Staging Parkinson’s Disease According to the MNCD (Motor/Non-motor/Cognition/Dependency) Classification Correlates with Disease Severity and Quality of Life
Abstract
Background:
Recently, a novel simple classification called MNCD, based on 4 axes (Motor; Non-motor; Cognition; Dependency) and 5 stages, has been proposed to classify Parkinson's disease (PD).
Objective:
Our aim was to apply the MNCD classification in a cohort of PD patients for the first time and also to analyze the correlation with quality of life (QoL) and disease severity.
Methods:
Data from the baseline visit of PD patients recruited from 35 centers in Spain from the COPPADIS cohort fromJanuary 2016 to November 2017 were used to apply the MNCD classification. Three instruments were used to assess QoL:1) the 39-item Parkinson's disease Questionnaire [PDQ-39]); PQ-10; the EUROHIS-QOL 8-item index (EUROHIS-QOL8).
Results:
Four hundred and thirty-nine PD patients (62.05±7.84 years old; 59% males) were included. MNCD stage was:stage 1, 8.4% (N = 37); stage 2, 62% (N = 272); stage 3, 28.2% (N = 124); stage 4-5, 1.4% (N = 6). A more advancedMNCD stage was associated with a higher score on the PDQ39SI (p < 0.0001) and a lower score on the PQ-10 (p< 0.0001) and EUROHIS-QOL8 (p< 0.0001). In many other aspects of the disease, such as disease duration, levodopa equivalent daily dose, motor symptoms, non-motor symptoms, and autonomy for activities of daily living, an association between the stage and severity was observed, with data indicating a progressive worsening related to disease progression throughout the proposed stages.
Conclusion:
Staging PD according to the MNCD classification correlated with QoL and disease severity. The MNCD could be a proper tool to monitor the progression of PD.
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disorder causing not only motor but also and non-motor symptoms (NMS) that result in loss of patient autonomy for activities of daily living (ADL) and quality of life (QoL) [1]. Since there is currently no cure for PD, the management is centered around the patient’s symptoms, aiming to provide the best possible QoL [2]. Therefore, QoL is a key factor to measure the impact that the disease has on the patient over time [3]. In the context of a clinically heterogeneous neurodegenerative disorder like PD, simple classifications that adequately inform clinicians about key symptoms at different stages of the disease would be crucial. Recently, a novel yet simple classification called MNCD has been proposed [4]. The MNCD is based on 4 axes: M, Motor; N, Non-motor; C, Cognition; D, Dependency. Motor and Non-motor axes include 4 sub-axes: “Motor fluctuations”, “Dyskinesia”, “Axial symptoms”, and “Tremor” for the Motor axis; “Neuropsychiatric symptoms”, “Autonomic dysfunction”, “Sleep disturbances and fatigue”, “Pain and sensory disorders” for the Non-motor axis. Regarding Cognition and Dependency, patients can be classified as with normal cognition, mild cognitive impairment, or dementia, and with independence for ADL, dependency for instrumental ADL, or dependency for basic ADL, respectively. According to the MNCD, 5 stages are considered, from stage 1 (no disabling motor symptoms or NMS with normal cognition and independency for ADL) to 5 (dementia and dependency for basic ADL) [4]. In summary, the MNCD classification includes 4 major axes and 5 stages to identify key symptoms and monitor the progression of PD. Importantly, this is the first classification that takes into account key aspects of the PD such as axial symptoms, NMS, cognition and autonomy for ADL, due to their prognostic value, their impact on the patient and/or caregiver and/or their importance when deciding on a specific therapeutic attitude. Currently, the MNCD classification is a proof of concept and a study{ to examine the usability and variability of this tool in PD patients is on-going.∥The objective of this study was to apply the MNCD classification in a cohort of patients with PD for the first time. Data were obtained from the COPPADIS cohort [5] and the criteria to apply over the data for different symptoms included in the MNCD classification were specifically defined. Our hypothesis was that patients’ QoL would be different between the different PD stages according to the MNCD classification, with a better QoL in stage 1 and a worse QoL at a higher advanced stage (i.e., a more advanced MNCD stage, a worse QoL). In other words, we wanted to know if the MNCD stage can be a good indicator of PD patient’s QoL. In addition, we analyzed disease severity regarding to the MNCD stage.
MATERIALS AND METHODS
Data from PD patients recruited from 35 hospitals in Spain from the COPPADIS cohort [5] from January 2016 to November 2017 were used in this study. Methodology about COPPADIS-2015 study can be consulted at https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-016-0548-9[6]. This is a multi-center, observational, 5-year follow-up study designed to analyze disease progression in a Spanish population of PD patients. All patients included were diagnosed according to UK PD Brain Bank criteria [7]. Exclusion criteria were: non-PD parkinsonism, dementia (Mini-Mental State Examination <26), age <18 or >75 years, inability to read or understand the questionnaires, to be receiving any advanced therapy (continuous infusion of levodopa or apomorphine, and/or with deep brain stimulation), and the presence of comorbidity, sequelae, or any disorder that could interfere with the assessment. For the present specific transversal and retrospective analysis, data from the baseline visit were used to apply the MNCD classification (axes and stages).
PD patient assessment
Information on sociodemographic aspects, factors related to PD, comorbidity, and treatment including levodopa equivalent daily dose (LEDD) [8] were collected at baseline. The evaluation included (1) motor assessment (Hoenh & Yahr [H&Y], Unified Parkinson’s Disease Rating Scale [UPDRS] part III and part IV, Freezing of Gait Questionnaire [FOGQ]), (2) NMS (Non-Motor Symptoms Scale [NMSS], Parkinson’s Disease Sleep Scale [PDSS], Visual Analog Scale-Pain [VAS-Pain], Visual Analog Fatigue Scale [VAFS]), (3) cognition (Parkinson's Disease Cognitive Rating Scale [PD-CRS]), (4) mood and neuropsychiatric symptoms (Beck Depression Inventory II [BDI-II], Neuropsychiatric Inventory [NPI], Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale [QUIP-RS]), (5) disability (Schwab and England Activities of daily living Scale [ADLS]), and (6) health-related (the 39-item Parkinson’s disease Questionnaire [PDQ-39]) and global QoL (PQ-10, the EUROHIS-QOL 8-item index [EUROHIS-QOL8]) [6]. In all the questionnaires/scales a higher score indicates a more severe affectation apart from PDSS, PD-CRS, ADLS, and EUROHIS-QOL8, which were the opposite. In patients with motor fluctuations, the motor evaluation was made during the OFF state (without medication in the last 12 h) and during the ON state whereas in patients without motor fluctuations, it was conducted without medication. The non-motor assessment was conducted after taking dopaminergic medication.
Three different instruments were used to assess QoL: 1) PDQ-39 [9], 2) a rating of global perceived QoL (PQ-10) on a scale from 0 (worst) to 10 (best) [10], and 3) EUROHIS-QOL8 [11]. The PDQ-39 is a questionnaire to assess specifically the patients’ health-related quality of life (HRQoL) in PD patients. It has 39 items grouped into 8 domains: (1) Mobility (items 1 to 10); (2) Activities of daily living (items 11 to 16); (3) Emotional well-being (items 17 to 22); (4) Stigma (items 23 to 26); (5) Social support (items 27 to 29); (6) Cognition (items 30 to 33); (7) Communication (items 34 to 36); (8) Pain and discomfort (items 37 to 39). For each item, the score may range from 0 (never) to 4 (always). The symptoms refer to the 4 weeks prior to assessment. Domain total scores are expressed as a percentage of the corresponding maximum possible score and a Summary Index is obtained as average of the domain scores (PDQ-39SI). The EUROHIS-QOL8 is an 8-item GQoL questionnaire (quality of life; health status; energy; autonomy for activities of daily living; self-esteem; social relationships; economic capacity; habitat) derived from the WHOQOL-BREF. For each item, the score ranges from 0 (not at all) to 5 (completely). The total score is expressed as the mean of the individual scores. A higher score indicates a better QoL.
MNCD classification
The MNCD classification has been designed with the idea that it can be applied by a neurologist in his/her clinical practice based on the symptoms detected with the anamnesis and examination and without the need to use specific scales, being the neurologist who scores the presence or absence of symptoms based on to whether they produce a truly significant impact on the patient (e.g., it is not the same dysthymia or minor depression than major depression). For this study, we defined the symptoms specifically according all the information collected from the patients from the COPPADIS cohort (Table 1).
