Management of Sleep Disturbances in Parkinson’s Disease
Abstract
Parkinson’s disease (PD) is defined by its motor symptoms rigidity, tremor, and akinesia. However, non-motor symptoms, particularly autonomic disorders and sleep disturbances, occur frequently in PD causing equivalent or even greater discomfort than motor symptoms effectively decreasing quality of life in patients and caregivers. Most common sleep disturbances in PD are insomnia, sleep disordered breathing, excessive daytime sleepiness, REM sleep behavior disorder, and sleep-related movement disorders such as restless legs syndrome. Despite their high prevalence, therapeutic options in the in- and outpatient setting are limited, partly due to lack of scientific evidence. The importance of sleep disturbances in neurodegenerative diseases has been further emphasized by recent evidence indicating a bidirectional relationship between neurodegeneration and sleep. A more profound insight into the underlying pathophysiological mechanisms intertwining sleep and neurodegeneration might lead to unique and individually tailored disease modifying or even neuroprotective therapeutic options in the long run. Therefore, current evidence concerning the management of sleep disturbances in PD will be discussed with the aim of providing a substantiated scaffolding for clinical decisions in long-term PD therapy.
INTRODUCTION
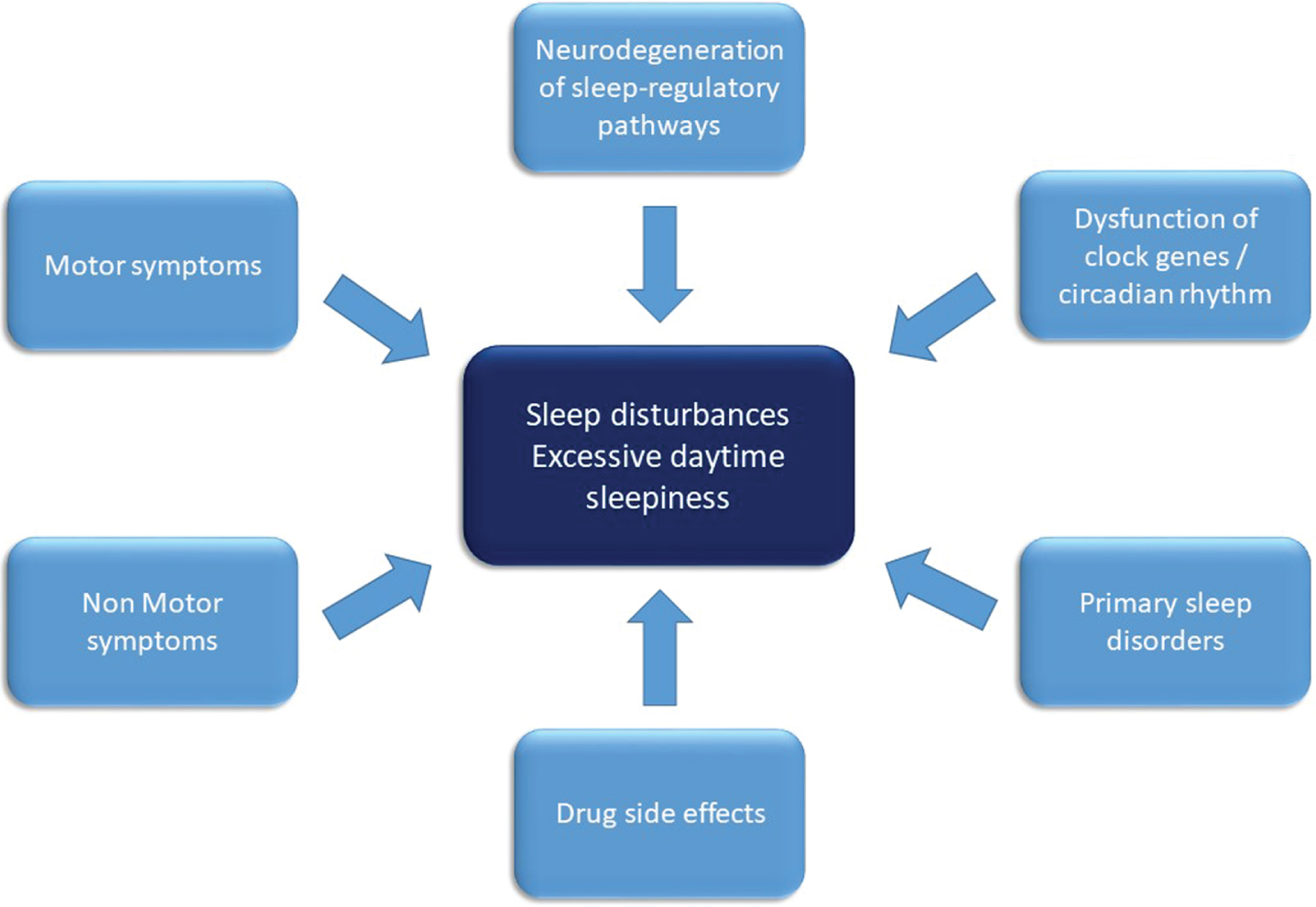
Parkinson’s disease (PD) is the second most common neurodegenerative disorder with a standardized prevalence of about 500/100000 people in the overall population further increasing with age [1, 2]. Although PD is defined by its motor symptoms bradykinesia, tremor, rigidity, and postural instability, non-motor symptoms (NMS) such as autonomic dysfunction, sleep disturbances, and neuropsychiatric symptoms may precede motor symptoms for years or even decades affecting nearly all PD patients over the disease course with deleterious effects on quality of life and activities of daily living [3–7]. However, NMS are still underreported and underestimated in clinical routine, partially due to a lack of knowledge regarding their association with PD as well as scarce therapeutic options [4]. Sleep disturbances are one of the most common NMS in PD affecting about 60% to 90% of patients [8–10] with insomnia, sleep disordered breathing disorders (SDB), excessive daytime sleepiness (EDS), rapid eye movement (REM) sleep behavior disorder (RBD), sleep-related movement disorders such as restless legs syndrome (RLS), and disturbances of circadian rhythm as the most prevalent sleep disturbances in PD with high inter individual and nightly variability [11–14]. Sleep disturbances compromise nighttime sleep quality as well as daytime activities and quality of life (QoL) of patients and caregivers [15–20] and may affect motor [21] as well as cognitive function [22, 23], mood [18, 24], and driving ability adversely [25, 26]. Although the pathophysiology of sleep disturbances in PD is complex involving multiple factors such as nighttime motor complications and adverse effects of PD treatment regimens, disintegration of sleep-regulatory pathways due to neurodegenerative processes affecting sleep regulatory circuits and neurotransmitters seem to play a key role particularly in the early phases of PD (Fig. 1) [27, 28]. The influence of neurodegenerative processes independent of motor complications and medication effects in PD is further validated by the occurrence of sleep disorders, especially EDS and RBD, in the prodromal phase of PD. In agreement with the current understanding of the pathophysiology and progression of PD, mainly alterations of sleep regulatory structures within the brainstem consistent with Braak stages 1 and 2 including the raphe nuclei and the locus coeruleus, seem to be responsible for these sleep disturbances [29]. However, recent findings in other neurodegenerative diseases, such as Alzheimer’s disease, suggest a bidirectional interaction of neurodegeneration and sleep, with consecutively aggravated neurodegenerative processes in patients with disruption of regular sleep pattern and circadian rhythm [30, 31]. Potential pathophysiological mechanisms, which might be responsible for these close link between sleep and neurodegeneration, include altered synaptic homeostasis, decreased slow wave sleep (SWS) and REM sleep as well as impaired function of the cerebral glymphatic system, which may both be caused by and also lead to enhanced accumulation of neurotoxic proteins such as amyloid-β and α-synuclein [32–34]. The linkage between sleep dysfunction and neurodegeneration is further facilitated by epidemiological data, which show an increased risk to develop neurodegenerative diseases in patients with insomnia [35]. In more detail, polysomnographic sleep recordings (PSG) of PD patients even in the early stages repeatedly demonstrated disrupted sleep micro- and macrostructures including reduction of total sleep time, sleep efficiency (proportion of time spent asleep of the overall amount of time in bed), proportion of SWS and REM sleep, whereas lighter sleep stages (N1, N2) and sleep latency were found to be increased [36–38]. Alterations of sleep structure, particularly the reduction of SWS, have been associated with impaired neurotoxin clearance by the glymphatic system [33, 39], cognitive impairment, and motor complications such as dyskinesias [40]. Accordingly, in PD patients with a higher percentage of SWS activity slower motor progression was documented [41, 42].
Fig. 1
Overview on factors contributing to sleep disruption in Parkinson’s disease. Factors contributing to the pathophysiology of sleep disturbances and excessive daytime sleepiness in Parkinson’s disease comprise of disintegration of sleep regulatory circuits and neurotransmitters as well as circadian rhythms by the neurodegenerative disease process. Nighttime sleep is disrupted by motor symptoms (such as akinesia, tremor), non-motor-symptoms such as autonomic or neuropsychiatric symptoms and drug side effects. Furthermore, primary sleep disorders such as restless legs syndrome and sleep disordered breathing contribute to nighttime and daytime impairment.
Although sleep disturbances and other NMS have recently been acknowledged as an integral part of PD [14, 43, 44], the main focus is often directed at motor symptoms in clinical routine. However, the deleterious impact of sleep disruption on nighttime sleep and daytime performance as well as overall morbidity and quality of life of PD patients and their caregivers was emphasized by recent publications, underlining the importance of correct and timely diagnosis as well as adequate management of sleep disturbances further emphasizing the need to include sleep management in the routine work-up of PD patients [18, 45]. However, the inter individual heterogeneity of sleep disorders as well as nightly variations in severity and presentation of sleep disturbances requires specialized knowledge to enable individualized diagnosis and treatment planning in PD patients. In our opinion, it is of critical importance to unravel pathophysiological mechanisms linking sleep and neurodegeneration in PD, which might lead to the invention of unique and individually tailored disease modifying or even neuroprotective therapeutic options in the future. This emphasizes the urgent need for further research on the pathophysiological mechanisms of sleep disturbances as well as multicenter trials focusing on treatment options of sleep disorders in PD. Furthermore, easily applicable, reliable, and cost-effective methods to detect and classify sleep disturbances are needed. This review aims at providing a comprehensive overview on sleep pathophysiology and management in PD patients.
METHODS
For this narrative review, we searched the PubMed database to obtain available literature of a time period between January 1980 and January 2021 using the following keywords: “Parkinson’s disease” in combination with either “sleep”, “sleep disorder”, “sleep disturbance”, “treatment of sleep disturbance”, “excessive daytime sleepiness”, “insomnia”, “sleep disordered breathing”, “sleep apnea”, “REM sleep behavior disorder”, “restless legs syndrome”, “periodic limb movements”, “circadian disorders”, “nocturnal dyskinesia”, “nocturia”, or “autonomic dysfunction”. The search retrieved more than 5000 results, of which we excluded duplicates, and favored most recently published manuscripts, randomized controlled clinical trials (RCT) and meta-analysis. Single-center trials as well as case reports were included, if no RCTs were available.
Sleep disorders were defined according to the current edition of the International Classification of Sleep Disorders (ICSD-3) [46]. Acute and chronic forms of sleep disorders were differentiated based on their frequency and duration. Thus, chronic sleep disturbances were defined as occurring at least 3 times a week for at least 3 months [46], whereas acute sleep disturbances occur for shorter durations not fulfilling the criteria for chronic sleep disorders. Accordingly, in this narrative review we specifically addressed the most common chronic sleep disorders in PD such as insomnia, excessive daytime sleepiness, SDB, RBD, and sleep related movement disorders such as RLS and periodic limb movements in sleep (PLM) as well as circadian rhythm disorders. Furthermore, PD related symptoms and complications like nocturnal motor symptoms and autonomic dysfunction likely to affect sleep quality were addressed specifically.
DIAGNOSTIC WORKUP OF SLEEP DISTURBANCES
Medical history
To detect and classify sleep disorders correctly, a detailed medical history should be obtained to evaluate actual and past subjective sleep quality and sleep characteristics including the patients as well as their caregivers’ opinion. Therefore, time in bed, total sleep time, sleep latency as well as sleep regularity and rhythm should be estimated by patients and caregivers. Subjective problems with sleep initiation or sleep maintenance and excessive daytime sleepiness including presence, frequency, and duration of planned or involuntary daytime napping as well as potential causes for sleep disruption such as nightmares, motor restlessness and involuntary leg movements, snoring, breathing problems and morning headaches should be evaluated. Furthermore, bedpartners should be encouraged to recollect occurrence of vocalization, dream enactment behavior, snoring or breathing abnormalities. Compliance with basic sleep hygiene rules including potential disturbance factors such as sleep environment and intake of alcoholic and caffeinated beverages should be evaluated. Of note, motor- and non-motor-symptoms and -fluctuations might cause or aggravate sleep disturbances in PD. Therefore, a detailed evaluation of nighttime motor symptoms including fluctuations as well as NMS such as anxiety, depression, and pain is highly recommended. Clinicians should obtain a detailed history of initial PD symptoms, course of the disease, treatment regimens and their relation to sleep quality. However, other conditions such as cardiovascular, pulmonary, or psychiatric diseases and their respective treatment might also cause sleep disruption and therefore need to be evaluated in regard to sleep quality. Sleep disturbances as well as their respective treatment may also have negative effects on sleep quality and quality of life of spouses/caregivers, which should be taken into account when treatment choices are made. To optimize the management of sleep disturbances clinicians should be aware of ‘sleep discrepancy’ to respond adequately to reported failure of a chosen treatment regimen. Sleep discrepancy is a term used to describe a discrepancy between objectively measured sleep parameters (e.g., by PSG) and (subjectively) perceived sleep quality [47]. Therefore, sleep disturbances should be evaluated integrating objective measures of sleep in combination with subjective sleep quality as reported by patients and bedpartners.
Questionnaires
The use of standardized questionnaires is recommended to screen for and to determine severity of sleep disturbances. However, questionnaires might be of limited use in cognitively impaired patients and clinicians should be aware of potential bias resulting from caregivers’ interferences in completing the questionnaires. In line with the recommendations of the Movement Disorder Society task force [48], the 19-item questionnaire Pittsburgh Sleep Quality Index (PSQI, cut-off-score between normal and abnormal sleep > 5) exploring sleep quality and sleep habits within the past four weeks is recommended to screen for the existence and severity of sleep disturbances [49]. Alternatively, two other scales, which were specifically designed to assess symptoms and possible causes for sleep disruption in PD, namely the Scales for Outcomes in PD Sleep (SCOPA-SLEEP) questionnaire and the Parkinson’s Disease Sleep Scale (PDSS-2) may be applied [48, 50]. The PDSS-2 addresses nocturnal problems over the past week incorporating PD specific symptoms such as akinesia [50]. The Scales for Outcomes in Parkinson’s Disease (SCOPA-SLEEP) assesses sleep in the last month by self-rating in three subscales addressing nighttime condition, quality of sleep, and specific sleep disturbances [51].
To screen for and to quantify specific sleep symptoms a variety of scales is used. For evaluating presence and severity of EDS, the Epworth Sleepiness Scale (ESS, cut-off ≥10) is recommended [48, 52, 53]. In contrast to the ESS, which covers a recent time span, the Stanford Sleepiness scale was established to measures a current state of EDS. Due to the lack of psychometric properties, the Stanford Sleepiness scale is less standardized than the ESS [48, 53]. While the RLS-diagnostic index (RLS-DI) is applied to support the diagnosis of RLS, the International Restless legs Symptoms Severity Scale (IRLSS) was designed to determine the severity of restless-legs-symptoms in RLS patients [54]. The IRLSS has been widely used in PD studies, although it has not been validated in PD populations, yet [53]. To screen for insomnia in PD, the PSQI is recommended [53]. The Insomnia Severity Index (cut-off ≥10) is used to screen and rate insomnia in primary insomnia patients [55, 56] and has already been utilized in PD [57], although it has not been validated in this population, yet. The REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ, cut-off ≥5) or the RBD single-question-questionnaire were designed to screen for symptoms of RBD, although their lack of specificity limits their usefulness for diagnostic purposes [58–61]. Morning and evening sleep protocols as well as sleep diaries might add insights on sleep habits, confounding disturbance factors as well as circadian rhythm, but might be of limited use in patients with cognitive impairment. To address NMS such as autonomic, neuropsychiatric, and mood related aspects of PD, which might contribute to sleep disturbances, the PD NMS questionnaire is recommended [62].
Actigraphy
Actigraphy is based on accelerometric measurements of movements allowing estimation of sleep and wake state by differentiating periods of activity and resting. Although this approach enables only a rough estimation of the amount of sleep and wake periods, actigraphy is favorable in case of suspected circadian rhythm disorders, irregular sleep rhythm or insufficient sleep hygiene. Since actigraphy devices imitate wristwatches, their application is non-invasive, easily accessible, and well tolerated. Therefore, main advantage and strength of actigraphy is the possibility to measure sleep for longer periods of up to weeks or even months without disruption at home. Efforts to overcome its main limitations, particularly distinguishing sleep and wake more accurately and even attempting to improve sleep staging, have already been stepped up using artificial intelligence approaches [63].
Polygraphy
Cardiorespiratory polygraphic recordings (PG) are feasible to screen for SDB/sleep apnea. These portable devices include sensors to record nasal airflow, thoracic and abdominal effort sensors to register breathing effort, a microphone to detect snoring, an oxygen desaturation sensor (SpO2), and a position sensor to detect supine related SDB. Optional additional sensors include electromyography (EMG) to detect limb movements and electrocardiogram. If electroencephalographic (EEG) sensors are added, the devices are applicable in the transition between at home screening tools and full PSG.
Polysomnography
Video polysomnography (vPSG) recordings are the gold standard to score sleep structure and detect sleep disorders. In accordance with the American Academy of Sleep Medicine guidelines, vPSG consists of EEG, electrooculography, and EMG of chin and limbs to enable scoring of sleep stages and limb movements as well as muscle tone, respiratory sensors including flow, effort, heart rate, and oxygen saturation to screen for SDB as well as video recording to screen for abnormal nighttime behaviors [64]. However, vPSG is performed mainly in sleep laboratories, often hospital-based and less likely at patients’ homes and therefore more expensive and time-consuming than PG or actigraphy. Broad application of vPSG is therefore restricted due to limited capacities and the relative invasiveness of the method. Thus, further research is already dedicated and should be intensified to invent easier accessible and applicable sleep recording methods.
GENERAL TREATMENT SUGGESTIONS
Treatment of sleep disturbances in PD is complex and often needs multifactorial approaches including non-pharmacological and pharmacological interventions. Multiple factors might be responsible for sleep disturbances in a certain patient, which need to be considered and addressed. However, these factors might be indistinctive in many cases, which might impede effective treatment. However, treating physicians should be aware of the mechanisms, which might be involved in sleep disruption in PD to aspire tailored approaches for the individual patient. Mechanisms involved in sleep disturbances in PD include neurodegeneration of structures involved in sleep regulation such as the hypothalamus and brainstem, primary sleep disorders such as SDB, side effects of dopaminergic or concurrent medication, nocturia, or impaired sleep hygiene.
