Pain and the Non-Pharmacological Management of Pain in People with Parkinson’s Disease
Abstract
Pain is a distressing and universal experience, yet everyone’s pain experience is influenced by a complex array of biological, psychological, and social factors. For people with Parkinson’s disease (PwP), these biopsychosocial factors include neurodegeneration and the psychological and social factors that accompany living with a chronic, neurodegenerative condition in addition to the factors experienced by those in the general population (e.g., living with co-morbidities such as osteoarthritis). The way these factors influence each individual is likely to determine which pain management strategies are optimal for them. This review first describes pain and the biopsychosocial model of pain. It explores how pain is classified in Parkinson’s disease (PD) and describes the three main types of pain: nociceptive, neuropathic, and nociplastic pain. This background provides context for a discussion of non-pharmacological pain management strategies that may aid in the management of pain in PwP; exercise, psychological strategies, acupuncture and massage. While there is little PD-specific research to inform the non-pharmacological management of pain, findings from current PD research are combined with that from chronic pain research to present recommendations for clinical practice. Recommendations include assessment that incorporates potential biopsychosocial contributors to pain that will then guide a holistic, multi-modal approach to management. As exercise provides overall benefits for PwP, those with chronic pain should be carefully monitored with exercise prescribed and adjusted accordingly. Research is needed to develop and evaluate multi-modal approaches to pain management that are delivered in a biopsychosocial framework.
INTRODUCTION
Chronic pain is an unpleasant and often distressing experience that occurs more frequently in people with Parkinson’s disease (PwP) than in the general population [1]. Recognizing and optimally managing pain therefore has the potential to improve health-related quality of life for many PwP. Contemporary research in chronic pain overall highlights the importance considering biological, psychological, and social influences of pain to provide an effective, holistic, individualized, and person-centered approach to pain management [2, 3]. However, there is little research in PwP that has adopted this biopsychosocial approach. Two recent reviews of the non-pharmacological management of pain in PwP [4, 5] have provided a comprehensive summary of the literature plus helpful insights into the types of non-pharmacological strategies that have been studied, but have not viewed pain in a biopsychosocial context. Therefore, this narrative review aims to provide new, holistic insights into the non-pharmacological management of pain in PwP by addressing the following aims within a biopsychosocial context:
1. Provide background information about pain generally and in Parkinson’s disease (PD), including the types of pain and factors that can contribute to pain.
2. Synthesize the literature about the non-pharmacological management of pain in PwP.
3. Make recommendations for clinical practice and highlight areas for future research based on currently available PD-specific literature combined with more general chronic pain literature.
METHODS
This review includes literature about non-pharmacological pain management that can be provided by allied health; therefore, deep brain stimulation is not included. For a comprehensive summary of deep brain stimulation for pain management, please refer to the previous reviews [4, 5]. Literature for inclusion in this review was identified by each author from personal libraries and resources. Additional literature was identified by searching the reference lists of previously published reviews and other included articles, as well as through informal database searches. Randomized controlled trials of exercise in PwP that included a pain outcome were also identified through a systematic search that was undertaken by the lead author (NEA) for a separate review yet to be published (Prospero registration CRD42019129154). Allied and Complementary Medicine Database (AMED), Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, EMBASE, MEDLINE, and Physiotherapy Evidence Database (PEDro) databases were searched for full text, peer reviewed articles from their inception until 15January 2023.
PAIN AND THE BIOPSYCHOSOCIAL MODEL OF PAIN
Pain is complex and is often described in terms of the biopsychosocial model. Pain is defined as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential issue damage” [6]. Acute pain serves as an important warning sign of disease or injury. However, chronic pain, that is pain that continues or recurs for more than 3 months, can occur even if the original condition is treated, whether that be optimally or inadequately [6]. A biopsychosocial approach to healthcare was proposed by Engel in 1977 [7] and was applied to a model of pain in the 1980s [8]. It has become widely accepted and provides a framework for considering contributors to pain, and therefore potential multidisciplinary treatments for pain [9]. This model considers that pain is due to dynamic interaction between overlapping biological (e.g., nociception, disease severity, co-morbidities), psychological (e.g., stress, anxiety, depression, expectations) and social/environmental (e.g., cultural beliefs, work environment, social support) factors, the latter being considered contextual factors within which the pain experience occurs [9]. However, while this framework is broadly accepted, in research to date these factors have rarely been comprehensively addressed in combination, nor have the individual’s unique combination of contributing factors been adequately considered [9, 10]. While research incorporating psychological and social factors in PwP is in its infancy, there is evidence that maladaptive cognitions (e.g., catastrophizing) [11, 12], anxiety and depression [13, 14] are associated with worse pain severity and that ethnic background influences analgesia use in PwP [15]. Therefore, taking a holistic, biopsychosocial approach to pain management is likely to be important for PwP, where pain is inevitably complex and multifactorial.
PAIN IN PARKINSON’S DISEASE
Pain is a frequent and problematic impairment experienced by PwP. A large observational study of people with mild to moderate disease found 85% reported pain, with over 40% reporting the pain was moderate to severe [13]. PwP are around twice as likely to report chronic pain than those in the general older population, even after adjusting for musculoskeletal joint problems [1]. Pain is inherently unpleasant and can negatively affect quality of life more so than the motor impairments [13, 16] and is associated with financial burden through factors such as reduced capacity to work and early retirement [17].
To aid pain management, systems have been developed for classifying pain in PwP, and these classification systems have evolved over the years. Initially, Ford (1998) divided pain into five categories: musculoskeletal pain, radicular or neuropathic pain, pain associated with dystonia, akathitic discomfort, and primary or central parkinsonian pain [18]. The first PD-specific pain assessment tool to be developed and validated, the King’s Parkinson’s Pain Scale, determines the severity and frequency of pain across seven domains: musculoskeletal, chronic, fluctuation-related, nocturnal, orofacial, discoloration/oedema/swelling and radicular pain [19]. More recently, the PD Pain Classification System was developed to guide a mechanistic approach to pain management [20]. This system involves first determining if the pain is PD-related or PD-unrelated [21], with PD-related pain considered to have started or worsened after PD diagnosis, be aggravated by motor impairments, associated with dyskinesia or improved with PD medication. PD-related pain is then classified as nociceptive, neuropathic or nociplastic and is graded in terms of intensity, frequency, and impact on daily living.
Nociceptive pain is the most common and is due to activation of nociceptors by actual or threatened non-neural tissue damage [6]. This type of pain is associated with musculoskeletal conditions such as osteoarthritis and other inflammatory conditions. In PwP the presence of motor impairments such as dystonia, dyskinesia [22] and rigidity [23], along with postural abnormalities (e.g., captocormia) [24, 25] and PD-associated changes in muscle tissue, (e.g., dystrophic processes and loss of muscle mass) [26, 27] also contribute to nociceptive pain. Neuropathic pain is due to a lesion or disease of the somatosensory nervous system, and is described as burning, tingling or shooting pain [6]. There is evidence that the PD process could contribute to central and peripheral neuropathic pain [28, 29], including through the deposition of alpha-synuclein in peripheral nerve fibers [30]. Postural abnormalities may also lead to increased rates of radicular neuropathic back pain through compression of spinal nerves [31]. Nociplastic pain is due to altered nociception despite no actual or threatened tissue damage activating peripheral nociceptors and no disease or lesion of the somatosensory system, reflecting a central pain mechanism [6, 32]. This type of pain is variable in presentation, can be difficult to localize and fluctuates in intensity [24]. Changes in the central nervous system [4, 28, 33] may contribute to nociplastic pain in PwP. It is important to note that while these pain types all have their own definitions, in practice many people demonstrate features consistent with more than one pain type and many people with chronic pain will have a nociplastic component [3].
While a useful and important development, the present review proposes that the mechanistic approach to diagnosing and managing pain in PwP designed by Mylius et al. [22] could be further enhanced by viewing all chronic pain experienced by PwP as influenced by the disease process, with PD-related degeneration known to occur in systems involved in the processing and modulation of pain [4, 28, 33]. It is believed that PD can impact pain processing at multiple levels, starting from the transmission of pain from peripheral structures to higher centers, the reception and interpretation of pain, and also affects the function of several anatomical structures involved in pain mechanisms [4, 34]. For example, the characteristic degeneration of dopaminergic neurons in the subcortical structures of the brain such as the striatum and mesolimbic system can lead to the hyperactivation of neurons responsible for pain processing and an increase in pain stimulation signals in the central nervous system [34, 35]. In addition, a lack of dopamine in the substantia nigra can lead to an increase in synaptic plasticity, which can contribute to a decrease in the pain sensitivity threshold. Furthermore, reduced dopamine contributes to non-motor impairments such as anxiety, depression, and insomnia [36–38], which are known to be associated with increased pain in PwP [13, 14]. Therefore, as the pathways of pain are similar in people with and without PD, all pain experienced by PwP is likely to be influenced by the presence of PD.
