Parkinson’s Disease Case Ascertainment in the Sister Study: A Cohort for Environmental Health Research
Abstract
Background:
Large prospective studies are essential for investigating the environmental causes of Parkinson’s disease (PD), but PD diagnosis via clinical exams is often infeasible in such studies.
Objective:
To present case ascertainment strategy and data collection in a US cohort of women.
Methods:
In the Sister Study (n = 50,884, baseline ages 55.6±9.0), physician-made PD diagnoses were first reported by participants or their proxies. Cohort-wide follow-up surveys collected data on subsequent diagnoses, medication usage and PD-relevant motor and nonmotor symptoms. We contacted self-reported PD cases and their treating physicians to obtain relevant diagnostic and treatment history. Diagnostic adjudication was made via expert review of all available data, except nonmotor symptoms. We examined associations of nonmotor symptoms with incident PD, using multivariable logistic regression models and reported odds ratio (OR) and 95% confidence intervals (CI).
Results:
Of the 371 potential PD cases identified, 242 diagnoses were confirmed. Compared with unconfirmed cases, confirmed cases were more likely to report PD diagnosis from multiple sources, medication usage, and motor and nonmotor features consistently during the follow-up. PD polygenic risk score was associated with confirmed PD (ORinter-quartile range = 1.74, 95% CI: 1.45–2.10), but not with unconfirmed cases (corresponding OR = 1.05). Hyposmia, dream-enacting behaviors, constipation, depression, unexplained weight loss, dry eyes, dry mouth, and fatigue were significantly related to PD risk, with ORs from 1.71 to 4.88. Only one of the eight negative control symptoms was associated with incident PD.
Conclusion:
Findings support our PD case ascertainment approach in this large cohort of women. PD prodromal presentation is likely beyond its well-documented profile.
INTRODUCTION
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease, affecting over a million older US adults [1]. PD clinical diagnosis requires experts’ evaluation of motor dysfunction via structured neurological exams [2]. Besides motor signs, PD patients often suffer from a wide range of nonmotor symptoms [3]. Some (e.g., hyposmia [4], constipation [5, 6], and REM sleep behavior disorder (RBD) [7]) may have developed years or decades before PD clinical diagnosis [8]. Others (e.g., unexplained weight loss, pain, and fatigue) have been seldom studied in prodromal PD. While PD motor signs are caused by the loss of dopaminergic neurons in the substantia nigra, prodromal symptoms may reflect the staged development and spreading of Lewy pathology from the olfactory bulb and gut enteric nerves to the brain [9]. Therefore, clinical PD is most likely the result of decades of slowly spreading pathogenesis, during which many genetic and nongenetic factors may come into play to initiate or modify disease progression [10].
This decades-long disease development presents substantial challenges for epidemiologists in their search for environmental causes for PD [11]. Retrospective case-control studies are efficient but prone to selection and recall biases and reverse causality [12–14]. Population-based cohorts with repeated exposure assessment and long follow-up may be less subject to these biases. However, most of these studies are large, not designed to study PD [15–18], and dependent on mailed surveys, phone interviews, or time-strained clinical visits for data collection, making study-based clinical exams for PD diagnosis infeasible. Often, alternative approaches were taken for field feasibility [5, 19–23]. Further, there is an apparent sex disparity in PD research with fewer and less robust nongenetic findings in women [15, 24–26]. We herein report the PD case identification strategy and assessment of nonmotor symptoms in the Sister Study of the National Institute of Environmental Health Sciences (NIEHS) [27]. This nationwide cohort is unique in its broad collection of environmental exposures and cohort-wide biospecimen samples (Supplementary Table 1) [27]. Further, in its regular follow-ups, the study has repeatedly asked all participants to report physician-made PD diagnosis, detailed medication usage, and the presence of PD-related motor and nonmotor symptoms. All these features make the Sister Study a desirable multipurpose cohort that can be readily adapted for PD environmental research in women.
MATERIALS AND METHODS
Study population
The Sister Study is an ongoing nationwide cohort established to investigate environmental and genetic risk factors for breast cancer and other chronic diseases [27, 28]. In 2003–2009, the study enrolled 50,884 women, aged 35–74, who had a sister with breast cancer. Since enrollment, the cohort has been followed with annual health updates (AHU) that ask for the occurrence of major chronic diseases and with detailed follow-ups (DFU) every 3 years. Follow-up rates have been consistently high (>90%). In addition, the study provides a help-desk hotline that participants may call to report disease diagnosis or study-related questions. Death surveillance has been carried out by linkage to the National Death Index to obtain the underlying cause of death. The study was approved by the Institutional Review Boards at the NIEHS/NIH and Michigan State University.
PD case ascertainment
The Sister Study routinely collects data about the diagnosis of chronic diseases, including physician-made PD diagnosis during AHU, DFU, and/or the help-desk contact, and the underlying cause of death if deceased. Potential PD cases were identified if a physician-made PD diagnosis was reported at any of the study’s follow-ups or contacts, or PD was listed as the underlying cause of death on the death certificate (International Disease Classification –G20). In addition to PD diagnosis, DFUs further collected the following information that was considered in the retrospective PD adjudication process: 1) the year of diagnosis and PD medication use; 2) separately on the questionnaire, current uses of any prescription or over-the-counter medications, using the inventory method; and for each reported medication use, the years of use, days of use per week or month, and times of use per day; 3) the presence of four motor symptoms in the past year—“a tremor or trembling in either of your hands”, “walking or other movements getting noticeably slower”, “handwriting getting noticeably smaller”, and “difficulty getting started when walking or making other movements”. These data were consistently collected in follow-up surveys over the years.
In 2018-2019, we contacted potential cases and asked them to provide additional information about PD diagnosis and care. We asked them to 1) confirm the diagnosis of PD, whether the diagnosis was current, the year or age of PD diagnosis, who made the diagnosis (neurologist, movement disorder specialist, or others); 2) major signs/symptoms; and 3) use of PD medications (brand and generic names listed), and whether they helped with PD symptoms and were still being used. Finally, for patients who consented, we contacted their treating physicians and asked about patients’ PD signs and symptoms and diagnostic history, neurological comorbidity, and alternative diagnosis, and a copy of relevant medical records. This data collection was completed in April 2019.
In 2021, we conducted a two-level retrospective PD adjudication. First, we reviewed the PD-relevant data from the cohort’s routine follow-ups alone. For each patient, longitudinal data were available for up to five time points during up to 15 years from study enrollment to the fourth DFU. We considered both evidence for and against PD diagnosis and the consistency of information within each survey and over time. We adjudicated the certainty of diagnosis as 1) yes: if there were multiple consistent reports of PD diagnosis, supported by motor symptoms and evidence of responsiveness to dopaminergic treatment; 2) probable: if the report(s) of PD diagnosis was supported by consistent motor symptoms over years or evidence of responsiveness to dopaminergic treatment, and there was no contradictory evidence against PD; 3) possible: if there were multiple reports of PD diagnosis but the evidence for motor symptoms and/or responsiveness to PD medication was insufficient, or there was only one report of PD diagnosis, supported by the presence of motor symptoms and/or responsiveness to PD medication; 4) uncertain: if the self-report was denied later, alternative neurodegenerative diseases was noted on death certificate, or there was only one report without consistent supportive information of motor symptoms or responsiveness to medications; 5) no: if there were indications of error in filling out the questionnaire or there was clear evidence against idiopathic PD. At this cohort-level review, we considered a valid PD clinical diagnosis if the expert adjudication was possible, probable, or yes. Admittedly, due to the complicated patterns of longitudinal data reporting, evidence for evaluation “probable” vs. “possible” cases was mostly subjective, and misclassification between these two categories was more likely than with other categories.
For PD patients who provided further diagnostic information and allowed us to contact their treating physicians, we performed a patient/physician level adjudication, independent of cohort data. Diagnosis was confirmed 1) if the treating physician or review of medical records confirmed the PD diagnosis; 2) if physician data were not available but the review of the patient-provided data confirmed a PD diagnosis. We compared the cohort-level vs. patient-physician level adjudications to evaluate whether we could obtain a reliable PD diagnosis by only reviewing pertinent longitudinal cohort data, circumventing the cost and nonresponses of further collecting data from the patients and their treating physicians.
