Recommendations for Deep Brain Stimulation Device Management During a Pandemic
Abstract
Most medical centers are postponing elective procedures and deferring non-urgent clinic visits to conserve hospital resources and prevent spread of COVID-19. The pandemic crisis presents some unique challenges for patients currently being treated with deep brain stimulation (DBS). Movement disorder (Parkinson’s disease, essential tremor, dystonia), neuropsychiatric disorder (obsessive compulsive disorder, Tourette syndrome, depression), and epilepsy patients can develop varying degrees of symptom worsening from interruption of therapy due to neurostimulator battery reaching end of life, device malfunction or infection. Urgent intervention to maintain or restore stimulation may be required for patients with Parkinson’s disease who can develop a rare but potentially life-threatening complication known as DBS-withdrawal syndrome. Similarly, patients with generalized dystonia can develop status dystonicus, patients with obsessive compulsive disorder can become suicidal, and epilepsy patients can experience potentially life-threatening worsening of seizures as a result of therapy cessation. DBS system infection can require urgent, and rarely emergent surgery. Elective interventions including new implantations and initial programming should be postponed. For patients with existing DBS systems, the battery status and electrical integrity interrogation can now be performed using patient programmers, and employed through telemedicine visits or by phone consultations. The decision for replacement of the implantable pulse generator to prevent interruption of DBS therapy should be made on a case-by-case basis taking into consideration battery status and a patient’s tolerance to potential therapy disruption. Scheduling of the procedures, however, depends heavily on the hospital system regulations and on triage procedures with respect to safety and resource utilization during the health crisis.
INTRODUCTION
The novel coronavirus (COVID-19) pandemic is rapidly changing how we live and practice medicine globally. Patients treated with deep brain stimulation (DBS) for movement disorders, neuropsychiatric disorders, and epilepsy face unique challenges. Highlighted in this article are practical management recommendations for DBS providers during COVID-19 pandemic. Since public health guidelines vary across countries, states and local municipalities and rapidly change, each medical center needs to be nimble and develop strategies to care for patients within the home setting. DBS practices range from large, tertiary centers to community clinics and vary widely in terms of workflow, resource availability, and level of expertise available on site. We provide general recommendations and workflow algorithm that centers can modify and adopt quickly while exercising compliance at multiple levels. This crisis highlights the challenge of postponing elective surgical procedures and transitioning to predominantly telemedicine for outpatient care in order to prevent virus spread in the general population, to protect healthcare workers, and to conserve hospital bed capacity, critical supplies and human resources. Decisions for allocation of scarce health care resources during a crisis must be fair and balanced across all patients requiring emergency medical care [1].
The DBS field was caught unprepared for patients with implantable pulse generators (IPG) requiring replacements and management of other hardware issues during the COVID-19 crisis. Some patients have rechargeable IPGs, but for many, the IPG needs to be replaced every 3–5 years depending on patient-specific energy requirements. Some patients, for example with essential tremor, may be able to defer the IPG replacement and to tolerate the re-emergence of symptoms. However, most patients with Parkinson’s disease and dystonia will not tolerate cessation of therapy [2, 3], and patients with obsessive compulsive disorder (OCD) and depression have a risk for re-emergence of neuropsychiatric symptoms including suicidal ideation [4]. Patients with epilepsy may experience an increase in seizure frequency [5, 6]. Additional hardware-related issues (infections, lead fractures, electrical malfunction) will also need to be promptly recognized and addressed. Failure to do so could lead to emergency department utilization, hospitalization, intensive care use, and increased disability - all leading to the possibility of more COVID-19 exposure and utilization of critically limited resources.
DBS DEVICE MANAGEMENT DURING A CRISIS
Patients who utilize DBS therapy for management of neuropsychiatric disorders require ongoing surgical care and outpatient clinic visits for device management. The implantation of de novo DBS leads (and preoperative workup of new patients) is considered an elective procedure and should not be scheduled while pandemic measures are in place.
