Detection of Dermal Alpha-Synuclein Deposits as a Biomarker for Parkinson’s Disease
Abstract
Alpha-synuclein deposits are detectable in skin biopsies of patients with Parkinson’s disease and other synucleinopathies like multiple system atrophy by immunohistochemical staining. As they are easily to obtain, they appear a promising tool for the pre-mortem histopathological confirmation of the disease and as a potential outcome measure in studies targeting alpha-synuclein aggregates. Good sensitivity, specificity, and practicability are the most important requirements of a biomarker. The review gives an overview on all three aspects, addresses methodological problems and the lack of standardized procedures as a major problem and gives an outlook on the future of skin biopsy as a potential diagnostic tool in synucleinopathies.
INTRODUCTION
Diagnosis of idiopathic Parkinson’s disease (iPD) is often difficult, especially at early stages of disease. Diagnosis is mostly based on clinical symptoms, comprising the motor symptoms tremor, rigor, akinesia and postural instability [1]. Research in the last decade has focused on the search of a feasible biomarker for iPD that allows a reliable diagnosis of the disease. As iPD has proven to be a multisystemic neurological disease with not only involvement of the brain but also neurons and nerve fibers of the peripheral nervous system, the latter has come into focus as it is easily accessible pre-mortem. Since the detection of Lewy bodies in neurons of iPD patients by F. H. Lewy in 1912 [2], this has been the gold standard of post-mortem neuropathological confirmation of the disease. In 1997, Spillantini et al. identified aggregated alpha-synuclein to be the major component of Lewy bodies [3]. Since then, immunohistochemical detection of alpha-synuclein deposits has been the neuropathological hallmark of iPD. Only in the last decades, alpha-synuclein deposits were detected in neurons and neurites of the peripheral nervous system (Fig. 1), rendering the detection of alpha-synuclein aggregates in tissues of the PNS a potential pre-mortem biomarker of the disease [4]. Alpha-synuclein deposits have so far been detected in nerve fibers of the enteric nervous system, submandibular gland and skin biopsies [5–14]. Studies detecting phosphorylated alpha-synuclein (p-alpha-syn) in biopsies of salivary glands of patients with iPD reported high specificity (100%) and sensitivity (56–100%) in submandibular glands [9, 15–17]. A relevant number of unilateral biopsies of the submandibular gland did not contain salivary gland tissue [16] but better tissue acquisition was achieved in bilateral submandibular biopsies or in biopsies of minor salivary glands [18, 19]. Studies on biopsies of gastrointestinal tissue included different biopsy sites and sensitivity and specificity very much varied between studies [5, 6, 20–23]. Skin biopsy seems to be a feasible tool as it is minimally-invasive, repeatable and the procedure does not require any special expertise. P-alpha-syn, that in contrast to native alpha-synuclein is highly specific for synucleinopathies, can be detected by immunohistochemical staining of skin sections [13, 14]. Even though the potential use of skin biopsy as a histopathological biomarker has been addressed in an increasing number of studies, it is still not an established tool in the diagnosis of iPD. In this review, four basic requirements of a biomarker in PD are addressed: Sensitivity, specificity, practicability and detection of prodromal stages of disease.
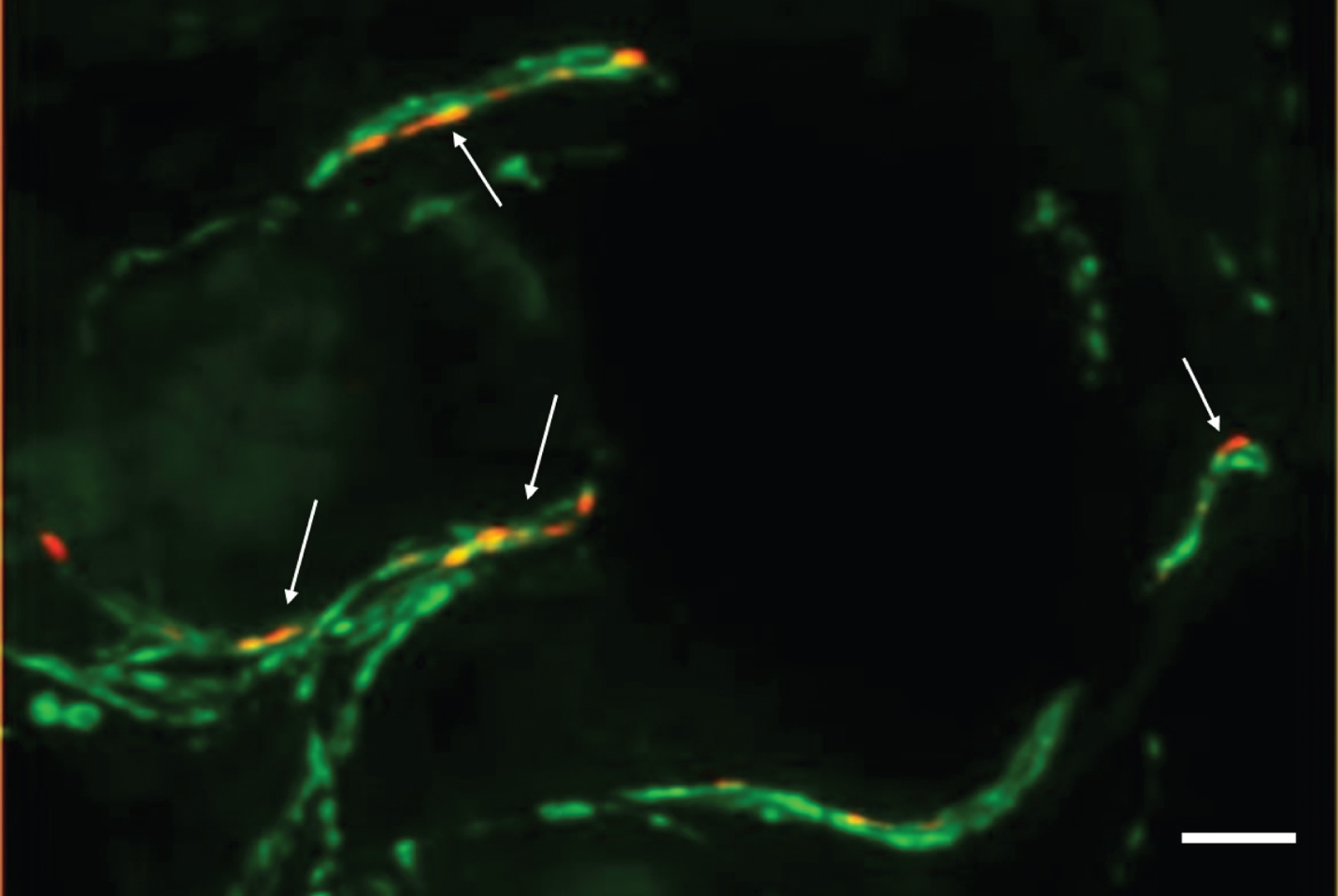
Fig. 1
Photomicrograph of a double-immunofluorescence staining with anti-phospho-alpha-synuclein (red, Covance, 1:500) and anti-protein-gene-product 9.5 (axonal marker, green, Zytomed Systems, 1:1000) of a skin biopsy of a patient with iPD. The biopsy was fixed with 4%paraformaldehyde prior to cryopreservation and 20-μm-sections were cut. Arrows indicate intraaxonal phospho-alpha-synuclein depositions. In samples of controls, no p-alpha-syn-positive depositions would be visible. Bar = 20μm.
METHODS
Pubmed was searched for the terms “skin biopsy”, “alpha-synuclein” and “Parkinson”/”RBD” and the results were checked for relevance. All pre-mortem studies on patients with Parkinson’s disease and/or iRBD that analyzed alpha-synuclein deposition within dermal nerve fibers were included.
Is dermal p-alpha-syn deposition a sensitive marker of PD?
