Oral Dysbiosis and Inflammation in Parkinson’s Disease
Abstract
Background:
Oral microbiota has largely escaped attention in Parkinson’s disease (PD), despite its pivotal role in maintaining oral and systemic health.
Objective:
The aim of our study was to examine the composition of the oral microbiota and the degree of oral inflammation in PD.
Methods:
Twenty PD patients were compared to 20 healthy controls. Neurological, periodontal and dental examinations were performed as well as dental scaling and gingival crevicular fluid sampling for cytokines measurement (interleukine (IL)-1β, IL-6, IL-1 receptor antagonist (RA), interferon-γ and tumor necrosis factor (TNF)-α). Two months later, oral microbiota was sampled from saliva and subgingival dental plaque. A 16S rRNA gene amplicon sequencing was used to assess bacterial communities.
Results:
PD patients were in the early and mid-stage phases of their disease (Hoehn & Yahr 2–2.5). Dental and periodontal parameters did not differ between groups. The levels of IL-1β and IL-1RA were significantly increased in patients compared to controls with a trend for an increased level of TNF-α in patients. Both saliva and subgingival dental plaque microbiota differed between patients and controls. Streptococcus mutans, Kingella oralis, Actinomyces AFQC_s, Veillonella AFUJ_s, Scardovia, Lactobacillaceae, Negativicutes and Firmicutes were more abundant in patients, whereas Treponema KE332528_s, Lachnospiraceae AM420052_s, and phylum SR1 were less abundant.
Conclusion:
Our findings show that the oral microbiome is altered in early and mid-stage PD. Although PD patients had good dental and periodontal status, local inflammation was already present in the oral cavity. The relationship between oral dysbiosis, inflammation and the pathogenesis of PD requires further study.
INTRODUCTION
The etiology of Parkinson’s disease (PD) is un-known in the vast majority of cases but probably involves a complex interaction between genetic predisposition, environmental contributors and age-related processes [1]. The hypothesis that the periphery of the body could be an early site of PD comes from the observation that phosphorylated (phos-) α-synuclein aggregates [2], the histopathological hallmark of PD, are found not only in the central nervous system (CNS) but also within neurons of the olfactory bulb and in the peripheral autonomic nervous system of the upper aerodigestive [3] and gastrointestinal tracts, very early in the course of the disease, before the classic motor symptoms emerge [4–6]. Consequently, PD patients frequently present a history of hyposmia, oral difficulties and/or gastrointestinal dysfunction [7], sometimes years before being diagnosed with PD.
Little attention has been paid to the role of the mouth in the PD literature. However, a rostro-caudal gradient of decreasing phos-α-synuclein histopa-thology has been demonstrated, with the highest density being found in the submandibular glands and the lower esophagus, and the lowest density in the colon and the rectum [8]. Hence, submandibu-lar gland needle biopsies have recently been proposed as a method for the diagnosis of PD [9–11]. Oral phos-α-synuclein deposits may underlie non-motor symptoms (NMS) [12] such as decreased salivary production and dysphagia [7]. These symptoms typically become troublesome in more advanced PD, but they can develop early, occasionally as the presenting feature [13, 14]. A higher frequency of poor oral health, caries and periodontal disease, and fewer remaining teeth have been reported in PD [15–17].
Recently, attention has increasingly been focused on the microbiota as a new player in PD pathogenesis because of its key role in the protection of the host from pathogenic organisms [18]. Several studies showed changes in PD patients’ gut microbiota with an important variation in the reported taxa [19–24]. Gut dysbiosis promotes intestinal inflammation and increased mucosal permeability [25–29], inducing or maintaining excessive phos-α-synuclein expression and misfolding [25, 30–35]. Phos-α-synuclein aggregates could then migrate, from the peripheral nervous system to the CNS through trans-synaptic transmission and from cell-to-cell in a prion-like fashion [36], eventually leading to PD degeneration.
In contrast to gut microbiota, data on oral microbiota in PD are scarce [37, 38]. However, the study of the oral microbiota may offer several advantages: 1) sampling is easy; 2) it represents the second largest and second most diverse microbiota after the gut, harboring over 700 species of bacteria [39]; 3) it is one of the microbiota which has the least intrapersonal variability, enabling broader conclusions [40]; 4) it is strategically located at the crossroads between the outside environment, the respiratory and digestive tracts. As such, the mouth is one of the first structures to be exposed to the external environment, such as airborne and diet-contained organisms, and is consequently one of the primary sites of entry for pathogenic microorganisms; and 5) oral microbiota is essential to maintain oral and systemic health [41]. Oral dysbiosis is associated with oral diseases such as dental caries and periodontal disease, but also with systemic diseases such as infective endocarditis, atherosclerosis [42] and with multifactorial neurodegenerative conditions such as glau-coma [43].
Considering the early involvement of the upper aerodigestive tract in terms of PD non-motor symptoms and α-synuclein deposits, we speculated that the study of the mouth could be central to improved understanding of PD pathophysiology. We studied the salivary and subgingival dental plaque microbiota, by comparing bacterial diversity and taxonomic composition in PD patients and matched healthy subjects. We also compared the level of oral inflammation between patients and controls, which was assessed both clinically and biologically.
METHODS
The study was approved by the Geneva Ethics Committee (EC-2016-01680). All participants gave written informed consent.
Study population
Patients were recruited from the Movement Disorders Unit of Geneva University Hospital. Selection criteria were a diagnosis of PD [44] made after 50 years of age (Supplementary Material A). Patients were compared with age-, sex-, and body mass index-matched healthy controls. Exclusion criteria included conditions that could independently affect the oral microbiome (Supplementary Material A).
Clinical data
The clinical assessment included the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale [45] part III for motor PD symptoms and the Hoehn & Yahr (H&Y) scale [46] for PD staging, the NMS Quest [47] for non-motor PD symptoms, the Hospital Anxiety and Depression scale [48] for the levels of anxiety and depression and the Montreal Cognitive Assessment (MoCA) [49] for the degree of cognitive impairment. The degree of drooling was assessed by the Sialorrhea Clinical Scale for PD [50], the degree of hyposialorrhea by the paraffin-stimulated salivary flow rate [51], the level of dysphagia by the Swallowing Disturbance Questionnaire [52] and the degree of constipation by the Bristol Stool Form Scale [53]. The dental and periodontal screening included an interview to assess oral hygiene, dental history, and socioeconomic background. The clinical oral assessment included counting the number of decayed, missing and filled teeth [54] (which determines the prevalence of dental caries as well as dental treatment needs) and the plaque index [55], which records the presence and quantity of dental biofilm that accumulates on the surface of the tooth. The periodontal inflammatory status was clinically assessed by probing pocket depth and the bleeding on probing [56]. A standardized dental scaling was performed at the end of the session in all participants (“reset” of the oral cavity) in order to control for oral hygiene. Advice about dental hygiene, teeth brushing and avoidance of oral antiseptics was given. All participants received the same toothbrush and toothpaste.
Cytokine dosage
Oral inflammation was also assessed by measuring interleukin (IL)-1β, IL-6, IL-1 receptor antagonist (IL-1RA), interferon (IFN)-γ and tumor necrosis factor (TNF)-α concentrations in the gingival crevicular fluid using Bio-Plex cytokine multiplex assays [57].
Analysis of oral microbiota
Two months after the “reset” of the oral cavity, microbiota sampling was performed from two different oral sites: the saliva and the subgingival dental plaque (i.e., tooth biofilm below the gum). Participants were asked to avoid teeth brushing the morning of the sampling. Unstimulated saliva was obtained by spitting into a sterile plastic 50 mL tube in the morning, at least 1.5 h after eating. Subgingival dental plaque was obtained by a pooled sample from the deepest pocket in each quadrant using sterile paper points. Within the following 3 h, samples were transferred for storage and kept at a temperature of –80°C until further processing. The principal steps in our analysis of bacterial communities in saliva and dental plaque were [58]: 1) DNA extraction; 2) bacterial DNA quantification using quantitative polymerase chain reaction (qPCR); and 3) Illumina sequencing of amplicons generated from the V3–4 region of the bacterial 16S ribosomal RNA genes. Merged sequence reads were clustered into zero-radius operational taxonomic units (zOTUs) with a method [59] resolving differences as small as one nucleotide and providing taxonomic resolution superior to conventional 97%OTUs [60]; 4) comparison of zOTUs to the EzBioCloud [61] 16S rRNA gene database for taxonomic assignments; 5) microbial communities comparisons in regards to ecological indices (richness, diversity) and abundance of taxa from phylum to zOTU level; and 6) assessment of correlations between microbial composition and clinical outcomes (Supplementary Material A).
