The Impact of the COVID-19 Pandemic on Psychological Distress, Physical Activity, and Symptom Severity in Parkinson’s Disease
Abstract
Background:
The ongoing COVID-19 pandemic has many consequences for people with Parkinson’s disease (PD). Social distancing measures complicate regular care and result in lifestyle changes, which may indirectly cause psychological stress and worsening of PD symptoms.
Objective:
To assess whether the COVID-19 pandemic was associated with increased psychological distress and decreased physical activity in PD, how these changes related to PD motor and non-motor symptom severity, and what frequency and burden of COVID-related stressors were.
Methods:
We sent an online survey to the Personalized Parkinson Project (PPP) cohort (n = 498 PD patients) in the Netherlands. In the survey, we distinguished between COVID-related stressor load, psychological distress, PD symptom severity, and physical activity. We related inter-individual differences to personality factors and clinical factors collected before the pandemic occurred.
Results:
358 PD patients completed the survey between April 21 and May 25, 2020 (response rate 71.9%). Patients with higher COVID-related stressor load experienced more PD symptoms, and this effect was mediated by the degree of psychological distress. 46.6% of PD patients were less physically active since the COVID-19 pandemic, and reduced physical activity correlated with worse PD symptoms. Symptoms that worsened most were rigidity, fatigue, tremor, pain and concentration. Presence of neuropsychiatric symptoms (anxiety, depression) before the pandemic, as well as cognitive dysfunction and several personality traits predicted increased psychological distress during the COVID-19 pandemic.
Conclusion:
Our findings show how an external stressor (the COVID-19 pandemic) leads to a worsening of PD symptoms by evoking psychological distress as well as lifestyle changes (reduced physical activity).
INTRODUCTION
The ongoing COVID-19 pandemic has an enormous impact on the physical as well as mental well-being of citizens across the world. Individuals who might have had contact with the SARS-CoV-2 virus are requested to isolate themselves. In addition, many countries have introduced different degrees of social distancing rules, including working from home, avoiding physical contact, closure of schools and restaurants, and travel restrictions.
As such, the COVID-19 pandemic has severely disrupted the lives of many people worldwide. Indeed, during earlier virus outbreak situations, many people reported psychological distress extending beyond the outbreak period [1], which underlines the importance of psychological care in susceptible individuals [2]. Importantly, the consequences reach even further in persons living with a chronic disease [3], and Parkinson’s disease (PD) is a prime example of this. The pandemic has markedly affected PD in several ways. First, although the risk of becoming infected is not increased for PD patients compared to the general population [4], those who do contract a SARS-CoV-2 infection are likely to experience a worsening of their symptoms [5]. It is not entirely clear whether the mortality is increased for PD patients with COVID-19, but patients with long-standing disease and those receiving an advanced treatment may well have a higher risk of dying [6]. Second, the care for PD patients has changed [7]: surgical treatments have been postponed [8], and access to outpatient clinics has been limited to prevent the spread of COVID-19 [9], making routine physical assessment and medication adjustments in non-emergency situations more difficult. Third, the social, economic, and medical consequences of the pandemic have led to profound lifestyle changes in PD patients, such as a reduced overall physical activity, inability to participate in exercise classes and increased levels of psychological distress. In PD, these lifestyle changes have been referred to as the “hidden sorrows” of the COVID-19 pandemic [10] since these may indirectly cause a worsening of symptoms: physical activity attenuates PD motor symptoms [11], so being grounded at home is particularly deleterious for this population; and psychological distress worsens a variety of motor symptoms [12, 13] and also induces or aggravates neuropsychiatric symptoms such as anxiety and depression [14].
The primary objective of this study was to test the hypothesis that the COVID-19 pandemic has led to an increased psychological distress in PD, thereby worsening PD symptoms. To this end, we distinguished between direct and indirect COVID-related stressors (such as respiratory symptoms, media coverage, or degree of social isolation) and perceived stress (psychological distress). This distinction is important: a similar set of stressors may lead to psychological distress in one patient but not in the other. Our second objective was to evaluate COVID-related changes in physical activities in PD, and to test whether this correlated with a worsening of PD symptoms. Third, we explored which stressors were most frequently encountered and most burdensome for patients, and which PD symptoms were affected most. Finally, we aimed to identify predictors of (increased) psychological distress in PD patients during the pandemic. This may help to identify vulnerable PD patients that could benefit from extra care in future crises, for example by offering telemedicine services [15, 16]. To test these predictions, we developed a focused online survey in an existing, well-defined longitudinal cohort of PD patients in the Netherlands (Personalized Parkinson Project; PPP [17]).
METHODS
Participants
A survey invitation was sent to all participants of the PPP, which currently consists of 498 PD patients. PPP is a single-center, longitudinal observational study with an observation period of two years, with three annual assessments at the Radboud university medical center in Nijmegen, the Netherlands [17]. All patients in the PPP had a disease duration of ≤5 years at inclusion. Measurements included extensive clinical assessments (including motor, cognitive and psychological tests), collection of biospecimens (stools, whole blood, and cerebrospinal fluid) and 3T MRI brain imaging. Subjects gave informed consent electronically to participate in this additional COVID-19 survey study.
