Alleviating Stress in Parkinson’s Disease: Symptomatic Treatment, Disease Modification, or Both?
Abstract
Psychological stress, a state of mental strain caused by mentally or physically threatening situations, plays a significant role in Parkinson’s disease (PD). Motor symptoms worsen during acute stress and common non-motor symptoms in PD, such as anxiety and depression, are linked to chronic stress. Although evidence in humans is lacking, animal models of PD suggest that chronic stress can accelerate dopaminergic cell death. This suggests that stress-reducing interventions have not only symptomatic, but perhaps also disease-modifying effects. Our objective was to identify the most promising strategies for stress-reduction in PD and to analyze their potential value for disease-modification. An unstructured literature search was performed, primarily focusing on papers published between 2020–2023. Several large clinical trials have tested the efficacy of aerobic exercise and mindfulness-based interventions on PD symptoms. The evidence is promising, but not definitive yet: some exercise trials found a reduction in stress-related symptoms, whereas others did not or did not report it. In the majority of trials, biological measures of stress and of disease progression are missing. Furthermore, follow-up periods were generally too short to measure disease-modifying effects. Hence, mechanisms underlying the intervention effects remain largely unclear. These effects may consist of attenuating progressive neurodegeneration (measured with MRI-markers of substantia nigra integrity or cortical thickness), or a strengthening of compensatory cerebral mechanisms (measured with functional neuroimaging), or both. Lifestyle interventions are effective for alleviating stress-related symptoms in PD. They hold potential for exerting disease-modifying effects, but new evidence in humans is necessary to fulfill that promise.
INTRODUCTION
Stress plays a prominent role in many societies and its detrimental effects on mental and physical health are well-established [1, 2]. In people with Parkinson’s disease (PD), stress is thought to play a particularly important role. Not only does acute stress aggravate the symptomatic manifestations of the disease, such as tremor, dyskinesia or freezing of gait [3–6], recent evidence in animals also suggests that chronic stress may influence the degree of nigro-striatal cell loss [7, 8]. Both these symptomatic as well as (potentially) disease-modifying effects of stress highlight the importance of employing stress-alleviating strategies in PD. Here, we review the evidence on such strategies, focusing both on symptomatic effects and disease-modifying effects. We will discuss clinical, neuroimaging, and biochemical evidence, to shed light on the impact of stress and stress-alleviation on clinical symptoms and on the pathophysiology in PD.
WHAT IS STRESS?
Stress refers to a process where environmental demands (i.e., stressors) exceed the adaptive capacity of an individual, resulting in psychological and biological responses (i.e., stress-response) [9]. The environmental demands may entail either physical (e.g., pain, injury) or psychological (e.g., life changes, abuse) stressors, or a combination. The extent of the stress-response depends on the nature of the stressor (magnitude and duration), as well as an individual’s biological and psychological vulnerability [9–11]. In healthy individuals, the biological stress-response restores homeostatic balance by initiating fast, sympathetic changes, and by activating the slower hypothalamic-pituitary-adrenal (HPA) axis that results in cortisol release [12]. Negative-feedback loops exist to protect against prolonged activity of the stress system. Accordingly, dysfunctions of the biological stress-response can be expressed as a failure of these feedback processes, and hence dysregulation of the HPA axis [13]. Chronic exposure to stressors can lead to such dysregulation, although its psychological effects are known to vary substantially between individuals. While some people develop stress-related symptoms, such as depression or anxiety disorders, the mental health of others quickly recovers, despite severe or chronic stress exposure, which is defined as stress-resilience [14]. Different mental health outcomes depend on personal as well as contextual factors, such as cognitive appraisal of the stressor, and these factors in turn determine the coping styles that are employed [15].
In the following, we will consider symptoms of anxiety and depression as maladaptive responses to stressful circumstances. In turn, we understand these symptoms as measures of the psychological stress-response (psychological stress), which may provide indications about the biological stress-response as well, albeit indirect.
STRESS IN PEOPLE WITH PARKINSON’S DISEASE
People with PD are often exposed to stressors, such as pain, sleep disturbances, cognitive decline, or dealing with an unknown future, and this may lead to stress. While many of these stressors also exist in other chronic disorders, evidence suggests that the pathophysiology in PD may specifically influence the appraisal of stressors (influencing the vulnerability to stress), as well as the stress-response itself [16]. For example, the presence of Lewy bodies in the hypothalamus and the adrenal glands, which are both key regulators of the HPA axis [17], may interfere with the biological stress-response. Furthermore, people with PD show elevated glucocorticoid levels [18], indicating disrupted feedback loops of the HPA axis. Degeneration of serotonergic and noradrenergic pathways [19] may further constitute a dysfunctional stress-response in PD, which might lead to elevated, or even chronic levels of stress. In line with this, stress-related symptoms, such as anxiety and depression, are highly prevalent in people with PD, with an estimated prevalence of 35% for depression [20] and 26% for anxiety disorders [21].
The pathophysiology of PD has also been associated with altered appraisal of stressors. Appraisal processes are needed to adequately cope with stressful situations, and therefore constitute a defining factor for an individual’s vulnerability to stressors. Importantly, they largely depend on adequate dopamine levels [22], which is why dopamine depletion in PD may considerably impair coping and behavioral flexibility [22]. The result of these different mechanisms is that people with PD are at an increased risk of developing stress-related symptoms during chronic stress. The biological stress-response during chronic stress (e.g., increased cortisol levels or increased inflammatory tone) may play a modifying role in progressive neurodegeneration, although the evidence so far is restricted to animal models of PD [7, 8]. This calls for more extensive studies in humans to measure the effects of stress and stress-alleviation on clinical symptoms and on relevant biomarkers of PD progression. In non-PD populations, effective stress-alleviation has been shown to reduce inflammatory tone [23]. This may also be relevant for PD [24], since biomarkers of inflammation are consistently increased in people with PD [25]. Whether or not inflammation contributes to nigral degeneration is unclear, but if it is the case, then this would be a potential mechanism for disease-modifying effects of stress-reducing interventions [16]. In recent years, evidence for the effect of nonpharmacologic treatments for PD, such as exercise, has accumulated [26, 27], but the evidence for stress-alleviating interventions is much less clear.
NONPHARMACOLOGIC TREATMENT STRATEGIES TO REDUCE PSYCHOLOGICAL STRESS
Evidence for the symptomatic effects of stress-reduction in PD operationally focuses on symptoms of anxiety, depression, or overall quality of life (QoL), or in other words, the psychological dimension of the stress-response. Only very few studies have measured effects of these interventions on the biological stress-response (e.g., hormonal changes). Consequently, the evidence presented in the current paper involves changes that are likely associated with stress-reduction, but there is some uncertainty about the specificity and neurobiological mechanisms underlying these effects. Treatment strategies thought to reduce stress, such as physical exercise (resistance training, dance, aerobic exercise), psychotherapy (remote and in-person cognitive behavioral therapy), and mind-body interventions (mindfulness-based interventions, yoga, Tai Chi) have received increasing attention in recent literature [28, 29]. Observational research corroborates their benefits, demonstrating that these strategies are indeed reported by many people with PD to reduce stress in daily life [3].