NON-PHARMACOLOGICAL MANAGEMENT OF PAIN IN PARKINSON’S DISEASE: INTERVENTIONS AND CONSIDERATIONS
This review explores non-pharmacological pain management strategies that can be provided by allied health, with a focus on exercise and psychological strategies. Readers should note that non-pharmacological management strategies should be implemented in the presence of optimal pharmacological management, including optimized doses and schedules of levodopa and/or other antiparkinsonian medications from different classes [4]. The evidence presented regarding the effect of non-pharmacological interventions on pain focuses predominantly on studies that had a stated aim to reduce pain. This is because there are many published studies that have measured a pain outcome as part of a broader battery of outcome measures, despite the intervention not being designed to reduce pain. The researchers judged that such studies are less likely to provide robust evidence about pain management. Therefore, only studies with a stated aim to reduce pain are presented in Table 1, where there are six studies of exercise interventions [39–43], two of acupuncture [44, 45], and one of massage [46]. The quality of the randomized controlled trials is presented using the PEDro scale [47]. This scale provides a score out of 10 where a higher score indicates better methodologicalquality.
Table 1
Trials of non-pharmacological interventions with an aim to reduce pain in people with Parkinson’s disease (excluding case reports)
| Author, year Study design PEDro Score* (/10) | Participants mean (SD) | Pain in inclusion criteria? | Intervention | Comparison | Outcomes related to pain and psychosocial factors |
| Exercise-based interventions | |||||
| Feital 2022 [39] Single group N/A | N=15 Age (y) = 67 (9) % female = 27% PD (y) = 9 (4) HY = 2.2 (0.8) UPDRS motor = 29.1 (6.4) | Y – low back pain | Pilates 60 min x2/wk×12 wks | nil | VAS ↓w# McGill Pain Questionnaire↓w# Roland-Morris Disability Questionnaire↓w# Beck Depression Inventory↓w Fatigue Severity Scale PDQ-39 |
| Gandolfi 2019 [51] RCT 7 | N = 37 Age (y) = 72 (6.5) % female = 35% PD (y) = 7.3 (5.1) HY = 2.3 (1.2) UPDRS motor = 34.2 (13.9) | N | Trunk-specific exercise and functional tasks 60 min×2/wk×4 wks | Stretching, strengthening, balance and gait training 60 min×2/wk×4 wks | VAS↓wboth Standing posture (forward trunk flexion)↓b# PDQ-8 quality of life↓b |
| Myers 2020 [40] RCT 5 | N = 26 Age (y) = 67.8 (8.7) % female = 42% PD (y) = NR HY = 2.3 (0.3) UPDRS motor = 28.1 (9.6) | N | Yoga 60 min x2/wk×12 wks | Usual care | Revised Oswestry Disability Index ↓w Beck Anxiety Inventory |
| Paolucci 2017 [43] RCT 8 | N = 36 (34 analyzed) Age (y) = 66.5 (12.3) % female = 44% PD (y) = 3 (1.2) HY = 1.5 (0.8) UPDRS motor = 10.5 (6.7) | N | Mézières Postural exercises 60 min×2/wk×5wks | Home exercise focused on posture, range of movement and functional activities 60 min×2/wk×5wks | VAS ↓w Trunk flexion flexibility ↑wboth SF-36quality of life (physical role functioning subscale)↑w* SF-36 quality of life (other subscales) |
| Perez de la Cruz 2017 [42] RCT 7 | N = 30 Age (y) = 67.2 (7.6) % female = 57% PD (y) = 6.5 (2.9) HY = 2.7 (0.6) UPDRS motor = 15.2 (7.3) | N | Aquatic therapy 45 min×2/wk×10 wks | Strengthening and aerobic exercises 45 min×2/wk×10 wks | VAS ↓b# |
| Perez de la Cruz 2019 [41] RCT 7 | N = 30 Age (y) = 67.2 (7.6) % female = 50% PD (y) = 7.4 (2.5) HY = 2.8 (0.6) MDS/UPDRS motor = NR | N | Aquatic therapy 45 min×2/wk×10 wks | Strengthening and aerobic exercises 45 min×2/wk×10 wks | VAS ↓b SF-36quality of life (physical functioning, general health and mental health subscores, physical and mental composite scores)↑*w SF-36quality of life (overall score)↑*b Geriatric Depression Scale(short form)↓b |
| Acupuncture | |||||
| Yaksi 2022 [45] RCT 5 | N = 40 (29 analyzed) Age (y) = 70.2 (3.4) % female = 40% PD (y) = 4.4 (1.6) HY = median 2 (range 1–4) MDS/UPDRS motor = NR | Y chronic neck pain | Acupuncture x 2/wk×5wks plus neck exercises x7/wk×5 wks | Neck exercises x 7/wk×5 wks | VAS ↓b Neck Disability Index ↓b |
| Yu 2019 [44] non-randomized trial 4 | N = 16 Age (y) = 64.9 (8.5) % female = 56% PD (y) = 9.5 (4.5) HY = NR MDS-UPDRS motor = 19.0 (5.2) | Y | Acupuncture 16 sessions over 8 wks (1 to 3 x/wk) | No intervention | King’s PD Pain Scale ↓b VAS Beck Depression Inventory Parkinson’s Disease Sleep Scale PDQ-39 quality of life |
| Massage | |||||
| Skogar 2013 [46] RCT 3 | N = 45 (44 analyzed) Age (y) = range 50–79 % female = 64% PD (y) = >2 HY = 2.2 (0.7) MDS/UPDRS motor = NR | Y – chronic PD-related pain | Massage 60 min, 10 sessions over 8 wks | Rest to music 60 min, 10 sessions over 8 wks | VAS ↓w SF-36quality of life (overall score)↑*wboth Parkinson’s disease sleep scale↑*w |
*Higher score is a better score; #Primary outcome; bBetween group change; w Within group change in the experimental group; wbothWithin group change in both the experimental and control groups; Bold significant improvement. C, control; Exp, experimental; NR, not reported; N/A, not applicable; VAS, visual analogue scale
Exercise
Exercise is a key management strategy for PwP and is often included as part of a non-pharmacological treatment strategy for pain [4, 5] even though the effect of exercise on chronic pain in PwP is uncertain. A survey of 125 PwP in the United Kingdom exploring pain management found exercise was the most frequently recommended strategy advised by healthcare professionals [48]. This exercise included walking, Pilates, and exercise classes. While the survey was not designed to explore the effectiveness of exercise interventions on managing pain, this result does suggest that healthcare professionals have an expectation that exercise may be beneficial for pain management. This is even though there is little research exploring the effect of exercise on pain in PwP, with the current research too broad and inconclusive to guide exercise prescription specifically for pain management. However, there is evidence from human and animal laboratory studies that exercise could lead to favorable changes related to the pathophysiology of pain in PD through mechanisms involved in neuroplasticity, neurorestoration, and neurogenesis which may improve the processing and modulation of pain signals [49]. Furthermore, exercise, particularly when provided as part of multidisciplinary care with a biopsychosocial focus, is effective in aiding pain management in other pain populations, such as chronic non-specific low back pain [50]. Therefore, exercise is a non-pharmacological pain management strategy that warrants attention with PwP in the current clinical setting, but also warrants further research to guide its clinicalapplication.
Exercise programs for pain
Six small studies (Table 1) with a specific aim to reduce pain in PwP have evaluated the effect of exercise interventions. Five of these were randomized controlled trials with moderate to high methodological quality (PEDro scores 5 to 8). Two evaluated exercise for PwP and low back pain, utilizing Pilates [39] and yoga [40]. Both studies found the interventions to be feasible. The single group Pilates study reported improvements in pain intensity, disability, and the sensory-affective impact. A randomized controlled trial of yoga compared to usual care [40] found back pain-related disability was reduced within the yoga group, though there were no between group differences. While favorable, the small sample sizes and within group improvements mean these results should be interpreted cautiously. Two randomized trials [41, 42] compared aquatic exercise with land-based exercise and used the Visual Analogue Scale (VAS) for pain intensity. Post intervention VAS score was improved in the aquatic group compared to the land-based group in both studies, suggesting aquatic exercise might be better than land-based for pain management.
