Effects of Levodopa-Carbidopa Intestinal Gel on Dyskinesia and Non-Motor Symptoms Including Sleep: Results from a Meta-Analysis with 24-Month Follow-Up
Abstract
Background:
In advanced Parkinson’s disease (PD), dyskinesias and non-motor symptoms such as sleep dysfunction can significantly impair quality of life, and high-quality management is an unmet need.
Objective:
To analyze changes in dyskinesia and non-motor symptoms (including sleep) among studies with levodopa-carbidopa intestinal gel (LCIG) in patients with advanced PD.
Methods:
A comprehensive literature review identified relevant studies examining LCIG efficacy. Outcomes of interest were dyskinesia (UDysRS, UPDRS IV item 32), overall non-motor symptoms (NMSS), mentation/behavior/mood (UPDRS I), and sleep/daytime sleepiness (PDSS-2, ESS). The pooled mean (95% confidence interval) change from baseline per outcome was estimated for each 3-month interval with sufficient data (i.e., reported by≥3 studies) up to 24 months using a random-effects model.
Results:
Seventeen open-label studies evaluating 1243 patients with advanced PD were included. All outcomes of interest with sufficient data for meta-analysis showed statistically significant improvement within 6 months of starting LCIG. There were statistically significant improvements in dyskinesia duration as measured by UPDRS IV item 32 at 6 months (–1.10 [–1.69, –0.51] h/day) and 12 months (–1.35 [–2.07, –0.62] h/day). There were statistically and clinically significant improvements in non-motor symptoms as measured by NMSS scores at 3 months (–28.71 [–40.26, –17.15] points). Significant reduction of NMSS burden was maintained through 24 months (–17.61 [–21.52, –13.70] points). UPDRS I scores significantly improved at 3 months (–0.39 [–0.55, –0.22] points). Clinically significant improvements in PDSS-2 and ESS scores were observed at 6 and 12 months in individual studies.
Conclusion:
Patients with advanced PD receiving LCIG showed significant sustained improvements in the burden of dyskinesia and non-motor symptoms up to 24 months after initiation.
INTRODUCTION
Parkinson’s disease (PD) is a chronic and progressive disorder primarily associated with the degeneration of brain dopamine neurons and depletion of striatal dopamine [1–3]. The gold standard treatment for PD is levodopa, which is converted to dopamine in the brain [4]. Administration of levodopa orally can lead to motor fluctuations due to its short half-life and delayed or variable gastric emptying [5]. In addition, intermittent or pulsatile dopamine receptor stimulation can lead to postsynaptic plasticity and further drive fluctuations and worsen the motor and non-motor symptoms of PD [2, 5, 6]. Continuous levodopa infusion can be provided in an effort to sustain plasma levels of levodopa, leading to improvement in both motor complications and the shortening response observed with oral levodopa as PD progresses [2, 7–9].
Levodopa-carbidopa intestinal gel (LCIG) is a stable gel suspension suitable for continuous delivery through percutaneous gastrojejunostomy via a portable pump [10]. By continuously delivering medication directly to the jejunum, LCIG can avoid blocks to oral absorption of dopaminergic drugs via delayed gastric emptying and other factors [11], and generate sustained dopamine release and receptor stimulation within a therapeutic window [4–6, 12].
Although motor symptoms traditionally define PD, patients with PD can experience both motor and non-motor symptoms. The development of dyskinesia, a motor symptom, is common in patients with advanced PD [2, 7]. While mild dyskinesia may minimally impact patients, moderate to severe dyskinesia can be disabling and associated with functional impairment, patient discomfort, and social limitations/stigma, as well as reduced quality of life and increased healthcare costs [13–16]. Non-motor symptoms include anxiety, mood disorders, fatigue, cognitive decline and dementia, autonomic dysfunction, and sleep-wake cycle regulation disorders and are an important feature of advanced PD and a chief therapeutic challenge [2, 17]. Approximately 28% of patients with PD rated fluctuations in non-motor symptoms as more disabling than fluctuations in motor symptoms [18]. The burden of non-motor symptoms is a key determinant of quality of life, overall disability progression, and nursing home placement in patients with PD [2, 19, 20]. In particular, sleep and mood are significant predictors of patients reporting poor quality of life [21].
Results from a previously conducted meta-analysis showed improvement in “off” time, activities of daily living (ADLs), and quality of life for patients with advanced PD treated with LCIG persisting up to 24 months [22]. LCIG has been shown to be safe and effective in clinical trials and real-world evidence studies in reducing dyskinesias in patients with advanced PD [6, 23–26], and open-label and global registry studies have shown consistent and robust improvement in several non-motor symptoms, including sleep quality improvement [25, 27–30]. However, the length of follow-up, inclusion criteria, study design, and outcome measures varied among these studies [31]. In addition, there is no previous meta-analysis on non-motor symptoms, sleep, and dyskinesia in advanced PD. Thus, the objective of the present analysis was to pool the available data to assess the impact of LCIG on dyskinesia and non-motor symptoms (such as sleep and mood/behavior) in patients with advanced PD.
MATERIALS AND METHODS
Study identification
We conducted a literature review to identify interventional, prospective observational, or retrospective studies published in the Embase and Medline databases through October 6, 2020. Studies of interest were single-arm or comparative evaluations of patients with advanced PD who were initiating LCIG treatment; studies had to report at least 1 outcome of interest and have a follow-up time of at least 3 months. Studies of patients with early PD, case reports, conference abstracts without a full-text publication, and trials or observational studies that did not examine the outcomes of interest were excluded. Following the literature search, 2 reviewers independently screened each title and abstract to identify relevant articles and reviewed the full-text articles for final study inclusion. Disagreements were resolved by discussion and consensus.
Outcome measures
Outcomes of interest for measuring dyskinesia were the Unified Dyskinesia Rating Scale (UDysRS) and item 32 (modified from percent improvement to hours per day) or items 32–34 (score) of the Unified Parkinson’s Disease Rating Scale (UPDRS) Part IV (UPDRS IV), depending on the study. Outcomes for measuring non-motor symptoms included the Non-Motor Symptoms Scale for Parkinson’s Disease (NMSS), Part I of the UPDRS (UPDRS I) for measuring mood/behavior, the Parkinson’s Disease Sleep Scale-2 (PDSS-2) for sleep, and the Epworth Sleepiness Scale Questionnaire (ESS) for daytime sleepiness.
Statistical analysis
Data for each outcome were grouped into 3-month intervals (e.g., 1–3 months [“3 months”], 4–6 months [“6 months”], 7–9 months [“9 months”]) up to 24 months. For each outcome of interest, the pooled mean change from baseline (CFB) with 95% CI was estimated for each time interval with sufficient data for meta-analysis (i.e., outcome reported by≥3 available studies). Data were pooled using random-effects methods to generate overall estimates for each outcome and time point (see the Interactive Appendix in the Supplementary Material for fixed-effects results). Heterogeneity among the studies was assessed for each outcome and time point reported by 3 or more studies using the I2 and Q statistics.
Outcomes in which the confidence intervals for mean change from baseline do not include zero are statistically significant. Mean changes from baseline that exceed the minimum clinically important differences (NMSS: –13.91 points [32]; PDSS-2: –3.44 points [33]) are considered clinically significant.
Evaluation of the associations between study outcomes and factors such as mean duration of PD, mean age at study entry, Hoehn and Yahr On score, or Hoehn and Yahr Off score showed no impact on differences among the studies; thus, no statistical adjustment for these factors was considered. Further, no formal risk of bias assessment was performed since the conduct of the studies is unlikely to affect the reported outcomes, which are measured with standard instruments and no association between baseline patient characteristics and outcomes was observed. Analyses were conducted using Microsoft Excel software.
RESULTS
Study selection and characteristics
A total of 17 studies evaluating a total of 1243 patients with advanced PD were included in the analysis (Fig. 1). No studies were excluded during the similarity assessment. At baseline, mean patient age in the individual studies ranged from 57.8 to 70.6 years and mean PD duration ranged from 10.0 to 16.1 years (Table 1).
Fig. 1
Study selection flow diagram. LCIG, levodopa-carbidopa intestinal gel; SLR, systematic literature review.
