Immunogenetic Determinants of Parkinson’s Disease Etiology
Abstract
Parkinson’s disease (PD) is increasingly recognised as a systemic disorder in which inflammation might play a causative role rather than being a consequence or an epiphenomenon of the neurodegenerative process. Although growing genetic evidence links the central and peripheral immune system with both monogenic and sporadic PD, our understanding on how the immune system contributes to PD pathogenesis remains a daunting challenge. In this review, we discuss recent literature aimed at exploring the role of known genes and susceptibility loci to PD pathogenesis through immune system related mechanisms. Furthermore, we outline shared genetic etiologies and interrelations between PD and autoimmune diseases and underlining challenges and limitations faced in the translation of relevant allelic and regulatory risk loci to immune-pathological mechanisms. Lastly, with the field of immunogenetics expanding rapidly, we place these insights into a future context highlighting the prospect of immune modulation as a promising disease-modifying strategy.
INTRODUCTION
Heterogeneous and multifactorial in nature, Parkinson’s disease (PD) follows a complex model of inheritance spanning the etiological spectrum ranging from monogenic disease (in a small proportion of affected individuals) to polygenic inheritance (in the vast majority of the cases) where environmental and genetic risk factors interact to induce PD pathology.
Emerging and compelling evidence supports chronic neuroinflammation, derived from impaired innate and/or adaptive immunity mechanisms, as among the main contributors to PD development, in which a pro-inflammatory state may trigger or promote neuronal loss [1]. A growing body of research recognises PD as a systemic disease characterised by the presence of central and peripheral inflammatory processes.
In recent years, extensive research in the PD genetics field has focused on unravelling both coding and non-coding genetic variation that could result in immune defects contributing to PD risk, onset, and progression. Indeed, the advent of high-throughput, high-resolution next-generation sequencing and genome-wide genotyping technologies has been instrumental in the identification of genes and regulatory genomic regions that play a prominent role in immune processes leading to the pathophysiology of the disease.
This review aims to provide an updated overview of the role of monogenic PD genes in the regulation of the immune system as well as our current knowledge on the contribution of recently identified immunogenetic risk factors leading to neurodegeneration in PD. We outline the challenges faced in the translation of relevant allelic and regulatory risk loci to immune-pathological mechanisms and discuss potential shared genetic etiologies between PD and autoimmune diseases. Furthermore, we illustrate the increasing need to generate harmonised large-scale omics, clinical and longitudinal data to enhance our understanding of the immune system involvement in PD etiology that could aid future development of immunomodulatory therapeutic interventions aimed to prevent or delay disease onset.
THE ROLE OF MONOGENIC PARKINSON’S DISEASE KNOWN GENES IN THE REGULATION OF THE IMMUNE SYSTEM
Monogenic PD —PD caused by a defined mutation in a single gene—constitutes about 5–10% of the total PD cases [2]. Mutations in several genes have been identified as causative for monogenic PD with different patterns of inheritance including autosomal dominant, autosomal recessive, and X-linked [3]. Scrutinising the molecular processes through which mutations in such genes culminate in the development of PD, modulation of immune-related pathways emerged as a potential pathogenic mechanism [4, 5]. This has stirred research to dissect the potential roles of monogenic PD-known genes in regulating the immune response [6].
LRRK2
The leucine-rich repeat kinase 2 (LRRK2) gene has been linked to monogenic as well as sporadic PD. LRRK2 mutations are the most frequently reported genetic factors in monogenic PD, representing 5–6% of familial PD cases and 1–2% of sporadic PD with variability in prevalence between different populations [7, 8]. The incomplete penetrance of LRRK2 indicates the involvement of other genetic/environmental components, which might act modifying the pathogenic effects of such mutations [9, 10]. LRRK2 gene encodes a multi-function protein with kinase and GTPase activity that is found associated with PD pathogenesis. The structural homology of LRRK2 with receptor-interacting protein kinases, a family of kinases with known implications in regulating the immune system, highlights the potential role of LRRKs in regulating immune-related pathways that might contribute to PD pathogenesis [11]. This has been further supported by the fact that LRRK2 expression in peripheral immune cells (such as B-cell, T-cell, and monocytes) is markedly upregulated in PD with a coinciding upsurge in the activity of these immune cells [12]. Results from numerous studies advocate that LRRK2 modulates immune responses to favor sustained inflammation and subsequent neurodegeneration in PD patients. For instance, mutated LRRK2 was found to promote neuroinflammation through increasing microglia and macrophages chemotaxis to the brain tissues via modulating pathways controlling cell adhesion, polarisation, and directional motility. LRRK2 is also implicated in modulating processes like phagocytosis, and the production of proinflammatory cytokines [13, 14]. Furthermore, LRRK2 mediates such effects by stimulating receptors like TLR2 and TLR4, and through several downstream kinases, mediators, and cytokines [4, 15–17]. It is noteworthy that LRRK2 effects on the immune response are genotype dependent. This has been observed in mouse models where a gain-of-function mutation (p.G2019S) in the LRRK2 kinase protein resulted in enhanced inflammatory responses, while the kinase inactivating mutation (p.D1994S) produced an opposite effect.
Additionally, studies have shown that LRRK2 mutants contribute to neurodegeneration through exacerbating neuroinflammation via peripheral proinflammatory cytokines. According to recent reports, LRRK2 maintains neuroinflammation through stimulating peripheral immune cells type II interferon responses rather than effects on central microglia [18]. Within the same context, LRRK2 was proved to influence myelopoiesis and peripheral myeloid cell differentiation [19]. Effects of LRRK2 on peripheral immune responses and its implication in PD pathogenesis are currently under intense investigation to analyse the interplay between central and peripheral immune responses and decipher the link between PD and peripheral inflammatory conditions like Crohn’s disease (CD), ulcerative colitis (UC), and leprosy [20, 21].
SNCA
The SNCA gene encodes alpha-synuclein, the histopathological hallmark of PD. Several mutations and genetic rearrangements in SNCA have been linked to autosomal dominant PD [22, 23]. Moreover, the dosage of SNCA has been found closely associated with PD onset, clinical phenotype, and patient’s survival [24]. Given that earlier onset and more rapid disease progression is observed in patients with SNCA gene triplications as compared to those with duplications, a dosage effect of the SNCA gene is expected. These observations indicate a neurotoxic effect of increased alpha-synuclein, even when point mutations are not present. The expression of alpha-synuclein in different immune cells, and the reported colocalization of neuroinflammatory spots at the sites of alpha-synuclein aggregation (Lewy neurites/Lewy bodies) indicate a potential implication of immune-mediated mechanisms regulated by alpha-synuclein in the pathogenesis of PD [25]. Alpha-synuclein has been proved to impact both the innate and adaptive immune responses in a way that favours neurodegeneration and PD development. Acting as a ligand of TLR-2 and TLR4 receptors or via metabolic reprogramming, alpha-synuclein is responsible for launching a proinflammatory response of microglia leading to neuronal demise probably through the release of neurotoxic mediators or phagocytosis of the neuronal cells in the region [26, 27]. Degenerated neurons release intracellularly aggregated alpha-synuclein to the extracellular space leading to further immune stimulation and neurodegeneration, a cascade that eventually leads to PD development. The neuroinflammation can be further aggravated by the microglial and astrocytes major histocompatibility complex class-II (MHC-II) presentation of alpha-synuclein leading to the recruitment and activation of other peripheral phagocytes (such as monocytes, macrophages, and lymphocytes) [28, 29]. Similar inflammatory responses mediated by alpha-synuclein were reported in the enteric nervous system, a finding that implies a potential role of gastrointestinal (GI) infections or inflammatory conditions in PD pathogenesis [30].
Moreover, alpha-synuclein has evident roles in regulating the magnitude and quality of adaptive immune responses in many aspects [31]. For instance, alpha-synuclein was found essential for the development and activation of humoral immunity, since SNCA knock-out mouse models demonstrated impaired B cells development and IgG production [32, 33]. In addition, alpha-synuclein activity is also important for T cell development and differentiation [32]. Deficiency of alpha-synuclein was accompanied by elevated IL2 and Th1 response and decreased IL4 and Th2 differentiation, a finding that further emphasises the role of alpha-synuclein in T cells differentiation and priming [32, 34]. Furthermore, in support of the notion that neurodegeneration in PD might be induced by autoimmune responses, it has been observed that alpha-synuclein induces substantia nigra (SN) neurons to display major histocompatibility complex class-I (MHC-1) presenting immunogenic alpha-synuclein epitopes that are recognized by T cells [35, 36]. Such alpha-synuclein reactive-T cells have been linked to the development of PD and are now proposed as early diagnostic markers of the disease [37].
VPS35
Mutations in the vacuolar protein sorting 35 ortholog (VPS35) gene have been reported in about 1% of monogenic PD and 0.2% of sporadic PD [38]. The VPS35 gene that has been linked to late-onset, autosomal dominant familial PD, encodes a core component of the retromer complex responsible for protein transmembrane sorting through the endosomes -trans-Golgi network pathway [38, 39]. VPS35 is expressed in neuronal immune cells, namely microglia, and astrocytes. Recent studies have reported the role of VPS35 in regulating innate system responses through modulating microglia and astrocytes activation [40, 41]. VPS35 regulates microglial functions and polarisation through modulating the trafficking and recycling of immunomodulating receptors/mediators. Scientific literature exploring the effects of VPS35 on microglia have been contradicting, probably due to variation in the brain anatomical sites and the inducer of neuroinflammation [41, 42]. One report indicated that VPS35 favours the priming of microglial response into a pro-inflammatory rather than an anti-inflammatory response following an ischemic brain injury in the brain cortex [41]. On the contrary, in vitro and murine model studies proved that VPS35 deficiency is associated with an enhanced microglial activation and excessive neuroinflammation through induction of microglial inflammatory mediators in the hippocampus [42, 43]. Furthermore, recent studies suggest that VPS35 interacts with LRRK2. It has been demonstrated that LRRK2 phosphorylates some RAB proteins, which are involved in vesicle trafficking, [44] and the D620N VPS35 mutation is implicated in hyper-activation of LRRK2 kinase pathway [45].
