Exploring the Complex Phenotypes of Impaired Finger Dexterity in Mild-to-moderate Stage Parkinson’s Disease: A Time-Series Analysis
Abstract
Background:
Impaired dexterity is an early motor symptom in Parkinson’s disease (PD) that significantly impacts the daily activity of patients; however, what constitutes complex dexterous movements remains controversial.
Objective:
To explore the characteristics of finger dexterity in mild-to-moderate stage PD.
Methods:
We quantitatively assessed finger dexterity in 48 mild-to-moderate stage PD patients and 49 age-matched controls using a simple alternating two-finger typing test for 15 seconds. Time-series analyses of various kinematic parameters with machine learning were compared between sides and groups.
Results:
Both the more and less affected hands of patients with PD had significantly lower typing frequency and slower typing velocity than the non-dominant and the dominant hands of controls (p = 0.019, p = 0.016, p < 0.001, p < 0.001). The slope of the typing velocity decreased with time, indicating a sequence effect in the PD group. A typing duration of 6 seconds was determined sufficient to discriminate PD patients from controls. Typing error, repetition, and repetition rate were significantly higher in the more affected hands of patients with PD than in the non-dominant hand of controls (p < 0.001, p = 0.03, p < 0.001). The error rate was constant, whereas the repetition rate was steep during the initiation of typing. A predictive model of the more affected hand demonstrated an accuracy of 70% in differentiating PD patients from controls.
Conclusion:
Our study demonstrated complex components of impaired finger dexterity in mild-to-moderate stage PD, namely bradykinesia with sequence effects, error, and repetition at the initiation of movement, suggesting that multiple neural networks may be involved in dexterity deficits in PD.
INTRODUCTION
Parkinson’s disease (PD) commonly impairs finger dexterity, with most patients exhibiting a deficit from the early stages of the disease [1–3]. It has been reported in both symptomatic and asymptomatic hands in untreated early-stage PD patients and leads to difficulties in several daily fine motor skill activities and is considered a disease burden [2–4]. The exact mechanism of manual dexterity impairment in patients with PD remains unknown. Most studies suggest that limb-kinetic apraxia plays a significant role, as loss of finger dexterity correlates with deficits in praxis function more so than with parkinsonian signs and is associated with somatosensory cortical dysfunction [5–7]. In addition, dexterous impairment responds poorly to dopaminergic medications compared to other motor symptoms [8, 9]. However, recent evidence has shown that fine motor deficits may be attributed to bradykinesia and nigrostriatal dopamine loss [2]. Dexterous movements also improved after deep brain stimulation of the subthalamic nucleus and globus pallidus interna [10]. This suggests that the underlying components of motor dexterity impairment may be complex and possibly involve multiple neuralnetworks.
Finger dexterity is usually clinically assessed using the finger tapping test, a semiquantitative rating of speed, amplitude, and decrement of repetitive finger tapping performed 10 times, as part of a motor examination in the Movement Disorder Society’s Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III [11]. However, this type of assessment is crude, largely affected by intra- and inter-rater variability, and may not reflect hand disability related to impaired dexterity [12]. Instrumental tests, including the Purdue pegboard test and coin rotation tasks, correlate with overall fine motor performance, but the outcomes of these tests are measured as a duration while performing a certain task [3, 9, 13]. Currently, alternating two-finger tapping performed using a computer keyboard, musical keyboard or mobile device is a technology-based test that has shown to be more sensitive than clinical assessments in distinguishing PD from controls even in the early stage of disease and may provide more detailed information about characteristics of finger dexterity impairment in PD [14–21]. Decreased tapping amplitude or inter-tap distance, reduced speed or inter-tap intervals, increased irregularity, and decrement of repetitive tapping or sequence effects have been documented in mild-to-moderate PD patients [14, 18, 19, 22–26]. However, the kinematic parameters of repetitive finger tapping were mostly demonstrated as the average speed, amplitude, rhythm, and asymmetry scores between the more affected side of PD patients [14–22]. However, other components of dexterity impairment, including error and repetition of movements, have rarely been explored. Thus, a comprehensive analysis of fine finger movements in multiple domains and the temporal evolution of each kinematic parameter over time may be necessary to provide insight into the complex phenotypes of impaired dexterity in mild-to-moderatestage PD.
The primary purpose of this study was to explore the characteristics of dexterous movements in mild-to-moderate stage PD using a simple alternating two-finger typing test on a computer keyboard. Various kinematic parameters, including typing frequency, velocity, digraph or keystroke duration, error, and repetition were analyzed in a time-series manner and compared between PD patients and healthy controls. In addition, we distinguished the typing characteristics between the less and more affected hands of patients with PD and the dominant and non-dominant hands of controls. The correlations between these parameters, disease severity, disease stage, and bradykinesia scores were also investigated. A machine learning-based approach was utilized to explore the digital characteristics that could differentiate PD patients from controls with good accuracy. We hypothesized that typing error and repetition would be more pronounce in the more affected hand of PD compared to controls. Our results could enhance our understanding of the clinical spectrum and underlying pathological mechanisms of impaired dexterity which could be utilized as a set of digital markers in early to mid-stage of PD and guide focused rehabilitation to improve hand function in patients with PD.
MATERIALS AND METHODS
Study population
A total of 48 patients with a clinical diagnosis of PD and between Hoehn and Yahr (H&Y) stages 1–2.5, indicating mild-to-moderate stage PD due to no axial involvement and 49 age-matched healthy controls without any complaints of impaired fine motor skills were recruited from the outpatient clinic of the Chulalongkorn Centre of Excellence for Parkinson’s Disease and Related Disorders (https://www.chulapd.org). Patients were diagnosed according to the United Kingdom Parkinson’s Disease Society Brain Bank (UKPDSBB) clinical criteria [27]. Any participants with impaired finger dexterities due to any neurological disorders, including stroke, neuromuscular disorders, peripheral neuropathy, ataxia, atypical parkinsonism, or other non-neurological causes (e.g., joint malformation), significant medical comorbidities, and cognitive impairment defined by Thai Mini-Mental State Examination (TMSE) scores below 26 were excluded from the study. All participants were right-handed, as determined using the Edinburgh Handedness Inventory [28]. The presence of motor disability was determined in all PD subjects using the MDS-UPDRS-III total scores and MDS-UPDRS bradykinetic sub-scores of each hand (sum of item 3.4–3.6). The more affected hands of patients with PD were defined according to higher MDS-UPDRS bradykinetic sub-scores compared to the contralateral hand. PD subtypes were classified according to the ratio of mean MDS-UPDRS tremor scores and postural instability/gait difficulty scores: tremor-dominant subtype (PD-TD; ratio ≥1.5), akinetic-rigid subtype (PD-AR; ratio ≤1.0), and mixed subtype (ratio >1.0 and <1.5) [29]. PD patients with severe action tremor (total score of more than 4 from 0–4 in the items 3.15–3.16) that could interfere with typing performances were excluded from our study. Clinical examinations including fingers tapping test, muscle tone examination and observation of rest and action tremor were evaluated in healthy controls to ensure that they did not have impaired finger dexterity and any signs of parkinsonism. This study was approved by the Human Subjects Ethics Committee of the Faculty of Medicine, Chulalongkorn University (IRB 535/61) and performed in accordance with the Helsinki Declaration of 1975. All participants provided written informed consent before participation in the study.