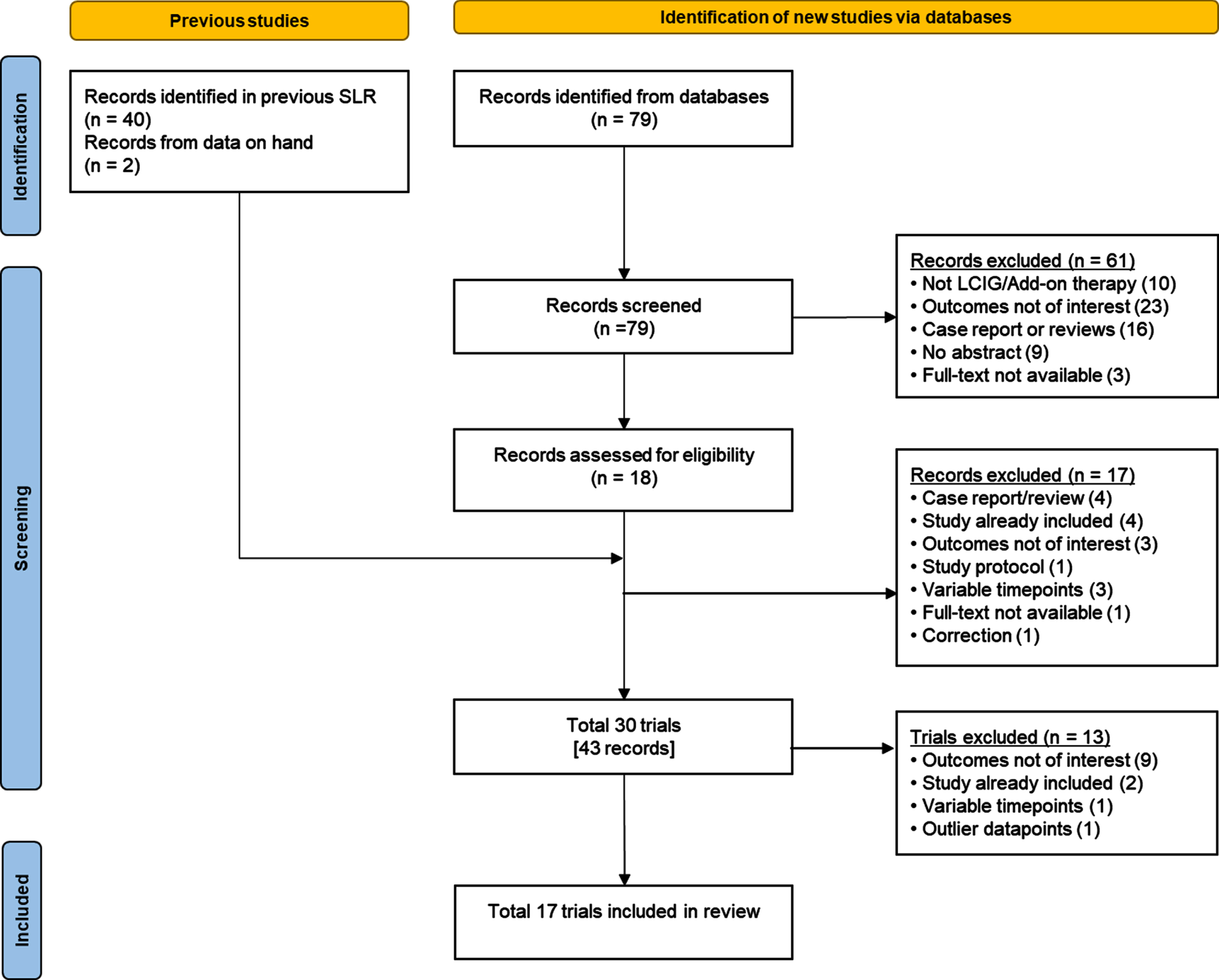
Table 1
Summary of included studies
| Author, Year | Data Source(s)a | Study Characteristics | Patient Baseline Characteristics, Mean (SD) | Patient Scores at Baseline, Mean (SD) | |||||||||||
| Study Design | Follow-up, mob | ITT, N | Age, y | PD Duration, y | Daily L-dopa Dose, mg | Hoehn and Yahr Score | UPDRS IV Item 32, h/d | UPDRS IV Items 32–34 | UDysRS | NMSS | UPDRS I | PDSS-2 | ESS | ||
| DUOGLOBE 2020 | Publication, CSR [28, 61] | Global, multicountry, single-arm, post-marketing observational analysis | 36 | 164 | 70.2 (8.17) | 11.2 (4.72) | 1432 (101.82) | On: 3 (0.83) Off: 3.6 (0.8) | 4.1 (3.7) | NA | 33.7 (21.14) | 88.2 (51.09) | NA | 26.6 (11.65) | 9.8 (5.26) |
| Alvarez 2020 | Publication, CSR [62, 63] | Open-label, randomized, multicenter interventional study | 3 | 25 | 69.3 (7.0) | 12.7 (4.2) | 1211.5 (374.89) | NA | NA | NA | 53.2 (12.24) | NA | NA | NA | NA |
| Antonini 2017 | Publication, CSR [27, 64] | Observational non-interventional | 24 | Retro-spective: 140 | 67.4 (8.1) | 12.6 (6.6) | 854.8 (513.3) | On: 2.9 (0.8) Off: 4.1 (0.8) | 4.1 (3.7) | NA | NA | 76.2 (47.9) | NA | NA | NA |
| Prospe-ctive: 189 | 66.1 (8.5) | 13.0 (6.1) | 899.7 (474.7) | On: 2.8 (0.8) Off: 3.9 (0.9) | 4.4 (3.8) | NA | NA | 66.5 (39.6) | NA | NA | NA | ||||
| Caceres-Redondo 2014 | Publication [65] | Observational | 24 | 16 | 66.5 (9.3) | 15.1 (5.4) | 1473.0 (449.0) | On: 2.4 (0.5) Off: 3.7 (0.8) | NA | NA | NA | 17.3 (4.7) | NA | NA | NA |
| De Fabregues 2017 | Publication [34] | Long-term, open-label, prospective, observational | 12 | 37 | 68.2 (6.8) | 13.5 (5.6) | NA | On: 2.5 Off: 3.8 | NA | NA | NA | NA | 3.2 (2.4) | NA | 5.6 (3.6) |
| Fasano 2012 | Publication [54] | Phase III, open-label | 24.9 (14.4) | 14 | 67.1 (11.5) | 12.9 (4.8) | 929.1 (682.2) | NA | NA | NA | NA | 126 (56.18) | 8.71 (3.15) | 39.08 (8.58) | NA |
| Fernandez 2015 | Publication, CSR, trial registry [66–68] | Phase III, open-label | 12 | 354 | 64.1 (9.1) | 12.5 (5.5) | 1082.9 (582.1) | NA | NA | 3.7 (2.4) | NA | NA | 2.2 (1.9) | NA | NA |
| Honig 2009 | Publication [25] | Prospective open-label observational | 6 | 22 | 58.6 (9.1) | 15.3 (5.9) | NA | Off: 3.8 | NA | NA | NA | 89.9 (56.5) | NA | NA | NA |
| Juhasz 2017 | Publication [26] | Prospective, multicenter, open-label cohort study | 12 | 34 | 67.0 (6.0) | 12.0 (5.0) | 1000.2 (577.6) | 3.0 | NA | NA | 45.9 (16.7) | 88.9 (40.3) | NA | 27.2 (10.5) | 9.1 (4.8) |
| Kruger 2017 | Publication, CSR [69, 70] | Phase III single-arm, open-label, baseline-controlled, multicenter study | 12 | 64 | 70.4 (7.8) | 13.9 (5.4) | NA | NA | NA | NA | NA | 95.5 (54.5) | NA | NA | NA |
| Martinez-Martin 2015 | Publication [30] | Prospective, multicenter, open-label cohort study | 6 | 44 | 62.7 (9.1) | 16.1 (6.7) | 1815.4 (771.5) | 4c | NA | NA | NA | 90.95 (45) | NA | NA | NA |
| Olanow 2014 | Publication, CSR, 2 trial registries [10, 71–73] | Phase III, double-blind RCT | 3 | 37 | 63.7 (9.5) | 10.0 (4.6) | 1005.4 (373.6) | On: 2.3 (0.6) | NA | 2.4 (1.6) | NA | NA | 1.8 (1.7) | NA | NA |
| Palhagen 2016 | Publication, CSR, trial registry [74–76] | Prospective, open-label, long-term study | 36 | 27 | 64.6 (6.4) | 10.7 | NA | On: 2.0 (0.9) Off: 3.6 (1.1) | NA | NA | NA | NA | 2.9 (1.9) | NA | NA |
| Reddy 2012 | Publication [77] | Single-center, real-life setting, matched based on PCT funding restrictions | 6 | 17 | 57.82 (7.71) | 16.12 (5.84) | 1996 (675) | On: 3.5 | NA | NA | NA | 113.88 (49.26) | NA | NA | NA |
| Sensi 2014 | Publication [78] | Prospective open-label cohort study | 24 | 17 | 67.6 (6.1) | 15.5 (4.0) | 1158.9 (334.5) | On: 3.2 (0.7) | NA | NA | NA | 51.8 (37.3) | NA | NA | NA |
| Slevin 2015 | Publication, trial registry [79, 80] | Phase III, open-label, multicenter | 12 | 29 | 64.8 (6.6) | 11.4 (5.7) | NA | NA | NA | NA | NA | NA | 1.2 (1.0) | NA | NA |
| Standaert 2017 | Publication, CSR, trial registry [6, 81, 82] | Phase IIIb, open-label | 12 | 38 | 64.3 (10.2) | 11.5 (5.3) | NA | NA | NA | NA | NA | 48.3 (35.6) | 1.6 (1.6) | NA | NA |
CSR, clinical study report; ESS, Epworth Sleepiness Scale; ITT, intent to treat; L-dopa, levodopa; N, number; NA, not available; NMSS, Non-Motor Symptoms Scale for Parkinson’s Disease; PCT, primary care trust; PD, Parkinson’s disease; PDSS-2, Parkinson’s Disease Sleep Scale-2; RCT, randomized controlled trial; UDysRS, Unified Dyskinesia Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale. aThe individual record(s) used per study are cited in this column. bValues in this table express the follow-up period defined in study publication(s). If a study did not report a prespecified follow-up period, but did report mean (SD) follow-up time, the mean (SD) follow-up time reported is presented in this table. cMedian score, not mean.
