Non-Pharmacological Treatment of Autonomic Dysfunction in Parkinson’s Disease and Other Synucleinopathies
Abstract
Symptoms of autonomic dysfunction are prevalent and can be very debilitating, reducing the quality of life in patients with Parkinson’s disease (PD) and other synucleinopathies such as dementia with Lewy bodies and multiple system atrophy. Non-pharmacological therapies are key to effective management and are frequently used alone in patients with mild autonomic symptoms, or in combination with pharmacological therapies in patients with moderate and severe symptoms. This article focuses on non-pharmacological approaches. Our objective was to review the non-drug and non-surgical approaches to treating autonomic symptoms in patients with PD and other synucleinopathies, focusing on cardiovascular, gastrointestinal, and genitourinary autonomic dysfunction. Evidence supporting the effectiveness of non-pharmacological treatment for the management of neurogenic orthostatic hypotension, supine hypertension, constipation, and bladder and sexual dysfunction is available. High-quality prospective trials are scarce, yet some non-pharmacological interventions (e.g., physical counter maneuvers) can be evaluated relatively quickly on an individual basis and often seem effective. The emerging variety of clinical presentations advocates for a stepwise, individualized, and non-pharmacological approach for the management of autonomic symptoms. Often, the first step is to reduce or discontinue drugs that cause or aggravate autonomic symptoms followed by lifestyle measures. While non-pharmacological and non-surgical treatments are available and, in many cases, effective to improve symptoms of autonomic dysfunction in PD and other synucleinopathies, they are often overlooked. Large randomized trials testing and comparing non-pharmacological approaches are warranted.
INTRODUCTION
Autonomic dysfunction in patients with Parkinson’s disease (PD) and other synucleinopathies such as dementia with Lewy bodies (DLB) and multiple system atrophy (MSA) is highly prevalent, and occurs at all stages, frequently emerging as the first manifestation of the disease during the prodromal stage, years or decades before any motor or cognitive symptoms are apparent. Indeed, autonomic manifestations are now part of the diagnostic criteria for prodromal PD and MSA [1, 2].
Symptoms of autonomic dysfunction are common and can be very debilitating and reduce the quality of life in affected patients. Dysfunction of the autonomic nervous system afflicts most patients with synucleinopathies affecting quality of life and mortality. For instance, slow gastric emptying and constipation can lead to impaired drug pharmacodynamics causing a worsening in motor function; orthostatic hypotension can cause syncope, falls, and fractures; and urinary dysfunction can increase the risk of sepsis and death.
Indeed, autonomic dysfunction is associated with a worse prognosis. In PD, orthostatic hypotension (OH) is part of a “malignant” phenotype characterized by faster progression [3, 4]; and in MSA, early and severe autonomic dysfunction predicts shorter survival [5, 6].
When identified, autonomic dysfunction can be treated with non-pharmacological and pharmacological therapies. Pharmacological approaches have been reviewed elsewhere [7]. Except for midodrine and droxidopa for the treatment of neurogenic orthostatic hypotension (nOH), lubiprostone for constipation, and sildenafil for erectile dysfunction, the level of evidence of pharmacological approaches for the treatment of autonomic dysfunction in patients with PD is very limited [7, 8].
While pharmacological therapies are often the go-to for treatment, non-pharmacological therapies are just as crucial in effectively managing symptoms and should be considered the first line of treatment. Non-pharmacological approaches can be used alone for patients with mild symptoms or in conjunction with pharmacological therapies for those with moderate to severe symptoms. Surprisingly, we have yet to come across an article specifically devoted to non-pharmacological approaches for autonomic dysfunction.
We here review the non-drug and non-surgical therapies for autonomic dysfunction in patients with PD and other synucleinopathies focusing on cardiovascular, gastrointestinal, and genitourinary autonomic symptoms due to the negative impact of these on the quality of life of affected patients and the availability of non-pharmacological therapies.
CARDIOVASCULAR AUTONOMIC DYSFUNCTION
Cardiovascular autonomic dysfunction occurs in most patients with synucleinopathies however this is symptomatic in only a minority of them [9]. The most disabling manifestation of cardiovascular autonomic dysfunction is OH, defined as a sustained fall in blood pressure (BP) of at least 20 mmHg systolic or 10 mmHg diastolic within 3 minutes of standing from the supine position [10, 11]. In patients with supine hypertension, the fall in systolic BP should be at least 30 mmHg [12]. When OH is caused by failure of compensatory baroreflex mechanisms and lack of appropriate norepinephrine-mediated vasoconstriction when standing up, it is referred to as neurogenic OH (nOH) [13]. nOH in PD, DLB, and patients with pure autonomic failure is due to postganglionic noradrenergic denervation whereas, in patients with MSA, nOH is related to the degeneration of central neurons involved in baroreflex control.
Approximately 30–50% of patients with PD have nOH; however, in most cases, it is asymptomatic, with only 15–20% of patients with PD having symptomatic nOH [14, 15]. The prevalence of OH in PD increases with age and disease duration, although it can sometimes be the presenting feature in the earlier pre-motor stages. nOH is highly prevalent in DLB (50–60%) [16] and MSA (70–80%) [17, 18], appearing typically in the earliest disease stages. nOH is a required feature for the diagnosis of pure autonomic failure, a synucleinopathy characterized by generalized autonomic dysfunction without motor or cognitive impairment [19].
