Vagus Nerve and Stomach Synucleinopathy in Parkinson’s Disease, Incidental Lewy Body Disease, and Normal Elderly Subjects: Evidence Against the “Body-First” Hypothesis
Abstract
Background:
Braak and others have proposed that Lewy-type α-synucleinopathy in Parkinson’s disease (PD) may arise from an exogenous pathogen that passes across the gastric mucosa and then is retrogradely transported up the vagus nerve to the medulla.
Objective:
We tested this hypothesis by immunohistochemically staining, with a method specific for p-serine 129 α-synuclein (pSyn), stomach and vagus nerve tissue from an autopsy series of 111 normal elderly subjects, 33 with incidental Lewy body disease (ILBD) and 53 with PD.
Methods:
Vagus nerve samples were taken adjacent to the carotid artery in the neck. Stomach samples were taken from the gastric body, midway along the greater curvature. Formalin-fixed paraffin-embedded sections were immunohistochemically stained for pSyn, shown to be highly specific and sensitive for α-synuclein pathology.
Results:
Median disease duration for the PD group was 13 years. In the vagus nerve none of the 111 normal subjects had pSyn in the vagus, while 12/26 ILBD (46%) and 32/36 PD (89%) subjects were pSyn-positive. In the stomach none of the 102 normal subjects had pSyn while 5/30 (17%) ILBD and 42/52 (81%) of PD subjects were pSyn-positive.
Conclusion:
As there was no pSyn in the vagus nerve or stomach of subjects without brain pSyn, these results support initiation of pSyn in the brain. The presence of pSyn in the vagus nerve and stomach of a subset of ILBD cases indicates that synucleinopathy within the peripheral nervous system may occur, within a subset of individuals, at preclinical stages of Lewy body disease.
INTRODUCTION
Since the discovery of α-synuclein as the major constituent of Lewy bodies, sensitive immunohistochemical (IHC) methods have demonstrated that the brain and peripheral nervous system (PNS) distribution and density of Lewy bodies and their associated abnormal neurites are much greater than formerly appreciated [1–4]. Furthermore, the common PNS occurrence of α-synuclein pathology has instigated a proliferation of research aimed at using PNS α-synuclein pathology as a biopsy biomarker as well as raising the critical question as to whether or not it begins in the brain or within the periphery [5–47]. The stimulus for the latter alternative, which might be called the “body-first” hypothesis, has come largely from clinical studies of PD that have found a wide range of non-motor signs and symptoms that accompany or may even precede the motor signs [48–50]. Many of these non-motor accompaniments are related to gastrointestinal (GI) dysfunction and therefore much attention has been focused on the stomach as the major “first stop” along the alimentary tract [43, 51, 52] and hence the most likely place for the initiation of synucleinopathy, perhaps by absorption of toxins or through microbial or inflammatory stimuli, followed by transmission to the brain through the vagus nerve.
Supporting this is the repeatedly-confirmed finding of a rostrocaudal GI gradient of synucleinopathy [1, 12, 14, 31], which may itself be related to the known distribution of vagal GI innervation [53]. A stomach-vagal-brain connection has been invoked to explain reports that subjects with prior vagotomy may have a lower prevalence of PD, although this has been disputed [54–59]. A stomach “inoculation” site has now been experimentally tested in multiple animal models, and a consensus seems to have emerged that in fact bidirectional spread is possible, both upwards from the stomach and downwards from the brainstem, including a possible hematogenous route [60–62].
A major piece of evidence for the body-first hypothesis, however, has been lacking, in that human autopsy studies have never found pathology-specific forms of α-synuclein in the stomach or other GI location in the absence of such in the brain. Also, previous autopsy studies have focused on the GI tract itself but not on the proposed gut-to-brain conduit, the vagus nerve. It has been rightfully argued that since nervous tissue is much less concentrated in enteric walls than in CNS tissue, the apparent primacy of brain α-synuclein pathology may only be due to its much greater endowment in this respect. The vagus nerve is, like the CNS, 100%nervous tissue, and if it does serve as the connection through which α-synuclein pathology makes its passage from gut to brain, it, as well as a GI location, should be affected in at least a small percentage of normal subjects as the only manifestation of α-synuclein pathology. About 25%of clinically normal elderly subjects, dependent on age, have a limited brain distribution of α-synuclein pathology, most often in the olfactory bulb, brainstem and/or amygdala. These “incidental Lewy body disease” (ILBD) subjects also have reduced striatal dopaminergic markers [63–65], suggesting that they represent prodromal PD or dementia with Lewy bodies (DLB). If α-synuclein pathology begins in the stomach and then passes through the vagus nerve to the brain, it might be expected that for a similar percentage of normal older people, α-synuclein pathology would be limited to the stomach and/or vagus nerve, but be lacking in the CNS.
MATERIALS AND METHODS
Human subjects
The study took place at Banner Sun Health Research Institute (BSHRI), which is part of Banner Health, a non-profit healthcare provider centered in Phoenix, Arizona. BSHRI and the Mayo Clinic Arizona are the principal members of the Arizona Parkinson’s Disease Consortium. Brain necropsies and neuropathological examinations were performed on elderly subjects who had volunteered for the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND)/BSHRI Brain and Body Donation Program (BBDP) [66]. Procedures involving experiments on human subjects are done in accord with the ethical standards of the BSHRI Institutional Review Boards and all subjects or their legally-authorized representatives signed a written informed consent. The majority of BBDP subjects are clinically characterized at BSHRI with annual standardized test batteries that include movement disorders and cognitive/neuropsychological components. Additionally, private medical records are requisitioned and reviewed for each subject and a postmortem questionnaire is conducted with subject contacts to help determine the presence or absence of dementia and parkinsonism for those subjects that did not have a recent standardized antemortem evaluation.
Subjects received complete neuropathological examinations while blinded to clinical diagnoses as described previously [66]. Specific consensus diagnostic criteria were used for PD, requiring 2 of 3 cardinal signs of rest tremor, rigidity or bradykinesia as well as substantia nigra α-synuclein pathology and pigmented neuron loss. Subjects with any brain α-synuclein pathology but who lacked dementia or parkinsonism were termed incidental Lewy body disease (ILBD).
Case selection was done by searching the BBDP database for all those that had died and had a full clinical evaluation and full autopsy including vagus nerve and/or stomach sampling with immunohistochemical staining for α-synuclein pathology, done with a method specific for α-synuclein phosphorylated at serine-129. Vagus nerve samples were taken adjacent to the midpoint of the carotid artery in the neck while stomach samples were taken midway along the greater curvature.
