Value of Clinical Signs in Identifying Patients with Scans without Evidence of Dopaminergic Deficit (SWEDD)
Abstract
Background:
In clinical trials that recruited patients with early Parkinson’s disease (PD), 4–15% of the participants with a clinical diagnosis of PD had normal dopamine transporter single photon emission computed tomography (DAT SPECT) scans, also called “scans without evidence of dopaminergic deficit” (SWEDD).
Objective:
To investigate in patients with a clinical diagnosis of PD, if specific clinical features are useful to distinguish patients with nigrostriatal degeneration from those that have no nigrostriatal degeneration.
Methods:
We performed a diagnostic test accuracy study. Patients that participated in the Levodopa in Early Parkinson’s disease trial, a clinical trial in patients with early PD, were asked to participate if they had not undergone DAT SPECT imaging earlier. The index tests were specific clinical features that were videotaped. A panel of six neurologists in training (NT), six general neurologists (GN), and six movement disorders experts (MDE) received a batch of ten videos consisting of all SWEDD subjects and a random sample of patients with abnormal DAT SPECT scans. The raters analyzed the videos for presence of specific signs and if they suspected the patient to have SWEDD. The reference test was visually assessed DAT SPECT imaging.
Results:
Of a total of 87 participants, three subjects were SWEDDs (3.4%). The overall intraclass correlation coefficient (ICC) of the Parkinsonian signs was poor to moderate with ICCs ranging from 0.14 to 0.67. NT correctly identified 50.0% of the SWEDD subjects, GN 33.3%, and MDE 66.7%.
Conclusion:
Our study suggests that the selected videotaped clinical features cannot reliably distinguish patients with a clinical diagnosis of PD and an abnormal DAT SPECT from patients with clinical PD and a SWEDD.
INTRODUCTION
Despite the significant advances in (nuclear) imaging and genetics to support the diagnosis of Parkinson’s disease (PD) in recent years, the diagnosis of PD remains mainly a clinical one. Bradykinesia is the cardinal symptom, which must be accompanied by tremor and/or rigidity [1–3]. In order to make the diagnosis, supportive signs are often present and exclusionary signs absent. An accurate diagnosis can be challenging in early stages, particularly when the clinical features are subtle [4, 5]. Using dopamine transporter single photon emission computed tomography (DAT SPECT) imaging, patients can be classified into two distinct groups; patients with nigrostriatal dysfunction, which can be degenerative (e.g., PD, multiple system atrophy, progressive supranuclear paralysis, dementia with Lewy bodies), and patients without nigrostriatal dysfunction. Among patients with clinically diagnosed PD whom are enrolled in trials or imaging studies for PD, 4–15% have been found to have normal DAT scans, also referred to as “scans without evidence of dopaminergic deficit” (SWEDD) [6, 7]. SWEDD cases do not develop abnormal DAT SPECT scans on long-term follow up [8]. In contrast, in early stages of PD, and even in preclinical stages, striatal DAT binding is significantly reduced [9–11]. Previous studies however suggest that a significant proportion of SWEDD cases may be related to an incorrect visual interpretation of DAT SPECT scans, rather than or in addition to an erroneous clinical diagnosis [12].
Reliable identification of diagnoses is paramount to individual patient care. As an adjunct, for clinical trials in early PD it is critical to ensure that the appropriate patients are included. The Levodopa in EArly Parkinson’s disease (LEAP) clinical trial provided a unique opportunity to investigate if patients clinically diagnosed with early PD and nigrostriatal dysfunction can reliably be differentiated from SWEDD subjects. Using a video assessment and raters with various levels of expertise [13], we explored the usefulness of selected clinical features to identify SWEDD subjects.
METHODS
This study was a diagnostic test accuracy study. The index tests were specific clinical features that were videotaped. The reference test was visually assessed DAT SPECT imaging. This study was ancillary to a multicenter, randomized, double-blind, placebo-controlled trial with a delayed-start design, the LEAP trial [13]. Patients for the LEAP trial were recruited by general neurologists from 50 community hospitals and by movement disorders specialists in seven academic hospitals in the Netherlands. The LEAP clinical trial and this ancillary study were approved by the ethics committee at the Amsterdam University Medical Centers in the Netherlands. The studies were conducted in accordance with the principles of the Declaration of Helsinki.