Timeline
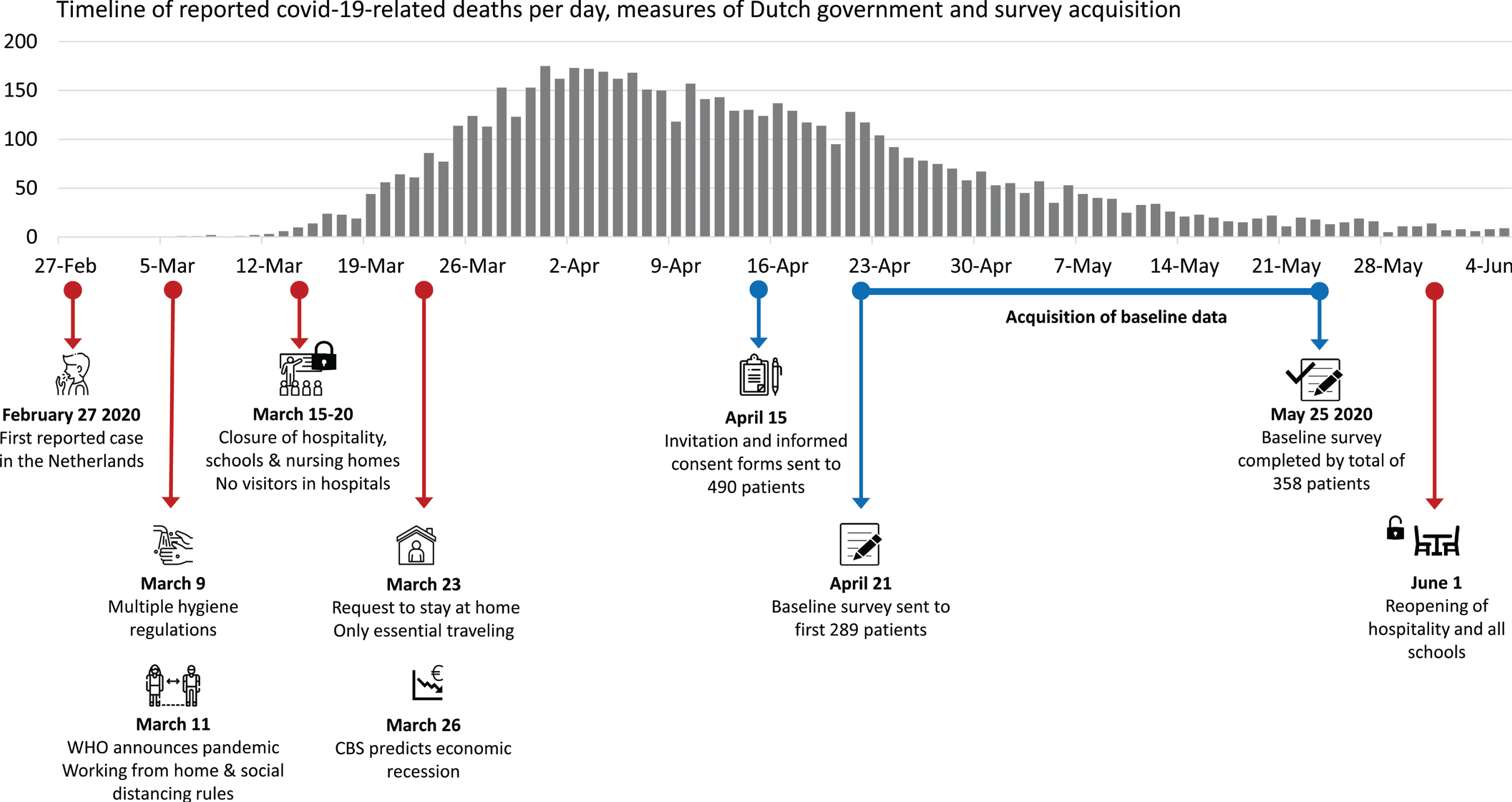
Figure 1 shows the timeline of important events and measures taken by the Dutch government since the first confirmed COVID-19 case in the Netherlands on February 27, 2020. In addition, the survey timeline is visualized. On April 15, participants of the PPP cohort were asked to participate in the additional COVID-19 survey study, for which approval was granted by the local medical ethical committee (file number 2016-2934). From April 21 onwards, after governmental measures had been in place for several weeks, the baseline survey was sent to all people who had given informed consent.
Fig. 1
Timeline: The relationship between the number of initially reported COVID-19 related deaths and measures taken in response. Also shown is the timeline of the baseline survey acquisition which is reported in the current article. WHO, World Health Organization; CBS, the Dutch ‘Central Bureau of Statistics’.
Survey content
The survey content partly overlapped with an ongoing global survey (DynaCORE-C, http://www.dynacore.info) assessing psychological resilience to the mental health consequences of the COVID-19 pandemic in healthy subjects, but our survey also contained PD-specific items. We distinguished between perceived stress, PD symptom severity, stressor load, physical activity and personality factors. Specifically, the Perceived Stress Scale (PSS) [18] was our measure of perceived stress (i.e., psychological distress): it evaluates how unpredictable, uncontrollable and overloading someone experienced the previous month, and their perceived ability to cope. We assessed PD symptom severity with the self-assessment part of the Unified Parkinson’s Disease Rating Scale part Ib and II (MDS-UPDRS-self) [19]. In addition, we added the subscale of the Parkinson Anxiety Scale (PAS) [20] measuring episodic anxiety and the brooding subscale of the Ruminative Response Scale (RRS) [21]. We also asked participants to rate to what extent PD symptoms were different in severity at this moment compared to the month preceding the start of the COVID-19 pandemic, on a 9-point scale (1 = much worse, 5 = no change, 9 = much improved) and evaluated self-reported changes in physical activity and minutes/hours of (moderate) intensive exercise per week. Next, we added a list of 18 potential external stressors asked participants which stressors of these they had been exposed to and if so, how burdensome they experienced this. Most of these stressors were related to changes due to the COVID-19 pandemic. Elements that we copied from DynaCORE-C were the SOZU-K-10 on perceived social support [22], the Brief Resilience Scale [23], the neuroticism subscale of the Big Five Inventory 10 [24], a selection of 10 items from the brief COPE [25] and 12 items from the Cognitive Emotion Regulation Questionnaire (CERQ) [26]. These selected items of COPE and CERQ were used to generate two measures: positive appraisal and behavioral coping. Positive appraisal avoids catastrophizing, pessimism and unnecessarily low self-efficacy or control perceptions, but at the same time avoids unrealistically positive (delusional) threat perceptions [27]. Behavioral coping assesses typical thinking processes that people perform when they are challenged.
