines contribute to care.8,9 They need to be able to adapt the intervention if the diagnosis turns out to be atypical parkinsonism. There are different initiatives and opportunities to gain PD- specific expertise. PD-specific training courses and resources for AHPs are provided by specialist health professional organizations, such as the International Parkinson and Movement Disorder Society, or by PD associations, such as Parkinson’s UK. Specialized treatment approaches are offered through courses that include certification, such as SPEAK OUT,10 LSVT LOUD®11 for speech-language therapists and LSVT BIG®12 for physical and occupational therapists. A step further is the provision of PD-specific training and certification as part of an infrastructure for specialized multidisciplinary network care, such as the ParkinsonNet model.13 Within ParkinsonNet, an initial criterion for expertise is the completion of a dedicated training program to understand the causes, clinical presentation, and impact of PD symptoms, as well as the specific treatment options for each problem or need, according to the latest scientific evidence. To maintain the specialist designation, AHPs are required to maintain a substantial caseload of people with PD, participate in regular training, and attend multidisciplinary meetings where they both learn and meet with ParkinsonNet professionals in the local area. The rationale is that professionals who meet these criteria will develop expertise and provide better care. However, there is no international consensus on the minimum curriculum or caseload requirements for an AHP with specific expertise in PD.
Second, specialized allied health care must be personalized to the needs and context of the individual. Personalized care is important in allied health care for all chronic diseases, but it is essential for people with PD because there is considerable inter-individual variation in the motor and non-motor symptoms. There is also a great deal of variation in the presentation of symptoms within an individual, even on a day-to-day basis. In addition, the impact of these symptoms on daily life varies from person to person, depending on the personal context and values of the person with PD.3 Recently, much attention has been given to the need to tailor care to the needs of the individual and to avoid a “one size fits all” approach.14,15 However, tailoring allied health care for PD is a complex due to the many disease and personal factors to consider. Furthermore, intervention research often excludes subgroups with cognitive problems or advanced disease. Consequently, there is still a knowledge gap in how to personalize allied health care for PD in the most effective way.
Third, specialized care must take into account the specific characteristics and complexity of PD in assessment and treatment. Motor symptoms such as bradykinesia, hypokinesia, and freezing require a PD-specific approach that promotes goal-directed movement, amplitude, and explicit feedback.8,16 The presence and impact of a potential wide range of non-motor symptoms, such as cognitive deficits, fatigue, orthostatic hypotension, urinary dysfunction, apathy, depression and anxiety must be considered or targeted in the interventions.8,17,18 Another specific feature in patients with advanced disease is fluctuation in motor and non-motor symptoms in response to levodopa.19 This needs to be considered in both the content and planning of assessment and interventions.
Fourth, it is essential to provide specialized in an appropriate treatment context. This is important because symptom presentation may be context-specific (e.g., freezing at a narrow basement door) and the transfer of newly acquired skills and strategies to a new context is generally impaired in PD.20,21 Consequently, if the goal is to (re)learn skills or to use new compensatory strategies, training should be task-oriented and (also) take place in the person’s real-life context. In the event that therapy sessions cannot be conducted in the home or community setting, then the AHP must fully consider the activity and environmental context (e.g., by requesting video/photo’s) and facilitate unsupervised practice at home.
A fifth criterion for specialized care is the provision of an appropriate dose of treatment to optimize physiological adaptation or to acquire and consolidate new skills and strategies. For exercise, the dose depends on the type of exercise and the functions being addressed. Recommendations for the appropriate dose of exercise are available.22 Consolidation of (motor) learning in people with PD requires greater intensity and repetition than in the healthy population.20,22 Successful PD-specific programs such as LSVT LOUD and LSVT BIG are based on high-intensity treatment (4 weeks; 4 times per week) to achieve improvement in daily functioning.12,23 An important gap exists in the evidence base regarding the optimal intensity, frequency, and duration of treatment for individuals with PD who are unable to adhere to such intensive programs.
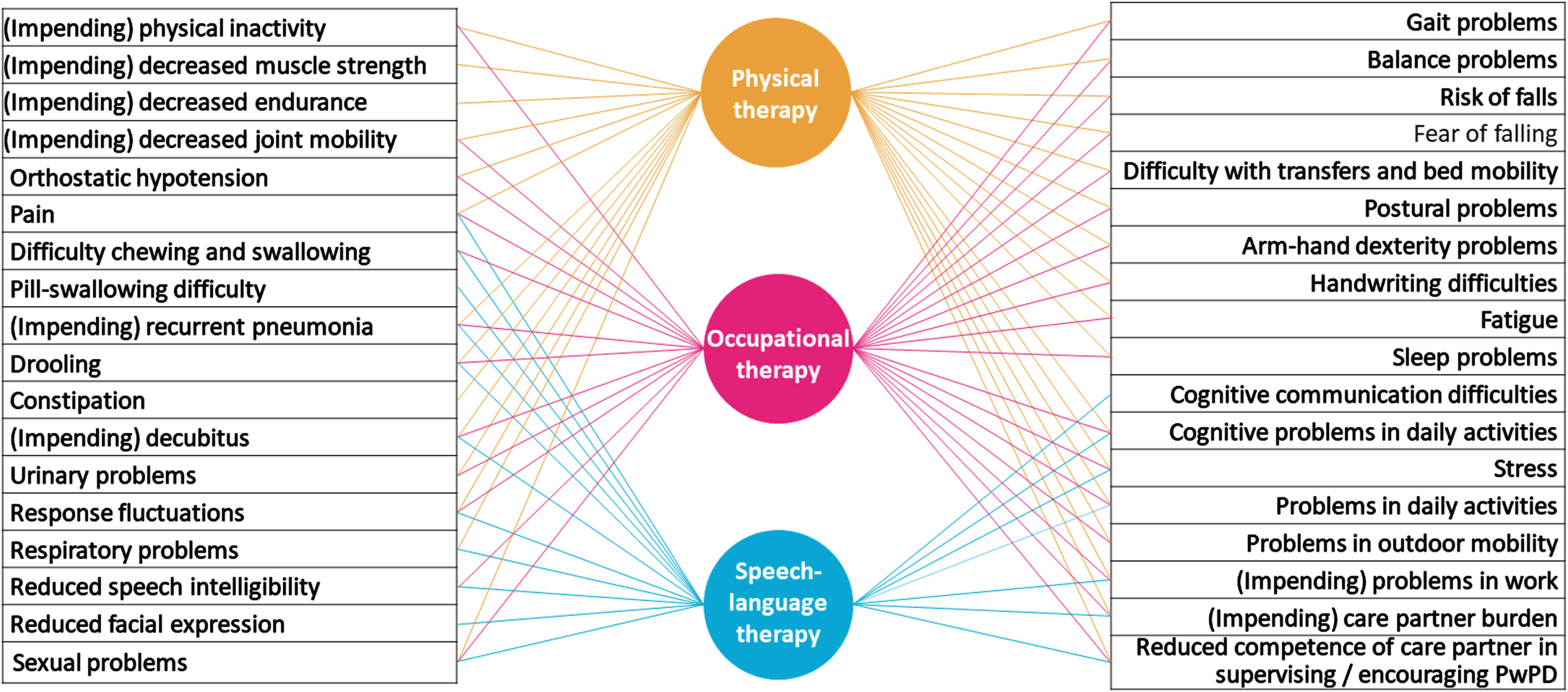