Histological methods
The process leading to the choice and evaluation of immunohistochemical methods for demonstrating pathological α-synuclein has been described in previous publications [16, 24, 67–69]. The standard method used at AZSAND employs proteinase K pretreatment, which not only results in superior epitope exposure but also may assist with the pathological specificity of the stain by digesting normal, non-aggregated α-synuclein, which is abundant in all nervous tissue. Using an antibody specific for α-synuclein phosphorylated at serine 129 (pSyn) [70–74] also helps identify stained structures as pathological since normal control subjects do not have pSyn-immunoreactive brain tissue elements [4, 75]. Complete details of the staining procedure have been previously described [76] and so only a brief description is given here.
From each postmortem subject, three sections from vagus nerve and three sections of stomach were stained and examined. Formalin-fixed, paraffin-embedded 5–7μm sections were deparaffinized and treated with 1:100 proteinase K (Enzo Life Sciences, Farmingdale, NY) at 37°C for 20 min, followed by suppression of endogenous peroxidase activity with 1%hydrogen peroxide for 30 min, incubation in primary antibody against α-synuclein phosphorylated at serine 129, diluted 1:10,000 [71–74], incubation in biotinylated secondary antibody, avidin-biotin peroxidase complex (ABC, Vector Laboratories; Burlingame, CA) and 3,3’-diaminobenzidine (DAB; Sigma, St. Louis, MO) with saturated nickel ammonium sulfate and imidazole. All solutions subsequent to proteinase K, and all wash steps, excluding DAB incubation, were carried out in 0.1 M PBS with 0.3%Triton X-100, pH 7.4. Sections were then counterstained in 1%Neutral Red. Positive neuronal perikarya and nerve fibers are bluish-black while background and negative tissue structures are red. Cases were staged with the Unified Staging System for Lewy Body Disorders (USSLB) [3, 4].
Statistical tests
Group proportions in the three diagnostic groups were compared with chi-square tests. Group means were compared with one-way analysis of variance and post-hoc Newman-Keuls tests or two-way, unpaired t-tests.
RESULTS
Subjects included 53 clinicopathologically diagnosed with PD, 33 with ILBD and 111 who were clinically non-demented without parkinsonism and had no CNS pSyn (Table 1). The subjects were predominantly of advanced age, with the mean age ranges for the diagnostic groups falling between 83 and 87 years. Median disease duration for PD cases was 13 years, ranging from 2 to 44 years. PD cases were significantly younger (p < 0.05) than normal or ILBD cases, were more likely to be male when compared to the normal group (p = 0.006) and had higher UPDRS scores than the other groups (p < 0.0001). The mean postmortem intervals ranged between 4.1 and 4.6 h and analysis of variance showed that there were no significant differences amongst groups. Vagus nerve was available for 26 ILBD, 36 PD and 111 normal control subjects. Stomach was available for 30 ILBD, 52 PD and 102 normal control subjects. Seven control cases had a family history of PD but as the pedigrees were not strongly suggestive of autosomal dominance, none were genetically tested for PD-associated mutations or polymorphisms. Additionally, none of the ILBD cases had a PD family history but two were tested for LRRK2 (both cases) and GBA (one case) and were negative for both markers; twelve PD cases had a PD family history and eight were tested but all were negative for both LRRK2 and GBA.
Table 1
Clinical characteristics of study subjects, by clinicopathological diagnosis, age, sex, last motor UPDRS score and disease duration. Means and standard deviations (SD) are given. ILBD, incidental Lewy body disease; PD, Parkinson’s disease; PMI, postmortem interval in hours; UPDRS, Unified Parkinson’s Disease Rating Scale, Part III; Dis Dur, disease duration in years. *PD cases were significantly younger (p < 0.05) than normal or ILBD cases, were more likely to be male when compared to the normal group (p < 0.05) and had higher UPDRS scores (p < 0.0001). The groups did not differ in terms of PMI
| Diagnosis (N) | Age at Death* | Sex (%male)* | PMI | UPDRS* | Dis Dur |
| Normal (111)1 | 85.1 (11.0) | 53.1 | 3.7 (2.5) | 7.6 (7.6)2 | N/A |
| ILBD (33)1 | 86.3 (8.2) | 57.6 | 4.5 (4.2) | 8.0 (6.2)3 | N/A |
| PD (53)1 | 79.1 (6.4) | 75.5 | 4.1 (2.6) | 38.8 (20.5)4 | 13.3 (7.3) |
1Not all subjects had both vagus nerve and stomach available. See text for details. 2For 15 normal cases, UPDRS scores were not available. 3For 5 ILBD cases, UPDRS scores were not available. 4For PD, 16 scores were done while on medication, 35 while off medication; 2 were not done.
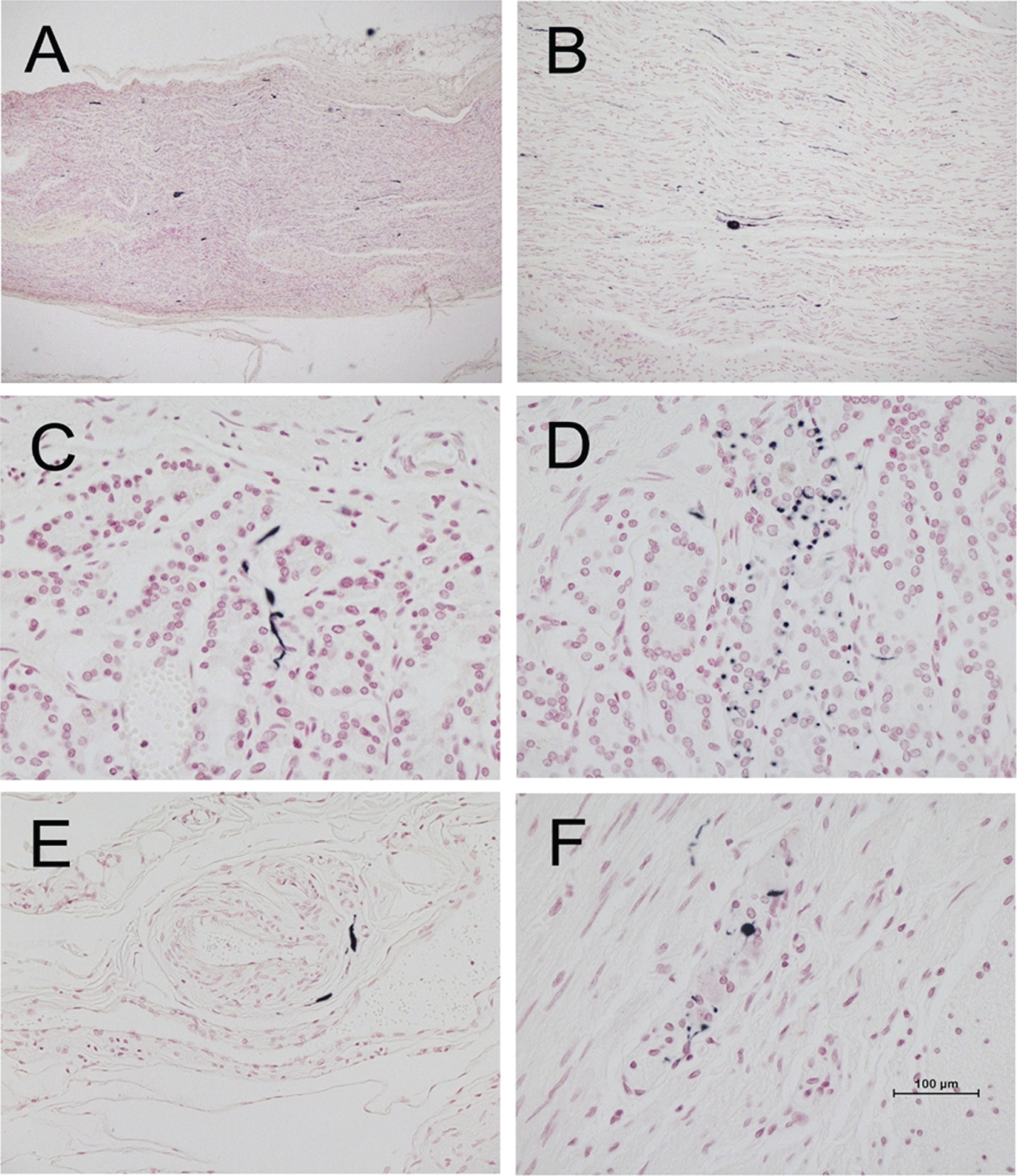
