Stemming the Tide: The Proactive Role of Allied Health Therapy in Parkinson’s Disease
Abstract
Motor and nonmotor symptoms occur in early Parkinson’s disease (PD), or even in the prodromal stage. Many of these symptoms can be addressed by allied health therapies, including physical therapy, occupational therapy, speech therapy, and psychological therapies. However, referrals to these services early in the disease are low. We provide a review summarizing the efficacy of proactive allied health interventions on motor and nonmotor symptoms and daily function in prodromal and early disease. We also highlight areas for additional research and provide recommendations to improve care for individuals with early PD within each discipline. We recognize the overlapping roles of the allied health disciplines and support integrated or transdisciplinary care beginning soon after diagnosis to help stem the tide in the progression of PD symptoms and disability.
Plain Language Summary
Many people with Parkinson’s disease start having symptoms years before their diagnosis. These symptoms can affect movement, communication, mood, work, and other aspects of daily life. Allied health therapies can be used soon after diagnosis, or even when diagnosis is suspected, to address these challenges proactively. This article reviews the roles of physical, occupational, speech, and psychological therapies. We highlight interventions for early Parkinson’s disease that are strongly supported by research, such as exercise and self-management.
INTRODUCTION
Neuronal damage in Parkinson’s disease (PD) begins in the decades prior to diagnosis, and can lead to early motor and nonmotor impairments, as well as functional limitations [1, 2]. Visible motor symptoms, including tremor, bradykinesia, and rigidity, lead to a PD diagnosis; however, other motor symptoms can present before or soon after diagnosis, including altered gait, handwriting, turning in bed, facial expressions, and speech [3]. Nonmotor symptoms such as cognitive dysfunction and neuropsychiatric symptoms (NPS; e.g., depression, anxiety) can also occur at or before diagnosis [4]. Both motor and nonmotor symptoms can have a significant impact on quality of life (QoL) [5]. Although symptoms are likely to be mild at the time of diagnosis, early screening can be an important form of secondary or tertiary prevention, such as secondary prevention of falls [6] and tertiary prevention of affective disorders through psychological therapies [7].
Allied health therapies, including rehabilitation and psychology, can support people with early PD by addressing the impairments and functional limitations identified at diagnosis. Multiple therapies have been identified as important in the care of people with PD (PwP) [8]. This review focuses on the roles of physical therapy (PT), occupational therapy (OT), speech and language therapy (ST), and psychological therapies in prodromal and early PD, as they have a robust body of evidence, including strong randomized controlled trials (RCT) [9–12], meta-analyses [7, 13–16], and systematic reviews [6, 17–25] that guide clinical practice recommendations [26–28].
Current utilization of allied health therapies in PD is low in early PD. An international analysis of rehabilitation referrals from 23 expert care centers showed that in early PD (Hoehn and Yahr [HY] Stage 1-2, n = 3088), referral rates were 18.8% for PT, 3.3% for ST, and less than 1% for OT [29]. Of all participants with early PD, 23% received referral to a single discipline whereas 6.3% received referrals to multiple disciplines [29]. Integrated multidisciplinary treatment programs are beneficial in early PD [30], but are not common due to access and reimbursement concerns. Given the presence of early motor and nonmotor challenges experienced by PwP [5, 31], care and outcomes may improve if allied health professionals increase their role in early assessment, education, and intervention compared to current utilization. There may also be a role for allied health therapies to screen for PD-relevant signs or symptoms when prodromal PD is suspected based on the presence of REM sleep behavior disorder (RBD) and/or hyposmia [32].
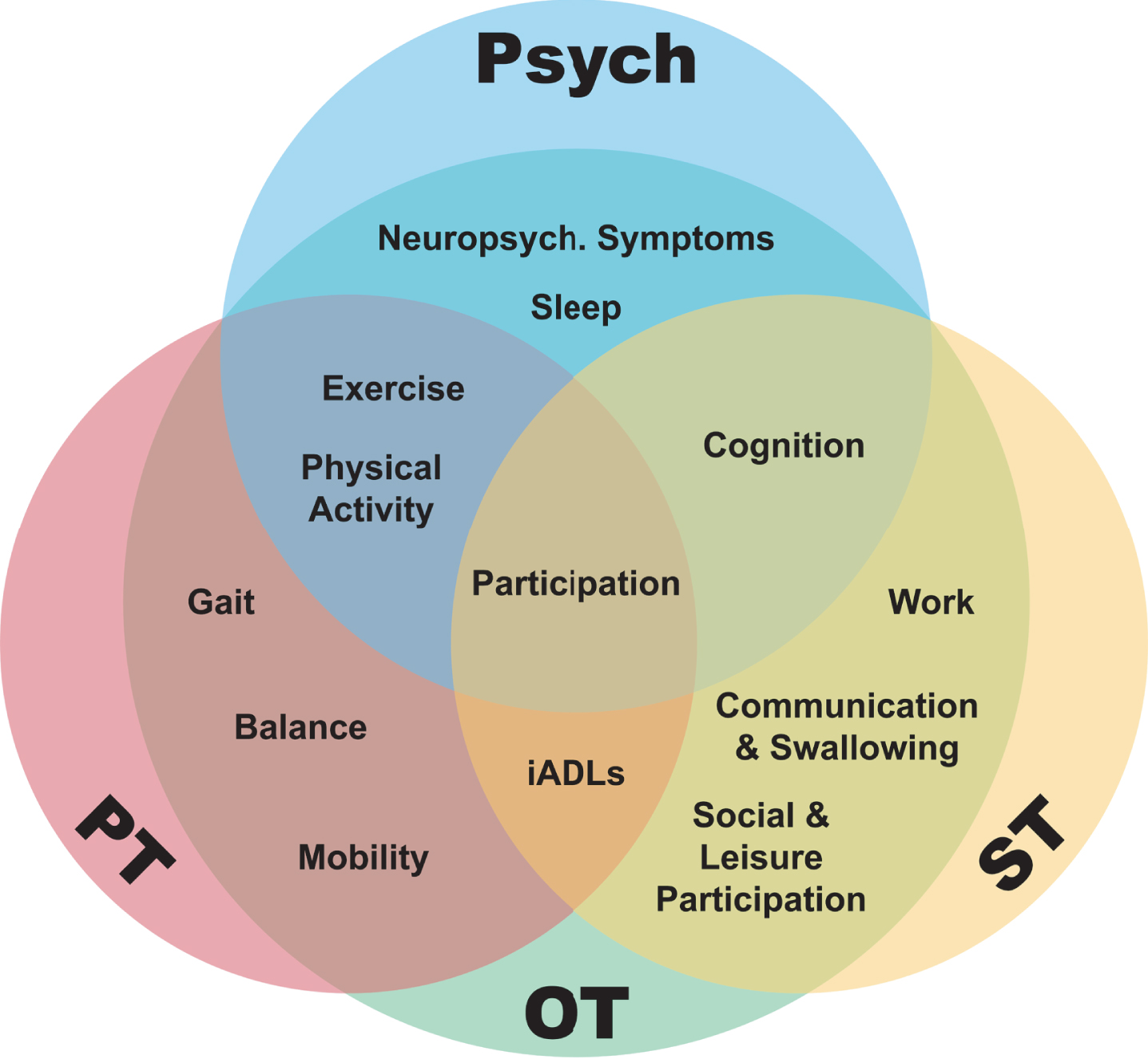
The purpose of this review was to outline the role of proactive PT, OT, ST, and psychological therapies in prodromal and early PD. We synthesized the evidence within each discipline, acknowledging that different disciplines can address similar targets with unique conceptual approaches. An opportunity for transdisciplinary care, or uniting frameworks and approaches across disciplines is demonstrated in Fig. 1 [33]. We identified knowledge gaps and made recommendations for improved integrated care of people with early PD.
Fig. 1
A transdisciplinary model demonstrating overlap between allied health disciplines and many of the target areas they address in early Parkinson’s disease. PT, physical therapy; OT, occupational therapy; ST, speech and language therapy; iADLs, instrumental activities of daily living.
