Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2022 Update
Abstract
Background:
As the international community dealt with the ongoing COVID-19 pandemic, important progress continued to be made in the development of new drug-based therapies for the neurodegenerative condition of Parkinson’s disease (PD) in 2021. This progress included both “symptomatic treatments” (ST – improves/reduces symptoms of the condition) and “disease modifying treatments” (DMT - attempts to delay/slow progression by addressing the underlying biology of PD), which can be categorised further based on their mechanisms of action and class of drug.
Objective:
This report continues previous efforts to provide an overview of the pharmacological therapies - both ST and DMT - in clinical trials for PD during 2021– 2022, with the aim of creating greater awareness and involvement in the clinical trial process. We also hope to stimulate collaboration amongst all stakeholders, including industry, academia, advocacy organizations, and most importantly patient community.
Methods:
We conducted a review of clinical trials of drug therapies for PD using trial data obtained from the ClinicalTrials.gov and World Health Organisation (WHO) registries, and performed a breakdown analysis of studies that were active as of January 31st 2022. We also assessed active drug development projects that had completed one clinical phase but were yet to start the next.
Results:
There was a total of 147 clinical trials registered on the ClinicalTrials.gov website as active during the period of analysis. Of these trials, 91 (62%)were investigating STs, while 56 (38%)focused on DMTs. Approximately 1/3 of the studies (34.7%; 51 trials) were in Phase 1, while over half of the trials were in Phase 2 (50.3%; 74 trials). Only 15% (22 trials) of the studies were in Phase 3, of which only 3 trials were evaluating DMTs. Novel therapeutics (42%)were the most common type of agents being tested across all phases of testing, followed by repurposed agents (34%)and reformulations (20%).
Conclusion:
Despite significant global health constraints, the development of new drug-based therapies for PD continued in 2021. Hopefully with a shift towards a post-pandemic world in which COVID-19 is better managed, we will see an increase in the number of clinical trials focused on drug development for PD. The need for more Phase 3 studies for DMTs remains acute.
INTRODUCTION
2021 was a remarkable year for medical research, highlighted by the global roll out of rapidly developed vaccines for COVID-19, the WHO recommendation of the first vaccine for malaria [1], clinical evidence of a genetic silencing method for Sickle cell disease [2], and a vastly improved new treatment for hepatitis C [3]. These and many other remarkable scientific milestones were achieved under the considerable constraints and limitations imposed by the ongoing global pandemic. Such successes bode well for future medical progress.
Important achievements were also made in the field of PD research with another $132million in basic research funding being awarded by the Aligning Science Across Parkinson’s initiative (ASAP [4]), the awarding of a grant to fund the setting up of the Accelerating Clinical Treatments for Parkinson’s Disease (Edmond J Safra ACT-PD) project - a Multi-Arm, Multi Stage clinical trial platform to rapidly accelerate the search for disease modifying therapies for PD [5], the roll out of the Parkinson’s Progression Marker Initiative (PPMI) 2.0 focusing on the identification of markers of disease progression for use in clinical trials of therapies to reduce progression of PD (NCT04477785), and a large number of biotech mergers and industrial collaborations further driving clinical development of novel therapeutics (for example, UCB and Novartis entered into a global agreement to develop novel alpha-synuclein targeting agents [6]).
Despite the pandemic-imposed restriction, a considerable amount of clinical trial activity focused on developing new therapies for PD was maintained over this period. With the goal of providing the PD research and patient communities with a better understanding of the current landscape of drug-based clinical trials, this annual report was initiated in 2020 [7]. In that first edition, the analysis was limited to active interventional drug-focused trials registered on the ClinicalTrials.gov website [8]. In 2021, we expanded the report to include clinical trials listed on the World Health Organisation (WHO) registries [9, 10]. And now, for a third year in a row, we provide another report on the drug development pipeline for PD intended to highlight areas of progress and hopefully stimulate greater awareness and involvement in the clinical trial process.
METHODS
The methods employed in this report were identical to those we have used previously [10]. Briefly, clinical trial data was downloaded from the ClinicalTrials.gov and WHO registries on the 31st January 2022, based on the following search criteria:
• Condition: Parkinson; Parkinson’s
• Study type: Interventional
• Phase: Early Phase 1, Phase 1, Phase 2, Phase 3
• Status parameter: “Recruiting”, “Not yet recruiting”, “Active, not recruiting”, or “Enrolling by invitation”.