Statistical analysis
Data were loaded into IBM SPSS Statistics 23 to calculate sum-scores for the validated scales and to perform statistical testing. Positive appraisal and behavioral coping were calculated as described by Veer et al. (2020) [28]: positive appraisal included the average of all 12 CERQ item scores and the 2 humor sub scores of COPE, for behavioral coping we took the average of the remaining 8 COPE items [28]. First, we conducted Pearson correlation analyses among cumulative stressor load, PSS and MDS-UPDRS-self, prior to performing a mediation analysis. Our hypothesis that PSS mediates the association between stressor load and symptom severity was tested using the PROCESS macro developed by Hayes (2013) [29], in which the 95% confidence interval of the indirect effects was obtained with 5,000 bootstrap samples. With a one-sample t-test we tested whether change in physical activity was significant different from 5 (no change). Change in physical activity was correlated with change in PD symptom severity and PSS scores using Spearman correlations, in which change in PD symptom severity was calculated as the average change score across all symptoms combined for every participant. We also assessed changes in separate PD symptoms since the COVID-19 pandemic, by merging responses into 3 classes: worsening (scores 1–4), no change (score 5) and improvement (scores 6–9). With a one-sample t-test we assessed whether change in PD symptom severity was significantly different from 5 (no change), and we calculated Pearson correlations with perceived stress (PSS) and symptom severity (MDS-UPDRS-self). We quantified stressor load by evaluating which stressors (from a list of 18) participants had been frequently exposed to, and which were most burdensome. We subsequently quantified individual cumulative stressor load by weighing each reported stressor by its burden rating (1–5) and formed a weighted sum. Additional correlations between PSS and hypothesized protective personality traits, clinical characteristics and stressor load were again calculated with Pearson correlations, and we corrected for multiple comparisons. Given the exploratory nature of this analysis, we corrected for multiple comparisons (n = 17) using a conservative Bonferroni correction (i.e., p < 0.0029 was considered significant).
Data handling and data availability
We used Castor (https://data.castoredc.com) to collect survey data. All data were stored with polymorphic encryptions and pseudonyms (PEP), to guarantee the privacy of participants but also to enable data sharing with interested researchers. This system allows qualified researchers to have access to the entire dataset or a subset of the dataset with specific keys for decryption. More information can be found on https://pep.cs.ru.nl.
RESULTS
Demographics
On April 15, 2020, all 498 enrolled PPP participants were invited to participate in this COVID-19 study (Table 1). 358 of them (response rate = 71.9%) completed the baseline survey (38.5% women), with a mean age of 62.8 years and a mean disease duration of 3.9 years. Table 1 also shows the general characteristics and total scores of the scales collected during the most recent PPP visit, which was on average 7.5 months prior to the COVID-survey, well before the start of the COVID-pandemic in the Netherlands. For these scales, non-responders scored higher on anxiety (STAI), depression (BDI), sleeping problems (SCOPA-sleep), motor and non-motor symptoms and aspects of experiences of daily living (MDS-UPDRS-I and MDS-UPDRS-II), and slightly lower on cognitive abilities (MoCA).
Table 1
Characteristics of the study population
| General characteristics | Responders (n = 358) | Non-responders (n = 140) | Difference | ||
| Mean (SD) or n (%) | Mean (SD) or n (%) | ||||
| Age (years) | 62.8 (9.0) | 63.3 (9.1) | p = 0.890 | ||
| Gender (% women) | 138 (38.5%) | 62 (44.3%) | p = 0.351 | ||
| Disease duration (years) | 3.9 (1.8) | 4.3 (2.0) | p = 0.059 | ||
| Use of dopaminergic medication (% yes) | 93.9% | 97.9% | p = 0.060 | ||
| Presence of other medical conditions (% yes) | 67.3% | 66.4% | p = 0.849 | ||
| Assessment at last PPP visit (on average 7.5 months prior to COVID-19 survey) | |||||
| Unified Parkinson’s Disease Rating Scale | |||||
| MDS-UPDRS I | 12.0 (5.4) | 13.6 (5.7) | p = 0.003* | ||
| MDS-UPDRS II | 8.3 (5.9) | 9.8 (6.8) | p = 0.024* | ||
| MDS-UPDRS self-assessment (Ib + II) | 17.7 (8.5) | 20.4 (9.5) | p = 0.003* | ||
| MDS-UPDRS III On PD medication | 29.5 (12.6) | 30.9 (12.5) | p = 0.234 | ||
| MDS-UPDRS III Off PD medication | 34.8 (12.9) | 35.0 (13.7) | p = 0.675 | ||
| Hoehn & Yahr | On state | Off state | On state | Off state | On state |
| 1 | 14 (3.9%) | 15 (4.2%) | 6 (4.3%) | 6 (4.3%) | p = 0.520 |
| 2 | 311 (86.9%) | 308 (86.0%) | 120 (85.7%) | 117 (83.6%) | Off state |
| 3 | 9 (2.5%) | 30 (8.4%) | 8 (5.7%) | 13 (9.3%) | p = 0.940 |
| 4 | 4 (1.1%) | 45(1.4%) | 2 (1.4%) | 3 (2.1%) | |
| Missing | 20 (5.6%) | 0 (0%) | 4 (2.9%) | 1 (0.7%) | |
| Montreal Cognitive Assessment (MoCA) | 26.8 (2.4) | 26.0 (3.4) | p = 0.003* | ||
| State Trait Anxiety Inventory (STAI) | 70.8 (18.1) | 79.4 (21.8) | p = 0.000* | ||
| Beck’s Depression Inventory II (BDI-II) | 9.9 (6.3) | 12.6 (7.7) | p = 0.000* | ||
| Scales for Outcomes in PD (SCOPA-sleep) | 7.6 (5.0) | 8.9 (5.6) | p = 0.020* | ||
| Years of education | 12.9 (2.7) | 12.9 (2.8) | p = 0.893 | ||
| Living situation | |||||
| With partner | 64.8% | 65.7% | p = 0.642 | ||
| With family | 24.3% | 20.3% | |||
| Alone | 7.5% | 9.1% | |||
| Other | 3.4% | 4.9% | |||
| Paid job (% yes) | 39.1% | 35.0% | p = 0.388 | ||
| Assessment during COVID-19 survey (on average 1.4 months after introduction of social distancing measures) | |||||
| MDS-UPDRS self-assessment (Ib+II) | 16.5 (9.4) | ||||
| Social Support (SOZU-K) | 31.4 (5.1) | ||||
| Brief Resilience Scale (BRS) | 3.5 (0.7) | ||||
| Optimism (5-point scale) | 4.0 (0.8) | ||||
| Positive appraisal style (CERQ &COPE) | 11.9 (2.6) | ||||
| Behavioral coping style (CERQ &COPE) | 20.1 (4.2) | ||||
| Perceived Stress Scale (PSS) | 9.9 (5.8) | ||||
| Parkinson Anxiety Scale (PAS part B) | 5.8 (2.0) | ||||
| Ruminative Response Scale (RRS) | 1.9 (2.0) | ||||
| Neuroticism (BFI-neuroticism) | 4.9 (1.9) | ||||
Mean (SD) or number (percentages) for demographic and clinical characteristics, and total scores of the scales used in our COVID-19 survey. Column 3 shows the p-values for differences between non-responders and responders, for continuous variables measured with t-tests and for categorical variables with χ2-tests. We indicated all significant differences of p < 0.05 with *.