Pain intensity was a secondary outcome for two randomized trials comparing the ‘Mézières’ rehabilitation method (postural exercises) [43] and trunk-specific exercises [51] with more general exercise. Each of the trials found no significant difference in pain between groups. However, the trial of the Mézières postural exercises found reductions in pain within the Mézières group, but not within the general exercise group (posture, range of motion and functional activities), despite both groups showing improvements in trunk flexibility [43]. In contrast, the trial of trunk specific exercise versus general exercise (stretching, strengthening and balance/gait exercises) [51] found within group reductions in pain in both groups, despite greater improvements in standing posture (trunk forward flexion) in the trunk exercise group. Further work is therefore required to better understand the relationship between posture, postural exercises, and pain in PwP.
Limitations in current evidence about exercise and pain
Interventional studies of exercise for PwP often do not require participants to have pain for inclusion [40–43, 51], and pain is not the primary outcome [40, 41, 43, 51]. Furthermore, pain is often measured in the broader context of quality of life (i.e., using the PDQ_39 bodily discomfort subscale [52]), making any effect on pain difficult to interpret. Consequently, there is limited evidence on the impact of exercise on pain management [23, 53]. Overall, both aerobic and isometric exercises have shown an immediate analgesic effect in individuals with PD and pain, although it’s noteworthy that some individuals do not experience this reduction in pain sensitivity [54]. The relationship between pain and physical activity in PwP is also unclear, with a cross-sectional study reporting those that were more active reported greater pain severity [14]. Studies suggest that exercise can be beneficial for those who feel capable of physical activity [55] but challenging for others due to intense pain [14]. It is also emphasized that pain can be a barrier to exercise [56, 57]. In some cases, poorly prescribed and monitored exercise might exacerbate pain, while in other cases, individuals with pain might engage in exercise to manage their pain.
Considering the complexity and variability of both PD symptoms and the pain experience, relying solely on exercise as a method for pain management in PwP is unlikely to be optimal. Effective exercise programs are likely to need careful individualization and combination with other interventions addressing biopsychosocial factors influencing pain. Further research is needed to better understand the relationship between pain and exercise in PwP and how exercise programs can be best tailored and combined with other interventions. These studies should take into account the impact of pain on daily life, as well as biological, psychological, and social factors related to pain [58, 59], and explore potential differentiated effects based on pain subtypes and possibly subtypes of PD where pain is present [23, 53].
Psychological strategies
People with low perceived control over their pain may find keeping active not acceptable or possible [60], therefore understanding the psychological components of pain is key when advocating exercise for both pain and PD impairments more broadly. For some PwP and pain, psychological strategies may therefore be required to help them to exercise as well as to improve their ability to manage theirpain.
Coping strategies and pain
Recently, the role of pain coping strategies in PwP has been explored. A cross sectional study of 52 PwP explored the prevalence of pain ‘active’ and ‘passive’ coping strategies and how these strategies were associated with pain ratings [11]. Active coping strategies such as taking control over and managing pain (e.g., taking medication, increasing activity levels) was associated with a lower overall pain severity. In contrast passive coping strategies such as avoiding activity and feeling hopeless was associated with a higher pain severity score. A similar result was found from interviews with PwP and pain [55]. While some PwP discussed the ability to manage the impact of pain and engage with strategies (i.e., active coping strategies), others felt helpless and to have no control over their pain (i.e., passive coping strategies).
Maladaptive cognitions in the form of catastrophizing have also been associated with worse pain in PwP [11, 12]. Additionally, a large cross-sectional study of 169 PwP found catastrophizing mediated the relationship between psychological distress (i.e., depression and anxiety) and pain and may predict a reduced response to pain management [12]. Interestingly, catastrophizing was viewed as a coping strategy within this study, albeit a maladaptive one. However, it must be noted that these cross-sectional studies do not demonstrate causation and future research is required to better understand the nature of the relationship between psychological factors and pain in PwP [11, 12].
Psychological interventions for pain
Within clinical practice, consideration of anxiety, depression, coping, and other strategies used by PwP and pain may provide a more holistic understanding of the experience and guide management. Cognitive behavioral therapy has been suggested as a potential treatment in the management of pain in PwP given the influence of psychological factors such as anxiety, depression and catastrophizing [12]. However, to our knowledge this has not yet been investigated in this group. An RCT evaluating self-management support for people living with pain and comorbidities used cognitive behavioral principles to support pain self-management and exercise. Results showed some promise with improvements in pain intensity and catastrophizing, but there were only twelve participants (out of 110) with a neurological condition, and it is not clear if PwP were included [61]. Notably, one small RCT did evaluate the effect of mindfulness training in PwP on the bodily discomfort subscore of the PDQ-39 [62]. Results indicated a small increase in bodily discomfort in the mindfulness group, suggesting that mindfulness may have increased awareness of discomfort without providing strategies for managing that discomfort.
Other non-pharmacological treatments
Both acupuncture and massage were highlighted in a survey of PwP as strategies for pain management [48]. However, current evidence in this area is limited. Acupuncture was trialed in two studies with moderate methodological quality (PEDro scores 4 and 5) aiming to reduce pain in PwP (Table 1). The first was a non-randomized trial [44] comparing acupuncture to no intervention in 16 people. An improvement in pain on the King’s Parkinson’s Pain Scale was found, but there were no improvements on the pain VAS or measures of depression, sleep, or quality of life. More recently, PwP and neck pain participated in a randomized controlled trial comparing acupuncture and neck exercises with neck exercises alone [45]. While both groups demonstrated a significant reduction in pain intensity as measured by the VAS, the acupuncture plus exercise group showed significantly more improvement than exercise alone. The results of both these trials should be interpreted with caution due to small sample sizes, a 27% drop-out rate in the second trial and a lack of longer term follow up.
Massage was compared to resting with music in an RCT of PwP and chronic pain [46]. Results did not show any differences between the groups, though there were within group improvements in pain and sleep in the massage group (Table 1). The trial was of poor methodological quality with a PEDro score of 3. A systematic review of massage for PwP [63] identified several other studies evaluating pain following massage, though none with a stated aim to reduce pain. While reduction in pain intensity was found in several, the authors highlight the methodological limitations of research in this area and the need for caution with generalization of results.
Non-pharmacological strategies to improve sleep are another potential pain management strategy for PwP. Strategies that improve sleep can help with pain management in the general population [64]; however, to our knowledge there are no trials that have evaluated the effects of interventions for sleep on pain in PwP. Strategies that could be considered include cognitive behavioral therapy and sleep hygiene [64–66]. Overall, acupuncture, massage and sleep therapies require methodologically robust investigation with consideration of the biopsychosocial nature of pain when designing the intervention and outcomes. The value of qualitative research must also be noted to further explore strategies used by PwP and to understand their impact and value.
Social considerations
Social factors are known to influence the pain experience in the general population. For example, people with chronic pain who have supportive spouses and social networks are better able to perform daily activities and manage their pain [67, 68]. Furthermore, among people with chronic pain and disability, social support was associated with less pain and improved functioning while inappropriate solicitous responses from significant others (e.g., general encouragement to avoid tasks or be less active) were associated with more pain and poorer function [69]. PwP have reported that chronic pain places stress on their relationships and leads to social isolation [55]. However, little is known about how to effectively address social contributors to chronic pain and research on this topic in PwP has not been reported. A 2021 review undertaken by the National Institute for Health and Care Excellence (NICE) did not find any evidence about the effectiveness of social interventions for improving quality of life, pain or associated outcomes in people with chronic pain [70]. Nonetheless, given the known associations between social factors and pain, involvement of significant others can be considered when implementing a holistic pain management program for PwP.
Pain, falls, and fear of falling
An emerging area of research, which has implications for rehabilitation for PwP and pain is the potential influence of pain on falls. Overall, PwP have an increased risk of falling and have twice as many falls than the general older population [71]. In relation to pain, older adults living with chronic pain have an increased prevalence of falls compared to older adults without pain [72, 73]. However, studies of falls in older adults often exclude, or include minimal numbers of PwP, e.g., [74]. Nonetheless, psychological influences are beginning to be considered in the context of falls, pain, and PD. A pilot study found fear of falling to be significantly associated with pain and proposed that improving pain may impact fear of falling and therefore falls in PwP [75]. Furthermore, self-reported balance confidence has been found to deteriorate more quickly in PwP with pain than those without [76]. As fear of falling can potentially increase activity avoidance and begin subsequent physical deconditioning [77] the relationships between pain, fear of falls, physical abilities, and rehabilitation strategies (including psychological strategies and exercise) warrants consideration.
