Effects of Blood Flow Restriction Resistance Training on Autonomic and Endothelial Function in Persons with Parkinson’s Disease
Abstract
Background:
Autonomic dysfunction precedes endothelial dysfunction in Parkinson’s disease (PD) and causes blood pressure and circulation abnormalities that are highly disruptive to one’s quality of life. While exercise interventions have proven helpful for motor symptoms of PD, improving associated non-motor symptoms is limited. Low-intensity resistance training with blood flow restriction (LIRT-BFR) improves autonomic dysfunction in non-PD patients and high-intensity resistance training (HIRT) is recommended for motor symptom improvements for people with PD (PwPD).
Objective:
To determine the effects of LIRT-BFR and HIRT on homocysteine and autonomic and endothelial function in PwPD and to determine the hemodynamic loads during LIRT-BFR and HIRT in PwPD using a novel exercise protocol.
Methods:
Thirty-eight PwPD were assigned LIRT-BFR, HIRT or to a control (CNTRL) group. The LIRT-BFR and HIRT groups exercised three days per week for four weeks. The LIRT-BFR protocol used 60% limb occlusion pressure (LOP) and performed three sets of 20 repetitions at 20% of the one-repetition maximum (1RM). The HIRT group performed three sets of eight repetitions at 80% 1RM. The CNTRL group was asked to continue their normal daily routines.
Results:
LIRT-BFR significantly improved orthostatic hypotension (p = 0.026), homocysteine levels (p < 0.001), peripheral circulation (p = 0.003), supine blood pressure (p = 0.028) and heart rate variability (p = 0.041); LIRT-BFR improved homocysteine levels (p < 0.018), peripheral circulation (p = 0.005), supine blood pressure (p = 0.007) and heart rate variability (p = 0.047) more than HIRT; and hemodynamic loads for LIRT-BFR and HIRT were similar.
Conclusions:
LIRT-BFR may be more effective than HIRT for autonomic and endothelial function improvements in PwPD and hemodynamic loads may be lessened in LIRT-BFR protocols using single-joint exercises with intermittent blood flow restriction. Further research is needed to determine if non-motor symptoms improve over time and if results are sustainable.
INTRODUCTION
Parkinson’s disease (PD) is a progressive, debilitating disease of the central nervous system that affects over six million worldwide [1]. The degeneration of dopaminergic neurons within the substantia nigra results in disrupted motor functioning through rigidity, bradykinesia, resting tremors and impaired postural reflexes [2]. Motor skills, muscle strength, endurance [3] and gait [4] are all negatively affected and result in disability, fragility [5] and increased risk of falling [6].
The most common drug prescribed for PD motor symptoms is carbidopa-levodopa (levodopa) [7]. While levodopa is effective in treating motor symptoms, it may exacerbate autonomic dysfunction through elevated homocysteine levels [8, 9]. Increased homocysteine levels in people with PD (PwPD) have been observed, well above healthy, age-matched controls and are strongly correlated with increased cardio- and cerebrovascular disease [8, 10] and cognitive impairment [11]. Elevated homocysteine provokes activation of the sympathetic nervous system [12] and is linearly correlated with cardiac autonomic nervous function disorders in patients with sleep apnea [13]. An overactive sympathetic nerve is detrimental to the blood vessels and may be why autonomic and endothelial dysfunction are closely related [14].
Autonomic dysfunction is a commonly overlooked feature of PD [15] and may disrupt quality of life and increase prevalence of falls, even more than the motor symptoms targeted with standard of care treatments [16]. Low heart rate variability (HRV) is an indication of autonomic dysfunction PwPD experience at much higher rates compared to controls [17]. Overstimulation of the sympathetic nervous system, as indicated by low HRV, is the major cause of autonomic-related blood flow dysregulation in PwPD and results in orthostatic hypotension (OH), supine hypertension, and increased resting heart rate [18, 19]. Prevalence of OH in PD populations have been reported to be over 65% [20] and associated with greater deterioration in activities of daily living, health care utilization and falls compared to patients without OH [21]. Other autonomic symptoms include gastrointestinal, urinary, pupillary and thermoregulatory system disruptions [21]. All of these conditions can be related to the disrupted interplay between the autonomic nervous system and communication with endothelial cells that are responsible for regulating blood flow for proper system metabolism and health [22].
At rest, autonomic dysfunction is most apparent during positional changes for PwPD [21]. Symptoms such as dizziness, light-headedness, pre-syncope and syncope may result from orthostatic and positional hypotension [23]. During exercise, autonomic dysfunction disrupts the ability to vasodilate and vasoconstrict vessels to reroute blood to the working muscles [24] and can cause PwPD to experience exercise-induced hypotension [25]. Recently, it has been determined that chronotropic incompetence may be present in early stages of PD and this may result in decreased aerobic capacity [26]. As PwPD progress in their disease, high-intensity exercises that demand increased motor control, heart rate, cardiac output and increased autonomic control of blood vessels may be more difficult to perform safely due to both motor symptom impairment, hemodynamic loads and autonomic dysfunction [24, 26–28].
High-intensity resistance training (HIRT) can decrease homocysteine levels more than aerobic exercise [29, 30] and is recommended over lower intensities for motor and non-motor symptom improvement in PD [31–33] though autonomic and endothelial adaptations are undetermined. Low-intensity resistance training with blood flow restriction (LIRT-BFR) is used widely in interventions to combat sarcopenia and increase strength, hypertrophy, endothelial function and peripheral circulation in older individuals who may have difficulty exercising at high intensities [28, 34, 35]. During blood flow restriction (BFR) exercise, hypoxic stress to skeletal muscles causes excessive secretion of neurohumoral factors [36, 37] and can moderately alter cardiac function and increase expression levels of proteins related to vascular endothelial function [38] and results in improved vessel reactivity and favorable artery structural adaptations, even when simple, isolated exercises such as handgrip and calf-raises are used [39, 40].
Literature pertaining to BFR exercise in PwPD is currently limited to two controlled case studies where improvements in restless leg syndrome were observed following six weeks of walking with BFR [41] and lower extremity strength improved following six weeks of LIRT-BFR [42]. Homocysteine levels following BFR exercise have not been reported. Exercise using LIRT-BFR improves endothelial function more than HIRT [43], results in less oxidative stress [37], does not alter arterial stiffness [44] and improves cardiac autonomic dysfunction, overall health and disease progression [45]. Therefore, the hypothesis of the study was that LIRT-BFR would more effectively improve endothelial and autonomic function compared to HIRT.
Hemodynamic loads during exercise are concerning factors for individuals with compromised cardiovascular health [46, 47]. During HIRT and LIRT-BFR, these loads are variable and dependent on the intensity, volume, type of exercise and duration of occlusion [46, 48–51]. In HIRT, arterial stiffness may be worsened in individuals with baseline arterial stiffness [52, 53], but may have a positive impact on endothelial function in young, healthy males [54]. Similarly, acute increases in arterial stiffness have been observed in LIRT-BFR exercise, but longitudinal studies have shown no changes in arterial stiffness in young and old people [47]. PwPD display endothelial and autonomic dysfunction, increased arterial stiffness and increased risk of cerebro- and cardiovascular events [9, 55, 56]. Interestingly, PD itself does not cause arterial stiffness, whereas autonomic blood pressure disturbances influence alterations in arterial stiffness and architectural changes in the arteries of PD patients [55].
Autonomic dysfunction causes blood pressure and circulation abnormalities in PwPD that are highly disruptive to one’s quality of life [57]. While exercise interventions have proven helpful for motor symptoms of PD, exercise focusing on improving non-motor symptoms associated with autonomic dysfunction is limited [18, 31]. Autonomic dysfunction precedes endothelial dysfunction in PD and may be exacerbated by increased homocysteine levels caused by levodopa [10, 55]. HIRT has been shown to decrease homocysteine levels and improve endothelial function and OH [30, 54, 58]. LIRT-BFR improves autonomic and endothelial function in non-PD populations [43, 45]. Therefore, the primary aim of this study was to determine the effects of LIRT-BFR and HIRT on homocysteine and autonomic and endothelial function in PwPD. The secondary aim for our study was to determine the hemodynamic loads during LIRT-BFR and HIRT in PwPD using a novel exercise protocol derived from previous study protocols aimed at decreasing such loads [49–51, 59, 60].
MATERIALS AND METHODS
After an initial phone screening, qualifying participants visited the laboratory on two separate occasions for pre- and post-assessments. The first visit included collection of anthropometric data and a fasted blood draw. The second visit included resting and supine blood pressures, an orthostatic tolerance test, reactive hyperemia index (RHI) assessments via finger plethysmography testing, peripheral circulation via transcutaneous oxygen monitoring (TcP02), and a strength assessment. After pre-assessments, participants were assigned to one of two exercise groups or a control group. Exercise sessions were held on three non-consecutive days per week for four weeks. After four weeks, all participants completed post-assessments on two separate occasions, mirroring the data collection protocol for pre-assessments.
This study was approved by the Institutional Review Board at Abilene Christian University in Abilene, TX, USA.