Impact on psychological distress and PD symptom severity
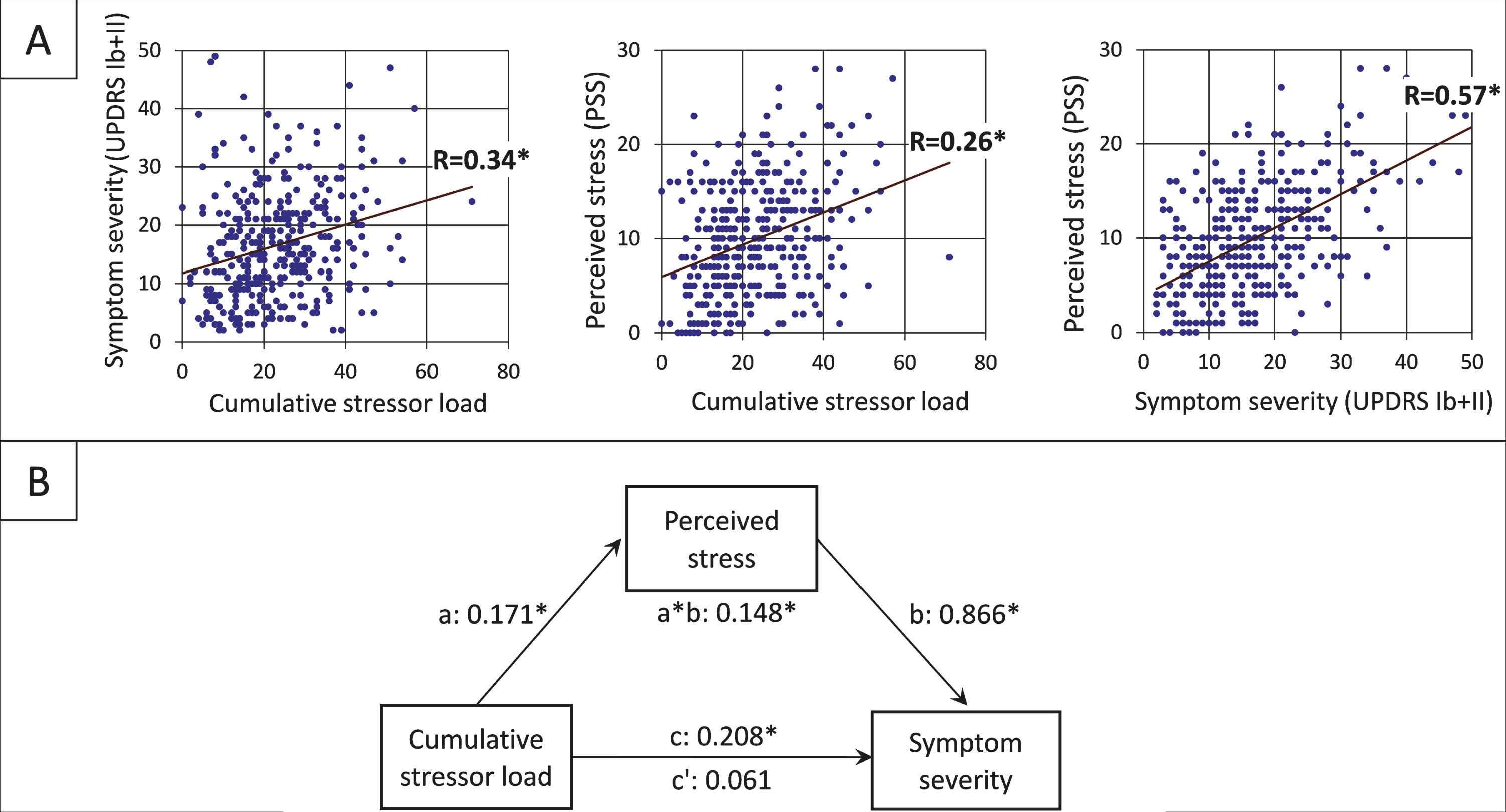
Higher stressor load was associated with higher scores on perceived stress (PSS) (R = 0.34, [95% CI 0.25, 0.43]), and MDS-UPDRS-self symptom severity (R = 0.26, [95% CI 0.16, 0.35]) (Fig. 2A). More specifically, the positive relationship between stressor load and increased PD symptom severity was mediated by increased levels of perceived stress (PSS; Fig. 2B): cumulative stressor load was a significant predictor of PD symptom severity (b = 0.208 [95% CI 0.127, 0.289]), but this relationship was no longer significant in the presence of the standardized indirect effect (b = 0.171*0.866 = 0.148 [95% CI 0.094, 0.206]) of perceived stress as a mediator in the model (b = 0.061 [95% CI –0.013, 0.134]).
Fig. 2
Relationship between stressor load, psychological distress, and PD symptom severity (A) The positive linear relationships between the three factors of the mediation analysis. (B) The standardized regression coefficients for the relationship between cumulative stressor load (total of experienced COVID-related stressors weighted by the experienced burden; range = 0–71) and self-assessed symptom severity (sum of MDS-UPDRS Ib and MSD-UPDRS II score; range = 2–53) as mediated by perceived stress (PSS score; range = 0–28) The direct effect is indicated by c’, the indirect effect by a*b, the total effect by c (*p < 0.001).