In general, all patients and caregivers should be instructed in regards to sleep hygiene rules favoring regular bedtimes, light exposure, and regular—preferably outdoor—activities and exercise. Exercise seems to improve motor function as well as NMS such as cognition, mood, and sleep in PD patients as well as in the general population [65, 66]. Among others, alterations of cerebral neurotransmitters as well as inflammatory, hormonal, and autonomic circuitries have been proposed to contribute to the positive effects of exercise on sleep [67, 68]. In the general population subjective sleep quality seems to be improved by a variety of exercise forms including cardio-training as well as resistance training [69]. Furthermore, immediate as well as prolonged effects of sustained training on objective sleep quality parameters such as SWS and sleep efficiency have been demonstrated [67, 68, 70]. In PD patients, high-intensity training 3 times per week for 16 weeks increased sleep efficiency, total sleep time as well as SWS in 27 PD patients compared to 28 patients receiving sleep hygiene information only in a small RCT [71]. Two RCTs demonstrated improved subjective sleep quality as measured by the PDSS/PDSS-2 during different exercise regimens including a combination of qigong and walking four times weekly compared to daily walking alone as well as a multidisciplinary approach including cardio-training, relaxation, and stretching with three one-hour sessions daily in 89 patients [72, 73]. However, most studies include only a limited number of patients and trials addressing the effects of exercise on specific sleep disorders in PD are missing [74]. Furthermore, pathophysiological mechanisms leading to these improvements have not been fully understood and recommendations for optimal training schedules are lacking. Thus, further research on the effects of exercise as well as lifestyle modifications on sleep disturbances in PD are needed.
SPECIFIC SLEEP DISORDERS: DIAGNOSTICS AND TREATMENT
Insomnia
Clinical presentation and pathophysiology
Insomnia is defined as subjective perception of non-restorative sleep with impaired daytime performance caused by difficulties to initiate and/or maintain sleep and/or early morning awakenings [46]. To fulfill the criteria for chronic insomnia symptoms have to occur at least three times per week for at least three months [46]. Current pathophysiological concepts of primary insomnia unrelated to PD include circadian rhythm disruption, partially attributed to clock gene malfunction, as well as neurotransmitter imbalances of the orexinergic, serotoninergic, and gamma-aminobutyric-acid (GABA) system as part of the sleep-wake-cycle. Furthermore, hyperarousal mechanisms including disturbances of cortisol secretion and persistence of fast EEG-frequencies assumingly leading to higher levels of awareness during sleep have been identified as mechanisms contributing to insomnia [75].
In PD, the frequency of insomnia varies widely from 37% up to 80%, depending on evaluation tools and definitions used, rendering insomnia one of the most common sleep disorders in PD [9, 43, 76, 77]. In contrast to patients with primary insomnia, in PD, sleep maintenance insomnia with sleep fragmentation is more common than sleep initiation problems [78]. Of note, subjective sleep quality and objective vPSG derived sleep measures often deviate from each other, a phenomenon called ‘sleep discrepancy’, which was already described in patients with primary insomnia (see section on ‘Medical history’ above) and might be caused or influenced by hyperarousal mechanisms [47]. Compared to the general population, neurodegenerative processes within sleep-wake regulatory centers such as the brainstem leading to pathophysiological and biochemical alterations are likely to contribute to insomnia in PD. As already mentioned, PD-related motor complications, particularly nighttime akinesia, dyskinesia, or motor fluctuations lead to insomnia symptoms such as sleep fragmentation [79, 80] with increasing symptoms in more advanced disease stages [81]. In accordance to findings in the general population, insomnia in PD is associated with female sex and age [81, 82]. Furthermore, insomnia in PD is associated with neuropsychiatric symptoms such as anxiety and autonomic symptoms like nocturia and blood pressure abnormalities [18, 80]. Furthermore, treatment with dopaminergics or amantadine in PD might lead to double-edged effects on sleep with primarily beneficial effects on sleep quality by improving nighttime motor symptoms such as akinesia but also adverse effects on sleep quality particularly when higher doses are necessary, which are able to induce or aggravate insomnia symptoms [83].
Diagnostic workup
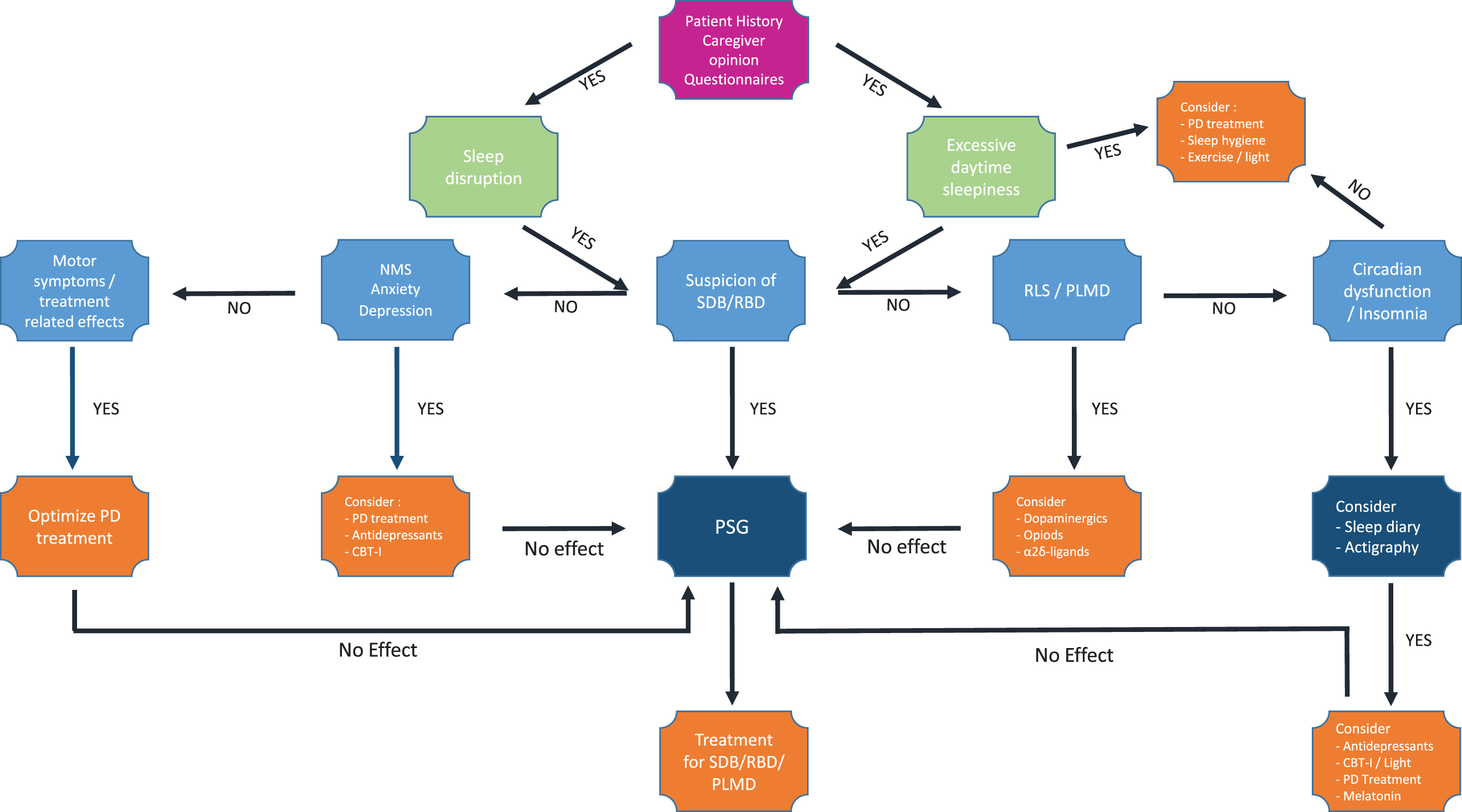
It is of the utmost importance to rule out treatable causes for insomnia symptoms, since a great variety of conditions including primary sleep disorders, concomitant conditions, and medications might disrupt or affect nighttime sleep adversely. For optimal treatment decisions, sound investigation of sleep and daytime symptoms as well as possible influencing factors and causes including nighttime motor as well as NMS, concomitant medication, and caregivers’/spouses’ opinion is strongly recommended [84, 85] as already mentioned. Treatment of PD related complications, which might influence sleep adversely, should be implemented, while executing further diagnostic and therapeutic steps to evaluate insomnia symptoms [43, 86] (also see section on ‘Medical history’ above). To score and monitor insomnia severity, the Insomnia Severity Index is recommended [55]. Chronic cases of insomnia unresponsive to treatment should be thoroughly investigated using questionnaires and further objective testing, if deemed necessary. Thus, if indicated by sleep history or questionnaires such as the PDSS-2 or PSQI treatable causes for insomnia symptoms such as primary sleep disorder including SDB need to be excluded by vPSG or PG (see sections on ‘Medical history’ and ‘Questionnaires’ above; Fig. 2). To gain objective information on sleep timing and a rough estimation of total sleep time and sleep efficiency, actigraphy can be used in combination with sleep diaries in cases of suspected circadian rhythm dysfunction or dysfunctional sleep hygiene [87]. Furthermore, the assessment of dim light melatonin excretion onset might be considered in specific sleep centers [88].
Fig. 2
Diagnostic and therapeutic algorithm for sleep disturbances in Parkinson’s disease. Flowchart suggesting a comprehensive approach towards pragmatic diagnostic and therapeutic options for sleep disturbances in Parkinson’s disease. CBT-I, Cognitive behavioral therapy for insomnia; NMS, non-motor symptoms; PD, Parkinson’s disease; PLMD, Periodic limb movement disorder; RBD, REM sleep behavior disorder; RLS, restless legs syndrome; SDB, sleep disordered breathing; vPSG, video polysomnography.
Therapeutic options
Optimization of PD related treatment regimens including effective treatment of potentially trouble-some PD related complications such as motor fluctuations should be considered first. Especially in case of nighttime akinesia or tremor application of extended-release formulations of dopamine agonists (DA) such as rotigotine and ropinirole might improve subjective and objective sleep quality as well as motor symptoms [88–91]. In contrast, controlled-release levodopa/carbidopa has not been shown to improve objectively measured sleep quality in a small single-center, randomized, placebo-controlled trial in 16 PD patients [92]. However, treatment with DA itself might also be responsible for sleep disturbances as already mentioned, particularly in higher doses and depending on the preferentially affected DA receptor type [91, 93, 94]. In a small, randomized, single-center trial, rasagiline treatment over 8 weeks led to improved sleep efficiency and sleep architecture not attributable to an improvement in motor symptoms, indicating other mechanisms such as alterations in neurotransmitter availability to be responsible for this improvement, which needs further research and confirmation [37].
Despite some small case series demonstrating improved subjective sleep quality patients after deep brain stimulation (DBS) partially attributed to improvement in motor symptoms, other studies failed to demonstrate effects on objective PSG-assessed sleep quality in PD [95, 96]. The effects of DBS on basal ganglia and motor pathways are complex and have only been partially understood. Thus, it is nearly impossible to fully estimate the impact of DBS on other regulatory networks, such as the sleep-wake-system, yet.
Apart from a number of case series and small randomized trials, the evidence for medical treatment of insomnia in PD is scarce [97]. Subsequently, treatment strategies are partially based on experiences in primary insomnia patients. Therefore, non-pharmacological approaches including instructions on sleep hygiene rules and restriction of bed times as well as exertion of relaxation techniques and music therapy seem to be favorable with little side effects [86, 98]. In addition, implementation of cognitive behavioral therapy (CBT-I) was found to be effective in primary insomnia [99]. Likewise, a couple of small interventional studies were able to demonstrate positive effects of CBT-I, sometimes combined with bright light therapy, on subjective sleep quality in PD patients [100, 101] as well as on sleep discrepancy [47]. Furthermore, application of bright light therapy with 1000–7500 lux for 30–90 minutes alone was able to improve insomnia in PD (Table 1) [102, 103].
Table 1
Overview on treatment options in Parkinson’s disease
| Insomnia | Consideration of optimization of dopaminergic treatment |
| Treatment of primary sleep disorders | |
| Behavioral approaches (e.g., sleep hygiene, exercise) | |
| CBT-I / bright light therapy* | |
| Z-substances (temporarily) | |
| Antidepressants* | |
| Melatonin* | |
| Excessive Daytime sleepiness | Consideration of optimization of dopaminergic treatment |
| Treatment of primary sleep disorders (e.g., SDB) | |
| Behavioral approaches (e.g., planned napping) | |
| Caffeine* | |
| Modafinil** / Atomoxetin** | |
| Sleep disordered breathing | PAP-treatment |
| Mandibular advancement therapy* | |
| Positional training* | |
| Stimulation of hypoglossal nerve** | |
| Restless-legs syndrome | Behavioral/physical approaches (e.g., cooling substances) |
| Evaluate treatable causes such as iron deficiency | |
| Evaluate interfering medication such as antidepressants | |
| Optimization of preexisting dopaminergic treatment | |
| •Levodopa | |
| •Dopamine agonists (ropinirole, pramipexole, rotigotine) | |
| α2δ-ligands* | |
| Opioids | |
| Periodic limb movements in sleep | Evaluate treatable causes such as sleep disordered breathing |
| Consider optimization of preexisting dopaminergic treatment | |
| •Levodopa | |
| •Dopamine agonists (ropinirole, pramipexole, rotigotine) | |
| REM Sleep Behavior Disorder | Clonazepam* |
| Melatonin* | |
| Sodium oxybate** | |
| Circadian rhythm disorder | Behavioral approaches (e.g., exercise / sleep schedule) |
| Bright light therapy* | |
| Melatonin* |
Primary/approved treatment options are not labelled. *labels therapeutic options without approval by FDA or other authorities for the specific sleep disorder (even if not specifically for PD), but in the authors opinion reliable scientific evidence for effectiveness and safety. **labels therapeutic options with questionable evidence or risk/safety profile. CBT-I, Cognitive behavioral therapy for insomnia; SDB, sleep disordered breathing.
Nonetheless, many PD patients require pharmacological strategies to improve insomnia symptoms. However, none of the drugs used to treat insomnia are FDA-approved for insomnia in PD. Therefore, treatment strategies for PD-related insomnia are mainly based on scientific evidence deduced from studies on primary insomnia. Treatment options for acute insomnia include z-substances, acting as GABA-agonists, which are favored for short-term use of up to 4 weeks. As one of these z-substances, eszopiclone was shown to be effective in a small, randomized, single-center trial in PD improving objective and subjective sleep quality over the 6-week treatment period [104]. However, relevant negative side effects such as confusion, dizziness, hypersomnia, and falls might occur with the use of z-substances. Furthermore, long-term use of z-substances or hypnotics is not recommended in insomnia in general due to its habituation effects and subsequent worsening of insomnia symptoms over time.
In cases of coexisting depression, application of antidepressants might be considered (Table 1). While small case series showed positive effects of some of the antidepressants (e.g., trazodone, doxepine, venlafaxine, and nortriptyline) on insomnia in PD, other substances such as duloxetine were ineffective in improving insomnia symptoms [105]. Despite the fact that melatonin showed small effects on subjective rather than objective sleep quality in PD patients [106], the evidence is not sufficient enough to justify a general recommendation [107, 108]. In contrast to quetiapine and clozapine, which were found to be ineffective, the novel neuroleptic agent pimavanserine demonstrated positive effects on insomnia symptoms in PD [105]. Other potential treatment options, such as orexin antagonists, have not yet been investigated in neurodegenerative diseases [109]. This lack of evidence is further complicated by case reports noting specific side effects of these new drugs such as the occurrence of troublesome RBD symptoms [110].
To sum up current treatment options for insomnia in PD: Evidence for treatment of insomnia in PD patients is scare. Therefore, multicenter trials to evaluate the effects of antidepressants and new treatment options such as orexin antagonists on sleep disturbances, specifically insomnia, in PD are needed to overcome treatment shortcomings. However, sleep hygiene should be optimized, and CBT-I and bright light therapy should be considered. Secondary causes for insomnia such as primary sleep disorders including SDB need to be diagnosed and treated. Motor complications such as nighttime akinesia need to be considered, e.g., by favoring extended-release formulations of PD medications. Pharmacological treatment options include antidepressants such as doxepine, trazodone, and venlafaxine, although their on-label use is restricted to cases of comorbid depression. Z-substances might be considered for short-term use. Furthermore, melatonin might be considered, preferentially in cases of insomnia related to circadian rhythm dysfunction, whereas promising results regarding pimavanserine and newer substances such as orexin antagonists need further evidence to confirm their value in PD patients.
Excessive daytime sleepiness
Clinical presentation and pathophysiology
EDS is defined as an increased propensity to fall asleep, particularly in monotonous situations despite a sufficient amount of total sleep time [46]. EDS may occur with or without sudden irresistible “sleep attacks” without warning signs, which were first described in the 1990s in conjunction with treatment initiation with dopamine agonists in PD [111]. The estimated prevalence of EDS in PD varies widely between 20 and 60%. This discrepancies in prevalence are partly explained by the usage of different definitions as well as screening instruments [112, 113]. Although EDS may occur prior to PD motor symptoms [114], the role of EDS as a prodromal sign or risk factor for neurodegeneration still needs to be clarified. While some case-control studies failed to detect an elevated frequency of EDS in drug naïve PD patients compared to controls [115], others showed a correlation of EDS with an increased risk to develop PD [116]. However, EDS in PD is associated with male sex and more advanced disease stages, faster motor progression and cognitive, autonomic and neuropsychiatric complications [117, 118] as well as aggravated dopaminergic deficits in the caudate nucleus depicted by FP-CIT SPECT [119].
Currently, EDS in PD is attributed to alterations in cerebral regions responsible for sleep-wake-regulation such as the hypothalamus and brainstem areas due to the neurodegenerative process. Thus, involving alterations of neuropeptide and neurotransmitter balances and interactions, particularly of the gabaergic, orexinergic, and serotonergic system [119–121]. However, the contribution of each solitary transmitter system to EDS has not been fully elucidated yet. While some studies demonstrated a decrease of hypothalamic orexin neurons, orexin levels in cerebrospinal fluid showed inconsistent results [121, 122]. Concurrently, sleep dysfunction in PD was found to be associated with reduced serotonergic signaling in the midbrain raphe, basal ganglia, and hypothalamus on serotonin transporter positron-emission tomography [120].
Several risk factors and treatable conditions should be excluded (Fig. 2), before EDS is considered “idiopathic” or only related to PD and its neurodegenerative processes itself [43]. The most common cause for EDS is a disruption of nighttime continuity resulting in non-restorative sleep, e.g., caused by primary sleep disorders. SDB is one of the most prevalent causes for EDS in the general population and also in PD. Furthermore, special emphasis should be placed on evaluating PD related nighttime motor complications such as akinesia as well as PD related non-motor complications such as depression and anxiety (Fig. 1). Furthermore, treatment regimens for PD, particularly the use of dopamine agonists and levodopa, are associated with EDS [118, 123]. While dopamine agonists such as rotigotine, pramipexiole, and ropinirole might be able to improve EDS by means of ameliorated nighttime motor function and improved sleep quality and duration, they were also associated with the onset or aggravation of EDS [124, 125]. About 18–25% of PD patients per patient-year reported of novel daytime sleepiness after the initiation of dopaminergic therapy in a prospective, open-label study [126]. Furthermore, withdrawal of dopaminergic medication resulted in mitigation or disappearance of EDS in some patients [127, 128]. However, in other studies no effect of DA initiation on EDS could be demonstrated [129]. These discrepancies might be partially explained by differences in dosage, timing, and kinetics of substances as well as preferentially affected dopamine receptor type as particularly substances affecting D3 receptors were accused of causing EDS and sleep attacks. In contrast, stimulation of the D1 receptor type seems to decrease sleep efficiency and increase arousals, whereas D2 receptors might be affected differentially depending on DA dosage [130].