Participants
Thirty-eight volunteers who were previously diagnosed with PD by a neurologist or movement disorder specialist and met the following criteria were admitted into the study: (1) mild to moderate PD using the modified Hoehn and Yahr (mHY) stages 1–3.5, (2) age over 50 years old, (3) currently on levodopa therapy (Sinemet, Rytary, Duopa, Sinemet CR) for at least the last six consecutive months, (4) free of any contraindication for BFR (cardiovascular disease, unstable hypertension, varicose veins, diabetes, cancer, musculoskeletal injury, post-surgical swelling, open wounds or on a medication that increases blood clotting risk) [46], (5) currently not participating in resistance training or high-intensity or long duration cardiovascular exercise [61], (6) free from orthopedic problems that would preclude seated lower- and upper-body exercises, (7) able to complete a Timed-Up-and-Go (TUG) assessment with or without assistance from a walker or cane, (8) able to complete four straight weeks of exercise, (9) and had a signed approval form (provided) by their doctor to participate in the study. The disease rating scale (mHY) was completed by a licensed and experienced physical therapist and results were used to determine each participants disease progression [62].
After thirty participants cleared the phone-screening, the preliminary musculoskeletal exam and received their doctors’ consent for participation, they were randomly assigned to one of two exercise groups: 1) low-intensity blood flow restriction resistance exercise (LIRT-BFR) or 2) high intensity resistance training without BFR (HIRT). A smaller subgroup of eight controls (CNTRL) were assigned after exercise groups were filled. The control group was asked to continue with their normal routine for four weeks and return for post-assessments. All participants completed pre- and post-assessments and are included in the data analysis. Sample sizes were determined based on a literature review and network meta-analysis analyzing exercise therapy for PD with an average sample of 26 participants that resulted in favorable outcomes, averaging a moderate effect size of > 0.5 [63]. We determined, a priori, that 15 participants would be randomly assigned to one of the exercise conditions and the remaining volunteers would serve as the control group for comparison.
Exercise protocols
The control group was asked to maintain activity and diet as usual and to return for post-assessments four weeks after pre-assessments. For the LIRT-BFR and HIRT groups, exercise was performed three days per week for four weeks, with one day of rest between the weekly exercise sessions and two days after the third. Participants performed leg extensions, seated biceps curls, seated leg curls, seated triceps extensions, seated calf raises and seated handgrips in this alternating upper and lower body exercise format. The LIRT-BFR group performed three straight sets of 20 repetitions at approximately 20% estimated one repetition maximum (1RM) with a 30 second rest between sets under 60% limb occlusion pressure (LOP). After the third set, the cuffs were deflated, and the participant transitioned to the next exercise with a 90 second rest between the completion of each exercise.
The HIRT group performed three straight sets of eight repetitions at approximately 80% estimated 1RM with a 1-min rest between sets. Participants remained seated for the duration of the exercise and only stood to transition between exercises. Transitions between exercises were approximately 90 seconds for both groups. The exercise protocol and ordering and a picture of the BFR cuffs during leg extension are displayed in Fig. 1. All participants had a research assistant guiding them through all exercise sessions. Only research personnel trained in BFR assisted those in the LIRT-BFR group.
Fig. 1
Exercise protocols for the high-intensity resistance training (HIRT) and low-intensity resistance training with blood flow restriction (LIRT-BFR) groups are summarized. The exercise format is pictured in the exact order performed by each group, starting with a warm-up and ending with a cool-down. A sample cuff placement for the blood flow restriction (BFR) protocol is also pictured.
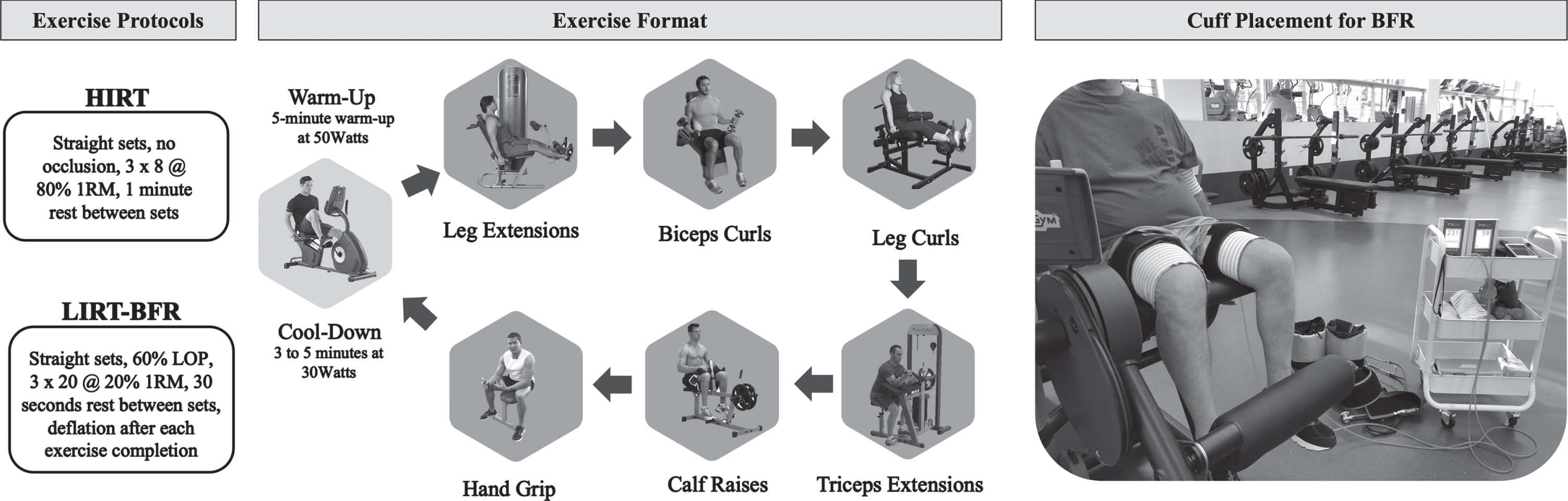
The LOP in the upper and lower body was determined automatically via the Delfi Personal Tourniquet System for Blood Flow Restriction (Owens Recovery Unit, Delfi Medical, Vancouver BC, Canada) in a seated and still position prior to each exercise session [64]. To determine LOP for the lower body, a 10 cm cuff was placed on each thigh. LOP was measured and recorded for exercise occlusion reference. To determine LOP for the upper body, a 7 cm cuff was placed on the upper proximal arm. Both cuffs were inflated to determine LOP. LOP was measured and recorded for exercise occlusion reference.
Vascular testing
All vascular testing occurred in the morning hours in the dopaminergic medication on-state, defined as a period of perceived maximal efficacy of dopaminergic medications, as indicated by good motor response [65] and at least three hours after a meal. Indicators of improved endothelial function were vessel reactivity, as indicated by the natural log rhythm reactive hyperemia index (LnRHI) measured through finger plethysmography, peripheral circulation measured through TcP02 and serum homocysteine levels. Arterial stiffness was assessed through finger plethysmography and reported as AIx75.
Reactive hyperemia index and arterial stiffness
Reactive hyperemia index (RHI) was measured in the supine position as a measure of vascular endothelial function using finger plethysmography. Participants came in the morning hours, free from exercise and in their dopaminergic medication on-state and laid supine for 20 min before baseline measurements. The test room temperature was set at 21–25°, as previously used in past studies [66]. Simultaneously, finger volume pulse waveforms were recorded in the index fingers of both hands non-invasively using the EndoPATTM device (Endo-PAT2000, Itamar Medical, Caesarea, Israel). The principle and methods of RHI have been previously described [67]. A blood pressure cuff was placed on the upper arm with the contralateral arm serving as a control. Fingertip probes were placed on both index fingers to measure arterial pulse wave amplitude. After a 5 min equilibrium period, the cuff was inflated to 60 mmHg above systolic blood pressure (SBP) or at least 200 mmHg for 5 min. The cuff was deflated to induce reactive hyperemia in the measured index finger, and the arterial pulse wave amplitude was recorded for 5 min. RHI was defined as the ratio of the arterial pulse wave amplitude measured for 1 min during reactive hyperemia to baseline amplitude during equilibration period [68]. Arterial stiffness was measured by the analysis of the shape of the arterial pressure waveform (pulse wave analysis), which provides an augmentation index (AIx) that is based on the reflection of the pulse wave from branch points in the arterial tree [69]. AIx was normalized to 75 bpm for analysis (AIx75), as described previously [70]. Both RHI and AIx75 were calculated by the instrument software from the same data set.
Peripheral blood circulation
Transcutaneous oxygen pressure (TcP02) was measured as an indicator of peripheral blood circulation in the foot using a TcP02 devise (TCM3, TINA, Radiometer). After subjects rested on a table in the supine position for 20 min, a probe was heated to 44.8°C and placed on the first intermetatarsal space on the dorsum of the right and left foot using the double-sided adhesive ring supplied by the manufacturers. Five minutes after probe attachment, TcP02 was recorded as stable if the value fluctuated within 2 mmHg during a 1-min measurement period. The RHI technique and peripheral circulation technique using TcP02 monitoring are well-established, non-invasive ways to determine vessel reactivity and peripheral blood circulation [43].
