Worsening of Parkinson’s Disease After Termination of COVID-19 Quarantine Cannot Be Reversed Despite Resumption of Physiotherapy
Abstract
In a retrospective analysis, we recently reported findings on the detrimental motor effects of interrupted physiotherapy following the COVID-19 pandemic in parkinsonian patients. Using an extended follow-up period, we investigated the beneficial effect of reinstated physiotherapy on patients’ disease severity and reversal of interruption-induced motor deterioration. Compared to before the COVID-19 outbreak, we observed persistence of motor disease worsening despite full resumption of state-of-the-art physical therapy suggesting that motor deterioration after discontinuation of physical therapy could not be compensated for. Therefore, and considering possible future crises, establishing means to safeguard continuation of physical therapy and to foster remote provision of care should be major goals.
The spread of SARS-CoV-2-related infections during the pandemic crisis caused serious health challenges associated with severe consequences for mortality and morbidity worldwide [1, 2]. As part of basic public health precautions, epidemic control measures were implemented to reduce viral spread, such as the stay-at-home mandate. Besides being an effective barrier for viral spread, this measure also resulted in the proliferation of several extra-SARS-CoV-2-related health challenges such as reductions in psychosocial wellbeing, psychiatric deterioration, and motor functioning on both general population but also patient-specific levels [1, 3–5]. This was not without consequences for patients suffering from Parkinson’s disease (PD) who were seriously affected by the loss and interruption of regular physical activity in the form of guided physiotherapy at hospitals and elsewhere. On the other hand, the pandemic, as dreadful as it undoubtedly is, represented an opportunity to investigate in real-life the effect of interrupted and reinstated physiotherapy on the well-being of patients. In a previous article, we presented results from a retrospective study revealing a worsening of motor function during the time of imposed pandemic restrictions comprising the suspension of physical therapy compared to the time period before the COVID-19 pandemic in patients suffering from PD [5]. Importantly, using data that was resampled and represented as mean motor symptom scores across years, the study found no involvement of medication-based alterations, hence, the motor worsening was pharmacotherapy-independent. Representing one of the most impactful and sudden interruptions, there is reason to believe that the confinement-based absence of physiotherapy in PD during the stay-at-home mandate was one of the main driving factors leading to such motor decrement [5].
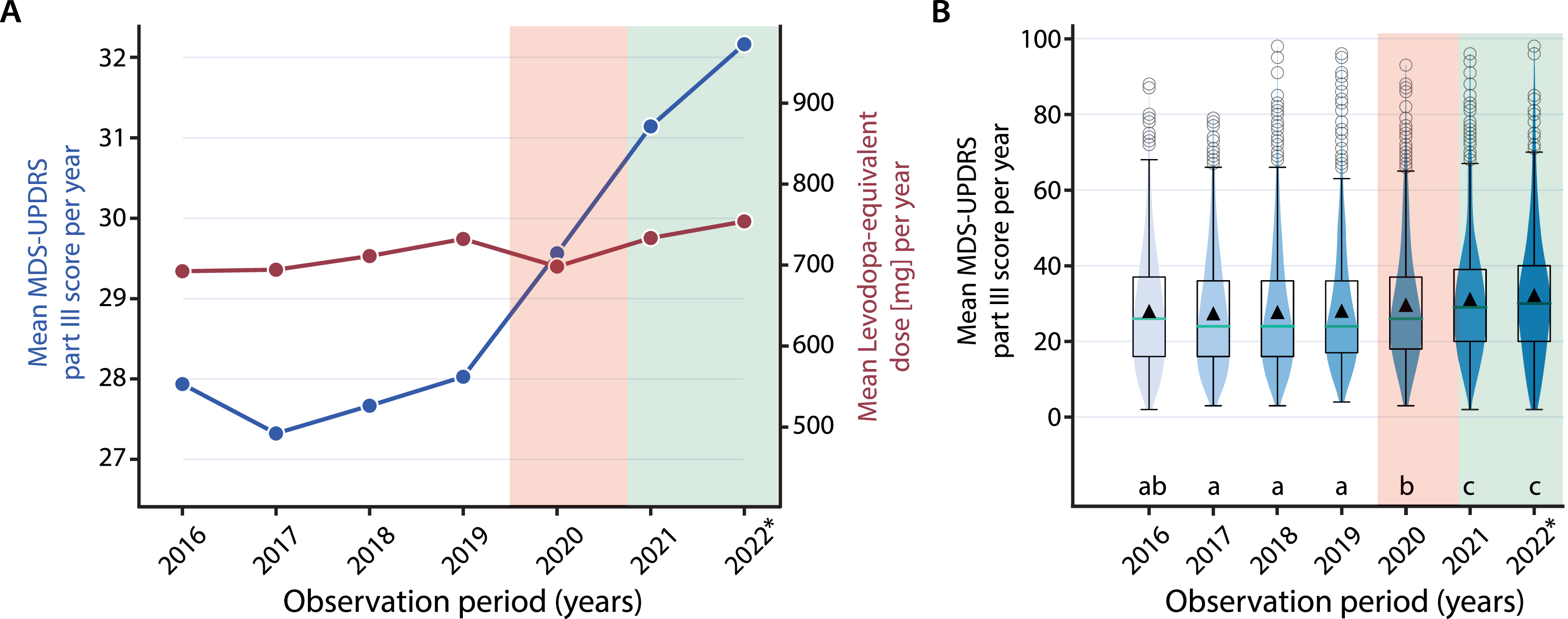