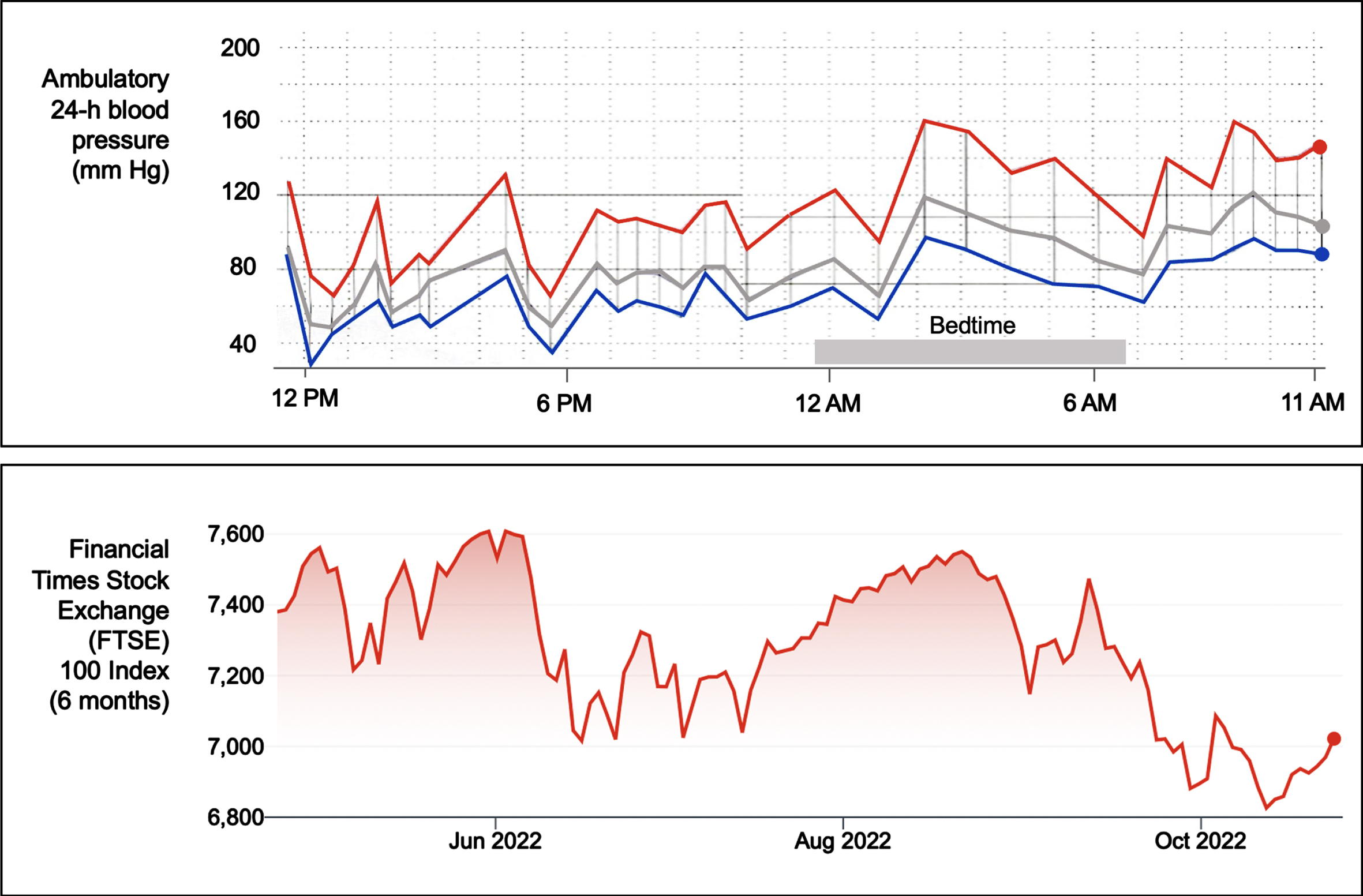
As mentioned, nOH can be asymptomatic or symptomatic. Lightheadedness, blurry vision, and feeling about to faint are the most frequently recognized symptoms of nOH; less frequently identified symptoms include tiredness, cognitive slowness, dyspnea, neck and shoulder discomfort (“coat hanger pain”), and chest oppression or pain. When severe, nOH can result in acute morbidity and hospitalizations related to syncope and falls [20]. To complicate matters, many patients with nOH also have the converse problem: hypertension in the supine position (SH) (i.e., systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or both) [12]. This makes their BP extremely labile, resembling a volatile stock market chart (Fig. 1) [21]. While SH is usually asymptomatic, it can, in the long-term, result in target organ damage (e.g., renal and heart failure, cerebral white matter hyperintensities, and, possibly, dementia), not unlike other forms of hypertension [6, 22].
Fig. 1
Blood pressure variability and stock market volatility. The upper panel shows an ambulatory 24-hour blood pressure (BP) monitor recording of a patient with Parkinson’s disease with neurogenic orthostatic hypotension and supine hypertension. The monitor takes a BP measurement every 30 minutes during the daytime and every 60 minutes during nighttime. The red line denotes systolic BP, the blue line diastolic BP, and the grey line mean BP. The tracing depicts high BP variability with low (as low as 65/32 mmHg at around 6 PM) and high blood pressure readings (as high as 160/100 mmHg at around 2 AM, during bedtime). Note that for several hours during bedtime (i.e., supine position), multiple BP readings were in the hypertensive range. The lower panel shows a 6-month tracing (May-October 2022) of the London Financial Times Stock Exchange (FTSE) 100 Index, depicting high variability.
Non-pharmacological management of nOH
Non-pharmacological interventions are the cornerstone of nOH management. It is important to realize that pharmacological treatments fail to normalize baroreflex function. Instead, pharmacological interventions augment BP independent of posture, frequently inducing or aggravating SH. Lifestyle measures, therefore, constitute the first line of treatment as they can improve orthostatic intolerance without inducing SH.
The goal of treating nOH in patients with PD and other synucleinopathies is not to normalize BP, but to reduce symptom burden, improve quality of life, and reduce morbidity and mortality associated with nOH. Consensus guidelines for the treatment of nOH are available [23]. Pharmacological therapies should only be considered if patient education and non-pharmacological measures fail to reduce symptom burden. If nOH is asymptomatic, treatment may not be required or may be limited to patient education and correction of aggravating factors.
Non-pharmacological interventions should be tailored to the individual. It is typical to implement multiple non-pharmacological measures simultaneously. There is, however, a lack of high-quality clinical trials that test non-pharmacological interventions for nOH. Furthermore, some clinical trials that tested non-pharmacological interventions did not specifically include patients with nOH caused by PD or other movement disorders, but rather patients with age-related OH or patients with vasovagal syncope [8, 24–27]. Despite this, many of these interventions are helpful in patients with nOH and will be discussed here. Since the impact of non-pharmacological interventions on BP and symptoms can be observed relatively quickly, they can be evaluated with individual patients as their control, similar to an n-of-1 trial design. The order of the non-pharmacological interventions described here is determined by the level of the available evidence, ease of implementation, and overall risk-benefit ratio.
Each non-pharmacological intervention operates at different time points: some measures like physical countermaneuvers can reduce symptoms of hypotension, whereas others may be used to prevent symptoms associated with specific events that can be predicted in advance (Table 1).
Patient education and lifestyle adjustments
It is of utmost importance for healthcare providers to take the necessary time and effort to educate patients and caregivers on the vital characteristics of nOH. Healthcare providers must also explain how BP can vary based on factors such as position, meals, hydration, weather, and other variables. Educating patients on warning signs and how to prevent triggers (e.g., standing for long periods, standing still after exercise, straining, eating large, carbohydrate-rich meals, drinking alcohol, and being in warm environments such as hot showers and saunas) is crucial.