Strength assessments
A 4–6 repetition maximum assessment was performed on exercises used in the experimental exercise protocol including leg extension, leg curl, triceps extension and biceps curl. Estimates of 1RM weight were calculated from the number of repetitions performed in the 4–6 range at a weight that resulted in maximum effort. The National Strength and Conditioning recommendations for 4–6 repetition maximum assessments were used [71]. These assessments were used to determine the proper exercise intensity and only the exercise groups performed 4–6 RM testing.
Blood pressure measurements
Participants refrained from caffeine and exercise before blood pressure measurements were taken in the morning in the on-state, as previously defined. Participants’ SBP and diastolic blood pressure (DBP) were measured by standard Welch-Allen manual sphygmomanometer. The cuff was placed on the left arm for all participants. Blood pressure was evaluated in the following conditions: (1) while sitting in a chair after at least 10 min of rest; (2) after a minimum of 10 min of supine rest; and (3) after 3 min of standing.
Orthostatic hypotension
Orthostatic hypotension was assessed by comparing a 10-min seated and quiet resting blood pressure to a 3-min standing blood pressure that followed. Orthostatic hypotension has been defined as a drop in systolic blood pressure by more than 20 mmHg or 10 mmHg diastolic within 3 min of standing [72]. Participants were asked if they felt any of the following symptoms while standing: dizziness, feeling faint, light headedness, blurred vision, disorientation/confusion, weakness/fatigue/falling, and/or chest pain.
Heart rate variability
Heart rate variability was measured using finger plethysmography (Endo-PAT2000, Itamar Medical, Caesarea, Israel) and the EndoPATTM HRV technique based on the European Society of Cardiology and North American Society of Pacing Electrophysiology task force stand was utilized (Heart Rate Variability-task force). Participants rested in a supine position for 20 min before the baseline measurement was taken. Finger volume pulse waveforms were recorded in the index fingers of both hands using the EndoPATTM device. The root mean square of successive normal-to-normal interval differences (RMSSD) from the EndoPATTM software was reported. This technique has been validated [73]. Short-term measurement of RMSSD for HRV has been validated in PwPD to determine such autonomic imbalances [17].
Exercise hemodynamic measurements
Measurements during exercise included blood pressure, ratings of perceived exertion and heart rate. Manual blood pressures were taken using a standard Welch-Allen upper arm cuff (12 cm wide internal bladder) and stethoscope by a trained research member after each upper and lower body exercise, during each session over the four weeks of exercise and were performed: (1) before exercise began, (2) immediately after each lower body exercise and/or immediately after lower cuffs were released, (3) after each upper-body exercise and/or immediately after upper cuffs were released, and (4) after cool-down. The use of manual recording of SBP and DBP has demonstrated smallest detectable differences of 7.6 and 7.0 mmHg, respectively, during rest conditions [74] and have been used to compare hemodynamic loads during BFR, LIRT and HIRT protocols [51]. The immediate post-upper and lower body exercises hemodynamic measurements were averaged and reported in the results section. Arterial oxygen saturation using pulse oximetry, rating of perceived exertion (RPE) using the modified Borg scale [75], and heart rate (HR) were recorded just before blood pressure and obtained at these time points.
Blood samples and biochemical analysis
Participants were asked to come in after an overnight fast, to not exercise and to sit for 5 min prior to their blood draw. Venous blood samples were obtained by a registered nurse from an antecubital vein by standard/sterile procedures [76]. Approximately 15 ml of blood was obtained before and after exercise intervention in two red top, no additive vacutainer tubes (BD Vacutainer®, Franklin Lakes, NJ). Blood samples were allowed to clot on ice for 30 min before being centrifuged at 3500 X g for 15 min with bench top centrifuge (Clinical 50™ , VWR®, Radnor, PA). Serum was extracted, combined, mixed and then aliquoted into 2.0 mL plastic ultracentrifuge tubes and stored at –80°C.
The concentrations of serum homocysteine were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits (MyBioSource, Inc., San Diego, CA, USA) with a microplate reader (X-Mark, Bio-Rad, Hercules, CA, USA). The absorbances were read in duplicate at a wavelength of 450 nm and unknown concentrations determined by linear regression against known standard curves using commercial software (Microplate Manager, Bio-Rad, Hercules, CA, USA).
Dietary analysis
Participants were asked to record their food and beverage consumption for three days leading up to pre- and post-assessments. All participants were asked to refrain from taking any supplements. Details such as food preparation, brand and portion size were recorded. Participants maintained a complete record of their diet by documenting the quantity and type of food or liquid consumed, as well as the time of day it was consumed. Participants turned in diet records prior to pre- and post-assessments. Participants were instructed to contact the research team if they had questions concerning their diet or meal composition during any record-keeping period. Dietary intake and macronutrient and micronutrient composition were estimated and analyzed when received using research-grade nutritional software (Food Processor SLQ ESHA, Salem, OR).
Statistics
Data were analyzed using the statistical package of Social Sciences (SPSS, IBM, v19) and presented in mean±standard deviation. A Shapiro-Wilk test was used to confirm normal distribution of the data. A one-way analysis of covariance (ANCOVA) was performed to analyze differences between the values of the post-test between groups for endothelial, autonomic and homocysteine variables, while the baseline values were considered as covariates. First, the assumption of ANCOVA included the normality of data (Shapiro-Wilk test), homogeneity of variance (box plot) and homogeneity of regression slopes was confirmed. To determine differences between groups, when a significant difference was found, Bonferroni post hoc tests were performed to analyze differences within groups. Paired sample tests were performed to analyze difference within groups. For hemodynamic responses, an independent samples test was performed to note differences between hemodynamic markers for all exercise sessions averaged between HIRT and LIRT-BFR. The alpha level was set at p < 0.05 for all analyses.
RESULTS
Basic characteristics and data of participants
All participants completed all exercise sessions over four weeks without the occurrence of any adverse events. Baseline data characteristics are presented in Table 1. Pre-assessment comparisons revealed group differences in age between CNTRL and LIRT-BFR groups (71.50±6.78 and 62.60±7.50 years, respectively, p = 0.032) and homocysteine between CNTRL and both LIRT-BFR and HIRT groups (14.91±2.02, 18.52±4.14 and 19.34±5.31μmol/L, respectively, p = 0.029 and p = 0.042). Thirty-one of the thirty-eight participants showed signs of orthostatic hypotension (OH), with blood pressure decreasing more than 20 mmHg upon standing with at least one common symptom of OH present such as dizziness, light-headedness, blurred vision, weakness and/or fatigue.
Table 1
Baseline anthropometric and physiological characteristics across groups
| Variable | HIRT | LIRT-BFR | CNTRL | Total |
| Number (n) | 15 | 15 | 8 | 38 |
| Age (y) | 64.07±8.00 | 62.60±7.50* | 71.50±6.78 | 65.05±8.13 |
| Males (n) (%) | 11 (73) | 13 (87) | 5 (63) | 29 (76) |
| Height (cm) | 174.42±12.72 | 175.34±9.80 | 168.76±15.54 | 173.58±12.90 |
| Weight (kg) | 195.56±52.72 | 178.39±30.66 | 182.63±31.14 | 186.06±40.67 |
| BMI (kg/m2) | 28.75±4.74 | 26.29±3.80 | 27.11±3.15 | 27.43±4.14 |
| SBP (mmHg) | 129±23 | 126±21 | 124±10 | 127±20 |
| DBP (mmHg) | 85±12 | 79±12 | 82±12 | 82±11 |
| mHY | 1.97±0.77 | 1.79±0.61 | 2.43±1.12 | 2.00±0.82 |
| Years Diagnosed | 6.67±4.73 | 6.33±5.12 | 9.75±6.76 | 7.18±5.37 |
| Years on C-Lev (y) | 5.80±3.61 | 5.20±3.93 | 9.00±6.91 | 6.24±4.69 |
| Dosage of C-Lev (mg) | 1032±693 | 1202±756 | 969±343 | 1086±656 |
| TcP02 (mmHg) | 50.83±6.24 | 52.80±7.79 | 45.50±11.56 | 50.49±8.40 |
| RHI | 0.73±0.18 | 0.42±0.48 | 0.50±0.23 | 0.56±0.38 |
| Homocysteine (μmol/L) | 19.34±5.31* | 18.52±4.14* | 14.91±2.02 | 18.07±4.56 |
| OH (n) (%) | 13 (87) | 12 (80) | 6 (75) | 31 (82) |
Average baseline characteristics by group. HIRT, high-intensity resistance training; LIRT-BFR, low-intensity resistance training with blood flow restriction; CNTRL, control groups; SBP, systolic blood pressure; DBP, diastolic blood pressure; C-Lev, Carbidopa-Levodopa; OH, orthostatic hypotension; TcPO2, transcutaneous oxygen; RHI, reactive hyperemia index; BMI, body mass index; mHY, modified Hoehn and Yahr. The *indicates significant differences compared to the CNTRL group at pre-assessments (p < 0.05).
Vascular function
Indicators of improved endothelial function were vessel reactivity, as indicated by the natural log rhythm reactive hyperemia index (LnRHI) measured through finger plethysmography, peripheral circulation measured through TcP02 and serum homocysteine levels. Arterial stiffness was assessed through finger plethysmography and reported as AIx75. Pre- and post-assessments values and statistical analysis can be seen in Table 2.
