Fatigue in Parkinson’s Disease Associates with Lower Ambulatory Diastolic Blood Pressure
Abstract
Background/Objective:
Fatigue is a common debilitating symptom in Parkinson’s disease (PD) of unclear etiology. Hypotension and blood pressure variability are common in PD though their relationship to other non-motor symptoms is less well understood.
Methods:
We conducted a cross-sectional study to explore differences in 24-hour ambulatory blood pressure measurements in PD subjects (n = 35) with and without fatigue. Subjects underwent hourly systolic (SBP) and diastolic (DBP) blood pressure testing in their home environment. The presence of fatigue was assessed using the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part 1. We compared blood pressure measurements in fatigued vs. non-fatigued PD subjects, assessed over 4 epochs: overnight, morning, midday, and evening.
Results:
PD subjects with symptoms of fatigue demonstrated lower mean DBP, compared to those without fatigue (67.8±4.8 mmHg vs. 75.6±9.4 t = 2.57, p = 0.014). These intergroup differences were most notable in the morning. The two groups did not differ in scoring on the Survey of Autonomic Symptoms or on an office-based blood assessment of SBP or DBP performed on the day of 24-hour monitor initiation.
Conclusions:
Fatigue in PD may be a clinical manifestation of low-grade systemic hypotension.
INTRODUCTION
Fatigue is a disabling non-motor symptom in Parkinson’s disease (PD)— distinct from daytime sleepiness, cognitive, or mood-related symptoms— that affects about 50% of all individuals with PD [1] and associates with worse health related quality of life [2]. The majority of people with PD consider fatigue, defined as diminished energy levels or increased perception of effort that is disproportionate to attempted activities [3], to be one of their three biggest symptomatic concerns [4]. Despite its high prevalence and disabling nature, we know relatively little about the underlying causes of fatigue in PD or about how to treat it clinically. Conventional PD medications including levodopa along with other newer approaches including stimulant medications, cognitive enhancers, caffeine, and exercise are largely unhelpful for improving PD fatigue [5]. The absence of any pharmacological or non-pharmacological treatment for fatigue [6] is a knowledge gap with clinical implications.
Although the exact pathophysiology of PD fatigue is unknown, different studies have suggested that striatal dopaminergic pathology, neuroinflammatory markers, regional serotoninergic dysfunction, and/or autonomic impairments may each be implicated in fatigue pathogenesis [3, 7, 8]. In PD, vascular autonomic dysfunction is usually measured by either an assessment of orthostatic vital signs or detailed autonomic research testing typically performed no more than once per day in an ambulatory care setting. Because of the once-daily nature of these types of assessments, inherent measurement imprecision and the presence of unmeasured confounding factors that vary by day may each influence hemodynamic outcome assessments. In addition, we know little about the clinical significance of normal diurnal hemodynamic variability in PD. This may be of particular relevance for PD fatigue which is known to have a diurnal component with substantial inter-subject variability [3, 9]. To better understand these topics, we explored differences between PD subjects with and without symptoms of fatigue using 24-hour ambulatory blood pressure assessments. We hypothesized that PD subjects with fatigue would show discrete time periods of relative hypotension compared to subjects without fatigue.
METHODS
We conducted a cross-sectional study of 35 subjects with idiopathic PD without dementia. All subjects were recruited from Neurology Movement Disorders clinics at the Veterans Affairs Ann Arbor Health System (VAAAHS), MI, USA. All subjects were men given the gender makeup of this clinical populace. All subjects met United Kingdom Brain Bank Clinical Diagnostic Criteria for the diagnosis of PD [10], were age ≥50 years, and had Hoehn and Yahr stage ≤3. All subjects provided written informed consent prior to study procedures.
