Experienced Respiratory Symptoms and the Impact on Daily Life from the Perspective of People with Parkinson’s Disease: A Grounded Theory
Abstract
Background:
Abnormal respiratory function tests can be observed early in the course of Parkinson’s disease (PD). A better understanding of the impact of respiratory dysfunction on daily life in PD is needed to prevent later occurring complications as a (aspiration) pneumonia.
Objective:
To explain which respiratory symptoms people with PD or a form of atypical parkinsonism experience and how these symptoms impact on their daily lives.
Methods:
This qualitative study used a grounded theory approach. A purposeful sample strategy was used to capture information-rich cases. Data were collected in semi-structured interviews with participants diagnosed with either PD (n = 11) or atypical parkinsonism (n = 3), all of whom had confirmed respiratory symptoms. Data were analyzed using grounded theory analysis by creating codes, categories, theoretical themes, and, ultimately, a conceptual model.
Results:
Four respiratory profiles emerged, describing different types of respiratory dysfunction, with various positive and negative influencing factors. First, a loss of breathing automatism was experienced. Second, episodes of breathlessness or a rapid, shallow breathing pattern were triggered by either physical exertion, fatigue, or postural deformities. Third, stress and anxiety also triggered episodes of breathlessness. Fourth, a decreased cough strength and frequent coughing. Based on these findings, we constructed a conceptual model that visualizes the relations between these four types of respiratory dysfunction and their impact on daily life, with ‘discomfort’ and ‘avoidance of social activities’ as crucial elements.
Conclusion:
A tailored approach for each profile of respiratory dysfunction is recommended to improve respiratory dysfunction and to reduce its social impact in people with PD.
INTRODUCTION
Respiratory dysfunction in people with Parkinson’s disease (PD) or a form of atypical parkinsonism (AP) is a complex phenomenon as different mechanisms and multiple factors can contribute [1]. Many studies describe both obstructive and restrictive patterns when using objective tests for respiratory function in people with PD [2–4]. Laryngeal muscle dysfunction and autonomic dysfunction, leading to a reduced ventilatory capacity and upper airway obstruction, have been suggested as possible mechanisms behind obstruction [5, 6]. Factors explaining the restrictive mechanism are chest wall rigidity, postural deformities, and respiratory muscle weakness. These factors contribute to a decrease in thoracic mobility, lung volume, and lung capacity [7, 8].
Research shows that respiratory function in terms of inspiratory muscle strength is already impaired early after diagnosis [9]. In addition, respiratory parameters such as forced vital capacity (FVC), maximum inspiratory capacity (MIP), maximum expiratory capacity (MEP), maximum voluntary ventilation (MVV), and forced expiratory volume in one second (FEV1) are also impaired early and slowly decline over time [10–12]. Dopaminergic treatment initially seems to improve respiratory function in many people with PD [13]. However, in more advanced stages of the disease, respiratory complications of dopaminergic medication have also been reported in some cases as well [14]. Importantly, respiratory dysfunction can have a serious impact towards the end stage of the disease, as (aspiration) pneumonia is the most common cause of death in people with PD or AP [15, 16]. However, little is known about how respiratory dysfunction impacts on daily life of people with PD in the earlier stages.
In order to optimize the clinical management of respiratory dysfunction in PD or AP, we need to better understand how people with PD or AP experience respiratory symptoms. In addition, it is important to explore how the impact of respiratory dysfunction could support clinically relevant questions such as when to adjust dopaminergic medication, when to start with respiratory training and how to prevent late stage respiratory complications. Therefore, this qualitative study aims to explain which respiratory symptoms people with PD and AP experience and how these symptoms impact on their daily life.
METHODS
Study design
A qualitative study was performed using the grounded theory method of Charmaz [17]. We chose a constructive, interpretative grounded theory approach to create a theory, a conceptual model in an interactive way, based on the most important respiratory symptoms experienced by people with PD or AP. The study protocol was approved by the ethics committee of the Radboud university medical center (CMO: 2018–4224). The Consolidated Criteria for Reporting Qualitative research (COREQ) checklist guided our reporting [18].
Setting
Participants were recruited from an elderly care institution (Liemerije) in the eastern part of the Netherlands and from the outpatient clinic of the Center of Expertise for Parkinson & Movement Disorders, Radboud university medical center in Nijmegen. Liemerije consists of seven nursing home locations providing nursing home care as well as day care services for community-dwelling people. These two settings were chosen to describe the full spectrum of the disease (from onset to late stage).
Participants and recruitment
A purposeful sample strategy was used to capture information-rich, heterogeneous cases [19, 20]. We selected a heterogeneous population to provide the richest information needed for a full understanding of respiratory dysfunction in people with PD or AP. Participants were included for the interviews one by one until theoretical saturation was reached. Theoretical saturation means that no new dimensions or properties of the categories were found during the previous interview [19].
A physical therapist, speech therapist and PD nurse from both Liemerije and Radboud university medical center were asked to identify people with PD or AP who confirmed respiratory symptoms such as breathlessness and coughing. These allied healthcare professionals were familiar with the management of PD and AP, as they all delivered specialized care through the Dutch ParkinsonNet approach. ParkinsonNet offers an innovative model of care, developed in the Netherlands, in which allied health interventions are delivered within integrated regional community networks that consist of specifically trained therapists that treat large numbers of individuals with AP [21].
Inclusion criteria were: diagnosis of PD or an AP (confirmed by a neurologist) and experiencing respiratory problems, including cough, as determined by the healthcare professional and confirmed subjectively by the people with PD or AP themselves. Exclusion criteria were: comorbidity score higher than one on the items cardiac, vascular or respiratory disorders rated by the Cumulative Illness Rating Scale (CIRS score) [22, 23]. The CIRS evaluated the severity of comorbid diseases rated on a scale from 0 to 4 (0 = none; 4 = extremely severe). Eligible participants were informed verbally by their healthcare professional and received an information letter. If an eligible participant was willing to participate, he or she contacted the researcher.
Data collection
Semi-structured interviews were held face-to-face in the participant’s own living environment.
The interviews were audio-recorded and the length of each interview was about 30 min. To gain theoretical sensitivity, sensitizing concepts were formulated based on both reviewing literature and the professional experience from a speech therapist (JGK) and a physical therapist (MN) [4, 24, 25]. JGK and MN both have over 15 years of working experience with people with PD. Those concepts were used as a starting point for the interview guide, to interpret the data and to examine our ideas during data-analysis. Initial sensitizing concepts were: respiratory problems, cough problems, perception of dyspnea (during exercise), sleep breathing disorders, coughing and swallowing problems, cough strength, anxiety as a result of respiratory problems, social impact and support, impact on quality of life, received treatment, and willingness to receive further treatment.
The first seven interviews were performed by a bachelor student Biomedical Sciences, who had no previous experience with qualitative research, but was supported by the interview guide and who had received training (from JGK and MN) in interviewing techniques (the use of ‘open end’ questioning and bracketing). The next seven interviews were held by VvdW, who is a physical therapy scientist (MSc) with five years of work experience at the Radboud university medical center neurological department and educated in qualitative research. From interview seven onwards, spouses were allowed to be present during the interviews, but were asked to give additional comments only.
The following baseline characteristics of participants were collected at the start of each interview: age, sex, Hoehn and Yahr stage, disease duration, diagnosis, disease duration, and living condition (community-dwelling or nursing home).
