Changes in Principal Caregiver Mood Affects the Mood of the Parkinson’s Disease Patient: The Vicious Cycle of Illness
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disorder causing motor and non-motor symptoms (NMS) that result in loss of patient autonomy for activities of daily-living (ADL) and quality of life (QoL) [1]. Given that symptoms progression with longer disease duration leads to a loss of independence, the majority of patients have a principal caregiver responsible for care throughout the course of the disease. However, PD symptoms impact not only the patient but on the principal caregiver too and can cause stress, burden, depression, and a worse QoL [2]. Persistent caregiver burden may lead to strain, an enduring change in the caregiver’s sense of well-being that predisposes to burnout [3]. Symptoms associated with caregiver burden have been identified in different studies, such as cognitive impairment, apathy, irritability, sleep disorders, falls, disability, and more advanced stage disease, among others [2–15]. Still, there is no knowledge about how the status of the caregiver impacts on the patient. This is an important association because an overworked caregiver might take worse care of the patient or have behavior changes (e.g., depression, irritability, etc.) that could negatively influence the patient directly, establishing a vicious cycle; the worse the patient’s condition, the worse the caregiver’s condition, and vice versa. In this context, it would be especially useful to analyze how the longitudinally changes experienced by the caregiver can impact on the PD patient.
Recently, we published the largest (N = 192) and longest (2-year follow-up) prospective study in which predictors of a change in burden, strain, mood, and QoL in the principal caregiver of PD patients were identified [16]. Mood changes in the patient were the main factors affecting mood in the caregiver and mood changes in the caregiver was identified as the main factor impacting on strain, burden and QoL of the caregiver. We hypothesized that changes in the status of the caregiver might influence the patient (Fig. 1). Under this approach, the aim of the present study was to analyze if a change in mood (BDI-II [Beck Depression Inventory-II]), burden (ZCBI [Zarit Caregiver Burden Inventory]), strain (CSI [Caregiver Strain Index]), and/or global QoL (EUROHIS-QOL8 [EUROHIS-QOL 8-item index]) of the principal caregiver of a patient with PD after a 2-year follow-up was associated with changes in the patient’s mood (BDI-II), autonomy for ADL (ADLS [Schwab & England Activities of Daily Living Scale]), health-related QoL (PDQ-39 [39-item Parkinson’s disease Questionnaire], and global QoL (EUROHIS-QOL8), independently to other PD related factors. In other words, the goal was to determine if a worse status of the caregiver impacts on the patient. More importantly, this would justify the necessity to identify and treat overworked caregivers as soon as possible, as has been previously suggested [3].
Fig. 1
Hypothesis proposed in this study. Regarding previous data published from the COPPADIS cohort [16], patient’s mood is the main factor influencing caregiver’s mood and caregiver’s mood the main factor influencing caregiver’s burden, strain and QoL. The question (question mark) is if the change in the long-term of these caregiver’s variables can impact over the change in patient’s variables (mood, QoL and autonomy for ADL). ADL, activities of daily living; ADLS, Schwab & England Activities of Daily Living Scale; CSI, Caregiver Strain Index; EUROHIS-QOL8, EUROHIS-QOL 8-item index; PD, Parkinson’s disease; PDQ-39, 39-item Parkinson’s disease Questionnaire; QoL, quality of life; ZCBI, Zarit Caregiver Burden Inventory.
![Hypothesis proposed in this study. Regarding previous data published from the COPPADIS cohort [16], patient’s mood is the main factor influencing caregiver’s mood and caregiver’s mood the main factor influencing caregiver’s burden, strain and QoL. The question (question mark) is if the change in the long-term of these caregiver’s variables can impact over the change in patient’s variables (mood, QoL and autonomy for ADL). ADL, activities of daily living; ADLS, Schwab & England Activities of Daily Living Scale; CSI, Caregiver Strain Index; EUROHIS-QOL8, EUROHIS-QOL 8-item index; PD, Parkinson’s disease; PDQ-39, 39-item Parkinson’s disease Questionnaire; QoL, quality of life; ZCBI, Zarit Caregiver Burden Inventory.](https://content.iospress.com:443/media/jpd/2023/13-2/jpd-13-2-jpd225014/jpd-13-jpd225014-g001.jpg)
MATERIALS AND METHODS
PD patients and their caregivers, who were recruited from 35 centers in Spain from the COPPADIS cohort [17] from January 2016 to November 2017 and evaluated again at 2-year follow-up, were included in the study. Methodology about COPPADIS-2015 study can be consulted at https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-016-0548-9 [83-016-0548-9] [18]. This is a multi-center, observational, longitudinal-prospective, 5-year follow-up study designed to analyze disease progression in a Spanish population of PD patients. All the patients included were diagnosed according to UK PD Brain Bank criteria [19]. The principal caregiver [20] of the patient was included if the patient had a caregiver who voluntarily agreed to participate and sign an informed consent. Patients had to have retained the same primary caregiver at both time points to be included in this analysis.
PD patient assessment
In PD subjects, information on sociodemographic aspects, factors related to PD, comorbidity, and treatment was collected at baseline (visit V0) and at 2 years±1 month (visit V2). V0 and V2 evaluations included motor assessment (Hoenh & Yahr [H&Y], Unified Parkinson’s Disease Rating Scale [UPDRS] part III and part IV, Freezing of Gait Questionnaire [FOGQ]), NMS (Non-Motor Symptoms Scale [NMSS], Parkinson’s Disease Sleep Scale [PDSS], Visual Analog Scale-Pain [VAS-Pain], Visual Analog Fatigue Scale [VAFS]), cognition (PD-CRS), mood and neuropsychiatric symptoms (BDI-II, Neuropsychiatric Inventory [NPI], Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale [QUIP-RS]), disability (ADLS), and health-related (PDQ-39) and global QoL (EUROHIS-QOL8) [18]. In all the scales/questionnaires a higher score indicates a more severe affectation apart from PD-CRS, PDSS, ADLS, and EUROHIS-QOL8, which were the opposite. In patients with motor fluctuations, the motor assessment was made during the OFF state (without medication in the last 12 hours) and during the ON state. The assessment was only performed without medication in patients without motor fluctuations.