These findings raise the question whether patients may have benefitted from reinstalling physiotherapy as a consequence of eased COVID-19 dependent restrictions, and whether motor deterioration could be compensated for. Hence, this comment presents data on patients’ disease severity after the reuptake of physiotherapy. For this, we repeated part of the retrospective analysis using the same cohort (longitudinally followed patients (n = 755) undergoing standardized neurological examination by a movement disorders specialist collecting MDS-UPDRS-III data during medication ON condition) and an extended follow-up comprising the time from May 2021 until October 19, 2022 during which healthcare provision was resumed and provided as before (neurological examinations (years 2021 and 2022): mean: 4.13, SD: 2.28; range: 1–12; characteristics of study population: mean age: 71.1 years; % females: 37.6; median Hoehn & Yahr stage: 2.5). The current analysis of the yearly data between 2016 and 2022 revealed exacerbated motor symptom severity during and after the pandemic-related confinement compared to before: whilst the mean MDS-UPDRS part III scores progressed only slowly from 2016 until the onset of the pandemic crisis (beginning of 2020; represented by the red transparent area in Fig. 1A; duration of COVID-dependent restrictions: 16 months), there is a steep, pharmacotherapy-independent increase from 2020 onwards (i.e., during and after the pandemic restrictions; effect size comparing 2019 and 2020, or Cohen’s dz: 0.16) as the mean levodopa equivalent daily dose (LEDD) remained unchanged between 2016 and 2022 (Fig. 1A). Interestingly, motor disease severity persisted at an elevated level compared to before the pandemic crisis, even when physiotherapy was relaunched (represented by the green transparent area in Fig. 1A; effect size comparing 2019 and 2022, or Cohen’s dz: 0.26), with all years before 2020 revealing lower motor disease progression compared to 2021/22 (Kruskal-Wallis test with Dunn’s post hoc test for multiple comparisons, main effect of time: χ2(6) = 74.73, p < 0.005; same analysis using total LEDD: n.s.) (Fig. 1B).
Fig. 1
A) Temporal trend analysis: Mean MDS-UPDRS part III scores (blue, left y-axis) and total LEDD (red, right y-axis) of all PD patients ascertained at the university hospital in Zurich resampled using a yearly time binning and comparing a time period ranging from 2016–2022 (with yearly means (and 95% confidence limits): 2016: 27.9 (26.9, 29.0); 2017: 27.3 (26.3, 28.3); 2018: 27.7 (26.7, 28.6); 2019: 28.0 (27.1, 29.0); 2020: 29.6 (28.6, 30.6); 2021: 31.1 (30.1, 32.2); 2022: 32.2 (30.6, 33.8)). B) Boxplots including violinplots of the mean yearly MDS-UPDRS part III scores across the same observation period reflecting the higher motor disease severity in 2020, 2021 and 2022 compared to the previous years. The red transparent area represents the time (16 months) during COVID-19-dependent restrictions whereas the green transparent area indicates the time (18.5 months) after the termination of COVID-19-dependent restrictions. The * in the year 2022 indicates that data was collected until October 19th 2022 only. Black triangles in B represent the mean. Years in B indicated by different letters were significantly different in Dunn’s posthoc test for multiple comparisons: e.g., a vs. b, a vs. c, p < 0.05 or lower.