During the interviews, observational memos were written to collect and visualize relevant solutions and thoughts. The memos were written out in the transcriptions and linked to the codes to explain interpretations and conclusions [26]. The last interview was completed on February 5, 2020. This was 22 days before the first COVID-19 outbreak in the Netherlands [27].
Data analysis
The analysis was performed stepwise as described by Charmaz (2006) [17], see Box 1. Transcripts were entered in the qualitative software ATLAS.ti (Scientific Software Development GmbH. Version 8, 2018). Initial coding and focused coding were done independently by the authors to enhance credibility. To develop the substantive theory, consensus meetings were organized to discuss the codes and to construct the main categories based on relationships between codes. During this meeting, reflective and analytic memos were kept to enhance theoretical sensitivity and to support the analytical interpretation regarding the participants’ experience of respiratory symptoms. Methodological memos were made during the analysis to reflect on the interview guide in a later stage and to consider changes in questioning regarding topics. After the completion of all interviews, theoretical themes were formulated together in a meeting. During this meeting, theoretical themes were discussed for their relevance, hierarchy, and quality. In a final meeting with all authors, the conceptual model providing insight in the relationships between the respiratory symptoms and the impact on daily life was developed.
Quality criteria
The following strategies were used to enhance trustworthiness. First, member checking was carried out by providing a verbal summary, including interpretations, at the end of each interview and asked if participants agreed [19]. Second, the constant comparison method was used after each interview by comparing the codes for duplications, contradictions, identifying the key message of each interview and to check if all relevant topics in the transcribed text were covered [28].
Third, investigator triangulation was established during the overall analytical process and by the reflective meetings of the research team to increase credibility by in-depth analysis and detect inappropriate subjectivity [19]. Finally, transferability of the outcomes was established by providing a detailed description of the characteristics of the participants, research group and setting.
Box 1: Analytic process
Data analysis:
1. Transcribed verbatim: of all interviews to become sensitive to the issues of importance (VvdW)
2. Initial coding: to identify relevant topics in the transcribed text (independently by VvdW, NK)
3. Focused coding: to construct categories that describe the type of respiratory problems people with PD experiences and the impact in their daily lives (independently by VvdW, MN, JGK)
4. Theoretical coding: Relationships between the categories and the relevance, hierarchy, and quality of the categories were discussed in a reflective meeting (VvdW, JGK, MN, and NK) until consensus in interpretation was reached and the theoretical codes were formulated.
5. Conceptual model: in a final meeting (VvdW, JGK, MN, NK, PvdW, BB) the theoretical codes were used to develop the conceptual model to gather insight in the relationships between the symptoms and the impact of this symptoms on daily life.
6. Based on Charmez [17]
RESULTS
Participants
Fourteen participants were included in the study (Table 1). Eleven participants were diagnosed with idiopathic PD. Three participants were diagnosed with a form of atypical parkinsonism: one participant was diagnosed with multiple system atrophy (MSA), with a disease duration of six years and her age was 70 years, one with vascular parkinsonism with a disease duration of 8 years and his age was 73 years, and one with corticobasal degeneration (CBD) with a disease duration of 2 years and his age was 59 years. When comparing the three participants with AP to the participants with PD, they experienced similar respiratory symptoms, as reflected by the fact that the constructed categories uses quotes from both participants with PD or AP (see Table 2). From the participants with PD, the age ranged between 50 and 86 years, time since diagnosis ranged between less than one year and 22 years and, six participants were in Hoehn and Yahr stage II, four were in stage III and one was in stage IV.
Table 1
Participant characteristics
| No.participant | Age (y) | Gender (M/W) | Living situation | H&Y stage | Diagnosis | Disease duration (y) | Date interview |
| 1 | 76 | W | Nursing home facility | 4 | Parkinson’s disease | 11 | 17-4-2018 |
| 2 | 73 | M | Community-dwelling | N.A. | Vascular parkinsonism | 8 | 23-4-2018 |
| 3 | 70 | W | Community-dwelling | N.A. | Multiple System Atrophy | 6 | 30-6-2018 |
| 4 | 71 | M | Community-dwelling | 3 | Parkinson’s disease | 19 | 12-6-2018 |
| 5 | 61 | M | Community-dwelling | 3 | Parkinson’s disease | 3 | 18-6-2018 |
| 6 | 86 | W | Community-dwelling | 2 | Parkinson’s disease | 22 | 18-6-2018 |
| 7 | 72 | W | Community-dwelling | 2 | Parkinson’s disease | 8 | 18-6-2018 |
| 8 | 56 | W | Community-dwelling | 2 | Parkinson’s disease | 3 | 19-6-2019 |
| 9 | 77 | W | Community-dwelling | 3 | Parkinson’s disease | 8 | 3-7-2019 |
| 10 | 70 | W | Community-dwelling | 2 | Parkinson’s disease | 0 | 24-7-2019 |
| 11 | 62 | W | Community-dwelling | 2 | Parkinson’s disease | 10 | 18-12-2019 |
| 12 | 64 | M | Community-dwelling | 3 | Parkinson’s disease | 11 | 18-12-2019 |
| 13 | 59 | M | Community-dwelling | N.A. | Corticobasal degeneration | 2 | 15-1-2020 |
| 14 | 50 | M | Community-dwelling | 2 | Parkinson’s disease | 9 | 5-2-2020 |
M, men; W, woman; H&Y stage, Hoehn & Yahr stage; N.A., not applicable.