Caregiver assessment
In caregivers, sociodemographic data were collected at baseline [16]. Four aspects were analyzed in the caregiver at V0 and at V2: mood (BDI-II); burden (ZCBI); strain (CSI); and global QoL (EUROHIS-QOL8). ZCBI [21] contains 22 items that rate the impact of the disease on the caregiver’s physical, emotional, and socioeconomic status. Responses are scored on a scale from 0 (never) to 4 (nearly always). The maximum total score, indicative of the highest burden, is 88. CSI [22] is a 13-item questionnaire designed to assess the level of stress experienced by caregivers. There are two possible responses for each item: “yes” or “no”. The total score is the result of adding all positive responses (from 0, no stress, to 13, maximum level of stress). Mood was assessed with the BDI-II [23]. This is a self-administered, 21 item instrument. It has been designed to assess the severity of depression symptoms in adults and adolescents with a minimum age of 13 years. The evaluated subject must choose one of four alternatives (ordered from lesser to greater severity), in each item, that best describes his/her status over the previous two weeks. The score ranges from 0 (minimum) to 63 (maximum). Higher scores will reflect, a priori, a worse mood. Finally, global QoL was measured with the EUROHIS-QOL8 [24]. This is an 8-item QoL questionnaire (QoL, health status, energy, autonomy in activities of daily living [ADL], self-esteem, social relationships, economic capacity, and habitat) derived from the WHOQOL-100 and the WHOQOL-BREF. For each item, the score ranges from 0 (not at all) to 5 (completely). The total score is expressed as the mean of the individual scores. A higher score indicates a higher QoL.
Data analysis
Data were processed using SPSS 20.0 for Windows. Only PD patients and their caregivers (the same caregiver after the 2-year follow-up) from the COPPADIS cohort with data of the BDI-II, ZCBI, CSI, and EUROHIS-QOL8 collected at both visits, V0 and V2, were included in the analysis [16].
With the aim to know the influence of the change from V0 to V2 of caregiver’s variables over the change in mood, QoL, and autonomy for ADL in the PD patient, linear regression models were conducted. The change in each variable from the patient and the caregiver was calculated as the difference between the value at V2 and at V0 (i.e., Δ BDI-II = BDI-IIV2 –BDI-IIV0). In all the models, the four caregiver’s variables were included (ΔBDI-II; ΔZCBI; ΔCSI; ΔEUROHIS-QOL8). Four models were defined as having an aspect of the patient to analyze as a dependent variable: 1) Model 1, change in mood (ΔBDI-II); 2) Model 2, change in health-related QoL (ΔPDQ-39); 3) Model 3, change in global QoL (ΔEUROHIS-QOL8); 4) Model 4, change in autonomy for ADL (ΔADLS). Covariates from the patient included in the models were the change from V0 to V2 (Δ) in LEDD [25], UPDRS-III-OFF, UPDRS-IV, FOGQ, PD-CRS, NMSS, BDI-II (except in Model 1 for being the dependent variable), PDSS, QUIP-RS, NPI, VAS-PAIN, VAFS, ADLS (except in Model 4 for being the dependent variable), PDQ-39 (except in Model 2 for being the dependent variable), and EUROHIS-QOL8 (except in Model 3 for being the dependent variable). Each model was adjusted to the value of the dependent variable at baseline too. Tolerance and variance inflation factor (VIF) were used to detect multicollinearity. Multicollinearity was considered problematic when tolerance was less than 0.2 and, simultaneously, the value of VIF was 10 and above. Spearman’s or Pearson’s correlation coefficient were also used as appropriate (distribution for variables was verified by a one-sample Kolmogorov-Smirnov test). Correlations were considered weak for coefficient values ≤0.29, moderate for values between 0.30 and 0.59, and strong for values ≥0.60. The p-value was considered significant (highly significant) when it was <0.001.
Standard protocol approvals, registrations, and patient consents
For this study, we received approval from the Comité de Ética de la Investigación Clínica de Galicia from Spain (2014/534; 02/DEC/2014). Written informed consents from all participants in this study were obtained. COPPADIS-2015 was classified by the AEMPS (Agencia Española del Medicamento y Productos Sanitarios) as a Post-authorization Prospective Follow-up study with the code COH-PAK-2014-01.
Data availability
The protocol and the statistical analysis plan are available on request. Deidentified participant data are not available for legal and ethical reasons.
RESULTS
The study included one hundred and ninety-two PD patients (63.96±8.74 years old; 63% males) and their principal caregiver. The mean age of the caregivers was 58.82±11.71 years old, and 69.3% were females. Clinical and sociodemographic details of patients and caregivers have been recently published [16] and are shown in Supplementary Table 1.
A significant moderate correlation was observed between the four caregiver’s variables (p < 0.0001 in all analyses): ΔBDI-II and ΔZCBI, r = 0.42; ΔBDI-II and ΔCSI, r = 0.39; ΔBDI-II and ΔEUROHIS-QOL8, r = –0.35; ΔZCBI and ΔCSI, r = 0.55; ΔZCBI and ΔEUROHIS-QOL8, r = –0.34; ΔCSI and ΔEUROHIS-QOL8, r = –0.31. Regarding PD-related variables, the strongest correlation was observed for the change from V0 to V2 in mood (ΔBDI-II) in the patient and the caregiver (r = 0.39; p < 0.0001) and in the global QoL (ΔEUROSHIS-QOL8) in the patient and the caregiver (r = 0.39; p < 0.0001) (Table 1).
Table 1
Correlation between the change in the stage of the principal caregiver (mood, burden, strain and QoL) and the change in the stage of the patient (mood, autonomy for ADL, and QoL) from baseline visit (V0) to 2-year follow-up visit (V2)
| Δ V2 –V0 | BDI-IIc | ZCBIc | CSIc | EUROSHIS-QOL8c |
| BDI-IIc | N. A | 0.42 (p < 0.0001) | 0.39 (p < 0.0001) | –0.35 (p < 0.0001) |
| ZCBIc | 0.42 (p < 0.0001) | N. A. | 0.55 (p < 0.0001) | –0.34 (p < 0.0001) |
| CSIc | 0.39 (p < 0.0001) | 0.55 (p < 0.0001) | N. A. | –0.31 (p < 0.0001) |
| EUROHIS-QOL8c | –0.35 (p < 0.0001) | –0.34 (p < 0.0001) | –0.31 (p < 0.0001) | N. A. |
| BDIp | 0.39 (p < 0.0001) | 0.19 (p = 0.007) | 0.18 (p = 0.010) | –0.23 (p = 0.001) |
| ADLSp | –0.08 (p = 0.271) | –0.18 (p = 0.012) | –0.14 (p = 0.042) | 0.09 (p = 0.184) |
| PDQ-39p | 0.15 (p = 0.037) | 0.13 (p = 0.065) | 0.10 (p = 0.163) | 0.02 (p = 0.740) |
| EUROHIS-QOL8p | –0.08 (p = 0.231) | –0.03 (p = 0.629) | –0.10 (p = 0.156) | 0.39 (p < 0.0001) |
Spearman correlation coefficient was applied (r and p value are shown). ADLS, Schwab & England Activities of Daily Living Scale; BDI-II, Beck Depression Inventory-II; CSI, Caregiver Strain Index; PDQ-39, the ZCBI, Zarit Caregiver Burden Inventory; QoL, quality of life. C in subscript, caregiver (i.e., BDI-IIc, change from V0 to V2 in the BDI-II score, etc.); P in subscript, patient.