Outcomes
All outcomes of interest with sufficient data for meta-analysis showed statistically significant improvement within 6 months of starting LCIG treatment (Table 2). Meta-analysis was possible for UPDRS IV modified item 32 (hours per day) outcome scores at 4 time points, NMSS scores at 5 time points, and UPDRS I scores at 2 time points. Even for time points where meta-analysis was not possible, the majority of results from individual studies showed improvement in the evaluated outcomes.
The results of our meta-analysis showed statistically significant improvements in dyskinesia duration as measured by UPDRS IV item 32 starting at 6 months (mean [95% CI] CFB, –1.10 [–1.69, –0.51] h/day) that was sustained through 24 months (–0.76 [–1.31, –0.22] h/day). Changes from baseline in UDysRS and UPDRS IV items 32–34 scores were not meta-analyzed, as there were no 3-month intervals in which 3 or more studies reported these outcomes. However, among the individual studies, improvements in UDysRS and UPDRS IV items 32–34 scores were observed at 3 months, 6 months, and 12 months.
Non-motor symptoms as measured on the NMSS scale showed statistically and clinically significant improvement starting at 3 months (mean [95% CI] CFB, –28.71 [–40.26, –17.15] points) that was sustained through 24 months (–17.61 [–21.52, –13.70] points) (Fig. 2).
Table 2
Impact of LCIG on dyskinesia, non-motor symptoms, and sleep outcomes in patients with advanced PD
| Symptom Domain | Outcome Measure | MCID | Score Range | Change From Baseline at Time Intervala | ||||
| 1 to 3 Months | 4 to 6 Months | 10 to 12 Months | 16 to 18 Months | 22 to 24 Months | ||||
| Dyskinesia | UPDRS IV, item 32 (h/day) | NA | 0 (best) to 24 (worst) | n = 99 | n = 422 | n = 363 | n = 140, n = 189 | n = 140, n = 189 |
| –0.7 (4.77)[28] ,c | –1.10 (–1.69, –0.51) | –1.35 (–2.07, –0.62) | –0.77 (–1.31, –0.22) | –0.76 (–1.31, –0.22) | ||||
| UPDRS IV, items 32–34 (score) | NA | 0 (best) to 12 (worst) | n = 34, n = 275 | n = 267 | n = 250 | |||
| –0.2 (1.9)[10] ,c –1.4 (0.2)[68] ,b | –1.2 (0.2)[68] ,b | –1.2 (0.2)[68] ,b | No data | No data | ||||
| UDysRS score | NA | 0 (best) to 104 (worst) | n = 24, n = 91 | n = 83 | n = 34, n = 26 | |||
| –17.4 (2.79)[63] ,b –11.9 (18.92)[28] ,c | –12.5 (19.91)[28] ,c | –13.8 (NR)[26] –9.9 (22.73)[28] ,c | –9.00 (21.69)[28] ,c | –8.1 (22.57)[28] ,c | ||||
| Non-motor symptoms | NMSS score | –13.91[32] | 0 (best) to 243 (worst) | n = 174 | n = 561 | n = 459 | n = 178, n = 189 | n = 376 |
| –28.71 (–40.26, –17.15) | –27.81 (–35.73, –19.89) | –20.97 (–25.85, –16.09) | –18.84 (–22.56, –15.12) | –17.61 (–21.52, –13.70) | ||||
| Mood/ Behavior | UPDRS I score | NA | 0 (best) to 16 (worst) | n = 407 | n = 267, n = 27 | n = 339 | n = 27 | n = 14, n = 27 |
| –0.39 (–0.55, –0.22) | –0.2 (0.1)[68] ,b –0.1 (1.7)[76] ,c | 0.11 (–0.20, 0.42) | 1.1 (3.1)[76] ,c | –1.92 (NR)[54] 1.0 (3.0)[76] ,c | ||||
| Sleep | PDSS-2 score | –3.44[33] | 0 (best) to 60 (worst) | n = 106 | n = 99 | n = 34, n = 37 | n = 14 | |
| –7.2 (13.35)[28] ,c | –8.1 (13.09)[28] ,c | –4.0 (NR)[26] –6.6 (12.19)[28] ,c | –5.9 (12.34)[28] ,c | –5.62 (NR)[54] –5.80 (13.15)[28] ,c | ||||
| ESS score | NA | 0 (best) to 24 (worst) | n = 107 | n = 37, n = 100 | n = 34, n = 36 | |||
| –1.5 (5.12)[28] ,c | –2.8 (NR)[34] –1.2 (5.66)[28] ,c | –1.0 (NR)[26] –1.0 (5.73)[28] ,c | –1.10 (6.08)[28] ,c | –1.40 (6.11)[28] ,c | ||||
ESS, Epworth Sleepiness Scale; LCIG, levodopa-carbidopa intestinal gel; MCID, minimum clinically important difference; NA, not available; NMSS, Non-Motor Symptoms Scale; NR, not reported; PD, Parkinson’s disease; PDSS-2, Parkinson’s Disease Sleep Scale-2; UDysRS, Unified Dyskinesia Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale. aBold values in these columns indicate meta-analysis outcomes, which are reported as mean (95% CI) change from baseline and were estimated using random effects analysis. When fewer than 3 studies were available, meta-analysis was not performed. Insufficient data for meta-analysis were available for any outcome for the 7–9 month, 13–15 month, and 19–21 month time intervals. bResults shown in table are mean (SE) values as reported in individual source publications. See citations in cells for specific source publication(s) referenced. Further information on values from individual source studies is presented in the Interactive Appendix. cResults shown are mean (SD) values as reported in individual source publications.
Fig. 2
Impact of LCIG on Non-Motor Symptoms Up to 24 Months After Initiationa. LCIG, levodopa-carbidopa intestinal gel; MCID, minimum clinically important difference; NMSS, Non-Motor Symptoms Scale. aBlack circles represent reported mean change from baseline. Diamonds represent pooled mean change from baseline with 95% CIs estimated using random effects meta-analysis.
