Physical Exercise as a Potential Treatment for Fatigue in Parkinson’s Disease? A Systematic Review and Meta-Analysis of Pharmacological and Non-Pharmacological Interventions
Abstract
Background:
Fatigue is one of the most common and debilitating non-motor symptoms among patients with Parkinson’s disease (PD) and significantly impacts quality of life. Therefore, effective treatment options are needed.
Objective:
To provide an update on randomized controlled trials (RCTs) including pharmacological and non-pharmacological (but non-surgical) treatments that examine the effects of fatigue on PD patients.
Methods:
We searched the MEDLINE, EMBASE, PsycINFO, CENTRAL, and CINAHL databases for (cross-over) RCTs on pharmacological and non-pharmacological interventions for treating fatigue in PD patients until May 2021. Meta-analyses for random-effects models were calculated when two or more studies on the same treatment option were available using standardized mean differences (SMDs) with 95% confidence intervals (CIs).
Results:
Fourteen pharmacological and 16 non-pharmacological intervention RCTs were identified. For pharmacological approaches, a meta-analysis could only be performed for modafinil compared to placebo (n = 2) revealing a non-significant effect on fatigue (SMD = – 0.21, 95% CI – 0.74–0.31, p = 0.43). Regarding non-pharmacological approaches, physical exercise (n = 8) following different training approaches versus passive or placebo control groups showed a small significant effect (SMD = – 0.37, 95% CI – 0.69‐ – 0.05, p = 0.02) which could not be demonstrated for acupuncture vs. sham-acupuncture (SMD = 0.16, 95% CI – 0.19–0.50, p = 0.37).
Conclusion:
Physical exercise may be a promising strategy to treat fatigue in PD patients. Further research is required to examine the efficacy of this treatment strategy and further interventions. Future studies should differentiate treatment effects on physical and mental fatigue as the different underlying mechanisms of these symptoms may lead to different treatment responses. More effort is required to develop, evaluate, and implement holistic fatigue management strategies for PD patients.
INTRODUCTION
In addition to the typical motor symptoms associated with Parkinson’s disease (PD), the wide variety of non-motor symptoms, including neuropsychiatric symptoms (e.g., depression, apathy), cognitive impairment, autonomic dysfunction, sleep and wakefulness disorders, pain, olfactory dysfunction, and ophthalmologic dysfunction, have attracted significant attention due to their frequency and impact on quality of life [1, 2]. One highly relevant but largely under investigated non-motor symptom is fatigue. PD patients use an extensive lexicon on fatigue that reflects the multidimensionality of this construct, consisting of emotional components (“overwhelming”), physical sensations (“heaviness”), and cognitive involvement (“foggy”, “unfocused”, or “numb”) with most patients experiencing more than one form of fatigue during the day [3]. The prevalence of PD-related fatigue varies greatly, ranging between 33–70%; this variance likely reflects the heterogeneous nature of populations and assessment methods [4, 5]. Fatigue is more frequently seen in later PD stages [6]; however, it can also manifest during premotor PD stages [7]. PD patients consider fatigue to be one of the most frequent and debilitating non-motor symptoms [8–10], and it can be more disruptive than motor symptoms [3, 11]. Fatigue impairs patients’ activities of daily living and limits their social participation and quality of life. Moreover, it is a major reason for early retirement [12]. Fatigue accounts for 5–10% of PD patients’ medical appointments in medical care [10].
The PD Foundation working group defines PD-related fatigue as a daily or near daily feeling of “significantly diminished energy level or increased perception of effort disproportionate to attempted activities or general activity level” [9]. Kluger, Krupp, and Enoka [13] proposed to differentiate between the subjective perception of fatigue and objective performance fatigability. The latter refers to a measurable decline in performance during a sustained task, while subjective fatigue is often divided into physical fatigue (i.e., a feeling of physical exhaustion and lack of energy), and mental fatigue (i.e., the cognitive effects that occur during and after prolonged periods of sustained mental effort) [14, 15]. Mental fatigue may also include fatigue with regard to emotional function [3, 11]. A distinction has been made between primary fatigue (directly related to PD pathophysiology) and secondary fatigue (related to the side effects of oral medication, comorbid medical conditions, sleep disorders, etc.) [10, 16].
To date, there is no clear understanding of the underlying pathophysiology of fatigue. Multifactorial interacting pathomechanisms have been discussed, including increased circulating proinflammatory cytokines, dysfunction in nigrostriatal and extrastriatal dopaminergic/serotonergic pathways, executive/prefrontal pathology, and autonomic nervous system involvement [9, 17–19].
Clinical treatment of fatigue is challenging, and evidence regarding treatment options is rare and inconsistent. To date, few systematic reviews have been conducted on the treatment of fatigue in PD patients. In the 2014 review by Franssen et al. [20], 14 studies (11 pharmacological interventions and 3 non-pharmacological interventions) were included; however, there was insufficient evidence regarding the efficacy of the included treatments. A Cochrane review and meta-analyses from 2015 by Elbers et al. [21] evaluated 11 pharmacological and 2 non-pharmacological interventions on subjective PD fatigue. The authors found evidence that rasagiline and modafinil were associated with improvements in physical fatigue, while doxepin was associated with improvement in general fatigue (both physical and mental). However, they concluded that due to limitations in the evidence quality, there was a lack of a clear basis for treatment decisions. Some reviews have only addressed specific intervention types, such as mind-body interventions [22], dance therapy [23], cognitive-behavioral therapy (CBT) [24], and MAO-B inhibitors [25], and have typically used general non-motor symptoms as outcomes. Some of the reviews included single fatigue scales [22–24] or included any type of clinical study [25]. These reviews found no [22–24] or unclear evidence [25] regarding treatment effects on fatigue. Notably, Cusso et al. [26] conducted a systematic review of physical activity as a broader category on non-motor symptoms in PD patients. This review included 5 randomized controlled trials (RCTs), one of which investigated Nordic walking, which demonstrated a significant improvement in fatigue. In their updated review on treatments for non-motor symptoms, conducted on behalf of the Movement Disorder Society, Seppi et al. [1] concluded that rasagiline is “efficacious” in treating fatigue in PD, while insufficient evidence existed regarding the efficacy of methylphenidate, modafinil, and acupuncture. The update search used for this review included studies published up to December 2016, and the literature search only involved the MEDLINE and CENTRAL databases.
Considering this background, and given the fact that the number of studies that have investigated treatment effects on PD fatigue has increased significantly in recent years, this systematic review aims to provide a comprehensive update on the RCTs, including pharmacological and non-pharmacological (but non-surgical) treatments, that have examined the effects on fatigue using various established fatigue scales in PD patients, and to conduct meta-analyses where possible.
MATERIALS AND METHODS
This systematic review was pre-registered in the international database of prospectively registered systematic reviews in health and social care (PROSPERO; CRD42021257853) and adhered to the PRISMA guidelines for reporting systematic reviews and meta-analyses [27]. No review protocol was published.
Search strategy
The MEDLINE, EMBASE, PsycINFO, CENTRAL, and CINAHL databases were searched for articles published up until May 15, 2021. Only English- and German-language articles were included. Supplementary Table 1 outlines the full search strategy for all databases. In general, we followed the PICOS framework [28] regarding the search string building. However, since we were not focused on specific pharmacological or non-pharmacological therapies, we filtered for clinical trials instead of searching for specific interventions and control groups. We manually searched the included studies of already published systematic reviews particularly focusing on treatment options for fatigue in PD [20, 21, 26] to source further literature.
Table 1
Main characteristics of included pharmacological and non-pharmacological trials
| Study Sample size (N) | Sample size (n); Sex ratio (F:M) | Age | H&Y; UPDRS-motor; duration of disease (y) | Fatigue baseline | Intervention and control group(s) | Frequency and duration | Fatigue outcome(s) and time points of measurement | Main fatigue results |