Table 2
Vascular and autonomic adaptations to four weeks of HIRT and LIRT-BFR
| Variable | Groups | Pre | Post | t-test p | Cohen’s d | ANCOVA p |
| LnRHI | HIRT | 0.67±0.25 | 0.50±0.35 | 0.051 | 0.296 | 0.338 |
| LIRT-BFR | 0.50±0.48 | 0.60±0.24 | 0.436 | 0.479 | ||
| CNTRL | 0.50±0.23 | 0.53±0.19 | 0.419 | 0.082 | ||
| TcP02 (mmHg) | HIRT | 49.93±6.26 | 50.07±5.69 | 0.941 | 6.85 | 0.005 |
| LIRT-BFR | 50.10±6.54 | 56.83±6.72*# | 0.003 | 7.14 | ||
| CNTRL | 44.88±11.26 | 45.19±12.82* | 0.788 | 3.16 | ||
| Homocysteine (μmol/L) | HIRT | 19.34±5.31 | 16.21±6.89 | 0.079 | 6.31 | 0.018 |
| LIRT-BFR | 18.52±4.14 | 11.34±5.90*# | <0.001 | 15.04 | ||
| CNTRL | 14.91±2.02 | 14.37±1.30* | 0.511 | 4.21 | ||
| AIx75 | HIRT | 8.93±22.89 | 9.87±33.11 | 0.911 | 20.35 | 0.389 |
| LIRT-BFR | 18.52±4.14 | 11.34±5.90 | 0.881 | 23.79 | ||
| CNTRL | 13.13±18.06 | 14.63±19.43 | 0.216 | 3.12 | ||
| Supine SBP (mmHg) | HIRT | 129±23 | 132±20 | 0.540 | 15.63 | 0.007 |
| LIRT-BFR | 127±21 | 113±16*# | 0.028 | 21.20 | ||
| CNTRL | 124±10 | 122±10 | 0.498 | 9.90 | ||
| Supine DBP (mmHg) | HIRT | 84±13 | 86±10 | 0.438 | 9.05 | 0.041 |
| LIRT-BFR | 81±12 | 75±13* | 0.180 | 17.19 | ||
| CNTRL | 82±77 | 77±10 | 0.144 | 8.37 | ||
| RMSSD (ms) | HIRT | 24.33±3.11 | 23.80±3.99 | 0.438 | 1.96 | 0.047 |
| LIRT-BFR | 23.60±3.33 | 26.87±5.07*# | 0.041 | 5.62 | ||
| CNTRL | 26.75±1.83 | 27.13±1.13 | 0.310 | 1.41 | ||
| Orthostatic SBP Drop (mmHg) | HIRT | 25±11 | 26±11 | 0.724 | 12.89 | 0.398 |
| LIRT-BFR | 30±12 | 24±12# | 0.026 | 9.58 | ||
| CNTRL | 31±17 | 27±10 | 0.243 | 8.88 |
Values are presented as means±standard deviation. HIRT, high-intensity resistance training; LIRT-BFR, low-intensity resistance training with blood flow restriction; CNTRL, control groups; SBP, systolic blood pressure; DBP, diastolic blood pressure; RMSSD, root mean square of successive normal-to-normal interval differences; Aix75, augmentation index corrected at 75 beats per minute. The *indicates significant differences compared to the HIRT group (p < 0.05). The #indicates significant difference compared to pre-assessment (p < 0.05).
Arterial stiffness measured through finger plethysmography was normalized for a heart rate of 75 beats per minute (bpm). Significant pre- and post-assessment values were not observed for groups. ANCOVA analysis revealed no significant differences between groups at post-assessments, after adjusting for pre-assessment differences (p = 0.389).
Vessel reactivity (LnRHI) was measured through finger plethysmography at pre- and post-assessments for each group to determine arterial stiffness. Significant pre- and post-assessment values were not observed for groups. ANCOVA analysis confirmed no significant difference between post-assessments among groups, after adjusting for pre-assessment differences (p = 0.338).
Figure 2a shows the average TcP02 measured at the first metatarsal of the right and left foot at zero degrees for pre- and post-assessments. Only the LIRT-BFR showed significant differences at post-assessments compared to pre-assessments (50.10±6.54 and 56.83±6.72 mmHg, respectively, p = 0.003). There was a significant difference at post-assessments between the groups for TcP02 (p = 0.043). Bonferroni post hoc tests revealed a significant difference between the LIRT-BFR and CNTRL groups (56.83±6.72 and 45.19±12.82 mmHg, respectively, p = 0.040).
Fig. 2
Homocysteine and peripheral circulation adaptations to four weeks of HIRT and LIRT-BFR. HIRT, high-intensity resistance training; LIRT-BFR, low-intensity resistance training with blood flow restriction; CNTRL, control. The *indicates a significant difference compared to HIRT at post-assessments (p < 0.05) and #indicates a significant difference compared to pre-assessments (p < 0.05). a) Plasma homocysteine levels. Elevated homocysteine is considered to be above 12μmol/L and is represented by the dotted line. b) Average peripheral circulation, as indicated by transcutaneous oxygen pressure (TcP02). The cutoff line is represented by the dotted line at 50 mmHg, with lower pressures indicating poor circulation in the foot.
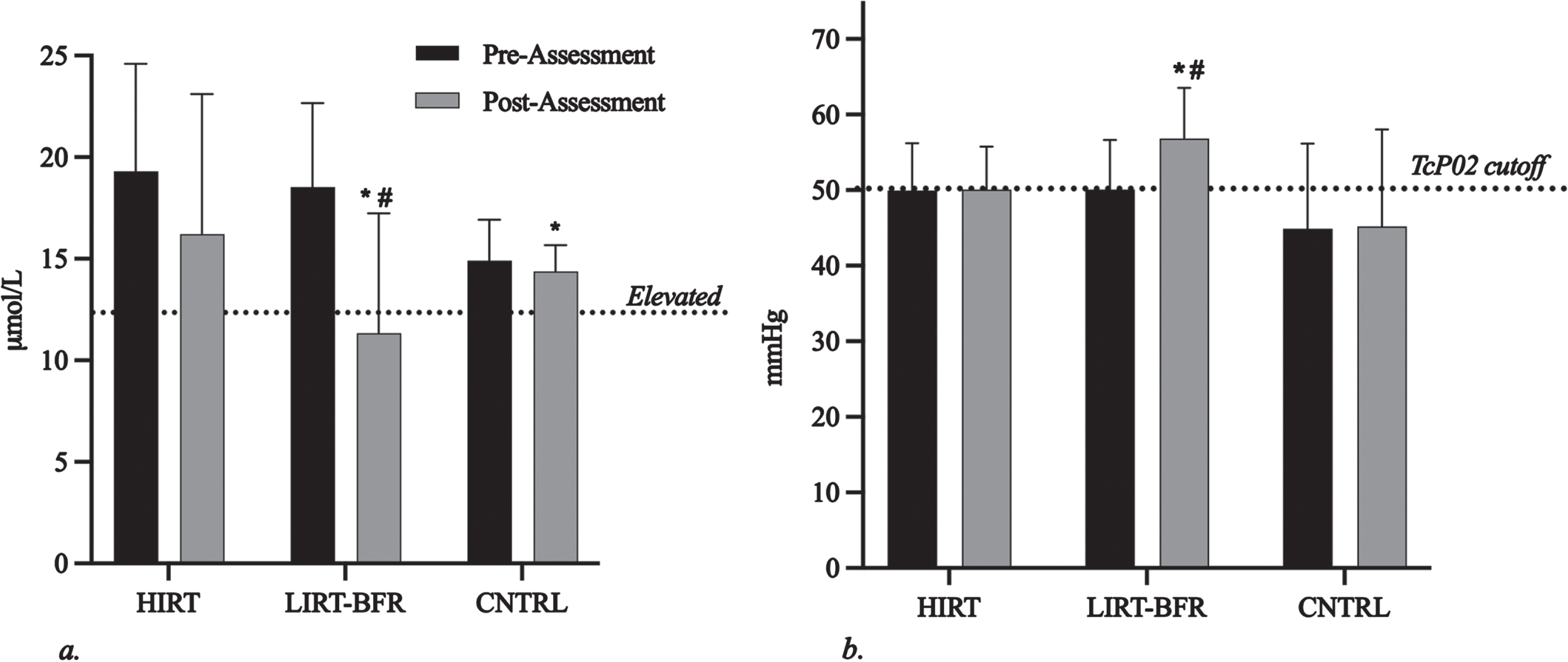
Figure 2b shows homocysteine levels at pre- and post-assessments for each group. There was a significant difference from pre- to post-assessment values for the LIRT-BFR group (18.52±4.14 and 11.34±5.90μmol/L, respectively, p < 0.001). Significant differences were found between groups (p = 0.005). Post-hoc analysis revealed significant differences in homocysteine levels for LIRT-BFR and CNTRL groups (11.34±5.90 and 14.37±1.30μmol/L, respectively, p = 0.019) and LIRT-BFR and HIRT group (11.34±5.90 and 16.21±6.89μmol/L, respectively, p = 0.015).