RECOMMENDATIONS FOR CLINICAL PRACTICE
There is a lack of PD-specific research to guide the management of pain in PwP in clinical practice. However, by combining the current PD research with that from the chronic pain literature, a biopsychosocial approach to pain management is proposed in Fig. 1. This approach begins with consideration of a broad battery of assessments. These can include the PD-specific tools for classifying and diagnosing pain (i.e., the PD Pain Classification System [20] and the King’s PD Pain Scale [19]), as well as tools for assessing pain severity and interference globally (e.g., Brief Pain Inventory [78]). However, additional tools that assess biological, psychological, and social factors should also be included (Table 2). This includes assessment of domains such as emotional dysregulation or pathology (e.g., anxiety/depression), maladaptive cognitions (e.g., pain catastrophizing or fear avoidance beliefs), and the socioenvironmental context (e.g., social/spousal support) [79]. Any contributing psychological and social factors that are identified can then be addressed alongside the more mechanistic pain contributors.
Fig. 1
Biopsychosocial approach to pain management for people with Parkinson’s disease.
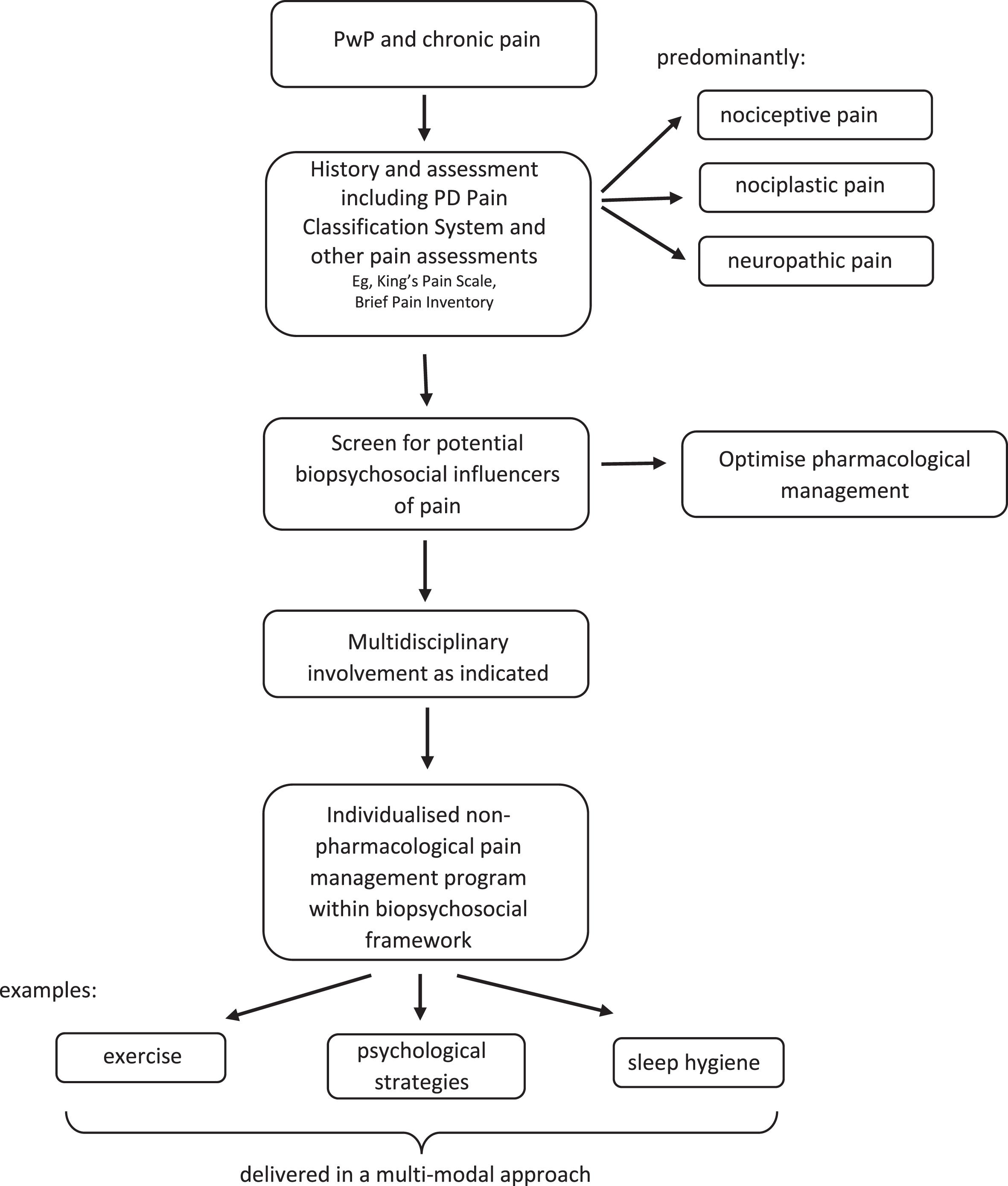
Table 2
Biopsychosocial factors influencing pain, their descriptions and examples of assessment.
| Biopsychosocial factors influencing pain | Description | Examples of assessment |
| Nociceptive input | Pain from nociception due to noxious mechanical, thermal or chemical stimuli as a result of injury or stress to tissues | History and assessment consistent with injury or tissue stress, with pain localized and proportionate to the mechanism. |
| Peripheral neuropathy | Pain from a lesion or disease affecting the peripheral nervous system | Self-report version of the Leeds Assessment of Neuropathic Signs and Symptoms [97] |
| Central nociplastic change | Pain due to central facilitation of nociceptive inputs producing hypersensitivity, pain with stimuli that would not normally be painful, and pain responses beyond an injured area. | Central Sensitization Inventory [98] |
| Sleep impairment | Pain can both contribute to and be exacerbated by poor sleep | Pittsburgh Sleep Quality Index [99] |
| Emotional dysregulation or pathology | Diagnosable psychological disorders or emotional distress such as anxiety and depression. | Patient Health Questionnaire – 9 item version [99]Parkinson’s Anxiety Scale [100]Hospital Anxiety and Depression Scale [101] |
| Maladaptive cognitions | Irrational or incorrect thoughts and beliefs about, or due to pain. | Pain Catastrophizing Scale [102]Fear Avoidance Beliefs Questionnaire [103]Tampa Scale for Kinesiophobia [104] |
| Sensorimotor disintegration | Mismatch between sensory inputs such that there is discordance between actual and perceived self | Difficulty locating painful areaJoint position sense errorTwo-point discrimination |
| Socioenvironmental context | Wide ranging contextual factors including emotional support, cultural beliefs, environmental demands and more. | Assessment of the social and environmental context, social support structures, work/employment arrangements, financial status, residential locationSpouse Response Inventory [105]Injustice Experience Questionnaire [106] |
Information based on Walton and Elliott, 2018 [79].
Current best practice for the management of chronic pain in the general population includes a multi-modal approach considering management within a biopsychosocial framework, optimal pharmacological management as required, physical activity/exercise and psychological strategies [3]. Individuals often have complex contributing factors to their pain experience, therefore a personalized approach involving the multidisciplinary team is important [3, 80]. The limited work exploring the pain experience has shown PwP have uncertainty about how to engage in exercise in the presence of pain [48, 55] as well as feelings of a lack of control over pain [55] and maladaptive coping strategies [12]. This reinforces the need for multi-modal interventions targeting the findings of holistic assessments, with the combination of exercise and psychological strategies potentially beneficial for many PwP and pain.
Exercise is an important part of overall management for all PwP. Exercise is known to improve mobility, muscle strength, balance, quality of life and reduce falls [81, 82]. Exercise guidelines for PwP recommend a combination of aerobic and progressive resistance training along with balance, agility, and multitasking [83, 84]. Expert input from a physical therapist can also incorporate task-specific training, including cueing and movement strategy training [85]. Therefore, all PwP, including those with chronic pain, should be prescribed individualized exercise programs within these guidelines, that address their personal impairments, activity limitations, goals, and preferences. However, the presence of chronic pain should be carefully monitored and exercise prescription and progression adjusted accordingly.
Figure 2 provides a framework for considering exercise prescription for PwP in the presence of chronic pain, informed by chronic pain best practice guidance [3, 86, 87] and exercise guidance for PwP [85]. The overlap between general exercise guidance for PwP and best practice for chronic pain is shown, with aerobic, progressive resistance, balance, and flexibility exercise recommended for both. However, this figure needs to be viewed in the knowledge that there is a paucity of information about the effect of aerobic and progressive resistance exercise on pain specifically in PwP. There is a small amount of evidence (summarized earlier in this review, Table 1) suggesting Pilates [39], yoga [40], and postural/trunk exercises [43, 51] are safe and feasible forms of balance and flexibility exercise for PwP that might reduce pain. Aquatic exercise is also an option that might help with pain management [41, 42] and can be a component of all exercise modes.
