Real-World Evaluation of the Feasibility, Acceptability and Safety of a Remote, Self-Management Parkinson’s Disease Care Pathway: A Healthcare Improvement Initiative
Abstract
Background:
There is significant unmet need for effective and efficiently delivered care for people with Parkinson’s disease (PwP). We undertook a service improvement initiative to co-develop and implement a new care pathway, Home Based Care (HBC), based on supported self-management, remote monitoring and the ability to trigger a healthcare contact when needed.
Objective:
To evaluate feasibility, acceptability and safety of Home Based Care.
Methods:
We evaluated data from the first 100 patients on HBC for 6 months. Patient monitoring, performed at baseline and 6-monthly, comprised motor (MDS-UPDRS II and accelerometer), non-motor (NMSQ, PDSS-2, HADS) and quality of life (PDQ) measures. Care quality was audited against Parkinson’s UK national audit standards. Process measures captured feasibility. Acceptability was assessed using a mixed-methods approach comprising questionnaires and semi-structured interviews.
Results:
Between October 2019 and January 2021, 108 PwP were enrolled onto HBC, with data from 100 being available at 6 months. Over 90% of all questionnaires were returned, 97% were complete or had < 3 missing items. Reporting and communications occurred within agreed timeframes. Compared with baseline, after 6m on HBC, PD symptoms were stable; more PwP felt listened to (90% vs. 79%) and able to seek help (79% vs. 68%). HBC met 93% of national audit criteria. Key themes from the interviews included autonomy and empowerment.
Conclusions:
We have demonstrated acceptability, feasibility and safety of our novel remotely delivered Parkinson’s care pathway. Ensuring scalability will widen its reach and realize its benefits for underserved communities, enabling formal comparisons with standard care and cost-effectiveness evaluation.
INTRODUCTION
Parkinson’s disease (PD) is the fastest growing neurological condition worldwide. Traditionally, care for people with Parkinson’s disease (PwP) comprises regular, in-person clinical review by a movement disorders specialist every 6–12 months [1], with increasing frequency as the diseases progresses. However, delivering these reviews within the UK National Health Service (NHS) is challenging due to increasing demands on PD services such that patients are sometimes seen annually or every 18 months [2] due to the lack of available specialist clinic appointments. Moreover, since symptoms vary both between days and throughout the same day, infrequent, one-shot clinical encounters may not give clinical teams representative information on which to base optimal treatment. They are largely dependent on patient recall, and as such many symptoms, not just in the motor, but also in the cognitive, neuropsychiatric, autonomic, gastrointestinal, and genitourinary domains often remain overlooked or undeclared. Clinic visits are often distant from where patients live, rendering them anxiety-provoking and burdensome on both PwP and care partner if one is present. Moreover, the current care model provides limited opportunity to educate PwP and their care partners on their condition. There is therefore a pressing need to deliver effective care more efficiently to the estimated 145,000 PwP in the UK, a number which is projected to rise to 170,000 by 2025 [3], equating to care costs totaling approximately £3–4 billion [4].
The UK NHS Long Term Plan emphasizes the need for self-management and technology-enabled, personalized care [5]. Empowered self-management and active involvement in treatment decisions is known to lead to better outcomes and promote independence. It relies centrally on supported self-efficacy, in tandem with interactive, tailored approaches to shared decision making.
In recognition of this significant unmet need and in line with the NHS Long Term Plan, our mission was to clearly characterize care priorities from the perspective of PwP and, guided by these, to co-produce and develop an improved care pathway [6]. To this end, we conducted a series of multi-stakeholder co-production workshops involving PwP, their families and care partners, multi-disciplinary HCPs and care service providers [7]. Four core themes around care priorities emerged from these workshops: need for knowledge and understanding of PD, personal involvement in care, the need for personalized care provision and the delivery of targeted care at time of need. Thus, the Home Based Care (HBC) care pathway evolved and grew in partnership with PwP from its inception. Its key functional elements are i) support for self-management of PD, ii) remote monitoring, and iii) the ability to request a healthcare contact when needed. Our aims were to develop and implement this pathway according to these functional elements, and to evaluate its feasibility, acceptability, and safety. The development of the pathway is described according to SQUIRE guidelines [8] and evaluated.