As in our previous investigations [1, 17, 76–78], only staining that was morphologically consistent with nerve fibers was considered to be specific for stomach or vagus nerve α-synuclein pathology. Immunoreactive nerve fibers were present within the stomach or vagus nerve of 42/52 (81%) and 32/36 (89%) PD subjects, as compared to 5/30 (17%) and 12/26 (46%) of ILBD subjects (Table 2). No pSyn was present in stomach (102 subjects) or vagus nerve (111 subjects) of control subjects. Immunoreactive nerve fibers, when present in PD and ILBD subjects, were mostly sparse but occasionally focally frequent in the vagus nerve, and sometimes had abnormally large swollen segments, consistent with dystrophic change (Fig. 1a, b). In the stomach, immunoreactive nerve fibers were found in all layers (Fig. 1c-f) but most frequently were found in the submucosa, where they were often closely applied to the external surface of arterioles or within small nerve fascicles.
Table 2
Neuropathological characteristics of Lewy body disease study subjects, by vagus and stomach pSyn status. Means and standard deviations (SD) are given. ILBD, incidental Lewy body disease; PD, Parkinson’s disease
| Diagnosis | USSLB Stage Mean (SD) | Brain pSyn Load Mean (SD) |
| ILBD: Vagus pSyn + (n = 12) | 2.33 (0.49) | 12.08 (8.57) |
| ILBD: Vagus pSyn-(n = 14)1 | 1.92 (0.64) | 6.08 (6.79) |
| ILBD: Stomach pSyn + (n = 5) | 2.6 (0.55) | 12.2 (3.96) |
| ILBD: Stomach pSyn-(n = 25)1 | 1.96 (0.69) | 7.92 (8.30) |
| PD: Vagus pSyn + (n = 32)1 | 3.44 (0.67) | 26.93 (6.28) |
| PD: Vagus pSyn-(n = 4) | 3.25 (0.96) | 25.75 (11.56) |
| PD: Stomach pSyn + (n = 42)2 | 3.62 (0.54) | 29.84 (5.83) |
| PD: Stomach pSyn-(n = 10) | 3.0 (0.67) | 22.33 (5.81) |
1Missing USSLB stage and brain pSyn load for one case. 2Missing brain pSyn load in three cases.
Fig. 1
Photomicrographs of vagus nerve and stomach from four different PD subjects, immunohistochemically stained for phosphorylated α-synuclein (black) and counterstained with Neutral Red (red). A and B are longitudinal sections of vagus nerve at low (A) and medium (B) magnification. C and D show short fibers and puncta in the stomach mucosa. E shows short fibers applied to the peripheral surface on an arteriole in the submucosa. F shows puncta within an intermyenteric ganglion. Calibration bar in F represents 100μm for C-F, 400μm for B and 800μm for A.
Cases, both ILBD and PD, that were pSyn-positive in stomach or vagus tended to have higher brain Unified LB stages and higher total brain pSyn loads than those that were pSyn negative, and, on average, ILBD cases with vagus or stomach pSyn were in USSLB Stage IIa or IIb (brainstem or limbic-predominant) while PD cases were most often in Stage III (brainstem and limbic), although some ILBD cases were in Stage III and some PD cases were in Stage IV (Table 2). All cases with vagus or stomach pSyn were in Unified Stage IIa (brainstem predominant), IIb (limbic predominant) or higher, with no olfactory bulb-only cases (Stage I).
DISCUSSION
This is the first comprehensive assessment of the prevalence of stomach and vagus nerve pSyn in PD, ILBD and control subjects. The results show that stomach pSyn was present in 81%and vagus nerve pSyn was present in 89%of autopsy confirmed PD subjects, while in ILBD, pSyn was present in stomach in only 17%and in vagus in only 46%of subjects. There was no pSyn found in either the stomach or vagus nerve from any of the more than 100 clinically normal control subjects without brain pSyn. The lack of pSyn in stomach and vagus nerve, in subjects without any brain pSyn, as well as the lesser prevalences of pSyn in stomach or vagus nerve as compared to brain in all of the PD and ILBD cases investigated suggest that the spread of pSyn to the stomach and PNS occurs subsequent to the establishment of threshold brain pSyn loads. Additional evidence for this conclusion is that USSLB Stages and total brain pSyn loads were higher in ILBD and PD cases with stomach or vagus pSyn.
A critical question has been whether or not pSyn begins in the brain or within elements of the PNS [79–81]. The present results do not support the concept that the initiation of α-synuclein pathology in Lewy body disorders begins in the PNS rather than the brain. Non-motor accompaniments of PD may predate or occur early in the motor progression [49, 82–85] but the results for ILBD subjects suggest that even in these clinically prodromal subjects, pSyn has already been established in the brain. Autopsy studies of relatively small numbers of subjects with ILBD have demonstrated a high prevalence of pSyn within the spinal cord, sympathetic ganglia, adrenal medulla and upper GI tract [1, 7, 11, 26, 42, 43] but no study to date has found pSyn in the spinal cord or in PNS sites in the absence of brain involvement, with the possible exception of Fumimura et al. [45] who reported one case out of 783 with adrenal medulla as the only site with pSyn. One other case report, by Miki et al. [86] exists of Lewy body pathology restricted to the heart and stellate ganglion. As we have tested only one potential “body-to-brain” route, this leaves open the possibility that isolated peripheral synuclein pathology might exist where it is has not yet been diligently searched for. For example, synuclein pathology might occur first in the sympathetic ganglia and from there retrogradely proceed to the CNS through preganglionic axons to the intermediolateral spinal cord, or may pass to the CNS by a hematogenous route [61].