Table 2
Key messages from the participants in relation to the categories and theoretical themes
| Theoretical theme | Categories | Key messages (quotes) |
| Loss of automatism in respiratory regulation result in feelings of helplessness and frustration | Dual tasking To forget to breath Less deep ventilation | “Yeah… for example when you’re watching television and there is something interesting on then it’s almost as if you… if you’re focused on television then it’s almost as if it… [gasps for air and aspiration is laboured]. Then you need to take a deep breath, because otherwise you run out of air.” [participant 13] |
| “And then I really had the idea that I was breathing in very high, very shallowly. And I, I could not change it by myself. Because I did do breathing exercises and such, but they simply did not work.” [participant 11] | ||
| “But I think it also combines with the uh... with being able to stay focused. When you see that your attention weakens… that it also uh, that the breathing somewhat.. yeah that it is annulled somewhat…” [participant 9] | ||
| “It is as if it becomes less natural, so less self-evident. So that you have to be more conscious of it.” [participant 14] | ||
| “What somebody then said too, that was already, when I lay in the operating room. On the uh, yeah on the table. Like are you uh… it almost looks like you have chronic hyperventilation. Are you familiar with that? I said no, that I uh… I am not familiar. Well she said, I think it’s increasingly obvious. And then she saw me again later and she said, then she said I hear it again.” [participant 11] | ||
| “That you then just don’t entirely have the uh… yeah ability let’s say to exhale.. because uh… I need to inhale again.” [participant 11] | ||
| Using conscious strategies | “Yeah, I needed to stop every time and then inhale for a moment and then I could go on my way. And that would otherwise go automatically of course and that… well I at least think that was the case. The automatic action of it, that that would fall away. That I wasn’t conscious of it.” [participant 11] | |
| “So when I was keenly aware of it and breathed well before the sentence, then it worked.” [participant 11] | ||
| “And then you’re suddenly without air. And then taking a few deep pulls and a taking a few deep breaths and then it’s alright again.” [participant 13] | ||
| “Inhaling and exhaling deeply and well.. then it gets a bit better.” [participant 9] | ||
| “Yeah and also with uh.. yeah my daughter said, just like with yoga. That is then a different form with your nose on the one side needing to be closed and then the other one again and then taking turns.. that exercise is pretty nice, actually. That breathing exercise.” [participant 10] | ||
| Feelings of helplessness and frustration | “Then you are confronted with your illness.. I get pretty angry about it sometimes. Because yeah, it does have an effect on you... on your uh... I don’t want to say character, but it does on your behavior. And on your mood, that’s what I wanted to say.” [participant 13] | |
| “They can’t do anything anyway…” [participant 3] | ||
| “You hear it right now too, right, that I uh, that I’m short of breath? You just live with it.” [participant 1] | ||
| “but uh.. yeah, it’s just a very unpleasant feeling.. very tiring.” [participant 10] | ||
| Breathlessness and a rapid, shallow breathing pattern resulting in feelings of insecurity showing a negative impact on participation in several social activities | Physical exertion | “And when I’m taking a walk, then I just have very little oxygen. And when I then walk and talk.. when I have company.. I already don’t do that anymore, because it’s not actually possible.” [participant 9] |
| “Yeah, less oxygen um yeah when you’ve walked for a while and... or when you need to do something you get tired more quickly that’s what I had earlier.” [participant 6] | ||
| “So when you just sit still or something then it goes pretty well, but uh.. when you either feel stressed or need to put in effort then breath starts to sit higher. So I can’t adequately keep on breathing.” [participant 14] | ||
| Postural deformities | “[Sir sits up straight, straightening his neck too] Yeah, like now I’m sitting up straight because then everything is free floating, or so I think [Sir bends his neck back downward] And when I sit like now then it still cuts something off, I think.” [participant 5] | |
| “But putting on a sock, that wrecks you. So it really is the pose, the bent-over folded pose.” [participant 13] | ||
| Tight feeling around throat and chest | “Yeah so when you want to say something or feel short of breath then it’s as if everything is stuck in here [participant points at throat].” [participant 1] | |
| “Yeah and.. sometimes it sits, it sometimes sits a bit down, but also a bit higher. And sometimes even higher and then those muscles over your lungs are all strained tight and then you just can’t take deep breaths.” [participant 11] | ||
| “A feeling like it’s being blocked off.. that there’s a blockage here [points at the bottom of the throat].” [participant 9] | ||
| “But that I then start breathing more shallowly, that is at that point something I hardly notice.” [participant 12] | ||
| Controversial medication effects | “When the medicine has worn off, then I have uh.. yea, then my whole face starts to tighten and uh.. [points at the jaws and the neck] and my jaw starts to uh.. cramp up. And then I get this oppressive feeling.” [participant 10] | |
| “And what I also notice more often is that at the moment I go towards… well I don’t know precisely whether it is when I go towards the direction of OFF or whether the medication just starts to work then that I sometimes inhale, hold my breath and then think to myself.. ho there, I also need to exhale let’s say.” [participant 14] | ||
| “When you have had too much, then it doesn’t regulate very nicely either. Because then you are a bit too tense or too alert and then it escalates. Then you’re too ON and then it automatically doesn’t work anymore. At least, for me it has to sit on that edge between not too little but not too much either.” [participant 14] | ||
| “Yeah yeah, well.. most of the time when I’ve taken my medicine again. I really do need that help, yeah.” [participant 11] | ||
| Sit and wait attitude | “Well, when I have that then uh.. I can’t really uh.. do something.. no, then I really must take it easy and uh.. kinda wait it out until that uh.. until the medicine starts working..” [participant 10] | |
| Speech difficulties due to lack of breathing support | “Yeah, since a few weeks back my speech has actually started getting worse and worse. And quieter too. I also talk very modestly I think.” [participant 4] | |
| “And speaking is difficult too, then. I sometimes don’t have the breath to pronounce my words.” [participant 3] | ||
| “And then uh I get short of breath such as now while talking yeah that sucks.” [participant 5] | ||
| “Yeah, especially over the telephone and such, then you actually try to throw out too much in one breath of air and then you take in insufficient oxygen to pronounce anything.. and then you’re at the end [makes a sound as if he’s short of breath] and then another part of the sentence comes out, but you have no breath left.” [participant 14] | ||
| Disturbed sleep | “What really bothers me is my... [Deep sigh] When at night I lie in bed I do hear myself breathing yeah.” [participant 2] | |
| “Sometimes at night I am woken up by uh... yeah, you don’t know the reason for it.. but then I feel it too, that oppressiveness..” [participant 10] | ||
| Avoidance of social activities | Yeah, then you try to... [unintelligible]then you sit together with a couple. And then you think I hope that say, I I can’t really participate well because of my shortness of breath.” [participant 1] | |
| Stress, anxiety and the feeling of losing independency as triggers for breathlessness | Worrying | “And in the evening when I’m sitting on the couch and I’m worrying a bit than [madam inhales quickly and deeply] than I go a bit [madam inhales quickly and deeply].” [participant 7] |
| “Well then I get a sort of panicky attack let’s say. Then I need to be careful with that, then I need to take it easier.” [participant 8] | ||
| Time pressure | “Yeah and when I need to do something with other people around: taking money out of my wallet or uh... you know, then you start worrying a bit about that. People are waiting in line for the register, at the register. Yeah, then I start to breath more shallowly. I notice that myself, then I need to take a few deep breaths.” [participant 11] | |
| Losing control on being independent | “And then trust on others that it will all you know.. be alright on such a day.. or that the medication on time… that… yeah that surrender is very hard for her.. and from that I can really see that it definitely has an effect on breathing.” [spouse of participant 9] | |
| “I would like to train my breathing because I think that it would make me feel safer.” [participant 7] | ||
| Coughing causing restlessness and avoidance of social activities | Increased urge to cough without being productive | “But coughing, I sometimes have the feeling that I get something in my throat and then you start coughing and that is well uh really annoying okay. For yourself, but it’s also something you find annoying for your environment.” [participant 6] |
| “Yeah yeah, the end… when you’ve gotten to the end of your breathing then it transitions into coughing.” [participant 13] | ||
| “A dry cough yeah. And yeah it is almost a bit like a frog in your throat, in a manner of speaking. And each time I blow away a layer of slime, in a manner of speaking.” [participant 4] | ||
| “I try to get rood of that loogey by coughing or [hawking sound] by hawking. And then it’s fine again.” [participant 2] | ||
| Increased coughing to clear phlegm or aspirated material Difficulty swallowing food, liquids, pills | “Did have a problem with uh.. that it uh... that I think that food sticks drier… in my throat and then a drink of water… but not really that I then think like it’s probably because of uh… because of the Parkinson’s.” [participant 10] | |
| “Uh when taking my medicine I have problems with coughing.” [participant 2] | ||
| “Yeah, nowadays I do it with a straw because just drinking out of a glass is not something I can do so well anymore.” [participant 5] | ||
| “No it does stay in the throat uh… really gotta hawk and do your best to get it out let’s say.. doesn’t sound very hygienic but..” [participant 13] | ||
| Coughing feels disturbing and unhygienic Avoidance of social activities | “No it does stay in the throat uh… really gotta hawk and do your best to get it out let’s say.. doesn’t sound very hygienic but..” [participant 13] | |
| “That was really gross… sitting there coughing and rasping [re-enacts], but you don’t get anything out.” [participant 8] | ||
| “A woman always comments on the coughing. I think it bothers her.” [participant 2] | ||
| “But coughing, I sometimes have the feeling that I get something in my throat and then you start coughing and that is well uh really annoying okay. For yourself, but it’s also something you find annoying for your environment.” [participant 6] | ||
| Reduced cough strength | “It’s less powerful, less powerful. I don’t have to cough heavily but when I cough it is certainly less.” [participant 3] | |
| “Last year, I caught uhh I caught pneumonia and I think that afterwards I got less uhh breath.” [participant 6] | ||
| Attempting to reduce cough frequency and intensity | “Well when I sit in the car to the taxibus to Liemerije then I try to cough as little as possible.” [participant 2] | |
| “But coughing, I sometimes have the feeling that I get something in my throat and then you start coughing and that is well uh really annoying okay. For yourself, but it’s also something you find annoying for your environment.” [participant 6] |
Initially, seven participants were recruited from the elderly care institution (Liemerije). One of them, diagnosed with PD, lived in a nursing home facility and six were living independently and visited the nursing home facility for treatment a few times per week. After this point with seven interviews, data saturation was evaluated by VvdW, MN, and JGK in a reflective meeting using the initial codes of all interviews. Theoretical sampling of new participants occurs along with the emergence of theoretical concepts. To enrich the data with a more heterogeneous sample, we decided to include another seven community-dwelling participants in the more early-stages of the disease from the outpatient clinic of the Radboud university medical center Nijmegen [29]. Afterwards, data saturation was reached by similarities in codes. Four spouses participated in the interviews and shared their observations about the respiratory function of their partner.