The necessary maintenance includes routine IPG replacements (for non-rechargeable devices), infection management, assessment of hardware-related complications, as well as outpatient stimulation adjustments (programming). Limited device programming and management can be handled through telemedicine (discussed in detail in a later section). The telemedicine visits and telephone consultations are also critical to promptly identify any potential surgical issues.
Surgical procedures can be classified into four categories according to the American College of Surgeons guidelines [7]: elective (those which can be postponed for 4 weeks or longer), time-sensitive (should be performed within 4 weeks), urgent (completed within 24 hours), and emergent (completed immediately due to threatening loss of life). Outpatient clinic visits can be classified more simply as elective (can be deferred) or urgent (there is a need for immediate in person care). We define an urgent clinic visit as one that would potentially prevent an emergency department visit, a hospitalization or significant disability. Pandemic measures may permit only emergent/urgent procedures and urgent outpatient visits. Depending on local regulations, virus spread, and available resources some elective and time-sensitive procedures may be liberalized based on need.
IPG depletion
The IPG battery status can be followed by the DBS provider and many devices have elective end of life battery replacement indicators for patients and family members (highlighted on their patient programmer device). In most cases, a battery indicator means there are a few weeks of electrical charge remaining. As a result, an IPG replacement procedure may initially be classified as elective according to the surgical triage criteria if expected depletion is more than 4 weeks away, but it can then progress to time-sensitive or urgent depending on patient-specific factors.
To avoid battery failure and adverse symptoms, a better understanding of IPG battery longevity and management is necessary. Existing methods of DBS battery life estimation utilize an interpolation of averaged current drains to calculate battery longevity. This technique only provides general approximations. Most expert centers will therefore check and track DBS battery status at every visit and plan for pre-emptive IPG change prior to depletion and re-emergence of symptoms [8], which is something that can now be accomplished via telemedicine using patient programmers.
In addition, clinical symptoms of the patient should be considered as in many cases symptoms can appear even before the battery has completely depleted. A cohort of 320 patients undergoing DBS battery replacement from 2002–2012 was studied for potential rebound symptoms prior to IPG replacement. In this series there were 38 cases reported where the symptoms improved following an IPG change, suggesting that the neurostimulator battery depletion was likely responsible for symptom worsening [9].
Hardware infection
DBS hardware infections are most common in the first few months following implantation, however they can occur at any time during the lifecycle of a device [10, 11]. A superficial infection can be treated conservatively with oral antibiotics. An aggressive infection requires explantation and IV antibiotics to avoid potentially life-threatening spread to the CNS and other organ systems. The presence and severity of infection or skin erosion may be initially assessed with telemedicine and through emailing (serial) photos, but also may warrant an urgent clinic visit. If an infection has progressed it may require a time-sensitive or even urgent IPG and extension wire removal or in select cases complete system removal.
Hardware malfunction
Hardware malfunction that may compromise optimal DBS therapy includes lead migrations, lead fractures and lead shorts. A limited check of the electrical system may be conducted remotely and we describe this procedure in the outpatient management section. An in-person visit may be necessary to localize the problem and perform ancillary tests (e.g., x-ray for lead breakage or CT for lead migration). The urgency depends largely on the risk of therapy disruption.
Surgical procedure considerations
A potential safety consideration may be to perform IPG replacement and other DBS-related procedures under local (with or without conscious sedation) rather than generalized anesthesia. This method may possibly reduce respiratory droplet spread during a pandemic, preserve hospital resources and shorten a patient’s post-anesthesia recovery. Performing these procedures at outpatient surgical centers rather than in hospitals should also be considered; however, many such centers may close operations during pandemics. Depending on local guidelines and availability, testing for COVID-19 may increasingly be performed during routine preoperative workup.
COVID-19 specific risk factors
Certain patients, regardless of their DBS-related diagnoses, have additional factors that put them at increased risk for severe disease and complications from COVID-19 infection which should be considered in DBS-related care. These include older age, male sex, cardiovascular and cerebrovascular disease, diabetes and immunosuppression [12, 13]. There is no evidence to date that patients with movement disorders are at increased risk from COVID-19, but patients with Parkinson’s disease and pneumonia have longer hospitalizations [14]. Additional consideration should be whether a patient resides in a nursing home or another group facility that puts them and other residents at increased risk of infection spread.