Physical exercise
There is increasing evidence for the efficacy of treatment programs for PD that use physical exercise. Initially, such treatment options were primarily targeted towards PD motor symptoms, where they have been proven very effective in attenuating motor symptom progression, as demonstrated in two large clinical trials [27, 30]. In addition, the effects of physical activity on non-motor symptoms in PD, particularly mental wellbeing, has received more and more attention [28, 31]. This focus is in part motivated by the anti-depressant effects of physical exercise in people with mood disorders without PD [32]. In line with that, about 50% of people with PD report that they engage in physical exercise several times a week in order to reduce stress, as shown in a large survey in the United States (N = 5,000) [3]. Below, we will summarize recent experimental studies that explored the effects of aerobic exercise, resistance training, Nordic walking, treadmill training, and dance on stress-related symptoms in people with PD.
Large trials that primarily focused on the effects of aerobic exercise on stress-related outcome measures are scarce and have mostly reported psychological rather than biological measures of stress. So far, the largest intervention trials investigating physical exercise (N > 100) have not found effects on depression, anxiety, or other stress-related symptoms [27, 30, 33]. Yet, these studies did not specifically target patients with stress-related issues at baseline, which could have caused a floor effect, masking potential intervention effects. In contrast, a number of smaller randomized controlled trials (RCTs) suggest positive effects of exercise on stress-related symptoms. For example, a recent RCT by Wu and colleagues [34] (N = 98) showed that a two-months home-based exercise program with moderate intensity improved QoL and stress-related symptoms (anxiety and depression) in people with PD, compared to a passive control group. Another trial (N = 55) recently demonstrated that high intensity agility training led to improvements in QoL and symptoms of depression, compared to an active control group [35]. A high intensity workout program significantly improved QoL as well, compared to a balance training [36]. Thus, relatively high-intensity exercise programs may reduce perceived stress in PD and improve resilience to stress. This has been established by a recent systematic review [31], confirming that aerobic exercise might be most effective in reducing depression in people with PD, compared to other forms of physical activity. In line with that, a small study investigating the effects of six months of high intensity treadmill exercise in people with PD with mild cognitive impairment (N = 8), showed a decrease in post-awakening salivary cortisol after the intervention [37]. This suggests that physical exercise may be able to attenuate HPA axis dysregulation in PD, but such findings need to be confirmed in larger samples. Other forms of exercise, e.g., resistance training or (Nordic) walking, have been indicated to improve motor symptoms, depressed mood, and QoL as well [38–40].
Apart from exercise, dance interventions have been increasingly investigated in PD. For example, Kalyani and colleagues [41] showed that after 12 weeks of biweekly dance classes (N = 33), symptoms of anxiety and depression were reduced and QoL improved. Other studies confirmed positive effects of dancing on stress-related symptoms [42, 43] and a recent network meta-analysis suggests that dance might be most effective in treating depression, among all nonpharmacologic treatment methods for PD [28]. However, two other meta-analyses do not support any significant effects of dance on stress-related symptoms in PD [44, 45], and the three studies on dance in the network meta-analysis [28] did not find effects on stress-related symptoms other than depression. To definitely say if and how physical exercise interventions may lead to stress-reduction and whether they exert an influence on the biological stress-response, the collection of biomarkers of stress in future trials is essential (see also Table 1).
Table 1
Overview of recommended stress-related outcome measures for future studies
| Stress-related outcome measure | Rationale | Limitations | |
| Clinical | Beck depression inventory, Beck anxiety inventory | Symptoms of anxiety and depression are expressions of a dysfunctional stress-response and chronic stress [10]. | (Self-report) measures of stress-related symptoms are indirect measures of stress. |
| Hamilton depression scale, Hamilton anxiety scale | Self-report questionnaires can capture the subjective psychological strain and the potential relief thereof as a consequence of lifestyle interventions [29]. | Reliability is limited. | |
| Hospital anxiety and depression scale | Validity and comparability of questionnaires might be population-dependent. | ||
| Perceived stress scale | |||
| Neuroimaging | Functional MRI: Balance and connectivity in areas involved in the stress-response | Stress-sensitivity and (dys)functional coping may be embodied in the cerebral stress-response. Activity and connectivity of the executive control and salience network could be important indicators [11]. | Complex stress-induction paradigms are required to measure functional reactivity to stress. Effectivity of such paradigms depend on the individual and might not be representative for real-life stress. |
| Structural MRI: Integrity of stress-related brain areas (locus coeruleus, amygdala, hippocampus) | Integrity of structures involved in the HPA axis are associated with the efficiency of the stress-response [93]. | MRI is expensive. | |
| Identifying structural changes in stress-related brain areas require trials with long follow-up periods. | |||
| Biophysiological, biochemical | Endocrine markers: hair and salivary cortisol | As a biomarker of HPA axis activity, cortisol has been reported as an indirect measure of chronic stress. | Saliva cortisol levels vary widely throughout the day and between individuals. |
| Saliva cortisol is optimal for short-term stress evaluation [90]. | Hair cortisol lacks sensitivity to short-term changes, and is largely affected by external factors (e.g., hair type, hair products, sunlight exposure). | ||
| Hair cortisol is better for assessing chronic stress levels, providing a retrospective measure of stress exposure. High hair cortisol has been correlated to depression and anxiety disorders [94]. | |||
| Cardiovascular markers: heart rate variability (HRV) | HRV (the fluctuation of the length of heart beat intervals) is highly sensitive to changes in autonomic nervous system activity. | HRV is also affected by physical fitness, therefore it is difficult to attribute HRV changes solely to stress. | |
| HRV reflects the ability to cope with stressors. Decreased HRV has been demonstrated in people with depression and anxiety disorders [95]. | |||
| Marker of sympathetic tone: Pupil diameter | Pupil dilation coincides with changes in noradrenergic and cholinergic signaling; changes in pupil dilation may relate to psychiatric symptoms [96]. | Pupil dilation is not specific to one neuronal circuit or behavioral state. | |
| Inflammation markers: C-reactive protein (CRP) | CRP is an important biomarker of inflammation, which can be triggered by stress. | CRP is a general marker for inflammation, which is not specific to stress alone. | |
| Enhanced CRP levels are associated with chronic stress conditions [97]. | |||
| Eccrine markers: Skin conductance response (SCR) | SCR directly reflects the body’s sympathetic nervous system activity. | SCR measures arousal, not exclusively stress, and is affected by environmental factors (e.g., humidity, temperature), leading to variability. | |
| Sympathetic stimulation during the stress-response leads to increased sweat gland activity, which increases the electrical conductivity of the skin [98]. |
To summarize, physical exercise interventions (with most evidence on aerobic exercise) may have direct, positive effects on symptoms of depression and anxiety, as well as QoL, which is in line with patients’ self-reported strategies to alleviate stress [3]. However, long-term effects and minimum dosage of exercise interventions are currently unclear, and large RCTs investigating stress-related symptoms, biomarkers of stress, and their relation to motor symptom severity, are missing.
