Levodopa-Carbidopa Intestinal Gel Monotherapy: GLORIA Registry Demographics, Efficacy, and Safety
Abstract
Background:
Continuous delivery of levodopa-carbidopa intestinal gel (LCIG) provides stable plasma levodopa concentrations and reduces motor fluctuations in advanced Parkinson’s disease (PD) patients.
Objective:
To compare the effectiveness and safety of LCIG monotherapy vs polytherapy in patients in the GLORIA registry.
Methods:
This was a post hoc analysis of a 24-month, multinational observational registry where advanced PD patients with persistent motor complications received LCIG (with adjunctive PD treatment, as necessary). Patients were categorized retrospectively into three stable treatment groups: LCIG monotherapy, LCIG in combination with oral levodopa only (“levodopa monotherapy” [including nighttime oral levodopa]), or LCIG in combination with any other antiparkinsonian medication (“LCIG polytherapy”).
Results:
Of 356 patients, 208 were on stable regimens (LCIG monotherapy n = 80; levodopa monotherapy n = 47; LCIG polytherapy n = 81). Baseline characteristics were similar across groups. LCIG monotherapy showed significant improvements until month 18 in activities of daily living and quality of life, and until month 24 for Unified Parkinson’s Disease Rating Scale (UPDRS) motor examination (p < 0.05), “Off” time (p < 0.001), “On” time with dyskinesia (p < 0.01), and non-motor symptoms (p < 0.01). More patients in the levodopa monotherapy and LCIG polytherapy groups experienced treatment-related adverse drug reactions (ADRs) including dyskinesias and serious ADRs than did patients in the LCIG monotherapy group. There were few polyneuropathy-related ADRs, of which one case of polyneuropathy led to discontinuation from the Levodopa monotherapy group.
Conclusions:
These data demonstrate that LCIG monotherapy is an effective treatment option in appropriate advanced PD patients; however, definitive baseline clinical predictors identifying patients who can discontinue concomitant oral therapy have not yet been defined.
INTRODUCTION
Long-term use of oral levodopa, the current standard of treatment for Parkinson’s disease (PD), is associated with motor fluctuations and dyskinesia that result in reduced patient quality of life (QoL) [1]. As PD progresses, levodopa dose and dosing frequency have to be increased and multiple adjunct therapies are needed to control motor complications [2]. An increase in number and frequency of anti-parkinsonian medications and complicated dosing schedules can negatively impact patient adherence, thus further compromising symptomatic control [3, 4].
Continuous administration of levodopa-carbidopa intestinal gel (LCIG) reduces variability in levodopa plasma levels and improves motor complications associated with chronic oral levodopa treatment [2]. Previously published data have demonstrated that LCIG therapy not only reduces “Off” time and dyskinesia, but also improves a patient’s ability to perform activities of daily living (ADL), treat his or her non-motor symptoms [5], and improve QoL [2, 6–8]. The improved efficacy of LCIG infusions over oral levodopa is believed to result from the continuous mode of delivery, although many patients on LCIG therapy in routine clinical practice continue with adjunct oral therapies.
GLORIA (global long-term registry on efficacy and safety of LCIG in patients with advanced PD in routine care) is a large, multicenter, multinational observational registry in patients with advanced PD who are receiving LCIG in routine clinical care with a follow-up period of 2 years [2, 9]. The objective of this post hoc analysis was to compare effectiveness and safety of LCIG monotherapy vs polytherapy in patients with advanced PD who were enrolled in the GLORIA registry.
METHODS
Study design and treatment
This was a 24-month, multinational, non-interventional, observational registry of patients with advanced PD and persistent motor complications who were eligible for LCIG treatment according to European Commission Summary Product Characteristics and national reimbursement criteria. Patients in this study received LCIG treatment at 75 movement disorder centers across 18 countries (Australia, Austria, Belgium, Bulgaria, Czech Republic, Denmark, France, Germany, Greece, Ireland, Italy, Netherlands, Norway, Romania, Slovenia, Spain, Switzerland, and United Kingdom) [2]. Enrollment began in June 2010 and the study was completed in June 2015 [9]. Informed consent was received from patients before enrollment. The study protocol was approved by the health authorities and national and/or local independent ethics committees at each participating institution and country [2].
LCIG (20 mg/mL) was administered via a temporary nasojejunal tube to verify drug efficacy and optimize dose before therapy was continued long term via a percutaneous endoscopic gastrostomy tube with jejunal extension (PEG-J) over 16 hours using a portable pump (CADD-Legacy®, Smiths Medical ASD, Inc., Minneapolis, MN) [2, 9]. Concomitant use of other PD medications was permitted at the discretion of the treating physician [2]. For this analysis, only those patients who were on stable LCIG regimens for the 24-month follow-up were categorized and analyzed in the following three types of treatments: stable LCIG monotherapy vs stable LCIG in combination with oral levodopa only (“levodopa monotherapy”; included nighttime oral levodopa) vs stable LCIG in combination with any other antiparkinsonian medication (LCIG polytherapy).
Efficacy assessments
Efficacy assessments were performed at baseline before LCIG initiation; on day 1 of LCIG treatment via the PEG-J tube (after titration via the nasojejunal tube); and at month 6, 12, 18, and 24 after LCIG initiation. Measurements included the results on mean change from baseline in the Unified Parkinson’s Disease Rating Scale (UPDRS) part II (ADL), part III (motor examination), part IV modified item 39 (total hours of “Off” time), part IV modified item 32 (“On” time with dyskinesia), part IV item 33 (dyskinesia disability), and part IV (dyskinesia pain). UPDRS IV items 39 and 32 were modified by using the rating instructions for the corresponding parts 4.3 and 4.1 of the Movement Disorder Society (MDS)-UPDRS to allow for calculation of actual hours of “Off” time and “On” time with dyskinesia. Non-motor symptoms were assessed using the Non-Motor Symptom Scale (NMSS) total score; QoL was assessed using the patient-reported PD Questionnaire (PDQ)-8 summary index [2].
Safety assessments
For the duration of the study, and for 28 days following a patient’s last reported study visit, adverse drug reactions (ADRs), which included all adverse events that had a reasonable possibility of being causally related to the treatment drug or device as determined by the investigator, were recorded. ADRs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA, version 14.0) [2] and categorized by the study investigator by seriousness; severity (mild, moderate, or severe); and as having an unlikely, possible, or probable relationship to LCIG treatment. Daily levodopa equivalent dose was calculated for the reported administration of LCIG and concomitant oral PD treatment for each study visit, according to published conversion factors [2].