CLASSIFYING THE RISK OF DBS THERAPY CESSATION
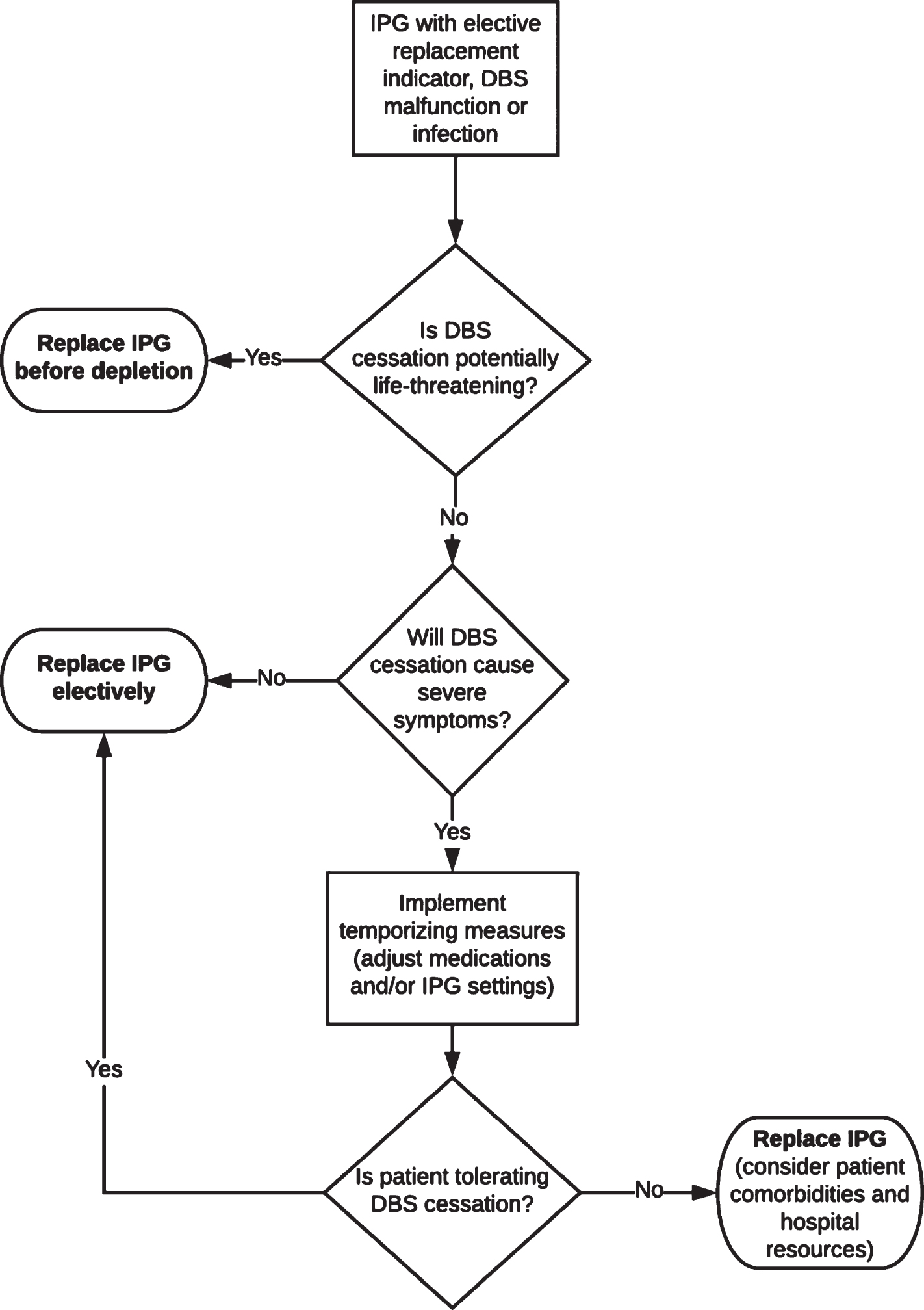
The risk of therapy cessation and proposed triage for DBS therapy maintenance and re-initiation varies by the disorder and patient’s characteristics (Table 1). We discuss conditions for which DBS has been FDA-approved (Parkinson’s disease, dystonia, tremor, OCD, epilepsy), as well as two other conditions with reasonably large numbers of treated patients (Tourette syndrome and depression). As a general rule, urgent DBS replacement should be performed in patients who may require hospitalization or may seek care via emergency services due to therapy cessation; with the highest priority given to those who may face life-threatening complications or require intensive care unit monitoring. A concise workflow algorithm is illustrated (Fig. 1), with more detailed workflow provided in the Supplementary Material.