PRKN, PINK1, DJ1
The Parkin RBR E3 ubiquitin-protein ligase (PRKN), PTEN-induced kinase 1 (PINK1), and DJ1 (also known as PARK7) genes are widely known to contribute to autosomal recessive PD and encode proteins that are essential regulators of mitochondrial homeostasis and quality control [46]. PINK1 and PARKIN control mitochondrial turnover via a selective autophagy process known as mitophagy [47]. They are also involved in regulating mitochondrial fusion and fission, local repair of impaired mitochondria, and the genesis of new mitochondria [48]. DJ1 works interactively with PARKIN and PINK1 in the mitochondrial quality control through regulating oxidative stress caused by ROS [49]. The three genes, which are known for their neuroprotective roles, are linked to recessive PD with their mutations reported in 13% of early-onset PD. The similarity in disease phenotype and clinicopathological features suggests similar pathogenic pathways through which the three genes culminate in PD development. Among the reported pathogenic mechanisms through which PRKN, PINK1, and DJ-1 genes contribute to PD is neuroinflammation. Neuroinflammation caused by loss-of-function mutations of these proteins can be mitochondrial-mediated, e.g., impaired mitophagy caused by PARKN/PINK1 deficiency results in the release of mitochondrial DNA and other reactive species that can stimulate innate immunity and trigger inflammation [50, 51]. Furthermore, PRKN/PINK1 loss of function mutations impair the proteins inhibitory effect on mitochondrial antigen presentation through the alternative mitochondrial-derived vesicles (MDV) pathway leading to excessive mitochondrial antigen presentation and stimulation of immune responses according to studies conducted in in vitro and in vivo models [52]. The unleashed mitochondrial antigen presentation through the MDV pathway caused by the loss of PRKN/PINK1 regulatory effect further supports the autoimmune basis of PD. It also supports the role of the brain/gut axis in PD pathogenesis, considering that PINK1-/- mouse models have demonstrated a propagated inflammatory response after bacterial infection with concomitant development of peripheral and neural cytotoxic mitochondrial-specific CD8 + T cells. The consequent decline in dopaminergic neuron density and development of motor impairment in the mice indicate that the PINK1-/- associated autoimmune response likely contributes to dopaminergic neurodegeneration and PD development [52]. Moreover, PRKN/PINK1 deficiency can lead to an exacerbated inflammatory response through inhibition on the NLRP3 inflammasome signalling [53, 54]. Similar to PRKN/PINK1 mutations, DJ-1 dysfunction was observed in CD4 + T cells to enhance neuroinflammation due to the loss of DJ-1 antioxidant effect and regulatory ROS mediated inflammation [55].
Furthermore, these proteins can also induce neuroinflammation through other mitochondrial-independent mechanisms, e.g., PINK1 deficiency was reported to promote innate immune responses through nitric oxide production by glia/astrocytes cells leading to inflammation-induced neuronal death [56]. Additionally, DJ-1 exerts anti-inflammatory effects independently from the ROS regulatory pathways, e.g., through inducing the synthesis of anti-inflammatory mediators like prostaglandin D2, stimulating the migration of CD3 + T cell, and CD4 + T cells differentiation [57, 58].
GBA
Mutations of the glucocerebrosidase gene (GBA), encoding for the lysosomal enzyme β-glucocerebrosidase, are the most prominent genetic risk factor recognized for PD. According to the records, GBA mutations, which are detected in 8–12% of total sporadic PD in the world, increase the risk of developing PD by 5–10 folds in a carrier compared to the general population [59]. GBA is expressed in immune cells including monocytes/macrophages and lymphocytes, and it has been associated with an aberrant inflammatory response mediated by monocytes/macrophages and B cells [60, 61]. In addition, the glucocerebrosidase enzyme activity is generally reduced in monocytes from PD patients compared to control cases according to published reports. Such reduction was found correlated with the disease’s clinical characteristics, which highlights the potential of peripheral monocytes glucocerebrosidase activity as an early marker of PD diagnosis [62, 63].
Moreover, GBA mutations demonstrated association with marked astrocytes and microglial activation indicating that GBA mutations are implicated in launching and/or aggravating neuroinflammatory responses [64, 65]. Such effect is detected early enough before neurodegeneration, a time where therapeutic intervention can be maximally beneficial. Hence, investigating the mechanisms and mediators through which GBA mutations initiate and propagate neuroinflammation is now a hot area of research [66]. Despite the mentioned association of GBA mutations with central and peripheral inflammatory responses, initial investigations of central and peripheral inflammatory cytokines as early markers of PD have returned with negative outcomes [67].
COMMON GENETIC RISK FACTORS CONTRIBUTING TO THE IMMUNE SYSTEM RESPONSE IN PARKINSON’S DISEASE
Since the development of advanced genotyping, sequencing technologies and complex analysis tools, large-scale genetic studies have expanded rapidly and have provided opportunities for a mechanistic elucidation of PD etiology. Since 2009, genome-wide association studies (GWAS) and meta-analyses have shown evidence for an implication of genetic contributors conferring moderate and low risk to PD susceptibility. The largest and latest GWAS meta-analyses conducted in Europeans, Asians, and Latino populations, have nominated a total of 92 loci predisposing to PD as well as several other suggestive genomic regions linked to age at disease onset and progression that warrant further study [68, 69]. PD risk loci such as LRRK2, MAPT, BST1, and HLA among others have been recognized as key players to the immune-mediated response involved in PD development (Table 1) [21], strengthening the hypothesis that common variation plays a crucial role in disease etiology. Furthermore, expression and methylation quantitative trait loci analyses have further nominated possible functional mechanisms by which genetic variants may be contributing to the immune system regulation [21].
Table 1
The implication of PD risk loci in the immune system regulation
| Chr | Nearest gene | Immune system implication | Reference |
| 1 | DJ-1 | By regulating oxidative stress caused by ROS, DJ1 interacts with PARKIN and PINK1 in the mitochondrial quality control pathway. As a result of DJ-1 loss-of-function, ROS are released, which can activate innate immunity and trigger inflammation. | PMID: 22403686, PMID: 19276172 |
| 1 | FCGR2A | FCGR2A is highly expressed in microglia and macrophages, and has prominent role in phagocytosis and debris cleaning (microglia-specific enhancer) | PMID: 34617105 |
| 1 | GBA | Several immune cells express GBA, including monocytes, macrophages, and lymphocytes. GBA deficiency has several immunological effects, such as multi-system inflammation, B-lymphocyte hyperproliferation, increased levels of proinflammatory cytokines, microglial activation, and astrogliosis. | PMID: 33935104, PMID: 26376862 |
| 1 | PINK1 | An analysis of gene expression profiles in PINK1-deficient mice indicated that the loss of PINK1 function modified the expression of immunomodulatory genes in the striatum. | PMID: 21249202 |
| 2 | IL-1 β | Proinflammatory cytokine that increases the degeneration of dopamine neurons in animal models of PD. IL-1 β has been also identified as a mediator of microglia activation. | PMID: 18304357 |
| 2 | IL1R2 | The IL1R2 gene is implicated in neurodegenerative diseases. IL-1R2 expression is suppressed by pro-inflammatory agents, such as LPS and IFN-γ. | PMID: 23195532 |
| 2 | STK39 | The association of STK39 variants with PD points to a potential of this gene on inflammation and oxidative stress. These functional consequences should be further investigated. | PMID: 26469904 |
| 3 | SATB1 | SATB1, a T-lymphocyte-enriched transcription factor and chromatin organiser, is crucial to the regulation of many genes involved in the T-lymphocyte development and activation. | PMID: 10716941, PMID: 17057718 |
| 3 | TLR9 | TLR9 is part of the toll-like receptor family which activates an inflammatory cascade by recognizing mitochondrial DNA as an endogenous danger-associated molecular pattern. The TLR9-mediated inflammatory response has been reported to increase the expression of pro-inflammatory cytokines, such as IL-1β. | PMID: 20347818 |
| 4 | BST1 | The BST1 gene encodes for the leukocyte surface protein CD157, which is highly expressed in bone marrow cells of patients with rheumatoid arthritis, suggesting that it may promote the growth of pre-B lymphocytes. | PMID: 12415565 |
| 4 | SCARB2/LIMP2 | SCARB2, which is highly expressed in plasmacytoid dendritic cells, plays a role in mediating GBA trafficking from endoplasmic reticulum to lysosome. SCARB2 expression regulates the production of IFN and the TLR9-mediated activation of IFN regulatory factor 7 (IRF7). | PMID: 24485911; PMID: 25862818 |
| 4 | SNCA | SNCA encodes alpha-synuclein which acts as a ligand for toll-like receptor 2 (TLR2) on microglia, linking it with the innate immune system. Moreover, TLR2 has been identified as being present on T lymphocytes, B lymphocytes, monocytes, and macrophages. alpha-synuclein epitopes can be presented on MHC molecules and can activate both helper and cytotoxic T-cells. | PMID: 23463005, PMID: 27358579 |