Patients
Patients were eligible for the LEAP clinical trial if they had received a clinical diagnosis of PD within the previous two years from a neurologist who based the diagnosis on standard clinical criteria [14, 15], if they had insufficient disability to warrant treatment with anti parkinson medication, if they were 30 years of age or older, and if they had a life expectancy of more than two years. Patients who had been treated previously with anti parkinson medication were excluded.
All LEAP participants were able to participate in this ancillary study unless they used medication or substances interfering with DAT SPECT imaging that could not be discontinued, in case of pregnancy, or if the patient underwent prior DAT SPECT imaging.
Study procedures
After inclusion in the LEAP clinical trial, but prior to randomization, a physical examination focused on Parkinsonism was video recorded and DAT SPECT imaging performed. There was no fixed sequence of study procedures (acquisition of the DAT SPECT scan and video recording). The results of the imaging had no influence on the participation in the LEAP clinical trial.
Physical examination
We used the following parameters to be assessed by the video panel (see Supplementary File 1 for video protocol): bradykinesia defined as a decreased amplitude and/or progressive deceleration of movement [17], re-emerging tremor in patients with a postural or rest tremor, reduced arm swing while walking, asymmetric arm swing during walking that normalizes during running, contra lateral mirror movements, reduced tremor in the most affected limb during finger tapping on the contra lateral side [18], and ten-step tandem gait test [19].
DAT SPECT imaging
DAT SPECT imaging was performed in seven hospitals (four tertiary referral hospitals and three community hospitals) in the Netherlands. Each parti-cipant was injected intravenously with approxi-mately 185 MBq 123I-N-omega-fluoropropyl-2ß-car-bomethoxy-3ß-(4-iodophenyl)nortropane ([123I]FP-CIT or [123I]ioflupane) and images were acquired 3 hours later [16]. Patients were pretreated with potassium iodide drops or tablets according to the standard protocol of the hospital. Images were acquired on 2-headed or brain-dedicated SPECT systems. Although all centers had experience in DAT imaging for routine clinical purposes, each participating center was asked to optimize the acquisition of the images by considering the EANM guidelines regarding the acquisition of DAT SPECT scans [20].
Classification and outcome of DAT SPECT
The DAT SPECT scans were visually assessed independently by two experts in neuroreceptor imaging (JB, HV). The experts were blinded to the initial assessment of the DAT SPECT and the clinical details aside from gender and date of birth scans. The images were analyzed in a familiar and consistent color scale on a HERMES workstation. The DAT SPECT images were classified as either “normal” or “abnormal”. This determination was based on the extent and intensity of the uptake of the radiotracer in the striatum. “Normal” DAT SPECT imaging was characterized by intense binding of the radiotracer in the putamen and caudate nuclei bilaterally, mostly symmetrical with almost equal intensity of the binding. Normal striatal binding looks comma- or crescent-shaped on transversal images (Fig. 1) [21]. The result of DAT SPECT imaging was considered “abnormal” when a decreased binding of the radiotracer was apparent in any of the striatal areas, in most cases asymmetrically. In the early phase reduced binding of the radiotracer is usually visible in the dorsal putamen and expands to the ventral putamen and caudate nucleus [21].
Fig. 1
DAT SPECT imaging. Normal (A) and abnormal (B) [123I]FP-CIT SPECT imaging of patients in the LEAP-cohort. Patient A is a 64-year-old male. Patient B is a 63-year-old female. DAT, dopamine transporter; SPECT; single-photon emission computed tomography; LEAP, Levodopa in EArly Parkinson’s disease.
![DAT SPECT imaging. Normal (A) and abnormal (B) [123I]FP-CIT SPECT imaging of patients in the LEAP-cohort. Patient A is a 64-year-old male. Patient B is a 63-year-old female. DAT, dopamine transporter; SPECT; single-photon emission computed tomography; LEAP, Levodopa in EArly Parkinson’s disease.](https://content.iospress.com:443/media/jpd/2020/10-4/jpd-10-4-jpd202090/jpd-10-jpd202090-g001.jpg)
Video assessment
Since the accuracy of video assessments of the specific symptoms may be dependent on experience [22], we formed a video panel of assessors with different levels of expertise. Six neurologists in training (NT), six general neurologists (GN), and six movement disorder experts (MDE) individually analyzed the videos (presence of signs tested during the comprehensive neurological examination) while blinded for the DAT SPECT imaging results. An example of the case record form for the video assessment can be found in Supplementary File 2. All raters received the same set of ten videos, which consisted of three SWEDD subjects and a random selection of seven patients with abnormal DAT SPECT imaging that participated in this ancillary study.