Although we used an immunohistochemical method that has been repeatedly demonstrated to be highly sensitive and specific for both CNS and PNS α-synuclein pathology, as found in multi-center blinded studies [16, 67–69, 78] and biochemical studies [74], it is possible that the initial form of peripheral α-synuclein pathology may differ from that commonly seen in the CNS. Alternate forms, among many possibilities, may include non-phosphorylated α-synuclein, α-synuclein oligomers, truncated α-synuclein [87–90] and α-synuclein aggregates [39, 91]. It is possible that conversion of normal α-synuclein to pathological varieties may occur more commonly in PNS locations other than stomach, perhaps on the basis of locally high normal α-synuclein concentrations [19], or due to inflammation [92–94]. It is possible that the initial “seeding” of synucleinopathy, whether from the environment or by spontaneous internal generation, is accomplished by transiently-existing forms that may not locally incite the more familiar forms of pSyn but do so in the CNS once transmitted there. Assessment of the PNS with α-synuclein seeding assays such as RT-QuIC or protein misfolding cyclic amplification (PMCA) may be more sensitive than IHC and may be more effective at uncovering PNS α-synuclein pathology [95] although direct comparisons so far have found IHC and seeding assays roughly equivalent in gastrointestinal tissue and skin [96–98].
As mentioned, our IHC method uses a highly-characterized antibody [74] and proteinase K pretreatment to eliminate non-aggregated α-synuclein. We have repeatedly compared our IHC method with multiple other methods, in studies that have used both autopsy and clinical gold standards, studies involving multiple centers and in studies with enforced, third-party blinding [4, 15–17, 67, 69, 78, 99]. Our IHC method always performed amongst the best methods in these studies. We are aware of the argument that has been advanced by some researchers that antibodies to p-serine 129 α-synuclein may be less sensitive to α-synuclein pathology than antibodies to unmodified synuclein because p-synuclein may be only a minor component in α-synuclein pathological aggregates, but again, in several multicenter studies, including some with third-party blinding, our pSyn IHC method has performed similarly as the best IHC methods using antibodies to unmodified synuclein. No other putative IHC biomarker of synuclein pathology has been so extensively and rigorously tested and therefore the suggestion that other methods might be significantly more sensitive and specific than our method remain speculative.
An issue we have not been able to explore is the possibility that a body-first stage might occur as long as 20 years or more before clinical signs. Although we expect that at least some of the control subjects in our study were likely to have been in a preclinical or prodromal phase when they died, a large autopsy series of much younger subjects might better address this possibility. However, as the rates of natural death and autopsy are much lower in these younger age groups, it would be necessary to have access to a forensic autopsy series and such access has become legally restrictive.
Borghammer and Van Den Berge [100] have posited the existence of two subtypes of PD, one that is “CNS-first” and one that is “PNS-first”. Somewhat similarly, Hallett et al. have suggested that CNS and PNS synucleinopathy might proceed in parallel [101]. However, as Borghammer and Van Den Berge admit, if there is a PNS-first subtype, or if PNS and CNS synucleinopathy occur more or less in parallel, and if these subjects are more than a rare occurrence, it should still be possible to find, in a large autopsy series, at least a few cases with verified PNS α-synuclein pathology in the absence of CNS synucleinopathy, and this has not occurred in several such autopsy series including ours. It must be admitted, as Borghammer and Van Den Berge suggest, that sampling of the GI tract and/or other PNS regions has likely not yet been adequate and that PNS α-synuclein pathological species might be transported through the vagus nerve over a short time window, although for the latter scenario it would have to also be assumed that vagal transport only occurs for a short time and then ceases, despite ongoing accumulation of synuclein pathology in the gut. We would add that bilateral asymmetry of CNS and PNS α-synuclein pathology might also complicate the issue although we have investigated this and found no asymmetry of peripheral synucleinopathy, at least for the submandibular gland [102]. Borghammer and Van Den Berge also cite studies that have found a high prevalence of aggregated synuclein in the GI tract of normal subjects including children [103–105], potentially evidence for the PNS-first hypothesis, but ordinarily the finding of a disease biomarker in large numbers of normal subjects would bring into question its specificity for the disease so the relevance to PD of the aggregated synuclein found with these methods is questionable unless one proposes that additional host or environmental factors are required to convert these aggregates into truly pathogenic forms. As with other alternative hypotheses, these scenarios are extremely difficult if not impossible to absolutely disprove.
In conclusion, the results presented here are most consistent with a first appearance of phosphorylated α-synuclein pathology in the CNS, with later spread, often at a premotor stage, to the PNS.
ACKNOWLEDGMENTS
This study was funded by grants from the National Institute of Neurological Disorders and Stroke (U24 NS072026) the National Institute on Aging (P30 AG19610), the Arizona Department of Health Services (contract 211002), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001) and the Michael J. Fox Foundation for Parkinson’s Research.
CONFLICT OF INTEREST
The authors declare the following potential conflicts of interest:
TGB: Consultant, scientific advisory board and stock options with Vivid Genomics; Honorarium for invited lecture from Roche Diagnostics.
CHA: Consultant, Amneal Pharmaceuticals; Eisai Pharmaceuticals; Jazz Pharmaceuticals; Neurocrine, Biosciences; Cionic Inc.
LIS: None
HAS: Advisory board, Acorda Therapeutics; Jazz Pharmaceuticals.