Diagnostic workup
The diagnostic workup of EDS should include a detailed medical history including PD related treatment and other medication such as hypnotics as well as exploration of nighttime motor- and NMS including patients’ and caregiver’s opinion. Further investigations similar to the exploration of insomnia symptoms should be executed, depending on sleep quality and symptoms (Fig. 2). Therefore, subsequent questionnaires for suspected sleep disorders including PDSS-2, SCOPA-Sleep, RBDSQ, and/or PSQI might be considered, as already mentioned above. Primary sleep disorders, particularly SDB, RBD, and RLS need to be ruled out preferably using PG or vPSG as deemed suitable (Fig. 2). However, quantification of EDS severity is not standardized yet. Thus, different methods have been recommended to assess EDS including questionnaires such as the ESS. For objective EDS assessment the PSG based multiple sleep latency test or the maintenance of wakefulness test might be considered [131]. However, usefulness of the multiple sleep latency test/maintenance of wakefulness test for clinical routine is limited due to the low grade of standardization of test execution resulting in low reproducibility for clinical routine. Furthermore, their application is restricted to sleep laboratories resulting in high costs and limited accessibility. However, objective quantification of EDS is not essential for treatment decisions in most cases, apart from clinical trials or assessment of driving ability, which might require further neuropsychological testing including vigilance tests in some cases [132].
Therapeutic options
Treatment of EDS should include treatment of nighttime motor- or non-motor complications, if present, e.g., by introducing extended release formulations of DA in case of nighttime akinesia. As elevated doses or inadequate timing of DA might also be responsible for EDS, adjustment of timing and DA dose should be considered. Furthermore, concomitant conditions and medication need to be evaluated and adjusted, if necessary. If EDS persists after adjustment of PD treatment, vPSG should be performed to search for primary sleep disorders, which should then be treated adequately (Fig. 2). In case of persistent EDS even with adequate treatment, non-pharmacological strategies should be considered before pharmacological approaches (Table 1), since pharmacological treatment options for EDS in PD are limited. Patients should be advised to follow sleep hygiene recommendations including the implementation of regular bedtime and sleep schedules. If necessary, structured napping periods might be helpful to overcome impaired daytime activity. Furthermore, it might be helpful to increase the level of activity in general, including exercising and exposition to natural light by outdoor activities. Consecutively, application of bright light therapy might also be favorable to entrain the circadian rhythm and improve nighttime sleep, mood, and EDS, probably via promoting dopamine secretion and regulation of melatonin secretion [43, 100].
The evidence for wake-promoting agents is generally low. While treatment with caffeine with doses of up to 200 mg twice per day was shown to improve EDS by 1.7 points in the ESS over 6 months, the amount of improvement is hardly clinically relevant [133, 134]. Istradefylline, acting as an adenosine-2A-receptor-antagonist, was recently approved by the Food and Drug Administration (FDA) as add-on therapy for dyskinesia in PD and was shown to reduce daytime sleepiness in PD as a secondary outcome parameter in a 3-month open-label study, but further evidence is needed before a general recommendation could be considered [135]. Methylphenidate, a derivate of amphetamine, established for the treatment of EDS in narcolepsy, was shown to improve EDS in a small open-label trial [136]. However, further evidence is lacking. Modafinil, a wake-promoting agent, approved for the treatment of EDS in narcolepsy, was assessed in three randomized-controlled trials in PD showing ambiguous results [137–139]. However, a meta-analysis demonstrated a reduction of subjective EDS symptoms as shown by a decrease of 2.24 points on the ESS score after initiation of modafinil treatment in PD [140], while no improvement of objective readouts for EDS was shown [141]. However, modafinil might lead to worsening of other sleep disturbances, particularly of insomnia symptoms [142]. Therefore, application of modafinil in PD patients cannot be recommended in general. Atomoxetine, a selective noradrenaline reuptake inhibitor, commonly used for the treatment of attention deficit hyperactivity disorder, demonstrated improved EDS and cognitive performance in a small, placebo-controlled, randomized, double-blind trial in PD [143]. However, further confirmatory evidence is needed. Sodium oxybate, also known as gamma hydroxyl butyric acid (GHB), improved subjective sleep quality and EDS as assessed by questionnaires in a small open-label trial with 38 PD patients demonstrated by a remarkable drop in the ESS score of 6.6 points [144]. However, the quality of the study was limited by the open-label trial design and also needs further confirmation. Furthermore, a small double-blind, placebo-controlled crossover trial including 12 PD patients, subjective and objective measures of EDS were improved using GHB [145]. A broad use of sodium oxybate is complicated by its laborious preparation and its side effects, particularly the risk of acute intoxication and evocation or worsening of SDB with potentially life-threatening consequences [145–147]. However, if sodium oxybate is cautiously applied in due consideration of security measures, its use is safe and effective as shown in narcolepsy patients.
To sum up, none of the above-mentioned drugs can be recommended as a standard treatment option of EDS in PD. Therefore, it is of utmost importance to identify treatable causes for EDS such as SDB. Furthermore, treatable causes for nighttime sleep disruption resulting from PD related motor and non-motor complications should be identified and treated accordingly. If necessary, sleep hygiene should be optimized including implementing strict sleep bedtime regimens, adding daytime napping, bright light therapy and exercise into daily routine.
Sleep disordered breathing
Clinical symptoms and pathophysiology
SDB, also known as sleep apnea, is the most common primary sleep disorder affecting up to 5 to 10% of the general population and up to 50% of males and 40% of females in the older population [148]. Up to 60 % of PD seem to suffer from SDB, rendering it also one of the most common sleep disorders in PD [149–151]. However, the exact prevalence of SDB in PD is uncertain, ranging widely in different studies [152, 153], but SDB prevalence seems not to be elevated in PD compared to the general population [152, 153].
SDB is defined by the occurrence of repetitive reduction of breathing flow during sleep and concurrent, though temporary, desaturation with subsequent reoxygenation [64], thus leading to sleep fragmentation and non-restorative sleep [154–156]. Based on the amount of flow reduction, breathing abnormalities are classified as apneas (90% reduction) or hypopneas (30% with associated desaturation or arousals). With regards to their most common apnea type, obstructive sleep apnea (OSA) and the less prevalent central sleep apnea (CSA) are differentiated. OSA is characterized by recurrent upper airway obstruction due to upper airway instability, often caused by obesity, anatomical abnormalities, or ineffective pharyngeal dilator muscle function during sleep [157]. Further factors contributing to SDB are the degree of airway collapsibility, loop gain (drive response to reduced airflow), arousal threshold and magnitude of compensatory mechanisms such as increase in airflow and ventilation response to arousal [158]. In addition, social inequalities seem to increase the risk for SDB in the general population [159]. In contrast, CSA originates from a disruption of the balance between peripheral and central CO2-receptor sensitivity resulting in apnea threshold disturbances frequently occurring in patients with heart or kidney failure as well as lung edema [160, 161].
Sleep apnea severity is classified based on the amount of apneas/hypopneas per hour sleep (AHI index) with > 5/h < 15/h as mild, ≥15/h < 30/h as moderate and ≥30/h as severe [46]. Whereas the body mass index is an important risk factor for OSA in the general population, it does not seem to play an important role in OSA related to PD [22]. In contrast, other factors such as insufficient pharyngeal dilatator muscle stability as well as impaired respiratory muscle strength, such as the maximal inspiratory pressure (PImax) and maximal expiratory pressure (PEmax) was shown to be impaired in PD, presumably contributing to OSA in PD [162].
Sleep fragmentation, repetitive arousals, and oxygen desaturation as a consequence of SDB seem to increase the risk for cardiovascular events by several mechanisms including non-dipping of nighttime blood pressure and generation of reactive oxygen species causing enhanced oxidative stress and endothelial dysfunction as well as impaired mitochondrial function, thus, resulting in atherosclerosis, increased risk of atrial fibrillation, end-organ damage, and cardiovascular events such as stroke [154, 163]. Mainly, oxygen desaturation seems to be responsible for cardiovascular health related issues [164] and may contribute to neurodegeneration in PD. However, controversies regarding the impact of SBD on cardiovascular events and the extent of preventive effects of treating SDB in the general population are ongoing [165].
Most patients with SDB, whether with or without PD, report sleep fragmentation, difficulties with sleep maintenance rather than sleep initiation and excessive daytime sleepiness resulting in daytime naps in monotonous (such as watching TV) or even active situations such as within conversations or during meals. SDB is associated with cognitive impairment, metabolic disturbances, and weight gain as well as neuropsychiatric symptoms in the general population [166, 167]. In PD patients, SDB was associated with impaired cognitive performance, particularly impaired vigilance/attention, executive function, and working memory [22, 168]. Associations of SDB with motor symptoms or NMS in PD are still discussed controversially. While some studies did not detect differences in motor function in PD patients with and without SDB in a smaller pilot study [152], others found an association of SDB with worse motor function [169]. Apart from these associations with PD-related symptoms, SDB affects quality of life of patients and caregivers to a relevant extent. In fact, SDB leads to relevant impairment of nighttime sleep quality and daytime activities partially by causing EDS.
Diagnostic workup
SDB might be suspected, if patients complain about sleep fragmentation, problems with sleep initiation or sleep maintenance, snoring or apneas reported by bed partners. However, EDS is one of the most common complaints in SDB in general as well as in PD. Of note, in contrast to the diagnostic work-up strategy in the general population, EDS in PD patients is often simply attributed to possible adverse side effects of PD drug regimen. Thus, diagnostic work-up strategies to rule out SDB are often not deemed necessary, resulting in occurrence of undetected and untreated sleep apnea in several of these patients [170, 171]. Due to the deleterious effects of sleep disturbances and EDS on quality of life of patients and caregivers as well as the negative consequences of SDB including cardiovascular events and worsening of motor and cognitive performance, diagnostic workup and therapy implementation in case of suspected SDB in PD is strongly recommended. Therefore, in case of suspected SDB vPSG or at least an at-home cardiorespiratory PG should be conducted (Fig. 2).
Therapeutic options
The gold standard for SDB is positive airway pressure treatment (PAP), mostly as continuous positive airway pressure treatment (CPAP), implemented in specialized sleep centers. However, in specific conditions, such as a combination of central and obstructive apneas, other methods of ventilation such as biphasic positive airway pressure treatment (BiPAP) might be considered by sleep specialists. The efficacy of PAP treatment has been demonstrated in the elderly, in PD and even in Alzheimer’s disease patients [172]. However, the contribution of PAP treatment of SDB to the prevention of cardiovascular diseases in the general population has been controversially disputed lately [165]. Nonetheless, considering the positive effects of improved sleep quality on patients and caregivers, effective treatment of SDB should be aspired. Unfortunately, PAP-treatment is not always well tolerated, particularly in those patients too incapacitated to handle the mask and device on their own. Long-term drop-out rates and adherence rates of PAP therapy in PD have not been comprehensively evaluated yet. However, an epidemiological study suggested lower OSA care rates and adherence in neurological conditions such as PD compared to the general population [173]. In a prospective trial, only 50% of PD patients with SDB agreed to start PAP treatment, of which 75% dropped out due to lack of CPAP tolerance [174]. One of the unresolved issues contributing to this gap in SDB care is the lack of sleep centers with special knowledge regarding the management of patients with disabling and care-dependent neurological conditions such as PD.
For OSA, other treatment options include positional training to avoid the supine position. Mandibular advancement devices have emerged as another potential treatment option for OSA, particularly in case of light to moderate sleep apnea. However, effectiveness [175–178] as well as OSA severity best suited for this treatment option still need to be confirmed for PD [178, 179] and further evidence of long-term outcome and adherence rates in PD patients are needed on this matter. This is also true for surgical approaches including hypoglossal nerve stimulation. In CSA, treatment of underlying causal conditions such as opioid treatment, heart, lung, or kidney diseases is recommended as first line therapy, while PAP treatment should be considered in moderate to severe CSA only [180]. However, CSA is a lot less common in PD patients than OSA.
In conclusion, identification of SDB in PD is of great importance due to its deleterious effects on general health, cognition, and alertness and therefore quality of life. Furthermore, missing the diagnosis of SDB might lead to avoidable changes of medication with consecutive side effects or negative impact on PD symptoms. However, considering treatment gaps of SDB in neurological conditions, particularly neurodegenerative diseases, it is necessitated to intensify efforts in gathering knowledge and developing concepts to treat SDB more effectively in patients with neurological disorders.
Rapid eye movement sleep behavior disorder
Clinical presentation and pathophysiology
RBD is characterized by dream enactment or complex-motor behavior often associated with vocalizations in the content of nightmares [181] and is proven by impaired muscle atonia (REM sleep without atonia, RSWA) and excessive movements demonstrated by vPSG. Movements show a high night-to-night variability ranging from small jerks to wide movements including kicking, boxing, and bed falls [182], endangering patients and bed-partners [183]. While patients sometimes complain about non-restorative sleep, EDS, and nightmares, many patients are unaware of their abnormal behaviors during sleep [184]. However, some patients might experience startling awakenings by sudden movements such as kicking or boxing associated with fitting dream content. Furthermore, caregivers’ burden is high with impact on quality of life and marital relationships [185]. The underlying pathophysiological mechanisms leading to RBD include neurodegenerative processes within the brainstem, the accumulation of potentially neurotoxic proteins such as α-synuclein as well as transmitter imbalances due to the neurodegenerative processes [186]. The key structures involved in REM sleep muscle atonia include the locus coeruleus/subcoeruleus complex in the mesopontine tegmentum, pontine glutamatergic neurons, and inhibitory neurons of the ventromedial medulla [187, 188]. Direct and indirect projections from these areas inhibit motoneurons of the spinal cord using glutamatergic, GABAergic, and glycinergic inputs [186]. However, REM sleep control is complex and further nuclei such as the dorsal raphe as well other transmitters such as serotonin seem to be involved in REM regulation.
RBD associated with use of antidepressants or narcolepsy type 1 is considered secondary, while patients with “idiopathic” RBD, recently named “clinically-isolated” RBD [189], are at impending risk of phenoconversion most frequently to one of the α-synucleinopathies such as PD, multiple system atrophy, or dementia with Lewy bodies (DLB) [183, 190–192]. RBD may precede the onset of motor symptoms or cognitive decline by years or decades. Conversion rates of RBD patients developing PD, multiple system atrophy, or DLB are high adding up to 92 to 100% [193]. Thus, in recent years RBD was recognized as part of the prodromal stage of α-synucleinopathies, particularly PD [183], and was included into the diagnostic criteria of the Movement Disorder Society for prodromal PD [194, 195].
The estimated prevalence of RBD in the general population lies between 0.5 and 2%, while adding up to 40 to 60% in PD [27]. In PD patients, presence of RBD is associated with age, higher LED doses, faster and more severe motor progression, falls, fluctuations, cognitive declinem and neuropsychiatric complications as well as impaired quality of life [182, 196–199]. However, in one study, the predictive value of RBD regarding motor progression was restricted to patients with low α-synuclein cerebrospinal fluid levels and low dopamine reuptake in the striatum as measured by [(123)I]FP-CIT [200]. The intensity of RBD symptoms seems to increase with progressing neurodegeneration over the disease course, indicated by increasing intensity of RSWA over time in iRBD patients [201]. This is in line with the description of gradually increasing intensity of RBD symptoms over time from isolated REM sleep behavioral events without RSWA towards the full clinical picture and vPSG features consisting of RBD symptoms with RSWA in approximately 40% of de novo PD patients over a time period of 2 years [202]. Therefore, REM sleep behavioral event detection might enable diagnosis of emergent alpha-synucleinopathy at an even earlier disease stage.
Diagnostic workup
The diagnosis of definite RBD requires a history of dream enactment or complex motor behaviors and should be confirmed by vPSG [46]. While RSWA is defined by abolished or diminished atonia within REM sleep measured by EMG during polysomnography, video recordings are necessary to identify movements in REM confirming the diagnosis of RBD [203]. Furthermore, video monitoring is essential to rule out other differential diagnosis and mimics of RBD such as NREM-parasomnias or epileptic seizures. However, other conditions like severe SDB or current use of antidepressants, which also lead to RSWA, must be ruled out by the sleep medicine specialist and if necessary, vPSG needs to be reevaluated after antidepressant discontinuation or treatment of severe SDB [204].
While normative values for RSWA still vary between different sleep centers, many researchers have complied with the SINBAR EMG montage and associated scoring criteria [203, 205]. The SINBAR montage includes simultaneous EMG recording of the M. mentalis, Mm. flexor dig. Superficialis, and Mm. extensor brevis muscles leading to a 94.4% sensitivity of motor and vocal events with a specificity of 47.2% [203, 206]. In clinical routine, these scoring methods are limited by its laborious and time-consuming exertion, while automatic scoring algorithms have not been widely implemented yet [207]. Several questionnaires such as the RBDSQ have been developed for screening and diagnostic purposes of RBD (see ‘Diagnostic Workup of Sleep Disturbances’ above). However, despite their relatively high sensitivities of up to 90%, usefulness of these questionnaires is limited by their relatively low specificities ranging from 56 to 92% [58, 59, 61, 208, 209]. Hereby, the main challenge remains to distinguish RBD from other primary sleep disorders such as NREM-parasomnias or PLMS (Fig. 2), thus further cementing vPSG as the gold standard for RBD diagnosis. Actigraphy algorithms are not yet developed sufficiently to solely base RBD diagnosis on but might represent a future diagnostic tool [210].
Therapeutic options
Establishing a safe sleep environment to prevent sleep-related injuries of patients or bed-partners is crucial for effective treatment of RBD [211]. Furthermore, the decision for medical treatment should not only consider RBD symptoms, but also include aspects of quality of life of patients and caregivers, which are often compromised by recurrent nighttime disruption and EDS due to vocalizations and complex motor behaviors [185]. Melatonin and clonazepam have been widely used to treat RBD symptoms, although multicenter randomized controlled trials for RBD and RBD in PD are lacking (Table 1). Furthermore, outcome in trials has not been standardized with some studies using subjective control of RBD symptoms as outcome parameter, while others defined objective outcome variables based on vPSG [212].
Despite current knowledge on the principal mode of action of benzodiazepines as GABA-A-receptor agonists, detailed knowledge on the pathophysiological mechanisms leading to improvement of RBD symptoms are lacking. Case series and open-label studies confirmed effective reduction of clinical events in many but not all iRBD patients treated with clonazepam [191]. However, the only randomized, placebo-controlled trial failed to show significant effects of 0.5 mg clonazepam on Global Clinical Impression scores regarding RBD symptoms in a cohort of PD patients with probable (not vPSG proven) RBD [213]. Limitations of the study were its small sample size (n = 20 per group) as well as lack of objective vPSG outcome parameters. In trials based on vPSG outcome measures such as RSWA severity or number of RBD episodes, no objective improvement could be documented neither in iRBD nor in PD patients with RBD [214]. Interestingly, one prospective observational study showed conflictive results with subjective improvement of RBD symptoms, while simultaneously worsening of objective vPSG based outcome parameters [214, 215]. In a recent review, the estimated effectiveness of 0.5 to 1.5 mg clonazepam on RBD symptoms and vPSG readouts irrespective of RBD etiology combining evidence of case reports, prospective open-label studies, and one RCT was calculated to reach 66.7% [214] with additionally 17.8% of patients reporting partially improved subjective symptoms. Given the known side effects of benzodiazepines, which include EDS, falls, confusion, and the possibility of aggravating preexisting SDB, therapy should be closely monitored and respiratory related measures extracted of the vPSG should be considered. In case of moderate to severe SDB at least an ambulatory apnea screening after treatment initiation with clonazepam should be performed [211, 214].