METHODS
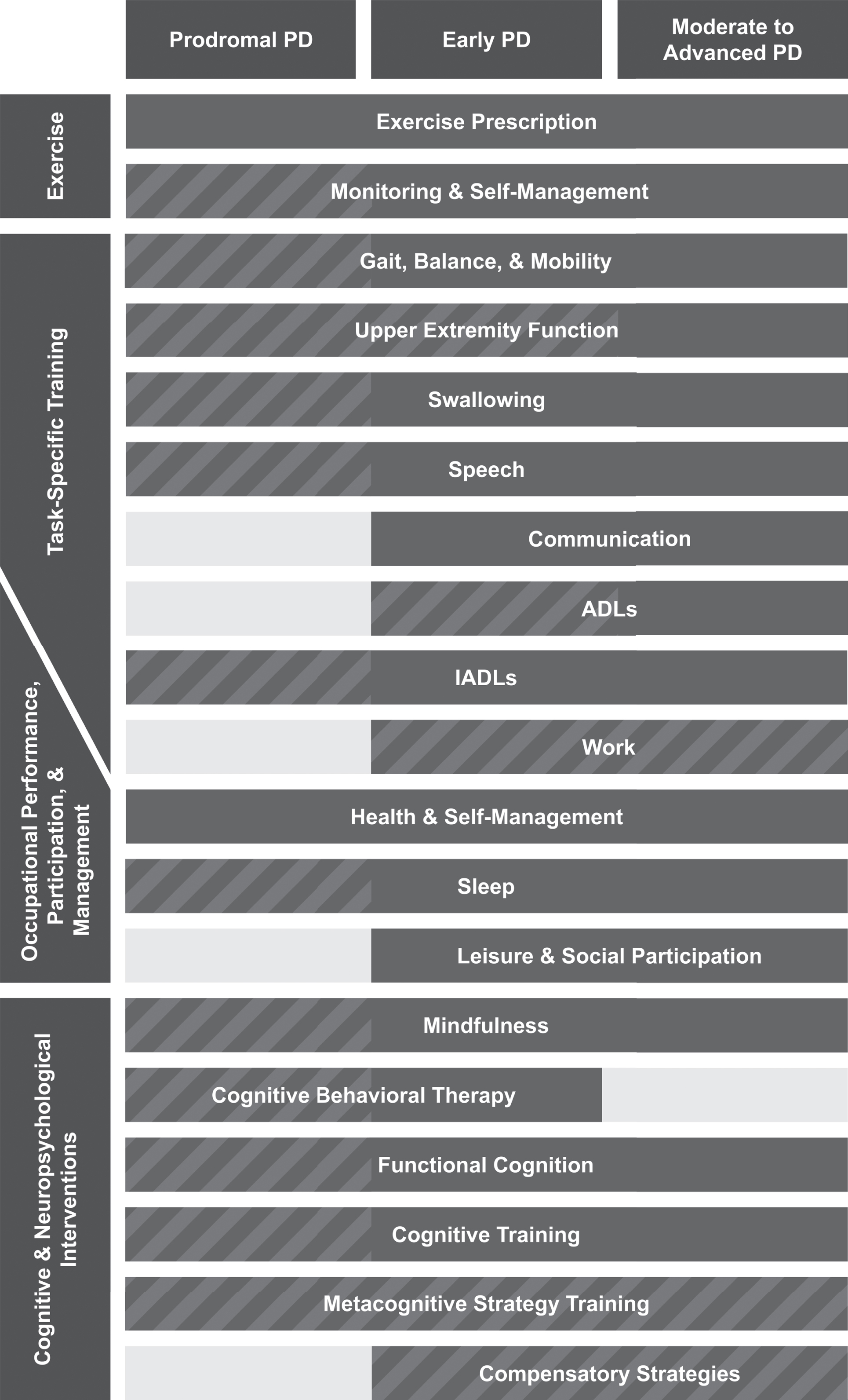
This narrative review began with two authors (MRR, RAL) determining the allied health fields with the strongest levels of evidence. Additional authors were identified (ERF, ACR) to ensure expertise in each selected field. The authors synthesized information from clinical practice guidelines (CPG), systematic reviews, RCT, and epidemiologic studies. Additional input was provided by an individual with a lived experience of PD (LLJ). Overlap identified across allied health fields is shown in Fig. 1. Recommendations were made based on consensus of the authors and are visualized in Fig. 2.
Fig. 2
Key Takeaways: Summary of recommendations on appropriate timing of allied health interventions for Parkinson’s disease (PD). Each allied health discipline has multiple key interventions that can benefit people with early, or even prodromal PD. The figure displays our recommended timing of allied health interventions through three time periods: prodromal PD, early PD (soon after diagnosis, mild symptoms), and moderate-advanced PD. Striped bars indicate where more research is needed. We recommend using an integrated care approach, where team members have open communication with each other and the people with PD regarding shared and overlapping goals and intervention strategies. ADL, activities of daily living; IADL, instrumental activities of daily living.
PHYSICAL THERAPY FOR EARLY PARKINSON’S DISEASE
The goal of PT includes “optimizing movement to improve the human experience” [34]. In early PD, movement limitations can begin with a general decrease in physical activity (steps per day) and exercise (minutes of moderate intensity walking per week), even before changes in walking speed or endurance can be measured [35]. Nonmotor symptoms including anxiety, apathy, and fatigue can also impact physical activity in prodromal or early PD [36–38]. Work in consultative PT programs for individuals with newly diagnosed PD suggests that some individuals have very early deficits in gait and balance [39]. Basic mobility, such as turning in bed, has also been reported as more difficult up to four years before diagnosis [36]. Other impairments that are common in people with prodromal or early PD include urinary dysfunction and pain (musculoskeletal, dystonic, radicular, central) [40, 41]. PT or other providers can address many of these symptoms (Fig. 1) [42, 43].
Exercise and/or PT in PD are supported by a CPG based on a systematic review [26], as well as independent meta-analyses [13, 18]. In early PD, studies describe moderate-vigorous intensity aerobic exercise as potentially neuroprotective and thus an important intervention that may slow functional decline [9, 44–46]. Moderate-vigorous aerobic and progressive resistance exercise are associated with improvements and long-term maintenance of motor function, QoL, and nonmotor features of PD like cognition and mood [9, 26, 46]. However, less than a third of PwP achieve recommended levels of exercise [47]. Thus, international guidelines recommend that neurologists consider referral to PT early in the disease process for personalised exercise progression [48–51]. PT can play an important role in helping PwP optimize guideline-concordant exercise participation through recognition of an individual’s unique barriers and facilitators to exercise, tailored exercise prescription, and long-term monitoring [39, 44]. The majority of PT and exercise trials include individuals with HY Stage 1–3 [26], but skilled exercise prescription would likely be beneficial in prodromal PD based on epidemiologic research [32]. Exercise prescription is complex, as it involves aerobic, strengthening, balance, and flexibility activities, which can be overwhelming without the guidance of a skilled PT or other experienced exercise professionals [26, 52]. There is high quality evidence that physical therapists should use behavior change approaches to help improve physical activity and QoL [26]. However, in a transdisciplinary model, other disciplines such as OT and psychology can also address exercise self-management and/or health behavior change (Fig. 1).
Evidence supports the role of PT in addressing several other challenges in people with mild-moderate PD (HY 1–3) [26]. For gait, balance and mobility challenges, strong evidence supports patient education, external cueing, task-specific functional training, and dual-task training [26, 53]. PT can address urinary dysfunction with behavioral and task-specific interventions [26, 40]. Finally, PT may be able to address pain through tailored exercise prescription [54].
A proactive approach to care in early PD should include PT delivery of exercise prescription, community-based exercise recommendations, and long-term exercise monitoring and progression. Additionally, it should include screening for early walking or mobility problems, urinary dysfunction, and pain that can occur even in prodromal PD [32]. Despite the predominance of research studies including PwP with mild symptoms [26], including publications supporting real-world implementation [55], there is a misperception among many medical professionals that PT is not warranted until a PwP experiences a substantial physical decline. Pragmatic clinical trials and implementation research are needed to evaluate effective interventions in prodromal or early PD.
OCCUPATIONAL THERAPY FOR EARLY PARKINSON’S DISEASE
The goal of OT is to promote performance of and participation in meaningful daily activities and roles in the home and community. Due to its broad scope, OT is well placed to screen for and address many deficits in early PD as part of a transdisciplinary team (Fig. 1), but utilization of OT is lower than other disciplines [29]. PwP experience performance and participation restrictions early in the disease course. Even in prodromal PD, people report limitations in activities of daily living (ADL), particularly instrumental ADL (IADL; e.g., handwriting, medication and financial management), and reduced health-related QoL compared with healthy controls [2, 56]. In early PD, while they are typically independent in ADL, difficulties or slowness in ADL, IADL, and social functioning are common. These difficulties can stem from symptoms such as fatigue, reduced psychomotor speed, cognitive dysfunction, subtle gait abnormalities, and decreased upper extremity strength and dexterity [38, 57–59]. Additionally, individuals with early PD report decreased engagement in instrumental, leisure and social activities [60, 61], which is associated with reduced health-related QoL [61] and may lead to further functional decline.