Table 1
Criteria for symptoms defined as clinically relevant symptoms in this study according to the MNCD classification; 0, the symptom is not present or if it is present is no clinically relevant; 1, the symptom is present and it is clinically relevant
| MOTOR SYMPTOMS |
| ⟶M1, Motor fluctuations. UPDRS-IV-item 39; 0 = 0 (no OFF time); from 1 (OFF time 1-2% of the waking day) to 4 (OFF time 76-100% of the waking day) = 1. |
| ⟶M2, Dyskinesia. UPDRS-IV-item 33; 0 = 0 (not disabling dyskinesia); from 1 (mildly disabling dyskinesia) to 4 (completely disabled dyskinesia) = 1. |
| ⟶M3, Axial symptoms: |
| *M3A, Dysphagia. NMSS-item 20; from 0 (absent) to 2 (often –l1/week–but mild or moderate but rarely –<1/week) = 0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe) = 1. |
| *M3B, Hypomimia. UPDRS-III-item 19; from 0 (normal) to 3 (moderate hypomimia; lips parted some of the time) = 0; 4 (masked or fixed facies with severe or complete loss of facial expression; lips parted 1/4 inch or more) = 1. |
| *M3C, FOG. FOGQ-item 3; from 0 (never) to 2 (rarely –about 1/week) = 0; from 3 (often –about 1/day) to 4 (always –about every time while walking) = 1. |
| *M3D, Falls. UPDRS-II-item 13; from 0 (none) to 1 (rare falling) = 0; from 2 (occasionally falls, less than once per day) to 4 (falls more than once daily) = 1. |
| *M3E, Abnormal posture. UPDRS-III-item 28; from 0 (normal erect) to 2 (moderately stooped posture, definitely abnormal; can be slightly leaning to one side) = 0; from 3 (severely stooped posture with kyphosis; can be moderately leaning to one side) to 4 (marked flexion with extreme abnormality of posture) = 1. |
| *M3F, Postural instability. UPDRS-III-item 30; from 0 (normal) to 1 (retropulsion, but recovers unaided) = 0; from 2 (absence of postural response; would fall if not caught by examiner) to 4 (unable to stand without assistance) = 1. |
| *M3G, Gait problems. UPDRS-II-item 15; from 0 (normal) to 2 (moderate difficulty, but requires little or no assistance) = 0; from 3 (severe disturbance of walking, requiring assistance) to 4 (cannot walk at all, even with assistance) = 1. |
| ⟶M4, Tremor. UPDRS-II-item 16, from 0 (absent) to 2 (moderate; bothersome to patient) = 0; from 3 (severe; interferes with many activities) to 4 (marked; interferes with most activities) = 1. |
| NON-MOTOR SYMPTOMS |
| ⟶N1, Neuropsychiatric symptoms: |
| *N1A, Major depression. No major depression = 0; major depression (DSM –V criteria [38])=1. |
| *N1B, Anxiety. NMSS-item 9; from 0 (absent) to 2 (often –1/week–but mild or moderate but rarely –<1/week)=0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe)=1. |
| *N1C, ICD and/or CB. Previously published cutoff points of the QUIP-RS were applied to define the case as 1: gambling ≥6, buying ≥8, sex ≥8, eating ≥7, hobbyism-punding ≥7 [39]. |
| *N1D, Apathy. NPI-item G; from 0 (absent) to 2 (sometimes –1/week–but mild or moderate but rarely –<1/week)=0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe)=1. |
| *N1E, Delusions. NPI-item A; from 0 (absent) to 2 (sometimes –1/week–but mild or moderate but rarely –<1/week)=0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe)=1. |
| *N1F, Hallucinations. NPI-item B; from 0 (absent) to 2 (sometimes –1/week––but mild or moderate but rarely –<1/week)=0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe)=1. |
| *N1G, Agitation. NPI-item C; from 0 (absent) to 2 (sometimes –1/week–but mild or moderate but rarely –<1/week)=0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe)=1. |
| ⟶N2, Autonomic dysfunction: |
| *N2A, Orthostatic dizziness. NMSS-item 1; from 0 (absent) to 2 (often –1/week–but mild or moderate but rarely –<1/week)=0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe)=1. |
| *N2B, Syncope. NMSS-item 2; from 0 (absent) to 2 (often –1/week–but mild or moderate but rarely –<1/week)=0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe)=1. |
| *N2C, Sweating. NMSS-item 30; from 0 (absent) to 2 (often –1/week–but mild or moderate but rarely –<1/week)=0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe)=1. |
| ⟶N3, Sleep disturbances and fatigue: |
| *N3A, Sleep disturbances. Previously published cutoff points of the PDSS were applied to define the case as 1: an overall score below 82 or a score below 5 on at least one item [40]. |
| *N3B, Fatigue. NMSS-item 4; from 0 (absent) to 2 (often –1/week–but mild or moderate but rarely –<1/week)=0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe)=1. |
| ⟶N4, Pain and sensory disorders: |
| *N4A, Pain. NMSS-item 27; from 0 (absent) to 2 (often –1/week–but mild or moderate but rarely –<1/week)=0; from 3 (rarely but severe or mild but frequent –several times per week) to 12 (very frequent –daily or all the time–and severe)=1. |
| *N4B, Cramps and/or spasms. PDQ-39-item 37; from 0 (never) to 2 (sometimes)=0; from 3 (often) to 4 (always)=1. |
| *N4C, Unpleasant hot or cold feeling. PDQ-39-item 39; from 0 (never) to 2 (sometimes)=0; from 3 (often) to 4 (always)=1. |
| COGNITION |
| ⟶C0, normal cognition. PD-CRS total score ≥81. |
| C1, mild cognitive impairment. PD-CRS total score <81 and >64. |
| C2, dementia. PD-CRS total score ≤64 and dependency for basic ADL (ADLS ≤50).* |
| *Patients with PD-CRS ≤64 but ADLS >50 were classified as C1. |
| DEPENDENCY |
| ⟶D0, independence for ADL. ADLS ≥80. |
| D1, dependency for instrumental ADL. ADLS >50 and <80. |
| D2, dependency for basic ADL. ADLS ≤50. |
ADL, activities of daily living; ADLS, Schwab and England Activities of daily living Scale; CB, compulsive behavior; FOG, freezing of gait; FOGQ, Freezing of Gait Questionnaire; ICD, impulse control disorder; NMSS, Non-Motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD, Parkinson's disease; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; PDQ-39, 39-item Parkinson’s disease Questionnaire; PDSS, Parkinson’s Disease Sleep Scale; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale.
Regarding the axes, patients were classified for each axis in groups [4]. For axis 1 (Motor): M0 (no sub-axis with symptoms); M1 (1 sub-axis with symptoms); M2 (2 sub-axes with symptoms); M3 (3 sub-axes with symptoms); M4 (all sub-axes with symptoms). For axis 2 (Non-motor): N0 (no sub-axis with symptoms); N1 (1 sub-axis with symptoms); N2 (2 sub-axes with symptoms); N3 (3 sub-axes with symptoms); N4 (all sub-axes with symptoms). For axis 3 (Cognition): C0, normal cognition; C1, mild cognitive impairment; C2, dementia. For axis 4, D0 (independency for ADL); D1 (dependency for instrumental ADL); D2 (dependency for basic ADL). A total sum (MNCD total score) was calculated with a range from 0 (M0N0C0D0) to 12 (M4N4C2D2).
Because the COPPADIS cohort includes a smaller number of advanced PD patients, patients with a MNCD stage 4 or 5 were included in the same category. MNCD stages [4] were: 1) Stage 1, if the patient has no any relevant motor and NMS, being independent for ADL and without cognitive impairment; 2) Stage 2, if there is at least 1 motor symptom or 1 NMS scoring in the MNCD classification, but there is neither cognitive impairment nor dependency for ADL; 3) Stage 3, if there is mild cognitive impairment (C = 1) and/or dependency for instrumental ADL (D = 1) and the score on axes 1 (Motor) and 2 (Non-Motor) could be from 0 to 4; Stage 4-5, if there is dementia (C = 2) and/or dependency for basic ADL (D = 2).
2.3Data analysis
Data were processed using SPSS 20.0 for Windows. For comparison of QoL and other disease related variables between patients with a different MNCD stage (all stages together or two consecutive stages), the Student’s t-test, Mann-Whitney U test, Chi-square test, Fisher test, ANOVA test, or Kuskal-Wallis tes were used as appropriate (distribution for variables was verified by one-sample Kolmogorov-Smirnov test). Spearman’s or Pearson’s correlation coefficient, as appropriate, were used for analyzing the relationship between the MNCD total score (from 0 to 12) and PDQ-39SI, EUROHIS-QOL8 and PQ-10 scores. Correlations were considered weak for coefficient values ≤0.29, moderate for values between 0.30 and 0.59, and strong for values ≥0.60.
2.4Standard protocol approvals, registrations, and patient consents
We received approval from the Comité de Ética de la Investigación Clínica de Galicia (2014/534; 02/DEC/2014) and a written informed consent from all participants in this study was obtained. COPPADIS-2015 was classified by the AEMPS (Agencia Española del Medicamento y Productos Sanitarios) as a Post-authorization Prospective Follow-up study with the code COH-PAK-2014-01.
2.5Data availability
The protocol and the statistical analysis plan are available on request. Deidentified participant data are not available for legal and ethical reasons.
3RESULTS
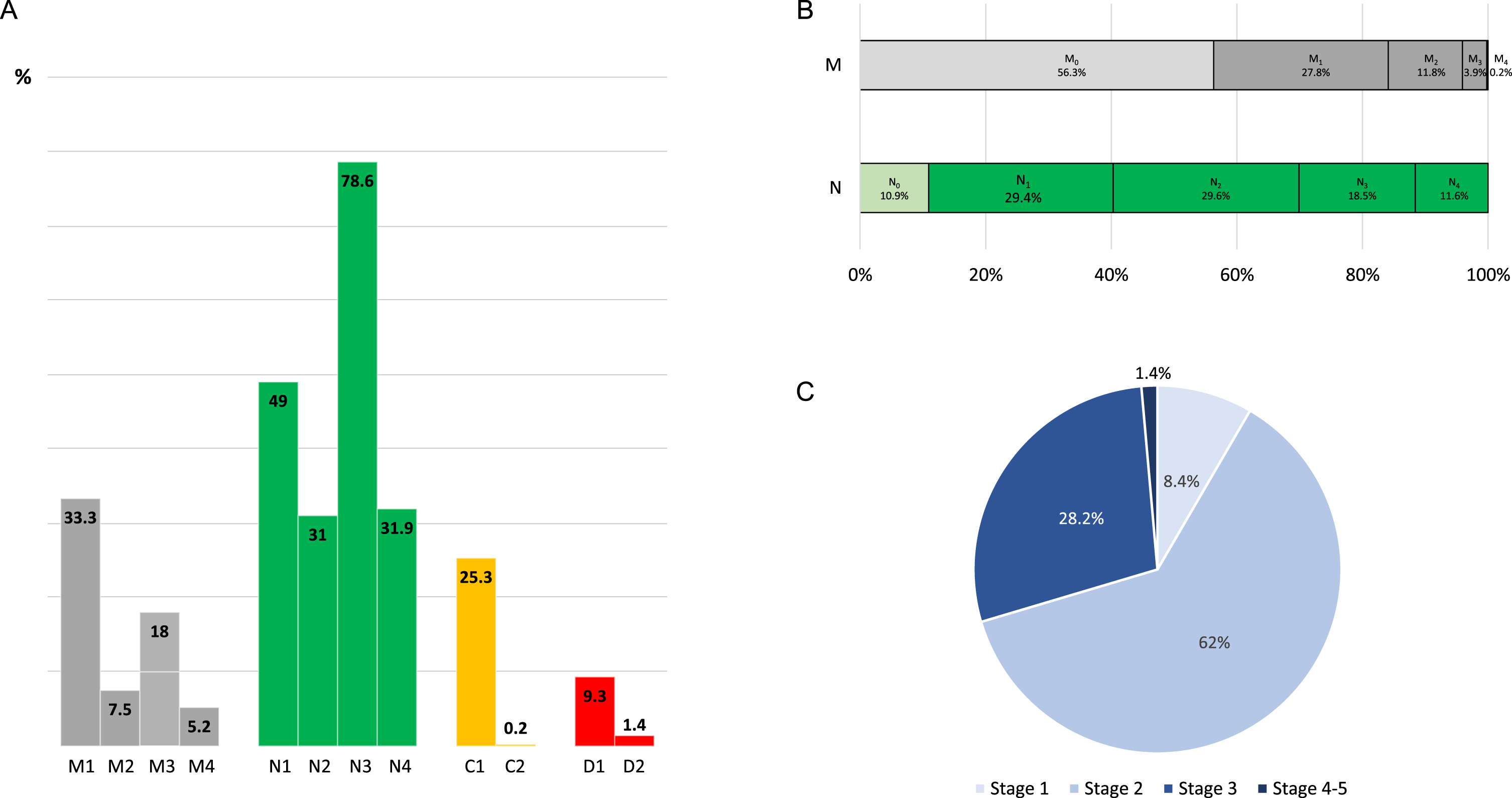
The study included 439 PD patients (62.05±7.84 years old; 59% males). Mean disease duration (year from symptoms onset) was 5.73±4.39, and only 10% of the patients had H&Y stage from 3 to 5. Up to 43.7% and 89.1% of the patients had at least one clinically relevant motor symptom (33.3% motor fluctuations; 7.5% disabling dyskinesia; 18% axial symptoms; 5.2% tremor) and NMS (49% neuropsychiatric symptoms; 31% autonomic dysfunction; 78.6% sleep disturbances and/or fatigue; 31.9% pain and sensory disorders), respectively (Table 2, Fig. 1A). Of axial symptoms, dysphagia was the most frequent (10%), whereas sleep disturbances (72%), fatigue (36%), and anxiety (23.7%) were the most frequent NMS. Of 439 PD patients, 111 (25.3%) had mild cognitive impairment, and only 1 patient had dementia. Regarding dependency for ADL, 41 (9.3%) were dependent for instrumental ADL and only 6 (1.4%) for basic ADL. Up to 56.3% of the patients didn’t suffer from any clinically relevant motor symptom (classified as M0) compared to only 10.9% with regard to NMS (N0) (Fig. 1B). Only 1 patient had relevant motor symptoms related to all sub-axes from axis 1 (motor fluctuations + dyskinesia + axial symptoms + tremor) (M4) compared to 51 patients with symptoms related to all sub-axes from axis 2 (N4) (neuropsychiatric symptoms + autonomic dysfunction + sleep disturbances and/or fatigue + pain and sensory disorders). Regarding MNCD stages (Fig. 1C), the distribution was; stage 1, 8.4% (N = 37); stage 2, 62% (N = 272); stage 3, 28.2% (N = 124); stage 4-5, 1.4% (N = 6; 5 patients with a stage 4 and only 1 patient with a stage 5 from the MNCD classification according to the original description [4]).
