Multimodal Balance Training Supported by Rhythmical Auditory Stimuli in Parkinson’s Disease: A Randomized Clinical Trial
Abstract
Background:
Balance impairment in Parkinson’s disease (PD) improves only partially with dopaminergic medication. Therefore, non-pharmacological interventions such as physiotherapy are important elements in clinical management. External cues are often applied to improve gait, but their effects on balance control are unclear.
Objective/Methods:
We performed a prospective, single-blind, randomized clinical trial to study the effectiveness of balance training with and without rhythmical auditory cues. We screened 201 volunteers by telephone; 154 were assigned randomly into three groups: (1) multimodal balance training supported by rhythmical auditory stimuli (n = 56) (RAS-supported multimodal balance training); (2) regular multimodal balance training without rhythmical auditory stimuli (n = 50); and (3) control intervention involving a general education program (n = 48). Training was performed for 5 weeks, two times/week. Linear mixed models were used for all outcomes. Primary outcome was the Mini-BESTest (MBEST) score immediately after the training period. Assessments were performed by a single, blinded assessor at baseline, immediately post intervention, and after one and 6-months follow-up.
Results:
Immediately post intervention, RAS-supported multimodal balance training was more effective than regular multimodal balance training on MBEST (difference 3.5 (95% Confidence Interval (CI) 2.2; 4.8)), p < 0.001). Patients allocated to both active interventions improved compared to controls (MBEST estimated mean difference versus controls 6.6 (CI 5.2; 8.0), p < 0.001 for RAS-supported multimodal balance training; and 3.0 (CI 2.7; 5.3), p < 0.001 for regular multimodal balance training). Improvements were retained at one-month follow-up for both active interventions, but only the RAS-supported multimodal balance training group maintained its improvement at 6 months.
Conclusion:
Both RAS-supported multimodal balance training and regular multimodal balance training improve balance, but RAS-supported multimodal balance training–adding rhythmical auditory cues to regular multimodal balance training–has greater and more sustained effects.
INTRODUCTION
Balance and gait impairments in Parkinson’s disease (PD) are both common and disabling [1–3], resulting in falls and fall-related injuries [4]. Because these axial symptoms only improve partially with dopaminergic medication [2, 5–8], non-pharmacological interventions such as physiotherapy are important to manage these problems [9–12]. There is growing evidence that physiotherapy interventions improve gait [13–19] and balance performance in PD [20–28]. Physiotherapy typically involves functional gait and balance exercises [9, 10, 24] that translate directly to daily life activities [29–31]. Moreover, compensation strategies such as rhythmic auditory cueing [32] have an immediate effect on walking speed, stride length and cadence [16, 17, 33]. However, their effects decline over time [16, 33]. In addition to their positive effect, rhythmic cues may also have a positive effect on balance performance. In contrast to gait performance, balance control is not rhythmic in nature. However, adding rhythmic auditory cueing might enhance the balance training effects by improving attention and task prioritization [34]. In this prospective randomized clinical trial, we aimed to: (1) investigate whether the effect of physiotherapy on balance performance in patients with PD would be maximized by adding rhythmical auditory stimuli to the training (RAS-supported multimodal balance training); and (2) investigate whether these effects would be retained in the long-term, after cessation of treatment. We hypothesized that, compared to control intervention (educational program) and regular multimodal balance training, adding rhythmical auditory stimuli would be more effective in improving balance performance, but that these effects would decline over time (as has been reported for gait performance).
METHOD
Study design and participants
We performed a prospective, single-blind, parallel group randomized clinical trial. The University of São Paulo Faculty Medicine Clinics Hospital received ethical approval for the study (Comissão de Ética para An
Study procedure
After screening for eligibility, subjects were randomly assigned (1:1:1) into either one of the two experimental groups, or one control intervention group (RAS-supported multimodal balance training, regular multimodal balance training and the educational program). A computerized block randomization procedure (block size 6) was performed by an independent study collaborator before the baseline assessment, with stratification for disease severity (H&Y stage). Group allocation was performed by the same study collaborator, who was not involved in interventions or assessments. This collaborator delivered a sealed envelope to the physiotherapist to ensure concealment. The trial was conducted between July 2015 and May 2017. Measurements were performed by five blinded assessors at four time points: baseline, i.e., 14 days prior to training; one day after the last 5th week training; at one-month follow-up; and 6-months follow-up. Both assessors and patients were instructed not to talk about the allocation. We did not formally test for the success of blinding. All participants were tested while they were on their usual Parkinson medication (ON-medication state), which was defined as maximally 1 hour after ingestion of their regular dose of dopaminergic medication (as self-reported by the patients) and when patients experienced a subjectively good On state. We chose exercises that are common in routine clinical treatment of gait and balance problems. These exercises involve 5 elements of posture, gait and balance:(a) sensory integration (e.g., walking tasks on varying surfaces); (b) anticipatory postural adjustments (e.g., voluntary arm, leg and trunk movements, postural transitions, and multidirectional stepping); (c) compensatory postural adjustments and motor agility (e.g., interlimb coordination under varying gait conditions and quick shifts of movement during predictable and unpredictable conditions); (d) performance at stability limits (e.g., controlled leaning tasks performed while standing with varying bases of support, stimulating weight shifts in multiple directions and turning); and (5) use of attentional strategies (maintenance of attention to the gait and balance task). No modification of the intervention protocol occurred.
Intervention
Interventions were delivered in at the University of São Paulo Clinics Hospital, Movement Disorders Clinic, Department of Neurology. The three interventions occurred on the same day and at the same location, but during different timeslots during the afternoon. This minimizes the risk of contamination. In addition, the physiotherapists were well trained to follow the different training protocols for the different training groups. Both experimental groups received multimodal balance training; one intervention group received all exercises combined with rhythmical auditory stimuli, provided by a metronome (RAS-supported multimodal balance training; Supplementary Video), whereas the other intervention group received balance training without rhythmical auditory stimuli (regular multimodal balance training). Both intervention groups also received gait training with visual cues (as this is part of routine physiotherapy care), but rhythmical auditory stimuli to maximize the balance exercises were only added in the RAS-supported multimodal balance training group on top of the training (Supplementary Video). The physicaltherapists gave instructions to the patients to perform the movements on the beat of the metronome. The training program was performed in group’s intervention of 10 participants supervised by 2 physiotherapists.
Training in both intervention groups involved 40 balance and gait exercises, provided during 10 sessions of 45 minutes (2 sessions/week over a 5-week period). The exercises, training progression and intensity are described in Supplementary Tables 1 and 2. The rhythmical auditory stimuli were delivered in an open-loop by a metronome (MA-1 KORG, which was amplified using a JBL GO Portable Wireless Speaker). Progression over time was facilitated by dividing the training period into two 5-week sessions. Each exercise component was introduced separately to the participants in week 1, with emphasis on the quality of performance rather than on difficulty level. In week 2, the level of difficulty for each exercise component was increased, whereas movement complexity was further increased in week 3, 4 and 5 by combining the exercise components and increasing the demands. To further promote training progression, the aim was to increase or decrease the speed throughout the parts of the training. The control intervention group received no functional balance and gait training, but received a general education program about PD, falls prevention and self-care, which also involved 10 sessions of 45 minutes (2 sessions/week over a 5-week period) (Supplementary Tables 1 and 2).
Outcome measures
Although the UPDRS was initially selected as the primary endpoint, the research team later changed the primary outcome to Mini-BESTest (MBEST) [40] because this better suits the aim of the intervention (namely to improve balance), as was also published in the study protocol [41]. Importantly, this adjustment was made before the end of patient recruitment, and hence also well before any analyses were performed. We acknowledge that this procedure is imperfect, but we certify that the decision to change the primary outcome was driven solely by scientific arguments and taken before any data analyses had been performed. The MBEST test has a maximum score of 28 points based on 14 items that are each scored from 0–2. “0” indicates the lowest level of functioning and “2” the highest level of functioning [42]. Secondary outcomes included measures of balance and gait: Berg Balance Scale (BBS) [43], retropulsion test of the UPDRS [44], push-and-release test [45], Timed Up and Go Test (TUG) [46]. Before the actual start of the trial, the following secondary outcomes were added to the original protocol: TUG-dual task test (14 domain in MBEST) [40] and Rapid Turns Test [39]. Also, the FOG-Questionnaire was replaced with the New Freezing of Gait Questionnaire (N-FOGQ) [38]. Activities of daily living and motor performance were assessed using the UPDRS [44]. Fear of falling were evaluated using the Falls Efficacy Scale-International (FES-I) [47]. We monitored adverse events only during the training, and did not address serious events because this was a low risk study. In addition, falls and serious adverse events were assessed through standardized weekly interviews.