A final criterion is that specialized allied health care for PD is delivered within an appropriate multidisciplinary or interdisciplinary team. Individuals with PD often have multiple problems that require the input of different healthcare disciplines, both within and beyond the scope of allied health care. Indeed, some symptoms or areas of activity or participation may necessitate the simultaneous treatment of several AHPs (Fig. 1). In the case of interdisciplinary overlap, the contribution of each AHP will often be different, depending on the discipline-specific perspective and the availability of appropriate interventions.24,25 An example is the management of a person with PD who has difficulty eating due to dysphagia and postural problems. For a speech-language therapist, upright posture is a prerequisite for efficient eating and swallowing. A physical therapist may provide an exercise program to train the muscles involved in postural control, or sensory cues or visual feedback strategies to improve sitting posture. Concurrently, an occupational therapist may integrate the utilization of these strategies into home mealtime activities, provide advice on meal timing, adjust positioning at the table, or provide additional external postural support while sitting. This example - albeit one of the many - illustrates how interdisciplinary care can have synergistic effects. In fact, there is currently widespread interest in developing training and guidelines for interdisciplinary care in PD with the aim of integrating knowledge and methods from different disciplines. In the Netherlands, an online, integrated allied health guideline with embedded decision support has recently been developed to facilitate interdisciplinary care.26
Fig. 1
Overview of interdisciplinary overlap between specialized Allied Health Professionals for Parkinson’s disease: Possible problem areas of people with PD that are relevant to physical therapy, occupational therapy and/or speech-language therapy are listed in this figure. The lines between the problem area and the disciplines indicate which discipline may be involved (to a greater or lesser extent) in providing interventions.
EVIDENCE FOR MONODISCIPLINARY SPECIALIZED ALLIED HEALTH CARE
Based on an unstructured review, the following sections summarize the current state of evidence for physical therapy, occupational therapy, and speech-language therapy in relation to the criteria for specialized allied health care.
Physical therapy
Physical therapy aims to improve movement-related limitations in functions, activities and participation. Core areas of physical therapy include physical capacity, transfers, manual activities, balance, gait and posture. The main intervention modalities include tailored education, exercise, skills practice and strategy training.9,24,27
There is a large and growing body of evidence on the effects of physical therapy. A multitude of studies have been conducted, and numerous systematic reviews and meta-analyses have been published on this subject. The majority of this research has focused on physical exercise interventions. While physical exercise is a part of physical therapy, and can be delivered according to the above mentioned criteria for specialization, it is often unclear in publications whether the exercise was delivered by specialized professionals, in a specific context, or as part of an individualized treatment plan. Moreover, specialized physical therapy includes not only exercise, but also focuses on specific motor- and non-motor symptoms of the disease. Examples of PD-specific treatment modalities include cueing, dual-task training, amplitude-oriented training and manual dexterity training.9,24,27 Cueing has been shown to have positive effects on gait initiation and the spatiotemporal parameters of gait (i.e. step- and stride length).28 However, the optimal cue (auditory, visual, tactile) and behavioral strategies to employ remain unclear. Dual-task training, which combines (physical) skill practice with a cognitive element, has been shown to have positive effects on multiple outcomes (e.g., spatiotemporal parameters, balance, and functional mobility) and does not seem to have serious adverse effects.29 The evidence regarding amplitude-oriented training (i.e., LSVT-BIG) remains inconclusive.30 Many physical therapy studies have combined different treatment modalities, such as exercise combined with strategy training. This is a common clinical practice, where the exact combination of physical therapy modalities depends on the specific symptoms, problems and goals of each individual patient. A comprehensive meta-analysis showed a positive effect of a combination of physical therapy treatment modalities on motor symptoms, fear of falling, and gait freezing, based on the pooled effects of 45 studies.31 As with other studies in the field, the extent to which the professionals involved were specialized remains unclear, and conclusions about the effectiveness of specialization cannot be drawn based on the available evidence from prospective trials. However, a detailed analysis of registry data revealed that specialized physical therapy given via ParkinsonNet is more effective (i.e., fewer complications) and cost-effective than regular physical therapy.32
Occupational therapy
Occupational therapy aims to maximize people’s participation in meaningful activities and roles at home, work and in the community.24,33,34 In PD, occupational therapists may specifically address activity limitations and participation restrictions due to changes in functional mobility, manual dexterity, functional cognition, and fatigue. The main intervention modalities include a combination of tailored education, skills practice, compensatory strategy training, adaptation of tasks and daily activity patterns, and adaptation of the environment.9,24,34 Given the specific motor symptoms associated with PD, it is essential that skills practice and strategy training to address functional mobility and manual dexterity must be PD-specific. While occupational therapy interventions to address functional cognition and fatigue are not unique to PD, it is important for treating therapists to be aware of all the factors that affect cognition and fatigue in people with PD in order to tailor education and strategies appropriately.