Table 1
Recommendations for DBS device replacement triage during pandemic measures
| Consequence of DBS cessation | Patient characteristics | Scheduling priority |
| At risk for life-threatening symptoms or hospitalization | Parkinson’s disease with DBS-withdrawal syndrome risk factors*; Generalized dystonia; Tourette syndrome with head snapping tics or self-injurious behavior; Depression depending on target+ | Highest priority for replacement |
| Severe symptoms with therapy cessation; may require hospitalization or seek care via emergency services | Non-generalized dystonias; Advanced Parkinson’s disease with meaningful DBS benefit; Severe essential tremor without caregivers to assist with activities of daily living; Tourette syndrome with severe tics; Obsessive compulsive disorder; Depression depending on target+; Epilepsy | High priority for replacement |
| Mild to moderate symptoms with therapy cessation; not requiring hospitalization | Most essential tremor; Parkinson’s disease, dystonia, OCD, depression or epilepsy with only mild DBS benefit; Mild Tourette syndrome | Moderate priority for replacement |
Fig. 1
Workflow algorithm for management of DBS-related issues in a pandemic.
Parkinson’s disease
For some Parkinson’s disease patients, the interruption of DBS therapy can provoke a life-threatening DBS-withdrawal syndrome [2]. For these patients, replacement of the IPG prior to depletion should be considered a very high priority. The DBS withdrawal syndrome is similar in presentation to parkinsonism-hyperpyrexia (and neuroleptic malignant syndrome) and has not been shown to be reversible with dopaminergic medications alone. The reason for dopamine unresponsiveness is unknown, but may be related to downregulation or absence of dopamine receptors in advanced Parkinson’s disease. The syndrome is rapid in its evolution with changes observed within hours to days of therapy cessation. Severe motor symptoms including akinesia, rigidity, dysarthria, dysphagia and autonomic instability have been observed and an ICU setting is usually necessary. The syndrome has been associated with death [15]. The syndrome has only been reported in patients with bilateral STN DBS with at least 5 years of DBS therapy and more than 15 years of disease duration [16]. Additional risk factors include early age at disease onset, advanced symptoms at the time of initial surgery, and excellent DBS benefit leading to significant dopaminergic medication reduction [2]. However, because reported cases are sparse we cannot assume that this represents the profile for all at risk patients and it is possible that GPi and other brain targets also are at risk. Medical intensivists and general neurologists may not be familiar with this emergent complication. One large expert DBS center reported it in 3.7% of all IPG changes so the number may be higher among patients with high risk characteristics [17].
For the majority of patients with Parkinson’s disease, and particularly those with advanced disease and those who are highly dependent on the therapy (e.g., severe tremor, dyskinesia), cessation of DBS may lead to severe disability. Even though symptoms do not threaten life, these patients may require hospitalization or seek care via emergency services if cessation is prolonged and they are unable to manage symptoms at home or perform activities of daily living. There is also a smaller subset of patients whose symptoms are less severe or who receive only mild to moderate benefit from DBS. These patients may be able to postpone IPG replacement until elective surgery restriction measures are lifted.