Research on early OT for PD shows promise. CPGs advise OT soon after diagnosis to address the needs of individuals early in the disease [27, 48, 51]. A recent meta-analysis reported growing evidence supporting the efficacy of OT for improving daily function and QoL in PD [14, 27]; however, specific evidence for proactive OT in prodromal or early PD is in its infancy. Perhaps the strongest support for early OT in this population comes from a large RCT in which 62% of PD participants had mild disease (HY 1-2) [11]. In this study, ten-weeks of home-based, tailored OT improved perceived performance and satisfaction in personally meaningful daily activities, positively influenced the QoL of caregivers, and led to significant cost-savings in terms of institutional care [11, 62]. Another group demonstrated improved finger, hand, upper extremity and daily activity function from an intensive four-week hospital-based multidisciplinary rehabilitation program for people with early PD (HY < 3) that included one hour of tailored OT five days/week [30, 63, 64]. Conversely, a large pragmatic RCT found no effect of outpatient OT and PT on IADLs and minimal therapy-related improvements in QoL in individuals with mild to moderate PD (67% HY 1-2) [65]. The small effects may have been due to a low dose of therapy (average four visits over eight weeks), lack of appropriate interventions (e.g., little use of home exercise), and measures that did not target the problems faced in early PD.
Despite emerging evidence supporting the early involvement of OT [59], referral to OT frequently occurs late in the disease when there is significant functional disability and caregiver strain. There is an opportunity for a transdisciplinary approach in early PD as there is significant overlap of OT scope of practice with other allied health providers (Fig. 1). Additionally, OT in early PD should address self-management, promotion and maintenance of current participation, and enhancement of activity performance [11, 27, 64]. For example, soon after diagnosis, PwP may experience challenges due to the impact of fatigue on cognitive performance, which could negatively affect complex activities such as work, driving, health management, and social participation. More research is needed to determine if there is efficacy for OT for prodromal or early PD to determine critical features, such as appropriate outcome measures, treatment approaches, intervention settings, dose, and intensity.
SPEECH AND LANGUAGE THERAPY FOR EARLY PARKINSON’S DISEASE
Often working within a transdisciplinary care model, the goal of ST is to provide education, counseling, assessment, and therapeutic approaches directed towards remediating communication and swallowing impairments that can result in risk to health and function. Symptoms like hypokinetic dysarthria and vocal fatigue are detectable in early disease stages [66]. Changes in voice and articulation can be detected acoustically even in prodromal disease [36, 67]. Language problems that disrupt the natural flow of speech (such as inappropriate pauses and word retrieval difficulties), difficulty processing and conveying emotions, and difficulty processing complex sentence structures can lead to less effective communication and increased frequency of conversation breakdowns [68, 69], PwP are aware of their communication and swallowing changes at the time of diagnosis, with speech symptoms perceived as progressing more rapidly in the first six years following diagnosis [70–72]. While often mild in early PD, these changes can interfere with communication participation and work activities [72]. Recent studies have also identified swallowing problems in early PD, including dysphagia and sialorrhea [73, 74]. These concerns highlight an important role for ST in providing early education and counseling that can promote timely self-referral at the onset of speech and swallowing concerns. However, there are no robust randomized trials addressing early dysphagia or speech therapies as a preventative strategy for delaying or lessening the severity of these symptoms.
Clinical guidelines, consensus statements, and expert opinion support early engagement of ST for education and management of communication and swallowing changes [8, 28, 75, 76]. Recent meta-analyses highlighted the effectiveness of high intensity, target-specific, exercise-based programs for improving voice loudness [15, 19], respiratory support [20], and speech intelligibility [19]. However, evidence for long-term maintenance of improvements, other than in sustained vowel volume, are less robust [21]. Recent evidence suggests that device-mediated interventions may have positive effects on vocal mechanism efficiency and improve communication compared to high intensity exercise programs [77]. Swallowing-related interventions, which can include transdisciplinary care models (ST, OT, and dieticians) (Fig. 1) in early PD may include pharmacotherapy and nutritional optimization, compensatory strategies for sensorimotor changes including sialorrhea management, and system-targeted oral, respiratory and pharyngeal exercises [16, 21, 75]. Although the need for diet modification may be less likely in early PD, there is international consensus that management should be initiated at the first onset of clinical or instrumental evidence of swallowing changes, regardless of disease stage [75]. A recent systematic review highlighted emerging evidence that respiratory muscle and cough strength training may be beneficial for targeting respiratory components of swallowing and airway protection in early PD [22, 75, 78]. The effectiveness of individualized dysphagia management plans in slowing or mitigating dysphagia in prodromal and early PD is unclear, as recommendation are based on PwP with moderate-advanced disease [22].
Despite the hypothetical benefit of early ST intervention, there is little experimental or clinical trial evidence in support of proactive or preventive ST in prodromal or early PD. In addition to screening and education targeting self-identification and self-management [8], early ST intervention should consider interventions to minimize risk of vocal hyperfunction [79], address early swallowing and saliva management changes [72, 76], and identify strategies targeting communication and functional cognitive challenges at work and home with consideration to minimizing stigma [76, 80]. These strategies may improve QoL, prolong time in the workforce, and reduce social isolation.
PSYCHOLOGICAL THERAPIES IN EARLY PARKINSON’S DISEASE
The goal of psychological therapies in prodromal or early PD includes evaluating changes in cognitive function, mood, and behavior. These commonly distressing symptoms are associated with reduced QoL, increased care burden, and early nursing home placement [81–83]. A recent survey highlighted that these nonmotor symptoms are unmet priority areas for PwP [84].
Cognitive dysfunction
At the time of diagnosis, approximately two-thirds of PwP demonstrate some cognitive impairment [85], and an epidemiologic study reported subjective cognitive changes prior to diagnosis in approximately one-third of PwP [37]. There are currently no disease modifying treatments for cognitive dysfunction in PD [86]. Several psychological specialties may be involved (e.g., neuropsychologists) to identify cognitive dysfunction and to provide recommendations to the medical team, such as starting pharmacological treatments for cognition or mood. However, there is growing evidence for the efficacy of psychological therapies to promote cognitive reserve, such as cognitive training, as secondary prevention of cognitive decline [87].
Cognitive-focused interventions may be transdisciplinary and implemented by neuropsychologists, OT, or ST (Fig. 1) [23]. Cognitive training involves repetitive guided practice on standard cognitive tasks, such as attention, executive function or visuospatial function [87]. An RCT in PwP with normal cognition or PD-MCI (HY 1–3) found that structured cognitive training was associated with improved short-term and working memory [12]. Findings suggest that cognitive training may prevent or delay cognitive dysfunction in PwP [88] and has been associated with improved cognitive performance over six months [89]. Strategy-based interventions, which involve teaching people metacognitive, compensatory, or adaptive techniques to circumvent cognitive limitations and accomplish ADL, are emerging as potentially effective for supporting functional cognitive performance (functional cognition) in mild-moderate PwP without dementia [90, 91]. Finally, a recent systematic review and meta-analysis [13] highlighted that exercise can improve cognitive performance [24], with growing interest in people with mild deficits or prodromal PD [86, 92]. Aerobic exercise, strength training, or general exercise could be facilitated by health psychologists, OT, or PT as part of a transdisciplinary approach (Fig. 1).
Neuropsychiatric symptoms
It is increasingly recognized that NPS occur early in disease [4], with a recent systematic review finding that some symptoms precede the onset of motor symptoms [93]. An epidemiology study reported that anxiety, low mood, and sleep dysfunction preceded motor symptoms by several years, while prodromal hallucinations preceded motor symptoms by an average of 12 months [37]. Additionally, use of psychological services increases in the years preceding PD diagnosis, suggesting that psychological function is altered in prodromal PD [94]. However, NPS are under-recognized and treatment options are limited, particularly early in the disease [95]. Neuropsychologists conduct comprehensive evaluations of mood and behavior changes early after diagnosis, which help establish a baseline and identify emerging symptoms, particularly those that may impact work and relationships. Other specialities, such as health psychologists, rehabilitation or vocational counsellors, sex therapists, and social workers, can also be involved in assessing and managing symptoms.