Occupational therapists have a distinct focus and approach, yet occupational therapy in PD is seldom delivered in isolation; it is naturally integrated into multidisciplinary programs. Some recent systematic reviews have focused on interventions that fall ‘within the scope of’ occupational therapy, namely those designed to improve activities and participation.35–37 However, other AHPs also aim to improve activity performance and participation.38 Consequently, these reviews also include interventions delivered by other professionals, which precludes a clear insight into the unique contribution of occupational therapy. Recent systematic reviews that included studies in which the intervention was (mainly) delivered by occupational therapists, can only be cautious in their conclusions due to the limited number of studies included, the heterogeneity of interventions, outcomes and study design.39,40 Three randomized controlled trials specifically examined occupational therapy as a single intervention. These are a trial of individualized, goal-directed home-based occupational therapy delivered within the context of ParkinsonNet,41 a dexterity-focused home-based exercise program,42 and a multicomponent PD-specific occupational therapy program focused on motor limitations in activities of daily living.43 The interventions in the trials were PD-specific and delivered by AHPs experienced in PD. The dose was only appropriate if participants did the home practice. The interventions were either home-based41,42 or focused on using what was learned at home.43 Overall, the results suggest an improvement in (perceived) activity performance41,43 and short-term improvements in dexterity-related ADL.42 The results showed no effect on patients’ quality of life. However, the trial on individualized home-based occupational therapy, showed improvement for caregivers’ quality of life.41 The study also showed that the intervention had no effect on total costs over six months, but there were significant savings in institutional care in the intervention group.44
A recent study assessed whether adding a task-oriented LSVT-BIG® program to general occupational therapy has beneficial effects.45 From the intervention description it is unclear what constituted “general occupational therapy”. However, the results suggest that adding PD-specific and task-oriented training may improve outcomes. More occupational therapy feasibility/ pilot studies have recently been done to address problems that affect daily life and participation in PD, such as functional cognition,46,47 fatigue,48 anxiety,49 and work.50 These studies advance the scientific evidence on occupational therapy in PD.
Speech-language therapy
Speech-language therapists focus on three oral motor domains that can deteriorate in PD: speech intelligibility (dysarthria), safe and efficient nutrition intake (dysphagia) and saliva control (drooling).9,51 They also provide treatment options to compensate for communication disorders caused by cognitive decline, in particular word finding difficulties and bradyphrenia.52,53 Speech-language therapists offer different types of treatment, including tailored education, exercise, skill training, and compensatory strategies; manuals and guidelines available for this.23,51
There is a growing body of evidence supporting the benefits of specialized speech-language therapy for PD. The first studies of speech-language therapy evaluated the Lee Silverman Voice Treatment (LSVT LOUD) three decades ago.54 A recent meta-analysis confirms the efficacy of this approach in improving voice loudness and functional communication.11 However, the success of such programs depends on their high intensity over a brief period (three to four times per week for four weeks), which is reflected in the inclusion of participants with mild to moderate PD.11 Meanwhile, large randomized controlled trials of personalized approaches are underway, including people with severe PD.55 Importantly, tele-rehabilitation has been shown to be non-inferior to face-to-face treatment sessions, facilitating the delivery of intensive speech-language therapy in the home.56 While speech-language therapy is generally delivered individually, PD-specific group treatments seem promising. A well-moderated weekly or monthly intensive therapeutic singing group, known as ParkinSong, requires a high level of respiratory and vocal effort and showed improved voice-related quality of life and well-being after 12 months.57 However, replication of this approach is necessary, as is comparison with intensive individual treatment to better interpret the individual benefit.
Moreover, the treatment of cognitive communication disorders, which further affect communicative efficacy, has attracted scientific interest. Studies suggest that approaches such as Communication Partner Training (CPT) may also be valuable for caregivers of people with PD,58–60 but clear results and the evaluation of specific approaches for PD are awaited.
Treatment of dysphagia in PD depends on the timely compensation to prevent aspiration or to facilitate swallowing.51 Specific exercises to practice swallowing skills or indirect exercises such as expiratory muscle strength training (EMST) may also be beneficial.61 Expiratory strength training may also improve coughing as a protective response to aspiration of liquids or food, but intensive skills training to improve cough efficiency seems to be more effective.62 Although the content of the treatments is not reported (e.g., exercise, compensations, or alterations of food consistencies), there is now convincing evidence that speech-language therapy in the community within the context of ParkinsonNet, reduces the rate of pneumonia in PD.63 Behavioral treatment of drooling in PD is primarily based on education and identifying the right cues to improve swallowing frequency. Given that saliva is swallowed throughout the day, wearable devices are being investigated,64 but results are pending.
The costs of specialized speech-language therapy in comparison to usual care have not been well studied. An exploratory economic evaluation conducted alongside the PD COMM pilot trial compared the costs and outcomes of LSVT LOUD, standard speech-language therapy, and no treatment. There were no differences in outcomes at 12 months, but the full study is awaited for a more definitive assessment of the cost-effectiveness of speech-language therapy for people with voice and communication problems due to PD.65
EVIDENCE FOR MULTI-OR INTERDISCIPLINARY SPECIALIZED ALLIED HEALTH INTERVENTIONS
In order to review multi- or interdisciplinary allied health programs for people with PD, we conducted a structured literature search on randomized controlled trials that evaluated the effectiveness of the combination of two or more allied health disciplines compared with a control group that did not receive care from multiple allied health disciplines. The level of specialization was evaluated according to the proposed criteria (Table 1) and evidence for effectiveness. The exact search terms and evaluation criteria are presented in the Supplementary Material A and B.
The literature search yielded only nine studies.66–74 The interventions, outcome measures, and results are summarized in Table 2. All trials involved physical therapy; four trials involved all three allied health disciplines.69–72 Most trials included participants with mild to moderate PD, whereas two trials included “de novo” PD.68,69 The number of participants per trial ranged from 36 to 762.