METHODS
HBC development
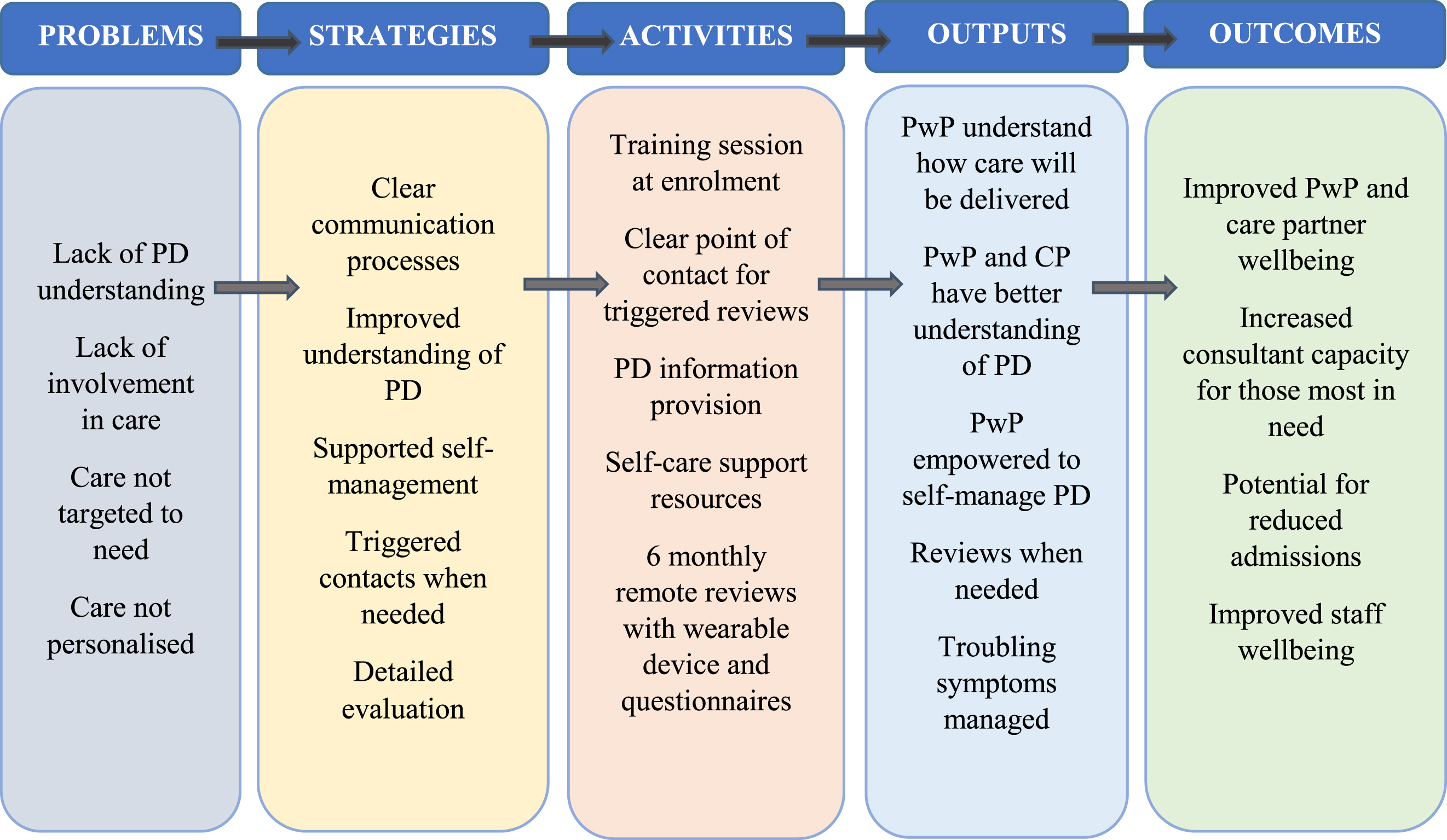
Between March and July 2019, we held four multi-stakeholder workshops with 20 PwP and care partners to map the HBC service and resource requirements, plan the detail of the resource components, co-create information content for different audiences and refine the patient facing materials in terms of content and format. Six months after HBC was implemented, we held a further multi-stakeholder workshop to evaluate the service and understand resource use. The workshops were facilitated by researchers (JL, RP, UA) and informed the development of a logic model (Fig. 1) which guided the co-production of the functional elements of HBC. Once launched, feedback and continuous evaluation by PwP and staff guided and informed future iterations.
Fig. 1
Logic model to guide the design of the Home Based Care intervention (CP, care partner; PD, Parkinson’s disease; PwP, person/people with Parkinson’s).
HBC implementation
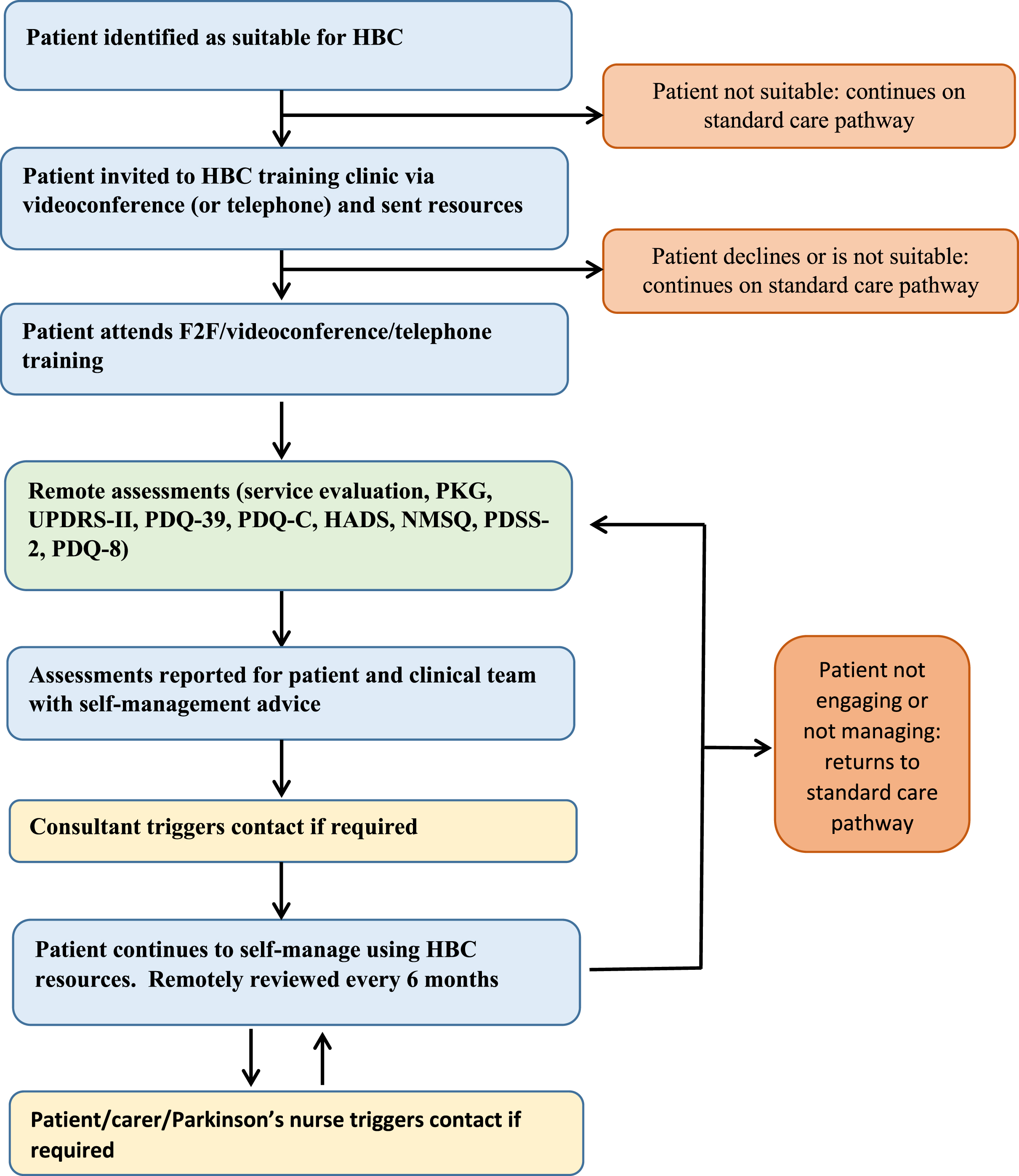
The implementation of the HBC pathway began in October 2019 by the Parkinson’s service within the Department of Neurology at University Hospitals Plymouth NHS Trust, Plymouth, UK. The project management group included the lead neurologist (CC), a Parkinson’s disease nurse specialist (PDNS (EE)), patient and care partner representatives (SW, MB, JR) and project collaborators. Figure 2 illustrates the HBC pathway. Its key functional elements are i) support for self-management of PD, ii) remote monitoring, and iii) the ability to request a healthcare contact when needed. Patients were considered suitable for HBC if they were ambulant, living in their own home (not supported living or nursing homes) and able to adhere to pathway requirements, particularly remote monitoring of symptoms. Patients were invited to enroll onto HBC by invitation letter or during routine clinic visits. Suitability for the pathway enrolment was determined by the patient’s clinical care team and assessed continually thereafter by the clinical care team and HBC pathway team.
Fig. 2
Home-based Care (HBC): clinical pathway. HADS, Hospital anxiety and depression scale; NMSQ, Non-motor symptoms questionnaire; PDSS-2, Parkinson’s disease sleep scale; PDQ-39, Parkinson’s disease questionnaire; PDQ-C, Parkinson’s disease-Carer questionnaire; PKGtrademark, Parkinson’s KinetiGraph; UPDRS II, MDS-Unified Parkinson’s disease rating scale part II.
Resources and training to support self-management
Prior to enrolment, PwP received a comprehensive resource pack that included information about PD, service provision and support available for PwP and care partners, details of a single point of contact for guidance on symptom monitoring and management, lifestyle advice and a patient passport capturing key aspects of living with PD (Fig. 3). Following receipt of the resource pack, PwP were invited to attend a two-hour HBC group training session, initially delivered in-person by the PDNS and converted to an