Melatonin seems to exert less side effects than benzodiazepines and was reported to be effective in open-label trials and case-series [216, 217]. However, a double-blind, placebo-controlled trial including 30 PD patients with RBD randomized to either 4 mg prolonged-release melatonin or placebo did not show an improvement of subjective RBD frequency as well as RSWA on vPSG over the 8-weeks treatment period [218]. Another randomized controlled trial failed to show improvement of subjective RBD symptoms in a cohort of iRBD patients [219]. A single center trial using a cross-over design in a small group consisting of only 8 patients with mixed diagnoses consisting of iRBD as well as PD and narcolepsy patients implied improvement of RSWA frequency on vPSG and global clinical impression over a 4-week treatment period [220], while an open-label trial in 15 RBD patients of different etiologies also claimed improvement of PSG measures after treatment with 3 to 9 mg melatonin [221]. However, the chosen trial designs, small patient numbers, and only moderate effects leave room for critical appraisal of these results. The overall calculated effectiveness of melatonin on RBD irrespective of RBD etiology based on this available evidence resided within the range of the effects calculated for clonazepam. Thus, melatonin was reported to be effective or partially effective in about 60% of patients, while ineffective in approximately 30% of patients [214]. However, both above mentioned recent randomized trials showed relevant placebo effects on subjective RBD symptoms, which might account for the putative effectiveness of melatonin [214]. In conclusion, evidence for the effectiveness of melatonin remains modest and questions remain unsolved, including differential effects of immediate versus prolonged release melatonin formulations as well as effectiveness of combination therapy regimens. However, due to its more favorable side effects profile compared to clonazepam, melatonin is still worth to be considered as first line therapy in RBD (Table 1).
In case of therapeutic failure of clonazepam or melatonin, evidence is scarce. Case series in small cohorts have reported effects of the combination of clonazepam and melatonin or clonazepam with other substances such as pramipexole or carbamazepine, but these data need further confirmation [214]. The evidence regarding other therapeutic options is also scarce. Small open label trials reported conflicting results in regard to ramelteon, a melatonin receptor agonists, which seems to improve subjective RBD symptoms in PD patients with probable RBD (not vPSG proven) [222] as well as in iRBD, but failed to improved vPSG parameters [223]. Conflicting results have also been reported regarding pramipexole. Whereas no effects were reported in a small open-label trial in PD patients with RBD [224], symptoms improved in a cohort of iRBD patients [214]. A very recently published small randomized pilot trial with nelotanserin, a selective 5-HT (2A) inverse agonist, failed to show treatment effects on RBD in patients with DLB or PD patients with dementia after treatment for 4 weeks on vPSG outcome parameters [212]. Small case series reported effectiveness of sodium oxybate and zopiclone on iRBD as well as rivastigmine on PD related RBD [225], while larger studies are missing.
Given the lack of scientific evidence, a pragmatic approach on RBD in PD is recommended, starting with the implementation of a regular sleep routine and a safe sleep environment. If necessary, pharmacological approaches should be considered, taking RBD severity, particularly in cases of either evident or impending self- or bedpartner-inflicted injuries, and its impact on sleep quality and quality of life of patients and caregivers into account. Furthermore, disease severity of PD and complications such as cognitive function, gait disturbances and frailty as well as comorbidities and medication leading to possible drug interactions should be accounted for. In conclusion, currently treatment starting with either melatonin or clonazepam in the lowest possible dosage (2 mg prolonged release melatonin or 0.5 mg clonazepam) should be considered first. If treatment is ineffective, melatonin might be increased up to 6 mg and clonazepam up to 1.5 mg. In case of persisting RBD symptoms, combination of clonazepam and melatonin might be considered, although the evidence on combination therapies is insufficient for general recommendations. If treatment with these substances is not sufficient, other strategies with less often used substances should be considered taking the individuals personal situation and comorbidities into account. Furthermore, seeking advice of a sleep medicine specialist with expertise in RBD might be considered.
Apart from positive effects of some of the above-mentioned substances on RBD symptoms, large doubled-blind, randomized, controlled, multicenter trials are needed to improve RBD treatment [226, 227]. However, standardized assessment regimens of subjective RBD symptoms, which are not compromised by bias due to selective recollection of RBD episodes or abrasive dissection of symptoms such as by the Global Clinical Impression are needed. Patient diaries might overcome some of the limitations of recollection bias. However, RBD symptoms may occur undetected, and patients are often unaware of symptoms, therefore implementation of objective vPSG, newly developed at-home PSG or actigraphy based outcome parameters for future clinical trials seems inevitable. However, RBD symptoms show high variance, further complicating the establishment of reliable outcome parameter for future trials [214].
Sleep related movement disorders
Restless legs syndrome
Clinical presentation and pathophysiology RLS, also known as Willis-Ekbom-Syndrome, is defined by unpleasant sensations, usually of the legs, but sometimes also arms or other body parts, with an uncontrollable urge to move. Symptoms occur primarily at rest in a circadian pattern, preferably in the evenings or at nighttime, and are at least temporarily relieved by movements as defined by the International Restless legs study group criteria (IRLSSG) [228]. The estimated prevalence of RLS varies widely depending on the selected study population between 1% and 24% [229]. Whether the prevalence of RLS is elevated in PD compared to the general population still needs to be finally determined [230–232]. However, current evidence suggests a similar prevalence of RLS in de novo PD patients compared to the general population with increasing numbers associated with dopaminergic treatment and disease progression [86, 229] as well as age and female sex [233]. However, other symptoms such as akinesia, NMS, and limb motor restlessness (LMR) resulting in unspecific distress and unpleasant feelings at nighttime, but not fulfilling the strict criteria for RLS, might be confused with RLS, thus leading to overestimation of RLS prevalence in PD [234].
RLS symptoms lead to disturbances of sleep initiation and sleep maintenance with recurring awakenings and sleep fragmentation, often resulting in increased daytime sleepiness, impaired QoL, and elevated risk of anxiety disorders and depression [235]. The pathophysiology of RLS has not been fully elucidated, yet and the pathophysiology of RLS in PD might differ from idiopathic RLS [236]. However, currently identified mechanism include disturbances of dopaminergic and iron metabolism, genetic risk factors (e.g., MEIS1, BTBD9) modified by environmental factors and hypoxia [229, 237, 238]. Other disturbances associated with or causing RLS are vitamin B12 and folic acid deficiency, diabetes, pregnancy, chronic kidney disease, spinal cord lesions or peripheral neuropathies, dopamine antagonists or treatment with selective serotonin reuptake inhibitors [229]. Iron deficiency and its link to the pathophysiology of RLS is an important issue in PD patients, in which nutritional status might be detrimental [239].
Diagnostic workup
RLS is a clinical diagnosis based on the criteria of the IRLSSG criteria [240, 241], which might be facilitated by a questionnaire supporting the diagnosis (RLS-DI). RLS severity might be monitored with the IRLSSG Severity Scale, although both have not been validated in PD populations yet [242, 243]. Furthermore, the immobilization test assessing subjective leg discomfort and notable leg movements during inactivity prior to bedtime supports RLS diagnosis [244]. If RLS symptoms are indistinct or treatment response is diminished or ambiguous, vPSG might be considered to screen for periodic limb movements (PLMS, see next section). However, the diagnostic value of PLMS detection is limited by the fact that PLMS are a rather non-specific condition, detectable in the majority of RLS patients, but also in SDB and a great variety of other sleep disorders and even in healthy persons, compromising its usefulness as a valid diagnostic marker for RLS [245]. Therefore, vPSG should be considered, if other primary sleep disorder are suspected or in case of difficulties of managing symptoms (Fig. 2).
Therapeutic options
Therapeutic strategies of RLS in PD align with idiopathic RLS combining pharmacological and non-pharmacological measures such as exercise, physical approaches including application of cold water or cooling sprays and reduction of intake of alcohol, nicotine, and caffeinated beverages (Table 1) [246]. Furthermore, preexisting drug regimens should be thoroughly evaluated hence a variety of substances such as antidepressants or dopamine antagonists might induce or aggravate RLS symptoms. However, reduction or cessation of antidepressants needs a cautious approach due to the close comorbidity of RLS, depression, and anxiety [106, 247]. Iron deficiency should be substituted, aiming at serum ferritin levels > 75μg/ml, although this quantitative recommendation is not specifically validated in PD patients [11]. On the basis of a consensus of RLS experts, patients with RLS and serum ferritin concentrations of 75μg/l or less and transferrin saturation below 45% should receive oral iron therapy. Of note, ferritin might be elevated in inflammation or in case of malignancy, therefore iron supplementation is recommended, if transferrin saturation is below 20% [248, 249]. In case of moderate to severe or therapy refractory RLS or if oral substitution is not tolerated, intravenous iron supplementation with two doses of 1 g iron dextran should be considered, although delayed clinical effects after up to three to five weeks might occur [86, 248].
The core element of pharmacological RLS treatment in general is dopaminergic substitution (Table 1) [250–252]. However, RCTs specifically aiming at pharmacological treatment options for RLS in PD, particularly regarding dopaminergic agents, have not been performed yet [253, 254]. Therefore, recommendations are based on data in idiopathic RLS populations. Levodopa and dopamine agonists such as rotigotine, pramipexole, and ropinirole improved RLS symptoms significantly in idiopathic RLS patients in multiple RCTs. In PD patients with LMR or RLS symptoms DA agonists such as rotigotine were shown to be effective in improving morning motor function and subjective sleep quality including LMR/RLS symptoms [14, 89, 90, 255]. Furthermore, PLM and awakenings were reduced in placebo-controlled trials in PD patients by nighttime continuous apomorphine infusion, especially in those with co-existing RLS [256, 257]. However, many PD patients are already treated with higher levodopa-equivalent doses (LED) and dopamine agonist therapy might be limited by older age, presence of EDS, presence or history of impulse control disorders, hallucinations, and cognitive decline. Use of higher doses of dopaminergics also increases the risk for augmentation, consisting of aggravated symptoms occurring already at daytime or involving previously unaffected body parts (such as the arms) [258]. Although the pathophysiological mechanisms of augmentation have not been fully elucidated yet, upregulation of the excitatory D1 receptor by long-term use of dopaminergics is suspected to contribute to this phenomenon [259]. As total LED in PD often exceeds doses used in RLS, other treatment options for RLS should be generously discussed, before dosages of dopaminergics are increased even further.
These therapeutic alternatives include alpha-2-delta ligands (α2δ-ligands) such as gabapentin, gabapentin enacarbil, and pregabalin, which are also known to be effective in the management of sleep disturbances associated with neuropathic pain [260]. In addition, they seem to reach similar efficacy in the treatment of RLS in PD patients compared to dopamine agonists with lower rates of augmentation [261]. Furthermore, their potentially sedative side effects might be beneficial towards associated sleep disturbances [253]. Adverse side effects include EDS, dizziness, and weight gain. Therefore, introduction und up-titration should be executed carefully. Further therapeutic options are low-potent opioids like oxycodone-naloxone combinations, which have also only been systemically evaluated in idiopathic RLS, but not in PD, yet [258, 262]. Although opioids were approved by the EMEA and FDA for the treatment of RLS and have shown potential benefits on chronic pain in PD patients, the increased risk of falls and augmentation of PD-related constipation should be acknowledged [263].
Periodic limb movements
Clinical presentation and pathophysiology
PLMS are characterized by repetitive, stereotype movements of the limbs—mostly the legs—during sleep and are classified as sleep-related movement disorders [46]. Although pathophysiological mechanisms of PLMS have only been partially understood, currently a dysfunction of a network including the spinal cord, brainstem, and supratentorial structures resulting in overactive bilateral spinal pattern generators with impact on spinal motoneurons is suspected. This network seems to be modulated by genetic risk factors, the autonomic system as well as iron and dopamine metabolism [230, 264, 265]. PLMS are frequently detectable in primary sleep disorders, most prominently in RLS patients, affecting approximately up to 80% of RLS patients [266, 267]. Furthermore, PLMS are associated with many clinical conditions such as heart insufficiency, primary sleep disorders such as SDB [245], age, and male sex, but are also detectable in up to 25 to 60% of healthy persons on vPSG, questioning their clinical relevance [266, 268, 269]. PLMS prevalence in PD is equally high with 30 to 80% of patients affected, even in de novo PD patients [38]. However, prevalence of PLMS varied widely between different cohorts, thus leaving the questions whether PLMS prevalence in PD is increased compared to the general population still open for debate [270, 271]. In early PD PLMS prevalence seems to be similar to controls [272]. However, PLM severity, as determined by the PLM-Index, increases with PD duration and disease severity [233].
PLMS were described to occur either unilaterally or bilaterally both in RLS and in PD, although asymmetry of PLMS did not correlate with motor symptom laterality in PD [230]. Despite the association of PLMS with RLS, an equal association between RLS and PLMS in PD patients has not been clearly determined but was discussed rather controversially [273, 274]. The occurrence of PLMS is associated with sleep fragmentation, non-restorative sleep, and EDS in the general population [86, 269]. However, the clinical relevance of PLMS was scrutinized by contradictory studies unable to demonstrate an association of PLMS with EDS [266]. The fact that many patients are unaware of the occurrence of PLMS, which are often reported by bed partner only [275] as well as their frequent occurrence in healthy persons [276], leads to reasonable doubts regarding the negative implications of PLMS on sleep, challenging the concept of PLMS as a relevant cause for sleep disruption and the importance of treatment of PLMS. Thus, PLMS without clinical symptoms are deemed an unspecific phenomenon without significance. However, some authors suggested that treatment of PLMS might benefit cardiovascular health due to the close relationship of PLM and the autonomic system [277]. In contrast, Periodic limb movement disorder (PLMD) is diagnosed, if the PLMS index (PLM-I) exceeds 15/h in adults in association with clinical symptoms such as sleep disturbances or daytime function impairment in absence of other primary sleep disorders or medication possibly accounting for PLMS occurrence [46].
Diagnostic workup
PLMS are detectable by surface EMG in vPSG (Fig. 2). According to the actual scoring rules of the American Academy of Sleep Medicine, limb movements are scored, if EMG activity exceeds baseline EMG for at least 0.5 s and up to 10 s [46]. PLM criteria require the periodically occurrence of at least four limb movements with an inter movement interval of 5 s to 90 s. PLM severity is determined as the PLM-Index, based on PLM frequency per hour sleep (n/h). In contrast to PLMS as a condition determined by PSG findings, the term PLMD requires a PLM-Index exceeding 15/h as well clinical symptoms such as disturbances of sleep or daytime function.
Therapeutic options
The evidence for treatment of isolated PLMS is non-existent, even in the general population. Mostly, PLMS frequency has been assessed in patients with comorbid RLS. Randomized controlled trials regarding treatment of isolated PLMS in PD are lacking, but dopaminergic treatment was shown to decrease PLMS frequency in RLS patients as well as in early PD [88, 247]. However, PLM time structure seems to differ in RLS and PD patients, showing effective suppression of PLMS in early, but not advanced PD patients after initiation of dopaminergic treatment [270]. Small cohort trials and case series reporting benefits of sleep quality in PLMD patients are not sufficient for general conclusions [278]. Therefore, treatment recommendations for isolated PLMS without RLS symptoms in PD are impossible due to lack of scientific evidence. Also, in our clinical experience, treatment of PLMD with dopaminergic drugs does not result in improvement of subjective sleep quality and/or daytime sleepiness. Thus, leading to the question, whether PLMD does in fact represent a distinct disease entity or should be considered as an incidental phenomenon instead. In conclusion, further studies including PD patients with PLMS and PLMD are needed to allow reliable recommendations for clinical practice.
Circadian rhythm disorders
Clinical presentation and pathophysiology
Neurodegenerative diseases such as PD and Alzheimer’s disease are associated with disturbances of the circadian rhythm. The circadian rhythm constitutes of alternating periods of sleep and wakefulness as well as a regulated order of physiological and behavioral processes including variations of blood pressure, heart rate and body temperature as well as metabolic and hormonal processes of which some are aligned to a 24-hour rhythm, others to longer or shorter time periods. The alignment of the circadian rhythm to its length of roughly 24 hours is accomplished by different mechanisms including light and darkness, expression of clock genes such as BMAL1 and PER2, and socio-behavioral inputs such as work life, mealtimes, social activities, and exercise. The suprachiasmatic nucleus, receiving visual input from the retina, is one of the main structures to entrain the circadian rhythm by communicating with the pineal gland responsible for melatonin secretion [279, 280]. In PD patients alterations of SCN regulation caused by neurodegeneration within dopaminergic retinal cells as well as reduced physical exercise and bright light exposure due to impaired mobility supposedly contribute to circadian rhythm dysfunction [281]. Furthermore, clock gene expression of BMAL1 was found to be decreased in PD patients [282], whereas the stress hormone cortisol was elevated compared to healthy controls [27]. Concomitantly, melatonin secretion was diminished, which was aggravated in advanced disease stages and associated with long-term dopaminergic treatment [280].
Circadian rhythm disorders lead to disruption of regular wake-sleep rhythm resulting in non-restorative sleep with consecutive EDS and thus episodes of daytime napping. Furthermore, increased nighttime activities as well as aggravation of neuropsychiatric complications such as anxiety, hallucinations, and challenging behaviors might occur. Disruption of the regular circadian rhythm is accompanied by an advanced or delayed melatonin secretion peak as well as alterations of physiological functions including body temperature, blood pressure, and heart rate [27]. Circadian rhythm disruption leading to advanced bed times resulting in early awakenings are labelled “phase advance”, while delayed bed times at night and sleeping late in the day is called “phase delay”. These alterations are associated with impaired daytime activities as well as social activities, possibly causing a disintegration of caregivers and patients’ sleep-wake schedule leading to further social and behavioral problems. Furthermore, alterations of circadian rhythm and consecutive sleep disruption may enhance the neurodegenerative process itself [279].
Diagnostic procedure
Diagnosis of circadian rhythm disturbances is based on the clinical interview and sleep diaries and may be supported by actigraphy over a few weeks alone or combined with the measurement of saliva melatonin concentrations (Fig. 2) [283]. vPSG might be considered, if other sleep disorders are suspected to contribute to disturbances of nighttime sleep or disruption of circadian rhythm (Fig. 2). Furthermore, neuropsychiatric symptoms such as anxiety, depression, hallucinations, or challenging behavior should be thoroughly assessed and treated, as such symptoms might occur in close correlation to sleep disorders either inducing or resulting from sleep disruption.
Therapeutic options
Therapeutic approaches for circadian sleep-wake disorders in PD should include educational training on sleep hygiene, thus admonishing patients and caregivers to establish regular bedtime routines, avoid daytime napping and increase the exposure to daylight participating in outdoor activities and exercise (Table 1). Eating and drinking habits, particularly regarding fatty and carbohydrate-saturated meals as well as caffeine and alcohol containing drinks should be reconsidered, particularly near bedtime in the evenings. If phase advance is present, exposure to daylight and exercise should be intensified and indoor light sources should be optimized using bright light sources to help re-establishing regular rhythm. If phase delay is present, bedtimes should be gradually shifted forwards and exposure to sunlight should be intensified in the mornings until reaching desired bedtimes. Furthermore, the application of bright light therapy was shown to restore circadian rhythmicity and to improve sleep quality and mood [284] as well as motor function in PD patients [27, 100]. Complementary, melatonin application was recommended to stabilize circadian rhythm [285, 286]. However, in a small double-blind, placebo-controlled, randomized, cross-over pilot trial administration of melatonin did not alter BMAL1 levels or subjective sleep quality significantly compared to placebo [287]. Thus, further research is needed to establish the role of application of external melatonin in circadian rhythm dysfunction in PD.
Other aspects associated with sleep disruption in PD
In PD patients with sleep disturbances, specific conditions and treatment regimens need to be considered in addition to primary sleep disorders. These conditions are already mentioned in some of the earlier sections. While long-acting dopamine agonists such as cabergoline, rotigotine, pramipexol, and others have been demonstrated to improve subjective sleep quality [88], the evidence regarding extended release levodopa is not sufficient to prove positive effects on sleep [79]. Amelioration of sleep quality was also reported after DBS as well as initiation of treatment with intestinal levodopa gel-infusions [288, 289]. Improvement of sleep quality seems to be partially exerted by simultaneous improvement of nighttime and early morning motor function [89]. However, the fraction of improvement attributable to enhanced nighttime mobility in contrast to direct effects on sleep are indistinct. The importance of nighttime mobility was stressed by a study demonstrating a strong correlation between perception of impaired mobility in bed with quantification of sleep-related body position changes and sleep efficiency [290]. Therefore, thorough analysis of nighttime problems including objective evaluation of sleep by vPSG and eventually nighttime mobility is essential to differentiate between PD associated symptoms such as akinesia, which will benefit from optimization of medication, and primary sleep disorders requiring adequate treatment. On the other hand, mostly dose-dependent adverse effects of dopaminergic drugs on sleep structure and maintenance have been described [83]. However, adverse effects of medication may vary widely depending on dosage and timing as well as receptor type stimulated preferentially as already mentioned. In conclusion, nighttime mobility and motor symptoms as well effects of medication regimens need to be carefully evaluated (see respective sections for certain sleep disorders).