| Pharmacological interventions (N = 14) | ||||||||
| Adler 2003 [46]N = 21 (rand.)N = 20 (eval.)cross-over trial | Arm 1: n = 11; NR | Study level: 65.0±12 | Study level: 2.0±0.5; 14.4±8.5; 7.4±4.9 | Arm 1: FSS: 4.6±1.8 | Arm 1: Modafinil 200mg | 1x/day7x/week3 weeks | FSSBaseline; 3 weeks after baseline (post-intervention) | No significance difference between EG and CG in FFS post-intervention. |
| Arm 2: n = 10; NR | Arm 2: FSS: 4.8±1.6 | Arm 2: Placebo | 1x/day7x/week3 weeks | |||||
| Büchele 2018 [47] N = 12 (rand.) N = 11 (eval.) cross-over trial | Study level: N = 12; 2:10 | Study level: 62.0±11.1 | Study level: range: 2-3; 29.8±11.5; 8.4 (4.6) | Arm 1: FSS: 4.1±1.0 | Arm 1: Sodium Oxybate (3.0 to 9.0g per night) | 1x/day7x/week6 weeks | FSS Baseline; 6 weeks after baseline (post-intervention) | No significance difference between EG and CG in FFS post-intervention. |
| Arm 2: FSS: 4.3±1.1 | Arm 2: Placebo | 1x/day7x/week6 weeks | ||||||
| Lim 2015 [31] N = 30 (rand. = eval.) | Arm 1: n = 16; 5:11 | Arm 1: 68.7±7.4 | Arm 1: NR; 31±NR; Median: 3 (IQR: 3–10) | Arm 1: FSS: Median: 52 (IQR: 45.25–57) | Arm 1: Rasagiline 1mg | 1x/day7x/week12 weeks | MFIS; FSS; MFIBaseline; 4 + 8 weeks after baseline (mid-intervention); 12 weeks after baseline (post-intervention) | Significant difference in MFIS change from baseline and post-intervention between EG and CG; significant difference between EG and CG in FSS post-intervention; trend for improvement in MFI for EG; trend for a difference between EG and CG post-intervention. |
| Arm 2: n = 14; 9:5 | Arm 2: 65.4±6.4 | Arm 2: NR; 27.5±NR; Median: 4 (IQR: 3–7.25) | Arm 2: FSS: Median: 47 (IQR: 42–50) | Arm 2: Placebo | 1x/day7x/week12 weeks | |||
| Lou 2009 [39] N = 22 (rand.) N = 19 (eval.) | Arm 1: n = 9; 0:9 | Arm 1: 64.0±9.0 | Arm 1: 1.9±0.6; NR; 4.0±3.0 | Arm 1: MFI: 55.8±5.1 (SE) | Arm 1: Modafinil 100mg | 2x/day7x/week8 weeks | MFIBaseline; 4 weeks after baseline (mid-intervention); 8 weeks after baseline (post-intervention) | No significance difference between EG and CG in MFI post-intervention. |
| Arm 2: n = 10; 5:5 | Arm 2: 69.0±8.0 | Arm 2: 2.3±0.6; NR; 8.0±6.0 | Arm 2: MFI: 63.5±4.8 (SE) | Arm 2: Placebo | 2x/day7x/week8 weeks | |||
| Mendonca 2007 [40] N = 36 (rand.) N = 34 (eval.) | Arm 1: n = 17; 1:16 | Arm 1: 66.3±7.6 | Arm 1: 2.4±0.3; 33.3±10.4; 72.2±61.2 (in months) | Arm 1: FSS: 43.8±6.7 | Arm 1: Methylphenidate 10mg | 3x/day7x/week6 weeks | FSS; MFIBaseline; 6 weeks after baseline (post-intervention) | Significant reductions in FSS and MFI for EG post-intervention; non-significant reduction for CG. |
| Arm 2: n = 19; 11:8 | Arm 2: 62.2±10.0 | Arm 2: 2.6±0.5; 32.3±8.0; 80.6±79.8 (in months) | Arm 2: FSS: 44.9±6.2 | Arm 2: Placebo | 3x/day7x/week6 weeks | |||
| Ondo 2005 [41] N = 40 (rand.) N = 37 (eval.) | Arm 1: n = 20; 7:13 | Arm 1: 64.4±10.4 | Arm 1: NR; 24.1±9.8; 6.5±5.5 | Arm 1: FSS: 37.6±14.1 | Arm 1: Modafinil up to 200mg | 2x/day7x/week4 weeks | FSSBaseline; 4 weeks after baseline (post-intervention) | No significance difference between EG and CG in FFS post-intervention. |
| Arm 2: n = 20; 7:13 | Arm 2: 65.1±12.3 | Arm 2: NR; 29.2±9.5; 7.0±4.6 | Arm 2: FSS: 36.8±12.8 | Arm 2: Placebo | 2x/day7x/week4 weeks | |||
| Ondo 2011 [42] N = 40 (rand.) N = 36 (eval.) | Arm 1: n = 20; 8:12 | Arm 1: 69.2±7.9 | Arm 1: 2.5±0.53; 24.3±14.3; NR | Arm 1: FSS: 37.6±14.2 | Arm 1: Memantine up to 10mg | 2x/day7x/week8 weeks | FSSBaseline; 8 weeks after baseline (post-intervention) | No significance difference between EG and CG in FSS post-intervention. |
| Arm 2: n = 20; 8:12 | Arm 2: 68.9±8.4 | Arm 2: 2.3±0.41; 19.0±10.5; NR | Arm 2: FSS: 37.2±14.3 | Arm 2: Placebo | 2x/day7x/week8 weeks | |||
| Pahwa 2015 [32] N = 83 (rand.) N = 80 (eval.) | Arm 1: n = 20; 10:10 | Arm 1: 66.4±9.4 | Arm 1: 2.4±0.8; NR; 9.0±3.5 | Arm 1: FSS: 4.8±1.1 | Arm 1: Amantadine 420mg | 1x/day7x/week8 weeks | FSSBaseline; 2 weeks after baseline (mid-intervention); 4 weeks after baseline (mid-intervention); 8 weeks after baseline (post-intervention) | No significant change in FSS in any of the EG. |
| Arm 2: n = 21; 8:13 | Arm 2: 64.7±10.0 | Arm 2: 2.5±0.6; NR; 9.3±4.9 | Arm 2: 4.8±1.4 | Arm 2: Amantadine 340mg | 1x/day 7x/week8 weeks | |||
| Arm 3: n = 20; 12:8 | Arm 3: 67.5±8.6 | Arm 3: 2.5±0.9; NR; 8.9±3.4 | Arm 3: FSS: 4.4±1.5 | Arm 3: Amantadine 260mg | 1x/day7x/week8 weeks | |||
| Arm 4: n = 22; 8:14 | Arm 4: 65.5±10.2 | Arm 4: 2.5±0.7; NR; 10.7±7.1 | Arm 4: FSS: 4.9±1.2 | Arm 4: Placebo | 1x/day7x/week8 weeks | |||
| Peball 2020 [43] N = 38 (rand. = eval.) | Arm 1: n = 19; 9:10 | Arm 1: 65.4±7.9 | Arm 1: 1.8±0.5; 26.0±13.3; 7.8±5.5 | Arm 1: FSS: 34.1±8.9 | Arm 1: Nabilone up to 1mg | up to 2x/day7x/week4 weeks | FSSBaseline; 4 weeks after baseline (post-intervention) | No significance difference between EG and CG in FSS post-intervention. |
| Arm 2: n = 19; 5:14 | Arm 2: 64.0±8.0 | Arm 2: 2.0±0.4; 27.9±10.0; 7.4±5.1 | Arm 2: FSS: 34.1±13.1 | Arm 2: Placebo | 1-2x/day7x/week4 weeks | |||
| Postuma 2012 [33] N = 61 (rand. = eval.) | Arm 1: n = 30; 5:25 | Arm 1: 65.2±8.3 | Arm 1: NR; 23.2±8.5; 7.8±3.5 | Arm 1: FSS: 39.9±12.2 | Arm 1: Caffeine 100mg, after 3 weeks increase to 200mg | 2x/day7x/week6 weeks | FSSBaseline; 6 weeks after baseline (post-intervention) | No significance difference between EG and CG in FSS post-intervention. |
| Arm 2: n = 31; 12:19 | Arm 2: 67.8±11.2 | Arm 2: NR; 22.5±11.5; 8.0±4.8 | Arm 2: FSS: 39.5±14.9 | Arm 2: Placebo | 2x/day7x/week6 weeks | |||
| Stocchi 2014 [34] N = 1176 (rand.) N = 1105 (eval.) | Arm 1: n = 277; NR | Arm 1: 62.2±9.7 | Arm 1: 1.5±0.5; 14.5±6.6; 4.6±4.6 (in months) | Arm 1: PFS: 2.2±0.9 | Arm 1: Rasagiline 2mg | 1x/day7x/week36 weeks | PFS-16Baseline; 36 weeks after baseline (post-intervention) | Significant greater deterioration from baseline to post-intervention for CG compared to both EG (1mg and 2mg). |
| Arm 2: n = 270; NR | Arm 2: 62.4±9.7 | Arm 2: 1.5±0.5; 14.6±6.3; 4.5±4.6 (in months) | Arm 2: PFS: 2.2±0.9 | Arm 2: Rasagiline 1mg | 1x/day7x/week36 weeks | |||
| Arm 3: n = 558; NR | Arm 3: 62.0±9.7 | Arm 3: 1.5±0.5; 13.9±6.2; 4.5±4.6 (in months) | Arm 3: PFS: 2.2±0.9 | Arm 3: Placebo | NA7x/week36 weeks | |||
| Ricciardi 2015 [44] N = 47 (rand.) N = 24 (eval.) | Arm 1: n = 11; 2:9 | Arm 1: 76.1±5.7 | Arm 1: NR; 32.3±6.4; 12.0±7.8 | Arm 1: FSS: 41.5±14.0 | Arm 1: Homotaurine 50mg | 2x/day7x/week24 weeks | FSSBaseline; 24 weeks after baseline (post-intervention) | No significant change from baseline to post-intervention for EG and CG in FSS. |
| Arm 2: n = 13; 4:9 | Arm 2: 69.6±9.0 | Arm 2: NR; 25.3±6.3; 13.0±7.9 | Arm 2: FSS: 26.1±15.2 | Arm 2: Placebo | 2x/day7x/week24 weeks | |||
| Schifitto 2008 [45] N = 361 (rand.) N = 349 (eval.) | Arm 3: n = 91; 29:62 | Arm 3: 65.2±10.7 | Arm 3: 1.9±0.6; 20.5±10.8; 6.0±6.1 (in months) | Arm 3: FSS: NR | Arm 3: Levodopa-Carbidopa 150/600 mg | 3x/day7x/week42 weeks | FSSBaseline; 3, 9, 24, 40 weeks after baseline (mid-intervention); and 42 weeks after baseline (post-intervention) | Significant greater increase in FSS for the CG compared to the three EGs post-intervention. |
| Arm 2: n = 88; 29:59 | Arm 3: 63.8±12.1 | Arm 2: 1.8±0.5; 18.9±8.8; 7.6±7.5 (in months) | Arm 2: FSS: NR | Arm 2: Levodopa-Carbidopa 75/300 mg | 3x/day7x/week42 weeks | |||
| Arm 1: n = 92; 34:58 | Arm 1: 64.3±10.6 | Arm 1: 1.9±0.6; 18.6±9.1; 5.7±6.1 (in months) | Arm 1: FSS: NR | Arm 1: Levodopa-Carbidopa 37.5/150 mg | 3x/day7x/week42 weeks | |||
| Arm 4: n = 90; 25:65 | Arm 4: 64.9±10.3 | Arm 4: 1.8±0.5; 18.8±8.9; 5.3±5.6 (in months) | Arm 4: FSS: NR | Arm 4: Placebo | 3x/day7x/week42 weeks | |||
| Tyne 2010 [37] N = 13 (rand. = eval.) | Arm 1: n = 6; NR | Arm 1: 57.0 (range: 49–64) | Arm 1: 2.5 (range: 1–3); NR; NR | Arm 1: FSS: Median: 6.1 (range: 2.0) | Arm 1: Modafinil up to 400mg/day | NR7x/week9 weeks | FSS; FSIBaseline; 4 weeks after baseline (mid-intervention); 9 weeks after baseline (post-intervention) | No significance difference between EG and CG in FSS and FSI post-intervention. |
| Arm 2: n = 7; NR | Arm 2: 61.0 (range: 51–74) | Arm 2: 2.0 (range: 1–2.5); NR; NR | Arm 2: FSS: Median: 5.4 (range: 3.0) | Arm 2: Placebo | NR7x/week9 weeks | |||
| Non-pharmacological interventions (N = 16)\\ Bogosian 2021 [48] N = 60 (rand. = eval.) | Arm 1: n = 30; 13:17 | Arm 1: 59.5±11.1 | Arm 1: NR; NR; 5.2±3.6 | Arm 1: FSS: 4.1±1.3 | Arm 1: Mindfulness-based intervention | 60 min1x/week8 weekstotal: 480 min | FSSBaseline; 4 weeks after baseline (mid-intervention); 8 weeks after baseline (post-intervention); 20 weeks after baseline (12 weeks follow-up) | No significance difference between EG and CG in FSS post-intervention. |
| Arm 2: n = 30; 17:13 | Arm 2: 62.2±9.0 | Arm 2: NR; NR; 6.4±3.9 | Arm 2: FSS: 4.0±1.6 | Arm 2: Passive CG/Wait-list | NA | |||
| Canning 2012 [49] N = 20 (rand.) N = 18 (eval.) | Arm 1: n = 10; 5:5 | Arm 1: 60.7±5.9 | Arm 1: range 1-2; 20.9±10.2; 6.1±4.0 | Arm 1: VAS: 3.3±1.6 | Arm 1: Semi-supervised home-based treadmill training | 30–40 min4x/week6 weekstotal: 840 min | Fatigue VAS (0–7 points) Baseline; 6 weeks after baseline (post-intervention); 12 weeks after baseline (6 weeks follow-up) | Significant difference between EG and CG in VAS post-intervention with the EG showing less fatigue. |
