The Universal Prescription for Parkinson’s Disease: Exercise
Abstract
Over the past two decades, aerobic exercise has emerged as a mainstream recommendation to aid in treating Parkinson’s disease (PD). Despite the acknowledgement of the benefits of exercise for people with PD (PwPD), frequently, exercise recommendations lack specificity in terms of frequency, intensity and duration. Additionally, conflating physical activity with exercise has contributed to providing vague exercise recommendations to PwPD. Therefore, the beneficial effects of exercise may not be fully realized in PwPD. Data provided by animal studies and select human trials indicate aerobic exercise may facilitate structural and functional changes in the brain. Recently, several large human clinical trials have been completed and collectively support the use of aerobic exercise, specifically high-intensity aerobic exercise, in improving PD motor symptoms. Data from these and other studies provide the basis to include aerobic exercise as an integral component in treating PD. Based on positive clinical findings and trials, it is advised that PwPD perform aerobic exercise in the following dose: 3x/week, 30–40-minute main exercise set, 60–80% of heart rate reserve or 70–85% of heart rate max. In lieu of heart rate, individuals can achieve an intensity of 14–17 on a 20-point RPE scale. Ongoing clinical trials, SPARX3 and CYCLE-II, have potential to further develop patient-specific exercise recommendations through prognostic modeling.
INTRODUCTION
Incidence of Parkinson’s disease (PD) has been estimated at 1.5% per year among adults 65+, with nearly a million Americans having been diagnosed with PD [1]. Recent data suggest that PD is the fastest growing neurological disease, outpacing even Alzheimer’s disease [2]. Despite advances in pharmaceutical and surgical approaches in PD symptomatic treatment, disease-modifying therapies have remained elusive [3, 4]. Data from animal [5–10] and human models [11, 12] indicate high intensity exercise is a promising approach to slow the neurodegenerative processes associated with PD.
The World Health Organization recommends, at minimum, older adults (those 65+ years) should completed at least 150 minutes of moderate intensity aerobic exercise per week or at least 75 minutes of vigorous intensity exercise [13]. Despite these recommendations, the vast majority of healthy older adults do not achieve these minimal activity levels on a weekly basis [14]. Following a diagnosis of PD, activity levels drop precipitously and are significantly less than healthy peers [15, 16]. While exercise and physical activity are often used interchangeably, they are distinct [17]. Physical activity is defined as any bodily movement that produces energy expenditure (e.g., gardening, household chores, etc.) while exercise, a subset of physical activity, is planned, structured, repetitive, and has a defined end point with a goal of improving physical fitness [18]. Both physical activity and exercise play a role in the management and quality of life of people with PD (PwPD); moreover, exercise is a broad term that incorporates a plethora of activity such as aerobic exercise, resistance training, flexibility training, and other types of exercise. The focus of this paper is to provide a balanced review of the aerobic exercise and PD literature and provide rationale for the use of aerobic exercise as medicine for the treatment of PD.
THE DARK AGES OF EXERCISE AND PARKINSON’S DISEASE
Jean-Martin Charcot, the Father of Neurology, may have been the first to recognize that movement, of sort, had potential in aiding PD patients (see Goetz 1986 for review [19]). Observing that his patients reported symptomatic relief following horse and carriage rides, he developed a vibratory chair and helmet to relive motor symptoms [20]. Positive symptom relief furthered his theory that muscular weakness was not the primary driver of motor symptoms, but rather the cause was central in nature. Goetz and colleagues developed a similar vibratory chair and were unable to replicate the initial observations of Charcot in a randomized trial [21]; it appears the initial observation may have been placebo. Nevertheless, Charcot’s suggestion that movement, albeit passive, may be useful in treating PD could have started a revolution in the use of exercise to treat PD.