| 6 | ER β | Estrogen has been suggested to have a neuroprotective role in PD, and regulate neuroinflammatory genes, including IL-6. | PMID: 11102464, PMID: 15635591 |
| 6 | HLA-DQA1 | HLA-DQA, is considered to be a key immune system player, and it is found to be increased in PD patients. | PMID: 27148593 |
| 6 | HLA-DRB6 | HLA-DRB6, a component of the major histocompatibility complex, is decreased in PD patients. The change of HLA alleles influences the expression of MHC which leads to the abnormal T cell immunity in PD patients. | PMID: 28892059; PMID: 34548497 |
| 6 | PARK2 | Parkin knockout mice show mitochondrial dysfunction and oxidative stress, which induces inflammatory factors production. Parkin deficient individuals show decreased lymphocyte mitochondrial complex I activity, providing additional evidence that loss of Parkin function causes mitochondrial dysfunction. | PMID: 27345367, PMID: 29665074 |
| 7 | GPNMB | GPNMB is found to be highly expressed in microglia. Inhibition of GPNMB by siRNA significantly reduces the expression of TNF-α, IL-1β and inducible nitric oxide synthase (iNOS) in activated mouse BV2 cells, suggesting that GPNMB is involved in microglia activation and the production of pro-inflammatory cytokines. | PMID: 24682924 |
| 7 | IL-6 | IL-6 is a multifunctional cytokine involved in immune response and inflammation and plays a crucial role in the central nervous system. Previous reports showed that IL-6 is induced by alpha-synuclein and PD-causing mutants in astrocytes and microglia cells. | PMID: 17012252 |
| 8 | PDLIM2 | PDLIM2 has been shown to inhibit the development of T-helper 17 (TH17) cells through the activator of transcription 3 (STAT3) pathway, known to have a pathogenic role in inflammatory disease. | PMID: 22155789 |
| 9 | SH3GL2 | SH3GL2 plays a role in endocytosis and it has been hypothesized that this protein acts downstream of LRRK2 to induce synaptic autophagosome formation and may be deregulated in PD. | PMID: 28282269 |
| 12 | LRRK2 | The rs76904798 variant, which regulates the expression of LRRK2, colocalizes with a peripheral monocyte eQTL. Accordingly, LRRK2 is strongly expressed in B-lymphocytes, T-lymphocytes, and monocytes from PD patients, and it is positively related to cytokine production in T-lymphocytes. | PMID: 30824768 |
| 13 | MBNL2 | The function of MBNL2 is related to postnatal splicing patterns in brains. A previous study showed that knockout MBNL2 related to the proinflammatory process by increasing microglia expression in specific brain regions, such as medial prefrontal cortex and hippocampus. | PMID: 30060068; PMID: 34617105 |
| 17 | MAPT | MAPT, which encodes the tau protein, has been shown to induce morphological transformation of microglia which results in the generation of NO, and proinflammatory cytokines (IL-1β, IL-6, TNF-α). | PMID: 21813771 |
| 17 | ATP6V0A1 | Microglia and their precursors express the ATP6V0A1 gene, involved in the acidification of intracellular compartments and in phagosomal fusion, which is essential for phagocytosis. | PMID: 18510934 |
| 20 | DDRGK1 | DDRGK1 depletion inhibits the expression of NF-κB target genes, suggesting that DDRGK1 is involved in the regulation of the NF-κB pathway through interaction with IκBα. | PMID: 23675531 |
| 21 | DYRK1A | DYRK1A plays an key role in the phosphorylation of several immune response mediators (neuron-specific enhancer). | PMID: 34617105 |
While GWAS meta-analyses have identified increasing numbers of novel genetic risk loci in myriad datasets, recent research in the PD genetics field has focused on understanding how genetic risk variants may disrupt biological processes and drive the underlying pathobiology of the disease. In this context, an exciting era for PD research has arisen with large-scale omics analyses supporting the role of the immune response in the pathophysiology of PD [21, 70, 71]. Based on an unbiased approach applied to large genetic and genomics datasets available to date, pathway-specific polygenic risk scores and transcriptomics analyses have identified that a cumulative effect of common genetic risk variants contribute to the innate and adaptive immune responses, as critical pathways in PD etiology [70]. In concordance with this study, heritability and gene-set enrichment methods aimed at exploring particular functional marks for regulatory activity and gene-set lists have supported the implication of the innate and adaptive immune system in PD etiology and an enrichment for sporadic PD genetic heritability [72].
Interestingly, a more recent study has shown a significant enrichment of PD risk heritability in microglia and monocytes [73]. As an effort to understand the contribution of the immune system in PD pathogenesis, the authors showed that the microglial signature gene, P2RY12, located near a PD GWAS signal colocalizes to a microglia open chromatin region and enhancer region [73]. P2RY12 has been found to be downregulated in Alzheimer’s disease (AD) brain sections of microglia as a marker of inflammation, hence supporting a possible similar role in PD as a targetable microglial gene candidate and pathogenic player [74]. Additionally, a recent genetic analysis of the human microglial transcriptome across brain regions, aging and disease pathologies has nominated microglia-specific enhancers, finding associations with microglial expression of USP6NL for AD and P2RY12 for PD [75].
Furthermore, much attention has been paid to explore the role of immune cells in PD pathogenesis from a genetics perspective. Interestingly, recent studies showed that genes within PD GWAS loci are specifically expressed in T-cells [72]. Of note, GWAS have nominated a transcription factor named SATB1, which is associated with T-cell function and the establishment of immune tolerance [76, 77]. Specifically, the number of infiltrated CD8 positive T-cells is elevated in the PD substantia nigra pars compacta which correlates to neuronal cell loss. In addition, half of CD8 positive T-cells express an immune activation marker named IFNγ [78].
Moreover, PD-associated variants, such as rs76904798 which regulates the expression of LRRK2, had been shown to colocalize with peripheral monocyte expression quantitative trait loci (eQTL) [71]. Recent publications supported the notion that LRRK2 levels were elevated in PD patient monocytes which tended to secret more inflammatory factors and cytokines than healthy subjects [12]. Although LRRK2 plays a regulatory role in immune cells and PD, the mechanism of LRRK2 regulating PD patient monocyte or T-cell gene expression has not been yet unravelled.
Besides immune responses nominated through polygenic risk of biological processes, additional pathways have also been shown to induce immune activation in neurodegenerative disease through common genetic variation, such as the alpha-synuclein pathway and the MAPK signalling pathway [70]. Previous studies demonstrated that microglia activation was induced by aggregated alpha-synuclein, and this phenomenon raised dopaminergic neurotoxicity [79]. An additional supportive study has shown that alpha-synuclein induced the inflammatory factor interleukin-6 (IL-6) through MAPK MEK1/2, JNK and p38 MAPK in human astrocytes [80]. Therefore, genetic variation in the SNCA locus may also be responsible for inducing innate and adaptive immunity [81, 82]. MAPKs are a group of serine/threonine protein kinases participating in the regulation of inflammatory mediator production, stress response and maintenance of the immune system, which contribute to PD pathology [83, 84]. In addition, MAPKs contribute to T-cell differentiation and maturation, as well as immune cell activation [85, 86].
The majority of nominated risk variants are located in intronic and intergenic regions, regulating gene expression. For example, rs1990622, a genetic variant thought to affect cognition in PD, has the ability to enhance long-range chromatin looping by promoting the interaction between the chromatin organising protein CCCTC-binding factor (CTCF) downstream of TMEM106B and distal regulatory elements that in turn act increasing the expression of TMEM106B [87, 88]. TMEM106B has been found to modulate inflammation in the central nervous system (CNS) of post-mortem aged brains [89, 90]. Whole-genome co-expression network analysis nominated five gene clusters derived from frontal cortex data analyses conducted in elder individuals and categorised CNS cell types where these clusters were more differentially expressed, including microglia, astroglia and other neuron-associated groups. Interestingly, the rs1990622 genotype showed a significant elevation in expression with increased risk allele load on the microglia-associated genes cluster [89]. Further analyses confirmed the relationship between microglia gene set expression levels and the rs1990622 risk variant [89]. In this study, authors demonstrated that TMEM106B modulates the polarisation of immune cells towards pro-inflammatory stage of gene expression signature in the risk allele (Thr185) compared with the protective allele (Ser185) of rs1990622 in human monocyte-derived dendritic cells [89]. This suggests that PD-associated SNPs participating in immune response or immune-associated genes might interact with CTCF binding sites, regulating gene expression in harmful neuronal cells, and increasing the risk of neurodegeneration. However, further verification and discussion are needed in PD studies.