Fig. 2
Exercise recommendations for people with Parkinson’s disease and pain, *Chronic pain guidance; # Neuropathic pain guidance.
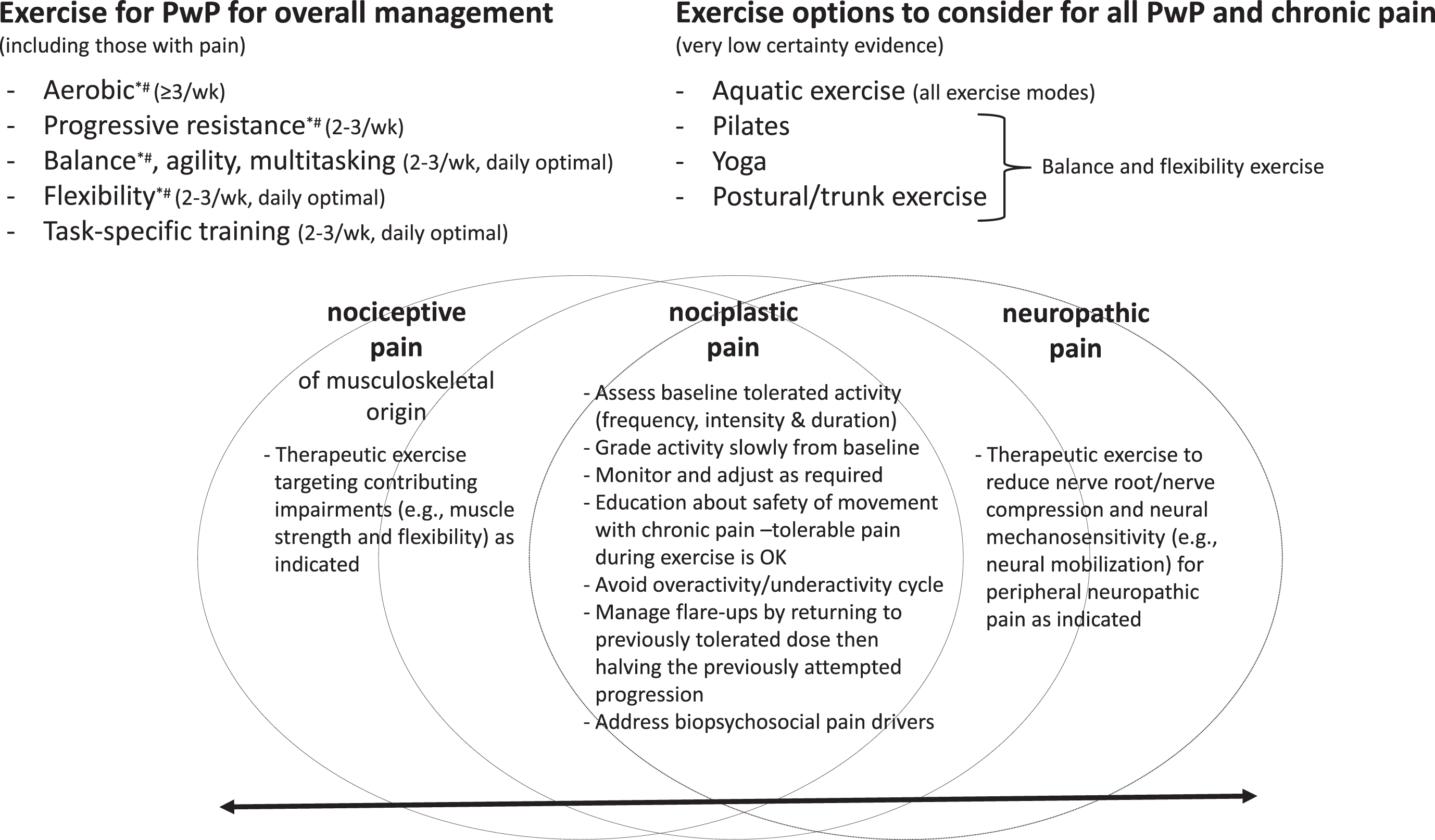
Alongside this general exercise guidance is the need to consider the type(s) of pain an individual is presenting with. As depicted in Fig. 2, nociceptive, nociplastic, and neuropathic pain occur along a continuum with individuals often presenting with more than one type [3]. Evidence from the chronic pain literature [2, 88–90] indicates that common to all pain types is the need to take an individualized and graded approach to exercise, progressing slowly as pain allows from baseline tolerated activity levels, with monitoring and adjustments (progression and regression) as required. Generally speaking, people with chronic pain should begin with aerobic exercise at least twice per week and resistance training two to three non-consecutive days per week at an intensity and duration tolerable for them [2]. An overactivity/underactivity cycle where too much exercise leads to flare-ups and is followed by no or very little exercise is best avoided. However, even with careful grading of exercise, progressions can lead to flare-ups. The impact of flare-ups can be minimized by having a flare-up plan, such as returning to the dose of exercise that was tolerated prior to the flare-up, then progressing by 50% of the previously attempted progression [2]. It is recommended that exercise is delivered within a biopsychosocial framework and includes education about the pathophysiology of chronic pain to provide reassurance that exercise with tolerable pain is safe [2, 88]. Elements of nociceptive pain of musculoskeletal origin and neuropathic pain can be addressed through specific therapeutic exercises targeting contributing impairments [91, 92].
FUTURE DIRECTIONS
While there has been much research on the non-pharmacological management of chronic pain in the general population, there is a paucity of studies specifically in PwP. There are promising interventions being trialed for chronic pain that warrant exploration of their effects on pain in PwP. These include virtual reality to provide distraction or delivery of psychological interventions [93], and the role of nutrition in optimizing weight and improving inflammatory joint pain and overall health [94]. The degeneration of neurological pathways involved in the processing and modulation of pain, the impairments of PD [4] and the psychological and social factors that come with adjusting to a long-term, neurodegenerative disorder, means that PD-specific research into the management of chronic pain is warranted. In order to provide robust and meaningful results, this research should be designed to explore the different types of pain (i.e., predominantly nociplastic, nociceptive or neuropathic) and include only PwP who have chronic pain of the targeted pain type(s). Furthermore, research that genuinely and holistically addresses each person’s biopsychosocial contributors to pain is required [10]. Such research would align with the emerging precision medicine approach, where alongside the clinical presentation, genes, environment, and lifestyle are considered in order to personalize management [95]. In this way, innovations in research could help determine which package of interventions is likely to be optimal for each PwP with chronic pain [96].
CONCLUSION
PD and pain are both complex and require individualized management. By viewing pain through a biopsychosocial framework, a holistic and multi-modal assessment and management of pain can be provided to individual PwP. Exercise is an important part of PD management; therefore, health professionals prescribing exercise should screen and monitor people for pain, making appropriate modifications as required. For many PwP and pain, exercise could be considered alongside management for other contributing factors, such as maladaptive cognitions. Research is required to develop and evaluate multidisciplinary pain management where strategies are matched to the type of pain as well as the psychological and social factors contributing to pain.