Dystonia
The highest priority for not delaying DBS neurostimulator changes should be offered to patients with generalized dystonia who may experience status dystonicus [3]. Status dystonicus or dystonic storm has been defined as continuous or increasingly frequent severe dystonic spasms requiring hospitalization for life-threatening complications including respiratory distress, bulbar dysfunction, metabolic derangements and pain [18]. The common triggers include medication changes, infection, fever and surgical procedures. The treatment includes supportive care and high doses of anti-dystonic medications, and in medically-refractory cases, DBS can be urgently performed [19]. An ICU setting is often necessary. Interruption of DBS therapy in patients with generalized dystonia (genetic or acquired) can precipitate status dystonicus and require urgent re-initiation of stimulation [3, 20, 21]. This complication can occur even with cessation of unilateral stimulation. The dystonia exacerbation may or may not be fully reversible [22].
Patients with other forms of dystonia are at risk for development of severe disability (e.g., cervical dystonia accompanied by severe pain or oromandibular dystonia leading to difficulty with eating or speaking). Patients who may not tolerate prolonged cessation due to symptom severity should be given high priority for IPG replacement. A small subset of patients whose symptoms are focal or who receive only mild to moderate benefit from DBS may be able to defer IPG replacement.
Essential tremor
In some patients with essential tremor, rebound tremor can be very severe, leading to increased sympathetic state with elevated heart rate, hypertension and excessive sweating, necessitating prompt IPG replacement. Most patients are unlikely to experience severe medical deterioration with DBS cessation unless tremor significantly impairs their ability to perform basic activities of daily living (e.g., eating, performing hygiene) and there is not a caregiver available to assist. Therefore majority of essential tremor patients should defer IPG replacement.
OCD and depression
Obsessive compulsive disorder [4] and depression patients are another high-risk population as they may experience worsening neuropsychiatric symptoms and suicidal ideation with DBS cessation. Therefore, they should receive high priority for IPG replacement. In DBS for depression, the reemergence of symptoms varies depending on the target of implantation. In subcallosal cingulate target, worsening of depression is usually gradual, occurring within weeks of therapy cessation [23–25]. The medial forebrain bundle has a more rapid return of symptoms and an observational report of 5 cases with involuntary or planned cessation of stimulation reported a fast deterioration within hours to a couple of weeks [26]. A randomized clinical trial of ventral capsule DBS for depression, where a blinded discontinuation of stimulation was conducted, demonstrated loss of antidepressant effects in all patients in less than two weeks [27]. While return of suicidality has been reported, and there have been suicides in studies of DBS for depression and OCD, no reports of completed suicides due to abrupt acute therapy cessation have been published [4].
Tourette syndrome
Patients with Tourette syndrome with head snapping tics behavior will likely require urgent IPG replacement. Those with severe tics and/or self-injurious behavior will likely experience increased disability but may not require hospitalization. In these cases, a high priority for replacement is indicated. Patients with mild symptoms or mild to moderate DBS benefit should possibly defer replacement.
Epilepsy
DBS for epilepsy was recently approved in the U.S., but has been widely available around the world since 2008. Device depletion has been reported to be associated with increase in seizures in some patients [5, 6], but there are no reports of status epilepticus or SUDEP. IPG replacement should be performed as an elective procedure in the absence of such reports, although increased numbers of generalized seizures or recurrent seizure-related injuries could increase the patient to time-sensitive status.
RECOMMENDATIONS IF THE NEUROSTIMULATOR (IPG) CANNOT BE REPLACED
If the neurostimulator (IPG) cannot be replaced prior to depletion, a telemedicine or phone consultation for patients can be useful to implement temporizing measures. Options for Parkinson’s disease patients include medication optimization (e.g., increase dopaminergic medication dose or decrease the time interval between dosages). Also, an important consideration is making sure patients have three month supplies of the increased medication regimen. For patients with STN DBS who are on minimal levodopa, the provider might consider re-introducing more levodopa over several days or weeks and if necessary decreasing DBS therapy in an effort to lessen symptoms at the time of complete battery cessation. It is theoretically possible, though it has not been formally tested, that this procedure may re-establish dopamine transmission in the basal ganglia and reduce the risk of DBS-withdrawal syndrome.