Qualitative analyses
The data from the fourteen interviews were analyzed and four theoretical themes emerged as profiles describing different types of respiratory symptoms and their impact on daily life. The four themes were: 1) Loss of automatism in respiratory regulation, resulting in feelings of helplessness and frustration, 2) Breathlessness and a rapid, shallow breathing pattern resulting in feelings of insecurity showing a negative impact on participation in several social activities, 3) Stress, anxiety and a feeling of losing independency as triggers for breathlessness, and 4) Coughing causing restlessness and avoidance of social activities. The categories related to the themes were underlined in the text below. Table 2 presents the quotes related to the categories and theoretical themes.
Loss of automatism in respiratory regulation result in feelings of helplessness and frustration
People with PD or AP said that the respiratory regulation, normally happening automatically, now was hampered leading to a less deep ventilation. Participants also mentioned that they sometimes ‘forgot’ to breath, particularly during the performance of a dual task. Forgetting to breath and less deep ventilation leads to feelings of helplessness and frustration, also because this confronted participants with the consequences of the disease. Participants also told that they could diminish a less deep ventilation and could overcome forgetting to breath by switching to more conscious breathing techniques. For instance, consciously taking a deep breath helped, and another conscious compensation strategy a participant talked about was to stop dual tasking and to start focusing only on their breathing pattern for a moment. Participants also used conscious breathing techniques learned from their healthcare professionals or by yoga classes.
Breathlessness and a rapid, shallow breathing pattern resulting in feelings of insecurity showing a negative impact on participation in several social activities
Participants mentioned episodes of breathlessness and a rapid, shallow breathing pattern. Feelings of breathlessness were portrayed by participants as a tight, obstructive feeling around the throat and chest while breathing. They mentioned these symptoms in relation to either their postural deformities, higher fatigue levels and physical exertion, medication, speech problems or disturbed sleeping.
Reported effects of dopaminergic medication on the breathing pattern were somewhat inconsistent. On the one hand, improvement of breathlessness was reported by participants during the “on” state of dopaminergic medication. On the other hand, participants also reported a clear relation between the moment of medication intake and the occurrence of breathlessness throughout all dopaminergic medication cycles. Episodes of breathlessness were reported after taking each medication, and just before taking the next dose similar breathlessness reappeared. One participant described that high doses of dopaminergic medication caused breathlessness.
Participants also brought up speech difficulties in relation to their respiratory symptoms. They mentioned terms like ‘mumbling’, ‘speak more softly’, ‘speaking indistinctly’, ‘a reduced breathing support when speaking’, and ‘the inability to create enough volume while speaking’.
Another symptom that bothered participants was their disturbed sleep. In their perception, it was caused by audible breathing, which woke them up at night. One spouse also told she observed a rapid, shallow breathing pattern while the participant himself was not aware of it.
Breathlessness did have an impact on social interaction as participants felt uncertain to go out as they described that they had to ‘sit and wait’ until medication started to work and their breathlessness improved. For others breathlessness was getting worse when visiting people and while having a conversation. For example, participants described they did not have enough breath to speak full sentences.
Stress, anxiety, and the feeling of losing independency as triggers for breathlessness
Stress and anxiety were indicated as triggers for respiratory discomfort by people with PD or AP. Participants talked about a rapid, shallow and chest breathing pattern after being exposed to a stressor. A stressor that was mentioned by participants was doing too much during the day without taking enough rest. In addition, participants felt that their speech difficulties caused others having to wait longer and that, in turn, the experienced (time) pressure triggered breathlessness. Time pressure was a stressor for another participant who described that taking money out of her wallet, when other people were waiting in line behind her, lead to more superficial breathing. One spouse illustrated that when their partner was faced with a loss of independency in a particular situation, the rapid, shallow breathing pattern started.
Coughing causing restlessness and avoidance of social activities
Participants experienced an increased coughing frequency at the end of a breathing cycle, described as an irritation or a dry cough. An increased cough frequency and a reduced coughing strength was experienced when trying to get rid of a lump in the throat caused by phlegm or aspirated food, liquids or saliva. Coughing related to difficulty in swallowing food, liquids, and pills was common in many participants. Some participants mentioned they had a pneumonia in the past.
Coughing also affected social activities, according to the participants. People with PD or AP described coughing as unhygienic and annoying for both themselves and others. In their opinion, coughing upsets other people as participants received comments from others on their coughing frequency. As a result, being with others, for example taking a bus ride, was avoided or participants tried to suppress both their cough frequency and intensity while being with others.