First promising results on the use of p-alpha-syn detection in skin biopsies as a biomarker were obtained by Ikemura et al. when studying autopsy samples with a sensitivity of 70%for iPD and specificity of 100%compared to patients without CNS Lewy body pathology [12]. However, a subsequent analysis of pre-mortem biopsy samples of PD patients only resulted in a low sensitivity of 10%[11]. Only in the last decade, further studies on cutaneous p-alpha-syn deposition with a high specificity of 100%compared to controls and a sensitivity of 55%to 100%were published [13, 14, 24, 25] (Table 1). Besides these studies with a moderate to high sensitivity of dermal p-alpha-syn detection there were also studies that did not succeed in detection of p-alpha-syn in skin biopsies or only with a very low sensitivity [26, 27] (Table 1). The use of different protocols of biopsy procedure, fixation, immunostaining and neuropathological assessment were discussed as possible reasons, disclosing the need of methodological studies comparing different protocols [25, 28, 29]. Whereas inter- and intra-laboratory reproducibility of analysis of skin sections was successfully demonstrated [30], studies comparing fixation and staining procedures are scarce [25, 29]. Recently, the Systemic Synuclein Sampling Study (S4), a multi-center observational study compared different staining procedures on paraffin-embedded tissue resulting in the establishment of an optimized protocol for paraffin sections [31]. They reported a sensitivity of 24.1%for skin biopsies that is much lower compared to former studies [32]. However, when taking a closer look at published studies (Table 1), the highest sensitivity was mostly reported in studies using cryosections. Unfortunately, systematic comparison of biopsy procedures in the S4 study was restricted to formalin-fixed paraffin-embedded tissue that indeed appears to be more feasible in clinical practice [31], but may result in lower sensitivity (Table 1). Although systematic comparison of formalin-fixed paraffin-embedded and freshly-fixed cryoconserved tissue is lacking, it becomes evident that in the majority of studies reporting a moderate to high sensitivity, skin biopsies were cryoconserved following the recommendations that were long ago established for the use of skin biopsies in the diagnosis of peripheral neuropathies [33, 34] and that are based on comparison of different fixatives for neuronal markers in skin biopsies and experience from studies establishing skin biopsy as a diagnostic tool for small fiber neuropathy [35, 36]. Furthermore, cryosections with a thickness of 10–50μm allow the analysis of nerve fibers in their length whereas paraffin sections with a thickness of 2–5μm only contain few fibers that cannot be studied in length and make it difficult to discriminate between specific staining and artefacts [37]. A very recent study focusing on optimal thickness of sample sections of skin biopsies for detection of dermal p-alpha-syn deposits confirmed these observations [38]. However, a very recent study reported a higher sensitivity of 70%in iPD subjects by using double-staining of p-alpha-syn and PGP9.5, an axonal marker of formalin-fixed-paraffin-embedded tissue, demonstrating that a moderate to high sensitivity can also be obtained when using paraffin sections [39]. Double-staining with an axonal marker, pretreatment of sections with protease and alkaline phosphatase and evaluation of additional sections in subjects with a low number of PGP9.5-positive nerve fibers were discussed as relevant points to increase sensitivity, but again direct comparison with other protocols is lacking [39]. Another aspect that may have a direct effect on sensitivity is the choice of the best site of biopsy. Different biopsy sites were studied (see Table 1) and paravertebral (mostly C7/8) sites and the leg are the biopsy sites that were most often chosen. However, large studies systematically comparing different biopsy sites are lacking. There is some evidence of a proximal-to-distal gradient of p-alpha-syn positivity in patients with iPD [13, 14, 40]. A recent study reported a distal-to-proximal gradient in patients with MSA whereas in an earlier study the proximal leg was mostly affected in patients with MSA [40, 41]. Both studies suggest that the distribution of p-alpha-syn in MSA may be different to iPD but larger studies are needed.