Final case adjudication was based on reviews at both levels. A PD diagnosis was confirmed if 1) adjudicated PD diagnosis at the patient-physician level; 2) if patient/physician data were not available, cohort-level adjudication was yes, probable, or possible. In all, we confirmed 242 PD diagnoses, as detailed in the Results. For the year or age of diagnosis, we used the earliest age or year from cohort data (n = 171), patient data (n = 45), or physician data (n = 26). Of these 242 confirmed cases, 132 had >5 years of cohort’s follow-up after diagnosis and 110 had ≤5 years of follow-up.
Nonmotor symptoms
The study assessed 18 PD nonmotor symptoms in its second and/or third DFU via structured questionnaires and self-reports (Supplementary Table 2). Several of these were assessed specifically for PD research (e.g., olfaction and dream-enacting behavior), others were for general research purposes (e.g., depression and pain). In addition, 8 symptoms that have not been reported in prodromal PD (e.g., swelling in joints/legs, shortness of breath) were asked side-by-side with some PD nonmotor symptoms, offering a unique opportunity to analyze them as “negative controls” to assess potential bias in symptom reporting. The timeframe asked varied by symptom, as detailed in Supplementary Table 2. Briefly, questions for poor olfaction, depression, anxiety, constipation, daytime sleepiness, insomnia, pain, and fatigue assessed the current/recent presence of symptoms. For dream-enacting behaviors, we asked for ever presence of the symptoms. The cognition questionnaire assessed symptoms in the last several years. All other symptoms were asked since the reference date (1/1/2009 for DFU-2 and 1/1/2012 for DFU-3). We did not consider any of these nonmotor symptoms in the PD diagnostic adjudication because they were mostly self-reported by the general population as part of the cohort-wide health follow-up surveys. Therefore, the data are not specific nor up to the level of rigor for PD diagnosis or differential diagnosis. Further, we also aimed to assess whether self-recognition of these symptoms in the general population was associated with future PD risk, and the inclusion of them in case adjudication would bias the analyses toward stronger associations.
PD polygenic risk score (PRS)
In a subpopulation to investigate olfaction and PD [29], we genotyped 3,722 participants along with all self-reported PD cases with DNA samples regardless of their final PD adjudication results. Detailed genetic analysis in this olfaction sub-study will be published in a separate manuscript. In this paper, we compared the associations of PRS with confirmed versus unconfirmed PD cases to validate our case adjudication. The PD PRS score was calculated based on 90 single nucleotide polymorphisms that were significantly associated with PD from the latest genome-wide association meta-analysis [30].
Statistical analyses
We first compared, between confirmed and unconfirmed cases, relevant medical/diagnostic data collected in cohort routine follow-ups (e.g., source of PD identification, motor symptoms, and medication uses) and in the additional case validation effort (e.g., details of symptoms and medication use and responses). Then, we presented the age-standardized prevalence of nonmotor symptoms from the cohort’s third follow-up across confirmed cases, unconfirmed cases, and non-PD cases who never reported a PD diagnosis using direct standardization with non-PD cases as the reference. Next, we validated PD case adjudication by examining PD PRS in relation to confirmed vs. unconfirmed PD cases. Furthermore, we investigated nonmotor vs. negative control symptoms at the cohort’s second DFU in relation to incident PD cases. Finally, we examined the association of PD with baseline age, race/ethnicity, education, smoking status, and daily caffeine intake. In these analyses, we examined outcomes of final adjudication versus cohort-level adjudication and presented corresponding odds ratios (ORs) and 95% confidence intervals (CIs) from multivariable logistic regression models. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary NC), and the type-I error rate was set at two-sided 0.05.
RESULTS
Figure 1 shows the adjudication process of the 371 potential PD patients identified. All data considered, a total of 242 potential PD diagnoses were confirmed, including 106 based on diagnostic information and/or medical records from patients’ treating physicians, 30 based on diagnostic history provided by the patients without additional information from their treating physicians, and 106 only based on reviewing routine cohort follow-up data. Of the 371 potential cases, 176 have adjudication results at both the cohort and patient/physician levels, and the kappa statistic coefficient between these two-levels of case-adjudication was 0.66 (95% CI: 0.52–0.80). Of these, 75 out of the 77 cohort-adjudicated “yes” were confirmed by additional reviewing of patient/physician level data, 46 out of the 51 “probable” were confirmed, 11 out of the 19 “possible” were confirmed, and 4 out of the 29 “no” were re-adjudicated as PD cases.
Fig. 1
Diagnostic adjudication of Parkinson’s diagnosis in the Sister Study.
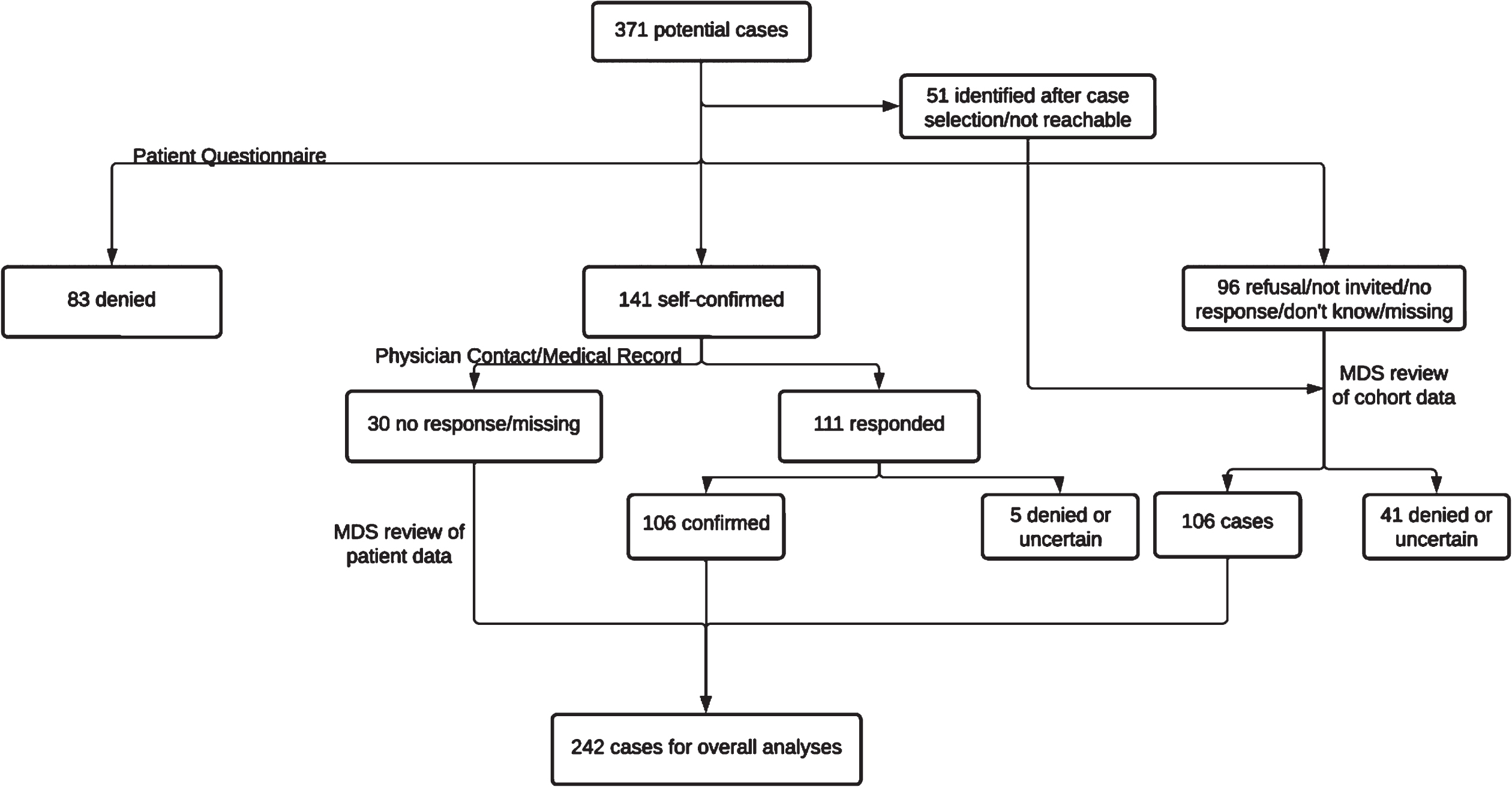
Table 1 compares the clinical features of confirmed versus unconfirmed PD cases at both the cohort-level and patient-physician level review. Based on cohort data, confirmed cases were older than those unconfirmed and more likely to report a later age of diagnosis. Further, 71.1% of the confirmed cases versus 10.1% of the unconfirmed cases were identified from multiple sources initially, and confirmed cases were more likely to report motor symptoms and PD medication usage, consistently from multiple surveys. In our additional data collection from self-reported patients to validate their PD diagnosis, the vast majority who denied the diagnosis did not provide detailed clinical information, but for the confirmed PD cases, clinical symptoms and treatment history are consistent with a clinical PD diagnosis.