Statistical analysis
Statistical analysis was performed according an intention-to-treat (ITT) which was better regarded as a complete trial strategy for our design. The intention-to-treat population was defined as all patients who received the intervention, provided baseline efficacy data, and from whom at least one measurement after baseline was obtained. All participants were analyzed as they were randomized, as there were no inconsistencies between the randomization and the actual treatment. Following discussions with an independent statistician (J.IH., PhD) who was not involved in the study design or data collection, the statistical plan was optimized, specifically, by replacing the originally planned repeated measures ANOVA with a mixed effects model to analyze the repeated measurements, which can handle data that are missing at random and is more flexible than a repeated measures ANOVA [48, 49]. The sample size calculation was based on the MBEST as outcome. Based on the results of a pilot study (unpublished data) with exactly the same methodology and intervention, we performed a power calculation. The pilot study included 10 participants in each group. We found a 3-point difference between standard training and multimodal training. Assuming a 3-point difference and an SD of 4 (∼ an effect size of 0.75), 37 patients per group would be enough for 90% power, using a two-sided significance level α of 0.05. Assuming a dropout rate of 25%, this resulted in 50 patients per group. We included 154 patients instead of 150 because we reasoned that several extra participants would correct for a potential higher drop-out rate. Linear mixed models were used for all outcomes. The primary endpoint was the MBEST immediately post-intervention. We used treatment (RAS-supported multimodal balance training vs regular multimodal balance training vs controls), visit (immediately post-intervention, one-month follow-up, and 6 months follow-up) and the interaction between visit and treatment group as fixed factors. The model was adjusted for baseline MBEST, UPDRS part 2 and 3 and levodopa equivalent daily dose (LEDD), and, if not yet included, for the baseline value of the dependent variable. We adjusted for these variables, despite the randomized design of the study, to correct for possible imbalances due to a somewhat higher dropout rate in the control group than in the training groups. Patient was included as a random factor. Berg Balance Scale, Retropulsion Test, Push and Release Test, Falls Efficacy Scale- International, Timed Up and Go, Timed up and Go Dual Task, New Freezing of Gait Questionnaire, Rapid Turns test were also analyzed with a linear mixed model, similar to the MBEST. A Bonferroni adjustment for multiple testing (three pairwise comparisons at 5 weeks follow-up) was applied, resulting in a significance threshold of 0.017 that was used for all tests. No interim analyses were performed before the recruitment target was reached.
RESULTS
We screened 201 potential participants of whom 154 were randomized (Fig. 1). The groups were similar on baseline characteristics (Table 1). A total of 21 patients dropped out of the study (14%, Fig. 1). The training compliance was determined by the number of sessions that the patients attended. Training compliance was 85% in the RAS-supported multimodal balance training group, 86% in the regular multimodal balance training group and 82% in the control intervention group. Reasons for not being compliant were lack of time, problems with transport, injuries, illness and fatigue not related to the intervention session. No adverse events occurred during the training.
Fig.1
Flow diagram of participants through the trial, number of participants.
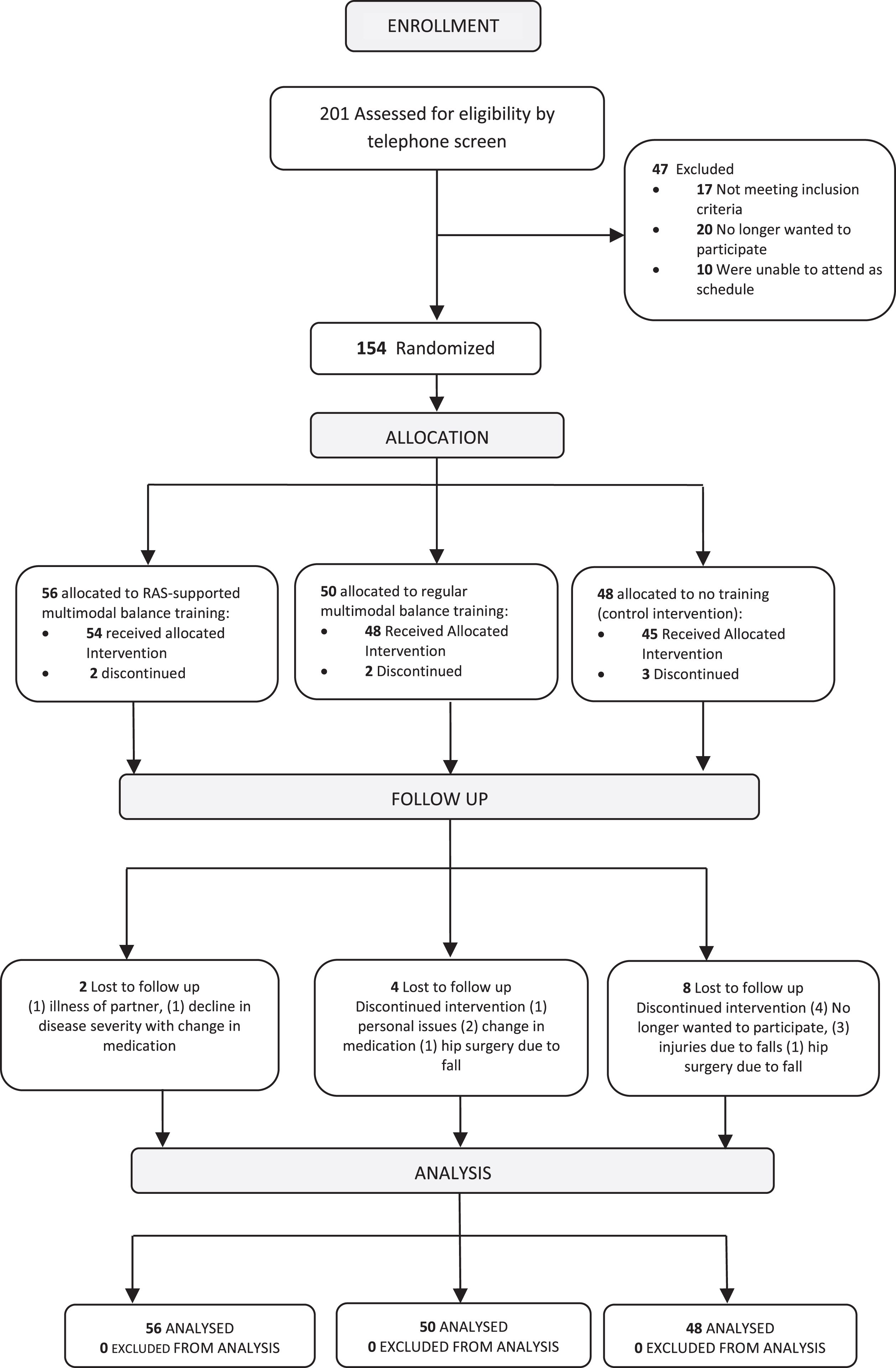
Table 1
Participants’characteristics at the baseline visit
| RAS-supported N = 56 | Regular N = 50 | Control N = 48 | |
| Age, years (mean (SD)) | 74 (8) | 67 (13) | 73 (10) |
| Gender, men(N (%)) | 27 (48%) | 32 (64%) | 29 (60%) |
| H&Y (N(%)), I | 11 (20%) | 11 (22%) | 7 (14%) |
| II | 17 (30%) | 16 (32%) | 18 (38%) |
| III | 28 (50%) | 23 (46%) | 23 (48%) |
| Time since first symptoms (median (IQR)) | 7 (4–13) | 8 (4–14) | 11 (4–20) |
| Disease duration (median (IQR)) | 5 (2–9) | 6 (2–10) | 8 (2–15) |
| Deep Brain Stimulation (N (%)) | 6 (10%) | 7 (14%) | 4 (8%) |
| Freezers of gait (N, %) | 17 (30%) | 15 (30%) | 16 (13%) |
| Recurrent Fallers (N, %) | 25 (45%) | 21 (38%) | 20 (36%) |
| Injuries before protocol (N, %) | 26 (46%) | 23 (46%) | 20 (42%) |
| LEDD, mg/day (mean (SD)) | 615 (424) | 701 (466) | 698 (389) |
| MMSE,score (mean (SD)) | 27 (2) | 26 (2) | 25 (2) |
| MoCA, score (mean (SD)) | 26 (3) | 25 (3) | 24 (3) |
| UPDRS 2, ADL score (mean (SD)) (ON) | 11 (7) | 14 (7) | 16 (8) |
| UPDRS 3, motor score (mean (SD)) (ON) | 15 (7) | 17 (9) | 19 (7) |
| Years of education (N, %) 2–8 (primary school) | 10 (18%) | 11 (22%) | 8 (17%) |
| 9–14 (secondary School) | 16 (29%) | 21 (42%) | 17 (35%) |
| >14 | 30 (53%) | 18 (36%) | 23 (48%) |
Group: Multimodal balance training supported by rhythmical auditory stimuli (RAS-supported), Multimodal balance training without rhythmical auditory stimuli (Regular), Control Intervention group (Control). N, number of participants; SD, Standard Deviation; IQR, Interquartile range are presented; ON (ON-medication); LEDD, levodopa equivalent daily dose; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment; H&Y, Hoehn & Yahr stage; UPDRS 2, ADL, Activities of daily living score; UPDRS 3, motor score.