Several psychological therapies have been proposed for the management of NPS to address adapting to and accepting changes in physical function, cognition and mood, as well as the fear surrounding these changes [6, 96–98]. Therapies can address changes in personal identity or family roles following diagnosis of a chronic disease, fear of anticipated and unanticipated changes in function, as well as perceived stigma related to PD [98–101]. These fears and changes in perceived identity can immediately follow diagnosis, negatively impacting QoL and potentially leading to less participation, increased isolation, and reduced mental wellbeing [6, 27, 80]. A meta-analysis found that the most common psychological interventions are cognitive behavioral therapy (CBT) and mindfulness [17], although acceptance and commitment therapy (ACT) and psychoeducation are increasingly being used in PwP to manage NPS [102].
Despite growing evidence highlighting the role of psychological therapies as part of a team-based approach, there is inconsistency in access and referrals, particularly in prodromal or early PD. Understanding cognitive dysfunction and NPS in prodromal PD is poor due to a lack of research in these areas [103]. Heterogeneous intervention delivery components, such as dose, duration, and setting (e.g., group or individual, in person or virtual) make it difficult to synthesize the evidence. Recent systematic and scoping reviews highlighted the limited availability of high-quality, consistent results on the efficacy of CBT, ACT, mindfulness, and psychoeducation for NPS management [25, 102]. Notably, existing studies focus on treating existing symptoms, not on preventing symptoms or determining the optimal time to initiate interventions.
RECOMMENDATIONS FOR CARE
The lack of consensus surrounding the efficacy of early engagement with allied health professions has resulted in CPGs that stop short of an explicit recommendation that clinicians should refer to allied health professions soon after diagnosis. Instead, CPGs state that clinicians “should consider” referrals [48–51]. This ambiguity lets referrers rely on clinical reasoning, taking into account Person-centered needs at different times in the disease process. Well-designed PD-specific RCTs demonstrate the efficacy of exercise [9, 10], self-management interventions [11], swallowing training [78], cognitive training [12], and CBT [102] in people with early PD or mild symptoms. Yet, the feasibility and effectiveness of delivering these evidence-based interventions at the appropriate dose to people in the real world with early PD is unknown. Thus, when PwP are newly diagnosed, the referrer or the PwP may question the benefits of participating in proactive allied health therapies. Based on the emerging evidence and our clinical and lived experience, we recommend initiating allied health therapies for screening, education, and intervention as soon as possible, particularly related to the importance of exercise. Since some individuals may not have accepted or adjusted to their diagnosis, or may lack the time to participate in allied health therapies soon after diagnosis, physicians should discuss the benefits of allied health therapies and exercise at least annually [104].
Figure 2 summarizes our recommendations on the timing of allied health interventions, recognizing that some areas of intervention have stronger evidence (solid bars) than others (striped bars). Solid bars are used in prodromal PD only for exercise and health-related self-management, as these have strong evidence in healthy older adult populations [105]. Additionally, regular exercise may be associated with reduced risk of developing PD [106]. Figure 2 focuses on the timing of interventions, recognizing that the disciplines delivering these interventions may overlap or vary from one region to another, as depicted in the transdisciplinary model in Fig. 1.
We recommend PwP receive regular follow-up assessments every 6-12 months by allied health professionals to screen for new functional concerns, provide education and interventions for the secondary prevention of declines in function, and to manage any new problems that are observed [44, 45]. We recommend this ‘dental model’ of rehabilitation [44, 47], so that allied health professionals can monitor change over time, coach on disease self-management, inform timely and relevant intervention, and adjust treatment goals. This long-term care approach also facilitates the early development of relationships between expert allied health professionals, their patients, and their families or care partners. It allows the care team to be respectful of and responsive to the PwP’s individual preferences, needs, values, and experiences. The allied health team can empower PwP to be active participants in their own health management after diagnosis. With proper education PwP can learn how to monitor and document the progression of their symptoms, including use of wearable devices, such as smart watches or other sensors, to monitor long-term symptoms and participation in real time, while measuring subtle changes between visits [107, 108]. The team can then help the individual address concerns early and adapt to the disease as it progresses and individual needs and priorities change.
LIMITATIONS OF CURRENT RESEARCH AND RECOMMENDATIONS FOR FUTURE RESEARCH
To support these recommendations, more research is needed on the effectiveness of allied health therapies in prodromal and early PD. Past effectiveness trials delivered OT and PT interventions at a low dose and failed to achieve great gains [65, 109]. Continued efficacy research is needed for many allied health interventions for PD specifically focused on prodromal or early PD. However, the more strongly supported interventions (e.g., exercise, health self-management) need to be moved into pragmatic clinical effectiveness or implementation studies. Additionally, short- and long-term cost-effectiveness research is needed to show the value of intervening early. Value needs to be shown related to clinical outcomes, such as QoL, improved patient coping with a chronic condition, satisfaction with care, and long-term cost-savings.
More research is also needed on measures with greater sensitivity in early disease to assist with screening, long-term monitoring and self-management of PD. Screening tools need to detect subtle impairments and functional changes that can be present early in PD [36]. For example, current tools to measure cognitive dysfunction and NPS are not sensitive enough to detect early subtle changes, particularly in prodromal PD [110]. Assessments that focus on higher level IADLs, or those that measure the concerns and goals of the PwP and their care partners should be used to guide patient-centered care in early PD [27]. The psychometric properties of such screening and outcome measures must be validated for the prodromal and early PD populations.
Additionally, research and allied health capacity building should address limited access to care in different countries and cultures. Limitations of this review include that the supporting research was primarily conducted in the United States and Europe and does not reflect the challenges to integrated care in low- and middle-income countries (LMIC). LMIC can lack specialist providers and centers and may require alternative healthcare models [111]. LMICs as well as traditional healthcare environments may improve access to therapies by utilizing a transdisciplinary approach (Fig. 1), allowing one lead allied health professional to address goals across disciplines due to a shortage of PD expert providers. Other allied health providers, including social workers and dieticians, also play a vital role in the care of PwP in different care centers. Similar to exercise, diet could play a potential neuroprotective role in prodromal PD based on epidemiologic research and should be considered in future proactive allied health research [112]. With the growth of efficacious telehealth models [113–115], local access to allied health providers would facilitate care but should not prevent referral to services. Additionally, digital health solutions are being developed that could increase access for PwP in LMIC, rural, or other underserved areas. Although promising, digital health technologies are still not widely adopted [116]. Additional research on implementation of technologies into real-world practice may improve self-management by people with early PD [117].
CONCLUSION
With the high prevalence of early and prodromal motor and nonmotor challenges experienced by PwP, allied health professionals should play a greater role in screenings, assessment, early education, and early intervention to improve care and outcomes. Through these activities, allied health professionals can play a key role in secondary prevention in PD, slowing future functional decline in multiple domains. They can also empower PwP to understand, manage, and maintain current performance and participation. Additionally, they can play a key role in education to help PwP and their care partners adapt to, cope with, and prepare for life changes as their PD is diagnosed and progresses. However, more research is needed to support the clinical and cost-effectiveness of early therapy, outcome measurement, self-management with long-term follow-up, and access concerns related to the use of allied health therapies in early and prodromal PD.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
MRR received grant support from the National Institute of Child Health & Human Development of the National Institutes of Health (R01HD110668), the United States Department of Defence Congressionally Directed Medical Research Programs (W81XWH-19-PRP-EIRA), and the Parkinson’s Foundation. ERF received grant support from the National Institute on Aging (R01AG065214). ACR’s research reported in this article was supported in part by the Canada Research Chairs program. RAL is supported by the National Institute for Health and Care Research Newcastle Biomedical Research Centre and by grants from Parkinson’s UK (F-1801). These contents, however, do not necessarily represent the policy or endorsement of any of the funding sources.
CONFLICT OF INTEREST
ACR received honoraria and travel reimbursements from the Movement Disorders Society and World Parkinson Congress. She also consults for Winterlight, Inc. and holds three patents on wireless medical sensors and methods. No other authors have conflicts of interest to report.