Impact on physical activity
Patients were significantly less active than before the pandemic when comparing against a score of 5 (no change): (MD = –0.50, [95% CI –0.67, –0.33]). Specifically, 46.6% of responders were less active compared to before the pandemic, 33.0% were equally active and 20.4% were more active. Patients with lower scores on this 9-point scale (i.e., less physical activity) experienced more worsening of PD symptoms (rs = 0.14 [95% CI 0.03, 0.25]). Furthermore, 38.8% of patients were physically active ≥4 hours per week. There was no relationship between time spent on physical activity and the degree of perceived stress (PSS) (rs = –0.08 [95% CI –0.18 0.05]).
Changes in PD symptoms
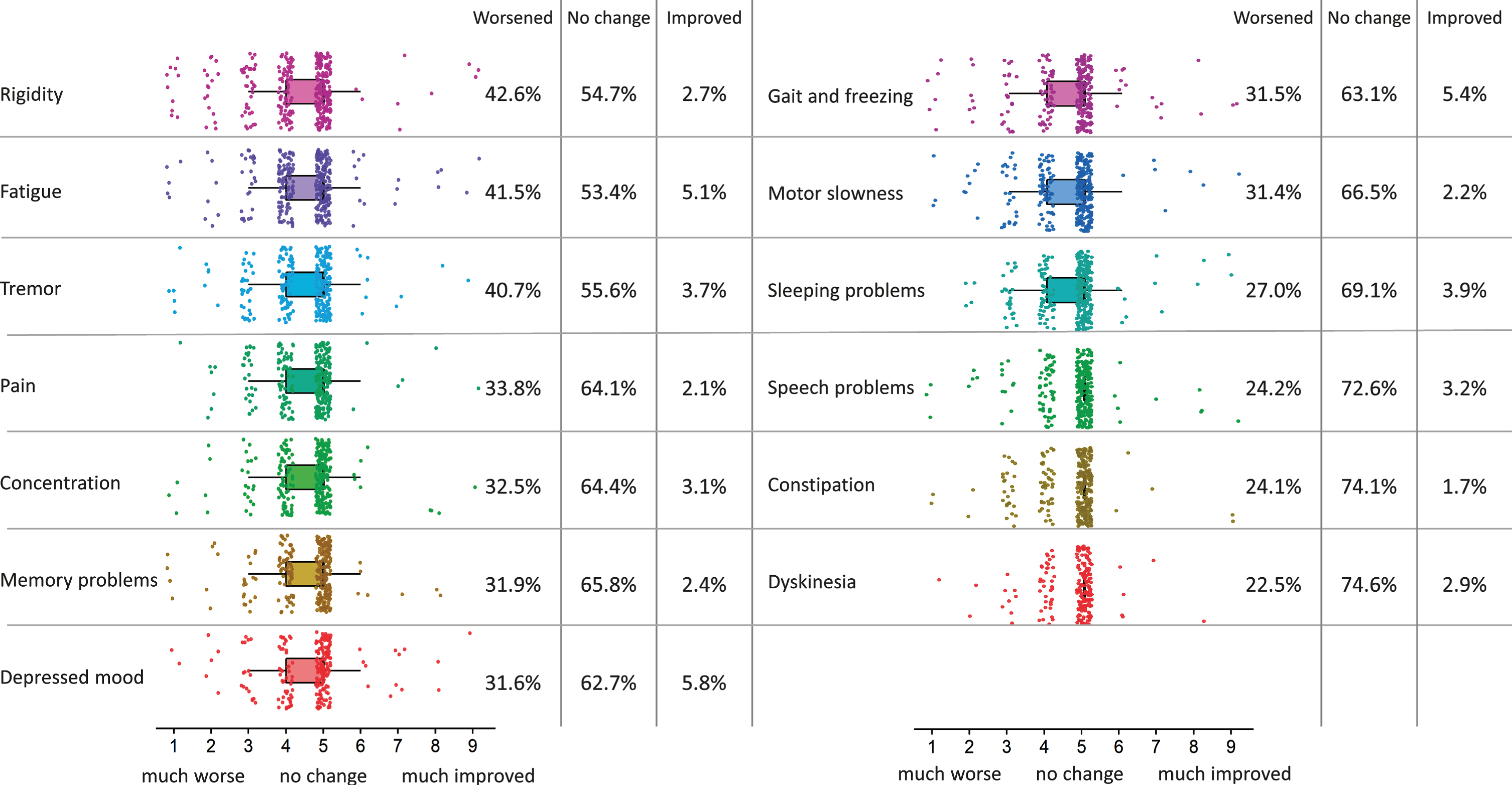
Figure 3 shows subjective changes in PD symptom severity during the COVID-19 pandemic, as compared to the month before the pandemic started. The average individual score of all 13 evaluated PD symptoms significantly worsened during the COVID-19 pandemic, when compared against a score of 5 (no change): (MD = –0.36, [95% CI –0.43, –0.29]). Patients with higher levels of perceived stress (PSS) experienced more worsening of PD symptoms (R = –0.37, [95% CI –0.46, –0.28]). Furthermore, patients with more severe PD symptoms (MDS-UPDRS-self) experienced more worsening of their symptoms during the pandemic (R = –0.46, [95% CI –0.54, –0.37]). Symptoms for which most worsening was perceived were rigidity, fatigue, tremor, pain and concentration.
Fig. 3
Change in PD symptom severity during COVID-19 pandemic. Changes in PD symptom severity of 13 problems which are common in PD during the COVID-19 pandemic, as compared to a month before the pandemic started (n = 358). The colored boxplots show responses on the 9-point scale (1 = much worse, 5 = no change, 9 = much improved). The percentages at the right side of the boxplots show percentages of people that experienced worsening (scores of 1–4), no change (scores of 5) and improvement (scores of 6–9) for every of these symptoms. Symptoms are ordered by how much they got worse during the COVID-19 pandemic.
Stressor exposure
Stressors that were most frequently experienced by PD patients were loss of social contact (93.1%), COVID-19 related media coverage (91.7%), not being able to perform physical activity or leisure activities as usual (78.7%) and feeling restricted to leave home (75.6%). Most burdensome (scale 1–5) for PD patients were not being able to attend the funeral of a loved one (3.6), being restricted in visiting loved ones in the hospital (3.4), loss of social contact (3.1) and not being able to perform physical activities or leisure activities as usual (3.0). An overview of all stressors can be found in the Supplementary Material.