online video platform during the COVID-19 pandemic. The training outlined the reasons for HBC, what it hoped to achieve, how the service would work, the resources available and how best to use them. Specific information was provided regarding rapid deterioration and emergence or worsening of red flag symptoms (falls, hallucinations, delusions, impulse control disorder symptoms). Anonymized feedback on the delivery and content of these training sessions, and recipients’ understanding was collected from PwP and informed training session iteration.
Fig. 3
Home-Based Care resource pack. (a) The Home-Based Care Pathway pack; (b) Parkinson’s patient passport; (c) Service and local information; (d) A card deck to support self-reflection; (e) A self-management support and general information package. The pack included information about Parkinson’s service provision and support available for PwP and care partner; details of a single point of contact; information about Parkinson’s disease; information and guidance for symptom monitoring and management; lifestyle advice; a Parkinson’s patient passport to capture key aspects of Parkinson’s important to the PwP.

Remote monitoring
Baseline remote monitoring was undertaken following training attendance, and then regularly every six months, using a wrist worn sensor and a set of questionnaires which were sent out in hard copy and returned to our center by post.
The medical device used to provide data on motor symptoms of bradykinesia, dyskinesia, tremor, and immobility and monitor motor function in relation to levodopa doses, was the Parkinson’s Kinetigraph (PKGtrademark). The PKGtrademark device is a wrist-worn accelerometer developed by Global Kinetics (GK)) [9], with sufficient memory for 6–10 days of continuous recording, which has previously been used to identify unmet need and inform treatment decisions between clinic visits at our center [10]. Its inclusion in the HBC pathway represents one of several use cases for this type of technology currently in the NHS. The PKGtrademark was requested via a web-portal, delivered to patients’ homes, worn for six days and returned to GK in a pre-paid envelope. Reports generated by the company were sent electronically to the lead neurologist.
In addition, questionnaires validated for use in PwP were used to assess motor and non-motor symptoms, and quality of life for PwP and their care partner; these included the Parkinson’s Disease Questionnaire 39 (PDQ-39), Parkinson’s Disease Questionnaire 8 (PDQ-8) and Parkinson’s Disease Questionnaire –Carer (PDQ-C), the Movement Disorder Society’s Unified Parkinson’s Disease Rating Scale Part Two on motor experiences of daily living (MDS-UPDRS II)), the Non Motor Symptoms Questionnaire (NMSQ), the Parkinson’s Disease Sleep Scale 2 (PDSS2), and the Hospital Anxiety and Depression Scale (HADS). A bespoke questionnaire on medications and the main concerns of PwP and their care partners was also created (see Questionnaire 1, Supplementary Material).