Table 1
Overview on studies on the detection of alpha-synuclein in dermal nerve fibers of patients with synucleinopathies by immunohistochemistry or RT-QuIC using skin biopsies of living subjects
| Authors | subjects | Biopsy diameter/sites | staining | Section thickness/number | fixation | embedding | Sens. (%) | Spec. (%) | |
| Miki et al., 2010 [11] | 20 PD | 6 mm, chest wall, lower limb | Immunostaining, p-alpha-syn Ser129 | 6μm, 3 | formalin | paraffin | 10 | n/a | |
| Wang et al., 2013 [42] | 20 PD, 14 HC | 3 mm, distal leg, proximal, distal thigh | Double-immunofluorescence, n-alpha-syn | 50μm, 20 | Zamboni | cryo | n/a | n/a | |
| Doppler et al., 2014 [14] | 31 PD, 35 HC | 5 mm, distal, proximal leg, Th12, finger | Double-immunofluorescence, p-alpha-syn Ser129 | 20μm, 3 (steps) | PFA 4% | cryo | 51.6 | 100 | |
| Donadio et al., 2014 [13] | 20 PD, 20 other parkinsonism, 20 HC | 3 mm, C8, thigh, distal leg (two biopsies each site) | Double-immunofluorescence, p-alpha-syn Ser129 | 10μm, 4 | Zamboni | cryo | 100 | 100 | |
| Navarro-Otano et al., 2015 [26] | 6 PD, 6 HC | 3 mm, distal leg | Immunostaining, n- and p-alpha-syn Ser129 | 5μm | formalin | paraffin | 0 | 0 | |
| Doppler et al., 2015 [41] | 30 PD, 12 MSA, 15 tauopathies, 39 HC | 5 mm, distal, proximal leg, Th12 | Double-immunofluorescence, p-alpha-syn Ser129 | 20μm, 5 (serial) | PFA 4% | cryo | 75 MSA, 73 PD | 100 | |
| Zange et al., 2015 [59] | 10 PD, 10 MSA, 6 ED | 3 mm, forearm | Immunostaining, p-alpha-syn Ser129 | 3μm, serial | formaldehyde | paraffin | 0 MSA, 100 PD (non-blinded evaluation, only autonomic fibers analyzed) | 100 | |
| Haga et al., 2015 [27] | 38 PD, 13 MSA | 6 mm, chest wall, lower limb | Double-immunofluorescence, p-alpha-syn Ser129 | 60μm, 4 | cryo | 5.3 PD, 0 MSA | n/a | ||
| Donadio et al., 2016 [24] | 16 PD, 14 PAF | 3 mm, C8, thigh, distal leg (two biopsies each site) | Double-immunofluorescence, p-alpha-syn Ser129, n-alpha-syn | 10μm, 4 | Zamboni | cryo | p-alpha-syn: 100 PD and PAF, n-alpha-syn: 100 | p-alpha-syn: 100, n-alpha-syn: 0 | |
| Doppler et al., 2017 [49] | 18 iRBD, 25 PD, 20 HC | 5 mm, distal, proximal leg, Th12, C7 | Double-immunofluorescence, p-alpha-syn Ser129 | 20μm, 5 (serial) | PFA 4% | cryo | iRBD: 55.6, PD: 80 | 100 | |
| Antelmi et al., 2017 [50] | 12 iRBD, 55 HC | 3 mm, C7 and leg (2x) | Double-immunofluorescence, p-alpha-syn Ser129 | 10μm, 4 | Zamboni | cryo | iRBD: 75 | 100 | |
| Donadio et al., 2017 [60] | 28 PD | 3 mm, C7 (2x) or C7 and Th12 | Double-immunofluorescence, p-alpha-syn Ser129 | 10μm, 4 | Zamboni | cryo | 100 | 100 | |
| Donadio et al., 2017 [46] | 18 DLB, 23 other dementia, 25 HC | 3 mm, C8, thigh, distal leg (two biopsies each site) | Double-immunofluorescence, p-alpha-syn Ser129 | 10μm, 4 | Zamboni | cryo | 100 | p-alpha-syn: 100, n-alpha-syn: | |
| Melli et al., 2018 [44] | 19 PD, 13 other parkinsonism, 17 HC | 3 mm, C8, thigh, distal leg (two per site) | Double-immunofluorescence, p-alpha-syn, aggregated alpha-syn 5G4, free-floating | 50μm, 8/biopsy | PLP2% | Cryo | p-alpha-syn: 56, 5G4:81 | p-alpha-syn: 100, 5G4:96 | |
| Doppler et al., 2018 [61] | 10 PD-GBA | 5 mm, distal and proximal leg, Th10, C7 | Double-immunofluorescence, p-alpha-syn | 20μm | PFA | cryo | 60 | 100 | |
| Donadio et al., 2018 [40] | 15 PD, 12 DLB, 5 PAF, 12 MSA, 10 HC | 3 mm, C7, distal and proximal leg (two from each site) | Double-immunofluorescence, native alpha-syn, p-alpha-syn Ser129, other posttranslational modifications | 10μm | PFA | cryo | p-syn: 100 for PD, DLB, PAF, 75 for MSA | 100 | |
| Kuzkina et al., 2019 [43] | 27 PD, 9 MSA, 21 HC | 5 mm, distal and proximal leg, Th10, C7 | Double-immunofluorescence, p-alpha-syn, truncated alpha-syn, aggregated alpha-syn (5G4) | 20μm | PFA | cryo | p-alpha-syn: 82, others lower (but stored sections) | 100 | |
| Antelmi et al., 2019 [62] | 30 iRBD, 17 RBD with narcolepsy | 3 mm, C7, leg (two from each site) | Double-immunofluorescence, p-alpha-syn Ser129 | 10μm | Zamboni | cryo | iRBD: 86.7 | 100 (compared to narcolepsy) | |
| Carmona-Abellan et al., 2020 [63] | 7 E46K-SNCA carrier (3 DLB, 2 PAF, 1 PD, 1 asymptomatic) 2 Park2, 2 HC | 4 mm, C7 | Immunostaining, p-alpha-Syn Ser129 | 5μm | formalin | paraffin | E46K-SNCA: 100, Park2:50 | 0 | |
| Donadio et al., 2020 [64] | 25 PD + OD, 25MSA | 3 mm, C7, thigh, leg (two from each site) | Double-immunofluorescence, p-alpha-syn Ser129 | 10μm | Zamboni | cryo | MSA: 72, PD + OD: 100 | n/a | |