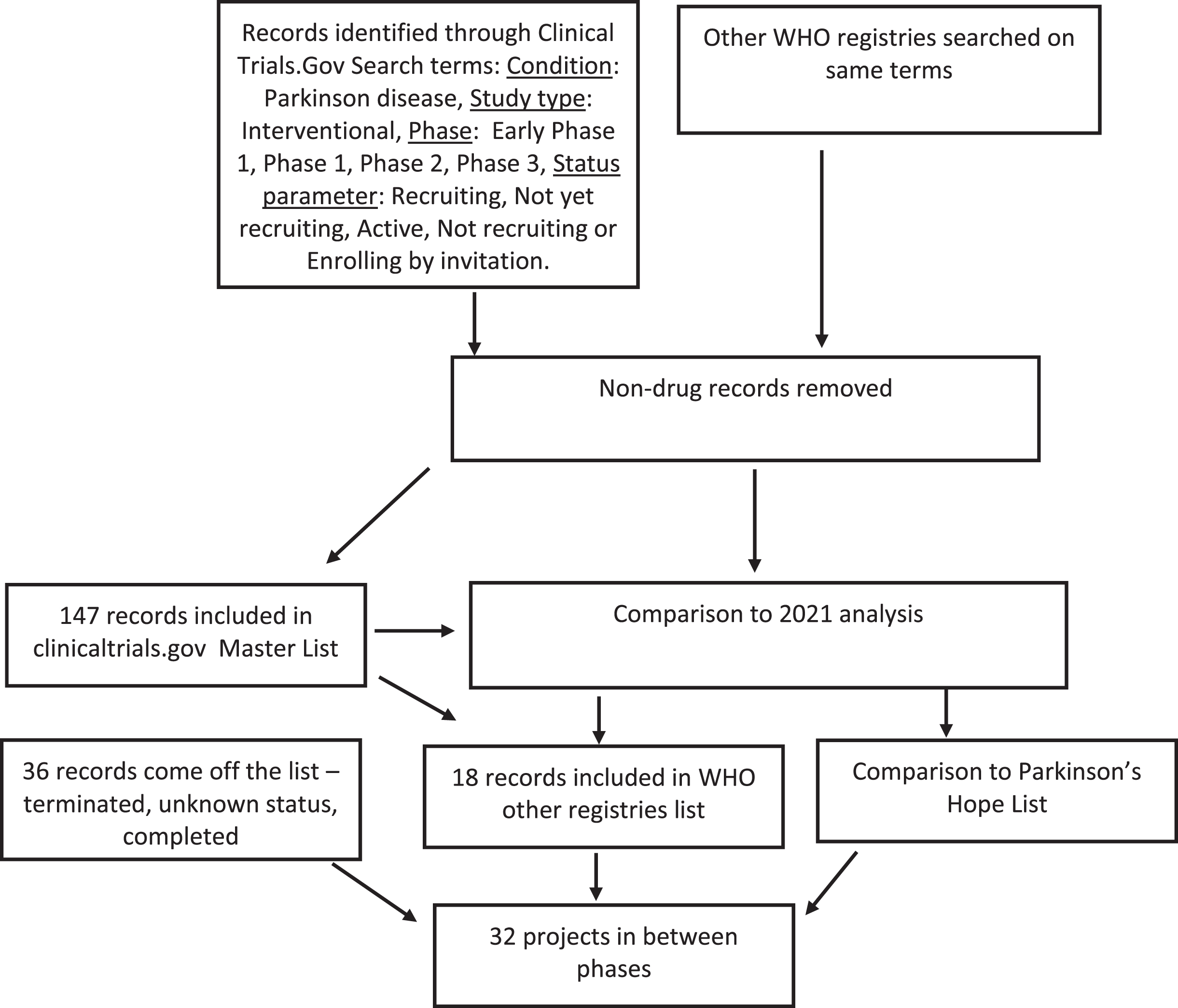
A breakdown analysis of the studies that were active was conducted, which involved dividing the therapies into “symptomatic treatments” (ST - improves / reduces symptoms of the condition) and “disease modifying treatments” (DMT - attempts to delay/slow progression by addressing the underlying biology of PD alleviating the features of the condition). These studies were then further assigned to one of 14 categories in accordance with our previously used methods [7]. Analyses were also conducted on the nature of the therapies, in terms of their origin – whether they were novel, repurposed, reformulated, or under a new claim. Those studies listed as Phase 1/2 were classified as Phase 1; and those listed as Phase 2/3 classified as Phase 2. A diagram of the workflow is presented in Fig. 1.
Fig. 1
A schematic outlining the data collection and analysis.
RESULTS
On January 31st 2022, the records of 226 interventional clinical trials were downloaded from the ClinicalTrials.gov (N = 183) and WHO ICTRP websites (N = 43). Unfortunately, many of the studies on the WHO ICTRP website did not have information regarding the phase of study and had to be excluded. Further assessment of each study downloaded from the ClinicalTrials.gov website resulted in the exclusion of a further 36 trials from this dataset as they did not meet our criteria. This led to a total dataset of 165 trials registered as ongoing and active for drug therapies targeting PD, 147 of which were from ClinicalTrials.gov and 18 from the WHO ICTRP registry collation. The full list is available in supplementary file 1.
Thirty-six trials that were in the dataset for the 2021 report were not present in the 2022 dataset. Some of these trials were no longer on the list because one phase of development had been completed successfully and although planning for the next trial is in progress, it had not yet been registered. These “in between” projects were added to the list of inbetweeners, also presented in supplementary file 1.
To allow for a comparison with our 2020 and 2021 reports, we focused our analysis on the clinicaltrials.gov list. We classified these trials by ST and DMT and compared these to our previous analyses (Table 1). An obvious increase in the number of ST trials at the Phase 2 stage was evident in 2022, but limited progress to Phase 3 is apparent across both categories.
Table 1
ST and DMT by Phase and year – ClinicalTrials.gov
| PHASE | ST | DMT | TOTAL TRIALS | ||||||
| 2020 | 2021 | 2022 | 2020 | 2021 | 2022 | 2020 | 2021 | 2022 | |
| 1 | 27 | 24 | 27 | 24 | 25 | 24 | 51 | 49 | 51 |
| 2 | 36 | 33 | 46 | 30 | 32 | 28 | 66 | 65 | 74 |
| 3 | 25 | 26 | 19 | 3 | 2 | 3 | 28 | 28 | 22 |
| Total | 88 | 83 | 93 | 57 | 59 | 54 | 145 | 142 | 147 |
Next, we focused on designating a therapeutic category for each trial (Fig. 2). Dopaminergic symptom relief (N = 39) and non-dopaminergic symptom relief (N = 38) were the largest categories based on the number of trials (Table 2). A comparison was made with the 2020 and 2021 category data, where an increase in the number of trials for GLP-1 R agonists and those targeting alpha-synuclein was a positive DMT trend (Supplemental Table 1).
Fig. 2
A schematic of all of the agents in active clinical trials for PD, registered on clinicaltrials.gov as of the 31st January 2022.