ED-D: Clinical trials, AbbVie Inc; Biogen Inc; UCB Pharma
SHM: Consultant, CNS Ratings; Adamas Pharmaceuticals; Abbott Laboratories
AJI: None
MJG: None
JEW: None
RA: None
CMN: None
GES: None
REFERENCES
[1] | Beach TG , Adler CH , Sue LI , Vedders L , Lue L , White Iii CL , Akiyama H , Caviness JN , Shill HA , Sabbagh MN , Walker DG ((2010) ) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119: , 689–702. |
[2] | Adler CH , Beach TG ((2016) ) Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov Disord 31: , 1114–1119. |
[3] | Adler CH , Beach TG , Zhang N , Shill HA , Driver-Dunckley E , Caviness JN , Mehta SH , Sabbagh MN , Serrano GE , Sue LI , Belden CM , Powell J , Jacobson SA , Zamrini E , Shprecher D , Davis KJ , Dugger BN , Hentz JG ((2019) ) Unified Staging System for Lewy body disorders: Clinicopathologic correlations and comparison to Braak staging. J Neuropathol Exp Neurol 78: , 891–899. |
[4] | Beach TG , Adler CH , Lue L , Sue LI , Bachalakuri J , Henry-Watson J , Sasse J , Boyer S , Shirohi S , Brooks R , Eschbacher J , Akiyama H , Caviness J , Shill HA , Connor DJ , Sabbagh MN , Walker DG ((2009) ) Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117: , 613–634. |
[5] | Serrano GE , Shprecher D , Callan M , Cutler B , Glass M , Zhang N , Walker J , Intorcia A , Adler CH , Shill HA , Driver-Dunckley E , Mehta SH , Belden CM , Zamrini E , Sue LI , Vargas D , Beach TG ((2020) ) Cardiac sympathetic denervation and synucleinopathy in Alzheimer’s disease with brain Lewy body disease. Brain Commun 2: , doi: 10.1093/braincomms/fcaa004. |
[6] | Travagli RA , Browning KN , Camilleri M ((2020) ) Parkinson disease and the gut: New insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 17: , 673–685. |
[7] | Bloch A , Probst A , Bissig H , Adams H , Tolnay M ((2006) ) Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 32: , 284–295. |
[8] | Braak H , Bohl JR , Muller CM , Rub U , de Vos RA , Del TK ((2006) ) Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21: , 2042–2051. |
[9] | Braak H , Ghebremedhin E , Rub U , Bratzke H , Del Tredici K ((2004) ) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318: , 121–134. |
[10] | Hishikawa N , Hashizume Y , Yoshida M , Sobue G ((2003) ) Clinical and neuropathological correlates of Lewy body disease. Acta Neuropathol 105: , 341–350. |
[11] | Klos KJ , Ahlskog JE , Josephs KA , Apaydin H , Parisi JE , Boeve BF , DeLucia MW , Dickson DW ((2006) ) Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology 66: , 1100–1102. |
[12] | Wakabayashi K , Takahashi H , Takeda S , Ohama E , Ikuta F ((1988) ) Parkinson’s disease: The presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76: , 217–221. |
[13] | Gelpi E , Navarro-Otano J , Tolosa E , Gaig C , Compta Y , Rey MJ , Marti MJ , Hernandez I , Valldeoriola F , Rene R , Ribalta T ((2014) ) Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord 29: , 1010–1018. |
[14] | Annerino DM , Arshad S , Taylor GM , Adler CH , Beach TG , Greene JG ((2012) ) Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol 124: , 665–680. |
[15] | Beach TG , Adler CH , Serrano G , Sue LI , Walker DG , Dugger BN , Shill HA , Driver-Dunckley E , Caviness JN , Intorcia A , Filon J , Scott S , Garcia A , Hoffman B , Belden CM , Davis KJ , Sabbagh MN ((2016) ) Prevalence of submandibular gland synucleinopathy in Parkinson’s disease, dementia with Lewy bodies and other Lewy body disorders. J Parkinsons Dis 6: , 153–163. |
[16] | Beach TG , Corbille AG , Letournel F , Kordower JH , Kremer T , Munoz DG , Intorcia A , Hentz J , Adler CH , Sue LI , Walker J , Serrano G , Derkinderen P ((2016) ) Multicenter assessment of immunohistochemical methods for pathological alpha-synuclein in sigmoid colon of autopsied Parkinson’s disease and control subjects. J Parkinsons Dis 6: , 761–770. |
[17] | Adler CH , Dugger BN , Hinni ML , Lott DG , Driver-Dunckley E , Hidalgo J , Henry-Watson J , Serrano G , Sue LI , Nagel T , Duffy A , Shill HA , Akiyama H , Walker DG , Beach TG ((2014) ) Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology 82: , 858–864. |
[18] | Fujishiro H , Frigerio R , Burnett M , Klos KJ , Josephs KA , Delledonne A , Parisi JE , Ahlskog JE , Dickson DW ((2008) ) Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord 23: , 1085–1092. |
[19] | Gray MT , Munoz DG , Gray DA , Schlossmacher MG , Woulfe JM ((2014) ) Alpha-synuclein in the appendiceal mucosa of neurologically intact subjects. Mov Disord 29: , 991–998. |
[20] | Hilton D , Stephens M , Kirk L , Edwards P , Potter R , Zajicek J , Broughton E , Hagan H , Carroll C ((2014) ) Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol 127: , 235–241. |
[21] | Ito S , Takao M , Hatsuta H , Kanemaru K , Arai T , Saito Y , Fukayama M , Murayama S ((2014) ) Alpha-synuclein immunohistochemistry of gastrointestinal and biliary surgical specimens for diagnosis of Lewy body disease. Int J Clin Exp Pathol 7: , 1714–1723. |
[22] | Lebouvier T , Neunlist M , Bruley d, V , Coron E , Drouard A , N’Guyen JM , Chaumette T , Tasselli M , Paillusson S , Flamand M , Galmiche JP , Damier P , Derkinderen P ((2010) ) Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One 5: , e12728. |
[23] | Lebouvier T , Chaumette T , Damier P , Coron E , Touchefeu Y , Vrignaud S , Naveilhan P , Galmiche JP , Bruley d, V , Derkinderen P , Neunlist M ((2008) ) Pathological lesions in colonic biopsies during Parkinson’s disease. Gut 57: , 1741–1743. |
[24] | Lee JM , Derkinderen P , Kordower JH , Freeman R , Munoz DG , Kremer T , Zago W , Hutten SJ , Adler CH , Serrano GE , Beach TG ((2017) ) The search for a peripheral biopsy indicator of alpha-synuclein pathology for Parkinson disease. J Neuropathol Exp Neurol 76: , 2–15. |
[25] | Lionnet A , Leclair-Visonneau L , Neunlist M , Murayama S , Takao M , Adler CH , Derkinderen P , Beach TG ((2018) ) Does Parkinson’s disease start in the gut? Acta Neuropathol 135: , 1–12. |