Statistical analysis
All patients who had a baseline efficacy evaluation and received ≥1 dose of LCIG and ≥1 post-baseline safety and efficacy evaluation were included in the primary efficacy analyses (full analysis set N = 329). Safety analyses included all patients who received ≥1 dose of LCIG and had ≥1 post-baseline safety evaluation (N = 356). Descriptive statistics were used to summarize UPDRS II, III, IV, and V, and NMSS data. QoL data were analyzed according the validated standard procedures defined for the PDQ-8 questionnaire [9]. Analysis of variance on matched pairs over time and paired t tests were performed to compare all efficacy outcomes to baseline. Missing data were accounted for using previously described survey methodology [2].
RESULTS
Overall therapy for the duration of the study
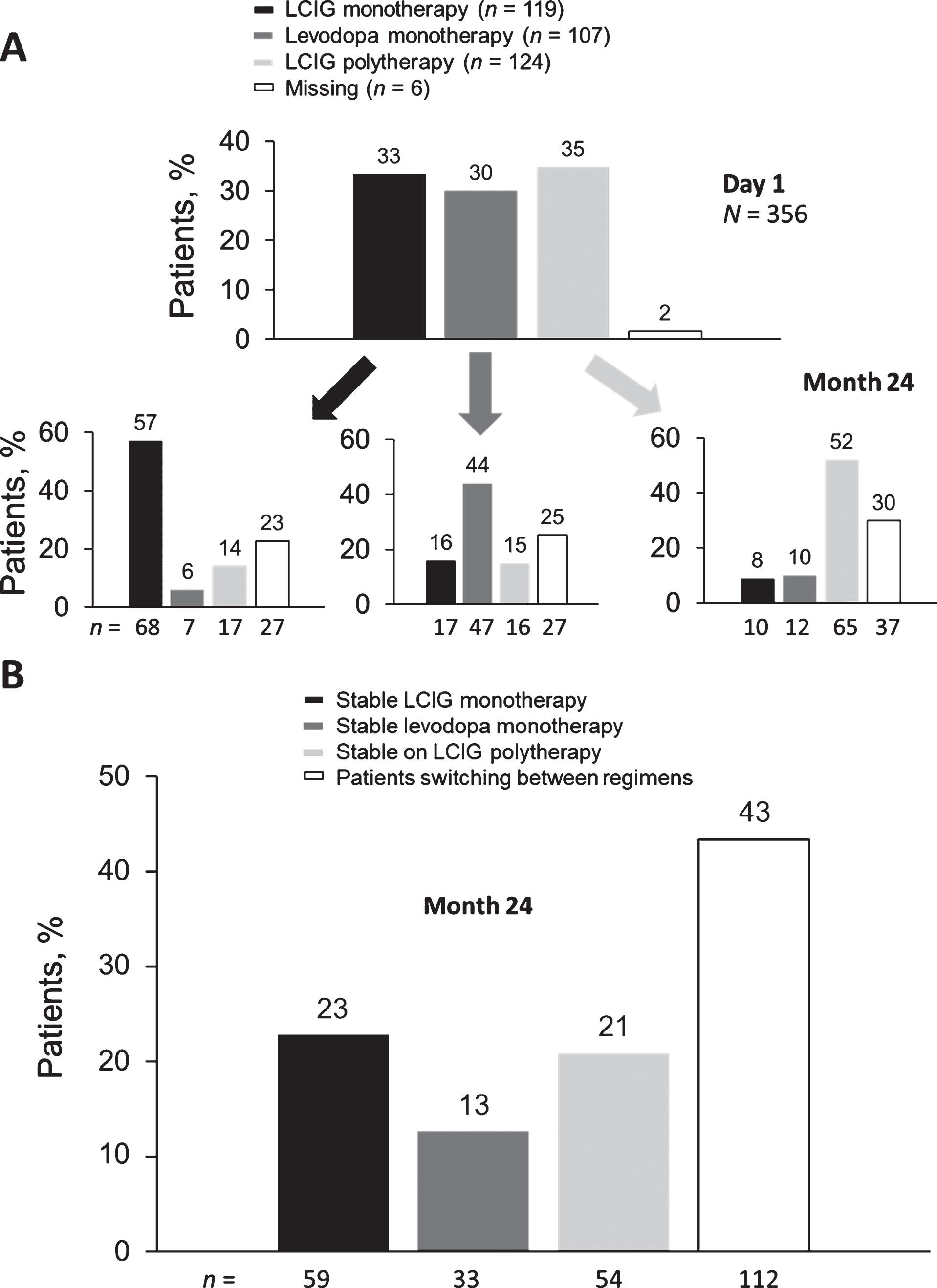
Of the 356 patients in the registry, approximately one-third were in each therapy group on day 1. Patients could have switched between therapy groups between day 1 and month 24. At month 24, of those patients who began treatment in each therapy group, 57% remained on LCIG monotherapy, 44% remained on levodopa monotherapy, and 52% remained on LCIG polytherapy (Fig. 1A). The analyses presented herein are only for the patients that remained stable in their original treatment group throughout the study, without switching regimens at all.
Fig.1
LCIG treatment status on day 1 and at month 24 (A); percentage of patients on stable therapeutic regimens (B). LCIG, levodopa-carbidopa intestinal gel.
Stable treatment groups
A total of 208 patients remained stable in their original therapeutic regimen until their last reported visit (LCIG monotherapy n = 80, levodopa monotherapy n = 47, and LCIG polytherapy n = 81); 148 patients switched treatments. At each single time point, between 36% and 40% of patients were on an LCIG monotherapy. At study completion (24 months of treatment), 59 patients (23%) had been on stable LCIG monotherapy, 33 (13%) had been on stable levodopa monotherapy, and 54 (21%) had been on stable LCIG polytherapy; the remainder of patients either discontinued the study, were lost to follow-up, or switched between treatment regimens (Fig. 1B).