ACKNOWLEDGMENTS
The authors would like to acknowledge the people with Parkinson’s disease and researchers who are contributing to the ongoing research into pain management.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report. Dr Jenni Naisby is the recipient of a Mid-career Pain Research Fellowship from the Medical Research Foundation.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
REFERENCES
[1] | Nègre-Pagès L , Regragui W , Bouhassira D , Grandjean H , Rascol O ((2008) ) Chronic pain in Parkinson’s disease: The cross-sectional French DoPaMiP survey. Mov Disord 23: , 1361–1369. |
[2] | Booth J , Moseley GL , Schiltenwolf M , Cashin A , Davies M , Hübscher M ((2017) ) Exercise for chronic musculoskeletal pain: A biopsychosocial approach. Musculoskeletal Care 15: , 413–421. |
[3] | Cohen SP , Vase L , Hooten WM ((2021) ) Chronic pain: An update on burden, best practices, and new advances. Lancet 397: , 2082–2097. |
[4] | Viseux FJF , Delval A , Simoneau M , Defebvre L ((2023) ) Pain and Parkinson’s disease: Current mechanism and management updates. Eur J Pain 27: , 553–567. |
[5] | Qureshi AR , Jamal M , Rahman E , Paul D , Shamli Oghli Y , Mulaffer M , Qureshi D , Danish A , Rana AQ ((2021) ) Non-pharmacological therapies for pain management in Parkinson’s disease: A systematic review. Acta Neurol Scand 144: . |
[6] | Raja SN , Carr DB , Cohen M , Finnerup NB , Flor H , Gibson S , Keefe FJ , Mogil JS , Ringkamp M , Sluka KA , Song X-J , Stevens B , Sullivan MD , Tutelman PR , Ushida T , Vader K ((2020) ) The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 161: , 1976–1982. |
[7] | Engel GL ((1977) ) The need for a new medical model: A challenge for biomedicine. Science 196: , 129–136. |
[8] | Waddell G , Bircher M , Finlayson D , Main CJ ((1984) ) Symptoms and signs: Physical disease or illness behaviour? Br Med J (Clin Res Ed) 289: , 739–741. |
[9] | Nicholas MK ((2022) ) The biopsychosocial model of pain 40 years on: Time for a reappraisal? Pain 163: , S3–S14. |
[10] | Cormack B , Stilwell P , Coninx S , Gibson J ((2023) ) The biopsychosocial model is lost in translation: From misrepresentation to an enactive modernization. Physiother Theory Pract 39: , 2273–2288. |
[11] | Prell T , Liebermann JD , Mendorf S , Lehmann T , Zipprich HM ((2021) ) Pain coping strategies and their association with quality of life in people with Parkinson’s disease: A cross-sectional study. PLoS One 16: , e0257966. |
[12] | Zimmers S , Robieux L , Bungener C ((2023) ) Towards a better comprehension and management of pain and psychological distress in Parkinson’s: The role of catastrophizing. J Geriatr Psychiatry Neurol 36: , 351–365. |
[13] | Silverdale MA , Kobylecki C , Kass-Iliyya L , Martinez-Martin P , Lawton M , Cotterill S , Chaudhuri KR , Morris H , Baig F , Williams N , Hubbard L , Hu MT , Grosset DG ((2018) ) A detailed clinical study of pain in 1957 participants with early/moderate Parkinson’s disease. Park Relat Disord 56: , 27–32. |
[14] | Nguy V , Barry BK , Moloney N , Hassett LM , Canning CG , Lewis SJG , Allen NE ((2020) ) The associations between physical activity, sleep, and mood with pain in people with Parkinson’s disease: An observational cross-sectional study. J Parkinsons Dis 10: , 1161–1170. |
[15] | Rukavina K , Ocloo J , Skoric MK , Sauerbier A , Thomas O , Staunton J , Awogbemila O , Trivedi D , Rizos A , Chaudhuri KR ((2022) ) Ethnic disparities in treatment of chronic pain in individuals with Parkinson’s disease living in the United Kingdom. Mov Disord Clin Pract 9: , 369–374. |
[16] | Martinez-Martin P , Rodriguez-Blazquez C , Kurtis MM , Chaudhuri KR ((2011) ) The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 26: , 399–406. |
[17] | Martínez-Martin P , Rodriguez-Blazquez C , Paz S , Forjaz MJ , Frades-Payo B , Cubo E , De Pedro-Cuesta J , Lizán L ((2015) ) Parkinson symptoms and health related quality of life as predictors of costs: A longitudinal observational study with linear mixed model analysis. PLoS One 10: , e0145310. |
[18] | Ford B ((1998) ) Pain in Parkinson’s disease. Clin Neurosci 5: , 63–72. |
[19] | Chaudhuri KR , Rizos A , Trenkwalder C , Rascol O , Pal S , Martino D , Carroll C , Paviour D , Falup-Pecurariu C , Kessel B , Silverdale M , Todorova A , Sauerbier A , Odin P , Antonini A , Martinez-Martin P ((2015) ) King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation. Mov Disord 30: , 1623–1631. |
[20] | Mylius V , Perez Lloret S , Cury RG , Teixeira MJ , Barbosa VR , Barbosa ER , Moreira LI , Listik C , Fernandes AM , de Lacerda Veiga D , Barbour J , Hollenstein N , Oechsner M , Walch J , Brugger F , Hägele-Link S , Beer S , Rizos A , Chaudhuri KR , Bouhassira D , Lefaucheur J-P , Timmermann L , Gonzenbach R , Kägi G , Möller JC , Ciampi de Andrade D ((2021) ) The Parkinson disease pain classification system: Results from an international mechanism-based classification approach. Pain 162: , 1201–1210. |
[21] | Lee MA , Walker RW , Hildreth TJ , Prentice WM ((2006) ) A survey of pain in idiopathic Parkinson’s disease. J Pain Symptom Manage 32: , 462–469. |
[22] | Mylius V , Möller JC , Bohlhalter S , Ciampi de Andrade D , Perez Lloret S ((2021) ) Diagnosis and management of pain in Parkinson’s disease: A new approach. Drugs Aging 38: , 559–577. |
[23] | Allen NE , Wong CM , Canning CG , Moloney N ((2016) ) The association between Parkinson’s disease motor impairments and pain. Pain Med 17: , 456–462. |
[24] | Alwardat M , Schirinzi T , Di Lazzaro G , Franco D , Sinibaldi Salimei P , Mercuri NB , Pisani A ((2019) ) The effect of postural deformities on back function and pain in patients with Parkinson’s disease. NeuroRehabilitation 44: , 419–424. |
[25] | Al-Wardat M , Geroin C , Schirinzi T , Etoom M , Tinazzi M , Pisani A , Natoli S ((2023) ) Axial postural abnormalities and pain in Parkinson’s disease. J Neural Transm 130: , 77–85. |
[26] | Li J , Zhu BF , Gu ZQ , Zhang H , Mei SS , Ji SZ , Liu SY , Han C , Chen HZ , Chan P ((2022) ) Musculoskeletal pain in Parkinson’s disease. Front Neurol 12: , 756538. |
[27] | Abuhasira R , Zlotnik Y , Horev A , Ifergane G ((2019) ) Fibromyalgia-like syndrome associated with Parkinson’s disease—a cohort study. J Clin Med 8: , 1118. |
[28] | Rukavina K , Leta V , Sportelli C , Buhidma Y , Duty S , Malcangio M , Ray Chaudhuri K ((2019) ) Pain in Parkinson’s disease: New concepts in pathogenesis and treatment. Curr Opin Neurol 32: , 579–588. |
[29] | Cortes-Altamirano JL , Reyes-Long S , Bandala C , Morraz-Varela A , Bonilla-Jaime H , Alfaro-Rodriguez A ((2022) ) Neuropathic pain in Parkinson’s disease. Neurol India 70: , 1879–1886. |
[30] | Kass-Iliyya L , Javed S , Gosal D , Kobylecki C , Marshall A , Petropoulos IN , Ponirakis G , Tavakoli M , Ferdousi M , Chaudhuri KR , Jeziorska M , Malik RA , Silverdale MA ((2015) ) Small fiber neuropathy in Parkinson’s disease: A clinical, pathological and corneal confocal microscopy study. Parkinsonism Relat Disord 21: , 1454–1460. |
[31] | Broetz D , Eichner M , Gasser T , Weller M , Steinbach JP ((2007) ) Radicular and nonradicular back pain in Parkinson’s disease: A controlled study. Mov Disord 22: , 853–856. |
[32] | Kosek E , Cohen M , Baron R , Gebhart GF , Mico J-A , Rice ASC , Rief W , Sluka AK ((2016) ) Do we need a third mechanistic descriptor for chronic pain states? Pain 157: , 1382–1386. |
[33] | Tracey I , Mantyh PW ((2007) ) The cerebral signature for pain perception and its modulation. Neuron 55: , 377–391. |
[34] | Tan Y , Tan J , Deng J , Cui W , He H , Yang F , Deng H , Xiao R , Huang Z , Zhang X , Tan R , Shen X , Liu T , Wang X , Yao D , Luo C ((2015) ) Alteration of basal ganglia and right frontoparietal network in early drug-naïve Parkinson’s disease during heat pain stimuli and resting state. Front Hum Neurosci 9: , 467. |
