Current Update on Clinically Relevant Sleep Issues in Parkinson’s Disease: A Narrative Review
Abstract
Sleep disturbances are among the common nonmotor symptoms in patients with Parkinson’s disease (PD). Sleep can be disrupted by nocturnal motor and nonmotor symptoms and other comorbid sleep disorders. Rapid eye movement sleep behavior disorder (RBD) causes sleep-related injury, has important clinical implications as a harbinger of PD and predicts a progressive clinical phenotype. Restless legs syndrome (RLS) and its related symptoms can impair sleep initiation. Excessive daytime sleepiness (EDS) is a refractory problem affecting patients’ daytime activities. In particular, during the COVID-19 era, special attention should be paid to monitoring sleep problems, as infection-prevention procedures for COVID-19 can affect patients’ motor symptoms, psychiatric symptoms and sleep. Therefore, screening for and managing sleep problems is important in clinical practice, and the maintenance of good sleep conditions may improve the quality of life of PD patients. This narrative review focused on the literature published in the past 10 years, providing a current update of various sleep disturbances in PD patients and their management, including RBD, RLS, EDS, sleep apnea and circadian abnormalities.
INTRODUCTION
Sleep-related symptoms, such as daytime sleepiness and insomnia, are among the typical common nonmotor symptoms in Parkinson’s disease (PD). It is important to understand that sleep disorders are comparable to motor symptoms and impair patient quality of life. A recent multicenter study showed that sleep problems were found in 66%of PD patients and were related to a worse quality of life and greater nonmotor symptom burden [1]. In addition to secondary sleep disorders caused by parkinsonism, the causes of sleep disorders include pathological degeneration in the sleep-wake regulatory center of the brainstem and hypothalamus. The sleep disorders associated with PD are diverse, and various factors overlap.
In the era of coronavirus disease 2019 (COVID-19), a respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which started in January 2020 [2], infection-prevention procedures such as lockdown, social distancing and isolation have been reported to affect sleep or mood in individuals who do not have COVID-19 [3, 4]. Neurologists who specialize in sleep disorders noticed increased sleep disorders during the COVID-19 pandemic, termed “COVID-somnia” [5]. Fear of infection, anxiety, and isolation, reduced exposure to sunlight, excessive daytime napping, and the excessive use of electronic media contribute to disrupted sleep patterns.
Among patients with PD, social isolation has been associated with greater disease severity and lower quality of life [6]. The COVID-19 pandemic may also worsen motor symptoms and nonmotor symptoms, including sleep problems, due to decreased physical activity and increased stress [7]. During the COVID-19 pandemic, sleep disturbances in PD patients were reported to be independently related to worsening motor symptoms and anxiety [8]. Therefore, sleep problems are currently the central issue regarding nonmotor symptoms in PD.
A literature search for this narrative review was conducted from August 2020 to October 2020 using the PubMed/Medline and Web of Science databases. English-language articles were identified, focusing on the literature published in the past 10 years. The term “Parkinson’s disease” was combined with the terms “sleep”, “rapid eye movement sleep disorder”, “restless legs syndrome”, “excessive daytime sleepiness”, “circadian abnormality”, and “sleep apnea”. The important studies published before 2010 that were cited in the recent studies identified in this search were also included in this review. I will review the updated findings, including those pertaining to treatment, regarding various sleep problems, such as excessive daytime sleepiness (EDS), rapid eye movement (REM) sleep behavior disorder (RBD), sleep apnea and restless legs syndrome (RLS), as well as circadian abnormalities in PD.
NIGHTTIME AND DAYTIME SLEEP PROBLEMS IN PD
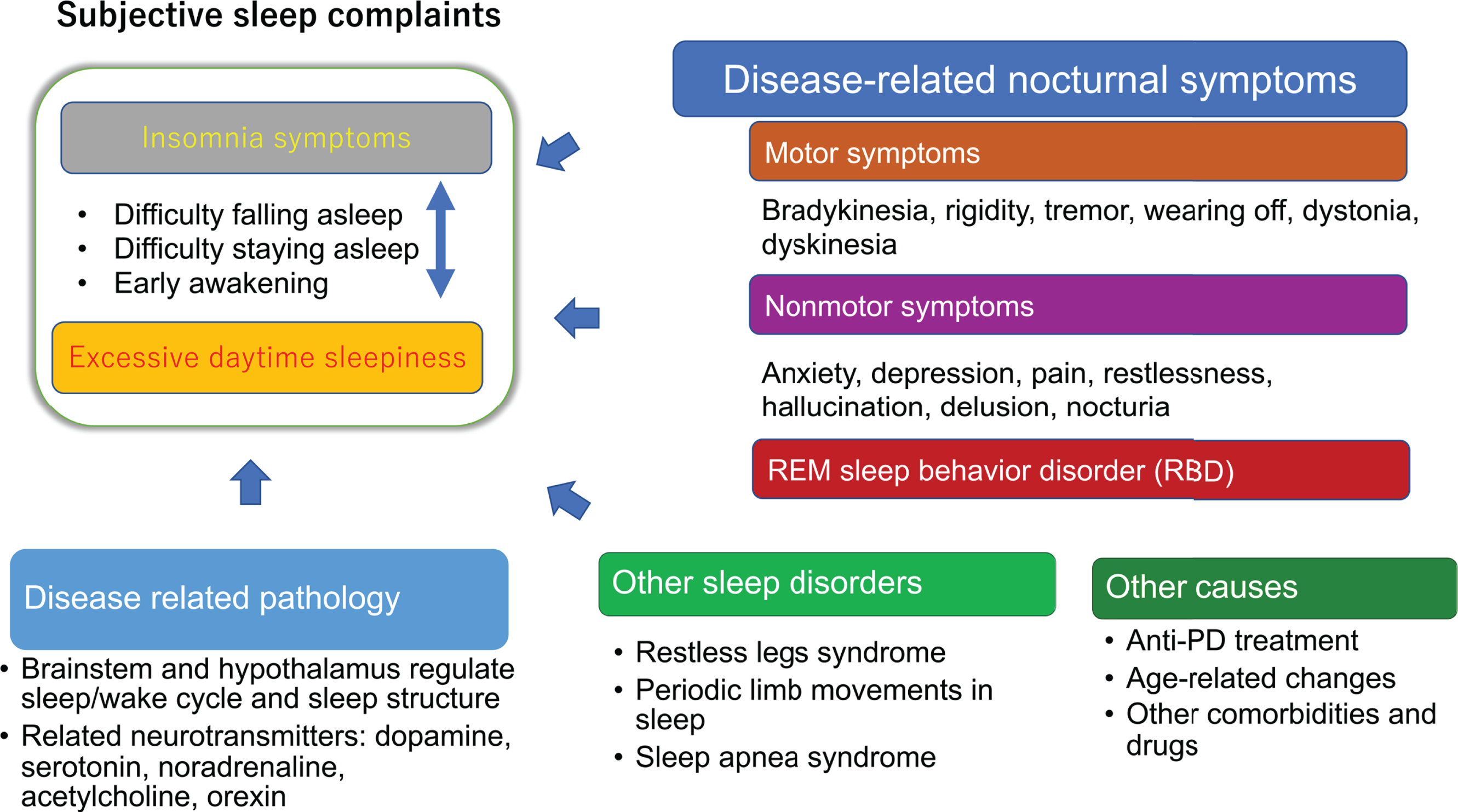
Figure 1 shows the cause of insomnia and EDS in PD. Insomnia symptoms (sleep-onset insomnia, sleep-maintenance insomnia and early awakening) and EDS have a bidirectional relationship. For example, disrupted or insufficient sleep in the previous night can cause EDS the following day, while too many daytime naps can cause short nighttime sleep and insomnia symptoms that night. However, there are some exceptions: narcolepsy phenotypes unrelated to sleep status have been found in 24–39%of PD patients, indicating impairment in arousal systems [9, 10]. Alternatively, many patients with insomnia experience a state of hyperarousal; they do not feel sleepy during the day and cannot nap [11]. Nocturnal motor and nonmotor symptoms, RBD and other sleep disorders, such as sleep apnea syndrome and RLS, and periodic limb movements in sleep can contribute to insomnia symptoms, and disease-related pathology in the brainstem and hypothalamus may play a role in EDS (Fig. 1).
Fig. 1
Sleep disturbances in patients with Parkinson’s disease. An arrow represents a possible causal influence, and a two-way arrow indicates a bidirectional relationship.
In a study using wearable sensors, compared with their spouses, PD patients turned less; their turns were characterized by a slower speed, less acceleration and fewer degrees; and they spent more time in the supine position. Nocturnal hypokinesia and getting out of bed due to nocturia were observed in patients with PD, particularly in the second half of sleep [12]. This observation suggests that nocturnal hypokinesia progresses during the course of sleep. A multicenter study including 436 patients with PD showed that approximately one-third of the patients suffered from one of three sleep-related symptoms (EDS, RBD, and PD-related sleep problems as assessed by the PD Sleep Scale (PDSS)-2); one-fifth of the patients had two of these symptoms, and one-eighth had all three symptoms [13] (Fig. 2). Additionally, the number of these sleep-related symptoms the patients experienced contributed to the worsening of disease-related disability, highlighting the need for the assessment of sleep problems.
Fig. 2
Prevalence and overlap of sleep-related symptoms in PD patients (reproduced from [13] with Creative Commons CC BY 4.0 license). EDS, excessive daytime sleepiness; PD-SP, PD-related sleep problems; pRBD, probable rapid eye movement sleep behavior disorder.
![Prevalence and overlap of sleep-related symptoms in PD patients (reproduced from [13] with Creative Commons CC BY 4.0 license). EDS, excessive daytime sleepiness; PD-SP, PD-related sleep problems; pRBD, probable rapid eye movement sleep behavior disorder.](https://content.iospress.com:443/media/jpd/2021/11-3/jpd-11-3-jpd202425/jpd-11-jpd202425-g002.jpg)
Insomnia symptoms include difficulty initiating sleep (sleep-onset insomnia), difficulty maintaining sleep (sleep-maintenance insomnia) and early awakening. Sleep-maintenance insomnia and early awakening, but not sleep-onset insomnia, have been described more commonly in PD patients than in healthy controls. In an analysis of prospective studies involving patients with PD, the risk factors for insomnia included depression, long disease duration, female sex, the use of dopamine agonists and cognitive impairment [14]. However, insomnia can be seen in the untreated early stage and has been shown to increase in frequency and severity after dopaminergic treatment. A 5-year prospective study involving untreated PD patients showed that the rate of overall insomnia (including sleep-onset and sleep-maintenance insomnia) did not significantly change over the 5-year follow-up; however, the proportion of patients with sleep-maintenance insomnia increased from 31%to 49%, whereas the proportion of patients with sleep-onset insomnia decreased from 21%to 7.4%after 5 years [15]. Up to 80%of PD patients complain of sleep-maintenance insomnia or early awakening [16], likely resulting from multiple factors, such as nocturnal motor and nonmotor symptoms, medication use and comorbid sleep disorders [17]. The worsening of nocturnal motor symptoms or wearing off of medication could be the main reasons for disturbed sleep continuity. Early-morning off periods can occur in up to 52%of PD patients with early-stage disease [18]. Therefore, even in patients with early-stage disease, the appearance of wearing off phenomenon and related nocturnal symptoms should be carefully monitored.
In addition to motor symptoms, nonmotor symptoms such as pain, depression and anxiety have been associated with sleep problems [19, 20]. PD is now recognized as a systemic disease, the manifestations of which include nonmotor symptoms throughout the body [21]. Since nonmotor symptoms include symptoms that may respond to dopaminergic treatment or other medications depending on symptoms, it is important to recognize nonmotor symptoms in addition to motor symptoms.
Screening tool for PD-related sleep problems
Regarding the scales used for the assessment of sleep problems, the PDSS, the Pittsburgh Sleep Quality Index (PSQI), and Scales for Outcomes in Parkinson’s Disease–Sleep (SCOPA-sleep) are recommended for rating overall sleep problems, and the Epworth Sleepiness Scale (ESS) is recommended for assessing daytime sleepiness [22]. The ESS and PSQI are widely used but were not developed for PD patients. The PDSS-2 is a revised version of the PDSS that evaluates 15 PD-related sleep and nocturnal problems using a frequency measure ranging from 0 (never) to 4 (very frequent) [23]. Total PDSS-2 scores range from 0 to 60, with higher scores indicating greater severity [24]. Cutoff values for the PDSS-2 total score, namely, ≥15 for the detection of poor sleep [25] and≥18 for clinically relevant sleep problems [26], have been reported. When sleep-maintenance insomnia or early awakening is identified by PDSS-2 subitems, screening for motor symptoms (wearing off) and other nonmotor symptoms and adjustment of the dopaminergic medication dosage should be performed.
Treatment of insomnia
The first step in the treatment of insomnia is screening for and managing nocturnal symptoms. In patients with sleep-onset insomnia, RLS should be ruled out. Sleep-maintenance insomnia can result from various problems, such as motor symptoms, pain, nocturia, sleep apnea, anxiety, and depression. Initially, sleep hygiene instructions should be given, such as refraining from consuming caffeine and alcohol; refraining from smoking; avoiding naps; adjusting the bedroom environment; and getting regular exercise. In a meta-analysis on the effects of exercise on sleep quality in PD patients, regular exercise at a moderate to maximum level of intensity improved subjective sleep quality, but exercise at a mild to moderate level of intensity showed nonsignificant effects [27]. In a randomized controlled trial with PD patients assigned to an exercise group, which performed supervised exercise 3 times a week for 16 weeks, and a no exercise group, which engaged in in-person discussions and monthly phone calls about sleep hygiene, significant improvements in sleep efficiency, total sleep time, wake after sleep onset, and slow-wave sleep (SWS) were observed in the exercise group compared with the no exercise group [28].
Long-acting dopamine agonists, such as rotigotine, are useful for treating nocturnal PD-related symptoms [29]. In a 6-week, randomized, placebo-controlled trial in 30 PD patients, eszopiclone did not increase the total sleep time but improved sleep quality and decreased the number of awakenings [30]. Prolonged-release melatonin [31] and melatonin [32] have also been shown to be effective as treatments for insomnia in patients with PD in randomized, double-blind, placebo-controlled trials. Dual orexin receptor antagonists such as suvorexant and lemborexant, which have different actions relative to GABAA agonists, had fewer next-day residual effects [33] and were safe for non-PD individuals with comorbid sleep apnea [34]. Because of their safety and tolerability profile and lack of dependence, dual orexin receptor antagonists may be useful and recommended for treating insomnia in patients with various psychiatric and neurodegenerative diseases [35]. The treatment of irregular sleep-wake rhythm disorder associated with mild-to-moderate Alzheimer’s disease with lemborexant is under investigation [36].