Predictors of psychological distress
Table 2 shows an overview of personality traits, clinical characteristics, and stressor load, and how these factors correlate to perceived stress (PSS) scores. In addition to the effects described above (Fig. 2), people with high perceived stress scores (PSS) had lower scores on social support, trait resilience, optimism, positive appraisal style, whereas they scored higher on anxiety, rumination and neuroticism (Table 2). We did not find a significant association (Bonferroni corrected) with age, disease duration, sex, time of physical activity, cognitive abilities or behavioral coping style.
Table 2
Associations between psychological distress (PSS) and hypothesized resilience factors
| Hypothesized factors influencing psychological distress | Correlation with psychological distress (PSS) | |
| Pearson’s R [95% CI] | P-value | |
| General characteristics | ||
| Age (years) | R = –0.01 [95% CI –0.12 0.09] | p = 0.809 |
| Biological sex | R = –0.04 [95% CI –0.14 0.06] | p = 0.451 |
| Physical activity (minutes per week) | R = –0.07 [95% CI –0.17 0.04] | p = 0.195 |
| Clinical characteristics | ||
| PD disease duration (years) | R = 0.01 [95% CI –0.09, 0.11] | p = 0.825 |
| PD symptom severity in daily life (MDS-UPDRS Ib + II) | R = 0.57 | p = 0.000 |
| Anxiety | ||
| Parkinson Anxiety Scale (PAS part B) | R = 0.62 [95% CI 0.55, 0.68] | p = 0.000 |
| State-Trait Anxiety Inventory (STAI)1 | R = 0.64 [95% CI 0.56, 0.69] | p = 0.000 |
| Ruminative Response Scale (RRS) | R = 0.57 [95% CI 0.50, 0.64] | p = 0.000 |
| Cognitive abilities (MoCA)1 | R = –0.14 [95% CI –0.24 –0.04] | p = 0.007 |
| Depression (BDI-II)1 | R = 0.51 [95% CI 0.43, 0.58] | p = 0.000 |
| Sleeping problems (SCOPA-sleep)1 | R = 0.30 [95% CI 0.20, 0.39] | p = 0.000 |
| Stressor load | ||
| Cumulative stressor load | R = 0.34 [95% CI 0.25, 0.43] | p = 0.000 |
| Personality traits | ||
| Perceived social support (SOZU-K) | R = –0.32 [95% CI –0.41, –0.22] | p = 0.000 |
| Brief Resilience Scale (BRS) | R = –0.56 [95% CI –0.63, –0.48] | p = 0.000 |
| Optimism (5-point scale) | R = –0.36 [95% CI –0.45, –0.27] | p = 0.000 |
| Neuroticism (BFI-neuroticism) | R = 0.52 [95% CI 0.44, 0.59] | p = 0.000 |
| Positive appraisal style (CERQ &COPE) | R = –0.31 [95% CI –0.40, –0.21] | p = 0.000 |
| Behavioral coping style (CERQ &COPE) | R =–0.10 [95% CI –0.20, 0.00] | p = 0.068 |
Bold values significant correlations at p < 0.05 (p < 0.0029 after Bonferroni correction). 1 measurement at last PPP assessment on average 7.5 months before the COVID-19 survey, not added in the survey. Pearson correlation values and their confidence intervals between psychological distress (score on Perceived Stress Scale (PSS)) and several general and clinical characteristics, stressor load and personality traits.
DISCUSSION
We report the results of the COVID-survey assessing the impact of the COVID-19 crisis in a large group of 358 PD patients, for whom we also had longitudinal baseline findings spanning the period prior to the pandemic. There are four main findings. First, patients with higher COVID-related stressor load experienced higher PD symptom severity, and this effect was mediated by the degree of psychological distress [30]. Second, 46.6% of PD patients in our sample were physically less active since the COVID-19 pandemic unfolded, and the reduction in physical activity correlated with worsening of PD symptoms. Third, we showed that the symptoms that worsened most were rigidity, fatigue, tremor, pain and concentration. Fourth, we found that PD patients with high perceived stress levels experienced more anxiety, rumination and neuroticism, and they scored lower on cognitive abilities, social support, trait resilience, optimism and positive appraisal style.
Our findings fit with a recent study in a much smaller group of 38 Egyptian PD patients, who reported higher levels of stress and anxiety during the pandemic than healthy controls, as well as reduced physical exercise [31]. Here we extend these previous findings by showing that physical activity and psychological distress increased symptom severity in the patients. Surprisingly, time of physical activity and perceived stress were not correlated to each other, despite some reports showing that physical activity lowers stress [32]. This may suggest that both lifestyle factors independently influence disease severity through different mechanisms and, consequently, that both should be targeted separately as part of a comprehensive treatment approach. Symptoms for which most PD patients experienced worsening were rigidity, fatigue, tremor and pain. The subjective increase in tremor and rigidity is in line with previous work showing that these symptoms are particularly sensitive to stress [12, 33]. Increased pain may similarly result from increased anxiety but could also be secondary to rigidity; this remains speculative. Fatigue is a well-known consequence of psychological distress [34, 35], also in non-PD populations. Dyskinesias increased only in 13.1% and gait difficulties in 24.6% of PD patients, which seems low when considering previous studies which showed that psychological distress clearly worsens these two symptoms [13, 36]. This may be explained by the fact that our sample consisted of relatively early PD patients (disease duration of 3.9 years), where these symptoms are relatively uncommon. The fact that even these relatively mildly affected patients were already affected substantially underscores the enormous impact of the COVID-19 pandemic; we suspect that the consequences are even greater in more severely affected patients with longstanding disease.
The COVID-19 related stressor that was most frequently experienced (by 93.9% of patients) was loss of social contacts. This is in line with recent interviews with German PD patients, 86.9% of whom reported having fewer social contacts and staying at home more because of the COVID-19 pandemic [37]. Others mentioned that due to their PD, they already had a low number of social contacts or were frequently homebound. The greatest fear amongst the interviewed German patients was becoming infected with COVID-19. This was different in our sample: even among the 19% of patients who experienced COVID-19 related symptoms, this was not experienced as burdensome (2.6 on a scale of 1–5). Healthy respondents to the DynaCORE survey resembled our PD sample regarding the most frequently experienced and most burdensome stressors. One striking difference is that 22.6% of patients were unable to attend the funeral of a loved one, whereas this was only 11.1% in the DynaCORE respondents. Another difference is that as many as 56.4% of our PD group belonged to a risk group for a serious disease course in case of a COVID-19 infection (average burden 2.5), and this was the case for 33.3% of the DynaCORE respondents (average burden 3.0).