Data from remote monitoring informed the creation of both a patient and healthcare professional (HCP) facing report by the neurologist (CC); both were shared with the patient and the care team. The patient-facing report, the template for which was co-designed with PwP, comprised personalized self-management guidance, including explanation of why management of reported symptoms was important, guidance on non-pharmacological interventions the patient could implement in the home environment, and signposting to other written or on-line resources. The HCP-facing report comprised the PKG report and its interpretation, as well as a summary of the questionnaire findings, highlighting actions required by members of the care team.
Triggered healthcare contacts
PwP, their families or care partners could trigger contact with a healthcare practitioner at any time either by using established channels, such as the community PDNS, or by the single point of contact process developed within the care pathway. Healthcare practitioners could also trigger a patient contact based on data received.
HBC evaluation
Feasibility
The feasibility of the HBC pathway was assessed using descriptive enrolment data and process measures. Due to the pragmatic nature of HBC enrolment within the service, data relating to non-participation were not systematically collected. Data for the first 100 patients who enrolled on the HBC pathway and underwent baseline and 6-month remote monitoring assessments were evaluated. Process measures included: time taken to enroll and train patients; time required to prepare the PKG reports, calculated as the number of days from the start of PKG recording to the production of the self-management guidance; the number of triggered contacts, both PDNS and consultant, and whether these were initiated by the patient or clinical team. Data relating to all triggered consultant reviews within the evaluation period were captured, along with the number of days from trigger to consultant review. Data relating to all PDNS contacts with the first 30 patients enrolled on the pathway for two periods of 12 weeks: May-July 2020 and August-November 2020 were also recorded. The feasibility of remote monitoring was assessed by evaluating engagement (number of patients who withdrew from the HBC pathway; number of patients who returned completed questionnaires and PKG assessment after 6 months of care) and data completeness (number of patients with 3 or more missing items on questionnaires completed at enrolment and after 6 months of care) in the first 100 patients to complete 6-month follow up.
Acceptability
Quantitative and qualitative approaches were undertaken to understand the acceptability of the HBC pathway among PwP, care partners and staff. PwP were invited to complete an anonymized service evaluation questionnaire after training and every six months thereafter comprising questions from the Parkinson’s UK National Audit Patient Reported Experience Survey (Questionnaire 3, Supplementary Material) as well as free-text response items. Descriptive data are presented from the first 100 PwP to complete 6-month follow up, at enrolment and after 6 months on HBC on overall rating of care, patient-centeredness of care and understanding of PD and self-management. Data from December 2019 to April 2021 were analyzed, corresponding to the timeframe in which the first 100 patients had received six months of care.
The qualitative evaluation (TN) involved semi-structured interviews with ten PwP and their care partners, purposively sampled to ensure representation of age, gender, and locality. These audio-recorded interviews were conducted either face-to-face at home or over the telephone and focused on the interviewee’s experience of the HBC pathway and its impact on their well-being and quality of care (see Supplementary Table 1). Approvals were obtained from the Health Research Authority (HRA) and Health and Care Research Wales (ref: 19/NW/0369). Interview transcripts and service evaluation questionnaire free-text comments (outlined above) were analyzed using an adapted Framework Analysis Approach (TN) [11]. Two further experienced qualitative researchers (DS, AAK) assessed the validity of the analysis.
Finally, all HBC service staff were invited to complete the Warwick-Edinburgh Mental Wellbeing Scale (WEMWBS) [12] at the commencement of the HBC pathway and approximately one year later.
Safety of care
The quality of care delivered in the HBC pathway was evaluated by retrospective audit of 20 patients’ notes against the 43 items of the 2019 Parkinson’s UK national service audit criteria. The Parkinson’s UK Audit tool uses evidence-based clinical guidelines as the basis for measuring the quality of care provided in the outpatient setting, with domains including management of motor and non-motor symptoms, education and multidisciplinary involvement. The first 10 patients enrolled on HBC for 12 months were automatically selected, then every second patient enrolled for at least six months was selected. Clinical data pertaining to symptom severity from the point of entry to the HBC pathway and after 6 months of care for the first 100 patients were also assessed. Assessments were excluded from analysis if questionnaires were not returned or if three or more questionnaire items were missing.
Findings
HBC pathway feasibility
Enrolment began in October 2019 but paused between March and May 2020 due to COVID-19 pandemic restrictions during which online training delivery was developed. Due to the pragmatic roll-out of the pathway within our service, we did not systematically collect data relating to reasons for non-participation. Training session adaptations in response to evaluation and feedback subsequently included delivery by a healthcare practitioner (JI), development of telephone training and self-paced training.