REFERENCES
[1] | Braak H , Del Tredici K , Rüb U , de Vos RA , Jansen Steur EN , Braak E ((2003) ) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: , 197–211. |
[2] | Foubert-Samier A , Helmer C , Le Goff M , Guillet F , Proust-Lima C , Jacqmin-Gadda H , Dartigues J-F , Amieva H , Tison F ((2020) ) Cognitive and functional changes in prediagnostic phase of Parkinson disease: A population-based study. Parkinsonism Relat Disord 79: , 40–46. |
[3] | Postuma RB , Lang AE , Gagnon JF , Pelletier A , Montplaisir JY ((2012) ) How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 135: , 1860–1870. |
[4] | Dlay JK , Duncan GW , Khoo TK , Williams-Gray CH , Breen DP , Barker RA , Burn DJ , Lawson RA , Yarnall AJ ((2020) ) Progression of neuropsychiatric symptoms over time in an Incident Parkinson’s Disease Cohort (ICICLE-PD). Brain Sci 10: , 78. |
[5] | Gulunay A , Cakmakli GY , Yon MI , Ulusoy EK , Karakoc M ((2020) ) Frequency of non-motor symptoms and their impact on the quality of life in patients with Parkinson’s disease: A prospective descriptive case series. Psychogeriatrics 20: , 206–211. |
[6] | Liu WY , Tung TH , Zhang C , Shi L ((2022) ) Systematic review for the prevention and management of falls and fear of falling in patients with Parkinson’s disease. Brain Behav 12: , e2690. |
[7] | Hong CT , Tan S , Huang TW ((2021) ) Psychotherapy for the treatment of anxiety and depression in patients with Parkinson disease: A meta-analysis of randomized controlled trials. J Am Med Dir Assoc 22: , 2289–2295e2282. |
[8] | Goldman JG , Volpe D , Ellis TD , Hirsch MA , Johnson J , Wood J , Aragon A , Biundo R , Di Rocco A , Kasman GS , Iansek R , Miyasaki J , McConvey VM , Munneke M , Pinto S , St. Clair KA , Toledo S , York MK , Todaro R , Yarab N , Wallock K ((2024) ) Delivering multidisciplinary rehabilitation care in Parkinson’s disease: An international consensus statement. J Parkinsons Dis 14: , 135–166. |
[9] | Schenkman M , Moore CG , Kohrt WM , Hall DA , Delitto A , Comella CL , Josbeno DA , Christiansen CL , Berman BD , Kluger BM , Melanson EL , Jain S , Robichaud JA , Poon C , Corcos DM ((2018) ) Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol 75: , 219–226. |
[10] | Corcos DM , Robichaud JA , David FJ , Leurgans SE , Vaillancourt DE , Poon C , Rafferty MR , Kohrt WM , Comella CL ((2013) ) A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord 28: , 1230–1240. |
[11] | Sturkenboom IH , Graff MJ , Hendriks JC , Veenhuizen Y , Munneke M , Bloem BR , Nijhuis-van der Sanden MW ((2014) ) Efficacy of occupational therapy for patients with Parkinson’s disease: A randomised controlled trial. Lancet Neurol 13: , 557–566. |
[12] | Petrelli A , Kaesberg S , Barbe MT , Timmermann L , Fink GR , Kessler J , Kalbe E ((2014) ) Effects of cognitive training in Parkinson’s disease: A randomized controlled trial. Parkinsonism Relat Disord 20: , 1196–1202. |
[13] | Tonkin PG , Miller TD , Hartmann TE , Skein M ((2023) ) The effects of exercise on non-motor experiences of daily living experienced in Parkinson’s disease: A systematic review and network meta-analysis. Clin Park Relat Disord 9: , 100203. |
[14] | Tofani M , Ranieri A , Fabbrini G , Berardi A , Pelosin E , Valente D , Fabbrini A , Costanzo M , Galeoto G ((2020) ) Efficacy of occupational therapy interventions on quality of life in patients with Parkinson’s disease: A systematic review and meta-analysis. Mov Disord Clin Pract 7: , 891–901. |
[15] | Xu H , Bao Z , Liang D , Li M , Wei M , Ge X , Liu J , Li J ((2020) ) Speech and language therapy for voice problems in Parkinson’s disease: A meta-analysis. J Neuropsychiatry Clin Neurosci 32: , 344–351. |
[16] | Cheng I , Sasegbon A , Hamdy S ((2023) ) Dysphagia treatments in Parkinson’s disease: A systematic review and meta-analysis. Neurogastroenterol Motil 35: , e14517. |
[17] | Hofmann SG , Asnaani A , Vonk IJJ , Sawyer AT , Fang A ((2012) ) The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive Ther Res 36: , 427–440. |
[18] | Ernst M , Folkerts AK , Gollan R , Lieker E , Caro-Valenzuela J , Adams A , Cryns N , Monsef I , Dresen A , Roheger M , Eggers C , Skoetz N , Kalbe E ((2023) ) Physical exercise for people with Parkinson’s disease: A systematic review and network meta-analysis. Cochrane Database Syst Rev 1: , Cd013856. |
[19] | Muñoz-Vigueras N , Prados-Román E , Valenza MC , Granados-Santiago M , Cabrera-Martos I , Rodríguez-Torres J , Torres-Sánchez I ((2021) ) Speech and language therapy treatment on hypokinetic dysarthria in Parkinson disease: Systematic review and meta-analysis. Clin Rehabil 35: , 639–655. |
[20] | Wen X , Liu Z , Liu X , Peng Y , Liu H ((2022) ) The effects of physiotherapy treatments on dysphagia in Parkinson’s disease: A systematic review of randomized controlled trials. Brain Res Bull 188: , 59–66. |
[21] | Saleem S , Miles A , Allen J ((2023) ) A systematic review of behavioural therapies for improving swallow and cough function in Parkinson’s disease. Int J Speech Lang Pathol, 10.1080/17549507.2023.2215488. |
[22] | van de Wetering-van Dongen VA , Kalf JG , van der Wees PJ , Bloem BR , Nijkrake MJ ((2020) ) The effects of respiratory training in Parkinson’s disease: A systematic review. J Parkinsons Dis 10: , 1315–1333. |
[23] | Hindle JV , Petrelli A , Clare L , Kalbe E ((2013) ) Nonpharmacological enhancement of cognitive function in Parkinson’s disease: A systematic review. Mov Disord 28: , 1034–1049. |
[24] | da Silva FC , Iop RDR , de Oliveira LC , Boll AM , de Alvarenga JGS , Gutierres Filho PJB , de Melo L , Xavier AJ , da Silva R ((2018) ) Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: A systematic review of randomized controlled trials of the last 10 years. PLoS One 13: , e0193113. |
[25] | Roper A , Pacas Fronza G , Dobkin RD , Beaudreau SA , Mitchell LK , Pachana NA , Thangavelu K , Dissanayaka NN ((2024) ) A systematic review of psychotherapy approaches for anxiety in Parkinson’s disease. Clin Gerontol 47: , 188–214. |
[26] | Osborne JA , Botkin R , Colon-Semenza C , DeAngelis TR , Gallardo OG , Kosakowski H , Martello J , Pradhan S , Rafferty M , Readinger JL , Whitt AL , Ellis TD ((2022) ) Physical therapist management of Parkinson disease: A clinical practice guideline from the American Physical Therapy Association. Phys Ther 102: , 1–36. |
[27] | Wood J , Henderson W , Foster ER ((2022) ) Occupational therapy practice guidelines for people with Parkinson’s disease. Am J Occup Ther 76: , 7603397010. |
[28] | Kalf JG , de Swart BJM , Bonnier M , Hofman M , Kanters J , Kocken J , Miltenburg M , Bloem BR , Munneke M ((2019) ) Guidelines for speech-language therapy in Parkinson’s disease. ParkinsonNet/NPF, Nijmegen, The Netherlands / Miami (FL), USA. |
[29] | Roberts AC , Rafferty MR , Wu SS , Miao G , Cubillos F , Simuni T ((2021) ) Patterns and predictors of referrals to allied health services for individuals with Parkinson’s disease: A Parkinson’s foundation (PF) QII study. Parkinsonism Relat Disord 83: , 115–122. |