Experimental procedure
An in-house typing kit consisting of a computer program connected to a keyboard was used to objectively determine the typing performance of patients with PD and healthy controls (Supplementary Figure 1). All subjects were instructed to type side-by-side as quickly and precisely as possible, alternating between the index and middle fingers, on a computer keyboard where the B/M keystrokes were measured for 15 s. Patients with PD were assessed using the MDS-UPDRS-III scales and the typing test during the OFF period, to avoid the effect of anti-parkinsonian medications on typing performance, by withholding their medications for at least 12 h before participating in the study. All participants were asked to start side-by-side typing with their right hand regardless of the most affected side, since all recruited subjects were right-handed. The test was repeated 3 times for each hand, with a rest of 2 min between each trial.
Typing performances were transformed into raw data and analyzed using Python software. The outcomes included the accumulative typing frequency (keys), typing velocity (keys/s), accumulative error (keys), error rate (accumulative error/frequency; keys/s), accumulative typing repetition (keys), repetition rate (accumulative repetition/frequency; keys/s), digraph or inter-tap intervals (s), and digraph rate (accumulative digraph/frequency). Accumulative typing frequency (keys) was the accumulative sum of typed keys. The slope of the accumulative typing frequency at each time point was calculated as the typing velocity (keys/s). Accumulative error was determined by counting the inaccurate keys, while repetition was computed by counting the accurate keys that subjects repetitively typed on the same key instead of alternately typing on the next accurate key. Repetition duration was an additional parameter that measured the total duration of typing repetition throughout the duration of the test. Digraph (inter-tap interval) was defined as the gap duration between two adjacent key sequences, indicating the typing rhythm (Supplementary Table 1). To demonstrate the changes in each kinematic parameter over time, all parameters were plotted as the mean of the three trials for each hand in time-series graphs. The slopes of typing velocity over time represent a sequenceeffect.
Statistical analysis
Baseline clinical characteristics and objective outcomes were summarized using either means and standard deviations (SD) or frequencies and percentages, as appropriate. The Shapiro-Wilk test was performed to evaluate the normality of demographics and typing parameters in each group. All three trials from each hand of each participant were pooled and used for comparison between each hand of patients with PD and healthy controls, with a total of four groups. The differences in the mean parameters between groups (the more affected hand of PD patients, the less affected hand of PD patients, the non-dominant hand of controls, and the dominant hand of controls) over time were analyzed using a generalized linear mixed model (GLMM) using the lmer function from the lme4 package [30]. In order to exclude the possible learning effect across three trials and the effect of handedness as a confounding factor, the order of trials and handedness were put into as a random effect into the original model for statistical analysis. Additional p values were calculated based on Satterthwaite’s approximation using the lmerTest package [31]. Stepwise regressions were conducted considering the Akaike information criterion (AIC) or log-likelihood, depending on the intraclass correlation of variances. Changing points in the mean, trend, and autocorrelation were analyzed using the EnvCpt function from the EnvCpt package [32]. To compare between PD subtypes (TD-PD, AR-PD), a GLMM with the lmer function from the lme6 package was also performed. The glht() function in the multcomp package with the Benjamini-Hochberg adjustment was applied for multiple comparisons to determine the differences between groups (PD vs. controls; the more affected hand of PD vs. the non-dominant hand of controls, the less affected hand of PD vs. the dominant hand of controls) and subgroups (TD-PD vs. AR-PD vs. controls; the more affected hand of TD-PD/AR-PD v.s the non-dominant hand of controls, the less affected hand of TD-PD/AR-PD vs. the dominant hand of controls). The mixed model repeated analysis of variance (ANOVA) was used to test the differences in repetitive duration among groups/subgroups using two within-subject factors (sides of hands; the less affected hand of PD patients/dominant hand of controls, the more affected hand of PD patients/non-dominant hand of controls) and two/three between-subject factors (groups: PD patients and controls, subgroups: PD-TD, PD-AR, controls) because this parameter did not change over time. Sides, group/subgroup, and interaction between sides and group/subgroup were fixed effects. A simple t-test was used for post hoc pairwise analysis, with Bonferroni’s adjustment for multiple comparisons. Spearman’s correlations across typing parameters of the more affected hand of PD patients, HY staging, and motor rating scales were evaluated with application of Benjamini-Hochberg adjustment. All analyses were performed using R version 4.2.1. The level of significance was set at p < 0.05(two-tailed).
A machine-learning based approach to differentiate between PD patients and controls
The machine-learning based approach is a method that uses data and algorithms to discover particular patterns from datasets. Two main categories of machine learning models are established. Supervised learning that uses labeled datasets to train predictive models to classify data or predict outcomes while unsupervised learning uses algorithms to analyze and cluster unlabeled datasets. In this study, a supervised machine learning model was constructed using the decision tree method to distinguish PD patients from controls. All three trials of each typing parameter from the more affected hand of PD and controls were included in this predictive model. Multiple random decision trees were created, and the particular features were selected based on the information gain or entropy, a measure of disorder or unpredictability in the system. We focused on the Specificity, Sensitivity (recall), Precision, and F1 scores. Specificity, the rate at which healthy controls are predicted to be healthy, is the number of true negatives divided by the sum of true negatives and false positives. Sensitivity, the rate of PD patients that are correctly identified as having impaired dexterity, is the number of true positives divided by the sum of true positives and false negatives. Precision, the rate of correctly predicted positive class events, is the number of true positives divided by the sum of true positives and false positives. The F1 score, a measure of a test’s accuracy, is the harmonic mean of precision and sensitivity, and is considered one of the most important parameters because it can aid medical decision-making to ensure PD patients receive appropriate treatment. A predictive model using certain typing parameters with the highest F1 score was selected to differentiate between patients with PD and controls.
RESULTS
Baseline characteristics
In our study, PD patients had a mean H&Y stage of 1.8 (0.5) with a mean disease duration of 3.7 (3) years. The mean OFF period MDS-UPDRS III and bradykinesia sub-scores were 23.3 (10.5) and 6.3 (3.3), respectively, indicating an early to mid-stage of disease with mild-to-moderate disease severity. The details of the demographic and clinical characteristics are presented in Table 1. About half of them (56%) were categorized in the PD-TD subtype. There were no significant differences in any baseline characteristics, namely H&Y stage, disease duration, MDS-UPDRS III, and bradykinesia sub-scores between PD subtypes. No impaired finger dexterity or signs of parkinsonism were noted in the healthy controls. All PD patients and controls were right-handed, but the most affected side of 22 out of 48 PD patients (46%) were non-dominant or left-handed.
Table 1
Clinical demographics of PD patients, PD subtypes, and control subjects
| PD (N = 48) | PD AR (N = 21) | PD TD (N = 27) | Controls (N = 49) | pa | pb | |
| Age (y) | 63.7±11.3 | 67.6±10.9 | 60.7±10.9 | 63.6±11.7 | 0.95 | 0.12 |
| Sex (N, % male) | 28 (58%) | 8 (38%) | 12 (44%) | 27 (55%) | 0.45 | 0.73 |
| H&Y stage (mean) | 1.8±0.5 | 2.07±0.6 | 1.8±0.6 | – | – | 0.16 |
| •H&Y stage 1 | 10 (21%) | |||||
| •H&Y stage 1.5 | 6 (12.5%) | |||||
| •H&Y stage 2 | 17 (35%) | |||||
| •H&Y stage 2.5 | 15 (31%) | |||||
| Disease duration (y) | 3.7±3.0 | 4.43±3.0 | 3.25±3.0 | – | – | 0.08 |
| MDS UPDRS-III total | 23.3±10.5 | 25.5±10.1 | 21.6±10.6 | – | – | 0.16 |
| MDS UPDRS bradykinesia sub-score | 6.3±3.3 | 7.3±3.5 | 5.5±3.0 | – | – | 0.08 |
| PD Subtype | ||||||
| •Tremor dominant (PD-TD) | 27 (56%) | – | – | |||
| •Akinetic-rigid (PD-AR) | 22 (44%) | – | – | |||
| TMSE | 28.4±2.2 | 28.0±2.0 | 28.1±2.0 | 28.5±1.6 | 0.21 | 0.45 |
| LED | 769.3±494 | 854.5±418 | 703.0±544 | – | – | 0.07 |
*p < 0.05, pa between all PD patients and controls, pb between AR-PD and TD-PD subtypes. PD, Parkinson’s disease; AR-PD, akinetic rigid PD subtype; TD-PD, tremor dominant PD subtype; C, controls; HY, Hoehn & Yahr; TMSE, Thai version of the Mini-Mental Status Examination; UPDRS, Unified Parkinson’s Disease Rating Scale; LED, Levodopa equivalent dose.