Table 1
Non-pharmacological interventions used to treat or prevent autonomic symptoms in patients with Parkinson disease and other synucleinopathies
| Orthostatic hypotension |
| General measures to ameliorate symptoms of orthostatic hypotension |
| •Patient education and lifestyle adjustments [28] |
| •Reducing or stopping blood pressure-lowering drugs [30] |
| •Increasing fluid and salt intake [37] |
| •Eating frequent small meals and decreasing alcohol intake [36] |
| •Promoting physical activity [30] |
| •Compression garments [24, 26, 27, 45, 47] |
| •Head-up sleeping [26, 49] |
| Interventions to prevent symptoms of orthostatic hypotension in the near future |
| •Bolus water drinking [24, 34] |
| Interventions to acutely combat symptoms of orthostatic hypotension |
| •Physical countermaneuvers [28, 31] |
| Supine hypertension |
| General measures to ameliorate supine hypertension |
| •Avoid the supine position during the day [35] |
| •Head-up sleeping [26, 49] |
| •Eating a high-calorie snack or alcoholic drink before bedtime [9, 30] |
| •Take the last dose of anti-hypotensive agents at least 3-4 h before sleep time [35] |
| Interventions to acutely reduce supine hypertension |
| •Application of a heating pad (40–42°C) in the abdomen [52] |
| •Nighttime use of continuous positive airway pressure (8–12 cm H2O) [53] |
| Gastrointestinal symptoms |
| General measures to alleviate gastroparesis |
| •Low-fat diet, frequent meals [57] |
| General measures to alleviate constipation |
| •Eating at regular times [75] |
| •Increase the intake of high-fiber fruits and vegetables, wholegrain bread and cereals, extra virgin olive oil, and remain |
| well-hydrated [75] |
| •Probiotic intake (e.g., fermented milk products like kefir) [58–61] |
| Urinary symptoms |
| General measures to alleviate symptoms of urinary dysfunction |
| •Self-monitoring with a diary [66, 67] |
| •Daily pelvic floor muscle exercises [66, 67] |
| •Squeezing the pelvic floor muscles immediately before events that trigger leakage (e.g., sneezing, coughing, bending) [66, 67] |
| •Minimize caffeine intake [66, 67] |
| •Treat constipation [66, 67] |
| •Head-up sleeping (can reduce nocturia) [49] |
| Sexual dysfunction |
| General measures to alleviate erectile dysfunction |
| •Reduce alcohol and tobacco use [72] |
| •Reduce or stop medications that can cause erectile dysfunction [72] |
| Acute interventions to treat erectile dysfunction |
| •Use of vacuum pump device [73] |
| General measures to manage female sexual dysfunction |
| •Vaginal lubrication [74] |
| •Psychotherapy [74] |
Encouraging simple lifestyle changes, such as sitting instead of standing, or making slower posture changes, can significantly alleviate orthostatic stress and prevent initial drops in BP when standing up [28–30]. For some patients, a small, portable, lightweight folding chair can be helpful to enable them to sit, and thereby ameliorate or end an episode of symptomatic nOH [31]. Straining during bowel movements can cause hypotensive syncope; if this is the case, constipation must be treated.
Teaching how to properly perform BP measurements at home, in the supine and standing positions, is also important. At the same time, the patient and their caregivers must understand that the management of nOH is geared toward improving symptoms and quality of life, rather than achieving a perfectly normal BP. To avoid SH, patients should avoid the supine position during the day and sleep with the head of the bed raised at night. Modifying the home environment, such as putting a chair in the bathroom to shower in a sitting position, can also help. Print (brochures, leaflets) and online materials (videos or instructional websites like Syncopedia) summarizing these aspects can be helpful for patients and caregivers.
Concomitant medication review
In patients with hypotension, all medications should be reviewed and reduced or discontinued if appropriate. Numerous classes of medication can exacerbate nOH by diminishing the compensatory cardiovascular responses needed to maintain BP in the upright posture. The unwanted cardiovascular effects are not restricted to BP-lowering drugs but also include medications to manage urinary symptoms (e.g., tamsulosin), erectile dysfunction (e.g., sildenafil), pain (e.g., opioids, benzodiazepines), and psychiatric symptoms (e.g., tricyclic antidepressants). L-dopa and dopaminergic agonists can also aggravate nOH by mechanisms that are not well understood (Table 2).
Physical counter maneuvers
Easy-to-apply physical counter maneuvers (e.g., leg-crossing, squatting, buttock-clenching) effectively ameliorate orthostatic intolerance [28, 31]. Respiratory maneuvers (e.g., pursed lips breathing) may also reduce symptoms [33]. These maneuvers can abort an episode of symptomatic nOH if applied immediately upon standing and may also prevent symptoms when applied preemptively to increase BP when standing. Applying these counter-maneuvers can be challenging in some patients with PD and other parkinsonism due to motor difficulties. A session with the patient evaluating the efficacy of the different maneuvers may therefore be pertinent to offer tailored approaches. Eventually, some patients learn to anticipate situations in which BP drops are likely to occur and apply these measures intuitively.
Bolus water drinking
Drinking 500 mL of cold water quickly (within 2-3 minutes) can cause a temporary increase in BP within 5 to 10 minutes, which can last for 30–45 minutes. This effect is triggered by a spinal reflex known as the osmopressor reflex, activated by a sudden drop in osmolality in the portal vein, which stimulates residual sympathetic efferent activity. Therefore, bolus water drinking may be recommended in advance of events triggering hypotension (e.g., right before getting up from bed in the morning) [27, 34].
Eating frequent small meals and decreasing alcohol intake
Postprandial hypotension refers to a drop in systolic BP of at least 20 mmHg within 30 minutes after a meal. For patients with nOH and postprandial hypotension, it is recommended to avoid meals rich in high-glycemic index carbohydrates and sugary drinks (e.g., soda, bottled juice). Low glycemic index carbohydrates are preferable, and frequent smaller meals should be implemented. Alcohol should be avoided during the daytime as it is a vasodilator [35, 36]. On the other hand, patients with coexisting supine hypertension can reduce their high BP by consuming a sweet snack (∼500 kcal) or having a glass of wine before going to bed [30].
Increasing salt and water intake
Salt supplementation promotes plasma volume expansion, therefore improving orthostatic tolerance. Patients with nOH are instructed to increase dietary salt intake to about 10 g sodium chloride per day, provided that there are no contraindications. Increases in salt intake can be achieved by generously adding salt to meals or taking salt tablets (0.5–1 g/day, which can cause abdominal discomfort). Water intake should be increased to 2–3 L/day [37]. The relative improvement in symptoms of nOH with this approach should be balanced against the potential to exacerbate supine hypertension.
Physical activity
Patients with nOH may experience symptoms that make it difficult to stand or perform physical activities. Patients with PD, even in the earliest stages of the disease, may develop chronotropic insufficiency during submaximal exercise possibly due to cardiac sympathetic denervation [38], which can, in turn, aggravate exercise intolerance [39–41]. Moreover, exercise can worsen hypotension in patients with PD [42]. This may all lead to physical immobility and muscle atrophy, which will worsen the severity of nOH in a vicious cycle [43]. To prevent this, patients need to continue exercising as much as possible. This can be facilitated by exercising in a recumbent or seated position, such as using a stationary bicycle or rowing machine. However, patients should be cautious of post-exercise hypotension, which may elicit syncope. For example, while cycling, patients should try to avoid freewheeling as it could provoke symptoms. Swimming is a good exercise option as it does not usually trigger symptoms, but patients should be aware that exiting the pool can worsen symptoms, particularly in warm temperatures. Patients with mild nOH can benefit from hiking with a walking stick or pole to mitigate orthostatic stress and tolerate longer trails. A small randomized trial in 30 patients with PD, showed that a 12-week resistance training program resulted in improved cardiovascular autonomic responses [44].