Mind-body exercises
Mind-body exercises like mindfulness, Tai Chi, Qigong and yoga have become increasingly popular for people with PD. In fact, 32% of 5,000 surveyed PD patients indicated to use relaxation exercises like Tai Chi or yoga at least several times per month to reduce stress [3]. Tai Chi and Qigong both describe traditional Chinese martial art forms of training, which include breathing and meditation exercises alongside controlled body movements. RCTs investigating the effects of Qigong and Tai Chi with regards to stress-related symptoms are limited, but overall show promising results. Both have been associated with improved QoL [46, 47] and mood (as measured by the UPDRS part I) [48] in people with PD. Yoga, a Hindu relaxation technique involving specific body postures and breathing exercises, has been studied in a recent meta-analysis [49]. The authors suggest positive, albeit limited, empirical evidence around the effects of yoga in PD, with regards to depression and anxiety. Effects on the biological stress-response have not been investigated in PD. Interestingly, a meta-analysis suggests that in healthy individuals, yoga may lead to reduced afternoon and evening cortisol levels, as well as a reduction of interleukin-6 (a marker for inflammation) [50]. This may point towards potential mechanistic underpinnings of such interventions. However, comparable evidence in PD is currently missing.
Mindfulness, originally a Buddhist tradition, describes the capacity to purposely experience the present moment, without judging any current emotions or thoughts [51]. Mindfulness-based interventions (MBIs) are focused around developing this capacity and are complemented by approaches of modern psychology, leading to well-documented programs like mindfulness-based stress reduction (MBSR) and mindfulness-based cognitive therapy (MBCT). MBIs have been applied and shown effective in many populations with regards to physical, as well as mental well-being [52]. With regards to PD, effects of MBIs on chronic and acute stress are very promising, as reviewed recently [16]. For example, a large RCT (N = 138) demonstrated significant effects of a mindfulness-yoga intervention, as compared to an active control condition (stretching and resistance), on symptoms of anxiety, depression, as well as QoL and motor symptom severity [53]. Importantly, the reported effects were consistent or even increased during a three-month follow-up period. This might indicate an advantage of MBIs as opposed to other treatment strategies, particularly with regards to stress-reduction: in MBIs, participants are encouraged to practice a skill rather than performing a particular activity (like exercise). Such skills are applicable in day-to-day life and various stressful situations, while an activity is likely unavailable during acute stress. Other RCTs have confirmed short- and long-term (3 months) benefits of MBSR in people with PD, particularly on QoL [54]. Interestingly, patients reported that those symptoms that were initially rated as inducing most stress in daily life (tremor, gait, loss of ability), were less bothersome after completing MBSR [54]. Bogosian and colleagues [55] recently corroborated positive effects of (video call-based) MBCT on QoL in PD. However, their results did not show any effects on symptoms of depression or anxiety. On the other hand, a small trial (N = 27) comparing MBCT to a waitlist control group showed no significant changes in QoL, but they did find improvements in anxiety and depressive symptoms [56]. To date, effects of MBIs on the biological stress-response have only been suggested in non-PD samples [23, 50, 57].
Stress-alleviation by MBIs might be effective for acute and long-term stress-reduction in PD, presumably because patients learn to better cope with stress. Indeed, improved emotion regulation via strengthening of prefrontal cognitive control mechanisms (and consequential downregulation of the limbic stress-response) might play a role in the benefits of MBIs, as suggested by evidence in healthy populations [58]. At the same time, MBIs may normalize dysfunctional activity in the HPA axis, resulting in reduced cortisol levels –as shown in non-PD populations [59]. Yet, large RCTs with longer follow-up periods are required to confirm this in PD populations. Such trials should take a comprehensive approach with regards to symptomatic effects and mechanistic underpinnings. Potential benefits of a larger focus on exercise- or yoga elements as part of MBIs might be worth exploring in PD samples, given the literature on those techniques reviewed above. The effects of MBIs on motor symptom severity are currently unclear.
Psychotherapy and others
It should be noted that psychotherapy has been investigated thoroughly with regards to its effect on depression in PD [60–62]. Furthermore, alternative treatment methods like sensory focused exercise [63], acupuncture [64, 65], bright light therapy [66], or therapeutic singing [67] have been suggested for stress-alleviation as well. Elaborate discussion of those methods would go beyond the scope of this manuscript.
STRESS-ALLEVIATION TO MODIFY PARKINSON’S DISEASE?
Available treatments for PD focus primarily on managing symptoms, and there is currently no known intervention that can stop or significantly delay the disease progression. As mentioned above (see Stress in people with Parkinson’s disease), animal models suggest that prolonged stressful circumstances might accelerate dopaminergic degeneration, associated with cellular, neuroendocrine, as well as inflammatory factors [7, 8, 68, 69]. The existing animal studies were performed in rodents with a (toxin-induced) PD-like phenotype and wildtype control groups. They were exposed to either unpredictable stressors [8], corticosterone [7], or chronic restraint and isolation [68, 69], which are well-established animal models for depression [70]. All these studies showed a worsened neuropathology, quantified by the amount of depleted dopaminergic neurons in the substantia nigra, specifically in the chronically stressed animals.
This raises an intriguing question: can strategies or interventions that effectively reduce stress also modify the course of PD? In this context, disease modification in PD refers to treatments that effectively alter the progression of the disease while maintaining or improving the quality of life [71]. This is not easy to answer, because it begs the question what disease progression is exactly, and how we can measure it. For instance, although α-synuclein pathology is a hallmark of PD, the extent and distribution of α-synuclein aggregates can vary among individuals [72]. Such variability in clinical presentation makes it challenging to establish a standardized biomarker that accurately reflects disease progression for all PD patients. Consequently, disease-modification may work either by slowing progressive nigro-striatal neurodegeneration and α-synucleinopathy, or by strengthening compensatory mechanisms, or both [73]. Furthermore, recent studies have shown that there are marked inter-individual differences in where neurodegeneration in PD first starts (body-first or brain-first PD), and how α-synucleinopathy propagates through the brain [72, 74]. Also, it has long been known that PD is not confined to the nigro-striatal system. Neuropsychiatric symptoms in particular have recently been associated with a more “diffuse-malignant” (probably a body-first) type of PD, suggesting that they are indeed not solely caused by dopamine depletion [74]. Thus, disease-modifying treatments may have various targets. So far, after decades of research, no disease-modifying treatment has been found in PD [75].