Table 2
Trials on effectiveness of multi- or interdisciplinary specialized allied health care in Parkinson’s disease
| Author, date | Population | Intervention | Meeting criteria for specialized allied health care | Primary outcome | Secondary outcome | Effectiveness: Significant difference |
| Clarke et al., 201666 | n = 762 people with mild-moderate PD | IG: PD REHAB •AHPs: PT and OT •Community outpatient or home-based setting •Mean duration of 8 weeks •Median number of therapy sessions was 4 (range, 1-21) •CG: no AHPs | 1. PD-trained AHPs: Not reported 2. Personalized: Yes 3. PD-specific: Uncertain 4. Appropriate Context: Yes 5. Appropriate dose: No 6. Appropriate multidisciplinary care: Yes | •Independence in Daily activities: (NEADL) Assessed at 3 months | •Health related quality of life (PDQ-39, EQ5D) •Adverse events •Caregiver quality of life. Assessed at 3, 9, and 15 months | •Primary outcome: No •Secondary outcomes: No |
| Frazzitta et al., 201267 | n = 50 people with PD, H&Y stage 3 | IG: Intensive rehabilitation treatment (IRT) •AHPs: PT+OT •Inpatient setting •4 weeks •15 sessions per week CG: Only pharmacological treatment, no AHPs | 1. PD-trained AHPs: Not reported 2. Personalized: Not fully reported 3. PD-specific: Not fully reported 4. Appropriate Context: Partly 5. Appropriate dose: Yes 6. Appropriate multidisciplinary care: Yes | •Limitations in daily tasks (UPDRS II) •Motor impairment (UPDRS III) •Disease severity (UPDRS total) Assessed at 4 weeks | •Primary outcomes: Yes | |
| Frazzitta et al., 201568 | n = 40 people with newly diagnosed PD, H&Y stage 1-1.5 | IG: multidisciplinary intensive rehabilitation treatments (MIRT) •AHPs: PT+OT •Inpatient setting •4 weeks •15 sessions per week CG: only pharmacological treatment, no AHPs | 1. PD-trained AHPs: Not reported 2. Personalized: Uncertain 3. PD-specific: Not fully reported 4. Appropriate Context: Partly 5. Appropriate dose: Yes 6. Appropriate multidisciplinary care: Yes | •Limitation in daily tasks (UPDRS II) •Motor impairments (UPDRS III) •Aerobic capacity (6MWT) •Mobility (TUG) •Self-reported disability (PDDS) Assessed at 6 months, 12 months, 18 months, and at 24 months | •Dosage of levodopa equivalent •Number of patients in monotherapy with Rasagiline Assessed at 6 months, 12 months, 18 months, and at 24 months | •Primary outcomes: Yes •Secondary outcomes: Yes |
| Ferrazzoli et al., 2018a69 | n = 36 “de-novo” PD, H&Y stage 1-2 | IG: Rotigotine plus multidisciplinary intensive rehabilitation treatments (MIRT) •AHPs: PT+OT+ST •Inpatient setting •4 weeks •21 sessions per week •CG: Rotigotine alone, no AHPs | 1. PD trained AHPs: Not reported 2. Personalized: Not fully reported 3. PD-specific: Partly 4. Appropriate Context: Partly 5. Appropriate dose: Yes 6. Appropriate multidisciplinary care: Yes | Disease severity (UPDRS total) •Assessed at 6 months, 12 months, 18 months, and at 24 months | Limitations in daily tasks (UPDRS II) •Motor impairment (UPDRS III) •Aerobic capacity (6MWT) •Mobility (TUG) •The amount of Rotigotine •Assessed at 6 months, 12 months, 18 months, and at 24 months | Primary outcome: No •Secondary outcomes, respectively: •No •No •Yes •Yes •Yes |
| Ferrazzoli et al., 2018b70 | n = 234 people with PD, H&Y stage 2-4 | IG: multidisciplinary intensive rehabilitation treatments (MIRT) •AHPs: PT+OT+ST •Outpatient setting •4 weeks •21 sessions per week CG: no AHPs | 1. PD-trained AHPs: Not reported 2. Personalized: Yes 3. PD-specific: Partly 4. Appropriate Context: Partly 5. Appropriate dose: Yes 6. Appropriate multidisciplinary care: Yes | •Quality of life (PDQ-39) Assessed at 4 weeks | •Disease severity (UPDRS total) •Self-reported disability (PDDS) •Mobility (TUG) •Balance (BBS) Only assessed in experimental group, at 4 weeks | •Primary outcome: Yes •Secondary outcomes: Yes |
| Tickle-Degnen et al., 201071 | n = 116 people with PD, H&Y stage 2, 2.5, or 3 when “on” | IG1: •AHPs: PT, OT, ST •Outpatient setting •6 weeks •3 hours per week •IG2: •AHPs: PT, OT, ST •Outpatient and home-based setting •6 weeks •4.5 hours per week CG: no AHPs | 1. PD-trained AHPs: Yes 2. Personalized: Yes 3. PD-specific: Yes 4. Appropriate Context: IG1 Partly; IG2 Yes 5. Appropriate dose: IG1 Uncertain; IG2 Yes 6. Appropriate multidisciplinary care: Yes | •Quality of life (PDQ-39) Assessed at 6 weeks, and at 2 and 6 months of follow-up | •Primary outcome: Yes, but no differences between IG1 and IG2 | |
| Wade et al., 200372 | n = 144 people with PD | IG: multidisciplinary rehabilitation program •AHPs: OT, PT, ST •6 weeks •1 day/week outpatient setting CG: no AHPs | 1. PD trained AHPs: Uncertain 2. Personalized: Yes 3. PD-specific: Not reported 4. Appropriate Context: No 5. Appropriate dose: No 6. Appropriate multidisciplinary care: Yes | •Quality of life (PDQ-39, SF-36, EQ-5D) •Leg strength and endurance (Stand-walk-sit test) •Finger dexterity (NHPT) •Anxiety and depression (HADS) •Speech (items from UPDRS) Assessed at 24 weeks, 48 weeks | Primary/secondary was not specified | •Primary outcomes: No |
| Stożek et al., 201673 | n = 64 people with PD, H&Y stage 1.5-3 | IG: rehabilitation program •AHPs: PT, ST •Outpatient setting •4 weeks •28 sessions in total CG: Only medication therapy, no AHPs | 1. PD trained AHPs: Not reported 2. Personalized: Not fully reported 3. PD-specific: Partly 4. Appropriate Context: No 5. Appropriate dose: Yes 6. Appropriate multidisciplinary care: Uncertain | •Balance (tandem stance and Pastor test) •Gait (10 m walk and 360 degree turn) •Motor function (PPT) •Trunk rotation Assessed post intervention and 1 month follow-up | Primary/secondary was not specified | •Primary outcomes: Yes |
| Monticone et al., 201574 | n = 70 people with PD, H&Y stage 2.5-4 | IG: multidisciplinary rehabilitative care •AHPs: PT, OT •Inpatient setting •8 weeks •Daily PT session •1 OT session per week CG: general physical therapy •AHPs: PT •Inpatient setting •8 weeks •Daily PT session | 1. PD-trained AHPs: Not fully reported 2. Personalized: Not fully reported 3. PD-specific: Yes 4. Appropriate Context: Partly 5. Appropriate dose: Partly 6. Appropriate multidisciplinary care: Yes | •Motor impairment (UPDRS III) Assessed 8 weeks later (post-treatment), and 1 year follow-up | •Balance (BBS) •Disability (FIM) •Quality of life (PDQ-39) Assessed 8 weeks later (post-treatment), and 1 year follow-up | •Primary outcome: Yes •Secondary outcomes: Yes |
AHPs, Allied health professionals; BBS, Berg Balance Scale; CG, control group; EQ-5D, EuroQol 5D; FIM, Functional Independence Measure; H& Y, Hoehn and Yahr; IG, intervention group; IG1, intervention group one; IG2, intervention group two; NEADL, NHPT, Nine hole peg test; Nottingham Extended Activities of Daily Living Index; OT, occupational therapy; PD, Parkinson’s disease; PDDS, Parkinson’s Disease Disability Scale; PDQ-39, Parkinson’s Disease Questionnaire-39; PT, physical therapy; SF-36, 36-Item Short Form Health Survey; 6MWT, six-minute walking test; ST, speech- language therapy; TUG, Timed Up-and-Go Test; UPDRS, Unified Parkinson’s Disease Rating Scale.