Primary outcome
Figure 2A shows the results of our primary outcome, the MBEST. Immediately post treatment, both the RAS-supported multimodal training group and regular multimodal training group had improved significantly on the MBEST score compared to controls (RAS-supported multimodal balance training 6.6 (95% Confidence Interval (CI) 5.2; 8.0)), p < 0.001; regular multimodal balance training 3.0 (CI 2.7;5.3), p < 0.001). Moreover, the RAS-supported multimodal balance training group improved significantly more than the regular training group (RAS-supported multimodal balance training difference 3.5 (95% CI 2.2; 4.8)), p < 0.001. These improvements were retained at one-month follow-up for both groups, but only the RAS-supported multimodal balance training group maintained its improvement at 6-month follow-up (Table 2A).
Fig.2
(A) Mini-BESTest (MBEST) at each test visit. The red line represents RAS-supported multimodal balance training, the black line represents regular multimodal balance training and the gray line represents the control intervention group. Error bars represent the 95% confidence intervals. **all groups are significantly different, *except regular multimodal balance training vs control. (B) TUG (Timed Up and Go Test) at each test visit. The red line represents RAS-supported multimodal balance training, the black line represents regular multimodal balance training and the gray line represents the control intervention group. Error bars represent the 95% confidence intervals.
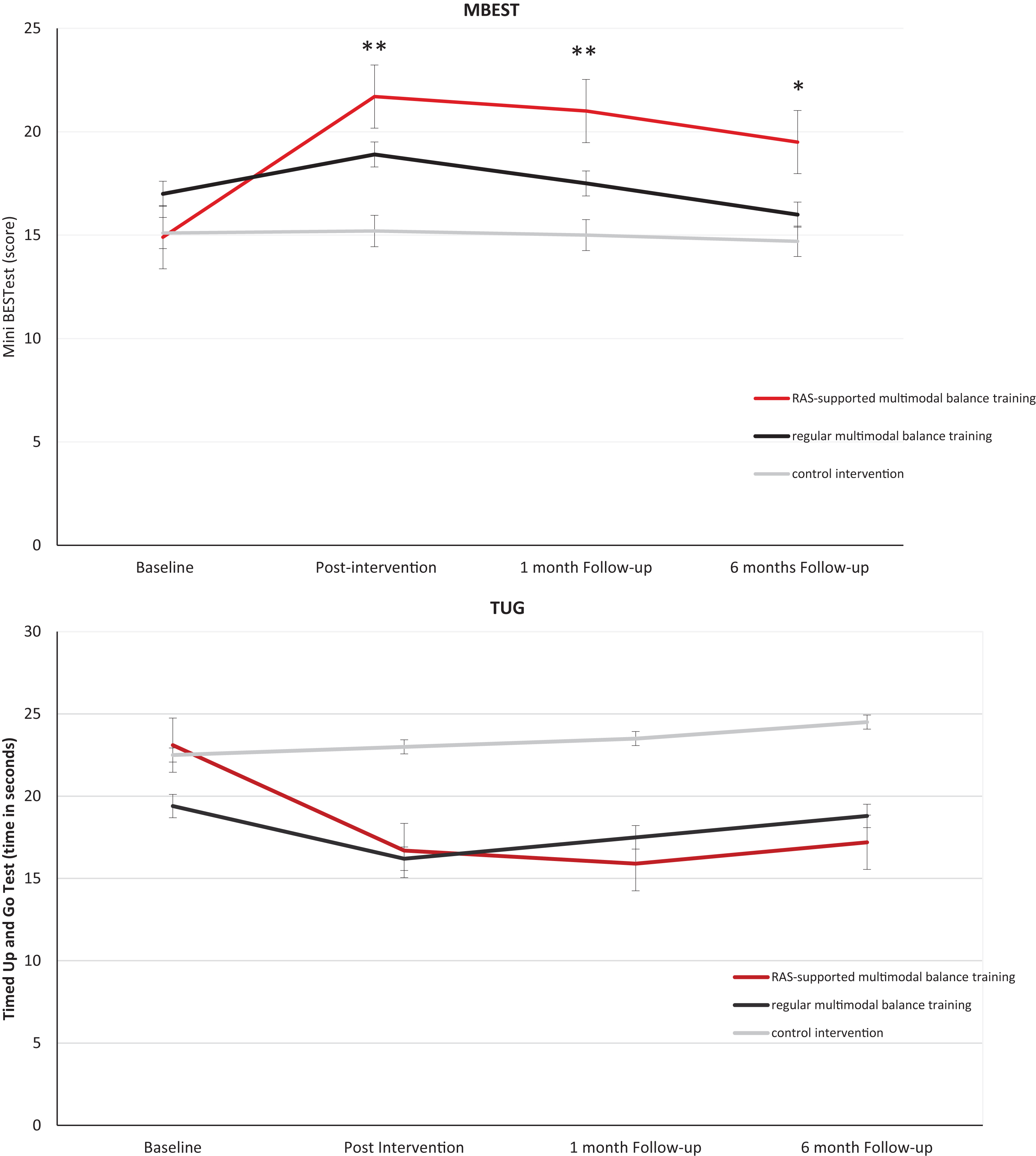
Table 2A
Observed mean values and estimated differences and 95% confidence intervals for balance primary and secondary outcome measures
| RAS-supported | Regular | Control | RAS-supported vs Regular | RAS-supported vs Control | Regular vs Control | ||
| 1 | 2 | 3 | 1-2 | 1-3 | 2-3 | ||
| Mini BESTest | Baseline | 14.8 (12.9; 16.6) | 17.3 (14.9; 19.7) | 15.1 (12.4; 17.7) | |||
| Post Intervention | 21.9 (21.0; 22.7) | 18.3 (17.3; 19.2) | 15.2 (14.2; 16.3) | 3.5 (2.2; 4.8), P < 0.001 | 6.6 (5.2; 8.0), P < 0.001 | 3.0 (2.7;5.3), P < 0.001 | |
| 1 month Follow-up | 21.1 (20.2; 22.0) | 17.0 (16.1; 18.0) | 15.0 (13.9; 16.0) | 4.0 (2.7; 5.3), P < 0.001 | 6.0 (4.7; 7.4) P < 0.001 | 2.0 (0.6; 3.4), P = 0.005 | |
| 6 month Follow-up | 19.6 (18.7; 20.4) | 15.8 (14.8; 16.7) | 14.7 (13.2; 15.7) | 3.7 (2.4; 5.1), P < 0.001 | 4.8 (3.4; 6.2) P < 0.001 | 1.0 (–0.3;2.4), P = 0.136 | |
| Berg Balance Scale | Baseline | 38.9 (35.6; 42.3) | 40.9 (37.0; 44.9) | 37.5 (33.0; 41.9) | |||
| Post Intervention | 47.0 (44.7; 48.4) | 41.7 (40.3; 43.2) | 38.3 (36.7; 39.92) | 5.2 (3.2; 7.3), P < 0.001 | 8.6 (6.; 10.8), P < 0.001 | 3.4 (1.2; 5.6), P = 0.002 | |
| 1 month Follow-up | 45.6 (44.2; 46.9) | 40.3 (38.8; 41.8) | 37.8 (36.1; 39.4) | 5.2 (3.2; 7.3), P < 0.001 | 7.8 (5.6; 9.4), P < 0.001 | 2.5 (0.3; 4.7), P = 0.024 | |
| 6 month Follow-up | 43.1 (41.7; 44.5) | 38.9 (37.5; 40.4) | 37.1 (35.5; 38.7) | 4.1 (2.1; 6.2), P < 0.001 | 6.0 (3.8; 8.1), P < 0.001 | 1.8 (–0.3; 4.0), P = 0.098 | |
| Retropulsion Test | Baseline | 2.3 (1.9; 2.7) | 2.1 (1.7; 2.4) | 2.4 (2.3; 2.8) | |||
| Post Intervention | 1.0 (0.9; 1.2) | 1.3 (1.0; 1.5) | 2.2 (2.0; 2.4) | –0.2 (–0.5; 0.0), P = 0.164 | –1.1 (–1.4; –0.8), P < 0.001 | –0.9 (–1.3; –0.6), P < 0.001 | |
| 1 month Follow-up | 1.2 (1.0; 1.4) | 1.8 (1.6; 2.1) | 2.3 (2.1; 2.6) | –0.6 (–0.9; –0.3), P < 0.001 | –1.1 (–1.4; –0.8), P < 0.001 | –0.5 (–0.8; –0.1), P = 0.002 | |
| 6 month Follow-up | 1.7 (1.5; 1.9) | 2.1 (1.9; 2.3) | 2.4 (2.1; 2.6) | –0.3 (–0.6; –0.0), P = 0.011 | –0.6 (–0.9; –0.3), P < 0.001 | –0.3 (–0.6; 0.0), P = 0.070 | |
| Push and Release Test | Baseline | 2.4 (2.0; 2.7) | 2.1 (1.8; 2.5) | 2.4 (2.0; 2.8) | |||
| Post Intervention | 1.1 (0.9; 1.2) | 1.4 (1.2; 1.5) | 2.3 (2.1; 2.5) | –0.3 (–0.5; –0.4), P = 0.020 | –1.2 (–1.4; –0.9), P < 0.001 | –0.9 (–1.1; –0,6), P < 0.001 | |