EXCESSIVE DAYTIME SLEEPINESS
EDS refers to a condition in which patients feel sleepy during the day and fall asleep in situations in which they should stay awake, such as when working and driving, which interferes with their daily activities. In PD, EDS arises from the involvement of dopaminergic and nondopaminergic systems due to PD pathology and their interaction with chronic dopaminergic treatment and other causes associated with sleep disruption. Approximately 15–50%of patients with PD suffer from EDS [17]; however, these numbers should be interpreted with caution, as the definition of EDS may differ across studies. In addition to the aforementioned ESS, which is a subjective assessment of sleepiness, an objective assessment can be performed with the multiple sleep latency test (MSLT), which assesses sleep latency during four or five 20-minute opportunities to nap and the presence of a sleep-onset REM period (patients with narcolepsy show 2 or more episodes of a sleep-onset REM period out of 4 or 5 MSLT sessions). Narcolepsy is a sleep disorder characterized by EDS, cataplexy, hypnagogic hallucinations and sleep paralysis with markedly decreased orexin-A levels in the cerebrospinal fluid (CSF) and 2 or more sleep-onset REM periods during the MSLT. As described later in this review, a subset of PD patients present with a narcoleptic phenotype, but they usually lack cataplexy.
Pathology and imaging findings relevant to EDS
In PD, dopamine neurons of the substantia nigra pars compacta (SNpc) and dopamine content in the striatum are reduced by 60%and 80%, respectively, by the time motor symptoms appear [37]. The SNpc dopaminergic neurons project directly to the thalamocortical circuitry that promotes arousal and feed back to the ascending reticular activating system along with the ventral tegmental area (VTA) and the ventral periaqueductal gray matter. In PD, it is possible that the degeneration of these dopaminergic neurons can cause EDS [38]. In a study of 21 patients with early PD who underwent FP-CIT SPECT, there was no correlation between dopamine transporter (DAT) binding and motor function or disease severity, but the ESS scores were negatively correlated with DAT binding in the striatum, caudate nucleus, and putamen [39]. In an MPTP-induced PD model, EDS and sleep-onset REM periods were reversible with levodopa [40]. These observations may support dopaminergic contributions to EDS.
In PD, in addition to the dopaminergic system, the brainstem and hypothalamic nuclei, which are involved in sleep-wake regulation, are also affected. The degree of neuronal depletion involved in sleep-wake regulation is 37–55%in the serotoninergic raphe nuclei, 32–46%in the basal forebrain cholinergic system of the nucleus basalis of Meynert, 46%in the pedunculopontine tegmental nucleus/laterodorsal tegmental nucleus, and 63–78%in the noradrenergic locus coeruleus [38]. These findings may support a link between PD-related pathology and EDS. However, the relevance of the involvement of the brainstem and hypothalamic nuclei in the clinical manifestations of EDS is still uncertain. EDS was found relatively more frequently in patients with PD and PD with dementia; however, CSF hypocretin-1 (orexin-A) levels did not differ among controls, patients with PD and patients with PD with dementia, and there was no correlation between CSF orexin levels and ESS scores or sleep latency on the MSLT [41]. Regarding the interpretation of these findings, it should also be noted that in animal studies, a 50%decrease in CSF orexin levels requires the degeneration of 70%or more of the hypothalamic orexin neurons [42].
The serotonin transporter availability of dorsal raphe nuclei measured with 123I-FP-CIT SPECT was reduced in PD patients with resting tremor compared with those without resting tremor, and serotonin transporter availability was correlated with tremor amplitude but not ESS or RBD screening questionnaire scores [43]. On the other hand, a study using [11C]DASB PET showed that serotonin transporter availability in the caudate nucleus, putamen, ventral striatum, thalamus, hypothalamus, and raphe nuclei was significantly reduced in PD patients with sleep disturbances (PDSS scores < 82) compared with PD patients without sleep disturbances [44]. Further brain imaging studies on EDS and the clinical correlates are warranted.
Natural course of and risk factors for EDS in PD
In a multicenter study, EDS was found in 165 (37.8%) of 436 PD patients, and logistic regression analysis revealed that male sex, Movement Disorder Society (MDS)-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) III scores (motor symptoms), hallucinations, and MDS-UPDRS II scores (aspects of motor symptoms experienced in daily life) were associated with EDS [13]. In the analysis by clinical subtype, ESS scores were significantly higher in the patients with the postural instability and gait disturbance (PIGD) type than in those with the tremor-dominant type. The prevalence of EDS increases after the onset of PD motor symptoms. In an 8-year follow-up study of 232 PD patients, the proportion of patients with EDS increased from 5.6%at the beginning of the study to 22.5%at 4 years and 40.8%at 8 years [45]. Logistic regression analysis showed that factors contributing to EDS were older age, male sex, and dopamine agonist use, but in a subanalysis of the patients who were never treated with dopamine agonists, the predictor of EDS was Hoehn-Yahr (HY) stage alone. This result indicates that EDS is related to disease severity, independent of the effects of dopamine agonists.
In a 5-year follow-up study of 413 PD patients, EDS (Scales for Outcomes in Parkinson’s Disease-Sleep-Daytime Sleepiness (SCOPA-SLEEP-DS) score≥5) was found in 43%of the patients, and 46%of the patients without EDS at the start of the study subsequently developed EDS [46]. The factors associated with the development of EDS over time included male sex, poor nighttime sleep quality, cognitive impairment, autonomic dysfunction, hallucinations, mild dyskinesia, dopamine agonist use, and antihypertensive use. The predictors of the future development of EDS in patients without EDS at baseline were a higher baseline SCOPA-SLEEP-DS score, a PIGD phenotype and urinary tract symptoms. Furthermore, in a follow-up study of untreated PD patients, the proportion of patients with EDS increased from 11%at the start of the study to 23.4%at 5 years. Risk factors for the development of EDS at 5 years included an ESS score > 5 at baseline, male sex, dopamine agonist use, depressive symptoms and high UPDRS-Activities of Daily Living (ADL) scores [47]. Similarly, a prospective study using data from the Parkinson’s Progression Markers Initiative, which included 423 untreated PD patients within 2 years of diagnosis, showed an increase in ESS scores from baseline to 3 years [48]. EDS was related to a non-tremor-dominant phenotype, autonomic dysfunction, depression, anxiety, probable RBD, and dopaminergic therapy in a dose-dependent manner.
In a review article analyzing the results of 18 longitudinal studies on nonmotor symptoms, the risk factors for EDS were male sex, dopamine agonist use, insomnia, impairments in activities of daily living, cognitive impairment, and depression [14]. Therefore, disease progression, the use of dopaminergic treatment, susceptibility to sleepiness, and cognitive and psychiatric symptoms may all interact synergistically and contribute to the emergence of EDS.
Sleep episodes (or sleep attacks)
Sudden-onset sleep episodes (or sleep attacks) are potentially life-threatening problems that occur abruptly during daily activities. In the first report by Frucht et al. [49] in 1999, car accidents caused by sleep episodes in eight patients with PD who were taking nonergot dopamine agonists, such as pramipexole and ropinirole, were described. Subsequent studies reported that regardless of the type of specific dopamine agonist, the use of multiple dopamine agonists and higher doses of dopaminergic drugs were associated with an increased risk of sleep episodes [50]. In a multicenter study, the prevalence of sleep episodes was higher in PD patients than in controls (6.4%vs. 0.7%), and sleep episodes were more likely to occur in patients with EDS than in those without EDS (22.5%vs. 2.0%) [51]. The factors associated with sleep episodes were UPDRS part 1 scores (mental status), high ESS scores, and the use of high doses of dopaminergic drugs; an ESS score≥10 was predictive of sleep episodes, with a sensitivity of 75%and specificity of 82.4%. Similarly, Tan et al. [52] reported that sudden-onset sleep episodes were present in 13.4%of 201 patients with PD, and an ESS score≥10 was useful for predicting sleep episodes (sensitivity, 71.4%; specificity, 88.4%). In a study performed by the Canadian Movement Disorders Group including 638 PD patients [53], 3.8%of 420 drivers with PD experienced sudden-onset sleep episodes while driving, and 0.7%had no warning signs. An ESS score > 7 had a sensitivity of 75%and specificity of 50%for predicting sudden sleep episodes related to motor vehicle accidents. In contrast, a systematic review of sleep attacks and dopaminergic medications showed that 13%of PD patients on dopaminergic medications experienced sleep attacks. However, there were no significant differences in the ESS scores between patients with and without sleep attacks [54]. Thus, it should be noted that patients with EDS tend to have sudden-onset sleep episodes, but subjective sleepiness cannot always predict sudden-onset sleep episodes.
Disease-dopaminergic drug interactions and EDS
In the advanced stages of PD, the dosage of dopaminergic agents increases with the severity of the disease, and therefore, EDS and sleep episodes should always be monitored. EDS or sleep episodes can occur not only when taking higher dopamine agonist dosages but also when switching between the same type of dopamine agonists at equivalent dosages [55]. In a study including 54 levodopa-treated PD patients complaining of EDS, the mean sleep latency (MSL) was 6.3±0.6 min, 50%had an MSL less than 5 min, and 39%had a narcolepsy phenotype (2 or more sleep-onset REM periods during the 5 sessions of the MSLT) [9]. There was no correlation between the MSL and the daily dose of dopamine agonists or the total levodopa equivalent daily dose (LEDD); however, the levodopa daily dose was positively correlated with the MSL. In clinical studies, all classes of dopaminergic medications have been associated with EDS [17, 56].
In animal studies, D1 receptor agonists induce an increase in wakefulness and reductions in SWS and REM sleep, and D3 receptor agonists induce increases in SWS and REM sleep [57]. D2 receptor agonists induce biphasic effects: lower doses reduce wakefulness and increase SWS and REM sleep predominantly through the activation of D2 autoreceptors, whereas higher doses induce the opposite effect predominantly through the facilitation of D2 postsynaptic receptors. However, some PD patients in clinical settings have experienced increases in daytime sleepiness with the administration of higher dosages of dopaminergic treatment, and the effect was dose-dependent [58, 59]. Interestingly, a study showed that in patients with PD, higher doses of dopamine agonists were associated with reduced daytime alertness, whereas higher doses of levodopa were associated with enhanced alertness [60].
A study of 46 levodopa-treated patients with advanced PD found abnormal objective sleepiness (MSL < 5 min) in 41.3%of the patients on MSLT. Although 70%of those with ESS scores≥10 (i.e., with subjective daytime sleepiness) had an MSL < 10 min, subjective sleepiness did not correlate with the MSL and tended to be underestimated. The intensity of sleepiness (Karolinska Sleepiness Scale) just before the MSLT did not predict sleep latency [10]. Liguori et al. [61] compared daytime sleepiness between PD patients treated with the nocturnal administration of rotigotine and those receiving a placebo for 6 to 10 weeks. Rotigotine improved sleep efficiency, shortened sleep latency and reduced wake time after sleep onset. However, daytime sleepiness did not change, regardless of whether it was measured subjectively (ESS) or objectively (MSLT). The results implied that daytime sleepiness may be caused by an impairment of wakefulness related to PD pathology that is unrelated to nocturnal symptoms or sleep status.
Taken together, it can be speculated that EDS increases along with progressive neurodegeneration in the brainstem and hypothalamus pathology that regulates sleep/wake states and is complexly related to increased dosages of dopaminergic medication over the disease course. Furthermore, it seems that some subsets of PD patients have a narcoleptic phenotype.
Treatment for EDS and sleep episodes
Patients with EDS or sleep episodes (sleep attacks) should be instructed not to drive or engage in potentially hazardous tasks until their symptoms improve. Motor and nonmotor symptoms during the nighttime and comorbid sleep disorders such as RBD, RLS and sleep apnea should be identified, as sleep disturbance can cause EDS. In patients taking dopamine agonists, a dose reduction should be considered. Especially in patients whose EDS is temporally associated with the addition of or change in dopamine agonists, adjustments should be made, such as reducing the dose or discontinuing the possible causative agent. In a 6-week placebo-controlled, randomized trial of PD patients complaining of daytime somnolence (ESS≥10), drowsiness on the clinical global impression scale was significantly improved in the caffeine-treated group compared to the placebo group, although the ESS scores did not significantly change [62]. Some reports have shown the efficacy of modafinil for EDS [63]. In a 3-month open-label study, the adenosine A2A receptor antagonist istradefylline improved motor symptoms and daytime sleepiness without worsening nighttime sleep disturbances [64]. In a 12-week open-label study, the addition of the MAO-B inhibitor selegiline, for which a major metabolite is amphetamine, allowed a reduction in the dose of dopamine agonist, resulting in improvement of EDS [65]. In a double-blind, placebo-controlled study of 30 PD patients with sleep disorders treated with 1 mg of rasagiline, an MAO-B inhibitor, or a placebo for 8 weeks, the rasagiline group showed a decrease in wake after sleep onset and stage N1 duration on polysomnography (PSG) and improvements in daytime sleepiness [66]. Additionally, in a small-sample study, another MAO-B inhibitor, safinamide, improved subjective daytime sleepiness [67]. Thus, although sufficient evidence is lacking, the addition of nondopaminergic drugs and decreased doses of dopamine agonists could be a potential treatment for EDS in patients with PD.
REM SLEEP BEHAVIOR DISORDER
RBD is a parasomnia that causes complex dream-related movements and behaviors during REM sleep. In patients with RBD, the normal physiologic suppression of motor activity is impaired, which is called REM sleep without atonia (RWA), resulting in dream-enacting behavior. RBD that is not associated with neurological diseases, brain lesions, or medications such as antidepressants is called idiopathic or isolated RBD (iRBD) [68]. The detection of RWA during REM sleep on PSG is essential for a definitive diagnosis of RBD.
In a recent general population study in Switzerland, among 1,997 valid responses, initial screening with RBD questionnaires was positive in 368 subjects (18.4%) who subsequently underwent PSG. In that study, the iRBD prevalence was 1.1%, and those with iRBD had higher rates of smoking and antipsychotic and antidepressant usage, but no differences were found between the sexes [69]. In a population-based iRBD survey in 2,858 adults aged 65 years or older in Japan, after initial screening with the RBD questionnaire (RBD1Q), subsequent telephone interviews and face-to-face interviews were performed. Twenty-nine subjects eventually underwent PSG, which identified 18 patients with iRBD (iRBD prevalence of 1.2%). In their study, the male:female ratio was 2 : 1, and half of the iRBD patients had orthostatic hypotension and olfactory impairments [70].