| Arm 2: n = 10; 4:6 | Arm 2: 62.9±9.9 | Arm 2: range 1-2; 17.9±7.1; 5.2±4.1 | Arm 2: VAS: 3.7±1.2 | Arm 2: Passive CG/Usual care | NA | |||
| Coe 2018 [50] N = 105 (rand.) N = 73 (eval.) | Arm 1: n = 29; 13:16 | Arm 1: 67.0±7.1 | Arm 1: NR; 15.1±10.0; 4.8±4.1 | Arm 1: FSS: 3.0±1.4 | Arm 1: Community-delivered endurance and resistance training | 60 min2x/week24 weekstotal: 2880 min | FSSBaseline; 24 weeks after baseline (post-intervention) | No significance difference between EG and CG in FSS post-intervention. |
| Arm 2: n = 36; 17:19 | Arm 2: 67.0±5.9 | Arm 2: NR; 19.0±10.7; 5.5±4.2 | Arm 2: FSS: 4.0±1.5 | Arm 2: Handwriting control intervention | 60 min2x/week24 weekstotal: 2880 min | |||
| Cugusi 2015 [51] N = 20 (rand. = eval.) | Arm 1: n = 10; 2:8 | Arm 1: 68.1±8.7 | Arm 1: 2.4±0.8; 25.3±11.1; 7.0±2.0 | Arm 1: PFS-16:52.1±11.2 | Arm 1: Nordic walking | 60 min2x/week12 weekstotal: 1440 min | PFS-16 Baseline; 12 weeks after baseline (post-intervention) | Significant decrease in PFS-16 in EG compared to CG post-intervention. |
| Arm 2: n = 10; 2:8 | Arm 2: 66.6±7.3 | Arm 2: 2.3±0.5; 25.0±11.8; 7.0±4.0 | Arm 2: PFS-16:48.2±14.7 | Arm 2: Passive CG/Conventional care | NA | |||
| Kluger 2016 [52] N = 94 (rand. = eval.) | Arm 1: n = 47; 17:30 | Arm 1: 64.4±10.3 | Arm 1: range: 1–3; 21.6±7.8; NR | Arm 1: MFIS: 48.7±10.5 | Arm 1: Acupuncture | 30 min2x/week6 weekstotal: 360 min | MFISBaseline; 6 weeks after baseline (post-intervention); 12 weeks after baseline (6 weeks follow-up) | EG and CG improved significantly post-intervention and at follow-up, but with no significant between-group differences. |
| Arm 2: n = 47; 18:29 | Arm 2: 63.0±13.0 | Arm 2: range: 1–4; 26.7±11.1; NR | Arm 2: MFIS: 50.0±12.9 | Arm 2: Sham-acupuncture | 30 min2x/week6 weekstotal: 360 min | |||
| Kong 2018 [53] N = 40 (rand.) N = 36 (eval.) | Arm 1: n = 20; 14:6 | Arm 1: 66.4±6.5 | Arm 1: NR; 27.1±13.7; 87.2±53.2 (in months) | Arm 1: MFI: 61.6±15.1 | Arm 1: Acupuncture | 20 min2x/week5 weekstotal: 200 min | MFIBaseline; 5 weeks after baseline (post-intervention); 9 weeks after baseline (4 weeks follow-up) | EG and CG improved significantly post-intervention and at follow-up, but with no significant between-group differences. |
| Arm 2: n = 20; 13:7 | Arm 2: 62.9±9.7 | Arm 2: NR; 23.8±10.9; 50.1±26.4 (in months) | Arm 2: MFI: 56.6±9.2 | Arm 2: Sham-acupuncture | 20 min2x/week5 weekstotal: 200 min | |||
| Michels 2018 [54] N = 13 (rand. = eval.) | Arm 1: n = 9; NR | Arm 1: 66.4±NR | Arm 1: 2.1±0.3; 27.6±11.6; NR | Arm 1: FSS: 34.2±10.9 | Arm 1: Dance therapy | 60 min1x/week10 weekstotal: 600 min | FSSBaseline; 10 weeks after baseline (post-intervention) | No change for FSS was found for the EG; the CG improved on the FSS; no analysis of possible significance presented. |
| Arm 2: n = 4; NR | Arm 2: 75.5±NR | Arm 2: 2.5±1.0; 40.8±8.7; NR | Arm 2: FSS: 33.0±8.5 | Arm 2: Support group | 60 min1x/week10 weekstotal: 600 min | |||
| Ortiz-Rubio 2018 [55] N = 46 (rand. = eval.) | Arm 1: n = 23; 6:17 | Arm 1: 74.2±5.8 | Arm 1: range: 2-3; 24.1±12.4; 4.0±2.2 | Arm 1: PFS: 3.6±2.2 | Arm 1: Group resistance training | 60 min2x/week8 weekstotal: 960 min | PFSBaseline; 8 weeks after baseline (post-intervention) | Significant difference in PFS for the EG in comparison to the CG post-intervention. |
| Arm 2: n = 23; 7:16 | Arm 2: 75.4±6.5 | Arm 2: range: 2-3; 26.4±14.5; 4.3±2.0 | Arm 2: PFS: 4.8±5.8 | Arm 2: Low-intensity exercise program (=placebo) | 60 min2x/week8 weekstotal: 960 min | |||
| Raymackers 2019 [35] N = 16 (rand.) N = 15 (eval.) cross-over trial | Arm 1: n = 8; 4:4 | Arm 1: 66.5±6.3 | Arm 1: NR; NR; 33.3±31.7 (in months) | Arm 1: FIS: 83.7±30.5 | Arm 1: Bright light therapy | 45 min7x/week4 weekstotal: 1260 min | FISBaseline; 4 weeks after baseline (post-assessment) | Non-significant decrease of the FIS scores for EG and CG; significant change from baseline to post-intervention for the EG. |
| Arm 2: n = 8; 2:6 | Arm 2: 68.9±5.9 | Arm 2: NR; NR; 33.5±20.0 (in months) | Arm 2: FIS: 59.8±36.9 | Arm 2: Sham bright light therapy (=placebo) | 45 min7x/week4 weekstotal: 1260 min | |||
| Rios Romenets 2013 [56] N = 20 (rand.) N = 18 (eval.) | Arm 1: n = 6; 0:6 | Arm 1: 64.5±16.3 | Arm 1: range: 1–3; 22.6±12.8; 5.2±1.8 | Arm 1: KFSS: 44.5±4.9 | Arm 1: Sleep hygiene training compared + cognitive behavioral therapy (CBT) + bright light therapy | 90 min1x/week6 weeks + daily 30 min bright light therapytotal: 1800 min | KFSSBaseline; 6 weeks after baseline (post-intervention) | Significant improvement in KFSS for the Doxepin group compared to sham bright light therapy; no further significant changes related to KFSS. |
| Arm 2: n = 6; 3:3 | Arm 2: 65.3±10.5 | Arm 2: range: 1–3; 30.9±7.6; 4.8±3.6 | Arm 2: KFSS: 46.5±10.9 | Arm 2: Doxepin 10mg | 1x/day7x/week6 weeks | |||
| Arm 3: n = 6; 1:5 | Arm 3: 69.5±10.5 | Arm 3: range: 1–3; 22.1±9.; 5.2±4.4 | Arm 3: KFSS: 34.0±16.6 | Arm 3: Sham bright light therapy (=placebo) | 30 min7x/week6 weekstotal: 1260 min | |||
| Solla 2019 [57] N = 20 (rand.) N = 19 (eval.) | Arm 1: n = 10; 4:6 | Arm 1: 67.8±5.9 | Arm 1: 2.1±0.6; 13.0±7.2; 4.4±4.5 | Arm 1: PFS-16:33.1±12.7 | Arm 1: Sardinian folk dance | 90 min2x/week12 weekstotal: 2160 min | PFS-16Baseline; 12 weeks after baseline (post-intervention) | No significance difference between EG and CG in PFS-16 post-intervention. |
| Arm 2: n = 10; 3:7 | Arm 2: 67.1±6.3 | Arm 2: 2.3±0.4; 14.7±7.0; 5.0±2.9 | Arm 2: PFS-16:34.1±16.0 | Arm 2: Passive CG/Usual care | NA | |||
| Sturkenboom 2014 [36] N = 191 (rand. = eval.) | Arm 1: n = 124; 46:78 | Arm 1: median: 71.0 (IQR: 63.3–76.0) | Arm 1: range: 1–5; median: 28 (IQR: 19.0–36.0); median: 6.0 (IQR: 3.0–11.0) | Arm 1: FSS: Median: 5.0 (IQR: 4.0–5.9) | Arm 1: Home-based occupational therapy | 60 minMax. 16 h10 weekstotal: 1600 min | FSSBaseline; 3 months after baseline (post-intervention); 6 months after baseline (follow-up) | No significance difference between EG and CG in FSS post-intervention. |
| Arm 2: n = 67; 26:41 | Arm 2: median: 70.0 (IQR: 63.0–75.0) | Arm 2: range: 1–4; median: 27 (IQR: 18.0–36.0); median: 6.0 (IQR: 4.0–10.0) | Arm 2: FSS: Median: 4.9 (IQR: 4.2–5.6) | Arm 2: Passive CG/Usual care | NA | |||
| Videnovic 2017 [58] N = 31 (rand. = eval.) | Arm 1: n = 16; 8:8 | Arm 1: 62.3±10.8 | Arm 1: range: 2-3; 24.8±11.3; 5.9±3.6 | Arm 1: FSS: 41.6±12.6 | Arm 1: Bright light therapy | 60 min2x/day2 weekstotal: 1680 min | FSSBaseline; 4 weeks after baseline (post-intervention); 6 weeks after baseline (follow-up) | No significance difference between EG and CG in FSS post-intervention. |
| Arm 2: n = 15; 10:5 | Arm 2: 64.1±8.9 | Arm 2: range: 2–4; 29.1±12.4; 8.4±3.7 | Arm 2: FSS: 37.0±9.1 | Arm 2: Sham bright light therapy (=placebo) | 60 min2x/day2 weekstotal: 1680 min | |||
| Walter 2019 [59] N = 30 (rand.) N = 27 (eval.) | Arm 1: n = 15; 5:10 | Arm 1: 65.5±6.1 | Arm 1: range: 1.5–3; 28.3±14.9; NR | Arm 1: PFS-16: NR | Arm 1: Yoga | 60 min2x/week8 weekstotal: 960 min | PFS-16Baseline; 8 weeks after baseline (post-intervention) | Significant improvement in PFS-16 for the EG, but no significant difference between EG and CG in FSS post-intervention. |
| Arm 2: n = 12; 5:7 | Arm 2: 70.5±4.4 | Arm 2: range: 2-3; 31.6±11.6; NR | Arm 2: PFS-16: NR | Arm 2: Passive CG/Wait-list | NA | |||
| Winward 2012 [60] N = 39 (rand.) N = 37 (eval.) | Arm 1: n = 20; 5:15 | Arm 1: 63.4±6.4 | Arm 1: range: 0–4; NR; 5.9±4.4 | Arm 1: FSS: 4.0±1.5 | Arm 1: Community gym-based cardiovascular, muscle strength and flexibility program | 30–45 minUp to 5 aerobic and 2 strength sessions/week12 weekstotal: 3360 min | FSSBaseline, 12 weeks after baseline (post-intervention) | No significance difference between EG and CG in FSS post-intervention. |
| Arm 2: n = 19; 3:16 | Arm 2: 64.9±9.6 | Arm 2: range: 0–4; NR; 5.7±4.2 | Arm 2: FSS: 4.2±1.5 | Arm 2: Passive CG/Usual care | NA | |||
| Wu 2021 [61] N = 98 (rand. = eval.) | Arm 1: n = 49; 23:26 | Arm 1: 63.7±6.0 | Arm 1: range: 1-2; 7.6±4.1; 5.0±3.9 | Arm 1: FSS: 31.9±14.4 | Arm 1: Self-monitored endurance and resistance training | 150min/week (30–50 min for 3x/week or 10–15 min daily) 8 weekstotal: 1200 min | FSSBaseline, 4 weeks after baseline (mid-intervention), 8 weeks after baseline (post-intervention) | Significant improvement in FSS for the EG compared to the CG post-intervention. |
| Arm 2: n = 49; 19:30 | Arm 2: 66.6±8.6 | Arm 2: range: 1-2; 10.1±5.4; 5.7±3.8 | Arm 2: FSS: 34.4±18.4 | Arm 2: Passive CG/Usual care | NA | |||
If not otherwise indicated, data is presented as mean±standard deviation or frequency. CG, control group; EG, experimental group; F, female; FIS, Fatigue Impact Scale; FSS, Fatigue Severity Scale, H&Y, Hoehn & Yahr stage; IQR, Interquartile range; KFSS, Krupp Fatigue Severity Scale; M, male; MFIS: Modified Fatigue Impact Scale; MFI, Multidimensional Fatigue Inventory; NA, not applicable; NR, not recorded; PFS, Revised Piper Fatigue Scale; PFS-16, Parkinson’s Fatigue Scale; VAS, Visual Analogue Scale.