[35] | Wang J , Sun J , Gao L , Zhang D , Chen L , Wu T ((2023) ) Common and unique dysconnectivity profiles of dorsal and median raphe in Parkinson’s disease. Hum Brain Mapp 44: , 1070–1078. |
[36] | Cao Y , Li G , Xue J , Zhang G , Gao S , Huang Y , Zhu A ((2021) ) Depression and related factors in patients with Parkinson’s disease at high altitude. Neuropsychiatr Dis Treat 17: , 1353–1362. |
[37] | Gao L , Huang W , Cai L , Li H ((2022) ) Association between sleep disturbances and pain subtypes in Parkinson’s disease. Neurol Sci 43: , 4785–4790. |
[38] | Zhou Z , Chen Q , Liu Q , Xu P , Lu J , Zhuo M ((2022) ) Cortical synaptic mechanism for chronic pain and anxiety in Parkinson’s disease. J Transl Int Med 10: , 300–303. |
[39] | Feital AMB de F , Gonçalves BM , Souza TR , Christo PP , Scalzo PL ((2022) ) Pilates method for low back pain in individuals with Parkinson’s disease: A feasibility study. J Bodyw Mov Ther 32: , 19–28. |
[40] | Myers PS , Harrison EC , Rawson KS , Horin AP , Sutter EN , McNeely ME , Earhart GM ((2020) ) Yoga improves balance and low-back pain, but not anxiety, in people with Parkinson’s disease. Int J Yoga Therap 30: , 41–48. |
[41] | Pérez-De la Cruz S ((2019) ) Mental health in Parkinson’s disease after receiving aquatic therapy: A clinical trial. Acta Neurol Belg 119: , 193–200. |
[42] | Pérez De La Cruz S ((2017) ) Effectiveness of aquatic therapy for the control of pain and increased functionality in people with Parkinson’s disease: A randomized clinical trial. Eur J Phys Rehabil Med 53: , 825–832. |
[43] | Paolucci T , Zangrando F , Piccinini G , Deidda L , Basile R , Bruno E , Buzi E , Mannocci A , Tirinelli F , Haggiag S , Lispi L , Villani C , Saraceni VM ((2017) ) Impact of Mézières rehabilitative method in patients with Parkinson’s disease: A randomized controlled trial. Parkinsons Dis 2017: , 2762987. |
[44] | Yu S-W , Lin S-H , Tsai C-C , Chaudhuri KR , Huang Y-C , Chen Y-S , Yeh B-Y , Wu Y-R , Wang J-J ((2019) ) Acupuncture effect and mechanism for treating pain in patients with Parkinson’s disease. Front Neurol 10: , 1114. |
[45] | Yaksi E , Yasar MF , Dogan N , Balci M ((2022) ) Is acupuncture effective against pain in patients with Parkinson s disease A randomized controlled study. Exp Biomed Res 5: , 204–216. |
[46] | Skogar Ö , Borg A , Larsson B , Robertsson L , Andersson L , Andersson L , Backstrom P , Fall P-A , Hallgren G , Bringer B , Carlsson M , Lennartsson U , Sandbjork H , Lökk J , Törnhage C-J ((2013) ) “Effects of Tactile Touch on pain, sleep and health related quality of life in Parkinson’s disease with chronic pain”: A randomized, controlled and prospective study. Eur J Integr Med 5: , 141–152. |
[47] | Maher CG , Sherrington C , Herbert RD , Moseley AM , Elkins M ((2003) ) Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 83: , 713–721. |
[48] | Naisby J , Amjad A , Ratcliffe N , Yarnall AJ , Rochester L , Walker R , Baker K ((2022) ) A survey of people with Parkinson’s and their carers: The management of pain in Parkinson’s. J Geriatr Psychiatry Neurol 35: , 613–621. |
[49] | Allen NE , Moloney N , Van Vliet V , Canning CG ((2015) ) The rationale for exercise in the management of pain in Parkinson’s disease. J Parkinsons Dis 5: , 229–239. |
[50] | Urits I , Burshtein A , Sharma M , Testa L , Gold PA , Orhurhu V , Viswanath O , Jones MR , Sidransky MA , Spektor B , Kaye AD ((2019) ) Low back pain, a comprehensive review: Pathophysiology, diagnosis, and treatment. Curr Pain Headache Rep 23: , 23. |
[51] | Gandolfi M , Tinazzi M , Magrinelli F , Busselli G , Dimitrova E , Polo N , Manganotti P , Fasano A , Smania N , Geroin C ((2019) ) Four-week trunk-specific exercise program decreases forward trunk flexion in Parkinson’s disease: A single-blinded, randomized controlled trial. Parkinsonism Relat Disord 64: , 268–274. |
[52] | Peto V , Jenkinson C , Fitzpatrick R , Greenhall R ((1995) ) The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual life Res 4: , 241–248. |
[53] | Ray Chaudhuri K , Leta V , Bannister K , Brooks DJ , Svenningsson P ((2023) ) The noradrenergic subtype of Parkinson disease: From animal models to clinical practice. Nat Rev Neurol 19: , 333–345. |
[54] | Nguy V , Barry BK , Moloney N , Hassett LM , Canning CG , Lewis SJG , Allen NE ((2019) ) Exercise-induced hypoalgesia is present in people with Parkinson’s disease: Two observational cross-sectional studies. Eur J Pain 23: , 1329–1339. |
[55] | Twomey D , Stuart S , Baker K ((2018) ) Pain in Parkinson’s disease: The lived experience. Int J Ther Rehabil 25: , 301–308. |
[56] | Wang J , Sun L , Chen L , Sun J , Xie Y , Tian D , Gao L , Zhang D , Xia M , Wu T ((2023) ) Common and distinct roles of amygdala subregional functional connectivity in non-motor symptoms of Parkinson’s disease. NPJ Parkinsons Dis 9: , 28. |
[57] | Allen NE , Song J , Paul SS , Sherrington C , Murray SM , O’Rourke SD , Lord SR , Fung VSC , Close JCT , Howard K , Canning CG ((2015) ) Predictors of adherence to a falls prevention exercise program for people with Parkinson’s disease. Mov Disord Clin Pract 2: , 395–401. |
[58] | Perez-Lloret S , Ciampi de Andrade D , Lyons KE , Rodríguez-Blázquez C , Chaudhuri KR , Deuschl G , Cruccu G , Sampaio C , Goetz CG , Schrag A , Martinez-Martin P , Stebbins G , Cubo E , Hall D , Luo S , Marinus J , Marsh L , Skorvanek M ((2016) ) Rating scales for pain in Parkinson’s disease: Critique and recommendations. Mov Disord Clin Pract 3: , 527–537. |
[59] | Walton DM , Elliott JM ((2018) ) A new clinical model for facilitating the development of pattern recognition skills in clinical pain assessment. Musculoskelet Sci Pract 36: , 17–24. |
[60] | Foster NE , Thomas E , Bishop A , Dunn KM , Main CJ ((2010) ) Distinctiveness of psychological obstacles to recovery in low back pain patients in primary care. Pain 148: , 398–406. |
[61] | Miller J , MacDermid JC , Walton DM , Richardson J ((2020) ) Chronic pain self-management support with pain science education and exercise (COMMENCE) for people with chronic pain and multiple comorbidities: A randomized controlled trial. Arch Phys Med Rehabil 101: , 750–761. |
[62] | Pickut B , Vanneste S , Hirsch MA , Van Hecke W , Kerckhofs E , Mariën P , Parizel PM , Crosiers D , Cras P ((2015) ) Mindfulness training among individuals with Parkinson’s disease: Neurobehavioral effects. Parkinsons Dis 2015: , 816404. |
[63] | Angelopoulou E , Anagnostouli M , Chrousos GP , Bougea A ((2020) ) Massage therapy as a complementary treatment for Parkinson’s disease: A systematic literature review. Complement Ther Med 49: , 102340. |
[64] | Whale K , Dennis J , Wylde V , Beswick A , Gooberman-Hill R ((2022) ) The effectiveness of non-pharmacological sleep interventions for people with chronic pain: A systematic review and meta-analysis. BMC Musculoskelet Disord 23: , 440. |
[65] | Gupta CC , Sprajcer M , Johnston-Devin C , Ferguson SA ((2023) ) Sleep hygiene strategies for individuals with chronic pain: A scoping review. BMJ Open 13: , e060401. |
[66] | Taximaimaiti R , Luo X , Wang X-P ((2021) ) Pharmacological and Non-pharmacological Treatments of Sleep Disorders in Parkinson’s Disease. Curr Neuropharmacol 19: , 2233–2249. |
[67] | Martire LM , Keefe FJ , Schulz R , Ready R , Beach SR , Rudy TE , Starz TW ((2006) ) Older spouses’ perceptions of partners’ chronic arthritis pain: Implications for spousal responses, support provision, and caregiving experiences. Psychol Aging 21: , 222–230. |
[68] | Peat G , Thomas E , Handy J , Croft P ((2004) ) Social networks and pain interference with daily activities in middle and old age. Pain 112: , 397–405. |
[69] | Jensen MP , Moore MR , Bockow TB , Ehde DM , Engel JM ((2011) ) Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: A systematic review. Arch Phys Med Rehabil 92: , 146–160. |