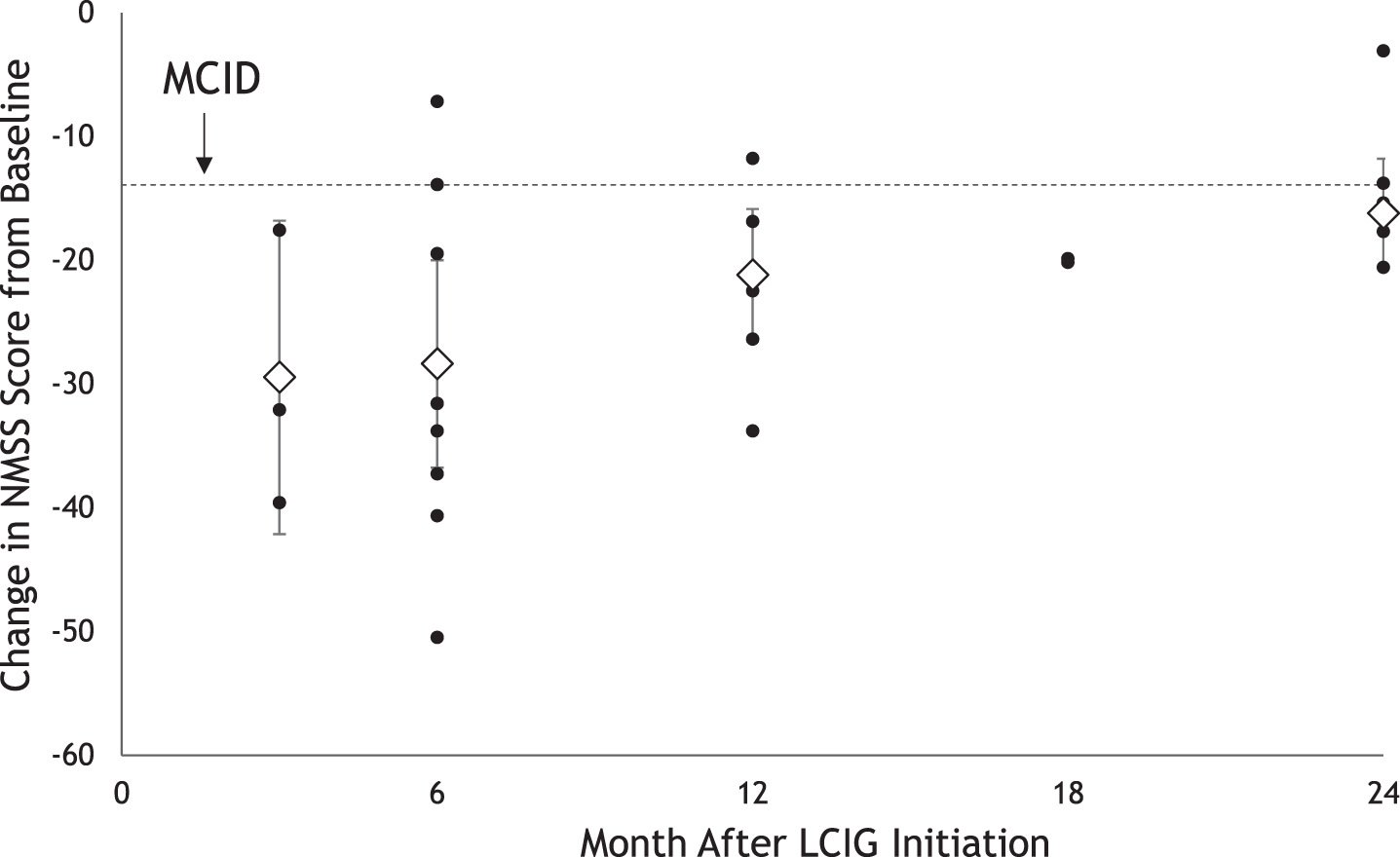
Mentation, behavior, and mood as measured by UPDRS I scores was significantly improved at 3 months (mean [95% CI] CFB, –0.39 [–0.55, –0.22] points). No statistically significant CFB in UPDRS I scores was observed at 12 months (0.11 [–0.20, 0.42] points). There were insufficient data for meta-analysis for the 4 to 6 month, 16 to 18 month, and 22 to 24 month intervals, but among the individual studies, improvements were observed at 6 months and 24 months, with no improvement observed at 18 months.
Sleep outcomes as measured by PDSS-2 and ESS scores were not meta-analyzed, as there were no 3-month intervals in which 3 or more studies reported these outcomes. However, among the individual studies, clinically significant improvements in PDSS-2 scores were observed at 3 months [28], 6 months [28], 12 months [26, 28], 18 months [28], and 24 months [28]. For ESS scores, improvements were observed at 3 months [28], 6 months [28, 34], 12 months [26, 28], 18 months [28], and 24 months [28], with clinically significant improvement at 6 months and 12 months.
Forest plots showing input data from individual studies along with the meta-analysis outcomes are presented in the Interactive Appendix.
DISCUSSION
Our analysis including data from 17 clinical and observational studies provides one of the most comprehensive assessments to date of the impact of LCIG therapy on non-motor symptom and dyskinesia outcomes among patients with advanced PD. Results of the meta-analysis suggest that the effects of LCIG on non-motor symptoms and dyskinesia are evident as early as 1 to 3 months and 4 to 6 months following treatment initiation, respectively, and are sustained over a period of 24 months. The improvement in non-motor symptoms from baseline was statistically significant and greater than the reported minimum clinically important difference for NMSS scores (–13.91) across 24 months [32]. Mood and behavior was significantly improved at 3 months. In most outcomes, the available data showed a pattern of initial improvement followed by a gradual worsening. This pattern was also observed in quality of life and ADL outcomes [22], which could be attributed to disease progression. However, most measures still remain improved or significantly improved over baseline at 24 months.
Although multiple treatments address dyskinesia in advanced PD, there is an unmet need for therapies to address the substantial burden of non-motor symptoms [2, 19, 20]. The results of our meta-analysis provide an important summary of the published evidence on the effect of LCIG on non-motor symptoms in advanced PD. Our analysis included 12 studies reporting NMSS scores in patients with PD. The statistically significant and clinically meaningful improvement in NMSS scores observed with LCIG in our analysis is consistent with the robust non-motor symptom effects observed in a systematic literature review of multiple large multicenter and observational studies, specifically in the sleep, mood, and fatigue domains [31]. Some recent studies such as INSIGHTS are not included in our analysis due to the study identification cut-off date. However, the changes from baseline to 6 months reported in this trial for NMSS and PDSS-2 were consistent with our estimates [35].
The results of our meta-analysis suggest the use of LCIG for sleep and other key non-motor symptoms of PD. While pathophysiology for improvements in non-motor symptoms is not fully understood, several domains may be partly driven by dopamine control [36]. These domains include depression, sleep dysfunction such as insomnia, restless legs syndrome, nocturia, pain, sexual dysfunction, apathy, and anhedonia [25, 36, 37]. The link between sleep dysfunction and dopamine pathophysiology can also be supported by reduced binding of 11 C-raclopride in the hypothalamus among PD patients, which is a key sleep and autonomic regulatory center [38]. Switching to LCIG would allow reduction in other anti-parkinsonian medications and potentially achieving monotherapy during the day [39]. As result, reduction in other dopaminergic medications may lead to improvements in other non-motor symptoms such as insomnia, sedation and urinary urgency [25]. Improvements in motor symptoms at night (as measured by PDSS-2) may also be correlated with a reduction in daytime sleepiness (as measured by ESS). Lastly, LCIG treatment can be maintained during the night when medically justified. This can lead to improvements in dopamine-driven non-motor symptoms including sleep [25, 40]. Therefore, improvement in dopamine-driven non-motor symptoms is likely to be associated with continuous and stable delivery of levodopa with LCIG compared to orals.
In observational studies, improvement in non-motor symptoms and dyskinesias was associated with improved quality of life in patients with advanced PD receiving LCIG treatment as well as their caregivers [19, 41]. In a cross-sectional epidemiologic study conducted in Italy, caregiver burden also tended to be lower when patients were treated with LCIG compared with continuous subcutaneous apomorphine infusion or continuation of standard of care [42]. Our previous meta-analysis showed that improvement in ADLs and quality of life for patients with advanced PD receiving LCIG persisted up to 24 months [22].