Statistical analysis
For univariate comparisons, we used the Wilcoxon rank sum test, unless otherwise indicated. For multivariate analyses of the microbiota, we constructed the Bray-Curtis similarity [62] matrix based on square root transformed relative abundance of zOTUs. In principal coordinates analysis (PCoA, PRIMER) each sample was visualized in a multidimensional space as a point whose location reflected its microbial composition (i.e., percentage of different zOTUs). To assess differences in overall microbiota taxonomic composition between groups defined by categorical variables (e.g., PD vs. controls), we used permutational multivariate analysis of variance (PERMANOVA; PRIMER v7, PRIMER-E Ltd, Plymouth, UK) with 9,999 permutations. Canonical analysis of principal coordinates (CAP, PRIMER) was used to test the success of allocating subjects to PD and controls from specific microbial profiles. This method maximizes the group differences in the multivariate cloud of points (constrained ordination) [63], and calculates the proportion of correct classifications of the groups according to the PD or control status. The permutational analysis of multivariate dispersions (PERMDISP, PRIMER) was performed (with 9,999 permutations) to examine homogeneity of multivariate dispersion (distances to centroids) of microbial communities between patients and controls. To analyze the relationship between bacterial community profiles and quantitative clinical continuous variables, we used a distance-based linear model (DISTLM, PRIMER) with 9,999 permutations.
To assess statistical significance of differences in the relative abundance of individual taxa according to the PD status, we used DESeq2 [64]. The taxa found in less than 25%(n = 10) of compared samples were excluded. FDR corrected p values < 0.05 were considered significant. DESeq2 models were additionally run with adjustment for age category and sex. Age was categorized in two groups: above-median and below-median.
RESULTS
Clinical data
A total of 40 participants were recruited, including 20 PD patients and 20 healthy controls. Demographic and clinical characteristics of each group are reported in Table 1 and Supplementary Material B. Participants were similar regarding the level of education, comorbidities, cognitive status and anxiety level. No clinical differences were identified in terms of dental or periodontal status. PD patients were predominantly within the early and mid-stage phases of their disease (median from PD diagnosis = 4.7 years, H&Y range from 2 to 2.5). As expected, PD patients had more severe motor and non-motor PD symptoms compared with controls. PD patients had significantly higher swallowing and saliva disturbances scores than controls, although their scores themselves did not reach pathological cut-offs. All but two PD patients were taking dopaminergic replacement therapy. One patient was treated with deep brain stimulation (Table 1 and Supplementary Material B).
Table 1
Selected demographic and clinical characteristics of participants including all parameters that showed significantly different distributions between groups
| PD Patients | Controls | p | |
| Demographics | |||
| Number of participants [men (%)] | 20 [9 (45%)] | 20 [9 (45%)] | 1 (F) |
| Age (y) | 62.8 (60.1; 67.0) | 64.3 (59.2; 68.3) | 0.8 |
| Education level I, II,≥III) | 0, 3, 17 (0%, 15%, 85%) | 1, 2, 17 (5%,10%,85%) | 0.5 (C) |
| Body Mass Index (kg/m2) | 24.8 (20.8; 28.8) | 25.8 (23.8; 26.9) | 0.8 |
| Current smokers (%) | 0 (0%) | 0 (0%) | 1 (F) |
| Former smokers (%) | 4 (20%) | 5 (25%) | 1 (F) |
| Parkinson’s disease duration (y) | 4.7 (3; 7.4) | ||
| Hoehn &Yahr stage (/4) | 2 (2; 2.5) | ||
| Clinical symptoms | |||
| Motor score MDS-UPDRS III (/132) | 15 (11.5; 20.5) | 0 (0; 0) | < 0.001 |
| Non-motor score NMSQ (/30) | 11 (6.7; 14) | 3 (2; 4.2) | < 0.001 |
| Swallowing Disturbance Questionnaire (SDQ) score (/45) | 3 (1.5; 5.5) | 0.5 (0.5; 0.5) | < 0.001 |
| Sialorrhea Clinical Scale score (/21) | 1.5 (0; 3.2) | 0 (0; 0) | < 0.001 |
| Paraffin-stimulated salivary flow rate (ml/min) | 1.6 (0.8; 1.7) | 6 (6; 6) | < 0.001 |
| Constipation level Bristol Stool Form Scale score (/7) | 3 (1.7; 3) | 4 (3; 4) | < 0.001 |
| Cognitive score MoCA (/30) | 29 (28.7; 30) | 30 (28; 30) | 0.4 |
| Anxiety HAD score (/21) | 4 (3; 7.2) | 4.5 (2.7; 6.2) | 0.5 |
| Depression HAD score (/21) | 3 (2.7; 6.2) | 2 (1; 4) | 0.02 |
| Dental and periodontal parameters | |||
| Participants with tooth brushing >once/day (%) | 20 (100%) | 20 (100%) | 1 (F) |
| Participants with dental visits ≥ once/year (%) | 20 (100%) | 20 (100%) | 1 (F) |
| Participants with decayed teeth (%) | 2 (10%) | 2 (10%) | 1 (F) |
| Percentage of filled teeth | 49.05 (33.3; 55.6) | 55.5 (34; 64.1) | 0.425 |
| PPD (mm) | 2.99 (2.9; 3.1) | 3 (2.8; 3.1) | 0.766 |
| Plaque index | 1.29 (0.8; 1.4) | 0.97 (0.8; 1.2) | 0.218 |
| BOP | 57.91 (34.1; 69.9) | 51.52 (34.6; 59.7) | 0.725 |
| Percentage of PPD ≥4 sites | 13.12 (7.6; 20.7) | 8.04 (5.4; 17.1) | 0.465 |
| Percentage of PPD ≥4 BOP + sites | 11.11 (5.6; 19.5) | 6.85 (1.8; 15.1) | 0.351 |
| Medication | |||
| Levodopa-equivalent daily dose (mg/d) | 522.5 (296.9; 720.6) | 0 (0; 0) | < 0.001 |
| Number of patients on Levodopa (%) | 16 (80%) | 0 | < 0.001 (F) |
| Number of patients on Dopamine agonist | 12 (60%) | 0 | < 0.001 (F) |
| Number of patients on MAO Inhibitor | 6 (30%) | 0 | 0.02 (F) |
For values expressed as median (Q1; Q3) scores, Wilcoxon rank sum test was used. When values were expressed as number of participants, %of the participant’s group was indicated in brackets and Fisher’s exact test (F) or Chi-square test (C) were used. Education level: Level I is defined as subjects who received a primary education, Level II a lower secondary education, and level 3 and above at least an upper secondary education. A total Swallowing Disturbance Questionnaire SDQ score of ≥11/45, a paraffin-stimulated salivary flow rate value < 0.7 ml/min and a HAD Depression score ≥8/21 were considered to be pathological cut-off values for dysphagia, hyposialorrhea, and depression respectively. BOP, bleeding on probing; BOP +, presence of BOP; HAD, Hospital Anxiety and Depression scale; MDS-UPDRS III score, motor scale of Movement Disorder Society Unified Parkinson’s Disease Rating Scale; MoCA, Montreal Cognitive Assessment; NMSQ, Non-Motor Symptoms Quest; PD, Parkinson’s disease; PPD, probing pocket depth.
Cytokines in the gingival crevicular fluid
The levels of cytokines IL-1β and IL-1RA were significantly increased in PD patients compared to controls, with a trend for an increased level of TNF-α in PD patients. No difference was found in the concentrations of IL-6 and IFN-γ between the two groups (Table 2).
Table 2
Levels of cytokines in the gingival crevicular fluid in PD patients and controls
| PD patients | Healthy controls | p | |
| IL-1β (pg/ml) | 61.6 (16.5; 107.4) | 22.4 (8.8; 35.4) | 0.05 |
| IL-6 (pg/ml) | 4.4 (2.3; 10.8) | 2.5 (1.6; 4.6) | 0.20 |
| IL-1RA (pg/ml) | 44,247.5 (28,736.7; 92,068.9) | 29,153.0 (20,227.3; 42,257.5) | 0.04 |
| IFN-γ (pg/ml) | 2.0 (1.3; 2.8) | 1.8 (1.4; 2.7) | 0.93 |
| TNF-α (pg/ml) | 16.5 (9.1; 38.1) | 9.1 (5.2; 18.8) | 0.06 |
Values are expressed as median (Q1; Q3) scores.