Time-series analysis of typing parameters between sides and groups
Accumulative typing frequency and typing velocity were significantly lower in both the more affected hand of PD patients compared to the non-dominant and the less affected hand of PD patients compared to the dominant hand of controls (p = 0.019, p = 0.016, p < 0.001, p < 0.001, respectively). There were significantly lower numbers of accumulative frequency and slower velocity in the more affected hands of PD patients and the non-dominant hands of controls compared to the less affected hands of PD patients and in the dominant hands of controls (all p < 0.001) (Fig. 1A, B). The slopes of velocity were high in the first 5.25 s and then decreased afterwards in all participants, demonstrating a fatigability of finger tapping in both PD patients and controls (Fig. 1B). The changing point that significantly discriminated PD patients from controls was 5.25 s. Digraph was significantly higher in the less affected hand of PD patients than in the dominant hands of controls (p < 0.001), while the digraph rate was significantly higher in both the more affected and less affected hands of PD patients than in the non-dominant and the dominant hands of controls (p = 0.009 and p < 0.001,respectively).
Fig. 1
Time-series plots of typing frequency (A), typing velocity (B), accumulative error (C), error rate (D), accumulative repetition (E), repetition rate (F), digraph (G), and digraph rate (H) of the less/the more affected hands of Parkinson’s disease (PD) and the dominant (dom)/the non-dominant (non-dom) hands of controls (C).
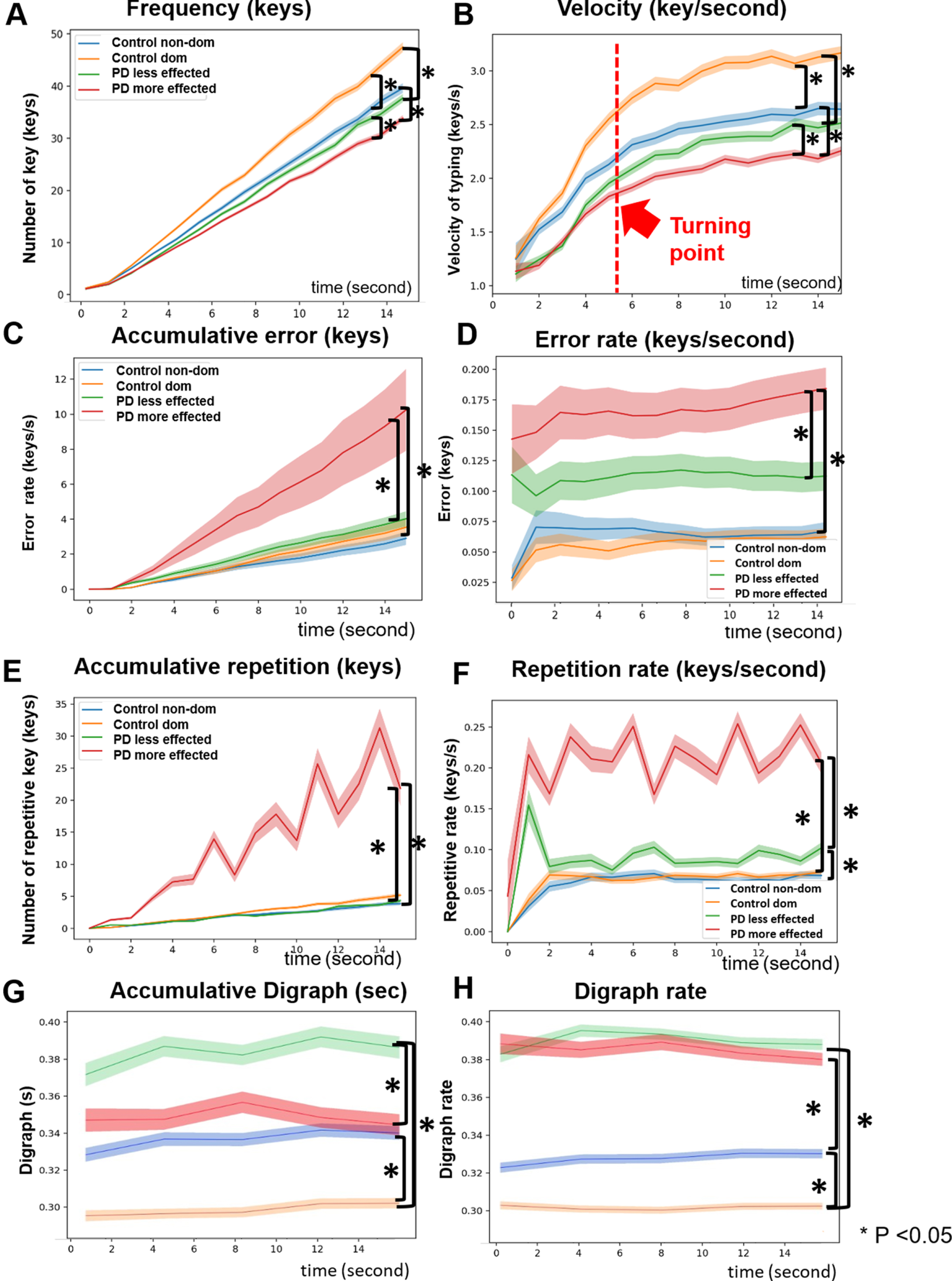
Accumulative typing error and error rate were significantly higher in the more affected hand in PD patients than in the non-dominant hand of controls (p < 0.001, p = 0.008) (Fig. 1C, D). The more affected hands of PD patients exhibited more typing errors and a higher error rate than the less affected hand (both p < 0.001), while the controls did not show any significant differences in error and error rate between sides. The error rate remained constant over time in the PD group. In addition, typing repetition was significantly increased in the more affected hand of PD patients than in the non-dominant hand of controls (p = 0.03) whereas the repetition rate was significantly greater in both the more and the less affected hands of PD compared to the non-dominant and dominant hands of controls (p < 0.001, p = 0.018, respectively) (Fig. 1E, F). Once again, there were significant differences in typing repetition and repetition rates between the more affected and less affected hands of PD patients (p < 0.001, p < 0.001), but not the controls. The repetition rate was steep in the first 1 s and then plateaued.
When repetitive duration was compared between the side and group with a mixed model repeated ANOVA, there were significant effects of side (F1,5.82, p = 0.02), group (F1,5.43, p = 0.02), and the interaction between condition and group (F1,6.54, p = 0.01) (Supplementary 3A). Post hoc pairwise comparison showed that the repetitive duration of the more affected hand of PD patients was higher than in the non-dominant hand of controls (p = 0.002).