Table 1
Clinical features of confirmed Parkinson’s disease (PD) cases versus those not confirmed
| Characteristics | Regular data collection | Additional data collection | ||
| from cohort follow-upsa | from potential patientsa | |||
| Confirmed | Unconfirmed | Confirmed | Unconfirmed | |
| (n = 242) | (n = 129) | (n = 141) | (n = 83) | |
| Age in January 2018 | 74.2 (7.2) | 70.3 (9.1) | 73.3 (7.1) | 69.5 (8.9) |
| Adjudicated age at diagnosis | 66.9 (8.8) | 63.6 (10.6) | 66.1 (8.2) | NA |
| Self-reported age at diagnosis | 67.3 (8.7) | 63.7 (10.6) | 66.4 (8.1) | NA |
| Regular data collection from cohort-wide follow-ups | ||||
| Source of PD identification | ||||
| From ≥2 sources | 172 (71.1%) | 13 (10.1%) | ||
| Reports from DFU only | 199 (82.6%) | 82 (78.1%) | ||
| Reports from AHU only | 190 (78.8%) | 28 (26.7%) | ||
| Other sources only | 51 (21.2%) | 11 (10.5%) | ||
| PD medication use | ||||
| Reported at any DFU | 177 (73.1%) | 25 (19.4%) | ||
| Reported at ≥2 DFUs | 99 (40.9%) | 11 (8.5%) | ||
| Any motor symptoms | ||||
| Reported at any DFU | 231 (97.9%) | 92 (73.6%) | ||
| Reported at ≥2 DFUs | 183 (77.5%) | 69 (55.2%) | ||
| Reported ≥2 motor symptoms | ||||
| Reported at any DFU | 213 (90.3%) | 74 (59.2%) | ||
| Reported at ≥2 DFUs | 144 (61.0%) | 46 (36.8%) | ||
| Additional data collection | ||||
| from potential patients in the diagnostic validation effort | ||||
| Who made or confirmed the Parkinson’s Diagnosis? | ||||
| Movement disorder specialist or neurologist | 132 (97.8%) | |||
| Other doctor or health care provider only | 3 (2.2%) | |||
| Have you ever had any of the following symptoms of Parkinson’s disease? | ||||
| Trembling or shaking on any part of the body | 113 (91.9%) | Data not | ||
| Did symptoms ever get better with medication? | 95 (92.2%) | provided as all | ||
| Slowness in moving, such as walking or performing a task | 95 (79.8%) | responses are | ||
| Did symptoms ever get better with medication? | 71 (84.5%) | smaller than 5 | ||
| Small handwriting than it once was | 100 (82.6%) | |||
| Did symptoms ever get better with medication? | 48 (57.1%) | |||
| Dragging a foot, shuffling feet, or taking smaller steps when walking compared to the past | 89 (73.0%) | |||
| Did symptoms ever get better with medication? | 55 (76.4%) | |||
| Difficulty getting up from a chair or sofa or getting out of a car | 91 (74.6%) | |||
| Did symptoms ever get better with medication? | 44 (66.7%) | |||
| Other symptoms | 57 (81.4%) | |||
| Did symptoms ever get better with medication? | 36 (75.0%) | |||
| Were any of the symptoms ever more severe on one side of the body? | 107 (84.3%) | |||
| Did you ever take the following Parkinson’s medication for more than 2 months? | ||||
| Carbidopa or levodopa such as Sinemet, Stalevo, Parcopa, Duodopa or Rytary | 110 (89.4%) | Data not | ||
| Did the medication ever help with Parkinson’s symptoms? | 101 (97.1%) | provided as all | ||
| Do you still take the medication? | 101 (96.2%) | responses are | ||
| Dopamine agonists such as pramipexole (Mirapex), ropinirole (Requip), pergolide (Permax), rotigotine (Neupro), or bromocriptine (Parlodel) | 52 (44.1%) | smaller than 5 | ||
| Did the medication ever help with Parkinson’s symptoms? | 45 (80.4%) | |||
| Do you still take the medication? | 39 (69.6%) | |||
| Mao-B inhibitors such as rasagiline (Azilect) or selegiline (Eldepryl or Zelapar) | 42 (35.0%) | |||
| Did the medication ever help with Parkinson’s symptoms? | 25 (69.4%) | |||
| Do you still take the medication? | 32 (72.7%) | |||
| Other PD prescribed PD medications (e.g., Artane or Amantadine) | 28 (24.3%) | |||
| Did the medication ever help with Parkinson’s symptoms? | 20 (69.0%) | |||
| Do you still take the medication? | 21 (61.8%) | |||
SD, standard deviation; DFU, detailed follow-up; AHU, annual health update. aMean (SD) for continuous variables and frequency count (percentage) for categorical variables are presented. The percentages for all clinical features were calculated with missing excluded.