Subjects underwent the Movement Disorder Society Unified Parkinson’s Disease Rating scale (MDS-UPDRS) [11] motor examination in the off-state after holding dopaminergic medications overnight. All other elements of testing, including blood pressure evaluations, were performed after subjects were instructed to take their usual dose of dopaminergic medications. The presence of fatigue symptoms was defined categorically by a score of ≥1 on the MDS-UPDRS part 1 non-motor aspects of experiences of daily living question 1.13. The 30-item Geriatric Depression Scale (GDS) was used to measure symptoms of depression [12], the Hamilton Anxiety Rating Scale (HAM-A) was used to measure symptoms of anxiety [13], the Survey of Autonomic Symptoms was used to measure symptoms of dysautonomia [14], and the Epworth Sleepiness Scale was used to measure symptoms of daytime sleepiness [15]— on each of these instruments, higher scores correspond with a greater burden of symptoms. The Montreal Cognitive Assessment was used to measure cognitive impairment [16].
All subjects underwent conventional systolic and diastolic blood pressure testing in the seated and standing positions with at least a 3-minute interval before blood pressure was checked following postural adjustment. Subjects wore the 24-hour Meditech ABPM-04 ambulatory blood pressure monitoring system, which collected systolic (SBP) and diastolic (DBP) blood pressure readings every hour for the next 24 hours. To explore associations between fatigue with different diurnal blood pressure epochs, we grouped mean blood pressure readings into 4 temporal categories: morning (600–1000), midday (1200–1600), evening (18000–2200), and overnight (2400–400). Each of these epochs consisted of the mean of available hourly blood pressure readings for subjects over these 5-hour time periods. Any missing readings or machine errors were omitted from averages or numerical analyses. Intergroup differences were compared using two-sample t-tests. All analyses were conducted using STATA 15 (College Station, TX).
RESULTS