In accordance with this, none of the treatments described above has successfully been translated into a clinically proven disease-modifying treatment. Nevertheless, evidence from animal models suggests that physical exercise may have a disease-modifying effect on PD, by increasing certain neurotropic factors [76]. Also, physical exercise can enhance brain neuroplasticity in people with PD [26]. This was corroborated by a recent publication from a Japanese research group showing that regular physical exercise was associated with a better clinical course of PD [77]. In another study, patients who underwent a 6-month aerobic exercise intervention did not have the progressive reduction in gray matter volume in the sensorimotor cortex observed in patients who received the active control intervention (stretching) [26], and instead the exercise group showed a strengthening of (putatively compensatory) cortico-striatal connectivity. However, effects of motor training on gray matter volume have been observed in other (non-PD) groups as well [78], so it is not clear if this is a disease-specific effect. Furthermore, in that same study, no intervention effects were observed on substantia nigra integrity. Whether these potentially disease-modifying effects of physical exercise in PD are (partly) related to stress-reduction is unclear, but should be investigated in the future. Apart from physical exercise, one mindfulness trial in a PD sample showed changes in gray matter volume in the hippocampus and amygdala after MBSR, which might be promising since hippocampal atrophy increases during the PD disease course [79]. No studies have followed up on that finding since, and underlying mechanisms of other mind-body interventions have not been evaluated yet. In summary, although there might be long-term positive effects of stress-alleviation, there are no studies available to confirm this.
There are several reasons why previous trials may lack evidence for disease-modifying effects of lifestyle interventions in PD [73]. In addition to the complex PD pathophysiology discussed above, it is noteworthy that by the time of diagnosis, 50–70% of nigrostriatal dopamine function has been lost [80]. This implies that the PD pathology is already quite advanced at that point, which makes it challenging for treatments to have a disease-modifying impact. Second, to date, there are no reliable markers of true disease progression in PD. To assess whether stress-alleviation interventions can contribute to disease modification, we need a marker that is objective, sensitive to subtle changes, as well as applicable and accurate across all stages of the disease. The Unified PD Rating Scale (MDS-UPDRS), which is the current state of the art to monitor disease progression, has the disadvantage that it relies on subjective assessments, which can introduce variability between different raters and assessments over time [81]. Also, the MDS-UPDRS cannot detect subtle changes, it is a snapshot in the clinic that may not represent disease severity in daily life, and it is not suitable to measure biological disease progression. Specifically in the context of stress-alleviation, motor symptom reduction in response to treatment (as measured by the MDS-UPDRS) could be purely symptomatic, rather than reflect altered disease progression.
Given these limitations and considering the critical need for disease-modifying treatments, more and more studies have been exploring biomarkers for PD progression (see [82] for a recent and comprehensive overview of different types of PD biomarkers). For example, α-synuclein seed amplification in cerebrospinal fluid has a high accuracy for diagnosing PD [83], but is currently not suitable to measure disease progression. The high heterogeneity in PD progression and the relatively long disease course will likely pose extra challenges for finding sensitive markers. Nevertheless, some promising markers have been proposed. For example, free-water imaging (a diffusion imaging technique) has been applied to track progression of PD in the substantia nigra, it was sensitive to changes over one year in early-stage PD, and was associated with changes in clinical scores [84]. Similarly, neuromelanin-sensitive magnetic resonance (MR) imaging in the substantia nigra and locus coeruleus are able to track progression in PD fairly well, but so far have only detected changes over a 2-year period [85]. Proteins like neurofilament light chain have been suggested as promising progression markers as well [86]. Notably, positron emission tomography of striatal dopamine transporters, although often used to aid diagnosis of PD, has been shown to correlate poorly with clinical function over time, especially in later disease stages [87]. It is therefore considered less suitable as a marker for PD progression. Overall, there are promising advances, but more research is needed to compare the utility of different markers and to evaluate how imaging outcomes translate to clinical measures and patient experience.
FUTURE DIRECTIONS
We propose the following future directions (see also Fig. 1). First, intervention trials aimed at stress-alleviation should be well-powered and include patients who have stress-related symptoms at baseline (to prevent a flooring effect). Existing studies are often underpowered and/or not focused towards individuals with psychiatric symptoms. Patients with stress-related symptoms will most likely benefit the most from stress-alleviating interventions, and such samples are therefore most suitable to demonstrate the efficacy of these interventions. Second, more comprehensive outcome measures should be investigated. For example, physiological measures such as cortisol, heart rate variability or inflammation markers could shed light on HPA axis (over)activity, albeit with limitations [88–90]. Measuring changes in cerebral stress reactivity by means of a laboratory stress induction like the socially evaluated cold pressor task [91], or the Trier social stress test [92] might be another promising way to assess effects of stress-reduction [64]. In Table 1, we provide an overview of suitable biomarkers of stress that could be incorporated in future research studying the effects of stress-reduction in PD. Furthermore, upcoming intervention trials may comprehensively evaluate both motor and non-motor symptoms when exploring the symptomatic effects, as most existing studies lack information on the impact of stress(-reduction) on motor functioning. Third, long-term follow-up periods of at least 12 months [87] are necessary to identify the most persistent intervention effects and to explore potential disease-modifying effects of stress-alleviating treatments. For the latter, suitable progression markers, e.g., free-water MRI or neuromelanin-sensitive MRI [82], could be included and associated with relevant clinical outcomes. Last, it would be valuable to establish the effect of stress-reducing interventions on compensatory mechanisms (or the lack thereof), and to define what these mechanisms entail. Altogether, although lifestyle interventions show great promise, their miscellaneous effects pose a challenge in deciphering target mechanisms and specifying relevant outcomes. Future studies could be more hypothesis-driven and would ideally involve outcomes that quantify intervention effects on the biological stress-response and on PD pathophysiology, to better understand the potential benefits of such treatments.