The most vulnerable patients in our study, i.e., those with the highest levels of psychological distress, were people with lower baseline cognitive abilities and increased neuropsychiatric symptoms at baseline (i.e., collected before the COVID-19 pandemic), as well as low (perceived) social support and specific personality characteristics such as low trait resilience, low optimism, and high neuroticism. This is in line with previous studies in healthy people [28, 38, 39]. Longitudinal follow-up of our cohort might reveal which factors remain correlated with changes in psychological distress over time. Some of these factors can possibly be addressed in vulnerable PD patients throughout a developing crisis, e.g., through individual remote counseling, or by distributing self-help material via social media.
When interpreting these effects, the specific sample that we investigated should be considered. The response rate (71.9%) was very high, but interestingly, the non-responders had higher baseline scores on anxiety, depression, subjective severity of PD symptoms, and lower cognitive abilities. In our analyses (Table 2), these factors predicted increased perceived stress, suggesting that our findings may be an underestimation of perceived stress in the entire PD population. This notion is further strengthened by the fact that our sample had relatively mild PD, given the inclusion criteria of the PPP (disease duration <5 years at inclusion). We acknowledge that the lack of a control group does not allow us to determine to what extent our findings are specific to PD. Indeed, as we outlined above, some of the observed effects, such as the relationship between personality characteristics and perceived stress, may generalize to the whole population. On the other hand, our primary findings concern the relationship with PD symptoms, for which a control group is not necessary.
Strengths of the current study are the large PD sample for which many baseline measures were collected prior to this present COVID-19 survey. Additionally, we were able to make a clear distinction between individual stressor load on the one hand and perceived stress or psychological distress on the other. However, there are some limitations regarding the degree to which these results can be generalized to all PD patients. First, the PPP cohort consists of patients with relatively early-stage PD. The impact of COVID-19 might well be even more dramatic for patients with late-stage PD. Second, due to the retrospective nature of the survey, responses might be less accurate, since for most questions we had no baseline data for the period from before the COVID-19 pandemic. Moreover, the correlations we found do not imply causality. The hypothesized protective personality traits for which we found relatively high correlations with psychological distress might be trained or strengthened in vulnerable PD patients, but they cannot be used to clinically identify which people will develop mental problems. Finally, stressor load should ideally be based on a more extensive list of (non-COVID related) items, although we already see strong correlations with other measures when using only the current items.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
A. van der Heide reports no disclosures. M. Meinders reports no disclosures. B. Bloem receives funding from the National Parkinson Foundation, the Netherlands organization for Scientific Research, International ParkinsonFonds and the Michael J. Fox Foundation. The Parkinson Center of the Radboud University Medical Center was supported by a center of excellence grant of the Parkinson’s Foundation. R. Helmich was supported by the Michael J. Foundation (grant #16048) and by the Netherlands Organization for Scientific Research (VENI grant #91617077). R. Helmich serves on the Clinical Advisory Board of Cadent Therapeutics. We would like to thank all PD patients for their participation in this study, and Mrs. Tessa van de Zande and Mr. Geert Schattenberg for their help in data processing.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-202251.
REFERENCES
[1] | Brooks SK , Webster RK , Smith LE , Woodland L , Wessely S , Greenberg N , Rubin GJ ((2020) ) The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 395: , 912–920. |
[2] | Duan L , Zhu G ((2020) ) Psychological interventions for people affected by the COVID-19 epidemic. Lancet Psychiatry 7: , 300–302. |
[3] | Adams ML , Katz DL , Grandpre J ((2020) ) Population-based estimates of chronic conditions affecting risk for complications from coronavirus disease, United States. Infect Dis 26: , 1831–1833. |
[4] | Fasano A , Cereda E , Barichella M , Cassani E , Ferri V , Zecchinelli AL , Pezzoli G ((2020) ) COVID-19 in Parkinson’s disease patients living in Lombardy, Italy. Disord 35: , 1089–1093. |
[5] | Cilia R , Bonvegna S , Straccia G , Andreasi NG , Elia AE , Romito LM , Devigili G , Cereda E , Eleopra R ((2020) ) Effects of COVID-19 on Parkinson’s disease clinical features: A community-based case-control study. Mov Disord, doi: 10.1002/mds.28170] |
[6] | Antonini A , Leta V , Teo J , Chaudhuri KR ((2020) ) Outcome of Parkinson’s disease patients affected by COVID-19. Disord 35: , 905–908. |
[7] | Schirinzi T , Cerroni R , Di Lazzaro G , Liguori C , Scalise S , Bovenzi R , Conti M , Garasto E , Mercuri NB , Pierantozzi M , Pisani A , Stefani A ((2020) ) Self-reported needs of patients with Parkinson’s disease during COVID-19 emergency in Italy. Sci 41: , 1373–1375. |
[8] | Moletta L , Pierobon ES , Capovilla G , Costantini M , Salvador R , Merigliano S , Valmasoni M ((2020) ) International guidelines and recommendations for surgery during Covid-19 pandemic: A systematic review. J Surg 79: , 180–188. |