Autonomic dysfunction measures
Changes in supine blood pressure, orthostatic blood pressure drops, and heart rate variability can be seen in Table 2.
Post-assessment SBP values differed from pre-assessment values only in the LIRT-BFR group (127±21 and 113±16 mmHg, respectively, p = 0.028). There were no significant pre- versus post-assessment values for supine DBP. There was a significant difference in groups at post-assessments for supine SBP and DBP (p = 0.007 and p = 0.041, respectively). Post-hoc analysis revealed significant differences between LIRT-BFR and HIRT groups for SBP (113±16 and 132±20 mmHg, respectively, p = 0.006) and DBP (75±13 and 86±10 mmHg, respectively, p = 0.046).
The average drop in SBP at 3 min of standing at pre-assessments, across all groups, was 27.63±11.84, indicating the presence of OH for our PD sample, on average. There were not significant time or group differences at post-assessments for improvements in the drop of systolic and diastolic blood pressure upon standing or number of reported symptoms upon standing.
Post-assessment HRV values differed from pre-assessment values only in the LIRT-BFR group (30±12 and 24±12 mmHg, respectively, p = 0.041). There was a significant difference between groups for root mean square of successive normal-to-normal interval differences (RMSSD) for HRV at post-assessments (p = 0.047). Post hoc analysis revealed a significant difference between LIRT-BFR and HIRT groups (26.87±5.07 and 23.80±3.99 ms, respectively, p = 0.042).
Exercise hemodynamic responses
The exercise time for the HIRT and LIRT-BFR groups was 54.85±4.23 min and 51.27±8.3 min, respectively. Average values for the HIRT and LIRT-BFR hemodynamic load averages over four weeks are noted in Table 3. An independent samples test was performed to note differences between hemodynamic markers for exercise sessions by using the average of all weeks combined. Significant differences were observed between lower body exercises average DBP immediately after exercise in the LIRT-BFR and HIRT groups (77.11±10.32 and 84.71±9.41 mmHg, p < 0.05, respectively). No other significant differences were found.
Table 3
Hemodynamic Response LIRT-BFR and HIRT
| Variable | HIRT | LIRT-BFR |
| Total SBP (mmHg) | 129±18 | 127±18 |
| Total DBP (mmHg) | 84±8 | 77±10 |
| Lower SBP (mmHg) | 129±18 | 129±18 |
| Lower DBP (mmHg) | 85±9 | 77±10* |
| Upper SBP (mmHg) | 128±19 | 128±20 |
| Upper DBP (mmHg) | 83±9 | 78±11 |
| MAP | 99±11 | 94±13 |
| Pulse Pressure | 45±13 | 50±11 |
| HR (bpm) | 89±12 | 90±11 |
| RPP | 115.29±25.19 | 115.33±24.04 |
| RPE | 7.57±0.98 | 6.97±2.11 |
Values are presented as means±standard deviation. HIRT, high-intensity resistance training; LIRT-BFR, low-intensity resistance training with blood flow restriction; CNTRL, control groups; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; RPP, rate pressure product; RPE, rating of perceived exertion. The *indicates significant differences compared to the HIRT group (p < 0.05). Lower indicates lower body exercises performed (leg extensions, leg curls and calf raises). Upper indicates upper body exercises performed (triceps extension, biceps curl and hand grip).
Nutritional analysis
There were no significant differences between conditions for total calories, macronutrients or estimated dietary folate levels at pre-assessments. No significant main effect for time were indicated for calories, carbohydrate, protein, fat or folate intake. Likewise, no significant group x time interaction was observed for nutrients previously mentioned.
DISCUSSION
The main findings of our study were that (1) LIRT-BFR significantly improves OH, homocysteine, peripheral circulation and HRV after four weeks in PwPD; (2) LIRT-BFR improves homocysteine, peripheral circulation and heart rate variability more than HIRT in PwPD and (3) hemodynamic loads for LIRT-BFR and HIRT are similar when using small muscle groups with intermittent BFR. Overall, LIRT-BFR was more effective than HIRT in improving autonomic and endothelial dysfunction in PwPD after four weeks of exercise.
LIRT-BFR had a more profound effect on endothelial function than HIRT in our study. A similar observation was made in healthy, older individuals who participated in four weeks of LIRT-BFR consisting of two upper and two lower body exercises [43]. A low TcP02 value (<46 mmHg) is a significant predictor of major adverse cardiovascular events [77] and is used as a predictive means of wound healing and decisions on amputation in diabetics [78]. While peripheral circulation is not a common clinical marker for PwPD, it is important to note that our participants, who did not have diabetes, had TcP02 values that paralleled middle-aged diabetics [79]. Our study was the first to measure peripheral circulation via TcP02 in PwPD. After four weeks of LIRT-BFR, our participants’ periperal circulation significantly improved and displayed values closer to non-diabetic, normal middle-aged individuals [79]. This was a significant difference compared to the HIRT group. This finding shows LIRT-BFR may be a promising intervention to help attenuate and possibly improve peripheral circulation in PwPD who experience poor circulation because of autonomic dysfunction.
Elevated levels of homocysteine are considered to be over 12μmol/L and are found in five to 10 percent of the general population and in up to 40 percent of patients with vascular disease [80]. In our study, all groups had elevated homocysteine levels at pre-assessment, averaging 18.07±4.56μmol/L and over 1000 mg of levodopa daily. Recommendations for levodopa dosage are between 300–1200 mg per day, divided into 3 to 12 doses [81]. Our participants’ homocysteine values were slightly higher than other PD participants studied who were treated with levodopa [8, 82, 83]. Our participants’ levodopa therapy was on the higher side of the recommended dosage, though more research is needed to determine if higher levodopa dosages linearly related to homocysteine levels. Our results were consistent with the current literature, where HIRT improved homocysteine levels [29], but LIRT-BFR had a more significant improvement compared to HIRT in our study. Homocysteine and cardiac autonomic dysfunction have not been linearly correlated in PwPD, but this correlation has been made in patients with obstructive sleep apnea and ischemic stroke patients with sleep apnea [13, 84]. This may indicate the clinical significance of testing homocysteine levels regularly in PwPD. To the authors’ knowledge, a study evaluating homocysteine in response to BFR exercise of any kind has not been done.
The autonomic and vascular state of our PwPD indicate a large deviation from normal, healthy older persons. HRV, RHI, homocysteine and OH rates for our participants were in agreeance with the current literature [8, 17, 19, 85] and indicate markers of autonomic dysfunction that can be used for clinical measures. Conversely, arterial stiffness measured just below standard cutoff values for our participants [69] and supports the observation that endothelial dysfunction in our participants precedes arterial stiffness in PwPD [55]. Typically, an individual with hypertension has altered endothelial function due to oxidative stress and subsequent atherosclerosis [86]. PwPD also experience endothelial dysfunction and atherosclerosis, but this is caused by autonomic dysfunction, rather than vascular oxidative stress [55]. PwPD experience high blood pressures when laying supine and low blood pressures when standing [18]. These variations in blood pressure are attributed to autonomic dysfunction rather than the diseased vessels seen in hypertensive individuals. In a study examining the effects of six weeks of walking in pre-hypertensive middle-aged men, significant improvements in SBP and HRV were observed only in the group who walked with BFR [87]. In our study, LIRT-BFR also resulted in improved supine SBP, HRV and OH while HIRT did not. While the mechanisms behind this are not fully understood, improved endothelial function, decreased sympathetic nerve activity and increased cardiac vagal tone may help to explain this improvement in these autonomic markers in response to BFR [38, 43, 45]. Further, a decrease in homocysteine can deactivate the angiotensin-renin-aldosterone system and thus, sympathetic nerve activity which results in improved autonomic function, as indicated by improved HRV [88].
Understanding the relationship between the autonomic nervous system and endothelial responses is crucial to help improve the quality of life in PwPD. Autonomic dysfunction is the culprit behind non-motor symptoms that disrupt the quality of life in PwPD and contribute to increased risk of falls associated with orthostatic intolerance [19, 21, 89]. Interactions exist between the autonomic nervous system and blood vessels. Neurotransmitters and endothelial cells communicate to change vascular tone in response to physiological conditions. This “cross-talk” is complex and endothelial dysfunction has been deemed to be the precursor to autonomic dysfunction in heart failure, diabetes and hypertension [90]. In our study, 31 of the 38 participants experienced OH. The inability for the autonomic nervous system to communicate effectively with the endothelial cells results in the steep drop in blood pressure. This disrupted autonomic function is also displayed through altered blood pressure responses during exercise in PwPD [27]. In our study, only LIRT-BFR resulted in significant improvements in HRV and OH. Further studies are needed to correlate improvements in autonomic function and subsequent improvements in endothelial function in PwPD, especially focusing on the cross-talk between the two systems in response to postural and exercise hemodynamic demands.