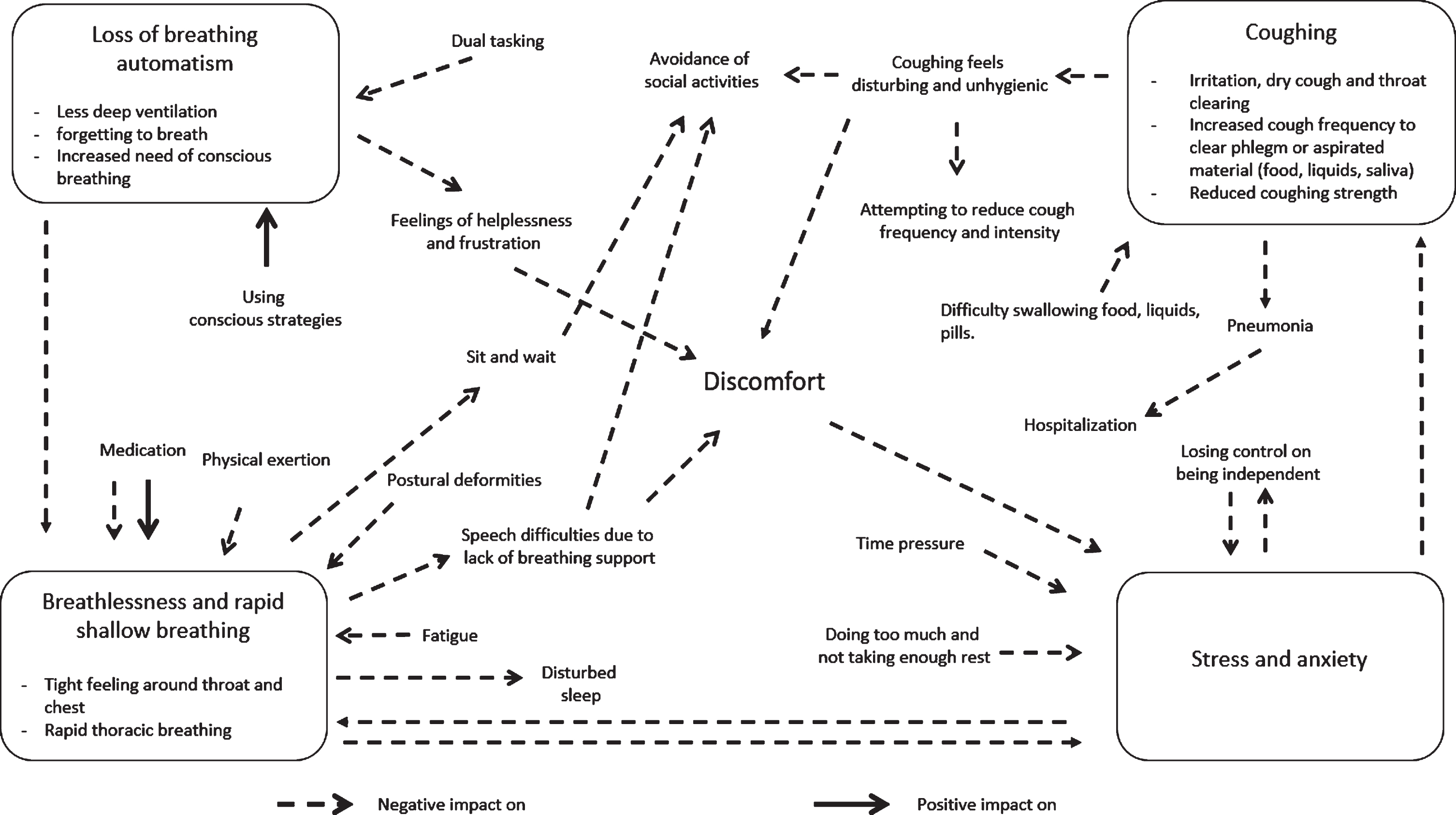
Conceptual model
With these results, we (VvdW, MN, NK, PvdW, BRB, and JGK) derived a conceptual model including four respiratory profiles that visualized the experience of discomfort due to respiratory symptoms, and the impact in daily life as it lead to the avoidance of social activities (Fig. 1). The causes of respiratory symptoms are visualized with inward-facing arrows in the direction of one of the four respiratory profiles. The consequences of respiratory symptoms are visualized with outward-facing arrows. We further outlined our interpretation of the profiles and the underlying relationships between the profiles in the discussion chapter. The loss of breathing automatism appeared to increase during dual tasking, but also seemed to benefit from compensation by using conscious breathing strategies. Loss of automatism also could trigger uncomfortable feelings in terms of breathlessness or a rapid, shallow breathing pattern. Other triggers for a rapid, shallow breathing were physical exertion, fatigue, postural deformities, stress, and anxiety. Dopaminergic treatment offered improvements; however, some participants also experienced an increase of breathlessness after they had taken levodopa medication. Time pressure, doing too much and not taking enough rest, or losing control over independency led to higher stress and anxiety levels. More frequently coughing seemed to be the result of difficulties in swallowing food, liquids, or pills and sometimes caused aspiration, an (aspiration) pneumonia or hospitalization. Coughing, breathlessness, and rapid, shallow breathing with their consequences all lead to the avoidance of social activities.
Fig. 1
Conceptual model visualizing the causes, consequences, and relationships between the four respiratory profiles and their impact on daily life. The inward-facing arrows visualizes the causes of respiratory symptoms and the outward-facing arrows visualize the consequences of respiratory symptoms.
DISCUSSION
This is the first qualitative study describing the complex construct of respiratory symptoms and its impact on daily activities and social participation using the perspectives of people with PD or AP. We constructed a conceptual model with four profiles of respiratory dysfunction and visualized how respiratory dysfunction had a wide range of consequences. We interpretated the term discomfort as an overarching, binding category, indirectly named by all participants which was related to all four themes. Loss of breathing automatism leads to helplessness and frustration and in turn to discomfort. Breathlessness triggered speech difficulties, which in result lead to discomfort during conversations. Discomfort was also named as a stressor that leads to breathlessness and a rapid, shallow breathing. Coughing felt disturbing and in turn caused discomfort. This study shows that, besides known late stage respiratory complications, the impact of respiratory dysfunction is considerable as it leads to limitations in participation and avoidance of social activities.
The loss of automatism in respiratory regulation, which can be unveiled by dual tasking, has not been described in previous studies. Not surprisingly, some people with PD and AP reported different attentional strategies that seem to compensate for their loss of automatism. The phenomenon of loss of automatism while dual tasking and the benefits from attentional strategies that introduce goal-directed behavior are well known in PD, particular for motor performance of activities such as gait [30, 31]. A recent study showed that dual tasking elicited blunting of the cough sensorimotor response, and hence on the ability to successfully protect the airway. This can may be critical for people with PD, due to their risk of airway invasion during swallowing [32]. From the perspective of people with PD or AP, the importance of this finding is that respiratory dysfunction worsens while dual tasking, but there are strategies to compensate for a part of this loss in automatism. A loss of breathing automatism unveiled by dual tasking, sometimes trigger breathlessness and rapid, shallow breathing.
A rapid, shallow breathing pattern was experienced by participants and has been described before in many other studies, where it has been suggested to result from a ventilation control impairment [33]. In more detail, brainstem dysregulation and autonomic dysfunction has been suggested as the central mechanisms influencing the central drive of ventilation in PD [1, 5, 34].