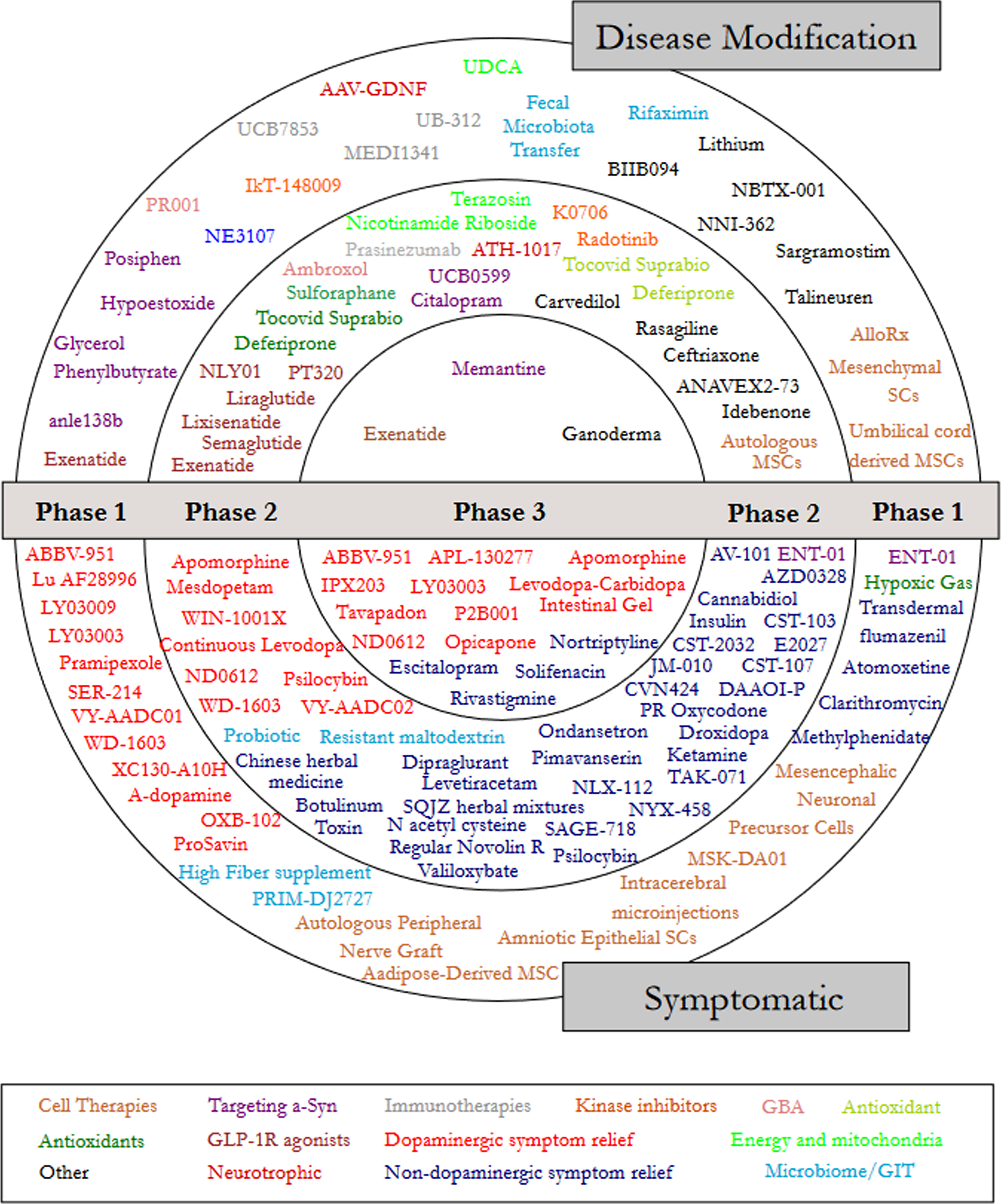
Table 2
Number of Trials by therapeutic category - ClinicalTrials.gov
| Category | Phase 1 | Phase 2 | Phase 3 | TOTAL | |||
| Anti-inflammatories | 1 | 2.0% | 0 | 0.0% | 0 | 0.0% | 1 |
| Antioxidants | 1 | 2.0% | 2 | 2.7% | 0 | 0.0% | 3 |
| Cell therapy | 9 | 17.6% | 2 | 2.7% | 0 | 0.0% | 11 |
| Dopaminergic symptom relief | 14 | 27.5% | 9 | 12.2% | 16 | 72.7% | 39 |
| Energy and mitochondria | 1 | 2.0% | 3 | 4.1% | 0 | 0.0% | 4 |
| GBA | 1 | 2.0% | 1 | 1.4% | 0 | 0.0% | 2 |
| GLP-1 R agonists | 1 | 2.0% | 6 | 8.1% | 1 | 4.5% | 8 |
| Immunotherapy | 3 | 5.9% | 2 | 2.7% | 0 | 0.0% | 5 |
| Kinase inhibitors | 1 | 2.0% | 2 | 2.7% | 0 | 0.0% | 3 |
| Microbiome/GIT | 4 | 7.8% | 2 | 2.7% | 0 | 0.0% | 6 |
| Neurotrophic factors | 2 | 3.9% | 1 | 1.4% | 0 | 0.0% | 3 |
| Non-dopaminergic symptom relief | 2 | 3.9% | 33 | 44.6% | 3 | 13.6% | 38 |