Lesions responsible for RBD
There are several neuronal populations in the brainstem that regulate REM sleep. During REM sleep, ventrolateral periaqueductal gray neurons, the locus coeruleus, the raphe nucleus and the tuberomammillary nucleus are inactive, whereas the sublaterodorsal nucleus (locus subcoeruleus) and cholinergic pedunculopontine systems are active [71]. The sublaterodorsal nuclei have a role in initiating REM sleep and transitioning from NREM sleep, and for normal REM atonia. Glutamatergic projections from the sublaterodorsal nuclei activate gamma-aminobutyric acid/glycinergic ventral gigantocellular nuclei in the medulla, resulting in suppression of spinal motor neurons [72, 73]. In RBD, degeneration of the sublaterodorsal nuclei results in an inability to activate ventral gigantocellular nuclei, allowing the activation of spinal motor neurons, which results in abnormal motor behavior during REM sleep. Studies using neuromelanin-sensitive MRI revealed a decrease in intensity in a region of the coeruleus/subcoeruleus complex in iRBD patients [74] and in PD patients with RBD [75]. In a study using 18F-fluoroethoxybenzovesamicol PET imaging, cholinergic alterations, i.e., compensatory cholinergic upregulation, were found in multiple brain areas, such as brainstem structures involved in REM sleep regulation and muscle atonia in iRBD patients [76].
RBD and neurodegeneration
In a study reported by McCarter et al. [77], RWA analysis of the submentalis muscles (SM) was positive in 58.2%of 53 patients with synucleinopathies (33 PD and 20 multiple system atrophy (MSA) patients) and in 3.2%of 24 patients with tauopathies (17 with progressive supranuclear palsy and 7 with corticobasal degeneration). This study reported a sensitivity of 70%and a specificity of 100%for phasic and tonic muscle activities in SM (“SM any”) and a sensitivity of 77%and a specificity of 100%for SM phasic activity for distinguishing synucleinopathies from tauopathies, but RWA analysis of the anterior tibialis muscle (AT) was not useful for differential diagnosis.
Different criteria have been proposed and discussed regarding the definition of RWA on PSG and the appropriate cutoff for RWA for the diagnosis of RBD. In a study performed by a Canadian group, a rate of tonic muscle activity≥30%or excessive phasic muscle activity≥15%during the entire REM sleep period based on submental electromyography was useful for distinguishing iRBD patients from healthy individuals [78]. An Austrian group reported that a criterion of≥27%combined tonic and excessive phasic muscle activity in the submental muscles and excessive phasic muscle activity in the forearm (flexor digitorum superficialis) by 30-second epoch judgments was useful for the diagnosis of RBD [79]. In a study including 60 patients with PSG-confirmed iRBD followed for at least 3 years, 43%developed parkinsonism or mild cognitive impairment. Kaplan-Meier analysis showed that the phenoconversion risk was 20%and 35%at 3 and 5 years, respectively. A cutoff of RWA in SM+AT any > 46.4%was associated with 25%and 45%phenoconversion risks at 3 and 5 years, and when applying this RWA cutoff in patients > 65 years of age at diagnosis, the phenoconversion risks increased to 30%and 55%at 3 and 5 years, respectively [80].
RBD has received considerable attention as a precursor to synucleinopathies such as PD, dementia with Lewy bodies (DLB) and MSA [81]. Schenck et al. [82] reported that 38%of 29 male patients with iRBD aged over 50 years developed Parkinsonian disorders at a mean follow-up of 3.7±1.4 years, and after 16 years of follow-up, 81%of the patients initially diagnosed with iRBD eventually developed neurodegenerative diseases such as PD and dementia [83]. Other studies from Canada and Spain have confirmed the risk of developing neurodegenerative disease in iRBD patients using the Kaplan-Meier method [84, 85]. A recent systematic review and meta-analysis showed that the risk of developing neurodegenerative diseases was 33.5%at 5 years, 82.4%at 10.5 years and 96.6%at 14 years of follow-up, with an average conversion rate of 32.0%after a mean duration of 4.8±2.4 years of follow-up [86]. Among the patients who converted to neurodegenerative diseases, the most common disease was PD (44%), followed by DLB (25%), unspecified dementia (7%), MSA (5%), Alzheimer’s disease (3%), mild cognitive impairment (3%) and others (0.5%). A clinicopathological study showed that among 170 patients with neurodegenerative disorders associated with RBD, 160 (94%) had synucleinopathies, and 83%had Lewy body disease (PD, PD with dementia, or DLB) [87]. Although RBD frequently co-occurs in patients with MSA (73–88%) when assessed in a cross-sectional manner [88], prospective studies have consistently shown that patients with RBD more frequently convert to PD or DLB than MSA among synucleinopathies [86, 89, 90]. Several clinical markers indicative of Lewy body diseases, such as cognitive impairment, olfactory impairment, visual impairment and reduced meta-iodobenzylguanidine (MIBG) myocardial scintigraphy accumulation, have been observed in iRBD patients [68, 91]. The frequency of olfactory impairment in iRBD patients who eventually developed MSA (50%) [92] seems to be lower than that in those who converted to PD or DLB (80%) [90].
In a study comparing annual changes in the precursor markers of neurodegeneration, including motor function, color vision, olfaction, cognition, autonomic dysfunction and glucocerebrosidase (GBA) mutation status, between patients with iRBD who had not progressed to neurodegenerative disease for more than 10 years (longstanding iRBD) and those with RBD who had progressed to neurodegenerative disease within 4 years (early converters), the rate of increase in the probability of prodromal PD (%), as calculated by the MDS research criteria for prodromal PD [93], was significantly greater in the early converter group than in the longstanding iRBD group. However, there were no differences between the two groups in terms of the baseline precursor markers, with the exception of olfactory impairments [94]. This suggests that neurodegeneration may insidiously progress at different rates in patients with iRBD.
Several studies have shown a higher rate of alpha-synuclein pathology outside the central nervous systems in patients with iRBD than in healthy controls. Iranzo et al. [95] reported that 50%of patients with iRBD, 54%of those with PD, 50%of those with DLB, and 3%of the controls had phosphorylated α-synuclein deposits in the labial salivary glands. Other studies of the submandibular gland [96] and skin biopsies [97] have shown that alpha-synuclein pathology was significantly more common in patients with iRBD than in healthy subjects. Of note, in a study of colon biopsies, immunostaining of tissue with serine 129-phosphorylated α-synuclein antibody was more specific than that with 15G7 α-synuclein antibody for differentiating iRBD patients from healthy subjects [98]. These biopsy studies provide pathological evidence that iRBD represents synucleinopathy and that a less invasive and more specific method of detecting synuclein pathology may be useful in future neuroprotective trials in patients with iRBD.
RBD with PD
When assessed in a cross-sectional manner, RBD is found in up to 50%of PD patients [68, 91]. Compared with those without RBD, PD patients with RBD have more rapid progression [99], a higher risk of dementia [100] and more severe synuclein pathology, including in the brainstem region [101]. Studies using a data-driven clustering approach identified 3 PD clinical subtypes: mild motor predominant, 49–53%; intermediate, 35–39%; and diffuse malignant, 9–16%. The mild motor predominant type had slow disease progression with a good response to dopaminergic treatment and was related to onset at a young age and mild motor symptoms, while the diffuse malignant type showed rapid disease progression and poor response to dopaminergic treatment and was associated with RBD, mild cognitive impairment, orthostatic impairment and severe motor symptoms [102, 103]. During 5 years of follow-up, the presence of RBD at baseline in early PD patients was associated with an increased risk of functional dependency as measured by the Schwab and England score [104]. In a meta-analysis addressing clinical differences between PD patients with and without RBD, PD patients with PSG-confirmed RBD were older, more likely to be male, and had a longer disease duration, higher HY scale scores (disease severity), and higher UPDRS III scores (motor signs) [105].
In a two-year prospective study of 123 early PD patients and 106 age-matched controls that monitored 30 candidate markers of disease progression, 10 progression markers in PD were identified, which included RBD symptoms and daytime sleepiness [106]. Figorilli et al. [107] prospectively studied 22 PD patients with PSG-confirmed RBD, and while the changes in RBD symptoms varied widely (27%, improved; 27%, worsened; 45%, unchanged), the rate of RWA increased in all patients. Changes in RWA were correlated with the degree of dyskinesia, motor fluctuations, and worsening of executive and visuospatial-cognitive functions. While RBD symptoms may fluctuate depending on the patient, RWA could be a marker of disease progression.
A close relationship between alpha-synuclein pathology and RBD has been recently shown using a novel prodromal PD mouse model. Taguchi et al. [108] successfully produced RWA, an essential RBD feature, and hyposmia using alpha-synuclein (SNCA) bacterial artificial chromosome transgenic mice, which showed no parkinsonism with only a 17.1%decrease in dopaminergic neurons at 18 months of age compared with wild-type mice.
Prodromal state of RBD
Nonviolent behaviors are observed in 18%of RBD patients [109], including jerky, simple, and minor movements of the limbs that are common in RBD patients [110]. It is possible that nonviolent mild motor behaviors may occur in the early phase of RBD or precede full-blown RBD. As a prodromal state of RBD, REM sleep behavioral events (RBE), involving seemingly purposeful motor behaviors and vocalizations with increased tonic and phasic electromyographic (EMG) activity during REM sleep that do not fulfill the RBD criteria, have been proposed [68, 111, 112]. RBE was found in half of de novo PD patients [111], and in early PD patients, 38%of patients with RBE developed RBD within two years [112].
Whether isolated RWA (iRWA), a condition in which no motor behaviors or vocalization are shown other than increased EMG tone during REM sleep, could be a very early state of RBD and precede RBE has attracted attention. In a study with 14 subjects with iRWA, 1 (7.3%) developed RBD after 8.6±0.9 years of follow-up. The rate of RWA significantly increased from 32.5±9.4%to 52.2±16.6%over time. Neurodegenerative markers, including mild cognitive impairment and impaired olfaction, were found in 72%of the patients [113]. In a prospective study including 31 iRBD and 67 iRWA subjects, 18 subjects with iRWA (26.8%) developed iRBD within 2.6±2.2 years, and 6 patients with iRWA (8.9%) developed neurodegenerative disorders (4 developed PD and 2 developed Alzheimer’s disease) within 2.4±1.5 years. In contrast, 8 (25.4%) patients with iRBD developed PD within 2.6±2.2 years. Phenoconversion was positively correlated with age and periodic limb movements in sleep in both the iRBD and iRWA groups [114]. However, a study comparing 49 iRWA subjects and 41 healthy controls found that compared with the healthy controls, iRWA subjects had higher REM density, which is reported to be lower in PD patients, and normal heart rate variability, which is known to be decreased in PD; these findings may weaken the hypothesis that iRWA is a prodromal state of PD [115].
Genetic similarities between RBD and PD
GBA mutations, which cause Gaucher disease, were more common in PD patients than in controls (7%vs. 1%), and GBA mutations were associated with a 5.43-fold higher risk of PD [116]. In addition, GBA mutations were more commonly found in idiopathic RBD patients than in healthy subjects (2.6%vs. 0.4%) [117], suggesting an association between RBD and GBA mutations. In other studies, PD patients with GBA mutations had more severe motor symptoms and higher rates of RBD, hallucinations and other nonmotor symptoms, such as depression and olfactory impairments, than PD patients without GBA mutations [118]. Thus, GBA mutations may affect the clinical features of PD.
A study showed a significant association between Lewy-type alpha-synucleinopathy (LTS) pathology and the prevalence of probable premortem RBD in various neurodegenerative diseases, including progressive supranuclear palsy (with LTS, 25.0%; without LTS, 6.1%) [119]. The association of RBD with MAPT is unclear [120]. Interestingly, carriers of different haplotypes of LRRK2 mutations may have different risks of RBD: the p.N551K-p.R1398H-p.K1423K haplotype was associated with a lower risk of RBD, and the common variant p. S1647T was associated with an increased risk of RBD [121]. Full sequencing and genotyping of SNCA in patients with iRBD, PD, and DLB and controls showed that a 5’-region SNCA variant (rs10005233) was associated with an increased risk of iRBD and probable RBD with PD [122]. Zhao et al. [123] recently found SNCA hypomethylation in leukocytes in patients with iRBD and PD.
Treatment of RBD
First, patients are asked to adjust their bedroom environment, including removing objects from the bedroom, such as stands, pens and furniture, that could cause trauma if abnormal behavior or movements occur, and the patient is instructed to sleep on a mattress on the floor to prevent him/her from falling out of bed. The patient’s bed partner should sleep in a separate room until the patient’s abnormal behavior is improved.
RBD can be induced by antidepressants such as tricyclic antidepressants and serotonin reuptake inhibitors; their use should be reviewed and, if possible, reduced or discontinued. A small dose of clonazepam (0.5 mg to 1 mg) or melatonin (3–12 mg) administered before bedtime is effective as a treatment for RBD [124]. A multicenter open-label study showed that the use of the melatonin receptor agonist ramelteon at 8 mg/day for 12 weeks improved RBD symptoms in patients with PD [125]. Although clonazepam is highly effective at reducing RBD symptoms, it should be noted that it may cause falls, next-day residual sleepiness and worsening of apnea in patients with comorbid sleep apnea syndrome. In addition, dopamine agonists (pramipexole and rotigotine [126]) and yi-gan san have been reported to be efficacious [124]. In a double-blind, crossover trial including 12 PD patients with PSG-confirmed RBD in whom RBD symptoms were refractory to melatonin and clonazepam, rivastigmine treatment (4.6 mg/24 hours) for 3 weeks significantly reduced RBD episodes compared with the placebo [127]. Another placebo-controlled, crossover pilot trial of rivastigmine in 25 consecutive patients with mild cognitive impairment who showed RBD symptoms refractory to first-line treatments such as melatonin and clonazepam was reported. The results showed that rivastigmine treatment resulted in a significant reduction in RBD episodes compared with the placebo [128]. Thus, cholinesterase inhibitors may be an alternative treatment for patients with refractory RBD.
RESTLESS LEGS SYNDROME
RLS is a sleep-related movement disorder that causes insomnia due to abnormal sensations in the lower extremities and is characterized by 1) an urge to move the lower extremities, 2) the appearance or exacerbation of symptoms at rest, 3) improvements in symptoms with exercise, and 4) the appearance or exacerbation of symptoms at night [129]. The diagnosis of RLS requires the exclusion of “RLS mimics,” which can cause RLS-like symptoms, such as myalgia, venous stasis, leg edema, arthritis, and muscle cramps. Akathisia, which is typically caused by exposure to neuroleptic medications, is an important differential diagnosis of RLS. It is characterized by inner restlessness affecting the whole body rather than only the legs without a circadian variation in symptoms [130].
Features supportive of a diagnosis of RLS include 1) a family history of RLS among first-degree relatives, 2) periodic limb movements in sleep (PLMS) and resting wakefulness, 3) responsiveness to dopaminergic drugs and 4) the lack of profound daytime sleepiness. PLMS, consisting of involuntary repetitive limb movements, frequently co-occurs in patients with RLS. It is worth noting that a cross-sectional study showed that one-third of RLS patients reported restless symptoms in body parts other than the legs, such as the arm and trunk, and among patients with restlessness in both in the legs and in other body parts, half developed symptoms in other body parts before they developed leg symptoms [131]. In addition, variants of RLS (restless face [132], arm [133], abdomen [134], or lower back [135]), which cause insomnia due to discomfort confined to, or predominantly involving, body parts other than the legs, have been reported, and careful interviewing/examination is necessary for its diagnosis [136].