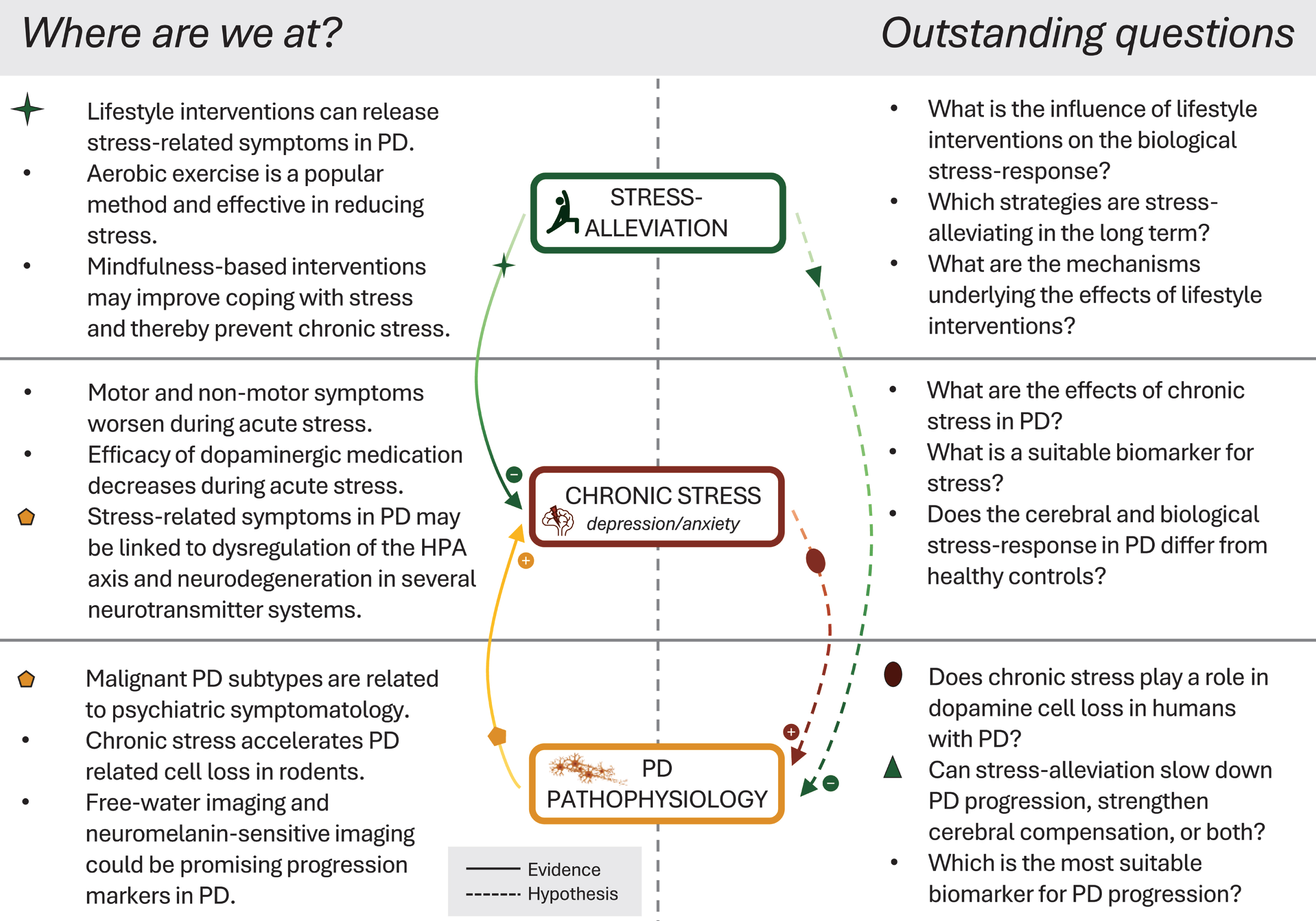
Fig. 1
Current advances and outstanding questions in the field. Left-hand panel illustrates the most crucial findings of recent evidence. Solid lines indicate the influence of stress-alleviation on chronic stress, and the pathophysiological link of Parkinson’s disease to stress and related non-motor symptoms. Right-hand panel and dashed lines illustrate outstanding questions and hypotheses regarding disease-modifying effects of stress and stress-alleviation in Parkinson’s disease. PD, Parkinson’s disease; HPA, hypothalamic-pituitary-adrenal.
CONCLUSION
Current evidence suggests that lifestyle interventions, specifically aerobic exercise and MBIs, are effective in reducing stress-related symptoms in PD, such as anxiety and depression. However, evidence for the effect of those interventions on the biological stress-response is missing. While disease-modifying effects of stress and of stress-reduction in PD are suggested by animal studies, there is currently no proof in humans. To answer these outstanding questions, larger trials with comprehensive outcome measures and reliable biomarkers for disease progression and stress are needed.
ACKNOWLEDGMENTS
The authors have no acknowledgements to report.
FUNDING
R. C. Helmich was supported by the Michael J. Foundation (grant ID: MJFF-021001) and by the Netherlands Organization for Scientific Research (grant ID: 09150172010044).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Bisht K , Sharma K , Tremblay M-È ((2018) ) Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress, Neurobiol Stress 9: , 9–21. |
[2] | Cohen S , Janicki-Deverts D , Doyle WJ , Miller GE , Frank E , Rabin BS , Turner RB ((2012) ) Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk, Proc Natl Acad Sci U S A 109: , 5995–5999. |
[3] | van der Heide A , Speckens AEM , Meinders MJ , Rosenthal LS , Bloem BR , Helmich RC ((2021) ) Stress and mindfulness in Parkinson’s disease –a survey in 5000 patients, NPJ Parkinsons Dis 7: , 7. |
[4] | Macht M , Kaussner Y , Möller JC , Stiasny-Kolster K , Eggert KM , Krüger H-P , Ellgring H ((2007) ) Predictors of freezing in Parkinson’s disease: A survey of 6,620 patients, Mov Disord 22: , 953–956. |
[5] | Zach H , Dirkx MF , Pasman JW , Bloem BR , Helmich RC ((2017) ) Cognitive stress reduces the effect of levodopa on Parkinson’s resting tremor, CNS Neurosci Ther 23: , 209–215. |
[6] | Durif F , Vidailhet M , Debilly B , Agid Y ((1999) ) Worsening of levodopa-induced dyskinesias by motor and mental tasks, Mov Disord 14: , 242–246. |
[7] | Burtscher J , Copin J-C , Rodrigues J , Kumar ST , Chiki A , Guillot de Suduiraut I , Sandi C , Lashuel HA ((2019) ) Chronic corticosterone aggravates behavioral and neuronal symptomatology in a mouse model of alpha-synuclein pathology, Neurobiol Aging 83: , 11–20. |
[8] | Hemmerle AM , Dickerson JW , Herman JP , Seroogy KB ((2014) ) Stress exacerbates experimental Parkinson’s disease, Mol Psychiatry 19: , 638–640. |
[9] | Cohen S , Kessler RC , Gordon UL (1995) Strategies for measuring stress in studies of psychiatric and physical disorder. In Measuring Stress: A Guide for Health and Social Scientists, Cohen S, Kessler RC, Gordon UL, eds. Oxford University Press, New York, NY, pp. 3-26. |
[10] | Tafet GE , Nemeroff CB ((2016) ) The links between stress and depression: Psychoneuroendocrinological, genetic, and environmental interactions, J Neuropsychiatry Clin Neurosci 28: , 77–88. |
[11] | Hermans EJ , Henckens MJAG , Joëls M , Fernández G ((2014) ) Dynamic adaptation of large-scale brain networks in response to acute stressors, Trends Neurosci 37: , 304–314. |
[12] | Ulrich-Lai YM , Herman JP ((2009) ) Neural regulation of endocrine and autonomic stress responses, Nat Rev Neurosci 10: , 397–409. |
[13] | Herman JP , McKlveen JM , Ghosal S , Kopp B , Wulsin A , Makinson R , Scheimann J , Myers B ((2016) ) Regulation of the hypothalamic-pituitary-adrenocortical stress response, Compr Physiol 6: , 603–621. |
[14] | Kalisch R , Baker DG , Basten U , Boks MP , Bonanno GA , Brummelman E , Chmitorz A , Fernàndez G , Fiebach CJ , Galatzer-Levy I , Geuze E , Groppa S , Helmreich I , Hendler T , Hermans EJ , Jovanovic T , Kubiak T , Lieb K , Lutz B , Müller MB , Murray RJ , Nievergelt CM , Reif A , Roelofs K , Rutten BPF , Sander D , Schick A , Tüscher O , Diest Van I , Harmelen van A-L , Veer IM , Vermetten E , Vinkers CH , Wager TD , Walter H , Wessa M , Wibral M , Kleim B ((2017) ) The resilience framework as a strategy to combat stress-related disorders, Nat Hum Behav 1: , 784–790. |
[15] | Folkman S , Lazarus RS , Dunkel-Schetter C , DeLongis A , Gruen RJ ((1986) ) Dynamics of a stressful encounter: Cognitive appraisal, coping, and encounter outcomes, J Pers Soc Psychol 50: , 992–1003. |
[16] | van der Heide A , Meinders MJ , Speckens AEM , Peerbolte TF , Bloem BR , Helmich RC ((2021) ) Stress and mindfulness in Parkinson’s disease: Clinical effects and potential underlying mechanisms, Mov Disord 36: , 64–70. |
[17] | Wakabayashi K , Takahashi H ((1997) ) Neuropathology of Autonomic nervous system in Parkinson’s disease, Eur Neurol 38: , 2–7. |
[18] | van den Heuvel LL , du Plessis S , Stalder T , Acker D , Kirschbaum C , Carr J , Seedat S ((2020) ) Hair glucocorticoid levels in Parkinson’s disease, Psychoneuroendocrinology 117: , 104704. |
[19] | Paulus W , Jellinger K ((1991) ) The neuropathologic basis of different clinical subgroups of Parkinson’s disease, J Neuropathol Exp Neurol 50: , 743–755. |