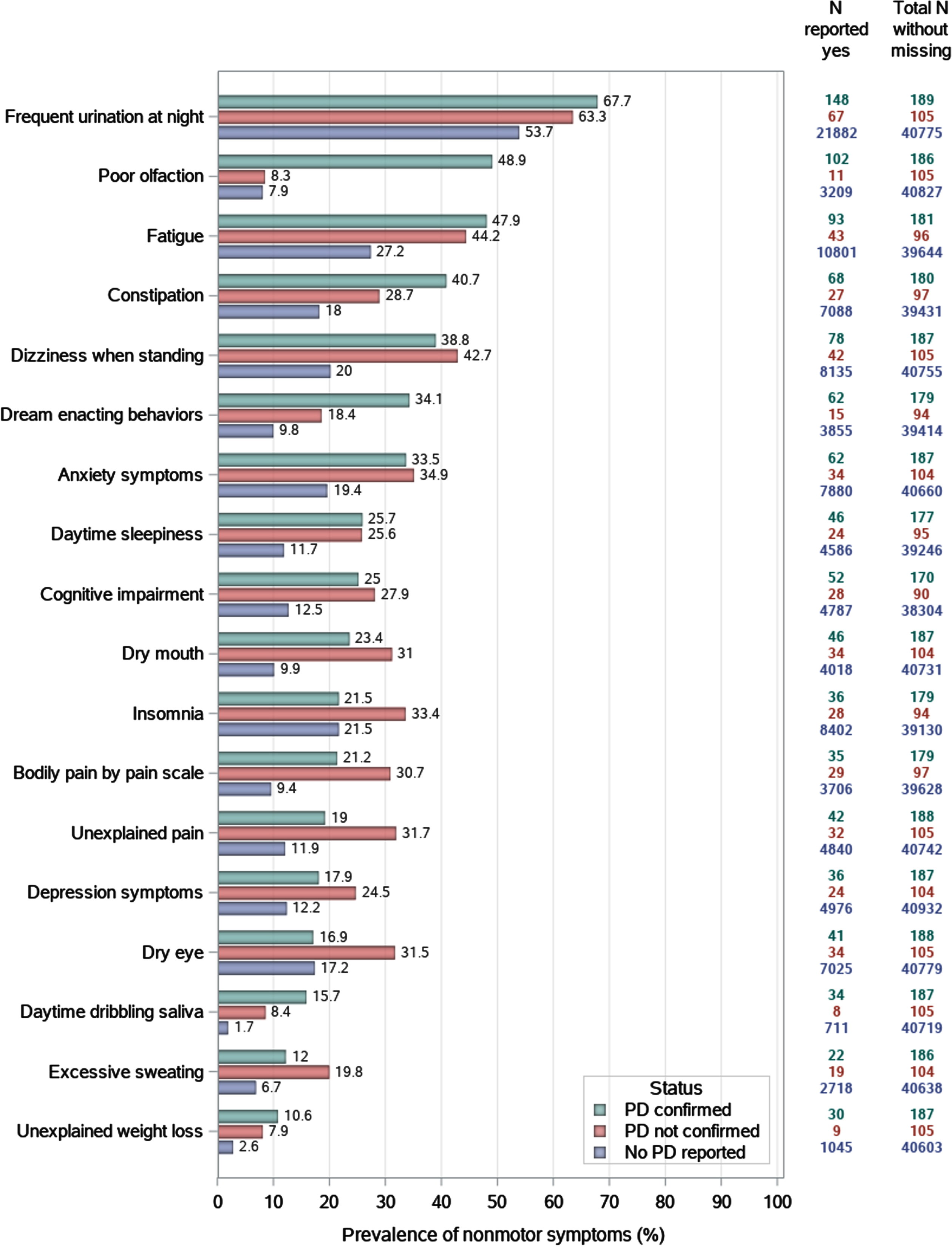
Figure 2 presents the age-standardized prevalence of nonmotor symptoms across PD self-reports and diagnostic confirmation results. As we used symptoms at the third DFU, most cases are prevalent. Confirmed cases were much more likely to report hyposmia, constipation, dream-enacting behaviors, daytime saliva dribbling, and unexplained weight loss than unconfirmed cases, and they both were much higher than that of non-PD cases (except for the comparable prevalence of hyposmia between unconfirmed cases and non-PD cases). For regular urination at night, fatigue, dizziness when standing, anxiety, daytime sleepiness, cognitive impairment, fatigue, and frequent urination at night, symptom prevalence was comparable between confirmed and unconfirmed cases, both much higher than non-PD cases. For dry mouth, pain severity, depression, unexplained pain, and excessive sweating, the prevalence was higher in unconfirmed cases than in confirmed cases, both higher than non-PD cases. For insomnia and dry eye, the prevalence was comparable between confirmed cases and non-PD cases, both lower than that in unconfirmed cases.
Fig. 2
Self-reported nonmotor symptoms of confirmed Parkinson’s disease cases, unconfirmed cases, versus controls, using data from the cohort’s third detailed follow-up.
PD PRS was strongly associated with being a confirmed case (ORinterquartilerange = 1.74, 95% CI: 1.45–2.10, p for trend <0.0001), but not with being an unconfirmed case (ORinterquartilerange = 1.05, 95% CI: 0.76–1.45) (Table 2). Incident PD risk was also associated with older age and higher education level (graduate vs. college), but lower in non-Hispanic Blacks (Supplementary Table 3). Smoking and coffee drinking were both associated with a modestly lower risk of PD, albeit statistically insignificant. Results were similar between analyses using final case adjudication versus using that at the cohort-level.
Table 2
Parkinson’s disease (PD) polygenic risk score in relation to confirmed and unconfirmed PD cases versus non-PD
| Polygenic risk score | Confirmed cases vs. Non-PD | Unconfirmed cases vs. Non-PD | ||||
| Cases | Non-PD | OR (95% CI) | Cases | Non-PD | OR (95% CI) | |
| (n = 234) | (n = 3,403) | (n = 85) | (n = 3,403) | |||
| All participants | ||||||
| Quartile 1 | 30 (12.8) | 874 (25.7) | Reference | 26 (30.6) | 874 (25.7) | Reference |
| Quartile 2 | 47 (20.1) | 864 (25.4) | 1.56 (0.95, 2.55) | 20 (23.5) | 864 (25.4) | 1.12 (0.58, 2.16) |
| Quartile 3 | 73 (31.2) | 840 (24.7) | 2.58 (1.62, 4.10) | 18 (21.2) | 840 (24.7) | 1.06 (0.54, 2.11) |
| Quartile 4 | 84 (35.9) | 825 (24.2) | 2.98 (1.89, 4.71) | 21 (24.7) | 825 (24.2) | 1.25 (0.64, 2.41) |
| Per inter-quartile range | 1.74 (1.45, 2.10) | 1.05 (0.76, 1.45) | ||||
| P for trend | <0.0001 | 0.5476 | ||||
| Non-Hispanic White participants | ||||||
| Quartile 1 | 26 (11.7) | 670 (22.1) | Reference | 22 (29.7) | 670 (22.1) | Reference |
| Quartile 2 | 44 (19.7) | 803 (26.5) | 1.45 (0.87, 2.41) | 17 (23.0) | 803 (26.5) | 0.90 (0.45, 1.81) |
| Quartile 3 | 72 (32.3) | 781 (25.8) | 2.53 (1.58, 4.05) | 15 (20.3) | 781 (25.8) | 0.87 (0.42, 1.79) |
| Quartile 4 | 81 (36.3) | 772 (25.5) | 2.89 (1.82, 4.61) | 20 (27.0) | 772 (25.5) | 1.14 (0.58, 2.23) |
| Per inter-quartile range | 1.70 (1.41, 2.06) | 1.05 (0.74, 1.47) | ||||
| P for trend | <0.0001 | 0.6968 | ||||
PD, Parkinson’s disease; OR, odds ratio; CI, confidence interval. Analysis adjusted for age, race, and the first 5 principal components.
Of the 242 confirmed PD cases, 99 were incident diagnoses after the cohort’s second DFU. With few exceptions, nonmotor features reported at the second DFU were associated with the risk of PD (Table 3). Moderate to strong associations were found for the well-documented PD prodromal symptoms of hyposmia (OR = 4.88), depression (OR = 2.53), dream-enacting behavior (OR = 2.20), cognitive impairment (OR = 2.08), and daytime sleepiness (OR = 2.00). Of the other nonmotor symptoms that have been rarely studied in prodromal PD, we found significant associations with unexplained weight loss (OR = 4.02), dry eyes (OR = 1.85), dry mouth (OR = 1.91), and fatigue (OR = 1.71). In contrast, only one of the eight negative control symptoms (i.e., shortness of breath while walking) was associated with incident PD. The findings were similar when using cohort-level confirmed cases as the analytic outcome.