Table 2
Drugs that cause or aggravate orthostatic hypotension
| Drug group | Mechanism of hypotension and comments |
| Diuretics | Extracellular fluid volume depletion. |
| Loop diuretics (e.g., furosemide, torasemide) or thiazides | |
| Adrenergic blockers | α1-adrenergic blockers produce vasodilation via direct effect in vascular smooth muscle. |
| α1-adrenergic blockers (e.g., alfuzosin, terazosin) | β-adrenergic blockers reduce cardiac output and renin release. May also reduce vascular peripheral resistance. |
| β-adrenergic blockers (e.g., propranolol) | Lipophilic β-adrenergic blockers cross the blood-brain barrier and may act via central mechanisms. |
| α2-adrenergic agonists (e.g., tizanidine, clonidine) | Vasodilation via central inhibition of sympathetic efferent activity. |
| Nitric oxide-mediated vasodilators | Vasodilation via direct effect in vascular smooth muscle. |
| Nitroglycerin | |
| Hydralazine | |
| Phosphodiesterase-5-inhibitors (e.g., sildenafil) | |
| Renin-angiotensin-aldosterone system blockers (e.g., captopril, lisinopril) | Vasodilation via central and peripheral inhibition of sympathetic efferent activity. |
| Calcium-channel blockers (e.g., verapamil, diltiazem) | Reduction of cardiac output, vasodilation via direct effect in vascular smooth muscle. |
| Dopamine antagonists Phenothiazines (e.g., chlorpromazine) | Vasodilation via central inhibition of sympathetic efferent activity. |
| Atypical antipsychotics (e.g., olanzapine, risperidone, quetiapine) | |
| Tricyclic antidepressants (e.g., trazodone, amytriptiline) | Vasodilation via central and peripheral inhibition of sympathetic efferent activity through stimulation of α2-adrenergic receptors. |
| Selective serotonin receptor reuptake inhibitors (e.g., paroxetine) | Unknown mechanism, possibly via central and peripheral inhibition of sympathetic efferent activity through stimulation of α2-adrenergic receptors. |
| Antiparkinsonian agents | Unknown mechanism. |
| Levodopa | |
| Dopaminergic agonists (e.g., pramipexole, ropirinole, apomorphine) | |
| Monoamine oxidase inhibitors (e.g., selegiline) | |
| Antiarrhythmic agents (e.g., procainamide, quinidine, sotalol) | Reduction of cardiac output, vasodilation via inhibition of peripheral sympathetic efferent activity. |
| Opioids (e.g., morphine, tramadol) | Reduction of cardiac output, vasodilation via inhibition of sympathetic efferent activity, reduced vasopressin release. |
| Benzodiazepines (e.g., diazepam) | Vasodilation via central inhibition of sympathetic efferent activity. |
| Tetrahydrocannabinol | Vasodilation via central and peripheral inhibition of sympathetic efferent activity. |
| First-generation histamine (H1) antagonists (e.g., promethazine) | Vasodilation via central inhibition of sympathetic efferent activity and renin-angiotensin inhibition. |
Compression garments
To combat symptomatic nOH, the most effective compression site is the abdomen, which reduces splanchnic venous pooling. There is substantial evidence that elastic abdominal binders are effective to attenuate symptoms of nOH in patients with PD [45, 46]. A servo-controlled abdominal binder that inflates automatically when standing was as effective as conventional pharmacotherapy in patients with nOH [47]. Because wearing compression stockings up to the upper thigh requires a lot of effort, particularly in patients with movement disorders, and only has a minor effect on standing BP, they should be deprioritized [27].
Elevation of the head of the bed
Patients with nOH may benefit from elevating the head end of their bed during sleep to improve orthostatic tolerance. This technique can alleviate supine hypertension, decrease nocturia, and prevent overnight volume depletion [48, 49]. It is recommended to elevate the head of the bed 20–30 cm, as small tilting angles do not provide significant improvement [50]. In our experience, the benefits of this technique can be significant enough for patients to tolerate any discomfort. An adjustable electric bed or mattress is the most practical solution for head-up sleeping, and placing a hard pillow under the mattress at thigh level can prevent sliding down. Additionally, a footboard can be helpful. A multicenter home-based double-blind phase II RCT to assess the impact of heads-up sleeping on nOH and supine hypertension is underway (ClinicalTrials.gov: NCT05551377).
Non-pharmacological management of supine hypertension
Managing symptoms of nOH and supine hypertension can be difficult since treating one can often worsen the other. There are consensus guidelines for treating supine hypertension [12]. Currently, the recommended approach is to prioritize treating nOH and tolerate mild supine hypertension. Severe supine hypertension in patients with synucleinopathies is linked to a higher risk of cardiovascular events and shorter life expectancy [6, 51]. To manage supine hypertension, it is important to avoid lying down during the day and to sleep with the head of the bed elevated. It is also recommended to take the last dose of anti-hypotensive agents at least 3-4 hours before sleep time. Additionally, eating a high-calorie snack and/or having an alcoholic drink before bedtime may help reduce supine BP.
Local passive heat may be used to lower BP. A heating pad set at 40–42°C placed over the abdomen for 2-h effectively lowered overnight BP in patients with supine hypertension [52]. A novel nonpharmacological approach to treating supine hypertension is the overnight use of continuous positive airway pressure (8–12 cm H2O), which lowered nighttime BP, was associated with lower nighttime diuresis, and improved symptoms of nOH in the morning[53].
GASTROINTESTINAL DYSFUNCTION
Patients with PD and other synucleinopathies experience gastrointestinal issues that affect all aspects of digestion, from chewing to defecation [54]. In PD and DLB, stool transit time is prolonged due to reduced colonic motility caused by abnormal intrinsic enteric and extrinsic vagal innervation. In MSA, gastrointestinal symptoms arise from neurodegeneration of brainstem nuclei and the intermediolateral cell column in the thoracic and lumbar spinal cord [55]. Gastroparesis, resulting in delayed gastric emptying, is common in patients leading to nausea, early satiety, gastric retention, and abdominal distension. Constipation is the most common autonomic and gastrointestinal symptom, affecting up to 90% of PD patients and 80% of MSA patients [18]. L-dopa can exacerbate some symptoms, such as delayed gastric emptying, by slowing down motility through its effect on dopaminergic enteric receptors [56].