The change in the caregiver from V0 to V2 in mood was associated with the change in the patient from V0 to V2 in mood (ΔBDI-II) after the adjustment to covariates (Model 1; R2 = 0.71): β= 0.32; p < 0.0001 (Table 2). The other factor associated with ΔBDI-II in the patient was the change in global QoL in the patient (β= –0.56; p < 0.0001). No caregiver’s variables were associated with the change in the patient from V0 to V2 in his/her health-related QoL (Table 3A), as the change in the patient from V0 to V2 in the NMSS total score the factor significantly associated with ΔPDQ-39 (β= 0.29; p < 0.0001) (Model 2; R2 = 0.51). However, regarding the patient’s change in global QoL, the change in the caregiver from V0 to V2 in the global QoL was identified as an associated factor (β= 0.39; p < 0.0001) together with the change in the own caregiver in mood (β= 0.55; p < 0.0001) (Model 3; R2 = 0.68; Table 3B). Finally, and again, no caregiver’s variables were associated with the change in the patient from V0 to V2 in the autonomy for ADL, being the change in the own patient in the health-related QoL the factor associated with ΔADLS (β= –0.42; p < 0.0001) (Model 4; R2 = 0.33; Table 4). Figure 2 shows the influence of caregiver’s variables (ΔBDI-II; ΔZCBI; ΔCSI; ΔEUROHIS-QOL8) over patient’s variables (ΔBDI-II; ΔPDQ-39; ΔEUROHIS-QOL8; ΔADLS) and the associations between patient’s variables. In all models, tolerance was less than 0.2 for all variables included.
Table 2
Effect of changes in the caregiver over the change in mood in PD patients from the COPPADIS cohort after 2-year follow-up (N = 192)
| Univariate analysis | Multivariate analysis | |||||
| β | 95% CI | p | β | 95% CI | p | |
| Caregiver | ||||||
| Δ BDI-II | 0.42 | 0.40 –0.77 | <0.0001 | 0.32 | 0.27 –0.67 | <0.0001 |
| Δ ZCBI | 0.19 | 0.05 –0.28 | 0.006 | 0.10 | –0.02–0.22 | 0.125 |
| Δ CSI | 0.14 | 0.03 –1.33 | 0.039 | –0.03 | –0.89–0.50 | 0.576 |
| Δ EUROHIS-QOL8 | –0.21 | –7.97––1.67 | 0.003 | 0.20 | 1.74–8.13 | 0.003 |
| Patient | ||||||
| Δ EUROHIS-QOL8 | 0.22 | 0.07–0.40 | 0.006 | –0.56 | –9.34––5.95 | <0.0001 |
| BDI-II at baseline | 0.19 | –0.14––0.01 | 0.035 | –0.36 | –0.64––0.32 | <0.0001 |
Dependent variable: change in the PD patient from V0 to V2 (Δ) in the BDI-II total score. β standardized coefficient and 95% IC are shown. a, univariate analysis; b, multivariate analysis (Durbin-Watson test = 2.11; R2 = 0.71). Only significant variables (p < 0.01) from the patient in the multivariate analysis are shown. Covariates from the patient included were the change from V0 to V2 (Δ) in LEDD, UPDRS-III-OFF, UPDRS-IV, FOGQ, PD-CRS, NMSS, PDSS, QUIP-RS, NPI, VAS-PAIN, VAFS, ADLS, PDQ-39SI, EUROHIS-QOL8, and the score on the BDI-II at baseline. ADLS, Schwab & England Activities of Daily Living Scale; BDI-II, Beck Depression Inventory-II; CSI, Caregiver Strain Index; FOGQ, Freezing Of Gait Questionnaire; LEDD, levodopa equivalent daily dose; Non-Motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; PDQ-39, the 39-item Parkinson’s disease Questionnaire Summary Index; PDSS, Parkinson’s Disease Sleep Scale; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale; VAS, Visual Analogue Scale; ZCBI, Zarit Caregiver Burden Inventory.
Table 3
Effect of changes in the caregiver over the change in health-related and global QoL in PD patients from the COPPADIS cohort after 2-year follow-up (N = 192)
| Univariate analysis | Multivariate analysis | |||||
| β | 95% CI | p | β | 95% CI | p | |
| A) Δ PDQ-39SI | ||||||
| Caregiver | ||||||
| Δ BDI-II | 0.19 | 0.10–0.62 | 0.006 | 0.16 | 0.01–0.54 | 0.047 |
| Δ ZCBI | 0.28 | 0.16–0.46 | <0.0001 | 0.03 | –0.13–0.20 | 0.671 |
| Δ CSI | 0.20 | 0.41–2.15 | 0.004 | 0.04 | –0.72–1.20 | 0.818 |
| Δ EUROHIS-QOL8 | 0.01 | –3.89–4.64 | 0.863 | 0.13 | –0.33–7.74 | 0.072 |
| Patient | ||||||
| Δ UPDRS-III | 0.41 | 0.37–0.73 | <0.0001 | 0.20 | 0.06–0.43 | 0.008 |
| Δ NMSS | 0.57 | 0.16–0.25 | <0.0001 | 0.29 | 0.05–0.15 | <0.0001 |
| Δ ADLS | –0.48 | –0.62––0.36 | <0.0001 | –0.25 | –0.39––0.10 | 0.001 |
| PDQ-39 at baseline | –0.20 | –0.32––0.05 | 0.005 | –0.20 | –0.29––0.06 | 0.002 |
| B) EUROSHIS-QOL8 | ||||||
| Caregiver | ||||||
| Δ BDI-II | –0.14 | –0.029––0.001 | 0.039 | 0.24 | 0.01–0.04 | 0.001 |
| Δ ZCBI | –0.04 | –0.011–0.006 | 0.530 | 0.02 | -0.00–0.01 | 0.679 |
| Δ CSI | –0.09 | –0.078–0.016 | 0.201 | –0.03 | –0.06–0.03 | 0.604 |
| Δ EUROHIS-QOL8 | 0.41 | 0.454–0.878 | <0.0001 | 0.39 | 0.49–0.89 | <0.0001 |
| Patient | ||||||
| Δ BDI-II | –0.63 | –0.053––0.037 | <0.0001 | –0.55 | –0.03––0.66 | <0.0001 |
| EUROHIS-QOL8 at baseline | 0.39 | 0.024–0.049 | <0.0001 | –0.37 | –0.65––0.36 | <0.0001 |
Dependent variable: change in the PD patient from V0 to V2 (Δ) in the PDQ-39 (A) and EROHIS-QOL8 (B). β standardized coefficient and 95% IC are shown. a, univariate analysis; b, multivariate analysis: A) Durbin-Watson test = 2.07; R2 = 0.51; B) Durbin-Watson test = 2.02; R2 = 0.68. Only significant variables (p < 0.01) from the patient in the multivariate analysis are shown. Covariates from the patient included were the change from V0 to V2 (Δ) in LEDD, UPDRS-III-OFF, UPDRS-IV, FOGQ, PD-CRS, NMSS, PDSS, QUIP-RS, NPI, VAS-PAIN, VAFS, ADLS, and the score on the PDQ-39SI (A) and EUROHIS-QOL8 (B) at baseline. ADLS, Schwab & England Activities of Daily Living Scale; BDI-II, Beck Depression Inventory-II; CSI, Caregiver Strain Index; FOGQ, Freezing Of Gait Questionnaire; LEDD, levodopa equivalent daily dose; Non-Motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; PDQ-39, the 39-item Parkinson’s disease Questionnaire Summary Index; PDSS, Parkinson’s Disease Sleep Scale; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale; VAS, Visual Analogue Scale; ZCBI, Zarit Caregiver Burden Inventory.