[26] | Minguez-Castellanos A , Chamorro CE , Escamilla-Sevilla F , Ortega-Moreno A , Rebollo AC , Gomez-Rio M , Concha A , Munoz DG ((2007) ) Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders?: A cohort study. Neurology 68: , 2012–2018. |
[27] | Mu L , Sobotka S , Chen J , Su H , Sanders I , Nyirenda T , Adler CH , Shill HA , Caviness JN , Samanta JE , Sue LI , Beach TG ((2013) ) Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol 72: , 614–623. |
[28] | Mu L , Sobotka S , Chen J , Su H , Sanders I , Adler CH , Shill HA , Caviness JN , Samanta JE , Beach TG ((2013) ) Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol 72: , 119–129. |
[29] | Mu L , Chen J , Sobotka S , Nyirenda T , Benson B , Gupta F , Sanders I , Adler CH , Caviness JN , Shill HA , Sabbagh M , Samanta JE , Sue LI , Beach TG ((2015) ) Alpha-synuclein pathology in sensory nerve terminals of the upper aerodigestive tract of Parkinson’s disease patients. Dysphagia 30: , 404–417. |
[30] | Orimo S , Uchihara T , Nakamura A , Mori F , Kakita A , Wakabayashi K , Takahashi H ((2008) ) Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 131: , 642–650. |
[31] | Pouclet H , Lebouvier T , Coron E , Des Varannes SB , Rouaud T , Roy M , Neunlist M , Derkinderen P ((2012) ) A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson’s disease. Neurobiol Dis 45: , 305–309. |
[32] | Sanchez-Ferro A , Rabano A , Catalan MJ , Rodriguez-Valcarcel FC , Fernandez DS , Herreros-Rodriguez J , Garcia-Cobos E , Alvarez-Santullano MM , Lopez-Manzanares L , Mosqueira AJ , Vela DL , Lopez-Lozano JJ , Lopez-Valdes E , Sanchez-Sanchez R , Molina-Arjona JA ((2015) ) In vivo gastric detection of alpha-synuclein inclusions in Parkinson’s disease. Mov Disord 30: , 517–524. |
[33] | Schneider SA , Boettner M , Alexoudi A , Zorenkov D , Deuschl G , Wedel T ((2016) ) Can we use peripheral tissue biopsies to diagnose Parkinson’s disease? A review of the literature. Eur J Neurol 23: , 247–261. |
[34] | Wakabayashi K ((2020) ) Where and how alpha-synuclein pathology spreads in Parkinson’s disease. Neuropathology 40: , 415–425. |
[35] | Shannon KM , Keshavarzian A , Dodiya HB , Jakate S , Kordower JH ((2012) ) Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord 27: , 716–719. |
[36] | Shannon KM , Keshavarzian A , Mutlu E , Dodiya HB , Daian D , Jaglin JA , Kordower JH ((2012) ) Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord 27: , 709–715. |
[37] | Sprenger FS , Stefanova N , Gelpi E , Seppi K , Navarro-Otano J , Offner F , Vilas D , Valldeoriola F , Pont-Sunyer C , Aldecoa I , Gaig C , Gines A , Cuatrecasas M , Hogl B , Frauscher B , Iranzo A , Wenning GK , Vogel W , Tolosa E , Poewe W ((2015) ) Enteric nervous system alpha-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology 85: , 1761–1768. |
[38] | Vilas D , Iranzo A , Tolosa E , Aldecoa I , Berenguer J , Vilaseca I , Marti C , Serradell M , Lomena F , Alos L , Gaig C , Santamaria J , Gelpi E ((2016) ) Assessment of alpha-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: A case-control study. Lancet Neurol 15: , 708–718. |
[39] | Visanji NP , Marras C , Kern DS , Al DA , Gao A , Liu LW , Lang AE , Hazrati LN ((2015) ) Colonic mucosal a-synuclein lacks specificity as a biomarker for Parkinson disease. Neurology 84: , 609–616. |
[40] | Wang N , Gibbons CH , Lafo J , Freeman R ((2013) ) alpha-Synuclein in cutaneous autonomic nerves. Neurology 81: , 1604–1610. |
[41] | Takeda S , Yamazaki K , Miyakawa T , Arai H ((1993) ) Parkinson’s disease with involvement of the parasympathetic ganglia. Acta Neuropathol 86: , 397–398. |
[42] | Braak H , Sastre M , Bohl JR , de Vos RA , Del TK ((2007) ) Parkinson’s disease: Lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 113: , 421–429. |
[43] | Braak H , de Vos RA , Bohl J , Del TK ((2006) ) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396: , 67–72. |
[44] | Koike Y , Takahashi A ((1997) ) Autonomic dysfunction in Parkinson’s disease. Eur Neurol 38: (Suppl 2), 8–12. |
[45] | Fumimura Y , Ikemura M , Saito Y , Sengoku R , Kanemaru K , Sawabe M , Arai T , Ito G , Iwatsubo T , Fukayama M , Mizusawa H , Murayama S ((2007) ) Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in Lewy body disease. J Neuropathol Exp Neurol 66: , 354–362. |
[46] | Hishikawa N , Hashizume Y , Hirayama M , Imamura K , Washimi Y , Koike Y , Mabuchi C , Yoshida M , Sobue G ((2000) ) Brainstem-type Lewy body disease presenting with progressive autonomic failure and lethargy. Clin Auton Res 10: , 139–143. |
[47] | den Hartog Jager WA , Bethlem J ((1960) ) The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry 23: , 283–290. |
[48] | Verbaan D , Marinus J , Visser M , van Rooden SM , Stiggelbout AM , van Hilten JJ ((2007) ) Patient-reported autonomic symptoms in Parkinson disease. Neurology 69: , 333–341. |
[49] | Siddiqui MF , Rast S , Lynn MJ , Auchus AP , Pfeiffer RF ((2002) ) Autonomic dysfunction in Parkinson’s disease: A comprehensive symptom survey. Parkinsonism Relat Disord 8: , 277–284. |
[50] | Adler CH ((2005) ) Nonmotor complications in Parkinson’s disease. Mov Disord 20: (Suppl 11), S23–S29. |
[51] | Edwards LL , Quigley EM , Pfeiffer RF ((1992) ) Gastrointestinal dysfunction in Parkinson’s disease: Frequency and pathophysiology. Neurology 42: , 726–732. |
[52] | Greene JG , Noorian AR , Srinivasan S ((2009) ) Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp Neurol 218: , 154–161. |
[53] | Hopkins DA , Bieger D , deVente J , Steinbusch WM ((1996) ) Vagal efferent projections: Viscerotopy, neurochemistry and effects of vagotomy. Prog Brain Res 107: , 79–96. |
[54] | Gray MT , Munoz DG , Schlossmacher MG , Gray DA , Woulfe JM ((2015) ) Protective effect of vagotomy suggests source organ for Parkinson’s disease. Ann Neurol 78: , 834–835. |
[55] | Borghammer P , Hamani C ((2017) ) Preventing Parkinson disease by vagotomy: Fact or fiction? Neurology 88: , 1982–1983. |