Data presented here correspond to a cohort of 108 PwPs, 100 of whom completed training, enrolled on the pathway and completed 6-month follow up (demographic data in Table 1). Two patients withdrew before 6 months on the pathway, one due to preference for face-to-face review, the other not stating a reason. A further four patients were withdrawn from the pathway by HCPs: three due to declining cognition that rendered them unsuitable for the pathway, and one due to terminal illness. Two patients deferred their 6-month assessment. From May to July 2020, one PDNS contact per week was triggered for every 5 patients on the pathway; this prompted a change in the patient-facing report to include much more explicit self-management advice. These changes were clearly effective as they led to one PDNS contact per week for every 11.1 patients over the subsequent 12-week evaluation period. Over the first 6 months on the pathway, 18 patients required consultant review, triggered by the patient (n = 9), care partner (n = 1), other HCP (n = 3) or the consultant (n = 5). The median time from trigger to consultant review was 7.5 working days (IQR = 10.5). On a standard care pathway over the same time period, with 6-monthly review, 200 consultant reviews would have been required for these 100 PwP. The median time to finalize the remote monitoring reports (patient- and HCP-facing), from the date of start of the PKG recording period to sign off, was 72 days (IQR = 71.5, n = 91) (this included the recording duration and time for return of questionnaires, data entry, data compilation, clinical interpretation, dictation, transcription, review and sign off); reports took about 20 min to dictate. Data completeness for almost all measures was > 97%, with > 90% questionnaire return rates, other than for the PDQ-39, for which there were > 2 missing items in 7% of 6-month assessments.
Table 1
Baseline demographics of the first 108 PwPs enrolled on the HBC pathway
| HBC patients (n = 108) | |
| Sex (F:M) | 34 : 74 |
| Age (y) | 71 (12.75) |
| Disease duration (y) | 5 (5) |
| *Index of multiple deprivation (IMD) | 6 (4) |
Data presented are median (interquartile range). *Index of multiple deprivation is the official measure of relative deprivation for small areas (or neighborhoods) in England, ranking every small area in deciles from 1 (most deprived) to 10 (least deprived), combining information from 7 different dimensions of deprivation [26].
Acceptability
Service evaluation questionnaires were received from 95 patients following enrolment on the HBC pathway, and 71 patients after 6 months on it. Most patients rated their care as “improving” or “staying the same –already good” at both enrolment (73%) and after 6 months on the HBC pathway (72%) (Supplementary Table 2), with the proportion of patients rating it as already good increasing from 29% to 46% at 6 months. After 6 months on the pathway, patients’ experience of feeling that they were listened to increased from 79% to 90% “always” and “mostly” (Supplementary Figure 1). In terms of understanding of PD and self-management, the most notable change after 6 months on HBC was patients’ self-reported ability to ask for help which increased from 68% to 79% “always” and “mostly” (Supplementary Figure 2).
Qualitative evaluation
Data from nine PwP and 10 care partners who participated in interviews and a focus group respectively, as well as free-text responses in the service evaluation questionnaire (Table 2) were thematically analyzed. PwP confirmed long delays in engaging with their clinical teams in standard care pathways.
Table 2
Demographics of participants in the qualitative study
| PwP | Care partners | |
| Age (y) | 69 (12.5) | 71.5 (10.5) |
| Sex (F:M) | 4 : 5 | 5 : 5 |
Data presented are median (interquartile range).
“I saw her [the neurologist] obviously when I was first diagnosed, then it was two years until I saw her again.” (Patient 9, baseline)
Interviews and written service evaluation feedback indicated that some PwP prefer in-person interactions with their clinical teams.
“I’m old fashioned enough to want more face-to-face contact with the doctors and the nurses, cos I feel just as much information can be swapped from patient to medic without the need of long questionnaires.” (patient 5, baseline)
“Since joining the homebased care pathway I have had no contact with anyone from the service. I preferred the situation as it was before when I was sent appointments even if they were often delayed” (service evaluation response, 6 months)
However, the experience of others on the HBC pathway was positive. Several PwPs elaborated on different aspects of their satisfaction with the pathway, which included timely responses to their clinical queries and needs, and a sense of feeling supported through more efficient communication with their clinical teams with whom they could now share new symptoms as they emerged and get timely input.
“Excellent care and team. No complaints. Fantastic support. The pathway is a good way to deploy resources to those most in need and to allow those who are able to manage their condition with appropriate support and information” (service evaluation response, 6 months)
“ . . . it’s only enhanced the care that I’m getting cos I’m having much more timely intervention, which is what I need” (Patient 4, 6 months)
This was echoed by a care partner “I feel that there’s people there that care” (Care partner 4, 6 months).
The overarching theme of self-empowerment emerged clearly through these narratives, as PwP shared both their improved understanding of their condition and their sense of enhanced agency with respect to managing their condition.
“It is not such an old person’s disease anymore, so I think there’s going to be more people that are able to sort of help themselves a bit more” (Patient 9, baseline)
“It empowers you if you like to dig in a bit more, and it’s just a different way of not kind of sitting back, it enables you to kind of self-evaluate almost where you are” (Patient 4, 6 months)
HBC may confer parallel benefits for care partners. Since the nature of PD symptoms is inherently unpredictable, frequent holistic assessment and the timely intervention it delivers can offer reassurance.
“It’s quite empowering as well cos it means that you’re managing the situation rather than waiting for them to come to you. You’re proactively saying something’s not quite right here, how are we going to deal with it, whereas before you limped along” (Care partner 4, 6 months)
Feedback on the main structural elements of the pathway focused primarily on questionnaires and the PKG. Care partners involved in the completion of these questionnaires commented on their acceptability and thoroughness, while one patient explained that the experience itself can be distressing due to the confronting or intrusive nature of some of the questionnaire items. The PKG watch itself was found by some to be cumbersome, while others viewed it as a valuable and interesting source of objective data on their condition. Comments on the HBC resource pack were overwhelmingly positive and highlighted the value of a succinct and curated body of information available to patients as aids to self-management.