| Giannoccaro et al., 2020 [65] | 7 DLB, 21 PD, 13 PAF, 13 MSA | C7, thigh, distal leg | Double-immunofluorescence, p-alpha-syn Ser129 | 10μm | Zamboni | cryo | 100 (DLB, PAF), 95.2 (PD), 69.2 (MSA) | n/a | (Continued) |
| Liu et al., 2020 [25] | 90 PD, 30 HC | C7, thigh, distal leg, forearm, 3 mm | Double-immunofluorescence, p-alpha-syn Ser129 | 15 and 50μm, 4 | Zamboni | cryo | 83.3 | 100 | |
| Wang et al., 2020 [38] | 29 PD, 21 HC | 3 mm, distal leg, distal and proximal thigh | Double-immunofluorescence, conventional for 10/20μm, free-floating for 50μm, p-alpha-syn Ser129 | 10, 20, 50μm, 5 | Zamboni | cryo | 50μm: 100, 20μm: 90, 10μm 73 | 100 | |
| Chahine et al., 2020 [32] | 58 PD, 21 HC | 3 mm, cervical, mid-thigh (2x) | Immunostaining, n-alpha-syn with Proteinase K | 4μm, mean 5.9 | formalin | paraffin | 24 | 100 | |
| Al-Qassabi et al., 2020 [39] | 28 iRBD, 20 PD, 10 atypical parkinsonism, 21 HC | 3 mm, C8 | Double-immunofluorescence, p-alpha-syn Ser129 | 4μm, 8 (step sections, two blocks) | formalin | paraffin | 82 iRBD, 70 PD, 20 atypical parkinsonism | 100 | |
| Wang et al., 2020 [58] | 20 PD, 21 HC (+autopsy samples) | 3–5 mm, leg or cervical | RT-QuIC, PMCA analysis | lysate | none | n/a | RT-QuIC: 95, PMCA: 80 | RT-QuIC: 100, PMCA: 90 |
Double-immunofluorescence=double-labeling with an axonal marker (mostly PGP9.5). (Sens., sensitivity; Spec., specificity; PD, Parkinson’s disease; HC, healthy control; n-alpha-syn, native alpha-synuclein; p-alpha-syn, phosphorylated alpha-synuclein; MSA, multiple system atrophy; ET, essential tremor; PAF, pure autonomic failure; (i) iRBD, isolated REM sleep behavior disorder; PFA, paraformaldehyde.
Is dermal p-alpha-syn deposition a specific marker of PD?
Studies using antibodies that specifically recognize p-alpha-syn unequivocally reported a specificity of 100%compared to controls [13, 14, 32]. Diffuse or granular p-alpha-syn staining that could be removed by pre-treatment with alkaline phosphatase and protease was also reported in controls, but discrete p-alpha-syn was restricted to patient samples [39]. Using an antibody against native alpha-synuclein, Wang et al. reported higher immunoreactivity in skin biopsies of patients with iPD compared to controls, but native alpha-synuclein was also detectable in dermal nerve fibers of healthy subjects [42]. Native alpha-synuclein was equally detected in dermal ann-exes’ innervation of patients with synucleinopathies and controls in other studies [24, 40]. In contrast, ant-ibodies specifically directed against aggregated alp-ha-synuclein (5G4) or the use of protein K digestion have also been described to allow differentiation between biopsies of patients with iPD and controls [31, 43, 44]. However, dermal p-alpha-syn deposition is not a specific marker of iPD but was also described in other synucleinopathies: In multiple system atrophy, p-alpha-syn was predominantly detected in somatosensory nerve fibers, in contrast to iPD where mainly autonomic fibers are affected [40, 41]. In pure autonomic failure and dementia with Lewy bodies, p-alpha-syn can also frequently be found in dermal autonomic nerve fibers [24, 45, 46].
Can dermal p-alpha-syn deposition detect prodromal stages of disease?
Prodromal stages of iPD are difficult to diagnose as there are no specific markers. Studies on prodromal stages of iPD often focused on isolated REM sleep behavior disorder (iRBD) which is one of the most specific early symptoms of a synucleinopathy with a conversion rate of more than 80%[47, 48]. Dermal p-alpha-syn could be detected in patients with iRBD and p-alpha-syn deposition correlated with other markers of prodromal PD like olfactory dysfunction, reduced dopamine transporter density measured by FP-CIT-SPECT or the likelihood ratio of prodromal PD based on the MDS criteria [49–51]. These findings give evidence that dermal p-alpha-syn deposition may be a marker of disease progression at prodromal stages. P-alpha-syn deposition was detectable in patients with normal FP-CIT-SPECT, thus indicating that it probably is a very early marker of a synucleinopathy [49, 50]. However, it needs to be taken into account that these data are based on patients with iRBD only. Studies focusing on dermal p-alpha-syn in iRBD-negative prodromal stages are needed but so far lacking because prodromal stages are difficult to diagnose.