| 1 month Follow-up | 1.4 (1.2; 1.5) | 2.0 (1.8; 2.1) | 2.4 (2.2; 2.6) | –0.6 (–0.8; –0.3), P < 0.001 | –1.0 (–1.2; –0.7), P < 0.001 | –0.4 (–0.7; –0.1), P = 0.001 | |
| 6 month Follow-up | 1.9 (1.7; 2.0) | 2.1 (2.0; 2.4) | 2.5 (2.3; 2.7) | –0.3 (–0.6; –0.1), P = 0.003 | –0.6 (–0.9; –0.3), P < 0.001 | –0.2 (–0.5; 0.0), P = 0.049 | |
| Falls Efficacy Scale - International | Baseline | 31.5 (26.8; 36.2) | 34.3 (30.0; 38.4) | 36.2 (31.0; 41.4) | |||
| Post Intervention | 27.8 (22.0; 29.3) | 31.4 (29.9; 32.9) | 36.2 (31.9; 35.3) | –3.6 (–5.7; –1.5), P = 0.001 | –5.7 (–8.0; –3.5), P < 0.001 | –2.1 (–4.4; –0.1), P = 0.062 | |
| 1 month Follow-up | 27.8 (22.1; 29.3) | 32.9 (31.4; 34.4) | 36.5 (32.4; 35.6) | –5.0 (–7.1; –2.9), P < 0.001 | –6.0 (–8.3; –3.8), P < 0.001 | –0.9 (–3.2; 1.3), P = 0.394 | |
| 6 month Follow-up | 30.0 (23.9; 31.7) | 33.6 (32.1; 35.2) | 38.7 (33.8; 37.2) | –3.6 (–5.7; –1.5), P < 0.001 | –5.5 (–7.7; –3.2), P < 0.001 | –1.8 (–4.1; –0.4), P = 0.113 |
Group: Multimodal balance training supported by rhythmical auditory stimuli (RAS-supported), Multimodal balance training without rhythmical auditory stimuli (Regular), Control Intervention group (Control). Confidence Intervals (CI) – Adjusted Mean Difference (95% CI) Between Baseline and 35-week). Primary analysis: Adjusted for baseline MBEST score; Secondary analyses: also adjusted for baseline LEDD, baseline UPDRS 2 and 3. Mini BESTest – 14 items, total 28 of points, scored 0–2 (higher score better balance). Berg Balance Scale - 14 items total 56 of points, scored 0–4 points (higher score better balance). Retropulsion test and Push-and-release test scored between 1–4 points, lower score means better balance. Falls Efficacy Scale International – 16 items, total 64 of points scored), higher scores greater fear of fallen.
Secondary outcomes
Balance
Similar effects were found for the secondary outcomes (Table 2A). When compared to controls, both the RAS-supported group and regular group improved immediately post-intervention on the BBS (RAS-supported vs control p < 0.001; regular vs control p = 0.002), retropulsion test (RAS-supported vs control p < 0.001; regular vs control p < 0.001), push-and-release test (multimodal vs control p < 0.001; standard vs control p < 0.001). Only RAS-supported group improved immediately post-intervention on FES-I when compared with the controls (RAS-supported vs control p < 0.001; regular vs control p = 0.062). The RAS-supported group improved significantly more than the regular group these secondary outcomes (BBS p < 0.001; push and release test p = 0.002; FES-I p = 0.001) except on retropulsion test (RAS-supported vs regular p = 0.164) (Table 2A). Improvements were retained at one-month follow-up (Table 2A), but only the RAS-supported multimodal training group maintained their improvement at 6-month follow-up. Fewer severe injuries outside the training were reported after the intervention by all groups.
Gait
Immediately post-intervention, both the RAS-supported multimodal balance training and regular multimodal balance training group showed a larger improvement on the TUG (Fig. 2B), TUG Dual Task, N-FOGQ, and rapid turns test than controls (Table 2B). No differences were found between both active interventions. The results were maintained at one month and 6 months follow-up in both groups. Except for the rapid turn test, for which the RAS-supported multimodal balance training showed better results than the regular multimodal training group and control intervention only in post-intervention (Table 2B). For the TUG Dual Task the RAS-supported multimodal balance training showed better results than the regular multimodal training group at one-month and 6-month follow-up (Table 2B).