Fig. 1
A) Frequency of patients with clinically relevant motor symptoms, NMS, cognitive problems and dependency for ADL according to the MNCD classification (M1, Motor fluctuations; M2, Dyskinesia; M3, Axial symptoms; M4, Tremor; N1, Neuropsychiatric symptoms; N2, Autonomic dysfunction; N3, Sleep disturbances and/or fatigue; N4, Pain and sensory disorders; C1, Mild cognitive impairment; C2, Dementia; D1, Dependency for instrumental ADL; D2, Dependency for basic ADL). B) Frequency of patients classified as M0, M1, M2, M3, M4 and N0, N1, N2, N3 and N4. C) Frequency of different stages of the MNCD classification.
A more advanced MNCD stage was associated with a longer disease duration (p = 0.001), to be older (p < 0.0001), a higher LEDD and number of non-antiparkinsonian drugs (p < 0.0001), and a worse status in terms of motor symptoms (H&Y; UPDRS-III; UPDRS-IV; FOGQ; p < 0.0001 for all analysis), NMS (PD-CRS, NMSS, BDI-II, NPI, PDSS, VAS-PAIN, VASF –physical, VASF –mental; p < 0.0001 for all analysis), and autonomy for ADL (p < 0.0001) (Table 3). Regarding QoL, both health-related and global QoL were related to the MNCD stage, such that the more advanced MNCD stage correlated to a higher score on the PDQ39SI and a lower score on the PQ-10 and the EUROHIS-QOL8 (Table 4). Considering the four MNCD stages (stage 1 vs stage 2 vs stage 3 vs stage 4-5), differences were significant in the three scales used to assess QoL: PDQ-39SI, 6.65±4.27 vs. 15.5±11.24 vs. 23.8±16.14 vs. 46.36±11.67 (p < 0.0001); PQ-10, 8±1.38 vs. 7.41±1.42 vs. 6.65±1.8 vs. 5.17±2.78 (p < 0.0001); EUROHIS-QOL8, 4.18±0.39 vs. 3.83±0.52 vs. 3.54±0.58 vs. 3.12±0.5 (p < 0.0001) (Table 4, Fig. 2A). By domains, significant differences were observed in all domains between groups when all the stages (from stage 1 to stage 4-5) were considered except in stigmatization (PDQ-39) and social relationships and habitat (EUROHIS-QOL8) (Table 4, Fig. 2B). When a MNCD stage was compared with its next consecutive stage, significant differences were detected in all comparisons for the PDQ-39SI: stage 1 vs. stage 2 (p < 0.0001); stage 2 vs. stage 3 (p < 0.0001); stage 3 vs. stage 4 (p = 0.002). For the PQ-10 and EUROHIS-QOL8, the only results that were not significant occurred when QoL in stage 3 was compared to QoL in stage 4 (Table 4).
Table 2
Frequency of patients presenting with clinically relevant symptoms collected according to the MNCD classification (N = 439).
| % | |
| MOTOR SYMPTOMS | 43.7 |
| Motor fluctuations | 33.3 |
| Dyskinesia | 7.5 |
| Axial symptoms | 18 |
| -Dysphagia | 10 |
| -Hypomimia | 0 |
| -FOG | 5.9 |
| -Falls | 5 |
| -Abnormal posture | 2.7 |
| -Postural instability | 2.7 |
| -Gait problems | 3 |
| Tremor | 5.2 |
| NON-MOTOR SYMPTOMS | 89.1 |
| Neuropsychiatric symptoms | 49 |
| -Major depression | 16.9 |
| -Anxiety | 23.7 |
| -ICD and/or CB | 18 |
| -Apathy | 15.7 |
| -Delusions | 2.7 |
| -Hallucinations | 3 |
| -Agitation | 4.8 |
| Autonomic dysfunction | 31 |
| -Orthostatic dizziness | 17.5 |
| -Syncope | 0.2 |
| -Sweating | 18.2 |
| Sleep disturbances and fatigue | 78.6 |
| -Sleep disturbances | 72 |
| -Fatigue | 36 |
| Pain and sensory disorders | 31.9 |
| -Pain | 18.7 |
| -Cramps and/or spasms | 13.4 |
| -Unpleasant hot or cold feeling | 11.8 |
| COGNITION | |
| Normal | 74.5 |
| Mild cognitive impairment | 25.3 |
| Dementia | 0.2 |
| DEPENDENCY | |
| Independence for ADL | 89.3 |
| Dependency for instrument ADL | 9.3 |
| Dependency for basic ADL | 1.4 |
The results represent percentage. ADL, activities of daily living; CB, compulsive behavior; FOG, freezing of gait; ICD, impulse control disorder.
Table 3
Disease related characteristics, motor and non-motor symptoms, autonomy for activities of daily living and quality of life in PD patients with different stage according to the MNCD classification (N = 439)
| Stage 1 | Stage 2 | Stage 3 | Stage 4-5 | Total | p | |
| (N = 37) | (N = 272) | (N = 124) | (N = 6) | (N = 439) | ||
| Age | 61.84±7.45 | 59.8±9.61 | 67.01±7.32 | 63.33±7.47 | 62.05±7.84 | <0.0001 |
| Males (%) | 56.8 | 60.7 | 55.6 | 66.7 | 59 | 0.775 |
| Weight (kg) | 77.51±16.28 | 75.62±13.91 | 76.74±12.41 | 70.41±10.08 | 76.02±13.67 | 0.716 |
| Disease duration (y) | 4.06±3.43 | 5.4±3.81 | 6.73±5.41 | 9.8±5.4 | 5.73±4.39 | 0.003 |
| Antiparkinsonian drugs: | ||||||
| - Levodopa | 45.9 | 67.6 | 83.1 | 83.3 | 70.4 | <0.0001 |
| - Dopamine agonist | 67.6 | 71.7 | 65.3 | 66.7 | 69.5 | 0.115 |
| - MAO-B inhibitor | 75.7 | 76.8 | 64.5 | 50 | 72.9 | 0.016 |
| - COMT inhibitor | 5.4 | 18.4 | 24.2 | 50 | 19.4 | 0.002 |
| - Amantadine | 5.4 | 8.8 | 11.3 | 16.7 | 9.3 | 0.099 |
| L-dopa eq. daily dose (mg) | 356.97±276.78 | 540.01±388.36 | 674.71±441.8 | 1057.2±762.12 | 569.48±413.15 | <0.0001 |
| Number of non antip. Drugs | 1 [0, 3] | 2 [1, 3] | 3 [1, 5.5] | 3 [1, 6] | 1 [0, 3] | <0.0001 |
| Motor phenotype (%) | 0.252 | |||||
| - Tremoric dominant | 45.9 | 44.9 | 38.7 | 0 | 42.6 | |
| - PIGD | 37.8 | 39.7 | 48.4 | 83.3 | 42.6 | |
| - Indeterminate | 16.2 | 15.4 | 12.9 | 16.7 | 14.8 | |
| Hoehn &Yahr - OFF | 2 [1.5, 2] | 2 [1.5, 2] | 2 [2, 2.5] | 3.5 [2, 4] | 2 [2, 2] | <0.0001 |
| - Stage from 3 to 5 (%) | 0 | 7.8 | 15.6 | 60 | 10 | 003C;0.0001 |
| UPDRS-III - OFF | 16.94±6.87 | 21.88±11.01 | 26.24±11.99 | 39.17±13.51 | 22.97±11.49 | <0.0001 |
| UPDRS-IV | 0.43±0.6 | 1.91±2.17 | 2.67±2.76 | 6±3.95 | 2.06±2.41 | <0.0001 |
| - Motor fluctuations (%) | 0 | 31.2 | 45.2 | 83.3 | 33.3 | <0.0001 |
| - Dyskinesia (%) | 0 | 7.4 | 8.9 | 33.3 | 7.5 | 0.024 |
| FOGQ | 0.95±1.29 | 3.2±3.96 | 5.62±5.15 | 16.83±4.07 | 3.87±4.65 | <0.0001 |
| - Patients with FOG (%) | 0 | 4.4 | 8.1 | 66.7 | 5.9 | <0.0001 |
| - Patients with falls (%) | 0 | 1.5 | 12.1 | 50 | 5 | <0.0001 |
| PD-CRS total score | 98.62±10.71 | 99.34±11.72 | 73.66±11.87 | 73.17±11.91 | 91.67±16.51 | <0.0001 |
| NMSS | 12.54±10.39 | 42.82±32.94 | 60.77±40.73 | 76.33±43.98 | 45.79±36.65 | <0.0001 |
| BDI-II | 3.14±2.93 | 8.32±6.97 | 13.09±8.49 | 16.5±8.59 | 9.34±7.77 | <0.0001 |
| - Major depression (%) | 0 | 13.6 | 27.4 | 50 | 16.9 | <0.0001 |
| NPI | 1.77±2.94 | 5.48±7.25 | 9.24±9.99 | 7.17±6.71 | 6.27±8.16 | <0.0001 |
| QUIP-RS | 1.24±3.57 | 5.16±9.31 | 4.25±8.02 | 3.5±8.57 | 4.55±8.65 | 0.015 |
| - ICD and/or CB (%) | 0 | 20.2 | 18.5 | 16.7 | 18 | 0.028 |
| PDSS | 139.82±9.52 | 112.26±27.62 | 108.53±24.68 | 82.33±34.78 | 113.12±27.28 | <0.0001 |
| VAS-PAIN | 1.08±2.24 | 2.61±2.86 | 3.48±3.34 | 5.19±3.02 | 3.15±2.83 | <0.0001 |
| VASF –physical | 0.93±1.7 | 3.02±2.74 | 3.91±2.84 | 6.86±2.98 | 3.15±2.83 | <0.0001 |
| VASF –mental | 0.63±1.13 | 2.21±2.61 | 2.72±2.86 | 3.66±2.67 | 2.24±2.65 | <0.0001 |
| ADLS | 94.05±4.97 | 90.73±6.72 | 82.41±12.77 | 41.66±11.69 | 87.99±11.1 | <0.0001 |
| PDQ-39SI | 6.65±4.27 | 15.5±11.24 | 23.8±16.14 | 46.36±11.67 | 17.52±13.76 | <0.0001 |
| EUROHIS-QOL8 | 4.18±0.39 | 3.83±0.52 | 3.54±0.58 | 3.12±0.5 | 3.76±0.56 | <0.0001 |
| PQ-10 | 8.08±1.38 | 7.41±1.42 | 6.65±1.8 | 5.17±2.78 | 7.22±1.63 | <0.0001 |
The results represent percentages, mean±SD or median [p25, p75]. Chi-squared, ANOVA and/or Kruskal-Wallis test were applied. Data about H&Y and UPDRS-III are during the OFF state (first thing in the morning without taking medication in the previous 12 h). ADLS, Schwab and England Activities of daily living Scale; BDI-II, Beck Depression Inventory-II; COMT, catechol-O-methyltransferase; CB, compulsive behavior; EUROHIS-QOL8, EUROHIS-QOL 8-item index; FOGQ, Freezing of Gait Questionnaire; ICD, impulse control disorder; MAO-B, Monoamine oxidase-B; NMSS, Non-Motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD, Parkinson's disease; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; PDQ-39, 39-item Parkinson’s disease Questionnaire; PDSS, Parkinson’s Disease Sleep Scale; PIGD, Postural Instability Gait Difficulty; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale; VAFS, Visual Analog Fatigue Scale; VAS-Pain, Visual Analog Scale-Pain.