Eligibility criteria
Study designs
RCTs including cross-over randomized trials, published in peer-reviewed journals were considered. Conference abstracts and trial registers were incorporated to provide further information regarding the included trials.
Participants
Studies investigating adults (≥18 years) of all sexes with a clinical PD diagnosis were eligible for review. There were no restrictions regarding disease duration or severity. Studies involving patients with PD dementia were excluded, as were trials involving subjects with atypical parkinsonism or Lewy body dementia.
Intervention and control groups
Articles involving surgical and invasive interventions (e.g., deep brain stimulation) were not eligible for including. All other therapy types involved in treating fatigue in PD patients, whether primarily or secondarily, were eligible. These approaches included pharmacological interventions (e.g., levodopa-carbidopa, memantine, rasagiline, caffeine, methylphenidate, modafinil, and doxepin) and non-pharmacological interventions (e.g., psychotherapy, psychosocial support, physical exercise, occupational therapy, cognitive interventions, brain stimulation techniques, arts/music, acupuncture, and bright light therapy), and combined approaches of pharmacological and non-pharmacological interventions. Non-pharmacological interventions had to include at least two supervised sessions. This was done to exclude experimental designs in which patients received an intervention or conducted a task only once (typically in a laboratory setting). No restrictions were defined in terms of the study settings; home-, individual- and group-based interventions were considered.
Studies comparing treatment effects against passive/placebo control groups or active control groups were included. Passive control groups were defined as no treatment, usual care, or wait-list control, which did not impact participants’ habitual routines. Placebo control groups included the administration of placebo pills in pharmacological trials, or the use of sham-programs (e.g., sham-acupuncture). Active control groups included comparisons of different pharmacological and non-pharmacological treatment options. However, studies comparing two interventions of the same type (e.g., two physical exercise approaches) were not considered for this review as they could not be included in the meta-analysis.
Outcomes
The outcome of interest was the change in fatigue assessed with standardized (i.e., quantitative) neuropsychological questionnaires including self- and proxy ratings. Fatigability was not investigated. The standardized instruments included the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Fatigue Severity Scale (FSS), Fatigue Assessment Inventory (FAI), Multidimensional Fatigue Inventory (MFI), Parkinson Fatigue Scale (PFS), Fatigue Impact Scale (FIS), Fatigue Severity Inventory (FSI), Fatigue Severity Questionnaire (FSQ), Profile of Mood Status (POMS) fatigue subscale, Fatigue Questionnaire (FQ), and visual analog scales. Qualitative outcomes (e.g., from interview studies) were not considered. Furthermore, standardized instruments that assessed fatigue with one item (e.g., the Non-Motor Symptoms Scale [NMSS]) were not included. Only short-term effects were considered (i.e., assessments conducted ≤4 weeks post-intervention) due to limited data and the high heterogeneity between the timing of follow-up assessments.
Study selection
Three reviewers (AKF, JN, and DS) independently screened the titles and abstracts. Two authors (AKF and JN) reviewed the full-text articles for eligibility. These processes were done using the Covidence Systematic Review software (Veritas Health Innovation, Melbourne, Australia. Available at http://www.covidence.org). Two authors (RG and AL) then extracted all relevant data using a standardized data extraction form and cross-checked all the information. All data were double-checked. Regarding discrepancies, the authors discussed or consulted another author (AKF) to reach a consensus. The extracted data consisted of general study information (e.g., author/s and publication date), study characteristics (e.g., trial design and setting), patient characteristics (e.g., eligibility criteria, baseline sociodemographic and clinical data, and the number of patients recruited/allocated/evaluated), information about the intervention (e.g., type, dose, frequency, and length) and control group (e.g., type, dose, frequency, and length), fatigue outcome (e.g., instruments used and assessments timings), and further notes (e.g., funding and conflicts of interest).
Risk of bias
Methodological quality was analyzed independently by two authors (AKF and RG) using the revised Cochrane Risk of Bias tool (RoB 2) for RCTs [29] for all studies included in the meta-analyses. In instances of discrepancies, the authors discussed or consulted another author (JN) until a consensus was reached. Five RoB domains were addressed in the following domains covering all types of bias that can affect RCT results: Bias arising from the randomization process; bias due to deviations from intended interventions; bias due to missing outcome data; bias in measurement of the outcome; and bias in selection of the reported result. The tool implements signaling questions for each RoB domain leading to low, high, or some concern for RoB.
Data analysis
RevMan Version 5.4 by the Cochrane Collaboration was used for the meta-analyses of the pre- and post-intervention comparisons of fatigue in PD patients and for the creation of forest plots. Data were extracted by three authors (AKF, RG, AL) and double-checked. The data extraction included post-treatment means, standard deviations, and the number of evaluated patients for the intervention and control group of each trial [30]. If data were missing, authors were contacted via e-mail and followed up after two weeks. Of the 7 authors contacted [31–37], 3 responded [32, 33, 35] and provided the requested data. In studies involving both intention-to-treat (ITT) and per-protocol (PP) analyses, only the ITT data were used in the meta-analyses. In trials reporting no ITT data, PP data were utilized.
Several meta-analyses were conducted, as two or more studies were available for each: modafinil vs. placebo, physical exercise vs. passive control group/placebo, and acupuncture vs. sham-acupuncture.
We used a random effects model with inverse variances and standardized mean differences (SMDs) due to diverse PD samples and fatigue outcomes, and because variations in the intervention effects of the included studies were expected. For the statistical analyses, SMDs and 95% CIs were computed to compare effect measures between intervention and control groups. Effects from 0.2 to >0.5 were categorized as small, effects from 0.5 to >0.8 were categorized as medium, and effects ≥0.8 were categorized as large [38]. The alpha level was set at 0.05 for all analyses. The p-value from the Chi2 test, generalized I2 statistic, and Tau2 were used to address the heterogeneity and inconsistency of the included studies. We interpreted the heterogeneity of the I2 statistic as recommended in the Cochrane Handbook for Systematic Reviews of Interventions [30], whereby 0–40% indicated unimportant/low heterogeneity, 30–60% indicated moderate heterogeneity, 50–90% indicated substantial heterogeneity, and 75–100% indicated considerable heterogeneity. Additionally, sensitivity analyses were conducted using fixed effect models and results from random effects model and fixed effect model analysis were compared. The Cochrane Handbook for Systematic Reviews of Interventions [30] recommends that funnel plots are used to identify possible publication bias for meta-analyses including at least 10 studies. The highest number of included studies in a meta-analysis included in this review was 8; therefore, no funnel plots are presented.
RESULTS
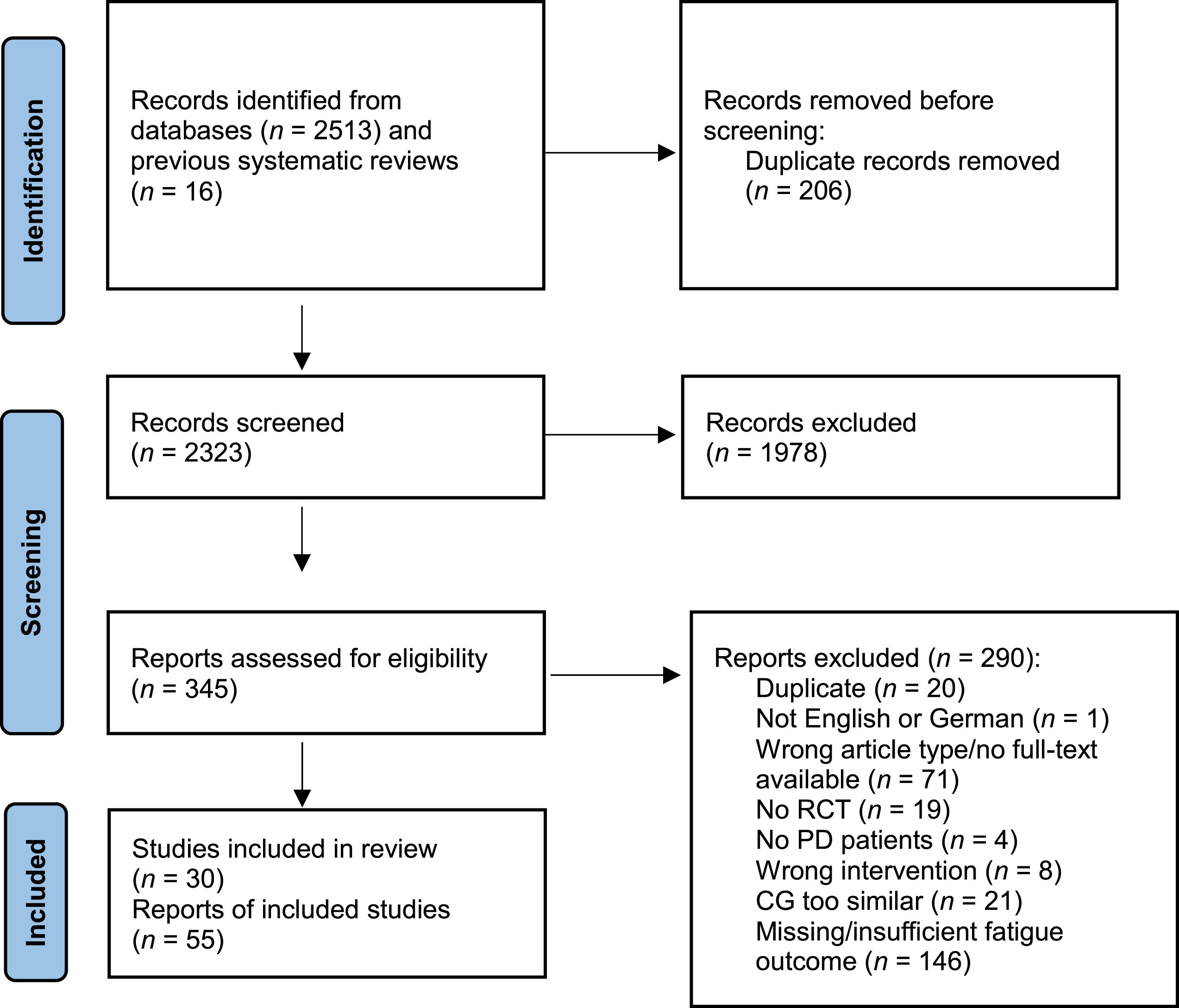
In total, 2,513 records were identified during the database searches, and 16 records were found by manually searching previously published reviews and meta-analyses. Two hundred and six duplicates were automatically removed prior to title and abstract screening. After screening 2,323 records, 1,978 records were excluded. Therefore, 345 full-text articles were assessed for eligibility. Two hundred and ninety articles were excluded, meaning 30 studies, published in 55 study articles were included in this systematic review. Supplementary Table 2 provides a full list of references for the included studies. Fourteen studies examined pharmacological interventions, and 16 analyzed the effects of non-pharmacological interventions. For the meta-analyses, 2 pharmacological and 10 non-pharmacological trials were included. Figure 1 depicts the PRISMA flow diagram.
Fig. 1
PRISMA flow diagram. CG, control group; PD, Parkinson’s disease; RCT, randomized controlled trial.