Is the use of skin biopsy a feasible tool for the diagnosis of PD?
Practicability of skin biopsy for the diagnosis of iPD is frequently discussed. It is cheaper and more broadly available compared to FP-CIT-SPECT. When comparing it with biopsies of gastrointestinal tissue that require endoscopic procedures or biopsies of the submandibular gland it is more easily to perform and skin biopsy is mostly well-tolerated by the patients [52]. However, processing and cryoconservation of biopsies requires laboratory equipment. Due to the low number of p-alpha-syn deposition, step sections and/or analysis of multiple biopsy sites are necessary and are rather time-consuming. Evaluation of skin sections at the microscope needs to be done by experienced examiners and is also time-consuming [31]. For these reasons, high-throughput biochemical procedures that allow objective and repeatable measurement of a large number of samples in a short time are needed. The most promising approach at the moment is RT-QuIC, an aggregation assay that was developed for the detection of prions in Creutzfeldt-Jakob disease [53]. As prion-like seeding activity can also be found in alpha-synuclein, small amounts of dermal alpha-synuclein become detectable by RT-QuIC [54]. Several studies detecting alpha-synuclein aggregates in cerebrospinal fluid of patients with iPD, dementia with Lewy bodies and iRBD showed promising results [54–56] and the first studies using RT-QuIC in skin lysates seem promising [57, 58]. However, larger studies including prodromal stages of iPD are needed to evaluate the use of dermal RT-QuIC as an early biomarker in iPD.
Where will the way of skin biopsy lead us?
More than ten years have passed since detection of p-alpha-syn in skin biopsies of patients with iPD. Numerous studies have confirmed the involvement of autonomic nerve fibers in alpha-synuclein pathology and improved protocols allow to detect p-alpha-syn with very good specificity and moderate to high sensitivity even at prodromal stages of disease [13, 14, 49, 50]. Nevertheless, skin biopsy is far from being an established diagnostic tool in PD. Immunofluorescence staining of serial section and the need of multiple biopsy sites to obtain a good sensitivity seem to be the major difficulties that hinder skin biopsy from getting an established biomarker in clinical routine. Most recently, RT-QuIC, a biochemical aggregation-based assay was studied as an efficient and more feasible method for the analysis of alpha-synuclein deposition in skin biopsies but also other tissues including CSF [54–58]. At the moment, RT QuIC appears a promising methodological approach for the detection of alpha-synuclein aggregates outside the brain but is still at an early stage of research. Future studies need to specify its sensitivity, specificity and reproducibility. Different tissues and biofluids need to be studied in comparison by immunohistochemistry as well as RT-QuIC to find the most appropriate biomarker for the future.
CONFLICT OF INTEREST
The author reports no conflicts of interest.
REFERENCES
[1] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[2] | Lewy FH ed. ((1912) ) agitans. I. Pathologische Anatomie. Lewandowsky’s Handbuch der Neurologie, Springer, Berlin. |
[3] | Spillantini MG , Schmidt ML , Lee VM , Trojanowski JQ , Jakes R , Goedert M ((1997) ) Alpha-synuclein in Lewy bodies. Nature 388: , 839–840. |
[4] | Beach TG , Adler CH , Sue LI , Vedders L , Lue L , White Iii CL , Akiyama H , Caviness JN , Shill HA , Sabbagh MN , Walker DG ((2010) ) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119: , 689–702. |
[5] | Shannon KM , Keshavarzian A , Mutlu E , Dodiya HB , Daian D , Jaglin JA , Kordower JH ((2012) ) Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord 27: , 709–715. |
[6] | Lebouvier T , Neunlist M , Bruley des Varannes S , Coron E , Drouard A , N’Guyen JM , Chaumette T , Tasselli M , Paillusson S , Flamand M , Galmiche JP , Damier P , Derkinderen P ((2010) ) Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One 5: , e12728. |
[7] | Braak H , de Vos RA , Bohl J , Del Tredici K ((2006) ) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396: , 67–72. |
[8] | Del Tredici K , Hawkes CH , Ghebremedhin E , Braak H ((2010) ) Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol 119: , 703–713. |
[9] | Adler CH , Dugger BN , Hentz JG , Hinni ML , Lott DG , Driver-Dunckley E , Mehta S , Serrano G , Sue LI , Duffy A , Intorcia A , Filon J , Pullen J , Walker DG , Beach TG ((2016) ) Peripheral synucleinopathy in early Parkinson’s disease: Submandibular gland needle biopsy findings. Mov Disord 31: , 250–256. |
[10] | Beach TG , Adler CH , Dugger BN , Serrano G , Hidalgo J , Henry-Watson J , Shill HA , Sue LI , Sabbagh MN , Akiyama H ((2013) ) Submandibular gland biopsy for the diagnosis of Parkinson disease. J Neuropathol Exp Neurol 72: , 130–136. |