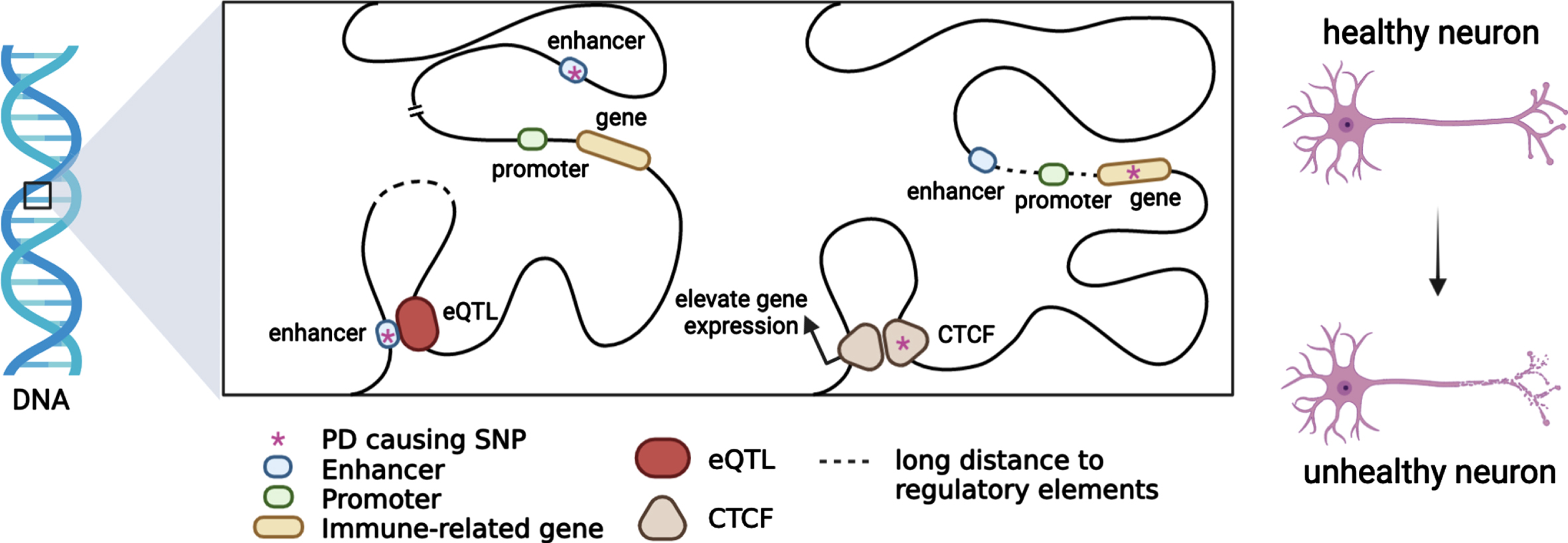
Heritable alterations contributing to regulatory gene expression, such as DNA methylation and histone modifications, which alter chromatin accessibility and gene regulation have been linked to PD etiology. Large-scale analysis also indicated that chromatin remodelling and organisation which is highly integrated in maintaining and controlling chromatin landscape and genome stability play a crucial role in PD pathogenesis [70]. A recent study revealed that BIN1, SORL1 and MEF2C show differentially methylated enhancers in the prefrontal cortex of Alzheimer’s patients [91]. In addition, evidence supports that a portion of genetic variants contributes to active and repressive chromatin states, such as heterochromatin and euchromatin [92]. Epigenetic regulation contributing to the immune system regulation affords an opportunity to develop potential therapeutic strategies in PD as neuroprotective targets in disease onset and progression (Fig. 1).
Fig. 1
Putative functional mechanisms for which PD genetic variants might contribute to the immune system regulation. Most PD risk loci span non-coding regions. When a PD risk SNP with an eQTL mechanism colocalizes with immune-related genes in topologically associating domains, immune-gene regulation will be affected by chromatin interaction. PD causing SNPs may be located in enhancers or promoters, and immune-gene regulation will be affected by trans-acting or cis-acting regulatory mechanisms.
GENETIC COMORBIDITIES BETWEEN PARKINSON’S DISEASE AND AUTOIMMUNE DISEASES
Several genetic factors have been found to be common between PD and autoimmune disorders. For instance, genome-wide analysis identified 17 shared loci adjacent to GAK, HLA-DRB5, LRRK2, and MAPT genes for PD and 7 autoimmune diseases, including rheumatoid arthritis, UCs, and CD, suggesting that moderate and low risk loci contribute to pleiotropy among these diseases [21].
The Human leukocyte antigen (HLA), also known as major histocompatibility complex (MHC) is a family of genes encoding membrane proteins responsible for antigen presentation to T cells, hence playing a crucial role in the adaptive immune response [93] and it is the most reported genetic risk factor associated with autoimmune diseases and has been widely linked to several neurological conditions [94]. The HLA region was first identified to be associated with late onset PD in 2010 and is commonly referred to as the PARK18 locus [95]. More recently, fine-mapping analyses have nominated four HLA types as associated with PD: HLA-DQA1*03:01, HLA-DQB1*03:02, HLA-DRB1*04:01, and HLA-DRB1*04:04 [96]. Furthermore, identification of new SNPs has raised the of PD having an association with regulatory elements effecting the HLA class II gene expression and classical HLA class II allele [97]. Since then, different approaches have been undertaken to further dissect the role of HLA in PD and autoimmune conditions.
Genetic characterization of the HLA-DRB1 locus have revealed shared genetic risk factors associated with rheumatoid arthritis (RA) and PD. Such association remains controversial since the directionality of effect for both diseases is sometimes inconsistent, suggesting either a predisposing (HLA-DRB1*01:01 allele) [98] or protective effect (HLA-DRB1*04 allele) [98, 99]. Therefore, there is an ongoing debate about the direction of the association between PD and RA [100]. In an attempt to further clarify such an association, research aimed at exploring genetic correlations between these diseases has been conducted in European and non-European populations. In a Taiwanese case-study, PD incidence was observably higher in RA patients than in controls [101] and a significant genetic correlation between PD and RA has been reported [102]. More recently, a Mendelian randomization study showed a genome-wide negative correlation, suggesting that RA has a protective effect on PD [103], a conclusion supported by other epidemiological case studies [104].
Likewise, the etiology of CD, an inflammatory bowel disorder, is thought to be partly explained by the contribution of variants in the LRRK2 gene, which is considered among the main genetic contributors to PD risk [105]. Exome sequencing and genotyping of CD cases and controls has recently suggested that the N551K variant in LRRK2 provides protection from CD, while the N2081D variant confers risk, reinforcing the notion that both protective and harmful influences contribute to the complex genetic architecture of immune contributors [20]. Additionally, one of the first identified shared polymorphisms between CD and PD was M2397T [106] which presumably enhances interferon-γ responses in monocytes thus modifying the immune response [107]. Besides LRRK2, other genes have also been studied in CD/PD. The Solute Carrier Family 39 Member 8 (SLC39A8) gene encodes for a membrane manganese transporter protein, and the missense variant A391T has been previously linked to both CD [108] and PD [109].
Additional candidate shared genes between PD and CD have recently come to attention. There are hints that both alpha-synuclein and tau proteins, encoded by the SNCA and MAPT genes respectively, and considered pathological hallmarks in PD, may also be involved in CD: increased expression levels of alpha-synuclein protein were detected in inflamed tissues of CD patients [110] and tau protein expression was found upregulated in the enteric nervous system in CD patients [111]. However, these studies were carried out at a protein level and do not provide enough evidence to assess genetic comorbidity.
Epigenetic changes have also been investigated between PD and autoimmune diseases. Estimation of Pearson’s correlation coefficient between PD and RA revealed that both traits share 337 gene pairs with co-methylation changes [112]. Also, a significant overlap in epigenetic patterns of risk genes for PD, RA, and CD was found in butyrate-associated methylation sites [113]. Investigating PD from an epigenetic angle may clarify the role of immune response and immune-mediated mechanisms.
FUTURE DIRECTIONS
Increasing evidence from recent literature suggests that PD presents genetically as more of a systemic disorder in which inflammation might play a causative role rather than being a consequence or an epiphenomenon of the neurodegenerative process. As discussed, this notion is reinforced by the overlapping genetic pleiotropy that seems to exist between PD and several autoimmune diseases. Additionally, genes implicated in monogenic forms of PD and genome-wide loci linked to idiopathic PD have been seen to be widely implicated in immune system related processes including antigen presentation, inflammation regulation and the complement system.
Despite efforts at dissecting genetic drivers and modifiers of PD etiology that modulate the immune response, gene-environment interactions merit further investigation. Neuroinflammation is triggered by immunological insults likely at the convergence of genetic and environmental factors for which the exact mechanisms of such association remain to be elucidated. The challenge ahead lies in our ability to further dissect immune targets by defining the complex genetic architecture of the immune system contributors to disease risk, onset and progression, as well as its functional implications, and the interplay that exists with the environment.
The field of immunogenetics is rapidly expanding but there are still caveats to be addressed. Most of our current knowledge on the role of the immune system in PD etiology comes from human studies conducted in European populations. As we move forward, shedding light on immunogenetic contributors in Non-European populations and its interaction to specific environmental factors is essential to provide generalised insights into disease pathophysiology. Looking to the future of immunogenetics in PD, multimodal data integration with harmonised large-scale, clinica-longitudinal data and collaborative worldwide initiatives will facilitate translation of immunogenetic factors to specific functional mechanisms. Although much work needs to be done from the genetics and genomics perspective to inform biology, future immune-based therapeutic approaches aimed at modifying the expression of PD risk loci in the right patients and at the right time may succeed.
ACKNOWLEDGMENTS
This work was carried out with the support and guidance of the ‘GP2 Trainee Network’ which is part of the Global Parkinson’s Genetics Program and funded by the Aligning Science Across Parkinson’s (ASAP) initiative.