However, discrepancies between subjective and objective measures of sleep quality (‘sleep discrepancy’) are well documented even in primary sleep disorders and might be influenced by mood disorders such as depression complicating management of sleep disorders even further [47, 79, 291]. This is underlined by a study showing a close correlation between subjective sleep quality and neuropsychiatric symptoms such as anxiety with daytime functioning, while objective sleep quality assessed by actigraphy did not predict daytime functioning [292]. It is of great importance to take sleep discrepancy into account in regard to initiating diagnostic and therapeutic measures.
Autonomic NMS, particularly in the advanced stages of PD, such as constipation, gastroesophageal reflux, and nocturia may also contribute or cause sleep fragmentation (Fig. 1) [293, 294]. Briefly, the management of these conditions includes behavioral and pharmacological approaches. Bladder dysfunction should be approached by establishing behavioral treatment, e.g., bladder training, restriction of fluid intake in the evenings, pelvic floor exercises, application of pads as well as introduction of specific medication such as solifenacin [102, 295]. In case of constipation, probiotics, macrogol, and lubiprostone are likely efficacious [102]. In conclusion, motor symptoms as well NMS should be evaluated regarding their impact on sleep and daytime functioning and treated adequately.
CONCLUSIONS
Sleep disorders are common NMS in PD with increasing frequencies during the disease course. Sleep disorders lead to negative effects on sleep quality, daytime function, mood, and quality of life of patients and caregivers. Furthermore, sleep disorders might contribute to the neurodegenerative process as a result of a bidirectional influence of sleep and neurodegeneration. Diagnostic guidelines are similar to the non-PD population including non-invasive and non-invasive measures. However, relevant aspects of sleep disturbances in PD ranging from pathophysiological mechanisms as well as treatment approaches still lack scientific evidence. Fortunately, in recent years, the importance of sleep in PD and other neurodegenerative disorders has been increasingly recognized, thus leading to increasing interest in sleep disorders bringing forth the initiation of treatment trials in PD. Still, the therapeutic management of sleep disturbances in PD remains off-label in many cases. Nonetheless, sleep-wake disorders in PD should be further addressed and evaluated in future attempts to optimize and personalize treatment strategies for the individual patient with PD. Effective treatments for the sleep disorders associated with neurodegenerative diseases are urgently needed, but current data are insufficient.
DISCLOSURES
W. Hermann reports financial support outside the submitted work from Bukwang Pharamceuticals CO/Contera Pharma (subinvestigator in a phase III clinical trial with financial support for the institution) as well as personal fees from DZNE (German Centre for Neurodegenrative Diseases). L. Schütz reports no conflicts of interest. F. Sixel-Döring reports financial support/honoraria for advisory boards and lectures outside the submitted work from Gruententahl, STADA-Pharm, Zambon, AbbVie, Bial, Desitin, EVER Pharma, Licher MT, Medtronic, MedizinMedien Austria, UCB, Suazio, University of Goettingen, Vitos Kliniken, Springer Medizin, Elsevier.
ACKNOWLEDGMENT
During the past five years Friederike Sixel-Döring has received honoraria for lectures from AbbVie, Bial, Desitin, EVER Pharma, Gruenenthal, Licher MT, Medtronic, MedizinMedien Austria, UCB, Universität Göttingen, Vitos Kliniken, Fa. Suazio. She served on an advisory board for Gruenenthal, STADA-Pharm, Zambon.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
[1] | Nerius M , Fink A , Doblhammer G ((2017) ) Parkinson’s disease in Germany: Prevalence and incidence based on health claims data. Acta Neurol Scand 136: , 386–392. |
[2] | Heinzel S , Berg D , Binder S , Ebersbach G , Hickstein L , Herbst H , Lorrain M , Wellach I , Maetzler W , Petersen G , Schmedt N , Volkmann J , Woitalla D , Amelung V ((2018) ) Do we need to rethink the epidemiology and healthcare utilization of Parkinson’s disease in Germany? Front Neurol 9: , 500. |
[3] | Chaudhuri KR , Prieto-Jurcynska C , Naidu Y , Mitra T , Frades-Payo B , Tluk S , Ruessmann A , Odin P , Macphee G , Stocchi F , Ondo W , Sethi K , Schapira AH , Martinez Castrillo JC , Martinez-Martin P ((2010) ) The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: An international study using the nonmotor symptoms questionnaire. Mov Disord 25: , 704–709. |
[4] | Hurt CS , Rixon L , Chaudhuri KR , Moss-Morris R , Samuel M , Brown RG ((2019) ) Barriers to reporting non-motor symptoms to health-care providers in people with Parkinson’s. Parkinsonism Relat Disord 64: , 220–225. |
[5] | Hurt CS , Rixon L , Chaudhuri KR , Moss-Morris R , Samuel M , Brown RG ((2019) ) Identifying barriers to help-seeking for non-motor symptoms in people with Parkinson’s disease. J Health Psychol 24: , 561–571. |
[6] | Chaudhuri KR , Martinez-Martin P , Schapira AH , Stocchi F , Sethi K , Odin P , Brown RG , Koller W , Barone P , MacPhee G , Kelly L , Rabey M , MacMahon D , Thomas S , Ondo W , Rye D , Forbes A , Tluk S , Dhawan V , Bowron A , Williams AJ , Olanow CW ((2006) ) International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov Disord 21: , 916–923. |
[7] | Martinez-Martin P , Schapira AH , Stocchi F , Sethi K , Odin P , MacPhee G , Brown RG , Naidu Y , Clayton L , Abe K , Tsuboi Y , MacMahon D , Barone P , Rabey M , Bonuccelli U , Forbes A , Breen K , Tluk S , Olanow CW , Thomas S , Rye D , Hand A , Williams AJ , Ondo W , Chaudhuri KR ((2007) ) Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord 22: , 1623–1629. |
[8] | Nausieda PA , Weiner WJ , Kaplan LR , Weber S , Klawans HL ((1982) ) Sleep disruption in the course of chronic levodopa therapy: An early feature of the levodopa psychosis. Clin Neuropharmacol 5: , 183–194. |
[9] | Lees AJ , Blackburn NA , Campbell VL ((1988) ) The nighttime problems of Parkinson’s disease. Clin Neuropharmacol 11: , 512–519. |
[10] | Chaudhuri KR , Healy DG , Schapira AH , National Institute for Clinical Excellence ((2006) ) Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol 5: , 235–245. |
[11] | Albers JA , Chand P , Anch AM ((2017) ) Multifactorial sleep disturbance in Parkinson’s disease. Pain Pract 35: , 41–48. |
[12] | Di Fabio N , Poryazova R , Oberholzer M , Baumann CR , Bassetti CL ((2013) ) Sleepwalking, REM sleep behaviour disorder and overlap parasomnia in patients with Parkinson’s disease. Eur Neurol 70: , 297–303. |
[13] | Gagnon JF , Petit D , Latreille V , Montplaisir J ((2008) ) Neurobiology of sleep disturbances in neurodegenerative disorders. Curr Pharm Des 14: , 3430–3445. |
[14] | Liu CF , Wang T , Zhan SQ , Geng DQ , Wang J , Liu J , Shang HF , Wang LJ , Chan P , Chen HB , Chen SD , Wang YP , Zhao ZX , Chaudhuri KR ((2018) ) Management recommendations on sleep disturbance of patients with Parkinson’s disease. Chin Med J (Engl) 131: , 2976–2985. |
[15] | Global Parkinson’s Disease Survey (GPDS) Steering Committee ((2002) ) Factors impacting on quality of life in Parkinson’s disease: Results from an international survey. Mov Disord 17: , 60–67. |
[16] | Scaravilli T , Gasparoli E , Rinaldi F , Polesello G , Bracco F ((2003) ) Health-related quality of life and sleep disorders in Parkinson’s disease. Neurol Sci 24: , 209–210. |
[17] | Smith MC , Ellgring H , Oertel WH ((1997) ) Sleep disturbances in Parkinson’s disease patients and spouses. J Am Geriatr Soc 45: , 194–199. |
[18] | Shafazand S , Wallace DM , Arheart KL , Vargas S , Luca CC , Moore H , Katzen H , Levin B , Singer C ((2017) ) Insomnia, sleep quality, and quality of life in mild to moderate Parkinson’s disease. Ann Am Thorac Soc 14: , 412–419. |
[19] | Havlikova E , van Dijk JP , Nagyova I , Rosenberger J , Middel B , Dubayova T , Gdovinova Z , Groothoff JW ((2011) ) The impact of sleep and mood disorders on quality of life in Parkinson’s disease patients. J Neurol 258: , 2222–2229. |
[20] | Barone P , Antonini A , Colosimo C , Marconi R , Morgante L , Avarello TP , Bottacchi E , Cannas A , Ceravolo G , Ceravolo R , Cicarelli G , Gaglio RM , Giglia RM , Iemolo F , Manfredi M , Meco G , Nicoletti A , Pederzoli M , Petrone A , Pisani A , Pontieri FE , Quatrale R , Ramat S , Scala R , Volpe G , Zappulla S , Bentivoglio AR , Stocchi F , Trianni G , Dotto PD , group Ps ((2009) ) The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 24: , 1641–1649. |
[21] | van Uem JM , Marinus J , Canning C , van Lummel R , Dodel R , Liepelt-Scarfone I , Berg D , Morris ME , Maetzler W ((2016) ) Health-related quality of life in patients with Parkinson’s disease–A systematic review based on the ICF model. Neurosci Biobehav Rev 61: , 26–34. |
[22] | Hermann W , Schmitz-Peiffer H , Kasper E , Fauser M , Franke C , Wienecke M , Otto K , Lohle M , Brandt MD , Reichmann H , Storch A ((2020) ) Sleep disturbances and sleep disordered breathing impair cognitive performance in Parkinson’s disease. Front Neurosci 14: , 689. |
[23] | Pushpanathan ME , Loftus AM , Thomas MG , Gasson N , Bucks RS ((2016) ) The relationship between sleep and cognition in Parkinson’s disease: A meta-analysis. Sleep Med Rev 26: , 21–32. |
[24] | Avidan A , Hays RD , Diaz N , Bordelon Y , Thompson AW , Vassar SD , Vickrey BG ((2013) ) Associations of sleep disturbance symptoms with health-related quality of life in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 25: , 319–326. |
[25] | Uc EY , Rizzo M , Anderson SW , Sparks JD , Rodnitzky RL , Dawson JD ((2007) ) Impaired navigation in drivers with Parkinson’s disease. Brain 130: , 2433–2440. |
[26] | Uc EY , Rizzo M , Anderson SW , Sparks JD , Rodnitzky RL , Dawson JD ((2006) ) Driving with distraction in Parkinson disease. Neurology 67: , 1774–1780. |
[27] | Videnovic A ((2018) ) Disturbances of sleep and alertness in Parkinson’s disease. Curr Neurol Neurosci Rep 18: , 29. |
[28] | Schrempf W , Brandt MD , Storch A , Reichmann H ((2014) ) Sleep disorders in Parkinson’s disease. J Parkinsons Dis 4: , 211–221. |
[29] | Braak H , Del Tredici K , Rub U , de Vos RA , Jansen Steur EN , Braak E ((2003) ) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: , 197–211. |
[30] | Sundaram S , Hughes RL , Peterson E , Muller-Oehring EM , Bronte-Stewart HM , Poston KL , Faerman A , Bhowmick C , Schulte T ((2019) ) Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson’s disease. Neurosci Biobehav Rev 103: , 305–315. |
[31] | Ju YE , Lucey BP , Holtzman DM ((2014) ) Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol 10: , 115–119. |
[32] | Iliff JJ , Lee H , Yu M , Feng T , Logan J , Nedergaard M , Benveniste H ((2013) ) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123: , 1299–1309. |
[33] | Xie L , Kang H , Xu Q , Chen MJ , Liao Y , Thiyagarajan M , O’Donnell J , Christensen DJ , Nicholson C , Iliff JJ , Takano T , Deane R , Nedergaard M ((2013) ) Sleep drives metabolite clearance from the adult brain. Science 342: , 373–377. |
[34] | Luk KC , Lee VM ((2014) ) Modeling Lewy pathology propagation in Parkinson’s disease. Parkinsonism Relat Disord 20: (Suppl 1), S85–87. |
[35] | Schrag A , Horsfall L , Walters K , Noyce A , Petersen I ((2015) ) Prediagnostic presentations of Parkinson’s disease in primary care: A case-control study. Lancet Neurol 14: , 57–64. |
[36] | Joy SP , Sinha S , Pal PK , Panda S , Philip M , Taly AB ((2014) ) Alterations in Polysomnographic (PSG) profile in drug-naive Parkinson’s disease. Ann Indian Acad Neurol 17: , 287–291. |
[37] | Schrempf W , Fauser M , Wienecke M , Brown S , Maass A , Ossig C , Otto K , Brandt MD , Lohle M , Schwanebeck U , Graehlert X , Reichmann H , Storch A ((2018) ) Rasagiline improves polysomnographic sleep parameters in patients with Parkinson’s disease: A double-blind, baseline-controlled trial. Eur J Neurol 25: , 672–679. |
[38] | Wetter TC , Collado-Seidel V , Pollmacher T , Yassouridis A , Trenkwalder C ((2000) ) Sleep and periodic leg movement patterns in drug-free patients with Parkinson’s disease and multiple system atrophy. Sleep 23: , 361–367. |
[39] | Hablitz LM , Nedergaard M ((2021) ) The glymphatic system: A novel component of fundamental neurobiology. J Neurosci 41: , 7698–7711. |
[40] | Amato N , Manconi M , Moller JC , Sarasso S , Stanzione P , Staedler C , Kaelin-Lang A , Galati S ((2018) ) Levodopa-induced dyskinesia in Parkinson disease: Sleep matters. Ann Neurol 84: , 905–917. |
[41] | Schreiner SJ , Imbach LL , Werth E , Poryazova R , Baumann-Vogel H , Valko PO , Murer T , Noain D , Baumann CR ((2019) ) Slow-wave sleep and motor progression in Parkinson disease. Ann Neurol 85: , 765–770. |
[42] | O’Dowd S , Galna B , Morris R , Lawson RA , McDonald C , Yarnall AJ , Burn DJ , Rochester L , Anderson KN ((2017) ) Poor sleep quality and progression of gait impairment in an incident Parkinson’s disease cohort. J Parkinsons Dis 7: , 465–470. |
[43] | Falup-Pecurariu C , Diaconu S ((2017) ) Sleep dysfunction in Parkinson’s disease. Int Rev Neurobiol 133: , 719–742. |
[44] | Raggi A , Bella R , Pennisi G , Neri W , Ferri R ((2013) ) Sleep disorders in Parkinson’s disease: A narrative review of the literature. Rev Neurosci 24: , 279–291. |
[45] | Yu RL , Tan CH , Wu RM ((2015) ) The impact of nocturnal disturbances on daily quality of life in patients with Parkinson’s disease. Neuropsychiatr Dis Treat 11: , 2005–2012. |
[46] | American Academy of Sleep Medicine (AASM) ((2014) ) International Classification of Sleep Disorders (ICSD-3). |
[47] | Chan WS , Dautovich ND , McNamara JPH , Stripling A , Dzierzewski JM , McCoy K , McCrae CS ((2021) ) Sleep discrepancy in a randomized controlled trial of brief behavioral therapy for chronic insomnia in older adults. Behav Sleep Med 19: , 221–231. |
[48] | Hogl B , Arnulf I , Comella C , Ferreira J , Iranzo A , Tilley B , Trenkwalder C , Poewe W , Rascol O , Sampaio C , Stebbins GT , Schrag A , Goetz CG ((2010) ) Scales to assess sleep impairment in Parkinson’s disease: Critique and recommendations. Mov Disord 25: , 2704–2716. |
[49] | Buysse DJ , Reynolds CF 3rd , Monk TH , Berman SR , Kupfer DJ ((1989) ) The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28: , 193–213. |
[50] | Trenkwalder C , Kohnen R , Hogl B , Metta V , Sixel-Doring F , Frauscher B , Hulsmann J , Martinez-Martin P , Chaudhuri KR ((2011) ) Parkinson’s disease sleep scale–validation of the revised version PDSS-2. Mov Disord 26: , 644–652. |
[51] | Marinus J , Visser M , van Hilten JJ , Lammers GJ , Stiggelbout AM ((2003) ) Assessment of sleep and sleepiness in Parkinson disease. Sleep 26: , 1049–1054. |
[52] | Johns MW ((1991) ) A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14: , 540–545. |
[53] | Kurtis MM , Balestrino R , Rodriguez-Blazquez C , Forjaz MJ , Martinez-Martin P ((2018) ) A review of scales to evaluate sleep disturbances in movement disorders. Front Neurol 9: , 369. |
[54] | Allen RP , Kushida CA , Atkinson MJ , Consortium RLSQ ((2003) ) Factor analysis of the International Restless Legs Syndrome Study Group’s scale for restless legs severity. Sleep Med 4: , 133–135. |
[55] | Bastien CH , Vallieres A , Morin CM ((2001) ) Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2: , 297–307. |
[56] | Morin CM , Belleville G , Belanger L , Ivers H ((2011) ) The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34: , 601–608. |
[57] | Jones J , Nielson SA , Trout J , Swenson M , Reiley J , Tanner J , Bowers D , Kay DB ((2021) ) A validation study of PROMIS Sleep Disturbance (PROMIS-SD) and Sleep Related Impairment (PROMIS-SRI) item banks in individuals with idiopathic Parkinson’s disease and matched controls. J Parkinsons Dis 11: , 877–883. |
[58] | Stiasny-Kolster K , Mayer G , Schafer S , Moller JC , Heinzel-Gutenbrunner M , Oertel WH ((2007) ) The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov Disord 22: , 2386–2393. |
[59] | Stiasny-Kolster K , Sixel-Doring F , Trenkwalder C , Heinzel-Gutenbrunner M , Seppi K , Poewe W , Hogl B , Frauscher B ((2015) ) Diagnostic value of the REM sleep behavior disorder screening questionnaire in Parkinson’s disease. Sleep Med 16: , 186–189. |
[60] | Halsband C , Zapf A , Sixel-Doring F , Trenkwalder C , Mollenhauer B ((2018) ) The REM Sleep Behavior Disorder Screening Questionnaire is not valid in de novo Parkinson’s disease. Mov Disord Clin Pract 5: , 171–176. |
[61] | Postuma RB , Arnulf I , Hogl B , Iranzo A , Miyamoto T , Dauvilliers Y , Oertel W , Ju YE , Puligheddu M , Jennum P , Pelletier A , Wolfson C , Leu-Semenescu S , Frauscher B , Miyamoto M , Cochen De Cock V , Unger MM , Stiasny-Kolster K , Fantini ML , Montplaisir JY ((2012) ) A single-question screen for rapid eye movement sleep behavior disorder: A multicenter validation study. Mov Disord 27: , 913–916. |
[62] | Chaudhuri KR , Sauerbier A , Rojo JM , Sethi K , Schapira AH , Brown RG , Antonini A , Stocchi F , Odin P , Bhattacharya K , Tsuboi Y , Abe K , Rizos A , Rodriguez-Blazquez C , Martinez-Martin P ((2015) ) The burden of non-motor symptoms in Parkinson’s disease using a self-completed non-motor questionnaire: A simple grading system. Parkinsonism Relat Disord 21: , 287–291. |