Participants named a wide range of triggers and consequences of a rapid, shallow breathing and breathlessness: postural deformities, physical exertion, speech problems, disturbed sleeping, medication, or stress and anxiety. It is known that kyphoscoliosis, which is prominent in many people with PD, decreases long volumes and lead to chest wall rigidity. This may explain some part of the restrictive pattern found in respiratory function test studies in PD [35, 36]. The contribution of physical exertion and fatigue to rapid, shallow breathing is somewhat controversial as some participants also report episodes of breathlessness or rapid shallow breathing more spontaneously in rest. The occurrence of rapid, shallow breathing and breathlessness in rest suggests no structural limitation in long volumes or capacity, in contrast with many cardiac and pulmonary diseases, and supports the hypothesis of an impaired central drive [37]. A factor that may influence breathlessness and rapid shallow breathing is dopaminergic medication. Monteiro et al. [13] reported a positive effect of dopaminergic treatment on respiratory symptoms, which we recognized by participants in this study. On the other hand, some participants described that their dopaminergic treatment was the trigger for rapid, shallow breathing pattern. Both levodopa-induced peak dose respiratory dyskinesias and also biphasic respiratory dysfunction have been described as motor complications in some case studies as well [14, 24]. Dyspnea while speaking and hypophonia reducing intelligibility of speech are well-known phenomena in PD and were mentioned by our participants as well as a consequence of a rapid, shallow breathing pattern [38]. The link between dyspnea and disturbed sleep that our participants mentioned have also been reported as a result of sleep apnea [25] and diaphragmatic weakness. The latter is suggested to contribute to nocturnal hypoventilation and dyspnea in other neuromuscular diseases such as amyotrophic lateral sclerosis [39, 40]. We believe that breathlessness and rapid shallow breathing could be experienced as a stressor as it confronts people with their illness. The other way around, stress or anxiety could lead to breathlessness or rapid, shallow breathing.
Anxiety is prevalent in people with PD and they also perceive more stress than controls [41, 42]. In a study by Baille et al. (2019), the multidimensional dyspnea profile, an instrument to distinguish sensory and emotional aspects of dyspnea, confirmed that dyspnea in PD seems to be related to anxiety and depression [33, 43]. Stress could also result in an increased coughing frequency.
There is mounting evidence showing that coughing (both reflex and voluntary cough) is disturbed in people with PD and results in lower peak expiratory flow rate and lower cough expired volume [15, 44–46]. The impact of reduced coughing strength in combination with swallowing difficulties is well known as a risk factor for aspiration pneumonia, which may lead to hospitalization and which is the major cause of death in advanced PD [16, 47, 48]. This cascade of swallowing difficulties, aspiration, pneumonia and hospitalization was also mentioned by our participants. However, the impact of coughing in terms of avoidance of social activities was a new finding of our study. This impact might be even larger these days during the COVID-19 pandemic, which results to exacerbating motor and non-motor symptoms in people with PD [49, 50].
Strengths and limitations
A first strength of this study is that we included a heterogeneous sample with participants in both early and advanced stages of the disease. This probably was the result of the purposeful sample strategy in both an elderly care institution and the outpatient clinic of the Radboud university medical center. Due to the known decline in respiratory parameters, this heterogeneous sample is important to gain a broad reflection of respiratory symptoms through the course of the disease. A second strength was that in order to increase credibility, all data were analyzed independently, and the conceptual model was developed in reflective meetings with all authors. A third strength was that, during the data collection, sensitizing concepts were evaluated by using the constant comparison method to be sure that all the relevant, various topics were covered by the interviews.
This study also had some limitations. First of all, the counter side of our purposeful sampling strategy is that the sampling aimed to provide rich information on a broad spectrum of the disease. We succeeded as at the time that theoretical saturation was achieved, a population with a wide range in disease duration and age were included. Additionally, also, three participants with different types of AP were included. However, the sample size is too small to answer potentially relevant questions such as what are the differences in the prevalence of the respiratory profiles regarding diagnosis (PD versus AP), age and disease duration. It is known that people diagnosed with AP have a considerably different course of the disease compared to people with PD [24, 51]. Although the experiences and impact of respiratory symptoms among the three people with AP appeared to be similar to those with PD, it is not possible to generalize the findings to the population of people with AP. Reviewing available literature, respiratory disorders in PD are more commonly described and categorized as upper airway obstruction, restrictive disorders, complications to medication intake or withdrawal, and aspiration pneumonia. For AP, laryngeal stridor or obstructive sleep apnea as upper airway obstruction, poor response to hypoxia/hypercapnia, and aspiration pneumonias in people with diagnosed with PSP are recognized as respiratory disorders [24].
A second limitation is that we only included Hoehn and Yahr stages and disease duration, but not the Movement Disorder Society unified Parkinson’s disease rating scale (MDS-UPDRS). Perhaps the MDS-UPDRS could have gained more insight in the severity of the disease of our participants. A third point of attention is that we initially included people with PD or AP in this study without their spouses. During the interviews we noticed that some participants were unaware of changes in their breathing pattern, while their spouses did noticed alterations like a more rapid, shallow breathing pattern. This phenomenon of reduced awareness is well-known in PD, e.g., not being fully aware of hypokinetic speech, which is described as a misperception of self-generated loudness due to the reduced sensorimotor integration [52, 53].
To overcome this limitation, we believe another, more quantitative, study design with a larger sample size of both persons with PD and AP is necessary for better recognition and further understanding of respiratory dysfunction in PD. In future studies, it would be interesting to investigate the link between the different stages of the disease and the respiratory symptoms. In addition, a quantitative study design also has the potential to calculate the prevalence for each respiratory profile, and to explore to what extent other determinants like age, gender, use of medication, speech and swallowing difficulties contribute to respiratory dysfunction.
Implications for clinical management
Positive effects of a wide range of attentional strategies or goal-directed exercises on respiratory function were mentioned by participants to compensate to some extent for the loss of automatism in the regulation of breathing. These findings open up opportunities for therapeutic interventions. Indeed, there are already evidence-based modalities of respiratory training that use this phenomenon, such as incentive spirometry or breath stacking [36]. In line with these findings, both voluntary cough and reflex cough seem to benefit from goal-directed coughing strategies including visual feedback performance [54, 55]. For allied healthcare professionals such as physical therapists and speech language therapists, respiratory function is currently not a separate core area in their clinical practice guidelines [56, 57]. However, we believe that they should inform people with PD about this phenomenon as many people with PD have the ability to benefit from compensation strategies. In addition, allied healthcare professionals should consider respiratory training to improve coughing and the loss of breathing automatism more often [58]. Thereby, allied healthcare professionals should not only look at symptoms in terms of an impaired voice or decreased long volume and function, but also pay attention to how these symptoms hamper social participation and how this can be improved. From the same perspective, it is also important for neurologists to take the impact of respiratory symptoms on daily functioning into account when reconsidering changes in dopaminergic treatment or advanced therapies.
Recommendations for future research
Altered respiratory function tests has been reported commonly in people with PD [10, 11]. However, it would be relevant to investigate whether alterations in respiratory function relate to the respiratory symptoms that people with PD experienced in this study. Another point of interest would be to investigate how dual tasking interferes with respiratory function (respiratory function tests, respiratory rate, chest wall volumes, diaphragmatic function, and arterial blood gas) and how attentional breathing techniques or consciously coughing techniques impact on these parameters [34]. For example, Tai Chi appeared to be very effective in PD in terms of gait velocity, balance, and improvement of bradykinesia [59]. It would be interesting to investigate whether consciously performed Tai Chi breathing exercises (focusing on deep ventilation) could alter abnormal hypoxic responses and consequently prevent the emergence of respiratory symptoms. Next to that, it might be interesting to investigate if cognitive behavioral strategies (e.g., copings strategies how to handle anxiety, to avoid time pressure or manage energy levels) or mindfulness-based interventions also reduce psychological distress and even prevent the occurrence of breathlessness or a rapid, shallow breathing [42, 60]. And finally, techniques to improve coughing are available (e.g., EMST), but long term effects on the consequences of the deterioration of swallowing in PD are awaited [34, 54, 55].
ACKNOWLEDGMENTS
The authors would like to thank all participants who took part in this study. We also thank Christijn Winkelmolen for his contribution during his internship of the bachelor Biomedical Sciences.