Despite Charcot’s observations, with the publication of Gower’s Manual of Diseases of the Nervous System in 1899, Neurology entered the Dark Ages of Exercise Recommendations for PD with the recommendation that, “life should be quiet and regular, freed, as far as may be, from care and work”[22]. As a result, exercise recommendations were largely focused on increasing range of motion or using low intensity chair-based resistance exercises [23, 24]. The emphasis on low intensity exercises persisted throughout the 1980s with predominating recommendations being experienced-based, rather than evidence-based [25, 26]. A notable advancement in PD management occurred in the 1975, when carbidopa/levodopa became commercially available [27]. The lifespan of PwPD increased following the introduction of the drug; Ahlskog suggested that the longevity may have been a result of increased mobilization on a generally sedentary population, as pharmacologic treatment has not demonstrated unequivocal disease-modifying properties [28].
THE GILDED AGE OF EXERCISE AND PARKINSON’S DISEASE
In the 2000s, positive results from animal studies ushered in the Gilded Age for use of exercise as an adjunct to pharmacological treatments for PwPD. Impactful animal studies indicated that high-intensity, but not low-intensity, exercise had neuroprotective properties [8, 9] and that exercise improved motor functioning in MPTP or 6-OHDA treated animals [6, 10, 29]. Results pointed toward exercise increasing brain derived neurotrophic factor (BDNF), glial derived neurotrophic factor (GDNF) and other proteins in the substantia nigra and striatum [8, 9]. Results from epidemiological studies demonstrated that individuals who regularly engaged in moderate to vigorous exercise in their third and fourth decade had a significantly reduced risk of developing PD [30, 31]. Assuming the physiological mechanism responsible for reducing risk of disease in midlife remains or is partially intact despite PD diagnosis, it is reasonable to hypothesize that vigorous exercise may trigger a similar, though possibly blunted, neural response and thereby slow disease progression. Support for this “sparing hypothesis” comes from the PD animal literature that has demonstrated high-intensity aerobic exercise, after the administration of MPTP or 6-OHDA, still increases BDNF, GDNF and availability of dopamine within the striatum and basal ganglia [5–9, 32].
There is evidence that aerobic exercise results in structural and functional CNS changes in healthy older adults [33–35] and PwPD [36–40]. After a single bout of high intensity forced-exercise, functional MRI data indicated altered CNS patterns of activation in the primary motor cortex, supplementary motor area, thalamus, globus pallidus and putamen in PwPD, similar to patterns seen following levodopa administration [36, 37]. In a subsequent study, individuals pedaling at a higher cadence during an 8-week stationary cycling intervention exhibited greater increases in cortico-subcortical connectivity during task performance compared to those who pedaled slower [38]. Importantly, results from these and other human imaging and mechanistic studies [39, 40] indicate that aerobic exercise is capable of improving CNS function, supporting that exercise is medicine for PD.
Building on the promising results of short-term human exercise studies [41–43], over the last several years, several large studies have examined the long-term effects of aerobic exercise on motor and non-motor symptoms in PwPD. Uc and colleagues reported improvements in aerobic fitness, motor symptoms, and quality of life following a 6-month walking program in PwPD [44]. The SPARX2 study examined the safety, feasibility, and potential efficacy of long-term high-intensity aerobic exercise in slowing PD progression in de-novo patients [45]. Results indicated that the high-intensity (4×/week, 30 minutes main exercise set, 80–85% of maximum heart rate (HR)), but not moderate-intensity (4×/week, 30 minutes main exercise set, 60–65% of max HR) exercise, met the non-futility threshold for moving forward with a large multi-center Phase III clinical trial to evaluate the disease-modifying capabilities of high intensity aerobic exercise on disease progression. If positive, results from the SPARX3 trial will be the first to identify any approach to effectively slow the progression of PD in de-novo PwPD, something that no pharmacological or surgical trials have yet to achieve.
The Park-in-Shape trial examined the effects of a home-based cycling program on motor symptoms of PD [46]. Following the 6-month intervention (3×/week, 30 minutes main exercise set, 50–80% of heart rate reserve (HRR), there was a between-group difference in the off-medication MDS-UPDRS III score favoring the aerobic exercise group compared with controls [11]. The attenuation of motor symptoms in Park-in-Shape is promising and further supports the potential of aerobic exercise as a disease-modifying intervention in those with mild to moderate disease. Building upon the success of Park-in-Shape, the Cyclical Lower Extremity Exercise (CYCLE-II) aims to investigate the disease-modifying effects of 12-month high intensity aerobic exercise in PD through the use of an in-home stationary bicycle. Through these previous and ongoing clinical trials, the field can move towards more specific exercise recommendations for PwPD.