Dystonia patients may consider reinstitution of oral medications in order to minimize dystonia symptoms (benzodiazepines, anticholinergics). Generalized dystonia patients should be warned about the possibility of continuous severe dystonic spasms which may require hospitalization and urgent IPG replacement.
In essential tremor, patients can be advised to turn the DBS off during night and as needed during daytime in order to save IPG charge prior to complete depletion. Reinstitution of oral medications may also reduce symptoms.
In DBS for psychiatric indications, medication adjustment or initiation of new psychotropics is advised, as well as supportive psychotherapy and close monitoring of symptoms. Patients with severe depression are eligible candidates to receive electroconvulsive therapy [28], or intravenous ketamine infusions, and should be hospitalized if depression or suicidality becomes intense. If IPG failure is expected to happen, and replacement will not be available, there is no evidence supporting cycling or reduction of stimulation intensity. Alternating stimulation between hemispheres may provide sustained antidepressant response in the subcallosal cingulate, and this could be a potential strategy to slow IPG depletion, but this has not been replicated [29].
For patients with epilepsy, daily anti-seizure medications can be optimized and patients can be given a home rescue medication to take in the event of worsening seizure severity or frequency. Parameters may be able to be adjusted to temporarily preserve battery; for example, most patients with DBS for epilepsy are being stimulated in cycling mode, so on time relative to off time can be decreased.
OUTPATIENT DBS MANAGEMENT
DBS patients require active programming for the first few months after the initial lead implantation in order to optimize DBS parameters. The initial programming visit is considered elective and can therefore be safely postponed. If the DBS device is activated with pre-programmed settings, this could enable telemedicine and telephone support for minor adjustments. For those already undergoing active programming, urgent clinic visits may be necessary to address stimulation-induced complications (e.g., severe dyskinesia). Overall, the need for clinic visits can be minimized by utilizing patient programmer advanced features and by allowing patients to make changes remotely, not initiating active programming, and postponing detailed programming optimization.
For patients on chronic DBS therapy, stimulation adjustments are infrequent and can be postponed. These patients require regular device interrogation to plan routine IPG replacements (for non-rechargeable IPG) and to address any sudden changes in the effectiveness of therapy. All three FDA-approved DBS device manufacturers (Medtronic, Abbott, and Boston Scientific) provide patient programmers that can be utilized to check therapy status (on/off), battery charge, and system electrical integrity (impedance). The details provided by the system integrity check vary between manufacturers, and this procedure is considered off-label for the Medtronic Activa system. These checks can be performed during telehealth visits or phone consultations, although it is unknown whether standard billing codes for DBS interrogation and programming can/will be utilized for these services. Important to take into consideration is that some patients may require the assistance of a caregiver as multiple steps may be necessary to use the patient programmer. Company representatives and technology support call centers can sometimes assist. We provide instructions for DBS providers within the Supplementary Material for this article.
Checking the device status and planning replacements
Important to also consider is that therapy interruption can occur due to inadvertent deactivation. This situation most commonly occurs when patients use their patient programmer to check or adjust therapy. Environmental causes of deactivation are less common (e.g., magnets, theft devices in stores). The device can also be damaged by a fall or other trauma (motor vehicle accident). Therefore, the first step during a telemedicine visit should be to check if the device is turned on. Additionally, we recommend that patients should check battery status every 3–6 months in order to prevent sudden interruption of DBS therapy and to plan for replacement before the end of battery life. The provider may instruct patients to perform the battery check once a month as the expiration date approaches. This attention can guide scheduling of IPG replacements. Finally, system integrity checks can be remotely performed if concerns about lead breakage or device malfunction emerge.