[56] | Liu B , Fang F , Pedersen NL , Tillander A , Ludvigsson JF , Ekbom A , Svenningsson P , Chen H , Wirdefeldt K ((2017) ) Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology 88: , 1996–2002. |
[57] | Svensson E , Horvath-Puho E , Thomsen RW , Djurhuus JC , Pedersen L , Borghammer P , Sorensen HT ((2015) ) Does vagotomy reduce the risk of Parkinson’s disease: The authors reply. Ann Neurol 78: , 1012–1013. |
[58] | Svensson E , Horvath-Puho E , Thomsen RW , Djurhuus JC , Pedersen L , Borghammer P , Sorensen HT ((2015) ) Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78: , 522–529. |
[59] | Tysnes OB , Kenborg L , Herlofson K , Steding-Jessen M , Horn A , Olsen JH , Reichmann H ((2015) ) Does vagotomy reduce the risk of Parkinson’s disease? Ann Neurol 78: , 1011–1012. |
[60] | Van Den Berge N , Ferreira N , Gram H , Mikkelsen TW , Alstrup AKO , Casadei N , Tsung-Pin P , Riess O , Nyengaard JR , Tamguney G , Jensen PH , Borghammer P ((2019) ) Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol 138: , 535–550. |
[61] | Arotcarena ML , Dovero S , Prigent A , Bourdenx M , Camus S , Porras G , Thiolat ML , Tasselli M , Aubert P , Kruse N , Mollenhauer B , Trigo D, I , Estrada C , Garcia-Carrillo N , Vaikath NN , El-Agnaf OMA , Herrero MT , Vila M , Obeso JA , Derkinderen P , Dehay B , Bezard E ((2020) ) Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain 143: , 1462–1475. |
[62] | Leclair-Visonneau L , Neunlist M , Derkinderen P , Lebouvier T ((2020) ) The gut in Parkinson’s disease: Bottom-up, top-down, or neither? Neurogastroenterol Motil 32: , e13777- |
[63] | Beach TG , Adler CH , Sue LI , Peirce JB , Bachalakuri J , Dalsing-Hernandez JE , Lue LF , Caviness JN , Connor DJ , Sabbagh MN , Walker DG ((2008) ) Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol 115: , 445–451. |
[64] | Delledonne A , Klos KJ , Fujishiro H , Ahmed Z , Parisi JE , Josephs KA , Frigerio R , Burnett M , Wszolek ZK , Uitti RJ , Ahlskog JE , Dickson DW ((2008) ) Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol 65: , 1074–1080. |
[65] | Dickson DW , Fujishiro H , Delledonne A , Menke J , Ahmed Z , Klos KJ , Josephs KA , Frigerio R , Burnett M , Parisi JE , Ahlskog JE ((2008) ) Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol 115: , 437–444. |
[66] | Beach TG , Adler CH , Sue LI , Serrano G , Shill HA , Walker DG , Lue L , Roher AE , Dugger BN , Maarouf C , Birdsill AC , Intorcia A , Saxon-Labelle M , Pullen J , Scroggins A , Filon J , Scott S , Hoffman B , Garcia A , Caviness JN , Hentz JG , Driver-Dunckley E , Jacobson SA , Davis KJ , Belden CM , Long KE , Malek-Ahmadi M , Powell JJ , Gale LD , Nicholson LR , Caselli RJ , Woodruff BK , Rapscak SZ , Ahern GL , Shi J , Burke AD , Reiman EM , Sabbagh MN ((2015) ) Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology 35: , 354–389. |
[67] | Beach TG , Serrano GE , Kremer T , Canamero M , Dziadek S , Sade H , Derkinderen P , Corbille AG , Letournel F , Munoz DG , White III CL , Schneider J , Crary JF , Sue LI , Adler CH , Glass MJ , Intorcia AJ , Walker JE , Foroud T , Coffey CS , Ecklund D , Riss H , Gossmann J , Konig F , Kopil CM , Arnedo V , Riley L , Linder C , Dave KD , Jennings D , Seibyl J , Mollenhauer B , Chahine L ((2018) ) Immunohistochemical method and histopathology judging for the Systemic Synuclein Sampling Study (S4). J Neuropathol Exp Neurol 77: , 793–802. |
[68] | Beach TG , White CL , Hamilton RL , Duda JE , Iwatsubo T , Dickson DW , Roncaroli F , Buttini M , Hladik CL , Sue LI , Noorigian JV , Adler CH ((2008) ) Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol 116: , 277–288. |
[69] | Corbille AG , Letournel F , Kordower JH , Lee J , Shanes E , Neunlist M , Munoz DG , Derkinderen P , Beach TG ((2016) ) Evaluation of alpha-synuclein immunohistochemical methods for the detection of Lewy-type synucleinopathy in gastrointestinal biopsies. Acta Neuropathol Commun 4: , 35. |
[70] | Saito Y , Kawashima A , Ruberu NN , Fujiwara H , Koyama S , Sawabe M , Arai T , Nagura H , Yamanouchi H , Hasegawa M , Iwatsubo T , Murayama S ((2003) ) Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol 62: , 644–654. |
[71] | Fujiwara H , Hasegawa M , Dohmae N , Kawashima A , Masliah E , Goldberg MS , Shen J , Takio K , Iwatsubo T ((2002) ) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4: , 160–164. |
[72] | Obi K , Akiyama H , Kondo H , Shimomura Y , Hasegawa M , Iwatsubo T , Mizuno Y , Mochizuki H ((2008) ) Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol 210: , 409–420. |
[73] | Lue LF , Walker DG , Adler CH , Shill H , Tran H , Akiyama H , Sue LI , Caviness J , Sabbagh MN , Beach TG ((2012) ) Biochemical increase in phosphorylated alpha-synuclein precedes histopathology of Lewy-type synucleinopathies. Brain Pathol 22: , 745–756. |
[74] | Walker DG , Lue LF , Adler CH , Shill HA , Caviness JN , Sabbagh MN , Akiyama H , Serrano GE , Sue LI , Beach TG ((2013) ) Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol 240: , 190–204. |
[75] | Beach TG , Adler CH ((2018) ) Importance of low diagnostic accuracy for early Parkinson’s disease. Mov Disord 33: , 1551–1554. |
[76] | Beach TG , Adler CH , Dugger BN , Serrano G , Hidalgo J , Henry-Watson J , Shill HA , Sue LI , Sabbagh MN , Akiyama H ((2013) ) Submandibular gland biopsy for the diagnosis of Parkinson disease. J Neuropathol Exp Neurol 72: , 130–136. |
[77] | Ornelas AS , Adler CH , Serrano GE , Curry JR , Shill HA , Kopyov O , Beach TG ((2020) ) Co-Existence of tau and α-synuclein pathology in fetal graft tissue at autopsy: A case report. Parkinsonism Relat Disord 71: , 36–39. |
[78] | Chahine LM , Beach TG , Brumm MC , Adler CH , Coffey CS , Mosovsky S , Caspell-Garcia C , Serrano GE , Munoz DG , White III CL , Crary JF , Jennings D , Taylor P , Foroud T , Arnedo V , Kopil CM , Riley L , Dave KD , Mollenhauer B ((2020) ) In vivo distribution of alpha-synuclein in multiple tissues and biofluids in Parkinson disease. Neurology 95: , e1267–e1284. |