[9] | Sharma A , Maxwell CR , Farmer J , Greene-Chandos D , LaFaver K , Benameur K ((2020) ) Initial experiences of US neurologists in practice during the COVID-19 pandemic via survey. Neurology 95: , 215–220. |
[10] | Helmich RC , Bloem BR ((2020) ) The impact of the COVID-19 pandemic on Parkinson’s disease: Hidden sorrows and emerging opportunities. J Parkinsons Dis 10: , 351–354. |
[11] | van der Kolk NM , de Vries NM , Kessels RPC , Joosten H , Zwinderman AH , Post B , Bloem BR ((2019) ) Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol 18: , 998–1008. |
[12] | Zach H , Dirkx M , Bloem BR , Helmich RC ((2015) ) The clinical evaluation of Parkinson’s tremor. J Parkinsons Dis 5: , 471–474. |
[13] | Macht M , Kaussner Y , Moller JC , Stiasny-Kolster K , Eggert KM , Kruger HP , Ellgring H ((2007) ) Predictors of freezing in Parkinson’s disease: A survey of 6,620 patients. Disord 22: , 953–956. |
[14] | Brown RG , Landau S , Hindle JV , Playfer J , Samuel M , Wilson KC , Hurt CS , Anderson RJ , Carnell J , Dickinson L , Gibson G , van Schaick R , Sellwood K , Thomas BA , Burn DJ , PROMS-PD Study Group ((2011) ) Depression and anxiety related subtypes in Parkinson’s disease. J Neurol Neurosurg Psychiatry 82: , 803–809. |
[15] | Dorsey E , Okun MS , Bloem BR ((2020) ) Care, convenience, comfort, confidentiality, and contagion: The 5 C’s that will shape the future of telemedicine. J Parkinsons Dis 10: , 893–897. |
[16] | Cilia R , Mancini F , Bloem BR , Eleopra R ((2020) ) Telemedicine for parkinsonism: A two-step model based on the COVID-19 experience in Milan, Italy. Relat Disord 75: , 130–132. |
[17] | Bloem B , Marks W , de Lima AS , Kuijf M , van Laar T , Jacobs B , Verbeek M , Helmich R , van de Warrenburg B , Evers L ((2019) ) The Personalized Parkinson Project: Examining disease progression through broad biomarkers in early Parkinson’s disease. BMC Neurol 19: , 160. |
[18] | Cohen S , Kamarck T , Mermelstein R ((1983) ) A global measure of perceived stress. J Health Soc Behav 24: , 385–396. |
[19] | Rodriguez-Blazquez C , Alvarez M , Arakaki T , Campos Arillo V , Chana P , Fernandez W , Garretto N , Martinez-Castrillo JC , Rodriguez-Violante M , Serrano-Duenas M , Ballesteros D , Rojo-Abuin JM , Ray Chaudhuri K , Merello M , Martinez-Martin P ((2017) ) Self-assessment of disability in Parkinson’s disease: The MDS-UPDRS Part II versus clinician-based ratings. Disord Clin Pract 4: , 529–535. |
[20] | Leentjens AF , Dujardin K , Pontone GM , Starkstein SE , Weintraub D , Martinez-Martin P ((2014) ) The Parkinson Anxiety Scale (PAS): Development and validation of a new anxiety scale. Disord 29: , 1035–1043. |
[21] | Nolen-Hoeksema S , Wisco BE , Lyubomirsky S ((2008) ) Rethinking Rumination. Psychol Sci 3: , 400–424. |
[22] | Dunkel D , Antretter E , Frohlich-Walser S , Haring C ((2005) ) Evaluation of the short-form social support questionnaire (SOZU-K-22) in clinical and non-clinical samples. Psychosom Med Psychol 55: , 266–277. |
[23] | Smith BW , Dalen J , Wiggins K , Tooley E , Christopher P , Bernard J ((2008) ) The brief resilience scale: Assessing the ability to bounce back. J Behav Med 15: , 194–200. |
[24] | Rammstedt B ((2007) ) The 10-item big five inventory. J Psychol Assess 23: , 193–201. |
[25] | Carver CS ((1997) ) You want to measure coping but your protocol’s too long: Consider the brief COPE. J Behav Med 4: , 92–100. |
[26] | Garnefski N , Kraaij V ((2006) ) Cognitive emotion regulation questionnaire–development of a short 18-item version (CERQ-short). Individ Dif 41: , 1045–1053. |
[27] | Kalisch R , Muller MB , Tuscher O ((2015) ) A conceptual framework for the neurobiological study of resilience. Brain Sci 38: , e92. |
[28] | Veer IM , Riepenhausen A , Zerban M , Wackerhagen C , Engen H , Puhlmann L , Köber G , Bögemann S , Weermeijer J , Uściłko A ((2020) ) Psycho-social factors associated with mental resilience in the Corona lockdown. PsyArXiv, April 22, doi:10.31234/osf.io/4z62t. |
[29] | Hayes AF , Rockwood NJ ((2017) ) Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Res Ther 98: , 39–57. |
[30] | Nielsen MG , Ornbol E , Vestergaard M , Bech P , Larsen FB , Lasgaard M , Christensen KS ((2016) ) The construct validity of the Perceived Stress Scale. J Psychosom Res 84: , 22–30. |
[31] | Shalash A , Roushdy T , Essam M , Fathy M , Dawood NL , Abushady EM , Elrassas H , Helmi A , Hamid E ((2020) ) Mental health, physical activity, and quality of life in Parkinson’s disease during COVID-19 pandemic. Disord 35: , 1097–1099. |
[32] | Zschucke E , Renneberg B , Dimeo F , Wüstenberg T , Ströhle A ((2015) ) The stress-buffering effect of acute exercise: Evidence for HPA axis negative feedback. Psychoneuroendocrinology 51: , 414–425. |
[33] | Boman K ((1971) ) Effect of emotional stress on spasticity and rigidity. J Psychosom Res 15: , 107–112. |
[34] | Chen MK ((1986) ) The epidemiology of self-perceived fatigue among adults. Med 15: , 74–81. |
[35] | Bültmann U , Kant I , Kasl SV , Beurskens AJ , van den Brandt PA ((2002) ) Fatigue and psychological distress in the working population: Psychometrics, prevalence, and correlates. J Psychosom Res 52: , 445–452. |
[36] | Durif F , Vidailhet M , Debilly B , Agid Y ((1999) ) Worsening of levodopa-induced dyskinesias by motor and mental tasks. Disord 14: , 242–246. |
[37] | Zipprich HM , Teschner U , Witte OW , Schonenberg A , Prell T ((2020) ) Knowledge, attitudes, practices, and burden during the COVID-19 pandemic in people with Parkinson’s disease in Germany. J Clin Med 9: , 1643. |
[38] | House JS , Landis KR , Umberson D ((1988) ) Social relationships and health. Science 241: , 540–545. |
[39] | Scheier MF , Carver CS ((1992) ) Effects of optimism on psychological and physical well-being: Theoretical overview and empirical update. Ther Res 16: , 201–228. |