Fig. 2
A) Health-related (PDQ-39SI) and global quality of life (PQ-10 and EUROHIS-QOL8) are represented in patients regarding to the MNCD stage, from stage 0 to stage 4-5. B) Comparison of the mean score on each domain of the PDQ-39SI and EUROHIS-QOL8 between patients regarding the MNCD stage (from 0 to 4-5). *p < 0.005. ADL, activities of daily living; EUROHIS-QOL8, EUROHIS-QOL 8-item index; PDQ-39SI; 39-item Parkinson’s Disease Quality of Life Questionnaire Summary Index.
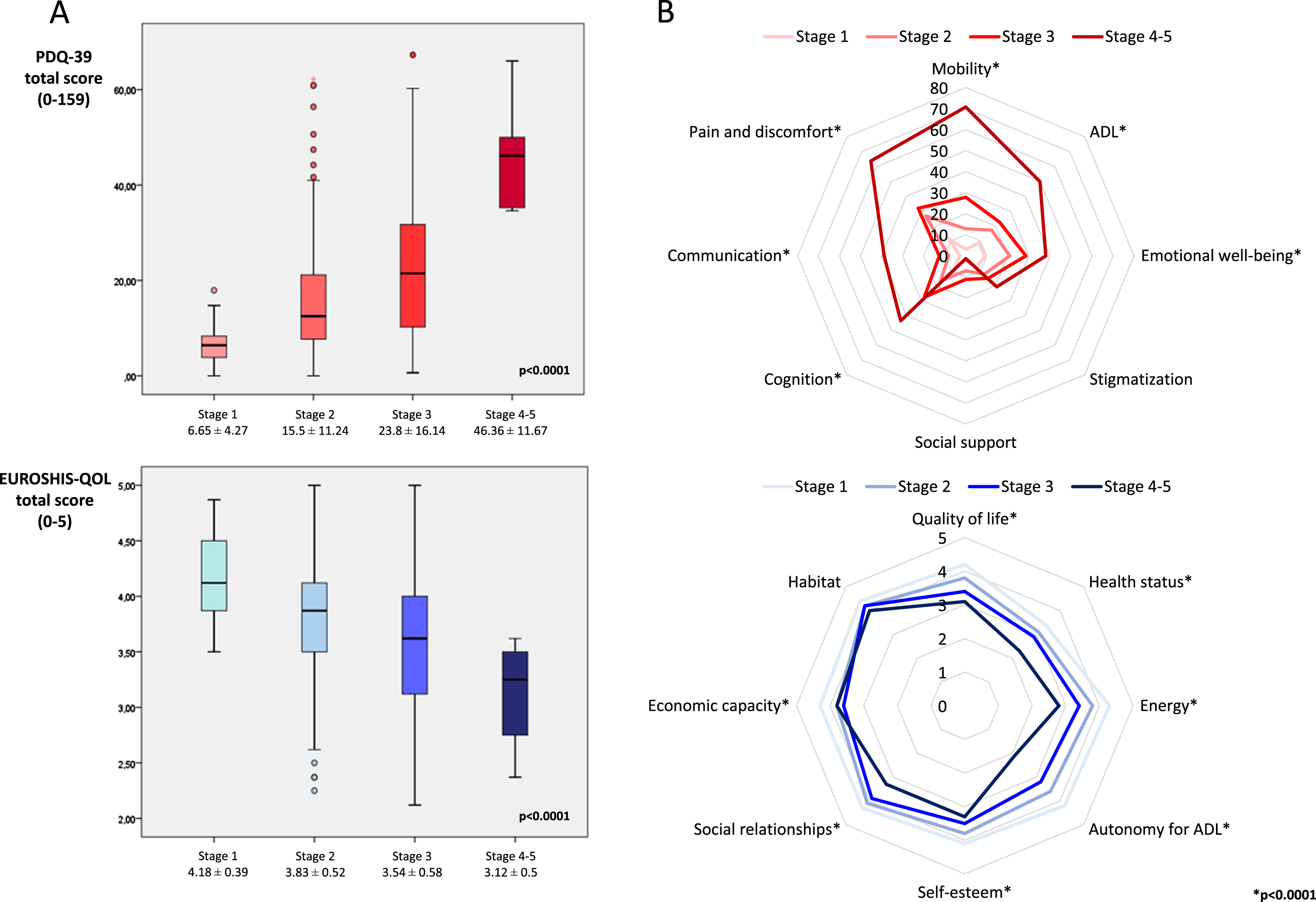
Table 4
Health-related and global quality of life in PD patients with different stage according to the MNCD classification (N = 439)
| Stage 1 | Stage 2 | Stage 3 | Stage 4-5 | pa | pb | pc | pd | |
| (N = 37) | (N = 272) | (N = 124) | (N = 6) | |||||
| HEALTH-RELATED QOL | ||||||||
| PDQ-39SI | 6.65±4.27 | 15.5±11.24 | 23.8±16.14 | 46.36±11.67 | <0.0001 | <0.0001 | <0.0001 | 0.002 |
| - Mobility | 3.91±6.33 | 12.97±14.67 | 27.98±24.32 | 70.83±14.8 | <0.0001 | <0.0001 | <0.0001 | 0.001 |
| - Activities of daily living | 8.77±10.04 | 17.33±18.25 | 22.79±21.15 | 49.97±29.11 | <0.0001 | 0.006 | 0.018 | 0.016 |
| - Emotional well-being | 9.1±10.13 | 20.99±20.04 | 28.6±23.94 | 38.15±19.23 | <0.0001 | <0.0001 | 0.007 | 0.290 |
| - Stigmatization | 7.59±12.67 | 12.42±18.25 | 15.16±22.36 | 20.8±26.39 | 0.545 | 0.283 | 0.842 | 0.390 |
| - Social support | 2.47±9.18 | 7.19±15.24 | 11.28±19.7 | 1.38±3.4 | 0.014 | 0.027 | 0.061 | 0.252 |
| - Cognition | 7.92±9.6 | 16.94±16.18 | 27.85±19.95 | 43.71±14.26 | <0.0001 | 0.001 | <0.0001 | 0.033 |
| - Communication | 2.92±6.85 | 8.26±13.26 | 12.69±17.52 | 38.86±24,51 | <0.0001 | 0.011 | 0.051 | 0.007 |
| - Pain and discomfort | 11.47±13.2 | 26.93±22.09 | 32.09±25.68 | 63.86±16.36 | <0.0001 | <0.0001 | 0.112 | 0.005 |
| GLOBAL QOL | ||||||||
| PQ-10 | 8±1.38 | 7.41±1.42 | 6.65±1.8 | 5.17±2.78 | <0.0001 | 0.015 | <0.0001 | 0.161 |
| EUROHIS-QOL8 | 4.18±0.39 | 3.83±0.52 | 3.54±0.58 | 3.12±0.5 | <0.0001 | <0.0001 | <0.0001 | 0.068 |
| - Quality of life | 4.22±0.58 | 3.87±0.68 | 3.48±0.75 | 3.17±0.98 | <0.0001 | 0.004 | 0.026 | 0.476 |
| - Health status | 3.46±0.86 | 3.14±0.89 | 2.91±0.95 | 2.33±0.81 | 0.001 | 0.022 | <0.0001 | 0.148 |
| - Energy | 4.3±0.7 | 3.81±0.78 | 3.42±0.88 | 2.83±0.75 | <0.0001 | <0.0001 | <0.0001 | 0.072 |
| - Autonomy for ADL | 4.22±0.67 | 3.69±0.83 | 3.23±0.91 | 2.17±0.41 | <0.0001 | <0.0001 | <0.0001 | 0.004 |
| - Self-esteem | 4.16±0.64 | 3.87±0.77 | 3.55±0.91 | 3.33±0.81 | <0.0001 | 0.035 | 0.001 | 0.501 |
| - Social relationships | 4.32±0.58 | 4.12±0.67 | 3.92±0.73 | 3.33±0.81 | 0.001 | 0.092 | 0.013 | 0.064 |
| - Economic capacity | 4.3±0.7 | 3.89±0.77 | 3.62±0.83 | 3.83±0.75 | <0.0001 | 0.001 | 0.002 | 0.642 |
| - Habitat | 4.49±0.51 | 4.28±0.71 | 4.23±0.63 | 4±0 | 0.149 | 0.137 | 0.296 | 0.245 |
The results represent mean±SD. ANOVA and/or Kruskal-Wallis and Mann-Whitney-Wilcoxon test were applied; pa, all groups; pb, stage 1 vs. stage 2; pc, stage 2 vs. stage 3; pd, stage 3 vs. stage 4. ADL, Activities of daily living; QoL, quality of life.