Three authors (BRM, IHWM, JGK) independently assessed whether the interventions in the studies met the six criteria of “specialized allied health care in PD” as listed in Table 1. In case of disagreement, consensus was reached by discussion. The results of the assessment are presented in Table 2; the detailed assessment in Supplementary Material C. If the characteristics of the interventions were not (clearly) described, we labeled it as uncertain or not (fully) reported. Only one study evaluated an intervention that met all the six criteria.71 In the other eight studies, the authors did not (clearly) specify whether the AHPs were trained in PD. Only in four studies did the description of the intervention indicate a personalized approach.66,70–72 Furthermore, although the programs were developed for the PD population, it was difficult to ascertain whether they were consistent with evidence-based interventions in most of the studies. Similarly, it was unclear whether the treatment context was appropriate in the majority of studies. Interventions were often provided in a clinical inpatient or outpatient setting. Only two interventions were (partly) delivered in the home-setting.66,71 In two trials, the intervention dose was insufficient to expect improvement in symptoms or the utilization of new skills and strategies.66,72
With regard to the selected outcomes, it is evident that the studies have made deliberate choices to limit the number of outcomes. Consequently, many studies employ an outcome that assesses the overall quality of life, such as the PDQ-39. In addition, several motor performance scores were selected. While speech-language therapy was incorporated into the treatment program in five studies, none of them included any outcome measures related to voice, speech, or communication, except for single items in the UPDRS.75
With respect to the effectiveness of the interventions, six trials demonstrated improvements in primary outcome measures in the intervention group in comparison to the control group.67,68,70,71,73,74 Thus, three trials showed no improvement.66,69,72 Of the nine studies, the two trials who did not meet the criterium of appropriate dose showed no improvement.66,72 We can only hypothesize that this played a role in the outcome, because other criteria might have played a role as well. Due to the heterogeneity of intervention characteristics and outcomes in the studies, it is difficult to determine other correlations between criteria and outcomes.
In addition to the evidence from randomized controlled trials, a recent large observational study in the Netherlands found that specialized occupational therapy was most effective in preventing PD-related complications, such as fractures, when combined with specialized physical therapy. The protective effects were larger than those observed in individuals who only received specialized physical therapy. These findings suggest that the combination of specialized AHPs may have synergistic protective effects.63
Gaps and future perspectives
Although there is a growing body of evidence for specialized physical therapy, occupational therapy and speech-language therapy in PD, there is a need for further research to address key remaining gaps in knowledge. Table 3 presents specific recommendations for high-priority research questions that should be addressed. It is important that future collaborative studies build upon the working criteria for specialized allied health care in PD as presented in this article and expand to incorporate other allied health disciplines, such as psychology and dietetics. Ideally, future refined criteria should include the perspectives of specialized AHPs across the world. In light of this, the recent publication of an international consensus statement on rehabilitation in PD is welcome development.9 The statement includes key principles of rehabilitative care in PD and recommendations for the content and organizational aspects of rehabilitative care. Future collaborative studies should integrate these principles into a refined set of criteria for specialized allied health care in PD. Once established, the criteria for specialized allied health treatment can serve as a foundation for intervention protocols, systematic review protocols and meta-analyses.
Table 3
Recommendations for future research on specialized allied health care for Parkinson’s disease
| Area of research | Recommended research questions |
| Criteria for AHPs with PD expertise | – What are minimum requirements for AHPs to be qualified as an expert in PD. (competencies, courses and experience)? – How should these requirements vary according to profession, setting, subgroups of PD seen? |
| Tailoring personalized intervention | – What are essential components in tailoring care? – What are helpful monitoring and decision making tools to support tailoring care in clinical practice? |
| Targeting Specific motor and non-motor characteristic features and complexity of PD | – What are the working mechanisms of successful AHP interventions in PD? – How can successful interventions optimally be adapted for subgroups of people with fatigue or cognitive problems? – When are successful interventions limited by disease severity and what are the best possible alternatives? |
| Treatment context | – What are the effects of multi-or interdisciplinary care in the community versus in secondary/tertiary care settings? – What are the utility and benefits of integrating telemedicine and novel technology (i.e., wearable devices) into personalized care? – What are the costs and cost-effectiveness when comparing specific interventions and models and settings of care. (i.e., community care versus secondary of tertiary care)? |
| Dose of treatment | – What are the optimal doses of treatment (intensity, frequency, duration) for exercise, skills practice and strategy training, depending on the treatment goal (high, moderate or low)? – What are effective novel methods that support reaching the appropriate dose? – What is the appropriate timing of booster follow-ups for different disease stages and different goals? |
| Multidisciplinary care | – Which are the most effective combination and sequencing of professionals to address specific goals? – What are the outcomes that provide insight in the effectiveness of each AHP versus the combined effectiveness. – What are effective infrastructures to deliver multi-or interdisciplinary care across settings? |
| Access | – How can access to specialized care for people with PD from different cultural or socioeconomic backgrounds within countries be improved? – How can access to specialized care for people with PD in low resource countries be improved? |
Aside from further research into its effectiveness, the implementation of specialized allied health programs in the care of people with PD across different populations will present significant challenges. Potential barriers may include both person-level and system-level factors.76 Future implementation studies should therefore also examine specific facilitators and barriers of implementation by country, region, setting, stage of disease, gender, socioeconomic status or racial and ethnic group.76–80 Due to the growing number of people living with PD worldwide,81,82 the demand for access to specialized allied health care for PD is likely to increase in the coming years. This highlights the need for studies that address the existing gaps in this area.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
Ingrid Sturkenboom has received research grants from The Netherlands Organisation for Health Research and Development.
Amir Talebi has no conflict of interest to report.
Bart Maas has no conflict of interest to report.
Nienke de Vries has received research grants from The Netherlands Organisation for Health Research and Development, the Michael J Fox Foundation and Verily Life Sciences. She serves as an associate editor of the Journal of Parkinson’s disease.