Results from this meta-analysis show statistically and clinically significant improvements in non-motor symptoms and a statistically significant reduction in dyskinesia duration, which contribute to LCIG’s overall impact in improving patients’ quality of life and ability to perform ADLs. Few studies assessed dyskinesia disability (UPDRS IV item 33, UDysRS), leading to limited evidence and an inability to evaluate these outcomes in meta-analysis. PD is associated with substantial economic burden to patients, caregivers, payers, and society [43–46]; improvements in patient quality of life could in turn reduce caregiver burden and improve caregiver quality of life [47]. Informal care costs are a large component of total costs [44, 46, 48], so potential reductions in caregiver burden and use of informal care could impact the total economic toll of PD.
However, while LCIG has been shown to improve dyskinesia, it is worth noting that breakthrough dyskinesia has been described in the long term, although this occurs only in a minority of patients [49]. Few patients may experience exacerbation of levodopa-induced dyskinesia after LCIG initiation [49–53], which can present in different forms including “peak of dose,” “on” period, or diphasic dyskinesia. Monitoring of patient symptoms and tailoring LCIG dose are warranted to insure optimal outcomes during LCIG initiation.
Although there were insufficient data to meta-analyze sleep outcomes in our analysis, clinically significant improvements with LCIG were observed in at least 1 study at 3, 6, 12, 18, and 24 months for PDSS-2 scores [26, 28, 54], and at 6 months and 12 months for ESS scores [26, 28, 34]. Improvements in sleep outcomes could also affect patients’ and caregivers’ daily lives, as patient sleep quality is significantly correlated with caregiver burden, quality of life, and sleep quality [47].
To our knowledge, there has been only one other meta-analysis to date evaluating LCIG in patients with advanced PD that included any of the outcomes used in our analysis. Liu et al. found significant improvement for subthalamic nucleus deep-brain stimulation versus LCIG for dyskinesia/motor fluctuation, measured using overall UPDRS IV scores [55]. These results are consistent with those of our meta-analysis, which evaluated UPDRS IV item 32 and items 32–34 and UDysRS total score change. However, our study focused on the efficacy of LCIG in improving outcomes versus baseline. The comparative efficacy of LCIG versus other treatment options for advanced PD was not assessed in this study.
Our meta-analysis presents a robust evaluation of the impact of LCIG on key PD-related outcomes over 24 months, overcoming the challenge of sample size in individual studies. However, as in all meta-analyses, our evaluation pools data from multiple studies with different study characteristics. We have closely examined the patient populations and outcome definitions to evaluate the impact these factors may have on LCIG treatment outcomes and to create a collection of studies with sufficient similarity to be suitable for meta-analytic combination. No studies were excluded during the similarity assessment.
A tailored, multidisciplinary approach to treatment decisions is recommended in advanced PD [4, 56–58], and the modern approach concentrates on personalized precise delivery of care using the circle of personalized medicine [59]. The choice of personalized therapy in PD is driven by motor and non-motor symptoms, comorbidities, age, caregiver support, and patient/caregiver preferences [60]. Improvements in patients’ daily functions and quality of life are a key component of treatment decision making [56], so the significant improvement in dyskinesia and non-motor symptoms with LCIG in this meta-analysis and the potential impact on patient and caregiver quality of life could influence healthcare providers’ treatment choices.
Conclusions
In this large-scale meta-analysis involving 1243 unique patients with advanced PD treated with LCIG, we found significant improvement in dyskinesia duration from 6 through 24 months and in non-motor symptoms from 3 through 24 months after treatment initiation. Improvement in NMSS scores was both statistically significant and clinically meaningful. Improvement in UPDRS IV and NMSS was rapid, appearing within the first 6 months, and remained consistent for up to 24 months. LCIG provided improvement in dyskinesia and NMSS including sleep and mood scores. The data available on other sleep endpoints were insufficient for meta-analysis, and additional studies are needed to evaluate the impacts of LCIG on sleep parameters in patients with PD.
ACKNOWLEDGMENTS
The authors would like to acknowledge Yash J. Jalundhwala, an employee of AbbVie, for assistance with study design and data collection and Vivek Chaudhuri, a former employee of AbbVie, for support in results interpretation and manuscript development. Assistance with development of the interactive appendix was provided by David Kratochvil and Michael Mersky, who are employees of OPEN Health. Medical writing support for this study was provided by Catherine Mirvis and Sarah Criddle, PharmD, who are employees of OPEN Health, and paid by AbbVie Inc.
Financial support for the study was provided by AbbVie. AbbVie participated in the interpretation of the data and the review and approval of the manuscript. All authors contributed to the development of the manuscript and maintained control over the final content.
CONFLICT OF INTEREST
K. Ray Chaudhuri has received educational funding from UCB and honoraria for sponsored symposiums from UCB, AbbVie, Britannia, US Worldmeds, Otsuka, Medtronic, and Zambon, and has acted as a consultant for AbbVie, UCB, Britannia, Medtronic, and Mundipharma. Angelo Antonini has received compensation for consultancy and speaker-related activities from UCB, Boehringer Ingelheim, Ever Pharma, General Electric, Britannia, AbbVie, Kyowa Kirin, Zambon, Bial, Theravance Biopharma, Jazz Pharmaceuticals, Roche, and Medscape; he receives research support from Bial, Lundbeck, Roche, Angelini Pharmaceuticals, Horizon 2020 - Grant 825785, Horizon2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Movement Disorders Society for NMS Scale validation, and Cariparo Foundation. He serves as a consultant for Boehringer Ingelheim for legal cases on pathological gambling. Rajesh Pahwa has received consulting fees from AbbVie, ACADIA, Acorda, Adamas, Cynapses, Global Kinetics, Lundbeck, Neurocrine, Pfizer, Sage, Sunovion, Teva Neuroscience, and US World Meds. He has received research grants from AbbVie, Adamas, Avid, Biotie, Boston Scientific, Civitas, Cynapses, Kyowa, National Parkinson Foundation, NIH/NINDS, and Parkinson Study Group. Per Odin has received compensations for consultancy and speaker-related activities from AbbVie, Bial, Britannia, Ever Pharma, Lobsor, Nordic Infucare, Stada, and Zambon. Dr. Odin has received royalties from Uni-Med Verlag. Nataliya Titova has no relevant disclosures. Sandeep Thakkar has received funding/grant support from Hoag Memorial Hospital Presbyterian as medical director for the Movement Disorders Program and honoraria for consultancy from Teva, AbbVie, Medtronic, Adamas, Amneal, and Neurocrine. Sonya J. Snedecor and Saket Hegde are employees of OPEN Health, which received funding from AbbVie for conducting the study. Ali Alobaidi, Juan Carlos Parra, Cindy Zadikoff, and Lars Bergmann are employees of AbbVie and may own stocks/shares in the company. David G. Standaert is a member of the faculty of the University of Alabama at Birmingham and is supported by endowment and university funds. Dr. Standaert is an investigator in studies funded by AbbVie, the American Parkinson Disease Association, the Michael J. Fox Foundation for Parkinson Research, Alabama Department of Commerce, the Department of Defense, and NIH grants P50NS108675, R25NS079188, and T32NS095775. He has a clinical practice and is compensated for these activities through the University of Alabama Health Services Foundation. In addition, since January 1, 2020, he has served as a consultant for or received honoraria from AbbVie, the Parkinson Study Group, Curium Pharma, the International Parkinson Disease and Movement Disorder Society, Theravance, McGraw Hill, Gray Matter Technologies, and Sanofi-Aventis.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-223295.