[30] | Frazzitta G , Maestri R , Bertotti G , Riboldazzi G , Boveri N , Perini M , Uccellini D , Turla M , Comi C , Pezzoli G , Ghilardi MF ((2015) ) Intensive rehabilitation treatment in early Parkinson’s disease: A randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair 29: , 123–131. |
[31] | Gaig C , Tolosa E ((2009) ) When does Parkinson’s disease begin? Mov Disord 24: (Suppl 2), S656–664. |
[32] | Summers RLS , Rafferty MR , Howell MJ , MacKinnon CD ((2021) ) Motor dysfunction in REM sleep behavior disorder: A rehabilitation framework for prodromal synucleinopathy. Neurorehabil Neural Repair 35: , 611–621. |
[33] | Rajan R , Brennan L , Bloem BR , Dahodwala N , Gardner J , Goldman JG , Grimes DA , Iansek R , Kovács N , McGinley J , Parashos SA , Piemonte MEP , Eggers C ((2020) ) Integrated care in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 35: , 1509–1531. |
[34] | American Physical Therapy Association, Vision, Mission, and Strategic Plans, https://www.apta.org/apta-and-you/leadership-and-governance/vision-mission-and-strategic-plan. |
[35] | Cavanaugh JT , Ellis TD , Earhart GM , Ford MP , Foreman KB , Dibble LE ((2012) ) Capturing ambulatory activity decline in Parkinson’s disease. J Neurol Phys Ther 36: , 51–57. |
[36] | Fereshtehnejad S-M , Yao C , Pelletier A , Montplaisir JY , Gagnon J-F , Postuma RB ((2019) ) Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain 142: , 2051–2067. |
[37] | Durcan R , Wiblin L , Lawson RA , Khoo TK , Yarnall AJ , Duncan GW , Brooks DJ , Pavese N , Burn DJ ((2019) ) Prevalence and duration of non-motor symptoms in prodromal Parkinson’s disease. Eur J Neurol 26: , 979–985. |
[38] | Morel T , Cleanthous S , Andrejack J , Barker RA , Blavat G , Brooks W , Burns P , Cano S , Gallagher C , Gosden L , Siu C , Slagle AF , Trenam K , Boroojerdi B , Ratcliffe N , Schroeder K ((2022) ) Patient experience in early-stage Parkinson’s disease: Using a mixed methods analysis to identify which concepts are cardinal for clinical trial outcome assessment. Neurol Ther 11: , 1319–1340. |
[39] | Rafferty MR , MacDonald J , Byskosh A , Sloan L , Toledo S , Marciniak C , Simuni T ((2019) ) Using implementation frameworks to provide proactive physical therapy for people with Parkinson disease: Case report. Phys Ther 99: , 1644–1655. |
[40] | Vaughan CP , Burgio KL , Goode PS , Juncos JL , McGwin G , Muirhead L , Markland AD , Johnson TM 2nd , ((2019) ) Behavioral therapy for urinary symptoms in Parkinson’s disease: A randomized clinical trial. Neurourol Urodyn 38: , 1737–1744. |
[41] | Valkovic P , Minar M , Singliarova H , Harsany J , Hanakova M , Martinkova J , Benetin J ((2015) ) Pain in Parkinson’s disease: A cross-sectional study of its prevalence, types, and relationship to depression and quality of life. PLoS One 10: , e0136541. |
[42] | Tueth LE , Duncan RP ((2021) ) Musculoskeletal pain in Parkinson’s disease: A narrative review. Neurodegener Dis Manag 11: , 373–385. |
[43] | Scott GD , Lim MM , Drake MG , Woltjer R , Quinn JF ((2021) ) Onset of skin, gut, and genitourinary prodromal Parkinson’s disease: A study of 1.5 million Veterans. Mov Disord 36: , 2094–2103. |
[44] | Ellis TD , Colón-Semenza C , DeAngelis TR , Thomas CA , Hilaire MS , Earhart GM , Dibble LE ((2021) ) Evidence for early and regular physical therapy and exercise in Parkinson’s disease. Semin Neurol 41: , 189–205. |
[45] | Quinn L , Morgan D ((2017) ) From disease to health: Physical therapy health promotion practices for secondary prevention in adult and pediatric neurologic populations. J Neurol Phys Ther 41: (Suppl 3), S46–s54. |
[46] | Rafferty MR , Schmidt PN , Luo ST , Li K , Marras C , Davis TL , Guttman M , Cubillos F , Simuni T ((2017) ) Regular exercise, quality of life, and mobility in Parkinson’s disease: A longitudinal analysis of National Parkinson Foundation Quality Improvement Initiative data. J Parkinsons Dis 7: , 193–202. |
[47] | Rafferty MR , Held Bradford EC , Fritz S , Hutchinson KJ , Miczak K , Resnick A , Billinger SA ((2022) ) Health promotion and wellness in neurologic physical therapy: Strategies to advance practice. J Neurol Phys Ther 46: , 103–117. |
[48] | Grimes D , Fitzpatrick M , Gordon J , Miyasaki J , Fon EA , Schlossmacher M , Suchowersky O , Rajput A , Lafontaine AL , Mestre T , Appel-Cresswell S , Kalia SK , Schoffer K , Zurowski M , Postuma RB , Udow S , Fox S , Barbeau P , Hutton B ((2019) ) Canadian guideline for Parkinson disease. CMAJ 191: , E989–E1004. |
[49] | Keus S , Munneke M , Graziano M , Paltamaa J , Pelosin E , Domingos J , Brühlmann S , Ramaswamy B , Prins J , Struiksma C , Rochester L , Nieuwboer A , Bloem B , on behalf of the Guideline Development Group ((2014) ) European physiotherapy guideline for Parkinson’s disease, KNGF/ParkinsonNet, the Netherlands. |
[50] | Arés Luque A , Baladia Rodríguez E , Bruna Rabassa O , Calvo Muñoz I , Chouza Insua M , Frutos Pérez-Surio A , Hernández Jaras MV , López del Val LJ , Martín Sánchez JI , Pilar Martínez Altarriba MC , Novo Porca A , Pintor Pérez L , Puyuelo Sanclemente M , Terrén Bescos R , Vázquez Sánchez F ((2014) ) Clinical practice guideline for the management of patients with Parkinson’s disease. Institute of Health Sciences of Aragon, Ministry of Health, Social Services, and Equality. |
[51] | National Institute for Health and Care Excellence, Non-pharmacological management of motor and non-motor symptoms, https://www.nice.org.uk/guidance/ng71/chapter/Recommendations#non-pharmacological-management-of-motor-and-non-motor-symptoms, Accessed January 22, 2024. |
[52] | Rafferty MR , Hoffman L , Feeney M , Schulte C , Hutber A , Galati T , Neric F , Ellis TD ((2023) ) Parallel development of Parkinson’s-specific competencies for exercise professionals and criteria for exercise education programs. Parkinsonism Relat Disord 112: , 105407. |
[53] | Xiao Y , Yang T , Shang H ((2023) ) The impact of motor-cognitive dual-task training on physical and cognitive functions in Parkinson’s disease. Brain Sci 13: , 1–11. |
[54] | Yu WY , Yang QH , Wang XQ ((2022) ) The mechanism of exercise for pain management in Parkinson’s disease. Front Mol Neurosci 15: , 1039302. |
[55] | MacDonald J , Doyle L , Moore JL , Rafferty MR ((2021) ) Sustainment of proactive physical therapy for individuals with early-stage Parkinson’s disease: A quality improvement study over 4 years. Implement Sci Commun 2: , 111. |
[56] | Hariz G-M , Forsgren L ((2011) ) Activities of daily living and quality of life in persons with newly diagnosed Parkinson’s disease according to subtype of disease, and in comparison to healthy controls. Acta Neurol Scand 123: , 20–27. |
[57] | Foster ER , Doty T ((2021) ) Cognitive correlates of instrumental activities of daily living performance in Parkinson disease without dementia. Arch Rehabil Res Clin Transl 3: , 100138. |
[58] | Staunton H , Kelly K , Newton L , Leddin M , Rodriguez-Esteban R , Chaudhuri KR , Weintraub D , Postuma RB , Martinez-Martin P ((2022) ) A patient-centered conceptual model of symptoms and their impact in early Parkinson’s disease: A qualitative study. J Parkinsons Dis 12: , 137–151. |