Table 4
Effect of changes in the caregiver over the change in autonomy for ADL in PD patients from the COPPADIS cohort after 2-year follow-up (N = 192)
| Univariate analysis | Multivariate analysis | |||||
| β | 95% CI | p | β | 95% CI | p | |
| Caregiver | ||||||
| Δ BDI-II | –0.05 | –0.35–0.16 | 0.465 | 0.25 | 0.07–0.81 | 0.018 |
| Δ ZCBI | -0.23 | -0.40––0.10 | 0.001 | –0.14 | –0.38–0.04 | 0.112 |
| Δ CSI | –0.18 | –1.99––0.28 | 0.009 | –0.05 | –1.55–0.79 | 0.526 |
| Δ EUROHIS-QOL8 | 0.05 | –2.60–5.72 | 0.461 | 0.06 | –3.62–7.61 | 0.484 |
| Patient | ||||||
| Δ FOGQ | –0.38 | –1.55––0.75 | <0.0001 | –0.25 | –1.28––0.33 | 0.001 |
| Δ PDQ39SI | –0.48 | –0.59––0.34 | <0.0001 | –0.42 | –0.62––0.24 | <0.0001 |
Dependent variable: change in the PD patient from V0 to V2 (Δ) in the ADLS score. β standardized coefficient and 95% IC are shown. a, univariate analysis; b, multivariate analysis (Durbin-Watson test = 1.956; R2 = 0.33). Only significant variables (p < 0.01) from the patient in the multivariate analysis are shown. Covariates from the patient included were the change from V0 to V2 (Δ) in LEDD, UPDRS-III-OFF, UPDRS-IV, FOGQ, PD-CRS, NMSS, PDSS, QUIP-RS, NPI, VAS-PAIN, VAFS, ADLS, PDQ-39SI, EUROHIS-QOL8, and the score on the ADLS at baseline. ADLS, Schwab & England Activities of Daily Living Scale; BDI-II, Beck Depression Inventory-II; CSI, Caregiver Strain Index; FOGQ, Freezing Of Gait Questionnaire; LEDD, levodopa equivalent daily dose; Non-Motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; PDQ-39, the 39-item Parkinson’s disease Questionnaire Summary Index; PDSS, Parkinson’s Disease Sleep Scale; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale; VAS, Visual Analogue Scale; ZCBI, Zarit Caregiver Burden Inventory.
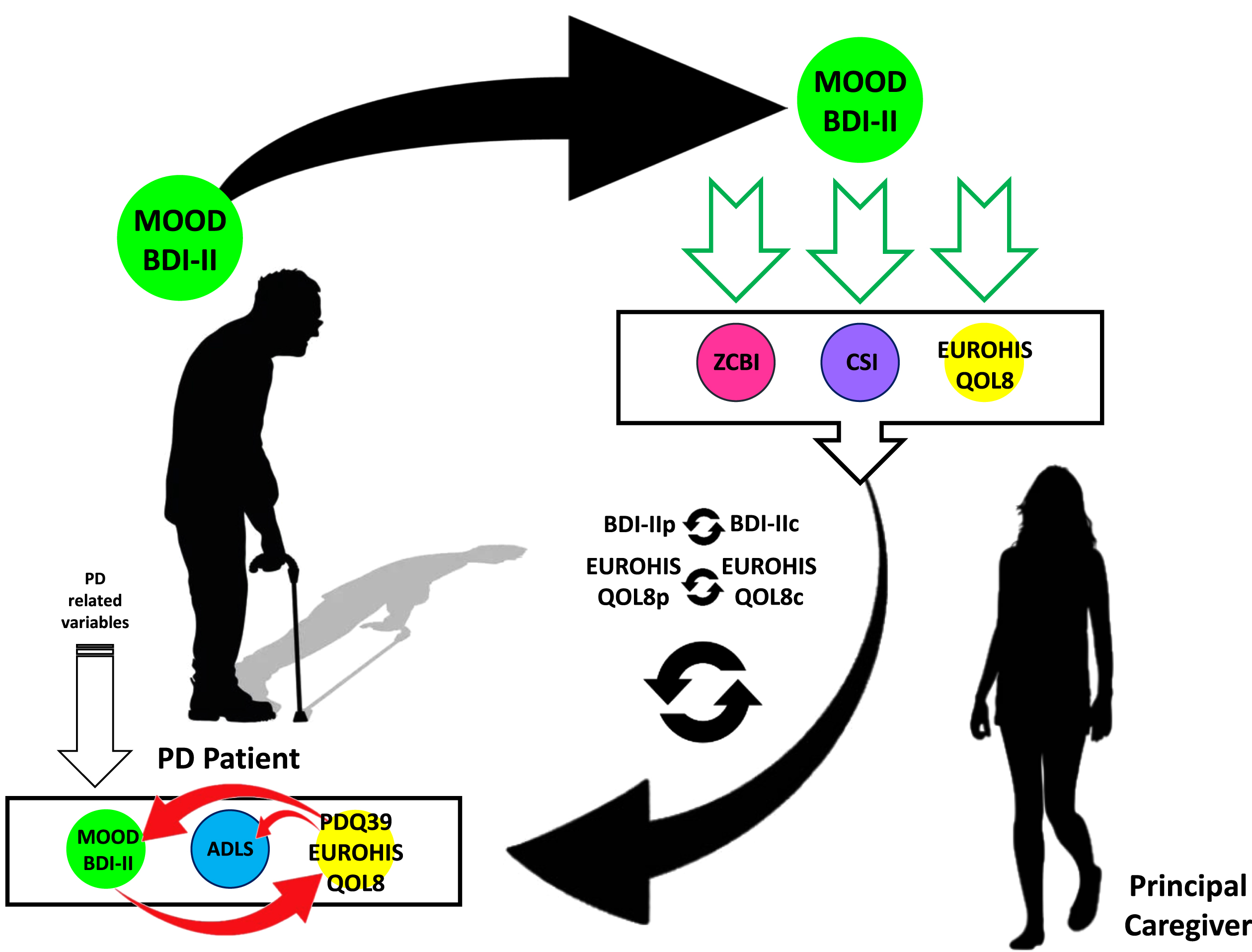
Fig. 2
The associations between the change from baseline visit (V0) to 2-year follow-up visit (V2) in caregiver’s and patient’s variables are shown. The change in caregiver’s mood influences the change in patient’s mood (ΔBDI-II; in bright green) whereas the change in caregiver’s global QoL is associated to the change in patient’s global QoL (ΔEUROHIS-QOL8; in bright yellow). Moreover, the change in patient’s mood influences the change in patient’s global QoL whereas the change in patient’s global QoL and health-related QoL is associated with the change in patient’s mood and autonomy for ADL, respectively (red arrows). ADL, activities of daily living; ADLS, Schwab & England Activities of Daily Living Scale; CSI, Caregiver Strain Index; EUROHIS-QOL8, EUROHIS-QOL 8-item index; PD, Parkinson’s disease; PDQ-39, 39-item Parkinson’s disease Questionnaire; QoL, quality of life; ZCBI, Zarit Caregiver Burden Inventory.
DISCUSSION
Unlike previously published studies [2–15] that analyze which factors of PD influence the status of the principal caregiver, the present study analyzes whether the progressive changes in the status of the caregiver have repercussions on the status of the patient. We found that the change in the caregiver’s mood predicted the change in the patient’s mood independently of other variables of the disease influencing the patient’s mood. We also found an association between the change in the global QoL in both the patient and the caregiver. This finding is novel and agrees with the idea of the vicious cycle of illness. Depressive symptoms in the patient impact the caregiver’s mood, and depressive symptoms in the caregiver have a negative impact on the patient’s mood as well. This could justify the necessity of early identification and proper management of depression and burden in the principal caregiver of a PD patient [2, 26].