Sirwan Darweesh has received funding from the Parkinson’s Foundation (PF-FBS-2026), ZonMW (09150162010183), ParkinsonNL (P2022-07 and P2021-14), Michael J Fox Foundation (MJFF-022767) and Edmond J Safra Foundation.
Johanna Kalf has received research grants from the Michael J Fox Foundation, The Netherlands Organisation for Health Research and Development and the Dutch Association for Speech and Language Therapy.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230307.
REFERENCES
1. | Ahn S , Springer K and Gibson JS. Social withdrawal in Parkinson’s disease: A scoping review, Geriatr Nurs (2022) ; 48: : 258–268. |
2. | van Uem JM , Marinus J , Canning C , et al. Health-Related Quality of Life in patients with Parkinson’s disease–A systematic review based on the ICF model, Neurosci Biobehav Rev (2016) ; 61: : 26–34. |
3. | Bloem BR , Okun MS and Klein C. Parkinson’s disease, Lancet (2021) ; 397: : 2284–2303. |
4. | Bloem BR , de Vries NM and Ebersbach G. Nonpharmacological treatments for patients with Parkinson’s disease, Mov Disord (2015) ; 30: : 1504–1520. |
5. | Duits A , van der Heijden C , van het Hoofd M , et al. Psychosocial needs of patients and spouses justify a position of psychosocial health professionals in the multidisciplinary care for Parkinson’s disease, Clin Park Relat Disord (2020) ; 3: : 100064. |
6. | Flanagan R , Rusch C , Lithander FE , et al. The missing piece of the puzzle – The key role of the dietitian in the management of Parkinson’s disease, Parkinsonism Relat Disord (2024) : 106021. |
7. | Hogden A , Foley G , Henderson RD , et al. Amyotrophic lateral sclerosis: improving care with a multidisciplinary approach, J Multidiscip Healthc (2017) ; 10: : 205–215. |
8. | van der Marck MA , Kalf JG , Faber MJ , et al. Nonpharmacological çnt of Parkinson’s disease. In: Jancovid J and Tolosa E (eds) Parkinson’s Disease & Movement Disorders (6th ed). Philadelphia: Wolters Kluwer, (2015) . |
9. | Goldman JG , Volpe D , Ellis TD , et al. Delivering multidisciplinary rehabilitation care in Parkinson’s disease: an international consensus statement. J Parkinsons Dis (2024) ; 14: : 135–166. |
10. | Behrman A , Cody J , Elandary S , et al. The Effect of SPEAK OUT! and The LOUD Crowd on Dysarthria Due to Parkinson’s Disease. Am J Speech Lang Pathol (2020) ; 29: : 1448–1465. |
11. | Pu T , Huang M , Kong X , et al. Lee Silverman voice treatment to improve speech in Parkinson’s disease: A systemic review and meta-analysis. Parkinsons Dis (2021) ; 2021: : 3366870. |
12. | McDonnell MN , Rischbieth B , Schammer TT , et al. Lee Silverman Voice Treatment (LSVT)-BIG to improve motor function in people with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil (2018) ; 32: : 607–618. |
13. | Bloem BR and Munneke M. Revolutionising management of chronic disease: the ParkinsonNet approach. BMJ (2014) ; 348: : g1838. |
14. | van den Heuvel L , Meinders MJ , Post B , et al. Personalizing decision-making for persons with Parkinson’s disease: where do we stand and what to improve? J Neurol (2022) ; 269: : 3569–3578. |
15. | Tosserams A and Nonnekes J. A Practical guide to the evaluation of compensation strategies for gait impairment in Parkinson’s Disease. J Parkinsons Dis (2022) ; 12: : 2005–2008. |
16. | Abbruzzese G , Marchese R , Avanzino L and Pelosin E. Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Parkinsonism Relat Disord (2016) ; 22 Suppl 1: : S60–64. |
17. | Sturkenboom I , Nott MT , Bloem BR , et al. Applied cognitive strategy behaviours in people with parkinson’s disease during daily activities: A cross-sectional study. J Rehabil Med (2019) ; 52: : jrm00010. |
18. | Barboza NM , Mancini M , Smaili SM , et al. Exploring mobility dysfunction in people with and without impaired cognition in Parkinson disease. Parkinsonism Relat Disord (2023) ; 115: : 105836. |
19. | Martinez-Fernandez R , Schmitt E , Martinez-Martin P , et al. The hidden sister of motor fluctuations in Parkinson’s disease: A review on nonmotor fluctuations. Mov Disord (2016) ; 31: : 1080–1094. |
20. | Marinelli L , Quartarone A , Hallett M , et al. The many facets of motor learning and their relevance for Parkinson’s disease. Clin Neurophysiol (2017) ; 128: : 1127–1141. |
21. | Cristini J , Parwanta Z , De Las Heras B , et al. Motor Memory consolidation deficits in Parkinson’s disease: a systematic review with meta-analysis. J Parkinsons Dis (2023) ; 13: : 865–892. |
22. | Corcos DM , Lamotte G , Luthra NS , et al. Advice to people with Parkinson’s in my clinic: exercise. J Parkinsons Dis (2024) ; 4: : 609–617. |
23. | Theodoros Dand Ramig L. Communication and Swallowing in Parkinson’s Disease. San Diego: Plural Publishing, (2011) . |
24. | Radder DLM , Sturkenboom IH , van Nimwegen M , et al. Physical therapy and occupational therapy in Parkinson’s disease. Int J Neurosci (2017) ; 127: : 930–943. |
25. | Tosserams A , de Vries NM , Bloem BR and Nonnekes J. Multidisciplinary care to optimize functional mobility in Parkinson disease. Clin Geriatr Med (2020) ; 36: : 159–172. |
26. | Sturkenboom IH , Langbroek-Amersfoort A , Munneke M and Bloem BR. Developing a living integrated guideline for allied health care in Parkinson’s disease with decision support: Lessons learned. (poster presentation). World Parkinson Congress. Barcelona, (2023) . |
27. | Domingos J , Keus SHJ , Dean J , et al. The European Physiotherapy Guideline for Parkinson’s Disease: implications for neurologists. J Parkinsons Dis (2018) ; 8: : 499–502. |
28. | Cosentino C , Putzolu M , Mezzarobba S , et al. One cue does not fit all: A systematic review with meta-analysis of the effectiveness of cueing on freezing of gait in Parkinson’s disease. Neurosci Biobehav Rev (2023) ; 150: : 105189. |
29. | Garcia-Lopez H , de Los Angeles Castillo-Pintor M , Castro-Sanchez AM , et al. Efficacy of dual-task training in patients with Parkinson’s disease: a systematic review with meta-analysis. Mov Disord Clin Pract (2023) ; 10: : 1268–1284. |