Table 3
Self-reported symptoms at the cohort’s second follow-up and future risk of Parkinson’s disease (PD)
| Symptoms | Finally adjudicated incident casesa | Incident cases adjudicated using cohort data onlya | ||||
| PD | Non-PD | OR (95% CI) | PD | Non-PD | OR (95% CI) | |
| (n = 99)/ | (n = 45,922) | (n = 109)/ | (n = 45,922) | |||
| PD nonmotor symptoms | ||||||
| Hyposmia | ||||||
| No | 68 (68.7) | 41,789 (91.0) | Reference | 77 (70.6) | 41,789 (91.0) | Reference |
| Yes | 28 (28.3) | 2,930 (6.4) | 4.88 (3.13, 7.62) | 28 (25.7) | 2,930 (6.4) | 4.36 (2.81, 6.74) |
| Dream-enacting behaviors | ||||||
| No | 78 (78.8) | 38,359 (83.5) | Reference | 87 (79.8) | 38,359 (83.5) | Reference |
| Yes | 16 (16.2) | 4,348 (9.5) | 2.20 (1.28, 3.78) | 17 (15.6) | 4,348 (9.5) | 2.05 (1.22, 3.47) |
| Constipation | ||||||
| No | 60 (60.6) | 31,622 (68.9) | Reference | 66 (60.6) | 31,622 (68.9) | Reference |
| Yes | 36 (36.4) | 10,980 (23.9) | 1.86 (1.23, 2.81) | 40 (36.7) | 10,980 (23.9) | 1.86 (1.25, 2.76) |
| Daytime sleepiness | ||||||
| No | 76 (76.8) | 37,429 (81.5) | Reference | 84 (77.1) | 37,429 (81.5) | Reference |
| Yes | 20 (20.2) | 5,088 (11.1) | 2.00 (1.22, 3.28) | 22 (20.2) | 5,088 (11.1) | 1.97 (1.23, 3.17) |
| Depression | ||||||
| No | 56 (56.6) | 33,491 (72.9) | Reference | 61 (56.0) | 33,491 (72.9) | Reference |
| Yes | 42 (42.4) | 11,385 (24.8) | 2.53 (1.69, 3.78) | 47 (43.1) | 11,385 (24.8) | 2.56 (1.74, 3.75) |
| Anxiety | ||||||
| No | 72 (72.7) | 37,471 (81.6) | Reference | 80 (73.4) | 37,471 (81.6) | Reference |
| Yes | 24 (24.2) | 7,128 (15.5) | 1.97 (1.24, 3.13) | 26 (23.9) | 7,128 (15.5) | 1.89 (1.21, 2.96) |
| Cognitive impairment | ||||||
| No | 68 (68.7) | 35,853 (78.1) | Reference | 74 (67.9) | 35,853 (78.1) | Reference |
| Yes | 23 (23.2) | 5,496 (12.0) | 2.08 (1.29, 3.36) | 27 (24.8) | 5,496 (12.0) | 2.23 (1.43, 3.49) |
| Dry eyes | ||||||
| No | 69 (69.7) | 37,884 (82.5) | Reference | 75 (68.8) | 37,884 (82.5) | Reference |
| Yes | 28 (28.3) | 6,784 (14.8) | 1.85 (1.19, 2.88) | 32 (29.4) | 6,784 (14.8) | 1.95 (1.29, 2.97) |
| Dry mouth | ||||||
| No | 80 (80.8) | 41,037 (89.4) | Reference | 87 (79.8) | 41,037 (89.4) | Reference |
| Yes | 16 (16.2) | 3,627 (7.9) | 1.91 (1.11, 3.28) | 19 (17.4) | 3,627 (7.9) | 2.08 (1.26, 3.44) |
| Weight loss | ||||||
| No | 86 (86.9) | 43,418 (94.5) | Reference | 96 (88.1) | 43,418 (94.5) | Reference |
| Yes | 10 (10.1) | 1,181 (2.6) | 4.02 (2.07, 7.81) | 10 (9.2) | 1,181 (2.6) | 3.51 (1.81, 6.80) |
| Excessive sweating | ||||||
| No | 86 (86.9) | 41,946 (91.3) | Reference | 93 (85.3) | 41,946 (91.3) | Reference |
| Yes | 9 (9.1) | 2,662 (5.8) | 1.63 (0.82, 3.26) | 12 (11.0) | 2,662 (5.8) | 1.99 (1.09, 3.65) |
| Insomnia | ||||||
| No | 78 (78.8) | 33,119 (72.1) | Reference | 85 (78.0) | 33,119 (72.1) | Reference |
| Yes | 17 (17.2) | 9,318 (20.3) | 0.84 (0.50, 1.42) | 20 (18.3) | 9,318 (20.3) | 0.90 (0.55, 1.47) |
| PD | Non-PD | OR (95% CI) | PD | Non-PD | OR (95% CI) | |
| (n = 99)/ | (n = 45,922) | (n = 109)/ | (n = 45,922) | |||
| Pain | ||||||
| None or mild | 81 (81.8) | 39,016 (85.0) | Reference | 89 (81.7) | 39,016 (85.0) | Reference |
| Moderate, severe or extremely severe | 13 (13.1) | 3,837 (8.4) | 1.73 (0.96, 3.12) | 15 (13.8) | 3,837 (8.4) | 1.77 (1.02, 3.07) |
| Fatigue | ||||||
| None or mild | 59 (59.6) | 31,317 (68.2) | Reference | 65 (59.6) | 31,317 (68.2) | Reference |
| Moderate, severe or extremely severe | 36 (36.4) | 11,605 (25.3) | 1.71 (1.12, 2.59) | 40 (36.7) | 11,605 (25.3) | 1.70 (1.14, 2.53) |
| Negative control symptoms | ||||||
| Swelling in joints | ||||||
| No | 81 (81.8) | 39,080 (85.1) | Reference | 89 (81.7) | 39,080 (85.1) | Reference |
| Yes | 15 (15.2) | 5,623 (12.2) | 1.14 (0.66, 1.99) | 17 (15.6) | 5,623 (12.2) | 1.18 (0.70, 1.98) |
| Joint stiffness in morning | ||||||
| No | 71 (71.7) | 34,538 (75.2) | Reference | 77 (70.6) | 34,538 (75.2) | Reference |
| Yes | 24 (24.2) | 10,121 (22.0) | 1.03 (0.65, 1.64) | 28 (25.7) | 10,121 (22.0) | 1.11 (0.72, 1.72) |
| Wheezing or whistling in chest | ||||||
| No | 83 (83.8) | 39,910 (86.9) | Reference | 91 (83.5) | 39,910 (86.9) | Reference |
| Yes | 12 (12.1) | 4,681 (10.2) | 1.21 (0.66, 2.22) | 14 (12.8) | 4,681 (10.2) | 1.27 (0.72, 2.24) |
| Shortness of breath (exercise) | ||||||
| No | 65 (65.7) | 32,300 (70.3) | Reference | 68 (62.4) | 32,300 (70.3) | Reference |
| Yes | 31 (31.3) | 12,363 (26.9) | 1.02 (0.66, 1.58) | 38 (34.9) | 12,363 (26.9) | 1.20 (0.80, 1.80) |
| Shortness of breath (at rest) | ||||||
| No | 92 (92.9) | 43,310 (94.3) | Reference | 99 (90.8) | 43,310 (94.3) | Reference |
| Yes | 3 (3.0) | 1,340 (2.9) | 1.08 (0.34, 3.44) | 6 (5.5) | 1,340 (2.9) | 1.98 (0.87, 4.55) |
| Shortness of breath (lying down) | ||||||
| No | 91 (91.9) | 43,210 (94.1) | Reference | 98 (89.9) | 43,210 (94.1) | Reference |
| Yes | 4 (4.0) | 1,402 (3.1) | 1.38 (0.50, 3.77) | 7 (6.4) | 1,402 (3.1) | 2.21 (1.02, 4.78) |
| Shortness of breath (walking) | ||||||
| No | 70 (70.7) | 38,146 (83.1) | Reference | 75 (68.8) | 38,146 (83.1) | Reference |
| Yes | 24 (24.2) | 6,457 (14.1) | 1.73 (1.08, 2.77) | 29 (26.6) | 6,457 (14.1) | 1.94 (1.25, 3.00) |
| Swelling or edema in legs | ||||||
| No | 75 (75.8) | 38,135 (83.0) | Reference | 81 (74.3) | 38,135 (83.0) | Reference |
| Yes | 19 (19.2) | 6,462 (14.1) | 1.27 (0.77, 2.12) | 23 (21.1) | 6,462 (14.1) | 1.43 (0.90, 2.28) |
PD, Parkinson’s disease; OR, odds ratio; CI, confidence interval. Frequency count (percentage) are presented. Number may not add up to total due to missing. Analyses adjusted for baseline age, race, education, smoking status, and daily caffeine intake.
DISCUSSION
In this nationwide cohort of women, we confirmed 242 PD diagnoses out of the 371 self-reports. Confirmed cases are clearly different from those unconfirmed in clinical features and known associations and features of PD further support the adjudication validity. For example, PD PRS was associated with confirmed cases in a dose-response manner, but not with unconfirmed cases. Further, the key nonmotor symptoms of poor olfaction, dream-enacting behaviors, and constipation were much more common in confirmed vs. unconfirmed cases. Finally, most nonmotor symptoms were associated with incident PD diagnosis in the study population as compared to none but one of the negative control symptoms. All support the validity of PD case ascertainment and relevant clinical feature data in this cohort.