Caregiving may have rewarding consequences, such as strengthening emotional ties, improving self-esteem, generating altruism, and making financial savings [27]. However, caring for ill family members, especially with a chronic degenerative disease in the long-term, can have negative impacts on caregivers’ mental health [28]. Caregiving burden, in terms of physical strain, has been found to predict caregivers’ health [29]. On the other hand, mental health, in terms of depression, could predict burden [30]. In fact, depression is one of the most common negative effects of caregiving [31], being major depression detected in this cohort in 13% and 15.1% of the caregivers at baseline and after the 2-year follow-up, respectively [16]. In this context, an important question arises: does the worsening of the caregiver’s condition worsen the care of the patient and secondarily perpetuate the problem since both factors feed off each other? Surprisingly, in PD and other pathologies including cancer, the literature focuses on identifying the causes of caregiver overload and the consequences on the caregiver but not on the patient [32, 33]. Even though there is literature about therapies aimed to treat caregiver burden, again the benefits for the caregiver are analyzed but not the positive consequences that they could have on the patient [2, 34, 35]. This is important because one of the consequences of caregiver burden is a reduction in care provision and in the quality of care provided [36]. A study by Given et al. [37] claims that the quality of care is reduced when a caregiver experiences burden, and it may be manifested due to a decreased coping ability and lack of emotional support for the care-recipient. Our study analyzes for the first time how short-term deterioration in the status of the caregiver can negatively influence the patient. It also detects that the worsening of the caregiver’s mood is a key factor that impacts on the patient’s mood after adjusting for the changes experienced in many other variables of the patient’s disease. Importantly, the model provided about 70% of the variance of the principal variable (patient’s mood change). This is a critical point given that the change in the patient’s mood is the most influential factor in the caregiver’s mood, and this, at the same time, generates overload, stress, and a worse QoL in the caregiver him/herself [16], which is associated with a worse QoL of the patient. However, the status of the caregiver did not influence the patient’s health-related QoL, which is more conditioned (PDQ-39) by the symptoms of the disease [38, 39], especially NMS as it has been found in the model. We also failed to demonstrate the impact of caregiver status on patient autonomy. Our findings are novel, and the next step should be to demonstrate if treating caregiver burden and depression can improve not only the status of the caregiver but also the patient indirectly as well. Different strategies could be tested, such as education and psychotherapy [40], rehabilitation [41], or multidisciplinary interventions [42]. Again, treatment of patient’s symptoms to improve the caregiver’s status has been analyzed [43] but the opposite has not.
Our study has some limitations, some of them previously reported in a recent publication [16], such as a loss to follow-up of nearly 30% of the subjects (patient and his/her caregiver) with respect to the baseline sample and the fact that caregiver’s treatment or other possible interventions were not collected. In the models, the relationship between some variables changed the sign after adjusting for the covariates, such as the relation between ΔBDI-II in the patient (dependent variable) and ΔEUROHIS-QOL8 in the caregiver in Model 1 (from negative to positive) and ΔEUROHIS-QOL8 in the patient (dependent variable) and ΔBDI-II in the caregiver in Model 3 (from negative to positive), contrary to expectation. This could be explained by the effect of including many covariates and the influence of altogether over the dependent variable. However, in all models the R2 was high, collinearity was excluded, and only results with very high significance (p < 0.001) were considered valid.
In conclusion, this is the first time that the change in the caregiver’s status demonstrated an influence on the change in patient’s status. So, depressive symptoms in the patient affect the caregiver but also vice versa. Moreover, the change in the caregiver’s global QoL seems to predict the change in the patient’s global QoL. With the aim to stop the vicious circle of illness in PD, detection of depression and burden in the principal caregiver of the patient is important and should be acted on as earlier as possible. In addition, more studies to replicate these findings and test this hypothesis are needed.