Time-series analysis of typing parameters between PD subtypes
Subgroup analysis between PD subtypes revealed that both PD-AR and PD-TD subtypes had a significantly lower typing frequency (p < 0.001, p < 0.001), slower typing velocity (p < 0.001, p < 0.001), and higher digraph (p = 0.003, p = 0.001) of the less affected hands than in the dominant hand of controls. In addition, there was asymmetry in typing frequency, velocity, and digraph between sides in both subtypes (all p < 0.001) (Supplementary Figure 2A, B). Typing errors and repetition were significantly higher in the more affected hand of PD-AR subtype patients than in the non-dominant hand of controls (p < 0.001, p = 0.007) (Supplementary Figure 2C, D). Both the more affected hands of AR-PD and TD-PD subtypes showed a greater rate of repetition compared to the non-dominant hands of controls (p < 0.001, p = 0.012 respectively). In addition, the more affected hand of the PD-AR patients exhibited higher tapping error than the more affected hand of PD-TD patients (p < 0.001). Repetitive duration was also higher in the more affected hand of the PD-AR subtype patients than in the non-dominant hand of controls (p = 0.006) (Supplementary Figure 3B).
Correlation between typing performances and disease stage/severity
To evaluate the alternating typing parameters that correlated with the clinical stage and disease severity scales in patients with PD, exploratory analysis was performed. A negative correlation between typing frequency, velocity, and HY stage (r = –0.45, p = 0.001, r = –0.4, p = 0.005), MDS-UPDRS part III total scores (r = –0.48, p = 0.001, r = –0.44, p = 0.002), and MDS-UPDRS bradykinesia subscores (r = –0.42, p = 0.003, r = –0.37, p = 0.009) in patients with PD was noted (Table 2). The repetition duration positively correlated with HY stage (r = 0.44, p = 0.001), the total MDS-UPDRS part III (r = 0.4, p = 0.005), but not with the MDS-UPRDS subscores.
Table 2
Spearman’s correlation between alternating typing parameters and clinical stage and clinical severity scales in patients with Parkinson’s disease
| Parameters | HY stage | MDS-UPDRS III | MDS-UPDRS bradykinesia | |||
| r | p | r | p | r | p | |
| Typing frequency (key) | –0.45 | 0.001* | –0.48 | 0.001* | –0.42 | 0.003* |
| Typing velocity (key/s) | –0.4 | 0.005* | –0.44 | 0.002* | –0.37 | 0.009* |
| Error rate (key/s) | 0.27 | 0.07 | 0.3 | 0.038 | 0.23 | 0.124 |
| Repetition rate (key/s) | 0.28 | 0.05 | 0.33 | 0.023 | 0.23 | 0.115 |
| Repetition duration (s) | 0.44 | 0.002* | 0.40 | 0.005* | 0.35 | 0.016 |
| Digraph rate (key/s) | 0.38 | 0.008* | 0.36 | 0.012 | 0.35 | 0.015 |
Significant correlations (bold). *Correlations that were significant (p-value <0.05) with applying Benjamini Hochberg correction. HY, Hoehn & Yahr; UPDRS, Unified Parkinson’s Disease Rating Scale.
A machine-learning analysis to distinguish PD patients from controls
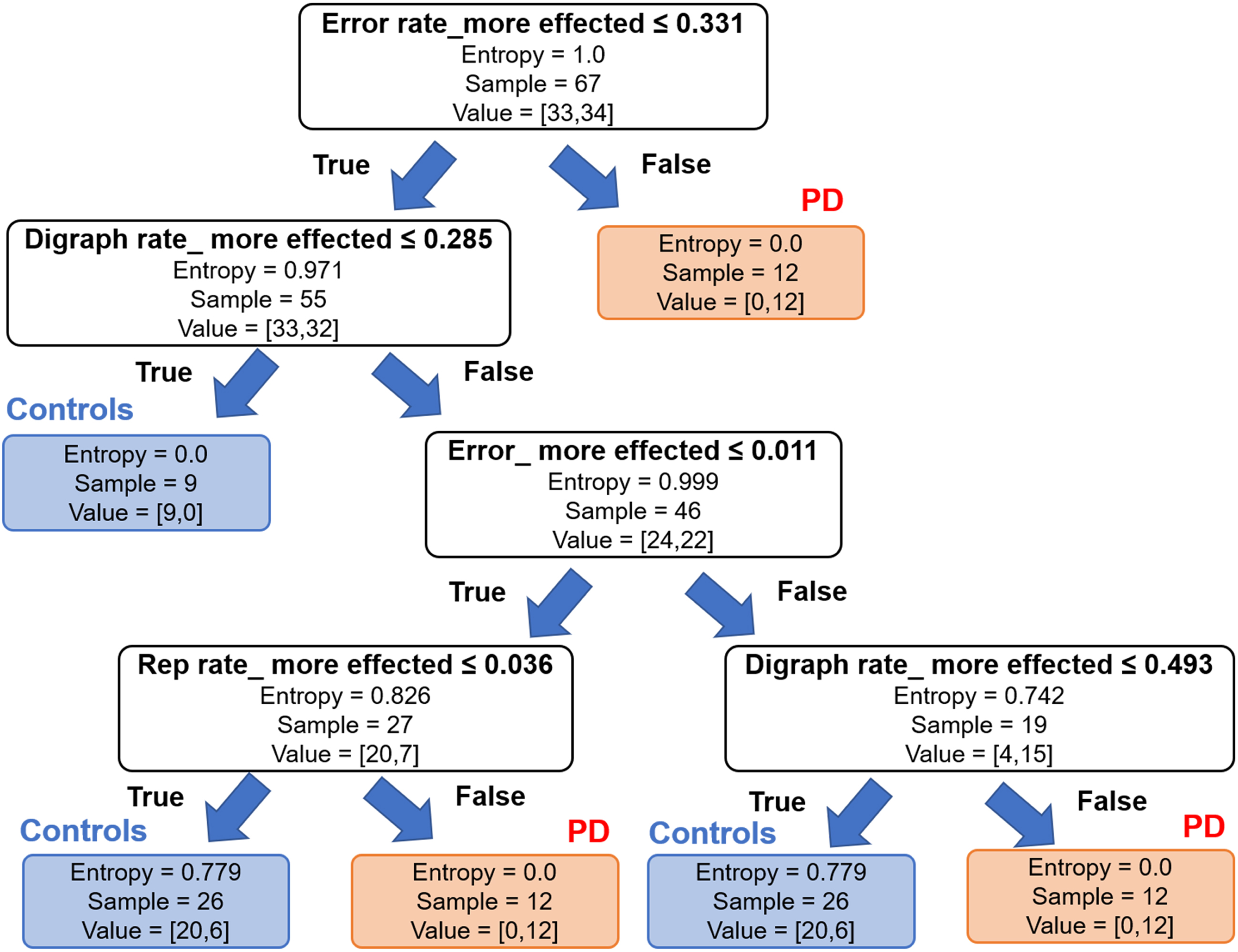
All typing parameters of the dominant hands of PD and controls were fed into machine learning for modeling. Multiple random decision trees were created and the features selected according to the information gain. The parameters of the more affected hand that were found to be significantly different across groups were accumulative error, error rate, repetition rate, and digraph rate. Based on these features, this model was able to distinguish impaired dexterity in PD from controls with a sensitivity of 77%, specificity of 65%, precision of 62.5, and F1 score (accuracy) of 70% (Fig. 2).
