T Lymphocytes in Parkinson’s Disease
Abstract
T cells are key mediators of both humoral and cellular adaptive immune responses, and their role in Parkinson’s disease (PD) is being increasingly recognized. Several lines of evidence have highlighted how T cells are involved in both the central nervous system and the periphery, leading to a profound imbalance in the immune network in PD patients. This review discusses the involvement of T cells in both preclinical and clinical studies, their importance as feasible biomarkers of motor and non-motor progression of the disease, and recent therapeutic strategies addressing the modulation of T cell response.
INTRODUCTION
There is growing evidence suggesting the crucial involvement of T cells in Parkinson’s disease (PD). T cells are essential mediators of humoral and cellular adaptive immune responses: highly specific receptor-mediated clonal selection and expansion of T cells allow both antigen-specific immunity and immunological memory against known pathogens [1]. It is known that the precursors of T cells migrate to the thymus and develop into two distinct subsets, CD4 + and CD8 + cells, according to their peculiar surface markers. Before their activation, T cells are in the naïve condition, and once in the circulation can interact with antigen-presenting cells displaying foreign or self-antigens. Previous studies have shown that T cells play a key role both in the central nervous system (CNS) and in the periphery, leading to a profound imbalance in the immune network of PD patients.
EVIDENCE OF T CELL INVOLVEMENT FROM ANIMAL MODELS AND NEUROPATHOLOGY: MORE CD4 + THAN CD8+?
In α-synuclein overexpression animal models, early infiltration of both CD4 + and CD8 + T cells was observed [2], and T cells enhanced the number of α-synuclein aggregates by promoting a pro-inflammatory M1 phenotype in CNS myeloid cells [3]. The crucial role of T cells was further supported by the examination of postmortem human PD brains: Brochard et al. found CD8 + and CD4 + T cells, but not B cells, either in close contact with blood vessels or near melanized dopamine-containing neurons [4]. Interestingly, T cell-mediated dopaminergic toxicity was almost exclusively arbitrated by CD4 + T cells [4], as also confirmed in a neurotoxic-driven animal model [5] and from in vitro and in vivo data [6]. Furthermore, in α-synuclein overexpression models, the genetic deletion of T cell receptor (TCR)β or CD4, as well as the use of the immunosuppressive drug fingolimod, reduced the CNS myeloid major histocompatibility complex (MHC)II response to α-synuclein, whereas the authors did not observe after the knockout of CD8 + T cells any significant effect on preventing the myeloid MHCII response or dopaminergic neuronal loss [7]. The interaction between CD8 + T cells and MHCI on neurons was also assessed, reporting increased MHCI expression in and around virally transduced neurons (including dopamine neurons) and in CNS myeloid cells, but not astrocytes [7].
α-SYNUCLEIN-SPECIFIC T CELL RESPONSES
A seminal study by Sulzer et al. explored whether T cells recognize epitopes derived from α-synuclein and found that the Y39 and S129 regions act as epitopes [8]. More in detail, epitopes derived from the Y39 region were displayed by two MHC class II beta chain alleles as well as an additional MHC class II allele and an MHC class I allele, with an immune response mostly mediated by interleukin (IL-5)-secreting CD4 + T cells and interferon (IFN)γ CD8 + cytotoxic T cells [8]. Furthermore, it was reported that α-synuclein-specific T cell activation was predominant in early-stage PD [9].
EVIDENCE OF T CELL INVOLVEMENT FROM ANIMAL MODELS AND NEUROPATHOLOGY: MORE CD8 + THAN CD4+?
Even though several lines of evidence point to the crucial role of CD4 + T cells in the pathogenesis of PD, the involvement of CD8 + T cells should be highlighted as well.
Firstly, it is known that dopamine neurons can express MHCI in response to IFN-γ, which makes them susceptible to cell death by cytotoxic CD8 + T cells [10]. In an experimental PINK1-/- mouse model of PD, the authors hypothesized that intestinal infection may act as the precipitating event in the establishment of a cytotoxic mitochondria-specific response both in the periphery and the brain [11]. Based on neuropathological evidence, a recent study [12] assessed T cell infiltration in human substantia nigra pars compacta (SNc) throughout different PD stages (one group with α-synuclein aggregates only in the olfactory bulb representing the earliest stage of the disease and the second group with α-synuclein aggregates in the SN). Nigral cytotoxic CD8 + T cell infiltration was robust in the earliest stage of the disease when no α-synuclein aggregation and dopaminergic neuronal death were present yet, whereas in the next stage neuronal loss was accompanied by a milder CD8 + T cell infiltration, thus suggesting that CD8 + T cell-mediated attack may trigger neuronal death and synucleinopathy.
CHANGES OF PERIPHERAL CD4 + AND CD8 + T CELLS IN PD PATIENTS
It is conceivable that the alteration of T cells in the CNS is mirrored in the periphery, likely as a consequence of blood-brain barrier disruption in PD patients [13].
Regarding CD8 + T cells, recent research by Yan et al. suggested that naïve CD8 + T cells were significantly decreased in the peripheral blood of PD patients, whereas IFN-γ–producing CD8 + T cells were increased [14]. An increase in peripheral CD8 + T cells was similarly observed in other studies [15, 16], but conflicting evidence detecting no significant differences compared with healthy controls was reported as well [17–19]. Another group [20] showed a reduction in CD8 + terminally differentiated effector memory re-expressing CD45RA (TEMRA) cells and a lower expression of the cell-aging marker p16, suggesting an attenuated shift towards CD8 + T cells senescence at the earliest stages of PD.
Furthermore, several studies found reduced levels of circulating CD3 + and CD4 + T cells [15, 16, 19, 21, 22]. A meta-analysis including 21 case-control studies and 943 PD patients confirmed that the numbers of CD3 + and CD4 + T cells were significantly decreased in PD [23]. In contrast with these results, another study found that PD patients had an increase in the percentage of CD3 + and CD4 + T s and the CD4 + /CD8 + ratio [24], whereas other groups did not find any significant difference in the percentage of both CD4 + and CD8 + between PD patients and controls [17, 18, 25]. Undoubtedly, the composition of peripheral T cells from PD patients in the reported studies was quite heterogeneous, which could be explained by the influence of ethnic variations or other relevant disease-related confounders. For example, a study by Bhatia et al. found that many factors, including age, sex, disease duration, and disease severity were associated with variations in T cell pathology, with disease severity being the most significant one [26].