This work received funding from the Principal Clinician Funds 2019-2022 of Maarten, J. Nijkrake, Radboud university medical center Nijmegen.
The Center of Expertise for Parkinson & Movement Disorders was supported by a center of excellence grant by the Parkinson Foundation.
CONFLICT OF INTEREST
The authors have no financial conflicts of interest related to this publication.
Prof. Bloem currently serves as co-Editor in Chief for the Journal of Parkinson’s disease, serves on the editorial of Practical Neurology and Digital Biomarkers, has received honoraria from serving on the scientific advisory board for Abbvie, Biogen and UCB, has received fees for speaking at conferences from AbbVie, Zambon, Roche, GE Healthcare and Bial, and has received research support from the Netherlands Organization for Scientific Research, the Michael J Fox Foundation, UCB, Abbvie, the Stichting Parkinson Fonds, the Hersenstichting Nederland, the Parkinson’s Foundation, Verily Life Sciences, Horizon 2020 and the Parkinson Vereniging. The Center of Expertise for Parkinson & Movement Disorders was supported by a center of excellence grant by the Parkinson Foundation.
Dr. Kalf has received research funding from the Michael J. Fox Foundation, and the Dutch Association of Logopedics and Phoniatrics.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-213121.
REFERENCES
[1] | Zhang W , Zhang L , Zhou N , Huang E , Li Q , Wang T , Ma C , Li B , Li C , Du Y , Zhang J , Lei X , Ross A , Sun H & Zhu X ((2019) ) Dysregulation of respiratory center drive (P0.1) and muscle strength in patients with early stage idiopathic Parkinson’s disease. Front Neurol 10: , 724. |
[2] | Haas BM , Trew M & Castle PC ((2004) ) Effects of respiratory muscle weakness on daily living function, quality of life, activity levels, and exercise capacity in mild to moderate Parkinson’s disease. . Am J Phys Med Rehabil 83: , 601–607. |
[3] | Izquierdo-Alonso JL , Jiménez-Jiménez FJ , Cabrera-Valdivia F & Mansilla-Lesmes M ((1994) ) Airway dysfunction in patients with Parkinson’s disease. Lung 172: , 47–55. |
[4] | Baille G , De Jesus AM , Perez T , Devos D , Dujardin K , Charley CM , Defebvre L & Moreau C ((2016) ) Ventilatory dysfunction in Parkinson’s disease. . J Parkinsons Dis 6: , 463–471. |
[5] | Vijayan S , Singh B , Ghosh S , Stell R & Mastaglia FL ((2020) ) Brainstem ventilatory dysfunction: A plausible mechanism for dyspnea in Parkinson’s disease? Mov Disord 35: , 379–388. |
[6] | Hammer MJ & Barlow SM ((2010) ) Laryngeal somatosensory deficits in Parkinson’s disease: Implications for speech respiratory and phonatory control. . Exp Brain Res 201: , 401–409. |
[7] | Sabaté M , Rodríguez M , Méndez E , Enríquez E & González I ((1996) ) Obstructive and restrictive pulmonary dysfunction increases disability in Parkinson disease. Arch Phys Med Rehabil 77: , 29–34. |
[8] | Pal PK , Sathyaprabha TN , Tuhina P & Thennarasu K ((2007) ) Pattern of subclinical pulmonary dysfunctions in Parkinson’s disease and the effect of levodopa. Mov Disord 22: , 420–424. |
[9] | Baille G , Perez T , Devos D , Deken V , Defebvre L & Moreau C ((2018) ) Early occurrence of inspiratory muscle weakness in Parkinson’s disease. PLoS One 13: , e0190400. |
[10] | Santos RBD , Fraga AS , Coriolano M , Tiburtino BF , Lins OG , Esteves ACF & Asano NMJ ((2019) ) Respiratory muscle strength and lung function in the stages of Parkinson’s disease. J Bras Pneumol 45: , e20180148. |
[11] | Guilherme EM , Padovez R , de Oliveira A , Ferro AM , di Lorenzo VAP & Gianlorenço ACL ((2021) ) Respiratory disorders in Parkinson’s disease. J Parkinsons Dis 11: , 993–1010. |
[12] | Kaminsky DA , Grosset DG , Kegler-Ebo DM , Cangiamilla S , Klingler M , Zhao P & Oh C ((2021) ) Natural history of lung function over one year in patients with Parkinson’s disease. Respir Med 182: , 106396. |
[13] | Monteiro L , Souza-Machado A , Valderramas S & Melo A ((2012) ) The effect of levodopa on pulmonary function in Parkinson’s disease: A systematic review and meta-analysis. Clin Ther 34: , 1049–1055. |
[14] | van de Wetering-van Dongen VA , Espay AJ , Marsili L , Sturchio A , Holter ST , Bloem BR & Nijkrake MJ ((2021) ) Biphasic (subtherapeutic) levodopa-induced respiratory dysfunction in Parkinson disease. Neurol Clin Pract 11: , e402–e406. |
[15] | Hegland KW , Okun MS & Troche MS ((2014) ) Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung 192: , 601–608. |
[16] | Fernandez HH & Lapane KL ((2002) ) Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit 8: , CR241–246. |
[17] | Charmaz K ((2006) ) Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. Sage Publications, Ltd. |
[18] | Tong A , Sainsbury P & Craig J ((2007) ) Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int J Qual Health Care 19: , 349–357. |
[19] | Holloway I & Wheeler S ((2013) ) Qualitative research in nursing and healthcare, 3rd ed. John Wiley & Sons. |
[20] | Palinkas LA , Horwitz SM , Green CA , Wisdom JP , Duan N & Hoagwood K ((2015) ) Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health 42: , 533–544. |
[21] | Bloem BR & Munneke M ((2014) ) Revolutionising management of chronic disease: The ParkinsonNet approach. BMJ 348: , g1838. |
[22] | Linn BS , Linn MW & Gurel L ((1968) ) Cumulative illness rating scale. J Am Geriatr Soc 16: , 622–626. |
[23] | Visser M , Marinus J , van Hilten JJ , Schipper RGB & Stiggelbout AM ((2004) ) Assessing comorbidity in patients with Parkinson’s disease. Mov Disord 19: , 824–828. |
[24] | Mehanna R & Jankovic J ((2010) ) Respiratory problems in neurologic movement disorders. Parkinsonism Relat Disord 16: , 628–638. |
[25] | Docu Axelerad A , Stroe AZ , Arghir OC , Docu Axelerad D & Gogu AE ((2021) ) Respiratory dysfunctions in Parkinson’s disease patients. Brain Sci 11: , 595. |
[26] | Boeije HR ((2009) ) Analysis in Qualitative Research, SAGE Publications. |
[27] | Rijksoverheid, Coronavirus Tijdlijn, https://www.rijksoverheid.nl/onderwerpen/coronavirus-tijdlijn/februari-2020-eerste-coronabesmetting-in-nederland. |
[28] | Boeije H ((2002) ) A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant 36: , 391–409. |
[29] | Moser A & Korstjens I ((2018) ) Series: Practical guidance to qualitative research. Part 3: Sampling, data collection and analysis. Eur J Gen Pract 24: , 9–18. |