Differences between PD subjects with and without symptoms of fatigue are shown in Table 1. In general, (+) fatigue subjects showed higher GDS and HAM-A scores (suggesting more depressive & anxiety symptoms respectively) and lower Epworth Sleepiness Scale scores (suggesting less symptoms of sleepiness). There were no differences between groups in the Survey of Autonomic Symptoms. Subjects acknowledging fatigue symptoms showed a non-significant trend towards higher proportional use of medications for treatment of depression or anxiety (χ2 = 2.92, p = 0.087) and no difference in the use of antihypertensive medications (χ2 = 1.46, p = 0.227). There were no significant differences between (+) fatigue vs. (–) fatigue subjects in systolic or diastolic blood pressures when measured at baseline in seated (SBP: 137.4±7.3 vs. 138.6±3.6; t = 0.17, p = 0.867; DBP: 77.6±2.55 vs.80.1±2.23; t = 0.67, p = 0.509) or standing (SBP: 122.2±9.7 vs. 133.8±4.4; t = 1.26, p = 0.216; DBP: 73.3±4.3 vs. 81.5±2.5; t = 1.75, p = 0.089) though there was a non-significant trend towards lower standing DBP measurements in (+) fatigue subjects.
Table 1
Cohort Characteristics
| (–) Fatigue n = 24; Mean (SD) | (+) Fatigue n = 11; Mean (SD) | Statistical test, p-value | |
| Age | 68.4 (6.6) | 71.2 (3.8) | t = – 1.31, p = 0.198 |
| Disease Duration | 5.5 (4.5) | 3.5 (3.8) | t = 1.33, p = 0.191 |
| MDS-UPDRS Motor Score | 33.3 (10.0) | 34.9 (16.2) | t = – 0.36, p = 0.719 |
| Levodopa dose Equivalents | 912.1 (439.3) | 669.9 (330.9) | t = 1.62, p = 0.114 |
| Montreal Cognitive Assessment | 25.1 (3.1) | 24.3 (3.6) | t = 0.72, p = 0.475 |
| Geriatric Depression Scale | 5.0 (3.5) | 9.9 (5.6) | t = – 3.21, p = 0.003 |
| Hamilton Anxiety Rating Scale | 6.8 (4.1) | 12.9 (7.0) | t = 3.29, p = 0.002 |
| Survey of Autonomic Symptoms | 10.2 (5.2) | 13.7 (10.0) | t = – 1.38, p = 0.177 |
| Epworth Sleepiness Scale | 8.9 (3.6) | 5.5 (4.1) | t = 2.49, p = 0.018 |
(+) Fatigue subjects showed a non-significant trend towards a greater degree of orthostatic decline in systolic (15.2±15.1 vs. 4.8±14.3; t = 1.96, p = 0.059) and diastolic (4.4±10.2 vs. – 1.3±7.1; t = 1.91, p = 0.065) blood pressure. Mean blood pressure values over the duration of 24-hour monitor testing showed lower DBP in (+) fatigue subjects (67.8±4.8 mmHg vs.75.6±9.4 t = 2.57, p = 0.014) and a non-significant trend towards lower SBP as well (124.2±13.5 vs. 132.5±12.0; t = 1.82, p = 0.078). PD subjects with fatigue showed numerically lower SBP and DBP readings through nearly every hour of the 24-hour period. Intergroup differences were most profound