Table 2B
Observed mean values and estimated differences and 95% confidence intervals for secondary outcome measures
| RAS-supported | Regular | Control | RAS-supported vs Regular | RAS-supported vs Control | Regular vs Control | ||
| 1 | 2 | 3 | 1-2 | 1-3 | 2-3 | ||
| Timed Up and Go | Baseline | 23.6 (19.2; 27.9) | 19.4 (14.8; 23.2) | 22.5 (17.3; 27.7) | |||
| Post Intervention | 16.7 (14.6; 18.5) | 16.2 (14.1; 18.3) | 23.0 (20.7; 25.3) | –0.3 (–2.5; 3.2); P = 0.820 | –6.4(–9.5; –3.3); P < 0.001 | –6.7 (–9.9; –3.6), P < 0.001 | |
| 1 month Follow-up | 15.7 (13.3; 17.6) | 17.7 (15.5; 19.7) | 23.5 (21.2; 25.8) | –1.9 (–4.8; 0.9); P = 0.189 | –7.8 (–10.9; –4.7); P < 0.001 | –5.8 (–9.0; –2.7), P < 0.001 | |
| 6 month Follow-up | 17.2 (15.2; 19.1) | 18.8 (16.7; 20.9) | 24.8 (22.4; 27.1) | –1.6 (–4.5; 1.2); P = 0.273 | –7.6 (–10.9; –4.5); P < 0.001 | –6.0 (–9.1; –4.5), P < 0.001 | |
| Timed up and Go Dual Task | Baseline | 28.3 (23.1; 33.4) | 25.0 (20.6; 30.6) | 31.6 (24.0; 39.1) | |||
| Post Intervention | 21.4 (19.3; 23.6) | 22.9 (20.6; 25.2) | 30.2 (27.6; 32.8) | –1.4 (–4.6; 1.7), P = 0.360 | –8.8 (–12.2; –5.3), P < 0.001 | –7.3 (–10.8; –3.6), P < 0.001 | |
| 1 month Follow-up | 20.6 (18.4; 22.7) | 24.2 (21.1; 26.5) | 30.8 (28.2; 33.4) | –3.6 (–6.8; –0.4), P = 0.027 | –10.1 (–13.6; –6.7), P < 0.001 | –6.5 (–10.0; –3.0), P = 0.001 | |
| 6 month Follow-up | 21.5 (19.4; 23.7) | 27.4 (25.0; 29.7) | 31.3 (28.7; 33.9) | –5.8 (–9.0; –2.6), P < 0.001 | –9.7 (–13.1; –6.3), P < 0.001 | –3.9 (–7.4; –0.4), P = 0.028 | |
| New Freezing of Gait Questionnaire | Baseline | 5.7 (3.6; 7.8) | 8.3 (4.8; 11.8) | 8.3 (5.5; 11.1) | |||
| Post Intervention | 7.1 (6.0; 8.2) | 6.6 (5.5; 7.8) | 8.7 (7.3; 10.0) | –0.4 (–1.1; 2.0); P = 0.594 | –1.5 (–3.3; –0.1), P < 0.076 | –2.0 (–3.8; –0.2), P = 0.026 | |
| 1 month Follow-up | 7.2 (6.0;8.3) | 7.0 (5.8; 8.2) | 9.0 (7.7; 10.4) | –0.1 (–1.4; 1.7); P = 0.831 | –1.8 (–3.6; –0.1), P < 0.036 | –2.0 (–3.8; 0.7), P = 0.024 | |
| 6 month Follow-up | 8.0 (6.9; 9.1) | 7.1 (5.9; 8.3) | 9.1 (7.7; 10.4) | 0.8 (–0.7; 2.4); P = 0.299 | –1.0 (–2.8; –0.6), P = 0.223 | –1.9 (–3.7; –0.1), P = 0.033 | |
| Rapid Turns test | Baseline | 0.4 (0.3; 0.5) | 0.3 (0.2; 0.5) | 0.5 (0.3; 0.7) | |||
| Post Intervention | 0.1 (0.0; 0.2) | 0.3 (0.2; 0.4) | 0.3 (0.2;0.4) | –0.2 (–0.3; –0.1), P < 0.001 | –0.2 (–0.3; –0.0), P < 0.001 | 0.0 (–0.1; 0.1), P = 0.883 | |
| 1 month Follow-up | 0.3 (0.2; 0.4) | 0.3 (0.3; 0.4) | 0.4 (0.3; 0.5) | –0.0 (–0.1; 0.0), P = 0.433 | –0.0 (–0.2; 0.0), P = 0.135 | –0.0 (–0.1;0.0), P = 0.458 | |
| 6 month Follow-up | 0.4 (0.3; 0.4) | 0.4 (0.2; 0.5) | 0.4 (0.3; 0.5) | 0.0 (–0.0; 0.1), P = 0.756 | –0.0 (–0.1; 0.1), P = 0.750 | –0.0 (–0.1; 0.0), P = 0.089 | |
| LEDD | Baseline | 680 (545;815) | 700 (556; 833) | 761 (633; 909) | |||
| Post Intervention | 695 (678; 712) | 704 (686;722) | 739 (699; 739) | –9.0 (–33.7; 15.7), P = 0.474 | –24.0 (–50.1; 2.1), P = 0.072 | –15.0 (–50.1; 2.1), P = 0.072 | |
| 1 month Follow-up | 699 (683; 716) | 710 (692; 728) | 765 (745; 785) | –10.9 (–35.6; 13.8), P = 0.385 | –65.4 (–91.6; –39.2), P < 0.001 | –54.5 (–81.1; –27.8), P < 0.001 | |
| 6 month Follow-up | 715 (699; 772) | 743 (726; 761) | 821 (801; 841) | –28.1 (–52.9; –3.4), P = 0.026 | –106.0 (–132.1; –79.8), P < 0.001 | –77.8 (–104.4; –51.2), P < 0.001 | |
| UPDRS 2 | Baseline | 12.6 (10.4; 14.8) | 14.5 (12.3; 16.8) | 17.0 (14.2; 19.7) | |||
| Post Intervention | 12.7 (12.3; 13.1) | 13.4 (13.0; 13.8) | 14.6 (14.2; 15.1) | –0.6 (–1.2; –0.0), P = 0.024 | –1.9 (–2.5; –1.2), P < 0.001 | –1.2 (–1.8; –0.6), P < 0.001 | |
| 1 month Follow-up | 13.1 (12.7; 13.5) | 14.0 (13.6; 13.8) | 14.8 (14.3; 15.3) | –0.9 (–1.5; –0.3), P = 0.003 | –1.7 (–2.3; –1.0), P < 0.001 | –0.8 (–1.4; –0.1), P = 0.013 | |
| 6 month Follow-up | 13.7 (13.5; 14.1) | 14.4 (13.9; 14.8) | 15.0 (14.5; 15.5) | –0.6 (–1.2; –0.0), P = 0.030 | –1.3 (–1.9; –0.6), P < 0.001 | –0.6 (–1.2; 0.0), P = 0.045 | |
| UPDRS 3 | Baseline | 15.2 (13.1; 17.0) | 17.4 (14.8; 20.1) | 18.9 (16.6; 21.2) | |||
| Post Intervention | 14.2 (13.7; 14.7) | 16.1 (15.5; 16.6) | 17.0 (16.4; 17.6) | –1.9 (–2.6; –1.1), P < 0.001 | –2.8 (–3.6; –2.0), P < 0.001 | –0.9 (–1.7; –0.1), P = 0.018 | |
| 1 month Follow up | 14.7 (14.2; 15.2) | 16.7 (16.2; 17.3) | 16.8 (16.2; 17.5) | –2.0 (–2.7; –1.2), P < 0.001 | –2.0 (–2.9; –1.3), P < 0.001 | –0.0 (–0.8; 0.7), P = 0.846 | |
| 6 month Follow up | 15.5 (14.9; 15.9) | 17.0 (16.5; 17.6) | 17.1 (16.6; 17.7) | –1.6 (–2.3; –0.8), P < 0.001 | –1.7 (–2.4; –0.9), P < 0.001 | –0.1 (–0.9; 0.6), P = 0.773 |
Group: Multimodal balance training supported by rhythmical auditory stimuli (RAS-supported), Multimodal balance training without rhythmical auditory stimuli (Regular), Control Intervention group (Control). Confidence Intervals (CI) – Adjusted Mean Difference (95% CI) Between Baseline and 35-week). Primary analysis: Adjusted for baseline MBEST score; Secondary analyses: also adjusted for baseline LEDD, baseline UPDRS 2 and 3. TUG and TUGDT were measured in time in seconds (time in seconds is the unit) with a range from 5–60 seconds (which is the range). A lower score means better mobility. N-FOGQ - 10 items, total 29 of points, scored 0–3 or 4. A lower score, less freezing problems. Rapid turns test was scoring 0 - no freezing and 1 - freezing. UPDRS 2–13 items, 52 of points, scored 0–4. A lower scores means better capacity in activities of daily living. UPDRS 3–18 items a total 108 points, scored 0–4. Lower scores means better motor performance.