As with non-pharmacological therapies for nOH, high-quality evidence of non-pharmacological measures for the treatment of gastrointestinal dysfunction in patients with PD is scarce, and, in clinical practice, we must rely on the evidence gathered in other patient groups.
Non-pharmacological treatment of gastroparesis
The goals of gastroparesis treatment are to alleviate symptom burden, correct malnutrition, and resume oral intake when possible [57]. Dietary modifications, including a low-fat diet with small frequent meals with preferably liquid nutrients, may facilitate gastric emptying.
Non-pharmacological treatment of constipation
There are various ways to manage constipation, including non-pharmacological methods like removing drugs that worsen the condition (such as opioids and anticholinergics), taking fiber supplements and stool softeners, and using medication. In some cases, patients may also need enemas or manual disimpactions.
Several randomized trials have shown that probiotics (nondigestible food ingredients that stimulate the growth and activity of beneficial bacteria in the colon) can be an effective non-pharmacological treatment for constipation in patients with PD [58, 59]. Probiotics (either administered as fermented milk products like kefir or in capsules) not only improve constipation but may have the potential to reduce inflammation and improve metabolism [60, 61]. Probiotics are safe and well-tolerated. A randomized clinical trial of fecal microbiota transplantation in PD is underway (ClinicalTrials.gov: NCT03808389).
A small randomized trial evaluated abdominal massage with lifestyle advice versus lifestyle advice alone for the treatment of constipation in PD, with negative results [62]. Additional recommendations not tested in clinical trials include dietary changes such as eating at regular times, increasing the intake of high-fiber fruits and vegetables, wholegrain bread and cereals, and extra virgin olive oil, as well as staying hydrated by drinking 2–2.5 L of fluids a day and engaging in physical activity.
URINARY DYSFUNCTION
Patients with PD and other synucleinopathies commonly experience urinary symptoms related to neurogenic bladder which, in turn, can be related to detrusor overactivity or underactivity [63, 64]. Up to 80% of patients with PD and DLB experience urinary symptoms, with nocturia being the most commonly reported symptom, followed by frequency, urgency, and urge incontinence; the severity of these symptoms is usually mild or moderate. Detrusor underactivity resulting in increased post-void residual volume is infrequent in PD and DLB disorders, and urinary catheterization is rarely required. In contrast, patients with MSA experience universal and severe urinary dysfunction, which is often one of the earliest presenting features. Difficulty voiding is the most frequently reported urinary symptom in MSA, followed by nocturia, urgency, and incontinence [18]. Detrusor underactivity occurs in 70% of patients with MSA, resulting in significant urinary retention. Managing bladder dysfunction adequately is crucial to avoid the risk of urosepsis and death, particularly in patients with MSA [7, 65].
Non-pharmacological treatment of urinary dysfunction
One effective non-pharmacological therapy for improving urinary symptoms in PD is “multi-component” behavioral therapy, which has been supported by small randomized trials [66–68]. The multiple components include self-monitoring with a diary, daily pelvic floor muscle exercises, and squeezing of the pelvic floor muscles immediately before a potential leakage triggered by sneezing, coughing, or bending. It also involves managing constipation, minimizing caffeine intake, and considering an individualized risk-benefit assessment for fluid restriction if the patient also has nOH. For those with supine hypertension, sleeping with the head of the bed raised can help with nocturia. Patients with nOH with nocturia must use a bedside commode or urinal rather than standing up to reduce the risk of syncopal events.
While infrequent in patients with PD and DLB, incomplete bladder emptying as a consequence of detrusor underactivity is common in MSA. A simple test to diagnose it is by measuring the post-void residual bladder volume with ultrasound echography; patients are usually unaware that their bladders do not empty. If the patient has a post-void residual bladder volume >100 ml, clean intermittent self-catheterization is usually recommended. Either the patient (if severe parkinsonism is not a limitation) or a caregiver can perform this after proper education is provided. When the technique is performed aseptically, the risk of urinary infections is minimal. In patients with advanced disease and severe neurological disability, a permanent indwelling catheter, sometimes suprapubic, may be required [7].
SEXUAL DYSFUNCTION
Up to 60% of men with PD and 80% with MSA acknowledge erectile dysfunction, ejaculation problems, and difficulties achieving orgasm. The severity of sexual problems increases with disease duration. Erectile dysfunction can manifest years before the diagnosis of PD or MSA, adding sexual dysfunction to the constellation of premotor autonomic manifestations of the synucleinopathies [69, 70]. Up to 75% of women with PD and MSA report sexual problems such as vaginal dryness, decreased libido, and difficulties reaching orgasm [71].
Non-pharmacologic treatment of erectile dysfunction
Psychogenic causes (anxiety, depression, stress), and excessive use of alcohol and tobacco can contribute to erectile dysfunction. Several medications can induce erectile dysfunction and decreased libido. These include but are not limited to hydrochlorothiazide and β-blockers (which can also cause or worsen nOH). Selective serotonin reuptake inhibitors and 5α-reductase inhibitors (finasteride) can also contribute to erectile dysfunction. Prostate cancer treatments (radical prostatectomy, radiotherapy, luteinizing hormone-releasing agonists, and antagonists) frequently have erectile dysfunction as an adverse event [72].
Vacuum pump devices are the only non-pharmacological therapies available for the treatment of erectile dysfunction, even though the available data from patients with synucleinopathies is limited to anecdotal evidence. These devices consist of clear, rigid, plastic chambers placed over the penis, tightened against the lower abdomen with a mechanism to create a vacuum inside the chamber. This results in increased blood flow into the penis. If an adequate erection occurs inside the chamber, the patient slips a small constriction band off the end of the vacuum pump device and onto the base of the penis to prevent venous return. An erection beyond 30-min with this method is not recommended. These devices can result a bit cumbersome, but are safe and tolerable, and patients can get used to them [73].
Non-pharmacological treatment for female sexual dysfunction
There is a major gap in understanding female sexual dysfunction in PD and related disorders and its treatment. Non-pharmacological therapeutic options for female sexual dysfunction are limited and understudied and include vaginal lubrication and psychotherapy [74].
CONCLUSIONS
Autonomic dysfunction is common among patients with synucleinopathies and can greatly impact their quality of life, morbidity, and mortality. For instance, gastrointestinal issues can affect drug effectiveness, leading to decreased motor function; nOH can cause syncope, falls, and fractures; and urinary problems can result in urosepsis. It is important to recognize and treat autonomic dysfunction in these patients. While non-pharmacological and non-surgical treatments are available and, in many cases, effective to improve symptoms, they are often overlooked. When feasible, the implementation of non-pharmacological approaches should be performed in a multi-disciplinary setting, e.g., involving physical therapists and dietitians, and other healthcare providers that are familiar with symptoms of autonomic dysfunction. Often, the first step is to reduce or discontinue drugs that cause or aggravate autonomic symptoms. A major gap is the lack of large randomized trials testing non-pharmacological approaches. Since the possibility of intellectual property protection for most non-pharmacological approaches is remote, it is unlikely that biotechnological and pharmaceutical companies will be interested in performing such trials. We, therefore, suggest that an important goal of not-for-profit foundations and patient advocacy groups should be research funding to gather high-quality evidence for non-pharmacological therapies.