in the morning epoch (Table 2, Fig. 1) where lower SBP and DBP associated with the presence of fatigue symptoms and in the evening epoch where DBP was lower in (+) fatigue subjects as well. The degree of nocturnal dipping was estimated by the ratio of BP during the overnight period divided by BP during the evening period. No differences in this nocturnal dipping ratio were seen in between (+) fatigue and (–) fatigue subjects in SBP (dipping ratio: 0.92±0.13 vs. 0.89±0.13; t = 0.68, p = 0.50) or DBP (dipping ratio: 0.87±0.12 vs. 0.86±0.13; t = 0.19, p = 0.85). PD subjects with fatigue were not more likely to report symptoms of lightheadedness on standing (score of ≥1) on MDS-UPDRS item 1.12 (χ2= 0.14, p = 0.71).
Fig. 1
Mean 24-hour systolic (top) and diastolic (bottom) in PD with and without fatigue.
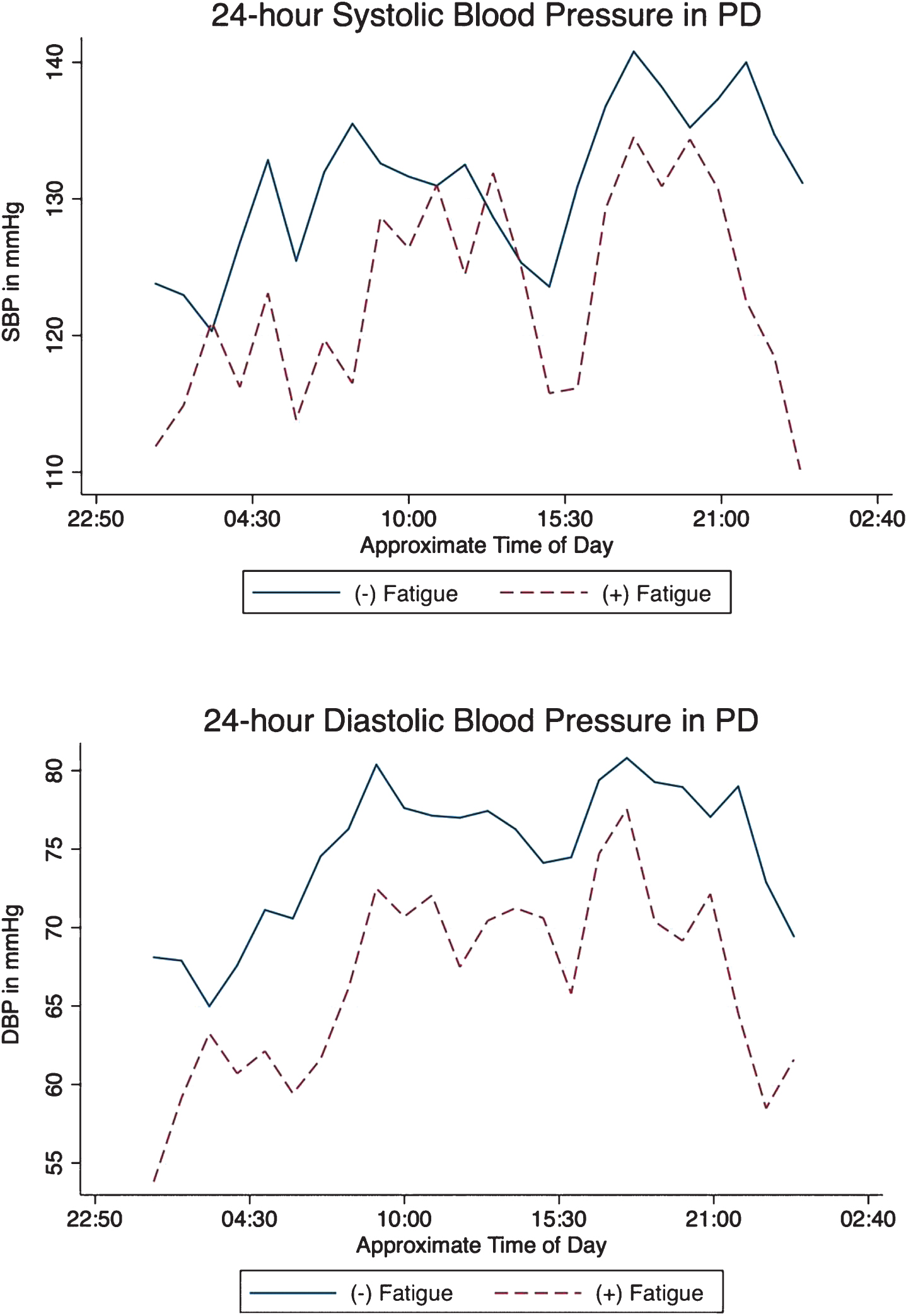
Table 2
Intergroup comparisons of blood pressure epochs
| Mean Systolic Blood Pressure (SD) | ||||
| Morning (600–1000) | Midday (1200–1600) | Evening (1800–2200) | Overnight (MN-400) | |
| (–) Fatigue | 131.4 (12.1) | 128.6 (13.4) | 138.7 (16.4) | 127.4 (20.9) |
| (+) Fatigue | 119.2 (18.8) | 123.4 (18.0) | 130.3 (14.5) | 115.8 (20.5) |
| t-test, p-value | t = 2.28, p = 0.029 | t = 0.95, p = 0.348 | t = 1.45, p = 0.157 | t = 1.53, p = 0.135 |
| Mean Diastolic Blood Pressure (SD) | ||||
| Morning | Midday | Evening | Overnight | |
| (–) Fatigue | 75.8 (10.7) | 76.0 (11.5) | 79.4 (10.9) | 68.8 (13.0) |
| (+) Fatigue | 65.5 (8.6) | 69.6 (8.5) | 71.2 (8.3) | 60.4 (7.2) |
| t-test, p-value | t = 2.79, p = 0.009 | t = 1.64, p = 0.110 | t = 2.23, p = 0.033 | t = 1.99, p = 0.055 |
DISCUSSION
These cross-sectional data suggest that symptoms of fatigue in PD may relate to differences in autonomic vascular tone as assessed by 24-hour blood pressure monitoring. Our data show the greatest difference in blood pressure between fatigued and non-fatigued subjects in the mornings. We also report that diastolic blood pressure measurements better distinguish (+) vs. (–) fatigued PD subjects than systolic blood pressure. Given that there is no accepted treatment strategy for PD fatigue and that low-grade hypotension may be modifiable through pharmacological or non-pharmacological interventions, these findings have implications for the clinical management of fatigue in PD.