Study discontinuations
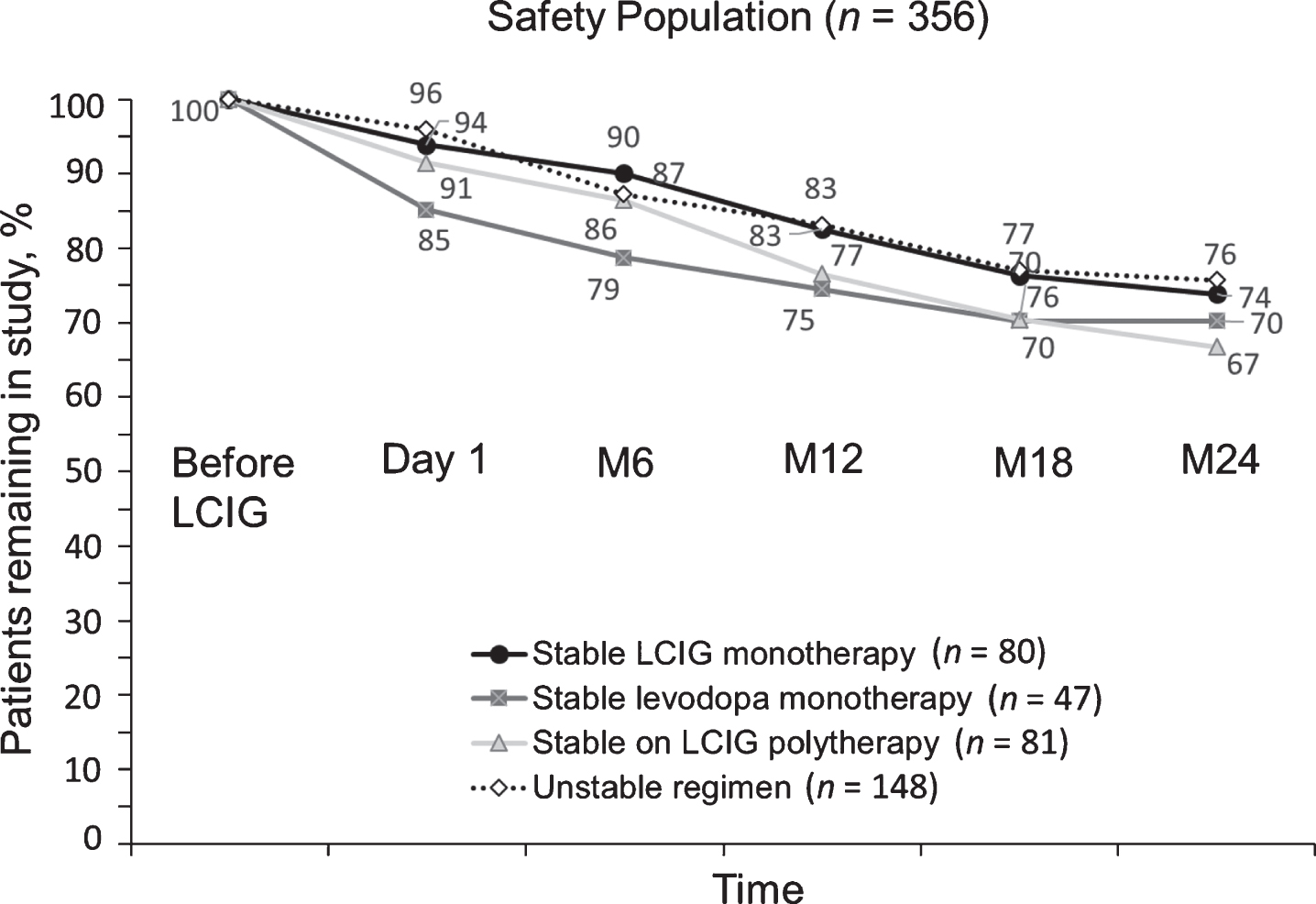
The discontinuation rate among the three stable treatment regimens was similar. The stable LCIG monotherapy group had the lowest numerical drop-out rate (26% at month 24), and the stable LCIG polytherapy group had the highest numerical drop-out rate (33% at month 24). Patients who switched treatment regimens during the study had the lowest overall drop-out rate of 24% at month 24 (Fig. 2).
Fig.2
Study discontinuations based on LCIG monotherapy vs combination therapies. LCIG, levodopa-carbidopa intestinal gel; M, month.
Baseline demographics and characteristics
Baseline data of the three groups of patients who remained stable in their original treatment regimens until last reported visit (n = 208) are summarized in Table 1. Overall, the groups were similar across a range of baseline variables, including UPDRS part II, UPDRS part III, “Off” time, NMSS, and PDQ-8 scores. However, there were a number of imbalances: “On” time with dyskinesia was highest in patients in the LCIG monotherapy group where more patients (71%) were started on LCIG because of uncontrollable dyskinesia compared with patients in the levodopa monotherapy (53%) or LCIG polytherapy (54%) groups; the baseline oral levodopa dose was lowest in the LCIG monotherapy group. More patients in the levodopa monotherapy group had dementia (17% compared with 8% and 7% of patients in the LCIG monotherapy and LCIG polytherapy groups, respectively).
Table 1
Demographics and baseline characteristics by stable therapy group
| LCIG monotherapy (n = 80) | Levodopa monotherapy (n = 47) | LCIG polytherapy* (n = 81) | |
| Mean (SD) age, years | 67.1 | 66.6 | 67.1 |
| Gender | |||
| Male, n (%) | 45 (56) | 28 (60) | 52 (64) |
| Female, n (%) | 35 (44) | 19 (40) | 29 (36) |
| Time since diagnosis, years | 13.8 | 12.9 | 12.9 |
| BMI, mean, kg/m2 | 24.3 | 25.0 | 25.6 |
| Dementia, n (%) | 6 (8) | 8 (17) | 6 (7) |
| Impulse disorder, n (%) | 8 (10) | 5 (11) | 11 (14) |
| Reason for starting LCIG, n (%) | |||
| “Off” periods | 77 (96) | 46 (98) | 76 (94) |
| Uncontrollable dyskinesia | 57 (71) | 25 (53) | 44 (54) |
| Other | 13 (16) | 10 (21) | 10 (12) |
| Last total oral levodopa dose, mg/day | 861.2 | 987.8 | 910.4 |
| Previous treatment, n (%) | |||
| Dopamine agonists | 65 (81) | 36 (77) | 75 (93) |
| COMT inhibitor | 51 (64) | 33 (70) | 59 (73) |
| MAO-B inhibitor | 41 (51) | 19 (40) | 41 (51) |
| Amantadine | 34 (43) | 20 (43) | 36 (44) |
| Apomorphine infusion | 2 (3) | 7 (15) | 10 (12) |
| DBS | 1 (1) | 2 (4) | 3 (4) |
| Baseline PD measurements, mean (SD) | |||
| UPDRS Part II score | 17.4±11.2 | 18.8±9.3 | 15.5±9.1 |
| UPDRS Part III score | 26.7±13.4 | 28.6±12.6 | 23.7±10.5 |
| UPDRS Part IV modified item 39, hours | 6.5±3.5 | 6.7±3.6 | 5.8±2.8 |
| UPDRS Part IV modified item 32, hours | 5.1±3.8 | 3.5±3.3 | 3.7±4.0 |
| NMSS score | 64.1±41.9 | 70.3±45.1 | 70.1±41.7 |
| PDQ-8 summary index | 47.9±19.0 | 47.8±18.1 | 45.8±19.3 |
BMI, body mass index; COMT, catechol-O-methytransferase; DBS, deep brain stimulation; LCIG, levodopa-carbidopa intestinal gel; MAO-B, monoamine oxidase B; NMSS, non-motor symptoms scale; PD, Parkinson’s disease; PDQ-8, Parkinson’s Disease Questionnaire-8; SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale. *LCIG + any other combination therapy.