Most late-onset sporadic clinical PD is likely the result of environmental exposures, alone or interacting with genetic factors, over years or decades of the disease’s prodromal development. While the past 25 years have seen multiple breakthroughs in understanding PD genetics, the search for nongenetic causes of PD has been largely stagnant [10]. This is particularly true for women among whom fewer robust associations have been observed. For example, the associations of PD with coffee drinking [31], dairy consumption [32], and urate [25] have been robustly reported in men, but data in women are less consistent. This may result from the biological complexity of PD development in women, for example, potential influences from hormonal changes [24]. Further, most occupational exposures of PD etiological research interest (e.g., pesticides and welding) are overwhelmingly represented by men [33–35]. In contrast, common chemical exposures in women (e.g., house cleaning and hair dye) are often less investigated [36]. Therefore, there is a need to expand the scope of environmental PD research in women.
The NIEHS Sister Study has multiple features desirable for PD environmental research in women [27]. First, study participants were from all 50 states of the US, representing potentially diverse environmental exposures. Study investigators have collected rich environmental data as summarized in Supplementary Table 1. Many of these are of substantial interest for PD research in women, and each should be thoroughly investigated. In addition, the cohort has blood, urine, house dust, and toenail samples from nearly all participants (≥98%), substantially enhancing environmental exposure assessments and expanding research scopes. Further, the study repeatedly assessed PD-relevant motor and nonmotor symptoms since its second DFU in 2012–2014, when the average age was 61.5 years. These features uniquely enable the study of PD environmental risk factors in the context of disease prodromal development. Therefore, Sister Study presents a rare opportunity to prospectively investigate environmental contributions to PD prodromal development in women, by leveraging its extensive environmental data collection and repeated and systematic assessments of nonmotor and motor symptoms.
Large multipurpose cohorts like the Sister Study were often established to study common chronic diseases such as cardiovascular diseases and cancer [37, 38]. In comparison, PD is much rarer and occurs predominately late in life. Its diagnosis relies on patients’ complaints of motor dysfunctions, followed by expert’s evaluations and a dopaminergic treatment trial. This often requires sequential clinical visits to neurology specialists, which are almost infeasible to implement in large population-based cohorts. With few exceptions [39, 40], investigators often seek alternative approaches to cost-efficiently identify and confirm PD cases, for example, adjudication of self-reported diagnosis with additional diagnostic data collection from potential patients and their treating physicians [5, 20, 23, 41, 42], solely based on routine cohort data collection (e.g., self-reports, medication uses, hospitalization/death surveillances) [19, 21], or via linkage to administrative data and electronic medical records [22].
While these strategies make PD case ascertainment in large cohorts feasible and affordable, they have some less-discussed limitations. For example, adjudications based on systematic cohort data collection suffer from reporting errors and lack of relevant diagnostic information (e.g., response to medication). Secondary administrative or hospitalization records are not systematically collected for research, are often incomplete, and are subject to coding errors [43, 44]. Requesting PD diagnostic history from patients or their treating physicians can address these concerns, but this approach is subject to non-responses from patients or their treating physicians. This is particularly true if case confirmation was conducted retrospectively with prevalent cases of poor health or deceased cases. For example, in the ARIC study [20] of the 293 potential cases identified from ∼20 years of follow-up, 95 (32.4%) did not respond when we contacted the study participants; and of the 88 self-confirmed cases that we contacted their treating physicians for medical records, 37 (42%) did not respond to the request.
Sister Study participants are predominantly highly educated and health-conscious women with a follow-up rate consistently 90% or higher. Even in this highly participatory population, 96 out of 320 (30%) women did not respond to our request to provide additional PD diagnostic information in our standalone diagnostic confirmation effort. Fortunately, as part of the cohort’s regular follow-ups, in addition to the self-reported physician-made diagnosis and age of diagnosis, we asked whether participants used medications to treat PD, details of current uses of all prescribed and over-the-counter medicines, and selected symptoms of motor dysfunction. These data were collected consistently every three years for up to 15 years, enabling more comprehensive case adjudication using longitudinal reports of symptoms and medication usages beyond self-reported physician-made diagnosis.
These detailed data collection in the Sister Study allows us to explore the validity of case adjudication solely using cohort data without considering further diagnostic data collection from patients and their treating physicians, circumventing additional non-responses and costs of data collection. Of those who provided both, cohort-based adjudication showed substantial agreement when compared with adjudication results using PD diagnostic data from patients and treating physicians. Inconsistencies possibly came from inconsistent/lack of PD medication use information from cohort data, newly diagnosed with little information, or reporting errors.
Empirical data support the validity of PD adjudication in this cohort. As expected, PD risk was age-dependent and higher among non-Hispanic White participants and participants with higher education level [45, 46]. Findings on smoking and caffeine intake are also supportive, albeit the associations were not statistically significant. Notably, prior studies found that the role of caffeine in PD might be complicated by female-specific factors such as hormonal use [24], and thus an inverse association of caffeine with PD might not be evident in women [31]. Of all potential risk factors for late-onset sporadic PD, genetic susceptibility is the most robust finding with the strongest causal insights [30]. In our analysis among a subgroup of women with genetic data, PD PRS was dose-responsively associated with confirmed PD cases but not with those unconfirmed, supporting that our case-adjudication results were specific to PD.
To the best of our knowledge, the Sister Study has one of the broadest assessments of PD nonmotor symptoms in population-based multipurpose cohorts, allowing us to compare a large set of diverse nonmotor symptoms of PD side-by-side in a large population. While some of the prodromal symptoms are well-documented in the literature (e.g., poor olfaction, RBD, and constipation) [4–7], others have not been extensively studied in prodromal PD, including dry eyes/mouth, dizziness, urinary symptoms, unexplained pain/weight loss, and excessive sweating/fatigue. The strongest association was found with poor olfaction, followed by unexplained weight loss, depression, and dream-enacting behaviors. Of these, weight loss has been less studied in prodromal PD, and our finding is consistent with our prior longitudinal cohort analysis [47, 48], suggesting that PD patients may start a persistent weight loss 2–4 years before PD clinical diagnosis. We also found moderate associations of incident PD with fatigue, dry eyes, and dry mouth, suggesting these less-studied symptoms may also be part of prodromal PD. In contrast, with one possible exception, none of the negative control symptoms (e.g., swelling in joints, shortness of breath, wheezing) were associated with incident PD, supporting the validity of both our PD ascertainment and assessments of the symptoms. Interestingly, some symptoms were more prevalent in unconfirmed cases than confirmed or non-cases such as depression and over-sweating. One possibility is that these individuals had other parkinsonism or age-related conditions that was mis-reported as PD by the study participants. Unfortunately, we did not have adequate information to make such differential diagnostic adjudication.
Our study has several limitations. First, study participants are mostly non-Hispanic White women volunteers with relatively high education levels. Their exposure profiles may not be nationally representative, and the incidence of PD is lower in women compared to men. Thus, study findings from this cohort may not be readily generalizable to populations with different sex/race/ethnicity compositions. Further, the LRRK2 G2019S mutation has been linked to both breast cancer and PD [49]. While participants of our study are sisters of breast cancer patients, this mutation is rare in people of European descents [50–52] and thus may little affect research on PD in this cohort. Second, case ascertainment mostly started with self-reported physician-made diagnosis, and our goal was mainly to confirm or refute the accuracy of these reports rather than re-diagnose patients with clinical exams. The clinical information we collected is not up to the standard for differential diagnosis, and therefore, our adjudication remains subject to errors, particularly mis-adjudication of other Parkinsonism as PD. Further, the clinical PD diagnostic criteria have evolved over time in the past several decades [2, 53, 54], and our diagnostic adjudication relied on the criteria that the patients’ treating physician made at the time of their diagnosis. Furthermore, some cases would be inevitably missed via our approach. However, with detailed triennial surveys and annual health updates, patients would have multiple opportunities to report their diagnoses. PD incidence (e.g., 99/100,000 person-years for women ages ≥65 years) in this cohort is comparable to that in women of other studies [55], indirectly supporting case ascertainment strategy in our study population. Third, while the study has collected more PD-relevant symptomatic information than most of the other general-purpose cohorts, it was not designed for PD research and relevant clinical data collection is limited. Finally, in DFUs, we asked about motor symptoms in terms that are sensible to the general public, and this inadvertently makes the questions less specific to PD. Further, both motor and nonmotor symptoms were mostly self-reported or assessed, subject to self-awareness and substantial measurement errors. For example, only about a third of Sister participants with hyposmia recognized that they had it [29], consistent with other population-based data [56, 57]. We therefore established a sub-cohort of ∼3,400 Sister participants with objectively assessed olfaction and genome-wide genotyping [29], hoping to prospectively follow up this sub-cohort with more detailed symptom assessment to study environmental contributions to PD in women.