30. | Ernst M , Folkerts AK , Gollan R , et al. Physical exercise for people with Parkinson’s disease: a systematic review and network meta-analysis. Cochrane Database of Systematic Reviews (2023) ; 1: : CD013856. |
31. | Radder DLM , Lígia Silva de Lima A , Domingos J , et al. Physiotherapy in Parkinson’s disease: a meta-analysis of present treatment modalities. Neurorehabil Neural Repair (2020) ; 34: : 871–880. |
32. | Ypinga JHL , de Vries NM , Boonen L , et al. Effectiveness and costs of specialised physiotherapy given via ParkinsonNet: a retrospective analysis of medical claims data. Lancet Neurol (2018) ; 17: : 153–161. |
33. | Sturkenboom I , Thijssen M , Gons-van Elsacker J , et al. Guidelines for occupational therapy in Parkinson’ s disease rehabilitation. Nijmegen, The Netherlands / Miami (FL), U.S.A: ParkinsonNet/NPF, (2011) . |
34. | Wood J , Henderson W and Foster ER. Occupational therapy practice guidelines for people with Parkinson’s disease. Am J Occup Ther (2022) ; 76: : 7603397010. |
35. | Foster ER , Carson LG , Archer J , et al. Occupational therapy interventions for instrumental activities of daily living for adults with Parkinson’s disease: a systematic review. Am J Occup Ther (2021) ; 75: : 7503190030p1–7503190030p24. |
36. | Doucet BM , Franc I and Hunter EG. Interventions within the scope of occupational therapy to improve activities of daily living, rest, and sleep in people with Parkinson’s disease: a systematic review. Am J Occup Ther (2021) ; 75: : 7503190020. |
37. | Boone AE , Henderson W and Hunter EG. Role of occupational therapy in facilitating participation among caregivers of people with Parkinson’s disease: a systematic review. Am J Occup Ther (2021) ; 75: : 7503190010. |
38. | Kossi O , Raats J , Wellens J , et al. Efficacy of rehabilitation interventions evaluated in common neurological conditions in improving participation outcomes: A systematic review. Clin Rehabil (2024) ; 38: : 47–59. |
39. | Tofani M , Ranieri A , Fabbrini G , et al. Efficacy of occupational therapy interventions on quality of life in patients with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord Clin Pract (2020) ; 7: : 891–901. |
40. | Welsby E , Berrigan S and Laver K. Effectiveness of occupational therapy intervention for people with Parkinson’s disease: Systematic review. Aust Occup Ther J (2019) ; 66: : 731–738. |
41. | Sturkenboom IH , Graff MJ , Hendriks JC , et al. Efficacy of occupational therapy for patients with Parkinson’s disease: a randomised controlled trial. Lancet Neurol (2014) ; 13: : 557–566. |
42. | Vanbellingen T , Nyffeler T , Nigg J , et al. Home based training for dexterity in Parkinson’s disease: A randomized controlled trial. Parkinsonism Relat Disord (2017) ; 41: : 92–98. |
43. | Schaeffer E , Streich S , Wurster I , et al. How to evaluate effects of occupational therapy – lessons learned from an exploratory randomized controlled trial. Parkinsonism Relat Disord (2019) ; 67: : 42–47. |
44. | Sturkenboom IHWM , Hendriks JCM , Graff MJL , et al. Economic evaluation of occupational therapy in Parkinson’s disease: A randomized controlled trial. Mov Disord (2015) ; 30: : 1059–1067. |
45. | Choi Y and Kim D. Effects of task-based LSVT-big intervention on hand function, activity of daily living, psychological function, and quality of life in Parkinson’s disease: a randomized control trial. Occup Ther Int (2022) ; 2022: : 1700306. |
46. | Davies SJ, Gullo HL and Doig E. Efficacy and feasibility of the CO-OP approach in Parkinson's disease: RCT study protocol. Can J Occup Ther (2023) ; 90: : 363–373. |
47. | Foster ER , Spence D and Toglia J. Feasibility of a cognitive strategy training intervention for people with Parkinson’s disease. Disabil Rehabil (2018) ; 40: : 1127–1134. |
48. | Alizadeh N , Packer TL , Sturkenboom I , et al. Managing fatigue in Parkinson’s disease: protocol for a pilot randomized controlled trial. Can J Occup Ther (2022) ; 89: : 180–189. |
49. | Lovegrove CJBK , Ingram W , Baily M , Aspinall P , Hoskings J , Sturkenboom I , Marsden J , et al. Evaluating the occupation-based complex intervention for living well with anxiety and Parkinson’s disease (OBtAIN-PD), a feasibility cluster randomized controlled trial protocol (poster presentation). In: World Parkinson Congress, Barcelona, July 6th (2023) . |
50. | Sturkenboom I , vanOmme-vanLaarhoven J , Noordegraaf M , et al. Both online and in-person JOBGRIP group training for people with Parkinson’s with paid employment is acceptable and perceived to be valuable (abstract). Mov Disord (2021) ; 36 suppl 1: : S5–S6. |
51. | Kalf JG , de Swart BJM , Bonnier MWJ , et al. NVLF Guidelines for speech-language therapy in Parkinson’s disease, Nijmegen, The Netherlands / Miami (FL), U.S.A: ParkinsonNet/NPF, (2011) . |
52. | Miller N , Noble E , Jones D , et al. Life with communication changes in Parkinson’s disease. Age Ageing (2006) ; 35: : 235–239. |
53. | Barnish MS , Whibley D , Horton SM , et al. Roles of cognitive status and intelligibility in everyday communication in people with Parkinson’s disease: a systematic review. J Parkinsons Dis (2016) ; 6: : 453–462. |
54. | Ramig LO , Countryman S , Thompson LL , et al. Comparison of two forms of intensive speech treatment for Parkinson disease. J Speech Hear Res (1995) ; 38: : 1232–1251. |
55. | Maas JJL , De Vries NM , Bloem BR , et al. Design of the PERSPECTIVE study: PERsonalized SPEeCh Therapy for actIVE conversation in Parkinson’s disease (randomized controlled trial). Trials (2022) ; 23: : 274. |
56. | Theodoros DG , Hill AJ and Russell TG. Clinical and quality of life outcomes of speech treatment for parkinson’s disease delivered to the home via telerehabilitation: A noninferiority randomized controlled trial. Am J Speech Lang Pathol (2016) ; 25: : 214–232. |