Pathophysiology of RLS
The pathogenesis of RLS is mainly brain iron dysfunction, in addition to dysfunction in various nervous system and neurotransmitter systems such as dopamine, glutamate, orexin and adenosine [137, 138], and the involvement of peripheral hypoxia [139] has been suggested. Serum levels of hepcidin, a hormone involved in iron regulation, were reported to be higher in an RLS group than in a healthy control group [140]. The effects of dopamine agonists suggest dopamine impairment in the central nervous system. However, brain imaging studies of the nigrostriatal system have not provided consistent results [136].
In an autopsy study of patients with idiopathic RLS, tyrosine hydroxylase (TH) staining did not reveal impairments in dopaminergic areas, including the hypothalamus, but iron and H-ferritin staining in the substantia nigra were lower than in controls [141]. However, other autopsy studies showed that the dopaminergic markers TH and phosphorylated TH were increased in the substantia nigra and putamen in RLS patients [142]. Furthermore, D2 receptor expression was negatively correlated with premortem RLS symptoms, and severe RLS symptoms were related to lower D2 receptor expression. These results are consistent with the iron deficiency model of RLS, and a decrease in D2/D3 receptors in the mesolimbic system and an increase in CSF levels of homovanillic acid, a dopamine metabolite, and 3-O-methyl dopa, a metabolite of levodopa [138], suggest activation of the dopaminergic system in RLS [137]. The possible explanation for the nighttime occurrence of RLS in a hyperdopaminergic state may be that it is a reflection of the circadian profile of dopamine, including decreased dopaminergic activity and the downregulation of dopamine receptors at night, which results in the occurrence of RLS symptoms predominantly at night [138].
RLS with PD
In a recent meta-analysis, the prevalence of RLS was 2.86 times higher in the PD group than in the healthy control group, with a prevalence of 15%in treated PD patients and 11%in drug-naïve PD patients [143]. Patients with comorbid RLS and PD have been reported to be less likely to have a family history of RLS than patients with idiopathic RLS, and they have an older age at onset, have mild RLS symptoms, and more often develop RLS after the onset of PD [144, 145]. In a cohort of 113 patients with PD, 24%had RLS, 61%of whom had RLS symptoms associated with wearing off phenomena, suggesting the importance of monitoring off-related symptoms [146]. An increase in the prevalence of RLS over time has been reported in a 4-year prospective study including untreated patients with PD at baseline [147].
Leg motor restlessness (LMR), which is characterized by the main symptom of RLS, “an urge to move”, but does not meet the diagnostic criteria for RLS, was reported to be three times more likely to occur in untreated patients with PD than in healthy subjects [148]. RLS variants and LMR are potential targets for treatment because they, similar to RLS, can cause insomnia. We investigated RLS, LMR and their variants in patients with PD-related disorders [149]. The results showed that in 63 PD patients, the proportions of patients with comorbid RLS, LMR, RLS variants and LMR variants were 12.7%, 11.1%, 0%, and 1.6%, respectively, with these RLS-related symptoms being associated with autonomic symptoms, sleep disturbances and depressive symptoms. Having any RLS-related symptoms was more prevalent in patients with PD (50.8%) or MSA (29.4%) than in patients with progressive supranuclear palsy (9.1%), with a similar trend observed in a subanalysis of the untreated group, suggesting that screening for RLS-related symptoms could be useful for differentiating PD or MSA from progressive supranuclear palsy.
The pathology of concomitant PD and RLS is distinct from that of idiopathic RLS. Patients with RLS with PD show degeneration of the nigrostriatal dopaminergic neuron and increased iron in the substantia nigra, in contrast to those with idiopathic RLS [130, 150]. In addition, it is important to note that dopaminergic treatment in PD may lead to an underestimation of RLS (dopamine agonists reduce RLS) or an overestimation of RLS (long-term treatment may cause augmentation and unmask subclinical RLS). In a study of 80 PD patients, 30 PD patients with RLS were older and had a longer duration of PD, higher nonmotor symptom scores (NMSQ), and a higher rate of impulse control disorders (50%vs. 26%) than those without RLS, and RLS was an independent risk factor for impulse control disorders (odds ratio 5.9) after adjusting for confounding factors [151]. Impairments in posterior hypothalamic A11 dopaminergic neurons in RLS have been suggested from animal studies [152]. The nucleus accumbens, which participates in the regulation of reward and motivation, receives dense projections from the paraventricular nucleus and dopaminergic fibers in the paraventricular nucleus originating from a group of hypothalamic dopamine cells, including A11 [153]. Therefore, impaired dopaminergic neurons in the hypothalamic A11 cell population in PD patients with RLS may show impaired projections from the paraventricular nucleus to the nucleus accumbens, which may contribute to the development of impulse control disorders. In a study of 143 PD patients, the association between sleep disturbance and impulsivity was investigated, and PD patients with impulsivity had more complaints of sleep disturbance and daytime sleepiness and a higher prevalence of RLS than PD patients without impulsivity [154]. In a hierarchical linear model, decreased sleep efficiency was most strongly associated with impulsivity. Therefore, the possibility of concomitant impulsivity in PD patients with RLS may deserve attention in clinical practice when adding dopamine agonists.
Treatment of RLS
Blood tests should be performed to screen for iron deficiency anemia, renal dysfunction and low serum ferritin. In patients with low serum ferritin (<50–75μg/L), iron supplementation is recommended. However, in patients with PD, whether iron should be added is debatable, as PD is related to oxidative damage via iron accumulation in the substantia nigra [155]. Alcohol or caffeine consumption, stress and intense exercise before bedtime can trigger RLS symptoms. Antidepressants, dopamine antagonists or antihistamines may induce RLS, and their use should be reduced or discontinued. As a nonpharmacological treatment, the efficacy of exercise with regard to the reduction in RLS symptoms has been reported. A randomized controlled trial showed that an exercise group engaging in aerobic and lower-body resistance training 3 days per week had significant improvements in RLS symptoms compared with a placebo group [156]. Additionally, exercise is considered likely to be efficacious for the treatment of RLS symptoms in uremic patients [157].
Long-acting dopamine agonists, such as rotigotine, should be added before bedtime for PD patients with RLS, LMR, or RLS symptoms related to the wear-off phenomenon. An alpha-2 δ ligand (gabapentin enacarbil at 300–600 mg) or clonazepam (0.5 mg) should also be considered in cases in which sufficient dopamine agonists are already being administered. In severe RLS, oxycodone–naloxone has been reported to be efficacious when used at low doses twice a day [158]. STN DBS may improve the symptoms of RLS, although the effect of concomitant changes in the dose of dopaminergic treatment should be considered [159].
SLEEP APNEA SYNDROME
In PD, in addition to obesity and craniofacial morphology, upper airway muscle impairment due to nocturnal muscle rigidity and bradykinesia may contribute to the occurrence of obstructive sleep apnea (OSA). There is no evidence supporting increased central sleep apnea in PD. During REM sleep, a significant decrease in muscle tone increases the propensity for upper airway collapse, possibly worsening OSA. REM-predominant OSA has been related to younger age, female sex, asthma [160] and lateral positioning during sleep [161].
The question of whether the likelihood of OSA is reduced in patients with RBD who have increased muscle tone during REM sleep has been raised. In a study of 239 patients with PD, 28%had OSA (apnea-hypopnea index (AHI)≥5/h), and logistic regression analyses showed that RBD and a higher LEDD were significant protective factors against OSA [162]. In contrast, Zhang et al [163] performed PSG in 46 PD patients and found that comorbid sleep apnea was significantly more common in the RBD-positive group than in the RBD-negative group (51.4%vs. 9.1%). The reduction in nocturnal oxygen saturation was more pronounced in the RBD-positive group than in the RBD-negative group. However, increased muscle tone in the chin muscle did not affect the severity of sleep apnea during REM sleep. The proportion of patients with RBD was significantly higher in the group of PD patients with sleep apnea than in the group with PD without sleep apnea (94.7%vs. 63%). A latest meta-analysis of PSG findings in PD patients showed that PD patients had decreased total sleep time, sleep efficiency, SWS and REM sleep and increased AHI, wake after sleep onset and PLM index compared with healthy controls [164]. A supplementary meta-analysis showed that PD with RBD was characterized by a decreased apnea index and increased PLM index and REM sleep.
Daytime sleepiness is an OSA-related symptom and has been associated with an increased oxygen desaturation index and a higher LF/HF power ratio (sympathetic-parasympathetic balance) in non-PD individuals [165]. However, in PD patients with OSA, EDS is not prevalent [166], possibly due to an impaired sympathetic response to apnea [167]. Although the clinical symptoms of OSA in PD patients seem to be milder than those in non-PD individuals, further observation is needed of the long-term effects of OSA on PD patients.
Sommerauer et al. [168] reported that PD patients spent twice as much time sleeping in the supine position than healthy subjects. Supine sleep was related to longer disease duration, daytime sleepiness and a higher AHI but not to total sleep time, sleep efficiency or sleep stage. Additionally, an AHI > 5/h and decreased sleep efficiency have been reported to contribute to impaired cognition, particularly attention, executive function/working memory, and semantic memory in patients with PD [169], implying that the treatment of OSA may be a potential target to improve cognition.
Treatment of obstructive sleep apnea
Oral appliances are used in mild cases, and continuous positive airway pressure (CPAP) is used in moderate or severe cases. Weight loss is recommended for obese patients. The significance of CPAP therapy for OSA has been highlighted by several studies: one study described associations among the narcoleptic phenotype, SOREMP on MSLT, and the presence of sleep-disordered breathing in 46 patients with advanced PD [10], and another prospective study that included 38 PD patients with a mean disease duration of 5.3 years showed that CPAP treatment improved OSA and daytime sleepiness measured by MSLT (baseline, MSL 8.7 min; 3 weeks, 11.3 min) [170]. Interestingly, positive effects of long-acting levodopa at bedtime on OSA have been reported [171]. Additionally, CPAP treatment of OSA in patients with PD with a mean usage of 3 hours 36 min was associated with improved sleep quality, anxiety, and cognitive function over a 12-month period [172]. In a study from a tertiary sleep center, 89.2%of PSG-confirmed RBD patients had concomitant OSA, and CPAP therapy improved RBD symptoms in 45.8%of those using CPAP [173]. Thus, in patients with RBD with OSA, CPAP should be tried first. However, long-term adherence to CPAP is a significant problem, considering the progressive motor and nonmotor symptoms in PD, particularly in the later stages. Cognitive impairment, motor disability, nocturia and RBD were reported to be factors influencing CPAP adherence [174].
CIRCADIAN ABNORMALITIES
According to a two-process model, sleep is regulated by a homeostatic process (process S), which depends on the duration of wakefulness and the level of behavioral states, and a circadian process (process C), controlled by the circadian pacemaker irrespective of process S. A number of sleep/wake state-regulating substances have been reported: adenosine and prostaglandin D2 accumulate in the daytime and are known to induce NREM sleep [71]. A preliminary study showed that the levels of plasma lipocalin-type prostaglandin D2 synthase (L-PGDS) were higher in patients with PD than in healthy controls. Although subjective sleepiness was not correlated with L-PGDS levels, prostaglandin D2 may play some role in sleep/wake dysregulation in PD [175]. Circadian rhythms over a period of approximately 24 hours are integrated with environmental and organismal cues by master pacemakers located in the suprachiasmatic nucleus of the hypothalamus, which synchronize subordinate organ and peripheral tissue clocks by electrical, endocrine, and metabolic signaling pathways and peripheral pacemakers [176].
Over 11 years of follow-up in community-dwelling older men, reduced circadian rhythmicity was associated with an increased risk of incident PD [177]. In PD, diurnal fluctuations in nonmotor and motor symptoms suggest circadian involvement [178]. The core body temperature rhythm may be altered in PD patients with depression [179]. Compared to healthy controls, thirty patients with early PD showed significantly increased serum cortisol levels, reduced circulating melatonin levels, and altered Bmal1 expression [180]. Twelve PD patients with EDS (ESS score≥10) exhibited a lower amplitude in the melatonin rhythm and 24-hour melatonin area under the curve compared with 8 PD patients without EDS [181]. Among autopsied cases with PD with a mean disease duration of 14.3 years, Lewy depositions were found in 9 of 13 suprachiasmatic nuclei in the hypothalamus (69.2%) and in 2 of 17 pineal glands (11.8%) examined [182], suggesting that PD-related pathology may participate in circadian abnormalities. However, a large-sample study is needed to confirm the relationship between hypothalamic and pineal impairments and the development of circadian rhythm disorders in patients with PD.
Treatment of circadian abnormalities
Exposure to one hour of bright light (5,000 lux) once a day during the evening hours for 3 months improved sleep problems assessed by PDSS-2 scores, with restoration of circadian phases based on the assessment of the expression levels of three clock genes (Per3, Nr1d1, and Nr1d2) in 16 patients with PD [183]. In a randomized trial including 31 treated PD patients with EDS (ESS≥12) who received 1 hour of bright light therapy (LT) (10,000 lux) or dim-red LT (control condition, <300 lux) in the morning and in the afternoon (twice daily) for 2 weeks, bright LT improved EDS, sleep quality, and sleep fragmentation and increased daily physical activity in patients with PD [184].
SLEEP BENEFIT
Sleep benefit has been used to describe the phenomenon in which PD patients experience a significant improvement in mobility upon awakening in the morning before taking their first PD medication. However, its definition, including the degree of improvement in motor symptoms, has varied across studies. Some patients experience sleep benefit after a short daytime nap. Several, but not all, studies have associated the following factors with sleep benefit: younger onset age, longer disease duration, and longer dopaminergic medication use [185]. Possible mechanisms include a circadian pattern of motor fluctuation [186] and increased dopamine storage in the nigrostriatal terminals during sleep [185]. In contrast, a study that utilized a quantitative motor assessment found that subjective sleep benefit is not related to improvements in quantitatively measured motor functioning, indicating the possibility that sleep benefit is a subjective feeling that is not related to an actual improvement in PD motor symptoms [187]. A recent systematic review and meta-analysis identified three factors related to sleep benefit in PD patients: long disease duration, low sleep efficiency and high MDS-UPDRS total scores [188]. Worse motor symptoms in patients who experience sleep benefit may indicate an association with motor fluctuations; however, many PD patients who do not experience a sleep benefit show motor fluctuations, and in the abovementioned meta-analysis, no association between sleep benefit and the duration of levodopa treatment was found. Hogl et al. [189] also reported lower sleep efficiency, increased sleep fragmentation, and less REM sleep in the sleep benefit group than in the non-sleep benefit group in their study. Favorable effects of REM sleep fragmentation on motor status in animal PD models have also been reported [190], and thus sleep benefit may not be associated with sleep quality.