As outlined in our initial publication, there is a strong body of evidence suggesting that physical activity, such as Qigong and aerobic exercise [6–12] improve motor (and non-motor) symptoms in PD. Of note, three recently published reviews and meta-analyses, one of which presenting data from nearly 8000 patients, attest the beneficial role of physical activity and conventional physiotherapy on motor symptoms, gait, quality of life [13, 14] and even freezing of gait [15], for which effective treatments are still missing, underscoring the importance of adopting physical therapy programs in PD. In addition, the interruption of physiotherapy during the COVID-19 pandemic was felt to be a major issue by PD patients [16]. A study recently replicated our findings providing further evidence for motor worsening based on, among others, physical inactivity [17]. Thus, the available evidence recommends promoting physical activity in PD to foster generic and motor health benefits by demonstrating that exercise and physiotherapy are efficacious means to achieve this goal.
In agreement with this body of evidence, our previous results highlight the effect the COVID-19 pandemic-related quarantine measures have had on patients’ motor well-being, allegedly due to discontinuation of physiotherapy. This outcome supports the view that interruption of physiotherapy cannot be performed without risking major health-related consequences. In the present work, we aimed at investigating whether the documented motor deterioration could be compensated for through the reuptake of physiotherapy: In the 18.5-month follow-up time after the termination of COVID-19 dependent restrictions, the resumption of conventional physiotherapy was not associated with an improvement in motor symptoms at an aggregated level. In contrary, motor disease severity remained high and tended to further increase in spite of reintroduction of physiotherapy, endorsing the view that interruption of physiotherapy not only leads to fast motor deterioration, but also to inexorable motor decrement. Hence, the observed higher rate of motor disease progression during the pandemic [5] was retained even after the end of isolation and reintroduction of physiotherapy. It attests to the difficulty in restoring motor functioning and indicates, once again, that interruption of physical activity should be prevented at all costs. If this development continues, it would signify an irreversible loss of motor functioning. Therefore, approaches dedicated to establishing remote provision of exercise, including telerehabilitation, should be intensified to anticipate and combat instability of care in case of future pandemics and other crises and to promote physical mobility and emotional well-being in patients suffering from PD [18]. This includes developing means and accelerating availability and accessibility of telemedicine.
Our study has several limitations, in particular the lack of a control group for the sequential intervention of stopping and later reinstalling of physiotherapy or the lack of control for additional factors, such as potentially altered delivery of physiotherapy after the removal of the stay-at-home mandate or persisting sequelae of psychosocial stress due to routine clinical care interruption. However, at the very least, our results show that the net result of all these and other changes induced by the pandemic has led to highly unwanted consequences. Identifying extra-motor symptoms that may present with persistence of severity aggravation secondary to quarantine measures should be the goal of future studies.
ACKNOWLEDGMENTS
This study was conducted in the absence of any financial support.
CONFLICT OF INTEREST
CB: Received competitive grants from the Swiss National Science Foundation, the Hochschulmedizin Zurich (Flagship Grant), the Novartis Foundation, and Parkinson Schweiz, and unrestricted grants from AbbVie Pharma, GE and and Roche. Is founder and shareholder of Tossoo AG, which invests into non-pharmacological sleep modulation technologies. Received speaker honoraria from AbbVie Pharma.
GD: has served as a consultant for Boston Scientific, Cavion and as DSMB-member for Functional Neuromodulation. He has received royalties from Thieme publishers and funding by the German Research Council (SFB 1261, T1)
CI: None.
HBV: Received a competitive grant from the Koetser Foundation Zurich.
MS: None.
The authors have no conflict of interest to report.