This research was supported, in part, by the Intramural Research Program of the National Institutes of Health (National Institute on Aging, National Institute of Neurological Disorders and Stroke: project numbers 1ZIA-NS003154, Z01-AG000949-02 and Z01-ES10198).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Tansey MG , Goldberg MS ((2010) ) Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 37: , 510–518. |
[2] | Reed X , Bandrés-Ciga S , Blauwendraat C , Cookson MR ((2019) ) The role of monogenic genes in idiopathic Parkinson’s disease. Neurobiol Dis 124: , 230–239. |
[3] | Bandres-Ciga S , Diez-Fairen M , Kim JJ , Singleton AB ((2020) ) Genetics of Parkinson’s disease: An introspection of its journey towards precision medicine. Neurobiol Dis 137: , 104782. |
[4] | Pierce S , Coetzee GA ((2017) ) Parkinson’s disease-associated genetic variation is linked to quantitative expression of inflammatory genes. PLoS One 12: , e0175882. |
[5] | Nalls MA , Blauwendraat C , Vallerga CL , Heilbron K , Bandres-Ciga S , Chang D , Tan M , Kia DA , Noyce AJ , Xue A , Bras J , Young E , von Coelln R , Simón-Sánchez J , Schulte C , Sharma M , Krohn L , Pihlstrøm L , Siitonen A , Iwaki H , Leonard H , Faghri F , Gibbs JR , Hernandez DG , Scholz SW , Botia JA , Martinez M , Corvol J-C , Lesage S , Jankovic J , Shulman LM , Sutherland M , Tienari P , Majamaa K , Toft M , Andreassen OA , Bangale T , Brice A , Yang J , Gan-Or Z , Gasser T , Heutink P , Shulman JM , Wood NW , Hinds DA , Hardy JA , Morris HR , Gratten J , Visscher PM , Graham RR , Singleton AB , 23andMe ResearchTeam, System Genomics of Parkinson’s Disease Consortium, International Parkinson’s Disease Genomics Consortium ((2019) ) Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol 18: , 1091–1102. |
[6] | Tan E-K , Chao Y-X , West A , Chan L-L , Poewe W , Jankovic J ((2020) ) Parkinson disease and the immune system - associations, mechanisms and therapeutics. Nat Rev Neurol 16: , 303–318. |
[7] | Dächsel JC , Farrer MJ ((2010) ) LRRK2 and Parkinson disease. Arch Neurol 67: , 542–547. |
[8] | Lesage S , Houot M , Mangone G , Tesson C , Bertrand H , Forlani S , Anheim M , Brefel-Courbon C , Broussolle E , Thobois S , Damier P , Durif F , Roze E , Tison F , Grabli D , Ory-Magne F , Degos B , Viallet F , Cormier-Dequaire F , Ouvrard-Hernandez A-M , Vidailhet M , Lohmann E , Singleton A , Corvol J-C , Brice A , French Parkinson disease Genetics Study Group (PDG) ((2020) ) Genetic and phenotypic basis of autosomal dominant Parkinson’s disease in a large multi-center cohort. Front Neurol 11: , 682. |
[9] | Marder K , Wang Y , Alcalay RN , Mejia-Santana H , Tang M-X , Lee A , Raymond D , Mirelman A , Saunders-Pullman R , Clark L , Ozelius L , Orr-Urtreger A , Giladi N , Bressman S , LRRK2 Ashkenazi Jewish Consortium ((2015) ) Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology 85: , 89–95. |
[10] | Lee AJ , Wang Y , Alcalay RN , Mejia-Santana H , Saunders-Pullman R , Bressman S , Corvol J-C , Brice A , Lesage S , Mangone G , Tolosa E , Pont-Sunyer C , Vilas D , Schüle B , Kausar F , Foroud T , Berg D , Brockmann K , Goldwurm S , Siri C , Asselta R , Ruiz-Martinez J , Mondragón E , Marras C , Ghate T , Giladi N , Mirelman A , Marder K , Michael J. Fox LRRK2 Cohort Consortium ((2017) ) Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non-Ashkenazi Jewish ancestry. Mov Disord 32: , 1432–1438. |
[11] | Rideout HJ , Re DB ((2017) ) LRRK2 and the “LRRKtosome” at the crossroads of programmed cell death: Clues from RIP kinase relatives. Adv Neurobiol 14: , 193–208. |
[12] | Cook DA , Kannarkat GT , Cintron AF , Butkovich LM , Fraser KB , Chang J , Grigoryan N , Factor SA , West AB , Boss JM , Tansey MG ((2017) ) LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Parkinsons Dis 3: , 11. |
[13] | Li T , Ning B , Kong L , Dai B , He X , Thomas JM , Sawa A , Ross CA , Smith WW ((2021) ) A LRRK2 GTP binding inhibitor, 68, reduces LPS-induced signaling events and TNF-α release in human lymphoblasts. Cells 10: , 480. |
[14] | Kim KS , Marcogliese PC , Yang J , Callaghan SM , Resende V , Abdel-Messih E , Marras C , Visanji NP , Huang J , Schlossmacher MG , Trinkle-Mulcahy L , Slack RS , Lang AE , Canadian Lrrk2 in Inflammation Team (CLINT), Park DS ((2018) ) Regulation of myeloid cell phagocytosis by LRRK2 via WAVE2 complex stabilization is altered in Parkinson’s disease. Proc Natl Acad Sci U S A 115: , E5164–E5173. |
[15] | Levy DR , Udgata A , Tourlomousis P , Symmons MF , Hopkins LJ , Bryant CE , Gay NJ ((2020) ) The Parkinson’s disease-associated kinase LRRK2 regulates genes required for cell adhesion, polarization, and chemotaxis in activated murine macrophages. J Biol Chem 295: , 10857–10867. |
[16] | Nazish I , Arber C , Piers TM , Warner TT , Hardy JA , Lewis PA , Pocock JM , Bandopadhyay R ((2021) ) Abrogation of LRRK2 dependent Rab10 phosphorylation with TLR4 activation and alterations in evoked cytokine release in immune cells. Neurochem Int 147: , 105070. |
[17] | Atashrazm F , Hammond D , Perera G , Bolliger MF , Matar E , Halliday GM , Schüle B , Lewis SJG , Nichols RJ , Dzamko N ((2019) ) LRRK2-mediated Rab10 phosphorylation in immune cells from Parkinson’s disease patients. Mov Disord 34: , 406–415. |
[18] | Kozina E , Sadasivan S , Jiao Y , Dou Y , Ma Z , Tan H , Kodali K , Shaw T , Peng J , Smeyne RJ ((2018) ) Mutant LRRK2 mediates peripheral and central immune responses leading to neurodegeneration in vivo. Brain 141: , 1753–1769. |
[19] | Park J , Lee J-W , Cooper SC , Broxmeyer HE , Cannon JR , Kim CH ((2017) ) Parkinson disease-associated transgene disrupts marrow myelopoiesis and peripheral Th17 response. J Leukoc Biol 102: , 1093–1102. |
[20] | Hui KY , Fernandez-Hernandez H , Hu J , Schaffner A , Pankratz N , Hsu N-Y , Chuang L-S , Carmi S , Villaverde N , Li X , Rivas M , Levine AP , Bao X , Labrias PR , Haritunians T , Ruane D , Gettler K , Chen E , Li D , Schiff ER , Pontikos N , Barzilai N , Brant SR , Bressman S , Cheifetz AS , Clark LN , Daly MJ , Desnick RJ , Duerr RH , Katz S , Lencz T , Myers RH , Ostrer H , Ozelius L , Payami H , Peter Y , Rioux JD , Segal AW , Scott WK , Silverberg MS , Vance JM , Ubarretxena-Belandia I , Foroud T , Atzmon G , Pe’er I , Ioannou Y , McGovern DPB , Yue Z , Schadt EE , Cho JH , Peter I ((2018) ) Functional variants in the gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci Transl Med 10: , eaai7795. |
[21] | Witoelar A , Jansen IE , Wang Y , Desikan RS , Gibbs JR , Blauwendraat C , Thompson WK , Hernandez DG , Djurovic S , Schork AJ , Bettella F , Ellinghaus D , Franke A , Lie BA , McEvoy LK , Karlsen TH , Lesage S , Morris HR , Brice A , Wood NW , Heutink P , Hardy J , Singleton AB , Dale AM , Gasser T , Andreassen OA , Sharma M , International Parkinson’s Disease Genomics Consortium (IPDGC), North American Brain Expression Consortium (NABEC), and United Kingdom Brain Expression Consortium (UKBEC) Investigators ((2017) ) Genome-wide pleiotropy between Parkinson disease and autoimmune diseases. JAMA Neurol 74: , 780–792. |
[22] | Polymeropoulos MH , Lavedan C , Leroy E , Ide SE , Dehejia A , Dutra A , Pike B , Root H , Rubenstein J , Boyer R , Stenroos ES , Chandrasekharappa S , Athanassiadou A , Papapetropoulos T , Johnson WG , Lazzarini AM , Duvoisin RC , Di Iorio G , Golbe LI , Nussbaum RL ((1997) ) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276: , 2045–2047. |
[23] | Proukakis C , Dudzik CG , Brier T , MacKay DS , Cooper JM , Millhauser GL , Houlden H , Schapira AH ((2013) ) A novel α-synuclein missense mutation in Parkinson disease. Neurology 80: , 1062–1064. |