ACKNOWLEDGMENTS
RDT is supported by the Michael J Fox Foundation for Parkinson’s Research (MJFF-020200) Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie, and The Netherlands Organisation for Health Research and Development (114025101).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Heinzel S , Berg D , Gasser T , Chen H , Yao C , Postuma RB , MDS Task Force on the Definition of Parkinson’s Disease ((2019) ) Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord 34: , 1464–1470. |
[2] | Wenning GK , Stankovic I , Vignatelli L , Fanciulli A , Calandra-Buonaura G , Seppi K , Palma JA , Meissner WG , Krismer F , Berg D , Cortelli P , Freeman R , Halliday G , Hoglinger G , Lang A , Ling H , Litvan I , Low P , Miki Y , Panicker J , Pellecchia MT , Quinn N , Sakakibara R , Stamelou M , Tolosa E , Tsuji S , Warner T , Poewe W , Kaufmann H ((2022) ) The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov Disord 37: , 1131–1148. |
[3] | Fereshtehnejad SM , Romenets SR , Anang JB , Latreille V , Gagnon JF , Postuma RB ((2015) ) New clinical subtypes of Parkinson disease and their longitudinal progression: A prospective cohort comparison with other phenotypes. JAMA Neurol 72: , 863–873. |
[4] | Goldstein DS , Holmes C , Sharabi Y , Wu T ((2015) ) Survival in synucleinopathies: A prospective cohort study. Neurology 85: , 1554–1561. |
[5] | Glasmacher SA , Leigh PN , Saha RA ((2017) ) Predictors of survival in progressive supranuclear palsy and multiple system atrophy: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 88: , 402–411. |
[6] | Palma JA , Redel-Traub G , Porciuncula A , Samaniego-Toro D , Millar Vernetti P , Lui YW , Norcliffe-Kaufmann L , Kaufmann H ((2020) ) The impact of supine hypertension on target organ damage and survival in patients with synucleinopathies and neurogenic orthostatic hypotension. Parkinsonism Relat Disord 75: , 97–104. |
[7] | Palma JA , Kaufmann H ((2018) ) Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov Disord 33: , 372–390. |
[8] | Seppi K , Ray Chaudhuri K , Coelho M , Fox SH , Katzenschlager R , Perez Lloret S , Weintraub D , Sampaio C; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee ((2019) ) Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 34: , 180–198. |
[9] | Palma JA , Kaufmann H ((2020) ) Orthostatic hypotension in Parkinson disease. Clin Geriatr Med 36: , 53–67. |
[10] | Wieling W , Kaufmann H , Claydon VE , van Wijnen VK , Harms MPM , Juraschek SP , Thijs RD ((2022) ) Diagnosis and treatment of orthostatic hypotension. Lancet Neurol 21: , 735–746. |
[11] | Lahrmann H , Cortelli P , Hilz M , Mathias CJ , Struhal W , Tassinari M ((2006) ) EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 13: , 930–936. |
[12] | Fanciulli A , Jordan J , Biaggioni I , Calandra-Buonaura G , Cheshire WP , Cortelli P , Eschlboeck S , Grassi G , Hilz MJ , Kaufmann H , Lahrmann H , Mancia G , Mayer G , Norcliffe-Kaufmann L , Pavy-Le Traon A , Raj SR , Robertson D , Rocha I , Struhal W , Thijs R , Tsioufis KP , van Dijk JG , Wenning GK ((2018) ) Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res 28: , 355–362. |
[13] | Kaufmann H , Norcliffe-Kaufmann L , Palma JA ((2020) ) Baroreflex dysfunction. N Engl J Med 382: , 163–178. |
[14] | Palma JA , Gomez-Esteban JC , Norcliffe-Kaufmann L , Martinez J , Tijero B , Berganzo K , Kaufmann H ((2015) ) Orthostatic hypotension in Parkinson disease: How much you fall or how low you go? Mov Disord 30: , 639–645. |
[15] | Velseboer DC , de Haan RJ , Wieling W , Goldstein DS , de Bie RM ((2011) ) Prevalence of orthostatic hypotension in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat Disord 17: , 724–729. |
[16] | Thaisetthawatkul P , Boeve BF , Benarroch EE , Sandroni P , Ferman TJ , Petersen R , Low PA ((2004) ) Autonomic dysfunction in dementia with Lewy bodies. Neurology 62: , 1804–1809. |
[17] | Pavy-Le Traon A , Piedvache A , Perez-Lloret S , Calandra-Buonaura G , Cochen-De Cock V , Colosimo C , Cortelli P , Debs R , Duerr S , Fanciulli A , Foubert-Samier A , Gerdelat A , Gurevich T , Krismer F , Poewe W , Tison F , Tranchant C , Wenning G , Rascol O , Meissner WG , European MSA Study Group ((2016) ) New insights into orthostatic hypotension in multiple system atrophy: A European multicentre cohort study. J Neurol Neurosurg Psychiatry 87: , 554–561. |
[18] | Palma JA , Krismer F , Meissner WG , Kuijpers M , Millar-Vernetti P , Perez MA , Fanciulli A , Norcliffe-Kaufmann L , Bower P , Wenning GK , Kaufmann H ((2022) ) Patient-reported symptoms in the global multiple system atrophy registry. Mov Disord Clin Pract 9: , 967–971. |
[19] | Kaufmann H , Norcliffe-Kaufmann L , Palma JA , Biaggioni I , Low PA , Singer W , Goldstein DS , Peltier AC , Shibao CA , Gibbons CH , Freeman R , Robertson D , Autonomic Disorders Consortium ((2017) ) Natural history of pure autonomic failure: A United States prospective cohort. Ann Neurol 81: , 287–297. |
[20] | Fanciulli A , Campese N , Goebel G , Ndayisaba JP , Eschlboeck S , Kaindlstorfer C , Raccagni C , Granata R , Bonuccelli U , Ceravolo R , Seppi K , Poewe W , Wenning GK ((2020) ) Association of transient orthostatic hypotension with falls and syncope in patients with Parkinson disease. Neurology 95: , e2854–e2865. |
[21] | Palma JA , Cortelli P ((2023) ) Blood pressure and risk of dementia in Parkinson disease and multiple system atrophy: Should you buy the dip in such a volatile market? Neurology 100: , 451–453. |
[22] | Kaufmann H , Palma JA ((2020) ) White matter hyperintensities in the synucleinopathies: Orthostatic hypotension, supine hypertension, or both? Mov Disord Clin Pract 7: , 595–598. |