ACKNOWLEDGMENTS
We would like to thank all patients and their caregivers who collaborated in this study. Many thanks also to Fundación Española de Ayuda a la Investigación en Enfermedades Neurodegenerativas y/o de Origen Genético (https://fundaciondegen.org/) and Alpha Bioresearch (https://www.alphabioresearch.com) and other institutions helping us.
FUNDING
COPPADIS and the present study were developed with the help of Fundación Española de Ayuda a la Investigación en Enfermedades Neurodegenerativas y/o de Origen Genético (https://fundaciondegen.org/) and Alpha Bioresearch (www.alphabioresearch.com). Also, we received grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Concesión de subvenciones de Proyectos de Investigación en Salud de la convocatoria 2020 de la Acción Estratégica en Salud 2017-2020 por el Proyecto “PROGRESIÓN NO MOTORA E IMPACTO EN LA CALIDAD DE VIDA EN LA ENFERMEDAD DE PARKINSON”) to develop a part of the COPPADIS project.
CONFLICT OF INTEREST
Santos García D. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, Teva, Archímedes, Esteve, Stada, Merz, and grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Concesión de subvenciones de Proyectos de Investigación en Salud de la convocatoria 2020 de la Acción Estratégica en Salud 2017-2020 por el proyecto “PROGRESIÓN NO MOTORA E IMPACTO EN LA CALIDAD DE VIDA EN LA ENFERMEDAD DE PARKINSON”).
De Deus Fonticoba T.: None.
Cores Bartolomé C. has received honoraria for educational presentations and advice service by Lundbeck and UCB Pharma.
Feal Painceiras M. J.: None.
Íñiguez Alvarado MC: None.
García Díaz I.: None.
Jesús S. has received honoraria from AbbVie, Bial, Merz, UCB, and Zambon and holds the competitive contract “Juan Rodés” supported by the Instituto de Salud Carlos III. She has received grants from the Spanish Ministry of Economy and Competitiveness (PI18/01898) and the Consejería de Salud de la Junta de Andalucía (PI-0459-2018).
Buongiorno M. T.: None.
Planellas LL.: None.
Cosgaya M.: None.
García Caldentey J. has received honoraria for educational presentations and advice service by Qualigen, Nutricia, Abbvie, Italfarmaco, UCB Pharma, Lundbeck, Zambon, Bial, and Teva.
Caballol N. has received honoraria from Bial, Italfármaco, Qualigen, Zambon, UCB, Teva and KRKA and sponsorship from Zambon, TEVA and Abbvie for attending medical conferences.
Legarda I. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva.
Hernández Vara J. has received travel bursaries and educational grants from Abbvie and has received honoraria for educational presentations from Abbvie, Teva, Bial, Zambon, Italfarmaco, and Sanofi-Genzyme.
Cabo I. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
López Manzanares L.: Compensated advisory services, consulting, research grant support, or speaker honoraria: AbbVie, Acorda, Bial, Intec Pharma, Italfarmaco, Pfizer, Roche, Teva, UCB, and Zambon.
González Aramburu I.: None.
Ávila Rivera MA. has received honoraria from Zambon, UCB Pharma, Qualigen, Bial, and Teva, and sponsorship from Zambon and Teva for attending conferences.
Gómez Mayordomo V.: None.
Nogueira V.: None.
Puente V. has served as consultant for Abbvie and Zambon; has received grant/research from Abbvie.
Dotor García-Soto J.: Compensated advisory services, consulting, research grant support, or speaker honoraria: Merck, Sanofi-Genzyme, Allergan, Biogen, Roche, UCB and Novartis.
Borrué C.: None.
Solano Vila B. has received honoraria for educational presentations and advice service by UCB, Zambon, Teva, Abbvie, Bial.
Álvarez Sauco M. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva.
Vela L. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva.
Escalante S. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
Cubo E.: Travel grants: Abbvie, Allergan, Boston; Lecturing honoraria: Abbvie, International Parkinson’s disease Movement Disorder Society.
Carrillo Padilla F. has received honoraria from Zambon (SEN Congress assistance).
Martínez Castrillo JC. has received research support from Lundbeck, Italfarmaco, Allergan, Zambon, Merz, and Abbvie. He has received speaking honoraria from AbbVie, Bial, Italfarmaco, Lundbeck, Krka, TEVA, UCB, Zambon, Allergan, Ipsen, and Merz.
Sánchez Alonso P. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva.
Alonso Losada M. G. has received honoraria for educational presentations and advice service by Zambon and Bial.
López Ariztegui N. has received honoraria for educational presentations and advice service by Abbvie, Italfarmaco, Zambon, and Bial.
Gastón I. has received research support from Abbvie and Zambon and has served as a consultant for Abbvie, Exelts, and Zambon.
Kulisevsky J.: (1) Consulting fees: Roche, Zambon; (2) Stock / allotment: No; (3) Patent royalties / licensing fees: No; (4) Honoraria (e.g. lecture fees): Zambon, Teva, Bial, UCB; (5) Fees for promotional materials: No; (6) Research funding: Roche, Zambon, Ciberned; Instituto de SaludCarlos III; FundacióLa Maratóde TV3; (7) Scholarship from corporation: No; (8) Corporate laboratory funding: No; (9) Others (e.g. trips, travel, or gifts): No.
Blázquez Estrada M. has received honoraria for educational presentations and advice service by Abbvie, Abbott, UCB Pharma, Allergan, Zambon, Bial, and Qualigen.
Seijo M. has received honoraria for educational services from KRKA, UCB, Zambon, Bial; travel grants from Daiichi and Roche.
Ruiz Martínez J. has received honoraria for educational presentations, attending medical conferences, and advice service by Abbvie, UCB Pharma, Zambon, Italfarmaco, Bial, and Teva.
Valero C. has received honoraria for educational services from Zambon, Abbvie and UCB.
Kurtis M. has received honoraria from Bial, the Spanish Neurology Society, and the International and Movement Disorders Society.
González Ardura J. has recieved honoraria for speking from italofarma, Krka, Genzyme, UCB, Esteve, Psyma iberica marketing research SL and Ferrer, course grant from Teva and travel grant from Merck.
Alonso Redondo R.: None.
Ordás C.: None.
López Díaz L. M. has received honoraria from UCB, Lundbeck, and KRKA.
McAfee D.: None.
Martínez-Martin P. has received honoraria from the International Parkinson and Movement Disorder Society (MDS) for management of the Clinical Outcome Assessments Program.