The random sample of seven videos was selected using the = RAND() formula in Microsoft Excel. This function generates a list with a random number per participant that can be sorted from low to high. The first seven subjects with the lowest numbers on the list were selected. The quality of the videos and neurological examinations were assessed (e.g., sufficient lighting, socks removed, whole body was filmed during execution of UPDRS items 18, 19, and 20, and all parts of the examination were performed correctly and long enough). If a video was considered to be of insufficient quality, the following video on the list was selected until there were seven videos of sufficient quality. The assessors were blinded for the number of SWEDD subjects, or the number of subjects with abnormal DAT SPECT imaging as well as any other clinical information.
Statistical analysis
Because of the unexpected low number of SWEDD subjects, the analysis of the data was mainly qualitative with limited statistical analysis. The inter-rater reliability per item of the comprehensive neurological examination was determined by calculating the intraclass correlation coefficient. We selected the two-way mixed model and tested the absolute agreement. The single measure coefficient was used. Based on the 95% confident interval (CI) of the ICC estimate, values less than 0.50, between 0.50 and 0.75, between 0.75 and 0.90, and greater than 0.90 are indicative of poor, moderate, good, and excellent reliability, respectively [23]. Analyses were performed with the use of SPSS software, version 25.
RESULTS
Patients
From August 2011 through May 2016, 446 patients were enrolled in the LEAP trial. The ancillary study was initiated after 85 had already been included in the LEAP trial and 271 participants declined participation or met exclusion criteria. One patient withdrew consent prior to DAT SPECT imaging and two patients were excluded due to technical issues with the DAT SPECT images. This left a total of 87 participants that underwent both DAT SPECT imaging and a videotaped examination (Fig. 2). Eighty-four patients had abnormal DAT SPECT imaging. Three patients (3.4%) had normal DAT SPECT imaging, which remained normal on rescanning 80 weeks after baseline imaging. There were no discrepancies between the two experts in neuroreceptor imaging who assessed the images. The baseline characteristics and demographics are shown in Table 1.