Finally, a strong positive correlation was observed between the MNCD total score and the PDQ-39SI (r = 0.693; p < 0.0001). Moderate negative correlations were detected between the MNCD total score and the PQ-10 score (r = –0.425; p < 0.0001) and the EUROHIS-QOL8 total score (r = –0.504; p < 0.0001).
4DISCUSSION
The present study applies the MNCD classification, a novel recently published classification for PD proposed by a Spanish group of experts on PD [4], for the first time in a cohort of PD patients. Interestingly, this transversal analysis observes that different stages for PD proposed in this novel classification correlated very clearly with disease severity and QoL. Moreover, a greater burden in symptoms defined in the MNCD classification (i.e., a higher MNCD total score), with 4 principal axes—Motor, Non-Motor, Cognition, Dependency, correlated with a poorer health-related and global QoL as well.
PD is an incredibly complex illness in which patients can suffer from a wide variety of motor and non-motor symptoms, which cause a progressive worsening in the long-term in QoL and loss of autonomy for ADL [12–14]. Furthermore, PD is very heterogeneous, with different subtypes described related to a variety of etiopathogenic mechanisms involved [15–17], which can explain the differences in the clinical presentation (motor and NMS) of the disease between patients even during the first years of disease duration [18–20]. In this context and taking into account that none of the previous classifications of PD encompasses the disease as a whole [21–24], the MNCD classification was proposed [4] with the idea of being a simple tool to identify key symptoms in PD and monitor the progression of the disease. The TNM classification [25], used in Oncology, was selected as a model and 4 major axes and 5 stages were considered in the design [4]. The four axes were key aspects in PD: Motor Symptoms; Non-Motor symptoms; Cognition; Dependency. Moreover, cognitive impairment and loss of autonomy for ADL were the key factors to define stages 4 and 5 of the MNCD classification. Data obtained from the application of this classification for the first time in a cohort of PD patients agree with great known variability in PD, even in a cohort in which 90% of the patients had a H&Y 1 or 2. The MNCD stage 2 was the most frequent (62%) but up to 28% of the patients had no clinically relevant motor and/or NMS (stage 1). On the contrary, close to 10% of the patients had cognitive impairment and/or dependency for ADL (stage from 3 to 5). Importantly, in many aspects of the disease such as disease duration, LEDD, motor symptoms, NMS, QoL, and autonomy for ADL, a relationship between the stage and the level of affectation was observed, with data indicating a progressive worsening related to disease progression throughout the proposed stages. It would be of great interest applying the MNCD classification in a longitudinal analysis with the aim to know if this tool could be useful to monitor the progression of PD, from the first moment (i.e., at diagnosis) to the end (i.e., at death). An adequate classification to use in a neurodegenerative disease such as PD should include symptoms, signs, or biomarkers that are key in decision-making for disease management [26]. Another important point is the high frequency of relevant symptoms and/or complications, such as motor fluctuations (33%), axial symptoms (18%) and especially NMS, with up to 89% of the patients suffering from at least 1 NMS. This aligns with data recently published about the COPPADIS cohort, demonstrating that NMS are very frequent even in patients with a stage 1 or 2 of H&Y, and their identification is very important because NMS impact the patient’s QoL independently of the motor stage [27]. Other studies have also observed a high frequency of motor fluctuations and NMS even in early PD patients and demonstrated the relationship between the two [28–33]. From a practical point of view, compared to the H&Y stage, the MNCD classification is also simple but provides much more information including key aspects such as cognitive status and dependency. Moreover, data of this analysis about motor severity assessed with the UPDRS-III and H&Y suggest that the MNCD stages could be useful to monitor changes in motor status along the time.
The principal objective of this study was to compare the QoL between different groups of PD patients from the COPPADIS cohort according to the MNCD stage. The results confirmed our hypothesis, with a very clear significant correlation between the stage and the QoL. The best perceived QoL corresponded to patients in stage 1 and a progressively worse QoL was observed at a more advanced stage of the disease, with stage 4-5 patients having the worst QoL. These results were found both when using the PDQ-39 to assess the health-related QoL and the PQ-10 and the EUROHIS-QOL8 to assess the global QoL. Moreover, significant differences were detected for all domains of the PDQ-39 (apart from except stigmatization and social support) and EUROHIS-QOL8 (apart from habitat). The strong correlation detected between the burden of symptoms defined in the MNCD classification (MNCD total score, from 0 to 12) and the QoL suggests that this scale could not only be useful for measuring disease progression but also as an indicator of the patient’s QoL. In a disease like PD for which there is no cure, improving QoL or at least slowing down its worsening is pivotal and is clearly related to the evolutionary stage [3, 34, 35], which is what the classification aims to measure.
This study has very important limitations. First, the MNCD classification was applied retrospectively using the data previously collected from the COPPADIS cohort PD patients at baseline visit. However, although it was not directly applied by the neurologist during a face-to-face assessment, the criteria for trying to define what symptoms could be considered as clinically relevant symptoms (e.g., major depression, ICD, etc.) were clearly defined for each symptom (Table 1). Second, there is a bias toward less advanced PD in this cohort and the COPPADIS cohort is not fully representative of PD due to inclusion/exclusion criteria at baseline. In fact, only 6 patients were classified as stage 4-5. Specifically and very important in relation with the application of the MNCD classification, patients at baseline with a MMSE <26 and dementia criteria were excluded, explaining why only 1 patient was in stage 5. Moreover, results about the comparison between patients with stage 3 (N = 124) and 4-5 (N = 6) were limited by the sample size. Third, the MNCD classification was applied through a cross-sectional analysis. Although the results are interesting and suggest that the MNCD classification may be useful for monitoring the progression of the disease in PD patients, it is important to be very cautious and the ideal propose would be to apply the MNCD classification in a cohort of early PD patients and follow up to observe the long-term change in the stage as the disease progresses. As an alternative, a cross-sectional analysis in a very large population including advanced or very advanced PD patients would be interesting as well. Even the MNCD classification could be an option to include in PD disease modifying treatment trials or in longitudinal prospective cohort studies [36]. Fourth, as it has been previously commented, the MNCD classification is a proof of concept purpose and a study to analyze the usability and variability of this tool in PD patients is on-going. In this sense, once again, we must still be very cautious when drawing clear conclusions about the classification, since it is necessary to verify beforehand that when it is applied in clinical practice at the discretion of the neurologist, this classification is useful and measures well what it intends. Fifth, a specific scale for the assessment of autonomic symptoms (e.g., SCOPA, etc.) has not been used in the COPPADIS cohort, unlike other cohorts [37], these symptoms may have been underrecognized. In contrast, strengths of our study are the large sample size as a whole (N = 439) and the extensive clinical and demographic information recorded. The results of this study are novel, as this analysis the first time that MNCD classification has been applied in a PD cohort.
In conclusion, we applied the MNCD classification in a PD cohort and observed that staging PD, according to this classification, correlates with QoL and disease severity. The MNCD could be a handy tool to monitor the progression of PD. However, firstly, a validation of the classification, and secondly, more studies designed to apply the MNCD classification in PD patients are needed.
ACKNOWLEDGMENTS
We would like to thank all patients and their caregivers who collaborated in this study. Many thanks also to Fundación Española de Ayuda a la Investigación en Enfermedades Neurodegenerativas y/o de Origen Genético (https://fundaciondegen.org/) and Alpha Bioresearch (www.alphabioresearch.com) and other institutions helping us.
COPPADIS and the present study were developed with the help of Fundación Española de Ayuda a la Investigación en Enfermedades Neurodegenerativas y/o de Origen Genético (https://fundaciondegen.org/) and Alpha Bioresearch (www.alphabioresearch.com). Also, we received grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Concesión de subvenciones de Proyectos de Investigación en Salud de la convocatoria 2020 de la Acción Estratégica en Salud 2017-2020 por el Proyecto “PROGRESIÓN NO MOTORA E IMPACTO EN LA CALIDAD DE VIDA EN LA ENFERMEDAD DE PARKINSON”) to develop a part of the COPPADIS project.
See the Supplementary Material for the full list of COPPADIS investigators.
CONFLICT OF INTEREST
Santos García D has received honoraria for educational presentations and/or advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, Teva, Archímedes, Esteve, Stada, Merz, and grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Concesión de subvenciones de Proyectos de Investigación en Salud de la convocatoria 2020 de la Acción Estratégica en Salud 2017–2020 por el proyecto “PROGRESIÓN NO MOTORA E IMPACTO EN LA CALIDAD DE VIDA EN LA ENFERMEDAD DE PARKINSON”).
De Deus Fonticoba T: None.
Cores Bartolomé C has received honoraria for educational presentations and advice service by Lundbeck and UCB Pharma.
Feal Painceiras MJ: None.
Íñiguez Alvarado MC: None.
García Díaz I: None.
Jesús S has received honoraria from AbbVie, Bial, Merz, UCB, and Zambon and holds the competitive contract “Juan Rodés” supported by the Instituto de Salud Carlos III. She has received grants from the Spanish Ministry of Economy and Competitiveness (PI18/01898) and the Consejería de Salud de la Junta de Andalucía (PI-0459-2018).
Buongiorno MT: None.
Planellas LL: None.
Cosgaya M: None.
García Caldentey J has received honoraria for educational presentations and advice service by Qualigen, Nutricia, Abbvie, Italfarmaco, UCB Pharma, Lundbeck, Zambon, Bial, and Teva.
Caballol N has received honoraria from Bial, Italfármaco, Qualigen, Zambon, UCB, Teva and KRKA and sponsorship from Zambon, TEVA and Abbvie for attending medical conferences.
Legarda I has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva.
Hernández Vara J has received travel bursaries and educational grants from Abbvie and has received honoraria for educational presentations from Abbvie, Teva, Bial, Zambon, Italfarmaco, and Sanofi-Genzyme.