[23] | Gibbons CH , Schmidt P , Biaggioni I , Frazier-Mills C , Freeman R , Isaacson S , Karabin B , Kuritzky L , Lew M , Low P , Mehdirad A , Raj SR , Vernino S , Kaufmann H ((2017) ) The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol 264: , 1567–1582. |
[24] | Frith J , Newton JL ((2020) ) Combination non-pharmacologic intervention for orthostatic hypotension in older people: A phase 2 study. Age Ageing 49: , 253–257. |
[25] | Eschlbock S , Wenning G , Fanciulli A ((2017) ) Evidence-based treatment of neurogenic orthostatic hypotension and related symptoms. J Neural Transm (Vienna) 124: , 1567–1605. |
[26] | Logan A , Freeman J , Pooler J , Kent B , Gunn H , Billings S , Cork E , Marsden J ((2020) ) Effectiveness of non-pharmacological interventions to treat orthostatic hypotension in elderly people and people with a neurological condition: A systematic review. JBI Evid Synth 18: , 2556–2617. |
[27] | Newton JL , Frith J ((2018) ) The efficacy of nonpharmacologic intervention for orthostatic hypotension associated with aging. Neurology 91: , e652–e656. |
[28] | Wieling W , Colman N , Krediet CT , Freeman R ((2004) ) Nonpharmacological treatment of reflex syncope. Clin Auton Res 14: (Suppl 1), 62–70. |
[29] | Palma JA , Kaufmann H ((2017) ) Epidemiology, diagnosis, and management of neurogenic orthostatic hypotension. Mov Disord Clin Pract 4: , 298–308. |
[30] | Palma JA , Kaufmann H ((2020) ) Management of orthostatic hypotension. Continuum (Minneap Minn) 26: , 154–177. |
[31] | Wieling W , van Dijk N , Thijs RD , de Lange FJ , Krediet CT , Halliwill JR ((2015) ) Physical countermeasures to increase orthostatic tolerance. J Intern Med 277: , 69–82. |
[32] | de Jong JSY , de Lange FJ , van Dijk N , Thijs RD , Wieling W , Syncopedia editorial board ((2018) ) Syncopedia: Training a new generation of syncope specialists. Clin Auton Res 28: , 173–176. |
[33] | Thijs RD , Wieling W , van den Aardweg JG , van Dijk JG ((2007) ) Respiratory countermaneuvers in autonomic failure. Neurology 69: , 582–585. |
[34] | May M , Jordan J ((2011) ) The osmopressor response to water drinking. Am J Physiol Regul Integr Comp Physiol 300: , R40–R46. |
[35] | Shibao CA , Biaggioni I ((2020) ) Management of orthostatic hypotension, postprandial hypotension, and supine hypertension. Semin Neurol 40: , 515–522. |
[36] | Jansen RW ((2005) ) Postprandial hypotension: Simple treatment but difficulties with the diagnosis. J Gerontol A Biol Sci Med Sci 60: , 1268–1270. |
[37] | Stock JM , Chelimsky G , Edwards DG , Farquhar WB ((2022) ) Dietary sodium and health: How much is too much for those with orthostatic disorders? Auton Neurosci 238: , 102947. |
[38] | Nakamura T , Hirayama M , Yamashita F , Uchida K , Hama T , Watanabe H , Sobue G ((2010) ) Lowered cardiac sympathetic nerve performance in response to exercise in Parkinson’s disease. Mov Disord 25: , 1183–1189. |
[39] | Strano S , Fanciulli A , Rizzo M , Marinelli P , Palange P , Tiple D , De Vincentis G , Calcagnini G , Censi F , Meco G , Colosimo C ((2016) ) Cardiovascular dysfunction in untreated Parkinson’s disease: A multi-modality assessment. J Neurol Sci 370: , 251–255. |
[40] | Palma JA , Carmona-Abellan MM , Barriobero N , Trevino-Peinado C , Garcia-Lopez M , Fernandez-Jarne E , Luquin MR ((2013) ) Is cardiac function impaired in premotor Parkinson’s disease? A retrospective cohort study. Mov Disord 28: , 591–596. |
[41] | Speelman AD , Groothuis JT , van Nimwegen M , van der Scheer ES , Borm GF , Bloem BR , Hopman MT , Munneke M ((2012) ) Cardiovascular responses during a submaximal exercise test in patients with Parkinson’s disease. J Parkinsons Dis 2: , 241–247. |
[42] | Low DA , Vichayanrat E , Iodice V , Mathias CJ ((2014) ) Exercise hemodynamics in Parkinson’s disease and autonomic dysfunction. Parkinsonism Relat Disord 20: , 549–553. |
[43] | Kaufmann H , Palma JA ((2017) ) Neurogenic orthostatic hypotension: The very basics. Clin Auton Res 27: , 39–43. |
[44] | Kanegusuku H , Silva-Batista C , Pecanha T , Nieuwboer A , Silva ND Jr , Costa LA , de Mello MT , Piemonte ME , Ugrinowitsch C , Forjaz CL ((2017) ) Effects of progressive resistance training on cardiovascular autonomic regulation in patients with Parkinson disease: A randomized controlled trial. Arch Phys Med Rehabil 98: , 2134–2141. |
[45] | Paschen S , Hansen C , Welzel J , Albrecht J , Atrsaei A , Aminian K , Zeuner KE , Romijnders R , Warmerdam E , Urban PP , Berg D , Maetzler W ((2022) ) Effect of lower limb vs. abdominal compression on mobility in orthostatic hypotension: A single-blinded, randomized, controlled, cross-over pilot study in Parkinson’s disease. J Parkinsons Dis 12: , 2531–2541. |
[46] | Fanciulli A , Goebel G , Metzler B , Sprenger F , Poewe W , Wenning GK , Seppi K ((2016) ) Elastic abdominal binders attenuate orthostatic hypotension in Parkinson’s disease. Mov Disord Clin Pract 3: , 156–160. |
[47] | Okamoto LE , Diedrich A , Baudenbacher FJ , Harder R , Whitfield JS , Iqbal F , Gamboa A , Shibao CA , Black BK , Raj SR , Robertson D , Biaggioni I ((2016) ) Efficacy of servo-controlled splanchnic venous compression in the treatment of orthostatic hypotension: A randomized comparison with midodrine. Hypertension 68: , 418–426 . |
[48] | Wieling W , Raj SR , Thijs RD ((2009) ) Are small observational studies sufficient evidence for a recommendation of head-up sleeping in all patients with debilitating orthostatic hypotension? MacLean and Allen revisited after 70 years. Clin Auton Res 19: , 8–12. |