Microbiome data
The number of reads obtained by sequencing of bacterial 16S rRNA gene amplicons is provided in Supplementary Material C. The relative abundance of 1,613 zOTUs identified was used to calculate similarities between bacterial communities, which we visualized using PCoA. Saliva and plaque samples were clearly separated in the first two PCo axes (Supplementary Material D, Supplementary Figure 1, Supplementary Table 1). The inter-site (i.e., saliva vs. plaque) microbiota Bray-Curtis similarity, in the same individual mouth was somewhat higher in patients than in controls [median (Q1; Q3) 47.9 (45.9; 56.9) vs. 43.7 (40.7; 50.5)], and this difference was close to the threshold of statistical significance (p = 0.056). Bacterial load expressed as the number of 16S rRNA copies in DNA extract did not show significant differences between PD and control groups in either saliva [median (Q1; Q3) 2.73 (1.17; 5.5)×107 vs. 4.81 (1.44; 15.72)×107; p = 0.14)] or plaque [2.4 (1.4; 4.13)×107 vs. 2.57 (1.84; 3.52)×107; p = 0.98)] samples.
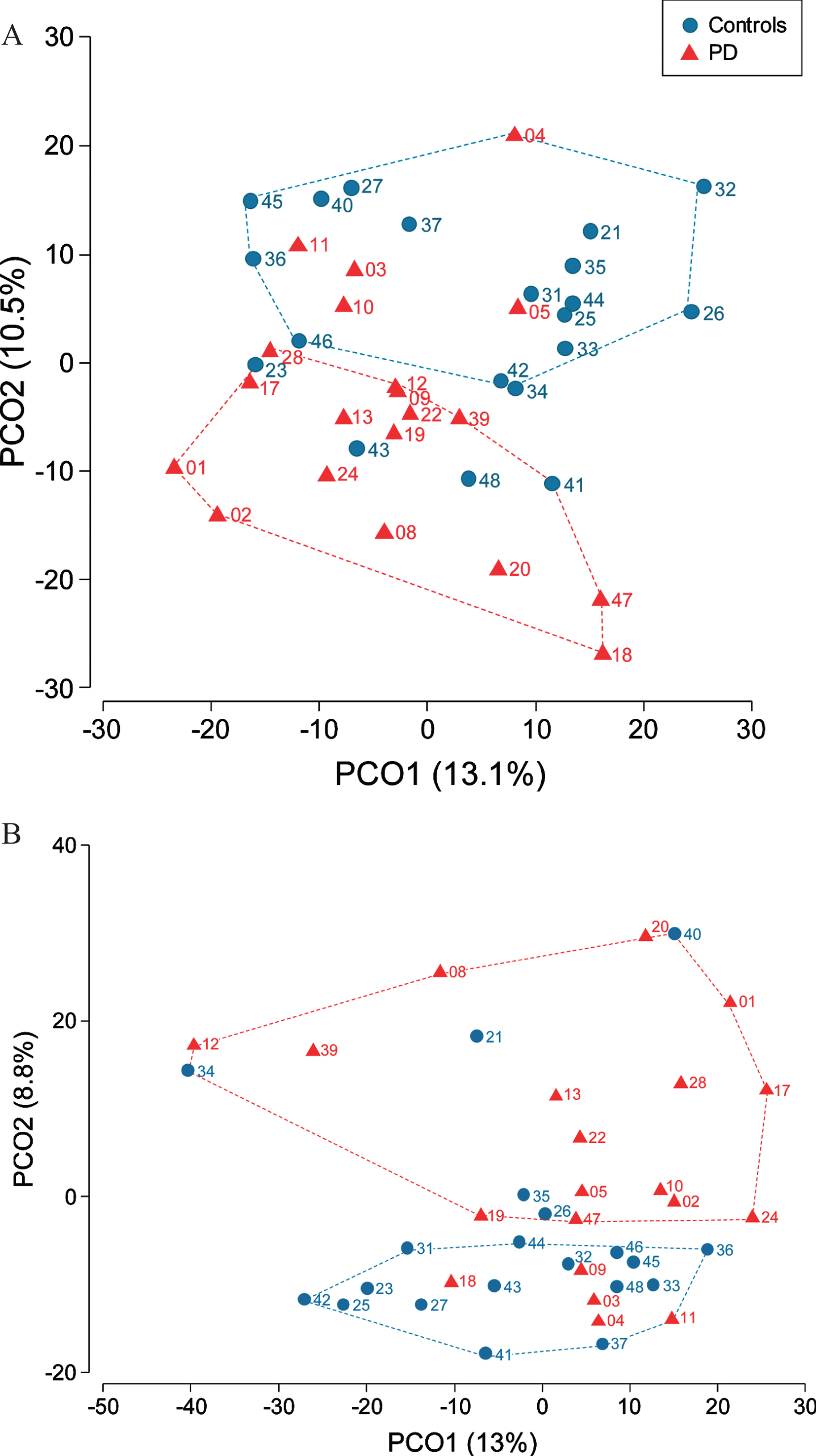
We assessed microbiota differences between controls and patients separately for saliva and dental plaque samples. Salivary microbiota profiles were different between patients and controls (PERMANOVA, p = 0.006) (Fig. 1A). The two arbitrarily defined non-overlapping clusters respectively contained 75%(15/20) of samples from patients and 80%(16/20) of control samples. Differences in dental plaque microbial communities between PD and control subjects were also present (PERMANOVA p = 0.03) with two separate clusters in PCoA, each formed by 75%(15/20) of samples from individuals with the same disease status (Fig. 1B). CAP, which is a constrained ordination method, allowed to allocate samples according to the PD status with 72.5%success for both saliva (correct classification of 75%PD patients and 70%controls) and plaque (correct classification of 65%PD patients and 80%controls) microbiota. Of note, saliva and plaque microbiota from 5 patients (#3, #4, #5, #10, #11) were misallocated to the control group using CAP. The results of the PERMDISP analysis showed that multivariate dispersion of microbial communities of patients and controls were not significantly different. No significant differences of oral microbiota were found between sexes in either sample type.
Fig. 1
Differential bacterial community composition between controls and PD patients assessed in saliva (A) and dental plaque samples (B). Participant’s number is indicated next to her/his location point.
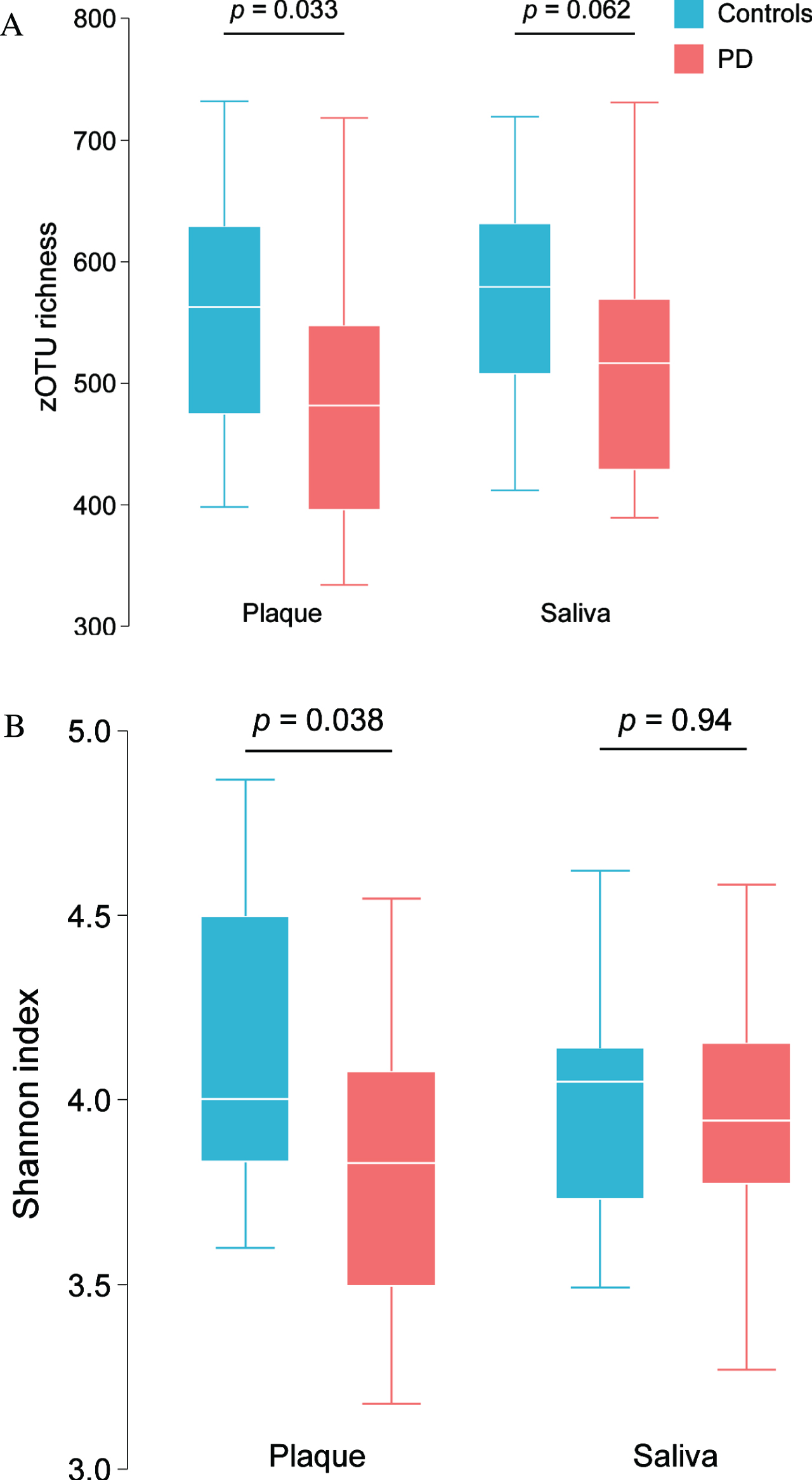
Compared with controls, PD patients presented with a lower alpha-diversity, as measured by species richness (Fig. 2A) and Shannon diversity index (Fig. 2B) in the dental plaque. The same trend was also observed in saliva samples.