Cabo I has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
López Manzanares L has received compensated advisory services, consulting, research grant support, or speaker honoraria from AbbVie, Acorda, Bial, Intec Pharma, Italfarmaco, Pfizer, Roche, Teva, UCB, and Zambon.
González Aramburu I: None.
Ávila Rivera MA. has received honoraria from Zambon, UCB Pharma, Qualigen, Bial, and Teva, and sponsorship from Zambon and Teva for attending conferences.
Gómez Mayordomo V: None.
Nogueira V: None.
Puente V has served as consultant for Abbvie and Zambon; has received grant/research from Abbvie.
Dotor García-Soto J: Compensated advisory services, consulting, research grant support, or speaker honoraria: Merck, Sanofi-Genzyme, Allergan, Biogen, Roche, UCB and Novartis.
Borrué C: None.
Solano Vila B has received honoraria for educational presentations and advice service by UCB, Zambon, Teva, Abbvie, Bial.
Álvarez Sauco M has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva.
Vela L has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva.
Escalante S has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
Cubo E has received travel grants form Abbvie, Allergan, Boston; Lecturing honoraria from Abbvie and International Parkinson's disease Movement Disorder Society.
Carrillo Padilla F has received honoraria from Zambon (SEN Congress assistance).
Martínez Castrillo JC has received research support from Lundbeck, Italfarmaco, Allergan, Zambon, Merz, and Abbvie. He has received speaking honoraria from AbbVie, Bial, Italfarmaco, Lundbeck, Krka, TEVA, UCB, Zambon, Allergan, Ipsen, and Merz.
Sánchez Alonso P has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva.
Alonso Losada MG has received honoraria for educational presentations and advice service by Zambon and Bial.
López Ariztegui N has received honoraria for educational presentations and advice service by Abbvie, Italfarmaco, Zambon, and Bial.
Gastón I has received research support from Abbvie and Zambon and has served as a consultant for Abbvie, Exelts, and Zambon.
Kulisevsky J has received consulting fees from Roche, Zambon; honoraria (e.g., lecture fees) from Zambon, Teva, Bial, UCB; and research funding from Roche, Zambon, Ciberned; Instituto de SaludCarlos III; FundacióLa Maratóde TV3.
Menéndez-González M has received honoraria for educational presentations and advice service by Eisai.
Seijo M has received honoraria for educational services from KRKA, UCB, Zambon, Bial; travel grants from Daiichi and Roche.
Ruiz Martínez J has received honoraria for educational presentations, attending medical conferences, and advice service by Abbvie, UCB Pharma, Zambon, Italfarmaco, Bial, and Teva.
Valero C has received honoraria for educational services from Zambon, Abbvie, and UCB.
Kurtis M has received honoraria from Bial, the Spanish Neurology Society, and the International and Movement Disorders Society.
González Ardura J has received honoraria for speaking from italofarma, Krka, Genzyme, UCB, Esteve, Psyma iberica marketing research SL and Ferrer, course grant from Teva and travel grant from Merck.
Alonso Redondo R: None.
Ordás C: None.
López Díaz LM has received honoraria from UCB, Lundbeck, and KRKA.
McAfee D: None.
Matilde Calopa M has received honoraria for lecturing or advisory boards from AbbVie, Bial and Zambon.
Fátima Carrillo F: None.
Escamilla Sevilla F has received honoraria as a speaker, support to attend scientific meetings and grants for conducting studies from Abbvie, Bial, Boston Scientifice, Medtronic, Merz Pharma, Teva, UCB Pharma and Zambon.
Freire E has received advisory, consulting and lecture fees from Abbvie, Teva, Bial, Zambon and Neu-raxpharm.
Gómez Esteban JC has received research support from Abbvie and speaking honoraria from AbbVie, Zambon and UCB.
Rocío García Ramos R has received honoraria as a speaker, support to attend scientific meetings and grants for conducting studies from Abbvie, Bial, Italfarmaco, Allergan, Merz Pharma, Teva, UCB Pharma and Zambon.
Rosario Isabel Luquín MR: None.
Martínez-Torres I has received honoraria from Abbvie, Bial, Merz, Ipsen, Insightec for educational and advice services.
Sesar Ignacio A has received honoraria from Bial, Ever Pharma Britannia, and Abbvie.
Martínez-Martin P has received honoraria from the International Parkinson and Movement Disorder Society (MDS) for management of the Clinical Outcome Assessments Program.
Mir P has received honoraria from AbbVie, Abbott, Allergan, Bial, Merz, UCB, and Zambon and have received grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Subdirección General de Evaluación y Fomento de la Investigación) and by Fondo Europeo de Desarrollo Regional (FEDER), the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía [CVI-02526, CTS-7685], the Consejería de Salud y Bienestar Social de la Junta de Andalucía [PI-0437-2012, PI-0471-2013], the Sociedad Andaluza de Neurología, the Jacques and Gloria Gossweiler Foundation, the Fundación Alicia Koplowitz, the Fundación Mutua Madrileña.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-225073.
REFERENCES
[1] | Bloem BR , Okun MS , Klein C ((2021) ) Parkinson’s disease. Lancet 397: , 2284–2303. |
[2] | Armstrong MJ , Okun MS ((2020) ) Diagnosis and treatment of Parkinson disease: A review. JAMA 323: , 548–560. |
[3] | Santos García D , de Deus Fonticoba T , Cores C , Muñoz G , PazGonzález JM , Martínez Miró C , Suárez E , Jesús S , Aguilar M , Pastor P , Planellas L , Cosgaya M , García Caldentey J , Caballol N , Legarda I , Hernández Vara J , Cabo I , LópezManzanares L , González Aramburu I , Ávila Rivera MA , Catalán MJ , Nogueira V , Puente V , Ruíz de Arcos M , Borrué C , Solano Vila B , Álvarez Sauco M , Vela L , Escalante S , Cubo E , Carrillo Padilla F , Martínez Castrillo JC , Sánchez Alonso P , Alonso Losada MG , López Ariztegui N , Gastón I , Clavero P , Kulisevsky J , Blázquez Estrada M , Seijo M , Rúiz Martínez J , Valero C , Kurtis M , de Fábregues O , González Ardura J , Ordás C , López Díaz LM , McAfee D , Martinez-Martin P , Mir P COPPADIS Study Group ((2021) ) Predictors ofclinically significant quality of life impairment in Parkinson’sdisease. NPJ Parkinsons Dis 7: , 118. |
[4] | Santos García D , Álvarez Sauco M , Calopa M , Carrillo F , Escamilla Sevilla F , Freire E , García Ramos R , Kulisevsky J , Gómez Esteban JC , Legarda I , Luquín MRI , Castrillo JCM , Martínez-Martin P , Martínez-Torres I , Mir P , Ignacio Á S ((2021) ) MNCD: A new tool for classifying Parkinson’s disease in dailyclinical practice. Diagnostics (Basel) 12: , 55. |
[5] | Santos García D , Jesús S , Aguilar M , Planellas LL , García Caldentey J , Caballol N , Legarda I , Hernández Vara J , Cabo I , López Manzanares L , González Aramburu I , ÁvilaRivera MA , Catalán MJ , López Díaz L , Puente V , García Moreno JM , Borrué C , Solano Vila B , Álvarez Sauco M , Vela L , Escalante S , Cubo E , Carrillo Padilla F , MartínezCastrillo JC , Sánchez Alonso P , Alonso Losada MG , LópezAriztegui N , Gastón I , Kulisevsky J , Menéndez González M , Seijo M , Rúiz Martínez J , Valero C , Kurtis M , deFábregues-Boixar O , González Ardura J , Prieto Jurczynska C , Martinez-Martin P , Mir P COPPADIS Study Group ((2019) ) COPPADIS-2015(COhort of Patients with PArkinson’s DIsease in Spain, 2015): Anongoing global Parkinson’s disease project about disease progressionwith more than 1000 subjects included. Results from the baseline evaluation. Eur J Neurol 26: , 1399–1407. |
[6] | Santos-García D , Mir P , Cubo E , Vela L , Rodríguez-Oroz MC , Martí MJ , Arbelo JM , Infante J , Kulisevsky J , Martínez-Martín P COPPADIS Study Group ((2016) ) COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015), a global–clinical evaluations, serum biomarkers, genetic studies and neuroimaging–prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression. BMC Neurol 16: , 26. |
[7] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[8] | Schade S , Mollenhauer B , Trenkwalder C ((2020) ) Levodopa equivalent dose conversion factors: An updated proposal including opicapone and safinamide. Mov Disord Clin Pract 7: , 343–345. |
[9] | Jenkinson C , Fitzpatrick R , Peto V , Greenhall R , Hyman N ((1997) ) The Parkinson's Disease Questionnaire (PDQ-39): Development and validation of a Parkinson's disease summary index score. Age Ageing 26: , 353–357. |
[10] | Santos García D , de la Fuente-Fernández R ((2013) ) Impact ofnon-motor symptoms on health-related and perceived quality of lifein Parkinson’s disease. J Neurol Sci 332: , 136–140. |
[11] | Da Rocha NS , Power MJ , Bushnell DM , Fleck MP ((2012) ) The EUROHIS-QOL 8-item index: Comparative psychometric properties to its parent WHOQOL-BREF. Value Health 15: , 449–457. |