REFERENCES
[1] | Antonini A , Moro E , Godeiro C , Reichmann H ((2018) ) Medical and surgical management of advanced Parkinson’s disease. Mov Disord 33: , 900–908. |
[2] | Coelho M , Ferreira JJ ((2017) ) The natural history of Parkinson’s disease. In Movement Disorders Curricula, Falup-Pecurariu C, Ferreira J, Martinez-Martin P, Chaud- huri KR, eds. Springer Vienna, Vienna, pp. 129–137. |
[3] | Mamelak M ((2018) ) Parkinson’s disease, the dopaminergic neuron and gammahydroxybutyrate. Neurol Ther 7: , 5–11. |
[4] | Virhammar J , Nyholm D ((2017) ) Levodopa-carbidopa enteral suspension in advanced Parkinson’s disease: Clinical evidence and experience. Ther Adv Neurol Disord 10: , 171–187. |
[5] | Thakkar S , Fung VSC , Merola A , Rollins M , Soileau MJ , Kovács N ((2021) ) 24-hour levodopa-carbidopa intestinalgel: Clinical experience and practical recommendations. CNS Drugs 35: , 137–149. |
[6] | Standaert DG , Rodriguez RL , Slevin JT , Lobatz M , Eaton S , Chatamra K , Facheris MF , Hall C , Sail K , Jalundhwala YJ , Benesh J ((2017) ) Effect of levodopa-carbidopa intestinal gel on non-motor symptoms in patients with advancedParkinson’s disease. Mov Disord Clin Pract 4: , 829–837. |
[7] | Ahlskog JE , Muenter MD ((2001) ) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16: , 448–458. |
[8] | Nyholm D , Odin P , Johansson A , Chatamra K , Locke C , Dutta S , Othman AA ((2013) ) Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease patients. AAPS J 15: , 316–323. |
[9] | Chaudhuri KR , Rizos A , Sethi KD ((2013) ) Motor and nonmotor complications in Parkinson’s disease: An argument for continuous drug delivery? J Neural Transm (Vienna) 120: , 1305–1320. |
[10] | Olanow CW , Kieburtz K , Odin P , Espay AJ , Standaert DG , Fernandez HH , Vanagunas A , Othman AA , Widnell KL , Robieson WZ , Pritchett Y , Chatamra K , Benesh J , Lenz RA , Antonini A ((2014) ) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13: , 141–149. |
[11] | Chaudhuri KR , Qamar MA , Rajah T , Loehrer P , Sauerbier A , Odin P , Jenner P ((2016) ) Non-oral dopaminergic therapies for Parkinson’s disease: Current treatments and the future. NPJ Parkinsons Dis 2: , 16023. |
[12] | Amjad F , Bhatti D , Davis TL , Oguh O , Pahwa R , Kukreja P , Zamudio J , Metman LV ((2019) ) Current practices for outpatient initiation of levodopa-carbidopa intestinal gel for management of advanced Parkinson’s disease in the United States. Adv Ther 36: , 2233–2246. |
[13] | Encarnacion EV , Hauser RA ((2008) ) Levodopa-induced dyskinesias in Parkinson’s disease: Etiology, impact on qualityof life, and treatments. Eur Neurol 60: , 57–66. |
[14] | Hechtner MC , Vogt T , Zöllner Y , Schröder S , Sauer JB , Binder H , Singer S , Mikolajczyk R ((2014) ) Quality of life in Parkinson’s disease patients with motor fluctuations and dyskinesias in five European countries. Parkinsonism Relat Disord 20: , 969–974. |
[15] | Manson A , Stirpe P , Schrag A ((2012) ) Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J Parkinsons Dis 2: , 189–198. |
[16] | Thanvi B , Lo N , Robinson T ((2007) ) Levodopa-induced dyskinesia in Parkinson’s disease: Clinical features, pathogenesis, prevention and treatment. Postgrad Med J 83: , 384–388. |
[17] | Seppi K , Weintraub D , Coelho M , Perez-Lloret S , Fox SH , Katzenschlager R , Hametner E-M , Poewe W , Rascol O , Goetz CG , Sampaio C ((2011) ) The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson’s disease. Mov Disorder 26: (Suppl 3), S42–S80. |
[18] | Witjas T , Kaphan E , Azulay JP , Blin O , Ceccaldi M , Pouget J , Poncet M , Chérif AA ((2002) ) Nonmotor fluctuationsin Parkinson’s disease. Neurology 59: , 408. |
[19] | Chaudhuri KR , Antonini A , Robieson WZ , Sanchez-Soliño O , Bergmann L , Poewe W , GLORIA study co-investigators ((2019) ) Burden of non-motor symptoms in Parkinson’s disease patients predicts improvement in quality of life during treatment with levodopa-carbidopa intestinal gel. Eur J Neurol 26: , 581–e543. |
[20] | Seppi K , Ray Chaudhuri K , Coelho M , Fox SH , Katzenschlager R , Perez Lloret S , Weintraub D , Sampaio C , The collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee ((2019) ) Update on treatments for nonmotor symptoms of Parkinson’s disease–an evidence-based medicine review. Mov Disord 34: , 180–198. |
[21] | Prakash KM , Nadkarni NV , Lye WK , Yong MH , Tan EK ((2016) ) The impact of non-motor symptoms on the quality of life of Parkinson’s disease patients: A longitudinal study. Eur J Neurol 23: , 854–860. |
[22] | Standaert DG , Patel V , Snedecor SJ , Thakkar S , Jalundhwala YJ , Kukreja P , Kratochvil D , Bao Y , Pahwa R ((2021) ) Impact of carbidopa-levodopa enteral suspension on quality of life and activities of daily living in patients with advanced Parkinson’s disease: Results from a pooled meta-analysis. Parkinsonism Relat Disord 86: , 52–57. |
[23] | Antonini A , Yegin A , Preda C , Bergmann L , Poewe W ((2015) ) Global long-term study on motor and non-motor symptoms and safety of levodopa-carbidopa intestinal gel in routine care of advanced Parkinson’s disease patients; 12-month interim outcomes. Parkinsonism Relat Disord 21: , 231–235. |
[24] | Antonini A , Fung VSC , Boyd JT , Slevin JT , Hall C , Chatamra K , Eaton S , Benesh JA ((2016) ) Effect of levodopa-carbidopa intestinal gel on dyskinesia in advanced Parkinson’s disease patients. Mov Disord 31: , 530–537. |
[25] | Honig H , Antonini A , Martinez-Martin P , Forgacs I , Faye GC , Fox T , Fox K , Mancini F , Canesi M , Odin P , Chaudhuri KR ((2009) ) Intrajejunal levodopa infusion in Parkinson’s disease: A pilot multicenter study of effects on nonmotor symptoms and quality of life. Mov Disord 24: , 1468–1474. |
[26] | Juhasz A , Aschermann Z , Acs P , Janszky J , Kovacs M , Makkos A , Harmat M , Tenyi D , Karadi K , Komoly S , Takats A , Toth A , Nagy H , Klivenyi P , Dibo G , Dezsi L , Zadori D , Annus A , Vecsei L , Varannai L , Kovacs N ((2017) ) Levodopa/carbidopa intestinal gel can improve both motor and non-motor experiences of daily living in Parkinson’s disease: An open-label study. Parkinsonism Relat Disord 37: , 79–86. |
[27] | Antonini A , Poewe W , Chaudhuri KR , Jech R , Pickut B , Pirtosek Z , Szasz J , Valldeoriola F , Winkler C , Bergmann L , Yegin A , Onuk K , Barch D , Odin P ((2017) ) Levodopa-carbidopa intestinal gel in advanced Parkinson’s: Final results of the GLORIA registry. Parkinsonism Relat Disord 45: , 13–20. |
[28] | Aldred J , Anca-Herschkovitsch M , Antonini A , Bajenaru O , Bergmann L , Bourgeois P , Cubo E , Davis TL , Iansek R , Kovács N , Kukreja P , Onuk K , Pontieri FE , Robieson W , Siddiqui MS , Simu M , Standaert DG , Chaudhuri KR ((2020) ) Application of the ‘5-2-1’ screening criteria in advanced Parkinson’s disease: Interim analysis of DUOGLOBE. Neurodegener Dis Manag 10: , 309–323. |