Fig. 2
zOTU richness (A) and Shannon bacterial diversity index (B) in saliva and dental plaque samples in PD patients and controls.
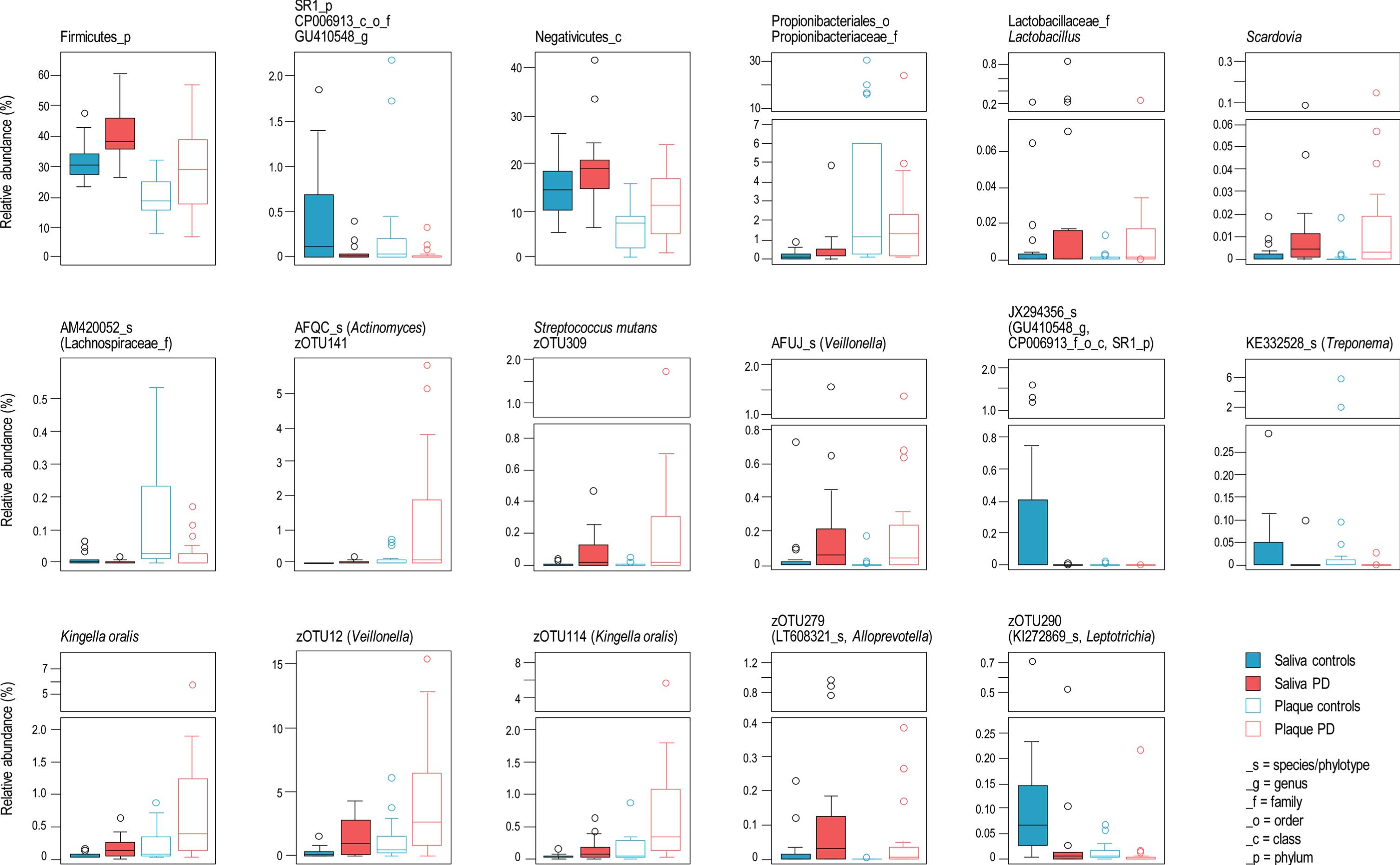
Relative bacterial taxa composition was significantly different between PD patients and controls. Supplementary Table 2 summarizes the taxa significantly different in abundance between patients and controls, from phylum to zOTU level. When a significant differential abundance according to the PD status was found in one sample type (i.e., saliva or dental plaque), the same trend (i.e., decrease or increase) was commonly (81.7%) observed in the other sample type. This was notably the case for 23 of 25 taxa (92%), whose abundance was significantly different between patients and controls in both saliva and dental plaque (Fig. 3). The phylum Firmicutes, class Negativicutes, family Lactobacillaceae/genus Lactobacillus and genus Scardovia (Actinobacteria) had higher relative abundance in PD patients. Of seven species or phylotypes (i.e., so far uncultured species) discriminating between PD and controls, four were more abundant in PD patients: Actinomyces AFQC_s, Veillonella AFUJ_s, Kingella oralis and Streptococcus mutans. Three zOTU from these species/phylotypes, as well as one from each unclassified Veillonella and Alloprevotella, also had increased proportion in PD patients. The taxa that had significantly lower relative abundance in PD patients included: zOTU290 (Leptotrichia), the phylotypes AM420052_s (Lachnospiraceae), KE332528_s (Treponema) and, JX294356_s and the higher-level taxa (up to the phylum SR1) to which the latter phylotype belongs. The observed associations between microbial taxa and PD status remained significant after adjustment for age category and sex (Supplementary Table 2) in the majority of cases.
Fig. 3
Bacterial taxa significantly different in abundance between patients and controls in both saliva and dental plaque.
We calculated the ‘absolute abundance’ of bacterial taxa, by combining their relative abundance (from the 16S sequencing data) with the number of 16S rRNA gene copies (determined by qPCR). Comparison between PD patients and controls revealed 194 differentially abundant taxa in either or both sample types (Supplementary Table 3). Importantly, of 44 taxa with significant differences (non-corrected p values) in both saliva and plaque, 41 had higher abundance in controls, while S. mutans, S. mutans zOTU309 and Streptococcus zOTU112 were more abundant in PD patients. After correction for multiple testing, the only differences that remained significant were decreased abundances of phyla Tenericutes and SR1 in saliva samples of PD patients.
Correlations between microbiota composition and clinical data
Regarding correlations between whole microbiota composition and clinical data (Supplementary Table 4), DISTLM analysis showed associations between salivary microbiota of PD patients and MoCA score, salivary flow rate, and parameters indicating an increased risk of periodontal disease. A trend for an association was found between PD salivary microbiota and NMS Quest score, anxiety and depression scores. PD dental plaque microbiota correlated with age at PD onset. In saliva samples from PD patients, Spearman correlations showed a positive correlation between Actinomycetales and tremor scores and between Veillonella and dysphagia (SDQ) score (Supplementary Table 5).