Different CD4 + T cell subsets orchestrate specific immune functions
Concerning CD4 + T cells, specific subsets are known to orchestrate different immune functions [27]: T helper (Th)1 and Th17 target bacterial and viral pathogens mainly through the release of IFN-γ, IL-17A, IL-21, and other pro-inflammatory cytokines. Th2 activity is focused on parasitic and allergic responses, in particular through IL-4, IL-5, and IL-13, which act as anti-inflammatory cytokines. Regulatory T cells (Tregs) modulate T cell activation and inflammation.
Imbalance of peripheral CD4 + T cell subsets in PD: Th1 and Th17
Chen et al. [21] observed in the peripheral blood of PD patients an increased proportion of circulating Th1 and Th17 cells and a decreased number of Th2 and Tregs. Compared with the control group, the Th1/Th2 and Th17/Treg ratios were significantly increased with a shift towards Th1 and Th17 subsets. The prominent role of pro-inflammatory Th1 and Th17 was further supported in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD: naïve CD4 + T cells treated with α-synuclein showed a polarization towards the Th1 or Th17 phenotype, thus causing cell death of dopaminergic neurons in the SN and exacerbating MPTP-induced cell death [5].
It was shown that Th1 cells may be relevant in the altered immune network of PD. This subgroup differentiates under the influence of IFN-γ and IL-12 released by antigen-presenting cells, and the release of Th1-derived pro-inflammatory cytokines is crucial for the activation of B cells and the phagocytosis of microbes [28]. Intriguingly, in PD patients, the shift towards Th1 cells was associated with motor function scores as assessed through the Unified Parkinson’s Disease Rating Scale (UPDRS)-part III [21]. Kustrimovic et al. reported no significant correlations between circulating CD4 + T cells, dopamine receptor (DR) expression, transcription factors mRNA levels, and demographic and clinical features of PD patients [22]. Nonetheless, the shift towards Th1 lineage was confirmed in both drug-naïve and drug-treated patients, and was associated with profound modifications of transcription factor genes expression and increased production of IFN-γ and tumor necrosis factor (TNF)-α. Modifications of the transcription factors network in CD4 + T cells occur early in PD, and the absence of correlations with patients’ characteristics suggests that the alteration of CD4 + T cell differentiation mechanisms is independent of PD progression and severity and antiparkinsonian treatment [22]. The imbalance in CD4 + T cells transcription factors could be of great interest since it represents a peculiar molecular signature shared by idiopathic REM sleep behavior disorder and PD patients [29] as well as potential biomarkers of motor complications [30].
The pro-inflammatory bias could be promoted by the Th17 subpopulation as well. This specific subset is mainly involved in host defense against extracellular pathogens and plays a central role in the pathophysiology of several autoimmune diseases through the production of IL-17, IL-17F, IL-21, IL-22, and granulocyte-macrophage colony-stimulating factor (GM-CSF) [31]. Increased levels of Th17 in early-stage PD patients were reported in several studies [14, 21, 32], even though conflicting results observing no differences or reduced levels of Th17 cells were described as well [22, 33]. A recent study also found that there were significant correlations between Th17 cells and the subscales I and II of the MDS-UPDRS [14].
Regarding in vitro evidence and animal models, the critical role of Th17-driven inflammation was further explored in a recent work [6] employing autologous co-cultures of activated T cells and induced pluripotent stem cells (iPSC)-derived midbrain neurons of 10 PD patients and 10 controls. After co-culture with T cells or the addition of IL-17, PD iPSC-derived midbrain neurons underwent increased neuronal death driven by upregulation of IL-17 receptor (IL-17R), whereas blockage of IL-17 or IL-17R prevented neuronal death. Furthermore, the co-culture of MPTP-treated neurons with Th17 cells further exacerbated neuronal cell death and increased IL-1α and TNF-α levels [34]: Liu et al. found that these effects were mediated via lymphocyte function-associated antigen 1 (LFA-1) and intracellular adhesion molecule-1 (ICAM-1), and the blocking of either LFA-1 in Th17 cells or ICAM-1 in ventral mesencephalic neurons abolished Th17-induced dopaminergic neuronal death. Taken together, these results suggest that counteracting Th17 development could represent a feasible therapeutic option in PD. The restriction of Th17 development and differentiation can be achieved through different compounds, for example, the peroxisome proliferator-activated receptor-gamma [35], or through the reduction of transcription factors RORγt and STAT3 via cytokines such as IL-4 or IL-32 [31].
Imbalance of peripheral CD4 + T cell subsets in PD: Th2 and Tregs
The prevalence of a pro-inflammatory phenotype in PD is also favored by an altered anti-inflammatory response promoted by Th2 and Treg cells. Th2 cells differentiate from naïve T cells under the influence of IL-4 and the activation of the GATA3 and STAT6 transcription factors. The cytokines most typically associated with Th2 cells are IL-4, IL-5, IL-9, and IL-13, and combinations of these cytokines drive B cell proliferation and immunoglobulin class-switching to immunoglobulin E (IgE), eosinophilia, mastocytosis, and macrophage polarization to an M2-like phenotype [36]. Several studies have observed lower absolute numbers and frequency of Th2 cells in PD compared with healthy controls [15, 22], with increased mRNA levels of both GATA3 and STAT6 [22]. Interestingly, increased levels of STAT6 were also reported in PD patients with motor fluctuations [30], suggesting the suitable targeting of Th2 cells in the complex stage of the disease. On the other side, Alvarez-Luquin et al. demonstrated no significant difference in Th2 cell counts in PD patients compared with controls, even though a significant increase in IL-13 levels was observed [33], and also significantly increased levels of IL-4-producing Th2 have been recently reported [14].
Regulatory T cells (Tregs) represent another T cell subset possibly involved in the disruption of immune mechanisms. Tregs are responsible for the preservation of immune tolerance and inhibition of autoimmunity. They act as negative regulators of inflammation [37] through the secretion of anti-inflammatory cytokines, in particular IL-10 and TGF-β, and express granzyme A to kill effector cells in a perforin-dependent manner [38]. It was previously reported that PD patients display an impaired ability to suppress effector T cell function [39] and reduced absolute numbers of Tregs have been found as well [15, 22, 33]. Intriguingly, dysregulation of the Treg compartment was also associated in PD patients with crucial non-motor symptoms, such as cognitive impairment [40] and constipation [41].