Characteristics of pharmacological intervention studies
Of the 14 studies that examined the effects of pharmacological interventions on fatigue in PD patients, 12 were RCTs [31–34, 37, 39–45], and 2 were cross-over RCTs [46, 47]. Table 1 provides an overview of these studies.
Among the studies that involved PD medications, 1 examined levodopa-carbidopa in 4 intervention groups that received a 112.5/450 mg, 225/900 mg, 450/1800 mg dose, or placebo daily for 42 weeks [45]. Rasagiline was used in 2 studies. One study involved a 1 mg/day dose versus placebo for 12 weeks [31], while the other involved a 1 or 2 mg/day dose versus placebo for 36 weeks [34]. One study assessed amantadine in 4 intervention groups that received a daily dose of 260 mg, 340 mg, or 420 mg, or a placebo for 8 weeks [32]. Mendonca et al. [40] examined the effects of a 30 mg/day dose of the dopamine re-uptake inhibitor methylphenidate versus placebo for 6 weeks, while Ondo et al. [42] analyzed the effects of a 40 mg/day dose of the antidementia drug and glutamate N-methyl-D-aspartate-receptor antagonist memantine versus placebo for 8 weeks. The effect of the central nervous system (CNS) stimulant modafinil was investigated in 4 studies. These studies involved a 200 mg/day dose versus placebo for 3 weeks [46], and 8 weeks [39] and a 400 mg/day dose versus placebo for 4 weeks [41], and 9 weeks [37]. Büchele et al. examined the effects of a 3–9 g/day dose of the CNS depressant sodium oxybate versus placebo for 6 weeks [47]. Other substances investigated included a 200 mg/day dose of caffeine for 3 weeks followed by 400 mg/day for further 3 weeks versus placebo [33], a 200 mg/day dose of homotaurine versus a placebo for 24 weeks [40], and a 1–2 mg/day dose of nabilone versus placebo for 4 weeks [43].
The sample sizes of PD patients were very heterogeneous and ranged from 12 [47] to 1176 [34], with all except 2 studies having sample sizes of 100 or lower. The mean age of PD participants ranged from 57.0 [37, 47] to 76.1 [44] years, with most studies having a mean participant age of 65 years. Most studies included more men than women; however, two studies did not report the sex distribution of their samples [37, 46]. Disease severity was typically mild to moderate, operationalized either with the Hoehn & Yahr Scale, with mean scores ranging between 1.5 [34] and 2.5 [32, 40], or the Unified PD Rating Scale (UPDRS) motor examination, with scores ranging between 14 [46] and 33 [40]. Mean disease duration ranged between less than 1 year [34, 45] to more than 12 years [44]; however, 2 studies did not indicate disease duration [37, 42].
All studies measured fatigue both pre- and post-intervention. Some studies included an additional point of measurement during the course of the intervention [31, 32, 37, 39, 45]. Most studies used the FSS to measure fatigue [31–33, 37, 40–47]. Other scales used included the PFS-16 [34], FSI [37], MFI [31, 39, 40], and the Modified Fatigue Impact Scale (MFIS) [31].
Characteristics of non-pharmacological intervention studies
Sixteen studies investigated the effects of non-pharmacological interventions on fatigue in PD; 15 were RCTs [36, 48–61], and 1 was a cross-over RCT [35]. An overview of these studies is provided in Table 1. Most of these studies examined the effect of physical exercise. Interventions included community-delivered endurance and resistance training versus handwriting training (control intervention) for 60 min twice a week for 24 weeks [50], a community gym-based cardiovascular, muscle strength, and flexibility program versus treatment as usual (TAU) for 30–45 min up to 7 times a week for 12 weeks [60], and self-monitored endurance and resistance training at home versus TAU for 150 min per week for 8 weeks [61]. Other studies employed more specialized training programs, such as group resistance training versus a low-intensity exercise program (placebo intervention) for 60 min twice per week for 8 weeks [55], Nordic walking versus TAU for 60 min twice per week for 12 weeks [51], and semi-supervised home-based treadmill training versus TAU for 30–40 min 4 times per week for 6 weeks [49]. Dancing interventions were also investigated. For instance, one study evaluated dance therapy versus a support group for 60 min once a week for 10 weeks [54], while another assessed Sardinian folk dance versus TAU for 90 min twice per week for 12 weeks [57]. The effects of mind-body exercises were also examined. One study investigated yoga versus a waiting list for 60 min twice per week for 8 weeks [59]. Sturkenboom et al. [36] compared the effects of home-based occupational therapy versus TAU, with a maximum intervention time of 16 h within 10 weeks. Two studies investigated the effects of acupuncture versus sham-acupuncture. One study involved acupuncture and sham-acupuncture for 30 min twice per week for 6 weeks [57], while the other occurred for 20 min twice per week for 5 weeks [53]. Videnovic et al. [58] assessed pure bright light therapy versus sham-light therapy for 60 min twice per day for 2 weeks. Raymackers et al. [35] used a more intensive light therapy regime involving 45 min of daily therapy for 4 weeks. Rios Romenets et al. [56] assessed sleep hygiene training plus CBT for 90 min once a week plus daily bright light therapy for 30 min for 6 weeks, sham-bright light therapy only, and 10 mg/day of doxepin. Bogosian et al. [48] investigated a mindfulness-based intervention offered once a week for 60 min for 8 weeks in contrast to a waiting list. The total time spent in the therapies used in the included studies ranged between 200 min [53] and 3360 min [60].
The sample sizes ranged from 13 [54] to 191 [36]. The mean age of PD patients ranged between 59.5 [48] and 75.5 [54] years. Similar to the studies examining pharmacological interventions, most studies had a mean participant age of approximately 65 years and included more men than women; however, one study did not report the sex distribution of its sample [54]. Disease severity was typically mild to moderate, operationalized using either the Hoehn & Yahr Scale, where scores typically ranged between stages 1–3, or the UPDRS motor examination, where scores ranged from less than 10 [61] to 33.4 [35]. Mean disease duration ranged between 2.8 [35] and 8.4 [58] years; however, several studies did not provide information on disease duration [52, 54, 59].
Fatigue was assessed pre- and post-intervention; only two studies reported an additional point of measurement for fatigue during the intervention [48, 61]. Similar to the pharmacological studies, the FSS was used most frequently to measure fatigue [36, 48, 50, 54, 58, 60, 61], followed by the PFS-16 [51, 57, 59]. Other scales used included a visual analog scale for measuring fatigue [49], the FIS [35], MFIS [52], MFI [53], the revised PFS [55], and the Krupp Fatigue Severity Scale (KFSS) [56].
Meta-analyses of pharmacological interventions
Meta-analyses were conducted for all drug groups that were investigated by two or more studies (i.e., for modafinil versus placebo only).
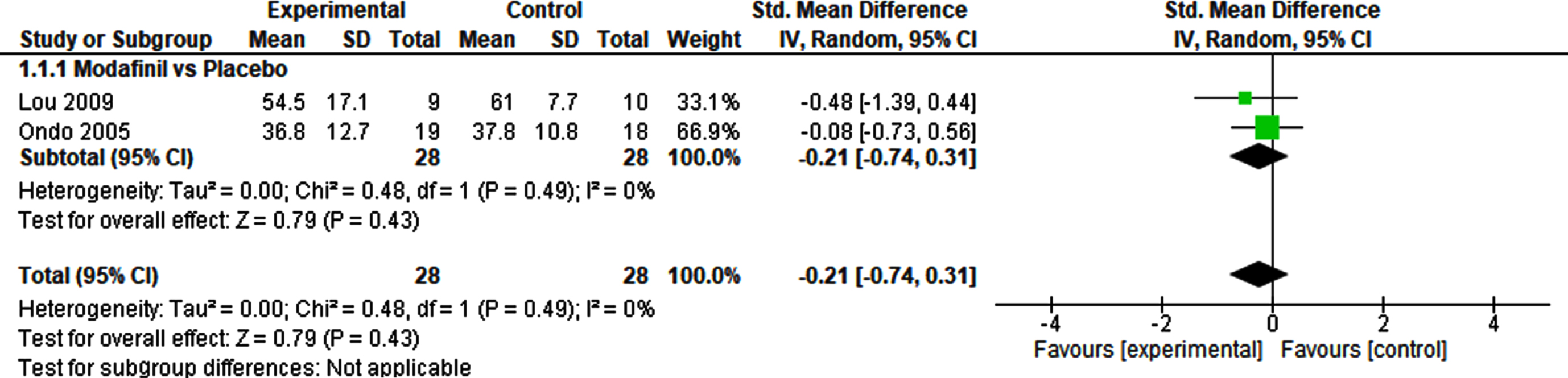
The meta-analysis of the effects of modafinil versus placebo on fatigue included two studies and a total of 56 patients (Fig. 2). The SMD was – 0.21 (95% CI – 0.74–0.31), and demonstrated no significant effect (p = 0.43). Heterogeneity was not detected (I2 = 0%). A sensitivity analysis using a fixed effect model revealed no difference, demonstrating a robust result (Supplementary Table 3).
Fig. 2
Pharmacological interventions vs. Placebo.
Meta-analyses of non-pharmacological interventions
Meta-analyses were conducted for physical exercise versus passive or placebo control groups, as well as acupuncture versus placebo.
The meta-analysis of the effects of physical exercise versus passive or placebo control groups on fatigue outcomes included 8 studies and 324 patients (Fig. 3). This was the largest meta-analysis in this review. The SMD was – 0.37 (95% CI – 0.69‐ – 0.05) and demonstrated a significant small effect (p = 0.02). Heterogeneity was moderate (I2 = 45%). The sensitivity analysis using a fixed effect model, confirmed the robustness of this result (Supplementary Table 3).
Fig. 3
Non-pharmacological interventions vs. Passive control groups/Placebo.