[20] | Reijnders JSAM , Ehrt U , Weber WEJ , Aarsland D , Leentjens AFG ((2008) ) A systematic review of prevalence studies of depression in Parkinson’s disease, Mov Disord 23: , 183–189. |
[21] | Broen MPG , Narayen NE , Kuijf ML , Dissanayaka NNW , Leentjens AFG ((2016) ) Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis, Mov Disord 31: , 1125–1133. |
[22] | Douma EH , de Kloet ER ((2020) ) Stress-induced plasticity and functioning of ventral tegmental dopamine neurons, Neurosci Biobehav Rev 108: , 48–77. |
[23] | Ng TKS , Fam J , Feng L , Cheah IK-M , Tan CT-Y , Nur F , Wee ST , Goh LG , Chow WL , Ho RC-M , Kua EH , Larbi A , Mahendran R ((2020) ) Mindfulness improves inflammatory biomarker levels in older adults with mild cognitive impairment: A randomized controlled trial, Transl Psychiatry 10: , 21. |
[24] | Darweesh SKL , De Vries NM , Helmich RC , Verbeek MM , Schwarzschild MA , Bloem BR ((2022) ) Inhibition of neuroinflammation may mediate the disease-modifying effects of exercise: Implications for Parkinson’s disease, J Parkinsons Dis 12: , 1419–1422. |
[25] | Qiu X , Xiao Y , Wu J , Gan L , Huang Y , Wang J ((2019) ) C-reactive protein and risk of Parkinson’s disease: A systematic review and meta-analysis, Front Neurol 10: , 384. |
[26] | Johansson ME , Cameron IGM , Vander Kolk NM , Vries NM , Klimars E , Toni I , Bloem BR , Helmich RC ((2022) ) Aerobic exercise alters brain function and structure in Parkinson’s disease: A randomized controlled trial, Ann Neurol 91: , 203–216. |
[27] | van der Kolk NM , de Vries NM , Kessels RPC , Joosten H , Zwinderman AH , Post B , Bloem BR ((2019) ) Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial, Lancet Neurol 18: , 998–1008. |
[28] | Wang Y , Sun X , Li F , Li Q , Jin Y ((2022) ) Efficacy of non-pharmacological interventions for depression in individuals with Parkinson’s disease: A systematic review and network meta-analysis, Front Aging Neurosci 14: , 1050715. |
[29] | Weintraub D , Aarsland D , Chaudhuri KR , Dobkin RD , Leentjens AF , Rodriguez-Violante M , Schrag A ((2022) ) The neuropsychiatry of Parkinson’s disease: Advances and challenges, Lancet Neurol 21: , 89–102. |
[30] | Schenkman M , Moore CG , Kohrt WM , Hall DA , Delitto A , Comella CL , Josbeno DA , Christiansen CL , Berman BD , Kluger BM , Melanson EL , Jain S , Robichaud JA , Poon C , Corcos DM ((2018) ) Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease, JAMA Neurol 75: , 219. |
[31] | Wu P-L , Lee M , Huang T-T ((2017) ) Effectiveness of physical activity on patients with depression and Parkinson’s disease: A systematic review, PLoS One 12: , e0181515. |
[32] | Agudelo LZ , Femenía T , Orhan F , Porsmyr-Palmertz M , Goiny M , Martinez-Redondo V , Correia JC , Izadi M , Bhat M , Schuppe-Koistinen I , Pettersson AT , Ferreira DMS , Krook A , Barres R , Zierath JR , Erhardt S , Lindskog M , Ruas JL ((2014) ) Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression, Cell 159: , 33–45. |
[33] | Shulman LM , Katzel LI , Ivey FM , Sorkin JD , Favors K , Anderson KE , Smith BA , Reich SG , Weiner WJ , Macko RF ((2013) ) Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease, JAMA Neurol 70: , 183. |
[34] | Wu P , Lee M , Wu S , Ho H , Chang M , Lin H , Huang T ((2021) ) Effects of home-based exercise on motor, non-motor symptoms and health-related quality of life in Parkinson’s disease patients: A randomized controlled trial, Jpn J Nurs Sci 18: , e12418. |
[35] | Tollár J , Nagy F , Kovács N , Hortobágyi T ((2018) ) A high-intensity multicomponent agility intervention improves Parkinson patients’ clinical and motor symptoms, Arch Phys Med Rehabil 99: , 2478–2484.e1. |
[36] | Cancela JM , Mollinedo I , Montalvo S , Vila Suárez ME ((2020) ) Effects of a high-intensity progressive-cycle program on quality of life and motor symptomatology in a Parkinson’s disease population: A pilot randomized controlled trial, Rejuvenation Res 23: , 508–515. |
[37] | Smyth N , Skender E , David FJ , Munoz MJ , Fantuzzi G , Clow A , Goldman JG , Corcos DM ((2019) ) Endurance exercise reduces cortisol in Parkinson’s disease with mild cognitive impairment, Mov Disord 34: , 1238–1239. |
[38] | Lima TA , Ferreira-Moraes R , Alves da WMGC , Alves TGG , Pimentel CP , Sousa EC , Abrahin O , Cortinhas-Alves EA ((2019) ) Resistance training reduces depressive symptoms in elderly people with Parkinson disease: A controlled randomized study, Scand J Med Sci Sports 29: , 1957–1967. |
[39] | Granziera S , Alessandri A , Lazzaro A , Zara D , Scarpa A ((2021) ) Nordic walking and walking in Parkinson’s disease: A randomized single-blind controlled trial, Aging Clin Exp Res 33: , 965–971. |
[40] | Cugusi L , Manca A , Dragone D , Deriu F , Solla P , Secci C , Monticone M , Mercuro G ((2017) ) Nordic walking for the management of people with Parkinson disease: A systematic review, PM&R 9: , 1157–1166. |
[41] | Kalyani HHN , Sullivan KA , Moyle G , Brauer S , Jeffrey ER , Kerr GK ((2019) ) Impacts of dance on cognition, psychological symptoms and quality of life in Parkinson’s disease, NeuroRehabilitation 45: , 273–283. |
[42] | Elpidoforou M , Bakalidou D , Drakopoulou M , Kavga A , Chrysovitsanou C , Stefanis L ((2022) ) Effects of a structured dance program in Parkinson’s disease. A Greek pilot study, Complement Ther Clin Pract 46: , 101528. |
[43] | Prewitt CM , Charpentier JC , Brosky JA , Urbscheit NL ((2017) ) Effects of dance classes on cognition, depression, and self-efficacy in Parkinson’s disease, Am J Dance Ther 39: , 126–141. |
[44] | Wang L , Sun C , Wang Y , Zhan T , Yuan J , Niu C-Y , Yang J , Huang S , Cheng L ((2022) ) Effects of dance therapy on non-motor symptoms in patients with Parkinson’s disease: A systematic review and meta-analysis, Aging Clin Exp Res 34: , 1201–1208. |