Hemodynamic loads
The safety of BFR has been questioned on a wide variety of platforms including abnormal blood pressure responses, blood clots, decreased venous return, altered muscle metaboreflex and cardiac output [46, 91, 92]. On the other hand, BFR has shown promising improvements in vulnerable populations, such as those with heart failure, chronic kidney disease, coronary artery disease and those who underwent cardiovascular surgery [45, 93–95]. It is important to note that no adverse events were reported in response to LIRT-BFR or HIRT over the course of four weeks in our study. Our BFR protocol was designed with these safety precautions in mind and was designed to avoid multi-joint, larger muscle group exercises, like leg press, where increased intra-abdominal pressures increased hemodynamic loads [51, 96]. Our LIRT-BFR protocol used seated, single-joint, small muscle group resistance exercises, like leg extensions, and resulted in similar hemodynamic loads compared to exercise-matched HIRT. The only significant difference was post-exercise DBP. The LIRT-BFR group’s exercise DBP was significantly lower than HIRT, on average. A decrease in DBP may indicate that vasodilation has occurred in the active muscular beds following exercise and may be an indicator of endothelial function [97] although post-exercise hypotension symptoms were not reported with this drop. While post-exercise hypotension following BFR exercise has been shown [98], BFR exercise that only uses single-joint exercises and is deflated after each exercise, alternating upper and lower body has not been studied until now.
Conclusions
The present study demonstrated a need for clinical autonomic and endothelial testing for PwPD and showed that LIRT-BFR may attenuate associated symptoms. Our participants had high homocysteine, decreased peripheral circulation and low vessel reactivity. They also displayed autonomic dysfunction through orthostatic hypotension and decreased HRV. Associated non-motor symptoms such as digestive issues and urinary incontinence are related to autonomic dysfunction, falls are associated with postural and OH and neuropathy is linked to poor peripheral circulation. Improving autonomic and endothelial dysfunction has the potential to improve these non-motor symptoms and increased the quality of life in PwPD. We demonstrated more favorable autonomic and endothelial adaptations after four weeks of LIRT-BFR compared to HIRT in PwPD displaying autonomic dysfunction. Additionally, using a LIRT-BFR protocol utilizing simple, single-joint, seated resistance exercises with intermittent BFR may be a solid solution to decrease hemodynamic loads, especially in those with diminished cardiovascular health. This study was limited by a small sample size and did not include persons with severe PD. Further, our study was only four weeks long, so it is unknown how long these training effects lasted or if these effects would remain favorable with continued exercise. Future research should aim to determine the residual and detraining effects on autonomic and endothelial function in PwPD after LIRT-BFR protocols and aim to test this LIRT-BFR protocol in persons with more advanced stages of PD. Further, studies examining the mechanisms behind the disrupted cross-talk between the autonomic and endothelial systems in PwPD are needed.
ACKNOWLEDGMENTS
This research study was done for completion of first author, Annie Bane’s, dissertation project at Baylor University. The entire dissertation project can be accessed on Baylor University’s graduate school website. Owen’s Recovery Science loaned the Delfi Personal Tourniquet System for Blood Flow Restriction system on an approved research loan.
FUNDING
Research funding was supplied by Baylor University dissertation allotment funds and by Abilene Christian University’s Department of Kinesiology & Nutrition.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
All datasets presented in this study are included in the article.
REFERENCES
[1] | GBD 2016 Parkinson’s Disease Collaborators ((2018) ) Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17: , 939–953. |
[2] | Janssens J , Malfroid K , Nyffeler T , Bohlhalter S , Vanbellingen T ((2014) ) Application of LSVT BIG intervention to address gait, balance, bed mobility, and dexterity in people with Parkinson disease: A case series. Phys Ther 94: , 1014–1023. |
[3] | Ahmed NN , Sherman SJ , Vanwyck D ((2008) ) Frailty in Parkinson’s disease and its clinical implications. Parkinsonism Relat Disord 14: , 334–337. |
[4] | Creaby MW , Cole MH ((2018) ) Gait characteristics and falls in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat Disord 57: , 1–8. |
[5] | Seiffert P , Derejczyk J , Kawa J , Marcisz C , Czernek M , Szymszal J , Kapko W , Bugdol M , Torbus A , Stępień-Wyrobiec O ((2017) ) Frailty phenotype and the role of levodopa challenge test in geriatric inpatients with mild parkinsonian signs. Biogerontology 18: , 641–650. |
[6] | Van Bladel A , Herssens N , Bouche K , Cambier D , Maes L , Lefeber N ((2023) ) Proportion of falls reported in persons with Parkinson’s disease: A meta-analysis. Clin Rehabil 37: , 1260–1277. |
[7] | ((2013) ) Drugs for Parkinson’s disease. Treat Guidel Med Lett 11: , 101–106. |
[8] | Rogers JD , Sanchez-Saffon A , Frol AB , Diaz-Arrastia R ((2003) ) Elevated plasma homocysteine levels in patients treated with levodopa: Association with vascular disease. Arch Neurol 60: , 59–64. |
[9] | Yoon JH , Lee JS , Yong SW , Hong JM , Lee PH ((2014) ) Endothelial dysfunction and hyperhomocysteinemia in Parkinson’s disease: Flow-mediated dilation study: Flow-Medicated Dilation and Hyperhomocysteinemia in PD. Mov Disord 29: , 1551–1555. |
[10] | Saadat P , Ahmadi Ahangar A , Samaei SE , Firozjaie A , Abbaspour F , Khafri S , Khoddami A ((2018) ) Serum homocysteine level in Parkinson’s disease and its association with duration, cardinal manifestation, and severity of disease. Parkinsons Dis 2018: , 5813084. |
[11] | Rodriguez-Oroz MC , Lage PM , Sanchez-Mut J , Lamet I , Pagonabarraga J , Toledo JB , García-Garcia D , Clavero P , Samaranch L , Irurzun C , Matsubara JM , Irigoien J , Bescos E , Kulisevsky J , Pérez-Tur J , Obeso JA ((2009) ) Homocysteine and cognitive impairment in Parkinson’s disease: A biochemical, neuroimaging, and genetic study. Mov Disord 24: , 1437–1444. |
[12] | Mendes RH , Mostarda C , Candido GO , Moraes-Silva IC , D’Almeida V , Belló-Klein A , Irigoyen MC , Rigatto K ((2014) ) Moderate hyperhomocysteinemia provokes dysfunction of cardiovascular autonomic system and liver oxidative stress in rats. Auton Neurosci 180: , 43–47. |
[13] | Liu L , Wu Q , Yan H , Zheng X , Zhou Q ((2020) ) Plasma homocysteine and autonomic nervous dysfunction: Association and clinical relevance in OSAS. Dis Markers 2020: , 4378505. |
[14] | Tiftikcioglu BI , Bilgin S , Duksal T , Kose S , Zorlu Y ((2016) ) Autonomic neuropathy and endothelial dysfunction in patients with impaired glucose tolerance or type 2 diabetes mellitus. Medicine (Baltimore) 95: , e3340. |
[15] | Liepelt-Scarfone I , Pilotto A , Müller K , Bormann C , Gauss K , Wurster I , Streffer J , Berg D ((2015) ) Autonomic dysfunction in subjects at high risk for Parkinson’s disease. J Neurol 262: , 2643–2652. |
[16] | Romagnolo A , Zibetti M , Merola A , Canova D , Sarchioto M , Montanaro E , Artusi CA , Vallelonga F , Maule S , Lopiano L ((2019) ) Cardiovascular autonomic neuropathy and falls in Parkinson disease: A prospective cohort study. J Neurol 266: , 85–91. |
[17] | Heimrich KG , Lehmann T , Schlattmann P , Prell T ((2021) ) Heart rate variability analyses in Parkinson’s disease: A systematic review and meta-analysis. Brain Sci 11: , 959. |
[18] | Amara AW , Memon AA ((2018) ) Effects of exercise on non-motor symptoms in Parkinson’s disease. Clin Ther 40: , 8–15. |
[19] | Goldstein DS ((2006) ) Orthostatic hypotension as an early finding in Parkinson’s disease. Clin Auton Res 16: , 46–54. |
[20] | Hiorth YH , Pedersen KF , Dalen I , Tysnes O-B , Alves G ((2019) ) Orthostatic hypotension in Parkinson disease. Neurology 93: , e1526–e1534. |
[21] | Merola A , Romagnolo A , Rosso M , Suri R , Berndt Z , Maule S , Lopiano L , Espay AJ ((2018) ) Autonomic dysfunction in Parkinson’s disease: A prospective cohort study. Mov Disord 33: , 391–397. |