[29] | Dafsari HS , Martinez-Martin P , Rizos A , Trost M , dos Santos Ghilardi MG , Reddy P , Sauerbier A , Petry-Schmelzer JN , Kramberger M , Borgemeester RWK , Barbe MT , Ashkan K , Silverdale M , Evans J , Odin P , Fonoff ET , Fink GR , Henriksen T , Ebersbach G , Pirtosek Z , Visser-Vandewalle V , Antonini A , Timmermann L , Ray Chaudhuri K , on behalf of EUROPAR and the International Parkinson Movement Disorders Society Non-Motor Parkinson’s Disease Study Group ((2019) ) EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord 34: , 353–365. |
[30] | Martinez-Martin P , Reddy P , Katzenschlager R , Antonini A , Todorova A , Odin P , Henriksen T , Martin A , Calandrella D , Rizos A , Bryndum N , Glad A , Dafsari HS , Timmermann L , Ebersbach G , Kramberger MG , Samuel M , Wenzel K , Tomantschger V , Storch A , Reichmann H , Pirtosek Z , Trost M , Svenningsson P , Palhagen S , Volkmann J , Chaudhuri KR ((2015) ) EuroInf: A multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord 30: , 510–516. |
[31] | Wirdefeldt K , Odin P , Nyholm D ((2016) ) Levodopa-carbidopa intestinal gel in patients with Parkinson’s disease: A systematic review. CNS Drugs 30: , 381–404. |
[32] | Martinez-Martin P , Rodriguez-Blazquez C , Abe K , Bhattacharyya KB , Bloem BR , Carod-Artal FJ , Prakash R , Esselink RAJ , Falup-Pecurariu C , Gallardo M , Mir P , Naidu Y , Nicoletti A , Sethi K , Tsuboi Y , van Hilten JJ , Visser M , Zappia M , Chaudhuri KR ((2009) ) International study on the psychometric attributes of the Non-Motor Symptoms Scale in Parkinson disease. Neurology 73: , 1584. |
[33] | Horváth K , Aschermann Z , Ács P , Deli G , Janszky J , Komoly S , Karádi K , Kovács M , Makkos A , Faludi B , Kovács N ((2015) ) Minimal clinically important difference on Parkinson’s Disease Sleep Scale 2nd Version. Parkinsons Dis 2015: , 970534. |
[34] | De Fabregues O , Dot J , Abu-Suboh M , Hernández-Vara J , Ferré A , Romero O , Ibarria M , Seoane JL , Raguer N , Puiggros C , Gómez MR , Quintana M , Armengol JR , Alvarez-Sabín J ((2017) ) Long-term safety and effectivenessof levodopa-carbidopa intestinal gel infusion.. Brain Behav 7: , e00758. |
[35] | AbbVie Inc., A Study to Examine the Effect of Levodopa-Carbidopa Intestinal Gel (LCIG) Therapy Relative to That of Optimized Medical Treatment (OMT) on Non-motor Symptoms (NMS) Associated With Advanced Parkinson’s Disease (PD) (NCT02549092), 2021https://clinicaltrials.gov/ct2/show/results/NCT02549092. |
[36] | Chaudhuri KR , Healy DG , Schapira AHV ((2006) ) Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol 5: , 235–245. |
[37] | Dhawan V , Healy DG , Pal S , Chaudhuri KR ((2006) ) Sleep-related problems of Parkinson’s disease. Age Ageing 35: , 220–228. |
[38] | Politis M , Piccini P , Pavese N , Koh S-B , Brooks DJ ((2008) ) Evidenceof dopamine dysfunction in the hypothalamus of patients withParkinson’s disease: An in vivo 11C-raclopride PET study. Exp Neurol 214: , 112–116. |
[39] | Soileau MJ , Pagan F , Fasano A , Rodriguez-Cruz R , Wang L , Kandukuri PL , Yan CH , Alobaidi A , Bao Y , Kukreja P , Oh M , Siddiqui MS ((2022) ) Comparative effectiveness of carbidopa-levodopa enteral suspension and deep brain stimulation on Parkinson’s disease-related pill burden reduction in advanced Parkinson’s disease: A retrospective real-world cohort study. Neurol Ther 11: , 851–861. |
[40] | Nyholm D , Jansson R , Willows T , Remahl IN ((2005) ) Long-term 24-hour duodenal infusion of levodopa: Outcome and dose requirements. Neurology 65: , 1506–1507. |
[41] | Ciurleo R , Corallo F , Bonanno L , Lo Buono V , Di Lorenzo G , Versaci R , Allone C , Palmeri R , Bramanti P , Marino S ((2018) ) Assessment of Duodopa® effects on quality of life of patients with advanced Parkinson’s diseaseand their caregivers. J Neurol 265: , 2005–2014. |
[42] | Tessitore A , Marano P , Modugno N , Pontieri FE , Tambasco N , Canesi M , Latorre A , Lopiano L , Sensi M , Quatrale R , Solla P , Defazio G , Melzi G , Costanzo AM , Gualberti G , di Luzio Paparatti U , Antonini A ((2018) ) Caregiver burdenand its related factors in advanced Parkinson’s disease: Data from the PREDICT study. J Neurol 265: , 1124–1137. |
[43] | Gomez-Inhiesto E , Acaiturri-Ayesta MT , Ustarroz-Aguirre I , Camahuali D , Urtaran-Laresgoiti M , Basabe-Aldecoa M , Nuño-Solinís R , Urizar E ((2020) ) Direct cost of Parkinson’s disease: A real-world data study of second-line therapies. Parkinsons Dis 2020: , 9106026 |
[44] | Gumber A , Ramaswamy B , Thongchundee O ((2019) ) Effects of Parkinson’s on employment, cost of care, and quality of life of people with condition and family caregivers in the UK: A systematic literature review. Patient Relat Outcome Meas 10: , 321–333. |
[45] | Martinez-Martin P , Macaulay D , Jalundhwala YJ , Mu F , Ohashi E , Marshall T , Sail K ((2019) ) The long-term direct and indirect economic burden among Parkinson’s disease caregivers in the United States. Mov Disord 34: , 236–245. |
[46] | Yang W , Hamilton JL , Kopil C , Beck JC , Tanner CM , Albin RL , Ray Dorsey E , Dahodwala N , Cintina I , Hogan P , Thompson T ((2020) ) Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Parkinsons Dis 6: , 15–15. |
[47] | Bartolomei L , Pastore A , Meligrana L , Sanson E , Bonetto N , Minicuci GM , Marsala SZ , Mesiano T , Bragagnolo L , Antonini A ((2018) ) Relevance of sleep quality on caregiver burden in Parkinson’s disease. Neurol Sci 39: , 835–839. |
[48] | Whetten-Goldstein K , Sloan F , Kulas E , Cutson T , Schenkman M ((1997) ) The burden of Parkinson’s disease on society, family, and the individual. J Am Geriatr Soc 45: , 844–849. |
[49] | Marano M , Naranian T , di Biase L , Di Santo A , Poon Y-Y , Arca R , Cossu G , Marano P , Di Lazzaro V , Fasano A ((2019) ) Complex dyskinesias in Parkinson patients on levodopa/carbidopa intestinal gel. Parkinsonism Relat Disord 69: , 140–146. |
[50] | Buongiorno M , Antonelli F , Cámara A , Puente V , de Fabregues-Nebot O , Hernandez-Vara J , Calopa M , Pascual-Sedano B , Campolongo A , Valldeoriola F , Tolosa E , Kulisevsky J , Martí MJ ((2015) ) Long-term response tocontinuous duodenal infusion of levodopa/carbidopa gel in patients with advanced Parkinson disease: The Barcelonaregistry. Parkinsonism Relat Disord 21: , 871–876. |
[51] | Catalán M-J , Escribano PM , Alonso-Frech F ((2017) ) Dyskinesias in levodopa-carbidopa intestinal gel infusionera: New challenges, new features. Mov Disord 32: , 624–625. |
[52] | Fabbri M , Zibetti M , Calandra-Buonaura G , Contin M , Sambati L , Mohamed S , Romagnolo A , Berchialla P , Imbalzano G , Giannini G , Rizzone MG , Artusi CA , Cortelli P , Lopiano L ((2020) ) Levodopa/carbidopa intestinal gel long-term outcome in Parkinson’s disease: Focus on dyskinesia. Mov Disord Clin Pract 7: , 930–939. |
[53] | Meloni M , Solla P , Mascia MM , Marrosu F , Cannas A ((2017) ) Diphasic dyskinesias during levodopa-carbidopa intestinal gel (LCIG) infusion in Parkinson’s disease. Parkinsonism Relat Disord 37: , 92–96. |
[54] | Fasano A , Ricciardi L , Lena F , Bentivoglio AR , Modugno N ((2012) ) Intrajejunal levodopa infusion in advanced Parkinson’s disease: Long-term effects on motor and non-motor symptoms and impact on patient’s and caregiver’s quality of life. Eur Rev Med Pharmacol Sci 16: , 79–89. |