[63] | Ludtke S , Hermann W , Kirste T , Benes H , Teipel S ((2021) ) An algorithm for actigraphy-based sleep/wake scoring: Comparison with polysomnography. Clin Neurophysiol 132: , 137–145. |
[64] | American Academy of Sleep Medicine (AASM). The AASM Manual for the Scoring of Sleep and Associated Events. |
[65] | Yang PY , Ho KH , Chen HC , Chien MY ((2012) ) Exercise training improves sleep quality in middle-aged and older adults with sleep problems: A systematic review. J Physiother 58: , 157–163. |
[66] | Memon AA , Coleman JJ , Amara AW ((2020) ) Effects of exercise on sleep in neurodegenerative disease. Neurobiol Dis 140: , 104859. |
[67] | Kredlow MA , Capozzoli MC , Hearon BA , Calkins AW , Otto MW ((2015) ) The effects of physical activity on sleep: A meta-analytic review. J Behav Med 38: , 427–449. |
[68] | Uchida S , Shioda K , Morita Y , Kubota C , Ganeko M , Takeda N ((2012) ) Exercise effects on sleep physiology. Front Neurol 3: , 48. |
[69] | Rubio-Arias JA , Marin-Cascales E , Ramos-Campo DJ , Hernandez AV , Perez-Lopez FR ((2017) ) Effect of exercise on sleep quality and insomnia in middle-aged women: A systematic review and meta-analysis of randomized controlled trials. Maturitas 100: , 49–56. |
[70] | Youngstedt SD ((2005) ) Effects of exercise on sleep. Clin Sports Med 24: , 355-365, xi. |
[71] | Amara AW , Wood KH , Joop A , Memon RA , Pilkington J , Tuggle SC , Reams J , Barrett MJ , Edwards DA , Weltman AL , Hurt CP , Cutter G , Bamman MM ((2020) ) Randomized, controlled trial of exercise on objective and subjective sleep in Parkinson’s disease. Mov Disord 35: , 947–958. |
[72] | Xiao CM , Zhuang YC ((2016) ) Effect of health Baduanjin Qigong for mild to moderate Parkinson’s disease. Geriatr Gerontol Int 16: , 911–919. |
[73] | Frazzitta G , Maestri R , Ferrazzoli D , Riboldazzi G , Bera R , Fontanesi C , Rossi RP , Pezzoli G , Ghilardi MF ((2015) ) Multidisciplinary intensive rehabilitation treatment improves sleep quality in Parkinson’s disease. J Clin Mov Disord 2: , 11. |
[74] | Ivy CC , Lockmiller MC , McKay M , Landess K , Manning J , Denney L ((2021) ) The impact of exercise on sleep in people with Parkinson’s disease a scoping review. J Clin Neurosci 86: , 223–229. |
[75] | Morin CM , Drake CL , Harvey AG , Krystal AD , Manber R , Riemann D , Spiegelhalder K ((2015) ) Insomnia disorder. Nat Rev Dis Primers 1: , 15026. |
[76] | Chahine LM , Amara AW , Videnovic A ((2017) ) A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev 35: , 33–50. |
[77] | Ylikoski A , Martikainen K , Sieminski M , Partinen M ((2015) ) Parkinson’s disease and insomnia. Neurol Sci 36: , 2003–2010. |
[78] | Factor SA , McAlarney T , Sanchez-Ramos JR , Weiner WJ ((1990) ) Sleep disorders and sleep effect in Parkinson’s disease. Mov Disord 5: , 280–285. |
[79] | Schaeffer E , Vaterrodt T , Zaunbrecher L , Liepelt-Scarfone I , Emmert K , Roeben B , Elshehabi M , Hansen C , Becker S , Nussbaum S , Busch JH , Synofzik M , Berg D , Maetzler W ((2021) ) Effects of Levodopa on quality of sleep and nocturnal movements in Parkinson’s disease. J Neurol 268: , 2506–2514. |
[80] | Zhu K , van Hilten JJ , Marinus J ((2016) ) The course of insomnia in Parkinson’s disease. Parkinsonism Relat Disord 33: , 51–57. |
[81] | Gjerstad MD , Wentzel-Larsen T , Aarsland D , Larsen JP ((2007) ) Insomnia in Parkinson’s disease: Frequency and progression over time. J Neurol Neurosurg Psychiatry 78: , 476–479. |
[82] | Mahale R , Yadav R , Pal PK ((2015) ) Quality of sleep in young onset Parkinson’s disease: Any difference from older onset Parkinson’s disease. Parkinsonism Relat Disord 21: , 461–464. |
[83] | Brunner H , Wetter TC , Hogl B , Yassouridis A , Trenkwalder C , Friess E ((2002) ) Microstructure of the non-rapid eye movement sleep electroencephalogram in patients with newly diagnosed Parkinson’s disease: Effects of dopaminergic treatment. Mov Disord 17: , 928–933. |
[84] | Franke C , Storch A ((2017) ) Nonmotor fluctuations in Parkinson’s disease. Int Rev Neurobiol 134: , 947–971. |
[85] | Storch A , Schneider CB , Wolz M , Sturwald Y , Nebe A , Odin P , Mahler A , Fuchs G , Jost WH , Chaudhuri KR , Koch R , Reichmann H , Ebersbach G ((2013) ) Nonmotor fluctuations in Parkinson disease: Severity and correlation with motor complications. Neurology 80: , 800–809. |
[86] | Loddo G , Calandra-Buonaura G , Sambati L , Giannini G , Cecere A , Cortelli P , Provini F ((2017) ) The treatment of sleep disorders in Parkinson’s disease: From research to clinical practice. Front Neurol 8: , 42. |
[87] | Maglione JE , Liu L , Neikrug AB , Poon T , Natarajan L , Calderon J , Avanzino JA , Corey-Bloom J , Palmer BW , Loredo JS , Ancoli-Israel S ((2013) ) Actigraphy for the assessment of sleep measures in Parkinson’s disease. Sleep 36: , 1209–1217. |
[88] | Hogl B , Rothdach A , Wetter TC , Trenkwalder C ((2003) ) The effect of cabergoline on sleep, periodic leg movements in sleep, and early morning motor function in patients with Parkinson’s disease. Neuropsychopharmacology 28: , 1866–1870. |
[89] | Trenkwalder C , Kies B , Rudzinska M , Fine J , Nikl J , Honczarenko K , Dioszeghy P , Hill D , Anderson T , Myllyla V , Kassubek J , Steiger M , Zucconi M , Tolosa E , Poewe W , Surmann E , Whitesides J , Boroojerdi B , Chaudhuri KR , Recover Study Group ((2011) ) Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: A double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord 26: , 90–99. |
[90] | Pagonabarraga J , Piñol G , Cardozo A , Sanz P , Puente V , Otermín P , Legarda I , Delgado T , Serrano C , Balaguer E , Aguirregomozcorta M , Álvarez R , Kulisevsky JJ ((2015) ) Transdermal rotigotine improves sleep fragmentation in Parkinson’s disease: Results of the multicenter, prospective SLEEP-FRAM Study. Parkinsons Dis 2015: , 131508. |
[91] | Pahwa R , Stacy MA , Factor SA , Lyons KE , Stocchi F , Hersh BP , Elmer LW , Truong DD , Earl NL , Investigators E-PAS ((2007) ) Ropinirole 24-hour prolonged release: Randomized, controlled study in advanced Parkinson disease. Neurology 68: , 1108–1115. |
[92] | Wailke S , Herzog J , Witt K , Deuschl G , Volkmann J ((2011) ) Effect of controlled-release levodopa on the microstructure of sleep in Parkinson’s disease. Eur J Neurol 18: , 590–596. |
[93] | Poewe WH , Rascol O , Quinn N , Tolosa E , Oertel WH , Martignoni E , Rupp M , Boroojerdi B , Investigators SP ((2007) ) Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson’s disease: A double-blind, double-dummy, randomised controlled trial. Lancet Neurol 6: , 513–520. |
[94] | Cantor CR , Stern MB ((2002) ) Dopamine agonists and sleep in Parkinson’s disease. Neurology 58: , S71–78. |
[95] | Amara AW , Walker HC , Joop A , Cutter G , DeWolfe JL , Harding SM , Standaert DG ((2017) ) Effects of subthalamic nucleus deep brain stimulation on objective sleep outcomes in Parkinson’s disease. Mov Disord Clin Pract 4: , 183–190. |
[96] | Choi JH , Kim HJ , Lee JY , Yoo D , Im JH , Paek SH , Jeon B ((2019) ) Long-term effects of bilateral subthalamic nucleus stimulation on sleep in patients with Parkinson’s disease. PLoS One 14: , e0221219. |
[97] | Seppi K , Weintraub D , Coelho M , Perez-Lloret S , Fox SH , Katzenschlager R , Hametner EM , Poewe W , Rascol O , Goetz CG , Sampaio C ((2011) ) The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord 26: (Suppl 3), S42–80. |
[98] | Jespersen KV , Koenig J , Jennum P , Vuust P ((2015) ) Music for insomnia in adults. Cochrane Database Syst Rev, CD010459. |
[99] | Eidelman P , Talbot L , Ivers H , Belanger L , Morin CM , Harvey AG ((2016) ) Change in dysfunctional beliefs about sleep in behavior therapy, cognitive therapy, and cognitive-behavioral therapy for insomnia. Behav Ther 47: , 102–115. |
[100] | Videnovic A , Klerman EB , Wang W , Marconi A , Kuhta T , Zee PC ((2017) ) Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease: A randomized clinical trial. JAMA Neurol 74: , 411–418. |
[101] | Fifel K , Videnovic A ((2020) ) Circadian and sleep dysfunctions in neurodegenerative disorders-an update. Front Neurosci 14: , 627330. |
[102] | Seppi K , Ray Chaudhuri K , Coelho M , Fox SH , Katzenschlager R , Perez Lloret S , Weintraub D , Sampaio C , the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee ((2019) ) Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 34: , 180–198. |
[103] | Rios Romenets S , Creti L , Fichten C , Bailes S , Libman E , Pelletier A , Postuma RB ((2013) ) Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson’s disease – a randomized study. Parkinsonism Relat Disord 19: , 670–675. |
[104] | Menza M , Dobkin RD , Marin H , Gara M , Bienfait K , Dicke A , Comella CL , Cantor C , Hyer L ((2010) ) Treatment of insomnia in Parkinson’s disease: A controlled trial of eszopiclone and placebo. Mov Disord 25: , 1708–1714. |
[105] | Amara AW , Chahine LM , Videnovic A ((2017) ) Treatment of sleep dysfunction in Parkinson’s disease. Curr Treat Options Neurol 19: , 26. |
[106] | Stefani A , Högl B ((2020) ) Sleep in Parkinson’s disease. Neuropsychopharmacology 45: , 121–128. |
[107] | Trotti LM , Karroum EG ((2016) ) Melatonin for sleep disorders in patients with neurodegenerative diseases. Curr Neurol Neurosci Rep 16: , 63. |
[108] | Dowling GA , Mastick J , Colling E , Carter JH , Singer CM , Aminoff MJ ((2005) ) Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med 6: , 459–466. |
[109] | Herring WJ , Connor KM , Snyder E , Snavely DB , Morin CM , Lines C , Michelson D ((2019) ) Effects of suvorexant on the Insomnia Severity Index in patients with insomnia: Analysis of pooled phase 3 data. Sleep Med 56: , 219–223. |
[110] | Tabata H , Kuriyama A , Yamao F , Kitaguchi H , Shindo K ((2017) ) Suvorexant-induced dream enactment behavior in Parkinson disease: A case report. J Clin Sleep Med 13: , 759–760. |
[111] | Frucht S , Rogers JD , Greene PE , Gordon MF , Fahn S ((1999) ) Falling asleep at the wheel: Motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology 52: , 1908–1910. |
[112] | Arnulf I , Neutel D , Herlin B , Golmard JL , Leu-Semenescu S , Cochen de Cock V , Vidailhet M ((2015) ) Sleepiness in idiopathic REM sleep behavior disorder and Parkinson disease. Sleep 38: , 1529–1535. |
[113] | Ondo WG , Dat Vuong K , Khan H , Atassi F , Kwak C , Jankovic J ((2001) ) Daytime sleepiness and other sleep disorders in Parkinson’s disease. Neurology 57: , 1392–1396. |
[114] | Pont-Sunyer C , Hotter A , Gaig C , Seppi K , Compta Y , Katzenschlager R , Mas N , Hofeneder D , Brucke T , Bayes A , Wenzel K , Infante J , Zach H , Pirker W , Posada IJ , Alvarez R , Ispierto L , De Fabregues O , Callen A , Palasi A , Aguilar M , Marti MJ , Valldeoriola F , Salamero M , Poewe W , Tolosa E ((2015) ) The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov Disord 30: , 229–237. |
[115] | Mollenhauer B , Zimmermann J , Sixel-Doring F , Focke NK , Wicke T , Ebentheuer J , Schaumburg M , Lang E , Trautmann E , Zetterberg H , Taylor P , Friede T , Trenkwalder C , DeNoPa Study G ((2016) ) Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology 87: , 168–177. |
[116] | Abbott RD , Ross GW , White LR , Tanner CM , Masaki KH , Nelson JS , Curb JD , Petrovitch H ((2005) ) Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 65: , 1442–1446. |
[117] | Amara AW , Chahine LM , Caspell-Garcia C , Long JD , Coffey C , Hogl B , Videnovic A , Iranzo A , Mayer G , Foldvary-Schaefer N , Postuma R , Oertel W , Lasch S , Marek K , Simuni T , Parkinson’s Progression Markers I ((2017) ) Longitudinal assessment of excessive daytime sleepiness in early Parkinson’s disease. J Neurol Neurosurg Psychiatry 88: , 653–662. |
[118] | Zhu K , van Hilten JJ , Marinus J ((2016) ) Course and risk factors for excessive daytime sleepiness in Parkinson’s disease. Parkinsonism Relat Disord 24: , 34–40. |
[119] | Yousaf T , Pagano G , Niccolini F , Politis M ((2018) ) Excessive daytime sleepiness may be associated with caudate denervation in Parkinson disease. J Neurol Sci 387: , 220–227. |
[120] | Wilson H , Giordano B , Turkheimer FE , Chaudhuri KR , Politis M ((2018) ) Serotonergic dysregulation is linked to sleep problems in Parkinson’s disease. Neuroimage Clin 18: , 630–637. |
[121] | Fronczek R , Overeem S , Lee SY , Hegeman IM , van Pelt J , van Duinen SG , Lammers GJ , Swaab DF ((2007) ) Hypocretin (orexin) loss in Parkinson’s disease. Brain 130: , 1577–1585. |
[122] | Poryazova R , Benninger D , Waldvogel D , Bassetti CL ((2010) ) Excessive daytime sleepiness in Parkinson’s disease: Characteristics and determinants. Eur Neurol 63: , 129–135. |
[123] | Frucht SJ , Greene PE , Fahn S ((2000) ) Sleep episodes in Parkinson’s disease: A wake-up call. Mov Disord 15: , 601–603. |
[124] | Giladi N , Boroojerdi B , Korczyn AD , Burn DJ , Clarke CE , Schapira AH , investigators SP ((2007) ) Rotigotine transdermal patch in early Parkinson’s disease: A randomized, double-blind, controlled study versus placebo and ropinirole. Mov Disord 22: , 2398–2404. |
[125] | Pal S , Bhattacharya KF , Agapito C , Chaudhuri KR ((2001) ) A study of excessive daytime sleepiness and its clinical significance in three groups of Parkinson’s disease patients taking pramipexole, cabergoline and levodopa mono and combination therapy. J Neural Transm (Vienna) 108: , 71–77. |
[126] | LeWitt PA , Boroojerdi B , Surmann E , Poewe W , Group SPS , Group SPS ((2013) ) Rotigotine transdermal system for long-term treatment of patients with advanced Parkinson’s disease: Results of two open-label extension studies, CLEOPATRA-PD and PREFER. J Neural Transm (Vienna) 120: , 1069–1081. |
[127] | Ferreira JJ , Galitzky M , Montastruc JL , Rascol O ((2000) ) Sleep attacks and Parkinson’s disease treatment. Lancet 355: , 1333–1334. |
[128] | Garcia-Borreguero D , Schwarz C , Larrosa O , de la Llave Y , Garcia de Yebenes J ((2003) ) L-DOPA-induced excessive daytime sleepiness in PD: A placebo-controlled case with MSLT assessment. Neurology 61: , 1008–1010. |
[129] | Roth T , Rye DB , Borchert LD , Bartlett C , Bliwise DL , Cantor C , Gorell JM , Hubble JP , Musch B , Olanow CW , Pollak C , Stern MB , Watts RL ((2003) ) Assessment of sleepiness and unintended sleep in Parkinson’s disease patients taking dopamine agonists. Sleep Med 4: , 275–280. |
[130] | Askenasy JJ , Yahr MD ((1985) ) Reversal of sleep disturbance in Parkinson’s disease by antiparkinsonian therapy: A preliminary study. Neurology 35: , 527–532. |
[131] | Sangal RB , Thomas L , Mitler MM ((1992) ) Maintenance of wakefulness test and multiple sleep latency test. Measurement of different abilities in patients with sleep disorders. Chest 101: , 898–902. |
[132] | Lloyd K , Gaunt D , Haunton V , Skelly R , Mann H , Ben-Shlomo Y , Henderson EJ ((2020) ) Driving in Parkinson’s disease: A retrospective study of driving and mobility assessments. Age Ageing 49: , 1097–1101. |
[133] | Postuma RB , Anang J , Pelletier A , Joseph L , Moscovich M , Grimes D , Furtado S , Munhoz RP , Appel-Cresswell S , Moro A , Borys A , Hobson D , Lang AE ((2017) ) Caffeine as symptomatic treatment for Parkinson disease (Cafe-PD): A randomized trial. Neurology 89: , 1795–1803. |
[134] | Postuma RB , Lang AE , Munhoz RP , Charland K , Pelletier A , Moscovich M , Filla L , Zanatta D , Rios Romenets S , Altman R , Chuang R , Shah B ((2012) ) Caffeine for treatment of Parkinson disease: A randomized controlled trial. Neurology 79: , 651–658. |
[135] | Suzuki K , Miyamoto M , Miyamoto T , Uchiyama T , Watanabe Y , Suzuki S , Kadowaki T , Fujita H , Matsubara T , Sakuramoto H , Hirata K ((2017) ) Istradefylline improves daytime sleepiness in patients with Parkinson’s disease: An open-label, 3-month study. J Neurol Sci 380: , 230–233. |
[136] | Devos D , Krystkowiak P , Clement F , Dujardin K , Cottencin O , Waucquier N , Ajebbar K , Thielemans B , Kroumova M , Duhamel A , Destee A , Bordet R , Defebvre L ((2007) ) Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 78: , 470–475. |
[137] | Adler CH , Caviness JN , Hentz JG , Lind M , Tiede J ((2003) ) Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson’s disease. Mov Disord 18: , 287–293. |
[138] | Hogl B , Saletu M , Brandauer E , Glatzl S , Frauscher B , Seppi K , Ulmer H , Wenning G , Poewe W ((2002) ) Modafinil for the treatment of daytime sleepiness in Parkinson’s disease: A double-blind, randomized, crossover, placebo-controlled polygraphic trial. Sleep 25: , 905–909. |
[139] | Ondo WG , Fayle R , Atassi F , Jankovic J ((2005) ) Modafinil for daytime somnolence in Parkinson’s disease: Double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 76: , 1636–1639. |
[140] | Rodrigues TM , Castro Caldas A , Ferreira JJ ((2016) ) Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson’s disease: Systematic review and meta-analysis. Parkinsonism Relat Disord 27: , 25–34. |
[141] | Knie B , Mitra MT , Logishetty K , Chaudhuri KR ((2011) ) Excessive daytime sleepiness in patients with Parkinson’s disease. CNS Drugs 25: , 203–212. |