AEROBIC EXERCISE PRESCRIPTION
Movement disorder and rehabilitation providers agree that exercise is beneficial for PwPD; however, the mode, frequency, and intensity necessary to achieve symptom management is not well-understood. It is acknowledged that aerobic exercise alone may not be the only type of exercise that PwPD need to manage their symptoms, and that multi-faceted exercise programs may have additional benefits. Nevertheless, the evidence supporting aerobic exercise mitigating global motor symptoms, measured primarily by the UPDRS, warrants further discussion. While optimal aerobic exercise parameters are not known for PwPD, using the best current evidence, it is advised that PwPD adhere to the following FITT (frequency, intensity, time, and type) principle for symptom attenuation.
Prior to starting any exercise program, PwPD should consult with their healthcare team to ensure they can safely engage and complete any exercise intervention. In an effort to facilitate safe exercise participation and minimize unnecessary medical testing, the American College of Sports Medicine’s Exercise Readiness Questionnaire presents a pre-exercise medical clearance decision-making chart [47]. Briefly, PwPD without symptoms of cardiovascular, metabolic, and renal disease, exercise can commence at appropriate intensity levels without medical clearance; those with symptoms require medical clearance. For PwPD who are not familiar with exercise and may need guidance developing a program, consultation with a physical therapist or exercise professional with disease-specific knowledge is recommended. Furthermore, formal exercise guidance may provide accountability and motivation necessary to overcome a lack of exercise self-efficacy and depression that may accompany the disease.
Frequency
The SPARX2 and Park-in-Shape studies provide guidance to achieve the potential disease-modifying effects of exercise [11, 12]. Based on these effective protocols, PwPD should exercise three times per week. It is not known if greater frequency of exercise would result in superior outcomes, although one would reason that exercise is unlikely to have linear benefit and at some point greater frequency of exercise would have no substantial increase in potential disease-modification. It is important to note that short-term exercise is not the panacea to treating PwPD; improvements in motor symptoms following short-term exercise dissipate after 4 weeks of inactivity [42], indicating that the exercise, similar to anti-parkinsonian medication, must be integrated into a PwPD’s routine to achieve consistent benefits.
Intensity
There are several studies indicating high intensity exercise, measured by HR [12, 43, 48] cadence [42], and metabolic equivalents (METs) [43], is a critical aspect of potential disease-modification. An advantage to measuring intensity by HR is that HR is not specific to a mode of exercise. In the event HR monitoring is not feasible, a RPE scale should be considered as a measure of exertion [49, 50]. It is advised that PwPD exercise at an intensity of 60–80% of HRR or 70–85% of maximum heart rate. Given the prevalence of autonomic dysfunction in this population [51], 14–17 on a 20 point RPE scale is also appropriate [50]. To put this into perspective, if one can exercise and hold a conversation, the intensity of the exercise should be increased.
Time
Exercise durations of 30–40 minutes for the main exercise set appear to be sufficient [11, 12, 41, 42]. Importantly, it is recommended that the main exercise set is flanked by a 5–10-minute warm-up and cool-down, particularly important given the prevalence of autonomic dysfunction [51].
Type
The most common modes of aerobic exercise reported in the PD literature are walking and stationary cycling. Treadmill training employs a specificity of training principle and facilitates relatively robust improvements in gait, including velocity and stride length [52]. However, in a population where the majority of individuals exhibit gait and balance deficits early in the disease process [53], stationary cycling may provide a safer option and mitigate the potential of falling while starting, performing and finishing the exercise. Regardless of mode, if the desired frequency and intensity can be achieved, it is likely one will experience the benefits associated with exercise. Importantly, this empowers PwPD to select of mode of exercise that they enjoy and is sustainable.