Fig. 2
Machine learning based approach with decision tree method using accumulative error, error rate, repetition rate and changing rate of keystroke duration or digraph rate to distinguish between Parkinson’s disease (PD) and controls with highest accuracy. Entropy is a measure of disorders or unpredictability in the system of the decision trees method. Lower entropy of the typing parameters including accumulative error, error rate, and repetition rate, but not digraph rate, would suggest higher information gain or the PD category.
DISCUSSION
Using an alternating two-finger typing test, our study demonstrated the characteristics of impaired finger dexterity in mild-to-moderate stage PD, included reduced velocity with decremental movements over time, increased keystroke duration or digraph, and increased typing error and repetition compared to controls. Slow typing speed was exhibited in both the more and less affected hands of PD patients, while typing error and repetition at the initiation of typing were more pronounced in the more affected hand of PD patients. A sequence effect was documented in both hands of the patients with mild-to-moderate stage PD. Subgroup analysis showed that finger movements were slow and asymmetrical between the more and less affected sides of PD patients regardless of subtype, whereas error and repetition of upper limb movements were more dominant in the more affected side of the PD-AR subtype. Typing velocity and repetition duration correlated with disease severity and stage. Our results suggest that impaired finger dexterity is not limited to bradykinesia but constitutes complex phenotypes, consisting of a sequence effect which is one of the main components of bradykinesia, error, and repetition in fine movements [33, 34].
Regardless of the degree of hand affectivity, PD patients showed a slower overall rate of repetitive alternating finger movements compared to controls, indicating that the less symptomatic hand still exhibited impaired dexterity in mild-to-moderate stage PD [18]. The magnitude of the differences in typing velocity between sides seemed to be greater in the control group than in the PD group, which might be explained by the small motor reserve in both hands in PD [35, 36]. Conversely, the faster speed of fine motor movements in the dominant hands compared to the non-dominant hands of controls could be due to the training effect of dexterity, implying that impaired dexterity could be retrained with a focused rehabilitation program to improve hand function in PD patients [37].
Consistent with previous studies involving early PD patients, a sequence effect was demonstrated in both hands in PD patients [22, 23, 25, 38]. Moreover, healthy participants showed this effect with a lower rate of decrement. Contrary to controls, in PD patients, a decrease in velocity was observed within the first 6 s which continued beyond this point. This phenomenon could be due to physiological fatigue in healthy people, which may be expressed in complex repetitive movements of the distal limb, such as alternating two-finger tapping, rather than proximal movements with alternating single-finger tapping or bimanual finger tapping [22, 23]. More complex motor tasks might increase activation at the motor, premotor, and sensory cortical areas, basal ganglia, and cerebellum compared to simple tasks, and possibly cause fatigue in the motor networks relating to maintenance of precise movements [39, 40]. The slopes of typing velocity in PD patients and controls were separated at the beginning of the task regardless of the extent of hand affectivity, suggesting that abnormal motor programming, deficits in movement initiation, and inappropriate scaling could be responsible for a true sequence effect in PD [40–43]. The changing point capable of discriminating PD from controls was 5.25 s, which implies that alternating two-finger tapping for 6 s, regardless of the number of consecutive taps, may be long enough to document bradykinesia with a sequence effect, differentiate between mild-to-moderate stage PD and controls, and avoid physiologic fatigue in controls. However, 6-s fatigue seems to be short compared to the time we regularly type in daily life. This could be due to the fact that repetitive typing on the same keys might be less physiologic than typing a sentence on a smartphone or keyboard. Future study focusing on typing pattern as well as time spent typing in daily activities would be considered as our next step.
Higher movement error and error rate were noted in the more affected hand of patients with mild-to-moderate stage PD compared to the less affected hand of PD patients and controls. The error in fine motor movements may have been due to abnormal motor feedforward and feedback controls and sensorimotor integration in PD [44–46]. Increased temporal discrimination thresholds in PD patients compared to controls may explain the sensory processing deficits up to the level of the somatosensory cortex that contributes to imprecise movements [47, 48]. Another possibility of movement error could be variability in the timing of repetitive movements of the distal limbs, which has previously been reported in the more affected hands in mild PD patients with abnormal activation of the cerebellum while performing finger movements as compensation for defective basal ganglia function [49–51]. The error rate was constant as movement continued, supporting the theory that abnormal motor programming is unchangeable over time and more pronounced on the more symptomatic side of PD [48]. Underlying neural networks and pathological mechanisms of movement error can overlap with limb-kinetic apraxia [5–7]. Apraxia has been used in different settings and, in the clinical setting of parkinsonism, it is often used as a red flag to suggest a diagnosis of atypical parkinsonian disorders such as corticobasal syndrome, which manifest with prominent praxis dysfunction [52–54].
The more affected hand of PD patients exhibited a greater typing repetition than that of controls. Both the more and the less affected hands of PD showed a higher rate of typing repetition than that of controls, and the slopes were steep during the initiation of finger movement. However, the magnitude of differences seemed to be more predominant in the more affected side. The nature of this typing repetition is unclear, but it could represent hesitations of finger movement causing repeated movements instead of alternating movement to another finger or mimicking freezing of upper limb. Freezing phenomenon refers to a significant reduction of movements while hesitations are currently defined as irregularities in movement timing in repetitive, alternating, and continuous movements of limbs [34, 55]. Implanted wearable sensors that measure the amplitude of movement during repetitive movements should be added to prove the nature of this observation. In addition, it would be interesting to follow these patients who displayed repetitive finger movements longitudinally and see if they exhibit freezing of the upper limb that might occur earlier than freezing of gait [56]. According to our hypothesis, impaired motor automaticity and motor timing leading to repetition of movement may occur prominently in the more symptomatic hand in the mild-to-moderate stage PD, particularly when performing fine finger movements.
Correlations between the computer-based analysis typing parameters and motor performance scores rated by the MDS-UPDRS part III in the more affected hand of PD patients were observed, as previously noted [16, 19]. In this study, we further revealed that the repetitive duration increased with disease severity, but not bradykinesia sub-score, suggesting that repetition of movements may not be grouped under the umbrella term of bradykinesia. Using a combination of digraph, error, and repetition rates, our machine learning-based analysis with the decision tree method was able to distinguish PD from controls with good accuracy. This supports the idea that impaired dexterity in mild-to-moderate stage PD is a complex motor phenotype that may comprise of bradykinesia, movement errors, and repetition of upper limb movements (Fig. 3A) [33]. The proposed neural network could be abnormal movement selections, abnormal scaling effects, and altered automaticity in the basal ganglia-cortical loop, as well as impaired feedback and feedforward loops as compensation during sensorimotor disintegration (Fig. 3B) [40]. However, further functional imaging of complex sequential motor movements should be performed to confirm this hypothesis.
Fig. 3
A) The proposed complex components of impaired dexterity in Parkinson’s disease including bradykinesia, error, and repetitive movements of the less and the more affected sides in different disease stages and (B) the proposed neural networks of each component of impaired dexterity in Parkinson’s disease.