[59] | Sadural A , MacDonald J , Johnson J , Gohil K , Rafferty M ((2022) ) Occupational therapy for people with early Parkinson’s disease: A retrospective program evaluation. Parkinsons Dis 2022: , 1931468. |
[60] | Foster ER , Hershey T ((2011) ) Everyday executive function is associated with activity participation in Parkinson disease without dementia. OTJR (Thorofare N J) 31: , S16–22. |
[61] | Connor L , Wolf T , Foster E , Hildebrand M , Baum C ((2013) ) Participation and engagement in occupation in adults with disabilities. In Occupational Science for Occupational Therapy Pierce DE, ed. Slack Incorporated, Thorofare, NJ, pp. 107–120. |
[62] | Sturkenboom IH , Hendriks JC , Graff MJ , Adang EM , Munneke M , Nijhuis-van der Sanden MW , Bloem BR ((2015) ) Economic evaluation of occupational therapy in Parkinson’s disease: A randomized controlled trial. Mov Disord 30: , 1059–1067. |
[63] | Franciotta M , Maestri R , Ortelli P , Ferrazzoli D , Mastalli F , Frazzitta G ((2019) ) Occupational therapy for parkinsonian patients: A retrospective study. Parkinsons Dis 2019: , 4561830. |
[64] | Milne-Ives M , Carroll C , Meinert E ((2022) ) Self-management interventions for people with Parkinson disease: Scoping review. J Med Internet Res 24: , e40181. |
[65] | Clarke CE , Patel S , Ives N , Rick CE , Dowling F , Woolley R , Wheatley K , Walker MF , Sackley CM , PD REHAB Collaborative Group ((2016) ) Physiotherapy and occupational therapy vs no therapy in mild to moderate Parkinson disease: A randomized clinical trial. JAMA Neurol 73: , 291–299. |
[66] | Rusz J , Tykalová T , Novotný M , Růžička E , Dušek P ((2021) ) Distinct patterns of speech disorder in early-onset and late-onset de-novo Parkinson’s disease. NPJ Parkinsons Dis 7: , 98. |
[67] | Rusz J , Hlavnicka J , Tykalova T , Novotny M , Dusek P , Sonka K , Ruzicka E ((2018) ) Smartphone allows capture of speech abnormalities associated with high risk of developing Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng 26: , 1495–1507. |
[68] | Wylie K , Carrier HM , Loftus AM , Thilakaratne R , Cocks N ((2022) ) Barriers and facilitators to conversation: A qualitative exploration of the experiences of people with Parkinson’s and their close communication partners. Brain Sci 12: , 944. |
[69] | Saldert C , Bauer M ((2017) ) Multifaceted communication problems in everyday conversations involving people with Parkinson’s disease. Brain Sci 7: , 123. |
[70] | Watts CR , Zhang Y ((2022) ) Progression of self-perceived speech and swallowing impairment in early stage Parkinson’s disease: Longitudinal analysis of the Unified Parkinson’s Disease Rating Scale. J Speech Lang Hear Res 65: , 146–158. |
[71] | Mammen JR , Speck RM , Stebbins GT , Müller M , Yang PT , Campbell M , Cosman J , Crawford JE , Dam T , Hellsten J , Jensen-Roberts S , Kostrzebski M , Simuni T , Barowicz KW , Cedarbaum JM , Dorsey ER , Stephenson D , Adams JL ((2023) ) Relative meaningfulness and impacts of symptoms in people with early-stage Parkinson’s disease. J Parkinsons Dis 13: , 619–632. |
[72] | Schalling E , Johansson K , Hartelius L ((2017) ) Speech and communication changes reported by people with Parkinson’s disease. Folia Phoniatr Logop 69: , 131–141. |
[73] | Pflug C , Bihler M , Emich K , Niessen A , Nienstedt JC , Flügel T , Koseki JC , Plaetke R , Hidding U , Gerloff C , Buhmann C ((2018) ) Critical dysphagia is common in Parkinson disease and occurs even in early stages: A prospective cohort study. Dysphagia 33: , 41–50. |
[74] | van Wamelen DJ , Leta V , Johnson J , Ocampo CL , Podlewska AM , Rukavina K , Rizos A , Martinez-Martin P , Chaudhuri KR ((2020) ) Drooling in Parkinson’s disease: Prevalence and progression from the non-motor international longitudinal study. Dysphagia 35: , 955–961. |
[75] | Schindler A , Pizzorni N , Cereda E , Cosentino G , Avenali M , Montomoli C , Abbruzzese G , Antonini A , Barbiera F , Benazzo M , Benarroch E , Bertino G , Clavè P , Cortelli P , Eleopra R , Ferrari C , Hamdy S , Huckabee M-L , Lopiano L , Marchese-Ragona R , Masiero S , Michou E , Occhini A , Pacchetti C , Pfeiffer RF , Restivo DA , Rondanelli M , Ruoppolo G , Sandrini G , Schapira A , Stocchi F , Tolosa E , Valentino F , Zamboni M , Zangaglia R , Zappia M , Tassorelli C , Alfonsi E ((2021) ) Consensus on the treatment of dysphagia in Parkinson’s disease. J Neurol Sci 430: , 120008. |
[76] | Ciucci MR , Grant LM , Rajamanickam ES , Hilby BL , Blue KV , Jones CA , Kelm-Nelson CA ((2013) ) Early identification and treatment of communication and swallowing deficits in Parkinson disease. Semin Speech Lang 34: , 185–202. |
[77] | Richardson K , Huber JE , Kiefer B , Snyder S ((2022) ) Perception of physical demand, mental demand, and performance: A comparison of two voice interventions for Parkinson’s disease. Am J Speech Lang Pathol 31: , 1963–1978. |
[78] | Saleem AF , Sapienza CM , Okun MS ((2005) ) Respiratory muscle strength training: Treatment and response duration in a patient with early idiopathic Parkinson’s disease. Neurorehabilitation 20: , 323–333. |
[79] | Countryman S , Hicks J , Ramig LO , Smith ME ((1997) ) Supraglottal hyperadduction in an individual with Parkinson disease. Am J Speech Lang Pathol 6: , 74–84. |
[80] | Prenger MTM , Madray R , Van Hedger K , Anello M , MacDonald PA ((2020) ) Social symptoms of Parkinson’s disease. Parkinsons Dis 2020: , 8846544. |
[81] | Schrag A , Hovris A , Morley D , Quinn N , Jahanshahi M ((2006) ) Caregiver-burden in Parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord 12: , 35–41. |
[82] | Mueller C , Ballard C , Corbett A , Aarsland D ((2017) ) The prognosis of dementia with Lewy bodies. Lancet Neurol 16: , 390–398. |
[83] | Bjoerke-Bertheussen J , Ehrt U , Rongve A , Ballard C , Aarsland D ((2012) ) Neuropsychiatric symptoms in mild dementia with Lewy bodies and Alzheimer’s disease. Dement Geriatr Cogn Disord 34: , 1–6. |
[84] | Port RJ , Rumsby M , Brown G , Harrison IF , Amjad A , Bale CJ ((2021) ) People with Parkinson’s disease: What symptoms do they most want to improve and how does this change with disease duration? J Parkinsons Dis 11: , 715–724. |
[85] | Yarnall AJ , Breen DP , Duncan GW , Khoo TK , Coleman SY , Firbank MJ , Nombela C , Winder-Rhodes S , Evans JR , Rowe JB , Mollenhauer B , Kruse N , Hudson G , Chinnery PF , O’Brien JT , Robbins TW , Wesnes K , Brooks DJ , Barker RA , Burn DJ , Group I-PS ((2014) ) Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD study. Neurology 82: , 308–316. |
[86] | Sun C , Armstrong MJ ((2021) ) Treatment of Parkinson’s disease with cognitive impairment: Current approaches and future directions. Behav Sci (Basel) 11: , 54. |
[87] | Leung IH , Walton CC , Hallock H , Lewis SJ , Valenzuela M , Lampit A ((2015) ) Cognitive training in Parkinson disease: A systematic review and meta-analysis. Neurology 85: , 1843–1851. |
[88] | Petrelli A , Kaesberg S , Barbe MT , Timmermann L , Rosen JB , Fink GR , Kessler J , Kalbe E ((2015) ) Cognitive training in Parkinson’s disease reduces cognitive decline in the long term. Eur J Neurol 22: , 640–647. |