[49] | MacLean AR , Allen EV ((1940) ) Orthostatic hypotension and orthostatic tachycardia. J Am Med Assoc 115: , 2162–2167. |
[50] | Fan CW , Walsh C , Cunningham CJ ((2011) ) The effect of sleeping with the head of the bed elevated six inches on elderly patients with orthostatic hypotension: An open randomised controlled trial. Age Ageing 40: , 187–192. |
[51] | Espay AJ , LeWitt PA , Hauser RA , Merola A , Masellis M , Lang AE ((2016) ) Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: Prioritisation of treatment targets. Lancet Neurol 15: , 954–966. |
[52] | Okamoto LE , Celedonio JE , Smith EC , Gamboa A , Shibao CA , Diedrich A , Paranjape SY , Black BK , Muldowney JAS , 3rd , Peltier AC , Habermann R , Crandall CG , Biaggioni I ((2021) ) Local passive heat for the treatment of hypertension in autonomic failure. J Am Heart Assoc 10: , e018979. |
[53] | Okamoto LE , Celedonio JE , Smith EC , Paranjape SY , Black BK , Wahba A , Park JW , Shibao CA , Diedrich A , Biaggioni I ((2023) ) Continuous positive airway pressure for the treatment of supine hypertension and orthostatic hypotension in autonomic failure. Hypertension 80: , 650–658. |
[54] | Chung KA , Pfeiffer RF ((2021) ) Gastrointestinal dysfunction in the synucleinopathies. Clin Auton Res 31: , 77–99. |
[55] | Gelpi E , Navarro-Otano J , Tolosa E , Gaig C , Compta Y , Rey MJ , Marti MJ , Hernandez I , Valldeoriola F , Rene R , Ribalta T ((2014) ) Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord 29: , 1010–1018. |
[56] | Pfeiffer RF , Isaacson SH , Pahwa R ((2020) ) Clinical implications of gastric complications on levodopa treatment in Parkinson’s disease. Parkinsonism Relat Disord 76: , 63–71. |
[57] | Camilleri M , Parkman HP , Shafi MA , Abell TL , Gerson L , American College of Gastroenterology ((2013) ) Clinical guideline: Management of gastroparesis. Am J Gastroenterol 108: , 18–37; quiz 38. |
[58] | Hong CT , Chen JH , Huang TW ((2022) ) Probiotics treatment for Parkinson disease: A systematic review and meta-analysis of clinical trials. Aging (Albany NY) 14: , 7014–7025. |
[59] | Chu C , Yu L , Li Y , Guo H , Zhai Q , Chen W , Tian F ((2023) ) Meta-analysis of randomized controlled trials of the effects of probiotics in Parkinson’s disease. Food Funct 14: , 3406–3422. |
[60] | Tan AH , Lim SY , Chong KK , MAA AM , Hor JW , Lim JL , Low SC , Chong CW , Mahadeva S , Lang AE ((2021) ) Probiotics for constipation in Parkinson disease: A randomized placebo-controlled study. Neurology 96: , e772–e782. |
[61] | Barichella M , Pacchetti C , Bolliri C , Cassani E , Iorio L , Pusani C , Pinelli G , Privitera G , Cesari I , Faierman SA , Caccialanza R , Pezzoli G , Cereda E ((2016) ) Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology 87: , 1274–1280. |
[62] | McClurg D , Walker K , Aitchison P , Jamieson K , Dickinson L , Paul L , Hagen S , Cunnington AL ((2016) ) Abdominal massage for the relief of constipation in people with Parkinson’s: A qualitative study. Parkinsons Dis 2016: , 4842090. |
[63] | Sakakibara R , Tateno F , Yamamoto T , Uchiyama T , Yamanishi T ((2018) ) Urological dysfunction in synucleinopathies: Epidemiology, pathophysiology and management. Clin Auton Res 28: , 83–101. |
[64] | Ogawa T , Sakakibara R , Kuno S , Ishizuka O , Kitta T , Yoshimura N ((2017) ) Prevalence and treatment of LUTS in patients with Parkinson disease or multiple system atrophy. Nat Rev Urol 14: , 79–89. |
[65] | Coon EA , Sletten DM , Suarez MD , Mandrekar JN , Ahlskog JE , Bower JH , Matsumoto JY , Silber MH , Benarroch EE , Fealey RD , Sandroni P , Low PA , Singer W ((2015) ) Clinical features and autonomic testing predict survival in multiple system atrophy. Brain 138: , 3623–3631. |
[66] | Vaughan CP , Burgio KL , Goode PS , Juncos JL , McGwin G , Muirhead L , Markland AD , Johnson TM 2nd ((2019) ) Behavioral therapy for urinary symptoms in Parkinson’s disease: A randomized clinical trial. Neurourol Urodyn 38: , 1737–1744. |
[67] | Hajebrahimi S , Chapple CR , Pashazadeh F , Salehi-Pourmehr H ((2019) ) Management of neurogenic bladder in patients with Parkinson’s disease: A systematic review. Neurourol Urodyn 38: , 31–62. |
[68] | Vaughan CP , Juncos JL , Burgio KL , Goode PS , Wolf RA , Johnson TM 2nd ((2011) ) Behavioral therapy to treat urinary incontinence in Parkinson disease. Neurology 76: , 1631–1634. |
[69] | Gao X , Chen H , Schwarzschild MA , Glasser DB , Logroscino G , Rimm EB , Ascherio A ((2007) ) Erectile function and risk of Parkinson’s disease. Am J Epidemiol 166: , 1446–1450. |
[70] | Raciti L , De Cola MC , Ortelli P , Corallo F , Lo Buono V , Morini E , Quattrini F , Filoni S , Calabro RS ((2020) ) Sexual dysfunction in Parkinson disease: A multicenter Italian cross-sectional study on a still overlooked problem. J Sex Med 17: , 1914–1925. |
[71] | Varanda S , Ribeiro da Silva J , Costa AS , Amorim de Carvalho C , Alves JN , Rodrigues M , Carneiro G ((2016) ) Sexual dysfunction in women with Parkinson’s disease. Mov Disord 31: , 1685–1693. |
[72] | Yafi FA , Jenkins L , Albersen M , Corona G , Isidori AM , Goldfarb S , Maggi M , Nelson CJ , Parish S , Salonia A , Tan R , Mulhall JP , Hellstrom WJ ((2016) ) Erectile dysfunction. Nat Rev Dis Primers 2: , 16003. |
[73] | Rocinante M ((2008) ) Living with autonomic failure. Clin Auton Res 18: , 48–51. |
[74] | Allahdadi KJ , Tostes RC , Webb RC ((2009) ) Female sexual dysfunction: Therapeutic options and experimental challenges. Cardiovasc Hematol Agents Med Chem 7: , 260–269. |
[75] | Fasano A , Visanji NP , Liu LW , Lang AE , Pfeiffer RF ((2015) ) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 14: , 625–639. |