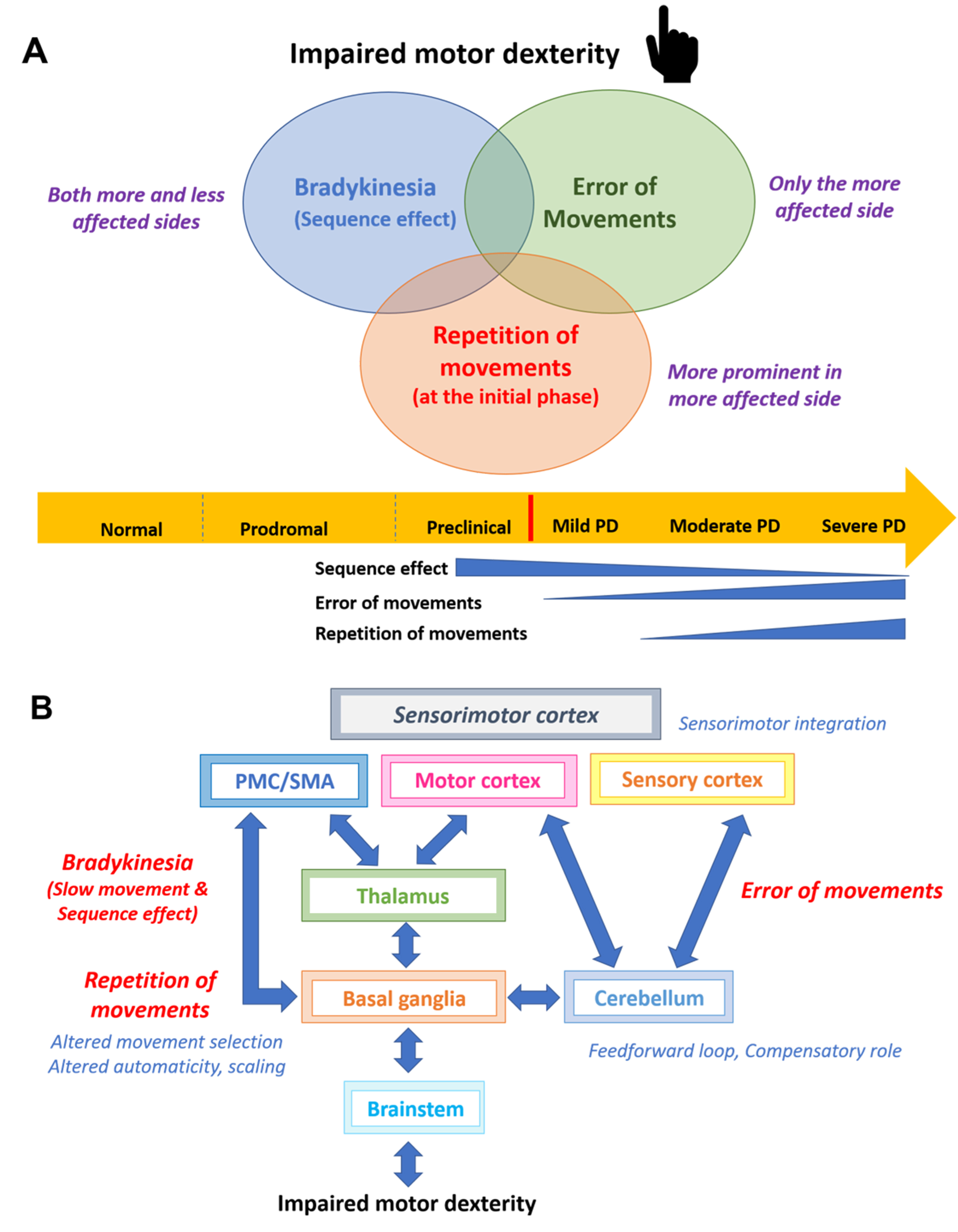
Our subgroup analysis between the PD subtypes showed that both AR-PD and TD-PD patients had slower finger movements compared to controls. Unsurprisingly, typing velocity seems to be slower in AR-PD than in TD-PD subtypes but it did not reach statistical significance [57]. Movement speed was asymmetrical between sides regardless of subtype, since our recruited PD patients were in the early-to-mid stages of the disease. The error and repetition of movements were prominent only on the more affected side of the AR subtype, implying that the AR-PD subtype has more basal ganglia involvement, greater abnormal sensorimotor integration, and less compensation from the cerebellum than the TD-PD subtype [58]. Functional imaging with objective tremor evaluation while performing repetitive motor tasks should be conducted in the future to assess the effect of tremor on the neural networks responsible for finger dexterity.
A strength of our study was the time-series analysis of fine finger movements and comparison between more and less affected sides, whereby we observed changes in each movement parameter over time in each hand and demonstrated the complex components of impaired dexterity in mild-to-moderate stage PD. However, a number of limitations should be mentioned. First, a relatively small number of patients, particularly for subgroup analysis between PD subtypes, were recruited compared to recently published studies [14, 18, 22]. However, our participants were asked to repeat the alternating finger-typing tests three times with each hand. Thus, all three trials of each typing parameter were put into the model, which increased the power for statistical and machine-learning analysis. Variation across trials of individual subject could also be addressed by putting all data in the model using the order of trials as a random effect. However, the learning effect of each typing feature should be further explored in both controls and PD to enhance our understanding of motor learning and motor reserve of fine movements. Secondly, the correlation between the typing performance and disease stage was assessed only once. Follow-up studies in PD patients with unilateral symptoms are needed to observe how these typing parameters change over time in order to represent the true progression of dexterous movements in PD. Thirdly, we included PD patients with H&Y stage 1–2.5 that may make the typing data quite heterogenous, as the complete asymptomatic hands were combined with the less affected hands. It would be interesting to study the differences in dexterity deficits between asymptomatic and less affected hands in very early PD patients with H&Y stage 1 and/or patients with less than 2 years of disease duration. In addition, further studies in patients with subtle motor symptoms in the prodromal stage should be considered to explore the spectrum of hand deficits at all disease stages. Capturing these unique characteristics may support early screening of patients who are at risk of PD. Finally, implanted wearable sensors could be added in the keyboard typing test to deliver more information such as amplitude of movements in the more or less affected hands of PD and compare to controls [59]. However, movement velocity is currently focused as a main component of newly defined bradykinesia [34]. Therefore, a quantitative motor test that provides a velocity of movements and a sequence effect could represent a core assessment of dexterity.
In conclusion, our study provides insight into the phenotypes of dexterity impairment in mild-to-moderate stage PD, comprising bradykinesia with sequence effect, movement error, and repetition of movement of the upper limb. These differ between the more and less affected sides of PD, depending on the severity of motor symptoms, such that the less affected hand demonstrates mainly bradykinesia, whereas the more affected hand exhibits additional errors and repetition. This suggests that the neural networks underlying impaired dexterity are more complex and widely involved in the advanced stage of PD. These characteristics of fine movement deficits may be served as digital markers for the screening of mild-to-moderate stage PD patients from healthy controls [60]. The current clinical diagnostic criteria for PD, as well as parkinsonism, only relies on bradykinesia as the single component of dexterity impairment, based on a standard finger tapping test, so is potentially insensitive as a measure for PD patients with mild motor signs or in patients at the prodromal stage [61]. Diagnostic accuracy of early PD has not improved over the past 25 years and is considered a major unmet need as diagnosis of PD should be early and timely for appropriate treatment [62]. We proposed that assessment of dexterous impairment with complex finger movements, such as repetitive and sequential 3–5 fingers movements, should be performed in clinical examinations and clinicians should pay attention to bradykinesia with a sequence effect, as well as error and repetition in sequential finger movements, for early diagnosis of PD. This quantitative motor testing could also be an option to document differences of dexterity between patients in clinical trials since the MDS-UPRDS-III bradykinesia sub-scores might not include all complex phenotypes of bradykinesia and being insensitive to capture small dexterous changes. In addition, further studies exploring the relationship between each dexterous parameter and level of difficulty in performing daily activities should also be conducted to create a focused rehabilitation program to improve the quality of life of patients with PD.