LCIG dose
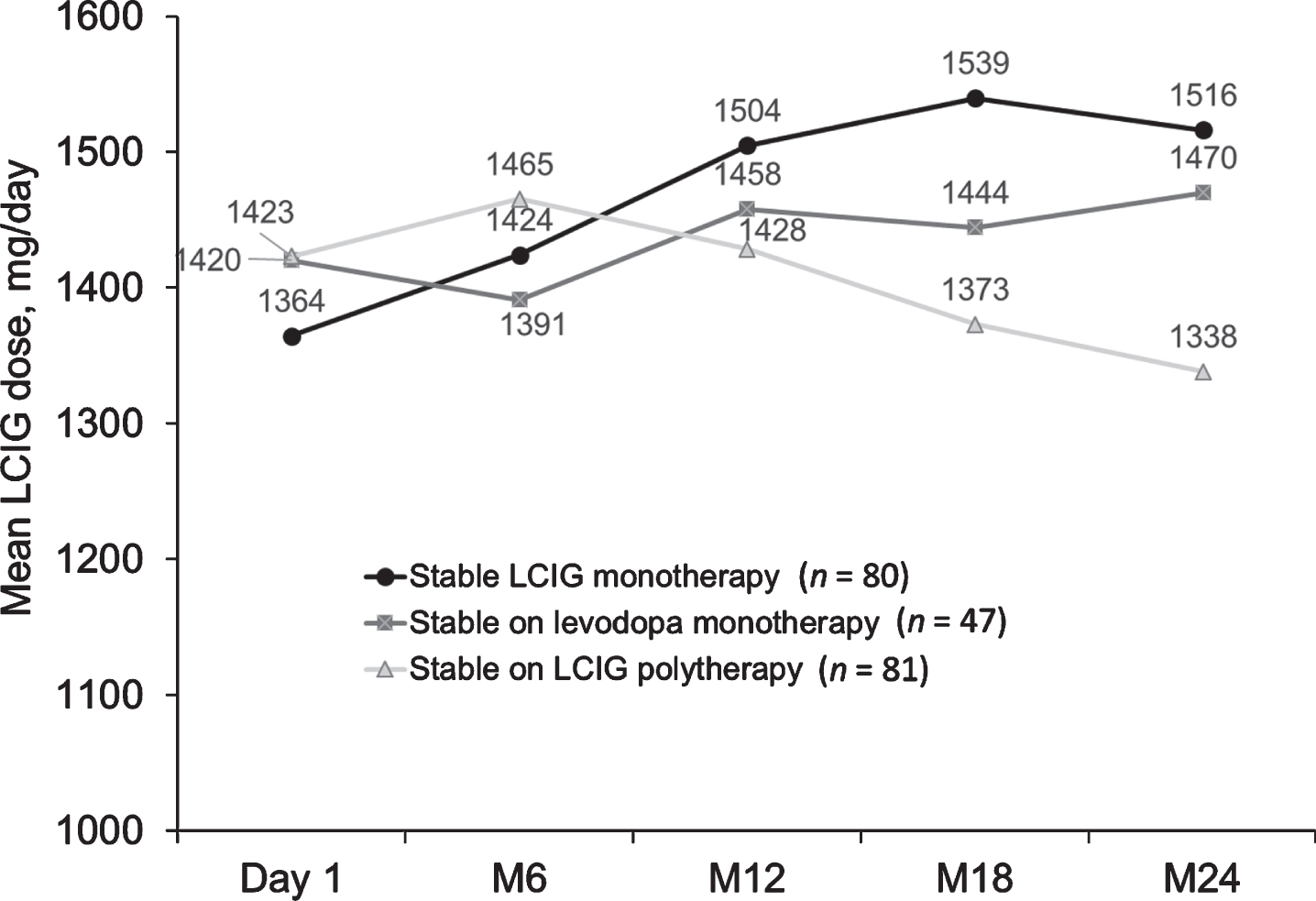
Over 24 months, mean LCIG dose increased from 1364 mg/day at day 1 to 1516 mg/day at month 24 in patients in the LCIG monotherapy group, while it slightly decreased in patients on LCIG polytherapy (1423 mg/day at day 1 to 1338 mg/day at month 24), and remained relatively consistent in patients on levodopa monotherapy (1420 mg/day at day 1 to 1470 mg/day at month 24) (Fig. 3).
Fig.3
Mean LCIG dose in patients on stable monotherapy or polytherapy at regularly scheduled visits. LCIG, levodopa-carbidopa intestinal gel; M, month.
Efficacy in stable treatment groups
UPDRS Part II (activities of daily living)
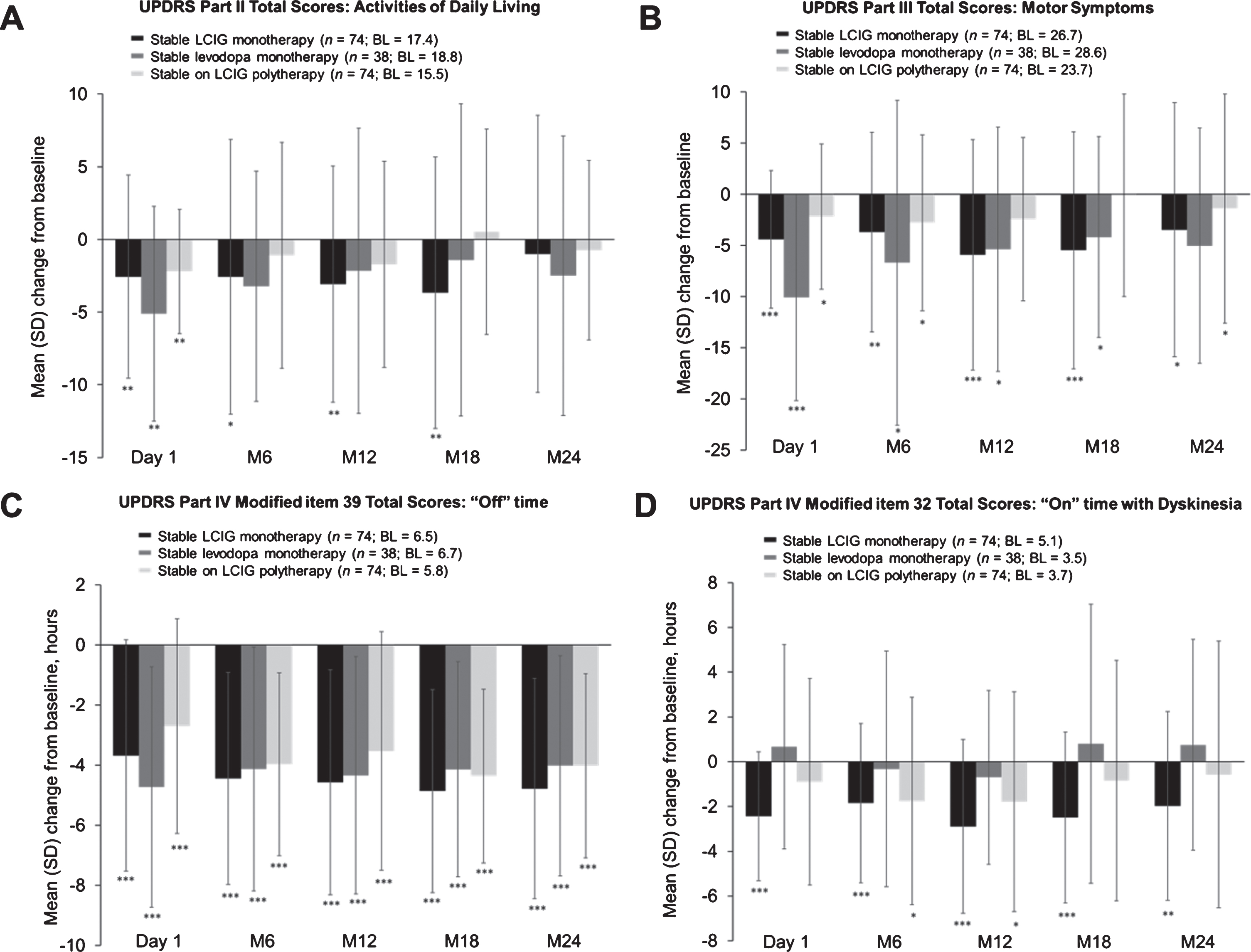
Patients on LCIG monotherapy (Fig. 4A) had significant improvements from baseline in their UPDRS II scores through month 18 (mean change from baseline±standard deviation [SD] –3.7±9.3, p≤0.01), while the other two groups showed significant improvements in ADL only after initiating LCIG, but not at further follow-up.