In summary, findings from this study support the case ascertainment strategy in a well-established nationwide environmental health cohort of women. The collection of a wide range of prodromal symptom data in this cohort will provide a unique opportunity to search for environmental triggers and accelerators of PD at the early stages of disease development.
ACKNOWLEDGMENTS
This study is supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Parkinson’s Research Program (W81XWH-17-1-0536) and the Parkinson’s Foundation (PF-IMP-1825), and in part by the Intramural Research Programs of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES044005) and National Institute on Aging (ZO1-AG000949). Dr. Chen is also supported by grants from NIA (R01AG071517) and NIEHS (R01ES029227). Dr. Huang is also supported by a NIH grant (R01ES019672) and the Penn State-College of Medicine Translational Brain Research Center. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense and the National Institutes of Health.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data that support findings from this study are available from the Sister Study, following appropriate approval procedures as detailed at https://sisterstudy.niehs.nih.gov/English/collaboration.htm.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230053.
REFERENCES
[1] | Poewe W , Seppi K , Tanner CM , Halliday GM , Brundin P , Volkmann J , Schrag AE , Lang AE ((2017) ) Parkinson disease. Nat Rev Dis Primers 3: , 17013. |
[2] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[3] | Chen H , Zhao EJ , Zhang W , Lu Y , Liu R , Huang X , Ciesielski-Jones AJ , Justice MA , Cousins DS , Peddada S ((2015) ) Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Transl Neurodegener 4: , 1. |
[4] | Ross GW , Petrovitch H , Abbott RD , Tanner CM , Popper J , Masaki K , Launer L , White LR ((2008) ) Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol 63: , 167–173. |
[5] | Gao X , Chen H , Schwarzschild MA , Ascherio A ((2011) ) A prospective study of bowel movement frequency and risk of Parkinson’s disease. Am J Epidemiol 174: , 546–551. |
[6] | Abbott RD , Ross GW , Petrovitch H , Tanner CM , Davis DG , Masaki KH , Launer LJ , Curb JD , White LR ((2007) ) Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 22: , 1581–1586. |
[7] | Postuma RB , Gagnon JF , Vendette M , Fantini ML , Massicotte-Marquez J , Montplaisir J ((2009) ) Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 72: , 1296–1300. |
[8] | Lang AE ((2011) ) A critical appraisal of the premotor symptoms of Parkinson’s disease: Potential usefulness in early diagnosis and design of neuroprotective trials. Mov Disord 26: , 775–783. |
[9] | Braak H , Bohl JR , Müller CM , Rüb U , de Vos RAI , Tredici KD ((2006) ) Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21: , 2042–2051. |
[10] | Chen H , Ritz B ((2018) ) The search for environmental causes of Parkinson’s disease: Moving forward. J Parkinsons Dis 8: , S9–S17. |
[11] | Chen H ((2018) ) The changing landscape of parkinson epidemiologic research. J Parkinsons Dis 8: , 1–12. |
[12] | Marras C , Hincapie CA , Kristman VL , Cancelliere C , Soklaridis S , Li A , Borg J , af Geijerstam JL , Cassidy JD ((2014) ) Systematic review of the risk of Parkinson’s disease after mild traumatic brain injury: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 95: , S238–244. |
[13] | Nelson LM ((2018) ) Physical activity and Parkinson disease risk: An intriguing link. JAMA Netw Open 1: , e182633. |
[14] | Ma C , Liu Y , Neumann S , Gao X ((2017) ) Nicotine from cigarette smoking and diet and Parkinson disease: A review. Transl Neurodegener 6: , 18. |
[15] | Ascherio A , Schwarzschild MA ((2016) ) The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol 15: , 1257–1272. |
[16] | Chen H , Ding D , Wang J , Zhao Q , Meng H , Li H , Gao YT , Shu XO , Tanner CM , Hong Z , Yang G ((2015) ) Parkinson’s disease research in a prospective cohort in China. Parkinsonism Relat Disord 21: , 1200–1204. |
[17] | Driver JA , Smith A , Buring JE , Gaziano JM , Kurth T , Logroscino G ((2008) ) Prospective cohort study of type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 31: , 2003–2005. |
[18] | Reedijk M , Huss A , Verheij RA , Peeters PH , Vermeulen RCH ((2020) ) Parkinson’s disease case ascertainment in prospective cohort studies through combining multiple health information resources. PLoS One 15: , e0234845. |
[19] | Jain S , Himali J , Beiser A , Ton TG , Kelly-Hayes M , Biggs ML , Delaney JA , Rosano C , Seshadri S , Frank SA ((2015) ) Validation of secondary data sources to identify Parkinson disease against clinical diagnostic criteria. Am J Epidemiol 181: , 185–190. |
[20] | Huang X , Alonso A , Guo X , Umbach DM , Lichtenstein ML , Ballantyne CM , Mailman RB , Mosley TH , Chen H ((2015) ) Statins, plasma cholesterol, and risk of Parkinson’s disease: A prospective study. Mov Disord 30: , 552–559. |
[21] | Chen H , Shrestha S , Huang X , Jain S , Guo X , Tranah GJ , Garcia ME , Satterfield S , Phillips C , Harris TB , Health ABC Study ((2017) ) Olfaction and incident Parkinson disease in US white and black older adults. Neurology 89: , 1441–1447. |
[22] | Gallo V , Brayne C , Forsgren L , Barker RA , Petersson J , Hansson O , Lindqvist D , Ruffmann C , Ishihara L , Luben R , Arriola L , Bergareche A , Gavrila D , Erro ME , Vanacore N , Sacerdote C , Bueno-de-Mesquita B , Vermeulen R , Seelen M , Sieri S , Masala G , Ramat S , Kyrozis A , Thricopolou A , Panico S , Mattiello A , Kaaks R , Teucher B , Katzke V , Kloss M , Curry L , Calboli F , Riboli E , Vineis P , Middleton L ((2015) ) Parkinson’s disease case ascertainment in the EPIC Cohort: The Neuro EPIC4PD Study. Neurodegener Dis 15: , 331–338. |
[23] | Shrestha S , Parks CG , Richards-Barber M , Chen H , Sandler DP ((2021) ) Parkinson’s disease case ascertainment in a large prospective cohort. PLoS One 16: , e0251852. |
[24] | Ascherio A , Chen H , Schwarzschild MA , Zhang SM , Colditz GA , Speizer FE ((2003) ) Caffeine, postmenopausal estrogen, and risk of Parkinson’s disease. Neurology 60: , 790–795. |
[25] | O’Reilly EJ , Gao X , Weisskopf MG , Chen H , Schwarzschild MA , Spiegelman D , Ascherio A ((2010) ) Plasma urate and Parkinson’s disease in women. Am J Epidemiol 172: , 666–670. |
[26] | Jiang W , Ju C , Jiang H , Zhang D ((2014) ) Dairy foods intake and risk of Parkinson’s disease: A dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol 29: , 613–619. |
[27] | Sandler DP , Hodgson ME , Deming-Halverson SL , Juras PS , D’Aloisio AA , Suarez LM , Kleeberger CA , Shore DL , DeRoo LA , Taylor JA , Weinberg CR , Sister Study Research Team ((2017) ) The Sister Study Cohort: Baseline methods and participant characteristics. Environ Health Perspect 125: , 127003. |
[28] | Weinberg CR , Shore DL , Umbach DM , Sandler DP ((2007) ) Using risk-based sampling to enrich cohorts for endpoints, genes, and exposures. Am J Epidemiol 166: , 447–455. |