[11] | Miki Y , Tomiyama M , Ueno T , Haga R , Nishijima H , Suzuki C , Mori F , Kaimori M , Baba M , Wakabayashi K ((2010) ) Clinical availability of skin biopsy in the diagnosis of Parkinson’s disease. Neurosci Lett 469: , 357–359. |
[12] | Ikemura M , Saito Y , Sengoku R , Sakiyama Y , Hatsuta H , Kanemaru K , Sawabe M , Arai T , Ito G , Iwatsubo T , Fukayama M , Murayama S ((2008) ) Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol 67: , 945–953. |
[13] | Donadio V , Incensi A , Leta V , Giannoccaro MP , Scaglione C , Martinelli P , Capellari S , Avoni P , Baruzzi A , Liguori R ((2014) ) Skin nerve alpha-synuclein deposits: A biomarker for idiopathic Parkinson disease. Neurology 82: , 1362–1369. |
[14] | Doppler K , Ebert S , Uceyler N , Trenkwalder C , Ebentheuer J , Volkmann J , Sommer C ((2014) ) Cutaneous neuropathy in Parkinson’s disease: A window into brain pathology. Acta Neuropathol 128: , 99–109. |
[15] | Adler CH , Dugger BN , Hinni ML , Lott DG , Driver-Dunckley E , Hidalgo J , Henry-Watson J , Serrano G , Sue LI , Nagel T , Duffy A , Shill HA , Akiyama H , Walker DG , Beach TG ((2014) ) Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology 82: , 858–864. |
[16] | Vilas D , Iranzo A , Tolosa E , Aldecoa I , Berenguer J , Vilaseca I , Marti C , Serradell M , Lomena F , Alos L , Gaig C , Santamaria J , Gelpi E ((2016) ) Assessment of alpha-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: A case-control study. Lancet Neurol 15: , 708–718. |
[17] | Shin J , Park SH , Shin C , Kim JH , Yun TJ , Kim HJ , Jeon B ((2019) ) Submandibular gland is a suitable site for alpha synuclein pathology in Parkinson disease. Parkinsonism Relat Disord 58: , 35–39. |
[18] | Adler CH , Serrano GE , Zhang N , Hinni ML , Lott DG , Mehta SH , Sue LI , Intorcia A , Beach TG ((2019) ) Feasibility of repeat and bilateral submandibular gland needle biopsies in Parkinson’s disease. Parkinsonism Relat Disord 68: , 69–72. |
[19] | Iranzo A , Borrego S , Vilaseca I , Marti C , Serradell M , Sanchez-Valle R , Kovacs GG , Valldeoriola F , Gaig C , Santamaria J , Tolosa E , Gelpi E ((2018) ) alpha-Synuclein aggregates in labial salivary glands of idiopathic rapid eye movement sleep behavior disorder. Sleep 41: , doi: 10.1093/sleep/zsy101 |
[20] | Pouclet H , Lebouvier T , Coron E , des Varannes SB , Rouaud T , Roy M , Neunlist M , Derkinderen P ((2012) ) A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson’s disease. Neurobiol Dis 45: , 305–309. |
[21] | Lebouvier T , Chaumette T , Damier P , Coron E , Touchefeu Y , Vrignaud S , Naveilhan P , Galmiche JP , Bruley des Varannes S , Derkinderen P , Neunlist M ((2008) ) Pathological lesions in colonic biopsies during Parkinson’s disease. Gut 57: , 1741–1743. |
[22] | Chung SJ , Kim J , Lee HJ , Ryu HS , Kim K , Lee JH , Jung KW , Kim MJ , Kim YJ , Yun SC , Lee JY , Hong SM , Myung SJ ((2016) ) Alpha-synuclein in gastric and colonic mucosa in Parkinson’s disease: Limited role as a biomarker. Mov Disord 31: , 241–249. |
[23] | Visanji NP , Marras C , Kern DS , Al Dakheel A , Gao A , Liu LW , Lang AE , Hazrati LN ((2015) ) Colonic mucosal a-synuclein lacks specificity as a biomarker for Parkinson disease. Neurology 84: , 609–616. |
[24] | Donadio V , Incensi A , Piccinini C , Cortelli P , Giannoccaro MP , Baruzzi A , Liguori R ((2016) ) Skin nerve misfolded alpha-synuclein in pure autonomic failure and Parkinson disease. Ann Neurol 79: , 306–316. |
[25] | Liu X , Yang J , Yuan Y , He Q , Gao Y , Jiang C , Li L , Xu Y ((2020) ) Optimization of the detection method for phosphorylated alpha-synuclein in Parkinson disease by skin biopsy. Front Neurol 11: , 569446. |
[26] | Navarro-Otano J , Casanova-Molla J , Morales M , Valls-Sole J , Tolosa E ((2015) ) Cutaneous autonomic denervation in Parkinson’s disease. J Neural Transm (Vienna) 122: , 1149–1155. |
[27] | Haga R , Sugimoto K , Nishijima H , Miki Y , Suzuki C , Wakabayashi K , Baba M , Yagihashi S , Tomiyama M ((2015) ) Clinical utility of skin biopsy in differentiating between Parkinson’s disease and multiple system atrophy. Parkinsons Dis 2015: , 167038. |
[28] | Doppler K , Volkmann J , Sommer C ((2016) ) Skin biopsies in the differential diagnosis of parkinsonism: Are we ready for simplified protocols? Brain 139: , e5. |
[29] | Tsukita K , Sakamaki-Tsukita H , Tanaka K , Suenaga T , Takahashi R ((2019) ) Value of in vivo alpha-synuclein deposits in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 34: , 1452–1463. |
[30] | Donadio V , Doppler K , Incensi A , Kuzkina A , Janzen A , Mayer G , Volkmann J , Rizzo G , Antelmi E , Plazzi G , Sommer C , Liguori R , Oertel WH ((2019) ) Abnormal alpha-synuclein deposits in skin nerves: Intra- and inter-laboratory reproducibility. Eur J Neurol 26: , 1245–1251. |