CONCLUSIONS
Public health measures due to the COVID-19 pandemic have forced a reassessment of how we manage patients with implantable devices such as DBS when device related complications or battery replacement issues emerge. The practical recommendations summarized in this article will hopefully help to guide the appropriate use of health care resources and will help to improve DBS patient safety. Expanded application of telemedicine and the use of patient-controlled programmers can enhance our ability to care for DBS patients during future pandemics and crises. Many of the lessons learned from the COVID-19 crisis can be utilized to guide future in the home management for DBS patients and to motivate technical innovations in rechargeable battery systems and remote care options.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-202072.
REFERENCES
[1] | Emanuel EJ , Persad G , Upshur R , Thome B , Parker M , Glickman A , Zhang C , Boyle C , Smith M , Phillips JP ((2020) ) Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. doi: 10.1056/NEJMsb2005114. |
[2] | Hariz M ((2016) ) Once STN DBS, always STN DBS?-Clinical, ethical, and financial reflections on deep brain stimulation for Parkinson’s disease, Mov Disord Clin Pract 3: , 285–287. |
[3] | Vercueil L , Pollak P , Fraix V , Caputo E , Moro E , Benazzouz A , Xie J , Koudsie A , Benabid AL ((2001) ) Deep brain stimulation in the treatment of severe dystonia, J Neurol 248: , 695–700. |
[4] | Vora AK , Ward H , Foote KD , Goodman WK , Okun MS ((2012) ) Rebound symptoms following battery depletion in the NIH OCD DBS cohort: Clinical and reimbursement issues, Brain Stimul 5: , 599–604. |
[5] | Cukiert A , Cukiert CM , Burattini JA , Lima Ade M ((2015) ) Seizure outcome after battery depletion in epileptic patients submitted to deep brain stimulation, Neuromodulation 18: , 439-441; discussion 441. |
[6] | Osorio I , Overman J , Giftakis J , Wilkinson SB ((2007) ) High frequency thalamic stimulation for inoperable mesial temporal epilepsy, Epilepsia 48: , 1561–1571. |
[7] | American College of Surgeons ((2020) ) COVID-19: Recommendations for Management of Elective Surgical Procedures, http://www.facs.org/about-acs/covid-19/information-for-surgeons/elective-surgery. Accessed 3/24/2020. |
[8] | Montuno MA , Kohner AB , Foote KD , Okun MS ((2013) ) An algorithm for management of deep brain stimulation battery replacements: Devising a web-based battery estimator and clinical symptom approach, Neuromodulation 16: , 147–153. |
[9] | Fakhar K , Hastings E , Butson CR , Foote KD , Zeilman P , Okun MS ((2013) ) Management of deep brain stimulator battery failure: Battery estimators, charge density, and importance of clinical symptoms, PLoS One 8: , e58665. |
[10] | Fernandez-Pajarin G , Sesar A , Ares B , Relova JL , Aran E , Gelabert-Gonzalez M , Castro A ((2017) ) Delayed complications of deep brain stimulation: 16-year experience in 249 patients, Acta Neurochir (Wien) 159: , 1713–1719. |
[11] | Sillay KA , Larson PS , Starr PA ((2008) ) Deep brain stimulator hardware-related infections: Incidence and management in a large series, Neurosurgery 62: , 360-366; discussion 366–367. |
[12] | Chen N , Zhou M , Dong X , Qu J , Gong F , Han Y , Qiu Y , Wang J , Liu Y , Wei Y , Xia J , Yu T , Zhang X , Zhang L ((2020) ) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study, Lancet 395: , 507–513. |
[13] | Li X , Xu S , Yu M , Wang K , Tao Y , Zhou Y , Shi J , Zhou M , Wu B , Yang Z , Zhang C , Yue J , Zhang Z , Renz H , Liu X , Xie J , Xie M , Zhao J ((2020) ) Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2020.04.006. |