[29] | Cao Z , Yang A , D’Aloisio AA , Suarez L , Deming-Halverson S , Li C , Luo Z , Pinto JM , Werder EJ , Sandler DP , Chen H ((2022) ) Assessment of self-reported sense of smell, objective testing, and associated factors in middle-aged and older women. JAMA Otolaryngol Head Neck Surg 148: , 408–417. |
[30] | Nalls MA , Blauwendraat C , Vallerga CL , Heilbron K , Bandres-Ciga S , Chang D , Tan M , Kia DA , Noyce AJ , Xue A , Bras J , Young E , von Coelln R , Simon-Sanchez J , Schulte C , Sharma M , Krohn L , Pihlstrom L , Siitonen A , Iwaki H , Leonard H , Faghri F , Gibbs JR , Hernandez DG , Scholz SW , Botia JA , Martinez M , Corvol JC , Lesage S , Jankovic J , Shulman LM , Sutherland M , Tienari P , Majamaa K , Toft M , Andreassen OA , Bangale T , Brice A , Yang J , Gan-Or Z , Gasser T , Heutink P , Shulman JM , Wood NW , Hinds DA , Hardy JA , Morris HR , Gratten J , Visscher PM , Graham RR , Singleton AB , 23 and Me Research Team; System Genomics of Parkinson’s Disease Consortium; International Parkinson’s Disease Genomics Consortium ((2019) ) Identification ofnovel risk loci, causal insights, and heritable risk for Parkinson’sdisease: A meta-analysis of genome-wide association studies. Lancet Neurol 18: , 1091–1102. |
[31] | Ascherio A , Zhang SM , Hernán MA , Kawachi I , Colditz GA , Speizer FE , Willett WC ((2001) ) Prospective study of caffeine consumption andrisk of Parkinson’s disease in men and women. Ann Neurol 50: , 56–63. |
[32] | Chen H , Zhang SM , Hernan MA , Willett WC , Ascherio A ((2002) ) Diet and Parkinson’s disease: A potential role of dairy products in men. Ann Neurol 52: , 793–801. |
[33] | Gamache PL , Haj Salem I , Roux-Dubois N , Le Bouthillier J , Gan-Or Z , Dupre N ((2019) ) Exposure to pesticides and welding hastens the age-at-onset of Parkinson’s disease. Can J Neurol Sci 46: , 711–716. |
[34] | Tanner CM , Kamel F , Ross GW , Hoppin JA , Goldman SM , Korell M , Marras C , Bhudhikanok GS , Kasten M , Chade AR , Comyns K , Richards MB , Meng C , Priestley B , Fernandez HH , Cambi F , Umbach DM , Blair A , Sandler DP , Langston JW ((2011) ) Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 119: , 866–872. |
[35] | Ritz BR , Paul KC , Bronstein JM ((2016) ) Of pesticides and men: A California story of genes and environment in Parkinson’s disease. Curr Environ Health Rep 3: , 40–52. |
[36] | Vedel-Krogh S , Nielsen SF , Schnohr P , Nordestgaard BG ((2016) ) Morbidity and mortality in 7,684 women according to personal hair dye use: The Copenhagen City Heart Study followed for 37 years. PLoS One 11: , e0151636. |
[37] | Colditz GA , Hankinson SE ((2005) ) The Nurses’ Health Study: Lifestyle and health among women. Nat Rev Cancer 5: , 388–396. |
[38] | Teo K , Chow CK , Vaz M , Rangarajan S , Yusuf S , Group PI-W ((2009) ) The Prospective Urban Rural Epidemiology (PURE) study: Examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J 158: , 1–7 e1. |
[39] | Ross GW , Abbott RD , Petrovitch H , Morens DM , Grandinetti A , Tung KH , Tanner CM , Masaki KH , Blanchette PL , Curb JD , Popper JS , White LR ((2000) ) Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 283: , 2674–2679. |
[40] | Darweesh SK , Koudstaal PJ , Stricker BH , Hofman A , Ikram MA ((2016) ) Trends in the incidence of Parkinson disease in the general population: The Rotterdam Study. Am J Epidemiol 183: , 1018–1026. |
[41] | Chen H , O’Reilly E , McCullough ML , Rodriguez C , Schwarzschild MA , Calle EE , Thun MJ , Ascherio A ((2007) ) Consumption of dairy products and risk of Parkinson’s disease. Am J Epidemiol 165: , 998–1006. |
[42] | Xu Q , Park Y , Huang X , Hollenbeck A , Blair A , Schatzkin A , Chen H ((2010) ) Physical activities and future risk of Parkinson disease. Neurology 75: , 341–348. |
[43] | Mues KE , Liede A , Liu J , Wetmore JB , Zaha R , Bradbury BD , Collins AJ , Gilbertson DT ((2017) ) Use of the Medicare database in epidemiologic and health services research: A valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol 9: , 267–277. |
[44] | Ilomaki J , Lai EC , Bell JS ((2020) ) Using clinical registries, administrative data and electronic medical records to improve medication safety and effectiveness in dementia. Curr Opin Psychiatry 33: , 163–169. |
[45] | Wright Willis A , Evanoff BA , Lian M , Criswell SR , Racette BA , Geographic and ethnic variation in Parkinson disease: A population-based study of US Medicare beneficiaries. Neuroepidemiology 34: , 143–151. |
[46] | Frigerio R , Elbaz A , Sanft KR , Peterson BJ , Bower JH , Ahlskog JE , Grossardt BR , de Andrade M , Maraganore DM , Rocca WA ((2005) ) Education and occupations preceding Parkinson disease: A population-based case-control study. Neurology 65: , 1575–1583. |
[47] | Chen H , Zhang SM , Hernan MA , Willett WC , Ascherio A ((2003) ) Weight loss in Parkinson’s disease. Ann Neurol 53: , 676–679. |
[48] | Song S , Luo Z , Li C , Huang X , Shiroma EJ , Simonsick EM , Chen H ((2021) ) Changes in body composition before and after Parkinson’s disease diagnosis. Mov Disord 36: , 1617–1623. |
[49] | Lee JYS , Ng JH , Saffari SE , Tan EK ((2022) ) Parkinson’s disease and cancer: A systematic review and meta-analysis on the influence of lifestyle habits, genetic variants, and gender. Aging (Albany NY) 14: , 2148–2173. |
[50] | Change N , Mercier G , Lucotte G ((2008) ) Genetic screening of the G2019S mutation of the LRRK2 gene in Southwest European, North African, and Sephardic Jewish subjects. Genet Test 12: , 333–339. |
[51] | Agalliu I , San Luciano M , Mirelman A , Giladi N , Waro B , Aasly J , Inzelberg R , Hassin-Baer S , Friedman E , Ruiz-Martinez J , Marti-Masso JF , Orr-Urtreger A , Bressman S , Saunders-Pullman R ((2015) ) Higher frequency of certain cancers in LRRK2 G2019S mutation carriers with Parkinson disease: A pooled analysis. JAMA Neurol 72: , 58–65. |
[52] | Correia Guedes L , Ferreira JJ , Rosa MM , Coelho M , Bonifati V , Sampaio C ((2010) ) Worldwide frequency of G2019S LRRK2 mutation in Parkinson’s disease: A systematic review. Parkinsonism Relat Disord 16: , 237–242. |
[53] | Hughes A , Daniel S , Kilford L , Lees A ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[54] | Gelb DJ , Oliver E , Gilman S ((1999) ) Diagnostic criteria for Parkinson disease. Arch Neurol 56: , 33–39. |
[55] | Willis AW , Roberts E , Beck JC , Fiske B , Ross W , Savica R , Van Den Eeden SK , Tanner CM , Marras C , Parkinson’s Foundation P4 Group ((2022) ) Incidence of Parkinson disease in North America. NPJ Parkinsons Dis 8: , 170. |
[56] | Dong J , Pinto JM , Guo X , Alonso A , Tranah G , Cauley JA , Garcia M , Satterfield S , Huang X , Harris T , Mosley TH Jr., Chen H ((2017) ) The prevalence of anosmia and associated factors among U.S. Black and white older adults. J Gerontol A Biol Sci Med Sci 72: , 1080–1086. |
[57] | Murphy C , Schubert CR , Cruickshanks KJ , Klein BE , Klein R , Nondahl DM ((2002) ) Prevalence of olfactory impairment in older adults. JAMA 288: , 2307–2312. |