Mir P. has received honoraria from AbbVie, Abbott, Allergan, Bial, Merz, UCB, and Zambon and have received grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Subdirección General de Evaluación y Fomento de la Investigación) and by Fondo Europeo de Desarrollo Regional (FEDER), the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía [CVI-02526, CTS-7685], the Consejería de Salud y Bienestar Social de la Junta de Andalucía [PI-0437-2012, PI-0471-2013], the Sociedad Andaluza de Neurología, the Jacques and Gloria Gossweiler Foundation, the Fundación Alicia Koplowitz, the Fundación Mutua Madrileña.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-225014.
REFERENCES
[1] | Shulman LM , Taback RL , Bean J , Weiner WJ ((2001) ) Comorbidity of the non-motor symptoms of Parkinson’s disease. Mov Disord 16: , 507–510. |
[2] | Mosley PE , Moodie R , Dissanayaka N ((2017) ) Caregiver burden in Parkinson disease: A critical review of recent literature. J Geriatr Psychiatry Neurol 30: , 235–252. |
[3] | Thornton M , Travis SS ((2003) ) Analysis of the reliability of the modified caregiver strain index. J Gerontol B Psychol Sci Soc Sci 58: , S127–S132. |
[4] | Smith MC , Ellgring H , Oertel WH ((1997) ) Sleep disturbances in Parkinson’s disease patients and spouses. J Am Geriatr Soc 45: , 194–199. |
[5] | Schrag A , Hovris A , Morley D , Quinn N , Jahanshahi M ((2006) ) Caregiver-burden in Parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord 12: , 35–44. |
[6] | Martínez-Martín P , Forjaz MJ , Frades-Payo B , Rusiñol AB , Fernández-García JM , Benito-León J , Arillo VC , Barberá MA , Sordo MP , Catalán MJ ((2007) ) Caregiver burden in Parkinson’s disease., . Mov disord 22: , 924–931. |
[7] | D’Amelio M , Terruso V , Palmeri B , Di Benedetto N , Famoso G , Cottone P , Aridon P , Ragonese P , Savettieri G ((2009) ) Predictors of caregiver burden in partners of patients with Parkinson’s disease. Neurol Sci 30: , 171–174. |
[8] | Martinez Martin P , Rodriguez Blazquez C , Forjaz MJ ((2012) ) Quality of life and burden in caregivers for patients with Parkinson’s disease: Concepts, assessment and related factors. Expert Rev Pharmacoecon Outcomes Res 12: , 221–230. |
[9] | Carod-Artal FJ , Mesquita HM , Ziomkowski S , Martinez-Martin P ((2013) ) Burden and health-related quality of life among caregivers of Bazilian Parkinson’s disease patients. Parkinsonism Relat Disord 19: , 943–948. |
[10] | Leroi I , McDonald K , Pantula H , Harbishettar V ((2012) ) Cognitive impairment in Parkinson disease: Impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol 25: , 208–214. |
[11] | Corallo F , De Cola MC , Lo Buono V , Di Lorenzo G , Bramanti P , Marino S ((2017) ) Observational study of quality of life of Parkinson’s patients and their caregivers. Psychogeriatrics 17: , 97–102. |
[12] | Rajiah K , Maharajan MK , Yeen SJ , Lew S ((2017) ) Quality of life and caregivers’ burden of Parkinson’s disease. Neuroepidemiology 48: , 131–137. |
[13] | Santos-García D , de la Fuente-Fernández R ((2015) ) Factors contributing to caregivers’ stress and burden in Parkinson’s disease. Acta Neurol Scand 131: , 203–210. |
[14] | Juneja A , Anand K , Chandra M , Deshpande S , Dhamija R , Kathuria P , Mahajan R , Neuropsychiatric symptoms and caregiver burden in Parkinson’s disease. Ann Indian Acad Neurol 23: , 656–660. |
[15] | Kumar H , Ansari S , Kumar V , Barry HD , Tahir A ((2019) ) Severity of caregiver stress in relation to severity of disease in persons with Parkinson’s. Cureus 11: , e4358. |
[16] | Santos-García D , de Deus Fonticoba T , Cores Bartolomé C , Íñiguez Alvarado MC , Feal Panceiras MJ , Suárez Castro E , Canfield H , Martínez Miró C , Jesús S , Aguilar M , Pastor P , Planellas L , Cosgaya M , García Caldentey J , Caballol N , Legarda I , Hernández Vara J , Cabo I , López Manzanares L , González Aramburu I , Ávila Rivera MA , Gómez Mayordomo V , Nogueira V , Puente V , Dotor García-Soto J , Borrué C , Solano Vila B , Álvarez Sauco M , Vela L , Escalante S , Cubo E , Carrillo Padilla F , Martínez Castrillo JC , Sánchez Alonso P , Alonso Losada MG , Ariztegui NL , Gastón I , Kulisevsky J , Blázquez Estrada M , Seijo M , Martínez JR , Valero C , Kurtis M , de Fábregues O , González Ardura J , Alonso Redondo R , Ordás C , López DíazL LM , McAfee D , Martinez-Martin P , Mir P ; COPPADIS Study Group ((2022) ) Predictors of the change in burden, strain, mood, and quality of life among caregivers of Parkinson’s disease patients. Int J Geriatr Psychiatry 37: –10.1002/gps.5761. |
[17] | Santos García D , Jesús S , Aguilar M , Planellas LL , García Caldentey J , Caballol N , Legarda I , Hernández Vara J , Cabo I , López Manzanares L , González Aramburu I , Ávila Rivera MA , Catalán MJ , López Díaz L , Puente V , García Moreno JM , Borrué C , Solano Vila B , Álvarez Sauco M , Vela L , Escalante S , Cubo E , Carrillo Padilla F , Martínez Castrillo JC , Sánchez Alonso P , Alonso Losada MG , López Ariztegui N , Gastón I , Kulisevsky J , Menéndez González M , Seijo M , Rúiz Martínez J , Valero C , Kurtis M , de Fábregues-Boixar O , González Ardura J , Prieto Jurczynska C , Martinez-Martin P , Mir P ; COPPADIS Study Group ((2019) ) COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015): An ongoing global Parkinson’s disease project about disease progression with more than 1000 subjects included. Results from the baseline evaluation. Eur J Neurol 26: , 1399–1407. |
[18] | Santos-García D , Mir P , Cubo E , Vela L , Rodríguez-Oroz MC , Martí MJ , Arbelo JM , Infante J , Kulisevsky J , Martínez-Martín P ; COPPADIS Study Group ((2016) ) COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015), a global–clinical evaluations, serum biomarkers, genetic studies and neuroimaging–prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression. BMC Neurol 16: , 26. |