Fig. 2
Selection of LEAP patients evaluated to participate in the ancillary study.
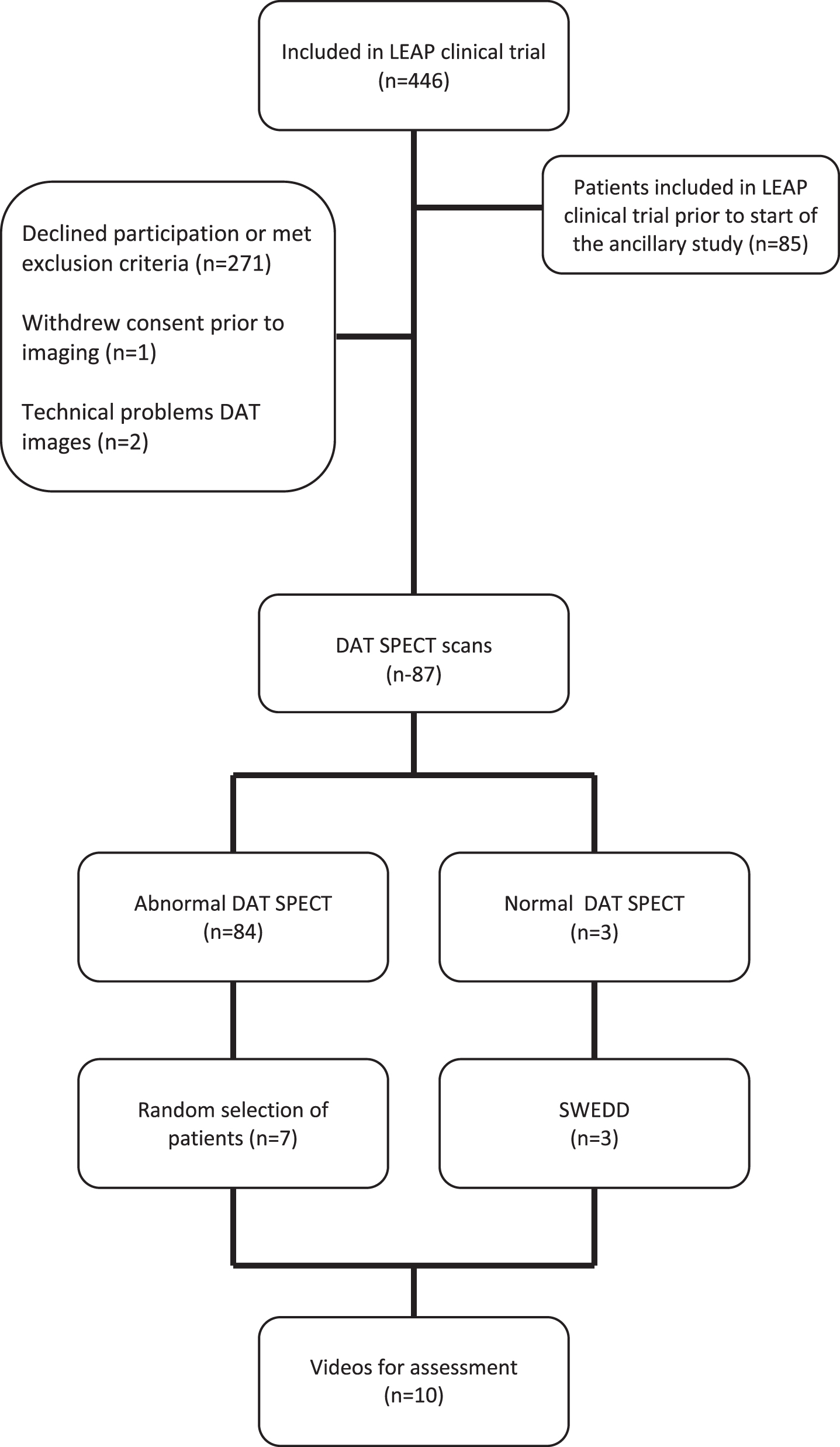
Table 1
Baseline characteristics and demographics
| Normal DAT SPECT imaging | Abnormal DAT SPECT imaging | |||
| Subject1 | Subject2 | Subject3 | PD subjects (n = 7) | |
| Age –y (mean±SD) | 62 | 75 | 68 | 64.7±5.7 |
| Gender (M/F) | Male | Female | Female | 6/1 |
| Symptom duration at imaging –(months, median, IQR) | 19 | 12 | 4 | 12 (4–109) |
| Clinically most affected side (Left/Right/symmetrical) | Right | Symmetrical | Right | 4/1/2 |
| First symptom (tremor/bradykinesia/pain/stiffness) | T | T/B/S | B/P | 5/2/1/2 |
| Total UPDRS score (0–176, mean±SD) | 19 | 29 | 22 | 23.7±10.4 |
| Part I (mean±SD) | 3 | 2 | 2 | 2.6±1.4 |
| Part II (mean±SD) | 5 | 9 | 4 | 7±4.2 |
| Part III (mean±SD) | 7 | 16 | 16 | 13.1±5.4 |
| Part IV (mean±SD) | 2 | 2 | 0 | 1±1.5 |
| Beck Depression inventory-II (0–33, median, IQR) | 11 | 15 | 5 | 7 (0–12) |
| Mini-Mental State Examination (0–30, median, IQR) | 29 | 30 | 28 | 29 (29–30) |
SWEDD Scan without evidence of dopaminergic deficit, PD Parkinson’s disease, UPDRS Unified Parkinson’s disease Rating Scale, IQR inter quartile range, SD Standard deviation.
SWEDD identification
One of the MDE did not fill out the question asking if he suspected the subjects to have a normal or abnormal DAT SPECT imaging due to misinterpretation of the question. Overall, SWEDD subjects were correctly identified in 41.2%. NT correctly identified 50.0% (median, range 0–70%), GN 33.3% (0–66.7%), and MDE 66.7% (0–100%) of the SWEDD subjects. The full dataset of the video assessments is provided in Supplementary File 3.
Patients with abnormal DAT imaging
Overall, the assessors identified 80.7% of the patients with abnormal DAT SPECT imaging correctly. The NT identified 71.4% (median, range 57.1–85.7%) of the patients with abnormal DAT SPECT imaging correctly compared to 85.7% (median, range 71.4–100%) of GN and 85.7% (median, range 71.4–85.7%) of MDE.
In contrast, one patient (Subject 5) was overall correctly identified in only 52.9% of the assessments. Interestingly, the raters that did not find an asymmetrical arm swing while walking (88.9%), suspected the patient frequently (55.6%) of having a SWEDD, even in presence of bradykinesia.