[30] | Redgrave P , Rodriguez M , Smith Y , Rodriguez-Oroz MC , Lehericy S , Bergman H , Agid Y , DeLong MR & Obeso JA ((2010) ) Goal-directed and habitual control in the basal ganglia: Implications for Parkinson’s disease. Nat Rev Neurosci 11: , 760–772. |
[31] | Nonnekes J , Ružicka E , Nieuwboer A , Hallett M , Fasano A & Bloem BR ((2019) ) Compensation strategies for gait impairments in Parkinson disease: A review. JAMA Neurol 76: , 718–725. |
[32] | Perry SE & Troche MS ((2021) ) Dual tasking influences cough reflex outcomes in adults with Parkinson’s disease: A controlled study. Dysphagia 36: , 959–973. |
[33] | Baille G , Perez T , Devos D , Machuron F , Dujardin K , Chenivesse C , Defebvre L & Moreau C ((2019) ) Dyspnea is a specific symptom in Parkinson’s disease. J Parkinsons Dis 9: , 785–791. |
[34] | Curtis JA , Dakin AE & Troche MS ((2020) ) Respiratory-swallow coordination training and voluntary cough skill training: A single-subject treatment study in a person with Parkinson’s disease. J Speech Lang Hear Res 63: , 472–486. |
[35] | Tamaki A , Matsuo Y , Yanagihara T & Abe K ((2000) ) Influence of thoracoabdominal movement on pulmonary function in patients with Parkinson’s disease: Comparison with healthy subjects. Neurorehabil Neural Repair 14: , 43–47 |
[36] | Ribeiro R , Brandão D , Noronha J , Lima C , Fregonezi G , Resqueti V & Dornelas de Andrade A ((2018) ) Breath-stacking and incentive spirometry in Parkinson’s disease: Randomized crossover clinical trial. Respir Physiol Neurobiol 255: , 11–16. |
[37] | Parshall MB , Schwartzstein RM , Adams L , Banzett RB , Manning HL , Bourbeau J , Calverley PM , Gift AG , Harver A , Lareau SC , Mahler DA , Meek PM , O’Donnell DE &American Thoracic Society Committee on Dyspnea ((2012) ) An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 185: , 435–452. |
[38] | Baille G , Chenivesse C , Perez T , Machuron F , Dujardin K , Devos D , Defebvre L & Moreau C ((2019) ) Dyspnea: An underestimated symptom in Parkinson’s disease. Parkinsonism Relat Disord 60: , 162–166. |
[39] | Fromageot C , Lofaso F , Annane D , Falaize L , Lejaille M , Clair B , Gajdos P & Raphaël JC ((2001) ) Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch Phys Med Rehabil 82: , 123–128. |
[40] | Bourke SC , Bullock RE , Williams TL , Shaw PJ & Gibson GJ ((2003) ) Noninvasive ventilation in ALS: Indications and effect on quality of life. Neurology 61: , 171–177. |
[41] | Broen MP , Narayen NE , Kuijf ML , Dissanayaka NN & Leentjens AF ((2016) ) Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 31: , 1125–1133. |
[42] | van der Heide A , Meinders MJ , Speckens AEM , Peerbolte TF , Bloem BR & Helmich RC ((2021) ) Stress and mindfulness in Parkinson’s disease: Clinical effects and potential underlying mechanisms. Mov Disord 36: , 64–70. |
[43] | Banzett RB , O’Donnell CR , Guilfoyle TE , Parshall MB , Schwartzstein RM , Meek PM , Gracely RH & Lansing RW ((2015) ) Multidimensional Dyspnea Profile: An instrument for clinical and laboratory research. Eur Respir J 45: , 1681–1691. |
[44] | Pitts T , Bolser D , Rosenbek J , Troche M & Sapienza C ((2008) ) Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia 23: , 297–301. |
[45] | Ebihara S , Saito H , Kanda A , Nakajoh M , Takahashi H , Arai H & Sasaki H ((2003) ) Impaired efficacy of cough in patients with Parkinson disease. Chest 124: , 1009–1015. |
[46] | Troche MS , Brandimore AE , Okun MS , Davenport PW & Hegland KW ((2014) ) Decreased cough sensitivity and aspiration in Parkinson disease. Chest 146: , 1294–1299. |
[47] | Langmore SE , Skarupski KA , Park PS & Fries BE ((2002) ) Predictors of aspiration pneumonia in nursing home residents. Dysphagia 17: , 298–307. |
[48] | Langmore SE , Terpenning MS , Schork A , Chen Y , Murray JT , Lopatin D & Loesche WJ ((1998) ) Predictors of aspiration pneumonia: How important is dysphagia? Dysphagia 13: , 69–81. |
[49] | Cartella SM , Terranova C , Rizzo V , Quartarone A & Girlanda P ((2021) ) Covid-19 and Parkinson’s disease: An overview. J Neurol 268: , 4415–4421. |
[50] | Jaiswal V , Alquraish D , Sarfraz Z , Sarfraz A , Nagpal S , Singh Shrestha P , Mukherjee D , Guntipalli P , Sánchez Velazco DF , Bhatnagar A , Savani S , Halilaj E , Ruxmohan S & Cueva W ((2021) ) The influence of coronavirus disease-2019 (COVID-19) on Parkinson’s disease: An updated systematic review. J Prim Care Community Health 12: , 21501327211039709. |
[51] | Armstrong MJ & Okun MS ((2020) ) Diagnosis and treatment of Parkinson disease: A review. JAMA 323: , 548–560. |
[52] | Mollaei F , Shiller DM & Gracco VL ((2013) ) Sensorimotor adaptation of speech in Parkinson’s disease. Mov Disord 28: , 1668–1674. |
[53] | Liu H , Wang EQ , Metman LV & Larson CR ((2012) ) Vocal responses to perturbations in voice auditory feedback in individuals with Parkinson’s disease. PloS One 7: , e33629–e33629. |
[54] | Pitts T , Bolser D , Rosenbek J , Troche M , Okun MS & Sapienza C ((2009) ) Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest 135: , 1301–1308. |
[55] | Reyes A , Castillo A & Castillo J ((2020) ) Effects of expiratory muscle training and air stacking on peak cough flow in individuals with Parkinson’s disease. Lung 198: , 207–211. |
[56] | Keus SH , Bloem BR , Hendriks EJ , Bredero-Cohen AB & Munneke M ((2007) ) Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord 22: , 451–460.quiz400. |
[57] | Kalf JG , Bloem BR & Munneke M ((2008) ) NVLF-richtlijn Logopedie bij de ziekte van Parkinson. Logopaedie Phoniatrie 81: , 101–107. |
[58] | van de Wetering-van Dongen VA , Kalf JG , van der Wees PJ , Bloem BR & Nijkrake MJ ((2020) ) The effects of respiratory training in Parkinson’s disease: A systematic review. J Parkinsons Dis 10: , 1315–1333. |
[59] | Yu X , Wu X , Hou G , Han P , Jiang L & Guo Q ((2021) ) The impact of tai chi on motor function, balance, and quality of life in Parkinson’s disease: A systematic review and meta-analysis. Evid Based Complement Alternat Med 2021: , 6637612. |
[60] | van der Heide A , Speckens AEM , Meinders MJ , Rosenthal LS , Bloem BR & Helmich RC ((2021) ) Stress and mindfulness in Parkinson’s disease - a survey in 5000 patients. NPJ Parkinsons Dis 7: , 7. |