DISCUSSION
PD patients exhibited a distinct microbiota profile in their saliva and subgingival dental plaque compared to controls. PD patients had a lower alpha-diversity in plaque samples with a similar trend observed in saliva. The level of pro-inflammatory cytokine IL-1β measured in the gingival crevicular fluid was significantly increased with a trend for an increased level of TNF-α in PD patients compared to controls, although PD patients had a good dental and periodontal status. Microbial profiles in saliva (but not in dental plaque) were associated with the cognitive status, salivary flow rate and indexes indicating an increased risk of periodontal disease. Dental plaque microbiota was related to PD motor symptoms severity.
To the best of our knowledge, this is the first time that the subgingival dental plaque has been studied in PD patients. To date, only two studies compared oral microbiota in PD patients and controls [37, 38]. Pereira et al. [37] studied samples originating from buccal and sublingual mucosa whereas Mihaila et al. [38] used salivary samples. We performed a detailed oral and periodontal examination, a good control of oral hygiene by means of the “reset” of the oral cavity, and cytokine measurement in the gingival crevicular fluid which reflects the level of local inflammation, all of which was not performed in the above mentioned studies. This allowed us to precisely assess the inflammatory state of the oral cavity. In accordance with the two previously mentioned studies, we demonstrated that PD patients had a different oral bacterial ecology than controls. We found similar results regarding increases in the relative abundance of S. mutans, Veillonella [37], Lactobacillaceae [37, 38] and Scardovia [38] in PD. In addition, we found that K. oralis and Negativicutes (a class withing the phylum Firmicutes, represented in particular by Veillonella), as well as the phylum Firmicutes were increased and that the phylum SR1 was decreased in PD patients. In vitro experiments have demonstrated that a species from the genus Veillonella reduces the growth inhibition of S. mutans [66] by antagonistic streptococcal species.
The bacteria found in increased proportions in our PD patients have been implicated in oral pathologies such as dental caries (S. mutans [67], Lactobacillus [68] and Scardovia [69]) and periodontitis (K. oralis [70] and Negativicutes [71]). Our patients did not present with more decayed teeth, nor more periodontitis than controls, probably because they were in the early and middle phases of their disease with low motor scores, allowing good fine hand motor skills [72] and no cognitive impairment nor significant depression, anxiety or apathy, all ensuring the capacity to maintain good oral hygiene. However, a higher frequency of caries and periodontal disease has been reported in PD [15–17]. In that light, our results could then suggest that the changes observed in PD oral microbiota composition could correspond to a transition phase in the development of oral diseases. More importantly, we believe that our data support the notion that an altered oral microbiome is unlikely a consequence of dental or periodontal complications, but rather may be a primary event, somehow related to the pathogenesis of PD. This hypothesis, however, requires further study for confirmation.
Interestingly, S. mutans, whose relative abundance was increased in our patients, is capable of amyloid formation [73]. Bacterial amyloid production contributes to the biology of numerous micro-organisms, with particular relevance for adhesion and biofilm formation. While the link between microbial amyloid formation and PD remains to be demonstrated [74], it has been shown in animal models that bacterial amyloids may play a role in alpha-synuclein production and aggregation, as well as cerebral inflammation. Rats orally exposed to amyloid-producing bacteria presented with enhanced alpha-synuclein production in the gut, as well as an increased production and aggregation of alpha-synuclein in the brain. Enhanced cerebral inflammation was also demonstrated in rats orally exposed to amyloid-producing E. coli when compared with animals exposed to isogenic E. coli mutant lacking the ability to produce bacterial amyloid protein [75]. Using a mice model for PD, Sampson et al. showed that gut microbiota was needed for motor impairment, alpha-synuclein pathology and microglia activation [30], and all of these were improved by the use of antibiotics and worsened by microbial re-colonization. The pathway of the innate immune system recognizing bacterial amyloid as a pathogen-associated molecular pattern [76], is also involved in the recognition of misfolded alpha-synuclein [77]. Fecal transplants from PD patients performed in alpha-synuclein-overexpressing mice enhanced physical impairments compared to microbiota transplants from healthy human donors [30]. Recently, a Finnish nationwide case-control study demonstrated that prior exposure to certain antibiotics was associated with an increased risk of PD with a delay consistent with the duration of the prodromal period. Changes in microbiota composition secondary to the exposure to certain antibiotics could explain the increased risk of PD [78].
Pro-inflammatory cytokine levels were significantly increased in PD gingival crevicular fluid. The level of IL-1RA was also increased. The level of this anti-inflammatory cytokine goes up in response to increased levels of IL-1 [79]. To the best of our knowledge, levels of cytokines measured in the subgingival fluid have never been studied in PD patients. A significant correlation has been demonstrated between the level of certain pro-inflammatory cytokines in the serum and the gingival crevicular fluid in periodontitis subjects [80]. Many studies have shown an increased peripheral and central inflammatory response in PD [81] with higher level of pro-inflammatory cytokines in the serum [82, 83], colon biopsies [27], the cerebrospinal fluid [84] and the brain [84, 85]. Inflammation has been suggested to mediate neurodegeneration [86] but the actual origin of the inflammation remains unclear. Putative laquo proinflammatory raquo Proteobacteria were more abundant in PD patients’ feces than controls, whereas putative laquo anti-inflammatory raquo butyrate-producing bacteria were more abundant in controls [20]. Dysbiosis in PD could promote immune activation and systemic inflammation, which could in turn exacerbate pathogenic processes (such as triggering and maintenance of excessive α-synuclein expression) by establishing a chronic neuroinflammatory milieu.
Many studies have reported gut dysbiosis in PD patients [87]. The oral microbiota may have a great impact on the composition of gut microbiota and the health of the gastrointestinal tract [88]. Indeed, oral acid-resistant bacteria can colonize the gut through swallowed saliva. Swallowed dead oral bacteria can also be a nutritional source for gut microbiota growth (necrotrophy). Oral bacteria are poor colonizers of the healthy digestive tract [89]. However, under pathological circumstances, it has been demonstrated that oral pathogens could colonize the gut, and influence colonic composition and functions, particularly in non-alcoholic fatty liver disease, rheumatoid arthritis, and periodontitis [90–93]. In PD, no studies have specifically examined the relationship between oral and gut dysbiosis. In accordance with several studies investigating gut microbiota [19, 21, 23, 94–96], we reported an increase in the family Lactobacillaceae. An increased relative abundance of the genus Prevotella and Prevotellaceae family in PD buccal and sublingual mucosa samples has been reported [37]. In line with these findings, we found significantly increased levels of four bacterial species of the family Prevotellaceae (Aloprevotella AM420222_s, Alloprevotella PAC001345_s, Prevotella PAC001346_s and Prevotella histicola) in the dental plaque samples of PD patients. In saliva, the only differentially abundant Prevotellaceae species was Prevotella shahii, but with a higher proportion in controls. A previous study performed on stool samples showed that members of the Prevotellaceae family were found at significantly lower levels in PD patients’ gut microbiota compared to controls [19]. Associations between microbial profiles and a given pathology are not necessarily expected to be the same across body locations. Differences in changes of the relative abundance of Prevotellaceae and/or their specific members, observed from different body sites when PD patients are compared to control subjects, may reflect differences in colonization of body sites by bacterial strains and species [37]. Mechanisms by which gut and oral dysbiosis are linked and may be a contributing factor to the pathophysiology of Parkinson’s disease remains a subject of active research.
Our study presents several limitations. Firstly, the sample size was relatively small to draw definitive conclusions. However, participants were well examined with a detailed neurological, dental and periodontal testings. Secondly, our patient sample included early and mid-stage PD patients. We cannot exclude the possibility of the microbiota profile being different in different stages of the disease and also potentially being associated with a different prognosis. Our sample was however homogeneous in terms of the motor and the non-motor clinical signs. In addition, it has been demonstrated that gut microbiota is remarkably stable in PD patients over a 2-year period [97, 98]. Thirdly, no information about the dietary habits of our subjects were presented in our study. We cannot exclude the possibility that diet may have influenced the oral microbiome composition and inflammation level. There is still conflicting evidence regarding the relationship between dietary factors, the oral microbiome and inflammation [99–101]. Some recent studies, employing microarray and metataxonomic approaches, concluded that diet has little influence on salivary bacterial profiles [102, 103]. The primary substrates for oral bacterial growth are endogenous nutrients provided by saliva, tissue exudates, crevicular fluids, degenerating host cells, or metabolites from other bacteria [104], not directly derived from the food ingested. However, dietary intake is an important factor that influences these endogenous nutritional environments through systemic circulation. No significant difference was found in body-mass-index between study groups, arguing against major dietary differences. Given the assortment of potential health effects of oral microbiome, an increase in knowledge concerning how host lifestyle factors influence human oral microbial composition is warranted. Fourthly, most of our patients were taking dopamine replacement therapy and we did not have adequate representation of unmedicated subjects to fully evaluate the consequences of treatment on the outcomes. Fifthly, while our study focused on the taxonomic composition of the microbiota, PD might be associated with changes in microbial metabolic pathways that can be investigated by means of metagenomics, metatranscriptomics, metaproteomics and metabolomics. However, the use of 16S rRNA-gene based metataxonomic approach made comparisons with previously published data easier, since the majority of published studies used this method. Finally, we only assessed the oral microbiome on a single occasion, and it would be interesting to follow patients over time in order to investigate the influence of PD progression on microbiota composition.