UPDRS
Both the RAS-supported multimodal balance training group and regular multimodal training group improved on the UPDRS part 2 and part 3 immediately post-intervention (Table 2B). The RAS-supported multimodal balance training group showed a significantly larger improvement than the regular multimodal training group on both outcomes. The results on the UPDRS part 2 were retained at one and 6-month follow-up in both groups. The results on the UPDRS part 3 were retained at one and 6-month follow-up for the RAS-supported multimodal balance training group (Table 2B).
Levodopa equivalent daily dose (LEDD)
The LEDD was not significantly different between groups for either of the follow-up moments post-intervention (Table 2B).
DISCUSSION
Here, we present the results of an RCT investigating the long-term effects of two balance training interventions in a large group of early to mid-stage PD patients. Both training groups improved balance performance, as measured using the MBEST. Compliance in both training groups was good. Adding auditory rhythmical cues (RAS-supported multimodal balance training) to the balance training was more effective than balance training without these cues (regular multimodal balance training). Only the RAS-supported multimodal balance training group retained the effect at long-term follow-up (6 month).
Our study reports two important new findings. First, we show that multimodal balance training using simultaneously applied rhythmical cues is more effective than multimodal balance training without cueing. We suspect that RAS-supported multimodal balance training is more effective than regular multimodal balance training because this stimulates residual motor-cognitive abilities in PD more effectively than regular multimodal balance training [14]. Specifically, RAS-supported multimodal balance training may improve attention and task prioritization (executive control) [34], thereby facilitating the selection of efficient balance compensatory strategies and enhancing the training effects.
A second important finding is that the training effects (particularly those of RAS-supported multimodal balance training) are maintained up to 6-months follow-up.
‘The improvements on the MBEST-scores were not only significant, but also exceeded the previously found standard error for a test-retest measurement (3 points) [50]. Moreover, two studies have examined the responsiveness of the Mini-BESTest. Tsang et al. [51] concluded that the minimal detectable change score is 3.0 for individuals with stroke. In a mixed population of people with balance impairments involving 25 PD patients, Godi et al. [52] concluded that the minimally important change score is 4.0 points, and we therefore expect our results to be clinically relevant. Although both studies were performed in non-PD populations, the effect size observed by us was in the range of what was considered minimally important there, and we therefore expect our results to also be clinically relevant for PD patients. However, comparable trials using the Mini-BESTest are sparse and data on the minimal clinically important difference (MCID) in PD is lacking. The only study that can be compared with ours was that by Conradson et al., 2015 [53], who found a clinical change of 3 points on the MBEST.
This MBEST improvement following RAS-supported multimodal balance training is consistent with similar improvements that we observed for all secondary balance outcomes. Earlier studies also found that physiotherapy improves balance in PD patients [18, 25, 54, 55]. Our results showed larger effect sizes on MBEST than other high challenging physiotherapy interventions [25, 53]. Possibly, this large effect might be ascribed to the addition of rhythmical auditory cues to the functional balance and gait exercises. Indeed, such large effects were not observed in the regular multimodal balance training group, where rhythmical auditory cues were not used. One possible explanation for this might be that we included patients who had never received specialized physicaltherapy before. It is likely that the earlier studies [25, 53]—which had been performed in the western world—included patients who had received physiotherapy before. We suspect that previously untreated patients might show greater improvements, perhaps because expectations and placebo effects contributed, but also because of the younger age of these patients, and perhaps also their milder disease stage (we included some patients with HY stage 1) in whom the physiologic reserve is greater and learning processes are better preserved, rendering patients more amenable to interventions.
The observed results cannot be explained by a differential increase in dopaminergic medication across the intervention groups. In fact, the control intervention group reported a significantly higher dose of dopaminergic medication during follow-up, yet their performance on functional tests was statistically worse that the groups receiving an active intervention. These findings suggest that the beneficial effects following the interventions resulted from the training itself.
In addition to balance, gait also improved with both trainings immediately after the intervention. This effect was retained at follow-up. However, the clinically relevant findings reported for the TUG only apply to the change between baseline and post-intervention, not for the follow-up periods. We found no differences between RAS-supported multimodal balance training and regular multimodal balance training. This can likely be explained by the fact that visual cueing was applied during gait training in both groups. We chose to apply visual cues during gait training in both groups, as this is usual care for gait training [9]. Our results indicate that addition of rhythmical auditory cueing does not have an additional effect over and above that afforded by the visual cues. The effects are clinically relevant, as the observed improvements for, e.g., the TUG-test exceeded the minimally clinically important difference of 3.5 seconds [56]. The long-term effects are promising, in contrast to the earlier, in contrast to the earlier Rehabilitation in Parkinson’s Disease: Strategies for Cueing (RESCUE) trial, where rhythmical auditory stimuli provided no long-term benefits on gait and gait-related activity [15]. We are not sure why our results are so different of RESCUE trial. Perhaps dosing (dose-response) contributed to these discrepant findings, as the treatment ‘dose’ was considerably higher in the present study (450 minutes) compared to the RESCUE trial (270 minutes). Recent work emphasized that dose can certainly affect the treatment response in Parkinson patients, at least when it comes to aerobic exercise [57]. Another strength of our trial was the structured training as delivered by skilled trained physiotherapists delivered in a clinical setting, whereas the RECUE intervention was performed at home. While training at home is convenient and reduces travel burden, dosing can be less well monitored during a home-based training [58]. The long-term effect of physiotherapy training with auditory cueing on the TUG has been reported before in a small group of 8 patients with a shorter follow-up (8-weeks) [59]; our study extends these findings. The auditory stimulus may even have guided attention away from, instead of towards, the movement (forcing implicit motor learning). However, it is plausible that the internal and external cueing strategies used in this study, likely had enabled patients to switch from automatic motor control to goal-directed motor control [60]. We hypothesize that the distinctions between fully automatic motor control and more goal-directed motor control are not strict [61], but that there is in fact a continuum of relatively more automatic motor control on the one hand (e.g., when the patient is performing gait or maintaining posture), and relatively more goal-directed motor control on the other hand (e.g., when subjects must perform the exercises focusing attention on the auditory cues). This might explain the long-term retention effects on RAS-supported group. Originally, we planned see only patients in H&Y stage 2 and 3. However, patients with H&Y1 stage were also included (it is not well-known which disease stages respond best to treatment), and the intervention appeared to be effective even for this now overall relatively mildly affected patient group. Future work could consider examining the effects of cued balanced training for separate subgroups.
Our study has several limitations. First, we studied patients with mild to moderate PD and without cognitive impairments. It therefore remains unclear whether the results are generalizable to those with later stage disease or those with cognitive impairment [62]. Second, there were more drop-outs among controls, and this could be a source of bias (attrition bias), especially because participants knew their group assignment. Third, activity monitors were not employed to determine the overall level of activity both during and after the intervention, which could have contributed to the differences between groups. Finally, we cannot make any statements about possible effects when patients are OFF medication. We purposely tested all patients while ON-medication, as this would best reflect the effect of the intervention over and above standard medical management in daily clinical practice. Also, dopaminergic medication usually does little to improve balance, [63] so we expect that testing patients in an OFF phase would not make a big difference. However, future studies should examine the effects of RAS-supported multimodal balance training on balance in patients tested while OFF-medication.
Taken together, our findings further support the importance of non-pharmacological intervention in the clinical management of balance and gait problems in patients with PD. Current physiotherapy guidelines [9, 64] provide no recommendations on how to apply balance training. The present results, indicating that RAS-supported multimodal balance training is more effective and longer lasting than routine training, help to fill this gap and contribute to an increasing evidence base for specialized physiotherapy, eventually leading to optimized care for patients with PD.