[89] | Nombela C , Bustillo PJ , Castell PF , Sanchez L , Medina V , Herrero MT ((2011) ) Cognitive rehabilitation in Parkinson’s disease: Evidence from neuroimaging. Front Neurol 2: , 82. |
[90] | Foster ER , Spence D , Toglia J ((2018) ) Feasibility of a cognitive strategy training intervention for people with Parkinson’s disease. Disabil Rehabil 40: , 1127–1134. |
[91] | Goedeken S , Potempa C , Prager EM , Foster ER ((2018) ) Encoding strategy training and self-reported everyday prospective memory in people with Parkinson disease: A randomized-controlled trial. Clin Neuropsychol 32: , 1282–1302. |
[92] | Kalbe E , Aarsland D , Folkerts AK ((2018) ) Cognitive interventions in Parkinson’s disease: Where we want to go within 20 years. J Parkinsons Dis 8: , S107–s113. |
[93] | Leite Silva ABR , Gonçalves de Oliveira RW , Diógenes GP , de Castro Aguiar MF , Sallem CC , Lima MPP , de Albuquerque Filho LB , Peixoto de Medeiros SD , Penido de Mendonça LL , de Santiago Filho PC , Nones DP , da Silva Cardoso PMM , Ribas MZ , Galvão SL , Gomes GF , Bezerra de Menezes AR , Dos Santos NL , Mororó VM , Duarte FS , Dos Santos JCC ((2023) ) Premotor, nonmotor and motor symptoms of Parkinson’s Disease: A new clinical state of the art. Ageing Res Rev 84: , 101834. |
[94] | Leentjens AF , Driessen G , Weber W , Drukker M , van Os J ((2008) ) Mental health care use in Parkinson’s disease: A record linkage study. Neuroepidemiology 30: , 71–75. |
[95] | Weintraub D , Aarsland D , Chaudhuri KR , Dobkin RD , Leentjens AFG , Rodriguez-Violante M , Schrag A ((2022) ) The neuropsychiatry of Parkinson’s disease: Advances and challenges. Lancet Neurol 21: , 89–102. |
[96] | Folkerts A-K , Haarmann L , Nielsen J , Saliger J , Eschweiler M , Karbe H , Allert N , Vida V , Trenkwalder C , Kruse A , Oelsner H , Ebersbach G , Kalbe E ((2022) ) Fear of progression is determined by anxiety and self-efficacy but not disease-specific parameters in patients with Parkinson’s disease: Preliminary data from a multicenter cross-sectional study. J Parkinsons Dis 12: , 2543–2553. |
[97] | Maffoni M , Pierobon A , Frazzitta G , Callegari S , Giardini A ((2019) ) Living with Parkinson’s— past, present and future: A qualitative study of the subjective perspective. Br J Nurs 28: , 764–771. |
[98] | Lawson RA , Collerton D , Taylor J-P , Burn DJ , Brittain KR ((2018) ) Coping with cognitive impairment in people with Parkinson’s disease and their carers: A qualitative study. Parkinsons Dis 2018: , 1362053. |
[99] | Santa Rosa Malcher CM , Roberto da Silva Gonçalves Oliveira K , Fernandes Caldato MC , Lopes dos Santos Lobato B , da Silva Pedroso J , de Tubino Scanavino M ((2021) ) Sexual disorders and quality of life in Parkinson’s disease. Sex Med 9: , 100280–100280. |
[100] | McDaniels B , Pontone GM , Keener AM , Subramanian I ((2023) ) A prescription for wellness in early PD: Just what the doctor ordered. J Geriatr Psychiatry Neurol 36: , 461–469. |
[101] | Eccles FJR , Sowter N , Spokes T , Zarotti N , Simpson J ((2023) ) Stigma, self-compassion, and psychological distress among people with Parkinson’s. Disabil Rehabil 45: , 425–433. |
[102] | Zarotti N , Eccles FJR , Foley JA , Paget A , Gunn S , Leroi I , Simpson J ((2021) ) Psychological interventions for people with Parkinson’s disease in the early 2020s: Where do we stand? Psychol Psychother 94: , 760–797. |
[103] | Fengler S , Liepelt-Scarfone I , Brockmann K , Schäffer E , Berg D , Kalbe E ((2017) ) Cognitive changes in prodromal Parkinson’s disease: A review. Mov Disord 32: , 1655–1666. |
[104] | Chou KL , Martello J , Atem J , Elrod M , Foster ER , Freshwater K , Gunzler SA , Kim H , Mahajan A , Sarva H , Stebbins GT , Lee E , Yang L ((2021) ) Quality improvement in neurology: 2020 Parkinson Disease Quality Measurement Set Update. Neurology 97: , 239–245. |
[105] | Hughes KC , Gao X , Molsberry S , Valeri L , Schwarzschild MA , Ascherio A ((2019) ) Physical activity and prodromal features of Parkinson disease. Neurology 93: , e2157–e2169. |
[106] | Crotty GF , Schwarzschild MA ((2020) ) Chasing protection in Parkinson’s disease: Does exercise reduce risk and progression? Front Aging Neurosci 12: , 186. |
[107] | Del Din S , Yarnall AJ , Barber TR , Lo C , Crabbe M , Rolinski M , Baig F , Hu MT , Rochester L ((2020) ) Continuous real-world gait monitoring in idiopathic REM sleep behavior disorder. J Parkinsons Dis 10: , 283–299. |
[108] | Debelle H , Packer E , Beales E , Bailey HGB , Mc Ardle R , Brown P , Hunter H , Ciravegna F , Ireson N , Evers J , Niessen M , Shi JQ , Yarnall AJ , Rochester L , Alcock L , Del Din S ((2023) ) Feasibility and usability of a digital health technology system to monitor mobility and assess medication adherence in mild-to-moderate Parkinson’s disease. Front Neurol 14: , 1111260. |
[109] | Clarke CE , Patel S , Ives N , Rick CE , Woolley R , Wheatley K , Walker MF , Zhu S , Kandiyali R , Yao G , Sackley CM ((2016) ) Clinical effectiveness and cost-effectiveness of physiotherapy and occupational therapy versus no therapy in mild to moderate Parkinson’s disease: A large pragmatic randomised controlled trial (PD REHAB). Health Technol Assess 20: , 1–96. |
[110] | Aarsland D , Batzu L , Halliday GM , Geurtsen GJ , Ballard C , Ray Chaudhuri K , Weintraub D ((2021) ) Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers 7: , 47. |
[111] | Schiess N , Cataldi R , Okun MS , Fothergill-Misbah N , Dorsey ER , Bloem BR , Barretto M , Bhidayasiri R , Brown R , Chishimba L , Chowdhary N , Coslov M , Cubo E , Di Rocco A , Dolhun R , Dowrick C , Fung VSC , Gershanik OS , Gifford L , Gordon J , Khalil H , Kühn AA , Lew S , Lim S-Y , Marano MM , Micallef J , Mokaya J , Moukheiber E , Nwabuobi L , Okubadejo N , Pal PK , Shah H , Shalash A , Sherer T , Siddiqui B , Thompson T , Ullrich A , Walker R , Dua T ((2022) ) Six action steps to address global disparities in Parkinson disease: A World Health Organization priority. JAMA Neurol 79: , 929–936. |
[112] | Molsberry S , Bjornevik K , Hughes KC , Healy B , Schwarzschild M , Ascherio A ((2020) ) Diet pattern and prodromal features of Parkinson disease. Neurology 95: , e2095–e2108. |
[113] | Theodoros D ((2021) ) Telerehabilitation for communication and swallowing disorders in Parkinson’s disease. J Parkinsons Dis 11: , S65–s70. |
[114] | Quinn L , Macpherson C , Long K , Shah H ((2020) ) Promoting physical activity via telehealth in people with Parkinson disease: The path forward after the COVID-19 pandemic? Phys Ther 100: , 1730–1736. |
[115] | Dobkin RD , Mann SL , Gara MA , Interian A , Rodriguez KM , Menza M ((2020) ) Telephone-based cognitive behavioral therapy for depression in Parkinson disease: A Randomized control trial. Neurology 94: , e1764–e1773. |
[116] | Riggare S , Stamford J , Hägglund M ((2021) ) A long way to go: Patient perspectives on digital health for Parkinson’s disease. J Parkinsons Dis 11: , S5–s10. |
[117] | Laar A , Silva de Lima AL , Maas BR , Bloem BR , de Vries NM ((2023) ) Successful implementation of technology in the management of Parkinson’s disease: Barriers and facilitators. Clin Park Relat Disord 8: , 100188. |