| Targeting alpha-synuclein | 5 | 9.8% | 4 | 5.4% | 1 | 4.5% | 10 |
| Other | 6 | 11.8% | 7 | 9.5% | 1 | 4.5% | 14 |
| TOTAL | 51 | 74 | 22 | 147 | |||
Therapy category analysis
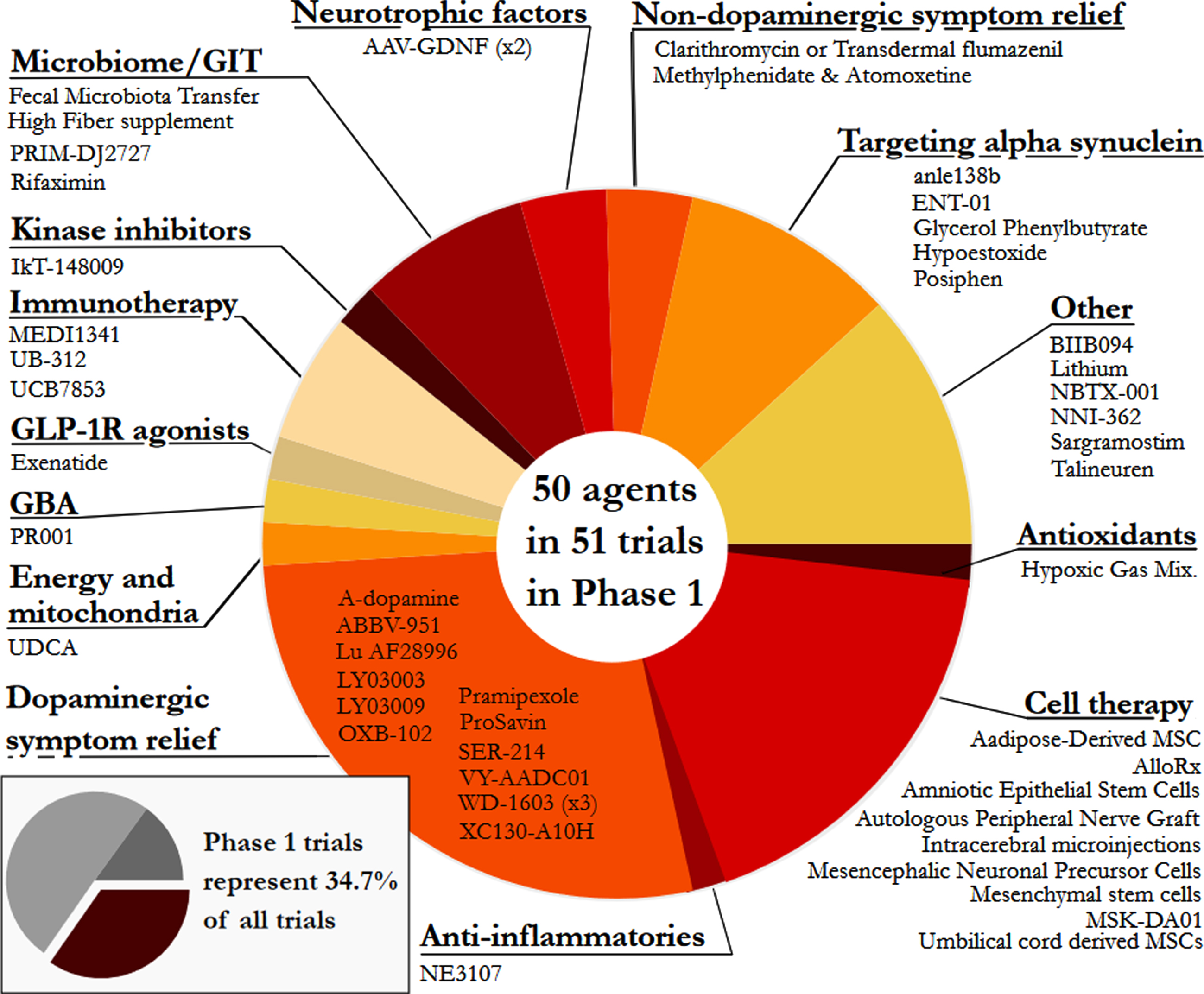
Phase 1 - Of the active trials registered on the clinicaltrials.gov website, 34.7% (51 trials of 50 agents) were listed as Phase 1 or Phase 1/2. Similar to our previous reports, the Phase 1 trials represented a broad range of different therapeutic approaches – every category was represented in Phase 1. The largest category in Phase 1 was dopaminergic symptom relief (27.5%), followed by cell therapies (17.6%), other (11.8%), and targeting alpha-synuclein (9.8%; Fig. 3).
Fig. 3
A pie chart of the agents in active Phase 1 trials for PD, registered on clinicaltrials.gov as of January 31st 2022.