[24] | Chartier-Harlin M-C , Kachergus J , Roumier C , Mouroux V , Douay X , Lincoln S , Levecque C , Larvor L , Andrieux J , Hulihan M , Waucquier N , Defebvre L , Amouyel P , Farrer M , Destée A ((2004) ) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364: , 1167–1169. |
[25] | Braak H , Sastre M , Del Tredici K ((2007) ) Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol 114: , 231–241. |
[26] | Kim C , Lee H-J , Masliah E , Lee S-J ((2016) ) Non-cell-autonomous Neurotoxicity of α-synuclein Through Microglial Toll-like Receptor 2. Exp Neurobiol 25: , 113–119. |
[27] | Sarkar S , Dammer EB , Malovic E , Olsen AL , Raza SA , Gao T , Xiao H , Oliver DL , Duong D , Joers V , Seyfried N , Huang M , Kukar T , Tansey MG , Kanthasamy AG , Rangaraju S ((2020) ) Molecular signatures of neuroinflammation induced by αSynuclein aggregates in microglial cells. Front Immunol 11: , 33. |
[28] | Rostami J , Fotaki G , Sirois J , Mzezewa R , Bergström J , Essand M , Healy L , Erlandsson A ((2020) ) Astrocytes have the capacity to act as antigen-presenting cells in the Parkinson’s disease brain. J Neuroinflammation 17: , 119. |
[29] | Ferreira SA , Romero-Ramos M ((2018) ) Microglia response during Parkinson’s disease: Alpha-synuclein intervention. Front Cell Neurosci 12: , 247. |
[30] | Stolzenberg E , Berry D , Yang D , Lee EY , Kroemer A , Kaufman S , Wong GCL , Oppenheim JJ , Sen S , Fishbein T , Bax A , Harris B , Barbut D , Zasloff MA ((2017) ) A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun 9: , 456–463. |
[31] | Olesen MN , Christiansen JR , Petersen SV , Jensen PH , Paslawski W , Romero-Ramos M , Sanchez-Guajardo V ((2018) ) CD4 T cells react to local increase of α-synuclein in a pathology-associated variant-dependent manner and modify brain microglia in absence of brain pathology. Heliyon 4: , e00513. |
[32] | Shameli A , Xiao W , Zheng Y , Shyu S , Sumodi J , Meyerson HJ , Harding CV , Maitta RW ((2016) ) A critical role for alpha-synuclein in development and function of T lymphocytes. Immunobiology 221: , 333–340. |
[33] | Xiao W , Shameli A , Harding CV , Meyerson HJ , Maitta RW ((2014) ) Late stages of hematopoiesis and B cell lymphopoiesis are regulated by α-synuclein, a key player in Parkinson’s disease. Immunobiology 219: , 836–844. |
[34] | Ettle B , Kuhbandner K , Jörg S , Hoffmann A , Winkler J , Linker RA ((2016) ) α-Synuclein deficiency promotes neuroinflammation by increasing Th1 cell-mediated immune responses. J Neuroinflammation 13: , 201. |
[35] | Cebrián C , Zucca FA , Mauri P , Steinbeck JA , Studer L , Scherzer CR , Kanter E , Budhu S , Mandelbaum J , Vonsattel JP , Zecca L , Loike JD , Sulzer D ((2014) ) MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun 5: , 3633. |
[36] | Sulzer D , Alcalay RN , Garretti F , Cote L , Kanter E , Agin-Liebes J , Liong C , McMurtrey C , Hildebrand WH , Mao X , Dawson VL , Dawson TM , Oseroff C , Pham J , Sidney J , Dillon MB , Carpenter C , Weiskopf D , Phillips E , Mallal S , Peters B , Frazier A , Lindestam Arlehamn CS , Sette A ((2017) ) T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546: , 656–661. |
[37] | Lindestam Arlehamn CS , Dhanwani R , Pham J , Kuan R , Frazier A , Rezende Dutra J , Phillips E , Mallal S , Roederer M , Marder KS , Amara AW , Standaert DG , Goldman JG , Litvan I , Peters B , Sulzer D , Sette A ((2020) ) α-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat Commun 11: , 1875. |
[38] | Hernandez DG , Reed X , Singleton AB ((2016) ) Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J Neurochem 139: (Suppl 1), 59–74. |
[39] | Williams ET , Chen X , Moore DJ ((2017) ) VPS35, the retromer complex and Parkinson’s disease. J Parkinsons Dis 7: , 219–233. |
[40] | Li J-G , Chiu J , Praticò D ((2020) ) Full recovery of the Alzheimer’s disease phenotype by gain of function of vacuolar protein sorting 35. Mol Psychiatry 25: , 2630–2640. |
[41] | Ye S-Y , Apple JE , Ren X , Tang F-L , Yao L-L , Wang Y-G , Mei L , Zhou Y-G , Xiong W-C ((2019) ) Microglial VPS35 deficiency regulates microglial polarization and decreases ischemic stroke-induced damage in the cortex. J Neuroinflammation 16: , 235. |
[42] | Yin J , Liu X , He Q , Zhou L , Yuan Z , Zhao S ((2016) ) Vps35-dependent recycling of Trem2 regulates microglial function. Traffic 17: , 1286–1296. |
[43] | Appel JR , Ye S , Tang F , Sun D , Zhang H , Mei L , Xiong W-C ((2018) ) Increased microglial activity, impaired adult hippocampal neurogenesis, and depressive-like behavior in microglial VPS35-depleted mice. J Neurosci 38: , 5949–5968. |
[44] | Steger M , Diez F , Dhekne HS , Lis P , Nirujogi RS , Karayel O , Tonelli F , Martinez TN , Lorentzen E , Pfeffer SR , Alessi DR , Mann M ((2017) ) Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife 6: , e31012. |
[45] | Mir R , Tonelli F , Lis P , Macartney T , Polinski NK , Martinez TN , Chou M-Y , Howden AJM , König T , Hotzy C , Milenkovic I , Brücke T , Zimprich A , Sammler E , Alessi DR ((2018) ) The Parkinson’s disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem J 475: , 1861–1883. |
[46] | Day JO , Mullin S ((2021) ) The genetics of Parkinson’s disease and implications for clinical practice. Genes 12: , 1006. |
[47] | Quinn PMJ , Moreira PI , Ambrósio AF , Alves CH ((2020) ) PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol Commun 8: , 189. |
[48] | Ge P , Dawson VL , Dawson TM ((2020) ) PINK1 and Parkin mitochondrial quality control: A source of regional vulnerability in Parkinson’s disease. Mol Neurodegener 15: , 20. |
[49] | Xu S , Yang X , Qian Y , Xiao Q ((2018) ) Parkinson’s disease-related DJ-1 modulates the expression of uncoupling protein 4 against oxidative stress. J Neurochem 145: , 312–322. |
[50] | Borsche M , König IR , Delcambre S , Petrucci S , Balck A , Brüggemann N , Zimprich A , Wasner K , Pereira SL , Avenali M , Deuschle C , Badanjak K , Ghelfi J , Gasser T , Kasten M , Rosenstiel P , Lohmann K , Brockmann K , Valente EM , Youle RJ , Grünewald A , Klein C ((2020) ) Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain 143: , 3041–3051. |
[51] | Sliter DA , Martinez J , Hao L , Chen X , Sun N , Fischer TD , Burman JL , Li Y , Zhang Z , Narendra DP , Cai H , Borsche M , Klein C , Youle RJ ((2018) ) Parkin and PINK1 mitigate STING-induced inflammation. Nature 561: , 258–262. |
[52] | Matheoud D , Sugiura A , Bellemare-Pelletier A , Laplante A , Rondeau C , Chemali M , Fazel A , Bergeron JJ , Trudeau L-E , Burelle Y , Gagnon E , McBride HM , Desjardins M ((2016) ) Parkinson’s disease-related proteins PINK1 and parkin repress mitochondrial antigen presentation. Cell 166: , 314–327. |
[53] | Mouton-Liger F , Rosazza T , Sepulveda-Diaz J , Ieang A , Hassoun S-M , Claire E , Mangone G , Brice A , Michel PP , Corvol J-C , Corti O ((2018) ) Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop. Glia 66: , 1736–1751. |
[54] | Lin Q , Li S , Jiang N , Shao X , Zhang M , Jin H , Zhang Z , Shen J , Zhou Y , Zhou W , Gu L , Lu R , Ni Z ((2019) ) PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol 26: , 101254. |
[55] | Zhou Y , Shi X , Chen H , Zhang S , Salker MS , Mack AF , Föller M , Mak TW , Singh Y , Lang F ((2017) ) DJ-1/Park7 sensitive Na /H exchanger 1 (NHE1) in CD4 T cells. J Cell Physiol 232: , 3050–3059. |
[56] | Sun L , Shen R , Agnihotri SK , Chen Y , Huang Z , Büeler H ((2018) ) Lack of PINK1 alters glia innate immune responses and enhances inflammation-induced, nitric oxide-mediated neuron death. Sci Rep 8: , 383. |
[57] | Choi D-J , An J , Jou I , Park SM , Joe E-H ((2019) ) A Parkinson’s disease gene, DJ-1, regulates anti-inflammatory roles of astrocytes through prostaglandin D synthase expression. Neurobiol Dis 127: , 482–491. |
[58] | Singh Y , Chen H , Zhou Y , Föller M , Mak TW , Salker MS , Lang F ((2015) ) Differential effect of DJ-1/PARK7 on development of natural and induced regulatory T cells. Sci Rep 5: , 17723. |