Concerning animal studies, Reynolds et al. demonstrated a neuroprotective role for Tregs in the MPTP mouse model of PD: the adoptive transfer of CD3-activated Tregs to MPTP-intoxicated mice protected the nigrostriatal system in a dose-dependent manner [42], probably by attenuating Th17-mediated neurodegeneration [5]. Also in the MPTP mouse model examined by Li et al., Treg transfer along with anti-TNFα antibody administration increased Tregs and reduced Th1 cells leading to an amelioration of PD severity [43].
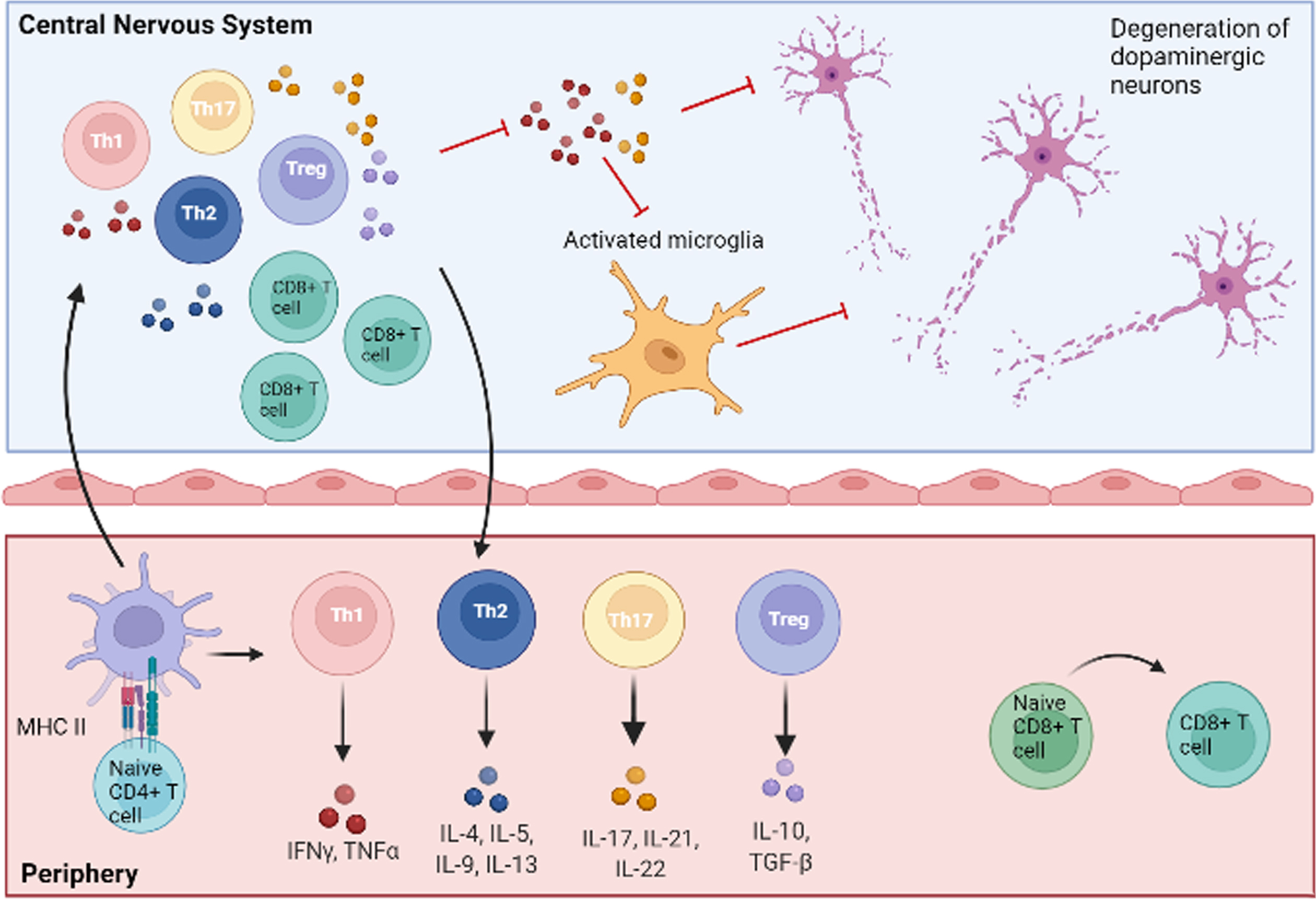
Alterations of CD8 + and CD4 + T cells in PD are summarized in Table 1 and Fig. 1.
Table 1
Summary of peripheral T changes in PD patients
| Peripheral blood alterations of T cells in PD | |||
| Finding | Population | Nation | Reference |
| ↓ Naïve CD4 + and naïve CD8 + T lymphocytes | 41 treated PD patients, 40 HC | USA | [14] |
| ↓ CD3 + and CD4 + T lymphocytes, no difference in CD8 + T lymphocytes | 127 treated PD patients, 148 HC | China | [19] |
| 32 drug-naïve PD patients, 20 HC | Mexico | [33] | |
| ↓ CD4 + T lymphocytes | 60 treated PD patients, 40 HC | China | [21] |
| 26 drug-naïve and 56 treated PD patients, 47 HC | Italy | [22] | |
| ↓ CD4 + and ↑ CD8 + T lymphocytes | 33 treated PD patients, 34 HC | Japan | [15] |
| ↑ CD3 + and CD4 + T lymphocytes, no difference in CD8 + T lymphocytes | 761 treated PD patients, 761 HC | China | [24] |
| No difference in CD4 + and CD8 + T lymphocytes | 10 treated PD patients, 13 HC | Germany | [17] |
| 268 PD patients, 268 HC | China | [18] | |
| 40 treated PD patients, 25 HC | Brazil | [25] | |
| ↑ Th1 and Th17, ↓ Th2 and Treg | 60 treated PD patients, 40 HC | China | [21] |
| ↑ Th1, ↓ Th2, Th1/17, Th17, Treg | 26 drug-naïve and 56 treated PD patients, 47 HC | Italy | [22] |
| ↑ Th17, Th2, no differences in Th1 and Treg | 41 treated PD patients, 40 HC | USA | [14] |
| ↓ Th2 and Treg, no difference in Th1 | 20 treated PD patients, 20 HC | Japan | [15] |
| ↑ Th17 | 18 drug-naïve PD patients and 18 HC | China | [32] |
| ↓ suppressor Treg, active Treg, type-1 regulatory T cells; no difference in Th1, Th2, Th17 | 32 drug-naïve PD patients, 20 HC | Mexico | [33] |
Fig. 1
Central and peripheral involvement of T cells in PD. Naïve CD4 + and CD8 + T lymphocytes are activated in the periphery after the interaction with antigen-presenting cells. CD4 + T cells then differentiate into pro-inflammatory (Th1, Th17) or anti-inflammatory (Th2, Treg) subtypes, characterized by the release of specific patterns of cytokines. Activated T cells can reach the central nervous system by crossing an altered blood-brain barrier, thus polarizing resident cells to pro-inflammatory or anti-inflammatory phenotypes. In particular, Th1 and Th17 subsets release pro-inflammatory molecules (TNF-α, IFN-γ, IL-17, IL-21, IL-22), which, in concert with other mechanisms, lead to neuronal damage and death. Detrimental pro-inflammatory pathways are indicated with red lines. Figure created with BioRender.com.