Fig.4
Mean (SD) change from baseline in scores for activities of daily living (A); motor symptoms (B); “Off” time (C); “On” time with dyskinesia at regularly scheduled visits (D). “Baseline” refers to the assessment at the start of LCIG therapy. Error bars indicate standard deviation. *** p≤0.001; **p≤0.01; *p≤0.05. BL, baseline; LCIG, levodopa-carbidopa intestinal gel; M, month; SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale.
UPDRS Part III (motor symptoms)
Significant improvements in motor symptoms from baseline were observed through month 24 in the patients on LCIG monotherapy (mean change from baseline±SD –5.5±11.6, p≤0.001 at month 18; –3.5±12.4, p≤0.05 at month 24) and through month 18 in the patients on levodopa monotherapy (–4.2±9.8, p≤0.05 at month 18) (Fig. 4B). The patients on LCIG polytherapy had the least improvement in motor symptoms, with significance through month 6 (–2.8±8.6, p≤0.05), and then at month 24 (mean change from baseline±SD –1.4±11.2, p≤0.05).
UPDRS Part IV Modified Item 39 (“Off” time)
All treatment groups demonstrated significant and sustained improvements in “Off” time from baseline through month 24 (p < 0.0001 for all treatment groups and time points) (Fig. 4C).
UPDRS Part IV Modified Item 32 (“On” time with dyskinesia)
Significant and sustained improvements in the patients on LCIG monotherapy were observed in “On” time with dyskinesia from baseline until month 18 (mean change from baseline±SD –2.5±3.8 hours, p≤0.001) and at month 24 (–2.0±4.2 hours, p≤0.01) (Fig. 4D). Significant improvements were seen in the patients on LCIG polytherapy at months 6 and 12 (1.8±4.6 hours, p≤0.05; –1.8±4.9 hours, p≤0.05 respectively), but not thereafter. The improvements in the LCIG monotherapy group were numerically higher compared with the LCIG polytherapy group. No significant improvements from baseline were noted in patients on levodopa monotherapy.
UPDRS Part IV Item 33 (Dyskinesia disability)
Significant and sustained improvements in dyskinesia disability were observed in patients on LCIG monotherapy from baseline through month 24 (mean change from baseline±SD –1.32±1.203, p≤0.001) (Supplementary Figure 1A). Significant improvements were seen in patients on levodopa monotherapy at months 6 (p≤0.001), 12 (p≤0.001), and 24 (p≤0.05), and in patients on LCIG polytherapy at months 6 (p≤0.01) and 12 (p≤0.01).
UPDRS Part IV Item 34 (Dyskinesia pain)
Patients on LCIG monotherapy exhibited significant and sustained improvements in dyskinesia pain from baseline through month 24 (mean change from baseline±SD: –0.89±1.43, p≤0.001) (Supplementary Figure 1B). Patients on levodopa monotherapy or LCIG polytherapy exhibited significant improvements at month 6 (p≤0.01 for both), but not thereafter.
Non-Motor Symptom Scale (non-motor symptoms)
Significant improvements from baseline in non-motor symptoms were observed through month 24 in patients on LCIG monotherapy (mean change from baseline±SD –13.8±31.7, p≤0.01) and patients on LCIG polytherapy (–22.1±40.8, p≤0.01) (Fig. 5A). Patients on levodopa monotherapy showed a significant improvement only at the start of LCIG therapy (–15.5±28.5, p≤0.01), but not thereafter.
Fig.5
Mean (SD) change from baseline in NMS (A); PDQ-8 total scores (B). “Baseline” refers to the assessment at the start of LCIG therapy. Error bars indicate standard deviation. ***p≤0.001; **p≤0.01; *p≤0.05. BL, baseline; LCIG, levodopa-carbidopa intestinal gel; M, month; NMS, non-motor symptoms; PDQ-8, Parkinson’s Disease Questionnaire-8; SD, standard deviation.