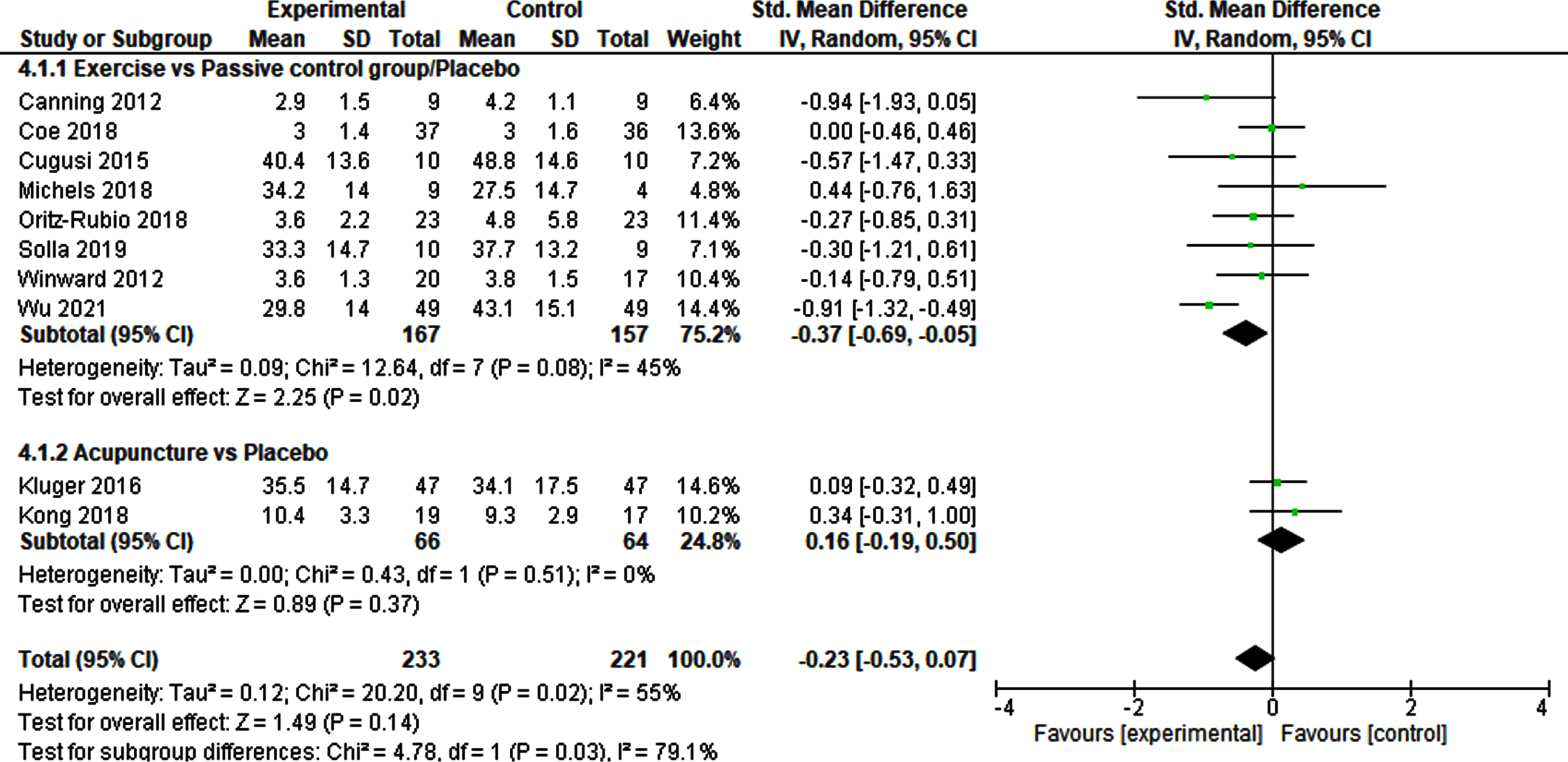
Two studies involving 130 patients were included in the meta-analysis of acupuncture versus sham-acupuncture (Fig. 3). The SMD was 0.16 (95% CI – 0.19–0.50), indicating no significant result (p = 0.37). No heterogeneity was detected (I2 = 0%). The robustness of this finding was indicated by the fixed effect model used for the sensitivity analysis (Supplementary Table 3).
Risk of bias
The evaluation of the RoB 2.0 of the included studies is shown in Figs. 4 and 5 and Supplementary Figures 1 and 2.
Fig. 4
Risk of bias 2.0 summary for pharmacological trials included in meta-analysis.
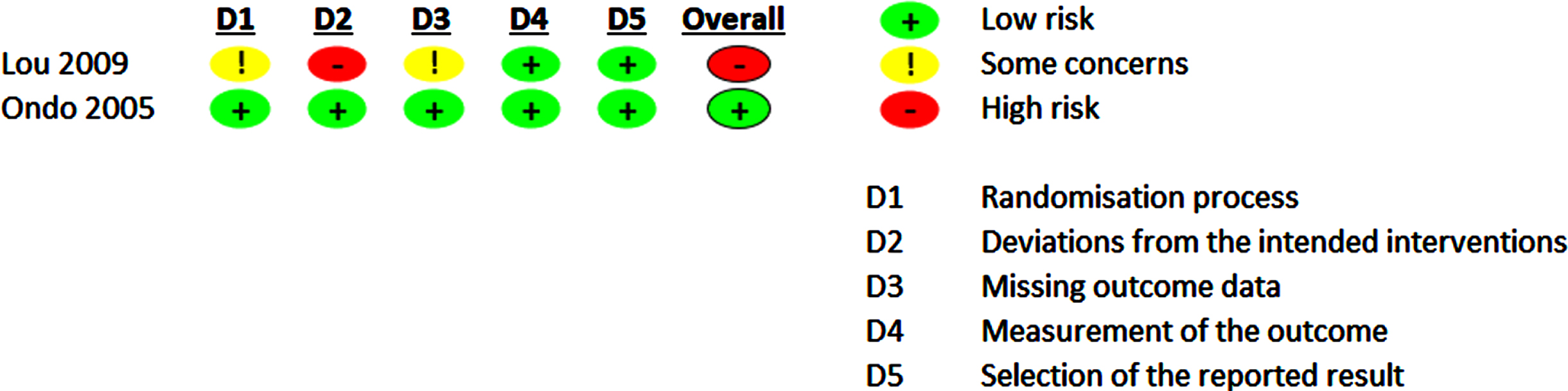
Fig. 5
Risk of bias 2.0 summary for non-pharmacological trials included in meta-analysis.
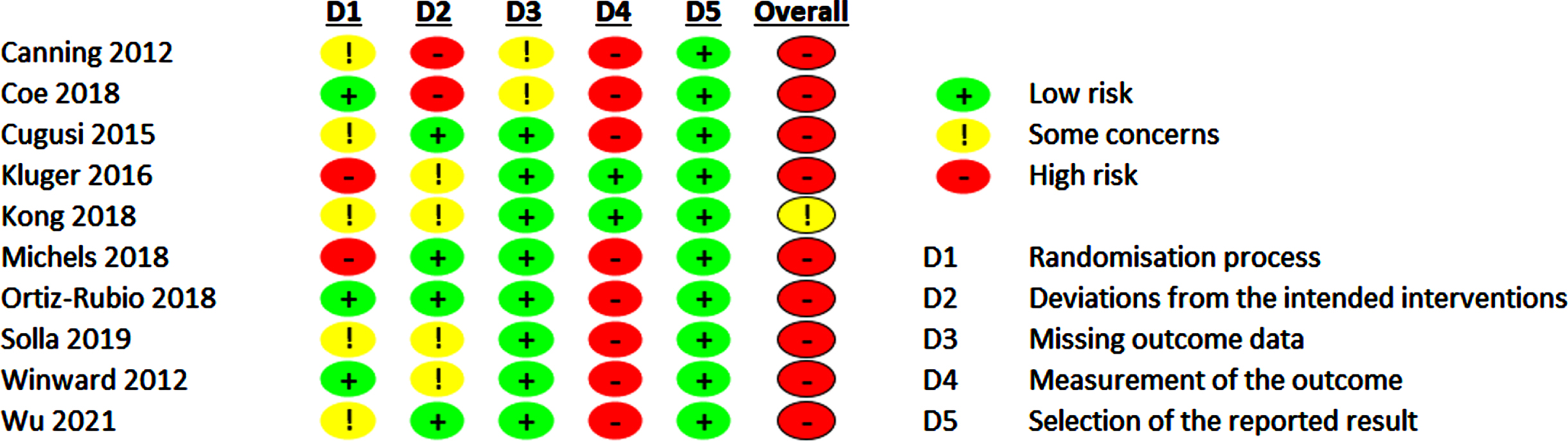
For pharmacological trials, the overall RoB rating, which included only two studies, led to low (50%) or high RoB (50%). Some concern regarding RoB was evident regarding the randomization process in one study, as the random sequence allocation and the allocation concealment was not precisely described in these study reports. One study [39] reported on dropout rates of 14% while not following an ITT analysis approach. This was the main driver for high RoB for deviations from the intended interventions and some concerns for RoB for missing outcome data leading to an overall high RoB.
For non-pharmacological trials, we found some concerns for RoB in one study (10%), and high RoB in 90%. The main driver for a possible RoB are insufficient and incomprehensible descriptions of the randomization procedures and the lack of blinding of the participants leading to RoB concerning measurement of the outcome as the included scales for operationalizing the fatigue outcomes are based on self-reports. However, lack of participants’ blinding is a challenge in non-pharmacological trials in general. However, in the acupuncture [52, 53] trials sham-interventions could be used. Therefore, participants were probably blinded regarding their group assignment. Dropout rates ≥10% were only reported in two trials [49, 50] leading to high RoB for deviations from the intended interventions and missing outcome data.
DISCUSSION
This study aimed to provide an updated systematic review of the RCTs that investigated the effects of pharmacological and non-pharmacological (but non-surgical) treatments on fatigue in PD patients. The main results are: (i) In this review, 14 pharmacological and 16 non-pharmacological RCTs were included. Although fatigue has rarely been defined as a primary outcome, the database of studies investigating fatigue among PD patients has improved compared to previously published reviews [20, 21, 26]. However, due to the high heterogeneity of the intervention approaches and study designs used, drawing precise conclusions remain difficult. (ii) Although the efficacy of 10 different medications for fatigue treatment was analyzed in the included studies, only modafinil could be included in the meta-analysis, and no significant effects were identified. (iii) Five different non-pharmacological intervention approaches were identified, including physical exercise, acupuncture, bright light therapy, psychosocial approaches, and occupational therapy. A meta-analysis could be conducted for physical exercise and acupuncture. A significant effect of physical exercise on fatigue was found; no other significant effects were detected.
Pharmacological treatment
The meta-analysis of modafinil vs. placebo indicated no significant effects of pharmacological treatments on fatigue among PD patients. The two included studies reported on small sample sizes, resulting in a total sample of only 56 patients in the analysis. As for only one trial data suitable for a meta-analysis was available, a meta-analysis on the effects of rasagiline could not be performed. However, The Cochrane review [21] concluded that rasagiline had a significant effect based only on the large trial by Stocchi et al. [34]. Therefore, more studies are needed to further explore rasagiline as a treatment option for fatigue in PD patients.
Within neurological diseases, most pharmacological treatment trials for fatigue have been conducted in multiple sclerosis (MS) patients However, the evidence remains limited, and more research is needed [62]. Recent meta-analyses have demonstrated the positive effects of amantadine and modafinil on fatigue in MS patients [63, 64]; these pharmacotherapeutic approaches were not included or to be effective in the current meta-analysis. However, the studies included in the current review had several methodological challenges, including small sample sizes. Therefore, further research is required to elucidate the potential of these treatment options in PD patients.
Non-pharmacological treatment
The highest number of RCTs investigating non-pharmacological treatments involved physical exercise (n = 8). Promising results were demonstrated in the meta-analysis of these trials. In the Cochrane review by Elbers et al. [21], only two physical exercise studies [49, 60] were included, and no significant effects were reported. The review by Cusso et al. [26] reported one Nordic walking RCT that led to significant effects on fatigue. Therefore, our findings suggest a substantial increase in the number of physical exercise trials that have included fatigue as an outcome parameter in recent years. However, the sample sizes of the included RCTs were small, and the types of physical exercise approaches were heterogenous in intensity and in type ranging from treadmill training to resistance training, dancing, and Nordic walking. Therefore, the significant result reported in the current study must be interpreted with caution, and recommendations for specific interventions cannot be derived. Future studies should further investigate physical exercise as a treatment option for fatigue in PD patients. A significant advantage of physical exercise compared to pharmacological therapy is that serious side effects are relatively rare, and further positive effects on health are often associated with such interventions. The current results are corroborated by previous studies on fatigue treatment in MS patients. Two meta-analyses [65, 66] revealed that non-pharmacological interventions (exercise and psychological/educational interventions) appeared to have stronger and more significant favorable effects regarding the reduction of fatigue severity or impact in MS patients compared to medication. Furthermore, a recent network meta-analysis involving 27 exercise studies ranked different physical exercise approaches and concluded that aquatic exercise was the most effective approach for treating fatigue in MS patients [67]. However, the practicality of transferring effective treatment interventions for MS patients to PD patients is limited, as PD patients are, on average, older than MS patients.