Although the exact pathogenesis of fatigue in PD is not well understood, several previous studies have suggested associations with different facets of autonomic dysfunction. Previous PD survey-based studies have shown a correlation between fatigue severity and the self-reported severity of autonomic symptoms [17], including the frequency and severity of symptomatic orthostatic hypotension [18]. PD (+)-fatigue compared to PD (–)-fatigue subjects have been reported to have a greater degree of sympathetic denervation on cardiac 123I-metaiodobenzylguanidine (MIBG) testing and a corresponding higher degree of vascular pressor response to sympathomimetic including dobutamine and norepinephrine [19]. Our findings expand on these data by highlighting sustained differences in normal range vascular tone between fatigued and non-fatigued subjects throughout the day. We reported differences between groups in 24-hour DBP but not SBP. In the context of aging, SBP elevations are thought to more closely reflect arterial stiffness and associated impaired vascular compliance [20]–one reason that SBP is commonly used as a primary outcome measure in antihypertensive drug trials. The variable presence of arteriosclerosis may make systolic hypotension a less reliable indicator of peripheral autonomic dysfunction in PD. Interestingly, pyridostigmine— a cholinesterase inhibitor thought to affect peripheral autonomic ganglia by augmenting nicotinic cholinergic neurotransmission— increased standing diastolic, but not systolic, blood pressure in clinical trial of subjects with neurogenic orthostatic hypotension [21]. Although these findings point at the autonomic system as a potential causal source of dysfunction for PD-fatigue, they do not shed light on why some subjects may be more likely to develop fatigue than others. It is possible that variable involvement of central or peripheral autonomic circuits causes the development of PD fatigue [22, 23]. Alternatively, PD fatigue may be a symptom that simply clusters with a more aggressive subtype of an alpha-synucleinopathy [24]. It is worth noting that (+) fatigue subjects in our cohort reported greater GDS and HAM-A scores but lower symptom burden on self-reported measures of daytime sleepiness and degree of dopaminergic medication use. Subclinical hypotension and orthostatic intolerance associate with depression and anxiety symptoms in non-PD cohorts [25]. In PD studies, orthostatic hypotension, but not relative differences in ambulatory blood pressure, have been correlated with depression symptoms as well [26]. Some investigators have also recently suggested that the GDS in PD may be a better measure of apathy, anxiety, and fatigue than of conventional depressive symptoms [27]. This cluster of symptoms may reflect a common central or peripheral nervous system pathology in PD. It is worth noting that the blood pressure values seen in this study generally fall within a normal (i.e., non-pathological) range and that (+) and (-) fatigue subjects did not show differences in the prevalence of symptomatic orthostatic lightheadedness. These findings raise the possibility that fatigue may be a manifestation of low-grade or “subclinical” autonomic dysfunction, below the threshold needed to cause symptomatic lightheadedness.
Previous studies of 24-hour blood pressure monitoring in PD suggest that relatively lower blood pressure values are expected in the overnight and morning time periods [28–30]. This is believed to represent a normal physiological response dependent on physical activity, regulation of sodium homeostasis, and autonomic function [31]. The clinical significance of these nocturnal and early morning blood pressure declines is less clear, given the likelihood of early autonomic neuropathology in many PD subjects [32]. We did not find a correlation between degree of nocturnal dipping and the presence of fatigue symptoms. It is possible that autonomic dysfunction giving rise to elevations in nocturnal blood pressure may be caused by different mechanisms than those underlying the presence of fatigue. Temporally speaking, it is possible that fatigue symptoms themselves do not wax and wane throughout the day with hypotension but rather are a correlate of a tendency towards low vascular tone in general— our findings may simply be measuring this latter possibility more precisely by collecting 24 different measurements in a single day. It is important to note that our dataset does not include recurrent fatigue symptom assessments needed to correlate diurnal variations in fatigue with similar temporal variations in relative hypotension. Even so, our data are comprised of hourly measurements and in this way, provide a uniquely precise estimate of the burden of ambulatory hypotension within a 24-hour period. There is a growing appreciation that conventional outpatient PD research assessments may not fully capture the true nature of PD motor and non-motor features compared to analogous assessments using new wearable technologies [33, 34] including ambulatory blood pressure monitors.
The localization of fatigue in PD is likely to be complex. It is possible that fatigue may be the end result of a neurovascular system unable to compensate for autonomic changes affecting any number of end-organ targets. Alternatively, it is possible that fatigue is a direct correlate of downstream fluctuations in cerebral blood flow [35]. Both seem like reasonable possibilities given that fatigue also associates with lower ambulatory blood pressures in chronic fatigue syndrome [36] and with autonomic neurovascular dysfunction in multiple sclerosis [37, 38].