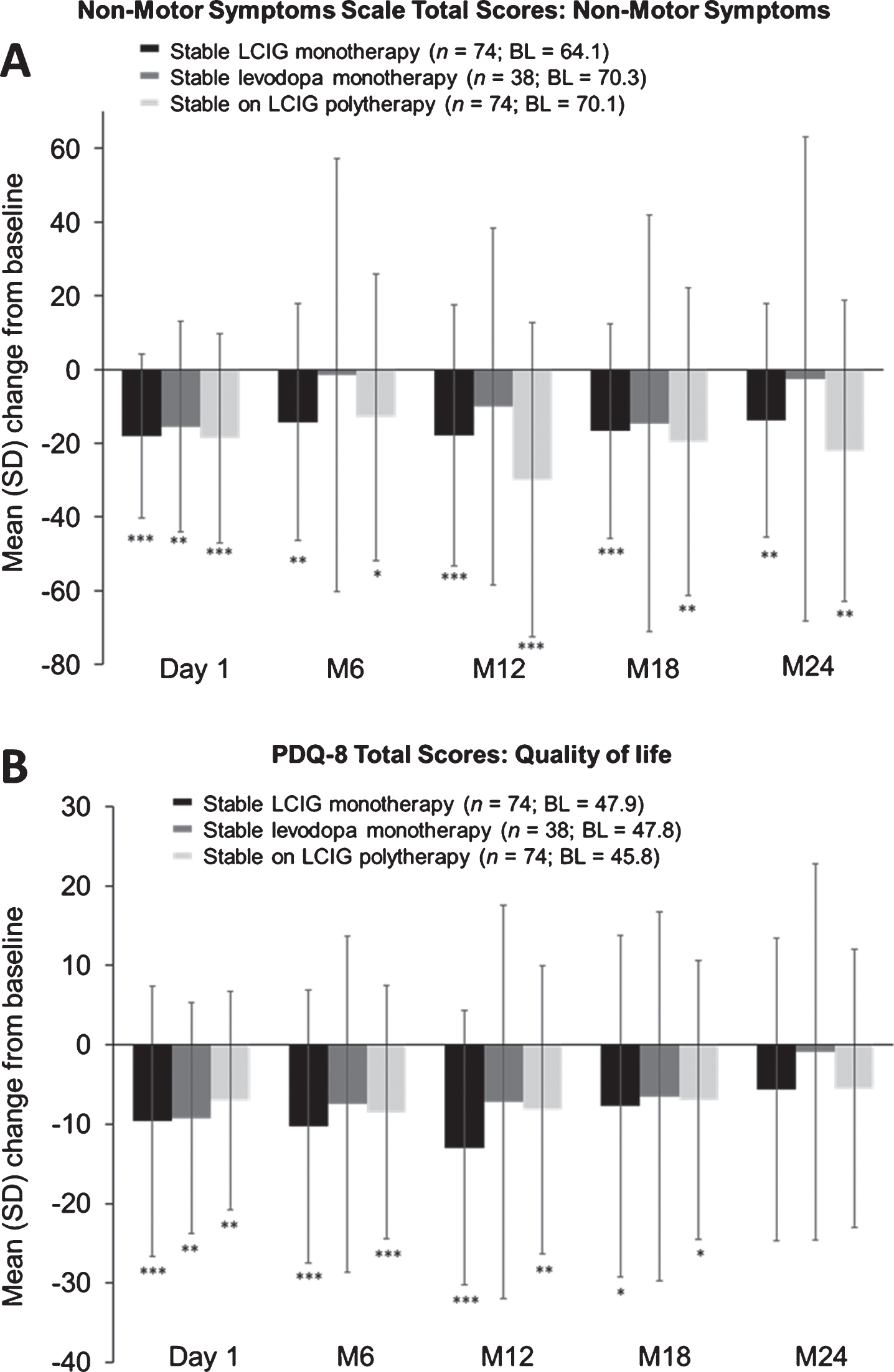
Patient-Reported PD Questionnaire (quality of life)
Patients receiving LCIG monotherapy and LCIG polytherapy demonstrated significant improvements in QoL through month 18 (mean change from baseline±SD –7.7±21.5, p≤0.05; –7.0±17.6, p≤0.05, respectively). Improvements for patients receiving levodopa monotherapy were only significant at day 1 (–9.2±14.5, p≤0.01) and not thereafter (Fig. 5B).
Safety in stable treatment groups
Overall, the safety profile was similar across the three treatment groups. Although fewer patients in the LCIG monotherapy group had serious ADRs than in the other two groups, a similar number of patients in each treatment group discontinued LCIG treatment because of an ADR (Table 2). ADRs leading to discontinuation did not occur in more than one patient in each individual treatment group; across treatment groups, only device dislocation led to discontinuation in more than one patient (n = 2). There were few gastrointestinal disorders in each group, with one case of small bowel perforation and peritonitis in a patient on levodopa monotherapy that led to the patient’s death. The most common non–device-related ADRs were hallucination, abdominal pain, and weight decrease in patients on levodopa monotherapy, and abdominal pain and weight decrease in patients on LCIG polytherapy. The incidence of polyneuropathy-related ADRs (polyneuropathy, peripheral sensory neuropathy, peripheral sensorimotor neuropathy, neuropathy peripheral, paresthesia, and demyelinating polyneuropathy) was ≤5% of patients in each treatment group; only one case of polyneuropathy led to discontinuation. Dyskinesia was reported as an ADR in two patients (4%) in the levodopa monotherapy group and two patients (3%) in the LCIG polytherapy group; dyskinesia was not reported as an ADR in the LCIG monotherapy group.
Table 2
Adverse drug reactions
| Patients, n (%) | |||
| Parameter | LCIG monotherapy (n = 80) | Levodopa monotherapy (n = 47) | LCIG polytherapy* (n = 81) |
| Patients with ≥1 ADR possibly or probably related | 31 (39) | 27 (57) | 40 (49) |
| Patients with ≥1 serious ADR | 19 (24) | 16 (34) | 28 (35) |
| Patients with ≥1 ADR leading to discontinuation | 5 (6) | 4 (9) | 5 (6) |
| Patients with ADRs that led to discontinuation | |||
| Device dislocation | 1 (1) | 1 (2) | — |
| Death due to peritonitis | — | — | 1 (1) |
| Medical device complication | 1 (1) | — | — |
| Cerebral hemorrhage | — | — | 1 (1) |
| Parkinson’s disease | 1 (1) | — | — |
| Polyneuropathy | — | 1 (2) | — |
| Pneumonia | — | — | 1 (1) |
| Postoperative wound infection | 1 (1) | — | — |
| Abnormal behavior | — | 1 (2) | — |
| Delirium | — | 1 (2) | — |
| Acute respiratory failure | — | — | 1 (1) |
| Pneumonia aspiration | 1 (1) | — | — |
| Gallbladder cancer | — | — | 1 (1) |
| Patients with device issues and general disorders | 11 (14) | 15 (32) | 18 (22) |
| Patients with common device issues and general disorders ADRs (occurring in > 5% of patients) | |||
| Device dislocation | 3 (4) | 4 (9) | 6 (7) |
| Device issue | 3 (4) | 2 (4) | 4 (5) |
| Device lead issue | 1 (1) | 4 (9) | 4 (5) |
| Medical device complication | 2 (3) | 4 (9) | 2 (3) |
| Device occlusion | 1 (1) | 1 (2) | 4 (5) |
| Device-related infection | 2 (3) | 4 (9) | 2 (3) |
| Patients with nervous system disorders (e.g., polyneuropathy, dyskinesia) | 19 (24) | 9 (19) | 15 (19) |
| Dyskinesia | — | 2 (4) | 2 (3) |
| Patients with polyneuropathy related ADRs | |||
| Polyneuropathy | 4 (5) | 2 (4) | 4 (5) |
| Peripheral sensory neuropathy | 3 (4) | — | — |
| Peripheral sensorimotor neuropathy | 3 (4) | — | — |
| Neuropathy peripheral | 1 (1) | 1 (2) | 3 (4) |
| Paresthesia | — | 1 (2) | 1 (1) |
| Demyelinating polyneuropathy | 1 (1) | — | — |
| Patients with psychiatric disorders (hallucinations, depression, confused state) | 5 (6) | 10 (21) | 5 (6) |
| Common psychiatric disorders (occurring in > 1 patient) | |||
| Hallucination | 1 (1) | 4 (9) | 2 (3) |
| Psychotic disorder | 1 (1) | — | 1 (1) |
| Delirium | 1 (1) | 1 (2) | 1 (1) |
| Confusional state | — | — | 2 (3) |
| Abnormal behavior | — | 1 (2) | 1 (1) |
| Dopamine dysregulation syndrome | — | 2 (4) | — |
| Jealous delusion | — | 2 (4) | — |
| Patients with gastrointestinal disorders | 1 (1) | 7 (15) | 13 (16) |
| Common gastrointestinal disorders (occurring in > 1 patient) | |||
| Abdominal pain | 1 (1) | 5 (11) | 5 (6) |
| Pneumoperitoneum | 1 (1) | — | 1 (1) |
| Vomiting | — | — | 2 (3) |
| Duodenal ulcer | — | — | 1 (1) |
| Acute abdomen | — | — | 1 (1) |
| Common non–device-related ADRs (occurring in > 5% of patients) | |||
| Hallucination | 1 (1) | 4 (9) | 2 (3) |
| Abdominal pain | 1 (1) | 5 (11) | 5 (6) |
| Weight decrease | 3 (4) | 4 (9) | 6 (7) |
ADR, adverse drug reactions; LCIG, levodopa-carbidopa intestinal gel. *LCIG + any other combination therapy.