“If it’s something unknown I tend to go to the pack first . . . it just covers everything to do with Parkinson’s, the various symptoms and causes, it’s just a great reference source” (Patient 2, 6 months)
Staff wellbeing
6 of the 13 HBC healthcare staff completed WEMWBS questionnaires at baseline (October 2019) and follow-up (December 2020). Mental wellbeing scores improved by 9.8% from baseline (mean = 53.4; SD 7.62) to follow-up (mean = 57.67; SD 5.61).
Safety of care
Audit data
The retrospective audit showed the HBC service was compliant with 93% of the Parkinson’s UK national audit criteria compared with the 77% national average for neurology services in 2019.
Clinical data
Paired data for the first 100 patients who enrolled, completed training and returned all assessments at baseline and after 6 months on the HBC pathway are presented in Table 3. Although no formal statistical comparison is feasible as the study was not powered to detect differences, these descriptive data show no deterioration in any of the remote assessment tools employed.
Table 3
Clinical data for the first 100 PwPs to enroll and complete the 6-month assessment on the Home-based care pathway
| Remote assessment tool | Baseline | 6-months | ||
| n | Median (IQR) | n | Median (IQR) | |
| Parkinson’s KinetiGraph (PKG) | ||||
| Bradykinesia Score (BKS) | 108 | 28.9 (23.4–33.9) | 100 | 29 (23–33.8) |
| Dyskinesia Score (DKS) | 108 | 1.0 (04–2.5) | 100 | 1.2 (0.5–2.7) |
| Percent Time Inert (PTI) | 108 | 6.9 (6–9.5) | 100 | 7.7 (3.8–11.4) |
| Percent Time with Tremor (PTT) | 108 | 3.6 (0.6–11.8) | 100 | 1.9 (0.5–9.1) |
| Non-Motor Symptoms Questionnaire (NMSQ) | 102 | 10 (5–13) | 96 | 8 (5–13) |
| Parkinson’s Disease Sleep Scale (PDSS-2) | 97 | 14 (9–23) | 99 | 14 (8–21) |
| Hospital Anxiety and Depression Scale-Anxiety (HADS-A) | 100 | 5 (2–8.5) | 98 | 5 (2–8) |
| Hospital Anxiety and Depression Scale-Depression (HADS-D) | 100 | 6 (3–8) | 98 | 5 (2.3–7) |
| Unified Parkinson’s Disease Rating Scale (UPDRS II) | 103 | 12 (5.5–19) | 93 | 12 (6–20.2) |
| Parkinson’s Disease Questionnaire (PDQ-8) | 98 | 18.8 (7–31.2) | 100 | 15.6 (6.3–32.8) |
| Parkinson’s Disease Questionnaire -Carer (PDQ-C) | 76 | 18.5 (5.6–43.7) | 74 | 18.6 (4.8–40.8) |
Data presented are median (interquartile range). At baseline, one PDSS-2 questionnaire and 2 PDQ-C datasets with more than 2 missing items were excluded. At 6 months, 3 NMSQ, 1 UPRS II and 2 PDQ-C datasets with more than 2 missing items were excluded.
DISCUSSION
The Home Based Care pathway represents a healthcare improvement initiative, co-developed with PwP and their care partners to help us, as their care team, deliver on their priorities in the face of increasing health service pressures. The aims of the pathway are to deliver excellent patient care while simultaneously supporting greater self-management through increasing knowledge and understanding of PD, involving patients in care decisions, and providing personalized care available at time of need. HBC captures the benefits of co-developing services with patients and their care partners which focus on patients’ priorities and encourage self-management of chronic conditions. Key to its implementation is the use of a digital health technology that provides objective, continuous motor data for each patient. To our knowledge, this is the first initiative of its kind for PD within the NHS to be co-designed by PwP and offer digitally enabled, personalized, responsive care while supporting self-management.
Data are presented on this early evaluation of the HBC pathway which is currently in its third year of implementation in the NHS. This supported self-management pathway with digitally-enabled remote monitoring and safety-netting is feasible to deliver within the NHS, acceptable to patients, care partners and the staff delivering it, and meets national quality standards. Acceptability was assessed in several ways and deemed to be good. PwP reported an increase in being able to ask for help when they needed it. They reported they felt involved in their care and listened to. The patient narratives contained strong themes of autonomy and empowerment, with benefits for both patients and care partners. PwP remained stable from the perspective of their PD symptoms. An improvement in staff wellbeing was found following implementation of the pathway, despite challenges in the healthcare sector related to the COVID-19 pandemic.
Practically, when requested, a triggered personalized review was offered within 7.5 working days by the patient’s consultant who knew them and their condition. Despite this, some patients preferred standard routine, albeit delayed, outpatient contacts; in one case, this was the reason given for withdrawal from the pathway. It is important to note that HBC is, by design, not suitable for patients with advanced frailty, cognitive impairment or significant neuropsychiatric problems, as it requires engagement with self-management activities. For some patients, completing the questionnaires was burdensome or upsetting, and some also reported that the PKG watch was uncomfortable to wear. Nonetheless, if scalable and successful, this pathway may be an important means of delivering the NHS Long Term plan: it holds promise for improving self-efficacy and self-management, enhances care partner support, delivers care closer to home and increases access to specialist clinical input, facilitating timely review and intervention.