CONFLICT OF INTEREST
T.T.C. Capato, E.R. Barbosa, J. InHout, and J. Nonnekes: None; N.M. Vries. received a research grant by The Netherlands Organisation for Health Research and Development; B.R. Bloem received grant funding from the Netherlands Organization for Scientific Research, Michael J Fox Foundation, Parkinson Vereniging, Parkinson’s Foundation, Gatsby Foundation, Verily Life Sciences, Horizon 2020, Topsector Life Sciences and Health, Stichting Parkinson Fonds, AbbVie. Consultancy from Biogen, Abbvie, Walk with Path, UCB. Speaker fees from AbbVie, Zambon, Bial.
ACKNOWLEDGMENTS
The Radboudumc Center of Expertise for Parkinson & Movement Disorders was supported by a Center of Excellence grant of the Parkinson’s Foundation.
We would like to thank the reviewers for their very careful and constructive appraisal of earlier versions of this manuscript. We thank Dr Joanna IntHout of the department of statistics of Radboudumc for her expert advice on performing all statistical analyses. We also thank to colleagues from University of São Paulo for the support to this study: Juliana Tornai, Patrícia Ávila, Amanda Ferreira, Izabel Kayo, Rúbia Rodrigues, Maria Elisa Piemonte, Renata Guimarães, Rayssa Firmino, Vivian Gianguiagi, Thalita Gabriele and Kátia Kawai.
SUPPLEMENTARY MATERIAL
[1] The Supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-191752.
REFERENCES
[1] | Bloem BR , Hausdorff JM , Visser JE , Giladi N ((2004) ) Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov Disord 19: , 871–884. |
[2] | Kalia LV , Lang AE ((2015) ) Parkinson’s disease. Lancet 386: , 896–912. |
[3] | Ellis TD , Cavanaugh JT , Earhart GM , Ford MP , Foreman KB , Thackeray A , Thiese MS , Dibble LE ((2016) ) Identifying clinical measures that most accurately reflect the progression of disability in Parkinson disease. Parkinsonism Relat Disord 25: , 65–71. |
[4] | van der Marck MA , Klok MP , Okun MS , Giladi N , Munneke M , Bloem BR ((2014) ) Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson’s disease. Parkinsonism Relat Disord 20: , 360–369. |
[5] | Curtze C , Nutt JG , Carlson-Kuhta P , Mancini M , Horak FB ((2015) ) Levodopa is a double-edged sword for balance and gait in people with Parkinson’s disease. Mov Disord 30: , 1361–1370. |
[6] | Connolly BS , Lang AE ((2014) ) Pharmacological treatment of Parkinson disease: A review. JAMA 311: , 1670–1683. |
[7] | Ginis P , Heremans E , Ferrari A , Bekkers EMJ , Canning CG , Nieuwboer A ((2017) ) External input for gait in people with Parkinson’s disease with and without freezing of gait: One size does not fit all. J Neurol 264: , 1488–1496. |
[8] | Rochester L , Baker K , Nieuwboer A , Burn D ((2011) ) Targeting dopa-sensitive and dopa-resistant gait dysfunction in Parkinson’s disease: Selective responses to internal and external cues. Mov Disord 26: , 430–435. |
[9] | Keus S , Munneke M , Graziano M , Paltamaa J , Pelosin E , Domingos J , Brühlmann S , Ramaswamy B , Prins J , Struiksma C ((2014) ) European physiotherapy guideline for Parkinson’s disease. KNGF/ParkinsonNet. |
[10] | Tomlinson CL , Patel S , Meek C , Herd CP , Clarke CE , Stowe R , Shah L , Sackley C , Deane KH , Wheatley K , Ives N ((2012) ) Physiotherapy intervention in Parkinson’s disease: Systematic review and meta-analysis. BMJ 345: , e5004. |
[11] | Smania N , Corato E , Tinazzi M , Stanzani C , Fiaschi A , Girardi P , Gandolfi M ((2010) ) Effect of balance training on postural instability in patients with idiopathic Parkinson’s disease. Neurorehabil Neural Repair 24: , 826–834. |
[12] | Fox SH , Katzenschlager R , Lim SY , Barton B , de Bie RMA , Seppi K , Coelho M , Sampaio C ((2018) ) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33: , 1248–1266. |
[13] | Strouwen C , Molenaar E , Munks L , Keus SHJ , Zijlmans JCM , Vandenberghe W , Bloem BR , Nieuwboer A ((2017) ) Training dual tasks together or apart in Parkinson’s disease: Results from the DUALITY trial. Mov Disord 32: , 1201–1210. |
[14] | Rochester L , Baker K , Hetherington V , Jones D , Willems AM , Kwakkel G , Van Wegen E , Lim I , Nieuwboer A ((2010) ) Evidence for motor learning in Parkinson’s disease: Acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res 1319: , 103–111. |
[15] | Morris ME , Iansek R , Kirkwood B ((2009) ) A randomized controlled trial of movement strategies compared with exercise for people with Parkinson’s disease. Mov Disord 24: , 64–71. |
[16] | Nieuwboer A , Kwakkel G , Rochester L , Jones D , van Wegen E , Willems AM , Chavret F , Hetherington V , Baker K , Lim I ((2007) ) Cueing training in the home improves gait-related mobility in Parkinson’s disease: The RESCUE trial. J Neurol Neurosurg Psychiatry 78: , 134–140. |
[17] | Ginis P , Nackaerts E , Nieuwboer A , Heremans E ((2018) ) Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives. Ann Phys Rehabil Med 61: , 407–413. |
[18] | Ginis P , Nieuwboer A , Dorfman M , Ferrari A , Gazit E , Canning CG , Rocchi L , Chiari L , Hausdorff JM , Mirelman A ((2016) ) Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: A pilot randomized controlled trial. Parkinsonism Relat Disord 22: , 28–34. |
[19] | Frazzitta G , Maestri R , Bertotti G , Riboldazzi G , Boveri N , Perini M , Uccellini D , Turla M , Comi C , Pezzoli G , Ghilardi MF ((2015) ) Intensive rehabilitation treatment in early Parkinson’s disease: A randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair 29: , 123–131. |
[20] | Morris ME , Menz HB , McGinley JL , Watts JJ , Huxham FE , Murphy AT , Danoudis ME , Iansek R ((2015) ) A randomized controlled trial to reduce falls in people with Parkinson’s disease. Neurorehabil Neural Repair 29: , 777–785. |
[21] | Shen X , Mak MK ((2015) ) Technology-assisted balance and gait training reduces falls in patients with Parkinson’s disease: A randomized controlled trial with 12-month follow-up. Neurorehabil Neural Repair 29: , 103–111. |
[22] | Schlenstedt C , Paschen S , Kruse A , Raethjen J , Weisser B , Deuschl G ((2015) ) Resistance versus balance training to improve postural control in Parkinson’s disease: A randomized rater blinded controlled study. PLoS One 10: , e0140584. |
[23] | Santos SM , da Silva RA , Terra MB , Almeida IA , de Melo LB , Ferraz HB ((2017) ) Balance versus resistance training on postural control in patients with Parkinson’s disease: A randomized controlled trial. Eur J Phys Rehabil Med 53: , 173–183. |
[24] | Mak MK , Wong-Yu IS , Shen X , Chung CL ((2017) ) Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol 13: , 689–703. |
[25] | Sparrow D , DeAngelis TR , Hendron K , Thomas CA , Saint-Hilaire M , Ellis T ((2016) ) Highly challenging balance program reduces fall rate in Parkinson disease. J Neurol Phys Ther 40: , 24–30. |
[26] | Goodwin VA , Richards SH , Taylor RS , Taylor AH , Campbell JL ((2008) ) The effectiveness of exercise interventions for people with Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 23: , 631–640. |
[27] | Wallen MB , Hagstromer M , Conradsson D , Sorjonen K , Franzen E ((2018) ) Long-term effects of highly challenging balance training in Parkinson’s disease-a randomized controlled trial. Clin Rehabil 32: , 1520–1529. |
[28] | Canning CG , Sherrington C , Lord SR , Close JC , Heritier S , Heller GZ , Howard K , Allen NE , Latt MD , Murray SM , O’Rourke SD , Paul SS , Song J , Fung VS ((2015) ) Exercise for falls prevention in Parkinson disease: A randomized controlled trial. Neurology 84: , 304–312. |