[45] | Zhang Q , Hu J , Wei L , Jia Y , Jin Y ((2019) ) Effects of dance therapy on cognitive and mood symptoms in people with Parkinson’s disease: A systematic review and meta-analysis, Complement Ther Clin Pract 36: , 12–17. |
[46] | Lee H-J , Kim S-Y , Chae Y , Kim M-Y , Yin C , Jung W-S , Cho K-H , Kim S-N , Park H-J , Lee H ((2018) ) Turo (Qi Dance) Program for Parkinson’s disease patients: Randomized, assessor blind, waiting-list control, partial crossover study, Explore 14: , 216–223. |
[47] | Nocera JR , Amano S , Hass CJ , VVallabhajosula S ((2013) ) Tai Chi exercise to improve non-motor symptoms of Parkinson’s disease, J Yoga Phys Ther 3: , doi:10.4172/2157-7595.1000137 . |
[48] | Choi H-J , Garber CE , Jun T-W , Jin Y-S , Chung S-J , Kang H-J ((2013) ) Therapeutic effects of Tai Chi in patients with Parkinson’s disease, ISRN Neurol 2013: , 548240. |
[49] | Suárez-Iglesias D , Santos L , Sanchez-Lastra MA , Ayán C ((2022) ) Systematic review and meta-analysis of randomised controlled trials on the effects of yoga in people with Parkinson’s disease, Disabil Rehabil 44: , 6210–6229. |
[50] | Pascoe MC , Thompson DR , Ski CF ((2017) ) Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis, Psychoneuroendocrinology 86: , 152–168. |
[51] | Kabat-Zinn J (1990) Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Dell Publishing, New York. |
[52] | Goldberg SB , Riordan KM , Sun S , Davidson RJ ((2022) ) The empirical status of mindfulness-based interventions: A systematic review of 44 meta-analyses of randomized controlled trials, Perspect Psychol Sci 17: , 108–130. |
[53] | Kwok JYY , Kwan JCY , Auyeung M , Mok VCT , Lau CKY , Choi KC , Chan HYL ((2019) ) Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease, JAMA Neurol 76: , 755. |
[54] | Shah-Zamora D , Allen AM , Rardin L , Ivancic M , Durham K , Hickey P , Cooney JW , Scott BL , Mantri S ((2021) ) Mindfulness based stress reduction in people with Parkinson’s disease and their care partners, Complement Ther Clin Pract 43: , 101377. |
[55] | Bogosian A , Hurt CS , Hindle JV , McCracken LM , Vasconcelos e Sa DA , Axell S , Tapper K , Stevens J , Hirani PS , Salhab M , Ye W , Cubi-Molla P ((2022) ) Acceptability and feasibility of a mindfulness intervention delivered via videoconferencing for people with Parkinson’s, J Geriatr Psychiatry Neurol 35: , 155–167. |
[56] | Rodgers SH , Schütze R , Gasson N , Anderson RA , Kane RT , Starkstein S , Morgan-Lowes K , Egan SJ ((2019) ) Modified mindfulness-based cognitive therapy for depressive symptoms in Parkinson’s disease: A pilot trial, Behav Cogn Psychother 47: , 446–461. |
[57] | Carlson LE , Speca M , Faris P , Patel KD ((2007) ) One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients, Brain Behav Immun 21: , 1038–1049. |
[58] | Tang Y-Y , Hölzel BK , Posner MI ((2015) ) The neuroscience of mindfulness meditation, Nat Rev Neurosci 16: , 213–225. |
[59] | Rogerson O , Wilding S , Prudenzi A , O’Connor DB ((2024) ) Effectiveness of stress management interventions to change cortisol levels: A systematic review and meta-analysis, Psychoneuroendocrinology 159: , 106415. |
[60] | Dobkin RD , Menza M , Allen LA , Gara MA , Mark MH , Tiu J , Bienfait KL , Friedman J ((2011) ) Cognitive-behavioral therapy for depression in Parkinson’s disease: A randomized, controlled trial, Am J Psychiatry 168: , 1066–1074. |
[61] | Kraepelien M , Schibbye R , Månsson K , Sundström C , Riggare S , Andersson G , Lindefors N , Svenningsson P , Kaldo V ((2020) ) Individually tailored internet-based cognitive-behavioral therapy for daily functioning in patients with Parkinson’s disease: A randomized controlled trial, J Parkinsons Dis 10: , 653–664. |
[62] | Wuthrich VM , Rapee RM ((2019) ) Telephone-delivered cognitive behavioural therapy for treating symptoms of anxiety and depression in Parkinson’s disease: A pilot trial, Clin Gerontol 42: , 444–453. |
[63] | Beck EN , Wang MTY , Intzandt BN , Almeida QJ , Ehgoetz Martens KA ((2020) ) Sensory focused exercise improves anxiety in Parkinson’s disease: A randomized controlled trial, PLoS One 15: , e0230803. |
[64] | Kong KH , Ng HL , Li W , Ng DW , Tan SI , Tay KY , Au WL , Tan LCS ((2018) ) Acupuncture in the treatment of fatigue in Parkinson’s disease: A pilot, randomized, controlled, study, Brain Behav 8: , e00897. |
[65] | Fan J , Lu W , Tan W , Liu X , Wang Y , Wang N , Zhuang L ((2022) ) Effectiveness of acupuncture for anxiety among patients with Parkinson disease, JAMA Netw Open 5: , e2232133. |
[66] | Rutten S , Vriend C , Smit JH , Berendse HW , van Someren EJW , Hoogendoorn AW , Twisk JWR , van der Werf YD , van den Heuvel OA ((2019) ) Bright light therapy for depression in Parkinson disease, Neurology 92: , e1145–e1156. |
[67] | Stegemöller EL , Zaman A , Shelley M , Patel B , Kouzi A El , Shirtcliff EA ((2021) ) The effects of group therapeutic singing on cortisol and motor symptoms in persons with Parkinson’s disease, Front Hum Neurosci 15: , 703382. |
[68] | Smith LK , Jadavji NM , Colwell KL , Katrina Perehudoff S , Metz GA ((2008) ) Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson’s disease, Eur J Neurosci 27: , 2133–2146. |
[69] | Lauretti E , Di Meco A , Merali S , Praticò D ((2016) ) Chronic behavioral stress exaggerates motor deficit and neuroinflammation in the MPTP mouse model of Parkinson’s disease, Transl Psychiatry 6: , e733–e733. |
[70] | Wang Q , Timberlake MA , Prall K , Dwivedi Y ((2017) ) The recent progress in animal models of depression, Prog Neuropsychopharmacol Biol Psychiatry 77: , 99–109. |