REFERENCES
[1] | Sachs JD , Karim SSA , Aknin L , Allen J , Brosbøl K , Colombo F , Barron GC , Espinosa MF , Gaspar V , Gaviria A , Haines A , Hotez PJ , Koundouri P , Bascuñan FL , Lee J , Pate MA , Ramos G , Reddy KS , Serageldin I , Thwaites J , Vike-Freiberga V , Wang C , Were WK , Xue L , Bahadur C , Bottazzi ME , Bullen C , Laryea-Adjei G , Amor YB , Karadag O , Lafortune G , Torres E , Barredo L , Bartels JGE , Joshi N , Hellard M , Huynh UK , Khandelwal S , Lazarus JV , Michie S ((2022) ) The Lancet Commission on lessons for the future from the COVID-19 pandemic. Lancet 400: , 1224–1280. |
[2] | Bhidayasiri R , Virameteekul S , Kim JM , Pal PK , Chung SJ ((2020) ) COVID-19: An early review of its global impact and considerations for Parkinson’s disease patient care. J Mov Disord 13: , 105. |
[3] | Xiong J , Lipsitz O , Nasri F , Lui LM , Gill H , Phan L , Chen-Li D , Iacobucci M , Ho R , Majeed A , McIntyre RS ((2020) ) Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J Affective Disord 277: , 55–64. |
[4] | Schirinzi T , Di Lazzaro G , Salimei C , Cerroni R , Liguori C , Scalise S , Alwardat M , Biagio Mercuri N , Pierantozzi M , Stefani A , Pisani A ((2020) ) Physical activity changes and correlate effects in patients with Parkinson’s disease during COVID-19 lockdown. Mov Disord Clin Pract 7: , 797–802. |
[5] | Ineichen C , Baumann-Vogel H , Sitzler M , Waldvogel D , Baumann CR ((2021) ) Worsened Parkinson’s disease progression: Impact of the COVID-19 pandemic. J Parkinsons Dis 11: , 1579–1583. |
[6] | Talebi AH , Ypinga JH , De Vries NM , Nonnekes J , Munneke M , Bloem BR , Heskes T , Ben-Shlomo Y , Darweesh SK ((2022) ) Specialized versus generic allied health therapy and the risk of Parkinson’s disease complications. Mov Disord 38: , 223–231. |
[7] | Chen S , Zhang Y , Wang YT , Liu X , Song W , Du X ((2020) ) The effect of Qigong-based therapy on patients with Parkinson’s disease: A systematic review and meta-analysis. Clin Rehabil 34: , 1436–1448. |
[8] | Schootemeijer S , van der Kolk NM , Bloem BR , de Vries NM ((2020) ) Current perspectives on aerobic exercise in people with Parkinson’s disease. Neurotherapeutics 17: , 1418–1433. |
[9] | Zhen K , Zhang S , Tao X , Li G , Lv Y , Yu L ((2022) ) A systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson’s disease. NPJ Parkinsons Dis 8: , 146. |
[10] | Kim Y , Lai B , Mehta T , Thirumalai M , Padalabalanarayanan S , Rimmer JH , Motl RW ((2019) ) Exercise training guidelines for multiple sclerosis, stroke, and Parkinson’s disease: Rapid review and synthesis. Am J Phys Med Rehabil 98: , 613. |
[11] | Templeton JM , Poellabauer C , Schneider S ((2021) ) Negative effects of COVID-19 stay-at-home mandates on physical intervention outcomes: A preliminary study. J Parkinsons Dis 11: , 1067–1077. |
[12] | Cusso ME , Donald KJ , Khoo TK ((2016) ) The impact of physical activity on non-motor symptoms in Parkinson’s disease: A systematic review. Front Med 3: , 35. |
[13] | Bouça-Machado R , Rosario A , Caldeira D , Castro Caldas A , Guerreiro D , Venturelli M , Tinazzi M , Schena F , Ferreira J ((2020) ) Physical activity, exercise, and physiotherapy in Parkinson’s disease: Defining the concepts. Mov Disord Clin Pract 7: , 7–15. |
[14] | Radder DL , Lígia Silva de Lima A , Domingos J , Keus SH , van Nimwegen M , Bloem BR , de Vries NM ((2020) ) Physiotherapy in Parkinson’s disease: A meta-analysis of present treatment modalities. Neurorehabil Neural Repair 34: , 871–880. |
[15] | Cosentino C , Baccini M , Putzolu M , Ristori D , Avanzino L , Pelosin E ((2020) ) Effectiveness of physiotherapy on freezing of gait in parkinson’s disease: A systematic review and meta-analyses. Mov Disord 35: , 523–536. |
[16] | Schirinzi T , Cerroni R , Di Lazzaro G , Liguori C , Scalise S , Bovenzi R , Conti M , Garasto E , Biavio Mercuri N , Pierantozzi M , Pisani A , Stefani A ((2020) ) Self-reported needs of patients with Parkinson’s disease during COVID-19 emergency in Italy. Neurol Sci 41: , 1373–1375. |
[17] | Shalash A , Helmy A , Salama M , Gaber A , El-Belkimy M , Hamid E ((2022) ) A 6-month longitudinal study on worsening of Parkinson’s disease during the COVID-19 pandemic. NPJ Parkinsons Dis 8: , 111. |
[18] | Langer A , Gassner L , Hasenauer S , Gruber J , Wizany L , Pokan R , Maetzler W , Zach H ((2021) ) How COVID-19 will boost remote exercise-based treatment in Parkinson’s disease: A narrative review. NPJ Parkinsons Dis 7: , 25. |