Limitations of our study include the relatively small sample size of our cohort and our categorical assessment of the presence of fatigue symptoms. A limitation of our analysis was the use of the single fatigue item on MDS-UPDRS to categorize subjects with and without fatigue symptoms. A disadvantage of this approach is its lack of granularity for correlative analyses. On the other hand, it is not known to what degree self-reported fatigue symptom scales progress linearly and/or are affected by floor and ceiling effects. Future studies aiming to better understand the natural history of PD fatigue should collect detailed self-reported fatigue assessments at different times per day to test the correlation between fatigue and fluctuations in levels of relative hypotension. Fatigue is a nuanced symptom-complex. It may reflect contributions from central & peripheral nervous system pathologies and may also be modulated by factors relevant to physiological and psychosocial reserve. It is possible that differences between fatigued and non-fatigued subjects in this cohort are influenced by other unmeasured variables including inter-groups differences in sleep symptoms, diet, medications, or basal physical activity levels. Understanding the mechanisms that underlie PD fatigue is an important step toward designing rationale therapeutic approaches. Future studies collecting data of diurnal fatigue and blood pressure fluctuations would also benefit from mechanistic biomarker assessments such as MIBG cardiac sympathetic imaging as well as cerebral perfusion imaging— both of which might provide the rationale for target-engagement PD-fatigue clinical trials. An increasing body of evidence implicating autonomic dysfunction as the etiology of PD fatigue has the potential to move the field forward, particularly as blood pressure is an easily assessable biomarker for future target modification trials aiming to improve fatigue in ambulatory settings.
AUTHOR CONTRIBUTIONS
VK, AS, RLA, and NIB designed the study, VK & AS acquired and analyzed the data. VK drafted the manuscript, which was reviewed and revised by all coauthors.
DISCLOSURES
Dr. Kotagal receives funding from the VA Health Systems (IK2CX0011 86 and AAVA GRECC) and serves as an associate editor for Movement Disorders.
Ms. Szpara has no relevant disclosures.
Dr. Albin serves on the editorial boards of Annals of Neurology, Neurology, Movement Disorders, and Neurobiology of Disease. He receives grant support from the NIH and MJFF. Dr. Albin serves on the data safety and monitoring boards of the PASSPORT (Biogen), TANGO (Biogen), and HTTRX (IONIS) trials.
Dr. Bohnen has research support from the NIH, the Michael J. Fox Foundation (MJFF) and the Department of Veteran Affairs.
ACKNOWLEDGMENTS
We thank all study participants who generously donated their time and effort.
This work was funded by the Department of Veterans Affairs (grant IK2CX001186) as well as NINDS (grant P50NS091856).
REFERENCES
[1] | Friedman JH , Brown RG , Comella C , Garber CE , Krupp LB , Lou JS , Marsh L , Nail L , Shulman L , Taylor CB ((2007) ) Fatigue in Parkinson’s disease: A review. Mov Disord 22: , 297–308. |
[2] | Herlofson K , Larsen JP ((2003) ) The influence of fatigue on health-related quality of life in patients with Parkinson’s disease. Acta Neurol Scand 107: , 1–6. |
[3] | Kluger BM , Herlofson K , Chou KL , Lou JS , Goetz CG , Lang AE , Weintraub D , Friedman J ((2016) ) Parkinson’s disease-related fatigue: A case definition and recommendations for clinical research. Mov Disord 31: , 625–631. |