In conclusion, our findings suggest that the oral microbiome is altered in PD. Oral inflammation is present in PD and probably precedes poor oral health. Additional studies are warranted to further elucidate the causal relationships between oral dysbiosis and the pathogenesis and clinical manifestations of PD, as well as the suitability of an analysis of oral microbiota as a potential biomarker or therapeutic target for PD.
CONFLICT OF INTEREST
The authors have no financial disclosures and no conflicts of interest concerning the research related to the manuscript.
ACKNOWLEDGMENTS
We would like to thank Lotfi Hatjas for his help during the recruitment phase of the study. We thank Dr Michael Nissen for his assistance for proofreading.
This study was supported by a grant from the Association Swiss Parkinson.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-202459.
REFERENCES
[1] | Poewe W , Seppi K , Tanner CM , Halliday GM , Brundin P , Volkmann J , Schrag AE , Lang AE ((2017) ) Parkinson disease. Nat Rev Dis Primers 3: , 17013. |
[2] | Spillantini MG , Schmidt ML , Lee VM , Trojanowski JQ , Jakes R , Goedert M ((1997) ) Alpha-synuclein in Lewy bodies. Nature 388: , 839–840. |
[3] | Mu L , Chen J , Sobotka S , Nyirenda T , Benson B , Gupta F , Sanders I , Adler CH , Caviness JN , Shill HA , Sabbagh M , Samanta JE , Sue LI , Beach TG , Arizona Parkinson’s Disease Consortium ((2015) ) Alpha-synuclein pathology in sensory nerve terminals of the upper aerodigestive tract of Parkinson’s disease patients. Dysphagia 30: , 404–417. |
[4] | Stokholm MG , Danielsen EH , Hamilton-Dutoit SJ , Borghammer P ((2016) ) Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol 79: , 940–949. |
[5] | Vilas D , Iranzo A , Tolosa E , Aldecoa I , Berenguer J , Vilaseca I , Marti C , Serradell M , Lomena F , Alos L , Gaig C , Santamaria J , Gelpi E ((2016) ) Assessment of alpha-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: A case-control study. Lancet Neurol 15: , 708–718. |
[6] | Hilton D , Stephens M , Kirk L , Edwards P , Potter R , Zajicek J , Broughton E , Hagan H , Carroll C ((2014) ) Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol 127: , 235–241. |
[7] | Fasano A , Visanji NP , Liu LW , Lang AE , Pfeiffer RF ((2015) ) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 14: , 625–639. |
[8] | Beach TG , Adler CH , Sue LI , Vedders L , Lue L , White Iii CL , Akiyama H , Caviness JN , Shill HA , Sabbagh MN , Walker DG , Arizona Parkinson’s Disease Consortium ((2010) ) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119: , 689–702. |
[9] | Adler CH , Dugger BN , Hentz JG , Hinni ML , Lott DG , Driver-Dunckley E , Mehta S , Serrano G , Sue LI , Duffy A , Intorcia A , Filon J , Pullen J , Walker DG , Beach TG ((2016) ) Peripheral synucleinopathy in early Parkinson’s disease: Submandibular gland needle biopsy findings. Mov Disord 31: , 250–256. |
[10] | Adler CH , Dugger BN , Hinni ML , Lott DG , Driver-Dunckley E , Hidalgo J , Henry-Watson J , Serrano G , Sue LI , Nagel T , Duffy A , Shill HA , Akiyama H , Walker DG , Beach TG ((2014) ) Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology 82: , 858–864. |
[11] | Manne S , Kondru N , Jin H , Anantharam V , Huang X , Kanthasamy A , Kanthasamy AG ((2020) ) alpha-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov Disord 35: , 268–278. |
[12] | Adler CH , Beach TG ((2016) ) Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov Disord 31: , 1114–1119. |
[13] | Cersosimo MG , Tumilasci OR , Raina GB , Benarroch EE , Cardoso EM , Micheli F , Pazo JH ((2009) ) Hyposialorrhea as an early manifestation of Parkinson disease. Auton Neurosci 150: , 150–151. |
[14] | Noyce AJ , Silveira-Moriyama L , Gilpin P , Ling H , Howard R , Lees AJ ((2012) ) Severe dysphagia as a presentation of Parkinson’s disease. Mov Disord 27: , 457–458. |
[15] | Hanaoka A , Kashihara K ((2009) ) Increased frequencies of caries, periodontal disease and tooth loss in patients with Parkinson’s disease. J Clin Neurosci 16: , 1279–1282. |
[16] | Müller T , Palluch R , Jackowski J ((2011) ) Caries and periodontal disease in patients with Parkinson’s disease. Spec Care Dentist 31: , 178–181. |
[17] | Zlotnik Y , Balash Y , Korczyn AD , Giladi N , Gurevich T ((2015) ) Disorders of the oral cavity in Parkinson’s disease and parkinsonian syndromes. Parkinsons Dis 2015: , 379482. |
[18] | Gill SR , Pop M , Deboy RT , Eckburg PB , Turnbaugh PJ , Samuel BS , Gordon JI , Relman DA , Fraser-Liggett CM , Nelson KE ((2006) ) Metagenomic analysis of the human distal gut microbiome. Science 312: , 1355–1359. |
[19] | Scheperjans F , Aho V , Pereira PA , Koskinen K , Paulin L , Pekkonen E , Haapaniemi E , Kaakkola S , Eerola-Rautio J , Pohja M , Kinnunen E , Murros K , Auvinen P ((2015) ) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30: , 350–358. |
[20] | Keshavarzian A , Green SJ , Engen PA , Voigt RM , Naqib A , Forsyth CB , Mutlu E , Shannon KM ((2015) ) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30: , 1351–1360. |
[21] | Hasegawa S , Goto S , Tsuji H , Okuno T , Asahara T , Nomoto K , Shibata A , Fujisawa Y , Minato T , Okamoto A , Ohno K , Hirayama M ((2015) ) Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One 10: , e0142164. |
[22] | Unger MM , Spiegel J , Dillmann KU , Grundmann D , Philippeit H , Burmann J , Fassbender K , Schwiertz A , Schafer KH ((2016) ) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32: , 66–72. |
[23] | Petrov VA , Saltykova IV , Zhukova IA , Alifirova VM , Zhukova NG , Dorofeeva YB , Tyakht AV , Kovarsky BA , Alekseev DG , Kostryukova ES , Mironova YS , Izhboldina OP , Nikitina MA , Perevozchikova TV , Fait EA , Babenko VV , Vakhitova MT , Govorun VM , Sazonov AE ((2017) ) Analysis of gut microbiota in patients with Parkinson’s disease. Bull Exp Biol Med 162: , 734–737. |
[24] | Bedarf JR , Hildebrand F , Coelho LP , Sunagawa S , Bahram M , Goeser F , Bork P , Wullner U ((2017) ) Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med 9: , 39. |
[25] | Forsyth CB , Shannon KM , Kordower JH , Voigt RM , Shaikh M , Jaglin JA , Estes JD , Dodiya HB , Keshavarzian A ((2011) ) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6: , e28032. |
[26] | Schwiertz A , Spiegel J , Dillmann U , Grundmann D , Burmann J , Fassbender K , Schafer KH , Unger MM ((2018) ) Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord 50: , 104–107. |
[27] | Devos D , Lebouvier T , Lardeux B , Biraud M , Rouaud T , Pouclet H , Coron E , Bruley des Varannes S , Naveilhan P , Nguyen JM , Neunlist M , Derkinderen P ((2013) ) Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50: , 42–48. |
[28] | Perez-Pardo P , Dodiya HB , Engen PA , Naqib A , Forsyth CB , Green SJ , Garssen J , Keshavarzian A , Kraneveld AD ((2018) ) Gut bacterial composition in a mouse model of Parkinson’s disease. Benef Microbes 9: , 799–814. |
[29] | Pott Godoy MC , Tarelli R , Ferrari CC , Sarchi MI , Pitossi FJ ((2008) ) Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain 131: , 1880–1894. |
[30] | Sampson TR , Debelius JW , Thron T , Janssen S , Shastri GG , Ilhan ZE , Challis C , Schretter CE , Rocha S , Gradinaru V , Chesselet MF , Keshavarzian A , Shannon KM , Krajmalnik-Brown R , Wittung-Stafshede P , Knight R , Mazmanian SK ((2016) ) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167: , 1469–1480 e1412. |