[22] | Amiya E , Watanabe M , Komuro I ((2014) ) The relationship between vascular function and the autonomic nervous system. Ann Vasc Dis 7: , 109–119. |
[23] | Claassen DO , Adler CH , Hewitt LA , Gibbons C ((2018) ) Characterization of the symptoms of neurogenic orthostatic hypotension and their impact from a survey of patients and caregivers. BMC Neurol 18: , 125. |
[24] | Sabino-Carvalho JL , Vianna LC ((2020) ) Altered cardiorespiratory regulation during exercise in patients with Parkinson’s disease: A challenging non-motor feature. SAGE Open Med 8: , 2050312120921603. |
[25] | Low DA , Vichayanrat E , Iodice V , Mathias CJ ((2014) ) Exercise hemodynamics in Parkinson’s disease and autonomic dysfunction. Parkinsonism Relat Disord 20: , 549–553. |
[26] | Griffith G , Lamotte G , Mehta N , Fan P , Nikolich J , Springman V , Suttman E , Joslin E , Balfany K , Dunlap M , Kohrt WM , Christiansen CL , Melanson EL , Josbeno D , Chahine LM , Patterson CG , Corcos DM ((2024) ) Chronotropic incompetence during exercise testing as a marker of autonomic dysfunction in individuals with early Parkinson’s disease. J Parkinsons Dis 14: , 121–133. |
[27] | Sabino-Carvalho JL , Fisher JP , Vianna LC ((2021) ) Autonomic function in patients with Parkinson’s disease: From rest to exercise. Front Physiol 12: , 626640. |
[28] | Rodrigo-Mallorca D , Loaiza-Betancur AF , Monteagudo P , Blasco-Lafarga C , Chulvi-Medrano I ((2021) ) Resistance training with blood flow restriction compared to traditional resistance training on strength and muscle mass in non-active older adults: A systematic review and meta-analysis. Int J Environ Res Public Health 18: , 11441. |
[29] | Deminice R , Ribeiro DF , Frajacomo FTT ((2016) ) The effects of acute exercise and exercise training on plasma homocysteine: A meta-analysis. Plos One 11: , e0151653. |
[30] | Vincent HK , Bourguignon C , Vincent KR ((2006) ) Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity 14: , 1921–1930. |
[31] | Morberg BM , Jensen J , Bode M , Wermuth L ((2014) ) The impact of high intensity physical training on motor and non-motor symptoms in patients with Parkinson’s disease (PIP): A preliminary study. Neurorehabilitation 35: , 291–298. |
[32] | Dibble LE , Hale TF , Marcus RL , Droge J , Gerber JP , LaStayo PC ((2006) ) High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov Disord 21: , 1444–1452. |
[33] | Hirsch MA , Toole T , Maitland CG , Rider RA ((2003) ) The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch Phys Med Rehabil 84: , 1109–1117. |
[34] | Letieri RV , Teixeira AM , Furtado GE , Lamboglia CG , Rees JL , Gomes BB ((2018) ) Effect of 16 weeks of resistance exercise and detraining comparing two methods of blood flow restriction in muscle strength of healthy older women: A randomized controlled trial. Exp Gerontol 114: , 78–86. |
[35] | Loenneke JP , Wilson GJ , Wilson JM ((2010) ) A mechanistic approach to blood flow occlusion. Int J Sports Med 31: , 1–4. |
[36] | Patterson SD , Leggate M , Nimmo MA , Ferguson RA ((2013) ) Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur J Appl Physiol 113: , 713–719. |
[37] | Takarada Y , Takazawa H , Sato Y , Takebayashi S , Tanaka Y , Ishii N ((2000) ) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol (1985) 88: , 2097–2106. |
[38] | Zhao Y , Lin A , Jiao L ((2021) ) Eight weeks of resistance training with blood flow restriction improve cardiac function and vascular endothelial function in healthy young Asian males. Int Health 13: , 471–479. |
[39] | Hunt JEA , Walton LA , Ferguson RA ((2012) ) Brachial artery modifications to blood flow-restricted handgrip training and detraining. J Appl Physiol (1985) 112: , 956–961. |
[40] | Hunt JEA , Galea D , Tufft G , Bunce D , Ferguson RA ((2013) ) Time course of regional vascular adaptations to low load resistance training with blood flow restriction. J Appl Physiol (1985) 115: , 403–411. |
[41] | Douris PC , Cogen ZS , Fields HT , Greco LC , Hasley MR , Machado CM , Romagnuolo PM , Stamboulis G , DiFrancisco-Donoghue J ((2018) ) The effects of blood flow restriction training on functional improvements in an active single subject with Parkinson disease. Int J Sports Phys Ther 13: , 247–254. |
[42] | Douris PC , D’Agostino N , Werner WG , Petrizzo J , DiFrancisco-Donoghue J ((2022) ) Blood flow restriction resistance training in a recreationally active person with Parkinson’s disease. Physiother Theory Pract 38: , 422–430. |
[43] | Shimizu R , Hotta K , Yamamoto S , Matsumoto T , Kamiya K , Kato M , Hamazaki N , Kamekawa D , Akiyama A , Kamada Y , Tanaka S , Masuda T ((2016) ) Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol 116: , 749–757. |
[44] | Ozaki H , Yasuda T , Ogasawara R , Sakamaki-Sunaga M , Naito H , Abe T ((2013) ) Effects of high-intensity and blood flow-restricted low-intensity resistance training on carotid arterial compliance: Role of blood pressure during training sessions. Eur J Appl Physiol 113: , 167–174. |
[45] | de Deus LA , Neves RVP , Corrêa H de L , Reis AL , Honorato FS , Silva VL , de Araújo TB , Souza MK , Sousa CV , Simões HG , Prestes J , Silva Neto LS , Rodrigues Santos CA , Melo GF , Stone WJ , Rosa TS ((2021) ) Improving the prognosis of renal patients: The effects of blood flow-restricted resistance training on redox balance and cardiac autonomic function. Exp Physiol 106: , 1099–1109. |
[46] | Cristina-Oliveira M , Meireles K , Spranger MD , O’Leary DS , Roschel H , Peçanha T ((2020) ) Clinical safety of blood flow-restricted training? A comprehensive review of altered muscle metaboreflex in cardiovascular disease during ischemic exercise. Am J Physiol Heart Circ Physiol 318: , H90–H109. |
[47] | Amorim S , Gaspar AP , Degens H , Cendoroglo MS , de Mello Franco FG , Ritti-Dias RM , Cucato GG , Rolnick N , de Matos LDNJ ((2022) ) The effect of a single bout of resistance exercise with blood flow restriction on arterial stiffness in older people with slow gait speed: A pilot randomized study. J Cardiovasc Dev Dis 9: , 85. |
[48] | Sardeli AV , do Carmo Santos L , Ferreira MLV , Gáspari AF , Rodrigues B , Cavaglieri CR , Chacon-Mikahil MPT ((2017) ) Cardiovascular responses to different resistance exercise protocols in elderly. Int J Sports Med 38: , 928–936. |
[49] | Horiuchi M , Okita K ((2012) ) Blood flow restricted exercise and vascular function. Int J Vasc Med 2012: , 543218–543218. |
[50] | Staunton CA , May AK , Brandner CR , Warmington SA ((2015) ) Haemodynamics of aerobic and resistance blood flow restriction exercise in young and older adults. Eur J Appl Physiol 115: , 2293–2302. |
[51] | Scott BR , Peiffer JJ , Thomas HJ , Marston KJ , Hill KD ((2018) ) Hemodynamic responses to low-load blood flow restriction and unrestricted high-load resistance exercise in older women. Front Physiol 9: , 1324. |
[52] | Miyachi M ((2013) ) Effects of resistance training on arterial stiffness: A meta-analysis. Br J Sports Med 47: , 393–396. |
[53] | Rossow LM , Fahs CA , Thiebaud RS , Loenneke JP , Kim D , Mouser JG , Shore EA , Beck TW , Bemben DA , Bemben MG ((2014) ) Arterial stiffness and blood flow adaptations following eight weeks of resistance exercise training in young and older women. Exp Gerontol 53: , 48–56. |
[54] | Rakobowchuk M , McGowan CL , de Groot PC , Hartman JW , Phillips SM , MacDonald MJ ((2005) ) Endothelial function of young healthy males following whole body resistance training. J Appl Physiol (1985) 98: , 2185–2190. |
[55] | Kim J-S , Lee S-H , Oh Y-S , Park J-W , An J-Y , Choi H-S , Lee K-S ((2017) ) Arterial stiffness and cardiovascular autonomic dysfunction in patients with Parkinson’s disease. Neurodegener Dis 17: , 89–96. |
[56] | Hong CT , Hu HH , Chan L , Bai CH ((2018) ) Prevalent cerebrovascular and cardiovascular disease in people with Parkinson’s disease: A meta-analysis. Clin Epidemiol 10: , 147–1154. |
[57] | Chen Z , Li G , Liu J ((2020) ) Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol Dis 134: , 104700. |
[58] | Brilla LR , Stephens AB , Knutzen KM , Caine D ((1998) ) Effect of strength training on orthostatic hypotension in older adults. J Cardiopulm Rehabil 18: , 295–300. |
[59] | Monahan KD , Tanaka H , Dinenno FA , Seals DR ((2001) ) Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation 104: , 1627–1632. |