The meta-analyses of acupuncture and bright light therapy did not reveal any significant effects. These therapeutic options were not considered in previous systematic reviews [20, 21]. Regarding psychosocial interventions, no meta-analysis could be performed. However, psychosocial treatments should be considered when developing further study designs for PD patients. The development and evaluation of a fatigue management program that utilizes a holistic approach combining pharmacological and non-pharmacological interventions could be a further step to improve fatigue management in PD patients. For MS, the National Institute for Health and Care Excellence guidelines [68] recommend a holistic and multidisciplinary approach for the management of fatigue, involving concurrent exercise therapy, self-management, education, and medication. There has been a growing interest among patients, their relatives, and health-care providers in these intervention approaches as a means to empower patients and improve symptoms and overall quality of life [66].
Strengths and limitations
A clear strength of this systematic review is that it follows the high standards set by the Cochrane Collaboration for conducting systematic reviews. Furthermore, this updated and comprehensive systematic review considered a broad range of pharmacological and non-pharmacological approaches.
However, when interpreting the results of this review, several few limitations must be considered. First, some publications could have been missed during the literature search, as not all databases were used (e.g., SCOPUS). However, with MEDLINE, EMBASE, and CENTRAL plus two further databases used in our study, we fulfilled the recommendations provided by the Cochrane Handbook for Systematic Reviews of Interventions [30]. Therefore, the current literature search can be regarded as strong. Second, the high heterogeneity of treatment approaches and study designs (e.g., participant eligibility criteria, intervention type, dosage/intensity of interventions, and outcome measurements) limited the ability to conduct meta-analyses for some intervention types and the validity of the meta-analyses that were performed. Third, the effects of surgical procedures such as deep brain stimulation were not considered in this review. Therefore, such procedures should be considered in further research. Fourth, conclusions regarding treatment effects on the subdomains of fatigue (i.e., physical and mental fatigue) cannot be derived from this review. Fatigability, a concept related to fatigue that can be objectively assessed, was also not considered. Further research is needed to investigate potential treatments for these aspects.
Conclusion
Although fatigue is a frequent and debilitating symptom in PD patients, the available evidence is too limited to form therapeutic recommendations. However, the current systematic review indicates that physical exercise may be a promising treatment approach, even though more studies are needed to further examine the effects of specific physical exercise interventions on fatigue. Furthermore, future studies should consider the multidimensionality of the fatigue construct (i.e., physical vs. mental fatigue and primary vs. secondary fatigue) [69], particularly as the different underlying mechanisms of these subtypes may result in different responses to various treatments. Moreover, future studies should focus on developing, evaluating, and implementing fatigue management strategies for PD patients.
ACKNOWLEDGMENTS
We thank Anne Adams for her statistical advice.
FUNDING
This systematic review was financed by budget resources of the participating study sites.
CONFLICT OF INTEREST
AKF has received honoraria from Springer Medizin Verlag GmbH, Heidelberg, Germany; Springer-Verlag GmbH, Berlin; ProLog Wissen GmbH, Cologne, Germany; Bundesverband Klinische Linguistik e.V., Coburg, Germany; Hochschule Fresenius, Düsseldorf, Germany; as well as Seminar- und Fortbildungszentrum Rheine, Germany; and has received grants from the German Parkinson Society; German Alzheimer’s Society; Federal Joint Committee (G-BA); and STADAPHARM GmbH.
JN has received honoraria from the Fresenius University of Applied Sciences, Düsseldorf, Germany, the MS Society Vienna, Austria, and grants from the Pharos Foundation, Dortmund, Germany.
RG declares no conflict of interests.
AL declares no conflict of interests.
DS declares no conflict of interests.
KEZ has received research support from the Christa and Hans-Peter Thomsen Foundation, the German Research Foundation (DFG 5919/4-1) and from Strathmann GmbH & Co. KG. She reports speaker’s honoraria from Bayer Vital GmbH, BIAL, Alexion, AbbVie Allergan and Merz outside the submitted work. She has served as a consultant and received fees from Merz, Ipsen, Alexion and the German Federal Institute for Drugs and Medical Devices (BfArM).
IM declares no conflict of interests.
NS declares no conflict of interests.
EK has received honoraria from ProLog Wissen GmbH, Cologne, Germany; Kyowa Kirin Services LTD, London, United Kingdom; AbbVie Inc., as well as from the Movement Disorders Society; and has received grants from German Ministry of Education and Research (BMBF); German Parkinson Society; German Alzheimer’s Society; Federal Joint Committee (G-BA); and STADAPHARM GmbH.
DATA AVAILABILTY
Data is available on request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-225116.
REFERENCES
[1] | Seppi K , Chaudhuri R , Coelho M , Fox SH , Katzenschlager R , Perez-Lloret S , Weintraub D , Sampaio C ((2019) ) Update on treatments for nonmotor symptoms of Parkinson’s disease – An evidence-based medicine review. Mov Disord 34: , 180–198. |
[2] | van Wemelen DJ , Sauerbier A , Leta V , Rodriguez-Blazquez C , Falup-Pecurariu C , Rodriguez-Violante M , Rizos A , Tsuboi Y , Metta V , Bhidayasiri R , Bhattacharya K , Borgohain R , Prashanth LK , Rosales R , Lewis S , Fung V , Behari M , Goyal V , Kishore A , Perez Lloret S , Martinez-Martin P , Chaudhuri KR ((2021) ) Cross-sectional analysis of the Parkinson’s disease Non-motor International Longitudinal Study baseline non-motor characteristics, geographical distribution and impact on quality of life. Sci Rep 11: , 9611. |
[3] | Mantri S , Klawson E , Albert S , Nabieva K , Lepore M , Kahl S , Daeschler M , Mamikonyan E , Kopil C , Marras C , Chahine LM ((2020) ) Understanding the Lexicon of fatigue in Parkinson’s Disease. J Parkinsons Dis 10: , 1185–1193. |
[4] | Friedman JH , Alves G , Hagell P , Marinus J , Marsh L , Martinez-Martin P , Goetz CG , Poewe W , Rascol O , Sampaio C , Stebbins G , Schrag A ((2010) ) Fatigue rating scales critique and recommendations by the Movement Disorders Society task force on rating scales for Parkinson’ disease. Mov Disord 25: , 805–822. |
[5] | Friedman JH , Abrantes A , Sweet LH ((2011) ) Fatigue in Parkinson’s disease. Exp Opinion Pharmacother 12: , 1999–2007. |
[6] | Metta V , Logishetty K , Martinez-Martin P , Gage HM , Schartau PE , Kaluarachchi TK , Martin A , Odin P , Barone P , Stocchi F , Antonini A , Chaudhuri KR ((2011) ) The possible clinical predictors of fatigue in Parkinson’s disease: A study of 135 patients as part of international nonmotor scale validation project. Parkinsons Dis 2011: , 125271. |
[7] | Friedmann JH , Friedman H ((2001) ) Fatigue in Parkinson’s disease: Nine-year follow-up. Mov Disord 16: , 1120–1122. |
[8] | Barone P , Antonini A , Colosimo C , Marconi R , Morgante L , Avarello TP , Bottacchi E , Cannas A , Ceravolo G , Ceravolo R , Cicarelli G , Gaglio RM , Giglia RM , Iemolo F , Manfredi M , Meco G , Nicoletti A , Pederzoli M , Petrone A , Pisani A , Pontieri FE , Quatrale R , Ramat S , Scala R , Volpe G , Zappulla S , Bentivoglio AR , Stocchi F , Trianni G , Dotto PD; PRIAMO study group ((2009) ) The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 24: , 1641–1649. |
[9] | Kluger BM , Herlofson K , Chou KL , Lou JS , Goetz CG , Lang AE , Weintraub D , Friedman J ((2016) ) Parkinson’s disease-related fatigue: A case definition and recommendations for clinical research. Mov Disord 31: , 625–631. |
[10] | Nassif DV , Pereira JS ((2018) ) Fatigue in Parkinson’s disease: Concepts and clinical approach. Psychogeriatrics 18: , 143–150. |
[11] | Ridder A , Chou KL ((2016) ) Managing fatigue in patients with Parkinson’s disease: A patient-focused perspective. J Parkinsonism Restless Legs Syndrome 6: , 65–72. |
[12] | Herlofson K , Kluger BM ((2017) ) Fatigue in Parkinson’s disease. J Neurol Sci 374: , 38–41. |
[13] | Kluger BM , Krupp LB , Enoka RM ((2013) ) Fatigue and fatigability in neurologic illnesses. Proposal for a unified taxonomy. Neurology 80: , 409–416. |
[14] | Lou JS ((2009) ) Physical and mental fatigue in Parkinson’s disease: Epidemiology, patho physiology and treatment. Drugs Aging 26: , 195–208. |
[15] | Friedman Jh , Beck JC , Chou KL , Clark G , Fagundes CP , Goetz CG , Herlofsen K , Kluger B , Krupp LB , Lang AE , Lou JS , Marsh L , Newbould A , Weintraub D ((2016) ) Fatigue in Parkinson’s disease: A report from a multidisciplinary symposium. NPJ Parkinson Dis 2: , 15025. |
[16] | Shulman LM , Taback RL , Bean J , Weiner WJ ((2001) ) Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord 16: , 507–510. |
[17] | Hagell P , Brundin L ((2009) ) Towards an understanding of fatigue in Parkinson disease. J Neurol Neurosurg Psychiatry 80: , 489–493. |
[18] | Kostic V , Tomic A , Jecmenica-Lukic M ((2016) ) The pathophysiology of fatigue in Parkinson’ disease and its pragmatic management. Mov Disord Clin Pract 3: , 323–330. |
[19] | Kotagal V , Szpara A , Albin RL , Bohnen N ((2019) ) Fatigue in Parkinson’s disease associates with lower ambulatory diastolic blood pressure. J Parkinson Dis 9: , 575–581. |
[20] | Franssen M , Winward C , Collett J , Wade D , Dawes H ((2014) ) Interventions for fatigue in Parkinson’s disease: A systematic review and Meta-analysis. Mov Disord 29: , 1675–1679. |
[21] | Elbers RG , Verhoef J , van Wegen EE , Berendse HW , Kwakkel G ((2016) ) Interventions for fatigue in Parkinson’s disease. Cochrane Database Syst Rev 2015: , CD010925. |
[22] | Wang K , Li K , Zhang P , Ge S , Wen X , Wu Z , Yao X , Jiao B , Sun P , Lv P , Lu L ((2021) ) Mind-body exercises for non-motor symptoms of patients with Parkinson’s disease: A systematic review and meta-analysis. Front Aging Neurosci 13: , 770920. |
[23] | Wang LL , Sun CJ , Wang Y , Zhan TT , Yuan J , Niu CY , Yang J , Huang S , Cheng L ((2022) ) Effects of dance therapy on non-motor symptoms in patients with Parkinson’s disease: A systematic review and meta-analysis. Aging Clin Exp Res 34: , 1201–1208. |