In a study by Kataoka et al. [191] that included 157 patients with PD using actigraphy, better objective sleep measures, including reduced wake after sleep onset, increased sleep efficiency, and a lower fragmentation index, were significantly related to improvements in early-morning mobility in PD patients. This study suggested that good, sustained sleep can improve early-morning motor symptoms by reducing sleep fragmentation and early-morning akinesia. In their study, improvements in early-morning off periods may be related to the presence of sleep benefit, although sleep benefit was not assessed.
DOES GOOD SLEEP REDUCE THE RISK OF NEURODEGENERATION?
During sleep, fluid-clearance pathways, called the glymphatic system, which acts to remove waste products from the brain, have been reported in animal studies. Especially during non-REM (NREM) sleep, the brain interstitial space substantially increases, resulting in increased convective exchange of CSF with interstitial fluid through the aquaporin-4 water channel. This facilitates the removal of waste products such as amyloid beta and tau protein from the brain [192, 193]. Relationships between the glymphatic pathways activated during sleep and neurological diseases that show a reduced influx of CSF into the brain or reduced removal of interstitial fluid in the brain have been observed in the following: Alzheimer’s disease, stroke, and idiopathic normal pressure hydrocephalus. Therefore, disturbed nocturnal sleep can facilitate the accumulation of abnormal proteins such as amyloid-beta, tau protein, and alpha synuclein, accelerating the underlying pathophysiology of neurodegenerative diseases [193]. In A53T mice overexpressing mutated human α-synuclein, the blockage of meningeal lymphatic drainage aggravated glymphatic dysfunction, resulting in more severe accumulation of α-synuclein, glial activation, inflammation and dopaminergic neuronal loss in the substantia nigra and motor impairments [194]. After traumatic brain injury, glymphatic pathway function is reduced, and the development of neurofibrillary pathology and neurodegeneration are promoted [195].
In a meta-analysis of non-PD individuals, sleep deprivation lasting 24 to 48 hours resulted in significantly reduced performance in all cognitive domains except for accuracy [196]. A study including a large sample of PD and non-PD individuals using a longitudinal database from UK primary care clinics reported that the incidence of insomnia increased 2 years before PD motor symptoms in PD patients compared with non-PD individuals [197]. Therefore, it is presumed that good sleep can accelerate the removal of abnormal proteins and waste products and can prevent neurodegenerative diseases, which should be investigated in future studies. However, the definition of good sleep also needs to be thoroughly characterized in further studies. Importantly, it has been reported that in 129 patients with PD, decreased SWS at baseline was associated with the worsening of motor symptoms after 5 years [198].
CONCLUSION
A current update of PD-related sleep problems such as EDS, RBD, RLS, and OSA as well as circadian rhythm abnormalities is provided. In the era of COVID-19, social isolation and reduced physical activity are altering sleep and wake patterns, and sleep disturbances, anxiety and depression may worsen, which could adversely affect motor symptoms. Therefore, screening for and managing sleep problems in clinical practice is important because the maintenance of good sleep conditions may improve the quality of life of PD patients.
ACKNOWLEDGMENTS
I sincerely appreciate Koichi Hirata, MD, PhD, an emeritus professor of the Department of Neurology, Dokkyo Medical University and Yasuo Haruyama, MD, PhD, Integrated Research Faculty for Advanced Medical Science, Dokkyo Medical University School of Medicine, for their helpful comments and suggestions.
CONFLICT OF INTEREST
Dr. Suzuki serves on the Editorial Board of BMC Neurology and Medicine and has received speaker honoraria from Kyowa Kirin Co., Ltd.; Otsuka Pharmaceutical, Co., Ltd.; Sumitomo Dainippon Pharma Co., Ltd.; Takeda Pharmaceuticals Co., Ltd.; Eisai Co., Ltd.; Ono Pharmaceutical Co., Ltd.; Novartis Pharma K.K.; MSD K.K.; AbbVie Inc.; and FP Pharmaceutical Corporation.
REFERENCES
[1] | Santos-Garcia D , Castro ES , de Deus Fonticoba T , Panceiras MJF , Enriquez JGM , Gonzalez JMP , Bartolome CC , Planellas LL , Caldentey JG , Caballol N , Legarda I , Lopez IC , Manzanares LL , Rivera MAA , Catalan MJ , Nogueira V , Borrue C , Sauco MA , Vela L , Cubo E , Castrillo JCM , Alonso PS , Losada MGA , Ariztegui NL , Gaston MI , Kulisevsky J , Pagonabarraga J , Seijo M , Martinez JR , Valero C , Kurtis M , Ardura JG , Prieto C , Mir P , Martinez-Martin P (2020) Sleep problems are related to a worse quality of life and a greater non-motor symptoms burden in Parkinson’s disease, J Geriatr Psychiatry Neurol, doi: 10.1177/0891988720964250. |
[2] | Wang C , Horby PW , Hayden FG , Gao GF ((2020) ) A novel coronavirus outbreak of global health concern. Lancet 395: , 470–473. |
[3] | Gualano MR , Lo Moro G , Voglino G , Bert F , Siliquini R ((2020) ) Effects of Covid-19 lockdown on mental health and sleep disturbances in Italy. Int J Environ Res Public Health 17: , 4779. |
[4] | Huang Y , Zhao N ((2020) ) Chinese mental health burden during the COVID-19 pandemic. Asian J Psychiatr 51: , 102052. |
[5] | Hurley D ; American Academy of Neurology ((2020) ) Sleep neurologists call it ‘COVID-somnia’—Increased sleep disturbances linked to the pandemic. Neurol Today 20: , 1–31. |
[6] | Subramanian I , Farahnik J , Mischley LK ((2020) ) Synergy of pandemics-social isolation is associated with worsened Parkinson severity and quality of life. NPJ Parkinsons Dis 6: , 28. |
[7] | Helmich RC , Bloem BR ((2020) ) The impact of the COVID-19 pandemic on Parkinson’s disease: Hidden sorrows and emerging opportunities. J Parkinsons Dis 10: , 351–354. |
[8] | Xia Y , Kou L , Zhang G , Han C , Hu J , Wan F , Yin S , Sun Y , Wu J , Li Y , Zhang Z , Huang J , Xiong N , Wang T ((2020) ) Investigation on sleep and mental health of patients with Parkinson’s disease during the Coronavirus disease 2019 pandemic. Sleep Med 75: , 428–433. |
[9] | Arnulf I , Konofal E , Merino-Andreu M , Houeto JL , Mesnage V , Welter ML , Lacomblez L , Golmard JL , Derenne JP , Agid Y ((2002) ) Parkinson’s disease and sleepiness: An integral part of PD. Neurology 58: , 1019–1024. |
[10] | Bargiotas P , Lachenmayer ML , Schreier DR , Mathis J , Bassetti CL ((2019) ) Sleepiness and sleepiness perception in patients with Parkinson’s disease: A clinical and electrophysiological study. Sleep 42: , zsz004. |
[11] | Bonnet MH , Arand DL ((2010) ) Hyperarousal and insomnia: State of the science. Sleep Med Rev 14: , 9–15. |
[12] | Sringean J , Anan C , Thanawattano C , Bhidayasiri R ((2017) ) Time for a strategy in night-time dopaminergic therapy? An objective sensor-based analysis of nocturnal hypokinesia and sleeping positions in Parkinson’s disease. J Neurol Sci 373: , 244–248. |
[13] | Suzuki K , Okuma Y , Uchiyama T , Miyamoto M , Sakakibara R , Shimo Y , Hattori N , Kuwabara S , Yamamoto T , Kaji Y , Hirano S , Kadowaki T , Hirata K , Kanto Ni ((2017) ) Impact of sleep-related symptoms on clinical motor subtypes and disability in Parkinson’s disease: A multicentre cross-sectional study. J Neurol Neurosurg Psychiatry 88: , 953–959. |
[14] | Marinus J , Zhu K , Marras C , Aarsland D , van Hilten JJ ((2018) ) Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol 17: , 559–568. |
[15] | Tholfsen LK , Larsen JP , Schulz J , Tysnes OB , Gjerstad MD ((2017) ) Changes in insomnia subtypes in early Parkinson disease. Neurology 88: , 352–358. |
[16] | Videnovic A ((2017) ) Management of sleep disorders in Parkinson’s disease and multiple system atrophy. Mov Disord 32: , 659–668. |
[17] | Suzuki K , Miyamoto M , Miyamoto T , Hirata K ((2015) ) Parkinson’s disease and sleep/wake disturbances. Curr Neurol Neurosci Rep 15: , 8. |
[18] | Ray Chaudhuri K , Poewe W , Brooks D ((2018) ) Motor and nonmotor complications of levodopa: Phenomenology, risk factors, and imaging features. Mov Disord 33: , 909–919. |
[19] | Martinez-Martin P , Rizos AM , Wetmore JB , Antonini A , Odin P , Pal S , Sophia R , Carroll C , Martino D , Falup-Pecurariu C , Kessel B , Andrews T , Paviour D , Trenkwalder C , Chaudhuri KR , Europar, EUROPAR & MDS Non-Motor PD Study Group ((2019) ) Relationship of nocturnal sleep dysfunction and pain subtypes in Parkinson’s disease. Mov Disord Clin Pract 6: , 57–64. |
[20] | Rana AQ , Qureshi ARM , Shamli Oghli Y , Saqib Y , Mohammed B , Sarfraz Z , Rana R ((2018) ) Decreased sleep quality in Parkinson’s patients is associated with higher anxiety and depression prevalence and severity, and correlates with pain intensity and quality. Neurol Res 40: , 696–701. |
[21] | Schapira AHV , Chaudhuri KR , Jenner P ((2017) ) Non-motor features of Parkinson disease. Nat Rev Neurosci 18: , 435–450. |
[22] | Hogl B , Arnulf I , Comella C , Ferreira J , Iranzo A , Tilley B , Trenkwalder C , Poewe W , Rascol O , Sampaio C , Stebbins GT , Schrag A , Goetz CG ((2010) ) Scales to assess sleep impairment in Parkinson’s disease: Critique and recommendations. Mov Disord 25: , 2704–2716. |
[23] | Suzuki K , Miyamoto M , Miyamoto T , Tatsumoto M , Watanabe Y , Suzuki S , Iwanami M , Sada T , Kadowaki T , Numao A , Trenkwalder C , Hirata K ((2012) ) Nocturnal disturbances and restlessness in Parkinson’s disease: Using the Japanese version of the Parkinson’s disease sleep scale-2. J Neurol Sci 318: , 76–81. |
[24] | Kurtis MM , Balestrino R , Rodriguez-Blazquez C , Forjaz MJ , Martinez-Martin P ((2018) ) A review of scales to evaluate sleep disturbances in movement disorders. Front Neurol 9: , 369. |
[25] | Suzuki K , Miyamoto T , Miyamoto M , Suzuki S , Numao A , Watanabe Y , Tatsumoto M , Sakuta H , Watanabe Y , Fujita H , Iwanami M , Sada T , Kadowaki T , Hashimoto K , Trenkwalder C , Hirata K ((2015) ) Evaluation of cutoff scores for the Parkinson’s disease sleep scale-2. Acta Neurol Scand 131: , 426–430. |
[26] | Muntean ML , Benes H , Sixel-Doring F , Chaudhuri KR , Suzuki K , Hirata K , Zimmermann J , Trenkwalder C ((2016) ) Clinically relevant cut-off values for the Parkinson’s Disease Sleep Scale-2 (PDSS-2): A validation study. Sleep Med 24: , 87–92. |
[27] | Cristini J , Weiss M , De Las Heras B , Medina-Rincon A , Dagher A , Postuma RB , Huber R , Doyon J , Rosa-Neto P , Carrier J , Amara AW , Roig M ((2020) ) The effects of exercise on sleep quality in persons with Parkinson’s disease: A systematic review with meta-analysis. Sleep Med Rev 55: , 101384. |
[28] | Amara AW , Wood KH , Joop A , Memon RA , Pilkington J , Tuggle SC , Reams J , Barrett MJ , Edwards DA , Weltman AL , Hurt CP , Cutter G , Bamman MM ((2020) ) Randomized, controlled trial of exercise on objective and subjective sleep in Parkinson’s disease. Mov Disord 35: , 947–958. |
[29] | Trenkwalder C , Kies B , Rudzinska M , Fine J , Nikl J , Honczarenko K , Dioszeghy P , Hill D , Anderson T , Myllyla V , Kassubek J , Steiger M , Zucconi M , Tolosa E , Poewe W , Surmann E , Whitesides J , Boroojerdi B , Chaudhuri KR , Recover Study G ((2011) ) Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: A double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord 26: , 90–99. |
[30] | Menza M , Dobkin RD , Marin H , Gara M , Bienfait K , Dicke A , Comella CL , Cantor C , Hyer L ((2010) ) Treatment of insomnia in Parkinson’s disease: A controlled trial of eszopiclone and placebo. Mov Disord 25: , 1708–1714. |
[31] | Ahn JH , Kim M , Park S , Jang W , Park J , Oh E , Cho JW , Kim JS , Youn J ((2020) ) Prolonged-release melatonin in Parkinson’s disease patients with a poor sleep quality: A randomized trial. Parkinsonism Relat Disord 75: , 50–54. |
[32] | Daneshvar Kakhaki R , Ostadmohammadi V , Kouchaki E , Aghadavod E , Bahmani F , Tamtaji OR , R JR , Mansournia MA , Asemi Z ((2020) ) Melatonin supplementation and the effects on clinical and metabolic status in Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin Neurol Neurosurg 195: , 105878. |
[33] | Moline M , Zammit G , Yardley J , Pinner K , Kumar D , Perdomo C , Cheng JY ((2021) ) Lack of residual morning effects of lemborexant treatment for insomnia: Summary of findings across 9 clinical trials. Postgrad Med 133: , 71–81. |
[34] | Cheng JY , Filippov G , Moline M , Zammit GK , Bsharat M , Hall N ((2020) ) Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: A randomized, double-blind, placebo-controlled, crossover study. J Sleep Res 29: , e13021. |