ACKNOWLEDGMENTS
This study was supported by a Grant for Innovation, Chula Research Scholar, Rachadapiseksomphot Endowment Fund (CU-GI-62-14-30-04), and Centre of Excellence grant of Chulalongkorn University (GCE 6100930004-1), Bangkok, Thailand.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose. Roongroj Bhidayasiri is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230029.
REFERENCES
[1] | Haaxma CA , Bloem BR , Overeem S , Borm GF , Horstink MW ((2010) ) Timed motor tests can detect subtle motor dysfunction in early Parkinson’s disease. Mov Disord 25: , 1150–1156. |
[2] | Lee SH , Lee MJ , Lyoo CH , Cho H , Lee MS ((2018) ) Impaired finger dexterity and nigrostriatal dopamine loss in Parkinson’s disease. J Neural Transm (Vienna) 125: , 1333–1339. |
[3] | Dan X , Liu J , Doyon J , Zhou Y , Ma J , Chan P ((2019) ) Impaired fine motor function of the asymptomatic hand in unilateral Parkinson’s disease. Front Aging Neurosci 11: , 266. |
[4] | Pohar SL , Allyson Jones C ((2009) ) The burden of Parkinson disease (PD) and concomitant comorbidities. Arch Gerontol Geriatr 49: , 317–321. |
[5] | Lee MS , Lyoo CH , Lee MJ , Sim J , Cho H , Choi YH ((2010) ) Impaired finger dexterity in patients with Parkinson’s disease correlates with discriminative cutaneous sensory dysfunction. Mov Disord 25: , 2531–2535. |
[6] | Foki T , Pirker W , Geißler A , Haubenberger D , Hilbert M , Hoellinger I , Wurnig M , Rath J , Lehrner J , Matt E , Fischmeister F , Trattnig S , Auff E , Beisteiner R ((2015) ) Finger dexterity deficits in Parkinson’s disease and somatosensory cortical dysfunction. Parkinsonism Relat Disord 21: , 259–265. |
[7] | Kübel S , Stegmayer K , Vanbellingen T , Pastore-Wapp M , Bertschi M , Burgunder JM , Abela E , Weder B , Walther S , Bohlhalter S ((2017) ) Altered praxis network underlying limb kinetic apraxia in Parkinson’s disease - an fMRI study. Neuroimage Clin 16: , 88–97. |
[8] | Gebhardt A , Vanbellingen T , Baronti F , Kersten B , Bohlhalter S ((2008) ) Poor dopaminergic response of impaired dexterity in Parkinson’s disease: Bradykinesia or limb kinetic apraxia? Mov Disord 23: , 1701–1706. |
[9] | Nozaki T , Asakawa T , Sugiyama K , Koda Y , Shimoda A , Mizushima T , Sameshima T , Namba H ((2018) ) Effect of subthalamic deep brain stimulation on upper limb dexterity in patients with Parkinson disease. World Neurosurg 115: , e206–e217. |
[10] | Nakamura K , Christine CW , Starr PA , Marks WJ Jr ((2007) ) Effects of unilateral subthalamic and pallidal deep brain stimulation on fine motor functions in Parkinson’s disease. Mov Disord 22: , 619–626. |
[11] | Goetz CG , Tilley BC , Shaftman SR , Stebbins GT , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stern MB , Dodel R , Dubois B , Holloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , Lewitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , Van Hilten JJ , Lapelle N ((2008) ) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23: , 2129–2170. |
[12] | Agostino R , Berardelli A , Currà A , Accornero N , Manfredi M ((1998) ) Clinical impairment of sequential finger movements in Parkinson’s disease. Mov Disord 13: , 418–421. |
[13] | Mendoza JE , Apostolos GT , Humphreys JD , Hanna-Pladdy B , O’Bryant SE ((2009) ) Coin rotation task (CRT): A new test of motor dexterity. Arch Clin Neuropsychol 24: , 287–292. |
[14] | Lee CY , Kang SJ , Hong SK , Ma HI , Lee U , Kim YJ ((2016) ) A Validation study of a smartphone-based finger tapping application for quantitative assessment of bradykinesia in Parkinson’s disease. PLoS One 11: , e0158852. |
[15] | Surangsrirat D , Sri-Iesaranusorn P , Chaiyaroj A , Vateekul P. Bhidayasiri R ((2022) ) Parkinson’s disease severity clustering based on tapping activity on mobile device. Sci Rep 12: , 3142. |
[16] | Taylor Tavares AL , Jefferis GS , Koop M , Hill BC , Hastie T , Heit G , Bronte-Stewart HM ((2005) ) Quantitative measurements of alternating finger tapping in Parkinson’s disease correlate with UPDRS motor disability and reveal the improvement in fine motor control from medication and deep brain stimulation. Mov Disord 20: , 1286–1298. |
[17] | Pal PK , Lee CS , Samii A , Schulzer M , Stoessl AJ , Mak EK , Wudel J , Dobko T , Tsui JK ((2001) ) Alternating two finger tapping with contralateral activation is an objective measure of clinical severity in Parkinson’s disease and correlates with PET. Parkinsonism Relat Disord 7: , 305–309. |
[18] | Lalvay L , Lara M , Mora A , Alarcon F , Fraga M , Pancorbo J , Marina JL , Mena MA , Lopez Sendon JL , Garcia De Yebenes J ((2017) ) Quantitative measurement of akinesia in Parkinson’s disease. Mov Disord Clin Pract 4: , 316–322. |
[19] | Louie S , Koop MM , Frenklach A , Bronte-Stewart H ((2009) ) Quantitative lateralized measures of bradykinesia at different stages of Parkinson’s disease: The role of the less affected side. Mov Disord 24: , 1991–1997. |
[20] | Arora S , Baig F , Lo C , Barber TR , Lawton MA , Zhan A , Rolinski M , Ruffmann C , Klein JC , Rumbold J , Louvel A , Zaiwalla Z , Lennox G , Quinnell T , Dennis G , Wade-Martins R , Ben-Shlomo Y , Little MA , Hu MT ((2018) ) Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology 91: , e1528–e1538. |
[21] | Hasan H , Athauda DS , Foltynie T , Noyce AJ ((2017) ) Technologies assessing limb bradykinesia in Parkinson’s disease. J Parkinsons Dis 7: , 65–77. |
[22] | Hasan H , Burrows M , Athauda DS , Hellman B , James B , Warner T , Foltynie T , Giovannoni G , Lees AJ , Noyce AJ ((2019) ) The BRadykinesia Akinesia INcoordination (BRAIN) tap test: Capturing the sequence effect. Mov Disord Clin Pract 6: , 462–469. |
[23] | Ling H , Massey LA , Lees AJ , Brown P , Day BL ((2012) ) Hypokinesia without decrement distinguishes progressive supranuclear palsy from Parkinson’s disease. Brain 135: , 1141–1153. |
[24] | Krupička R , Krýže P , Net’uková S , Duspivová T , Klempíř O , Szabó Z , Dušek P , Šonka K , Rusz J , Růžička E ((2020) ) Instrumental analysis of finger tapping reveals a novel early biomarker of parkinsonism in idiopathic rapid eye movement sleep behaviour disorder. Sleep Med 75: , 45–49. |
[25] | Bologna M , Leodori G , Stirpe P , Paparella G , Colella D , Belvisi D , Fasano A , Fabbrini G , Berardelli A ((2016) ) Bradykinesia in early and advanced Parkinson’s disease. J Neurol Sci 369: , 286–291. |