[71] | Vijiaratnam N , Simuni T , Bandmann O , Morris HR , Foltynie T ((2021) ) Progress towards therapies for disease modification in Parkinson’s disease, Lancet Neurol 20: , 559–572. |
[72] | Horsager J , Andersen KB , Knudsen K , Skjærbæk C , Fedorova TD , Okkels N , Schaeffer E , Bonkat SK , Geday J , Otto M , Sommerauer M , Danielsen EH , Bech E , Kraft J , Munk OL , Hansen SD , Pavese N , Göder R , Brooks DJ , Berg D , Borghammer P ((2020) ) Brain-first versus body-first Parkinson’s disease: A multimodal imaging case-control study, Brain 143: , 3077–3088. |
[73] | Lang AE , Espay AJ ((2018) ) Disease modification in Parkinson’s disease: Current approaches, challenges, and future considerations, Mov Disord 33: , 660–677. |
[74] | Johansson ME , van Lier NM , Kessels RPC , Bloem BR , Helmich RC ((2023) ) Two-year clinical progression in focal and diffuse subtypes of Parkinson’s disease, NPJ Parkinsons Dis 9: , 29. |
[75] | Mari Z , Mestre TA ((2022) ) The disease modification conundrum in Parkinson’s disease: Failures and hopes, Front Aging Neurosci 14: , 810860. |
[76] | Ahlskog JE ((2011) ) Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 77: , 288–294. |
[77] | Tsukita K , Sakamaki-Tsukita H , Takahashi R ((2022) ) Long-term effect of regular physical activity and exercise habits in patients with early Parkinson disease, Neurology 98: , e859–e871. |
[78] | Draganski B , Gaser C , Busch V , Schuierer G , Bogdahn U , May A ((2004) ) Changes in grey matter induced by training, Nature 427: , 311–312. |
[79] | Pickut BA , Van Hecke W , Kerckhofs E , Mariën P , Vanneste S , Cras P , Parizel PM ((2013) ) Mindfulness based intervention in Parkinson’s disease leads to structural brain changes on MRI, Clin Neurol Neurosurg 115: , 2419–2425. |
[80] | Fearnley JM , Lees AJ ((1991) ) Ageing and Parkinson’s disease: Substantia nigra regional selectivity, Brain 114: , 2283–2301. |
[81] | AlMahadin G , Lotfi A , Zysk E , Siena FL , Carthy MM , Breedon P ((2020) ) Parkinson’s disease: Current assessment methods and wearable devices for evaluation of movement disorder motor symptoms - a patient and healthcare professional perspective, BMC Neurol 20: , 419. |
[82] | Vijiaratnam N , Foltynie T ((2023) ) How should we be using biomarkers in trials of disease modification in Parkinson’s disease? Brain 146: , 4845–4869. |
[83] | Siderowf A , Concha-Marambio L , Lafontant D-E , Farris CM , Ma Y , Urenia PA , Nguyen H , Alcalay RN , Chahine LM , Foroud T , Galasko D , Kieburtz K , Merchant K , Mollenhauer B , Poston KL , Seibyl J , Simuni T , Tanner CM , Weintraub D , Videnovic A , Choi SH , Kurth R , Caspell-Garcia C , Coffey CS , Frasier M , Oliveira LMA , Hutten SJ , Sherer T , Marek K , Soto C ((2023) ) Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: A cross-sectional study, Lancet Neurol 22: , 407–417. |
[84] | Zhou G , Ren J , Rong D , Zhou H , Ning H , Wang H , Pan C , Wang Y , Zhang R , Guo Z , Huang P , Liu W ((2023) ) monitoring substantia nigra degeneration using free water imaging across prodromal and clinical Parkinson’s disease, Mov Disord 38: , 774–782. |
[85] | Biondetti E , Gaurav R , Yahia-Cherif L , Mangone G , Pyatigorskaya N , Valabrègue R , Ewenczyk C , Hutchison M , François C , Arnulf I , Corvol J-C , Vidailhet M , Lehéricy S ((2020) ) Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease, Brain 143: , 2757–2770. |
[86] | Liu Y , Dou K , Xue L , Li X , Xie A ((2022) ) Neurofilament light as a biomarker for motor decline in Parkinson’s disease, Front Neurosci 16: , 959261. |
[87] | Mitchell T , Lehéricy S , Chiu SY , Strafella AP , Stoessl AJ , Vaillancourt DE ((2021) ) Emerging neuroimaging biomarkers across disease stage in Parkinson disease, JAMA Neurol 78: , 1262. |
[88] | Noushad S , Ahmed S , Ansari B , Mustafa U-H , Saleem Y , Hazrat H ((2021) ) Physiological biomarkers of chronic stress: A systematic review, Int J Health Sci (Qassim) 15: , 46–59. |
[89] | Marsland AL , Walsh C , Lockwood K , John-Henderson NA ((2017) ) The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis, Brain Behav Immun 64: , 208–219. |
[90] | Hellhammer DH , Wüst S , Kudielka BM ((2009) ) Salivary cortisol as a biomarker in stress research, Psychoneuroendocrinology 34: , 163–171. |
[91] | Schwabe L , Haddad L , Schachinger H ((2008) ) HPA axis activation by a socially evaluated cold-pressor test, Psychoneuroendocrinology 33: , 890–895. |
[92] | Allen AP , Kennedy PJ , Dockray S , Cryan JF , Dinan TG , Clarke G ((2017) ) The Trier Social Stress Test: Principles and practice, Neurobiol Stress 6: , 113–126. |
[93] | McEwen BS ((2005) ) Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain, Metabolism 54: , 20–23. |
[94] | Staufenbiel SM , Penninx BWJH , Spijker AT , Elzinga BM , van Rossum EFC ((2013) ) Hair cortisol, stress exposure, and mental health in humans: A systematic review, Psychoneuroendocrinology 38: , 1220–1235. |
[95] | Kim H-G , Cheon E-J , Bai D-S , Lee YH , Koo B-H ((2018) ) Stress and heart rate variability: A meta-analysis and review of the literature, Psychiatry Investig 15: , 235–245. |
[96] | Larsen RS , Waters J ((2018) ) Neuromodulatory correlates of pupil dilation, Front Neural Circuits 12: , 21. |
[97] | Hapuarachchi JR , Chalmers AH , Winefield AH , Blake-Mortimer JS ((2003) ) Changes in clinically relevant metabolites with psychological stress parameters, Behav Med 29: , 52–59. |
[98] | Vahey R , Becerra R ((2015) ) Galvanic Skin response in mood disorders: A critical review, Int J Psychol Psychol Ther 15: , 275–304. |