[35] | Muehlan C , Vaillant C , Zenklusen I , Kraehenbuehl S , Dingemanse J ((2020) ) Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders. Expert Opin Drug Metab Toxicol 16: , 1063–1078. |
[36] | Scott LJ ((2020) ) Lemborexant: First approval. Drugs 80: , 425–432. |
[37] | Gaig C , Tolosa E ((2009) ) When does Parkinson’s disease begin? Mov Disord 24: (Suppl 2), S656–664. |
[38] | Freeman AAH ((2015) ) Neurochemistry of the sleep-wake cycle in Parkinson’s disease In Disorders of Sleep and Circadian Rhythms in Parkinson’s Disease, Videnovic A, Högl B, eds. Springer, pp. 19–34. |
[39] | Happe S , Baier PC , Helmschmied K , Meller J , Tatsch K , Paulus W ((2007) ) Association of daytime sleepiness with nigrostriatal dopaminergic degeneration in early Parkinson’s disease. J Neurol 254: , 1037–1043. |
[40] | Rye DB , Jankovic J ((2002) ) Emerging views of dopamine in modulating sleep/wake state from an unlikely source: PD. Neurology 58: , 341–346. |
[41] | Compta Y , Santamaria J , Ratti L , Tolosa E , Iranzo A , Munoz E , Valldeoriola F , Casamitjana R , Rios J , Marti MJ ((2009) ) Cerebrospinal hypocretin, daytime sleepiness and sleep architecture in Parkinson’s disease dementia. Brain 132: , 3308–3317. |
[42] | Al-Kuraishy HM , Abdulhadi MH , Hussien NR , Al-Niemi MS , Rasheed HA , Al-Gareeb AI ((2020) ) Involvement of orexinergic system in psychiatric and neurodegenerative disorders: A scoping review. Brain Circ 6: , 70–80. |
[43] | Qamhawi Z , Towey D , Shah B , Pagano G , Seibyl J , Marek K , Borghammer P , Brooks DJ , Pavese N ((2015) ) Clinical correlates of raphe serotonergic dysfunction in early Parkinson’s disease. Brain 138: , 2964–2973. |
[44] | Wilson H , Giordano B , Turkheimer FE , Chaudhuri KR , Politis M ((2018) ) Serotonergic dysregulation is linked to sleep problems in Parkinson’s disease. Neuroimage Clin 18: , 630–637. |
[45] | Gjerstad MD , Alves G , Wentzel-Larsen T , Aarsland D , Larsen JP ((2006) ) Excessive daytime sleepiness in Parkinson disease: Is it the drugs or the disease? Neurology 67: , 853–858. |
[46] | Zhu K , van Hilten JJ , Marinus J ((2016) ) Course and risk factors for excessive daytime sleepiness in Parkinson’s disease. Parkinsonism Relat Disord 24: , 34–40. |
[47] | Tholfsen LK , Larsen JP , Schulz J , Tysnes OB , Gjerstad MD ((2015) ) Development of excessive daytime sleepiness in early Parkinson disease. Neurology 85: , 162–168. |
[48] | Amara AW , Chahine LM , Caspell-Garcia C , Long JD , Coffey C , Hogl B , Videnovic A , Iranzo A , Mayer G , Foldvary-Schaefer N , Postuma R , Oertel W , Lasch S , Marek K , Simuni T , Parkinson’s Progression Markers Initiative ((2017) ) Longitudinal assessment of excessive daytime sleepiness in early Parkinson’s disease. J Neurol Neurosurg Psychiatry 88: , 653–662. |
[49] | Frucht S , Rogers JD , Greene PE , Gordon MF , Fahn S ((1999) ) Falling asleep at the wheel: Motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology 52: , 1908–1910. |
[50] | Paus S , Brecht HM , Koster J , Seeger G , Klockgether T , Wullner U ((2003) ) Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson’s disease. Mov Disord 18: , 659–667. |
[51] | Suzuki K , Miyamoto T , Miyamoto M , Okuma Y , Hattori N , Kamei S , Yoshii F , Utsumi H , Iwasaki Y , Iijima M , Hirata K ((2008) ) Excessive daytime sleepiness and sleep episodes in Japanese patients with Parkinson’s disease. J Neurol Sci 271: , 47–52. |
[52] | Tan EK , Lum SY , Fook-Chong SM , Teoh ML , Yih Y , Tan L , Tan A , Wong MC ((2002) ) Evaluation of somnolence in Parkinson’s disease: Comparison with age- and sex-matched controls. Neurology 58: , 465–468. |
[53] | Hobson DE , Lang AE , Martin WR , Razmy A , Rivest J , Fleming J ((2002) ) Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: A survey by the Canadian Movement Disorders Group. JAMA 287: , 455–463. |
[54] | Yeung EYH , Cavanna AE ((2014) ) Sleep attacks in patients with Parkinson’s disease on dopaminergic medications: A systematic review. Mov Disord Clin Pract 1: , 307–316. |
[55] | SuzukiK, MiyamotoT, MiyamotoM, NishibayashiM, HirataK ((2008) ) Daytime sleepiness in Parkinson’s disease patient worsened by changing medication between same types of dopamine agonists. Sleep Biol Rhythms 6: , 180–182. |
[56] | Videnovic A ((2018) ) Disturbances of sleep and alertness in Parkinson’s disease. Curr Neurol Neurosci Rep 18: , 29. |
[57] | Monti JM , Monti D ((2007) ) The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev 11: , 113–133. |
[58] | Gros P , Videnovic A ((2020) ) Overview of sleep and circadian rhythm disorders in Parkinson disease. Clin Geriatr Med 36: , 119–130. |
[59] | Stefani A , Hogl B ((2020) ) Sleep in Parkinson’s disease. Neuropsychopharmacology 45: , 121–128. |
[60] | Bliwise DL , Trotti LM , Wilson AG , Greer SA , Wood-Siverio C , Juncos JJ , Factor SA , Freeman A , Rye DB ((2012) ) Daytime alertness in Parkinson’s disease: Potentially dose-dependent, divergent effects by drug class. Mov Disord 27: , 1118–1124. |
[61] | Liguori C , Mercuri NB , Albanese M , Olivola E , Stefani A , Pierantozzi M ((2019) ) Daytime sleepiness may be an independent symptom unrelated to sleep quality in Parkinson’s disease. J Neurol 266: , 636–641. |
[62] | Postuma RB , Lang AE , Munhoz RP , Charland K , Pelletier A , Moscovich M , Filla L , Zanatta D , Rios Romenets S , Altman R , Chuang R , Shah B ((2012) ) Caffeine for treatment of Parkinson disease: A randomized controlled trial. Neurology 79: , 651–658. |
[63] | Rodrigues TM , Castro Caldas A , Ferreira JJ ((2016) ) Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson’s disease: Systematic review and meta-analysis. Parkinsonism Relat Disord 27: , 25–34. |
[64] | Suzuki K , Miyamoto M , Miyamoto T , Uchiyama T , Watanabe Y , Suzuki S , Kadowaki T , Fujita H , Matsubara T , Sakuramoto H , Hirata K ((2017) ) Istradefylline improves daytime sleepiness in patients with Parkinson’s disease: An open-label, 3-month study. J Neurol Sci 380: , 230–233. |
[65] | Lyons KE , Friedman JH , Hermanowicz N , Isaacson SH , Hauser RA , Hersh BP , Silver DE , Tetrud JW , Elmer LW , Parashos SA , Struck LK , Lew MF , Pahwa R ((2010) ) Orally disintegrating selegiline in Parkinson patients with dopamine agonist-related adverse effects. Clin Neuropharmacol 33: , 5–10. |
[66] | Schrempf W , Fauser M , Wienecke M , Brown S , Maass A , Ossig C , Otto K , Brandt MD , Lohle M , Schwanebeck U , Graehlert X , Reichmann H , Storch A ((2018) ) Rasagiline improves polysomnographic sleep parameters in patients with Parkinson’s disease: A double-blind, baseline-controlled trial. Eur J Neurol 25: , 672–679. |
[67] | Liguori C , Stefani A , Ruffini R , Mercuri NB , Pierantozzi M ((2018) ) Safinamide effect on sleep disturbances and daytime sleepiness in motor fluctuating Parkinson’s disease patients: A validated questionnaires-controlled study. Parkinsonism Relat Disord 57: , 80–81. |
[68] | Hogl B , Stefani A , Videnovic A ((2018) ) Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat Rev Neurol 14: , 40–55. |
[69] | Haba-Rubio J , Frauscher B , Marques-Vidal P , Toriel J , Tobback N , Andries D , Preisig M , Vollenweider P , Postuma R , Heinzer R ((2018) ) Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep 41: , zsx197. |
[70] | Sasai-Sakuma T , Takeuchi N , Asai Y , Inoue Y , Inoue Y ((2020) ) Prevalence and clinical characteristics of REM sleep behavior disorder in Japanese elderly people. Sleep 43: , zsaa024. |
[71] | Schneider L ((2020) ) Neurobiology and neuroprotective benefits of sleep. Continuum (Minneap Minn) 26: , 848–870. |
[72] | Luppi PH , Clement O , Valencia Garcia S , Brischoux F , Fort P ((2013) ) New aspects in the pathophysiology of rapid eye movement sleep behavior disorder: The potential role of glutamate, gamma-aminobutyric acid, and glycine. Sleep Med 14: , 714–718. |
[73] | Roguski A , Rayment D , Whone AL , Jones MW , Rolinski M ((2020) ) A neurologist’s guide to REM sleep behavior disorder. Front Neurol 11: , 610. |
[74] | Ehrminger M , Latimier A , Pyatigorskaya N , Garcia-Lorenzo D , Leu-Semenescu S , Vidailhet M , Lehericy S , Arnulf I ((2016) ) The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain 139: , 1180–1188. |
[75] | Garcia-Lorenzo D , Longo-Dos Santos C , Ewenczyk C , Leu-Semenescu S , Gallea C , Quattrocchi G , Pita Lobo P , Poupon C , Benali H , Arnulf I , Vidailhet M , Lehericy S ((2013) ) The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain 136: , 2120–2129. |
[76] | Bedard MA , Aghourian M , Legault-Denis C , Postuma RB , Soucy JP , Gagnon JF , Pelletier A , Montplaisir J ((2019) ) Brain cholinergic alterations in idiopathic REM sleep behaviour disorder: A PET imaging study with (18)F-FEOBV. Sleep Med 58: , 35–41. |
[77] | McCarter SJ , Feemster JC , Tabatabai GM , Sandness DJ , Timm PC , McCarter AR , Talley HN , Junna MR , Savica R , Singer W , Coon EA , Benarroch EE , Josephs KA , Boeve BF , Silber MH , St Louis EK ((2019) ) Submentalis rapid eye movement sleep muscle activity: A potential biomarker for synucleinopathy. Ann Neurol 86: , 969–974. |
[78] | Montplaisir J , Gagnon JF , Fantini ML , Postuma RB , Dauvilliers Y , Desautels A , Rompre S , Paquet J ((2010) ) Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord 25: , 2044–2051. |
[79] | Frauscher B , Iranzo A , Gaig C , Gschliesser V , Guaita M , Raffelseder V , Ehrmann L , Sola N , Salamero M , Tolosa E , Poewe W , Santamaria J , Hogl B , Group S ((2012) ) Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep 35: , 835–847. |
[80] | McCarter SJ , Sandness DJ , McCarter AR , Feemster JC , Teigen LN , Timm PC , Boeve BF , Silber MH , St Louis EK ((2019) ) REM sleep muscle activity in idiopathic REM sleep behavior disorder predicts phenoconversion. Neurology 93: , e1171–e1179. |
[81] | Gan-Or Z , Alcalay RN , Rouleau GA , Postuma RB ((2018) ) Sleep disorders and Parkinson disease; lessons from genetics. Sleep Med Rev 41: , 101–112. |
[82] | Schenck CH , Bundlie SR , Mahowald MW ((1996) ) Delayed emergence of a parkinsonian disorder in 38%of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 46: , 388–393. |
[83] | Schenck CH , Boeve BF , Mahowald MW ((2013) ) Delayed emergence of a parkinsonian disorder or dementia in 81%of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: A 16-year update on a previously reported series. Sleep Med 14: , 744–748. |
[84] | Iranzo A , Molinuevo JL , Santamaria J , Serradell M , Marti MJ , Valldeoriola F , Tolosa E ((2006) ) Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: A descriptive study. Lancet Neurol 5: , 572–577. |
[85] | Postuma RB , Gagnon JF , Vendette M , Fantini ML , Massicotte-Marquez J , Montplaisir J ((2009) ) Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 72: , 1296–1300. |
[86] | Galbiati A , Verga L , Giora E , Zucconi M , Ferini-Strambi L ((2019) ) The risk of neurodegeneration in REM sleep behavior disorder: A systematic review and meta-analysis of longitudinal studies. Sleep Med Rev 43: , 37–46. |
[87] | Boeve BF , Silber MH , Ferman TJ , Lin SC , Benarroch EE , Schmeichel AM , Ahlskog JE , Caselli RJ , Jacobson S , Sabbagh M , Adler C , Woodruff B , Beach TG , Iranzo A , Gelpi E , Santamaria J , Tolosa E , Singer C , Mash DC , Luca C , Arnulf I , Duyckaerts C , Schenck CH , Mahowald MW , Dauvilliers Y , Graff-Radford NR , Wszolek ZK , Parisi JE , Dugger B , Murray ME , Dickson DW ((2013) ) Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med 14: , 754–762. |
[88] | Palma JA , Fernandez-Cordon C , Coon EA , Low PA , Miglis MG , Jaradeh S , Bhaumik AK , Dayalu P , Urrestarazu E , Iriarte J , Biaggioni I , Kaufmann H ((2015) ) Prevalence of REM sleep behavior disorder in multiple system atrophy: A multicenter study and meta-analysis. Clin Auton Res 25: , 69–75. |
[89] | Miyamoto T , Miyamoto M ((2018) ) Phenoconversion from idiopathic rapid eye movement sleep behavior disorder to Lewy body disease. Mov Disord Clin Pract 5: , 506–511. |
[90] | Postuma RB , Iranzo A , Hu M , Hogl B , Boeve BF , Manni R , Oertel WH , Arnulf I , Ferini-Strambi L , Puligheddu M , Antelmi E , Cochen De Cock V , Arnaldi D , Mollenhauer B , Videnovic A , Sonka K , Jung KY , Kunz D , Dauvilliers Y , Provini F , Lewis SJ , Buskova J , Pavlova M , Heidbreder A , Montplaisir JY , Santamaria J , Barber TR , Stefani A , St Louis EK , Terzaghi M , Janzen A , Leu-Semenescu S , Plazzi G , Nobili F , Sixel-Doering F , Dusek P , Bes F , Cortelli P , Ehgoetz Martens K , Gagnon JF , Gaig C , Zucconi M , Trenkwalder C , Gan-Or Z , Lo C , Rolinski M , Mahlknecht P , Holzknecht E , Boeve AR , Teigen LN , Toscano G , Mayer G , Morbelli S , Dawson B , Pelletier A ((2019) ) Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 142: , 744–759. |