[12] | Bjornestad A , Tysnes OB , Larsen JP , Alves G ((2016) ) Loss of independence in early Parkinson disease: A 5-year population-based incident cohort study. Neurology 87: , 1599–1606. |
[13] | Auyeung M , Tsoi TH , Mok V , Cheung CM , Lee CN , Li R , Yeung E ((2012) ) Ten year survival and outcomes in a prospective cohort of new onset Chinese Parkinson’s disease patients. J Neurol Neurosurg Psychiatry 83: , 607–611. |
[14] | Bjornestad A , Pedersen KF , Tysnes OB , Alves G ((2017) ) Clinical milestones in Parkinson’s disease: A 7-year population-based incident cohort study. Parkinsonism Relat Disord 42: , 28–33. |
[15] | Aasly JO ((2020) ) Long-term outcomes of genetic Parkinson’s disease. J Mov Disord 13: , 81–96. |
[16] | Horsager J , Andersen KB , Knudsen K , Skjærbæk C , Fedorova TD , Okkels N , Schaeffer E , Bonkat SK , Geday J , Otto M , Sommerauer M , Danielsen EH , Bech E , Kraft J , Munk OL , Hansen SD , Pavese N , Göder R , Brooks DJ , Berg D , Borghammer P ((2020) ) Brain-firstversus body-first Parkinson’s disease: A multimodal imagingcase-control study. Brain 143: , 3077–3088. |
[17] | Sauerbier A , Jenner P , Todorova A , Chaudhuri KR ((2016) ) Non motor subtypes and Parkinson’s disease. Parkinsonism Relat Disord 22: , (Suppl 1), S41–S46. |
[18] | Fereshtehnejad SM , Romenets SR , Anang JB , Latreille V , Gagnon JF , Postuma RB ((2015) ) New clinical subtypes of Parkinson disease and their longitudinal progression: A prospective cohort comparison with other phenotypes. JAMA Neurol 72: , 863–873. |
[19] | van Rooden SM , Colas F , Martínez-Martín P , Visser M , Verbaan D , Marinus J , Chaudhuri RK , Kok JN , van Hilten JJ ((2011) ) Clinical subtypes of Parkinson’s disease. Mov Disord 26: , 51–58. |
[20] | Erro R , Picillo M , Vitale C , Palladino R , Amboni M , Moccia M , Pellecchia MT , Barone P ((2016) ) Clinical clusters and dopaminergic dysfunction in de-novo Parkinson disease. Parkinsonism Relat Disord 28: , 137–140. |
[21] | Hoehn MM , Yahr MD ((1967) ) Parkinsonism: Onset, progression and mortality. Neurology 17: , 427–442. |
[22] | Ray Chaudhuri K , Rojo JM , Schapira AH , Brooks DJ , Stocchi F , Odin P , Antonini A , Brown RG , Martinez-Martin P ((2013) ) A proposal for a comprehensive grading of Parkinson’s disease severity combining motor and non-motor assessments: Meeting an unmet need, PLoS One 8: , e57221. |
[23] | Martinez-Martin P , Kulisevsky J , Mir P , Tolosa E , García-Delgado P , Luquin MR ((2018) ) Validation of a simple screening tool for early diagnosis of advanced Parkinson’s disease in daily practice: The CDEPA questionnaire. NPJ Parkinsons Dis 4: , 20. |
[24] | Santos-García D , de Deus Fonticoba T , Suárez Castro E , Aneiros Díaz A , McAfee D ((2020) ) 5-2-1 Criteria: A simplescreening tool for identifying advanced PD patients who need anoptimization of Parkinson’s treatment. Parkinsons Dis 2020: , 7537924. |
[25] | Sobin LH , Wittekind CH (1997) TNM classification of malignant tumors, 5th edition. International Union Against Cancer (UICC). John Wiley & Sons, Inc., New York. |
[26] | Burciu RG , Ofori E , Archer DB , Wu SS , Pasternak O , McFarland NR , Okun MS , Vaillancourt DE ((2017) ) Progression marker of Parkinson’s disease: A 4-year multi-site imaging study. Brain 140: , 2183–2192. |
[27] | Santos García D , De Deus Fonticoba T , Paz González JM , CoresBartolomé C , Valdés Aymerich L , Muñoz Enríquez JG , Suárez E , Jesús S , Aguilar M , Pastor P , Planellas LL , Cosgaya M , García Caldentey J , Caballol N , Legarda I , Hernández Vara J , Cabo I , López Manzanares L , GonzálezAramburu I , Ávila Rivera MA , Catalán MJ , Nogueira V , Puente V , García Moreno JM , Borrué C , Solano Vila B , ÁlvarezSauco M , Vela L , Escalante S , Cubo E , Carrillo Padilla F , Martínez Castrillo JC , Sánchez Alonso P , Alonso Losada MG , López Ariztegui N , Gastón I , Kulisevsky J , BlázquezEstrada M , Seijo M , Rúiz Martínez J , Valero C , Kurtis M , deFábregues O , González Ardura J , Ordás C , LópezDíaz L , Mir P , Martinez-Martin P Coppadis Study Group ((2021) ) Staging Parkinson’s disease combining motor and nonmotor symptoms correlates with disability and quality of life. Parkinsons Dis 2021: , 8871549. |
[28] | Stocchi F , Antonini A , Barone P , Tinazzi M , Zappia M , Onofrj M , Ruggieri S , Morgante L , Bonuccelli U , Lopiano L , Pramstaller P , Albanese A , Attar M , Posocco V , Colombo D , Abbruzzese G DEEP study group((2014) ) Early DEtection of wEaring off in Parkinson disease: The DEEP study. Parkinsonism Relat Disord 20: , 204–211. |
[29] | Zis P , Rizos A , Martinez-Martin P , Pal S , Silverdale M , Sharma JC , Sauerbier A , Chaudhuri KR ((2014) ) Non-motor symptoms profile and burden in drug naïve versus long-term Parkinson’s disease patients. J Parkinsons Dis 4: , 541–547. |
[30] | Storch A , Schneider CB , Wolz M , Stürwald Y , Nebe A , Odin P , Mahler A , Fuchs G , Jost WH , Chaudhuri KR , Koch R , Reichmann H , Ebersbach G ((2013) ) Nonmotor fluctuations in Parkinson disease: Severity and correlation with motor complications. Neurology 80: , 800–809. |
[31] | Martínez-Fernández R , Schmitt E , Martinez-Martin P , Krack P ((2016) ) The hidden sister of motor fluctuations in Parkinson’sdisease: A review on nonmotor fluctuations., Mov Disord 31: , 1080–1094. |
[32] | Santos-García D , de Deus Fonticoba T , Suárez Castro E , Aneiros Díaz A , McAfee D , Catalán MJ , Alonso-Frech F , Villanueva C , Jesús S , Mir P , Aguilar M , Pastor P , GarcíaCaldentey J , Esltelrich Peyret E , Planellas LL , Martí MJ , Caballol N , Hernández Vara J , Martí Andrés G , Cabo I , Ávila Rivera MA , López Manzanares L , Redondo N , Martinez-Martin P COPPADIS Study Group; McAfee D ((2020) ) Non-motorsymptom burden is strongly correlated to motor complications inpatients with Parkinson’s disease. Eur J Neurol 27: , 1210–1223. |
[33] | Santos-García D , de Deus Fonticoba T , Bartolomé CC , Painceiras MJF , Castro ES , Canfield H , Miró CM , Jesús S , Aguilar M , Pastor P , Planellas L , Cosgaya M , Caldentey JG , Caballol N , Legarda I , Hernández-Vara J , Cabo I , Manzanares LL , Aramburu IG , Rivera MAÁ , Mayordomo VG , Nogueira V , Puente V , García-Soto JD , Borrué C , Vila BS , Sauco MÁ , Vela L , Escalante S , Cubo E , Padilla FC , Castrillo JCM , Alonso PS , Losada MGA , Ariztegui NL , Gastón I , Kulisevsky J , Estrada MB , Seijo M , Martínez JR , Valero C , Kurtis M , de Fábregues O , Ardura JG , Redondo RA , Ordás C , Díaz LML , McAfee D , Martinez-Martin P , Mir P , Coppadis Study Group ((2022) ) Motor fluctuations development isassociated with non-motor symptoms burden progression in Parkinson’sdisease patients: A 2-year follow-up study. Diagnostics(Basel) 12: , 1147. |
[34] | Martinez-Martin P , Kurtis MM ((2012) ) Health-related quality of life as an outcome variable in Parkinson’s disease. Ther Adv Neurol Disord 5: , 105–117. |
[35] | Li T , Zou S , Zhang Z , Liu M , Liang Z ((2022) ) Efficacy of pramipexole on quality of life in patients with Parkinson’s disease: A systematic review and meta-analysis. BMC Neurol 22: , 320. |
[36] | Heinzel S , Lerche S , Maetzler W , Berg D ((2017) ) Global, yet incomplete overview of cohort studies in Parkinson’s disease. J Parkinsons Dis 7: , 423–432. |
[37] | Rodriguez-Blazquez C , Forjaz MJ , Frades-Payo B , de Pedro-Cuesta J , Martinez-Martin P Longitudinal Parkinson's Disease Patient Study, Estudio Longitudinal dePacients con Enfermedad da Parkinson Group ((2010) ) Independent validation of the scales for outcomes in Parkinson’s disease-autonomic (SCOPA-AUT). Eur J Neurol 17: , 94–201. |
[38] | American Psychiatric Association (1994) Diagnostic and Statical Manual of Mental Disorders, Fourth ed. American Psychiatric Association, Washington, DC. |
[39] | Weintraub D , Mamikonyan E , Papay K , Shea JA , Xie SX , Siderowf A ((2012) ) Questionnaire for impulsive-compulsive disorders in Parkinson’s Disease-Rating Scale. Mov Disord 27: , 242–247. |
[40] | Santos-García D , Castro ES , de Deus Fonticoba T , Panceiras MJF , Enriquez JGM , González JMP , Bartolomé CC , Planellas LL , Caldentey JG , Caballol N , Legarda I , López IC , Manzanares LL , Rivera MAÁ , Catalán MJ , Nogueira V , Borrué C , Sauco MÁ , Vela L , Cubo E , Castrillo JCM , Alonso PS , Losada MGA , Ariztegui NL , Gastón MI , Kulisevsky J , Pagonabarraga J , Seijo M , Martínez JR , Valero C , Kurtis M , Ardura JG , Prieto C , Mir P , Martinez-Martin P ((2021) ) Sleep problems are related to a worsequality of life and a greater non-motor symptoms burden inParkinson’s disease. J Geriatr Psychiatry Neurol 34: , 642–658. |
Appendices
Appendix 1. COPPADIS STUDY GROUP.