Intraclass correlation coefficient
The overall intraclass correlation coefficient (ICC) of the individual items was poor to moderate with ICCs ranging from 0.14 to 0.67 (Table 2). Re-emerging tremor (0.62, 95% confidence interval (CI) 0.40–0.88), arm swing while walking (0.52, 95% CI 0.32–0.79), reduced tremor after immobilization (0.54, 95% CI 0.33–0.82), and tandem gait test (0.67, 95% CI 0.44–0.91) were the only items with an overall ICC above 0.5. All other items had ICCs below 0.5.
Table 2
Intra class correlation coefficient with confidence intervals
| Neurologists in Training | General Neurologists | Movement Disorders Experts | Overall | |
| Deceleration of pace | 0.19 (0.00–0.55) | 0.26 (0.06–0.61) | 0.22 (0.04–0.58) | 0.26 (0.11–0.56) |
| Acceleration of pace | 0.21 (0.02–0.58) | 0.08 (–0.05–0.41) | 0.29 (0.07–0.66) | 0.27 (0.09–0.59) |
| Reduced amplitude | 0.43 (0.19–0.75) | 0.16 (–0.02–0.52) | 0.17 (–0.01–0.53) | 0.37 (0.18–0.68) |
| Number of arrests | –0.02 (–0.09–0.21) | 0.19 (–0.00–0.57) | 0.24 (0.05–0.67) | 0.15 (0.04–0.51) |
| Tandem gait | 0.72 (0.49–0.98) | 0.48 (0.23–0.78) | 0.40 (0.14–0.77) | 0.67 (0.44–0.91) |
| Re-emerging phenomenon | 0.44 (0.18–0.76) | 0.71 (0.45–0.92) | 0.54 (0.29–0.82) | 0.62 (0.40–0.88) |
| Asymmetrical arm swing while walking | 0.46 (0.22–0.77) | 0.56 (0.30–0.83) | 0.55 (0.30–0.82) | 0.52 (0.32–0.79) |
| Normalization arm swing while running | 0.13 (–0.04–0.48) | 0.09 (–0.05–0.42) | 0.05 (–0.05–0.33) | 0.14 (0.04–0.41) |
| Contra lateral mirror movement | 0.16 (0.00–0.50) | 0.21 (0.02–0.57) | 0.29 (0.07–0.66) | 0.33 (0.15–0.67) |
| Reduced tremor | 0.58 (0.32–0.84) | 0.45 (0.21–0.76) | 0.62 (0.35–0.87) | 0.54 (0.33–0.82) |
| Micrography | 0.24 (0.05–0.59) | 0.30 (0.09–0.65) | 0.25 (0.04–0.66) | 0.36 (0.16–0.73) |
| Unstable writing pattern | 0.21 (0.01–0.58) | 0.43 (0.19–0.75) | 0.41 (0.14–0.80) | 0.45 (0.22–0.81) |
| DAT-deficiency | 0.31 (0.08–0.66) | 0.30 (0.08–0.65) | 0.26 (0.03–0.63)* | 0.31 (0.15–0.63) |
*Intra class correlation coefficient with confidence intervals are based on five assessments instead of six.
DISCUSSION
This analysis showed that video-based assessments of clinical features might be insufficient to accurately distinguish individuals with SWEDD from patients with abnormal DAT SPECT imaging. The inter-rater agreement of interpreting clinical features in patients with suspected PD is poor to moderate, independent of the level of expertise.
Our panel for the video assessment was not able to reliably differentiate SWEDD subjects from patients with neurodegenerative parkinsonism based on videos. However, two MDE were able to identify all three SWEDD subjects correctly. One of these MDE was even able to classify all patients correctly. This rater scored the individual items of the examination similarly to the other raters, but had a different conclusion if the patient had normal or abnormal DAT SPECT imaging. This was the only patient in which the same ratings led to a different conclusion. These findings may suggest that a “custom weighted compound score” of all findings is more reliable rather than the individual features of the neurological examination. However, due to the small number of SWEDD subjects in this study it was not possible to determine whether individual items or a combination thereof were critical in correctly identifying the subjects. Furthermore, we had expected that the accuracy of SWEDD identification would increase with increasing level of expertise and experience, which was not the case.
This study showed that the overall inter-rater agreement regarding the presence or absence of clinical features is poor to moderate. In contrast to Fearon et al. [22] we did not find that MDE had a higher inter-rater agreement compared to non-MDE (NT and GN). However, we did find that NT had the lowest inter-rater agreement in 7 (out 13 items) compared to 3 for GN and MDE.