MOVING TO A PATIENT-SPECIFIC EXERCISE PRESCRIPTION FOR PARKINSON’S DISEASE
Just as the medical management of PD is not a “one-size-fits-all” response, it is likely that an effective exercise prescription should be unique to each PwPD. Hence, if exercise is truly to be treated as medicine, we need to provide a patient-specific “dose” with potentially known effects. For example, a 45-year-old male with a 2-year history of tremor-dominant PD and a 78-year-old female with a 10-year history of PIGD-dominate PD will likely require a different dose of aerobic exercise to achieve a level of disease-modification. If exercise is to be an effective adjunct to the treatment and slowing of PD, a prognostic model considering patient phenotype and exercise parameters must be established to aid clinicians in their recommendations to PwPD. A personalized model with predicted PD progression under different exercise conditions would improve PD treatment through patient-specific exercise recommendations and will be developed following completion of the CYCLE-II trial.
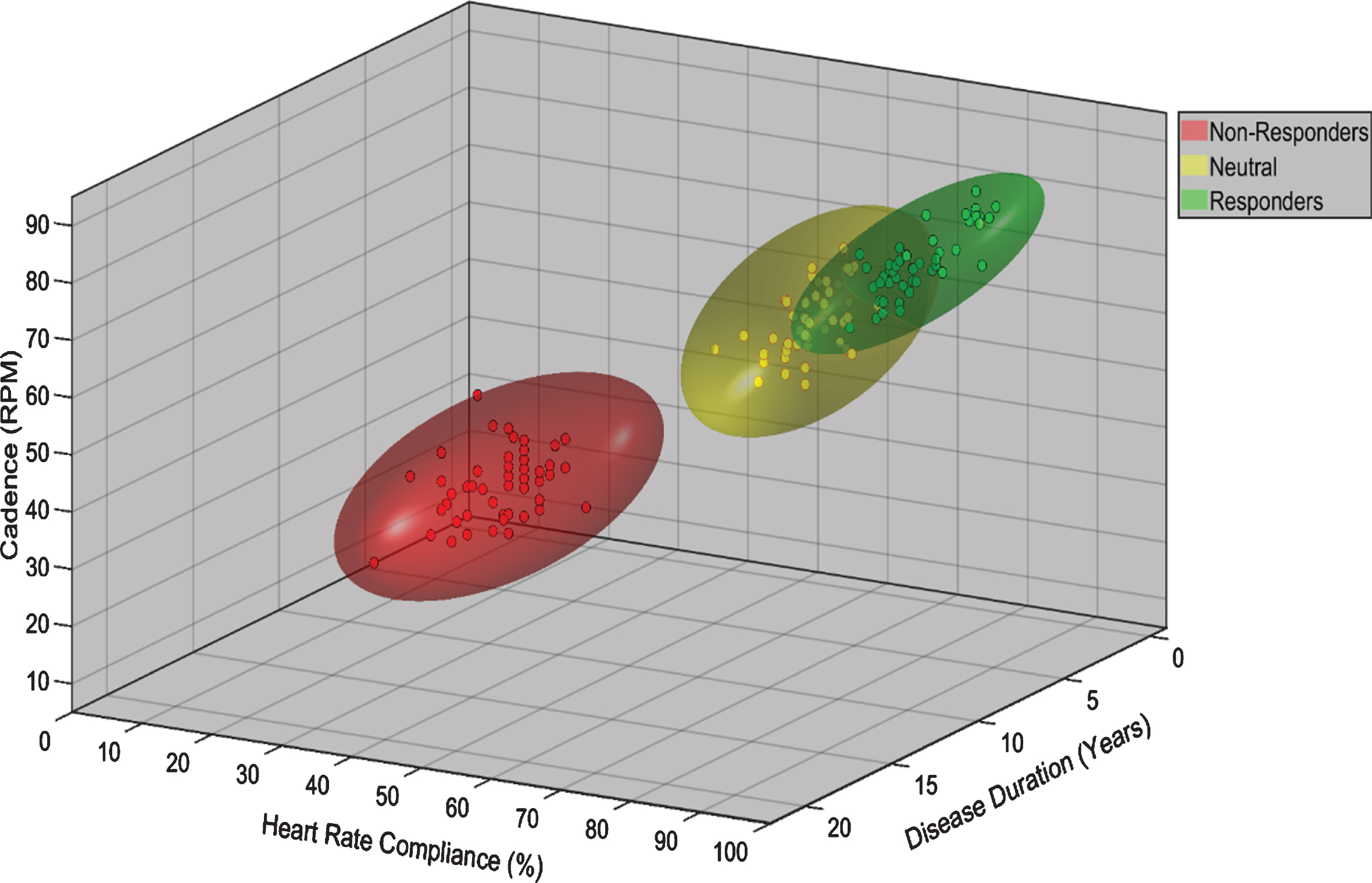
Figure 1 is a graphical representation of a mathematical model from a classification analysis relating MDS-UPDRS III improvement to exercise compliance and performance variables such as pedaling cadence (cycling), and disease demographics. In this hypothesized model, individuals with greater cadence, percent of HR max, and shorter disease duration experienced improved symptom management as measured by the MDS-UPDRS III. Personal prediction models utilizing a modern, prediction-motivated, validity-focused approach [54], as proposed in the CYCLE-II trial, present a potential method of developing personalized exercise prescription with a potentially known outcome. Combinations of patient-specific demographic, disease variables, and/or compliance data, with accompanying nomograms that predict MDS-UPDRS III after a given period (i.e., 12 months) of high intensity aerobic exercise program would be a significant advancement in the treatment of PD. These types of analyses will empower PwPD with a predictive model to increase motivation to exercise and compliance.
Fig. 1