[79] | Langston JW ((2006) ) The Parkinson’s complex: Parkinsonism is just the tip of the iceberg. Ann Neurol 59: , 591–596. |
[80] | Braak H , Rub U , Gai WP , Del TK ((2003) ) Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110: , 517–536. |
[81] | Hawkes CH , Del TK , Braak H ((2007) ) Parkinson’s disease: A dual-hit hypothesis. Neuropathol Appl Neurobiol 33: , 599–614. |
[82] | Kaufmann H , Nahm K , Purohit D , Wolfe D ((2004) ) Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology 63: , 1093–1095. |
[83] | Petrovitch H , Abbott RD , Ross GW , Nelson J , Masaki KH , Tanner CM , Launer LJ , White LR ((2009) ) Bowel movement frequency in late-life and substantia nigra neuron density at death. Mov Disord 24: , 371–376. |
[84] | Abbott RD , Petrovitch H , White LR , Masaki KH , Tanner CM , Curb JD , Grandinetti A , Blanchette PL , Popper JS , Ross GW ((2001) ) Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57: , 456–462. |
[85] | Pfeiffer RF ((2003) ) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 2: , 107–116. |
[86] | Miki Y , Mori F , Wakabayashi K , Kuroda N , Orimo S ((2009) ) Incidental Lewy body disease restricted to the heart and stellate ganglia. Mov Disord 24: , 2299–2301. |
[87] | Zhang J , Li X , Li JD ((2019) ) The roles of post-translational modifications on alpha-synuclein in the pathogenesis of Parkinson’s Diseases (2019). Front Neurosci 13: , 381. |
[88] | Sorrentino ZA , Giasson BI ((2020) ) The emerging role of alpha-synuclein truncation in aggregation and disease. J Biol Chem 295: , 10224–10244. |
[89] | Ahn EH , Kang SS , Liu X , Chen G , Zhang Z , Chandrasekharan B , Alam AM , Neish AS , Cao X , Ye K ((2020) ) Initiation of Parkinson’s disease from gut to brain by delta-secretase. Cell Res 30: , 70–87. |
[90] | Prasad K , Beach TG , Hedreen J , Richfield EK ((2012) ) Critical role of truncated alpha-synuclein and aggregates in Parkinson’s disease and incidental Lewy body disease. Brain Pathol 22: , 811–825. |
[91] | Bourdenx M , Nioche A , Dovero S , Arotcarena ML , Camus S , Porras G , Thiolat ML , Rougier NP , Prigent A , Aubert P , Bohic S , Sandt C , Laferriere F , Doudnikoff E , Kruse N , Mollenhauer B , Novello S , Morari M , Leste-Lasserre T , Damas IT , Goillandeau M , Perier C , Estrada C , Garcia-Carrillo N , Recasens A , Vaikath NN , El-Agnaf OMA , Herrero MT , Derkinderen P , Vila M , Obeso JA , Dehay B , Bezard E ((2020) ) Identification of distinct pathological signatures induced by patient-derived alpha-synuclein structures in nonhuman primates. Sci Adv 6: , eaaz9165. |
[92] | Resnikoff H , Metzger JM , Lopez M , Bondarenko V , Mejia A , Simmons HA , Emborg ME ((2019) ) Colonic inflammation affects myenteric alpha-synuclein in nonhuman primates. J Inflamm Res 12: , 113–126. |
[93] | Devos D , Lebouvier T , Lardeux B , Biraud M , Rouaud T , Pouclet H , Coron E , Bruley d, V , Naveilhan P , Nguyen JM , Neunlist M , Derkinderen P ((2013) ) Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50: , 42–48. |
[94] | Rolli-Derkinderen M , Leclair-Visonneau L , Bourreille A , Coron E , Neunlist M , Derkinderen P ((2020) ) Is Parkinson’s disease a chronic low-grade inflammatory bowel disease? J Neurol 267: , 2207–2213. |
[95] | Manne S , Kondru N , Jin H , Serrano GE , Anantharam V , Kanthasamy A , Adler CH , Beach TG , Kanthasamy AG ((2020) ) Blinded RT-QuIC analysis of α-synuclein biomarker in skin tissue from Parkinson’s disease patients. Mov Disord 35: , 2230–2239. |
[96] | Fenyi A , Duyckaerts C , Bousset L , Braak H , Del Tredici K , Melki R ((2021) ) Seeding propensity and characteristics of pathogenic alphaSyn assemblies in formalin-fixed human tissue from the enteric nervous system, olfactory bulb, and brainstem in cases staged for Parkinson’s disease. Cells 10: , 139. |
[97] | Fenyi A , Leclair-Visonneau L , Clairembault T , Coron E , Neunlist M , Melki R , Derkinderen P , Bousset L ((2019) ) Detection of alpha-synuclein aggregates in gastrointestinal biopsies by protein misfolding cyclic amplification. Neurobiol Dis 129: , 38–43. |
[98] | Donadio V , Wang Z , Incensi A , Rizzo G , Fileccia E , Vacchiano V , Capellari S , Magnani M , Scaglione C , Maserati M , Avoni P , Liguori R , Zou W ((2021) ) In vivo diagnosis of synucleinopathies: A comparative study of skin biopsy and RT-QuIC. Neurology 96: , e2513–e2524. |
[99] | Beach TG , White CL , 3rd, Hladik CL , Sabbagh MN , Connor DJ , Shill HA , Sue LI , Sasse J , Bachalakuri J , Henry-Watson J , Akiyama H , Adler CH ((2009) ) Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol 117: , 169–174. |
[100] | Borghammer P , Van Den Berge N ((2019) ) Brain-first versus gut-first Parkinson’s disease: A hypothesis. J Parkinsons Dis 9: , S281–S295. |
[101] | Hallett PJ , Engelender S , Isacson O ((2019) ) Lipid and immune abnormalities causing age-dependent neurodegeneration and Parkinson’s disease. J Neuroinflammation 16: , 153. |
[102] | Adler CH , Serrano GE , Zhang N , Hinni, ML , Lott, DG , Mehta, SH , Sue, LI , Intorcia, A , Beach, TG , ((2019) ) Feasibility of repeat and bilateral submandibular gland needle biopsies in Parkinson’s disease. Parkinsonism Relat Disord 68: , 69–72. |
[103] | Stokholm MG , Danielsen EH , Hamilton-Dutoit SJ , Borghammer P ((2106) ) Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol 79: , 940–949. |
[104] | Killinger BA , Madaj Z , Sikora JW , Rey N , Haas AJ , Vepa Y , Lindqvist D , Chen H , Thomas PM , Brundin P , Brundin L , Labrie V ((2018) ) The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci Transl Med 10: , eaar5280. |
[105] | Stolzenberg E , Berry D , Yang D , Lee EY , Kroemer A , Kaufman S , Wong GCL , Oppenheim JJ , Sen S , Fishbein T , Bax A , Harris B , Barbut D , Zasloff MA ((2017) ) A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun 9: , 456–463. |