Imbalance of peripheral CD4 + T cell subsets in PD: Role of dopaminergic treatment
Several works have also explored whether dopaminergic drugs may play a significant role in regulating lymphocyte subsets in PD. Kustrimovic et al. [22, 44] did not suggest relevant effects of antiparkinsonian treatment on the peripheral immune system of PD patients. Similarly, Chen et al. found a weak association between the percentage of CD4 + T cells and the levodopa equivalent daily dose [24]. In another study [45], the negative correlation between the levels of T cytotoxic cells 1 (CD8 + Tbet+IFN-γ+) and T cytotoxic cells 2 (CD8 + GATA3 + IL-13+) with the Hoehn and Yahr scale score was observed only in patients receiving treatment with levodopa, thus suggesting that levodopa could affect T cytotoxic cells. Furthermore, it should be noticed that human and murine lymphocytes express all the five subtypes of DR, and the DRD2 agonist sumanirole was able to inhibit the shift to the Th1 and Th17 phenotypes of CD4 + T cells obtained from MPTP-intoxicated mice [46].
T CELL IMMUNITY AND GUT MICROBIOTA
Whether the peculiar immune profile observed in PD patients arises from the periphery, favoring subsequent neuroinflammation, or is a consequence of peripheral leakage of CNS-derived antigens, has not been fully clarified. Among peripheral sources, intestinal immune activation and dysbiosis could represent one potential driver of PD inflammatory state. There is increasing research interest in the gut-brain axis: several studies have suggested in PD an association between gastrointestinal inflammation and the accumulation of α-synuclein in the enteric nervous system [47]. Moreover, a relationship between inflammatory bowel diseases (IBD) and PD has been reported [48, 49], and a recent study showed a significant reduction in the risk of developing PD in IBD patients receiving early treatment with anti-TNF-α therapy [50].
Regarding animal models, chronic mild focal intestinal inflammation accelerated brain neuropathology and motor dysfunction in α-synuclein mutant mice [51]. Additionally, when α-synuclein overexpressing mice were colonized with microbiota from PD patients, enhanced physical impairment and neuroinflammation were observed compared with microbiota transplants from healthy human donors [52].
It was shown that PD patients display an altered composition of several gut microbiome taxa [53]. Among these, Lactobacillaceae may induce Th1-type immune responses [54], whereas Prevotellaceae abundance was associated with augmented Th17-mediated mucosal inflammation [55]. Another study evaluating fecal DNA samples from 69 PD patients and 244 controls reported that, among the microbiota-associated epitopes involved in inflammatory pathways, two were involved in T cell responses [56]. Based on these observations, it could be speculated that T cell-related immunity, triggered by the aggregation of α-synuclein in the gut mucosa, may promote further CNS neuroinflammation and neurodegeneration. Nonetheless, the complex interaction between intestinal mechanisms, the enteric nervous system, the immune system, the CNS, and environmental factors, is yet to be fully elucidated.
THE CONNECTION BETWEEN PD GENETIC FACTORS AND T CELLS
Finally, in this complex scenario, genetic factors should be considered as well: the association between human leukocyte antigen genes and PD was explored in several studies [57, 58] and a large-scale meta-analysis including more than 100,000 subjects [59]. Other lines of evidence found that the knockout of the α-synuclein gene affected IL-2 production by CD4 + T cells and the frequency of Tregs in mice [60]. The role of α-synuclein deficiency in promoting a pro-inflammatory immune response was also observed in experimental autoimmune encephalomyelitis models of multiple sclerosis [61, 62]. The LRRK2 G2019S gene altered myeloid cell differentiation in transgenic rats, leading to decreased Th17 cell activity [63]. Furthermore, PINK1–/– T cells exhibited a reduced suppressive function despite normal FoxP3 expression kinetics [64]. A recent study [11] reported that the intestinal infection with gram-negative bacteria in PINK1–/– mice leads to autoimmune mechanisms eliciting cytotoxic mitochondria-specific CD8 + T cells, thus highlighting the role of PINK1 as a repressor of the immune system and supporting the relevance of the gut-brain axis as a triggering event in PD. Taken together, these results provide evidence that PD-associated genetic mutations could influence the immune network and suggest that specific subsets of patients with a genetic predisposition could be more suitable for immune-targeted therapies.