[142] | Sheng P , Hou L , Wang X , Wang X , Huang C , Yu M , Han X , Dong Y ((2013) ) Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: A systematic review and meta-analysis. PLoS One 8: , e81802. |
[143] | Weintraub D , Mavandadi S , Mamikonyan E , Siderowf AD , Duda JE , Hurtig HI , Colcher A , Horn SS , Nazem S , Ten Have TR , Stern MB ((2010) ) Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology 75: , 448–455. |
[144] | Ondo WG , Perkins T , Swick T , Hull KL Jr , Jimenez JE , Garris TS , Pardi D ((2008) ) Sodium oxybate for excessive daytime sleepiness in Parkinson disease: An open-label polysomnographic study. Arch Neurol 65: , 1337–1340. |
[145] | Buchele F , Hackius M , Schreglmann SR , Omlor W , Werth E , Maric A , Imbach LL , Hagele-Link S , Waldvogel D , Baumann CR ((2018) ) Sodium oxybate for excessive daytime sleepiness and sleep disturbance in Parkinson disease: A randomized clinical trial. JAMA Neurol 75: , 114–118. |
[146] | Kuting T , Kramer M , Bicker W , Madea B , Hess C ((2019) ) Case report: Another death associated to gamma-hydroxybutyric acid intoxication. Forensic Sci Int 299: , 34–40. |
[147] | Raposo Pereira F , McMaster MTB , de Vries Y , Polderman N , van den Brink W , van Wingen GA ((2019) ) Influence of gamma-hydroxybutyric acid-use and gamma-hydroxybutyric acid-induced coma on affect and the affective network. Eur Addict Res 25: , 173–181. |
[148] | Heinzer R , Vat S , Marques-Vidal P , Marti-Soler H , Andries D , Tobback N , Mooser V , Preisig M , Malhotra A , Waeber G , Vollenweider P , Tafti M , Haba-Rubio J ((2015) ) Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir Med 3: , 310–318. |
[149] | Arnulf I , Konofal E , Merino-Andreu M , Houeto JL , Mesnage V , Welter ML , Lacomblez L , Golmard JL , Derenne JP , Agid Y ((2002) ) Parkinson’s disease and sleepiness: An integral part of PD. Neurology 58: , 1019–1024. |
[150] | Zoccolella S , Savarese M , Lamberti P , Manni R , Pacchetti C , Logroscino G ((2011) ) Sleep disorders and the natural history of Parkinson’s disease: The contribution of epidemiological studies. Sleep Med Rev 15: , 41–50. |
[151] | Cochen De Cock V , Abouda M , Leu S , Oudiette D , Roze E , Vidailhet M , Similowski T , Arnulf I ((2010) ) Is obstructive sleep apnea a problem in Parkinson’s disease? Sleep Med 11: , 247–252. |
[152] | Beland SG , Postuma RB , Latreille V , Bertrand JA , Panisset M , Chouinard S , Wolfson C , Gagnon JF ((2015) ) Observational study of the relation between Parkinson’s disease and sleep apnea. J Parkinsons Dis 5: , 805–811. |
[153] | da Silva-Junior FP Jr , do Prado GF , Barbosa ER , Tufik S , Togeiro SM ((2014) ) Sleep disordered breathing in Parkinson’s disease: A critical appraisal. Sleep Med Rev 18: , 173–178. |
[154] | Spicuzza L , Caruso D , Di Maria G ((2015) ) Obstructive sleep apnoea syndrome and its management. Ther Adv Chronic Dis 6: , 273–285. |
[155] | Berry RB , Brooks R , Gamaldo C , Harding SM , Lloyd RM , Quan SF , Troester MT , Vaughn BV ((2017) ) AASM Scoring Manual Updates for 2017 (Version 2.4). J Clin Sleep Med 13: , 665–666. |
[156] | Berry RB , Budhiraja R , Gottlieb DJ , Gozal D , Iber C , Kapur VK , Marcus CL , Mehra R , Parthasarathy S , Quan SF , Redline S , Strohl KP , Davidson Ward SL , Tangredi MM , American Academy of Sleep M ((2012) ) Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8: , 597–619. |
[157] | Osman AM , Carter SG , Carberry JC , Eckert DJ ((2018) ) Obstructive sleep apnea: Current perspectives. Nat Sci Sleep 10: , 21–34. |
[158] | Op de Beeck S , Dieltjens M , Azarbarzin A , Willemen M , Verbraecken J , Braem MJ , Wellman A , Sands SA , Vanderveken OM ((2021) ) Mandibular advancement device treatment efficacy is associated with polysomnographic endotypes. Ann Am Thorac Soc 18: , 511–518. |
[159] | Petrovic D , Haba-Rubio J , Carmeli C , Vollenweider P , Heinzer R , Stringhini S ((2019) ) Social inequalities in sleep-disordered breathing: Evidence from the CoLaus|HypnoLaus study. J Sleep Res 28: , e12799. |
[160] | Lavie L ((2015) ) Oxidative stress in obstructive sleep apnea and intermittent hypoxia–revisited–the bad ugly and good: Implications to the heart and brain. Sleep Med Rev 20: , 27–45. |
[161] | Jenner P ((2003) ) Oxidative stress in Parkinson’s disease. Ann Neurol 53: (Suppl 3), S26–36; discussion S36-28. |
[162] | Zhang W , Zhang L , Zhou N , Huang E , Li Q , Wang T , Ma C , Li B , Li C , Du Y , Zhang J , Lei X , Ross A , Sun H , Zhu X ((2019) ) Dysregulation of respiratory center drive (P0.1) and muscle strength in patients with early stage idiopathic Parkinson’s disease. Front Neurol 10: , 724. |
[163] | Haba-Rubio J , Marques-Vidal P , Andries D , Tobback N , Preisig M , Vollenweider P , Waeber G , Luca G , Tafti M , Heinzer R ((2015) ) Objective sleep structure and cardiovascular risk factors in the general population: The HypnoLaus Study. Sleep 38: , 391–400. |
[164] | Hausler N , Marques-Vidal P , Heinzer R , Haba-Rubio J ((2019) ) How are sleep characteristics related to cardiovascular health? Results from the population-based HypnoLaus study. J Am Heart Assoc 8: , e011372. |
[165] | Mansukhani MP , Somers VK , Shafazand S ((2017) ) PAP and cardiovascular events in adults with sleep apnea: Is PAP useful? J Clin Sleep Med 13: , 1487–1489. |
[166] | Haba-Rubio J , Marti-Soler H , Tobback N , Andries D , Marques-Vidal P , Waeber G , Vollenweider P , von Gunten A , Preisig M , Castelao E , Tafti M , Heinzer R , Popp J ((2017) ) Sleep characteristics and cognitive impairment in the general population: The HypnoLaus study. Neurology 88: , 463–469. |
[167] | Hausler N , Heinzer R , Haba-Rubio J , Marques-Vidal P ((2019) ) Does sleep affect weight gain? Assessing subjective sleep and polysomnography measures in a population-based cohort study (CoLaus/HypnoLaus). Sleep 42: , zsz077. |
[168] | Redline S , Strauss ME , Adams N , Winters M , Roebuck T , Spry K , Rosenberg C , Adams K ((1997) ) Neuropsychological function in mild sleep-disordered breathing. Sleep 20: , 160–167. |
[169] | Meng L , Benedetti A , Lafontaine AL , Mery V , Robinson AR , Kimoff J , Gros P , Kaminska M ((2020) ) Obstructive sleep apnea, CPAP therapy and Parkinson’s disease motor function: A longitudinal study. Parkinsonism Relat Disord 70: , 45–50. |
[170] | Shpirer I , Miniovitz A , Klein C , Goldstein R , Prokhorov T , Theitler J , Pollak L , Rabey JM ((2006) ) Excessive daytime sleepiness in patients with Parkinson’s disease: A polysomnography study. Mov Disord 21: , 1432–1438. |
[171] | Braga-Neto P , da Silva-Junior FP , Sueli Monte F , de Bruin PF , de Bruin VM ((2004) ) Snoring and excessive daytime sleepiness in Parkinson’s disease. J Neurol Sci 217: , 41–45. |
[172] | Neikrug AB , Liu L , Avanzino JA , Maglione JE , Natarajan L , Bradley L , Maugeri A , Corey-Bloom J , Palmer BW , Loredo JS , Ancoli-Israel S ((2014) ) Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep 37: , 177–185. |
[173] | Dunietz GL , Chervin RD , Burke JF , Braley TJ ((2020) ) Obstructive sleep apnea treatment disparities among older adults with neurological disorders. Sleep Health 6: , 534–540. |
[174] | Terzaghi M , Spelta L , Minafra B , Rustioni V , Zangaglia R , Pacchetti C , Manni R ((2017) ) Treating sleep apnea in Parkinson’s disease with C-PAP: Feasibility concerns and effects on cognition and alertness. Sleep Med 33: , 114–118. |
[175] | Guimaraes TM , Poyares D , Oliveira ESL , Luz G , Coelho G , Dal Fabbro C , Tufik S , Bittencourt L ((2021) ) The treatment of mild OSA with CPAP or mandibular advancement device and the effect on blood pressure and endothelial function after one year of treatment. J Clin Sleep Med 17: , 149–158. |
[176] | E Silva LO , Guimarães TM , Pontes G , Coelho G , Badke L , Fabbro CD , Tufik S , Bittencourt L , Togeiro S ((2021) ) The effects of continuous positive airway pressure and mandibular advancement therapy on metabolic outcomes of patients with mild obstructive sleep apnea: A randomized controlled study. Sleep Breath 25: , 797–805. |
[177] | Qin L , Li N , Tong J , Hao Z , Wang L , Zhao Y ((2021) ) Impact of mandibular advancement device therapy on cerebrovascular reactivity in patients with carotid atherosclerosis combined with OSAHS. Sleep Breath 25: , 1543–1552. |
[178] | Trzepizur W , Cistulli PA , Glos M , Vielle B , Sutherland K , Wijkstra PJ , Hoekema A , Gagnadoux F ((2021) ) Health outcomes of continuous positive airway pressure versus mandibular advancement device for the treatment of severe obstructive sleep apnea: An individual participant data meta-analysis. Sleep 44: , zsab015. |
[179] | Castel M , Cochen De Cock V , Leon H , Dupuy-Bonafe I ((2020) ) Mandibular advancement device in Parkinson’s disease: A pilot study on efficacy and usability. Sleep Med 66: , 78–81. |
[180] | Carmo J , Araujo I , Marques F , Fonseca C ((2017) ) Sleep-disordered breathing in heart failure: The state of the art after the SERVE-HF trial. Rev Port Cardiol 36: , 859–867. |
[181] | Schenck CH , Bundlie SR , Ettinger MG , Mahowald MW ((1986) ) Chronic behavioral disorders of human REM sleep: A new category of parasomnia. Sleep 9: , 293–308. |
[182] | Sixel-Doring F , Schweitzer M , Mollenhauer B , Trenkwalder C ((2011) ) Intraindividual variability of REM sleep behavior disorder in Parkinson’s disease: A comparative assessment using a new REM sleep behavior disorder severity scale (RBDSS) for clinical routine. J Clin Sleep Med 7: , 75–80. |
[183] | Boeve BF ((2010) ) REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci 1184: , 15–54. |
[184] | Fernandez-Arcos A , Iranzo A , Serradell M , Gaig C , Santamaria J ((2016) ) The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: A study in 203 consecutive patients. Sleep 39: , 121–132. |
[185] | Lam SP , Wong CC , Li SX , Zhang JH , Chan JW , Zhou JY , Liu YP , Yu MW , Wing YK ((2016) ) Caring burden of REM sleep behavior disorder - spouses’ health and marital relationship. Sleep Med 24: , 40–43. |
[186] | Iranzo A ((2018) ) The REM sleep circuit and how its impairment leads to REM sleep behavior disorder. Cell Tissue Res 373: , 245–266. |
[187] | Valencia Garcia S , Luppi PH , Fort P ((2018) ) A particular medullary-spinal inhibitory pathway is recruited for the expression of muscle atonia during REM sleep. J Exp Neurosci 12: , 1179069518808744. |
[188] | Garcia-Lorenzo D , Longo-Dos Santos C , Ewenczyk C , Leu-Semenescu S , Gallea C , Quattrocchi G , Pita Lobo P , Poupon C , Benali H , Arnulf I , Vidailhet M , Lehericy S ((2013) ) The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain 136: , 2120–2129. |
[189] | Hogl B , Stefani A , Videnovic A ((2018) ) Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat Rev Neurol 14: , 40–55. |
[190] | Claassen DO , Josephs KA , Ahlskog JE , Silber MH , Tippmann-Peikert M , Boeve BF ((2010) ) REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 75: , 494–499. |
[191] | Schenck CH , Mahowald MW ((2002) ) REM sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 25: , 120–138. |
[192] | Iranzo A , Santamaria J , Tolosa E ((2009) ) The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev 13: , 385–401. |
[193] | Boeve BF , Silber MH , Ferman TJ , Lucas JA , Parisi JE ((2001) ) Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord 16: , 622–630. |
[194] | Berg D , Postuma RB , Adler CH , Bloem BR , Chan P , Dubois B , Gasser T , Goetz CG , Halliday G , Joseph L , Lang AE , Liepelt-Scarfone I , Litvan I , Marek K , Obeso J , Oertel W , Olanow CW , Poewe W , Stern M , Deuschl G ((2015) ) MDS research criteria for prodromal Parkinson’s disease. Mov Disord 30: , 1600–1611. |
[195] | Heinzel S , Berg D , Gasser T , Chen H , Yao C , Postuma RB , MDS Task Force on the Definition of Parkinson’s Disease ((2019) ) Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord 34: , 1464–1470. |
[196] | Postuma RB , Bertrand JA , Montplaisir J , Desjardins C , Vendette M , Rios Romenets S , Panisset M , Gagnon JF ((2012) ) Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: A prospective study. Mov Disord 27: , 720–726. |
[197] | Nomura T , Inoue Y , Kagimura T , Nakashima K ((2013) ) Clinical significance of REM sleep behavior disorder in Parkinson’s disease. Sleep Med 14: , 131–135. |
[198] | Zhang L , Xu Y , Zhuang J , Peng H , Wu H , Zhao Z , He B , Zhao Z ((2016) ) Metabolic abnormality of pontine tegmentum in patients with REM sleep behavior disorder analyzed using magnetic resonance spectroscopy. Clin Neurol Neurosurg 148: , 137–141. |
[199] | Chahine LM , Xie SX , Simuni T , Tran B , Postuma R , Amara A , Oertel WH , Iranzo A , Scordia C , Fullard M , Linder C , Purri R , Darin A , Rennert L , Videnovic A , Del Riva P , Weintraub D ((2016) ) Longitudinal changes in cognition in early Parkinson’s disease patients with REM sleep behavior disorder. Parkinsonism Relat Disord 27: , 102–106. |
[200] | Pagano G , De Micco R , Yousaf T , Wilson H , Chandra A , Politis M ((2018) ) REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology 91: , e894–e905. |
[201] | Iranzo A , Ratti PL , Casanova-Molla J , Serradell M , Vilaseca I , Santamaria J ((2009) ) Excessive muscle activity increases over time in idiopathic REM sleep behavior disorder. Sleep 32: , 1149–1153. |
[202] | Sixel-Doring F , Zimmermann J , Wegener A , Mollenhauer B , Trenkwalder C ((2016) ) The evolution of REM sleep behavior disorder in early Parkinson disease. Sleep 39: , 1737–1742. |
[203] | Frauscher B , Iranzo A , Gaig C , Gschliesser V , Guaita M , Raffelseder V , Ehrmann L , Sola N , Salamero M , Tolosa E , Poewe W , Santamaria J , Hogl B , Group S ((2012) ) Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep 35: , 835–847. |
[204] | Iranzo A , Santamaria J ((2005) ) Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep 28: , 203–206. |
[205] | Fernandez-Arcos A , Iranzo A , Serradell M , Gaig C , Guaita M , Salamero M , Santamaria J ((2017) ) Diagnostic value of isolated mentalis versus mentalis plus upper limb electromyography in idiopathic REM sleep behavior disorder patients eventually developing a neurodegenerative syndrome. Sleep 40: , doi: 10.1093/sleep/zsx025. |
[206] | Iranzo A , Frauscher B , Santos H , Gschliesser V , Ratti L , Falkenstetter T , Sturner C , Salamero M , Tolosa E , Poewe W , Santamaria J , Hogl B , SINBAR (Sleep Innsbruck Barcelona) Group ((2011) ) Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med 12: , 284–288. |
[207] | Figorilli M , Ferri R , Zibetti M , Beudin P , Puligheddu M , Lopiano L , Cicolin A , Durif F , Marques A , Fantini ML ((2017) ) Comparison between automatic and visual scorings of REM sleep without atonia for the diagnosis of REM sleep behavior disorder in Parkinson disease. Sleep 40: , doi: 10.1093/sleep/zsw060. |
[208] | Li SX , Wing YK , Lam SP , Zhang J , Yu MW , Ho CK , Tsoh J , Mok V ((2010) ) Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK). Sleep Med 11: , 43–48. |
[209] | Boeve BF , Molano JR , Ferman TJ , Lin SC , Bieniek K , Tippmann-Peikert M , Boot B , St Louis EK , Knopman DS , Petersen RC , Silber MH ((2013) ) Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med 9: , 475–480. |
[210] | Stefani A , Heidbreder A , Brandauer E , Guaita M , Neier LM , Mitterling T , Santamaria J , Iranzo A , Videnovic A , Trenkwalder C , Sixel-Doring F , Wenning GK , Chade A , Poewe W , Gershanik OS , Hogl B ((2018) ) Screening for idiopathic REM sleep behavior disorder: Usefulness of actigraphy. Sleep 41: , zsy053. |
[211] | Dauvilliers Y , Schenck CH , Postuma RB , Iranzo A , Luppi PH , Plazzi G , Montplaisir J , Boeve B ((2018) ) REM sleep behaviour disorder. Nat Rev Dis Primers 4: , 19. |
[212] | Stefani A , Santamaria J , Iranzo A , Hackner H , Schenck CH , Hogl B ((2021) ) Nelotanserin as symptomatic treatment for rapid eye movement sleep behavior disorder: A double-blind randomized study using video analysis in patients with dementia with Lewy bodies or Parkinson’s disease dementia. Sleep Med 81: , 180–187. |
[213] | Shin C , Park H , Lee WW , Kim HJ , Kim HJ , Jeon B ((2019) ) Clonazepam for probable REM sleep behavior disorder in Parkinson’s disease: A randomized placebo-controlled trial. J Neurol Sci 401: , 81–86. |
[214] | Gilat M , Marshall NS , Testelmans D , Buyse B , Lewis SJG ((2022) ) A critical review of the pharmacological treatment of REM sleep behavior disorder in adults: Time for more and larger randomized placebo-controlled trials. J Neurol 269: , 125–148. |
[215] | Li SX , Lam SP , Zhang J , Yu MW , Chan JW , Liu Y , Lam VK , Ho CK , Zhou J , Wing YK ((2016) ) A prospective, naturalistic follow-up study of treatment outcomes with clonazepam in rapid eye movement sleep behavior disorder. Sleep Med 21: , 114–120. |
[216] | Boeve BF , Silber MH , Ferman TJ ((2003) ) Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: Results in 14 patients. Sleep Med 4: , 281–284. |
[217] | McGrane IR , Leung JG , St Louis EK , Boeve BF ((2015) ) Melatonin therapy for REM sleep behavior disorder: A critical review of evidence. Sleep Med 16: , 19–26. |
[218] | Gilat M , Coeytaux Jackson A , Marshall NS , Hammond D , Mullins AE , Hall JM , Fang BAM , Yee BJ , Wong KKH , Grunstein RR , Lewis SJG ((2020) ) Melatonin for rapid eye movement sleep behavior disorder in Parkinson’s disease: A randomised controlled trial. Mov Disord 35: , 344–349. |