[29] | Paul SS , Dibble LE , Peterson DS ((2018) ) Motor learning in people with Parkinson’s disease: Implications for fall prevention across the disease spectrum. Gait Posture 61: , 311–319. |
[30] | Ferrazzoli D , Ortelli P , Madeo G , Giladi N , Petzinger GM , Frazzitta G ((2018) ) Basal ganglia and beyond: The interplay between motor and cognitive aspects in Parkinson’s disease rehabilitation. Neurosci Biobehav Rev 90: , 294–308. |
[31] | Yarnall A , Rochester L , Burn DJ ((2011) ) The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord 26: , 2496–2503. |
[32] | Morris ME ((2006) ) Locomotor training in people with Parkinson disease. Phys Ther 86: , 1426–1435. |
[33] | Lim I , van Wegen E , de Goede C , Deutekom M , Nieuwboer A , Willems A , Jones D , Rochester L , Kwakkel G ((2005) ) Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: A systematic review. Clin Rehabil 19: , 695–713. |
[34] | Rochester L , Burn DJ , Woods G , Godwin J , Nieuwboer A ((2009) ) Does auditory rhythmical cueing improve gait in people with Parkinson’s disease and cognitive impairment? A feasibility study. Mov Disord 24: , 839–845. |
[35] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[36] | Hoehn MM , Yahr MD ((1967) ) Parkinsonism: onset, progression and mortality. Neurology 17: , 427–442. |
[37] | Tombaugh TN , McIntyre NJ ((1992) ) The mini-mental state examination: A comprehensive review. J Am Geriatr Soc 40: , 922–935. |
[38] | Nieuwboer A , Rochester L , Herman T , Vandenberghe W , Emil GE , Thomaes T , Giladi N ((2009) ) Reliability of the new freezing of gait questionnaire: Agreement between patients with Parkinson’s disease and their carers. Gait Posture 30: , 459–463. |
[39] | Snijders AH , Haaxma CA , Hagen YJ , Munneke M , Bloem BR ((2012) ) Freezer or non-freezer: Clinical assessment of freezing of gait. Parkinsonism Relat Disord 18: , 149–154. |
[40] | Franchignoni F , Horak F , Godi M , Nardone A , Giordano A ((2010) ) Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J Rehabil Med 42: , 323–331. |
[41] | Capato T , Tornai J , Avila P , Barbosa ER , Piemonte ME ((2015) ) Randomized controlled trial protocol: Balance training with rhythmical cues to improve and maintain balance control in Parkinson’s disease. BMC Neurol 15: , 162. |
[42] | King L , Horak F ((2013) ) On the mini-BESTest: Scoring and the reporting of total scores. Phys Ther 93: , 571–575. |
[43] | Berg KO , Wood-Dauphinee SL , Williams JI , Maki B ((1992) ) Measuring balance in the elderly: Validation of an instrument. Can J Public Health 83 Suppl 2: , S7–11. |
[44] | Fahn S , EltonRL, UPDRS Development Committee ((1987) ) Unified Parkinson’s Disease Rating Scale. In Recent Developments inParkinson’s Disease, FahnS, MarsdenCD, CalneDB, GoldsteinM, eds. Macmillan, Florham Park, NJ, pp. 153–163. |
[45] | Jacobs JV , Horak FB , Van Tran K , Nutt JG ((2006) ) An alternative clinical postural stability test for patients with Parkinson’s disease. J Neurol 253: , 1404–1413. |
[46] | Podsiadlo D , Richardson S ((1991) ) The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39: , 142–148. |
[47] | Yardley L , Beyer N , Hauer K , Kempen G , Piot-Ziegler C , Todd C ((2005) ) Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 34: , 614–619. |
[48] | Chakraborty H , Gu H ((2009) ) A mixed model approach for intent-to-treat analysis in longitudinal clinical trials with missing values. RTI 2009 Press publication No. MR-0009-0903. Research Triangle Park, NC: RTI International. Retrieved from http://www.rti.org/rtipress. |
[49] | Mallinckrodt CH WJ , Molenberghs G , Carroll RJ ((2004) ) Choice of the primary analysis in longitudinal clinical trials. Pharm Stat 3: , 161–169. |
[50] | Lofgren N , Lenholm E , Conradsson D , Stahle A , Franzen E ((2014) ) The Mini-BESTest–a clinically reproducible tool for balance evaluations in mild to moderate Parkinson’s disease? BMC Neurol 14: , 235. |
[51] | Tsang CS , Liao LR , Chung RC , Pang MY ((2013) ) Psychometric properties of the Mini-Balance Evaluation Systems Test (Mini-BESTest) in community-dwelling individuals with chronic stroke. Phys Ther 93: , 1102–1115. |
[52] | Godi M , Franchignoni F , Caligari M , Giordano A , Turcato AM , Nardone A ((2013) ) Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys Ther 93: , 158–167. |
[53] | Conradsson D , Lofgren N , Nero H , Hagstromer M , Stahle A , Lokk J , Franzen E ((2015) ) The effects of highly challenging balance training in elderly with Parkinson’s disease: A randomized controlled trial. Neurorehabil Neural Repair 29: , 827–836. |
[54] | Ni M , Signorile JF , Mooney K , Balachandran A , Potiaumpai M , Luca C , Moore JG , Kuenze CM , Eltoukhy M , Perry AC ((2016) ) Comparative effect of power training and high-speed yoga on motor function in older patients with Parkinson disease. Arch Phys Med Rehabil 97: , 345–354.e315. |
[55] | Duncan RP , Earhart GM ((2012) ) Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair 26: , 132–143. |
[56] | Huang SL , Hsieh CL , Wu RM , Tai CH , Lin CH , Lu WS ((2011) ) Minimal detectable change of the timed “up & go” test and the dynamic gait index in people with Parkinson disease. Phys Ther 91: , 114–121. |
[57] | Schenkman M , Moore CG , Kohrt WM , Hall DA , Delitto A , Comella CL , Josbeno DA , Christiansen CL , Berman BD , Kluger BM , Melanson EL , Jain S , Robichaud JA , Poon C , Corcos DM ((2018) ) Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol 75: , 219–226. |
[58] | Chivers Seymour K , Pickering R , Rochester L , Roberts HC , Ballinger C , Hulbert S , Kunkel D , Marian IR , Fitton C , McIntosh E , Goodwin VA , Nieuwboer A , Lamb SE , Ashburn A ((2019) ) Multicentre, randomised controlled trial of PDSAFE, a physiotherapist-delivered fall prevention programme for people with Parkinson’s. J Neurol Neurosurg Psychiatry 90: , 774–782. |
[59] | Kadivar Z , Corcos DM , Foto J , Hondzinski JM ((2011) ) Effect of step training and rhythmic auditory stimulation on functional performance in Parkinson patients. Neurorehabil Neural Repair 25: , 626–635. |
[60] | Redgrave P , Rodriguez M , Smith Y , Rodriguez-Oroz MC , Lehericy S , Bergman H , Agid Y , DeLong MR , Obeso JA ((2010) ) Goal-directed and habitual control in the basal ganglia: Implications for Parkinson’s disease. Nat Rev Neurosci 11: , 760–772. |
[61] | Capato TTC , Nonnekes J , Barbosa ER , Bloem BR ((2019) ) Internal and external compensation strategies to alleviate upper limb freezing in Parkinson’s disease. Parkinsonism Relat Disord 64: , 335–336. |
[62] | Domingos JM , Godinho C , Dean J , Coelho M , Pinto A , Bloem BR , Ferreira JJ ((2015) ) Cognitive impairment in fall-related studies in Parkinson’s disease. J Parkinsons Dis 5: , 453–469. |
[63] | Bloem BR , Marinus J , Almeida Q , Dibble L , Nieuwboer A , Post B , Ruzicka E , Goetz C , Stebbins G , Martinez-Martin P , Schrag A ((2016) ) Measurement instruments to assess posture, gait, and balance in Parkinson’s disease: Critique and recommendations. Mov Disord 31: , 1342–1355. |
[64] | Capato T. DJ , Almeida L. ((2015) ) Versāo em Português da Diretriz Europeia de Fisioterapia para Doença de Parkinson. 1 Ed. Ominifarma, São Paulo. |