[31] | Peelaerts W , Bousset L , Van der Perren A , Moskalyuk A , Pulizzi R , Giugliano M , Van den Haute C , Melki R , Baekelandt V ((2015) ) alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522: , 340–344. |
[32] | Holmqvist S , Chutna O , Bousset L , Aldrin-Kirk P , Li W , Bjorklund T , Wang ZY , Roybon L , Melki R , Li JY ((2014) ) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128: , 805–820. |
[33] | Pan-Montojo F , Schwarz M , Winkler C , Arnhold M , O’Sullivan GA , Pal A , Said J , Marsico G , Verbavatz JM , Rodrigo-Angulo M , Gille G , Funk RH , Reichmann H ((2012) ) Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2: , 898. |
[34] | Breen DP , Halliday GM , Lang AE ((2019) ) Gut-brain axis and the spread of alpha-synuclein pathology: Vagal highway or dead end? Mov Disord 34: , 307–316. |
[35] | Hawkes CH , Del Tredici K , Braak H ((2007) ) Parkinson’s disease: A dual-hit hypothesis. Neuropathol Appl Neurobiol 33: , 599–614. |
[36] | Dunning CJR J.F. R , Steiner JA , Brundin P ((2012) ) Can Parkinson’s disease pathology be propagated from one neuron to another? Progr Neurobiol 97: , 205–219. |
[37] | Pereira PAB , Aho VTE , Paulin L , Pekkonen E , Auvinen P , Scheperjans F ((2017) ) Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat Disord 38: , 61–67. |
[38] | Mihaila D , Donegan J , Barns S , LaRocca D , Du Q , Zheng D , Vidal M , Neville C , Uhlig R , Middleton FA ((2019) ) The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function. PLoS One 14: , e0218252. |
[39] | Lamont RJ , Koo H , Hajishengallis G ((2018) ) The oral microbiota: Dynamic communities and host interactions. Nat Rev Microbiol 16: , 745–759. |
[40] | Ursell LK , Clemente JC , Rideout JR , Gevers D , Caporaso JG , Knight R ((2012) ) The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J Allergy Clin Immunol 129: , 1204–1208. |
[41] | Wade WG ((2013) ) The oral microbiome in health and disease. Pharmacol Res 69: , 137–143. |
[42] | Kumar PS ((2013) ) Oral microbiota and systemic disease. Anaerobe 24: , 90–93. |
[43] | Astafurov K , Elhawy E , Ren L , Dong CQ , Igboin C , Hyman L , Griffen A , Mittag T , Danias J ((2014) ) Oral microbiome link to neurodegeneration in glaucoma. PLoS One 9: , e104416. |
[44] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[45] | Goetz CG , Tilley BC , Shaftman SR , Stebbins GT , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stern MB , Dodel R , Dubois B , Holloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , LeWitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , van Hilten JJ , LaPelle N , Movement Disorder Society UPDRS Revision Task Force ((2008) ) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23: , 2129–2170. |
[46] | Goetz CG , Poewe W , Rascol O , Sampaio C , Stebbins GT , Counsell C , Giladi N , Holloway RG , Moore CG , Wenning GK , Yahr MD , Seidl L , Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease ((2004) ) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov Disord 19: , 1020–1028. |
[47] | Chaudhuri KR , Martinez-Martin P , Schapira AH , Stocchi F , Sethi K , Odin P , Brown RG , Koller W , Barone P , MacPhee G , Kelly L , Rabey M , MacMahon D , Thomas S , Ondo W , Rye D , Forbes A , Tluk S , Dhawan V , Bowron A , Williams AJ , Olanow CW ((2006) ) International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov Disord 21: , 916–923. |
[48] | Zigmond AS , Snaith RP ((1983) ) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: , 361–370. |
[49] | Nasreddine ZS , Phillips NA , Bedirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[50] | Perez Lloret S , Piran Arce G , Rossi M , Caivano Nemet ML , Salsamendi P , Merello M ((2007) ) Validation of a new scale for the evaluation of sialorrhea in patients with Parkinson’s disease. Mov Disord 22: , 107–111. |
[51] | Laine M , Pienihakkinen K , Leimola-Virtanen R ((1999) ) The effect of repeated sampling on paraffin-stimulated salivary flow rates in menopausal women. Arch Oral Biol 44: , 93–95. |
[52] | Manor Y , Giladi N , Cohen A , Fliss DM , Cohen JT ((2007) ) Validation of a swallowing disturbance questionnaire for detecting dysphagia in patients with Parkinson’s disease. Mov Disord 22: , 1917–1921. |
[53] | Lewis SJ , Heaton KW ((1997) ) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32: , 920–924. |
[54] | Becker T , Levin L , Shochat T , Einy S ((2007) ) How much does the DMFT index underestimate the need for restorative care? J Dent Educ 71: , 677–681. |
[55] | Silness J , Loe H ((1964) ) Periodontal disease in pregnancy. Ii. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 22: , 121–135. |
[56] | Newbrun E ((1996) ) Indices to measure gingival bleeding. J Periodontol 67: , 555–561. |
[57] | Zekeridou A , Giannopoulou C , Cancela J , Courvoisier D , Mombelli A ((2017) ) Effect of initial periodontal therapy on gingival crevicular fluid cytokine profile in subjects with chronic periodontitis. Clin Exp Dent Res 3: , 62–68. |
[58] | Everard A , Lazarevic V , Gaia N , Johansson M , Stahlman M , Backhed F , Delzenne NM , Schrenzel J , Francois P , Cani PD ((2014) ) Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J 8: , 2116–2130. |
[59] | Edgar RC (2016) UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. BioRxiv, doi: https://doi.org/10.1101/081257. |
[60] | Edgar RC ((2013) ) UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10: , 996–998. |
[61] | Schloss PD , Westcott SL , Ryabin T , Hall JR , Hartmann M , Hollister EB , Lesniewski RA , Oakley BB , Parks DH , Robinson CJ , Sahl JW , Stres B , Thallinger GG , Van Horn DJ , Weber CF ((2009) ) Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75: , 7537–7541. |
[62] | Bray JR , Curtis JT ((1957) ) An ordination of upland forest communities of southern Wisconsin. Ecol Monogr 27: , 325–349. |
[63] | Anderson MJ , Willis TJ ((2003) ) Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 84: , 511–525. |
[64] | Love MI , Huber W , Anders S ((2014) ) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: , 550–550. |
[65] | Kennedy MA , Rosen S , Paulson GW , Jolly DE , Beck FM ((1994) ) Relationship of oral microflora with oral health status in Parkinson’s disease. Spec Care Dentist 14: , 164–168. |
[66] | Liu J , Wu C , Huang IH , Merritt J , Qi F ((2011) ) Differential response of Streptococcus mutans towards friend and foe in mixed-species cultures. Microbiology 157: , 2433–2444. |
[67] | Loesche WJ ((1986) ) Role of Streptococcus mutans in human dental decay. Microbiol Rev 50: , 353–380. |
[68] | Schulze-Schweifing K , Banerjee A , Wade WG ((2014) ) Comparison of bacterial culture and 16S rRNA community profiling by clonal analysis and pyrosequencing for the characterization of the dentine caries-associated microbiome. Front Cell Infect Microbiol 4: , 164. |
[69] | Tanner AC ((2015) ) Anaerobic culture to detect periodontal and caries pathogens. J Oral Biosci 57: , 18–26. |
[70] | Chen C ((1996) ) Distribution of a newly described species, Kingella oralis, in the human oral cavity. Oral Microbiol Immunol 11: , 425–427. |