FUTURE PERSPECTIVES
A deeper understanding of the peripheral immune system in PD has widened research avenues to explore whether it is a suitable target for disease-modifying therapies. In particular, the possibility of immune escape mechanisms in PD has built the premise of re-establishing immunological tolerance as a key strategy. In this context, compounds acting on the Treg compartment, i.e., vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), and GM-CSF, have been explored in recent literature [65]. VIP-receptor 2 peptide agonist (LBT-3627) attenuated neuroinflammation by promoting the restoration of Treg activity in both 6-hydroxydopamine (6-OHDA) and α-synuclein overexpression rat models [66]. Similarly, PACAP exerted a neuroprotective effect in the rotenone-induced snail and 6-OHDA-induced rat models of PD. [67]. The adoptive transfer of GM-CSF-induced Tregs to MPTP mice was able to protect nigral neurons through the activation of immune-based neuronal protection pathways linked to the upregulation of IL-27 [68]. Further evidence was provided in a study carried out by Thome et al., who found that ex vivo expansion of dysfunctional Tregs restored suppressive function by diminishing multiple pro-inflammatory pathways in myeloid cells and inhibiting responder T cell proliferation [69]. Regarding clinical trials, the subcutaneous administration of sargramostim (a human recombinant GM-CSF) at 6μg/kg/day for 56 days, increased the numbers of Tregs and determined modest improvement in the UPDRS-III after 6 and 8 weeks of treatment when compared with placebo [70]. Since some adverse events were noticed, another study [71] explored long-term sargramostim treatment at 3μg/kg/day in 5 PD patients. Reductions in adverse events, as well as an increase in peripheral blood Treg numbers, function, and hypomethylation of upstream FoxP3 DNA elements, were observed. Furthermore, there was no worsening of motor function scores for any subject during the course of treatment. An alternative approach to enhance the Treg compartment is to isolate and purify Tregs from peripheral blood, expand them in vitro, and administer autologous infusions of expanded Tregs, as reported in a recent phase I trial involving patients with amyotrophic lateral sclerosis [72]. Another feasible strategy could be represented by targeting T cells through immunosuppressant drugs, i.e., azathioprine. Azathioprine is a pro-drug of 6-mercaptopurine, a purine antagonist that inhibits leukocyte proliferation by interfering with nucleotide synthesis [73]. A phase 2 trial is currently exploring whether the suppression of the peripheral immune system using azathioprine has a disease-modifying effect in PD [74]. Additionally, glatiramer acetate, an FDA-approved treatment for multiple sclerosis which improves Th2 and Treg function, was investigated as a potential disease-modifying treatment in PD: in the MPTP murine model, this compound was able to reverse motor dysfunction, promote the recovery of tyrosine hydroxylase protein expression in the striatum and the levels of brain derived neurotrophic factor, and reduce the microglial activation marker IBA1 [75].
CONCLUSION
The present review highlighted how the dysregulation of central and peripheral T cells may play a key role in PD. Nonetheless, several unanswered questions remain: 1) Is the peripheral activation of T cells a primary event leading to neurodegeneration, or is it a secondary response caused by neuronal injury? 2) What is the exact relationship between the alteration of T cell subsets in the blood and the CNS of PD patients? 3) Which are the potential applications of T cell changes as diagnostic and therapeutic biomarkers? 4) What is the role of genetic stratification in identifying PD subjects susceptible to T cell impairment and T cell-targeted therapies? Moreover, a thorough understanding of the role of PD medication and the use of comparable methodologies (i.e., use of standardized markers for the identification of T cell subsets) are warranted to avoid contradictory findings. If these issues will be correctly tackled, the modulation of T cell response could hopefully slow or even halt neuronal damage through the restoration of immune balance, thus providing new therapeutic avenues in the management of PD patients.
ACKNOWLEDGMENTS
This research was supported by Fondazione CARIPLO http://www.fondazionecariplo.it (Project 2011-0504), and by the AGING PROJECT—Department of Excellence—Università del Piemonte Orientale. This paper is part of the PhD program of Clinical and Experimental Medicine and Medical Humanities (University of Insubria) for L.M. and Medical Science and Biotechnology for E.C. These PhD programs and therefore this paper constitute an important part of the project Recruiting and training Physician-Scientists to empower translational research, funded by Fondazione Cariplo.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Lee H-G , Cho M-Z , Choi J-M ((2020) ) Bystander CD4+T cells: Crossroads between innate and adaptive immunity. Exp Mol Med 52: , 1255–1263. |
[2] | Sanchez-Guajardo V , Febbraro F , Kirik D , Romero-Ramos M ((2010) ) Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One 5: , e8784. |
[3] | Sommer A , Fadler T , Dorfmeister E , Hoffmann A-C , Xiang W , Winner B , Prots I ((2016) ) Infiltrating T lymphocytes reduce myeloid phagocytosis activity in synucleinopathy model. J Neuroinflammation 13: , 174. |
[4] | Brochard V , Combadière B , Prigent A , Laouar Y , Perrin A , Beray-Berthat V , Bonduelle O , Alvarez-Fischer D , Callebert J , Launay J-M , Duyckaerts C , Flavell RA , Hirsch EC , Hunot S ((2009) ) Infiltration of CD4+lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119: , 182–192. |
[5] | Reynolds AD , Stone DK , Hutter JAL , Benner EJ , Mosley RL , Gendelman HE ((2010) ) Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol 184: , 2261–2271. |