Potential phenotyping of participant response to exercise based on exercise performance and demographic 3D model variables. Individuals are classified as non-responders, neutral, and responders based on change in the MDS-UPDRS III score over the course of the exercise intervention. The model postulates that individuals with greater cadence, HR compliance, and shorter disease duration will experience improved symptom management as measured by the MDS-UPDRS III.
CONCLUSION
Exercise is empowering. PD slowly steals motor and non-motor function which impacts every aspect of life; exercise provides a way for PwPD to exert control. Individuals with PD can exercise consistently at high intensities, resulting in symptom improvement. Large exercise trials examining the role of disease-modification are ongoing and are poised to provide a clear path forward, including using predictive modeling to create a patient-specific exercise prescription. Until these studies are completed, PwPD should be encouraged to integrate high-intensity aerobic exercise on a regular basis into their exercise routine. Exercise is medicine for PD, one must take the medicine to realize its benefits. In the case of PD, exercise your brain, get moving.
“Take Home” Points
• Research in animal models of PD and results from human studies demonstrate aerobic exercise improves brain function.
• Enhanced brain function associated with high intensity exercise in human models makes it a candidate for slowing disease progression.
• Based on current evidence, it is recommended that PwPD engage in aerobic exercise 3×/week, 30–40-minute main exercise set, 60–80% of heart rate reserve or 70–85% of heart rate max. In lieu of heart rate, individuals can achieve an intensity of 14–17 on a 20-point RPE scale.
• Ongoing clinical trials will provide patient-specific exercise prescriptions to facilitate clinical utilization and patient compliance.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
Support for this manuscript included the Davis Phinney Foundation, National Institute of Health (2R01NS073717), the Bell Family Chair, and the Farmer Family Foundation. The funding sources had no role in the manuscript content.