The items of the physical examination that were selected merit some discussion. Bradykinesia is one of the cardinal features of Parkinsonism so it has to be present in patients with any Parkinsonism. SWEDD subjects however may not have true bradykinesia [17, 24]. The re-emerging rest tremor is seen in the majority of patients with PD and is reported in other forms of neurodegenerative Parkinsonism. This phenomenon may be absent in SWEDD subjects [25]. Patients with PD nearly always (92%) have a reduced asymmetric arm swing during walking; this or a bilateral reduction of arm swing is recognized in about two-thirds of subjects with SWEDD [25]. We also included normalization of the arm swing while running. There is no published literature on this phenomenon. However, we observed that many PD patients with an asymmetric arm swing during walking have a normal or markedly improved arm swing while running. We hypothesized that for running a change in motor program is initiated, therefore in patients with psychogenic Parkinsonism the arm swing could remain reduced. A reduced tremor in the most affected limb during finger tapping on the contra lateral side is found in patients without dopaminergic degeneration [18]. Tandem gait performance was included since patients with PD have a normal tandem gait, therefore we expected this to be abnormal in patients with a normal DAT SPECT scan [19]. The included patients were patients without impairment in daily life and therefore we hypothesized that possible patients with MSA or PSP would still have a normal tandem gait.
One of the shortcomings of this study, as with any video study, is that clinical features like rigidity cannot be appreciated, and other items assessed clinically can vary from individual to individual; e.g., sequential handwriting. Furthermore, most patients were visited at home, which led to improvising to obtain the best videos possible. For example, in some cases the walking distance had to be reduced due to the living situation of the patient. Moreover, the lighting varied among the videos, which could have influenced the assessments.
One could argue that erroneous visual assessment of DAT SPECT imaging contributes to the SWEDD percentage. However, previous studies have shown that visual assessment of DAT SPECT imaging by experts and even non-experts is highly reliable [26, 27]. Additionally, all three SWEDD cases were rescanned approximately 80 weeks later, and also all three follow-up scans were rated as being normal by the two expert readers who analyzed the scans independently.
One of the strengths of this study is the fact that these were all patients who were referred to participate in the LEAP-clinical trial. To our knowledge this is the first study in which the included SWEDDs were initially referred by a neurologist who had no clinical doubt and diagnosis was made on clinical grounds only. We agree the number of SWEDDs is low, however these are the exact type of SWEDDs we wanted to evaluate.
In conclusion, our findings suggest that it is very difficult to reliably identify SWEDD subjects from patients with PD based solely on a video assessment of a neurological examination focused on parkinsonism [6]. Interestingly, the level of expertise of the video assessors did not appear to play a significant role in the inter-rater agreement as well as in the correct identification of the patients. As mentioned above the sample size was considerably smaller than anticipated, therefore we cannot draw firm conclusions. Until other reliable diagnostic and mechanistic biomarkers become available, DAT imaging should be used to confirm appropriate patient selection in clinical trials on disease-modifying drugs.
CONFLICT OF INTEREST
The authors report no conflicts of interest
Financial Disclosures for the Previous 12 Months: Susan Fox has received clinic support from the Edmond J Safra Foundation for Parkinson Research; National Parkinson Foundation and the Toronto Western and General Foundation. Salary from UHN Dept of Medicine Practice Plan. She has received Research Funding from the Michael J Fox Foundation for Parkinson Research, NIH (Dystonia Coalition), CIHR and Parkinson Canada. She has received Honoraria from the International Parkinson, Movement Disorder Society and American Academy of Neurology; served as site PI for Clinical Trials for Biotie, Cynapsus, Eisai; Revance and received consultancy/Speaker fees from Acadia; Atuka; CHDI; Lundbeck; Merz; Kyowa; Palidan, Sequirus; Sunovion; Teva; Zambon; and Royalties from Oxford University Press.
Carsten Eggers has received grant support paid to the institution from the German Research Foundation (DFG), the European Union (Horizon 2020), the German Ministry of Science, Education (BMBF), the German Parkinson Association (DPV) and the Parkinson’s Association. He has received personal compensation as a consultant/scientific advisory board member for Abbvie, Medtronic, Philyra; and honoraria for lectures/speaks from Abbvie, Bayer Vital, Bial, Daiichi Sankyo, UCB, Zambon.
Mike Samuel has received educational support from Medtronic (paid to the institution), Parkinson’s UK (via the UK DBS network), acts as a consultant for Abbott, and received honoraria from the Movement Disorders Society.