[14] | Papa SM , Brundin P , Fung VSC , Kang UJ , Burn DJ , Colosimo C , Chiang HL , Alcalay RN , Trenkwalder C , and the MDS-Scientific Issues Committee ((2020) ) Impact of the COVID-19 pandemic on Parkinson’s disease andmovement disorders. Mov Disord. doi: 10.1002/mds.28067. |
[15] | Reuter S , Deuschl G , Falk D , Mehdorn M , Witt K ((2015) ) Uncoupling of dopaminergic and subthalamic stimulation: Life-threatening DBS withdrawal syndrome, Mov Disord 30: , 1407–1413. |
[16] | Azar J , Elinav H , Safadi R , Soliman M ((2019) ) Malignant deep brain stimulator withdrawal syndrome, BMJ Case Rep 12: , e229122. |
[17] | Reuter S , Deuschl G , Berg D , Helmers A , Falk D , Witt K ((2018) ) Life-threatening DBS withdrawal syndrome in Parkinson’s disease can be treated with early reimplantation, Parkinsonism Relat Disord 56: , 88–92. |
[18] | Ruiz-Lopez M , Fasano A ((2017) ) Rethinking status dystonicus, Mov Disord 32: , 1667–1676. |
[19] | Vidailhet M , Jutras MF , Roze E , Grabli D ((2013) ) Deep brain stimulation for dystonia, Handb Clin Neurol 116: , 167–187. |
[20] | Cheung T , Flatow V , Ben-Haim S , Osborn I , Cho C , Tagliati M , Alterman R ((2012) ) Status dystonicus following deep brain stimulation surgery in DYT1 dystonia patients, Neurology 78: , P01.227. |
[21] | Rohani M , Munhoz RP , Shahidi G , Parvaresh M , Miri S ((2017) ) Fatal status dystonicus in tardive dystonia due to depletion of deep brain stimulation’s pulse generator, Brain Stimul 10: , 160–161. |
[22] | Sobstyl M , Zabek M , Kmiec T , Slawek J , Budohoski KP ((2014) ) Status dystonicus due to internal pulse generator depletion in a patient with primary generalized dystonia, Mov Disord 29: , 188–189. |
[23] | Crowell AL , Riva-Posse P , Holtzheimer PE , Garlow SJ , Kelley ME , Gross RE , Denison L , Quinn S , Mayberg HS ((2019) ) Long-term outcomes of subcallosal cingulate deep brain stimulation for treatment-resistant depression, Am J Psychiatry 176: , 949–956. |
[24] | Kennedy SH , Giacobbe P , Rizvi SJ , Placenza FM , Nishikawa Y , Mayberg HS , Lozano AM ((2011) ) Deep brain stimulation for treatment-resistant depression: Follow-up after 3 to 6 years, Am J Psychiatry 168: , 502–510. |
[25] | Merkl A , Aust S , Schneider GH , Visser-Vandewalle V , Horn A , Kuhn AA , Kuhn J , Bajbouj M ((2018) ) Deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: A double-blinded randomized controlled study and long-term follow-up in eight patients, J Affect Disord 227: , 521–529. |
[26] | Kilian HM , Meyer DM , Bewernick BH , Spanier S , Coenen VA , Schlaepfer TE ((2019) ) Discontinuation of superolateral medial forebrain bundle deep brain stimulation for treatment-resistant depression leads to critical relapse, Biol Psychiatry 85: , e23–e24. |
[27] | Bergfeld IO , Mantione M , Hoogendoorn ML , Ruhe HG , Notten P , van Laarhoven J , Visser I , Figee M , de Kwaasteniet BP , Horst F , Schene AH , van den Munckhof P , Beute G , Schuurman R , Denys D ((2016) ) Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: A randomized clinical trial, JAMA Psychiatry 73: , 456–464. |
[28] | Vila-Rodriguez F , McGirr A , Tham J , Hadjipavlou G , Honey CR ((2014) ) Electroconvulsive therapy in patients with deep brain stimulators, J ECT 30: , e16–e18. |
[29] | Guinjoan SM , Mayberg HS , Costanzo EY , Fahrer RD , Tenca E , Antico J , Cerquetti D , Smyth E , Leiguarda RC , Nemeroff CB ((2010) ) Asymmetrical contribution of brain structures to treatment-resistant depression as illustrated by effects of right subgenual cingulum stimulation, J Neuropsychiatry Clin Neurosci 22: , 265–277. |