[6] | Sommer A , Marxreiter F , Krach F , Fadler T , Grosch J , Maroni M , Graef D , Eberhardt E , Riemenschneider MJ , Yeo GW , Kohl Z , Xiang W , Gage FH , Winkler J , Prots I , Winner B ((2018) ) Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson’s disease. Cell Stem Cell 23: , 123–131.e6. |
[7] | Williams GP , Schonhoff AM , Jurkuvenaite A , Gallups NJ , Standaert DG , Harms AS ((2021) ) CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 144: , 2047–2059. |
[8] | Sulzer D , Alcalay RN , Garretti F , Cote L , Kanter E , Agin-Liebes J , Liong C , McMurtrey C , Hildebrand WH , Mao X , Dawson VL , Dawson TM , Oseroff C , Pham J , Sidney J , Dillon MB , Carpenter C , Weiskopf D , Phillips E , Mallal S , Peters B , Frazier A , Lindestam Arlehamn CS , Sette A ((2017) ) T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546: , 656–661. |
[9] | Lindestam Arlehamn CS , Dhanwani R , Pham J , Kuan R , Frazier A , Rezende Dutra J , Phillips E , Mallal S , Roederer M , Marder KS , Amara AW , Standaert DG , Goldman JG , Litvan I , Peters B , Sulzer D , Sette A ((2020) ) α-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat Commun 11: , 1875. |
[10] | Cebrián C , Zucca FA , Mauri P , Steinbeck JA , Studer L , Scherzer CR , Kanter E , Budhu S , Mandelbaum J , Vonsattel JP , Zecca L , Loike JD , Sulzer D ((2014) ) MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun 5: , 3633. |
[11] | Matheoud D , Cannon T , Voisin A , Penttinen A-M , Ramet L , Fahmy AM , Ducrot C , Laplante A , Bourque M-J , Zhu L , Cayrol R , Le Campion A , McBride HM , Gruenheid S , Trudeau L-E , Desjardins M ((2019) ) Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1-/- mice. Nature 571: , 565–569. |
[12] | Galiano-Landeira J , Torra A , Vila M , Bové J ((2020) ) CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson’s disease. Brain 143: , 3717–3733. |
[13] | Kortekaas R , Leenders KL , van Oostrom JCH , Vaalburg W , Bart J , Willemsen ATM , Hendrikse NH ((2005) ) Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57: , 176–179. |
[14] | Yan Z , Yang W , Wei H , Dean MN , Standaert DG , Cutter GR , Benveniste EN , Qin H ((2021) ) Dysregulation of the adaptive immune system in patients with early-stage Parkinson disease. Neurol Neuroimmunol Neuroinflamm 8: , e1036. |
[15] | Baba Y , Kuroiwa A , Uitti RJ , Wszolek ZK , Yamada T ((2005) ) Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord 11: , 493–498. |
[16] | Wang P , Yao L , Luo M , Zhou W , Jin X , Xu Z , Yan S , Li Y , Xu C , Cheng R , Huang Y , Lin X , Ma K , Cao H , Liu H , Xue G , Han F , Nie H , Jiang Q ((2021) ) Single-cell transcriptome and TCR profiling reveal activated and expanded T cell populations in Parkinson’s disease. Cell Discov 7: , 52. |
[17] | Schröder JB , Pawlowski M , Meyer zu Hörste G , Gross CC , Wiendl H , Meuth SG , Ruck T , Warnecke T ((2018) ) Immune cell activation in the cerebrospinal fluid of patients with Parkinson’s disease. Front Neurol 9: , 1081. |
[18] | Cen L , Yang C , Huang S , Zhou M , Tang X , Li K , Guo W , Wu Z , Mo M , Xiao Y , Chen X , Yang X , Huang Q , Chen C , Qu S , Xu P ((2017) ) Peripheral lymphocyte subsets as a marker of Parkinson’s disease in a Chinese population. Neurosci Bull 33: , 493–500. |
[19] | Sun C , Zhao Z , Yu W , Mo M , Song C , Si Y , Liu Y ((2019) ) Abnormal subpopulations of peripheral blood lymphocytes are involved in Parkinson’s disease. Ann Transl Med 7: , 637–637. |
[20] | Kouli A , Jensen M , Papastavrou V , Scott KM , Kolenda C , Parker C , Solim IH , Camacho M , Martin-Ruiz C , Williams-Gray CH ((2021) ) T lymphocyte senescence is attenuated in Parkinson’s disease. J Neuroinflammation 18: , 228. |
[21] | Chen Y , Qi B , Xu W , Ma B , Li L , Chen Q , Qian W , Liu X , Qu H ((2015) ) Clinical correlation of peripheral CD4+-cell sub-sets, their imbalance and Parkinson’s disease. Mol Med Rep 12: , 6105–6111. |
[22] | Kustrimovic N , Comi C , Magistrelli L , Rasini E , Legnaro M , Bombelli R , Aleksic I , Blandini F , Minafra B , Riboldazzi G , Sturchio A , Mauri M , Bono G , Marino F , Cosentino M ((2018) ) Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: Cross-sectional studies of CD4+Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients. J Neuroinflammation 15: , 205. |
[23] | Jiang S , Gao H , Luo Q , Wang P , Yang X ((2017) ) The correlation of lymphocyte subsets, natural killer cell, and Parkinson’s disease: A meta-analysis. Neurol Sci 38: , 1373–1380. |
[24] | Chen X , Feng W , Ou R , Liu J , Yang J , Fu J , Cao B , Chen Y , Wei Q , Shang H ((2021) ) Evidence for peripheral immune activation in Parkinson’s disease. Front Aging Neurosci 13: , 617370. |
[25] | Rocha NP , Assis F , Scalzo PL , Vieira ÉLM , Barbosa IG , de Souza MS , Christo PP , Reis HJ , Teixeira AL ((2018) ) Reduced activated T lymphocytes (CD4+CD25+) and plasma levels of cytokines in Parkinson’s disease. Mol Neurobiol 55: , 1488–1497. |
[26] | Bhatia D , Grozdanov V , Ruf WP , Kassubek J , Ludolph AC , Weishaupt JH , Danzer KM ((2021) ) T-cell dysregulation is associated with disease severity in Parkinson’s disease. J Neuroinflammation 18: , 250. |
[27] | Fang P , Li X , Dai J , Cole L , Camacho JA , Zhang Y , Ji Y , Wang J , Yang X-F , Wang H ((2018) ) Immune cell subset differentiation and tissue inflammation. J Hematol Oncol 11: , 97. |
[28] | MacMahon Copas AN , McComish SF , Fletcher JM , Caldwell MA ((2021) ) The pathogenesis of Parkinson’s disease: A complex interplay between astrocytes, microglia, and T lymphocytes? Front Neurol 12: , 666737. |
[29] | De Francesco E , Terzaghi M , Storelli E , Magistrelli L , Comi C , Legnaro M , Mauri M , Marino F , Versino M , Cosentino M ((2021) ) CD4+T-cell transcription factors in idiopathic REM sleep behavior disorder and Parkinson’s disease. Mov Disord 36: , 225–229. |