REFERENCES
[1] | Parkinson’s disease Foundation, Statistics on Parkinson’s, https://www.parkinson.org/Understanding-Parkinsons/Statistics, Accessed March 30, 2020. |
[2] | GBD Neurological Disorders Collaborator Group ((2017) ) Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16: , 877–897. |
[3] | Parkinson Study Group ((2002) ) Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA 287: , 1653–1661. |
[4] | Fahn S , Parkinson Study Group ((2005) ) Does levodopa slow or hasten the rate of progression of Parkinson’s disease?. J Neurol 252: (Suppl 4), IV37–IV42. |
[5] | Petzinger GM , Fisher B , Hogg E , Abernathy A , Arevalo P , Nixon K , Jakowec MW ((2006) ) Behavioral motor recovery in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned squirrel monkey (Saimiri sciureus): Changes in striatal dopamine and expression of tyrosine hydroxylase and dopamine transporter proteins. J Neurosci Res 83: , 332–347. |
[6] | Petzinger GM , Fisher BE , Van Leeuwen JE , Vukovic M , Akopian G , Meshul CK , Holschneider DP , Nacca A , Walsh JP , Jakowec MW ((2010) ) Enhancing neuroplasticity in the basal ganglia: The role of exercise in Parkinson’s disease. Mov Disord 25: (Suppl 1), S141–145. |
[7] | Petzinger GM , Walsh JP , Akopian G , Hogg E , Abernathy A , Arevalo P , Turnquist P , Vuckovic M , Fisher BE , Togasaki DM , Jakowec MW ((2007) ) Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci 27: , 5291–5300. |
[8] | Zigmond MJ , Cameron JL , Leak RK , Mirnics K , Russell VA , Smeyne RJ , Smith AD ((2009) ) Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat Disord 15: (Suppl 3), S42–45. |
[9] | Tajiri N , Yasuhara T , Shingo T , Kondo A , Yuan W , Kadota T , Wang F , Baba T , Tayra JT , Morimoto T , Jing M , Kikuchi Y , Kuramoto S , Agari T , Miyoshi Y , Fujino H , Obata F , Takeda I , Furuta T , Date I ((2010) ) Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res 1310: , 200–207. |
[10] | Tillerson JL , Caudle WM , Reveron ME , Miller GW ((2003) ) Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 119: , 899–911. |
[11] | van der Kolk NM , de Vries NM , Kessels RPC , Joosten H , Zwinderman AH , Post B , Bloem BR ((2019) ) Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol 18: , 998–1008. |
[12] | Schenkman M , Moore CG , Kohrt WM , Hall DA , Delitto A , Comella CL , Josbeno DA , Christiansen CL , Berman BD , Kluger BM , Melanson EL , Jain S , Robichaud JA , Poon C , Corcos DM ((2018) ) Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol 75: , 219–226. |
[13] | World Health Organization, Physical Activity and Older Adults, https://www.who.int/dietphysicalactivity/factsheet_olderadults/en/, Accessed April 14, 2020. |
[14] | Hallal PC , Andersen LB , Bull FC , Guthold R , Haskell W , Ekelund U , Lancet Physical Activity Series Working Group ((2012) ) Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 380: , 247–257. |
[15] | van Nimwegen M , Speelman AD , Hofman-van Rossum EJ , Overeem S , Deeg DJ , Borm GF , van der Horst MH , Bloem BR , Munneke M ((2011) ) Physical inactivity in Parkinson’s disease. J Neurol 258: , 2214–2221. |
[16] | Cavanaugh JT , Ellis TD , Earhart GM , Ford MP , Foreman KB , Dibble LE ((2015) ) Toward understanding ambulatory activity decline in Parkinson disease. Phys Ther 95: , 1142–1150. |
[17] | Bouca-Machado R , Rosario A , Caldeira D , Castro Caldas A , Guerreiro D , Venturelli M , Tinazzi M , Schena F , Ferreira JJ ((2020) ) Physical activity, exercise, and physiotherapy in Parkinson’s disease: Defining the concepts. Mov Disord Clin Pract 7: , 7–15. |
[18] | Caspersen CJ , Powell KE , Christenson GM ((1985) ) Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep 100: , 126–131. |