[19] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[20] | Martinez Martin P , Arroyo S , Rojo-Abuin JM , Rodrigues-Blazquez C , Frades B , de Pedro Cuesta J , the Longitudinal Parkinson’s disease Patients Study Group ((2008) ) Burden, perceived health status, and mood among caregivers of Parkinson’s disease patients. Mov Disord 23: , 1673–1780. |
[21] | Novak M , Guest C ((1989) ) Application of a multidimensional caregiver burden inventory. Gerontol 29: , 798–803. |
[22] | Robinson BC ((1983) ) Validation of a Caregiver Strain Index. J Gerontol 38: , 344–348. |
[23] | Beck AT , Steer RA , Brown GK ((1996) ) Beck Depression Inventory-second edition. Manual. The Psychological Corporation, San Antonio. |
[24] | Da Rocha NS , Power MJ , Bushnell DM , Fleck MP ((2012) ) The EUROHIS-QOL 8-item index: Comparative psychometric properties to its parent WHOQOL-BREF. Value Health 15: , 449–457. |
[25] | Schade S , Mollenhauer B , Trenkwalder C ((2020) ) Levodopa equivalent dose conversion factors: An updated proposal including opicapone and safinamide. Mov Disord Clin Pract 7: , 343–345. |
[26] | Chang HY , Chiou CJ , Chen NS ((2010) ) Impact of mental health and caregiver burden on family caregivers’ physical health. Arch Gerontol Geriatr 50: , 267–271. |
[27] | Tarlow BJ , Wisniewski SR , Belle SH , Rubert M , Ory MG , Gallagher-Thompson D ((2004) ) Positive aspects of caregiving: Contributions of the REACH project to the development of new measures for Alzheimer’s caregiving. Res Aging 26: , 429–453. |
[28] | Schulz R , Visintainer P , Williamson GM ((1990) ) Psychiatric and physical morbidity effects of caregiving. J Gerontol 45: , P181–P191. |
[29] | Beach SR , Schulz R , Yee JL , Jackson S ((2000) ) Negative and positive health effects of caring for a disabled spouse: Longitudinal findings from the caregiver health effects study. Psychol Aging 15: , 259–271. |
[30] | Stommel M , Given CW , Given B ((1999) ) Depression as an overriding variable explaining caregiver burdens. J Aging Health 2: , 80–103. |
[31] | Schulz R , Sherwood PR ((2008) ) Physical and mental health effects of family caregiving. Am J Nurs 108: (9 Suppl), 23–7; quiz 27. |
[32] | Liu Z , Heffernan C , Tan J ((2020) ) Caregiver burden: A concept analysis. Int J Nurs Sci 7: , 438–445. |
[33] | Bhimani R ((2014) ) Understanding the burden on caregivers of people with Parkinson’s: A scoping review of the literature. Rehabil Res Pract 2014: , 718527. |
[34] | Secker DL , Brown RG ((2005) ) Cognitive behavioural therapy (CBT) for carers of patients with Parkinson’s disease: A preliminary randomised controlled trial. J Neurol Neurosurg Psychiatry 76: , 491–497. |
[35] | Shah SP , Glenn GL , Hummel EM , Hamilton JM , Martine RR , Duda JE , Wilkinson JR ((2015) ) Caregiver tele-support group for Parkinson’s disease: A pilot study. Geriatr Nurs 36: , 207–211. |
[36] | Bastawrous M ((2013) ) Caregiver burden–a critical discussion. Int J Nurs Stud 50: , 431–441. |
[37] | Given BA , Sherwood P , Given CW ((2011) ) Support for caregivers of cancer patients: Transition after active treatment. Cancer Epidemiol Biomarkers Prev 20: , 2015–2021. |
[38] | Santos García D , de Deus Fonticoba T , Suárez Castro E , Borrué C , Mata M , Solano Vila B , Cots Foraster A , Álvarez Sauco M , Rodríguez Pérez AB , Vela L , Macías Y , Escalante S , Esteve P , Reverté Villarroya S , Cubo E , Casas E , Arnaiz S , Carrillo Padilla F , Pueyo Morlans M , Mir P , Martinez-Martin P ; Coppadis Study Group ((2019) ) Non-motor symptoms burden, mood, and gait problems are the most significant factors contributing to a poor quality of life in non-demented Parkinson’s disease patients: Results from the COPPADIS Study Cohort. Parkinsonism Relat Disord 66: , 151–157. |
[39] | Santos García D , de Deus Fonticoba T , Cores C , Muñoz G , Paz González JM , Martínez Miró C , Suárez E , Jesús S , Aguilar M , Pastor P , Planellas L , Cosgaya M , García Caldentey J , Caballol N , Legarda I , Hernández Vara J , Cabo I , López Manzanares L , González Aramburu I , Ávila Rivera MA , Catalán MJ , Nogueira V , Puente V , Ruíz de Arcos M , Borrué C , Solano Vila B , Álvarez Sauco M , Vela L , Escalante S , Cubo E , Carrillo v F , Martínez Castrillo JC , Sánchez Alonso P , Alonso Losada MG , López Ariztegui N , Gastón I , Clavero P , Kulisevsky J , Blázquez Estrada M , Seijo M , Rúiz Martínez J , Valero C , Kurtis M , de Fábregues O , González Ardura J , Ordás C , López Díaz LM , McAfee D , Martinez-Martin P , Mir P ; COPPADIS Study Group ((2021) ) Predictors of clinically significant quality of life impairment in Parkinson’s disease. NPJ Parkinsons Dis 7: , 118. |
[40] | A’Campo LE , Spliethoff-Kamminga NG , Macht M , EduPark C , Roos RA ((2010) ) Caregiver education in Parkinson’s disease: Formative evaluation of a standardized program in seven European countries. Qual Life Res 19: , 55–64. |
[41] | Oguh O , Eisenstein A , Kwasny M , Simuni T ((2014) ) Back to the basics: Regular exercise matters in Parkinson’s disease: Results from the National Parkinson Foundation QII registry study. Parkinsonism Relat Disord 20: , 1221–1225. |
[42] | Bruno V , Mancini D , Ghoche R , Arshinoff R , Miyasaki JM ((2016) ) High prevalence of physical and sexual aggression to caregivers in advanced Parkinson’s disease. Experience in the palliative care program. Parkinsonism Relat Disord 24: , 141–142. |
[43] | Leroi I , Baker P , Kehoe P , Daniel E , Byrne EJ . A pilot randomized controlled trial of sleep therapy in Parkinson’s disease: Effect on patients and caregivers ((2010) ). Int J Geriatr Psychiatry 25: , 1073–1079. |