Adarmes AD, Almeria M, Alonso Losada MG, Alonso Cánovas A, Alonso Frech F, Alonso Redondo R, Álvarez I, Álvarez Sauco M, Aneiros Díaz A, Arnáiz S, Arribas S, Ascunce Vidondo A, Aguilar M, Ávila MA, Bernardo Lambrich N, Bejr-Kasem H, Blázquez Estrada M, Botí M, Borrue C, Buongiorno MT, Cabello González C, Cabo López I, Caballol N, Cámara Lorenzo A, Canfield Medina H, Carrillo F, Carrillo Padilla FJ, Casas E, Catalán MJ, Clavero P, Cortina Fernández A, Cosgaya M, Cots Foraster A, Crespo Cuevas A, Cubo E, de Deus Fonticoba T, de Fábregues-Boixar O, Díez-Fairen M, Dotor García-Soto J, Erro E, Escalante S, Estelrich Peyret E, Fernández Guillán N, Gámez P, Gallego M, García Caldentey J, García Campos C, García Díez C, García Moreno JM, Gastón I, Gómez Garre MP, Gómez Mayordomo V, González Aloy J, González-Aramburu I, González Ardura J, González García B, González Palmás MJ, González Toledo GR, Golpe Díaz A, Grau Solá M, Guardia G, Hernández Vara J, Horta-Barba A, Idoate Calderón D, Infante J, Jesús S, Kulisevsky J, Kurtis M, Labandeira C, Labrador MA, Lacruz F, Lage Castro M, Lastres Gómez S, Legarda I, López Ariztegui N, López Díaz LM, López Domínguez D, López Manzanares L, López Seoane B, Lucas del Pozo S, Macías Y, Mata M, Martí Andres G, Martí MJ, Martínez Castrillo JC, Martinez-Martin P, McAfee D, Meitín MT, Mendoza Plasencia Z, Menéndez González M, Méndez del Barrio C, Mir P, Miranda Santiago J, Morales Casado MI, Moreno Diéguez A, Nogueira V, Novo Amado A, Novo Ponte S, Ordás C, Pagonabarraga J, Pareés I, Pascual-Sedano B, Pastor P, Pérez Fuertes A, Pérez Noguera R, Planas-Ballvé A, Planellas L, Prats MA, Prieto Jurczynska C, Puente V, Pueyo Morlans M, Puig Daví A, Redondo Rafales N, Rodríguez Méndez L, Rodríguez Pérez AB, Roldán F, Ruíz De Arcos M, Ruíz Martínez J, Sánchez Alonso P, Sánchez-Carpintero M, Sánchez Díez G, Sánchez Rodríguez A, Santacruz P, Santos García D, Segundo Rodríguez JC, Seijo M, Sierra Peña M, Solano Vila B, Suárez Castro E, Tartari JP, Valero C, Vargas L, Vela L, Villanueva C, Vives B
| Name (Last Name, First Name) | Location | Role | Contribution |
| Astrid Adarmes, Daniela | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Almeria, Marta | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Alonso Losada, Maria Gema | Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo (CHUVI), Vigo, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Alonso Cánovas, Araceli | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Alonso Frech, Fernando | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Alonso Redondo, Ruben | Hospital Universitario Lucus Augusti (HULA), Lugo, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Aneiros Díaz, Ángel | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Álvarez, Ignacio | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Álvarez Sauco, María | Hospital General Universitario de Elche, Elche, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Arnáiz, Sandra | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Arribas, Sonia | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Ascunce Vidondo, Arancha | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Aguilar, Miquel | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Ávila Rivera, Maria Asunción | Consorci Sanitari Integral, Hospital General de L'Hospitalet, L'Hospitalet de Llobregat, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Bernardo Lambrich, Noemí | Hospital de Tortosa Verge de la Cinta (HTVC), Tortosa, Tarragona, Spain | Site investigator | Evaluation of participants and/or data management |
| Bejr-Kasem, Helena | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Blázquez Estrada, Marta | Hospital Universitario Central de Asturias, Oviedo, Spain | Site investigator | Evaluation of participants and/or data management |
| Botí González, Maria Ángeles | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Borrué, Carmen | Hospital Infanta Sofía, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Buongiorno, Maria Teresa | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Nurse study coordinator |
| Cabello González, Carolina | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Scheduling of evaluations |
| Cabo López, Iria | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Caballol, Nuria | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain. | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Cámara Lorenzo, Ana | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Nurse study coordinator |
| Canfield Medina, Héctor | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Evaluation of participants and/or data management |
| Carrillo, Fátima | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Carrillo Padilla, Francisco José | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Casas, Elena | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Catalán, Maria José | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Clavero, Pedro | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cortina Fernández, A | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Coordination of blood extractions |
| Cosgaya, Marina | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cots Foraster, Anna | Institut d’Assistència Sanità ria (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Crespo Cuevas, Ane | Hospital del Mar, Barcelona, Spain. | Site investigator | Evaluation of participants and/or data management |
| Cubo, Esther | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| De Deus Fonticoba, Teresa | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Nurse study coordinator Evaluation of participants and/or data management |
| De Fábregues-Boixar, Oriol | Hospital Universitario Vall d'Hebron, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Díez Fairen, M | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Dotor García-Soto, Julio | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator / PI | Evaluation of participants and/or data management |
| Erro, Elena | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Escalante, Sonia | Hospital de Tortosa Verge de la Cinta (HTVC), Tortosa, Tarragona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Estelrich Peyret, Elena | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Fernández Guillán, Noelia | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Gámez, Pedro | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Gallego, Mercedes | Hospital La Princesa, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| García Caldentey, Juan | Centro Neurológico Oms 42, Palma de Mallorca, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| García Campos, Cristina | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| García Díez, Cristina | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator (from MAY/22) | neuropsychologist; evaluation of participants |
| García Moreno, Jose Manuel | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator / PI (until MAR/21) | Coordination at the center Evaluation of participants and/or data management |
| Gastón, Itziar | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Gómez Garre, María del Pilar | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Genetic studies coordination |
| Gómez Mayordomo, Víctor | Hospital Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| González Aloy, Javier | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| González Aramburu, Isabel | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| González Ardura, Jessica | Hospital Universitario Lucus Augusti (HULA), Lugo, Spain | Site investigator / PI (until FEB/21) | Evaluation of participants and/or data management |
| González García, Beatriz | Hospital La Princesa, Madrid, Spain | Site investigator | Nurse study coordinator |
| González Palmás, Maria Josefa | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Evaluation of participants and/or data management |
| González Toledo, Gabriel Ricardo | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Golpe Díaz, Ana | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Laboratory analysis coordination |
| Grau Solá, Mireia | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Guardia, Gemma | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Hernández Vara, Jorge | Hospital Universitario Vall d'Hebron, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Horta Barba, Andrea | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Idoate Calderón, Daniel | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigaor (until MAY/22) | neuropsychologist; evaluation of participants |
| Infante, Jon | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Jesús, Silvia | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Kulisevsky, Jaime | Hospital de Sant Pau, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Kurtis, Mónica | Hospital Ruber Internacional, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Labandeira, Carmen | Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo (CHUVI), Vigo, Spain | Site investigator | Evaluation of participants and/or data management |
| Labrador Espinosa, Miguel Ángel | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Neuroimaging data analysis |
| Lacruz, Francisco | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Lage Castro, Melva | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Evaluation of participants and/or data management |
| Lastres Gómez, Sonia | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Legarda, Inés | Hospital Universitario Son Espases, Palma de Mallorca, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| López Ariztegui, Nuria | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator / PI | Evaluation of participants and/or data management |
| López Díaz, Luis Manuel | Hospital Da Costa de Burela, Lugo, Spain | Site investigator | Evaluation of participants and/or data management |
| López Domínguez, Daniel | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| López Manzanares, Lydia | Hospital La Princesa, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| López Seoane, Balbino | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Lucas del Pozo, Sara | Hospital Universitario Vall d'Hebron, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Macías, Yolanda | Fundación Hospital de Alcorcón, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Mata, Marina | Hospital Infanta Sofía, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Martí Andres, Gloria | Hospital Universitario Vall d'Hebron, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Martí, Maria José | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Martínez Castrillo, Juan Carlos | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator /PI | Coordination at the center Evaluation of participants and/or data management |
| Martinez-Martin, Pablo | Centro Nacional de Epidemiología y CIBERNED, Instituto de Salud Carlos III. Madrid | Collaborator in statistical and methods analysis | Methods and statistical reviewer |
| McAfee, Darrian | University of Pennsylvania, Philadelphia | Collaborator in english style | English style reviewer |
| Meitín, Maria Teresa | Hospital Da Costa de Burela, Lugo, Spain | Site investigator | Evaluation of participants and/or data management |
| Menéndez González, Manuel | Hospital Universitario Central de Asturias, Oviedo, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Méndez del Barrio, Carlota | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Mendoza Plasencia, Zebenzui | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Mir, Pablo | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Miranda Santiago, Javier | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Morales Casado, Maria Isabel | Complejo Hospitalario de Toledo, Toledo, Spain. | Site investigator | Evaluation of participants and/or data management |
| Moreno Diéguez, Antonio | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Nogueira, Víctor | Hospital Da Costa de Burela, Lugo, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Novo Amado, Alba | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Novo Ponte, Sabela | Hospital Universitario Puerta de Hierro, Madrid, Spain. | Site investigator | Evaluation of participants and/or data management |
| Ordás, Carlos | Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Spain. | Site Investigator | Evaluation of participants and/or data management |
| Pagonabarraga, Javier | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Pareés, Isabel | Hospital Ruber Internacional, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Pascual-Sedano, Berta | Hospital de Sant Pau, Barcelona, Spain | Site Investigator | Evaluation of participants and/or data management |
| Pastor, Pau | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Pérez Fuertes, Aída | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Blood analysis |
| Pérez Noguera, Rafael | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Planas-Ballvé, Ana | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Planellas, Lluís | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator (until DEC/19) | Evaluation of participants and/or data management |
| Prats, Marian Ángeles | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Prieto Jurczynska, Cristina | Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Puente, Víctor | Hospital del Mar, Barcelona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Pueyo Morlans, Mercedes | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Puig Daví, Arnau | Hospital de Sant Pau, Barcelona, Spain | Site einvestigator | Evaluation of participants and/or data management |
| Redondo, Nuria | Hospital La Princesa, Madrid, Spain | Site Investigator | Evaluation of participants and/or data management |
| Rodríguez Méndez, Luisa | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Blood analysis |
| Rodríguez Pérez, Amparo Belén | Hospital General Universitario de Elche, Elche, Spain | Site investigator | Evaluation of participants and/or data management |
| Roldán, Florinda | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Neuroimaging studies |
| Ruíz de Arcos, María | Hospital Universitario Virgen Macarena, Sevilla, Spain. | Site investigator | Evaluation of participants and/or data management |
| Ruíz Martínez, Javier | Hospital Universitario Donostia, San Sebastián, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez Alonso, Pilar | Hospital Universitario Puerta de Hierro, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez-Carpintero, Macarena | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Sánchez Díez, Gema | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez Rodríguez, Antonio | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| Santacruz, Pilar | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Santos García, Diego | CHUAC, Complejo Hospitalario Universitario de A Coruña | Coordinator of the Project | Coordination of the COPPADIS-2015 |
| Segundo Rodríguez, José Clemente | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator | Evaluation of participants and/or data management |
| Seijo, Manuel | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Sierra, María | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| Solano, Berta | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Suárez Castro, Ester | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Evaluation of participants and/or data management |
| Tartari, Juan Pablo | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Valero, Caridad | Hospital Arnau de Vilanova, Valencia, Spain | Site investigator | Evaluation of participants and/or data management |
| Vargas, Laura | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Vela, Lydia | Fundación Hospital de Alcorcón, Madrid, Spain | Site investigator / PI | Coordination at the center Evaluation of participants and/or data management |
| Villanueva, Clara | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Vives, Bárbara | Hospital Universitario Son Espases, Palma de Mallorca, Spain | Site investigator | Evaluation of participants and/or data management |