[31] | Beach TG , Serrano GE , Kremer T , Canamero M , Dziadek S , Sade H , Derkinderen P , Corbille AG , Letournel F , Munoz DG , White CL 3rd , Schneider J , Crary JF , Sue LI , Adler CH , Glass MJ , Intorcia AJ , Walker JE , Foroud T , Coffey CS , Ecklund D , Riss H , Gossmann J , Konig F , Kopil CM , Arnedo V , Riley L , Linder C , Dave KD , Jennings D , Seibyl J , Mollenhauer B , Chahine L ((2018) ) Immunohistochemical method and histopathology judging for the Systemic Synuclein Sampling Study (S4). J Neuropathol Exp Neurol 77: , 793–802. |
[32] | Chahine LM , Beach TG , Brumm MC , Adler CH , Coffey CS , Mosovsky S , Caspell-Garcia C , Serrano GE , Munoz DG , White CL 3rd , Crary JF , Jennings D , Taylor P , Foroud T , Arnedo V , Kopil CM , Riley L , Dave KD , Mollenhauer B ((2020) ) In vivo distribution of alpha-synuclein in multiple tissues and biofluids in Parkinson disease. Neurology 95: , e1267–e1284. |
[33] | Lauria G , Cornblath DR , Johansson O , McArthur JC , Mellgren SI , Nolano M , Rosenberg N , Sommer C ((2005) ) EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 12: , 747–758. |
[34] | Lauria G , Hsieh ST , Johansson O , Kennedy WR , Leger JM , Mellgren SI , Nolano M , Merkies IS , Polydefkis M , Smith AG , Sommer C , Valls-Sole J ((2010) ) European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 17: , 903–912, e944-909. |
[35] | Ljungberg A , Johansson O ((1993) ) Methodological aspects on immunohistochemistry in dermatology with special reference to neuronal markers. Histochem J 25: , 735–745. |
[36] | McCarthy BG , Hsieh ST , Stocks A , Hauer P , Macko C , Cornblath DR , Griffin JW , McArthur JC ((1995) ) Cutaneous innervation in sensory neuropathies: Evaluation by skin biopsy. Neurology 45: , 1848–1855. |
[37] | Donadio V ((2019) ) Skin nerve alpha-synuclein deposits in Parkinson’s disease and other synucleinopathies: A review. Clin Auton Res 29: , 577–585. |
[38] | Wang N , Garcia J , Freeman R , Gibbons CH ((2020) ) Phosphorylated alpha-synuclein within cutaneous autonomic nerves of patients with Parkinson’s disease: The implications of sample thickness on results. J Histochem Cytochem 68: , 669–678. |
[39] | Al-Qassabi A , Tsao TS , Racolta A , Kremer T , Canamero M , Belousov A , Santana MA , Beck RC , Zhang H , Meridew J , Pugh J , Lian F , Robida MD , Ritter M , Czech C , Beach TG , Pestic-Dragovich L , Taylor KI , Zago W , Tang L , Dziadek S , Postuma RB (2020) Immunohistochemical detection of synuclein pathology in skin in idiopathic rapid eye movement sleep behavior disorder and parkinsonism. Mov Disord, doi: 10.1002/mds.28399 |
[40] | Donadio V , Incensi A , El-Agnaf O , Rizzo G , Vaikath N , Del Sorbo F , Scaglione C , Capellari S , Elia A , Stanzani Maserati M , Pantieri R , Liguori R ((2018) ) Skin alpha-synuclein deposits differ in clinical variants of synucleinopathy: An in vivo study. Sci Rep 8: , 14246. |
[41] | Doppler K , Weis J , Karl K , Ebert S , Ebentheuer J , Trenkwalder C , Klebe S , Volkmann J , Sommer C ((2015) ) Distinctive distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Mov Disord 30: , 1688–1692. |
[42] | Wang N , Gibbons CH , Lafo J , Freeman R ((2013) ) alpha-Synuclein in cutaneous autonomic nerves. Neurology 81: , 1604–1610. |
[43] | Kuzkina A , Schulmeyer L , Monoranu CM , Volkmann J , Sommer C , Doppler K ((2019) ) The aggregation state of alpha-synuclein deposits in dermal nerve fibers of patients with Parkinson’s disease resembles that in the brain. Parkinsonism Relat Disord 64: , 66–72. |
[44] | Melli G , Vacchi E , Biemmi V , Galati S , Staedler C , Ambrosini R , Kaelin-Lang A ((2018) ) Cervical skin denervation associates with alpha-synuclein aggregates in Parkinson disease. Ann Clin Transl Neurol 5: , 1394–1407. |
[45] | Shishido T , Ikemura M , Obi T , Yamazaki K , Terada T , Sugiura A , Saito Y , Murayama S , Mizoguchi K ((2010) ) alpha-synuclein accumulation in skin nerve fibers revealed by skin biopsy in pure autonomic failure. Neurology 74: , 608–610. |
[46] | Donadio V , Incensi A , Rizzo G , Capellari S , Pantieri R , Stanzani Maserati M , Devigili G , Eleopra R , Defazio G , Montini F , Baruzzi A , Liguori R ((2017) ) A new potential biomarker for dementia with Lewy bodies: Skin nerve alpha-synuclein deposits. Neurology 89: , 318–326. |
[47] | Iranzo A , Tolosa E , Gelpi E , Molinuevo JL , Valldeoriola F , Serradell M , Sanchez-Valle R , Vilaseca I , Lomena F , Vilas D , Llado A , Gaig C , Santamaria J ((2013) ) Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: An observational cohort study. Lancet Neurol 12: , 443–453. |
[48] | Iranzo A , Fernandez-Arcos A , Tolosa E , Serradell M , Molinuevo JL , Valldeoriola F , Gelpi E , Vilaseca I , San-chez-Valle R , Llado A , Gaig C , Santamaria J ((2014) ) Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: Study in 174 patients. PLoS One 9: , e89741. |