Monty Silverdale has received grants from Michael J Fox Foundation for Parkinson Research (paid to the institution), Parkinson’s UK (paid to the institution) and NIHR (paid to the institution) as well as conference expenses from Medtronic (paid to the institution).
Joke M. Dijk has received unrestricted grants from Medtronic (paid to the institution).
Alberto J. Espay has received grant support from the NIH and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Neuroderm, Neurocrine, Amneal, Adamas, Acadia, Acorda, Sunovion, Lundbeck, Osmotica Pharmaceuticals, and US World Meds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from US World Meds, Acadia, Sunovion, the American Academy of Neurology, and the Movement Disorders Society.
Anthony E. Lang has received grants from Brain Canada, Canadian Institutes of Health Research, Corticobasal Degeneration Solutions, Edmond J Safra Philanthropic Foundation, Michael J. Fox Foundation, the Ontario Brain Institute, Parkinson Foundation, Parkinson Canada, and W. Garfield Weston Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, AFFiRis, Biogen, Janssen, Lilly, Lundbeck, Merck, Paladin, Roche, Sun Pharma, Theravance, Corticobasal Degeneration Solutions, Jazz Pharma, Photo Pharmics, Sunovion; publishing royalties from Elsevier, Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press.
Jan Booij has received unrestricted research grants from GE Health (paid to the institution).
Rob M.A. de Bie received unrestricted research grants from GE Health (paid to the institution) and Lysosomal Therapeutics (paid to the institution).
ACKNOWLEDGMENTS
We thank all the participating centers for the acquisition of the DAT SPECT imaging.
The LEAP clinical trial was supported by unrestricted grants from the Netherlands Organization for Health Research and Development (Dutch governmental fund for health research, project number 0-82310-97-11031), Parkinson Vereniging (Dutch patient association), Stichting Parkinson fonds (Charity foundation for Parkinson’s disease research funding), and Stichting Parkinson Nederland (Charity foundation for Parkinson’s disease research funding). This ancillary study was supported by General Electric Healthcare.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-202090.
REFERENCES
[1] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[2] | Berg D , Adler CH , Bloem BR , Chan P , Gasser T , Goetz CG , Halliday G , Lang AE , Lewis S , Li Y , Liepelt-Scarfone I , Litvan I , Marek K , Maetzler C , Mi T , Obeso J , Oertel W , Olanow CW , Poewe W , Rios-Romenets S , Schäffer E , Seppi K , Heim B , Slow E , Stern M , Bledsoe IO , Deuschl G , Postuma RB ((2018) ) Movement disorder society criteria for clinically established early Parkinson’s disease. Mov Disord 33: , 1643–1646. |
[3] | Postuma RB , Poewe W , Litvan I , Lewis S , Lang AE , Halliday G , Goetz CG , Chan P , Slow E , Seppi K , Schaffer E , Rios-Romenets S , Mi T , Maetzler C , Li Y , Heim B , Bledsoe IO , Berg D ((2018) ) Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 33: , 1601–1608. |
[4] | Litvan I , Agid Y , Calne D , Campbell G , Dubois B , Duvoisin RC , Goetz CG , Golbe LI , Grafman J , Growdon JH , Hallett M , Jankovic J , Quinn NP , Tolosa E , Zee DS ((1996) ) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology 47: , 1–9. |
[5] | Rajput AH , Rozdilsky B , Rajput A ((1991) ) Accuracy of clinical diagnosis in parkinsonism–a prospective study. Can J Neurol Sci 18: , 275–278. |
[6] | Bajaj NP , Gontu V , Birchall J , Patterson J , Grosset DG , Lees AJ ((2010) ) Accuracy of clinical diagnosis in tremulous parkinsonian patients: A blinded video study. J Neurol Neurosurg Psychiatry 81: , 1223–1228. |
[7] | Marek K , Seibyl J , Eberly S , Oakes D , Shoulson I , Lang AE , Hyson C , Jennings D , Parkinson Study Group PRECEPT Investigators ((2014) ) Longitudinal follow-up of SWEDD subjects in the PRECEPT Study. Neurology 82: , 1791–1797. |