[59] | Avenali M , Blandini F , Cerri S ((2020) ) Glucocerebrosidase defects as a major risk factor for Parkinson’s disease. Front Aging Neurosci 12: , 97. |
[60] | Panicker LM , Miller D , Park TS , Patel B , Azevedo JL , Awad O , Masood MA , Veenstra TD , Goldin E , Stubblefield BK , Tayebi N , Polumuri SK , Vogel SN , Sidransky E , Zambidis ET , Feldman RA ((2012) ) Induced pluripotent stem cell model recapitulates pathologic hallmarks of Gaucher disease. Proc Natl Acad Sci U S A 109: , 18054–18059. |
[61] | Mizukami H , Mi Y , Wada R , Kono M , Yamashita T , Liu Y , Werth N , Sandhoff R , Sandhoff K , Proia RL ((2002) ) Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Invest 109: , 1215–1221. |
[62] | Hughes LP , Pereira MMM , Hammond DA , Kwok JB , Halliday GM , Lewis SJG , Dzamko N ((2021) ) Glucocerebrosidase activity is reduced in cryopreserved Parkinson’s disease patient monocytes and inversely correlates with motor severity. J Parkinsons Dis 11: , 1157–1165. |
[63] | Atashrazm F , Hammond D , Perera G , Dobson-Stone C , Mueller N , Pickford R , Kim WS , Kwok JB , Lewis SJG , Halliday GM , Dzamko N ((2018) ) Reduced glucocerebrosidase activity in monocytes from patients with Parkinson’s disease. Sci Rep 8: , 15446. |
[64] | Mullin S , Stokholm MG , Hughes D , Mehta A , Parbo P , Hinz R , Pavese N , Brooks DJ , Schapira AHV ((2021) ) Brain microglial activation increased in glucocerebrosidase (GBA) mutation carriers without Parkinson’s disease. Mov Disord 36: , 774–779. |
[65] | Sanyal A , DeAndrade MP , Novis HS , Lin S , Chang J , Lengacher N , Tomlinson JJ , Tansey MG , LaVoie MJ ((2020) ) Lysosome and inflammatory defects in GBA1-mutant astrocytes are normalized by LRRK2 inhibition. Mov Disord 35: , 760–773. |
[66] | Keatinge M , Bui H , Menke A , Chen Y-C , Sokol AM , Bai Q , Ellett F , Da Costa M , Burke D , Gegg M , Trollope L , Payne T , McTighe A , Mortiboys H , de Jager S , Nuthall H , Kuo M-S , Fleming A , Schapira AHV , Renshaw SA , Highley JR , Chacinska A , Panula P , Burton EA , O’Neill MJ , Bandmann O ((2015) ) Glucocerebrosidase 1 deficient Danio rerio mirror key pathological aspects of human Gaucher disease and provide evidence of early microglial activation preceding alpha-synuclein-independent neuronal cell death. Hum Mol Genet 24: , 6640–6652. |
[67] | Thaler A , Omer N , Giladi N , Gurevich T , Bar-Shira A , Gana-Weisz M , Goldstein O , Kestenbaum M , Shirvan JC , Cedarbaum JM , Orr-Urtreger A , Regev K , Shenhar-Tsarfaty S , Mirelman A ((2021) ) Mutations in GBA and LRRK2 are not associated with increased inflammatory markers. J Parkinsons Dis 11: , 1285–1296. |
[68] | Foo JN , Chew EGY , Chung SJ , Peng R , Blauwendraat C , Nalls MA , Mok KY , Satake W , Toda T , Chao Y , Tan LCS , Tandiono M , Lian MM , Ng EY , Prakash K-M , Au W-L , Meah W-Y , Mok SQ , Annuar AA , Chan AYY , Chen L , Chen Y , Jeon BS , Jiang L , Lim JL , Lin J-J , Liu C , Mao C , Mok V , Pei Z , Shang H-F , Shi C-H , Song K , Tan AH , Wu Y-R , Xu Y-M , Xu R , Yan Y , Yang J , Zhang B , Koh W-P , Lim S-Y , Khor CC , Liu J , Tan E-K ((2020) ) Identification of risk loci for Parkinson disease in Asians and comparison of risk between Asians and Europeans: A genome-wide association study. JAMA Neurol 77: , 746–754. |
[69] | Loesch DP , Horimoto ARVR , Heilbron K , Sarihan EI , Inca-Martinez M , Mason E , Cornejo-Olivas M , Torres L , Mazzetti P , Cosentino C , Sarapura-Castro E , Rivera-Valdivia A , Medina AC , Dieguez E , Raggio V , Lescano A , Tumas V , Borges V , Ferraz HB , Rieder CR , Schumacher-Schuh A , Santos-Lobato BL , Velez-Pardo C , Jimenez-Del-Rio M , Lopera F , Moreno S , Chana-Cuevas P , Fernandez W , Arboleda G , Arboleda H , Arboleda-Bustos CE , Yearout D , Zabetian CP , 23andMe Research Team, Cannon P , Thornton TA , O’Connor TD , Mata IF , Latin American Research Consortium on the Genetics of Parkinson’s Disease (LARGE-PD) ((2021) ) Characterizing the genetic architecture of Parkinson’s disease in Latinos. Ann Neurol 90: , 353–365. |
[70] | Bandres-Ciga S , Saez-Atienzar S , Kim JJ , Makarious MB , Faghri F , Diez-Fairen M , Iwaki H , Leonard H , Botia J , Ryten M , Hernandez D , Gibbs JR , Ding J , Gan-Or Z , Noyce A , Pihlstrom L , Torkamani A , Soltis AR , Dalgard CL , American Genome Center, Scholz SW , Traynor BJ , Ehrlich D , Scherzer CR , Bookman M , Cookson M , Blauwendraat C , Nalls MA , Singleton AB , International Parkinson Disease Genomics Consortium ((2020) ) Large-scale pathway specific polygenic risk and transcriptomic community network analysis identifies novel functional pathways in Parkinson disease. Acta Neuropathol 140: , 341–358. |
[71] | Li YI , Wong G , Humphrey J , Raj T ((2019) ) Prioritizing Parkinson’s disease genes using population-scale transcriptomic data. Nat Commun 10: , 994. |
[72] | Gagliano SA , Pouget JG , Hardy J , Knight J , Barnes MR , Ryten M , Weale ME ((2016) ) Genomics implicates adaptive and innate immunity in Alzheimer’s and Parkinson’s diseases. Ann Clin Transl Neurol 3: , 924–933. |
[73] | Andersen MS , Bandres-Ciga S , Reynolds RH , Hardy J , Ryten M , Krohn L , Gan-Or Z , Holtman IR , Pihlstrøm L , International Parkinson’s Disease Genomics Consortium ((2021) ) Heritability enrichment implicates microglia in Parkinson’s disease pathogenesis. Ann Neurol 89: , 942–951. |
[74] | Walker DG , Tang TM , Mendsaikhan A , Tooyama I , Serrano GE , Sue LI , Beach TG , Lue L-F ((2020) ) Patterns of expression of purinergic receptor P2RY12, a putative marker for non-activated microglia, in aged and Alzheimer’s disease brains. Int J Mol Sci 21: , 678. |
[75] | Lopes de K P , Snijders GJL , Humphrey J , Allan A , Sneeboer MAM , Navarro E , Schilder BM , Vialle RA , Parks M , Missall R , van Zuiden W , Gigase FAJ , Kübler R , van Berlekom AB , Hicks EM , Böttcher C , Priller J , Kahn RS , de Witte LD , Raj T ((2022) ) Genetic analysis of the human microglial transcriptome across brain regions, aging and disease pathologies. Nat Genet 54: , 4–17. |
[76] | Chang D , Nalls MA , Hallgrímsdóttir IB , Hunkapiller J , van der Brug M , Cai F , International Parkinson’s Disease Genomics Consortium, 23andMe Research Team, Kerchner GA , Ayalon G , Bingol B , Sheng M , Hinds D , Behrens TW , Singleton AB , Bhangale TR , Graham RR ((2017) ) A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet 49: , 1511–1516. |
[77] | Kondo M , Tanaka Y , Kuwabara T , Naito T , Kohwi-Shigematsu T , Watanabe A ((2016) ) SATB1 plays a critical role in establishment of immune tolerance. J Immunol 196: , 563–572. |
[78] | Galiano-Landeira J , Torra A , Vila M , Bové J ((2020) ) CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson’s disease. Brain 143: , 3717–3733. |
[79] | Zhang W , Wang T , Pei Z , Miller DS , Wu X , Block ML , Wilson B , Zhang W , Zhou Y , Hong J-S , Zhang J ((2005) ) Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J 19: , 533–542. |
[80] | Klegeris A , Giasson BI , Zhang H , Maguire J , Pelech S , McGeer PL ((2006) ) Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J 20: , 2000–2008. |
[81] | Allen Reish HE , Standaert DG ((2015) ) Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J Parkinsons Dis 5: , 1–19. |
[82] | Grozdanov V , Bousset L , Hoffmeister M , Bliederhaeuser C , Meier C , Madiona K , Pieri L , Kiechle M , McLean PJ , Kassubek J , Behrends C , Ludolph AC , Weishaupt JH , Melki R , Danzer KM ((2019) ) Increased immune activation by pathologic α-synuclein in Parkinson’s disease. Ann Neurol 86: , 593–606. |
[83] | Saporito MS , Thomas BA , Scott RW ((2000) ) MPTP activates c-Jun NH(2)-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J Neurochem 75: , 1200–1208. |