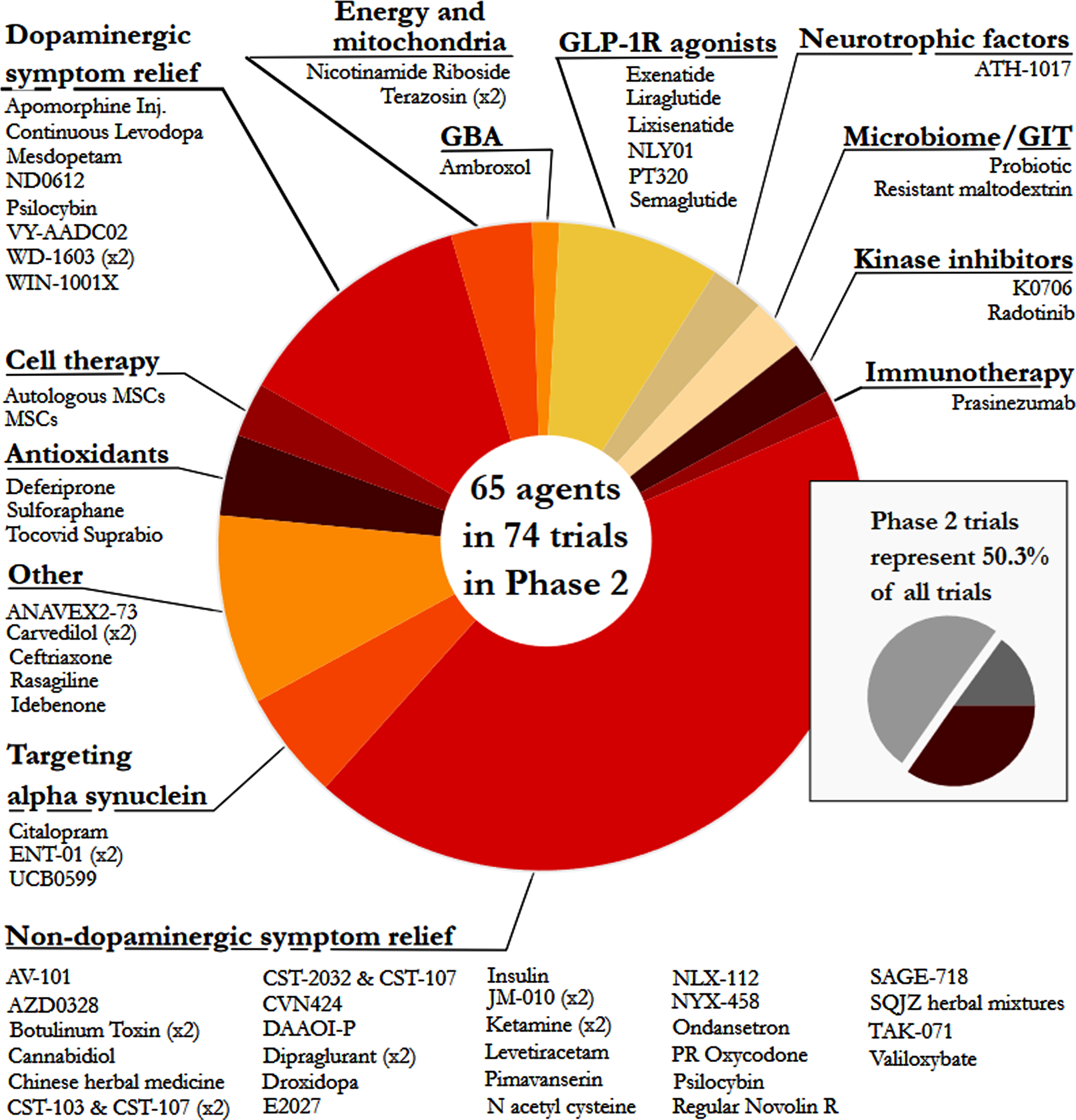
Phase 2 – Similar to our previous reports, Phase 2 made up the largest number of clinical trials in our dataset (50.3% of the total number of trials, including 74 studies involving 65 agents; Fig. 4). The main observation at this stage of drug development was the large number of trials for non-dopaminergic symptom relief (44.6%). The next largest segment was dopaminergic symptom relief (12.2%), then other (9.5%)and GLP-1 R agonists (8.1%).
Fig. 4
A pie chart of the agents in active Phase 2 trials for PD, registered on clinicaltrials.gov as of January 31st 2022.
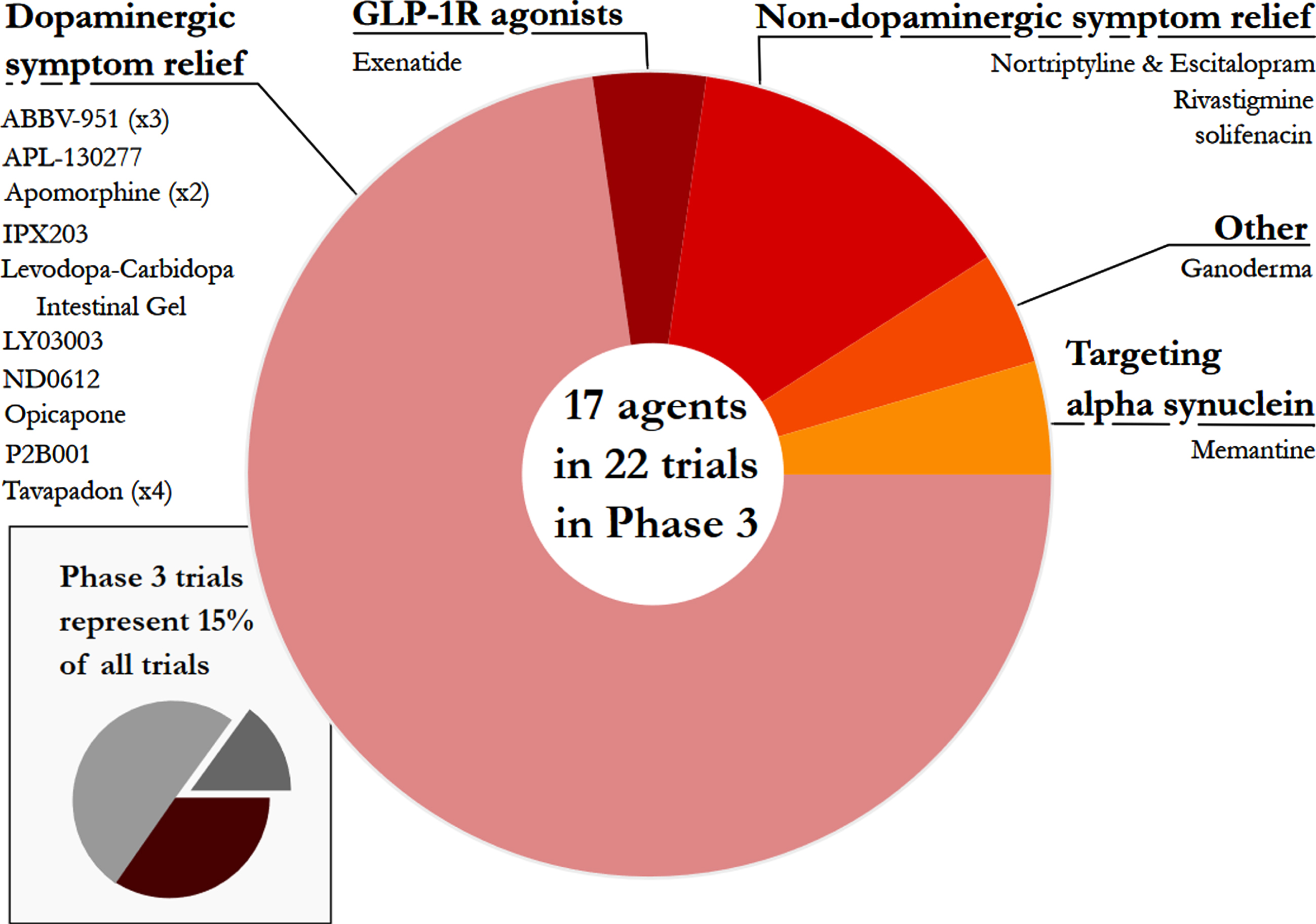
Phase 3 – For a third year, Phase 3 was the smallest set of clinical trials (only 15% of the total number) and the least diverse - only 5 categories were represented (Fig. 5). Of these, dopaminergic symptom relief (72.7%)made up the bulk of the active studies. Phase 3 is the final stage of the clinical development process, involving very large studies that require significant financial resources, but the lack of transition between Phase 2 to Phase 3 studies was a disappointing observation over the three-year period that these reports have been conducted. It is appreciated, however, that there has been a global pandemic during that period of time, which may have hampered progress.
Fig. 5
A pie chart of the agents in active Phase 3 trials for PD, registered on clinicaltrials.gov as of January 31st 2022.
3.2Studies completing in 2022
There were 96 trials listed in the clinicaltrials.gov dataset as completing before the end of 2022 (38 Phase 1 trials, 49 Phase 2 trials, and 9 Phase 3 trials). This accounts for 65% of the 147 active trials, and 33% of the trials due to complete are classified as DMT. Of the 32 DMT trials, 18 are in Phase 1, 13 in Phase 2 and 1 is in Phase 3 (Table 3).
Table 3
DMT clinical trials that are due to complete by end 2022 - Clinicaltrials.gov
| Phase 1 | Phase 2 | Phase 3 |
| Rifaximin | WIN-1001X | Ganoderma |
| Glycerol Phenylbutyrate | Rasagiline | |
| Exenatide | Ambroxol | |
| anle138b | Lixisenatide | |
| NE3107 | Deferiprone | |
| Talineuren | Carvedilol | |
| NNI-362 | ANAVEX2-73 | |
| Mesenchymal stem cells | Radotinib HCl | |
| Sargramostim | Carvedilol | |
| UB-312 | PT320 | |
| IkT-148009 | Autologous mesenchymal stem cells | |
| AAV2-GDNF | Liraglutide | |
| MEDI1341 | Exenatide | |
| Hypoestoxide | ||
| UDCA (Ursodeoxycholic acid) | ||
| Posiphen | ||
| Lithium | ||
| Injection of Umbilical cord derived MSCs | ||
Nature of agents being tested