[49] | Doppler K , Jentschke HM , Schulmeyer L , Vadasz D , Janzen A , Luster M , Hoffken H , Mayer G , Brumberg J , Booij J , Musacchio T , Klebe S , Sittig-Wiegand E , Volkmann J , Sommer C , Oertel WH ((2017) ) Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol 133: , 535–545. |
[50] | Antelmi E , Donadio V , Incensi A , Plazzi G , Liguori R ((2017) ) Skin nerve phosphorylated alpha-synuclein deposits in idiopathic REM sleep behavior disorder. Neurology 88: , 2128–2131. |
[51] | Berg D , Postuma RB , Adler CH , Bloem BR , Chan P , Dubois B , Gasser T , Goetz CG , Halliday G , Joseph L , Lang AE , Liepelt-Scarfone I , Litvan I , Marek K , Obeso J , Oertel W , Olanow CW , Poewe W , Stern M , Deuschl G ((2015) ) MDS research criteria for prodromal Parkinson’s disease. Mov Disord 30: , 1600–1611. |
[52] | Chahine LM , Beach TG , Seedorff N , Caspell-Garcia C , Coffey CS , Brumm M , Adler CH , Serrano GE , Linder C , Mosovsky S , Foroud T , Riss H , Ecklund D , Seibyl J , Jennings D , Arnedo V , Riley L , Dave KD , Mollenhauer B ((2018) ) Feasibility and safety of multicenter tissue and biofluid sampling for alpha-synuclein in Parkinson’s disease: The Systemic Synuclein Sampling Study (S4). J Parkinsons Dis 8: , 517–527. |
[53] | Atarashi R , Satoh K , Sano K , Fuse T , Yamaguchi N , Ishibashi D , Matsubara T , Nakagaki T , Yamanaka H , Shirabe S , Yamada M , Mizusawa H , Kitamoto T , Klug G , McGlade A , Collins SJ , Nishida N ((2011) ) Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med 17: , 175–178. |
[54] | Fairfoul G , McGuire LI , Pal S , Ironside JW , Neumann J , Christie S , Joachim C , Esiri M , Evetts SG , Rolinski M , Baig F , Ruffmann C , Wade-Martins R , Hu MT , Parkkinen L , Green AJ ((2016) ) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 3: , 812–818. |
[55] | van Rumund A , Green AJE , Fairfoul G , Esselink RAJ , Bloem BR , Verbeek MM ((2019) ) alpha-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann Neurol 85: , 777–781. |
[56] | Rossi M , Candelise N , Baiardi S , Capellari S , Giannini G , Orru CD , Antelmi E , Mammana A , Hughson AG , Calandra-Buonaura G , Ladogana A , Plazzi G , Cortelli P , Caughey B , Parchi P ((2020) ) Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol 140: , 49–62. |
[57] | Manne S , Kondru N , Jin H , Serrano GE , Anantharam V , Kanthasamy A , Adler CH , Beach TG , Kanthasamy AG ((2020) ) Blinded RT-QuIC analysis of alpha-synuclein biomarker in skin tissue from Parkinson’s disease patients. Mov Disord 35: , 2230–2239. |
[58] | Wang Z , Becker K , Donadio V , Siedlak S , Yuan J , Rezaee M , Incensi A , Kuzkina A , Orru CD , Tatsuoka C , Liguori R , Gunzler SA , Caughey B , Jimenez-Capdeville ME , Zhu X , Doppler K , Cui L , Chen SG , Ma J , Zou WQ ((2021) ) Skin alpha-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol 78: , 30–40. |
[59] | Zange L , Noack C , Hahn K , Stenzel W , Lipp A ((2015) ) Phosphorylated alpha-synuclein in skin nerve fibres differentiates Parkinson’s disease from multiple system atrophy. Brain 138: , 2310–2321. |
[60] | Donadio V , Incensi A , Rizzo G , Scaglione C , Capellari S , Fileccia E , Avoni P , Liguori R ((2017) ) Spine topographical distribution of skin alpha-synuclein deposits in idiopathic Parkinson disease. J Neuropathol Exp Neurol 76: , 384–389. |
[61] | Doppler K , Brockmann K , Sedghi A , Wurster I , Volkmann J , Oertel WH , Sommer C ((2018) ) Dermal phospho-alpha-synuclein deposition in patients with Parkinson’s disease and mutation of the glucocerebrosidase gene. Front Neurol 9: , 1094. |
[62] | Antelmi E , Pizza F , Donadio V , Filardi M , Sosero YL , Incensi A , Vandi S , Moresco M , Ferri R , Marelli S , Ferini-Strambi L , Liguori R , Plazzi G ((2019) ) Biomarkers for REM sleep behavior disorder in idiopathic and narcoleptic patients. Ann Clin Transl Neurol 6: , 1872–1876. |
[63] | Carmona-Abellan M , Gabilondo I , Murueta-Goyena A , Khurana V , Tijero B , Luquin MR , Acera M , Del Pino R , Gardeazabal J , Martinez-Valbuena I , Sanchez-Pernaute R , Gomez-Esteban JC ((2019) ) Small fiber neuropathy and phosphorylated alpha-synuclein in the skin of E46K-SNCA mutation carriers. Parkinsonism Relat Disord 65: , 139–145. |
[64] | Donadio V , Incensi A , Rizzo G , De Micco R , Tessitore A , Devigili G , Del Sorbo F , Bonvegna S , Infante R , Magnani M , Zenesini C , Vignatelli L , Cilia R , Eleopra R , Tedeschi G , Liguori R ((2020) ) Skin biopsy may help to distinguish multiple system atrophy-parkinsonism from Parkinson’s disease with orthostatic hypotension. Mov Disord 35: , 1649–1657. |
[65] | Giannoccaro MP , Donadio V , Giannini G , Devigili G , Rizzo G , Incensi A , Cason E , Calandra-Buonaura G , Eleopra R , Cortelli P , Liguori R ((2020) ) Comparison of 123I-MIBG scintigraphy and phosphorylated alpha-synuclein skin deposits in synucleinopathies. Parkinsonism Relat Disord 81: , 48–53. |