[91] | Dauvilliers Y , Schenck CH , Postuma RB , Iranzo A , Luppi PH , Plazzi G , Montplaisir J , Boeve B ((2018) ) REM sleep behaviour disorder. Nat Rev Dis Primers 4: , 19. |
[92] | Stefani A , Ferini-Strambi L , Postuma RB , Iranzo A , Videnovic A , Hogl B , Wenning GK ((2020) ) Olfaction in patients with isolated REM sleep behavior disorder who eventually develop multiple system atrophy. Sleep 43: , zsz303. |
[93] | Berg D , Postuma RB , Adler CH , Bloem BR , Chan P , Dubois B , Gasser T , Goetz CG , Halliday G , Joseph L , Lang AE , Liepelt-Scarfone I , Litvan I , Marek K , Obeso J , Oertel W , Olanow CW , Poewe W , Stern M , Deuschl G ((2015) ) MDS research criteria for prodromal Parkinson’s disease. Mov Disord 30: , 1600–1611. |
[94] | Yao C , Fereshtehnejad SM , Dawson BK , Pelletier A , Gan-Or Z , Gagnon JF , Montplaisir JY , Postuma RB ((2018) ) Longstanding disease-free survival in idiopathic REM sleep behavior disorder: Is neurodegeneration inevitable? Parkinsonism Relat Disord 54: , 99–102. |
[95] | Iranzo A , Borrego S , Vilaseca I , Marti C , Serradell M , Sanchez-Valle R , Kovacs GG , Valldeoriola F , Gaig C , Santamaria J , Tolosa E , Gelpi E ((2018) ) alpha-Synuclein aggregates in labial salivary glands of idiopathic rapid eye movement sleep behavior disorder. Sleep 41: , doi: 10.1093/sleep/zsy101 |
[96] | Vilas D , Iranzo A , Tolosa E , Aldecoa I , Berenguer J , Vilaseca I , Marti C , Serradell M , Lomena F , Alos L , Gaig C , Santamaria J , Gelpi E ((2016) ) Assessment of alpha-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: A case-control study. Lancet Neurol 15: , 708–718. |
[97] | Doppler K , Jentschke HM , Schulmeyer L , Vadasz D , Janzen A , Luster M , Hoffken H , Mayer G , Brumberg J , Booij J , Musacchio T , Klebe S , Sittig-Wiegand E , Volkmann J , Sommer C , Oertel WH ((2017) ) Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol 133: , 535–545. |
[98] | Sprenger FS , Stefanova N , Gelpi E , Seppi K , Navarro-Otano J , Offner F , Vilas D , Valldeoriola F , Pont-Sunyer C , Aldecoa I , Gaig C , Gines A , Cuatrecasas M , Hogl B , Frauscher B , Iranzo A , Wenning GK , Vogel W , Tolosa E , Poewe W ((2015) ) Enteric nervous system alpha-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology 85: , 1761–1768. |
[99] | Fereshtehnejad SM , Romenets SR , Anang JB , Latreille V , Gagnon JF , Postuma RB ((2015) ) New clinical subtypes of Parkinson disease and their longitudinal progression: A prospective cohort comparison with other phenotypes. JAMA Neurol 72: , 863–873. |
[100] | Postuma RB , Bertrand JA , Montplaisir J , Desjardins C , Vendette M , Rios Romenets S , Panisset M , Gagnon JF ((2012) ) Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: A prospective study. Mov Disord 27: , 720–726. |
[101] | Postuma RB , Adler CH , Dugger BN , Hentz JG , Shill HA , Driver-Dunckley E , Sabbagh MN , Jacobson SA , Belden CM , Sue LI , Serrano G , Beach TG ((2015) ) REM sleep behavior disorder and neuropathology in Parkinson’s disease. Mov Disord 30: , 1413–1417. |
[102] | Armstrong MJ , Okun MS ((2020) ) Diagnosis and treatment of Parkinson disease: A review. JAMA 323: , 548–560. |
[103] | Fereshtehnejad SM , Postuma RB ((2017) ) Subtypes of Parkinson’s disease: What do they tell us about disease progression? Curr Neurol Neurosci Rep 17: , 34. |
[104] | Kim R , Yoo D , Im JH , Kim HJ , Jeon B ((2019) ) REM sleep behavior disorder predicts functional dependency in early Parkinson’s disease. Parkinsonism Relat Disord 66: , 138–142. |
[105] | Zhu RL , Xie CJ , Hu PP , Wang K ((2017) ) Clinical variations in Parkinson’s disease patients with or without REM sleep behaviour disorder: A meta-analysis. Sci Rep 7: , 40779. |
[106] | Mollenhauer B , Zimmermann J , Sixel-Doring F , Focke NK , Wicke T , Ebentheuer J , Schaumburg M , Lang E , Trautmann E , Zetterberg H , Taylor P , Friede T , Trenkwalder C , DeNoPa Study Group ((2016) ) Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology 87: , 168–177. |
[107] | Figorilli M , Marques AR , Vidal T , Delaby L , Meloni M , Pereira B , Lambert C , Puligheddu M , Durif F , Fantini ML ((2020) ) Does REM sleep behavior disorder change in the progression of Parkinson’s disease? Sleep Med 68: , 190–198. |
[108] | Taguchi T , Ikuno M , Hondo M , Parajuli LK , Taguchi K , Ueda J , Sawamura M , Okuda S , Nakanishi E , Hara J , Uemura N , Hatanaka Y , Ayaki T , Matsuzawa S , Tanaka M , El-Agnaf OMA , Koike M , Yanagisawa M , Uemura MT , Yamakado H , Takahashi R ((2020) ) alpha-Synuclein BAC transgenic mice exhibit RBD-like behaviour and hyposmia: A prodromal Parkinson’s disease model. Brain 143: , 249–265. |
[109] | Oudiette D , De Cock VC , Lavault S , Leu S , Vidailhet M , Arnulf I ((2009) ) Nonviolent elaborate behaviors may also occur in REM sleep behavior disorder. Neurology 72: , 551–557. |
[110] | Hogl B , Santamaria J , Iranzo A , Stefani A ((2019) ) Precision medicine in rapid eye movement sleep behavior disorder. Sleep Med Clin 14: , 351–362. |
[111] | Sixel-Doring F , Trautmann E , Mollenhauer B , Trenkwalder C ((2014) ) Rapid eye movement sleep behavioral events: A new marker for neurodegeneration in early Parkinson disease? Sleep 37: , 431–438. |
[112] | Sixel-Doring F , Zimmermann J , Wegener A , Mollenhauer B , Trenkwalder C ((2016) ) The evolution of REM sleep behavior disorder in early Parkinson disease. Sleep 39: , 1737–1742. |
[113] | Stefani A , Gabelia D , Hogl B , Mitterling T , Mahlknecht P , Stockner H , Poewe W , Frauscher B ((2015) ) Long-term follow-up investigation of isolated rapid eye movement sleep without atonia without rapid eye movement sleep behavior disorder: A pilot study. J Clin Sleep Med 11: , 1273–1279. |
[114] | Dede HO , Benbir Senel G , Karadeniz D ((2019) ) Rapid eye movement sleep without atonia constitutes increased risk for neurodegenerative disorders. Acta Neurol Scand 140: , 399–404. |
[115] | Dijkstra F , Viaene M , De Volder I , Fransen E , Cras P , Crosiers D ((2020) ) Polysomnographic phenotype of isolated REM sleep without atonia. Clin Neurophysiol 131: , 2508–2515. |
[116] | Sidransky E , Nalls MA , Aasly JO , Aharon-Peretz J , Annesi G , Barbosa ER , Bar-Shira A , Berg D , Bras J , Brice A , Chen CM , Clark LN , Condroyer C , De Marco EV , Durr A , Eblan MJ , Fahn S , Farrer MJ , Fung HC , Gan-Or Z , Gasser T , Gershoni-Baruch R , Giladi N , Griffith A , Gurevich T , Januario C , Kropp P , Lang AE , Lee-Chen GJ , Lesage S , Marder K , Mata IF , Mirelman A , Mitsui J , Mizuta I , Nicoletti G , Oliveira C , Ottman R , Orr-Urtreger A , Pereira LV , Quattrone A , Rogaeva E , Rolfs A , Rosenbaum H , Rozenberg R , Samii A , Samaddar T , Schulte C , Sharma M , Singleton A , Spitz M , Tan EK , Tayebi N , Toda T , Troiano AR , Tsuji S , Wittstock M , Wolfsberg TG , Wu YR , Zabetian CP , Zhao Y , Ziegler SG ((2009) ) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361: , 1651–1661. |
[117] | Barber TR , Lawton M , Rolinski M , Evetts S , Baig F , Ruffmann C , Gornall A , Klein JC , Lo C , Dennis G , Bandmann O , Quinnell T , Zaiwalla Z , Ben-Shlomo Y , Hu MTM ((2017) ) Prodromal parkinsonism and neurodegenerative risk stratification in REM sleep behavior disorder. Sleep 40: , zsx071. |
[118] | Thaler A , Bregman N , Gurevich T , Shiner T , Dror Y , Zmira O , Gan-Or Z , Bar-Shira A , Gana-Weisz M , Orr-Urtreger A , Giladi N , Mirelman A ((2018) ) Parkinson’s disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat Disord 55: , 45–49. |
[119] | Shprecher DR , Adler CH , Zhang N , Hentz JG , Serrano GE , Dugger BN , Shill HA , Savica R , Caviness JN , Sabbagh MN , Belden CM , Driver-Dunckley E , Mehta SH , Sue LI , Davis KJ , Zamrini E , Beach TG ((2018) ) Predicting alpha-synuclein pathology by REM sleep behavior disorder diagnosis. Parkinsonism Relat Disord 55: , 92–96. |
[120] | Li J , Ruskey JA , Arnulf I , Dauvilliers Y , Hu MTM , Hogl B , Leblond CS , Zhou S , Ambalavanan A , Ross JP , Bourassa CV , Spiegelman D , Laurent SB , Stefani A , Charley Monaca C , Cochen De Cock V , Boivin M , Ferini-Strambi L , Plazzi G , Antelmi E , Young P , Heidbreder A , Labbe C , Ferman TJ , Dion PA , Fan D , Desautels A , Gagnon JF , Dupre N , Fon EA , Montplaisir JY , Boeve BF , Postuma RB , Rouleau GA , Ross OA , Gan-Or Z ((2018) ) Full sequencing and haplotype analysis of MAPT in Parkinson’s disease and rapid eye movement sleep behavior disorder. Mov Disord 33: , 1016–1020. |
[121] | Ouled Amar Bencheikh B , Ruskey JA , Arnulf I , Dauvilliers Y , Monaca CC , De Cock VC , Gagnon JF , Spiegelman D , Hu MTM , Hogl B , Stefani A , Ferini-Strambi L , Plazzi G , Antelmi E , Young P , Heidbreder A , Mollenhauer B , Sixel-Doring F , Trenkwalder C , Oertel W , Montplaisir JY , Postuma RB , Rouleau GA , Gan-Or Z ((2018) ) LRRK2 protective haplotype and full sequencing study in REM sleep behavior disorder. Parkinsonism Relat Disord 52: , 98–101. |
[122] | Krohn L , Wu RYJ , Heilbron K , Ruskey JA , Laurent SB , Blauwendraat C , Alam A , Arnulf I , Hu MTM , Dauvilliers Y , Hogl B , Toft M , Bjornara KA , Stefani A , Holzknecht E , Monaca CC , Abril B , Plazzi G , Antelmi E , Ferini-Strambi L , Young P , Heidbreder A , Cochen De Cock V , Mollenhauer B , Sixel-Doring F , Trenkwalder C , Sonka K , Kemlink D , Figorilli M , Puligheddu M , Dijkstra F , Viaene M , Oertel W , Toffoli M , Gigli GL , Valente M , Gagnon JF , Nalls MA , Singleton AB , andMe Research T, Desautels A , Montplaisir JY , Cannon P , Ross OA , Boeve BF , Dupre N , Fon EA , Postuma RB , Pihlstrom L , Rouleau GA , Gan-Or Z ((2020) ) Fine-mapping of SNCA in rapid eye movement sleep behavior disorder and overt synucleinopathies. Ann Neurol 87: , 584–598. |
[123] | Zhao A , Li Y , Niu M , Li G , Luo N , Zhou L , Kang W , Liu J ((2020) ) SNCA hypomethylation in rapid eye movement sleep behavior disorder is a potential biomarker for Parkinson’s disease. J Parkinsons Dis 10: , 1023–1031. |
[124] | Aurora RN , Zak RS , Maganti RK , Auerbach SH , Casey KR , Chowdhuri S , Karippot A , Ramar K , Kristo DA , Morgenthaler TI , Standards of Practice Committee; American Academy of Sleep Medicine ((2010) ) Best practice guide for the treatment of REM sleep behavior disorder (RBD). J Clin Sleep Med 6: , 85–95. |
[125] | Kashihara K , Nomura T , Maeda T , Tsuboi Y , Mishima T , Takigawa H , Nakashima K ((2016) ) Beneficial effects of ramelteon on rapid eye movement sleep behavior disorder associated with Parkinson’s disease - results of a multicenter open trial. Intern Med 55: , 231–236. |
[126] | Wang Y , Yang Y , Wu H , Lan D , Chen Y , Zhao Z ((2016) ) Effects of rotigotine on REM sleep behavior disorder in Parkinson disease. J Clin Sleep Med 12: , 1403–1409. |
[127] | Di Giacopo R , Fasano A , Quaranta D , Della Marca G , Bove F , Bentivoglio AR ((2012) ) Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson’s disease. Mov Disord 27: , 559–561. |
[128] | Brunetti V , Losurdo A , Testani E , Lapenta L , Mariotti P , Marra C , Rossini PM , Della Marca G ((2014) ) Rivastigmine for refractory REM behavior disorder in mild cognitive impairment. Curr Alzheimer Res 11: , 267–273. |
[129] | Allen RP , Picchietti DL , Garcia-Borreguero D , Ondo WG , Walters AS , Winkelman JW , Zucconi M , Ferri R , Trenkwalder C , Lee HB , International Restless Legs Syndrome Study Group ((2014) ) Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med 15: , 860–873. |
[130] | Suzuki K , Miyamoto M , Miyamoto T , Hirata K ((2015) ) Restless legs syndrome and leg motor restlessness in Parkinson’s disease. Parkinsons Dis 2015: , 490938. |
[131] | Suzuki K , Suzuki S , Miyamoto M , Miyamoto T , Matsubara T , Nozawa N , Arikawa T , Nakajima I , Hirata K ((2019) ) Involvement of legs and other body parts in patients with restless legs syndrome and its variants. J Neurol Sci 407: , 116519. |
[132] | Suzuki K , Suzuki H , Suzuki Y , Hirata K ((2017) ) “Restless face” as a variant of restless legs syndrome. Parkinsonism Relat Disord 41: , 130–131. |
[133] | Horvath J , Landis T , Burkhard PR ((2008) ) Restless arms. Lancet 371: , 530. |
[134] | Perez-Diaz H , Iranzo A , Rye DB , Santamaria J ((2011) ) Restless abdomen: A phenotypic variant of restless legs syndrome. Neurology 77: , 1283–1286. |
[135] | Suzuki K , Miyamoto M , Miyamoto T , Hirata K ((2013) ) Restless “lower back” in a patient with Parkinson’s disease.. Tremor Other Hyperkinet Mov (N Y) 3: . |