[71] | Griffen AL , Beall CJ , Campbell JH , Firestone ND , Kumar PS , Yang ZK , Podar M , Leys EJ ((2012) ) Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J 6: , 1176–1185. |
[72] | van Stiphout MAE , Marinus J , van Hilten JJ , Lobbezoo F , de Baat C ((2018) ) Oral health of Parkinson’s disease patients: A case-control study. Parkinsons Dis 2018: , 9315285. |
[73] | Oli MW , Otoo HN , Crowley PJ , Heim KP , Nascimento MM , Ramsook CB , Lipke PN , Brady LJ ((2012) ) Functional amyloid formation by Streptococcus mutans. Microbiology 158: , 2903–2916. |
[74] | Friedland RP , Chapman MR ((2017) ) The role of microbial amyloid in neurodegeneration. PLoS Pathog 13: , e1006654. |
[75] | Chen SG , Stribinskis V , Rane MJ , Demuth DR , Gozal E , Roberts AM , Jagadapillai R , Liu R , Choe K , Shivakumar B , Son F , Jin S , Kerber R , Adame A , Masliah E , Friedland RP ((2016) ) Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci Rep 6: , 34477. |
[76] | Tukel C , Nishimori JH , Wilson RP , Winter MG , Keestra AM , van Putten JP , Baumler AJ ((2010) ) Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell Microbiol 12: , 1495–1505. |
[77] | Daniele SG , Beraud D , Davenport C , Cheng K , Yin H , Maguire-Zeiss KA ((2015) ) Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci Signal 8: , ra45. |
[78] | Mertsalmi TH , Pekkonen E , Scheperjans F ((2020) ) Antibiotic exposure and risk of Parkinson’s disease in Finland: A nationwide case-control study. Mov Disord 35: , 431–442. |
[79] | Arend WP , Gabay C ((2000) ) Physiologic role of interleukin-1 receptor antagonist. Arthritis Res 2: , 245–248. |
[80] | Zekeridou A , Mombelli A , Cancela J , Courvoisier D , Giannopoulou C ((2018) ) Systemic inflammatory burden and local inflammation in periodontitis: What is the link between inflammatory biomarkers in serum and gingival crevicular fluid? Clin Exp Dent Res 5: , 128–135. |
[81] | Schonhoff AM , Williams GP , Wallen ZD , Standaert DG , Harms AS ((2020) ) Innate and adaptive immune responses in Parkinson’s disease. Prog Brain Res 252: , 169–216. |
[82] | Kim R , Kim HJ , Kim A , Jang M , Kim A , Kim Y , Yoo D , Im JH , Choi JH , Jeon B ((2018) ) Peripheral blood inflammatory markers in early Parkinson’s disease. J Clin Neurosci 58: , 30–33. |
[83] | Qin XY , Zhang SP , Cao C , Loh YP , Cheng Y ((2016) ) Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol 73: , 1316–1324. |
[84] | Nagatsu T , Mogi M , Ichinose H , Togari A (2000) Cytokines in Parkinson’s disease. J Neural Transm Suppl, pp. 143-151. |
[85] | Sawada M , Imamura K , Nagatsu T (2006) Role of cytokines in inflammatory process in Parkinson’s disease. J Neural Transm Suppl, pp. 373-381. |
[86] | Singh SS , Rai SN , Birla H , Zahra W , Rathore AS , Singh SP ((2020) ) NF-kappaB-mediated neuroinflammation in Parkinson’s disease and potential therapeutic effect of polyphenols. Neurotox Res 37: , 491–507. |
[87] | Elfil M , Kamel S , Kandil M , Koo BB , Schaefer SM ((2020) ) Implications of the gut microbiome in Parkinson’s disease. Mov Disord 35: , 921–933. |
[88] | Olsen I , Yamazaki K ((2019) ) Can oral bacteria affect the microbiome of the gut? J Oral Microbiol 11: , 1586422. |
[89] | Seedorf H , Griffin NW , Ridaura VK , Reyes A , Cheng J , Rey FE , Smith MI , Simon GM , Scheffrahn RH , Woebken D , Spormann AM , Van Treuren W , Ursell LK , Pirrung M , Robbins-Pianka A , Cantarel BL , Lombard V , Henrissat B , Knight R , Gordon JI ((2014) ) Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 159: , 253–266. |
[90] | Arimatsu K , Yamada H , Miyazawa H , Minagawa T , Nakajima M , Ryder MI , Gotoh K , Motooka D , Nakamura S , Iida T , Yamazaki K ((2014) ) Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep 4: , 4828. |
[91] | Qin N , Yang F , Li A , Prifti E , Chen Y , Shao L , Guo J , Le Chatelier E , Yao J , Wu L , Zhou J , Ni S , Liu L , Pons N , Batto JM , Kennedy SP , Leonard P , Yuan C , Ding W , Chen Y , Hu X , Zheng B , Qian G , Xu W , Ehrlich SD , Zheng S , Li L ((2014) ) Alterations of the human gut microbiome in liver cirrhosis. Nature 513: , 59–64. |
[92] | Zhang X , Zhang D , Jia H , Feng Q , Wang D , Liang D , Wu X , Li J , Tang L , Li Y , Lan Z , Chen B , Li Y , Zhong H , Xie H , Jie Z , Chen W , Tang S , Xu X , Wang X , Cai X , Liu S , Xia Y , Li J , Qiao X , Al-Aama JY , Chen H , Wang L , Wu QJ , Zhang F , Zheng W , Li Y , Zhang M , Luo G , Xue W , Xiao L , Li J , Chen W , Xu X , Yin Y , Yang H , Wang J , Kristiansen K , Liu L , Li T , Huang Q , Li Y , Wang J ((2015) ) The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med 21: , 895–905. |
[93] | Lourenvarsigmao TGB , Spencer SJ , Alm EJ , Colombo APV ((2018) ) Defining the gut microbiota in individuals with periodontal diseases: An exploratory study. J Oral Microbiol 10: , 1487741. |
[94] | Hopfner F , Kunstner A , Muller SH , Kunzel S , Zeuner KE , Margraf NG , Deuschl G , Baines JF , Kuhlenbaumer G ((2017) ) Gut microbiota in Parkinson disease in a northern German cohort. Brain Res 1667: , 41–45. |
[95] | Hill-Burns EM , Debelius JW , Morton JT , Wissemann WT , Lewis MR , Wallen ZD , Peddada SD , Factor SA , Molho E , Zabetian CP , Knight R , Payami H ((2017) ) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32: , 739–749. |
[96] | Lin CH , Chen CC , Chiang HL , Liou JM , Chang CM , Lu TP , Chuang EY , Tai YC , Cheng C , Lin HY , Wu MS ((2019) ) Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflammation 16: , 129. |
[97] | Aho VTE , Pereira PAB , Voutilainen S , Paulin L , Pekkonen E , Auvinen P , Scheperjans F ((2019) ) Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine 44: , 691–707. |
[98] | Minato T , Maeda T , Fujisawa Y , Tsuji H , Nomoto K , Ohno K , Hirayama M ((2017) ) Progression of Parkinson’s disease is associated with gut dysbiosis: Two-year follow-up study. PLoS One 12: , e0187307. |
[99] | Kato I , Vasquez A , Moyerbrailean G , Land S , Djuric Z , Sun J , Lin HS , Ram JL ((2017) ) Nutritional correlates of human oral microbiome. J Am Coll Nutr 36: , 88–98. |
[100] | Murtaza N , Burke LM , Vlahovich N , Charlesson B , O’Neill HM , Ross ML , Campbell KL , Krause L , Morrison M ((2019) ) Analysis of the effects of dietary pattern on the oral microbiome of elite endurance athletes. Nutrients 11: . |
[101] | Adler CJ , Dobney K , Weyrich LS , Kaidonis J , Walker AW , Haak W , Bradshaw CJ , Townsend G , Soltysiak A , Alt KW , Parkhill J , Cooper A ((2013) ) Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat Genet 45: , 450–455 455e451. |
[102] | Belstrom D , Holmstrup P , Nielsen CH , Kirkby N , Twetman S , Heitmann BL , Klepac-Ceraj V , Paster BJ , Fiehn NE ((2014) ) Bacterial profiles of saliva in relation to diet, lifestyle factors, and socioeconomic status. J Oral Microbiol 6: , doi: 10.3402/jom.v6.23609 |
[103] | De Filippis F , Vannini L , La Storia A , Laghi L , Piombino P , Stellato G , Serrazanetti DI , Gozzi G , Turroni S , Ferrocino I , Lazzi C , Di Cagno R , Gobbetti M , Ercolini D ((2014) ) The same microbiota and a potentially discriminant metabolome in the saliva of omnivore, ovo-lacto-vegetarian and Vegan individuals. PLoS One 9: , e112373. |
[104] | Morhart RE , Fitzgerald RJ ((1976) ) Nutritional determinants of the ecology of the oral flora. Dent Clin North Am 20: , 473–489. |