The majority of the therapies being tested in our dataset were novel (42.2%)or repurposed (34%). Novel agents dominated Phase 1 while repurposed therapies were more apparent at Phase 2 & 3 (Table 4). It is encouraging to note that some of the novel agents being evaluated are the result of ‘pathfinding studies’ with repurposed therapies. For example, NLY-01 is a new GLP-1 R agonist that has resulted from previous research with repurposed GLP-1R agonists like exenatide, liraglutide and lixisenatide.
Table 4
Nature of agents being clinically tested - Clinicaltrials.gov
| PHASE | NOVEL | REPURPOSED | REFORMULATION | NEW CLAIM | TOTAL |
| 1 | 25 | 12 | 13 | 1 | 51 |
| 2 | 32 | 33 | 6 | 3 | 74 |
| 3 | 5 | 5 | 10 | 2 | 22 |
| TOTAL | 62 | 50 | 29 | 6 | 147 |
| % | 42.2% | 34.0% | 19.7% | 4.1% |
Trials registered on the WHO ICTRP database
A total of 18 trials registered on WHO ICTRP list of other registries reached the final criteria for inclusion in our analysis. There are a large number of active clinical trials for PD on the WHO registries, but in most cases the information provided was lacking key details required for inclusion here (such as Phase of testing). Of the included studies, there were 8 trials in Phase 1 or 1/2, 9 studies in Phase 2, and only 1 Phase 3 trial (which explores curcumin supplementation). Half of these studies were categorised as DMT.
DISCUSSION
The 2022 drug development pipeline review represents a testament to the dedication of the PD community to addressing the need for new therapies. Despite significant obstacles presented by the restrictions of the COVID-19 response, clinical trials were initiated, continued and completed during this period. It is particularly encouraging that across the multi-year period of our analysis that we have not seen a major reduction in the amount of clinical trial activity. Of the trials that have completed and reported results since our previous update, there have been some very interesting findings. In 2021, the biotech company Denali Therapeutics completed their Phase 1b trial of their LRRK2 inhibitor DNL-151 (NCT04056689) in patients with PD, and announced that the safety and biomarker goals were met. They also reported data demonstrating target engagement and reduction of both LRRK2 and substrate activity [11]. The company is now collaborating with the pharmaceutical company Biogen to advance into a late-stage clinical trial of the drug (now referred to as BIIB122/DNL151). They plan to start two clinical studies this year, firstly the LIGHTHOUSE Study (a global Phase 3 trial expected to enroll approximately 400 individuals with PD who carry a LRRK2 mutation), and the LUMA study (a global Phase 2b trial hoping to enroll approximately 640 individuals with PD who do not carry a LRRK2 mutation [12]).