DISCUSSION
Polypharmacy and regimen complexity may lead to patient non-adherence and, thus, poorly controlled symptoms [3, 10–13]. There is a need for a simplified regimen for advanced PD that is effective in treating both motor symptoms and non-motor symptoms and improving ADL and QoL, as treating non-motor symptoms are key in enhancing QoL [14].
The use of LCIG monotherapy as an appropriate treatment option has not been previously described in a dedicated analysis in routine clinical care. This post hoc analysis of the GLORIA observational registry, representing the largest cohort of advanced PD patients followed on LCIG in a routine clinical care setting, provides evidence for efficacy of LCIG monotherapy, the regimen closest to providing continuous dopaminergic stimulation in the current analysis—in terms of reducing dyskinesias and improving ADL function. Overall, the baseline characteristics were similar across the three treatment groups for UPDRS II, UPDRS III, “Off” time; NMSS, and PDQ-8. At baseline, the prevalence of uncontrollable dyskinesia was the highest in the patients on LCIG monotherapy; higher LCIG doses in patients on LCIG monotherapy suggests that patients with the narrowest therapeutic window may have been treated preferably with LCIG monotherapy, as this allowed titration by changing LCIG dose as the only parameter. The higher baseline dyskinesia burden and subsequent greater improvement in this patient group reinforces the concept of continuous dopaminergic delivery as a strategy to reduce motor complications, particularly for levodopa-induced dyskinesias. In line with this, the reduction of dyskinesia duration by 2 hours in the LCIG monotherapy group and reductions in dyskinesia-associated disability and pain was achieved despite increasing the total levodopa equivalent dose over time. Further, dyskinesia was rarely reported as an ADR in the registry. These data are consistent with those reported in previous studies showing that continuous levodopa delivery reduces pre-existing dyskinesia, in addition to improving motor fluctuations [2], even though switching from oral PD medications to LCIG is usually associated with an increase in daily levodopa equivalent dose [2].
All treatment groups experienced significant reductions in “Off” time through 24 months of treatment; patients on LCIG monotherapy had a considerable reduction of 5 hours from baseline, which was sustained through month 24, and is consistent with the findings of other open-label studies and randomized controlled trials on LCIG [2]. This magnitude of response is also far above the 1-hour reduction considered clinically relevant [15].
LCIG monotherapy also led to significant improvements in measures of ADL, motor symptoms, “On” time with dyskinesia, dyskinesia-related disability and pain, QoL, and non-motor symptoms. Unlike patients in the other treatment groups, the patients on LCIG monotherapy had sustained significant improvements in ADL until month 18; the LCIG monotherapy group was the only treatment group that showed sustained and significant improvements in motor symptoms at each time point, including month 24. Patients on LCIG monotherapy showed a stable improvement in QoL until month 18, which is consistent with findings in other studies on LCIG [2]. The patients on LCIG polytherapy showed greater improvement in NMSS scores than did the patients on LCIG monotherapy at months 12 through 24, despite similar baseline scores. A possible explanation might be that concomitant medications (e.g., dopamine agonists) in a complex treatment regimen may address specific non-motor symptoms like sleep, mood, and others. Results from previous studies have shown that dopamine agonists improve depression [16].
The patients on LCIG monotherapy experienced the fewest discontinuations, ADRs, and serious ADRs during the study compared with patients on polytherapy. The discontinuation rate in patients on LCIG monotherapy was comparable to that reported in previous studies on patients receiving LCIG monotherapy and polytherapy [8]. The most frequently reported ADRs in patients on LCIG polytherapy were consistent with complications identified and reported in the established safety profile of LCIG [8]. The lowest incidence was seen in the LCIG monotherapy group for gastrointestinal disorders, device issues/general disorders, and psychiatric disorders as compared with the other treatment groups.
LCIG polytherapy exhibited considerable efficacy and safety and remains an appropriate treatment option in some patients. Although levodopa monotherapy led to improvements in “Off” time, it exhibited the least favorable safety/tolerability profile, and may not be considered as a favorable treatment option for most patients.
Study limitations
The information in the GLORIA registry provides important clinical data on the effectiveness and safety of LCIG monotherapy vs polytherapy in patients with advanced PD in a real-world setting. However, this is an observational study without a control group or randomization, which is a limitation when comparing the effectiveness and safety profiles among the treatment groups. The type of information collected in the registry was also limited; for example, details on the type and severity of polyneuropathy cases were not reported. Another limitation is the post hoc nature of this study; further studies are needed to prospectively assess the efficacy of LCIG monotherapy. Because this was an observational study that occurred in a real-world clinical setting, investigators could switch patients between treatment methods as needed for proper control of PD symptoms. As such, only patients with stable regimens through the entire course of the study were included in the analysis, which represents a selection of patients. Separating the stable LCIG monotherapy and levodopa monotherapy groups could also be considered a limitation that resulted in a small group of patients on levodopa monotherapy. This separation may have prevented some of the outcomes from becoming significant; however, there were a large number of patients on LCIG monotherapy, which provides valuable information about this treatment option. Because of the post hoc nature of the analysis and the small group sizes, we were unable to determine if there are any baseline predictors for which type of therapy would be best for patients. As a registry, the goal was to observe the treatment practices, so the protocol did not give any guidance or preferences for monotherapy vs other treatment regimens. Although LCIG monotherapy was not a declared goal of the study, it represents a more simplified treatment regimen that may be appropriate for some patients.