[19] | Goetz CG ((1986) ) Charcot on Parkinson’s disease. Mov Disord 1: , 27–32. |
[20] | Charcot JM ((1892) ) Vibratory therapeutics.–The application of rapid and continuous vibrations to the treatment of certain diseases of the nervous system. J Nerv Ment Dis 17: , 880–886. |
[21] | Kapur SS , Stebbins GT , Goetz CG ((2012) ) Vibration therapy for Parkinson’s disease: Charcot’s studies revisited. J Parkinsons Dis 2: , 23–27. |
[22] | Gowers WR ((1892) ) A Manual of Diseases of the Nervous System, 2nd Ed, Vol. 1, P. Blakiston, Son & Co., Philadelphia. |
[23] | Clark EC , Mulder DW , Erickson DJ , Clements BG , Maccarty CS ((1957) ) Parkinson’s disease: Therapeutic exercises in its management. Postgrad Med 21: , 301–308. |
[24] | Clark EC , Clements BG , Erickson DJ , Maccarty CS , Mulder DW ((1956) ) Therapeutic exercises in management of paralysis agitans. J Am Med Assoc 162: , 1041–1043. |
[25] | Van Oteghen SL ((1987) ) An exercise program for those with Parkinson’s disease. Geriatr Nurs 8: , 183–184. |
[26] | Wroe M , Greer M ((1973) ) Parkinson’s disease and physical therapy management. Phys Ther 53: , 849–855. |
[27] | Tolosa E , Marti MJ , Valldeoriola F , Molinuevo JL ((1998) ) History of levodopa and dopamine agonists in Parkinson’s disease treatment. Neurology 50: , S2-10; discussion S44–18. |
[28] | Ahlskog JE ((2011) ) Does vigorous exercise have a neuroprotective effect in Parkinson disease?. Neurology 77: , 288–294. |
[29] | Cohen AD , Tillerson JL , Smith AD , Schallert T , Zigmond MJ ((2003) ) Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: Possible role of GDNF. J Neurochem 85: , 299–305. |
[30] | Thacker EL , Chen H , Patel AV , McCullough ML , Calle EE , Thun MJ , Schwarzschild MA , Ascherio A ((2008) ) Recreational physical activity and risk of Parkinson’s disease. Mov Disord 23: , 69–74. |
[31] | Chen H , Zhang SM , Schwarzschild MA , Hernan MA , Ascherio A ((2005) ) Physical activity and the risk of Parkinson disease. Neurology 64: , 664–669. |
[32] | Fisher BE , Petzinger GM , Nixon K , Hogg E , Bremmer S , Meshul CK , Jakowec MW ((2004) ) Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- lesioned mouse basal ganglia. J Neurosci Res 77: , 378–390. |
[33] | Erickson KI , Leckie RL , Weinstein AM ((2014) ) Physical activity, fitness, and gray matter volume. Neurobiol Aging 35: (Suppl 2), S20–28. |
[34] | Dougherty RJ , Ellingson LD , Schultz SA , Boots EA , Meyer JD , Lindheimer JB , Van Riper S , Stegner AJ , Edwards DF , Oh JM , Koscik RL , Dowling MN , Gallagher CL , Carlsson CM , Rowley HA , Bendlin BB , Asthana S , Hermann BP , Sager MA , Johnson SC , Okonkwo OC , Cook DB ((2016) ) Meeting physical activity recommendations may be protective against temporal lobe atrophy in older adults at risk for Alzheimer’s disease. Alzheimers Dement (Amst) 4: , 14–17. |
[35] | Voss MW , Erickson KI , Prakash RS , Chaddock L , Malkowski E , Alves H , Kim JS , Morris KS , White SM , Wojcicki TR , Hu L , Szabo A , Klamm E , McAuley E , Kramer AF ((2010) ) Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition?. Neuropsychologia 48: , 1394–1406. |
[36] | Alberts JL , Phillips M , Lowe MJ , Frankemolle A , Thota A , Beall EB , Feldman M , Ahmed A , Ridgel AL ((2016) ) Cortical and motor responses to acute forced exercise in Parkinson’s disease. Parkinsonism Relat Disord 24: , 56–62. |
[37] | Beall EB , Lowe MJ , Alberts JL , Frankemolle AM , Thota AK , Shah C , Phillips MD ((2013) ) The effect of forced-exercise therapy for Parkinson’s disease on motor cortex functional connectivity. Brain Connect 3: , 190–198. |