Anavex Life Sciences announced positive results for their Phase 2 trial (NCT04575259) evaluating ANAVEX 2-73 (blarcamesine) in patients with PD dementia (PDD). The reported results showed clinically meaningful, and statistically significant, dose-dependent improvements in the Cognitive Drug Research (CDR) assessment analysis. The company is now in discussions with regulators regarding how to advance late stage clinical testing of the drug. In a Phase 2 clinical trial (NCT03713957) of GRF6021, in patients with PD and cognitive impairment, the biotech firm Alkahest reported that the drug demonstrated positive effects on cognitive endpoints and was safe and well tolerated [13]. The company plans to further study its efficacy as a PD DMT.
Another set of very recent trial results came from the Phase 2b KARMET study that was conducted by the biotech company Enterin. They are developing a derivative of squalamine, called ENT-01, which suppresses aggregation of alpha-synuclein in models of PD. The KARMET study involved 150 individuals with PD who experience constipation. The randomized, double-blind study found that ENT-01 was safe and tolerable, but also significantly improved gastrointestinal passage. Unexpectedly, they reported encouraging data on psychosis-related secondary endpoints [14]. These completed trial results indicate exciting potential developments in the coming years.
In 2022, we are looking forward to the results of additional clinical trials. These include the Phase 2 studies of DMTs such as the mitochondrial targeting UDCA (which has been tested in the “UP study” - NCT03840005) and the iron chelator deferiprone trial (NCT02655315). There are also a number of GLP-1 R agonist trials that will complete this year, such as with liraglutide (NCT02953665), lixisenatide (NCT03439943), and PT320, which is a slow-release formulation of exenatide that has been developed by the biotech company Peptron (NCT04269642).
This third edition of the drug development pipeline for PD provides the opportunity to start exploring trends across years, and several stark patterns stand out. As an estimated 10 million people are living with PD, it is important to see more transition from Phase 2 to Phase 3 in the clinical testing of new therapies for PD. There were only 22 Phase 3 studies in our analysis, 19 of which are ST. Of these, six use sub-cutaneous (SC) infusion, with the objective of providing a continuous delivery of the therapeutic agent to minimise the incidence of motor fluctuations. Abbvie’s ABBV-951 uses phosphorylated versions of levodopa and carbidopa (LD/CD) as the pro-drugs have greater solubility, with SC infusion. Neuroderm’s ND0612 also uses SC infused LD/CD. A similar principle applies to IPX203 from Impax (now owned by Amneal Pharmaceuticals) with an extended release tablet version of LD/CD. Supernus are testing SC infusion of apomorphine, a dopamine agonist, and Rennes University are assessing the use of apomorphine infusion in early PD. Cerevel’s tavapadon is a dopamine 1/5 partial agonist and is being investigated in four Phase 3 studies, covering both early PD and those patients enduring motor fluctuations.
In addition to the lack of phase transition, the slow speed of clinical development also stands out in our multi-year analysis. There are efforts to address this issue, however, as research teams explore new clinical trial designs and platforms. One exciting example of this in the PD field is the development of Multi-Arm studies that explore multiple drugs at the same time, drastically reducing patient numbers and costs by unifying a single placebo comparator arm across multiple treatment arms. The Australian Federal government has awarded an AUS$30 million grant to establish a series of DMT clinical trials in PD, evaluating three agents prioritised by the international Linked Clinical Trials committee [15, 16]. The multi-arm design for clinical trials has also evolved to become more adaptive [17]. Using interim analysis of data as the trial is active, investigators will be able to identify treatment arms that are having no impact and discontinue them. Likewise, such a dynamic platform will allow for seamless transitioning from Phase 2 safety and efficacy studies to larger Phase 3 trials. In 2021, the Edmond J Safra Foundation awarded a significant grant to build a Multi-Arm, Multi-stage platform for PD to evaluate DMT [5].
SUMMARY
Although COVID-19 has represented a significant obstacle during 2021-2022, important progress has still been made in terms of the clinical development of STs and DMTs for PD. The total number of trials and the mix of STs and DMTs has remained similar across the timeframe. While many projects have dropped out of the list, progression to Phase 1 and from Phase 1 to 2 appears to continue. The lack of movement of DMTs from Phase 2 to Phase 3 remains a concern and is a major rate-limiting step to the provision of new therapies to those people living with PD. There are, however, a number of Phase 3 trials in the planning stages and 13 DMT Phase 2 trials due to complete by the end of 2022 (almost two-thirds of the 147 trials in our primary dataset are planned to complete in 2022). This will hopefully result in an increase in the number of DMT Phase 3 trials in the coming years. We look forward to commenting on the outcomes in our future reviews.