[136] | Turrini A , Raggi A , Calandra-Buonaura G , Martinelli P , Ferri R , Provini F ((2018) ) Not only limbs in atypical restless legs syndrome. Sleep Med Rev 38: , 50–55. |
[137] | Garcia-Borreguero D , Cano-Pumarega I ((2017) ) New concepts in the management of restless legs syndrome. BMJ 356: , j104. |
[138] | Wijemanne S , Ondo W ((2017) ) Restless legs syndrome: Clinical features, diagnosis and a practical approach to management. Pract Neurol 17: , 444–452. |
[139] | Salminen AV , Rimpila V , Polo O ((2014) ) Peripheral hypoxia in restless legs syndrome (Willis-Ekbom disease). Neurology 82: , 1856–1861. |
[140] | Dauvilliers Y , Chenini S , Vialaret J , Delaby C , Guiraud L , Gabelle A , Lopez R , Hirtz C , Jaussent I , Lehmann S ((2018) ) Association between serum hepcidin level and restless legs syndrome. Mov Disord 33: , 618–627. |
[141] | Connor JR , Boyer PJ , Menzies SL , Dellinger B , Allen RP , Ondo WG , Earley CJ ((2003) ) Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 61: , 304–309. |
[142] | Connor JR , Wang XS , Allen RP , Beard JL , Wiesinger JA , Felt BT , Earley CJ ((2009) ) Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain 132: , 2403–2412. |
[143] | Yang X , Liu B , Shen H , Li S , Zhao Q , An R , Hu F , Ren H , Xu Y , Xu Z ((2018) ) Prevalence of restless legs syndrome in Parkinson’s disease: A systematic review and meta-analysis of observational studies. Sleep Med 43: , 40–46. |
[144] | Nomura T , Inoue Y , Miyake M , Yasui K , Nakashima K ((2006) ) Prevalence and clinical characteristics of restless legs syndrome in Japanese patients with Parkinson’s disease. Mov Disord 21: , 380–384. |
[145] | Ondo WG , Vuong KD , Jankovic J ((2002) ) Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol 59: , 421–424. |
[146] | Peralta CM , Frauscher B , Seppi K , Wolf E , Wenning GK , Hogl B , Poewe W ((2009) ) Restless legs syndrome in Parkinson’s disease. Mov Disord 24: , 2076–2080. |
[147] | Moccia M , Erro R , Picillo M , Santangelo G , Spina E , Allocca R , Longo K , Amboni M , Palladino R , Assante R , Pappata S , Pellecchia MT , Barone P , Vitale C ((2016) ) A four-year longitudinal study on restless legs syndrome in Parkinson disease. Sleep 39: , 405–412. |
[148] | Gjerstad MD , Tysnes OB , Larsen JP ((2011) ) Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology 77: , 1941–1946. |
[149] | Matsubara T , Suzuki K , Fujita H , Watanabe Y , Sakuramoto H , Matsubara M , Hirata K ((2018) ) Restless legs syndrome, leg motor restlessness and their variants in patients with Parkinson’s disease and related disorders. J Neurol Sci 393: , 51–57. |
[150] | Iranzo A , Comella CL , Santamaria J , Oertel W ((2007) ) Restless legs syndrome in Parkinson’s disease and other neurodegenerative diseases of the central nervous system. Mov Disord 22 Suppl 18: , S424–430. |
[151] | Marques A , Figorilli M , Pereira B , Derost P , Debilly B , Beudin P , Vidal T , Durif F , Fantini ML ((2018) ) Impulse control disorders in Parkinson’s disease patients with RLS: A cross sectional-study. Sleep Med 48: , 148–154. |
[152] | Clemens S , Rye D , Hochman S ((2006) ) Restless legs syndrome: Revisiting the dopamine hypothesis from the spinal cord perspective. Neurology 67: , 125–130. |
[153] | Li S , Shi Y , Kirouac GJ ((2014) ) The hypothalamus and periaqueductal gray are the sources of dopamine fibers in the paraventricular nucleus of the thalamus in the rat. Front Neuroanat 8: , 136. |
[154] | Scullin MK , Sollinger AB , Land J , Wood-Siverio C , Zanders L , Lee R , Freeman A , Goldstein FC , Bliwise DL , Factor SA ((2013) ) Sleep and impulsivity in Parkinson’s disease. Parkinsonism Relat Disord 19: , 991–994. |
[155] | Cochen De Cock V ((2019) ) Therapies for restless legs in Parkinson’s disease. Curr Treat Options Neurol 21: , 56. |
[156] | Aukerman MM , Aukerman D , Bayard M , Tudiver F , Thorp L , Bailey B ((2006) ) Exercise and restless legs syndrome: A randomized controlled trial. J Am Board Fam Med 19: , 487–493. |
[157] | Winkelmann J , Allen RP , Hogl B , Inoue Y , Oertel W , Salminen AV , Winkelman JW , Trenkwalder C , Sampaio C ((2018) ) Treatment of restless legs syndrome: Evidence-based review and implications for clinical practice (Revised 2017)(section sign). Mov Disord 33: , 1077–1091. |
[158] | Trenkwalder C , Allen R , Hogl B , Clemens S , Patton S , Schormair B , Winkelmann J ((2018) ) Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol 17: , 994–1005. |
[159] | Zuzuarregui JRP , Ostrem JL ((2020) ) The impact of deep brain stimulation on sleep in Parkinson’s disease: An update. J Parkinsons Dis 10: , 393–404. |
[160] | Bahammam RA , Al-Qahtani KM , Aleissi SA , Olaish AH , Almeneessier AS , Bahammam AS ((2020) ) The associations of gender, menopause, age, and asthma with REM-predominant obstructive sleep apnea: A prospective observational study. Nat Sci Sleep 12: , 721–735. |
[161] | Peregrim I , Gresova S , Pallayova M , Fulton BL , Stimmelova J , Bacova I , Mikulakova A , Tomori Z , Donic V ((2013) ) Does obstructive sleep apnea worsen during REM sleep? Physiol Res 62: , 569–575. |
[162] | Shen Y , Shen Y , Dong ZF , Pan PL , Shi HC , Liu CF ((2020) ) Obstructive sleep apnea in Parkinson’s disease: A study in 239 Chinese patients. Sleep Med 67: , 237–243. |
[163] | Zhang LY , Liu WY , Kang WY , Yang Q , Wang XY , Ding JQ , Chen SD , Liu J ((2016) ) Association of rapid eye movement sleep behavior disorder with sleep-disordered breathing in Parkinson’s disease. Sleep Med 20: , 110–115. |
[164] | Zhang Y , Ren R , Sanford LD , Yang L , Zhou J , Tan L , Li T , Zhang J , Wing YK , Shi J , Lu L , Tang X ((2020) ) Sleep in Parkinson’s disease: A systematic review and meta-analysis of polysomnographic findings. Sleep Med Rev 51: , 101281. |
[165] | Zhang S , Meng Z , Zhang X , Huang M , Xu J (2020) The rate of decrease in oxygen desaturation during severe obstructive sleep apnea syndrome is correlated with subjective excessive daytime sleepiness. Sleep Breath, doi: 10.1007/s11325-020-02223-w |
[166] | Nomura T , Inoue Y , Kobayashi M , Namba K , Nakashima K ((2013) ) Characteristics of obstructive sleep apnea in patients with Parkinson’s disease. J Neurol Sci 327: , 22–24. |
[167] | Valko PO , Hauser S , Werth E , Waldvogel D , Baumann CR ((2012) ) Heart rate variability in patients with idiopathic Parkinson’s disease with and without obstructive sleep apnea syndrome. Parkinsonism Relat Disord 18: , 525–531. |
[168] | Sommerauer M , Werth E , Poryazova R , Gavrilov YV , Hauser S , Valko PO ((2015) ) Bound to supine sleep: Parkinson’s disease and the impact of nocturnal immobility. Parkinsonism Relat Disord 21: , 1269–1272. |
[169] | Hermann W , Schmitz-Peiffer H , Kasper E , Fauser M , Franke C , Wienecke M , Otto K , Lohle M , Brandt MD , Reichmann H , Storch A ((2020) ) Sleep disturbances and sleep disordered breathing impair cognitive performance in Parkinson’s disease. Front Neurosci 14: , 689. |
[170] | Neikrug AB , Liu L , Avanzino JA , Maglione JE , Natarajan L , Bradley L , Maugeri A , Corey-Bloom J , Palmer BW , Loredo JS , Ancoli-Israel S ((2014) ) Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep 37: , 177–185. |
[171] | Gros P , Mery VP , Lafontaine AL , Robinson A , Benedetti A , Kimoff RJ , Kaminska M ((2016) ) Obstructive sleep apnea in Parkinson’s disease patients: Effect of Sinemet CR taken at bedtime. Sleep Breath 20: , 205–212. |
[172] | Kaminska M , Mery VP , Lafontaine AL , Robinson A , Benedetti A , Gros P , Kimoff RJ ((2018) ) Change in cognition and other non-motor symptoms with obstructive sleep apnea treatment in Parkinson disease. J Clin Sleep Med 14: , 819–828. |
[173] | Gabryelska A , Roguski A , Simpson G , Maschauer EL , Morrison I , Riha RL ((2018) ) Prevalence of obstructive sleep apnoea in REM behaviour disorder: Response to continuous positive airway pressure therapy. Sleep Breath 22: , 825–830. |
[174] | Lajoie AC , Lafontaine AL , Kaminska M ((2021) ) The spectrum of sleep disorders in Parkinson’s disease: A review. Chest 159: , 818–827. |
[175] | Suzuki K , Suzuki S , Ishii Y , Okamura M , Matsubara T , Fujita H , Nozawa N , Kobayashi S , Hirata K ((2020) ) Plasma prostaglandin D2 synthase levels in sleep and neurological diseases. J Neurol Sci 411: , 116692. |
[176] | Albrecht U ((2012) ) Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 74: , 246–260. |
[177] | Leng Y , Blackwell T , Cawthon PM , Ancoli-Israel S , Stone KL , Yaffe K (2020) Association of circadian abnormalities in older adults with an increased risk of developing Parkinson disease. JAMA Neurol, e201623. |
[178] | Videnovic A , Willis GL ((2016) ) Circadian system - A novel diagnostic and therapeutic target in Parkinson’s disease? Mov Disord 31: , 260–269. |
[179] | Suzuki K , Miyamoto T , Miyamoto M , Kaji Y , Takekawa H , Hirata K ((2007) ) Circadian variation of core body temperature in Parkinson disease patients with depression: A potential biological marker for depression in Parkinson disease. Neuropsychobiology 56: , 172–179. |
[180] | Breen DP , Vuono R , Nawarathna U , Fisher K , Shneerson JM , Reddy AB , Barker RA ((2014) ) Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 71: , 589–595. |
[181] | Videnovic A , Noble C , Reid KJ , Peng J , Turek FW , Marconi A , Rademaker AW , Simuni T , Zadikoff C , Zee PC ((2014) ) Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol 71: , 463–469. |
[182] | De Pablo-Fernandez E , Courtney R , Warner TT , Holton JL ((2018) ) A histologic study of the circadian system in Parkinson disease, multiple system atrophy, and progressive supranuclear palsy. JAMA Neurol 75: , 1008–1012. |
[183] | Endo T , Matsumura R , Tokuda IT , Yoshikawa T , Shigeyoshi Y , Node K , Sakoda S , Akashi M ((2020) ) Bright light improves sleep in patients with Parkinson’s disease: Possible role of circadian restoration. Sci Rep 10: , 7982. |
[184] | Videnovic A , Klerman EB , Wang W , Marconi A , Kuhta T , Zee PC ((2017) ) Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease: A randomized clinical trial. JAMA Neurol 74: , 411–418. |
[185] | van Gilst MM , Bloem BR , Overeem S ((2013) ) “Sleep benefit” in Parkinson’s disease: A systematic review. Parkinsonism Relat Disord 19: , 654–659. |
[186] | Factor SA , Weiner WJ ((1998) ) ‘Sleep benefit’ in Parkinson’s disease. Neurology 50: , 1514–1515. |
[187] | van Gilst MM , van Mierlo P , Bloem BR , Overeem S ((2015) ) Quantitative motor performance and sleep benefit in Parkinson disease. Sleep 38: , 1567–1573. |
[188] | Rui Z , Qingling C , Xinyue Z , Xin Z , Weihong L ((2019) ) The related factors of sleep benefit in Parkinson’s disease: A systematic review and meta-analysis. PLoS One 14: , e0212951. |
[189] | Hogl BE , Gomez-Arevalo G , Garcia S , Scipioni O , Rubio M , Blanco M , Gershanik OS ((1998) ) A clinical, pharmacologic, and polysomnographic study of sleep benefit in Parkinson’s disease. Neurology 50: , 1332–1339. |
[190] | Andrade LA , Lima JG , Tufik S , Bertolucci PH , Carlini EA ((1987) ) REM sleep deprivation in an experimental model of Parkinson’s disease. Arq Neuropsiquiatr 45: , 217–223. |
[191] | Kataoka H , Saeki K , Yamagami Y , Sugie K , Obayashi K ((2020) ) Quantitative associations between objective sleep measures and early-morning mobility in Parkinson’s disease: Cross-sectional analysis of the PHASE study. Sleep 43: , zsz203. |
[192] | Xie L , Kang H , Xu Q , Chen MJ , Liao Y , Thiyagarajan M , O’Donnell J , Christensen DJ , Nicholson C , Iliff JJ , Takano T , Deane R , Nedergaard M ((2013) ) Sleep drives metabolite clearance from the adult brain. Science 342: , 373–377. |
[193] | Rasmussen MK , Mestre H , Nedergaard M ((2018) ) The glymphatic pathway in neurological disorders. Lancet Neurol 17: , 1016–1024. |
[194] | Zou W , Pu T , Feng W , Lu M , Zheng Y , Du R , Xiao M , Hu G ((2019) ) Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated alpha-synuclein. Transl Neurodegener 8: , 7. |
[195] | Iliff JJ , Chen MJ , Plog BA , Zeppenfeld DM , Soltero M , Yang L , Singh I , Deane R , Nedergaard M ((2014) ) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34: , 16180–16193. |
[196] | Lim J , Dinges DF ((2010) ) A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull 136: , 375–389. |
[197] | Schrag A , Horsfall L , Walters K , Noyce A , Petersen I ((2015) ) Prediagnostic presentations of Parkinson’s disease in primary care: A case-control study. Lancet Neurol 14: , 57–64. |
[198] | Schreiner SJ , Imbach LL , Werth E , Poryazova R , Baumann-Vogel H , Valko PO , Murer T , Noain D , Baumann CR ((2019) ) Slow-wave sleep and motor progression in Parkinson disease. Ann Neurol 85: , 765–770. |