57. | Tamplin J , Morris ME , Marigliani C , et al. ParkinSong: Outcomes of a 12-month controlled trial of therapeutic singing groups in Parkinson’s disease. J Parkinsons Dis (2020) ; 10: : 1217–1230. |
58. | Theodoros D. Telerehabilitation for communication and swallowing disorders in Parkinson’s disease. J Parkinsons Dis (2021) ; 11: : S65–S70. |
59. | Eriksson K , Forsgren E , Hartelius L , et al. Communication partner training of enrolled nurses working in nursing homes with people with communication disorders caused by stroke or Parkinson’s disease. Disabil Rehabil (2016) ; 38: : 1187–1203. |
60. | Clay P , Beeke S , Volkmer A , et al. A Communication partner training program delivered via telehealth for people living with Parkinson’s (Better Conversations With Parkinson’s): protocol for a feasibility study. JMIR Res Protoc (2023) ; 12: : e41416. |
61. | Troche MS , Okun MS , Rosenbek JC , et al. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: a randomized trial. Neurology (2010) ; 75: : 1912–1919. |
62. | Troche MS , Curtis JA , Sevitz JS , et al. Rehabilitating cough dysfunction in Parkinson’s disease: a randomized controlled trial. Mov Disord (2023) ; 38: : 201–211. |
63. | Talebi AH , Ypinga JHL , De Vries NM , et al. Specialized versus generic allied health therapy and the risk of Parkinson’s disease complications. Mov Disord (2023) ; 38: : 223–231. |
64. | Dismore L , Montague K , Carvalho L , et al. A protocol for the evaluation of a wearable device for monitoring of symptoms, and cueing for the management of drooling, in people with Parkinson’s disease. PLoS One (2023) ; 18: : e0280727. |
65. | Scobie S , Jowett S , Lambe T , et al. Lee Silverman Voice Treatment versus standard speech and language therapy versus control in Parkinson’s disease: preliminary cost-consequence analysis of the PD COMM pilot randomised controlled trial. Pilot Feasibility Stud (2021) ; 7: : 154. |
66. | Clarke CE , Patel S , Ives N , et al. Physiotherapy and occupational therapy vs no therapy in mild to moderate Parkinson disease: a randomized clinical trial. JAMA Neurol (2016) ; 73: : 291–299. |
67. | Frazzitta G , Bertotti G , Riboldazzi G , et al. Effectiveness of intensive inpatient rehabilitation treatment on disease progression in parkinsonian patients: a randomized controlled trial with 1-year follow-up. Neurorehabil Neural Repair (2012) ; 26: : 144–150. |
68. | Frazzitta G , Maestri R , Bertotti G , et al. Intensive rehabilitation treatment in early Parkinson’s disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair (2015) ; 29: : 123–131. |
69. | Ferrazzoli D , Ortelli P , Riboldazzi G , et al. Effectiveness of Rotigotine plus intensive and goal-based rehabilitation versus Rotigotine alone in ``de-novo’’ Parkinsonian subjects: a randomized controlled trial with 18-month follow-up. J Neurol (2018) ; 265: : 906–916. |
70. | Ferrazzoli D , Ortelli P , Zivi I , et al. Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry (2018) ; 89: : 828–835. |
71. | Tickle-Degnen L , Ellis T , Saint-Hilaire MH , et al. Self-management rehabilitation and health-related quality of life in Parkinson’s disease: A randomized controlled trial. Mov Disord (2010) ; 25: : 194–204. |
72. | Wade DT , Gage H , Owen C , et al. Multidisciplinary rehabilitation for people with Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry (2003) ; 74: : 158–162. |
73. | Stożek J, Rudzińska M, Pustułka-Piwnik U, et al. In-patient multidisciplinary rehabilitation for Parkinson’s disease: A randomized controlled trial. Mov Disord (2015) ; 30: : 1050–1058. |
74. | Monticone M , Ambrosini E , Laurini A , et al. In-patient multidisciplinary rehabilitation for Parkinson’s disease: A randomized controlled trial. Mov Disord (2015) ; 30: : 1050–1058. |
75. | Gage H , Grainger L , Ting S , et al. Specialist rehabilitation for people with Parkinson’s disease in the community: a randomised controlled trial. NIHR Journals Library, Southampton (UK), (2014) . |
76. | Zaman MS , Ghahari S and McColl MA. Barriers to accessing healthcare services for people with Parkinson’s disease: a scoping review. J Parkinsons Dis (2021) ; 11: : 1537–1553. |
77. | Roberts AC , Rafferty MR , Wu SS , et al. Patterns and predictors of referrals to allied health services for individuals with Parkinson’s disease: A Parkinson’s foundation (PF) QII study. Parkinsonism Relat Disord (2021) ; 83: : 115–122. |
78. | Hamid E , Ayele BA , Massi DG , et al. Availability of therapies and services for Parkinson’s disease in Africa: a continent-wide survey. Mov Disord (2021) ; 36: : 2393–2407. |
79. | Lubomski M , Rushworth RL , Lee W , et al. A cross-sectional study of clinical management, and provision of health services and their utilisation, by patients with Parkinson’s disease in urban and regional Victoria. J Clin Neurosci (2013) ; 20: : 102–106. |
80. | Schrag A , Khan K , Hotham S , et al. Experience of care for Parkinson’s disease in European countries: a survey by the European Parkinson’s Disease Association. Eur J Neurol (2018) ; 25: : 1410–e1120. |
81. | Dorsey ER and Bloem BR. The Parkinson pandemic-a call to action. JAMA Neurol (2018) ; 75: : 9–10. |
82. | Ben-Shlomo Y , Darweesh S , Llibre-Guerra J , et al. The epidemiology of Parkinson’s disease. Lancet (2024) ; 403: : 283–292. |
83. | Jellish J , Abbas JJ , Ingalls TM , et al. A system for real-time feedback to improve gait and posture in Parkinson’s disease. IEEE J Biomed Health Inform (2015) ; 19: : 1809–1819. |
84. | Ho AK , Bradshaw JL , Iansek R , et al. Speech volume regulation in Parkinson’s disease: effects of implicit cues and explicit instructions. Neuropsychologia (1999) ; 37: : 1453–1460. |
85. | El Hayek M , Lobo Jofili Lopes JLM , LeLaurin JH , et al. Type, timing, frequency, and durability of outcome of physical therapy for Parkinson disease: A systematic review and meta-analysis. JAMA Netw Open (2023) ; 6: : e2324860. |