[84] | Huang G , Shi LZ , Chi H ((2009) ) Regulation of JNK and p38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine 48: , 161–169. |
[85] | Zhang Y , Nallaparaju KC , Liu X , Jiao H , Reynolds JM , Wang Z-X , Dong C ((2015) ) MAPK phosphatase 7 regulates T cell differentiation via inhibiting ERK-mediated IL-2 expression. J Immunol 194: , 3088–3095. |
[86] | Bachstetter AD , Xing B , de Almeida L , Dimayuga ER , Watterson DM , Van Eldik LJ ((2011) ) Microglial p38α MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Aβ). J Neuroinflammation 8: , 79. |
[87] | Gallagher MD , Posavi M , Huang P , Unger TL , Berlyand Y , Gruenewald AL , Chesi A , Manduchi E , Wells AD , Grant SFA , Blobel GA , Brown CD , Chen-Plotkin AS ((2017) ) A dementia-associated risk variant near TMEM106B alters chromatin architecture and gene expression. Am J Hum Genet 101: , 643–663. |
[88] | Tropea TF , Mak J , Guo MH , Xie SX , Suh E , Rick J , Siderowf A , Weintraub D , Grossman M , Irwin D , Wolk DA , Trojanowski JQ , Van Deerlin V , Chen-Plotkin AS ((2019) ) TMEM106B Effect on cognition in Parkinson disease and frontotemporal dementia. Ann Neurol 85: , 801–811. |
[89] | Rhinn H , Abeliovich A ((2017) ) Differential aging analysis in human cerebral cortex identifies variants in TMEM106B and GRN that regulate aging phenotypes. Cell Syst 4: , 404–415.e5. |
[90] | Ren Y , van Blitterswijk M , Allen M , Carrasquillo MM , Reddy JS , Wang X , Beach TG , Dickson DW , Ertekin-Taner N , Asmann YW , Rademakers R ((2018) ) TMEM106B haplotypes have distinct gene expression patterns in aged brain. Mol Neurodegener 13: , 35. |
[91] | Li P , Marshall L , Oh G , Jakubowski JL , Groot D , He Y , Wang T , Petronis A , Labrie V ((2019) ) Epigenetic dysregulation of enhancers in neurons is associated with Alzheimer’s disease pathology and cognitive symptoms. Nat Commun 10: , 2246. |
[92] | Sharma A , Osato N , Liu H , Asthana S , Dakal TC , Ambrosini G , Bucher P , Schmitt I , Wüllner U ((2019) ) Common genetic variants associated with Parkinson’s disease display widespread signature of epigenetic plasticity. Sci Rep 9: , 18464. |
[93] | Zakharova MY , Belyanina TA , Sokolov AV , Kiselev IS , Mamedov AE ((2019) ) The contribution of major histocompatibility complex class II genes to an association with autoimmune diseases. Acta Naturae 11: , 4–12. |
[94] | Muñiz-Castrillo S , Vogrig A , Honnorat J ((2020) ) Associations between HLA and autoimmune neurological diseases with autoantibodies. Auto Immun Highlights 11: , 2. |
[95] | Hamza TH , Zabetian CP , Tenesa A , Laederach A , Montimurro J , Yearout D , Kay DM , Doheny KF , Paschall J , Pugh E , Kusel VI , Collura R , Roberts J , Griffith A , Samii A , Scott WK , Nutt J , Factor SA , Payami H ((2010) ) Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet 42: , 781–785. |
[96] | Yu E , Ambati A , Andersen MS , Krohn L , Estiar MA , Saini P , Senkevich K , Sosero YL , Sreelatha AAK , Ruskey JA , Asayesh F , Spiegelman D , Toft M , Viken MK , Sharma M , Blauwendraat C , Pihlstrøm L , Mignot E , Gan-Or Z ((2021) ) Fine mapping of the HLA locus in Parkinson’s disease in Europeans. NPJ Parkinsons Dis 7: , 84. |
[97] | Hill-Burns EM , Factor SA , Zabetian CP , Thomson G , Payami H ((2011) ) Evidence for more than one Parkinson’s disease-associated variant within the HLA region. PLoS One 6: , e27109. |
[98] | Hollenbach JA , Norman PJ , Creary LE , Damotte V , Montero-Martin G , Caillier S , Anderson KM , Misra MK , Nemat-Gorgani N , Osoegawa K , Santaniello A , Renschen A , Marin WM , Dandekar R , Parham P , Tanner CM , Hauser SL , Fernandez-Viña M , Oksenberg JR ((2019) ) A specific amino acid motif of mediates risk and interacts with smoking history in Parkinson’s disease. Proc Natl Acad Sci U S A 116: , 7419–7424. |
[99] | Chuang Y-H , Lee P-C , Vlaar T , Mulot C , Loriot M-A , Hansen J , Lill CM , Ritz B , Elbaz A ((2017) ) Pooled analysis of the HLA-DRB1 by smoking interaction in Parkinson disease. Ann Neurol 82: , 655–664. |
[100] | Noyce AJ , Bandres-Ciga S , Kim J , Heilbron K , Kia D , Hemani G , Xue A , Lawlor DA , Smith GD , Duran R , Gan-Or Z , Blauwendraat C , Gibbs JR , 23andMe Research Team5, International Parkinson’s Disease Genomics Consortium (IPDGC), Hinds DA , Yang J , Visscher P , Cuzick J , Morris H , Hardy J , Wood NW , Nalls MA , Singleton AB ((2019) ) The Parkinson’s Disease Mendelian Randomization Research Portal. Mov Disord 34: , 1864–1872. |
[101] | Chang C-C , Lin T-M , Chang Y-S , Chen W-S , Sheu J-J , Chen Y-H , Chen J-H ((2018) ) Autoimmune rheumatic diseases and the risk of Parkinson disease: A nationwide population-based cohort study in Taiwan. Ann Med 50: , 83–90. |
[102] | Li CY , Yang TM , Ou RW , Wei QQ , Shang HF ((2021) ) Genome-wide genetic links between amyotrophic lateral sclerosis and autoimmune diseases. BMC Med 19: , 27. |
[103] | Li C , Ou R , Shang H ((2021) ) Rheumatoid arthritis decreases risk for Parkinson’s disease: A Mendelian randomization study. NPJ Parkinsons Dis 7: , 17. |
[104] | Bacelis J , Compagno M , George S , Pospisilik JA , Brundin P , Naluai ÅT , Brundin L ((2021) ) Decreased risk of Parkinson’s disease after rheumatoid arthritis diagnosis: A nested case-control study with matched cases and controls. J Parkinsons Dis 11: , 821–832. |
[105] | Derkinderen P , Neunlist M ((2018) ) Crohn’s and Parkinson disease: Is LRRK2 lurking around the corner? Nat Rev Gastroenterol Hepatol 15: , 330–331. |
[106] | Liu Z , Lee J , Krummey S , Lu W , Cai H , Lenardo MJ ((2011) ) The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol 12: , 1063–1070. |
[107] | Ikezu T , Koro L , Wolozin B , Farraye FA , Strongosky AJ , Wszolek ZK ((2020) ) Crohn’s and Parkinson’s disease-associated LRRK2 mutations alter type II interferon responses in human CD14+blood monocytes. J Neuroimmune Pharmacol 15: , 794–800. |
[108] | Li D , Achkar J-P , Haritunians T , Jacobs JP , Hui KY , D’Amato M , Brand S , Radford-Smith G , Halfvarson J , Niess J-H , Kugathasan S , Büning C , Schumm LP , Klei L , Ananthakrishnan A , Aumais G , Baidoo L , Dubinsky M , Fiocchi C , Glas J , Milgrom R , Proctor DD , Regueiro M , Simms LA , Stempak JM , Targan SR , Törkvist L , Sharma Y , Devlin B , Borneman J , Hakonarson H , Xavier RJ , Daly M , Brant SR , Rioux JD , Silverberg MS , Cho JH , Braun J , McGovern DPB , Duerr RH ((2016) ) A pleiotropic missense variant in SLC39A8 is associated with Crohn’s disease and human gut microbiome composition. Gastroenterology 151: , 724–732. |
[109] | Pickrell JK , Berisa T , Liu JZ , Ségurel L , Tung JY , Hinds DA ((2016) ) Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet 48: , 709–717. |
[110] | Prigent A , Lionnet A , Durieu E , Chapelet G , Bourreille A , Neunlist M , Rolli-Derkinderen M , Derkinderen P ((2019) ) Enteric alpha-synuclein expression is increased in Crohn’s disease. Acta Neuropathol 137: , 359–361. |
[111] | Prigent A , Chapelet G , De Guilhem de Lataillade A , Oullier T , Durieu E , Bourreille A , Duchalais E , Hardonnière K , Neunlist M , Noble W , Kerdine-Römer S , Derkinderen P , Rolli-Derkinderen M ((2020) ) Tau accumulates in Crohn’s disease gut. FASEB J 34: , 9285–9296. |
[112] | Tang G , Pan H , Xu L , Feng R , Jiang Y , Kong F , Hu S ((2018) ) A comparison of co-methylation relationships between rheumatoid arthritis and Parkinson’s disease. Front Neurosci 12: , 1001. |
[113] | Xie A , Ensink E , Li P , Gordevičius J , Marshall LL , George S , Andrew Pospisilik J , Aho VTE , Houser MC , Pereira PAB , Rudi K , Paulin L , Tansey MG , Auvinen P , Brundin P , Brundin L , Labrie V , Scheperjans F (2021) Butyrate and related epigenetic changes link Parkinson’s disease to inflammatory bowel disease and depressive symptoms. medRxiv 2021.09.17.21263343. |