[24] | Luo F , Ye M , Lv T , Hu B , Chen J , Yan J , Wang A , Chen F , He Z , Ding Z , Zhang J , Qian C , Liu Z ((2021) ) Efficacy of cognitive behavioral therapy on mood disorders, sleep, fatigue, and quality of life in Parkinson’s disease: A systematic review and meta-analysis. Front Psychiatry 12: , 793804. |
[25] | Tsuboi T , Satake Y , Hiraga K , Yokoi K , Hattori M , Suzuki M , Hara K , Ramirez-Zamora A , Okun MS , Katsuno M ((2022) ) Effects of MAO-B inhibitors on non-motor symptoms and quality of life in Parkinson’s disease: A systematic review. NPJ Parkinsons Dis 8: , 75. |
[26] | Cusso ME , Donald KJ , Khoo TK ((2016) ) The impact of physical activity on non-motor symptoms in Parkinson’s disease: A systematic review. Front Med (Lausanne) 3: , 35. |
[27] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , Shamseer L , Tetzlaff JM , Akl EA , Brennan SE , Chou R , Glanville J , Grimshaw JM , Hróbjartsson A , Lalu MM , Li T , Loder EW , Mayo-Wilson E , McDonald S , McGuinness LA , Stewart LA , Thomas J , Tricco AC , Welch VA , Whiting P , Moher D ((2021) ) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: , n71. |
[28] | da Costa Santos CM , de Mattos Pimenta CA , Nobre MR ((2007) ) The PICO strategy for the research question construction and evidence search. Rev Lat Am Enferm 15: , 508–511. |
[29] | Sterne JA , Savović J , Page MJ , Elbers RG , Blencowe NS , Boutron I , Cates CJ , Cheng HY , Corbett MS , Eldridge SM , Emberson JR , Hernán MA , Hopewell S , Hróbjartsson A , Junqueira DR , Jüni P , Kirkham JJ , Lasserson T , Li T , McAleenan A , Reeves BC , Shepperd S , Shrier I , Stewart LA , Tilling K , White IR , Whiting PF , Higgins JPT ((2019) ) RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366: , l4898. |
[30] | Higgins JPT , Thomas J , Chandler J , Cumpston M , Li T , Page MJ , Welch VA ((2022) ) (Version 6.3),, Accessed on April 1st. Cochrane Handbook for Systematic Reviews of Interventions 2022–https://training.cochrane.org/handbook. |
[31] | Lim TT , Kluger BM , Rodriguez RL , Malaty IA , Palacio R , Ojo OO , Patel S , Gujrati Y , Nutter B , Swartz C , Hennessy C , Fernandez HH ((2015) ) Rasagiline for the symptomatic treatment of fatigue in Parkinson’s disease. Mov Disord 30: , 1825–1830. |
[32] | Pahwa R , Tanner CM , Hauser RA , Sethi K , Isaacson S , Truong D , Struck L , Ruby AE , McClure NL , Went GT , Stempien MJ ((2015) ) Amantadine extended release for levodopa-induced dyskinesia in Parkinson’s disease (EASED Study). Mov Disord 30: , 788–795. |
[33] | Postuma RB , Lang AE , Munhoz RP , Charland K , Pelletier A , Moscovich M , Filla L , Zanatta D , Rios Romenets S , Altman R , Chuang R , Shah B ((2012) ) Caffeine for treatment of Parkinson disease: A randomized controlled trial. Neurology 79: , 651–658. |
[34] | Stocchi F; ADAGIO investigators ((2014) ) Benefits of treatment with rasagiline for fatigue symptoms in patients with early Parkinson’s disease. Eur J Neurol 21: , 357–360. |
[35] | Raymackers JM , Andrade M , Baey E , Vanneste M , Evrard F ((2019) ) Bright light therapy with a head-mounted device for anxiety, depression sleepiness and fatigue in patients with Parkinson’s disease. Acta Neurol Belg 119: , 607–613. |
[36] | Sturkenboom IHWM , Graff MJL , Hendriks JCM , Veenhuizen Y , Munneke M , Bloem BR , Nijhuis-van der Sanden MW ((2014) ) Efficacy of occupational therapy for patients with Parkinson’s disease: A randomized controlled trial. Lancet Neurol 13: , 557–566. |
[37] | Tyne HL , Taylor J , Baker GA , Steiger MJ ((2010) ) Modafinil for Parkinson’s disease fatigue. J Neurol 257: , 452–456. |
[38] | Cohen J ((1988) ) Statistical Power Analysis for the Behavioral Sciences (Second Edition). Lawrence Erlbaum Associates, Hillsdale, NJ. |
[39] | Lou JS , Dimitrova DM , Park BS , Johnson SC , Eaton R , Arnold G , Nutt JG ((2009) ) Using modafinil to treat fatigue in Parkinson disease: A double-blind, placebo-controlled pilot study. Clin Neuropharmacol 32: , 305–310. |
[40] | Mendonca DA , Menezes K , Jog MS ((2007) ) Methylphenidate improves fatigue scores in Parkinson disease: A randomized controlled trial. Mov Disord 22: , 2070–2076. |
[41] | Ondo WG , Fayle R , Atassi F , Jankovic J ((2005) ) Modafinil for daytime somnolence in Parkinson’s disease: Double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 76: , 1636–1639. |
[42] | Ondo WG , Shinawi L , Davidson A , Lai D ((2011) ) Memantine for non-motor features of Parkinson’s disease: A double-blind placebo controlled exploratory pilot trial. Parkinsonism Relat Disord 17: , 156–159. |
[43] | Peball M , Krismer F , Knaus HG , Djamshidian A , Werkmann M , Carbone F , Ellmerer P , Heim B , Marini K , Valent D , Goebel G , Ulmer H , Stockner H , Wenning GK , Stolz R , Krejcy K , Poewe W , Seppi K ((2020) ) Non-motor symptoms in Parkinson’s disease are reduced by Nabilone. Ann Neurol 88: , 712–722. |
[44] | Ricciardi L , De Nigris F , Specchia A , Fasano A ((2015) ) Homotaurine in Parkinson’s disease. Neurol Sci 36: , 1581–1587. |
[45] | Schifitto G , Friedman JH , Oakes D , Shulman L , Comella CL , Marek K , Fahn S ((2008) ) Fatigue in levodopa-naive subjects with Parkinson disease. Neurology 71: , 481–485. |
[46] | Adler CH , Caviness JN , Hentz JG , Lind M , Tiede J ((2003) ) Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson’s disease. Mov Disord 18: , 287–293. |
[47] | Büchele F , Hackius M , Schreglmann SR , Omlor W , Werth E , Maric A , Imbach LL , Hagele-Link S , Waldvogel D , Baumann CR ((2018) ) Sodium oxybate for excessive daytime sleepiness and sleep disturbance in Parkinson disease: A randomized clinical trial. JAMA Neurol 75: , 114–118. |
[48] | Bogosian A , Hurt CS , Hindle JV , McCracken LM , Vasconcelos e Sa DA , Axell S , Tapper K , Stevens J , Hirani PS , Salhab M , Ye W , Cubi-Molla P ((2021) ) Acceptability and feasibility of a mindfulness intervention delivered via videoconferencing for people with Parkinson’s. J Geriatr Psychiatry Neurol 35: , 155–167. |
[49] | Canning CG , Allen NE , Dean CM , Goh L , Fung VSC ((2012) ) Home-based treadmill training for individuals with Parkinson’s disease: A randomized controlled trial. Clin Rehabil 26: , 817–826. |
[50] | Coe S , Franssen M , Collett J , Boyle D , Meaney A , Chantry R , Esser P , Izadi H , Dawes H ((2018) ) Physical activity, fatigue, and sleep in people with Parkinson’s disease: A secondary per protocol analysis from an intervention trial. Parkinsons Dis 2018: , 1517807. |
[51] | Cugusi L , Solla P , Serpe R , Carzedda T , Piras L , Oggianu M , Gabba S , Di Blasio A , Bergamin M , Cannas A , Marrosu F , Mercuro G ((2015) ) Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation 37: , 245–254. |
[52] | Kluger BM , Rakowski D , Christian M , Cedar D , Wong B , Crawford J , Uveges K , Berk J , Abaca E , Corbin L , Garvan C ((2016) ) Randomized, controlled trial of acupuncture for fatigue in Parkinson’s disease. Mov Disord 31: , 1027–1032. |
[53] | Kong KH , Ng HL , Li W , Ng DW , Tan SI , Tay KY , Au WL , Tan LCS ((2018) ) Acupuncture in the treatment of fatigue in Parkinson’s disease: A pilot, randomized, controlled study. Brain Behav 8: , e00897. |
[54] | Michels K , Dubaz O , Hornthal E , Bega D ((2018) ) “Dance Therapy” as a psychotherapeutic movement intervention in Parkinson’s disease. Complement Ther Med 40: , 248–252. |
[55] | Ortiz-Rubio A , Cabrera-Martos I , Torres-Sánchez I , Casilda-López J , López-López L , Valenza MC ((2018) ) Effects of a resistance training program on balance and fatigue perception in patients with Parkinson’s disease: A randomized controlled trial. Med Clin (Barc) 150: , 460–464. |
[56] | Rios Romenets S , Creti L , Fichten C , Bailes S , Libman E , Pelletier A , Postuma RB ((2013) ) Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson’s disease – a randomized study. Parkinsonism Relat Disord 19: , 670–675. |
[57] | Solla P , Cugusi L , Bertoli M , Cereatti A , Della Croce U , Pani D , Fadda L , Cannas A , Marrosu F , Defazio G , Mercuro G ((2019) ) Sardinian folk dance for individuals with Parkinson’s disease: A randomized controlled pilot trial. J Altern Complement Med 25: , 305–316. |
[58] | Videnovic A , Klerman EB , Wang W , Marconi A , Kuhta T , Zee PC ((2017) ) Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease a randomized clinical trial. JAMA Neurol 74: , 411–418. |
[59] | Walter AA , Adams EV , Van Puymbroeck M , Crowe BM , Urrea-Mendoza E , Hawkins BL , Sharp J , Woschkolup K , Revilla FJ , Schmid AA ((2019) ) Changes in nonmotor symptoms following an 8-week yoga intervention for people with Parkinson’s disease. Int J Yoga Therap 29: , 91–99. |
[60] | Winward C , Sackley C , Meek C , Izadi H , Barker K , Wade D , Dawes H ((2012) ) Weekly exercise does not improve fatigue levels in Parkinson’s disease. Mov Disord 27: , 143–146. |
[61] | Wu PL , Lee M , Wu SL , Ho HH , Chang MH , Lin HS , Huang TT ((2021) ) Effects of home-based exercise on motor, non-motor symptoms and health-related quality of life in Parkinson’s disease patients: A randomized controlled trial., e. Jpn J Nurs Sci 12418. |
[62] | Penner IK , Paul F ((2017) ) Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol 13: , 662–675. |
[63] | Perez DQ , Espiritu AI , Jamora RDG ((2020) ) Efficacy and safety of amantadine for the treatment of fatigue in multiple sclerosis: A systematic review and meta-analysis. Neurodegener Dis Manag 10: , 383–395. |
[64] | Shangyan H , Kuiqing Li , Yumin Xu , Jie C , Weixiong L ((2018) ) Meta-analysis of the efficacy of modafinil versus placebo in the treatment of multiple sclerosis fatigue. Mult Scler Relat Disord 19: , 85–89. |
[65] | Asano M , Finlayson ML ((2014) ) Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: Exercise, education, and medication. Mult Scler Int 2014: , 798285. |
[66] | Khan F , Amatyra B , Galea M ((2014) ) Management of fatigue in persons with multiple sclerosis. Front Neurol 5: , 177. |
[67] | Chen Y , Shanshan X , Shen J , Yang H , Xu W , Shao M , Pan F ((2021) ) Effect of exercise on fatigue in multiple sclerosis patients: A network meta-analysis. Int J Sports Med 42: , 1250–1259. |
[68] | National Institute for Health and Care Excellence (NICE) ((2022) ) ,, Accessed December 18. Multiple sclerosis in adults: Management 2022–https://www.nice.org.uk/guidance/ng220. |
[69] | Lou JS , Kearns G , Oken B , Sexton G , Nutt J ((2001) ) Exacerbated physical fatigue and mental fatigue in Parkinson’s disease. Mov Disord 16: , 190–196. |