The need for alternative care delivery models in this space is well described, as studies have shown that standard PD care is driven by the clinician, PwP lack information on their condition and self-management, and feel insufficiently involved in treatment decisions [13–15]. However, evidence to inform selection or implementation of alternative care models is scarce. Nevertheless, a recent meta-analysis of integrated care models for PwP recognized two major types of studies, on care coordination and on delivery of multidisciplinary rehabilitation in various settings, demonstrating a modest but significant improvement in PwP quality of life with integrated care [16]. Bloem et al. define patient-centered integrated care as health services that are managed, discussed, and delivered so that patients can make health-related and disease-related choices according to their needs throughout their life course [17]. Our care model comprises the core elements suggested for inclusion in such an integrated and personalized care management model for PwP [18], by using remote monitoring to support care delivered close to home, participatory healthcare and patient empowerment achieved through patient and care partner triggered contacts and extensive training on self-management resulting in timely and proactive delivery of care [17]. Similar to the iCARE-PD approach [10, 11], multi-stakeholder participatory co-design was employed for the development of this technology-enabled care pathway [4], in which PwP and care partners were equal contributing partners; this approach may have been key to its successful implementation [19].
In contrast to standard care models, the HBC pathway encourages PwP to become active members of the care team, using the training and HBC resource pack to understand and monitor their symptoms themselves. After 6 months, PwP felt more involved in their care, and importantly, more of them felt that they knew when to report new or exacerbated symptoms. HBC also featured a single first point of contact which has been identified as a key priority for care for PwP [19]. Currently, standard care requires PwP to travel to clinics whereas HBC remote monitoring means proactive, triggered care can be provided in response to the symptoms PwP experience in their home environment. This may also reduce bias associated with the overt monitoring of patients [20]. Use of remote monitoring technologies such as the PKGtrademark can enhance patient care through the provision of objective continuous data and remove the challenges associated with the subjective reporting of symptoms or one-shot clinical encounters [21].
Healthcare resource implications
With HBC there was a dramatic reduction in consultant clinic requirements with a short waiting time from trigger to consultant clinic (median 7.5 days), which could lead to substantial cost savings and reduce prolonged waiting lists for patients. The HBC pathway requires robust processes for the ordering, distribution and collection of remote monitoring data and personnel to deliver training and distribute educational resources, as well as clinician time to review and interpret symptom questionnaires and PKG reports.
Limitations of this evaluation
This was a real-world evaluation following the implementation of a new care pathway as a service improvement initiative. Data gathered here suffer from the familiar limitations of missing data which may have influenced our findings. For instance, it is possible that PwP may have been more likely to complete the service evaluation questionnaire if they had felt more connected to the HBC team. Blinding was not feasible so performance bias may also have influenced our findings. Importantly, there are limits to the generalizability of our findings as PwP and care partners involved in the co-design and end-users of the service were all White British. HBC was developed and rolled out within a largely rural patient population who may have greater difficulty in accessing specialist secondary care compared with other patient populations [21] and therefore may be more receptive. COVID-19 restrictions may also have disproportionately influenced PwP attitudes to outpatient care and particularly to in-person clinic reviews.
Future priorities
Given the promise of the HBC pathway, the next step is to streamline pathway processes to reduce the administrative burden for patients and healthcare teams, thereby facilitating its delivery at scale. To address this, we have been commissioned by NHS Digital Transformation to investigate how digital products and services can support delivery of the pathway [22]. To realize the full potential of HBC, it is likely that additional components to support self-care will be required as the intervention evolves, for instance by stratifying patients according to amount of support required for self-management and delivering coaching and enhanced support for less activated patients. It is envisaged that incorporating agile feedback loops into the pathway will enable continuous rapid improvements to a pathway pragmatically tailored to and responsive to patient need. The current evaluation captured data from PwP who had received care over a 6-month period, but long-term outcomes of the HBC pathway should be considered and evaluated. Specifically, it is important to understand whether adherence to pathway processes remains strong and whether the changes in PwP understanding of PD and their symptoms is sustained. Previous trials of interventions designed to educate PwP and enhance self-management have been limited in their assessment of long-term outcomes or failed to demonstrate sustained behavior change [23–25].
To enable formal health technology assessment of its clinical and cost effectiveness, it is important to evaluate the pathway directly against current standard care and generate evidence for a robust health economic evaluation, that could include impacts on hospital admission rates, falls, hip fractures and prolonged independence, as well as impact on outpatient management of PwP with more healthcare needs. Evidence of impacts on clinical indices and health-related quality of life in both PwP and care partners should be captured. Future research priorities should also address specifically whether the HBC model of supported self-management is suitable for and could confer benefits to other under-served groups including people from diverse minority ethnic and socioeconomic backgrounds.
Conclusion
We have co-designed with PwP and care partners a novel care pathway, which empowers patients in their self-care, and delivers digitally supported remote monitoring and timely reviews when needed. We have demonstrated this pathway is feasible and acceptable to deliver within the NHS.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The service improvement initiative and evaluation was co-funded by the Health Foundation, UK (ref: 1119597) and a Parkinson’s UK service improvement grant (ref: SIG2018).
CONFLICT OF INTEREST
CC has received honoraria for consultancy services and/or service grants from AbbVie, Bial, Britannia, GKC, Kyowa Kirin, Lundbeck and Medscape. She has received research grants from Parkinson’s UK, Cure Parkinson’s, National Institute for Health and Care Research, and the Edmond J Safra Foundation. CC is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review. MHVV is currently employed by AstraZeneca. At the time of undertaking the qualitative research TN was a Non-Executive Director with Cornwall Partnership NHS Foundation Trust. No other authors have conflicts to declare.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230205.