[219] | Jun JS , Kim R , Byun JI , Kim TJ , Lim JA , Sunwoo JS , Lee ST , Jung KH , Park KI , Chu K , Kim M , Lee SK , Jung KY ((2019) ) Prolonged-release melatonin in patients with idiopathic REM sleep behavior disorder. Ann Clin Transl Neurol 6: , 716–722. |
[220] | Kunz D , Mahlberg R ((2010) ) A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res 19: , 591–596. |
[221] | Takeuchi N , Uchimura N , Hashizume Y , Mukai M , Etoh Y , Yamamoto K , Kotorii T , Ohshima H , Ohshima M , Maeda H ((2001) ) Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci 55: , 267–269. |
[222] | Kashihara K , Nomura T , Maeda T , Tsuboi Y , Mishima T , Takigawa H , Nakashima K ((2016) ) Beneficial effects of ramelteon on rapid eye movement sleep behavior disorder associated with Parkinson’s disease - results of a multicenter open trial. Intern Med 55: , 231–236. |
[223] | Esaki Y , Kitajima T , Koike S , Fujishiro H , Iwata Y , Tsuchiya A , Hirose M , Iwata N ((2016) ) An open-labeled trial of ramelteon in idiopathic rapid eye movement sleep behavior disorder. J Clin Sleep Med 12: , 689–693. |
[224] | Kumru H , Iranzo A , Carrasco E , Valldeoriola F , Marti MJ , Santamaria J , Tolosa E ((2008) ) Lack of effects of pramipexole on REM sleep behavior disorder in Parkinson disease. Sleep 31: , 1418–1421. |
[225] | Di Giacopo R , Fasano A , Quaranta D , Della Marca G , Bove F , Bentivoglio AR ((2012) ) Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson’s disease. Mov Disord 27: , 559–561. |
[226] | Videnovic A , Ju YS , Arnulf I , Cochen-De Cock V , Hogl B , Kunz D , Provini F , Ratti PL , Schiess MC , Schenck CH , Trenkwalder C , Treatment, Trials Working Group of the International RBDSG ((2020) ) Clinical trials in REM sleep behavioural disorder: Challenges and opportunities. J Neurol Neurosurg Psychiatry 91: , 740–749. |
[227] | Anderson KN , Shneerson JM ((2009) ) Drug treatment of REM sleep behavior disorder: The use of drug therapies other than clonazepam. J Clin Sleep Med 5: , 235–239. |
[228] | Allen RP , Picchietti DL , Garcia-Borreguero D , Ondo WG , Walters AS , Winkelman JW , Zucconi M , Ferri R , Trenkwalder C , Lee HB , International Restless Legs Syndrome Study Group ((2014) ) Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med 15: , 860–873. |
[229] | Trenkwalder C , Allen R , Hogl B , Paulus W , Winkelmann J ((2016) ) Restless legs syndrome associated with major diseases: A systematic review and new concept. Neurology 86: , 1336–1343. |
[230] | Hermann W , Flemming T , Brandt MD , Langner S , Reichmann H , Storch A ((2020) ) Asymmetry of periodic leg movements in sleep (PLMS) in Parkinson’s disease. J Parkinsons Dis 10: , 255–266. |
[231] | Gjerstad MD , Tysnes OB , Larsen JP ((2011) ) Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology 77: , 1941–1946. |
[232] | Bhalsing K , Suresh K , Muthane UB , Pal PK ((2013) ) Prevalence and profile of Restless Legs Syndrome in Parkinson’s disease and other neurodegenerative disorders: A case-control study. Parkinsonism Relat Disord 19: , 426–430. |
[233] | Young A , Home M , Churchward T , Freezer N , Holmes P , Ho M ((2002) ) Comparison of sleep disturbance in mild versus severe Parkinson’s disease. Sleep 25: , 573–577. |
[234] | Zhu XY , Liu Y , Zhang XJ , Yang WH , Feng Y , Ondo WG , Tan EK , Wu YC ((2015) ) Clinical characteristics of leg restlessness in Parkinson’s disease compared with idiopathic Restless Legs Syndrome. J Neurol Sci 357: , 109–114. |
[235] | Fereshtehnejad SM , Shafieesabet M , Shahidi GA , Delbari A , Lokk J ((2015) ) Restless legs syndrome in patients with Parkinson’s disease: A comparative study on prevalence, clinical characteristics, quality of life and nutritional status. Acta Neurol Scand 131: , 211–218. |
[236] | Ylikoski A , Martikainen K , Partinen M ((2015) ) Parkinson’s disease and restless legs syndrome. Eur Neurol 73: , 212–219. |
[237] | Patton SM , Ponnuru P , Snyder AM , Podskalny GD , Connor JR ((2011) ) Hypoxia-inducible factor pathway activation in restless legs syndrome patients. Eur J Neurol 18: , 1329–1335. |
[238] | Connor JR , Ponnuru P , Wang XS , Patton SM , Allen RP , Earley CJ ((2011) ) Profile of altered brain iron acquisition in restless legs syndrome. Brain 134: , 959–968. |
[239] | Fereshtehnejad SM , Ghazi L , Sadeghi M , Khaefpanah D , Shahidi GA , Delbari A , Lokk J ((2014) ) Prevalence of malnutrition in patients with Parkinson’s disease: A comparative study with healthy controls using Mini Nutritional Assessment (MNA) questionnaire. J Parkinsons Dis 4: , 473–481. |
[240] | Allen RP ((2014) ) Restless legs syndrome/Willis Ekbom disease: Evaluation and treatment. Int Rev Psychiatry 26: , 248–262. |
[241] | Allen RP , Picchietti D , Hening WA , Trenkwalder C , Walters AS , Montplaisi J , Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health; International Restless Legs Syndrome Study Group ((2003) ) Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 4: , 101–119. |
[242] | Walters AS , LeBrocq C , Dhar A , Hening W , Rosen R , Allen RP , Trenkwalder C , International Restless Legs Syndrome Study G ((2003) ) Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 4: , 121–132. |
[243] | Walters AS , Frauscher B , Allen R , Benes H , Chaudhuri KR , Garcia-Borreguero D , Lee HB , Picchietti DL , Trenkwalder C , Martinez-Martin P , Stebbins GT , Schrag A , Scales MDSCoR ((2014) ) Review of diagnostic instruments for the restless legs syndrome/Willis-Ekbom Disease (RLS/WED): Critique and recommendations. J Clin Sleep Med 10: , 1343–1349. |
[244] | De Cock VC , Bayard S , Yu H , Grini M , Carlander B , Postuma R , Charif M , Dauvilliers Y ((2012) ) Suggested immobilization test for diagnosis of restless legs syndrome in Parkinson’s disease. Mov Disord 27: , 743–749. |
[245] | Chervin RD ((2001) ) Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med 164: , 1454–1458. |
[246] | Fox SH , Katzenschlager R , Lim SY , Ravina B , Seppi K , Coelho M , Poewe W , Rascol O , Goetz CG , Sampaio C ((2011) ) The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson’s disease. Mov Disord 26: (Suppl 3), S2–41. |
[247] | Garcia-Borreguero D , Silber MH , Winkelman JW , Hogl B , Bainbridge J , Buchfuhrer M , Hadjigeorgiou G , Inoue Y , Manconi M , Oertel W , Ondo W , Winkelmann J , Allen RP ((2016) ) Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: A combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med 21: , 1–11. |
[248] | Allen RP , Picchietti DL , Auerbach M , Cho YW , Connor JR , Earley CJ , Garcia-Borreguero D , Kotagal S , Manconi M , Ondo W , Ulfberg J , Winkelman JW , International Restless Legs Syndrome Study Group ((2018) ) Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: An IRLSSG task force report. Sleep Med 41: , 27–44. |
[249] | Silber MH , Buchfuhrer MJ , Earley CJ , Koo BB , Manconi M , Winkelman JW , Scientific and Medical Advisory Board of the Restless Legs Syndrome Foundation ((2021) ) The management of restless legs syndrome: An updated algorithm. Mayo Clin Proc 96: , 1921–1937. |
[250] | Benes H , Kurella B , Kummer J , Kazenwadel J , Selzer R , Kohnen R ((1999) ) Rapid onset of action of levodopa in restless legs syndrome: A double-blind, randomized, multicenter, crossover trial. Sleep 22: , 1073–1081. |
[251] | Bliwise DL , Freeman A , Ingram CD , Rye DB , Chakravorty S , Watts RL ((2005) ) Randomized, double-blind, placebo-controlled, short-term trial of ropinirole in restless legs syndrome. Sleep Med 6: , 141–147. |
[252] | Oertel WH , Benes H , Bodenschatz R , Peglau I , Warmuth R , Happe S , Geisler P , Cassel W , Leroux M , Kohnen R , Stiasny-Kolster K ((2006) ) Efficacy of cabergoline in restless legs syndrome: A placebo-controlled study with polysomnography (CATOR). Neurology 67: , 1040–1046. |
[253] | Winkelmann J , Allen RP , Hogl B , Inoue Y , Oertel W , Salminen AV , Winkelman JW , Trenkwalder C , Sampaio C ((2018) ) Treatment of restless legs syndrome: Evidence-based review and implications for clinical practice (Revised 2017)(section sign). Mov Disord 33: , 1077–1091. |
[254] | Oertel WH , Benes H , Garcia-Borreguero D , Hogl B , Poewe W , Montagna P , Ferini-Strambi L , Sixel-Doring F , Trenkwalder C , Partinen M , Saletu B , Polo O , Fichtner A , Schollmayer E , Kohnen R , Cassel W , Penzel T , Stiasny-Kolster K ((2010) ) Rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome: A randomized, placebo-controlled polysomnographic study. Sleep Med 11: , 848–856. |
[255] | Wang Y , Yang YC , Lan DM , Wu H , Zhao ZX ((2017) ) An observational clinical and video-polysomnographic study of the effects of rotigotine in sleep disorder in Parkinson’s disease. Sleep Breath 21: , 319–325. |
[256] | Priano L , Albani G , Brioschi A , Guastamacchia G , Calderoni S , Lopiano L , Rizzone M , Cavalli R , Gasco MR , Fraschini F , Bergamasco B , Mauro A ((2003) ) Nocturnal anomalous movement reduction and sleep microstructure analysis in parkinsonian patients during 1-night transdermal apomorphine treatment. Neurol Sci 24: , 207–208. |
[257] | Reuter I , Ellis CM , Ray Chaudhuri K ((1999) ) Nocturnal subcutaneous apomorphine infusion in Parkinson’s disease and restless legs syndrome. Acta Neurol Scand 100: , 163–167. |
[258] | Trenkwalder C , Winkelmann J , Inoue Y , Paulus W ((2015) ) Restless legs syndrome-current therapies and management of augmentation. Nat Rev Neurol 11: , 434–445. |
[259] | Trenkwalder C , Allen R , Hogl B , Clemens S , Patton S , Schormair B , Winkelmann J ((2018) ) Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol 17: , 994–1005. |
[260] | Jost WH , Buhmann C ((2019) ) The challenge of pain in the pharmacological management of Parkinson’s disease. Expert Opin Pharmacother 20: , 1847–1854. |
[261] | Allen RP , Chen C , Garcia-Borreguero D , Polo O , DuBrava S , Miceli J , Knapp L , Winkelman JW ((2014) ) Comparison of pregabalin with pramipexole for restless legs syndrome. N Engl J Med 370: , 621–631. |
[262] | Silber MH , Becker PM , Buchfuhrer MJ , Earley CJ , Ondo WG , Walters AS , Winkelman JW , Scientific and Medical Advisory Board, Restless Legs Syndrome Foundation ((2018) ) The appropriate use of opioids in the treatment of refractory restless legs syndrome. Mayo Clin Proc 93: , 59–67. |
[263] | Trenkwalder C , Chaudhuri KR , Martinez-Martin P , Rascol O , Ehret R , Valis M , Satori M , Krygowska-Wajs A , Marti MJ , Reimer K , Oksche A , Lomax M , DeCesare J , Hopp M , PANDA study group ((2015) ) Prolonged-release oxycodone-naloxone for treatment of severe pain in patients with Parkinson’s disease (PANDA): A double-blind, randomised, placebo-controlled trial. Lancet Neurol 14: , 1161–1170. |
[264] | Trenkwalder C , Bucher SF , Oertel WH ((1996) ) Electrophysiological pattern of involuntary limb movements in the restless legs syndrome. Muscle Nerve 19: , 155–162. |
[265] | Ferri R , Koo BB , Picchietti DL , Fulda S ((2017) ) Periodic leg movements during sleep: Phenotype, neurophysiology, and clinical significance. Sleep Med 31: , 29–38. |
[266] | Leary EB , Moore HEt , Schneider LD , Finn LA , Peppard PE , Mignot E ((2018) ) Periodic limb movements in sleep: Prevalence and associated sleepiness in the Wisconsin Sleep Cohort. Clin Neurophysiol 129: , 2306–2314. |
[267] | Montplaisir J , Boucher S , Poirier G , Lavigne G , Lapierre O , Lesperance P ((1997) ) Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: A study of 133 patients diagnosed with new standard criteria. Mov Disord 12: , 61–65. |
[268] | Haba-Rubio J , Marti-Soler H , Marques-Vidal P , Tobback N , Andries D , Preisig M , Waeber G , Vollenweider P , Kutalik Z , Tafti M , Heinzer R ((2016) ) Prevalence and determinants of periodic limb movements in the general population. Ann Neurol 79: , 464–474. |
[269] | Claman DM , Ewing SK , Redline S , Ancoli-Israel S , Cauley JA , Stone KL , Study of Osteoporotic Fractures Research G ((2013) ) Periodic leg movements are associated with reduced sleep quality in older men: The MrOS Sleep Study. J Clin Sleep Med 9: , 1109–1117. |
[270] | Puligheddu M , Figorilli M , Arico D , Raggi A , Marrosu F , Ferri R ((2014) ) Time structure of leg movement activity during sleep in untreated Parkinson disease and effects of dopaminergic treatment. Sleep Med 15: , 816–824. |
[271] | Covassin N , Neikrug AB , Liu L , Corey-Bloom J , Loredo JS , Palmer BW , Maglione J , Ancoli-Israel S ((2012) ) Clinical correlates of periodic limb movements in sleep in Parkinson’s disease. J Neurol Sci 316: , 131–136. |
[272] | Bargiotas P , Schuepbach MW , Bassetti CL ((2016) ) Sleep-wake disturbances in the premotor and early stage of Parkinson’s disease. Curr Opin Neurol 29: , 763–772. |
[273] | Loo HV , Tan EK ((2008) ) Case-control study of restless legs syndrome and quality of sleep in Parkinson’s disease. J Neurol Sci 266: , 145–149. |
[274] | Prudon B , Duncan GW , Khoo TK , Yarnall AJ , Anderson KN ((2014) ) Primary sleep disorder prevalence in patients with newly diagnosed Parkinson’s disease. Mov Disord 29: , 259–262. |
[275] | Carrier J , Frenette S , Montplaisir J , Paquet J , Drapeau C , Morettini J ((2005) ) Effects of periodic leg movements during sleep in middle-aged subjects without sleep complaints. Mov Disord 20: , 1127–1132. |
[276] | Frauscher B , Mitterling T , Bode A , Ehrmann L , Gabelia D , Biermayr M , Walters AS , Poewe W , Hogl B ((2014) ) A prospective questionnaire study in 100 healthy sleepers: Non-bothersome forms of recognizable sleep disorders are still present. J Clin Sleep Med 10: , 623–629. |
[277] | Figorilli M , Puligheddu M , Congiu P , Ferri R ((2017) ) The clinical importance of periodic leg movements in sleep. Curr Treat Options Neurol 19: , 10. |
[278] | Kunz D , Bes F ((2001) ) Exogenous melatonin in periodic limb movement disorder: An open clinical trial and a hypothesis. Sleep 24: , 183–187. |
[279] | Hood S , Amir S ((2017) ) Neurodegeneration and the circadian clock. Front Aging Neurosci 9: , 170. |
[280] | Videnovic A , Noble C , Reid KJ , Peng J , Turek FW , Marconi A , Rademaker AW , Simuni T , Zadikoff C , Zee PC ((2014) ) Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol 71: , 463–469. |
[281] | Zuzuarregui JRP , During EH ((2020) ) Sleep issues in Parkinson’s disease and their management. Neurotherapeutics 17: , 1480–1494. |
[282] | Cai Y , Liu S , Sothern RB , Xu S , Chan P ((2010) ) Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson’s disease. Eur J Neurol 17: , 550–554. |
[283] | Baumann CR ((2019) ) Sleep-wake and circadian disturbances in Parkinson disease: A short clinical guide. J Neural Transm (Vienna) 126: , 863–869. |
[284] | Rutten S , Vriend C , van den Heuvel OA , Smit JH , Berendse HW , van der Werf YD ((2012) ) Bright light therapy in Parkinson’s disease: An overview of the background and evidence. Parkinsons Dis 2012: , 767105. |
[285] | Ono K , Mochizuki H , Ikeda T , Nihira T , Takasaki J , Teplow DB , Yamada M ((2012) ) Effect of melatonin on alpha-synuclein self-assembly and cytotoxicity. Neurobiol Aging 33: , 2172–2185. |
[286] | Pandi-Perumal SR , BaHammam AS , Brown GM , Spence DW , Bharti VK , Kaur C , Hardeland R , Cardinali DP ((2013) ) Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox Res 23: , 267–300. |
[287] | Delgado-Lara DL , Gonzalez-Enriquez GV , Torres-Mendoza BM , Gonzalez-Usigli H , Cardenas-Bedoya J , Macias-Islas MA , de la Rosa AC , Jimenez-Delgado A , Pacheco-Moises F , Cruz-Serrano JA , Ortiz GG ((2020) ) Effect of melatonin administration on the PER1 and BMAL1 clock genes in patients with Parkinson’s disease. Biomed Pharmacother 129: , 110485. |
[288] | Zhang X , Xie A ((2019) ) Improvement of subthalamic nucleus deep brain stimulation in sleeping symptoms in Parkinson’s disease: A meta-analysis. Parkinsons Dis 2019: , 6280896. |
[289] | Kamel WA , Al-Hashel JY ((2020) ) LCIG in treatment of non-motor symptoms in advanced Parkinson’s disease: Review of literature. Brain Behav 10: , e01757. |
[290] | Louter M , van Sloun RJ , Pevernagie DA , Arends JB , Cluitmans PJ , Bloem BR , Overeem S ((2013) ) Subjectively impaired bed mobility in Parkinson disease affects sleep efficiency. Sleep Med 14: , 668–674. |
[291] | Antczak JM , Rakowicz MJ , Banach M , Derejko M , Sienkiewicz J , Zalewska U , Wieclawska M , Jakubczyk T , Jernajczyk W ((2013) ) Negative influence of L-dopa on subjectively assessed sleep but not on nocturnal polysomnography in Parkinson’s disease. Pharmacol Rep 65: , 614–623. |
[292] | Wu JQ , Cronin-Golomb A ((2020) ) Temporal associations between sleep and daytime functioning in Parkinson’s disease: A smartphone-based ecological momentary assessment. Behav Sleep Med 18: , 560–569. |
[293] | Edwards LL , Quigley EM , Pfeiffer RF ((1992) ) Gastrointestinal dysfunction in Parkinson’s disease: Frequency and pathophysiology. Neurology 42: , 726–732. |
[294] | Bjornara KA , Dietrichs E , Toft M ((2014) ) Clinical features associated with sleep disturbances in Parkinson’s disease. Clin Neurol Neurosurg 124: , 37–43. |
[295] | Demaagd GA , Davenport TC ((2012) ) Management of urinary incontinence. P T 37: , 345–361H. |