[70] | National Guideline Centre (UK). Evidence review for social interventions for chronic pain (chronic primary pain and chronic secondary pain): Chronic pain (primary and secondary) in over 16s: Assessment of all chronic pain and management of chronic primary pain: Evidence review D. London: National Institute for Health and Care Excellence (NICE); 2021 Apr. (NICE Guideline, No. 193.), https://www.ncbi.nlm.nih.gov/books/NBK569981/ |
[71] | Canning CG , Paul SS , Nieuwboer A ((2014) ) Prevention of falls in Parkinson’s disease: A review of fall risk factors and the role of physical interventions. Neurodegener Dis Manag 4: , 203–221. |
[72] | Patel K V , Phelan EA , Leveille SG , Lamb SE , Missikpode C , Wallace RB , Guralnik JM , Turk DC ((2014) ) High prevalence of falls, fear of falling, and impaired balance in older adults with pain in the United States: Findings from the 2011 National Health and Aging Trends Study. J Am Geriatr Soc 62: , 1844–1852. |
[73] | Stubbs B , Binnekade T , Eggermont L , Sepehry AA , Patchay S , Schofield P ((2014) ) Pain and the risk for falls in community-dwelling older adults: Systematic review and meta-analysis. Arch Phys Med Rehabil 95: , 175–187.e9. |
[74] | Leveille SG , Jones RN , Kiely DK , Hausdorff JM , Shmerling RH , Guralnik JM , Kiel DP , Lipsitz LA , Bean JF ((2009) ) Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA 302: , 2214–2221. |
[75] | Khalil H , Alissa N , Al-Sharman A , E’leimat I , Qawasmeh M Al , El-Salem K ((2021) ) Understanding the influence of pain and fatigue on physical performance, fear of falling and falls in people with Parkinson’s disease: A pilot study. Neurodegener Dis Manag 11: , 113–124. |
[76] | Naisby J , Lawson RA , Galna B , Alcock L , Burn DJ , Rochester L , Yarnall AJ ((2021) ) Trajectories of pain over 6 years in early Parkinson’s disease: ICICLE-PD. J Neurol 268: , 4759–4767. |
[77] | Landers M , Nilsson M ((2023) ) A theoretical framework for addressing fear of falling avoidance behavior in Parkinson’s disease. Physiother Theory Pract 39: , 895–911. |
[78] | Cleeland CS , Ryan KM ((1994) ) Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 23: , 129–138. |
[79] | Walton DM , Elliott JM ((2018) ) A new clinical model for facilitating the development of pattern recognition skills in clinical pain assessment. Musculoskelet Sci Pract 36: , 17–24. |
[80] | Valentijn PP , Schepman SM , Opheij W , Bruijnzeels MA ((2013) ) Understanding integrated care: A comprehensive conceptual framework based on the integrative functions of primary care. Int J Integr Care 13: , e010. |
[81] | Pang MY ((2021) ) Physiotherapy management of Parkinson’s disease. J Physiother 67: , 163–176. |
[82] | Allen NE , Canning CG , Almeida LRS , Bloem BR , Keus SH , Löfgren N , Nieuwboer A , Verheyden GS , Yamato TP , Sherrington C ((2022) ) Interventions for preventing falls in Parkinson’s disease. Cochrane Database Syst Rev 6: , CD011574. |
[83] | Parkinson’s UK Parkinson’s Exercise Framework, https://www.parkinsons.org.uk/information-and-support/parkinsons-exercise-framework, Last updated October 2017, Accessed on July 24, 2023. |
[84] | Parkinson’s Foundation, Parkinson’s Today Blog, https://www.parkinson.org/blog/awareness/exercise-recommendations, Last updated May 25, 2021, Accessed on July 24, 2023. |
[85] | Osborne JA , Botkin R , Colon-Semenza C , DeAngelis TR , Gallardo OG , Kosakowski H , Martello J , Pradhan S , Rafferty M , Readinger JL , Whitt AL , Ellis TD ((2022) ) Physical therapist management of Parkinson disease: A clinical practice guideline from the American Physical Therapy Association. Phys Ther 102: , pzab302. |
[86] | Leitzelar BN , Koltyn KF ((2021) ) Exercise and neuropathic pain: A general overview of preclinical and clinical research. Sports Med Open 7: , 21. |
[87] | Zhang Y-H , Hu H-Y , Xiong Y-C , Peng C , Hu L , Kong Y-Z , Wang Y-L , Guo J-B , Bi S , Li T-S , Ao L-J , Wang C-H , Bai Y-L , Fang L , Ma C , Liao L-R , Liu H , Zhu Y , Zhang Z-J , Liu C-L , Fang G-E , Wang X-Q ((2021) ) Exercise for neuropathic pain: A systematic review and exert consensus. Front Med 8: , 756940. |
[88] | Cashin AG , Booth J , McAuley JH , Jones MD , Hübscher M , Traeger AC , Fried K , Moseley GL ((2022) ) Making exercise count: Considerations for the role of exercise in back pain treatment. Musculoskeletal Care 20: , 259–270. |
[89] | Caneiro JP , Smith A , Bunzli S , Linton S , Moseley GL , O’Sullivan P ((2022) ) From fear to safety: A roadmap to recovery from musculoskeletal pain. Phys Ther 102: , pzab271. |
[90] | Ferro Moura Franco K , Lenoir D , dos Santos Franco YR , Jandre Reis FJ , Nunes Cabral CM , Meeus M ((2021) ) Prescription of exercises for the treatment of chronic pain along the continuum of nociplastic pain: A systematic review with meta-analysis. Eur J Pain 25: , 51–70. |
[91] | Cuenca-Martínez F , La Touche R , Varangot-Reille C , Sardinoux M , Bahier J , Suso-Martí L , Fernández-Carnero J ((2022) ) Effects of neural mobilization on pain intensity, disability, and mechanosensitivity: An umbrella review with meta-meta-analysis. Phys Ther 102: , pzac040. |
[92] | Raposo F , Ramos M , Lúcia Cruz A ((2021) ) Effects of exercise on knee osteoarthritis: A systematic review. Musculoskeletal Care 19: , 399–435. |
[93] | Goudman L , Jansen J , Billot M , Vets N , De Smedt A , Roulaud M , Rigoard P , Moens M ((2022) ) Virtual reality applications in chronic pain management: Systematic review and meta-analysis. JMIR Serious Games 10: , e34402. |
[94] | Brain K , Burrows TL , Rollo ME , Chai LK , Clarke ED , Hayes C , Hodson FJ , Collins CE ((2019) ) A systematic review and meta-analysis of nutrition interventions for chronic noncancer pain. J Hum Nutr Diet 32: , 198–225. |
[95] | Ryden LE , Lewis SJG ((2019) ) Parkinson’s disease in the era of personalised medicine: One size does not fit all. Drugs Aging 36: , 103–113. |
[96] | Mackey S , Greely HT , Martucci KT ((2019) ) Neuroimaging-based pain biomarkers: Definitions, clinical and research applications, and evaluation frameworks to achieve personalized pain medicine. Pain Rep 4: , e762. |
[97] | Bennett MI , Smith BH , Torrance N , Potter J ((2005) ) The S-LANSS score for identifying pain of predominantly neuropathic origin: Validation for use in clinical and postal research. J Pain 6: , 149–158. |
[98] | Neblett R , Cohen H , Choi Y , Hartzell MM , Williams M , Mayer TG , Gatchel RJ ((2013) ) The Central Sensitization Inventory (CSI): Establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain 14: , 438–445. |
[99] | Buysse DJ , Reynolds CF 3rd , Monk TH , Berman SR , Kupfer DJ ((1989) ) The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28: , 193–213. |
[100] | Leentjens AFG , Dujardin K , Pontone GM , Starkstein SE , Weintraub D , Martinez-Martin P ((2014) ) The Parkinson Anxiety Scale (PAS): Development and validation of a new anxiety scale. Mov Disord 29: , 1035–1043. |
[101] | Bjelland I , Dahl AA , Haug TT , Neckelmann D ((2002) ) The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52: , 69–77. |
[102] | Walton DM , Mehta S , Seo W , MacDermid JC ((2020) ) Creation and validation of the 4-item BriefPCS-chronic through methodological triangulation. Health Qual Life Outcomes 18: , 124. |
[103] | Waddell G , Newton M , Henderson I , Somerville D , Main CJ ((1993) ) A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 52: , 157–168. |
[104] | Hudes K ((2011) ) The Tampa Scale of Kinesiophobia and neck pain, disability and range of motion: A narrative review of the literature. J Can Chiropr Assoc 55: , 222–232. |
[105] | Schwartz L , Jensen MP , Romano JM ((2005) ) The development and psychometric evaluation of an instrument to assess spouse responses to pain and well behavior in patients with chronic pain: The Spouse Response Inventory. J Pain 6: , 243–252. |
[106] | Sullivan MJL , Adams H , Horan S , Maher D , Boland D , Gross R ((2008) ) The role of perceived injustice in the experience of chronic pain and disability: Scale development and validation. J Occup Rehabil 18: , 249–261. |