[38] | Shah C , Beall EB , Frankemolle AM , Penko A , Phillips MD , Lowe MJ , Alberts JL ((2016) ) Exercise therapy for Parkinson’s disease: Pedaling rate is related to changes in motor connectivity. Brain Connect 6: , 25–36. |
[39] | Fisher BE , Li Q , Nacca A , Salem GJ , Song J , Yip J , Hui JS , Jakowec MW , Petzinger GM ((2013) ) Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport 24: , 509–514. |
[40] | Duchesne C , Gheysen F , Bore A , Albouy G , Nadeau A , Robillard ME , Bobeuf F , Lafontaine AL , Lungu O , Bherer L , Doyon J ((2016) ) Influence of aerobic exercise training on the neural correlates of motor learning in Parkinson’s disease individuals. Neuroimage Clin 12: , 559–569. |
[41] | Herman T , Giladi N , Gruendlinger L , Hausdorff JM ((2007) ) Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson’s disease: A pilot study. Arch Phys Med Rehabil 88: , 1154–1158. |
[42] | Ridgel AL , Vitek JL , Alberts JL ((2009) ) Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair 23: , 600–608. |
[43] | Fisher BE , Wu AD , Salem GJ , Song J , Lin CH , Yip J , Cen S , Gordon J , Jakowec M , Petzinger G ((2008) ) The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil 89: , 1221–1229. |
[44] | Uc EY , Doerschug KC , Magnotta V , Dawson JD , Thomsen TR , Kline JN , Rizzo M , Newman SR , Mehta S , Grabowski TJ , Bruss J , Blanchette DR , Anderson SW , Voss MW , Kramer AF , Darling WG ((2014) ) Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology 83: , 413–425. |
[45] | Moore CG , Schenkman M , Kohrt WM , Delitto A , Hall DA , Corcos D ((2013) ) Study in Parkinson disease of exercise (SPARX): Translating high-intensity exercise from animals to humans. Contemp Clin Trials 36: , 90–98. |
[46] | van der Kolk NM , Overeem S , de Vries NM , Kessels RP , Donders R , Brouwer M , Berg D , Post B , Bloem BR ((2015) ) Design of the Park-in-Shape study: A phase II double blind randomized controlled trial evaluating the effects of exercise on motor and non-motor symptoms in Parkinson’s disease. BMC Neurol 15: , 56. |
[47] | Riebe D , Franklin BA , Thompson PD , Garber CE , Whitfield GP , Magal M , Pescatello LS ((2015) ) Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med Sci Sports Exerc 47: , 2473–2479. |
[48] | Nadeau A , Pourcher E , Corbeil P ((2014) ) Effects of 24 wk of treadmill training on gait performance in Parkinson’s disease. Med Sci Sports Exerc 46: , 645–655. |
[49] | Penko AL , Barkley JE , Koop MM , Alberts JL ((2017) ) Borg scale is valid for ratings of perceived exertion for individuals with Parkinson’s disease. Int J Exerc Sci 10: , 76–86. |
[50] | American College of Sports Medicine (ACSM) (2018) ACSM’s Guidelines for Exercise Testing and Prescription, Lippincott Williams & Wilkins, Philadelphia. |
[51] | Klanbut S , Phattanarudee S , Wongwiwatthananukit S , Suthisisang C , Bhidayasiri R ((2018) ) Symptomatic orthostatic hypotension in Parkinson’s disease patients: Prevalence, associated factors and its impact on balance confidence. J Neurol Sci 385: , 168–174. |
[52] | Mehrholz J , Kugler J , Storch A , Pohl M , Hirsch K , Elsner B ((2015) ) Treadmill training for patients with Parkinson’s disease. Cochrane Database Syst Rev, CD007830. |
[53] | Kang GA , Bronstein JM , Masterman DL , Redelings M , Crum JA , Ritz B ((2005) ) Clinical characteristics in early Parkinson’s disease in a central California population-based study. Mov Disord 20: , 1133–1142. |
[54] | Harrell FE ((2015) ) Regression Modeling Strategies with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis, 2nd Ed., Springer, Heidelberg. |