[60] | Poton R , Polito MD ((2016) ) Hemodynamic response to resistance exercise with and without blood flow restriction in healthy subjects. Clin Physiol Funct Imaging 36: , 231–236. |
[61] | Ito S ((2019) ) High-intensity interval training for health benefits and care of cardiac diseases – The key to an efficient exercise protocol. World J Cardiol 11: , 171–188. |
[62] | Zhao YJ , Wee HL , Chan Y-H , Seah SH , Au WL , Lau PN , Pica EC , Li SC , Luo N , Tan LCS ((2010) ) Progression of Parkinson’s disease as evaluated by Hoehn and Yahr stage transition times. Mov Disord 25: , 710–716. |
[63] | Uhrbrand A , Stenager E , Pedersen MS , Dalgas U ((2015) ) Parkinson’s disease and intensive exercise therapy – a systematic review and meta-analysis of randomized controlled trials. J Neurol Sci 353: , 9–19. |
[64] | Weatherholt AM , Vanwye WR , Lohmann J , Owens JG ((2019) ) The effect of cuff width for determining limb occlusion pressure: A comparison of blood flow restriction devices. Int J Exerc Sci 12: , 136–143. |
[65] | Masood N , Jimenez-Shahed J ((2023) ) Effective management of “off” episodes in Parkinson’s disease: Emerging treatment strategies and unmet clinical needs. Neuropsychiatr Dis Treat 19: , 247–266. |
[66] | Liu W , Meng M , Chen J , Wang L , Sun Z , Li X , Zhou J , Gao C , Zhou J , Chu H , Fan W , Bai Y , Yang J ((2017) ) Reactive hyperemia index in patients on maintenance hemodialysis: Cross-sectional data from a cohort study. Sci Rep 7: , 45757. |
[67] | Rozanski A , Qureshi E , Bauman M , Reed G , Pillar G , Diamond GA ((2001) ) Peripheral arterial responses to treadmill exercise among healthy subjects and atherosclerotic patients. Circulation 103: , 2084–2089. |
[68] | Kuvin JT , Mammen A , Mooney P , Alsheikh-Ali AA , Karas RH ((2007) ) Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med 12: , 13–16. |
[69] | Perrault R , Omelchenko A , Taylor CG , Zahradka P ((2019) ) Establishing the interchangeability of arterial stiffness but not endothelial function parameters in healthy individuals. BMC Cardiovasc Disord 19: , 190. |
[70] | Weber T , Auer J , O’Rourke MF , Kvas E , Lassnig E , Berent R , Eber B ((2004) ) Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 109: , 184–189. |
[71] | Sands WA , Wurth JJ , Hewit JK ((2013) ), The National Strength and Conditioning Association’s (NSCA) Basics of Strength and Conditioning Manual.. |
[72] | Lahrmann H , Cortelli P , Hilz M , Mathias CJ , Struhal W , Tassinari M ((2006) ) EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 13: , 930–936. |
[73] | Linder JR , Stauss HM , Gindes H , Pierce GL , Von Bergen NH , Haynes WG , Fiedorowicz JG ((2014) ) Finger volume pulse waveforms facilitate reliable assessment of heart rate variability, but not blood pressure variability or baroreflex function. BMC Cardiovasc Disord 14: , 180. |
[74] | Keavney B , Bird R , Caiazza A , Casadei B , Conway J ((2000) ) Measurement of blood pressure using the auscultatory and oscillometric methods in the same cuff deflation: Validation and field trial of the A & D TM2421 monitor. J Hum Hypertens 14: , 573–579. |
[75] | Morishita S , Tsubaki A , Takabayashi T , Fu JB ((2018) ) Relationship between the rating of perceived exertion scale and the load intensity of resistance training. Strength Cond J 40: , 94–109. |
[76] | ((2010) ) WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. World Health Organization, Geneva. |
[77] | Gazzaruso C , Coppola A , Falcone C , Luppi C , Montalcini T , Baffero E , Gallotti P , Pujia A , Solerte SB , Pelissero G , Giustina A ((2013) ) Transcutaneous oxygen tension as a potential predictor of cardiovascular events in type 2 diabetes: Comparison with ankle-brachial index. Diabetes Care 36: , 1720–1725. |
[78] | Wütschert R , Bounameaux H ((1997) ) Determination of amputation level in ischemic limbs. Reappraisal of the measurement of TcPo2. Diabetes Care 20: , 1315–1318. |
[79] | de Meijer VE , van’t Sant HP , Spronk S , Kusters FJ , den Hoed PT ((2008) ) Reference value of transcutaneous oxygen measurement in diabetic patients compared with nondiabetic patients. J Vasc Surg 48: , 382–388. |
[80] | Stanger O , Herrmann W , Pietrzik K , Fowler B , Geisel J , Dierkes J , Weger M ((2004) ) Clinical use and rational management of homocysteine, folic acid, and B vitamins in cardiovascular and thrombotic diseases. Z Kardiol 93: , 439–453. |
[81] | Gandhi KR , Saadabadi A ((2024) ) Levodopa (L-Dopa). In StatPearls. StatPearls Publishing, Treasure Island (FL). |
[82] | Kocer B , Guven H , Comoglu SS ((2016) ) Homocysteine levels in Parkinson’s disease: Is entacapone effective? Biomed Res Int 2016: , 7563705. |
[83] | Nevrly M , Kanovsky P , Vranova H , Langova K , Hlustik P ((2010) ) Effect of entacapone on plasma homocysteine levels in Parkinson’s disease patients. Neurol Sci 31: , 565–569. |
[84] | Chen M-G , Zhu W-S , Yin Q , Wu B-N , Wang Q-Z , Ma M-M , Liu D-Z , Li Y-K , Liu C-L , Huang X-J , Chen Z-Y , Wang W-X , Xu G-L , Liu X-F ((2011) ) [Plasma homocysteine levels in ischemic stroke patients with obstructive sleep apnea]. Zhonghua Yi Xue Za Zhi 91: , 1753–1756. |
[85] | Fukui Y , Hishikawa N , Shang J , Sato K , Nakano Y , Morihara R , Ohta Y , Yamashita T , Abe K ((2016) ) Peripheral arterial endothelial dysfunction of neurodegenerative diseases. J Neurol Sci 366: , 94–99. |
[86] | Puddu P , Puddu GM , Zaca F , Muscari A ((2000) ) Endothelial dysfunction in hypertension. Acta Cardiol 55: , 221–232. |
[87] | Junior AF , Schamne JC , Perandini LAB , Chimin P , Okuno NM ((2019) ) Effects of walking training with restricted blood flow on HR and HRV kinetics and HRV recovery. Int J Sports Med 40: , 585–591. |
[88] | Li T , Yu B , Liu Z , Li J , Ma M , Wang Y , Zhu M , Yin H , Wang X , Fu Y , Yu F , Wang X , Fang X , Sun J , Kong W ((2018) ) Homocysteine directly interacts and activates the angiotensin II type I receptor to aggravate vascular injury. Nat Commun 9: , 11. |
[89] | Kotagal V , Lineback C , Bohnen NI , Albin RL ((2016) ) Orthostatic hypotension predicts motor decline in early Parkinson disease. Parkinsonism Relat Disord 32: , 127–129. |
[90] | Sheng Y , Zhu L ((2018) ) The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol 10: , 17–28. |
[91] | Patterson SD , Hughes L , Warmington S , Burr J , Scott BR , Owens J , Abe T , Nielsen JL , Libardi CA , Laurentino G , Neto GR , Brandner C , Martin-Hernandez J , Loenneke J ((2019) ) Blood flow restriction exercise position stand: Considerations of methodology, application, and safety. Front Physiol 10: , 533–533. |
[92] | Warmington SA , Staunton CA , May AK , Brandner CR ((2016) ) Blood flow restriction exercise: Acute versus chronic safety. Eur J Appl Physiol 116: , 861–862. |
[93] | Goennebaek T , Sieljacks P , Nielson R , Pryds K , Jespersen N , Wang J , Carlsen CR , Schmidt M , de Paoli FV , Miller BF , Vissing K , Botker HE ((2019) ) Effect of blood flow restricted resistance exercise and remote ischemic conditioning on functional capacity and myocellular adaptations in patients with heart failure | Circulation: Heart Failure. Circ Heart Fail 12: , e006427. |
[94] | Kambič T , Novaković M , Tomažin K , Strojnik V , Jug B ((2019) ) Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: A pilot randomized controlled trial. Front Physiol 10: , 656. |
[95] | Ogawa H , Nakajima T , Shibasaki I , Nasuno T , Kaneda H , Katayanagi S , Ishizaka H , Mizushima Y , Uematsu A , Yasuda T , Yagi H , Toyoda S , Hortobágyi T , Mizushima T , Inoue T , Fukuda H ((2021) ) Low-intensity resistance training with moderate blood flow restriction appears safe and increases skeletal muscle strength and size in cardiovascular surgery patients: A pilot study. J Clin Med 10: , 547. |
[96] | Blazek D , Stastny P , Maszczyk A , Krawczyk M , Matykiewicz P , Petr M ((2019) ) Systematic review of intra-abdominal and intrathoracic pressures initiated by the Valsalva manoeuvre during high-intensity resistance exercises. Biol Sport 36: , 373–386. |
[97] | Brett Sally E , Ritter James M , Chowienczyk Philip J. ((2000) ) Diastolic blood pressure changes during exercise positively correlate with serum cholesterol and insulin resistance. Circulation 101: , 611–615. |
[98] | Araújo JP , Silva ED , Silva JCG , Souza TSP , Lima EO , Guerra I , Sousa MSC ((2014) ) The acute effect of resistance exercise with blood flow restriction with hemodynamic variables on hypertensive subjects. J Hum Kinet 43: , 79–85. |