Conclusion
In conclusion, this study demonstrated the effectiveness and safety of LCIG monotherapy in patients in routine clinical care over a long period of time (2 years). Although direct statistical comparisons of the three treatment groups were not performed, LCIG monotherapy showed significant improvements from baseline in all efficacy parameters until month 18 in ADL and in QoL, and until month 24 in motor symptoms, “Off” time, and “On” time with dyskinesia. Although no definitive baseline clinical predictors indicating which patients may benefit from LCIG monotherapy were identified, data from this study suggest that LCIG monotherapy can be considered as an effective treatment option that provides a reduced pill burden, potentially leading to greater compliance, and an acceptable safety profile in patients with advanced PD.
CONFLICTS OF INTEREST
WP was a study investigator and has received compensation from AbbVie, Astra Zeneca, Teva, Novartis, BIAL, Biogen, Britannia, Neuroderm, UCB, Orion Pharma, Takeda, Roche, Zambon, and Merz Pharmaceuticals (for consultancy and lecture fees in relation to clinical drug development programs for Parkinson’s disease) outside the submitted work. He has also received royalties from Thieme, Wiley-Blackwell, and Oxford University Press. AA was a study investigator and has received compensation for consultancy and speaker-related activities from UCB, Boston Scientific, Boehringer Ingelheim, AbbVie, Mundipharma, BIAL, Acadia, Lundbeck and Zambon. PK, LB, and WZR are employees of AbbVie and own company stock or stock options.
Ethical compliance statement
The study protocol was approved by national and/or local independent ethics committees and health authorities at each participating institution and country. All patients provided written informed consent before enrollment in the registry. We have read the journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Data sharing
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. Access is provided to anonymized patient and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports) from AbbVie-sponsored Phase II-IV global interventional clinical trials conducted in patients (completed as of May 2004, for products and indications approved in either the United States or the European Union), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
Access to this clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
ACKNOWLEDGMENTS
This study was funded by AbbVie, Inc. AbbVie participated in the study design, research, data collection, analysis, and interpretation of data, and writing, reviewing, and approving this manuscript for publication. Medical writing assistance (funded by AbbVie) was provided by Bhawana Bariar, MS, PhD, and Kelly M Cameron, PhD, ISMPP Certified Medical Publication Professional™ of JB Ashtin, who, on behalf of the authors, assisted in writing the first draft and implemented author revisions throughout the editorial process.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JPD-191605.
REFERENCES
[1] | Hauser RA ((2009) ) Levodopa: Past, present, and future. Eur Neurol 62: , 1–8. |
[2] | Antonini A , Poewe W , Chaudhuri KR , Jech R , Pickut B , Pirtosek Z , Szasz J , Valldeoriola F , Winkler C , Bergmann L , Yegin A , Onuk K , Barch D , Odin P , GLORIA study co-investigators ((2017) ) Levodopa-carbidopa intestinal gel in advanced Parkinson’s: Final results of the GLORIA registry. Parkinsonism Relat Disord 45: , 13–20. |
[3] | Fleisher JE , Stern MB ((2013) ) Medication nonadherence in Parkinson’s disease. Curr Neurol Neurosci Rep 13: , 382. |
[4] | Davis KL , Edin HM , Allen JK ((2010) ) Prevalence and cost of medication nonadherence in Parkinson’s disease: Evidence from administrative claims data. Mov Disord 25: , 474–480. |
[5] | Standaert DG , Rodriguez RL , Slevin JT , Lobatz M , Eaton S , Chatamra K , Facheris MF , Hall C , Sail K , Jalundhwala YJ , Benesh J ((2017) ) Effect of Levodopa-carbidopa intestinal gel on non-motor symptoms in patients with advanced Parkinson’s disease. Mov Disord Clin Pract 4: , 829–837. |
[6] | Olanow CW , Kieburtz K , Odin P , Espay AJ , Standaert DG , Fernandez HH , Vanagunas A , Othman AA , Widnell KL , Robieson WZ , Pritchett Y , Chatamra K , Benesh J , Lenz RA , Antonini A , Group LHS ((2014) ) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13: , 141–149. |
[7] | Slevin JT , Fernandez HH , Zadikoff C , Hall C , Eaton S , Dubow J , Chatamra K , Benesh J ((2015) ) Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: An open-label extension of the double-blind pivotal study in advanced Parkinson’s disease patients. J Parkinsons Dis 5: , 165–174. |
[8] | Fernandez HH , Standaert DG , Hauser RA , Lang AE , Fung VS , Klostermann F , Lew MF , Odin P , Steiger M , Yakupov EZ , Chouinard S , Suchowersky O , Dubow J , Hall CM , Chatamra K , Robieson WZ , Benesh JA , Espay AJ ((2015) ) Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: Final 12-month, open-label results. Mov Disord 30: , 500–509. |
[9] | Antonini A , Yegin A , Preda C , Bergmann L , Poewe W , Gs investigators, coordinators ((2015) ) Global long-term study on motor and non-motor symptoms and safety of levodopa-carbidopa intestinal gel in routine care of advanced Parkinson’s disease patients; 12-month interim outcomes. Parkinsonism Relat Disord 21: , 231–235. |
[10] | Daley DJ , Myint PK , Gray RJ , Deane KHO ((2014) ) Interventions for improving medication adherence in patients with idiopathic Parkinson’s disease. Cochrane Database Syst Rev, Issue 8. Art. No.: CD011191. |
[11] | Grosset KA , Bone I , Grosset DG ((2005) ) Suboptimal medication adherence in Parkinson’s disease. Mov Disord 20: , 1502–1507. |
[12] | Daley DJ , Myint PK , Gray RJ , Deane KH ((2012) ) Systematic review on factors associated with medication non-adherence in Parkinson’s disease. Parkinsonism Relat Disord 18: , 1053–1061. |
[13] | Malek N , Grosset DG ((2015) ) Medication adherence in patients with Parkinson’s disease. CNS Drugs 29: , 47–53. |
[14] | Chaudhuri KR ((2011) ) Parkinson’s disease and quality of life – a clinician’s perspective. Eur Neurol Rev 6: , 9–12. |
[15] | Hauser RA , Auinger P , Parkinson Study Group ((2011) ) Determination of minimal clinically important change in early and advanced Parkinson’s disease. Mov Disord 26: , 813–818. |
[16] | Antonini A , Barone P , Ceravolo R , Fabbrini G , Tinazzi M , Abbruzzese G ((2010) ) Role of pramipexole in the management of Parkinson’s disease. CNS Drugs 24: , 829–841. |