[4] | Friedman J , Friedman H ((1993) ) Fatigue in Parkinson’s disease. Neurology 43: , 2016–2018. |
[5] | Elbers RG , Verhoef J , van Wegen EE , Berendse HW , Kwakkel G ((2015) ) Interventions for fatigue in Parkinson’s disease. Cochrane Database Syst Rev, CD010925. |
[6] | Elbers RG , Berendse HW , Kwakkel G ((2016) ) Treatment of fatigue in Parkinson disease. JAMA 315: , 2340–2341. |
[7] | Lindqvist D , Kaufman E , Brundin L , Hall S , Surova Y , Hansson O ((2012) ) Non-motor symptoms in patients with Parkinson’s disease - correlations with inflammatory cytokines in serum. PLoS One 7: , e47387. |
[8] | Pavese N , Metta V , Bose SK , Chaudhuri KR , Brooks DJ ((2010) ) Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain 133: , 3434–3443. |
[9] | van Hilten JJ , Hoogland G , van der Velde EA , Middelkoop HA , Kerkhof GA , Roos RA ((1993) ) Diurnal effects of motor activity and fatigue in Parkinson’s disease. J Neurol Neurosurg Psychiatry 56: , 874–877. |
[10] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[11] | Goetz CG , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stebbins GT , Stern MB , Tilley BC , Dodel R , Dubois B , Holloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , LeWitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , Van Hilten JJ , LaPelle N ((2007) ) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord 22: , 41–47. |
[12] | Williams JR , Hirsch ES , Anderson K , Bush AL , Goldstein SR , Grill S , Lehmann S , Little JT , Margolis RL , Palanci J , Pontone G , Weiss H , Rabins P , Marsh L ((2012) ) A comparison of nine scales to detect depression in Parkinson disease: Which scale to use? Neurology 78: , 998–1006. |
[13] | Kummer A , Cardoso F , Teixeira AL ((2010) ) Generalized anxiety disorder and the Hamilton Anxiety Rating Scale in Parkinson’s disease. Arq Neuropsiquiatr 68: , 495–501. |
[14] | Zilliox L , Peltier AC , Wren PA , Anderson A , Smith AG , Singleton JR , Feldman EL , Alexander NB , Russell JW ((2011) ) Assessing autonomic dysfunction in early diabetic neuropathy: The Survey of Autonomic Symptoms. Neurology 76: , 1099–1105. |
[15] | Hogl B , Arnulf I , Comella C , Ferreira J , Iranzo A , Tilley B , Trenkwalder C , Poewe W , Rascol O , Sampaio C , Stebbins GT , Schrag A , Goetz CG ((2010) ) Scales to assess sleep impairment in Parkinson’s disease: Critique and recommendations. Mov Disord 25: , 2704–2716. |
[16] | Skorvanek M , Goldman JG , Jahanshahi M , Marras C , Rektorova I , Schmand B , van Duijn E , Goetz CG , Weintraub D , Stebbins GT , Martinez-Martin P ((2018) ) Global scales for cognitive screening in Parkinson’s disease: Critique and recommendations. Mov Disord 33: , 208–218. |
[17] | Chou KL , Gilman S , Bohnen NI ((2017) ) Association between autonomic dysfunction and fatigue in Parkinson disease. J Neurol Sci 377: , 190–192. |
[18] | Claassen DO , Adler CH , Hewitt LA , Gibbons C ((2018) ) Characterization of the symptoms of neurogenic orthostatic hypotension and their impact from a survey of patients and caregivers. BMC Neurol 18: , 125. |
[19] | Nakamura T , Hirayama M , Hara T , Hama T , Watanabe H , Sobue G ((2011) ) Does cardiovascular autonomic dysfunction contribute to fatigue in Parkinson’s disease? Mov Disord 26: , 1869–1874. |
[20] | Osher E , Stern N ((2008) ) Diastolic pressure in type 2 diabetes: Can target systolic pressure be reached without “diastolic hypotension”? Diabetes Care 31: Suppl 2, S249–254. |
[21] | Singer W , Sandroni P , Opfer-Gehrking TL , Suarez GA , Klein CM , Hines S , O’Brien PC , Slezak J , Low PA ((2006) ) Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol 63: , 513–518. |