ABBREVIATIONS
AMSC Autologous Mesenchymal stem cells
ASAP Aligning Science Across Parkinson’s
COVID-19 Coronavirus disease 2019
CNS Central Nervous system
CSF Cerebrospinal fluid
DMT Disease Modifying Therapies
FDA Food and Drug Administration
GBA Glucocerebrosidase
GDNF Glial cell-derived neurotrophic factor
GIT Gastrointestinal Tract
GLP-1 R Glucagon-like peptide 1 receptor
ICTRP International Clinical Trials Registry Platform
LD/CD Levodopa and carbidopa
LID Levodopa-induced dyskinesia
LRRK2 Leucine-rich repeat kinase 2
MAMS Multi-Arm Multi-Stage
MAO-B Monoamine oxidase type B
MCI Mild Cognitive Impairment
MOA Mechanisms of Action
MRI Magnetic Resonance Imaging
NIH National Institutes of Health
NINDS National Institute of Neurological Disorders and Stroke
NMDA N-methyl-D-aspartate
NMS Non-motor symptoms
PD Parkinson’s Disease
SC Sub-cutaneous
ST Symptomatic Therapy
UDCA Ursodeoxycholic acid
WHO World Health Organization
AUTHOR CONTRIBUTION
KMcF, GR, MB, LM, RF, RKW & SRWS performed the trial categorization & helped write/edit the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Prof Tanya Simuni of Northwestern University and Helen Matthews of Cure Parkinson’s for reading the manuscript and providing constructive feedback. The authors would also like to thank all of the trial participants and their families, and the researchers involved in the ongoing clinical research for PD.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-229002.
REFERENCES
[1] | WHO, recommends groundbreaking malaria vaccine for children at risk, http://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk |
[2] | Esrick EB , Lehmann LE , Biffi A , Achebe M , Brendel C , Ciuculescu MF , Daley H , MacKinnon B , Morris E , Federico A , Abriss D , Boardman K , Khelladi R , Shaw K , Negre H , Negre O , Nikiforow S , Ritz J , Pai S-Y , London WB , Dansereau C , Heeney MM , Armant M , Manis JP , Williams DA ((2021) ) Post-Transcriptional Genetic Silencing of BCL11A to Treat Sickle Cell Disease. N Engl J Med 384: (3), 205–215. |
[3] | Andrieux-Meyer I , Tan SS , Thanprasertsuk S , Salvadori N , Menétrey C , Simon F , Cressey TR , Said HRHM , Hassan MRA , Omar H , Tee HP , Chan WK , Kumar S , Thongsawat S , Thetket K , Avihingsanon A , Khemnark S , Yerly S , Ngo-Giang-Huong N , Siva S , Swanson A , Goyal V , Bompart F , Pécoul B , Murad S ((2021) ) Efficacy and safety ofravidasvir plus sofosbuvir in patients with chronic hepatitis Cinfection without cirrhosis or with compensated cirrhosis(STORM-C-1): Interim analysis of a two-stage, open-label,multicentre, single arm, phase 2/3 trial. Lancet GastroenterolHepatol 6: (6), 448–458. |
[4] | Grantees for Circuitry and Brain-Body Interactions, http://parkinsonsroadmap.org/research-network/circuitry/ |
[5] | Edmond J. Safra Foundation awards £1.375 million to rapidly accelerate Parkinson’s drug search http://www.ucl.ac.uk/campaign/news/2021/jun/edmond-j-safra-foundation-awards-ps1375-million-rapidly-accelerate-parkinsons-drug |
[6] | |
[7] | McFarthing K , Buff S , Rafaloff G , Dominey T , Wyse RK , Stott SRW ((2020) ) Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020. J Parkinsons Dis 10: (3), 757–774. |
[8] | The ClinicalTrials.gov website, http://clinicaltrials.gov |
[9] | The World Health Organisation (WHO) , International Clinical Trials Registry Platform http://www.who.int/clinical-trials-registry-platform |
[10] | McFarthing K , Rafaloff G , Baptista MAS , Wyse RK , Stott SRW ((2021) ) Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2021 Update. J Parkinsons Dis 11: (3), 891–903. |
[11] | Denali Therapeutics Presents Positive Results from Phase 1 and Phase1b Studies of Its LRRK2 Inhibitor, BIIB122/DNL151, SupportingLate-Stage Development Plans in Parkinson’s Disease, www.denalitherapeutics.com/investors/press-release?id=8141&type=api |
[12] | Denali Therapeutics Announces Progression and Expansion of Broad Therapeutic Portfolio for Neurodegeneration and Expected Key Milestones in 2022, http://www.globenewswire.com/news-release/2022/01/10/2363990/0/en/Denali-Therapeutics-Announces-Progression-and-Expansion-of-Broad-Therapeutic-Portfolio-for-Neurodegeneration-and-Expected-Key-Milestones-in-2022.html |
[13] | Alkahest to Present Positive Data from Completed Phase 2 ClinicalTrial at the 15th International Conference on Alzheimer’s andParkinson’s Disease, http://www.globenewswire.com/news-release/2021/03/08/2188969/0/en/Alkahest-to-Present-Positive-Data-from-Completed-Phase-2-Clinical-Trial-at-the-15th-International-Conference-on-Alzheimer-s-and-Parkinson-s-Disease.html |
[14] | Enterin Meets Study Endpoints for the Phase 2b (KARMET) Study Involving Patients With Parkinson’s Disease, http://www.globenewswire.com/news-release/2022/01/27/2374392/0/en/Enterin-Meets-Study-Endpoints-for-the-Phase-2b-KARMET-Study-Involving-Patients-With-Parkinson-s-Disease.html |
[15] | Brundin P , Wyse RK ((2019) ) The Linked Clinical Trials initiative (LCT) for Parkinson’s disease. Eur J Neurosci 49: (3), 307–315. |
[16] | Stott SRW , Wyse RK , Brundin P ((2021) ) Drug Repurposing for Parkinson’s Disease: The International Linked Clinical Trials experience. Front Neurosci 15: , 653377. |
[17] | Zeissler ML , Li V , Parmar MKB , Carroll CB ((2020) ) Is It Possible to Conduct a Multi-Arm Multi-Stage Platform Trial in Parkinson’s Disease: Lessons Learned from Other Neurodegenerative Disorders and Cancer. J Parkinsons Dis 10: (2), 413–428. |