[8] | Suwijn SR , van Boheemen CJ , de Haan RJ , Tissingh G , Booij J , de Bie RM ((2015) ) The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson’s disease or clinically uncertain parkinsonism: A systematic review. EJNMMI Res 5: , 12. |
[9] | Booij J , Speelman JD , Horstink MW , Wolters EC ((2001) ) The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med 28: , 266–272. |
[10] | Jennings DL , Seibyl JP , Oakes D , Eberly S , Murphy J , Marek K ((2004) ) (123I) beta-CIT and single-photon emission computed tomographic imaging vs clinical evaluation in Parkinsonian syndrome: Unmasking an early diagnosis. Arch Neurol 61: , 1224–1229. |
[11] | Ponsen MM , Stoffers D , Booij J , van Eck-Smit BL , Wolters E , Berendse HW ((2004) ) Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol 56: , 173–181. |
[12] | Nicastro N , Garibotto V , Badoud S , Burkhard PR ((2016) ) Scan without evidence of dopaminergic deficit: A 10-year retrospective study. Parkinsonism Relat Disord 31: , 53–58. |
[13] | Verschuur CVM , Suwijn SR , Boel JA , Post B , Bloem BR , van Hilten JJ , van Laar T , Tissingh G , Munts AG , Deuschl G , Lang AE , Dijkgraaf MGW , de Haan RJ , de Bie RMA , LEAP Study Group ((2019) ) Randomized delayed-start trial of levodopa in Parkinson’s disease. N Engl J Med 380: , 315–324. |
[14] | Gelb DJ , Oliver E , Gilman S ((1999) ) Diagnostic criteria for Parkinson disease. Arch Neurol 56: , 33–39. |
[15] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[16] | Booij J , Hemelaar TG , Speelman JD , de Bruin K , Janssen AG , van Royen EA ((1999) ) One-day protocol for imaging of the nigrostriatal dopaminergic pathway in Parkinson’s disease by [123I]FPCIT SPECT. J Nucl Med 40: , 753–761. |
[17] | Bologna M , Paparella G , Fasano A , Hallett M , Berardelli A ((2020) ) Evolving concepts on bradykinesia. Brain 143: , 727–750. |
[18] | Jankovic J ((2011) ) Diagnosis and treatment of psychogenic parkinsonism. J Neurol Neurosurg Psychiatry 64: , 184–189. |
[19] | Abdo WF , Borm GF , Munneke M , Verbeek MM , Esselink RA , Bloem BR ((2006) ) Ten steps to identify atypical parkinsonism. J Neurol Neurosurg Psychiatry 77: , 1367–1369. |
[20] | Darcourt J , Booij J , Tatsch K , Varrone A , Vander Borght T , Kapucu OL , Någren K , Nobili F , Walker Z , Van Laere K ((2010) ) EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging 37: , 443–450. |
[21] | Djang DS , Janssen MJ , Bohnen N , Booij J , Henderson TA , Herholz K , Minoshima S , Rowe CC , Sabri O , Seibyl J , Van Berckel BN , Wanner M ((2012) ) SNM practice guideline for dopamine transporter imaging with 123I-ioflupane SPECT 1.0. J Nucl Med 53: , 154–163. |
[22] | Fearon C , Espay AJ , Lang AE , Lynch T , Martino D , Morgante F , Quinn NP , Vidailhet M , Fasano A ((2019) ) Soft signs in movement disorders: Friends or foes? J Neurol Neurosurg Psychiatry 90: , 961–962. |
[23] | Koo TK , Li MY ((2017) ) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15: , 155–163. |
[24] | Schneider SA , Edwards MJ , Mir P , Cordivari C , Hooker J , Dickson J , Quinn N , Bhatia KP ((2007) ) Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov Disord 22: , 2210–2215. |
[25] | Schwingenschuh P , Ruge D , Edwards MJ , Terranova C , Katschnig P , Carrillo F , Silveira-Moriyama L , Schneider SA , Kägi G , Palomar FJ , Talelli P , Dickson J , Lees AJ , Quinn N , Mir P , Rothwell JC , Bhatia KP ((2010) ) Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson’s disease: A clinical and electrophysiological study. Mov Disord 25: , 560–569. |
[26] | Meyer PT , Winz OH , Dafotakis M , Werner CJ , Krohn T , Schafer WM ((2011) ) Improved visual [(123)I]FP-CIT SPECT interpretation for evaluation of parkinsonism by visual rating of parametric distribution volume ratio images. Q J Nucl MedMol Imaging 55: , 301–309. |
[27] | Suwijn SR , Verschuur CVM , Slim MA , Booij J , De Bie RMA ((2019) ) Reliability of visual assessment by nonexpert nuclear medicine physicians and appropriateness of indications of [123I]FPCIT SPECT imaging by neurologists in patients with early drug-naive Parkinson’s disease. EJNMMI Res 9: , 63. |