[22] | Pyatigorskaya N , Mongin M , Valabregue R , Yahia-Cherif L , Ewenczyk C , Poupon C , Debellemaniere E , Vidailhet M , Arnulf I , Lehericy S ((2016) ) Medulla oblongata damage and cardiac autonomic dysfunction in Parkinson disease. Neurology 87: , 2540–2545. |
[23] | Leclair-Visonneau L , Magy L , Volteau C , Clairembault T , Le Dily S , Preterre C , Peyre A , Damier P , Neunlist M , Pereon Y , Derkinderen P ((2018) ) Heterogeneous pattern of autonomic dysfunction in Parkinson’s disease. J Neurol 265: , 933–941. |
[24] | Zuo LJ , Yu SY , Wang F , Hu Y , Piao YS , Du Y , Lian TH , Wang RD , Yu QJ , Wang YJ , Wang XM , Chan P , Chen SD , Wang Y , Zhang W ((2016) ) Parkinson’s disease with fatigue: Clinical characteristics and potential mechanisms relevant to alpha-synuclein oligomer. J Clin Neurol 12: , 172–180. |
[25] | Perlmuter LC , Sarda G , Casavant V , Mosnaim AD ((2013) ) A review of the etiology, associated comorbidities, and treatment of orthostatic hypotension. Am J Ther 20: , 279–291. |
[26] | Park HE , Kim JS , Oh YS , Park IS , Park JW , Song IU , Lee KS ((2016) ) Autonomic nervous system dysfunction in patients with Parkinson disease having depression. J Geriatr Psychiatry Neurol 29: , 11–17. |
[27] | Lopez FV , Split M , Filoteo JV , Litvan I , Moore RC , Pirogovsky-Turk E , Liu L , Lessig S , Schiehser DM ((2018) ) Does the Geriatric Depression Scale measure depression in Parkinson’s disease? Int J Geriatr Psychiatry 33: , 1662–1670. |
[28] | Stuebner E , Vichayanrat E , Low DA , Mathias CJ , Isenmann S , Haensch CA ((2013) ) Twenty-four hour non-invasive ambulatory blood pressure and heart rate monitoring in Parkinson’s disease. Front Neurol 4: , 49. |
[29] | Plaschke M , Trenkwalder P , Dahlheim H , Lechner C , Trenkwalder C ((1998) ) Twenty-four-hour blood pressure profile and blood pressure responses to head-up tilt tests in Parkinson’s disease and multiple system atrophy. J Hypertens 16: , 1433–1441. |
[30] | Oka H , Nakahara A , Umehara T ((2018) ) Rotigotine improves abnormal circadian rhythm of blood pressure in Parkinson’s disease. Eur Neurol 79: , 281–286. |
[31] | Agarwal R ((2010) ) Regulation of circadian blood pressure: From mice to astronauts. Curr Opin Nephrol Hypertens 19: , 51–58. |
[32] | Palma JA , Kaufmann H ((2014) ) Autonomic disorders predicting Parkinson’s disease. Parkinsonism Relat Disord 20: Suppl 1, S94–98. |
[33] | Del Din S , Godfrey A , Mazza C , Lord S , Rochester L ((2016) ) Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov Disord 31: , 1293–1313. |
[34] | Maetzler W , Domingos J , Srulijes K , Ferreira JJ , Bloem BR ((2013) ) Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov Disord 28: , 1628–1637. |
[35] | Abe K , Takanashi M , Yanagihara T ((2000) ) Fatigue in patients with Parkinson’s disease. Behav Neurol 12: , 103–106. |
[36] | Newton JL , Sheth A , Shin J , Pairman J , Wilton K , Burt JA , Jones DE ((2009) ) Lower ambulatory blood pressure in chronic fatigue syndrome. Psychosom Med 71: , 361–365. |
[37] | Flachenecker P , Rufer A , Bihler I , Hippel C , Reiners K , Toyka KV , Kesselring J ((2003) ) Fatigue in MS is related to sympathetic vasomotor dysfunction. Neurology 61: , 851–853. |
[38] | Krbot Skoric M , Crnosija L , Adamec I , Barun B , Gabelic T , Smoljo T , Stanic I , Pavicic T , Pavlovic I , Drulovic J , Pekmezovic T , Habek M ((2018) ) Autonomic symptom burden is an independent contributor to multiple sclerosis related fatigue. Clin Auton Res, doi: 10.1007/s10286-018-0563-6 |