REFERENCES
[1] | National Institute for Health and Care Excellence, Parkinson’s disease in adults. NICE guideline [NG71], https://www.nice.org.uk/guidance/ng71, Accessed 10 March 2023.. |
[2] | Parkinson’s UK, Parkinson’s UK Audit –Transforming Care, https://www.parkinsons.org.uk/media/34696. |
[3] | Parkinson’s UK (2022), Written evidence submitted by Parkinson’s UK (RTR0067). https://committees.parliament.uk/writtenevidence/42721/pdf/. |
[4] | Weir S , Samnaliev M , Kuo TC , Tierney TS , Walleser Autiero S , Taylor RS , Schrag A ((2018) ) Short- and long-term cost and utilization of health care resources in Parkinson’s disease in the UK, Mov Disord 33: , 974–981. |
[5] | NHS England, The NHS Long Term Plan., https://www.longtermplan.nhs.uk/, . |
[6] | NHS England, Parkinson’s patients benefit from revolutionary watch on the NHS to manage care at home, https://www.england.nhs.uk/2022/04/parkinsons-patientsbenefit-from-revolutionary-watch-on-the-nhs-to-managecare-at-home/, April 2022. |
[7] | Langley J , Partridge R , Ankeny U , Wheeler G , Carroll C (2022) Co-designing resources for knowledge-based selfreflection for people living with Parkinson’s disease to better enable independent living. In Developments in Design Research and Practice, Duarte E, Rosa C, eds. Springer, pp. 237-251. |
[8] | Ogrinc G , Davies L , Goodman D , Batalden P , Davidoff F , Stevens D ((2016) ) SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines From a Detailed Consensus Process. J Nurs Care Qual 31: , 1–8. |
[9] | Griffiths RI , Kotschet K , Arfon S , Xu ZM , Johnson W , Drago J , Evans A , Kempster P , Raghav S , Horne MK ((2012) ) Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J Parkinsons Dis 2: , 47–55. |
[10] | Dominey T , Kehagia AA , Gorst T , Pearson E , Murphy F , King E , Carroll C ((2020) ) Introducing the Parkinson’s KinetiGraph into routine parkinson’s disease care: A 3-year single centre experience. J Parkinsons Dis 10: , 1827–1832. |
[11] | Pope C , Ziebland S , Mays N ((2000) ) Qualitative research in health care. Analysing qualitative data. BMJ 320: , 114–116. |
[12] | Tennant R , Hiller L , Fishwick R , Platt S , Joseph S , Weich S , Parkinson J , Secker J , Stewart-Brown S ((2007) ) The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): Development and UK validation. Health Qual Life Outcomes 5: , 63. |
[13] | Hayes C ((2002) ) Identifying important issues for people with Parkinson’s disease. Br J Nurs 11: , 91–97. |
[14] | Bloem BR , Stocchi F ((2015) ) Move for Change Part III: A European survey evaluating the impact of the EPDA Charter for People with Parkinson’s Disease, Eur J Neurol 22: , 133–141, e138-139. |
[15] | van der Eijk M , Faber MJ , Post B , Okun MS , Schmidt P , Munneke M , Bloem BR ((2015) ) Capturing patients’ experiences to change Parkinson’s disease care delivery: A multicenter study. J Neurol 262: , 2528–2538. |
[16] | Rajan R , Brennan L , Bloem BR , Dahodwala N , Gardner J , Goldman JG , Grimes DA , Iansek R , Kovacs N , McGinley J , Parashos SA , Piemonte MEP , Eggers C ((2020) ) Integrated care in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 35: , 1509–1531. |
[17] | Bloem BR , Henderson EJ , Dorsey ER , Okun MS , Okubadejo N , Chan P , Andrejack J , Darweesh SKL , Munneke M ((2020) ) Integrated and patient-centred management of Parkinson’s disease: A network model for reshaping chronic neurological care. Lancet Neurol 19: , 623–634. |
[18] | van Halteren AD , Munneke M , Smit E , Thomas S , Bloem BR , Darweesh SKL ((2020) ) Personalized care management for persons with Parkinson’s disease, J Parkinsons Dis 10: , S11–S20. |
[19] | Vlaanderen FP , Rompen L , Munneke M , Stoffer M , Bloem BR , Faber MJ ((2019) ) The voice of the Parkinson customer. J Parkinsons Dis 9: , 197–201. |
[20] | Dorsey ER , Vlaanderen FP , Engelen LJ , Kieburtz K , Zhu W , Biglan KM , Faber MJ , Bloem BR ((2016) ) Moving Parkinson care to the home. Mov Disord 31: , 1258–1262. |
[21] | Krause E , Randhawa J , Mehanna R ((2021) ) Comparing subjective and objective response to medications in Parkinson’s disease patients using the Personal KinetiGraph. Parkinsonism Relat Disord 87: , 105–110. |
[22] | NHS England, Digital Health Partnership Awards, NHS England Tranformation Directorate, https://transform.england.nhs.uk/key-tools-and-info/digital-health-partnership-award/, Accessed November 22, 2023. |
[23] | A’Campo LE , Spliethoff-Kamminga NG , Roos RA ((2011) ) An evaluation of the patient education programme for Parkinson’s disease in clinical practice. Int J Clin Pract 65: , 1173–1179. |
[24] | Kessler D , Liddy C ((2017) ) Self-management support programs for persons with Parkinson’s disease: An integrative review. Patient Educ Couns 100: , 1787–1795. |
[25] | Hellqvist C , Bertero C , Dizdar N , Sund-Levander M , Hagell P ((2020) ) Self-management education for persons with Parkinson’s disease and their care partners: A quasi-experimental case-control study in clinical practice. Parkinsons Dis 2020: , 6920943. |
[26] | McLennan D , Noble S , Noble M , Plunkett E , Wright G , Gutacker N The English indices of deprivation 2019. Technical report. Ministry of Housing Communities and Local Government. https://assets.publishing.service. gov.uk/government/uploads/system/uploads/attachment_data/file/833951/IoD2019_Technical_Report.pdf. |