[30] | Contaldi E , Magistrelli L , Milner AV , Cosentino M , Marino F , Comi C ((2021) ) Expression of transcription factors in CD4+T cells as potential biomarkers of motor complications in Parkinson’s disease. J Parkinsons Dis 11: , 507–514. |
[31] | Prots I , Winner B ((2019) ) Th17 cells: A promising therapeutic target for Parkinson’s disease? Expert Opin Ther Targets 23: , 309–314. |
[32] | Chen S , Liu Y , Niu Y , Xu Y , Zhou Q , Xu X , Wang J , Yu M ((2017) ) Increased abundance of myeloid-derived suppressor cells and Th17 cells in peripheral blood of newly-diagnosed Parkinson’s disease patients. Neurosci Lett 648: , 21–25. |
[33] | Álvarez-Luquín DD , Arce-Sillas A , Leyva-Hernández J , Sevilla-Reyes E , Boll MC , Montes-Moratilla E , Vivas-Almazán V , Pérez-Correa C , Rodríguez-Ortiz U , Espinoza-Cárdenas R , Fragoso G , Sciutto E , Adalid-Peralta L ((2019) ) Regulatory impairment in untreated Parkinson’s disease is not restricted to Tregs: Other regulatory populations are also involved. J Neuroinflammation 16: , 212. |
[34] | Liu Z , Huang Y , Cao B-B , Qiu Y-H , Peng Y-P ((2017) ) Th17 cells induce dopaminergic neuronal death via LFA-1/ICAM-1 interaction in a mouse model of Parkinson’s disease. Mol Neurobiol 54: , 7762–7776. |
[35] | de Oliveira Boldrini V , Dos Santos Farias A , Degasperi GR ((2019) ) Deciphering targets of Th17 cells fate: From metabolism to nuclear receptors. Scand J Immunol 90: , e12793. |
[36] | Walker JA , McKenzie ANJ ((2018) ) TH2 cell development and function. Nat Rev Immunol 18: , 121–133. |
[37] | Duffy SS , Keating BA , Perera CJ , Moalem-Taylor G ((2018) ) The role of regulatory T cells in nervous system pathologies. J Neuro Res 96: , 951–968. |
[38] | Grossman WJ , Verbsky JW , Barchet W , Colonna M , Atkinson JP , Ley TJ ((2004) ) Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 21: , 589–601. |
[39] | Saunders JAH , Estes KA , Kosloski LM , Allen HE , Dempsey KM , Torres-Russotto DR , Meza JL , Santamaria PM , Bertoni JM , Murman DL , Ali HH , Standaert DG , Mosley RL , Gendelman HE ((2012) ) CD4+regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol 7: , 927–938. |
[40] | Magistrelli L , Storelli E , Rasini E , Contaldi E , Comi C , Cosentino M , Marino F ((2020) ) Relationship between circulating CD4+T lymphocytes and cognitive impairment in patients with Parkinson’s disease. Brain Behav Immun 89: , 668–674. |
[41] | Chen Y , Yu M , Liu X , Qu H , Chen Q , Qian W , Wei D , Xu W , Ma B , Wu W ((2015) ) Clinical characteristics and peripheral T cell subsets in Parkinson’s disease patients with constipation. Int J Clin Exp Pathol 8: , 2495–2504. |
[42] | Reynolds AD , Banerjee R , Liu J , Gendelman HE , Lee Mosley R ((2007) ) Neuroprotective activities of CD4+CD25+regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol 82: , 1083–1094. |
[43] | Li W , Luo Y , Xu H , Ma Q , Yao Q ((2021) ) Imbalance between T helper 1 and regulatory T cells plays a detrimental role in experimental Parkinson’s disease in mice. J Int Med Res 49: , 300060521998471. |
[44] | Kustrimovic N , Rasini E , Legnaro M , Bombelli R , Aleksic I , Blandini F , Comi C , Mauri M , Minafra B , Riboldazzi G , Sanchez-Guajardo V , Marino F , Cosentino M ((2016) ) Dopaminergic receptors on CD4+T naive and memory lymphocytes correlate with motor impairment in patients with Parkinson’s disease. Sci Rep 6: , 33738. |
[45] | Álvarez-Luquín DD , Guevara-Salinas A , Arce-Sillas A , Espinosa-Cárdenas R , Leyva-Hernández J , Montes-Moratilla EU , Adalid-Peralta L ((2021) ) Increased Tc17 cell levels and imbalance of naïve/effector immune response in Parkinson’s disease patients in a two-year follow-up: A case control study. J Transl Med 19: , 378. |
[46] | Liu Z , Zhai X-R , Du Z-S , Xu F-F , Huang Y , Wang X-Q , Qiu Y-H , Peng Y-P ((2021) ) Dopamine receptor D2 on CD4+T cells is protective against neuroinflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain Behav Immun 98: , 110–121. |
[47] | Chen Q-Q , Haikal C , Li W , Li J-Y ((2019) ) Gut inflammation in association with pathogenesis of Parkinson’s disease. Front Mol Neurosci 12: , 218. |
[48] | Wan Q-Y , Zhao R , Wu X-T ((2020) ) Older patients with IBD might have higher risk of Parkinson’s disease. Gut 69: , 193–194. |
[49] | Zhu F , Li C , Gong J , Zhu W , Gu L , Li N ((2019) ) The risk of Parkinson’s disease in inflammatory bowel disease: A systematic review and meta-analysis. Dig Liver Dis 51: , 38–42. |
[50] | Peter I , Dubinsky M , Bressman S , Park A , Lu C , Chen N , Wang A ((2018) ) Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol 75: , 939–946. |
[51] | Kishimoto Y , Zhu W , Hosoda W , Sen JM , Mattson MP ((2019) ) Chronic mild gut inflammation accelerates brain neuropathology and motor dysfunction in α-synuclein mutant mice. Neuromolecular Med 21: , 239–249. |
[52] | Sampson TR , Debelius JW , Thron T , Janssen S , Shastri GG , Ilhan ZE , Challis C , Schretter CE , Rocha S , Gradinaru V , Chesselet M-F , Keshavarzian A , Shannon KM , Krajmalnik-Brown R , Wittung-Stafshede P , Knight R , Mazmanian SK ((2016) ) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167: , 1469–1480.e12. |
[53] | Hill-Burns EM , Debelius JW , Morton JT , Wissemann WT , Lewis MR , Wallen ZD , Peddada SD , Factor SA , Molho E , Zabetian CP , Knight R , Payami H ((2017) ) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32: , 739–749. |
[54] | de Azevedo MSP , Innocentin S , Dorella FA , Rocha CS , Mariat D , Pontes DS , Miyoshi A , Azevedo V , Langella P , Chatel J-M ((2013) ) Immunotherapy of allergic diseases using probiotics or recombinant probiotics. J Appl Microbiol 115: , 319–333. |
[55] | Larsen JM ((2017) ) The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 151: , 363–374. |
[56] | Li Z , Lu G , Li Z , Wu B , Luo E , Qiu X , Guo J , Xia Z , Zheng C , Su Q , Zeng Y , Chan WY , Su X , Cai Q , Xu Y , Chen Y , Wang M , Poon WS , Luo X ((2021) ) Altered actinobacteria and firmicutes phylum associated epitopes in patients with Parkinson’s disease. Front Immunol 12: , 632482. |