[26] | Růžička E , Krupička R , Zárubová K , Rusz J , Jech R , Szabó Z ((2016) ) Tests of manual dexterity and speed in Parkinson’s disease: Not all measure the same. Parkinsonism Relat Disord 28: , 118–123. |
[27] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[28] | Oldfield RC ((1971) ) The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: , 97–113. |
[29] | Stebbins GT , Goetz CG , Burn DJ , Jankovic J , Khoo TK , Tilley BC ((2013) ) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov Disord 28: , 668–670. |
[30] | Bates D , Mächler M , Bolker B , Walker S ((2014) ) Fitting linear mixed-effects models using lme4. arXiv, https://doi.org/10.48550/arXiv.1406.5823 |
[31] | Kunzetsova A , Brockhoff P , Christensen R ((2017) ) lmerTest package: Tests in linear mixed effect models. J Stat Softw 82: , 1–26. |
[32] | Killick R , Beaulieu C , Taylor S , Hullait H ((2021) ) Envcpt: Detection of structural changes in climate and environment time series. r package version 1.1. 3. https://cran.r-project.org/web/packages/EnvCpt/index.html |
[33] | Hallett M ((2011) ) Bradykinesia: Why do Parkinson’s patients have it and what trouble does it cause? Mov Disord 26: , 1579–1581. |
[34] | Bologna M , Espay AJ , Fasano A , Paparella G , Hallett M , Berardelli A ((2023) ) Redefining bradykinesia. Mov Disord 38: , 551–557. |
[35] | Chung SJ , Yoo HS , Lee YH , Lee HS , Lee PH , Sohn YH ((2020) ) Initial motor reserve and long-term prognosis in Parkinson’s disease. Neurobiol Aging 92: , 1–6. |
[36] | Chung SJ , Lee JJ , Lee PH , Sohn YH ((2020) ) Emerging concepts of motor reserve in Parkinson’s disease. J Mov Disord 13: , 171–184. |
[37] | Kang SY , Sohn YH ((2020) ) Effectiveness of exercise on the sequence effect in Parkinson’s disease. J Mov Disord 13: , 213–217. |
[38] | Kang SY , Wasaka T , Shamim EA , Auh S , Ueki Y , Lopez GJ , Kida T , Jin SH , Dang N , Hallett M ((2010) ) Characteristics of the sequence effect in Parkinson’s disease. Mov Disord 25: , 2148–2155. |
[39] | Sobinov AR , Bensmaia SJ ((2021) ) The neural mechanisms of manual dexterity. Nat Rev Neurosci 22: , 741–757. |
[40] | Bologna M , Paparella G , Fasano A , Hallett M , Berardelli A ((2020) ) Evolving concepts on bradykinesia. Brain 143: , 727–750. |
[41] | Wu T , Zhang J , Hallett M , Feng T , Hou Y , Chan P ((2016) ) Neural correlates underlying micrographia in Parkinson’s disease. Brain 139: , 144–160. |
[42] | Tinaz S , Pillai AS , Hallett M ((2016) ) Sequence effect in Parkinson’s disease is related to motor energetic cost. Front Neurol 7: , 83. |
[43] | Lee E , Lee JE , Yoo K , Hong JY , Oh J , Sunwoo MK , Kim JS , Jeong Y , Lee PH , Sohn YH , Kang SY ((2014) ) Neural correlates of progressive reduction of bradykinesia in de novo Parkinson’s disease. Parkinsonism Relat Disord 20: , 1376–1381. |
[44] | Koop MM , Hill BC , Bronte-Stewart HM ((2013) ) Perceptual errors increase with movement duration and may contribute to hypokinesia in Parkinson’s disease. Neuroscience 243: , 1–13. |
[45] | Conte A , Khan N , Defazio G , Rothwell JC , Berardelli A ((2013) ) Pathophysiology of somatosensory abnormalities in Parkinson disease. Nat Rev Neurol 9: , 687–697. |
[46] | Wu T , Hallett M ((2005) ) A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 128: , 2250–2259. |
[47] | Conte A , Leodori G , Ferrazzano G , De Bartolo MI , Manzo N , Fabbrini G , Berardelli A ((2016) ) Somatosensory temporal discrimination threshold in Parkinson’s disease parallels disease severity and duration. Clin Neurophysiol 127: , 2985–2989. |
[48] | Lee MS , Lee MJ , Conte A , Berardelli A ((2018) ) Abnormal somatosensory temporal discrimination in Parkinson’s disease: Pathophysiological correlates and role in motor control deficits. Clin Neurophysiol 129: , 442–447. |
[49] | Trager MH , Velisar A , Koop MM , Shreve L , Quinn E , Bronte-Stewart H ((2015) ) Arrhythmokinesis is evident during unimanual not bimanual finger tapping in Parkinson’s disease. J Clin Mov Disord 2: , 8. |
[50] | Yu H , Sternad D , Corcos DM , Vaillancourt DE ((2007) ) Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 35: , 222–233. |
[51] | Cerasa A , Hagberg GE , Peppe A , Bianciardi M , Gioia MC , Costa A , Castriota-Scanderbeg A , Caltagirone C , Sabatini U ((2006) ) Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson’s disease. Brain Res Bull 71: , 259–269. |
[52] | Denes G , Mantovan MC , Gallana A , Cappelletti JY ((1998) ) Limb-kinetic apraxia. Mov Disord 13: , 468–476. |
[53] | Heilman KM ((2021) ) Upper limb apraxia. Continuum (Minneap Minn) 27: , 1602–1623. |
[54] | Leiguarda RC , Merello M , Nouzeilles MI , Balej J , Rivero A , Nogués M ((2003) ) Limb-kinetic apraxia in corticobasal degeneration: Clinical and kinematic features. Mov Disord 18: , 49–59. |
[55] | Nieuwboer A , Vercruysse S , Feys P , Levin O , Spildooren J , Swinnen S ((2009) ) Upper limb movement interruptions are correlated to freezing of gait in Parkinson’s disease. Eur J Neurosci 29: , 1422–1430. |
[56] | Vercruysse S , Gilat M , Shine JM , Heremans E , Lewis S , Nieuwboer A ((2014) ) Freezing beyond gait in Parkinson’s disease: A review of current neurobehavioral evidence. Neurosci Biobehav Rev 43: , 213–227. |
[57] | Choi SM , Kim BC , Cho BH , Kang KW , Choi KH , Kim JT , Lee SH , Park MS , Kim MK , Cho KH ((2018) ) Comparison of two motor subtype classifications in de novo Parkinson’s disease. Parkinsonism Relat Disord 54: , 74–78. |
[58] | Lewis MM , Du G , Sen S , Kawaguchi A , Truong Y , Lee S , Mailman RB , Huang X ((2011) ) Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson’s disease. Neuroscience 177: , 230–239. |
[59] | Syeda HB , Glover A , Pillai L , Kemp AS , Spencer H , Lotia M , Larson-Prior LJ , Virmani T ((2022) ) Amplitude setting and dopamine response of finger tapping and gait are related in Parkinson’s disease. Sci Rep 12: , 4180. |
[60] | Alfalahi H , Khandoker AH , Chowdhury N , Iakovakis D , Dias SB , Chaudhuri KR , Hadjileontiadis LJ ((2022) ) Diagnostic accuracy of keystroke dynamics as digital biomarkers for fine motor decline in neuropsychiatric disorders: A systematic review and meta-analysis. Sci Rep 12: , 7690. |
[61] | Rizzo G , Copetti M , Arcuti S , Martino D , Fontana A , Logroscino G ((2016) ) Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology 86: , 566–576. |
[62] | Beach TG , Adler CH ((2018) ) Importance of low diagnostic Accuracy for early Parkinson’s disease. Mov Disord 33: , 1551–1554. |