[55] | Liu XD , Bao Y , Liu Gj ((2019) ) Comparison between levodopa-carbidopa intestinal gel infusion and subthalamic nucleus deep-brain stimulation for advanced Parkinson’s disease: A systematic review and meta-analysis. Front Neurol 10: , 934. |
[56] | Volkmann J , Albanese A , Antonini A , Chaudhuri KR , Clarke CE , de Bie RMA , Deuschl G , Eggert K , Houeto J-L , Kulisevsky J , Nyholm D , Odin P , Østergaard K , Poewe W , Pollak P , Rabey JM , Rascol O , Ruzicka E , Samuel M , Speelman H , Sydow O , Valldeoriola F , van der Linden C , Oertel W ((2013) ) Selecting deep brain stimulation or infusion therapies in advanced Parkinson’s disease: An evidence-based review. J Neurol 260: , 2701–2714. |
[57] | Burack M , Aldred J , Zadikoff C , Vanagunas A , Klos K , Bilir B , Fernandez HH , Standaert DG ((2018) ) Implementing levodopa-carbidopa intestinal gel for Parkinson disease: Insights from US practitioners. Mov Disord Clin Pract 5: , 383–393. |
[58] | Weernink MGM , van Til JA , van Vugt JPP , Movig KLL , Groothuis-Oudshoorn CGM , Ijzerman MJ ((2016) ) Involving patients in weighting benefits and harms of treatment in Parkinson’s disease. PLoS One 11: , e0160771 |
[59] | Titova N , Chaudhuri KR ((2017) ) Personalized medicine in Parkinson’s disease: Time to be precise. Mov Disord 32: , 1147–1154. |
[60] | Odin P , Ray Chaudhuri K , Slevin JT , Volkmann J , Dietrichs E , Martinez-Martin P , Krauss JK , Henriksen T , Katzenschlager R , Antonini A , Rascol O , Poewe W ((2015) ) Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: Consensus from an international survey and discussion program. Parkinsonism Relat Disord 21: , 1133–1144. |
[61] | AbbVie Inc. Data on File. Clinical Study Report: DUOGLOBE, |
[62] | AbbVie Inc. Data on File. Clinical Study Report: DYSCOVER, |
[63] | Alvarez EF , Spanaki C , Pekkonen E , Manzanares L , Liu Y , Sánchez-Soliño O , Barbato L ((2020) ) Effect oflevodopa-carbidopa intestinal gel versus optimized medical treatment on dyskinesia in advanced Parkinson’sdisease patients: Final results of the randomized 12-week DYSCOVER study [abstract].Abstr. No. Mov Disord 35: , 867 |
[64] | AbbVie Inc. Data on File. Clinical Study Report: GLORIA, |
[65] | Cáceres-Redondo MT , Carrillo F , Lama MJ , Huertas-Fernández I , Vargas-González L , Carballo M , Mir P ((2014) ) Long-term levodopa/carbidopa intestinal gel in advanced Parkinson’s disease. J Neurol 261: , 561–569. |
[66] | AbbVie Inc., Levodopa-Carbidopa Intestinal Gel Open-Label Study in Advanced Parkinson’s Disease (NCT00335153),2015, https://clinicaltrials.gov/ct2/show/NCT00335153. |
[67] | Abbot Laboratories, Data on File. Clinical Study Report: An Open-Label, 12-Month Safety and Efficacy Study of Levodopa-Carbidopa Intestinal Gel in Levodopa-Responsive Subjects with Advanced Parkinson’s Disease and Severe Motor Fluctuations Despite Optimized Treatment with Available Parkinson’s Disease Medications. (2012). |
[68] | Fernandez HH , Standaert DG , Hauser RA , Lang AE , Fung VS , Klostermann F , Lew MF , Odin P , Steiger M , Yakupov EZ , Chouinard S , Suchowersky O , Dubow J , Hall CM , Chatamra K , Robieson WZ , Benesh JA , Espay AJ ((2015) ) Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: Final 12-month, open-label results. Mov Disord 30: , 500–509. |
[69] | AbbVie Inc. Data on File. Clinical study report: MONOTREAT, |
[70] | Krüger R , Lingor P , Doskas T , Henselmans JML , Danielsen EH , de Fabregues O , Stefani A , Sensken SC , Parra JC , Onuk K , Yegin A , Antonini A ((2017) ) An observational study of the effect of levodopa-carbidopa intestinal gel on activities of daily living and quality of life in advanced Parkinson’s disease patients. Adv Ther 34: , 1741–1752. |
[71] | Abbott Laboratories, Data on File. Clinical Study Report: A Randomized, Double-Blind, Double-Dummy, Efficacy, Safety, and Tolerability Study of Levodopa-Carbidopa Intestinal Gel in Levodopa-Responsive Parkinson’s Subjects Receiving Optimized Treatments with Parkinson Medicinal Products Who Continue to Experience Persistent Motor Fluctuations. (2012). |
[72] | AbbVie Inc., Study of Efficacy, Safety and Tolerability of Levodopa-Carbidopa Intestinal Gel in Levodopa-Responsive Parkinson’s Subjects (NCT00660387), 2015, https://clinicaltrials.gov/ct2/show/NCT00660387. |
[73] | AbbVie Inc., Study of Efficacy, Safety and Tolerability of Levodopa-Carbidopa Intestinal Gel in Levodopa-Responsive Parkinson’s Subjects (NCT00357994), 2015, https://clinicaltrials.gov/ct2/show/NCT00357994. |
[74] | Abbott Products AG, Data on File. Clinical Study Report: A Long-term Health Economics Study of Intraduodenal Levodopa (Duodopa®) in Routine Care for Subjects with Advanced Idiopathic Parkinson’s Disease with Severe motor Fluctuations and Hyper-/dyskinesiaDAPHNE(Duodopa in Advanced Parkinson’s: Health outcomes&Net Economic impact), (2012). |
[75] | Solvay Pharmaceuticals GmbH, Clinical Trial Results: A long-term health economics study of intraduodenal levodopa (Duodopa®) in routine care for patients with advanced idiopathic Parkinson’s disease with severe motor fluctuations and hyper-/dyskinesia. (EudraCT 2005- 002654-21). 2016. https://www.clinicaltrialsregister.eu/ctr-search/trial/2005-002654-21/results. |
[76] | Pålhagen SE , Sydow O , Johansson A , Nyholm D , Holmberg B , Widner H , Dizdar N , Linder J , Hauge T , Jansson R , Bergmann L , Kjellander S , Marshall TS ((2016) ) Levodopa-carbidopa intestinal gel(LCIG) treatment in routine care of patients with advancedParkinson’s disease: An open-label prospective observational studyof effectiveness, tolerability and healthcare costs. Parkinsonism Relat Disord 29: , 17–23. |
[77] | Reddy P , Martinez-Martin P , Rizos A , Martin A , Faye GC , Forgacs I , Odin P , Antonini A , Chaudhuri KR ((2012) ) Intrajejunal levodopa versus conventional therapy in Parkinson disease: Motor and nonmotor effects. Clin Neuropharmacol 35: , 205–207. |
[78] | Sensi M , Preda F , Trevisani L , Contini E , Gragnaniello D , Capone JG , Sette E , Golfre-Andreasi N , Tugnoli V , Tola MR , Quatrale R ((2014) ) Emerging issues on selection criteria of levodopa carbidopa infusion therapy: Considerations on outcome of 28 consecutive patients. J Neural Transm (Vienna) 121: , 633–642. |
[79] | AbbVie Inc., Safety/Efficacy Study of Levodopa-Carbidopa Intestinal Gel in Parkinson’s Subjects (NCT00360568), 2015. https://clinicaltrials.gov/ct2/show/NCT00360568. |
[80] | Slevin JT , Fernandez HH , Zadikoff C , Hall C , Eaton S , Dubow J , Chatamra K , Benesh J ((2015) ) Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: An open-label extension of the double-blind pivotal study in advanced Parkinson’s disease patients. J Parkinsons Dis 5: , 165–174. |
[81] | AbbVie Inc., Data on File. Clinical Study Report: M12-920. |
[82] | AbbVie Inc., A Study to Assess the Safety and Efficacy of Levodopa-Carbidopa Intestinal Gel (LCIG) for the Treatment of Non-motor Symptoms in Patients With Advanced Parkinson’s Disease (NCT01736176), 2017. https://clinicaltrials.gov/ct2/show/NCT01736176. |