[57] | Hamza TH , Zabetian CP , Tenesa A , Laederach A , Montimurro J , Yearout D , Kay DM , Doheny KF , Paschall J , Pugh E , Kusel VI , Collura R , Roberts J , Griffith A , Samii A , Scott WK , Nutt J , Factor SA , Payami H ((2010) ) Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet 42: , 781–785. |
[58] | Puschmann A , Verbeeck C , Heckman MG , Soto-Ortolaza AI , Lynch T , Jasinska-Myga B , Opala G , Krygowska-Wajs A , Barcikowska M , Uitti RJ , Wszolek ZK , Ross OA ((2011) ) Human leukocyte antigen variation and Parkinson’s disease. Parkinsonism Relat Disord 17: , 376–378. |
[59] | International Parkinson’s Disease Genomics Consortium (IPDGC), Parkinson’s Study Group (PSG) Parkinson’s Research: The Organized GENetics Initiative (PROGENI), 23andMe, GenePD, NeuroGenetics Research Consortium (NGRC), Hussman Institute of Human Genomics (HIHG), The Ashkenazi Jewish Dataset Investigator, Cohorts for Health and Aging Research in Genetic Epidemiology (CHARGE), North American Brain Expression Consortium (NABEC), United Kingdom Brain Expression Consortium (UKBEC), Greek Parkinson’s Disease Consortium, Alzheimer Genetic Analysis Group, Nalls MA , Pankratz N , Lill CM , Do CB , Hernandez DG , Saad M , DeStefano AL , Kara E , Bras J , Sharma M , Schulte C , Keller MF , Arepalli S , Letson C , Edsall C , Stefansson H , Liu X , Pliner H , Lee JH , Cheng R , Ikram MA , Ioannidis JPA , Hadjigeorgiou GM , Bis JC , Martinez M , Perlmutter JS , Goate A , Marder K , Fiske B , Sutherland M , Xiromerisiou G , Myers RH , Clark LN , Stefansson K , Hardy JA , Heutink P , Chen H , Wood NW , Houlden H , Payami H , Brice A , Scott WK , Gasser T , Bertram L , Eriksson N , Foroud T , Singleton AB ((2014) ) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 46: , 989–993. |
[60] | Shameli A , Xiao W , Zheng Y , Shyu S , Sumodi J , Meyerson HJ , Harding CV , Maitta RW ((2016) ) A critical role for alpha-synuclein in development and function of T lymphocytes. Immunobiology 221: , 333–340. |
[61] | Ettle B , Kuhbandner K , Jörg S , Hoffmann A , Winkler J , Linker RA ((2016) ) α-Synuclein deficiency promotes neuroinflammation by increasing Th1 cell-mediated immune responses. J Neuroinflammation 13: , 201. |
[62] | Trudler D , Levy-Barazany H , Nash Y , Samuel L , Sharon R , Frenkel D ((2020) ) Alpha synuclein deficiency increases CD4+T-cells pro-inflammatory profile in a Nurr1-dependent manner. J Neurochem 152: , 61–71. |
[63] | Park J , Lee J-W , Cooper SC , Broxmeyer HE , Cannon JR , Kim CH ((2017) ) Parkinson disease-associated LRRK2 G2019S transgene disrupts marrow myelopoiesis and peripheral Th17 response. J Leukoc Biol 102: , 1093–1102. |
[64] | Ellis GI , Zhi L , Akundi R , Büeler H , Marti F ((2013) ) Mitochondrial and cytosolic roles of PINK1 shape induced regulatory T-cell development and function: Molecular immunology. Eur J Immunol 43: , 3355–3360. |
[65] | Nasrolahi A , Safari F , Farhoudi M , Khosravi A , Farajdokht F , Bastaminejad S , Sandoghchian Shotorbani S , Mahmoudi J ((2019) ) Immune system and new avenues in Parkinson’s disease research and treatment. Rev Neurosci 30: , 709–727. |
[66] | Mosley RL , Lu Y , Olson KE , Machhi J , Yan W , Namminga KL , Smith JR , Shandler SJ , Gendelman HE ((2019) ) A synthetic agonist to vasoactive intestinal peptide receptor-2 induces regulatory T cell neuroprotective activities in models of Parkinson’s disease. Front Cell Neurosci 13: , 421. |
[67] | Maasz G , Zrinyi Z , Reglodi D , Petrovics D , Rivnyak A , Kiss T , Jungling A , Tamas A , Pirger Z ((2017) ) Pituitary adenylate cyclase-activating polypeptide (PACAP) has a neuroprotective function in dopamine-based neurodegeneration in rat and snail parkinsonian models. Dis Model Mech 10: , 127–139. |
[68] | Kosloski LM , Kosmacek EA , Olson KE , Mosley RL , Gendelman HE ((2013) ) GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice. J Neuroimmunol 265: , 1–10. |
[69] | Thome AD , Atassi F , Wang J , Faridar A , Zhao W , Thonhoff JR , Beers DR , Lai EC , Appel SH ((2021) ) Ex vivo expansion of dysfunctional regulatory T lymphocytes restores suppressive function in Parkinson’s disease. NPJ Parkinsons Dis 7: , 41. |
[70] | Gendelman HE , Zhang Y , Santamaria P , Olson KE , Schutt CR , Bhatti D , Shetty BLD , Lu Y , Estes KA , Standaert DG , Heinrichs-Graham E , Larson L , Meza JL , Follett M , Forsberg E , Siuzdak G , Wilson TW , Peterson C , Mosley RL ((2017) ) Evaluation of the safety and immunomodulatory effects of sargramostim in a randomized, double-blind phase 1 clinical Parkinson’s disease trial. NPJ Parkinsons Dis 3: , 10. |
[71] | Olson KE , Namminga KL , Lu Y , Schwab AD , Thurston MJ , Abdelmoaty MM , Kumar V , Wojtkiewicz M , Obaro H , Santamaria P , Mosley RL , Gendelman HE ((2021) ) Safety, tolerability, and immune-biomarker profiling for year-long sargramostim treatment of Parkinson’s disease. EBioMedicine 67: , 103380. |
[72] | Thonhoff JR , Beers DR , Zhao W , Pleitez M , Simpson EP , Berry JD , Cudkowicz ME , Appel SH ((2018) ) Expanded autologous regulatory T-lymphocyte infusions in ALS: A phase I, first-in-human study. Neurol Neuroimmunol Neuroinflamm 5: , e465. |
[73] | Broen JCA , van Laar JM ((2020) ) Mycophenolate mofetil, azathioprine and tacrolimus: Mechanisms in rheumatology. Nat Rev Rheumatol 16: , 167–178. |
[74] | Greenland JC , Cutting E , Kadyan S , Bond S , Chhabra A , Williams-Gray CH ((2020) ) Azathioprine immunosuppression and disease modification in Parkinson’s disease (AZA-PD): A randomised double-blind placebo-controlled phase II trial protocol. BMJ Open 10: , e040527. |
[75] | Churchill MJ , Cantu MA , Kasanga EA , Moore C , Salvatore MF , Meshul CK ((2019) ) Glatiramer acetate reverses motor dysfunction and the decrease in tyrosine hydroxylase levels in a mouse model of Parkinson’s disease. Neuroscience 414: , 8–27. |




